Stabilization of Anthocyanins from Coffee (Coffea arabica L.) Husks and In Vivo Evaluation of Their Antioxidant Activity
Abstract
1. Introduction
2. Results and Discussion
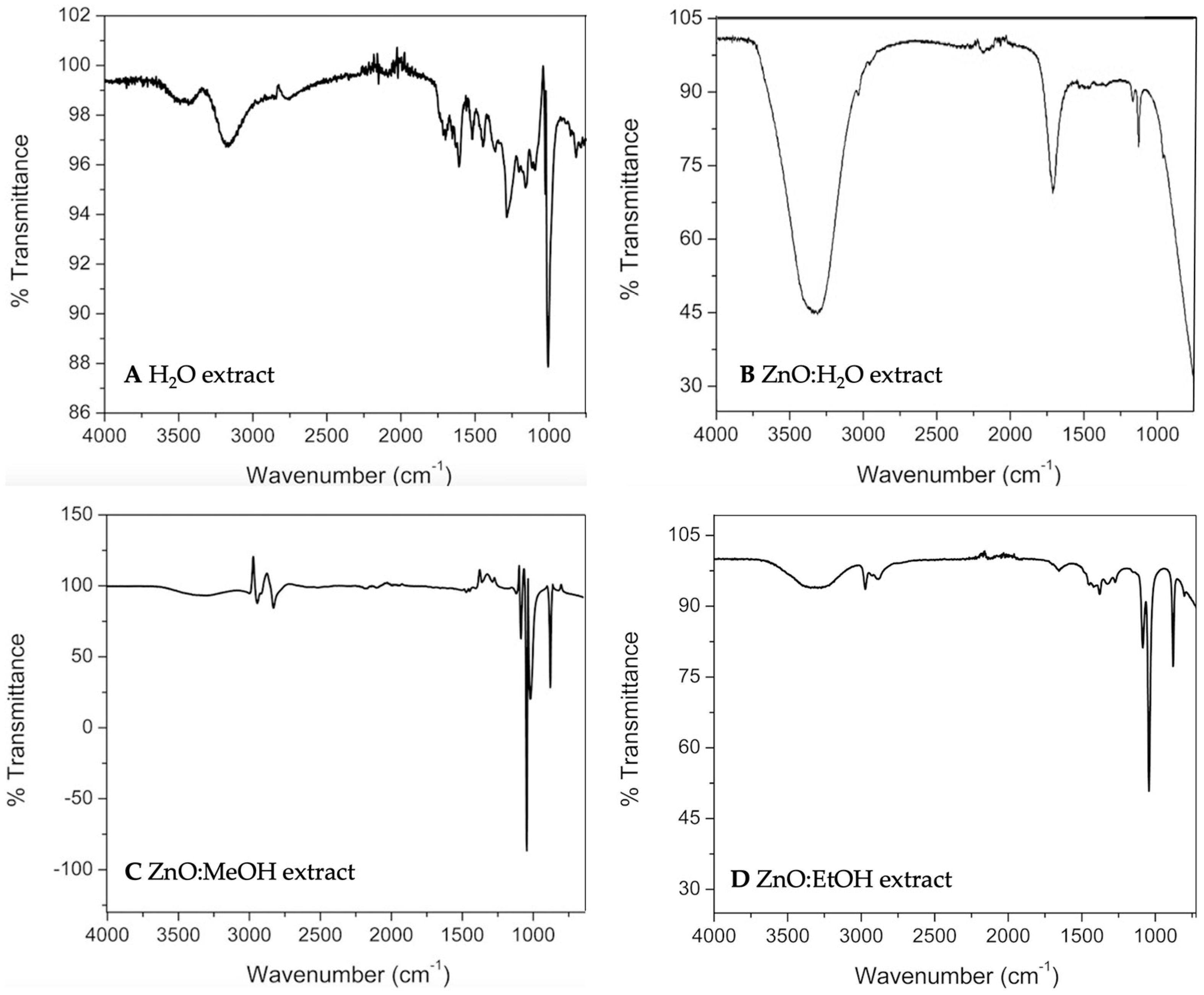
2.1. Extraction and Phenolic Compounds’ Determination
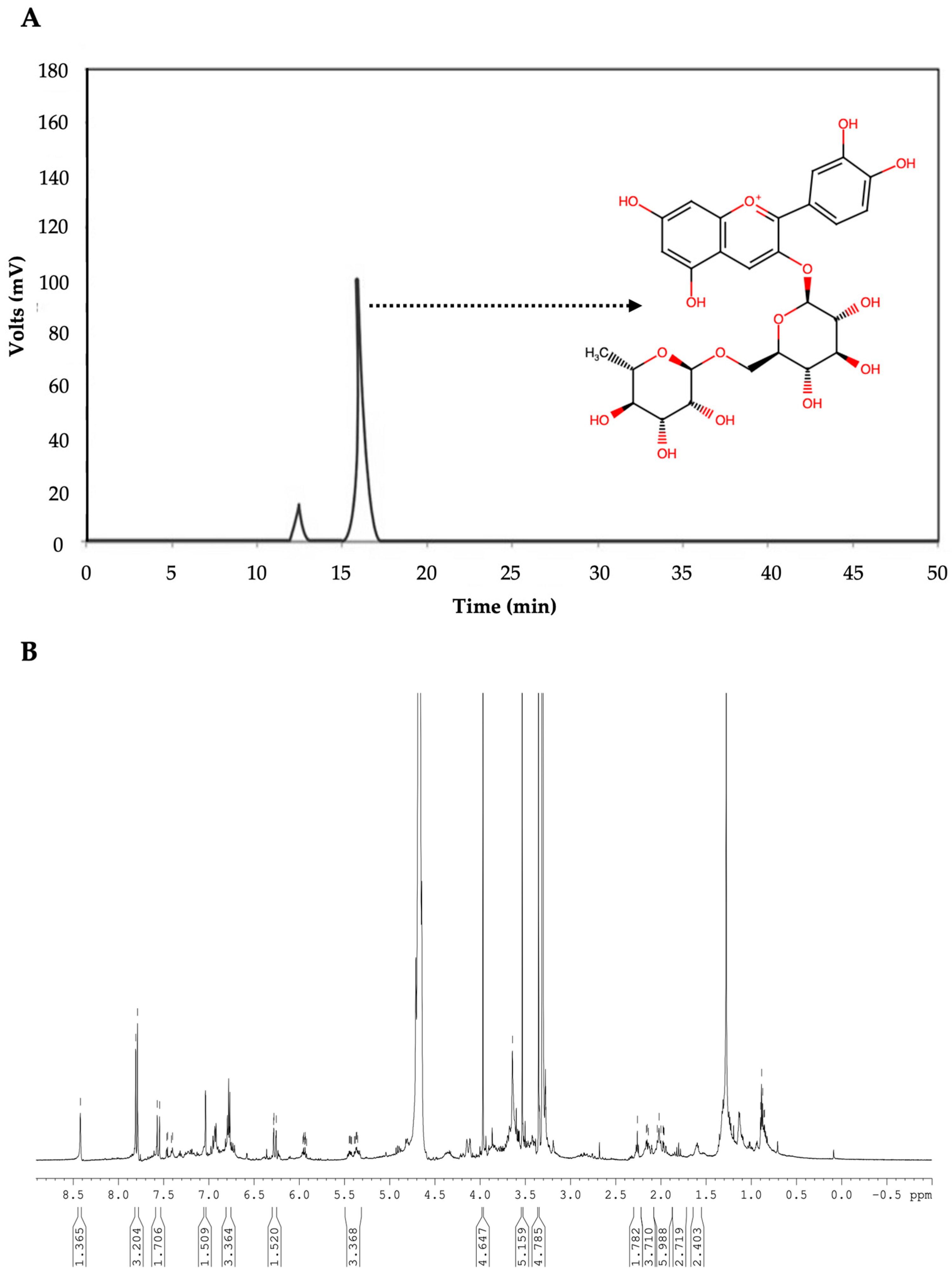
2.2. Anthocyanins’ Determination
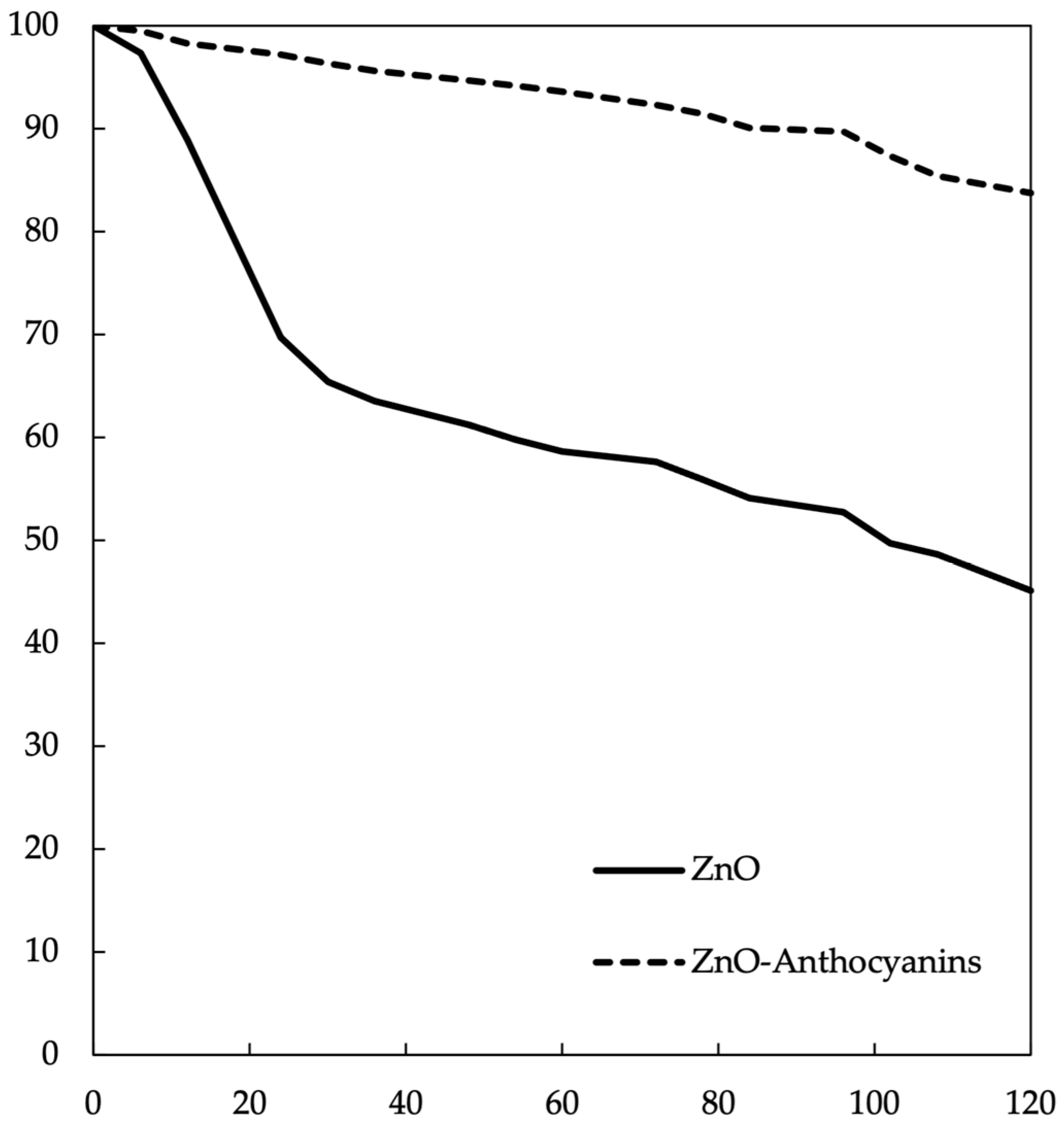
2.3. Measurements of Stabilized Nanoparticles
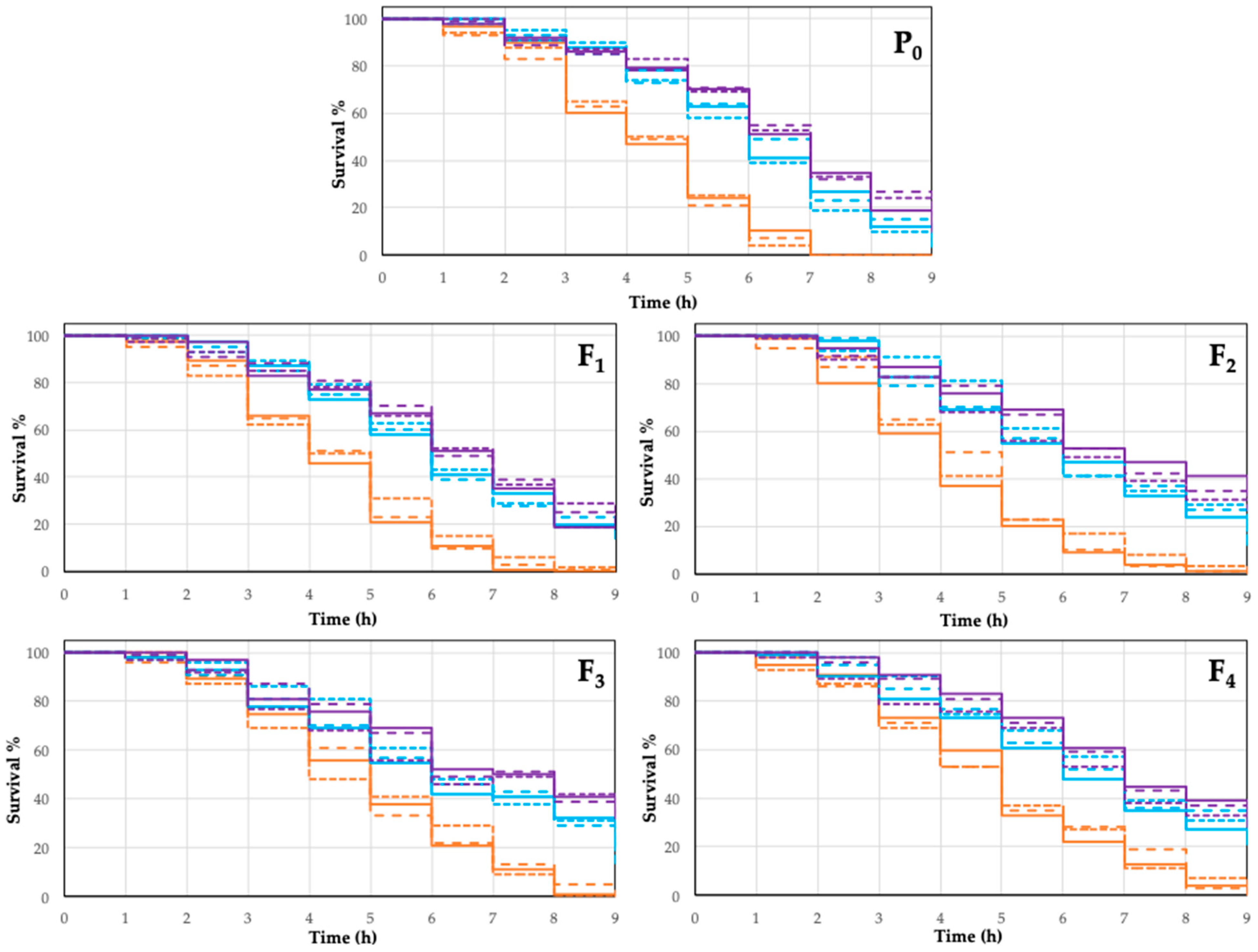
2.4. Biological Assay
3. Materials and Methods
3.1. Materials
3.2. Anthocyanin Extraction
3.3. Anthocyanin Purification
3.4. Anthocyanin Separation and Identification
3.5. Quantification of Total Phenolic Compounds
3.6. Total Monomeric Anthocyanins
3.7. Antioxidant Activity
3.8. Preparation of ZnO Nanoparticles
3.9. Anthocyanin Stabilization Using ZnO Nanoparticles
3.10. Quantification of Anthocyanins Stabilized with ZnO Nanoparticles
3.11. Caenorhabditis Elegans Culture, Maintenance, and Syncronization
3.12. Biological Assay
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| NF-κB | (nuclear factor kappa-light-chain-enhancer of activated B cells) |
| COX-2 | (cyclooxygenase-2) |
| MAPK | (mitogen-activated protein kinase) |
| PPAR | (peroxisome proliferator-activated receptor) |
| CD36 | (cluster of differentiation 36) |
| LDL | (low-density lipoprotein) |
| GLUT4 | (glucose transporter type 4) |
| AMPK | (AMP-activated protein kinase) |
| PEPCK | (phosphoenolpyruvate carboxykinase) |
| CPT1A | (carnitine palmitoyl transferase 1A) |
| ACO | (acyl-CoA oxidase) |
| FKBPs | (FK506 binding proteins) |
| CaCo-2 cells | (colorectal adenocarcinoma cells) |
| Hep3B cells | (human hepatocyte carcinoma cells) |
| GSK3β | (glycogen synthase kinase 3 beta) |
| GAE | (gallic acid equivalents) |
| EtOH | (ethanol) |
| MeOH | (methanol) |
| HPLC | (high-performance liquid chromatography) |
| NMR | (nuclear magnetic resonance) |
| FTIR | (Fourier transform infrared) |
| AAS | (atomic absorption spectrometry) |
| ZnO | (zinc oxide) |
| C3G | (cyanidin-3-glucoside) |
| ABTS | (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| NGM | (nematode growth medium); LBM (luria–bertani medium) |
References
- Nieber, K. The Impact of Coffee on Health. Planta Med. 2017, 83, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Pham, Y.; Reardon-Smith, K.; Mushtaq, S.; Cockfield, G. The Impact of Climate Change and Variability on Coffee Production: A Systematic Review. Clim. Chang. 2019, 156, 609–630. [Google Scholar] [CrossRef]
- International Coffee Organization. Annual Review 2019 Addressing the Coffee Price Crisis; International Coffee Organization: London, UK, 2020. [Google Scholar]
- Dos Santos, É.M.; Macedo, L.M.D.; Tundisi, L.L.; Ataide, J.A.; Camargo, G.A.; Alves, R.C.; Oliveira, M.B.P.P.; Mazzola, P.G. Coffee By-Products in Topical Formulations: A Review. Trends Food Sci. Technol. 2021, 111, 280–291. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Franca, A.S. An Overview of the Potential Uses for Coffee Husks. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 283–291. ISBN 978-0-12-409517-5. [Google Scholar]
