The Construction of Polycyclic Pyridones via Ring-Opening Transformations of 3-hydroxy-3,4-dihydropyrido[2,1-c][1,4]oxazine-1,8-diones
Abstract
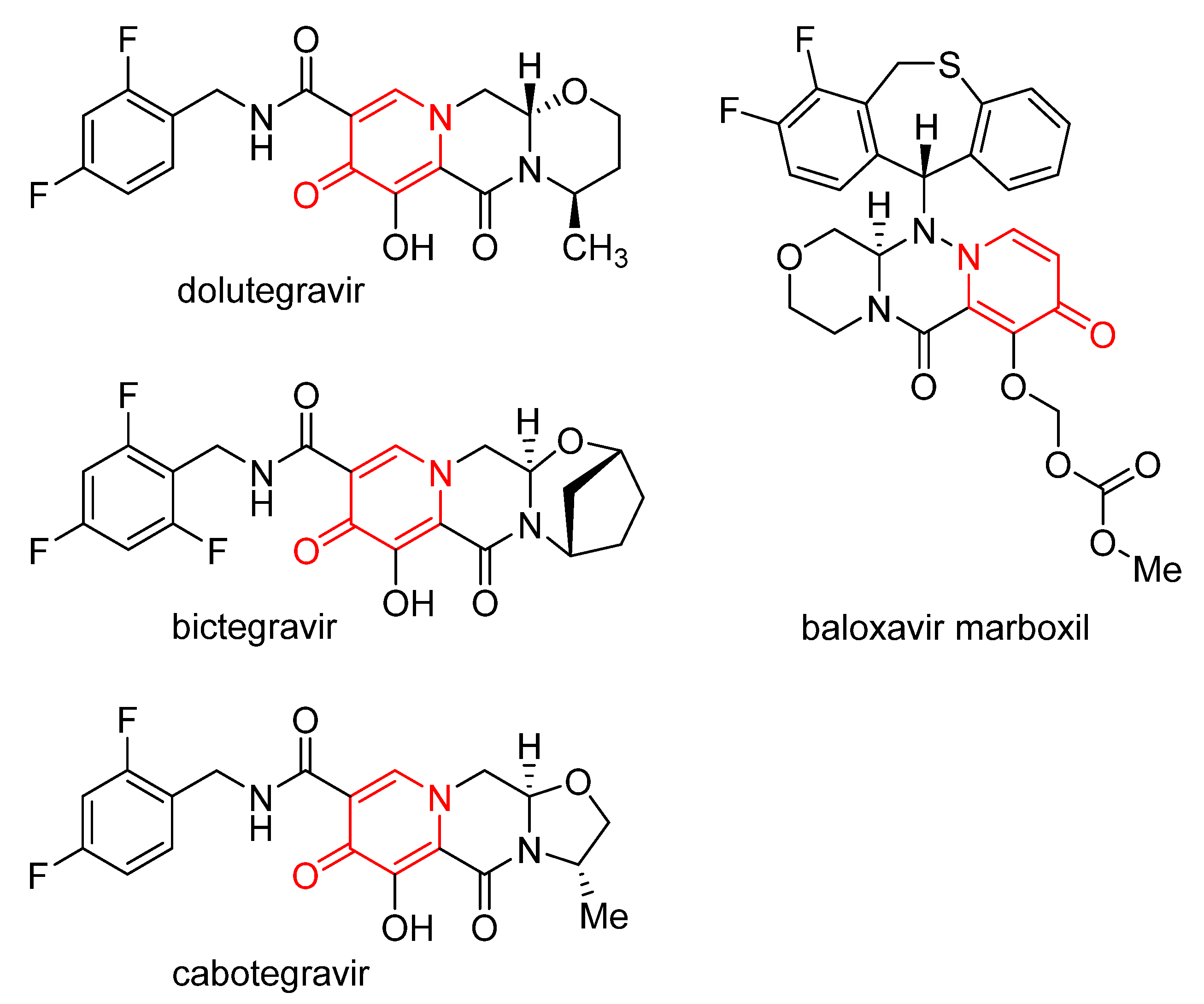
1. Introduction
2. Results and Discussion
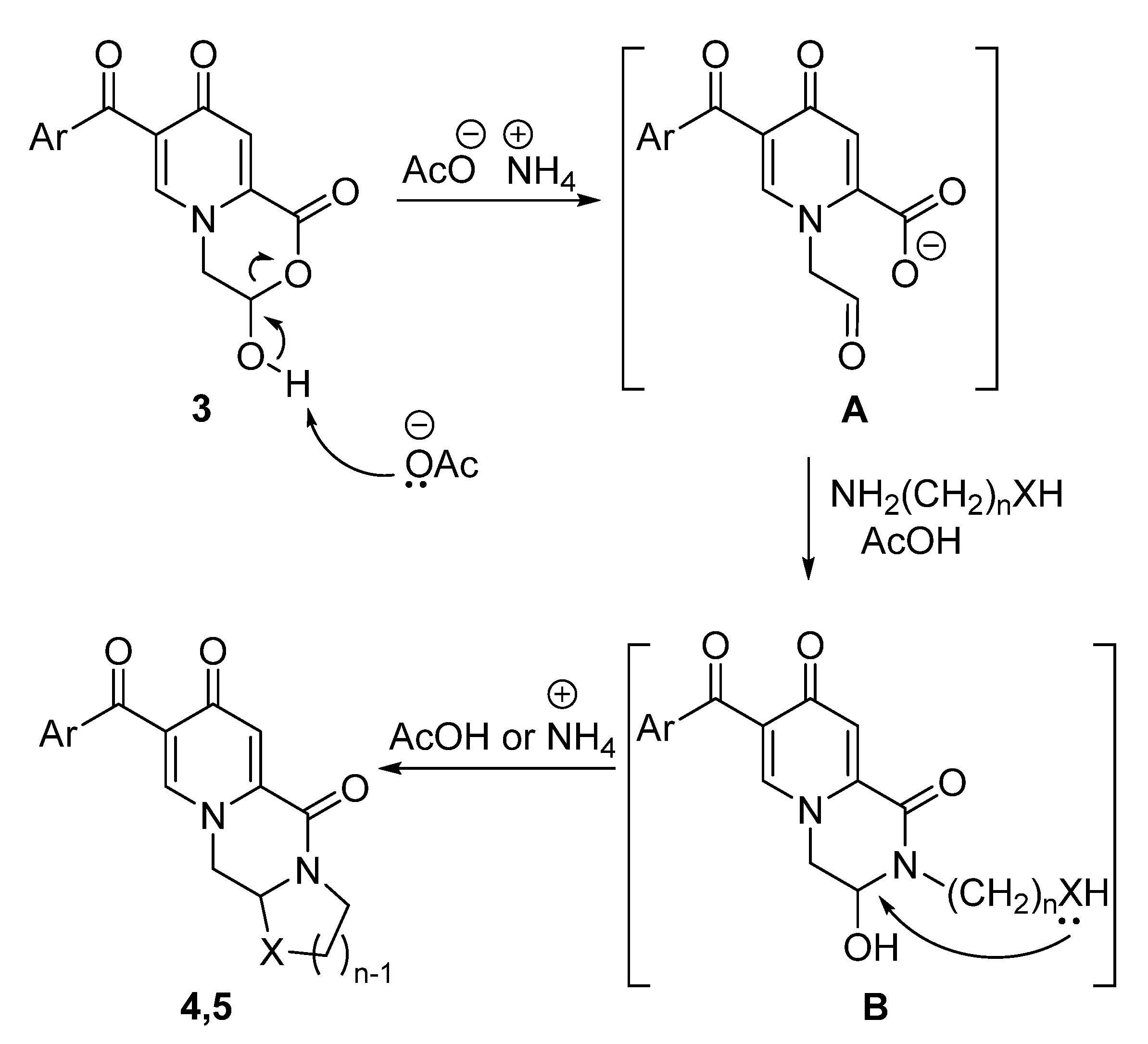
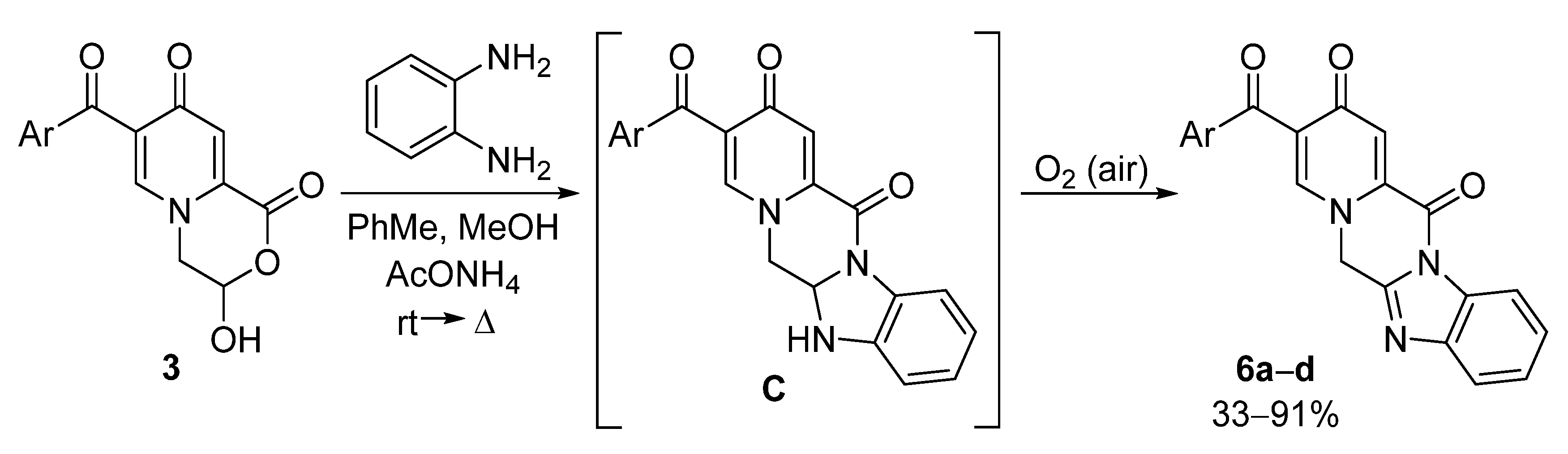
Synthesis of 3-hydroxy-3,4-dihydropyrido[2,1-c][1,4]oxazine-1,8-diones 3 and Their Chemical Properties
3. Materials and Methods
3.1. General Procedure for the Preparation of 1-(2,2-dimethoxyethyl)-4-pyridones 2
3.2. General Method for the Preparation of dihydropyrido[2,1-c][1,4]oxazine-1,8-diones 3
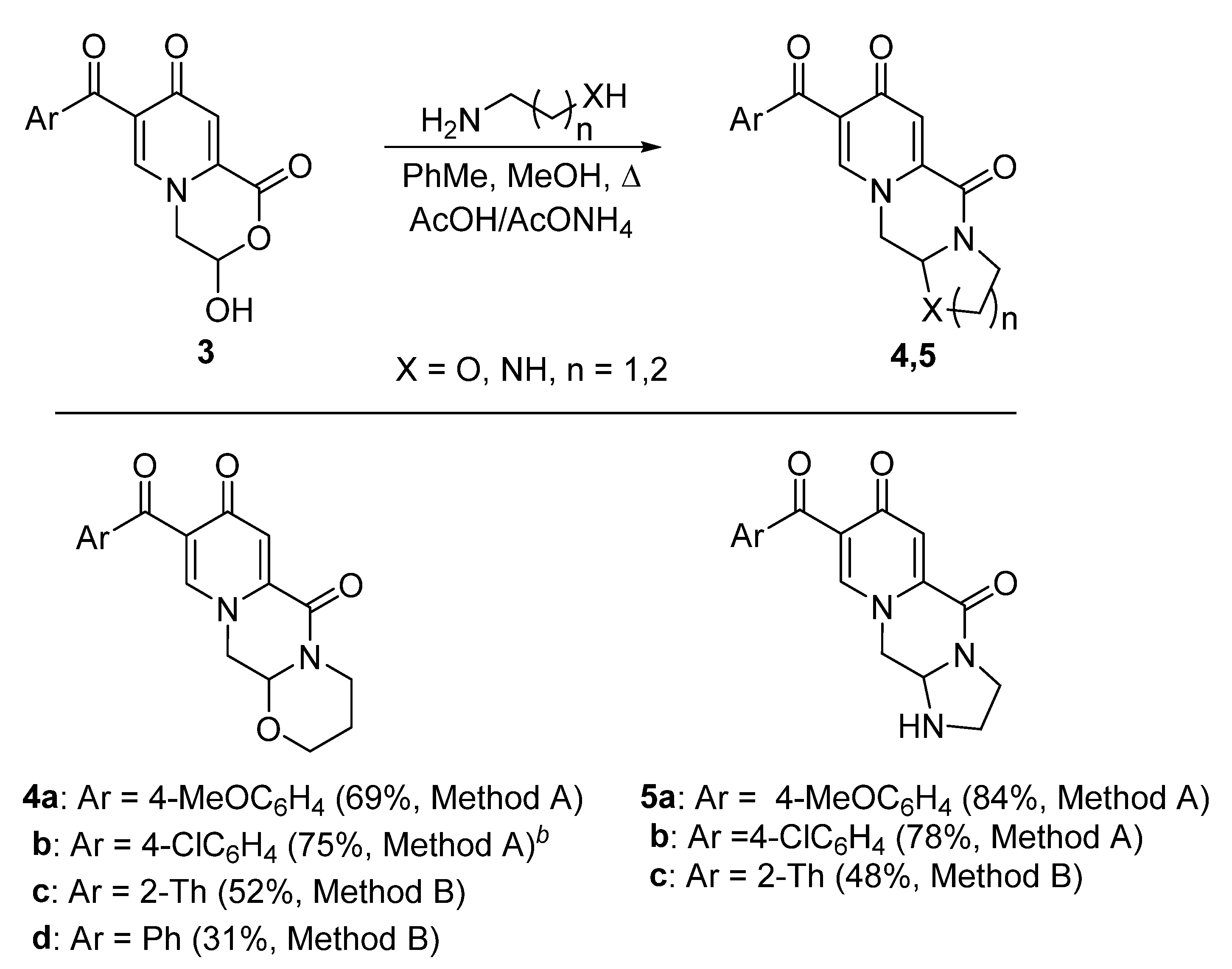
3.3. General Method for the Preparation of Compounds 4 and 5
3.4. General Method for the Preparation of Compound 6
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hughes, D.L. Review of synthetic routes and final forms of integrase inhibitors dolutegravir, cabotegravir, and bictegravir. Org. Process Res. Dev. 2019, 23, 716–729. [Google Scholar] [CrossRef]
- Hughes, D.L. Review of the patent literature: Synthesis and final forms of antiviral drugs tecovirimat and baloxavir marboxil. Org. Process Res. Dev. 2019, 23, 1298–1307. [Google Scholar] [CrossRef]
- Schreiner, E.; Richter, F.; Nerdinger, S. Development of synthetic routes to dolutegravir. Top. Heterocycl. Chem. 2016, 44, 187–208. [Google Scholar] [CrossRef]
