Synthesis, Photoswitching Behavior and Nonlinear Optical Properties of Substituted Tribenzo[a,d,g]coronene
(This article belongs to the Section Materials Chemistry)
Abstract
1. Introduction
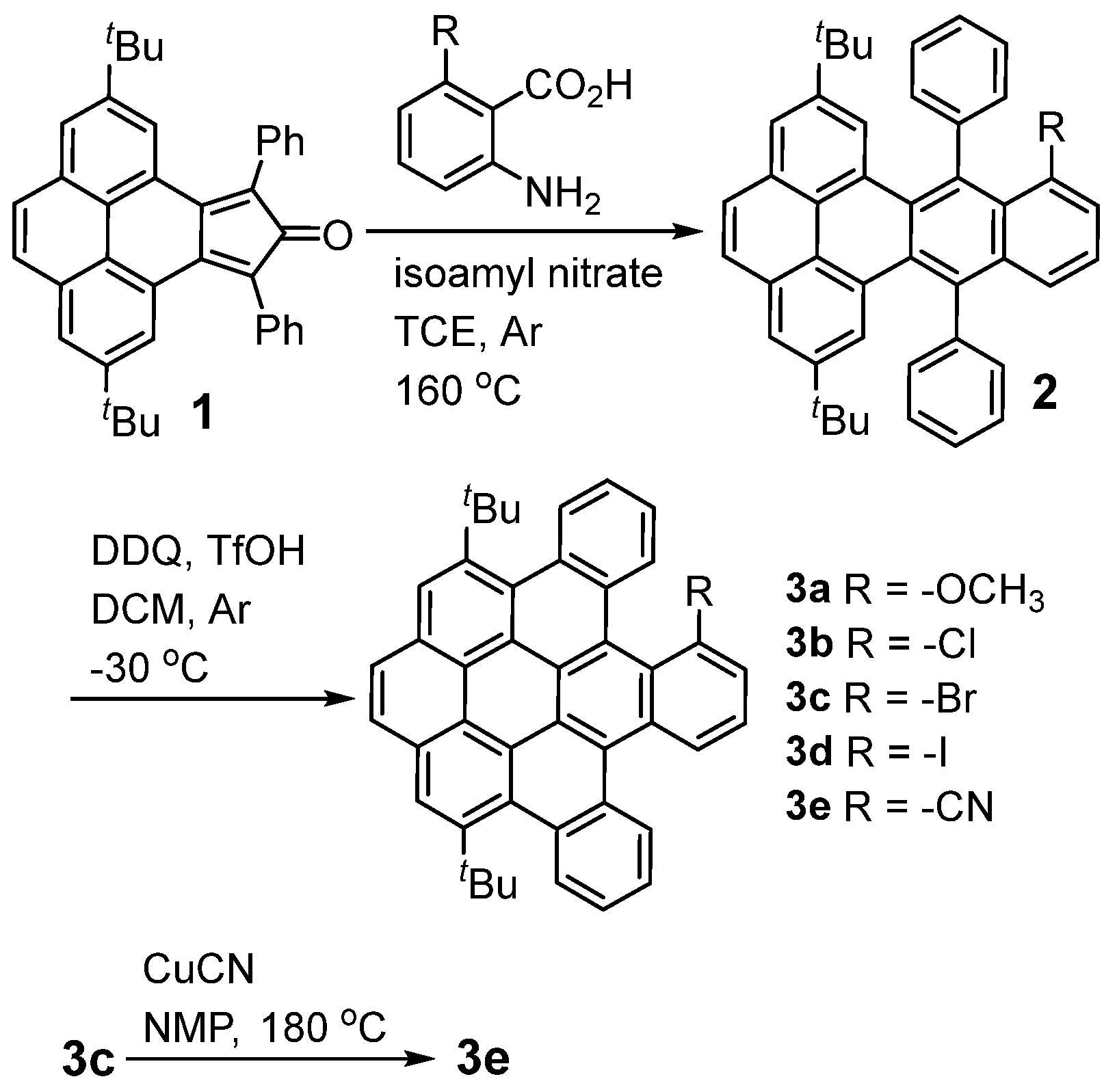
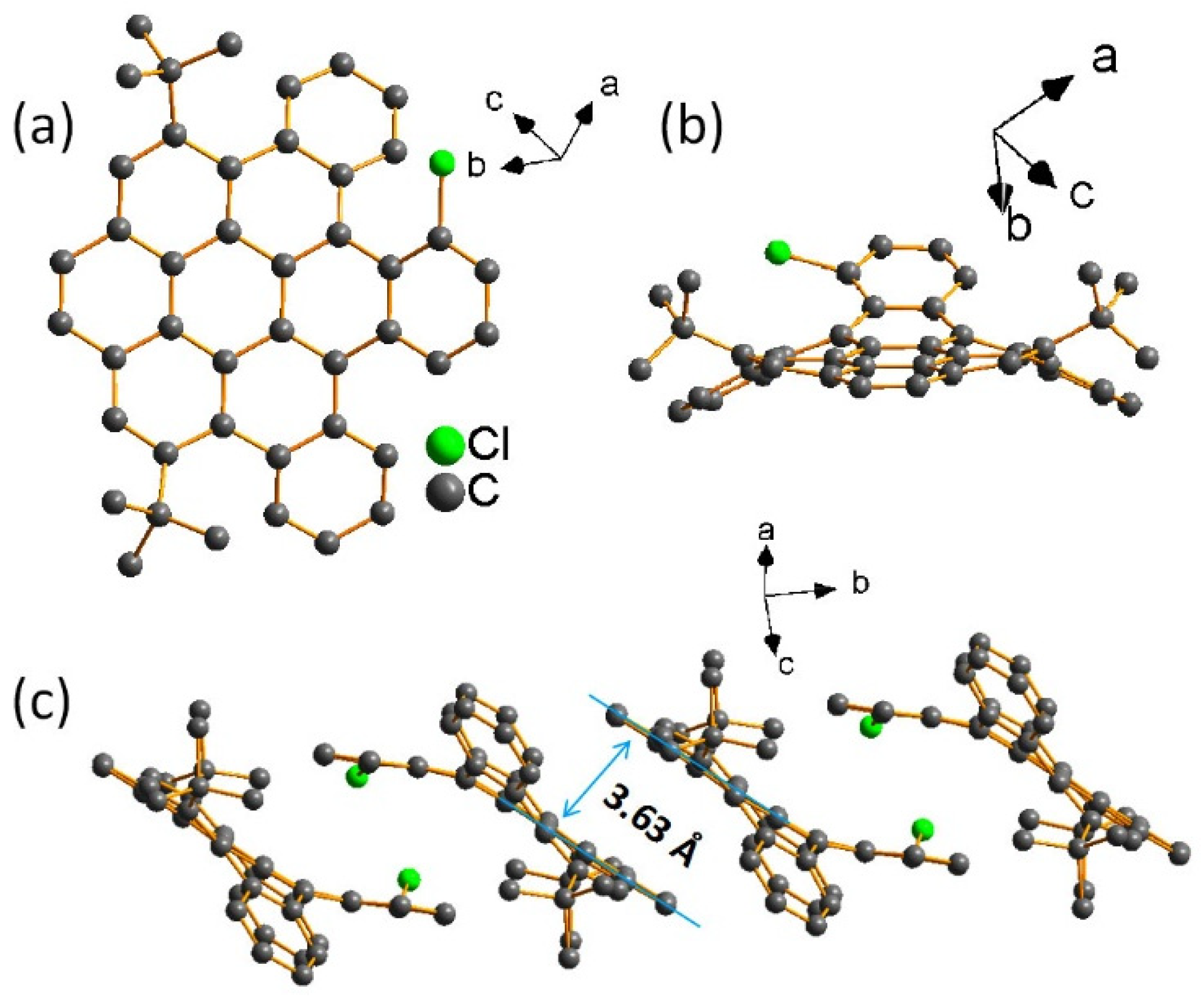
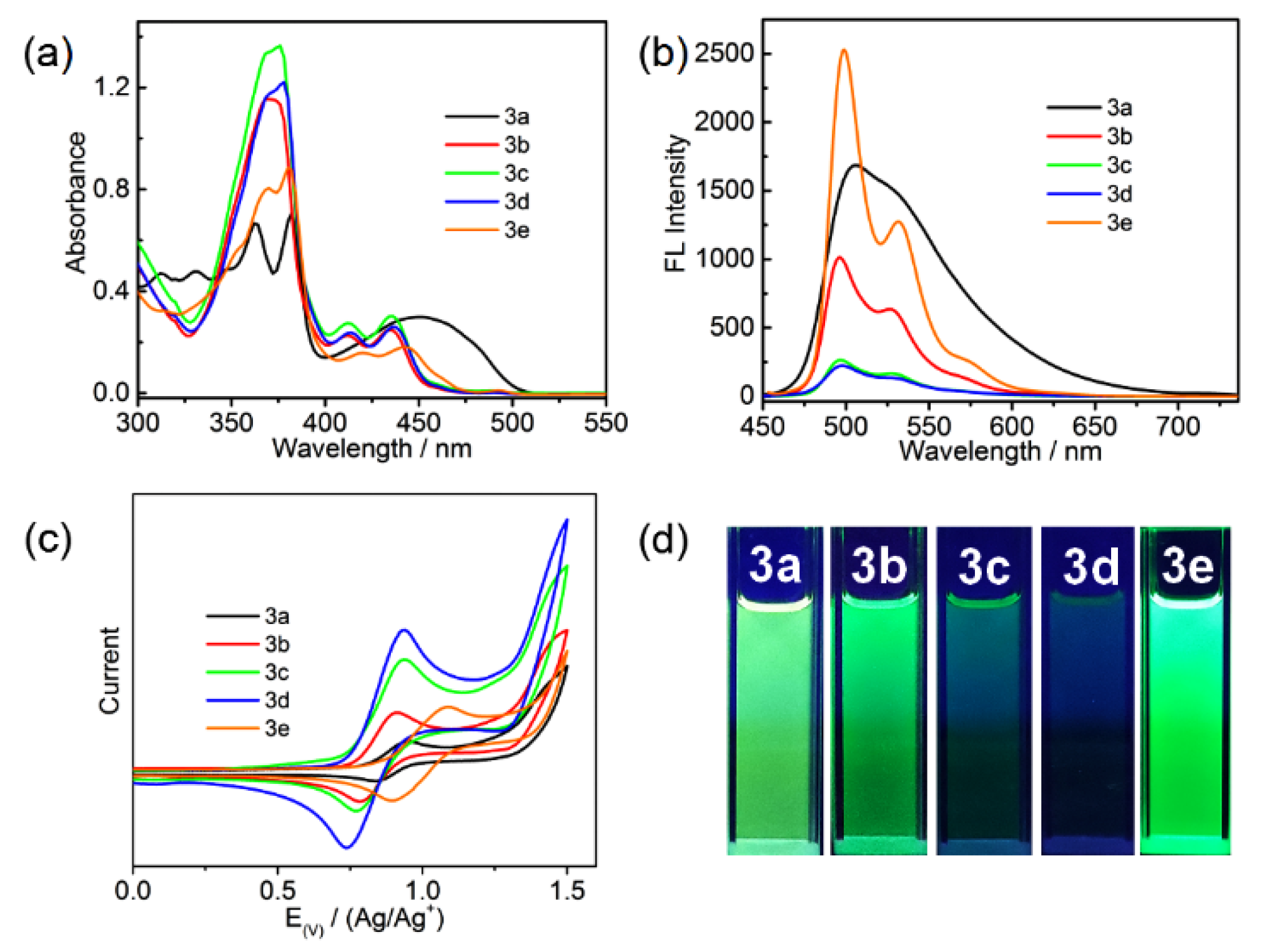
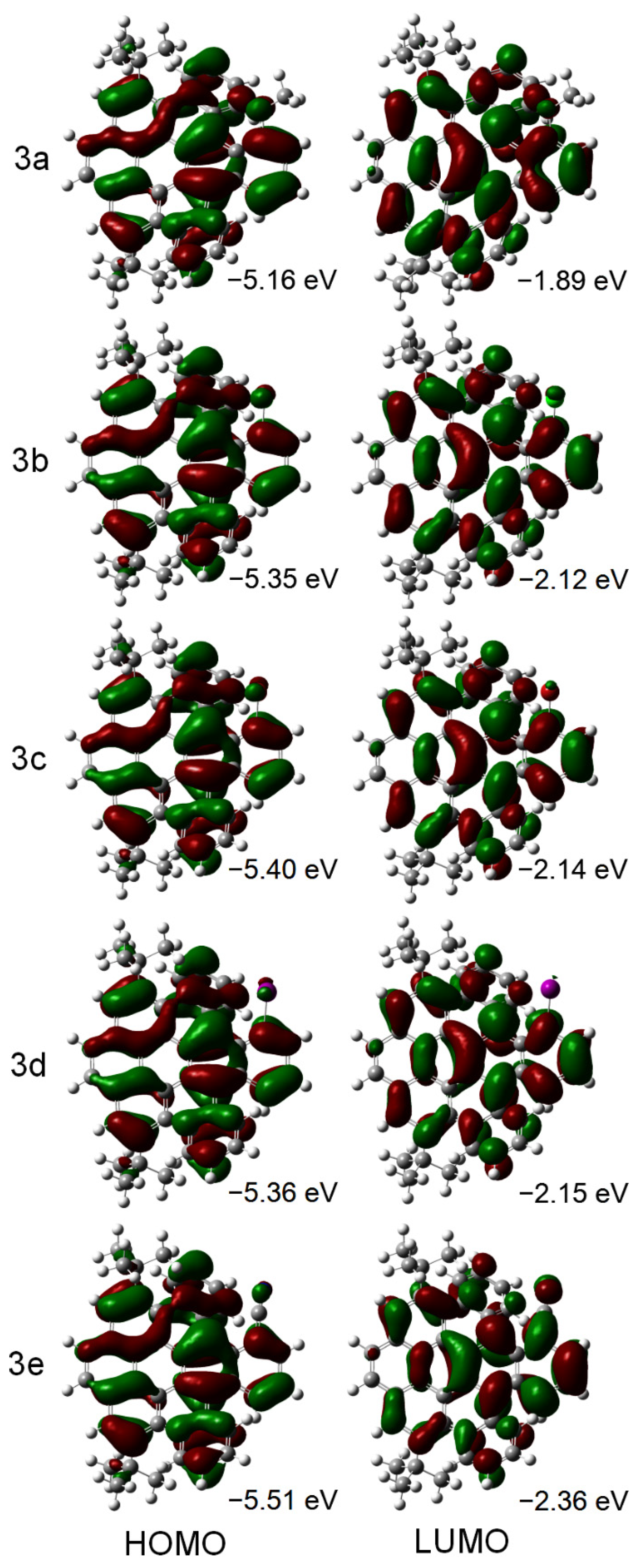
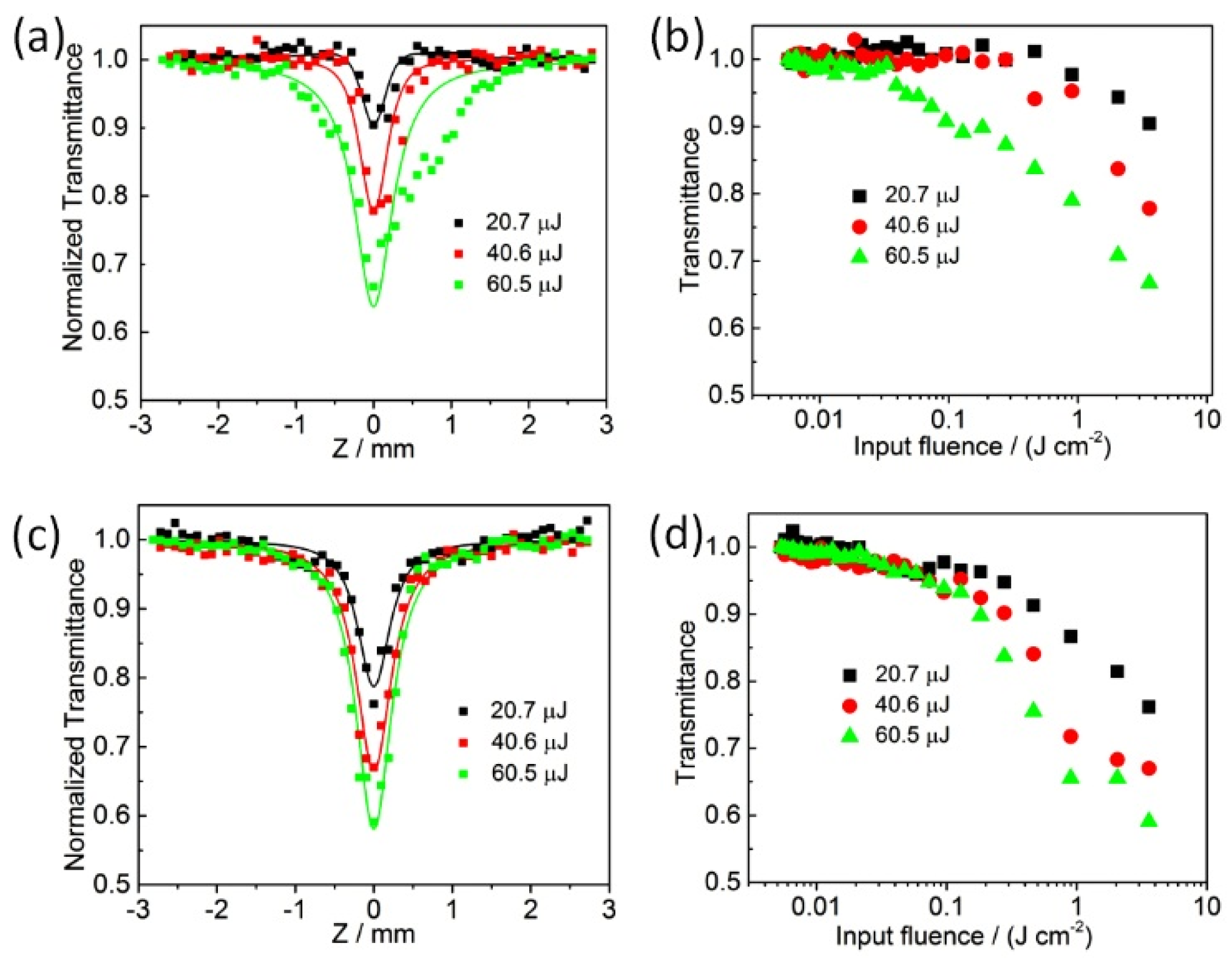
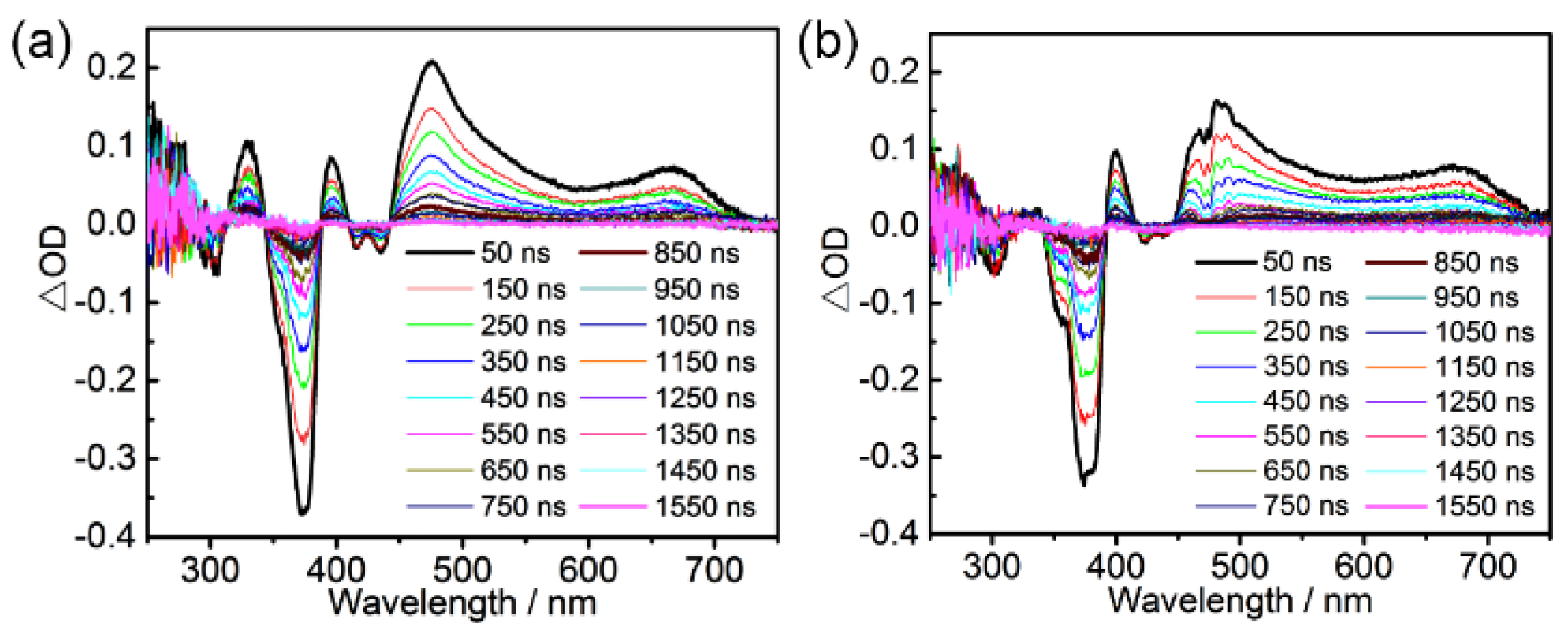
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of 3a
3.2. Synthesis of 2b
3.3. Synthesis of 3b
3.4. Synthesis of 2d
3.5. Synthesis of 3d
3.6. Synthesis of 3e
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Narita, A.; Wang, X.; Feng, X.; Müllen, K. New advances in nanographene chemistry. Chem. Soc. Rev. 2015, 44, 6616. [Google Scholar] [CrossRef] [PubMed]
- Senese, A.D.; Chalifoux, W.A. Nanographene and Graphene Nanoribbon Synthesis via Alkyne Benzannulations. Molecules 2019, 24, 118. [Google Scholar] [CrossRef] [PubMed]
- Kurpanik, A.; Matussek, M.; Lodowski, P.; Szafraniec-Gorol, G.; Krompiec, M.; Krompiec, S. Diels–Alder Cycloaddition to the Bay Region of Perylene and Its Derivatives as an Attractive Strategy for PAH Core Expansion: Theoretical and Practical Aspects. Molecules 2020, 25, 5373. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Song, T.; Xiao, J.; Zhang, Q. Recent Progress in Using Pyrene-4,5-diketones and Pyrene-4,5,9,10-tetraketones as Building Blocks to Construct Large Acenes and Heteroacenes. Asian J. Org. Chem. 2018, 7, 2046. [Google Scholar] [CrossRef]
