Heteroaromatic Diazirines Are Essential Building Blocks for Material and Medicinal Chemistry
Abstract
1. Introduction
2. Diazirinyl-Substituted Pyridines and Pyrimidines
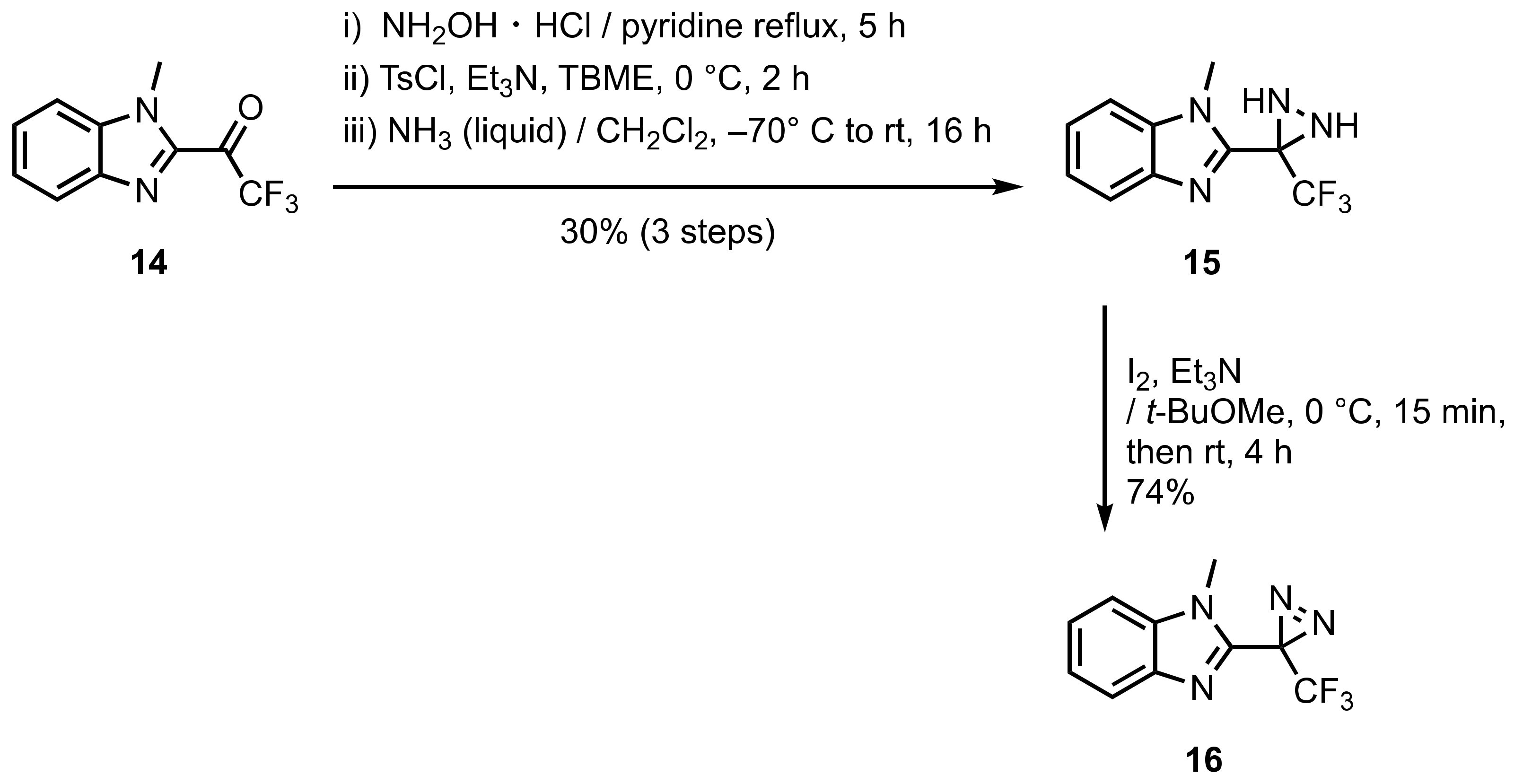
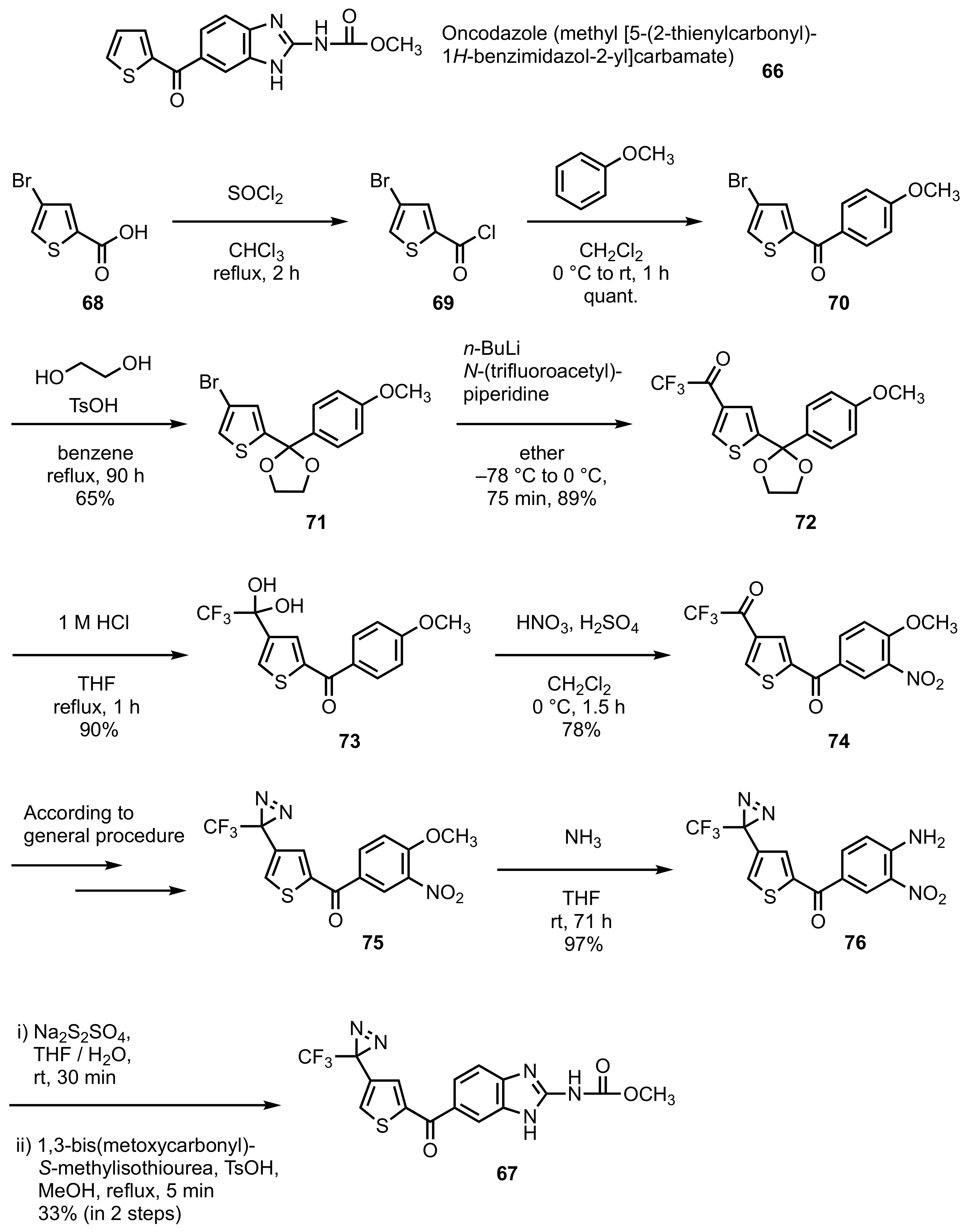
3. Diazirinyl-Substituted Benzimidazoles
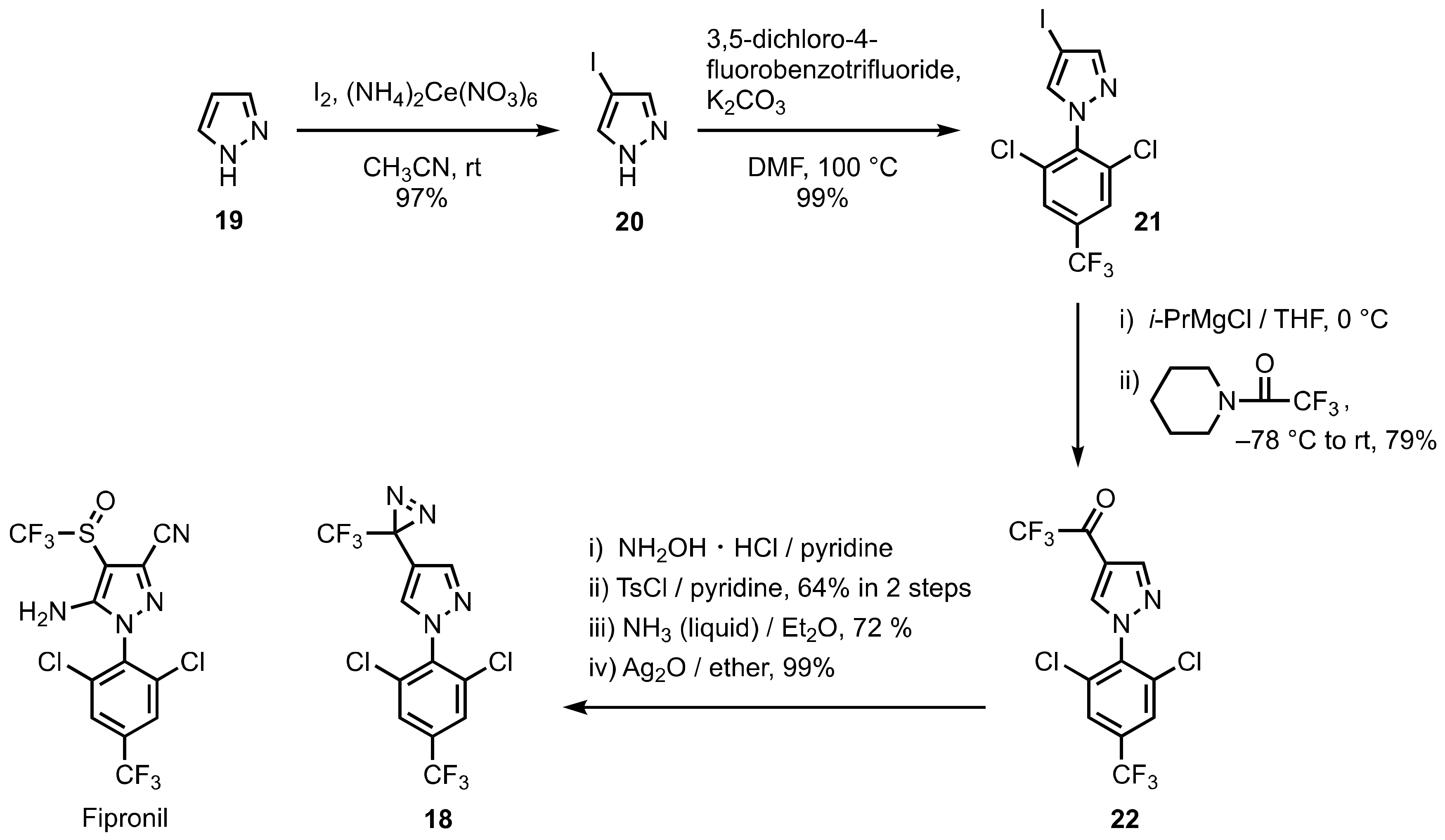
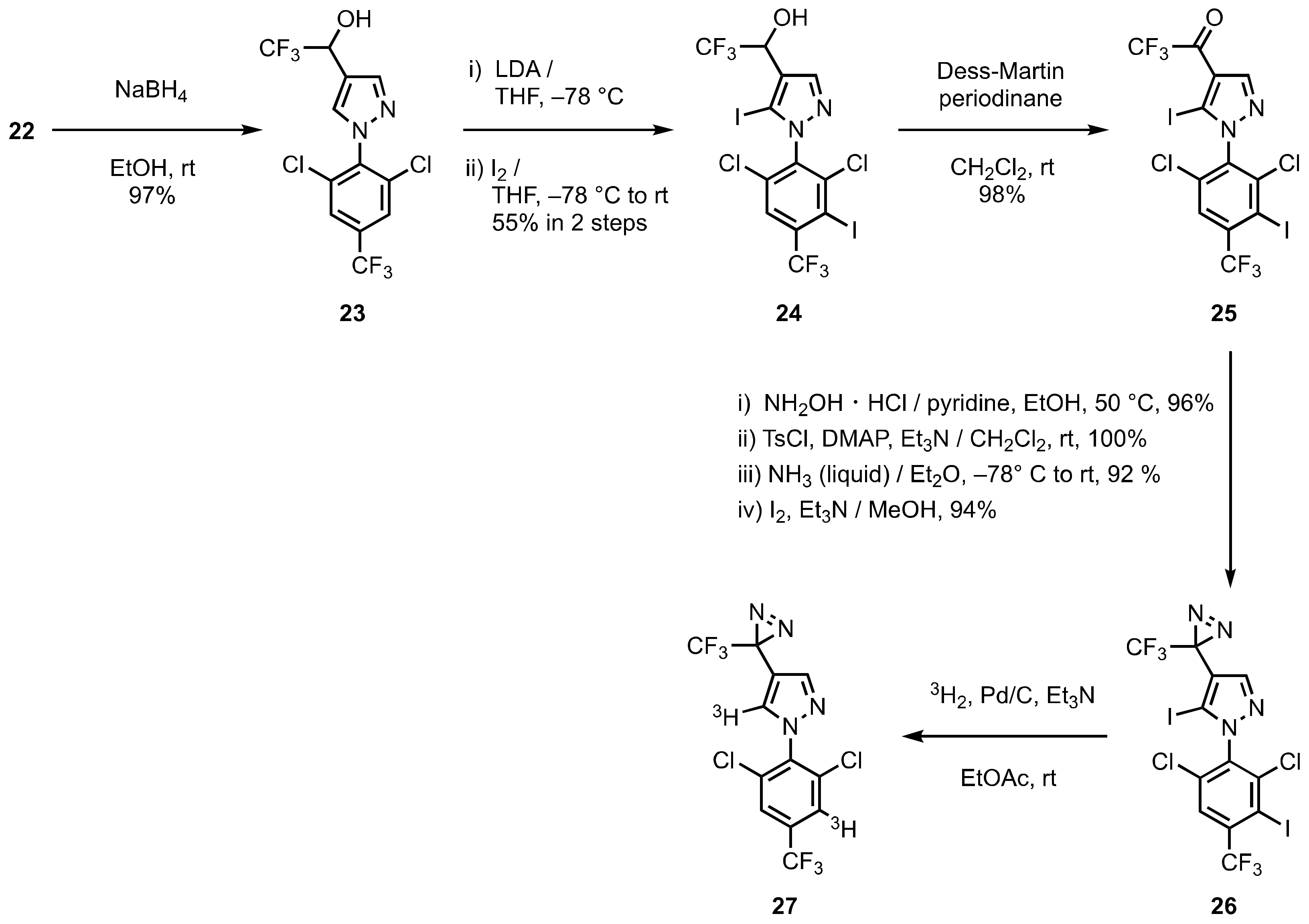
4. Diazirinyl-Substituted Pyrazoles
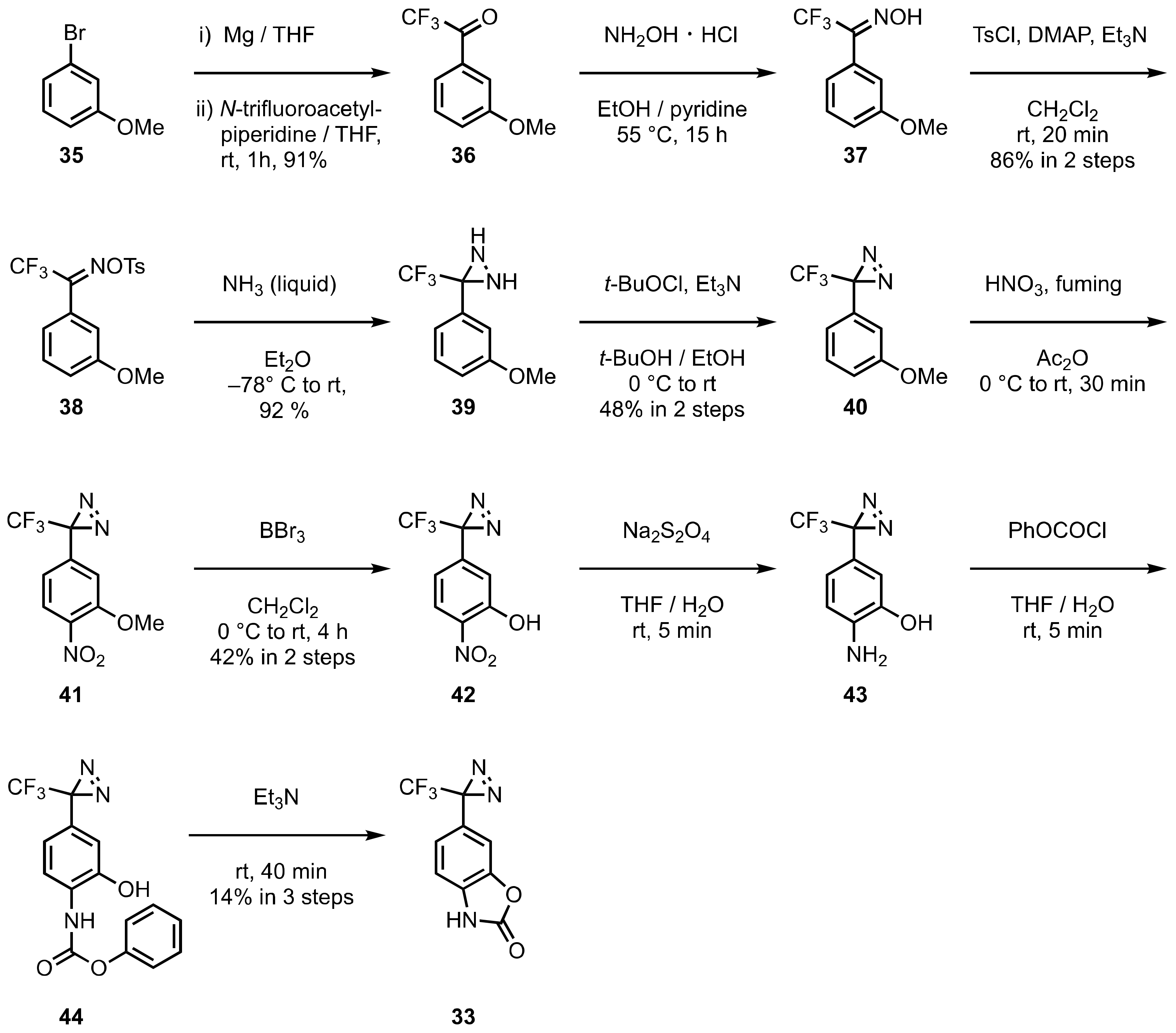
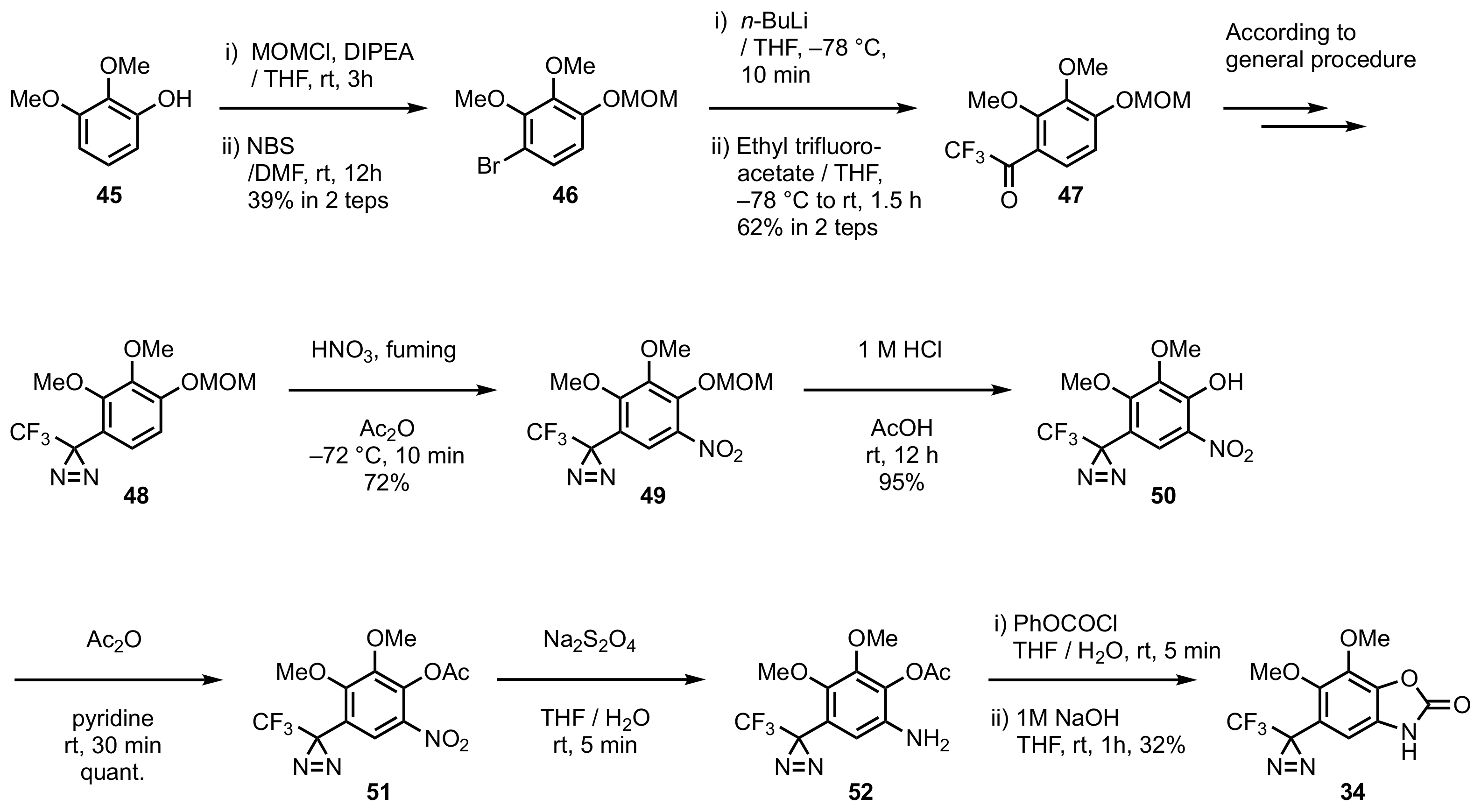
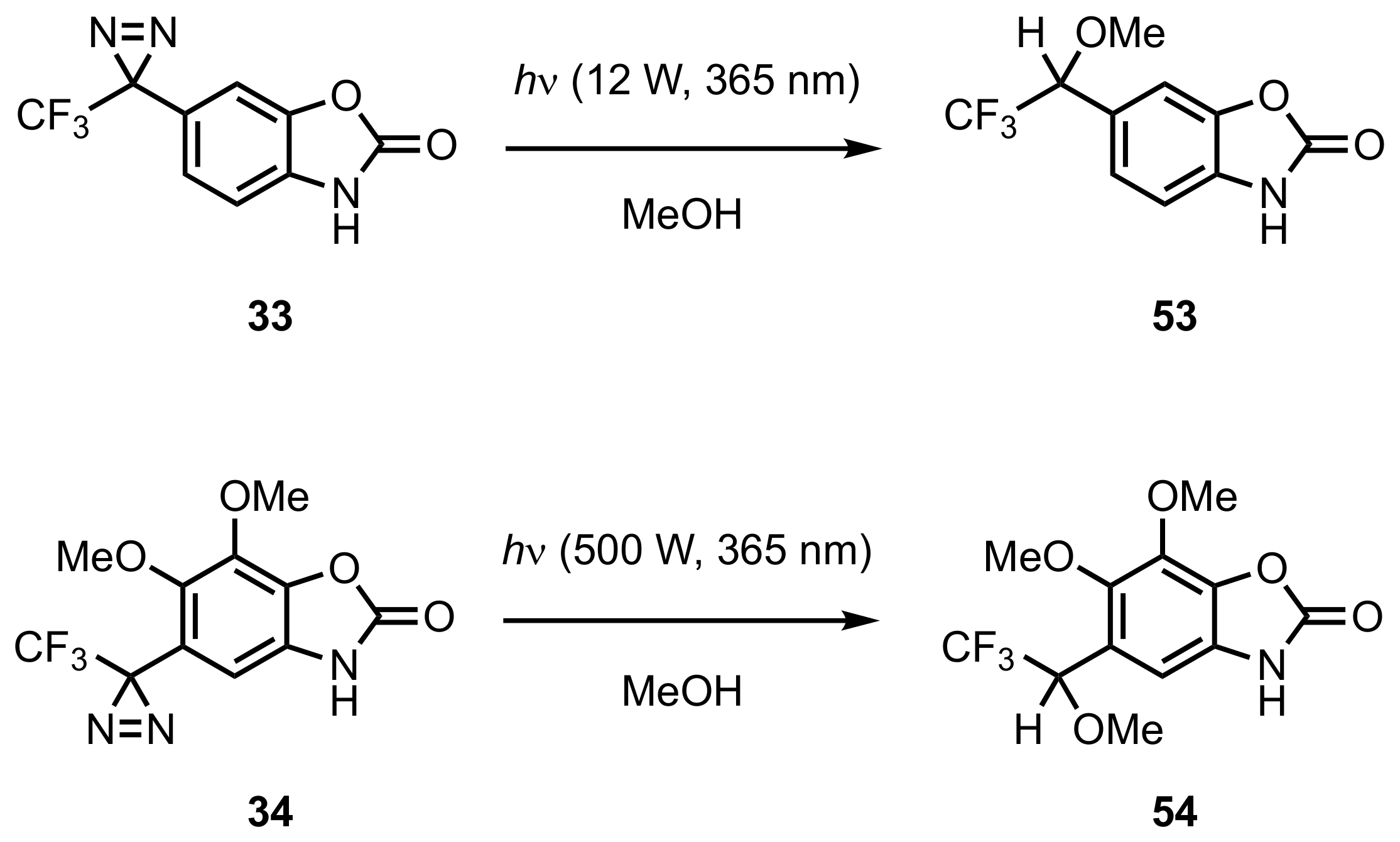
5. Diazirinyl-Substituted Benzoxazolinone
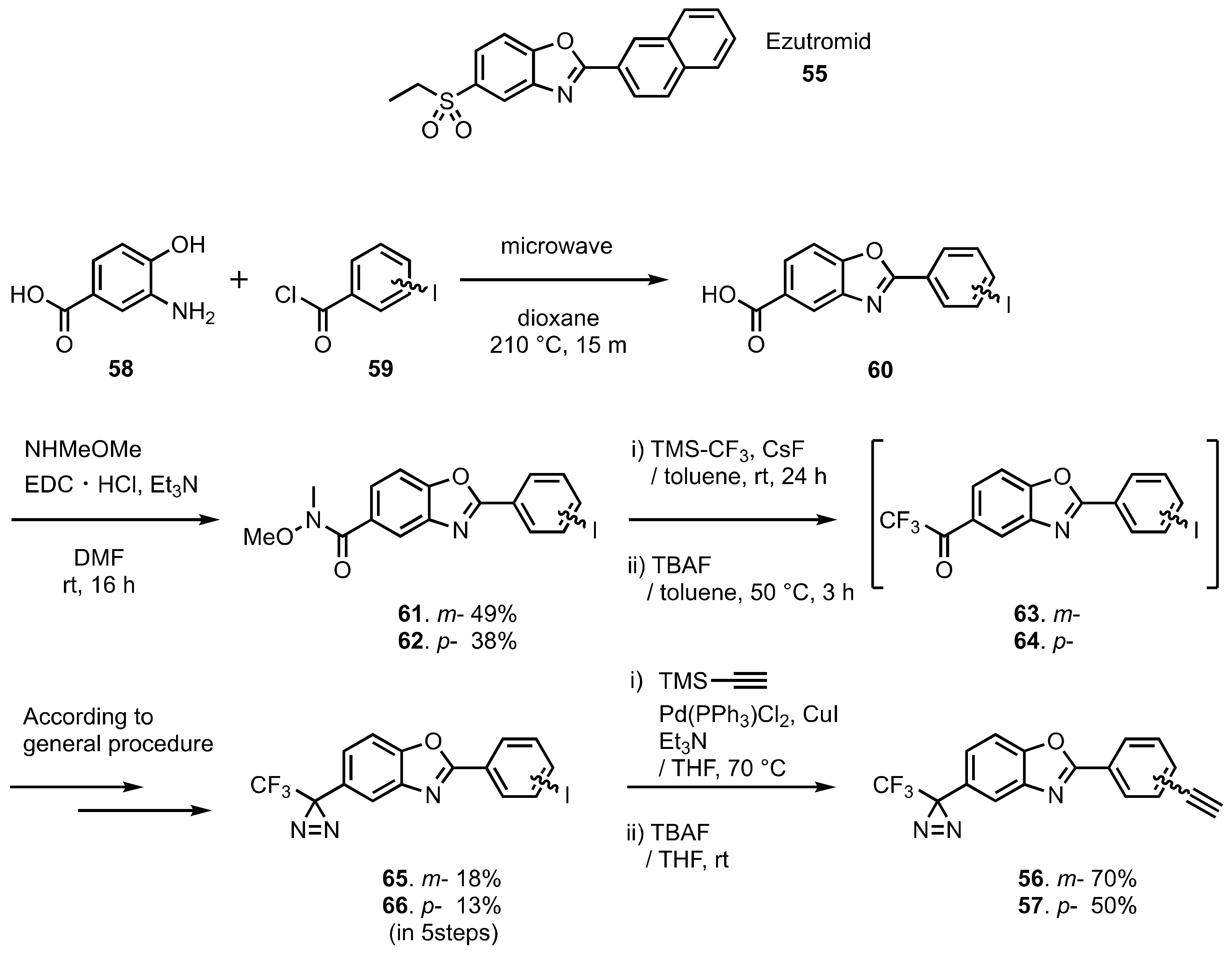
6. Diazirinyl-Substituted Benzoxazole
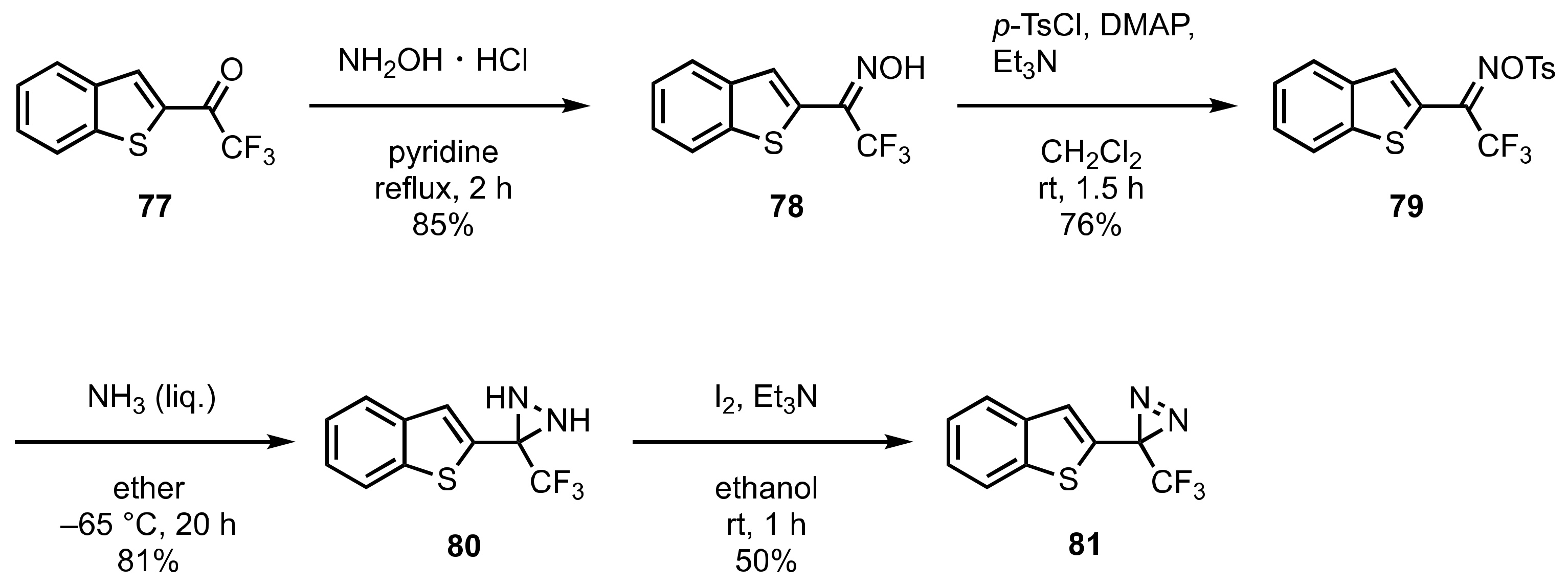
7. Diazirinyl-Substituted (Benzo)thiophene
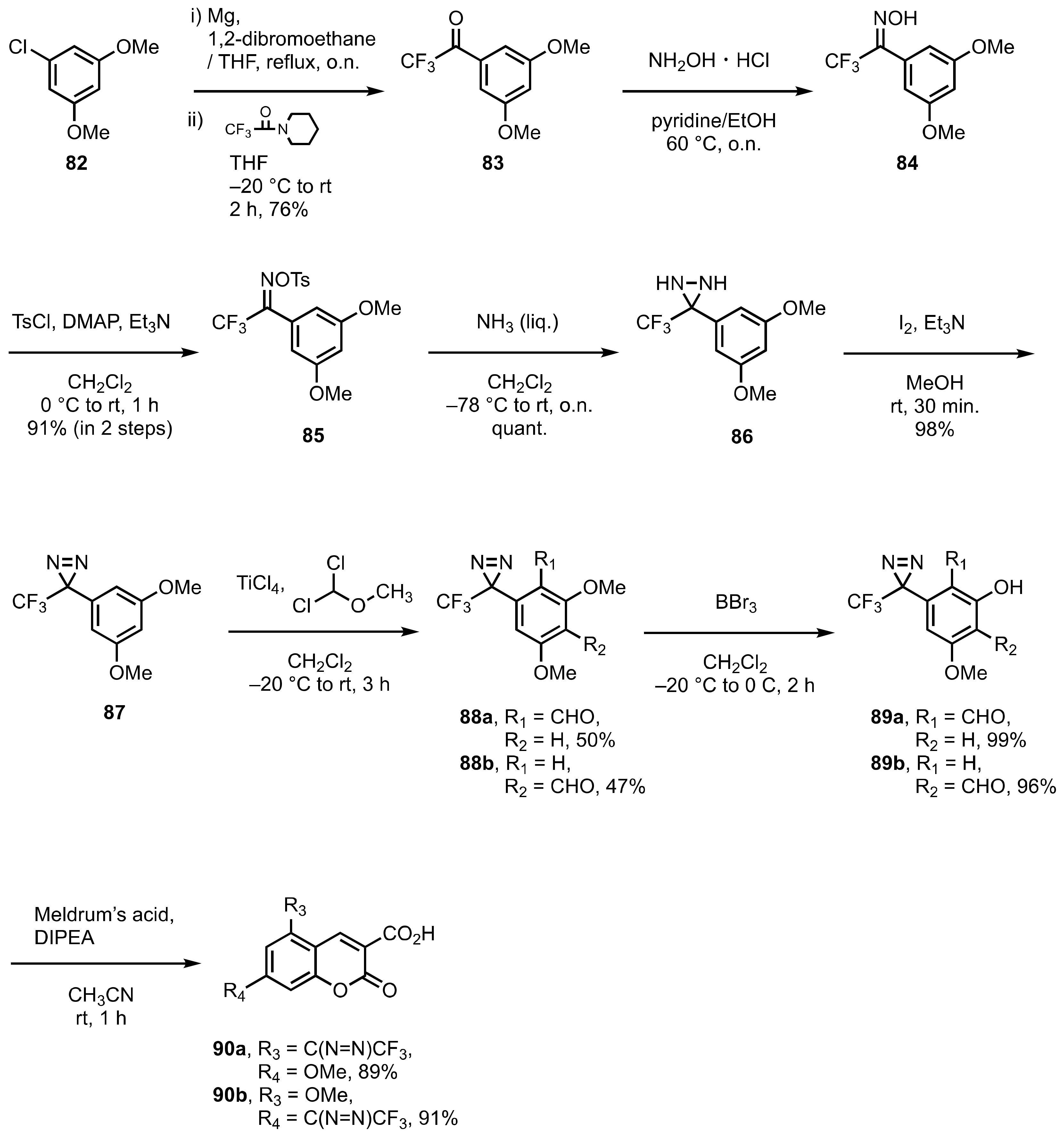
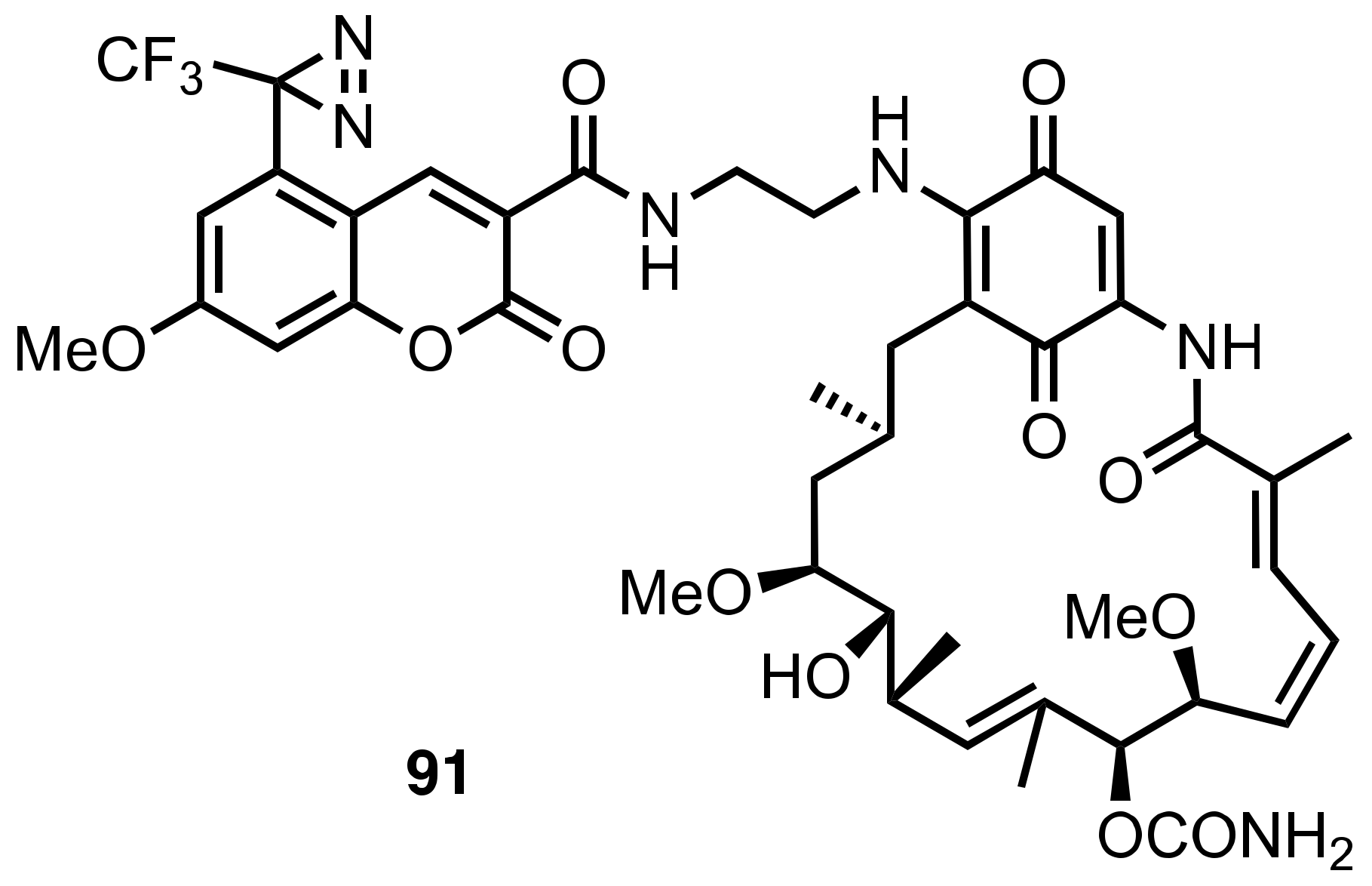
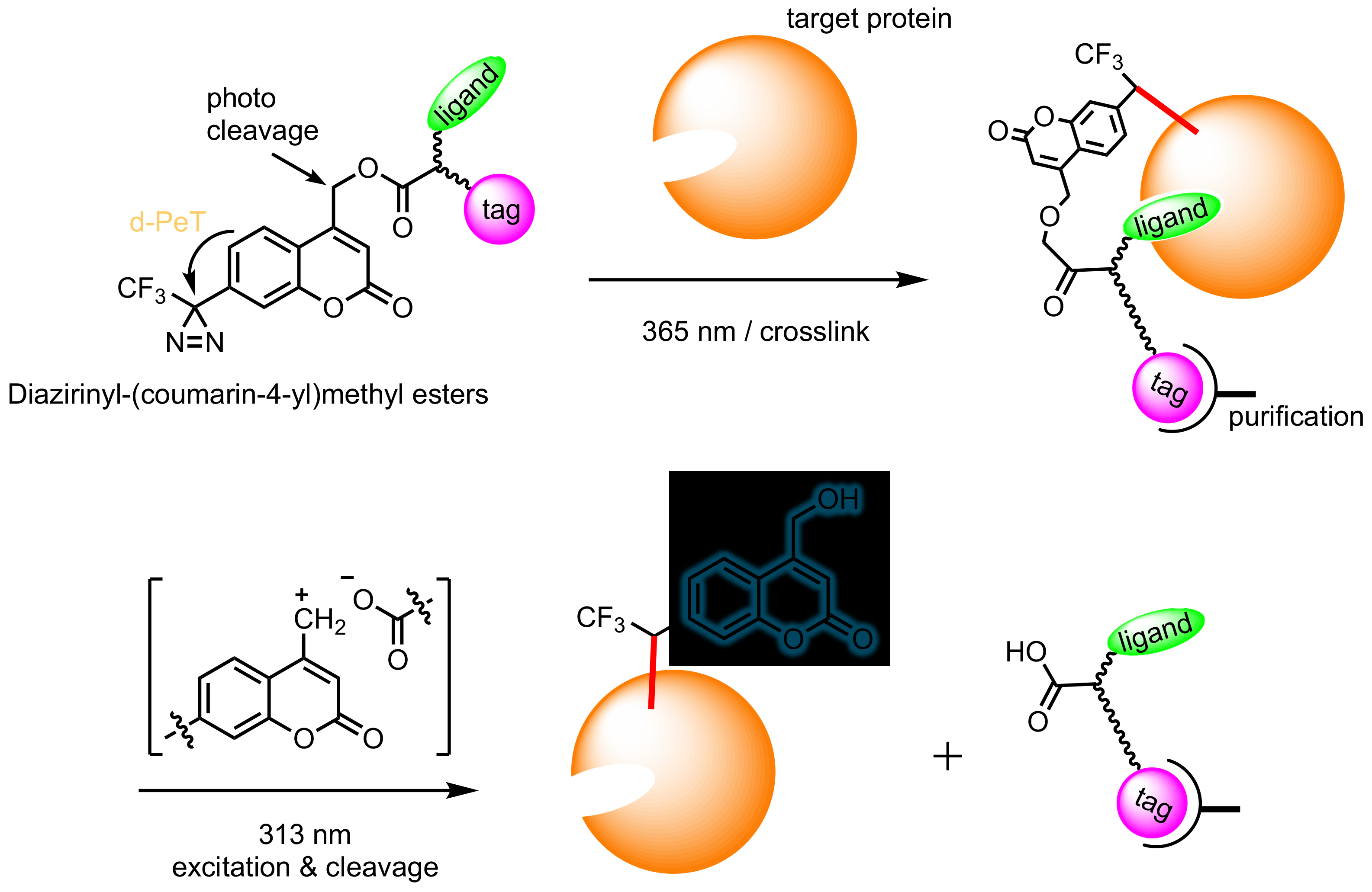
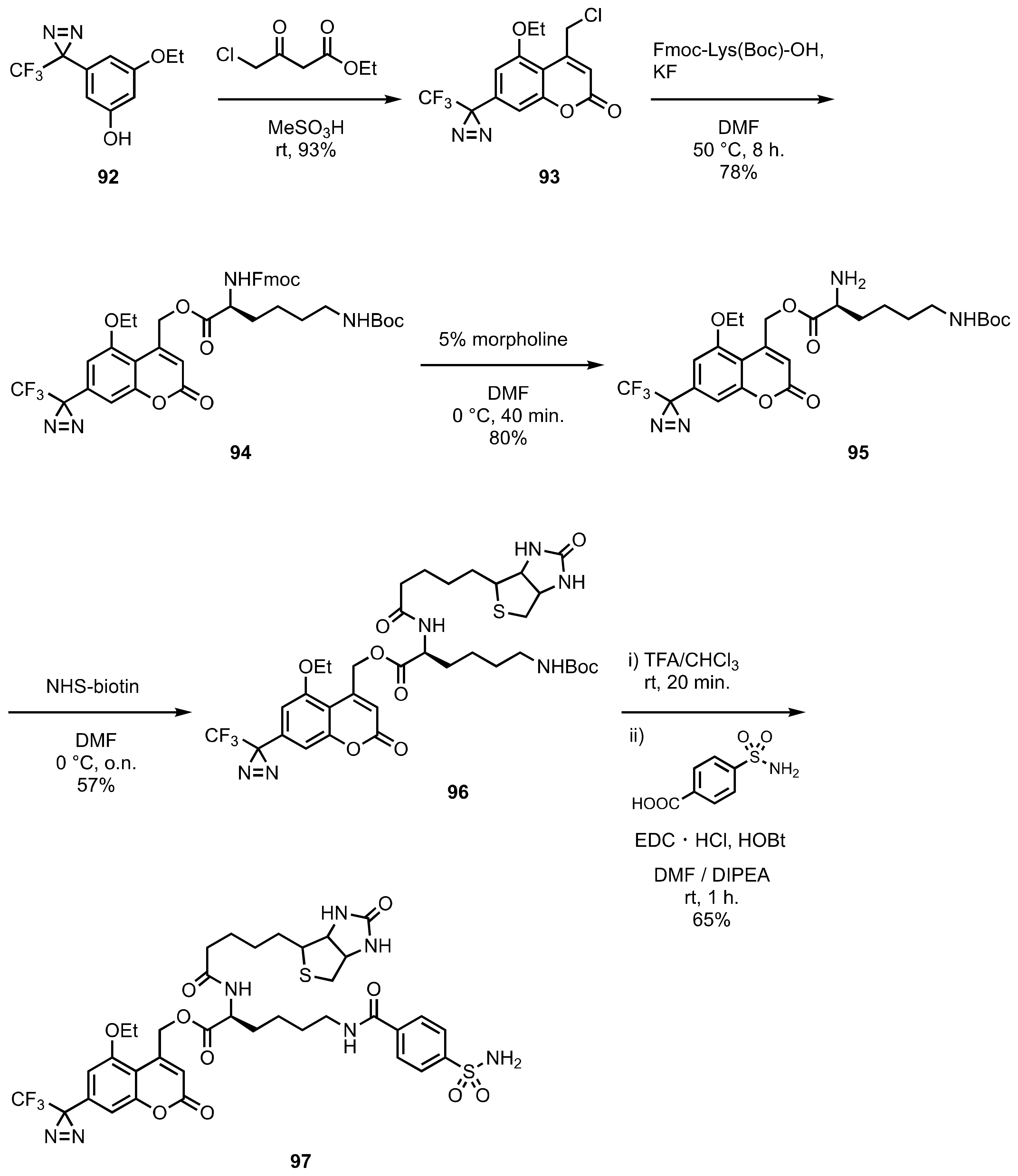
8. Diazirinyl-Substituted Coumarin
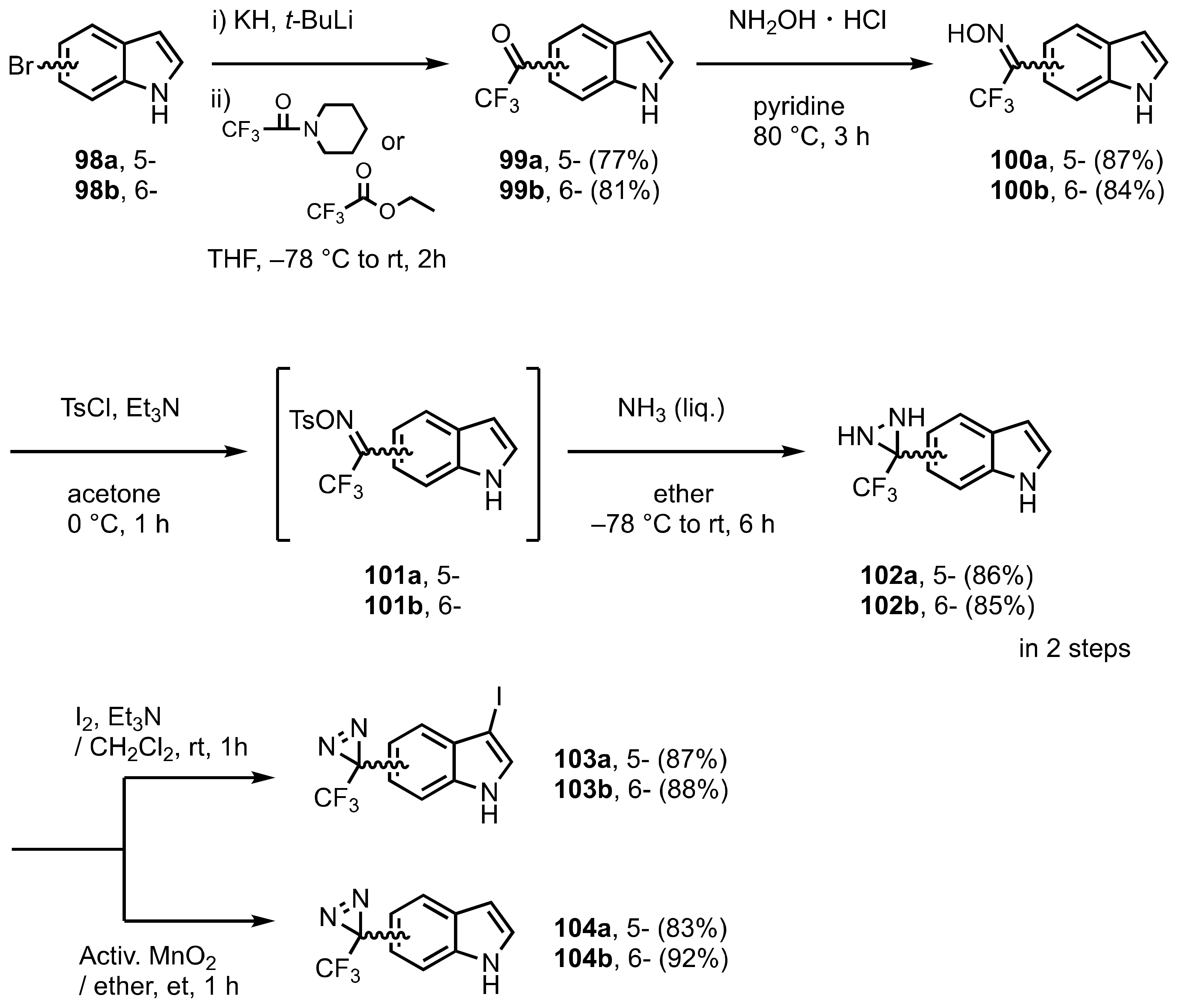
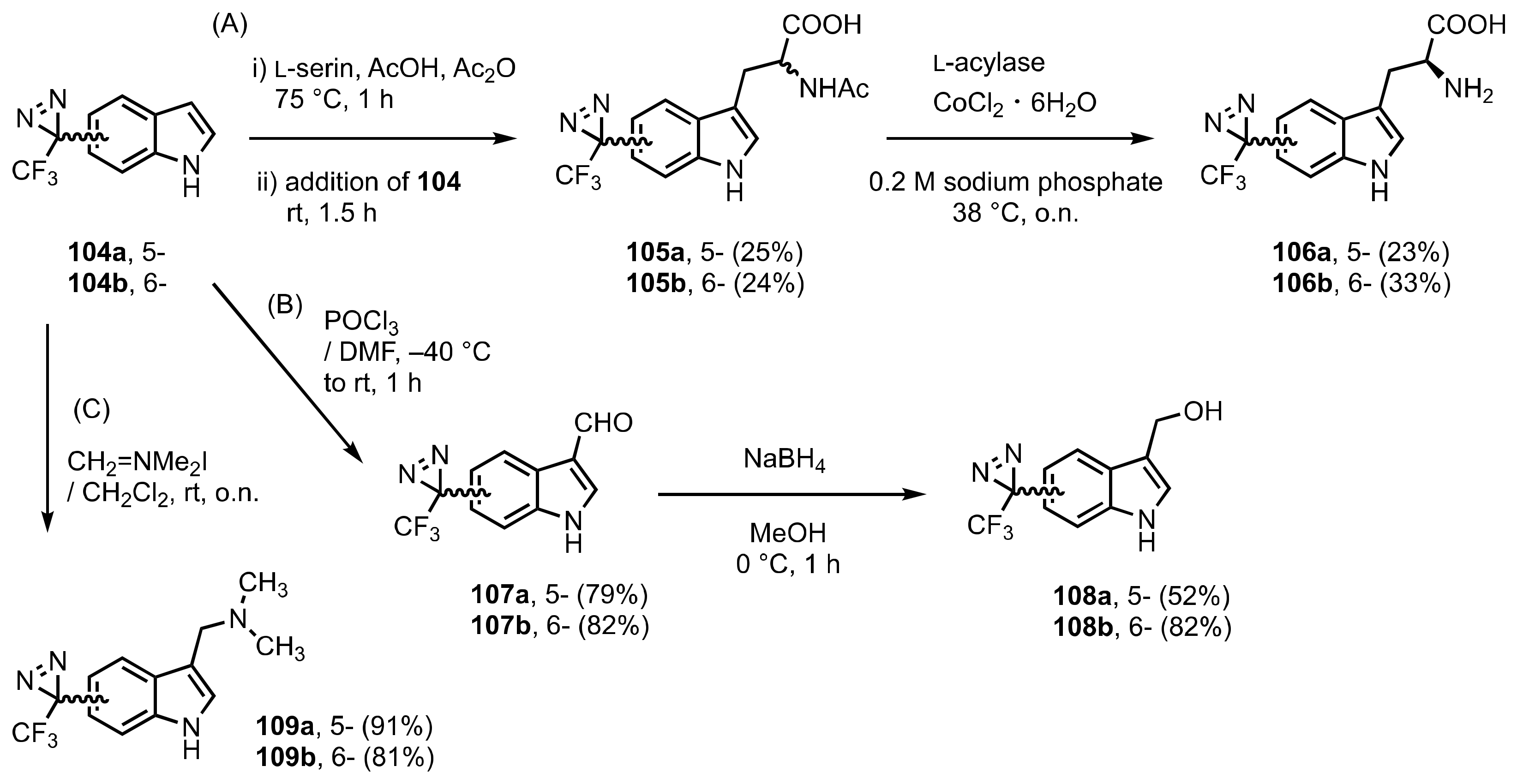
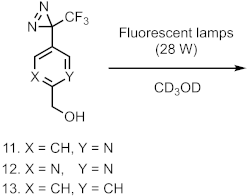
9. Diazirinyl-Substituted Indoles