- He, M.; Fan, M.; Peng, Z.; Wang, G. An overview of hydroxypyranone and hydroxypyridinone as privileged scaffolds for novel drug discovery. Eur. J. Med. Chem. 2021, 221, 113546. [Google Scholar] [CrossRef]
- Hayat, F.; Sonavane, M.; Makarov, M.V.; Trammell, S.A.J.; McPherson, P.; Gassman, N.R.; Migaud, M.E. The biochemical pathways of nicotinamide-derived pyridones. Int. J. Mol. Sci. 2021, 22, 1145. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-L.; Zhang, J.-Z.; Luo, D.-Q. The taxonomy, biology and chemistry of the fungal Pestalotiopsis genus. Nat. Prod. Rep. 2012, 29, 622–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kowalski, M.D.; Lakdawala, A.S.; Vogt, F.G.; Wu, L. An efficient and highly diastereoselective synthesis of GSK1265744, a potent HIV integrase inhibitor. Org. Lett. 2015, 17, 564–567. [Google Scholar] [CrossRef]
- Johns, B.A.; Kawasuji, T.; Weatherhead, J.G.; Taishi, T.; Temelkoff, D.P.; Yoshida, H.; Akiyama, T.; Taoda, Y.; Murai, H.; Kiyama, R.; et al. Carbamoyl pyridone HIV-1 integrase inhibitors 3. A diastereomeric approach to chiral nonracemic tricyclic ring systems and the discovery of dolutegravir (S/GSK1349572) and (S/GSK1265744). J Med. Chem. 2013, 56, 5901–5916. [Google Scholar] [CrossRef]
- Kawasuji, T.; Johns, B.A.; Yoshida, H.; Weatherhead, J.G.; Akiyama, T.; Taishi, T.; Taoda, Y.; Mikamiyama-Iwata, M.; Murai, H.; Kiyama, R.; et al. Carbamoyl pyridone HIV-1 integrase inhibitors. 