- Fernández-García, J.; Evans, P.J.; Filippone, S.; Herranz, M.Á.; Martín, N. Chiral Molecular Carbon Nanostructures. Acc. Chem. Res. 2019, 52, 1565. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, J.; Evans, P.J.; Rivero, S.M.; Fernández, I.; García-Fresnadillo, D.; Perles, J.; Casado, J.; Martín, N. π-Extended Corannulene-Based Nanographenes: Selective Formation of Negative Curvature. J. Am. Chem. Soc. 2018, 140, 17188. [Google Scholar] [CrossRef]
- Kirschbaum, T.; Rominger, F.; Mastalerz, M. A Chiral Polycyclic Aromatic Hydrocarbon Monkey Saddle. Angew. Chem. Int. Ed. 2020, 59, 270. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Elbert, S.M.; Rominger, F.; Mastalerz, M. A Series of Soluble Thieno-Fused Coronene Nanoribbons of Precise Lengths. J. Am. Chem. Soc. 2022, 144, 9883. [Google Scholar] [CrossRef]
- Borissov, A.; Kumar, Y.; Moshniaha, L.; Wong, W.; Żyła-Karwowska, M.; Stępień, M. Recent Advances in Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds. Chem. Rev. 2022, 122, 565. [Google Scholar] [CrossRef]
- Stępień, M.; Gońka, E.; Żyła, M.; Sprutta, N. Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds: Synthetic Routes, Properties, and Applications. Chem. Rev. 2017, 117, 3479. [Google Scholar] [CrossRef]
- Chen, W.; Yu, F.; Xu, Q.; Zhou, G.; Zhang, Q. Recent Progress in High Linearly Fused Polycyclic Conjugated Hydrocarbons (PCHs, n > 6) with Well-Defined Structures. Adv. Sci. 2020, 7, 1903766. