The Potential Value of Debarking Water as a Source of Polyphenolic Compounds for the Specialty Chemicals Sector
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of the Polyphenolic Compounds by HPLC-DAD-MS/MS
2.2. Polyphenolic Compounds in Debarking Water, Bark Press Water, and Bark Extracts
2.3. DPPH Tests
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Sample Collection
3.3. Sample Collection
3.4. LC-MS/MS Analysis
3.5. Radical Scavenging Activity Measured Using DPPH Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CEPI. Confideration of European Paper Industry Key Statistics 2020: European Pulp & Paper Industry. 2021. Available online: https://www.cepi.org/wp-content/uploads/2021/07/Key-Stats-2020-FINAL.pdf (accessed on 6 July 2022).
- Bajpai, P. Environmentally Friendly Production of Pulp and Paper; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; p. 365. [Google Scholar]
- Sjöström, E. Wood Chemistry: Fundamentals and Applications, 2nd ed.; Academic Press: San Diego, CA, USA, 1993; p. 293. [Google Scholar]
- Kylliäinen, O.; Holmbom, B. Chemical composition of components in spruce bark waters. Pap. Puu 2004, 86, 289–292. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Food 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Lea, A.G.H. Flavor, color, and stability in fruit products: The effect of polyphenols. In Plant Polyphenols; Hemingway, R.W., Laks, P.E., Eds.; Springer Science + Business Media, LCC: New York, NY, USA, 1992; pp. 827–847. [Google Scholar]
- Bajpai, P. Green Chemistry and Sustainability in Pulp and Paper Industry; Springer International Publishing: Cham, Switzerland, 2015; p. 258. [Google Scholar]
- Sadeer, N.B.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Foti, M.C. Use and abuse of the DPPH• radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Coșarcă, S.-L.; Moacă, E.-A.; Tanase, C.; Muntean, D.L.; Pavel, I.Z.; Dehelean, C.A. Spruce and beech bark aqueous extracts: Source of polyphenols, tannins and antioxidants correlated to in vitro antitumor potential on two different cell lines. Wood Sci. Technol. 2019, 53, 313–333. [Google Scholar] [CrossRef]
- Mulat, D.G.; Latva-Mäenpää, H.; Koskela, H.; Saranpää, P.; Wähälä, K. Rapid chemical characterisation of stilbenes in the root bark of Norway spruce by off-line HPLC/DAD-NMR. Phytochem. Anal. 2014, 5, 529–536. [Google Scholar] [CrossRef]
- Co, M.; Fagerlund, A.; Engman, L.; Sunnerheim, K.; Sjöberg, P.J.R.; Turner, C. Extraction of antioxidants from spruce (Picea abies) bark using eco-friendly solvents. Phytochem. Anal. 2012, 23, 1–11. [Google Scholar] [CrossRef]