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borissov, A.; Maurya, Y.K.; Moshniaha, L.; Wong, W.S.; Żyła-Karwowska, M.; Stępień, M. Recent Advances in Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds. Chem. Rev. 2022, 122, 565–788. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhang, F.; Wu, Y.; Chen, M.; Yao, C.; Nan, J.; Shu, D.; Zeng, R.; Zeng, H.; Chou, S.L. Heteroaromatic Organic Compound with Conjugated Multi-Carbonyl as Cathode Material for Rechargeable Lithium Batteries. Sci. Rep. 2016, 11, 23515. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.; Uzzaman, M. A Review on Biological and Medicinal Impact of Heterocyclic Compounds. Results Chem. 2022, 4, 100606. [Google Scholar] [CrossRef]
- Davison, E.K.; Sperry, J. Natural Products with Heteroatom-Rich Ring Systems. J. Nat. Prod. 2017, 80, 3060–3079. [Google Scholar] [CrossRef]
- Lepage, M.L.; Simhadri, C.; Liu, C.; Takaffoli, M.; Bi, L.; Crawford, B.; Milani, A.S.; Wulff, J.E. A Broadly Applicable Cross-Linker for Aliphatic Polymers Containing C-H Bonds. Science 2019, 366, 875–878. [Google Scholar] [CrossRef]