- Sumardiono, S.; Jos, B.; Dewanti, A.A.E.; Mahendra, I.; Cahyono, H. Biogas Production from Coffee Pulp and Chicken Feathers Using Liquid- and Solid-State Anaerobic Digestions. Energies 2021, 14, 4664. [Google Scholar] [CrossRef]
- Esquivel, P.; Viñas, M.; Steingass, C.B.; Gruschwitz, M.; Guevara, E.; Carle, R.; Schweiggert, R.M.; Jiménez, V.M. Coffee (Coffea arabica L.) by-Products as a Source of Carotenoids and Phenolic Compounds—Evaluation of Varieties with Different Peel Color. Front. Sustain. Food Syst. 2020, 4, 590597. [Google Scholar] [CrossRef]
- Prata, E.R.B.A.; Oliveira, L.S. Fresh Coffee Husks as Potential Sources of Anthocyanins. LWT Food Sci. Technol. 2007, 40, 1555–1560. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.-L.; Liang, N.-N.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- Pina, F.; Oliveira, J.; de Freitas, V. Anthocyanins and Derivatives Are More than Flavylium Cations. Tetrahedron 2015, 71, 3107–3114. [Google Scholar] [CrossRef]
- Cruz, L.; Basílio, N.; Mateus, N.; de Freitas, V.; Pina, F. Natural and Synthetic Flavylium-Based Dyes: The Chemistry Behind the Color. Chem. Rev. 2022, 122, 1416–1481. [Google Scholar] [CrossRef]
- Oancea, S.; Oprean, L. Anthocyanins, from Biosynthesis in Plants to Human Health Benefits. Acta Univ. Cibiniensis Ser. E Food Technol. 2011, 15, 3–16. [Google Scholar]
- Li, S.; Wu, B.; Fu, W.; Reddivari, L. The Anti-Inflammatory Effects of Dietary Anthocyanins against Ulcerative Colitis. Int. J. Mol. Sci. 2019, 20, 2588. [Google Scholar] [CrossRef] [PubMed]
- Hassimotto, N.M.A.; Moreira, V.; do Nascimento, N.G.; Souto, P.C.M.D.C.; Teixeira, C.; Lajolo, F.M. Inhibition of Carrageenan-Induced Acute Inflammation in Mice by Oral Administration of Anthocyanin Mixture from Wild Mulberry and Cyanidin-3-Glucoside. BioMed Res. Int. 2013, 2013, 146716. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Klimis-Zacas, D. Anti-Inflammatory Effect of Anthocyanins via Modulation of Nuclear Factor-ΚB and Mitogen-Activated Protein Kinase Signaling Cascades. Nutr. Rev. 2015, 73, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Kao, E.-S.; Tseng, T.-H.; Lee, H.-J.; Chan, K.-C.; Wang, C.-J. Anthocyanin Extracted from Hibiscus Attenuate Oxidized LDL-Mediated Foam Cell Formation Involving Regulation of CD36 Gene. Chem. Biol. Interact. 2009, 179, 212–218. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health Benefits of Anthocyanins and Molecular Mechanisms: Update from Recent Decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Aboonabi, A.; Meyer, R.R.; Gaiz, A.; Singh, I. Anthocyanins in Berries Exhibited Anti-Atherogenicity and Antiplatelet Activities in a Metabolic Syndrome Population. Nutr. Res. 2020, 76, 82–93. [Google Scholar] [CrossRef]
- Xie, L.; Su, H.; Sun, C.; Zheng, X.; Chen, W. Recent Advances in Understanding the Anti-Obesity Activity of Anthocyanins and Their Biosynthesis in Microorganisms. Trends Food Sci. Technol. 2018, 72, 13–24. [Google Scholar] [CrossRef]