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q. Linearly Fused Azaacenes: Novel Approaches and New Applications Beyond Field-Effect Transistors (FETs). ACS Appl. Mater. Interfaces 2015, 7, 28049. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Yang, H.; Yin, Z.; Guo, J.; Boey, F.; Zhang, H.; Zhang, Q. Preparation, characterization, and photoswitching/light-emitting behaviors of coronene nanowires. J. Mater. Chem. C 2011, 21, 1423. [Google Scholar] [CrossRef]
- Deng, X.; Yu, X.; Xiao, J.; Zhang, Q. Our research progress in heteroaggregation and homoaggregationof organic π-conjugated systems. Aggregate 2021, 2, e35. [Google Scholar] [CrossRef]
- Chi, X.; Besnard, C.; Thorsmølle, V.K.; Butko, V.Y.; Taylor, A.J.; Siegrist, T.; Ramirez, A.P. Structure and Transport Properties of the Charge-Transfer Salt Coronene-TCNQ. Chem. Mater. 2004, 16, 5751. [Google Scholar] [CrossRef]
- Yoshida, Y.; Kumagai, Y.; Mizuno, M.; Saito, G. Structure–Property Relationship of Supramolecular Rotators of Coronene in Charge-Transfer Solids. Cryst. Growth. Des. 2015, 15, 1389. [Google Scholar] [CrossRef]
- Zhu, W.; Zhu, L.; Zou, Y.; Wu, Y.; Zhen, Y.; Dong, H.; Fu, H.; Wei, Z.; Shi, Q.; Hu, W. Deepening Insights of Charge Transfer and Photophysics in a Novel Donor–Acceptor Cocrystal for Waveguide Couplers and Photonic Logic Computation. Adv. Mater. 2016, 28, 5954. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, F.; Chen, W.; Wang, J.; Liu, J.; Yao, C.; Zhao, J.; Dong, H.; Hu, W.; Zhang, Q. Rational Control of Charge Transfer Excitons toward High-Contrast Reversible Mechanoresponsive Luminescent Switching. Angew. Chem. Int. Ed. 2020, 59, 17580. [Google Scholar] [CrossRef]