- Musolino, S.F.; Mahbod, M.; Nazir, R.; Bi, L.; Graham, H.A.; Milani, A.S.; Wulff, J.E. Electronically Optimized Diazirine-based Polymer Crosslinkers. Polym. Chem. 2022, 13, 3833–3839. [Google Scholar] [CrossRef]
- Brunner, J. New hotolabeling and crosslinking methods. Annu. Rev. Biochem. 1993, 62, 483–514. [Google Scholar] [CrossRef]
- Tomohiro, T.; Hashimoto, M.; Hatanaka, Y. Cross-Linking Chemistry and Biology: Development of Multifunctional Photoaffinity Probes. Chem. Rec. 2005, 5, 385–395. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hatanaka, Y. Recent Progress in Diazirine-Based Photoaffinity Labeling. Eur. J. Org. Chem. 2008, 2008, 2513–2523. [Google Scholar] [CrossRef]
- Hatanaka, Y. Development and Leading-Edge Application of Innovative Photoaffinity Labeling. Chem. Pharm. Bull. 2015, 63, 1–12. [Google Scholar] [CrossRef]
- Platz, M.S. Comparison of Phenylcarbene and Phenylnitrene. Acc. Chem. Res. 1995, 28, 487–492. [Google Scholar] [CrossRef]
- Karney, W.L.; Borden, W.T. Why Does o-Fluorine Substitution Raise the Barrier to Ring Expansion of Phenylnitrene? J. Am. Chem. Soc. 1997, 119, 3347–3350. [Google Scholar] [CrossRef]