2. Bi- and tricyclic derivatives result in superior antiviral and pharmacokinetic profiles. J. Med. Chem. 2013, 56, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Sankareswaran, S.; Mannam, M.; Chakka, V.; Mandapati, S.R.; Kumar, P. Identification and control of critical process impurities: An improved process for the preparation of dolutegravir sodium. Org. Process Res. Dev. 2016, 20, 1461–1468. [Google Scholar] [CrossRef]
- Sumino, Y.; Okamoto, K.; Masui, M.; Yamada, D.; Ikarashi, F. Process for Preparing Compound Having HIV Integrase Inhibitory Activity. WO Patent 018065, 9 February 2012. [Google Scholar]
- Maras, N.; Selic, L.; Cusak, A. Processes for Preparing Dolutegravir and Cabotegravir and Analogues Thereof. U.S. Patent 0368040, 11 July 2017. [Google Scholar]
- Srinivasachary, K.; Subbareddy, D.; Ramadas, C.; Balaji, S.K.K.; Somannavar, Y.S.; Ramadevi, B. Practical and efficient route to dolutegravir sodium via one-pot synthesis of key intermediate with controlled formation of impurities. Russ. J. Org. Chem. 2022, 58, 526–535. [Google Scholar] [CrossRef]
- Ziegler, R.E.; Desai, B.K.; Jee, J.-A.; Gupton, B.F.; Roper, T.D.; Jamison, T.F. 7-Step flow synthesis of the HIV integrase inhibitor dolutegravir. Angew. Chem. Int. Ed. 2018, 57, 7181–7185. [Google Scholar] [CrossRef] [PubMed]
- Dietz, J.-P.; Lucas, T.; Groß, J.; Seitel, S.; Brauer, J.; Ferenc, D.; Gupton, B.F.; Opatz, T. Six-step gram-scale synthesis of the human immunodeficiency virus integrase inhibitor dolutegravir sodium. Org. Process Res. Dev. 2021, 25, 1898–1910. [Google Scholar] [CrossRef]