- Scalora, M.; Dowling, J.P.; Bowden, C.M.; Bloemer, M.J. Optical Limiting and Switching of Ultrashort Pulses in Nonlinear Photonic Band Gap Materials. Phys. Rev. Lett. 1994, 73, 1368. [Google Scholar] [CrossRef]
- Makri, E.; Ramezani, H.; Kottos, T.; Vitebskiy, I. Concept of a reflective power limiter based on nonlinear localized modes. Phys. Rev. A 2014, 89, 031802. [Google Scholar] [CrossRef]
- Pascal, S.; David, S.; Andraud, C.; Maury, O. Near-infrared dyes for two-photon absorption in the short-wavelength infrared: Strategies towards optical power limiting. Chem. Soc. Rev. 2021, 50, 6613. [Google Scholar] [CrossRef] [PubMed]
- Tutt, L.W.; Kost, A. Optical limiting performance of C60 and C70 solutions. Nature 1992, 356, 225. [Google Scholar] [CrossRef]
- Shirk, J.S.; Pong, R.G.S.; Bartoli, F.J.; Snow, A.W. Optical limiter using a lead phthalocyanine. Appl. Phys. Lett. 1993, 63, 1880. [Google Scholar] [CrossRef]
- Balapanuru, J.; Yang, J.; Xiao, S.; Bao, Q.; Jahan, M.; Polavarapu, L.; Wei, J.; Xu, Q.; Loh, K.P. A Graphene Oxide–Organic Dye Ionic Complex with DNA-Sensing and Optical-Limiting Properties. Angew. Chem. Int. Ed. 2010, 49, 6549. [Google Scholar] [CrossRef]
- Xu, L.; Sun, J.; Tang, T.; Zhang, H.; Sun, M.; Zhang, J.; Li, J.; Huang, B.; Wang, Z.; Xie, Z.; et al. Metallated Graphynes as a New Class of Photofunctional 2D Organometallic Nanosheets. Angew. Chem. Int. Ed. 2021, 60, 11326. [Google Scholar] [CrossRef]