- Galardy, R.E.; Craig, L.C.; Printz, M.P. Benzophenone Triplet: A New Photochemical Probe of Biological Ligand-Receptor Interactions. Nat. New Biol. 1973, 242, 127–128. [Google Scholar] [CrossRef]
- Brunner, J.; Senn, H.; Richards, F.M. 3-Trifluoromethyl-3-Phenyldiazirine. A New Carbene Generating Group for Photolabeling Reagents. J. Biol. Chem. 1980, 255, 3313–3318. [Google Scholar] [CrossRef]
- Dey, K.; Roy Chowdhury, S.; Dykstra, E.; Koronatov, A.; Lu, H.P.; Shinar, R.; Shinar, J.; Anzenbacher, P., Jr. Diazirine-Based Photo-Crosslinkers for Defect Free Fabrication of Solution Processed Organic Light-Emitting Diodes. J. Mater. Chem. C 2020, 8, 11988–11996. [Google Scholar] [CrossRef]
- Chowdhury, S.; Jana, A.; Mandal, A.K.; Choudhary, R.J.; Phase, D.M. Time Evolution of the Structural, Electronic, and Magnetic Phases in Relaxed SrCoO3 Thin Films. ACS Appl. Electron. Mater. 2021, 3, 3060–3071. [Google Scholar] [CrossRef]