- Kong, J.; Xia, H.; He, R.; Chen, H.; Yu, Y. Preparation of the key dolutegravir intermediate via MgBr2-promoted cyclization. Molecules 2021, 26, 2850. [Google Scholar] [CrossRef] [PubMed]
- Yasukata, T.; Masui, M.; Ikarashi, F.; Okamoto, K.; Kurita, T.; Nagai, M.; Sugata, Y.; Miyake, N.; Hara, S.; Adachi, Y.; et al. Practical synthetic method for the preparation of pyrone diesters: An efficient synthetic route for the synthesis of dolutegravir sodium. Org. Process Res. Dev. 2019, 23, 565–570. [Google Scholar] [CrossRef]
- Stojanović, M.; Bugarski, S.; Baranac-Stojanović, M. Synthesis of 2,3-dihydro-4-pyridones and 4-pyridones by the cyclization reaction of ester-tethered enaminones. J. Org. Chem. 2020, 85, 13495–13507. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Xiao, L.-Y.; Peng, Q.-Q.; Zhao, Y.-L. Thermally induced formal [4+2] cycloaddition of 3-aminocyclobutenones with electron-deficient alkynes: Facile and efficient synthesis of 4-pyridones. Chem. Commun. 2018, 54, 8229–8232. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, J.; Lin, J.; Liu, F.; Liu, T.; Huang, C. Substituent-controlled chemoselective synthesis of multi-substituted pyridones via a one-pot three-component cascade reaction. Org. Biomol. Chem. 2020, 18, 1130–1134. [Google Scholar] [CrossRef]