- Hirata, S.; Vacha, M. Large Reverse Saturable Absorption at the Sunlight Power Level Using the Ultralong Lifetime of Triplet Excitons. J. Phys. Chem. Lett. 2017, 8, 3683. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Wang, M. The organic co-crystals formed using naphthalenediimide-based triangular macrocycles and coronene: Intermolecular charge transfers and nonlinear optical properties. Phys. Chem. Chem. Phys. 2022, 24, 29747. [Google Scholar] [CrossRef]
- Wei, L.; Deng, X.; Yu, X.; Li, X.; Wang, W.; Zhang, C.; Xiao, J. Double π-Extended Helicene Derivatives Containing Pentagonal Rings: Synthesis, Crystal Analyses, and Photophysics. J. Org. Chem. 2021, 86, 17535. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Cyrański, M.; Ciesielski, A.; Świrska, B.; Leszczyński, P. Separation of the Energetic and Geometric Contributions to Aromaticity. 2. Analysis of the Aromatic Character of Benzene Rings in Their Various Topological Environments in the Benzenoid Hydrocarbons. Crystal and Molecular Structure of Coronene. J. Chem. Inf. Comput. Sci. 1996, 36, 1135. [Google Scholar] [CrossRef]
- Pan, H.; Song, T.; Yin, X.; Jin, P.; Xiao, J. Synthesis, Crystal Analysis, and Optoelectronic Properties of Diazole-Functionalized Acenes and Azaacenes. Chem. Eur. J. 2018, 24, 6572. [Google Scholar] [CrossRef]