- Nazir, R.; Bi, L.; Musolino, S.F.; Margoto, O.H.; Çelebi, K.; Mobuchon, C.; Takaffoli, M.; Milani, A.S.; Falck, G.; Wulf, J.E. Polyamine−Diazirine Conjugates for Use as Primers in UHMWPE−Epoxy Composite Materials. ACS Appl. Polym. Mater. 2022, 4, 1728–1742. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, D.; Lin, Z.; Wang, P.; Cao, B.; Ren, H.; Song, F.; Wan, C.; Wang, L.; Zhou, J.; et al. Highly Stretchable van der Waals Thin Films for Adaptable and Breathable Electronic Membranes. Science 2022, 375, 852–859. [Google Scholar] [CrossRef]
- Kumar, A.B.; Tipton, J.D.; Manetsch, R. 3-Trifluoromethyl-3-Aryldiazirine Photolabels with Enhanced Ambient Light Stability. Chem. Commun. 2016, 52, 2729–2732. [Google Scholar] [CrossRef]
- Cross, R.M.; Monastyrskyi, A.; Mutka, T.S.; Burrows, J.N.; Kyle, D.E.; Manetsch, R. Endochin Optimization: Structure-Activity and Structure-Property Relationship Studies of 3-Substituted 2-Methyl-4(1H)-quinolones with Antimalarial Activity. J. Med. Chem. 2010, 53, 7076–7094. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, X.; Xiong, S.; Xiong, R.; Liu, J.; Zou, L.; Lei, X.; Cao, X.; Xie, Z.; Chen, Y.; et al. Design, Synthesis and Biological Evaluation of Chrysin Benzimidazole Derivatives as Potential Anticancer Agents. Nat. Prod. Res. 2018, 32, 2900–2909. [Google Scholar] [CrossRef] [PubMed]