- Fedin, V.V.; Usachev, S.A.; Obydennov, D.L.; Sosnovskikh, V.Y. Reactions of trifluorotriacetic acid lactone and hexafluorodehydroacetic acid with amines: Synthesis of trifluoromethylated 4-pyridones and aminoenones. Molecules 2022, 27, 7098. [Google Scholar] [CrossRef]
- Zantioti-Chatzouda, E.-M.; Kotzabasaki, V.; Stratakis, M. Synthesis of γ-pyrones and N-methyl-4-pyridones via the Au nanoparticle-catalyzed cyclization of skipped diynones in the presence of water or aqueous methylamine. J. Org. Chem. 2022, 87, 8525–8533. [Google Scholar] [CrossRef]
- Ropero, B.P.F.D.; Elsegood, M.R.J.; Fairley, G.; Pritchard, G.J.; Weaver, G.W. Pyridone functionalization: Regioselective deprotonation of 6-methylpyridin-2(1H)- and -4(1H)-one derivatives. Eur. J. Org. Chem. 2016, 2016, 5238–5242. [Google Scholar] [CrossRef]
- Obydennov, D.L.; El-Tantawy, A.I.; Sosnovskikh, V.Y. Synthesis of multifunctionalized 2,3-dihydro-4-pyridones and 4-pyridones via the reaction of carbamoylated enaminones with aldehydes. J. Org. Chem. 2018, 83, 13776–13786. [Google Scholar] [CrossRef] [PubMed]
- Obydennov, D.L.; Chernyshova, E.V.; Sosnovskikh, V.Y. Acyclic enaminodiones in the synthesis of heterocyclic compounds. Chem. Heterocycl. Comp. 2020, 56, 1241–1253. [Google Scholar] [CrossRef]
- Diesel, J.; Finogenova, A.M.; Cramer, N. Nickel-catalyzed enantioselective pyridone C−H functionalizations enabled by a bulky N-heterocyclic carbene ligand. J. Am. Chem. Soc. 2018, 140, 4489–4493. [Google Scholar] [CrossRef] [PubMed]