- Li, Z.; Tian, J.; Cheng, Z.; Teng, W.; Zhang, W.; Bao, Y.; Wang, Y.; Song, B.; Chen, Y.; Li, B. Hypoglycemic Bioactivity of Anthocyanins: A Review on Proposed Targets and Potential Signaling Pathways. Crit. Rev. Food Sci. Nutr. 2022, 1–18. [Google Scholar] [CrossRef]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary Anthocyanin-Rich Bilberry Extract Ameliorates Hyperglycemia and Insulin Sensitivity via Activation of AMP-Activated Protein Kinase in Diabetic Mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef]
- Mehmood, A.; Zhao, L.; Wang, Y.; Pan, F.; Hao, S.; Zhang, H.; Iftikhar, A.; Usman, M. Dietary Anthocyanins as Potential Natural Modulators for the Prevention and Treatment of Non-Alcoholic Fatty Liver Disease: A Comprehensive Review. Food Res. Int. 2021, 142, 110180. [Google Scholar] [CrossRef]
- Hung, T.-C.; Chang, T.-T.; Fan, M.-J.; Lee, C.-C.; Chen, C.Y.-C. In Silico Insight into Potent of Anthocyanin Regulation of FKBP52 to Prevent Alzheimer’s Disease. Evid. Based Complement. Altern. Med. ECAM 2014, 2014, 450592. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Liu, F.; Tong, L.; Chen, Z.; Chen, J.; He, H.; Xu, R.; Ma, Y.; Huang, C. Neuroprotective Effects of Anthocyanins and Its Major Component Cyanidin-3-O-Glucoside (C3G) in the Central Nervous System: An Outlined Review. Eur. J. Pharmacol. 2019, 858, 172500. [Google Scholar] [CrossRef]
- Forester, S.C.; Choy, Y.Y.; Waterhouse, A.L.; Oteiza, P.I. The Anthocyanin Metabolites Gallic Acid, 3-O-Methylgallic Acid, and 2,4,6-Trihydroxybenzaldehyde Decrease Human Colon Cancer Cell Viability by Regulating pro-Oncogenic Signals. Mol. Carcinog. 2014, 53, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, Y.-K.; Lee, W.S.; Park, O.J.; Kim, Y.-M. The Involvement of AMPK/GSK3-Beta Signals in the Control of Metastasis and Proliferation in Hepato-Carcinoma Cells Treated with Anthocyanins Extracted from Korea Wild Berry Meoru. BMC Complement. Altern. Med. 2014, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, D.; Jude, B.; Morand, C. MiRNA as Molecular Target of Polyphenols Underlying Their Biological Effects. Free Radic. Biol. Med. 2013, 64, 40–51. [Google Scholar] [CrossRef]
- Wei, J.; Yu, W.; Hao, R.; Fan, J.; Gao, J. Anthocyanins from Aronia melanocarpa Induce Apoptosis in Caco-2 Cells through Wnt/β-Catenin Signaling Pathway. Chem. Biodivers. 2020, 17, e2000654. [Google Scholar] [CrossRef]
- Fu, X.; Shen, X.; Yin, X.; Zhang, Y.H.; Wang, X.; Han, Z.; Lin, Q.; Fan, J. Antioxidant and Pro-Apoptosis Activities of Coffee Husk (Coffea arabica) Anthocyanins. Int. Food Res. J. 2021, 28, 1187–1195. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Wrolstad, R.E. Extraction, Isolation, and Purification of Anthocyanins. Curr. Protoc. Food Anal. Chem. 2001, 1, F1.1–F1.11. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Chai, Z.; Beta, T.; Feng, J.; Huang, W. Blueberry Anthocyanins: An Updated Review on Approaches to Enhancing Their Bioavailability. Trends Food Sci. Technol. 2021, 118, 808–821. [Google Scholar] [CrossRef]