- Curtis, M.D.; Cao, J.; Kampf, J.W. Solid-State Packing of Conjugated Oligomers: From π-Stacks to the Herringbone Structure. J. Am. Chem. Soc. 2004, 126, 4318. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Khan, S.U.D.; Rana, U.A.; Janjua, M.R.S.A.; Tahir, M.H.; Nazar, M.F.; Song, Y. Effect of thiophene rings on UV/visible spectra and non-linear optical (NLO) properties of triphenylamine based dyes: A quantum chemical perspective. J. Phys. Org. Chem. 2015, 28, 418. [Google Scholar] [CrossRef]
- Mahmood, A.; Khan, S.U.D.; Rehman, F.U. Assessing the quantum mechanical level of theory for prediction of UV/Visible absorption spectra of some aminoazobenzene dyes. J. Saudi Chem. Soc. 2015, 19, 436. [Google Scholar] [CrossRef]
- Khalid, M.; Khan, M.U.; Shafiq, I.; Hussain, R.; Mahmood, K.; Hussain, A.; Jawaria, R.; Hussain, A.; Imran, M.; Assiri, M.A.; et al. NLO potential exploration for D–π–A heterocyclic organic compounds by incorporation of various π-linkers and acceptor units. Arab. J. Chem. 2021, 14, 103295. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Mahmood, A.; Abdullah, M.I.; Nazar, M.F. Quantum Chemical Designing of Novel Organic Non-Linear Optical Compounds. Bull. Korean Chem. Soc. 2014, 35, 1391. [Google Scholar] [CrossRef]
- Mahmood, A.; Khan, S.U.D.; Rana, U.A.; Tahir, M.H. Red shifting of absorption maxima of phenothiazine based dyes by incorporating electron-deficient thiadiazole derivatives as π-spacer. Arab. J. Chem. 2019, 12, 1447. [Google Scholar] [CrossRef]