- Kea, D.; Wu, Y.; Zhang, L.; Shao, J.; Yu, Y.; Chen, W. Group-assisted-purification chemistry strategy for the efficient assembly of cyclic fused pyridinones. Synthesis 2022, 54, 1765–1774. [Google Scholar] [CrossRef]
- Obydennov, D.L.; Roeschenthaler, G.-V.; Sosnovskikh, V.Y. An improved synthesis and some reactions of diethyl 4-oxo-4H-pyran-2,5-dicarboxylate. Tetrahedron Lett. 2013, 54, 6545–6548. [Google Scholar] [CrossRef]
- Obydennov, D.L.; Khammatova, L.R.; Steben’kov, V.D.; Sosnovskikh, V.Y. Synthesis of novel polycarbonyl Schiff bases by ring-opening reaction of ethyl 5-acyl-4-pyrone-2-carboxylates with primary mono- and diamines. RSC Adv. 2019, 9, 40072–40083. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, L.; Zhou, Y.; Zha, D.; Hai, Y.; You, L. Dynamic covalent reactions controlled by ring-chain tautomerism of 2-formylbenzoic acid. Eur. J. Org. Chem. 2022, 2022, e202101461. [Google Scholar] [CrossRef]
- Osyanin, V.A.; Osipov, D.V.; Semenova, I.A.; Korzhenko, K.S.; Lukashenko, A.V.; Demidov, O.P.; Klimochkin, Y.N. Eco-friendly synthesis of fused pyrano[2,3-b]pyrans via ammonium acetate-mediated formal oxa-[3 + 3] cycloaddition of 4H-chromene-3-carbaldehydes and cyclic 1,3-dicarbonyl compounds. RSC Adv. 2020, 10, 34344–34354. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; Wan, Y.; Huang, Y.; Huang, G.; Zhang, G. Ammonium acetate-promoted one-pot tandem aldol condensation/aza-addition reactions: Synthesis of 2,3,6,7-tetrahydro-1H-pyrrolo[3,2-c]pyridin-4(5H)-ones. ACS Omega 2017, 2, 6844–6851. [Google Scholar] [CrossRef]