- Spinelli, S.; Costa, C.; Conte, A.; La Porta, N.; Padalino, L.; Del Nobile, M.A. Bioactive compounds from Norway spruce bark: Comparison among sustainable extraction techniques for potential food applications. Foods 2019, 8, 524. [Google Scholar] [CrossRef]
- Strižincová, P.; Ház, A.; Burčová, Z.; Feranc, J.; Kreps, F.; Šurina, I.; Jablonský, M. Spruce Bark—A source of polyphenolic compounds: Optimizing the operating conditions of supercritical carbon dioxide extraction. Molecules 2019, 24, 4049. [Google Scholar] [CrossRef] [PubMed]
- Jablonský, M.; Vernarecová, M.; Ház, A.; Dubinyová, L.; Škulcová, A.; Sladková, A.; Šurina, I. Extraction of phenolic and lipophilic compounds from spruce (Picea abies) bark using accelerated solvent extraction by ethanol. Wood Res. 2015, 60, 583–590. [Google Scholar]
- Ghitescu, R.-E.; Volf, I.; Carausu, C.; Bühlmann, A.-M.; Gilca, I.A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef]
- Burcova, Z.; Kreps, F.; Strižincová, P.; Ház, A.; Jablonský, M.; Šurina, I.; Schmidt, Š. Spruce bark as a source of antioxidant active substances. BioResources 2019, 14, 5980–5987. [Google Scholar]
- Hammerbacher, A.; Paetz, C.; Wright, L.P.; Fischer, T.C.; Bohlmann, J.; Davis, A.J.; Fenning, T.M.; Gershenzon, J.; Schmidt, A. Flavan-3-ols in Norway spruce: Biosynthesis, accumulation, and function in response to attack by the bark beetle-associated fungus Ceratocystis polonica. Plant Physiol. 2014, 164, 2107–2122. [Google Scholar] [CrossRef] [PubMed]
- Francezon, N.; Romaric Meda, N.-S.-B.; Stevanovic, T. Optimization of bioactive polyphenols extraction from Picea mariana bark. Molecules 2017, 22, 2118. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, M.-E.; Royer, M.; Herbette, G.; Desjardins, Y.; Pouliot, R.; Stevanovic, T. Picea mariana bark: A new source of trans-resveratrol and other bioactive polyphenols. Food Chem. 2012, 135, 1173–1182. [Google Scholar] [CrossRef]
- Krogell, J.; Holmbom, B.; Pranovich, A.; Hemming, J.; Willför, S. Extraction and chemical characterization of Norway spruce inner and outer bark. Nord. Pulp Pap. Res. J. 2012, 27, 6–17. [Google Scholar] [CrossRef]
- Bianchi, S.; Glöss, A.N.; Kroslakova, I.; Mayer, I.; Pichelin, F. Analysis of the structure of condensed tannins in water extracts from bark tissues of Norway spruce (Picea abies [Karst.]) and Silver fir (Abies alba [Mill.]) using MALDI-TOF mass spectrometry. Ind. Crops Prod. 2014, 61, 430–437. [Google Scholar] [CrossRef]
- Bianchi, S.; Kroslakova, I.; Janzon, R.; Mayer, I.; Saake, B.; Pichelin, F. Characterization of condensed tannins and carbohydrates in hot water bark extracts of European softwood species. Phytochemistry 2015, 120, 53–61. [Google Scholar] [CrossRef]