- Khalid, M.; Ali, A.; Din, Z.U.; Tahir, M.N.; Morais, S.F.A.; Braga, A.C.; Akhtar, M.N.; Imran, M.; Rodrigues-Filho, E. β-Hydroxy Carbonyl compounds via aldol reaction: Single crystal investigation and quantum chemical exploration for the unveiling of supramolecular behavior. J. Mol. Struct. 2021, 1241, 130650. [Google Scholar] [CrossRef]
- Sheik-Bahae, M.; Said, A.A.; Wei, T.H.; Hagan, D.J.; Van Stryland, E.W. Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Elect. 1990, 26, 760. [Google Scholar] [CrossRef]
- He, G.S.; Tan, L.S.; Zheng, Q.; Prasad, P.N. Multiphoton Absorbing Materials: Molecular Designs, Characterizations, and Applications. Chem. Rev. 2008, 108, 1245. [Google Scholar] [CrossRef]
- Wu, X.; Xiao, J.; Sun, R.; Jin, T.; Yang, J.; Shi, G.; Wang, Y.; Zhang, X.; Song, Y. Spindle-Type Conjugated Compounds Containing Twistacene Unit: Synthesis and Ultrafast Broadband Reverse Saturable Absorption. Adv. Opt. Mater. 2017, 5, 1600712. [Google Scholar] [CrossRef]
- Liu, R.; Dandu, N.; Chen, J.; Li, Y.; Li, Z.; Liu, S.; Wang, C.; Kilina, S.; Kohler, B.; Sun, W. Influence of Different Diimine (N∧N) Ligands on the Photophysics and Reverse Saturable Absorption of Heteroleptic Cationic Iridium(III) Complexes Bearing Cyclometalating 2-{3-[7-(Benzothiazol-2-yl)fluoren-2-yl]phenyl}pyridine (C∧N) Ligands. J. Phys. Chem. C 2014, 118, 23233. [Google Scholar] [CrossRef]
- Li, Y.; Dandu, N.; Liu, R.; Li, Z.; Kilina, S.; Sun, W. Effects of Extended π-Conjugation in Phenanthroline (N∧N) and Phenylpyridine (C∧N) Ligands on the Photophysics and Reverse Saturable Absorption of Cationic Heteroleptic Iridium(III) Complexes. J. Phys. Chem. C 2014, 118, 6372. [Google Scholar] [CrossRef]