- Obydennov, D.L.; Roeschenthaler, G.-V.; Sosnovskikh, V.Y. Synthesis of 6-aryl- and 5-aroylcomanic acids from 5-aroyl-2-carbethoxy-4-pyrones via a deformylative rearrangement and ring-opening/ring-closure sequence. Tetrahedron Lett. 2014, 55, 472–474. [Google Scholar] [CrossRef]
- Obydennov, D.L.; Goncharov, A.O.; Sosnovskikh, V.Y. Preparative synthesis of ethyl 5-acyl-4-pyrone-2-carboxylates and 6-aryl-, 6-alkyl-, and 5-acylcomanic acids on their basis. Russ. Chem. Bull. 2016, 65, 2233–2242. [Google Scholar] [CrossRef]

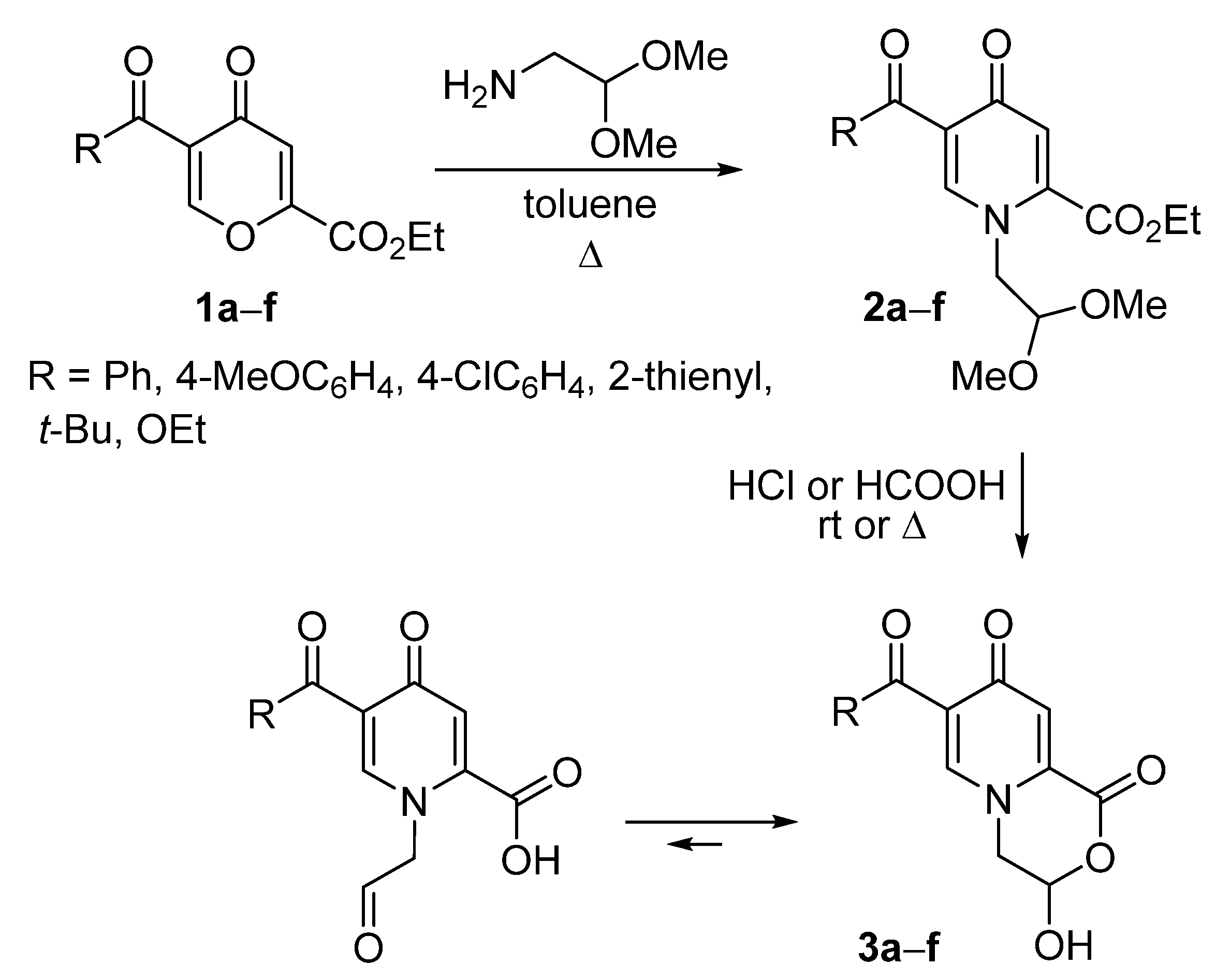
| Entry | Compound 2,3 | R | Yield of 2, % | Yield of 3, % |
|---|---|---|---|---|
| 1 | a | Ph | 90 | 53 |
| 2 | b | 4-MeOC6H4 | 63 | 85 |
| 3 | c | 4-ClC6H4 | 73 | 59 |
| 4 | d | 2-Th | 72 | 84 |
| 5 | e | t-Bu | 42 | 41 |
| 6 | f | OEt | 30 | 74 |
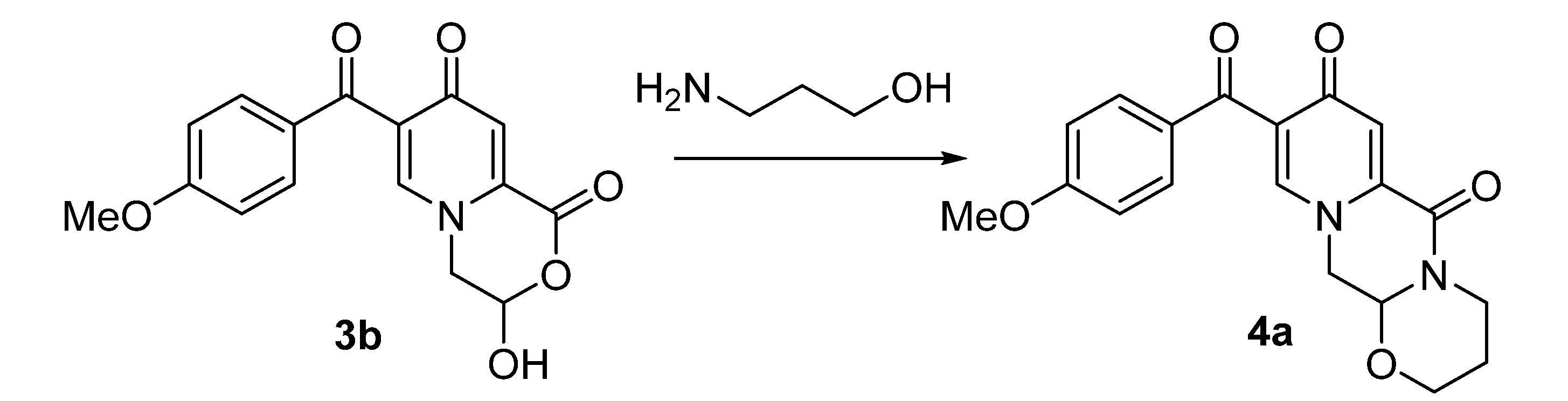 | ||||
| Entry | Catalyst, Equiv. | Time, h | Temp., °C | Yield of 4a, % |
|---|---|---|---|---|
| 1 | – | 12 | reflux | – |
| 2 | AcOH, 1.2 | 12 | reflux | 48 |
| 3 | AcOH, 0.2 | 12 | reflux | – |
| 4 | AcOH, 1.2 | 12 | room temperature | – |
| 5 | AcONH4, 1.0 | 12 | reflux | 69 |
| 6 | AcONH4, 1.0 | 12 | room temperature | – |
| 7 | NH4Cl, 1.0 | 12 | reflux | – |