- Osvaldt Rosales, T.K.; Pessoa da Silva, M.; Lourenço, F.R.; Aymoto Hassimotto, N.M.; Fabi, J.P. Nanoencapsulation of Anthocyanins from Blackberry (Rubus spp.) through Pectin and Lysozyme Self-Assembling. Food Hydrocoll. 2021, 114, 106563. [Google Scholar] [CrossRef]
- Katsaboxakis, K.; Papanicolaou, D.; Melanitou, M. Stability of Pigmented Orange Anthocyanins in Model and Real Food Systems [Food Colourants]. Ital. J. Food Sci. 1998, 10, 17–25. [Google Scholar]
- Tseng, K.-C.; Chang, H.-M.; Wu, J.S.-B. Degradation Kinetics of Anthocyanin in Ethanolic Solutions. J. Food Process. Preserv. 2006, 30, 503–514. [Google Scholar] [CrossRef]
- Mahadevan, S.; Karwe, M.V. Effect of High-Pressure Processing on Bioactive Compounds. In High Pressure Processing of Food: Principles, Technology and Applications; Balasubramaniam, V.M., Barbosa-Cánovas, G.V., Lelieveld, H.L.M., Eds.; Food Engineering Series; Springer: New York, NY, USA, 2016; pp. 479–507. ISBN 978-1-4939-3234-4. [Google Scholar]
- Thuy, N.M.; Han, D.H.N.; Minh, V.Q.; Tai, N.V. Effect of Extraction Methods and Temperature Preservation on Total Anthocyanins Compounds of Peristrophe bivalvis L. Merr Leaf. J. Appl. Biol. Biotechnol. 2022, 10, 146–153. [Google Scholar] [CrossRef]
- Garzón, G.A. Las antocianinas como colorantes naturales y compuestos bioactivos: Revisión. Acta Biológica Colomb. 2008, 13, 27–36. [Google Scholar]
- Yu, Y.; Lv, Y. Degradation Kinetic of Anthocyanins from Rose (Rosa rugosa) as Prepared by Microencapsulation in Freeze-Drying and Spray-Drying. Int. J. Food Prop. 2019, 22, 2009–2021. [Google Scholar] [CrossRef]
- Righi da Rosa, J.; Nunes, G.L.; Motta, M.H.; Fortes, J.P.; Cezimbra Weis, G.C.; Rychecki Hecktheuer, L.H.; Muller, E.I.; Ragagnin de Menezes, C.; Severo da Rosa, C. Microencapsulation of Anthocyanin Compounds Extracted from Blueberry (Vaccinium spp.) by Spray Drying: Characterization, Stability and Simulated Gastrointestinal Conditions. Food Hydrocoll. 2019, 89, 742–748. [Google Scholar] [CrossRef]
- Acosta, E. Bioavailability of Nanoparticles in Nutrient and Nutraceutical Delivery. Curr. Opin. Colloid Interface Sci. 2009, 14, 3–15. [Google Scholar] [CrossRef]
- Chen, R.; Ratnikova, T.A.; Stone, M.B.; Lin, S.; Lard, M.; Huang, G.; Hudson, J.S.; Ke, P.C. Differential Uptake of Carbon Nanoparticles by Plant and Mammalian Cells. Small 2010, 6, 612–617. [Google Scholar] [CrossRef]
- Roger, E.; Lagarce, F.; Garcion, E.; Benoit, J.-P. Biopharmaceutical Parameters to Consider in Order to Alter the Fate of Nanocarriers after Oral Delivery. Nanomedicine 2010, 5, 287–306. [Google Scholar] [CrossRef]
- He, B.; Ge, J.; Yue, P.; Yue, X.; Fu, R.; Liang, J.; Gao, X. Loading of Anthocyanins on Chitosan Nanoparticles Influences Anthocyanin Degradation in Gastrointestinal Fluids and Stability in a Beverage. Food Chem. 2017, 221, 1671–1677. [Google Scholar] [CrossRef]
- Ju, M.; Zhu, G.; Huang, G.; Shen, X.; Zhang, Y.; Jiang, L.; Sui, X. A Novel Pickering Emulsion Produced Using Soy Protein-Anthocyanin Complex Nanoparticles. Food Hydrocoll. 2020, 99, 105329. [Google Scholar] [CrossRef]