- Liu, B.; Jabed, M.A.; Kilina, S.; Sun, W. Synthesis, Photophysics, and Reverse Saturable Absorption of trans-Bis-cyclometalated Iridium(III) Complexes (C^N^C)Ir(R-tpy) + (tpy = 2,2′:6′,2″-Terpyridine) with Broadband Excited-State Absorption. Inorg. Chem. 2020, 12, 8532. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; de Castro, A.R.B.; Shen, Y.R. Surface-Enhanced Second-Harmonic Generation. Phys. Rev. Lett. 1981, 46, 145. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhao, J.; Wang, W.; Li, Y.; Li, Y.; Zhou, S.; Xiao, J. Synthesis, Photoswitching Behavior and Nonlinear Optical Properties of Substituted Tribenzo[a,d,g]coronene. Molecules 2023, 28, 1419. https://doi.org/10.3390/molecules28031419
Li X, Zhao J, Wang W, Li Y, Li Y, Zhou S, Xiao J. Synthesis, Photoswitching Behavior and Nonlinear Optical Properties of Substituted Tribenzo[a,d,g]coronene. Molecules. 2023; 28(3):1419. https://doi.org/10.3390/molecules28031419
Chicago/Turabian StyleLi, Xueqing, Jie Zhao, Wei Wang, Yiming Li, Yunfei Li, Shuyun Zhou, and Jinchong Xiao. 2023. "Synthesis, Photoswitching Behavior and Nonlinear Optical Properties of Substituted Tribenzo[a,d,g]coronene" Molecules 28, no. 3: 1419. https://doi.org/10.3390/molecules28031419
APA StyleLi, X., Zhao, J., Wang, W., Li, Y., Li, Y., Zhou, S., & Xiao, J. (2023). Synthesis, Photoswitching Behavior and Nonlinear Optical Properties of Substituted Tribenzo[a,d,g]coronene. Molecules, 28(3), 1419. https://doi.org/10.3390/molecules28031419