| 8 | NBu4Br, 1.0 | 12 | reflux | – |
| 9 | NH2Et2OAc, 1.0 | 12 | reflux | – |
 |
| Entry | Compound 6 | Ar | Yield of 6, % |
|---|---|---|---|
| 1 | a | Ph | 71 |
| 2 | b | 4-MeOC6H4 | 91 |
| 3 | c | 4-ClC6H4 | 33 |
| 4 | d | 2-Th | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viktorova, V.V.; Steparuk, E.V.; Obydennov, D.L.; Sosnovskikh, V.Y. The Construction of Polycyclic Pyridones via Ring-Opening Transformations of 3-hydroxy-3,4-dihydropyrido[2,1-c][1,4]oxazine-1,8-diones. Molecules 2023, 28, 1285. https://doi.org/10.3390/molecules28031285
Viktorova VV, Steparuk EV, Obydennov DL, Sosnovskikh VY. The Construction of Polycyclic Pyridones via Ring-Opening Transformations of 3-hydroxy-3,4-dihydropyrido[2,1-c][1,4]oxazine-1,8-diones. Molecules. 2023; 28(3):1285. https://doi.org/10.3390/molecules28031285
Chicago/Turabian StyleViktorova, Viktoria V., Elena V. Steparuk, Dmitrii L. Obydennov, and Vyacheslav Y. Sosnovskikh. 2023. "The Construction of Polycyclic Pyridones via Ring-Opening Transformations of 3-hydroxy-3,4-dihydropyrido[2,1-c][1,4]oxazine-1,8-diones" Molecules 28, no. 3: 1285. https://doi.org/10.3390/molecules28031285
APA StyleViktorova, V. V., Steparuk, E. V., Obydennov, D. L., & Sosnovskikh, V. Y. (2023). The Construction of Polycyclic Pyridones via Ring-Opening Transformations of 3-hydroxy-3,4-dihydropyrido[2,1-c][1,4]oxazine-1,8-diones. Molecules, 28(3), 1285. https://doi.org/10.3390/molecules28031285