- Cai, D.; Li, X.; Chen, J.; Jiang, X.; Ma, X.; Sun, J.; Tian, L.; Vidyarthi, S.K.; Xu, J.; Pan, Z.; et al. A Comprehensive Review on Innovative and Advanced Stabilization Approaches of Anthocyanin by Modifying Structure and Controlling Environmental Factors. Food Chem. 2022, 366, 130611. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Targeted Pharmaceutical Nanocarriers for Cancer Therapy and Imaging. AAPS J. 2007, 9, E128–E147. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Zhang, Z.; Jing, P. Black Rice Anthocyanins Embedded in Self-Assembled Chitosan/Chondroitin Sulfate Nanoparticles Enhance Apoptosis in HCT-116 Cells. Food Chem. 2019, 301, 125280. [Google Scholar] [CrossRef]
- Guo, C.; Sun, J.; Dong, J.; Cai, W.; Zhao, X.; Song, B.; Zhang, R. A Natural Anthocyanin-Based Multifunctional Theranostic Agent for Dual-Modal Imaging and Photothermal Anti-Tumor Therapy. J. Mater. Chem. B 2021, 9, 7447–7460. [Google Scholar] [CrossRef]
- Pons, E.; Alquézar, B.; Rodríguez, A.; Martorell, P.; Genovés, S.; Ramón, D.; Rodrigo, M.J.; Zacarías, L.; Peña, L. Metabolic Engineering of β-Carotene in Orange Fruit Increases Its In vivo Antioxidant Properties. Plant Biotechnol. J. 2014, 12, 17–27. [Google Scholar] [CrossRef]
- Bouyanfif, A.; Jayarathne, S.; Koboziev, I.; Moustaid-Moussa, N. The Nematode Caenorhabditis elegans as a Model Organism to Study Metabolic Effects of ω-3 Polyunsaturated Fatty Acids in Obesity. Adv. Nutr. 2019, 10, 165–178. [Google Scholar] [CrossRef] [PubMed]
- González-Peña, M.A.; Lozada-Ramírez, J.D.; Ortega-Regules, A.E. Trolox Protection against Oxidative Stress in Caenorhabditis elegans. In Technology, Science and Culture—A Global Vision, Volume II; Picazo-Vela, S., Herández, L.R., Eds.; InTech: London, UK, 2020; pp. 73–82. ISBN 978-1-78984-306-4. [Google Scholar]
- Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C. Caenorhabditis elegans as a Model Organism to Evaluate the Antioxidant Effects of Phytochemicals. Molecules 2020, 25, 3194. [Google Scholar] [CrossRef]
- Hilty, F.; Arnold, M.; Hilbe, M.; Teleki, A.; Knijnenburg, J.T.N.; Ehrensperger, F.; Hurrell, R.F.; Pratsinis, S.E.; Langhans, W.; Zimmermann, M.B. Iron from nanocompounds containing iron and zinc is highly bioavailable in rats without tissue accumulation. Nat. Nanotechnol. 2010, 5, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Faleni, N.; Moloto, M.J. Effect of glucose as stabilizer of ZnO and CdO nanoparticles on the morphology and optical properties. IJRRAS 2013, 14, 127–135. [Google Scholar]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, e1062562. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, S.; Ashtami, J.; Mohanan, P.V. Biomedical Application and Hidden Toxicity of Zinc Oxide Nanoparticles. Mater. Today Chem. 2018, 10, 175–186. [Google Scholar] [CrossRef]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Biomedical Applications of Zinc Oxide Nanomaterials. Curr. Mol. Med. 2013, 13, 1633–1645. [Google Scholar] [CrossRef]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef]