- Bianchi, S.; Koch, G.; Janzon, R.; Mayer, I.; Saake, B.; Pichelin, F. Hot water extraction of Norway spruce (Picea abies [Karst.]) bark: Analyses of the influence of bark aging and process parameters on the extract composition. Holzforschung 2016, 70, 619–631. [Google Scholar] [CrossRef]
- Routa, J.; Brännström, H.; Anttila, P.; Mäkinen, M.; Jänis, J.; Asikainen, A. Wood extractives of Finnish pine, spruce and birch—Availability and optimal sources of compounds: A literature review. In Natural Resources and Bioeconomy Studies 73/2017; Natural Resources Institute Finland: Helsinki, Finland, 2017; p. 55. [Google Scholar]
- Tanase, C.; Cosarcă, S.; Muntean, D.L. A critical review of phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef]
- Multia, E. Potential and Utilization of Water Extracts from Spruce Bark. Master’s Thesis, Aalto University, Espoo, Finland, 16 April 2018. [Google Scholar]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of polyphenols from agri-food by-products: The olive oil and winery industries cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Gamaleldin, M.; Karar, E.; Kuhnert, N. UPLC-ESI-Q-TOF-MS/MS Characterization of phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) leaves, fruits and their herbal derived drops (Crataegutt Tropfen). J. Chem. Biol. Ther. 2015, 1, 102. [Google Scholar]
- Zhang, B.; Cai, J.; Duan, C.-Q.; Reeves, M.J.; He, F. A review of polyphenolics in oak woods. Int. J. Mol. Sci. 2015, 16, 6978–7014. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.A.; Pereira do Nascimento, E.S.; Cardoso, D.R.; Wagner Franco, D. Coumarins and phenolic fingerprints of oak and Brazilian woods extracted by sugarcane spirit. J. Sep. Sci. 2009, 32, 3681–3691. [Google Scholar] [CrossRef]
- Karna, K.K.; Choi, B.R.; You, J.H.; Shin, Y.S.; Cui, W.S.; Lee, S.W.; Kim, J.H.; Kim, C.Y.; Kim, H.K.; Park, J.K. The ameliorative effect of monotropein, astragalin, and spiraeoside on oxidative stress, endoplasmic reticulum stress, and mitochondrial signaling pathway in varicocelized rats. BMC Complement. Altern. Med. 2019, 19, 333. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, L.; Ye, Y.; Zhu, M.; Li, J.; Tu, Z.; Yang, S.; Liao, H. Phytochemical profiles and screening of α-glucosidase inhibitors of four Acer species leaves with ultra-filtration combined with UPLC-QTOF-MS/MS. Ind. Crops Prod. 2019, 129, 156–168. [Google Scholar] [CrossRef]