- Morais, G.R.; Palma, E.; Marques, F.; Gano, L.; Oliveira, M.C.; Abrunhosa, A.; Miranda, H.V.; Outeiro, T.F.; Santos, I.; Paulo, A. Synthesis and Biological Evaluation of Novel 2-Aryl Benzimidazoles as Chemotherapeutic Agents. J. Heterocycl. Chem. 2017, 54, 255–267. [Google Scholar] [CrossRef]
- Shaker, Y.M.; Omar, M.A.; Mahmoud, K.; Elhallouty, S.M.; El-Senousy, W.M.; Ali, M.M.; Mahmoud, A.E.; Abdel-Halim, A.H.; Soliman, S.M.; El Diwani, H.I. Synthesis, in Vitro and in Vivo Aantitumor and Antiviral Activity of Novel 1-Substituted Benzimidazole Derivatives. J. Enzyme Inhib. Med. Chem. 2015, 30, 826–845. [Google Scholar] [CrossRef] [PubMed]
- Onnis, V.; Demurtas, M.; Deplano, A.; Balboni, G.; Baldisserotto, A.; Manfredini, S.; Pacifico, S.; Liekens, S.; Balzarini, J. Design, Synthesis and Evaluation of Antiproliferative Activity of New Benzimidazolehydrazones. Molecules 2016, 21, 579. [Google Scholar] [CrossRef] [PubMed]