- Vandebriel, R.J.; De Jong, W.H. A Review of Mammalian Toxicity of ZnO Nanoparticles. Nanotechnol. Sci. Appl. 2012, 5, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc oxide particles: Synthesis, properties and applications. Chem. Eng. J. 2012, 185, 1–22. [Google Scholar] [CrossRef]
- Metivier, R.P.; Francis, F.J.; Clydesdale, F.M. Solvent Extraction of Anthocyanins from Wine Pomace. J. Food Sci. 1980, 45, 1099–1100. [Google Scholar] [CrossRef]
- Szajdek, A.; Borowska, E.J. Bioactive Compounds and Health-Promoting Properties of Berry Fruits: A Review. Plant Foods Hum. Nutr. 2008, 63, 147–156. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total Phenolics and Total Flavonoids in Bulgarian Fruits and Vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Su, M.-S.; Chien, P.-J. Antioxidant Activity, Anthocyanins, and Phenolics of Rabbiteye Blueberry (Vaccinium ashei) Fluid Products as Affected by Fermentation. Food Chem. 2007, 104, 182–187. [Google Scholar] [CrossRef]
- Hečimović, I.; Belščak-Cvitanović, A.; Horžić, D.; Komes, D. Comparative Study of Polyphenols and Caffeine in Different Coffee Varieties Affected by the Degree of Roasting. Food Chem. 2011, 129, 991–1000. [Google Scholar] [CrossRef]
- Murthy, P.S.; Manjunatha, M.R.; Sulochannama, G.; Naidu, M.M. Extraction, Characterization and Bioactivity of Coffee Anthocyanins. Eur. J. Biol. Sci. 2012, 4, 13–19. [Google Scholar] [CrossRef]
- Li, S.; Xiao, J.; Chen, L.; Hu, C.; Chen, P.; Xie, B.; Sun, Z. Identification of A-Series Oligomeric Procyanidins from Pericarp of Litchi chinensis by FT-ICR-MS and LC-MS. Food Chem. 2012, 135, 31–38. [Google Scholar] [CrossRef]
- Sarni-Manchado, P.; Le Roux, E.; Le Guernevé, C.; Lozano, Y.; Cheynier, V. Phenolic Composition of Litchi Fruit Pericarp. J. Agric. Food Chem. 2000, 48, 5995–6002. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, Y. Litchi Flavonoids: Isolation, Identification and Biological Activity. Molecules 2007, 12, 745–758. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of Antioxidant Phenolic Compounds Extracted from Spent Coffee Grounds by Freeze-Drying and Spray-Drying Using Different Coating Materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef]
- Setiawan, T.; Subekti, W.Y.; Nur’Adya, S.S.; Ilmiah, K.; Ulfa, S.M. Performance of Natural Dye and Counter Electrode from Robusta Coffee Beans Peel Waste for Fabrication of Dye-Sensitized Solar Cell (DSSC). IOP Conf. Ser. Mater. Sci. Eng. 2018, 299, 012073. [Google Scholar] [CrossRef]
- Santillán-Urquiza, E.; Arteaga-Cardona, F.; Hernandez-Herman, E.; Pacheco-García, P.F.; González-Rodríguez, R.; Coffer, J.L.; Mendoza-Alvarez, M.E.; Vélez-Ruiz, J.F.; Méndez-Rojas, M.A. Inulin as a Novel Biocompatible Coating: Evaluation of Surface Affinities toward CaHPO4, α-Fe2O3, ZnO, CaHPO4@ZnO and α-Fe2O3@ZnO Nanoparticles. J. Colloid Interface Sci. 2015, 460, 339–348. [Google Scholar] [CrossRef]
- Santos, M.C.P.; Gonçalves, É.C.B.A. Effect of different extracting solvents on antioxidant activity and phenolic compounds of a fruit and vegetable residue flour. Sci. Agropecu. 2016, 7, 7–14. [Google Scholar] [CrossRef]