- Mansikkala, T.; Patanen, M.; Karkonen, A.; Korpinen, R.; Pranovich, A.; Ohigashi, T.; Swaraj, S.; Seitsonen, J.; Ruokolainen, J.; Huttula, M.; et al. Lignans in knotwood of Norway spruce: Localisation with soft X-ray microscopy and scanning transmission electron microscopy with energy dispersive X-ray spectroscopy. Molecules 2020, 25, 2997. [Google Scholar] [CrossRef]
- Metsämuuronen, S.; Sirén, H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019, 18, 623–664. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A. Green and sustainable valorization of bioactive phenolic compounds from Pinus by-products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Fritsch, C.; Cosgun, S.; Dumarcay, S.; Colin, F.; Gérardin, P. Quantitative and qualitative composition of bark polyphenols changes longitudinally with bark maturity in Abies alba Mill. Ann. For. Sci. 2020, 77, 9. [Google Scholar] [CrossRef]
- Sut, S.; Baldan, V.; Faggian, M.; Ferrarese, I.; Maccari, E.; Teobaldo, E.; De Zordi, N.; Bertoni, P.; Peron, G.; Dall’Acqua, S. The bark of Picea abies L., a waste from sawmill, as a source of valuable compounds: Phytochemical investigations and isolation of a novel pimarane and a stilbene derivative. Plants 2021, 10, 2106. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Long, J.; Li, Y.; Ye, L.; Yin, B.; France, L.J.; Dong, J.; Zheng, L.; He, H.; Liu, S.; et al. Selective production of diethyl maleate via oxidative cleavage of lignin aromatic unit. Chem 2019, 5, 2365–2377. [Google Scholar] [CrossRef]
- Zeppetzauer, F.; Nadányi, R.; Kamm, B.; Putz, R.; Lisý, A.; Šurina, I. Investigation of selective extraction of phenolic compounds and of saccharides from Picea abies bark using organosolv solvents. Preprints 2021, 1, 2021100095. [Google Scholar]
- Talmaciu, A.I.; Volf, I.; Popa, V.I. supercritical fluids and ultrasound assisted extractions applied to spruce bark conversion. Environ. Eng. Manag. J. 2015, 14, 615–623. [Google Scholar]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Routa, J.; Brännström, H.; Hellström, J.; Laitila, J. Influence of storage on the physical and chemical properties of Scots pine bark. BioEnergy Res. 2021, 14, 575–587. [Google Scholar] [CrossRef]
- Chen, Z.; Bertin, R.; Froldi, G. EC50 estimation of antioxidant activity in DPPH· assay using several statistical programs. Food Chem. 2013, 138, 414–420. [Google Scholar] [CrossRef]
- Žegura, B.; Dobnik, D.; Niderl, M.H.; Filipič, M. Antioxidant and antigenotoxic effects of rosemary (Rosmarinus officinalis L.) extracts in Salmonella typhimurium TA98 and HepG2 cells. Environ. Toxicol. Pharmacol. 2011, 32, 296–305. [Google Scholar] [CrossRef]
- Alfei, B.; Pannelli, G.; Ricci, A. Olivicoltura; Edizione Agricole de il Sole: Milan, Italy, 2013; p. 429. [Google Scholar]
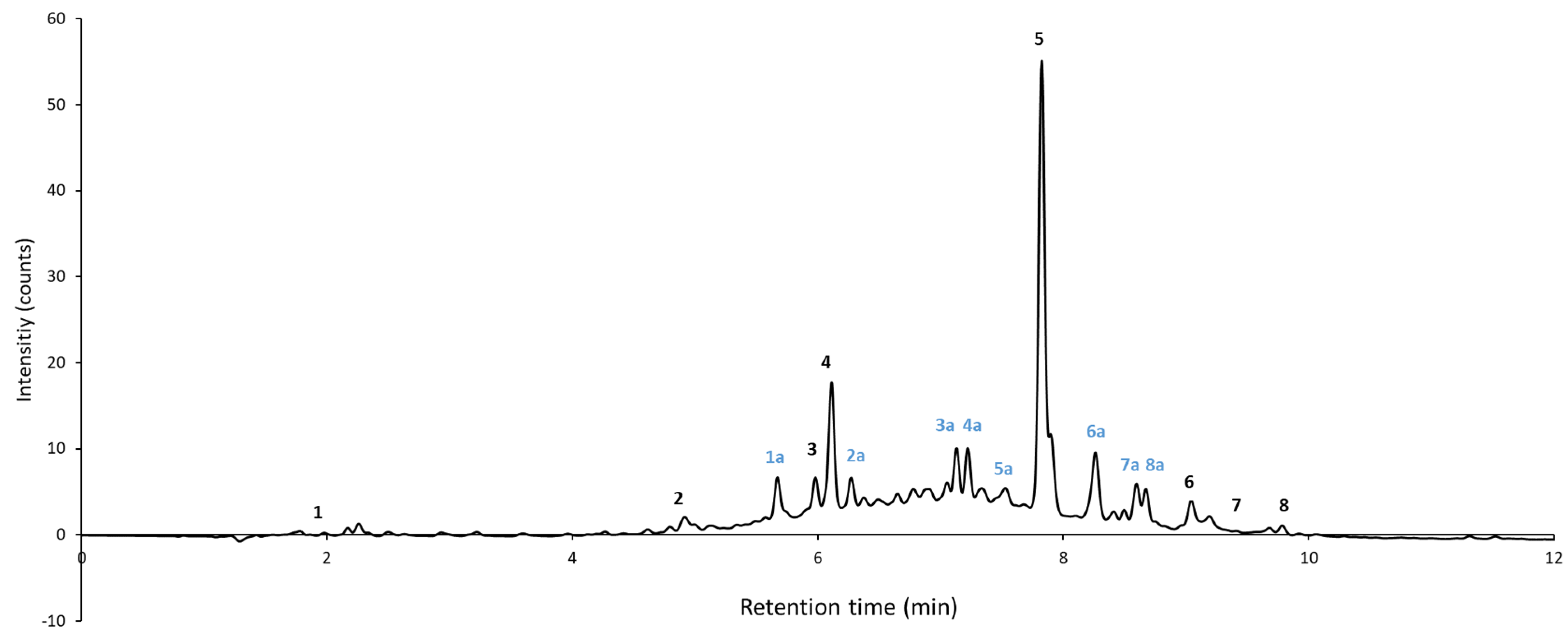