- Çevik, U.A.; Sağlık, B.N.; Korkut, B.; Özkay, Y.; Ilgınc, S. Antiproliferative, Cytotoxic, and Apoptotic Effects of New Benzimidazole Derivatives Bearing Hydrazone Moiety. J. Heterocycl. Chem. 2018, 55, 138–148. [Google Scholar] [CrossRef]
- Abd el Al, S.N.; Soliman, F.M.A. Synthesis, Some Reactions, Cytotoxic Evaluation and Antioxidant Study of Novel Benzimidazole Derivatives. Der Pharma Chem. 2015, 7, 71–84. [Google Scholar]
- Bellam, M.; Gundluru, M.; Sarva, S.; Chadive, S.; Netala, V.R.; Tartte, V.; Cirandur, S.R. Synthesis and Antioxidant Activity of Some New N-Alkylated Pyrazole Containing Benzimidazoles. Chem. Heterocycl. Compd. 2017, 53, 173–178. [Google Scholar] [CrossRef]
- Sharma, R.; Bali, A.; Chaudhari, B.B. Synthesis of Methanesulphonamido-Benzimidazole Derivatives as Gastro-Sparing Antiinflammatory Agents with Antioxidant Effect. Bioorg. Med. Chem. Lett. 2017, 27, 3007–3013. [Google Scholar] [CrossRef]
- Sethi, P.; Bansal, Y.; Bansal, G. Synthesis and PASS-Assisted Evaluation of Coumarin–Benzimidazole Derivatives as Potential Anti-Inflammatory and Anthelmintic Agents. Med. Chem. Res. 2018, 27, 61–71. [Google Scholar] [CrossRef]