- Luna-Vital, D.; Li, Q.; West, L.; West, M.; Gonzalez de Mejia, E. Anthocyanin Condensed Forms Do Not Affect Color or Chemical Stability of Purple Corn Pericarp Extracts Stored under Different PHs. Food Chem. 2017, 232, 639–647. [Google Scholar] [CrossRef]
- Andrade, J.C.; Rodrigues, C.F.; Martins, N. Nanoencapsulation of Anthocyanins for Drug Delivery Systems. In Functional Bionanomaterials: From Biomolecules to Nanoparticles; Nanotechnology in the Life Sciences; Thangadurai, D., Sangeetha, J., Prasad, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 145–163. ISBN 978-3-030-41464-1. [Google Scholar]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2019, 11, 39. [Google Scholar] [CrossRef]
- Nardini, M. Phenolic Compounds in Food: Characterization and Health Benefits. Molecules 2022, 27, 783. [Google Scholar] [CrossRef] [PubMed]
- Yazhen, S.; Wenju, W.; Panpan, Z.; Yuanyuan, Y.; Panpan, D.; Wusen, Z.; Yanling, W. Anthocyanins: Novel Antioxidants in Diseases Prevention and Human Health. In Flavonoids—A Coloring Model for Cheering up Life; Badria, F.A., Ananga, A., Eds.; InTech: London, UK, 2019; ISBN 978-1-83968-504-0. [Google Scholar] [CrossRef]
- Gagman, H.A.; Him, N.A.I.I.N.; Ahmad, H.; Sulaiman, S.F.; Zakaria, R.; Termizi, F.H.M. In Vitro Efficacy of Aqueous and Methanol Extract of Cassia siamea Against the Motility of Caenorhabditis elegans. Trop. Life Sci. Res. 2020, 31, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Ersus, S.; Yurdagel, U. Microencapsulation of Anthocyanin Pigments of Black Carrot (Daucus carota L.) by Spray Drier. J. Food Eng. 2007, 80, 805–812. [Google Scholar] [CrossRef]
- Lao, F.; Giusti, M.M. Quantification of Purple Corn (Zea mays L.) Anthocyanins Using Spectrophotometric and HPLC Approaches: Method Comparison and Correlation. Food Anal. Methods 2016, 9, 1367–1380. [Google Scholar] [CrossRef]
- Guzmán-Figueroa, M.D.P.; Ortega-Regules, A.E.; Bautista-Ortín, A.B.; Gómez-Plaza, E.; Anaya-Berríos, C. New Pyranoanthocyanins Synthesized from Roselle (Hibiscus sabdariffa L.) Anthocyanins. J. Mex. Chem. Soc. 2016, 60, 13–18. [Google Scholar] [CrossRef]
- Camelo-Méndez, G.A.; Jara-Palacios, M.J.; Escudero-Gilete, M.L.; Gordillo, B.; Hernanz, D.; Paredes-López, O.; Vanegas-Espinoza, P.E.; Del Villar-Martínez, A.A.; Heredia, F.J. Comparative Study of Phenolic Profile, Antioxidant Capacity, and Color-Composition Relation of Roselle Cultivars with Contrasting Pigmentation. Plant Foods Hum. Nutr. 2016, 71, 109–114. [Google Scholar] [CrossRef]
- Yousef, H.M.A.; Amina, M. Essential Oil of Coffee arabica L. Husks: A Brilliant Source of Antimicrobial and Antioxidant Agents. Biomed. Res. 2018, 29, 174–180. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Michiu, D.; Socaciu, M.-I.; Fogarasi, M.; Jimborean, A.M.; Ranga, F.; Mureşan, V.; Semeniuc, C.A. Implementation of an Analytical Method for Spectrophotometric Evaluation of Total Phenolic Content in Essential Oils. Molecules 2022, 27, 1345. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Francis, F.J.; Markakis, P.C. Food Colorants: Anthocyanins. Crit. Rev. Food Sci. Nutr. 1989, 28, 273–314. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Food Sci. Technol. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Brenner, S. The Genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Stiernagle, T. Maintenance of C. elegans; WormBook. 2006. Available online: http://www.wormbook.org/chapters/www_strainmaintain/strainmaintain (accessed on 10 October 2022).