| Peak No. | Retention Time | Identity Compound | m/z | Detected Fragments | Fragments Found in Literature |
|---|---|---|---|---|---|
| 1 | 2.0 | Quinic acid | 191.0555 | 173, 127, 111, 93, 87, 85 | 173, 127, 111, 85 |
| 2 | 4.9 | Protocatechuic acid | 153.0182 | 153, 109 | 153, 109 |
| 3 | 6.0 | Catechin | 289.0713 | 289, 245, 205, 203, 179, 125, 109 | 289, 271, 245 |
| 4 | 6.1 | Ferulic acid | 193.05 | 193, 178, 149, 134 | 178, 149, 134 |
| 5 | 7.8 | Dihydrorobinetin | 303.0506 | 303, 285 | 303, 285 |
| 6 | 9.0 | Quercitrin | 447.0927 | 301 | 300, 301, 271, 255, 151 |
| 7 | 9.4 | Spiraeoside | 463.0873 | 301 | 301 |
| 8 | 9.8 | Isorhamnetin rhamnoside | 461.1086 | 315 | 315 |
| Peak No. | Retention Time | m/z | Detected Fragments | Possible Identity Compound |
|---|---|---|---|---|
| 1a | 5.7 | 371.0970 | 325, 205, 163, 119, 45 | Oxomatairesinol |
| 2a | 6.3 | 443.1320 | 443, 113, 101, 89, 71, 59 | Unknown |
| 3a | 7.3 | 451.1248 | 406, 405, 243, 201, 199, 173, 159 | (Epi)catechin-O-glucoside |
| 4a | 7.3 | 441.0954 | 441, 405, 243, 201, 159, 113, 112, | (Epi)catechin monogallate |
| 5a | 7.5 | 525.1978 | 327, 179, 167, 161, 146, 134, 119, 89 | Isorhamnetin glucoside |
| 6a | 8.3 | 373.1506 | 373, 281, 161, 143, 101, 45 | (Iso)hdydroxymatairesinol |
| 7a | 8.5 | 647.1764 | 647, 605, 600, 485,435, 401, 361, 309, 241 | Piceatannol derivative |
| 8a | 8.8 | 171.0734 | 171, 145, 127, 103, 83 | Diethyl maleate |
| Retention Time | Identity Compound | m/z | Detected Fragments | Fragments in Literature |
|---|---|---|---|---|
| 4.8 | Syringaldehyde | 181.0499 | 181, 151 | 181, 166, 151 |
| 5.2 | Dihydroxyphenylacetic acid | 167.0342 | 123 | 123 |
| 6.7 | Vanillin | 151.0391 | 151, 136, 108, 107 | 151, 137, 136, 123, 108, 107 |
| 6.2 and 7.2 | Oxyresveratrol | 243.0652 | 225, 199, 157 | 225, 199, 157, 133, 115 |
| 7.8 | P-coumaric acid | 163.0395 | 163, 119 | 163, 119, 94 |
| 7.8 and 8.1 | Aromadendrin-rhamnoside | 433.1148 | 269, 179, 151 | 287, 269, 259, 180, 179, 151 |
| 9.2 | Isorhamnetin-O-hexoside | 477.1043 | 315 | 315 |
| 10.4 | Robinetin | 301.0359 | 301, 273, 245 | 301, 273, 245, 229, 135, 91 |
| 13.2 | Eugenol | 209.0808 | 163 | 163, 149, 147, 137 |
| Compound (RT in min) | PC | PW | DC | DW |
|---|---|---|---|---|
| Quinic acid | 85,989 ± 1912 | 81,331 ± 2084 | 83,169 ± 2719 | |
| Protocatechuic acid (isomer 1) | <LOQ | <LOQ | ||
| Syringaldehyde | 6539 ± 581 | <LOQ | <LOQ | <LOQ |
| Protocatechuic acid (isomer 2) | 16,204 ± 1184 | 23,408 ± 695 | <LOQ | 11,451 ± 1292 |
| Catechin | 8461 ± 1419 | >LOQ | ||
| Ferulic acid | 13,006 ± 523 | 15,998 ± 767 | 9151 ± 1304 | |
| Vanillin | <LOQ | |||
| Oxyresveratrol | <LOQ | |||
| P-coumaric acid | 10,106 ± 2511 | <LOQ | ||
| Isorhamnetin-O-hexoside | <LOQ | |||
| Spiraeoside | <LOQ | |||
| Eugenol | 6431 ± 727 | 9070 ± 247 | ||
| Total polyphenol concentration (mg/mL) | 12.99 ± 0.22 | 15.55 ± 0.81 | 1.95 ± 0.02 | 4.76 ± 0.35 |
| Compound | Bark Cold Water Extraction | Bark Ethanol/Water (1:1) Extraction | Bark Ethanol Extraction | Bark Hot Water Extraction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (RT in min) | 2 min | 5 min | 30 min | 2 min | 5 min | 30 min | 2 min | 5 min | 30 min | 2 min | 5 min | 30 min |
| Quinic acid | 91,055 ± 5082 | 88,490 ± 4899 | 97,538 ± 5933 | 73,973 ± 4246 | 80,280 ± 1328 | 81,208 ± 2909 | 3734 ± 817 | 6093 ± 451 | 8290 ± 633 | 87,926 ± 2482 | 84,382 ± 4714 | 90,067 ± 570 |
| Syringaldehyde | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | |||||||
| Protocatechuic acid | 8274 ± 778 | 9894 ± 1117 | 11,650 ± 581 | 9432 ± 886 | 10,745 ± 214 | 10,132 ± 93 | 1011 ± 14 | 2090 ± 381 | 3696 ± 871 | 10,773 ± 361 | 11,477 ± 424 | 14,670 ± 1067 |
| Dihydroxyphenylacetic acid | <LOQ | |||||||||||
| Catechin | 17,701 ± 729 | 17,458 ± 1583 | 18,348 ± 1169 | 10,004 ± 670 | 13,353 ± 567 | 16,185 ± 105 | <LOQ | |||||
| Ferulic acid | 7771 ± 857 | 7388 ± 416 | 9443 ± 220 | 7557 ± 261 | 6633 ± 871 | 7490 ± 85 | <LOQ | <LOQ | 7731 ± 80 | 8325 ± 763 | 8043 ± 57 | |
| Aromadendrin-rhamnoside | <LOQ | |||||||||||
| Dihydrorobinetin | 8214 ± 65 | 9153 ± 2554 | 16,081 ± 522 | 42,783 ± 4072 | 47,511 ± 3313 | 49,642 ± 894 | 20,044 ± 1785 | 27,636 ± 3964 | 44,938 ± 1006 | 10,325 ± 3840 | 14,068 ± 5642 | 14,914 ± 2224 |
| Oxyresveratrol | <LOQ | 15,509 ± 2885 | 17,221 ± 3324 | |||||||||
| Quercitrin | 3582 ± 911 | 3902 ± 784 | 5816 ± 419 | 15,565 ± 1036 | 16,300 ± 732 | 17,460 ± 296 | 5003 ± 834 | 6667 ± 323 | 9063 ± 596 | 4549 ± 299 | 5775 ± 192 | 7951 ± 94 |
| Isorhamnetin-hexoside | <LOQ | <LOQ | <LOQ | |||||||||
| Spiraeoside | <LOQ | 29,498 ± 1639 | 30,412 ± 4680 | 30,695 ± 1565 | 14,107 ± 2418 | 17,537 ± 695 | 22,268 ± 671 | <LOQ | ||||
| Isorhamnetin-O-rhamnoside | <LOQ | < LOQ | 9333 ± 179 | 9493 ± 503 | <LOQ | 3243 ± 24 | 3873 ± 53 | 5688 ± 88 | ||||
| Robinetin | 1089 ± 217 | 1404 ± 139 | 1528 ± 136 | |||||||||
| Total polyphenol concentration (mg/mL) | 7.24 ± 1.11 | 7.07 ± 1.75 | 9.21 ± 0.55 | 15.86 ± 2.27 | 16.35 ± 1.62 | 16.74 ± 0.30 | 6.84 ± 0.41 | 9.39 ± 0.47 | 13.18 ± 2.03 | 7.54 ± 0.05 | 7.29 ± 0.01 | 9.41 ± 0.64 |
| Sample | 0.2 µm Filtered Samples | 3000 Da Filtered Samples | ||
|---|---|---|---|---|
| EC50 (µg/mL) | mg GAE/g (Dry Mass) | EC50 (µg/mL) | mg GAE/g (Dry Mass) | |