- Solis, G.M.; Petrascheck, M. Measuring Caenorhabditis elegans Life Span in 96 Well Microtiter Plates. J. Vis. Exp. JoVE 2011, 49, e2496. [Google Scholar] [CrossRef]
- Colmenares, D.; Sun, Q.; Shen, P.; Yue, Y.; McClements, D.J.; Park, Y. Delivery of Dietary Triglycerides to Caenorhabditis elegans Using Lipid Nanoparticles: Nanoemulsion-Based Delivery Systems. Food Chem. 2016, 202, 451–457. [Google Scholar] [CrossRef]
- Porta-de-la-Riva, M.; Fontrodona, L.; Villanueva, A.; Cerón, J. Basic Caenorhabditis elegans Methods: Synchronization and Observation. J. Vis. Exp. JoVE 2012, 64, e4019. [Google Scholar] [CrossRef]
- Pang, S.; Curran, S.P. Adaptive Capacity to Bacterial Diet Modulates Aging in C. elegans. Cell Metab. 2014, 19, 221–231. [Google Scholar] [CrossRef]
- Chen, W.; Rezaizadehnajafi, L.; Wink, M. Influence of Resveratrol on Oxidative Stress Resistance and Life Span in Caenorhabditis elegans. J. Pharm. Pharmacol. 2013, 65, 682–688. [Google Scholar] [CrossRef]
- Chen, F.; Wei, C.; Chen, Q.; Zhang, J.; Wang, L.; Zhou, Z.; Chen, M.; Liang, Y. Internal Concentrations of Perfluorobutane Sulfonate (PFBS) Comparable to Those of Perfluorooctane Sulfonate (PFOS) Induce Reproductive Toxicity in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2018, 158, 223–229. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozada-Ramírez, J.D.; Guerrero-Moras, M.C.; González-Peña, M.A.; Silva-Pereira, T.S.; Anaya de Parrodi, C.; Ortega-Regules, A.E. Stabilization of Anthocyanins from Coffee (Coffea arabica L.) Husks and In Vivo Evaluation of Their Antioxidant Activity. Molecules 2023, 28, 1353. https://doi.org/10.3390/molecules28031353
Lozada-Ramírez JD, Guerrero-Moras MC, González-Peña MA, Silva-Pereira TS, Anaya de Parrodi C, Ortega-Regules AE. Stabilization of Anthocyanins from Coffee (Coffea arabica L.) Husks and In Vivo Evaluation of Their Antioxidant Activity. Molecules. 2023; 28(3):1353. https://doi.org/10.3390/molecules28031353
Chicago/Turabian StyleLozada-Ramírez, José Daniel, María Cristina Guerrero-Moras, Marco Antonio González-Peña, Taisa Sabrina Silva-Pereira, Cecilia Anaya de Parrodi, and Ana E. Ortega-Regules. 2023. "Stabilization of Anthocyanins from Coffee (Coffea arabica L.) Husks and In Vivo Evaluation of Their Antioxidant Activity" Molecules 28, no. 3: 1353. https://doi.org/10.3390/molecules28031353
APA StyleLozada-Ramírez, J. D., Guerrero-Moras, M. C., González-Peña, M. A., Silva-Pereira, T. S., Anaya de Parrodi, C., & Ortega-Regules, A. E. (2023). Stabilization of Anthocyanins from Coffee (Coffea arabica L.) Husks and In Vivo Evaluation of Their Antioxidant Activity. Molecules, 28(3), 1353. https://doi.org/10.3390/molecules28031353