| CW2 | 154 ± 25 | 3.23 ± 0.97 | 560 ± 28 | 1.26 ± 0.06 |
| CW5 | 203 ± 16 | 2.24 ± 0.16 | 920 ± 46 | 0.48 ± 0.02 |
| CW30 | 213 ± 13 | 1.96 ± 0.22 | 971 ± 49 | 0.42 ± 0.02 |
| CWE2 | 77 ± 24 | 6.90 ± 2.06 | 425 ± 21 | 1.02 ± 0.05 |
| CWE5 | 105 ± 4 | 4.27 ± 1.70 | 450 ± 23 | 0.49 ± 0.02 |
| CWE30 | 85 ± 16 | 4.54 ± 1.27 | 432 ± 22 | 0.51 ± 0.03 |
| E2 | 317 ± 21 | 1.26 ± 0.05 | 332 ± 17 | 1.38 ± 0.07 |
| E5 | 238 ± 81 | 1.31 ± 0.81 | 166 ± 8 | 2.26 ± 0.11 |
| E30 | 161 ± 13 | 1.84 ± 0.99 | 153 ± 8 | 1.41 ± 0.07 |
| HW2 | 108 ± 5 | 5.27 ± 0.90 | 584 ± 29 | 0.86 ± 0.04 |
| HW5 | 113 ± 16 | 4.13 ± 0.23 | 901 ± 45 | 0.51 ± 0.03 |
| HW30 | 167 ± 66 | 2.40 ± 0.62 | 1030 ± 52 | 0.34 ± 0.02 |
| PC | 134 ± 1 | 4.02 ± 0.70 | 391 ± 20 | 1.73 ± 0.09 |
| PW | 136 ± 5 | 3.53 ± 0.15 | 391 ± 20 | 1.04 ± 0.05 |
| DC | 3333 ± 590 | 0.17 ± 0.04 | 3006 ± 150 | 0.18 ± 0.01 |
| DW | 169 ± 18 | 1.62 ± 0.12 | 1340 ± 67 | 0.24 ± 0.01 |
| Sample | Temperature | Time (min) | Solvent |
|---|---|---|---|
| CW2 | TR | 2 | Distilled water |
| CW5 | TR | 5 | Distilled water |
| CW30 | TR | 30 | Distilled water |
| CWE2 | TR | 2 | Deionised water and ethanol (50:50) |
| CWE5 | TR | 5 | Deionised water and ethanol (50:50) |
| CWE30 | TR | 30 | Deionised water and ethanol (50:50) |
| E2 | TR | 2 | Ethanol |
| E5 | TR | 5 | Ethanol |
| E30 | TR | 30 | Ethanol |
| HW2 | 80 °C | 2 | Deionised water |
| HW5 | 80 °C | 5 | Deionised water |
| HW30 | 80 °C | 30 | Deionised water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peeters, K.; Esakkimuthu, E.S.; Tavzes, Č.; Kramberger, K.; Miklavčič Višnjevec, A. The Potential Value of Debarking Water as a Source of Polyphenolic Compounds for the Specialty Chemicals Sector. Molecules 2023, 28, 542. https://doi.org/10.3390/molecules28020542
Peeters K, Esakkimuthu ES, Tavzes Č, Kramberger K, Miklavčič Višnjevec A. The Potential Value of Debarking Water as a Source of Polyphenolic Compounds for the Specialty Chemicals Sector. Molecules. 2023; 28(2):542. https://doi.org/10.3390/molecules28020542
Chicago/Turabian StylePeeters, Kelly, Esakkiammal Sudha Esakkimuthu, Črtomir Tavzes, Katja Kramberger, and Ana Miklavčič Višnjevec. 2023. "The Potential Value of Debarking Water as a Source of Polyphenolic Compounds for the Specialty Chemicals Sector" Molecules 28, no. 2: 542. https://doi.org/10.3390/molecules28020542
APA StylePeeters, K., Esakkimuthu, E. S., Tavzes, Č., Kramberger, K., & Miklavčič Višnjevec, A. (2023). The Potential Value of Debarking Water as a Source of Polyphenolic Compounds for the Specialty Chemicals Sector. Molecules, 28(2), 542. https://doi.org/10.3390/molecules28020542