- Yang, H.; Ren, Y.; Gao, X.; Gao, Y. Synthesis and Anticoagulant Bioactivity Evaluation of 1,2,5-Trisubstituted Benzimidazole Fluorinated Derivatives. Chem. Res. Chin. Univ. 2016, 32, 973–978. [Google Scholar] [CrossRef]
- Wang, F.; Ren, Y.-J. Design, Synthesis, Biological Evaluation and Molecular Docking of Novel Substituted 1-Ethyl-1H-Benzimidazole Fluorinated Derivatives as Thrombin Inhibitors. J. Iran. Chem. Soc. 2016, 13, 1155–1166. [Google Scholar] [CrossRef]
- Vandeputte, M.M.; Van Uytfanghe, K.; Layle, N.K.; St Germaine, D.M.; Iula, D.M.; Stove, C.P. Synthesis, Chemical Characterization, and μ-Opioid Receptor Activity Assessment of the Emerging Group of “Nitazene” 2-Benzylbenzimidazole Synthetic Opioids. ACS Chem. Neurosci. 2021, 12, 1241–1251. [Google Scholar] [CrossRef]
- Raimer, B.; Wartmann, T.; Jones, P.G.; Lindel, T. Synthesis, Stability, and Photoreactivity of Diazirinyl-Substituted N-Heterocycles Based on Indole, Benzimidazole, and Imidazole. Eur. J. Org. Chem. 2014, 2014, 5509–5520. [Google Scholar] [CrossRef]
- Cheng, H.; Pei, Y.; Leng, F.; Li, J.; Liang, A.; Zou, D.; Wu, Y.; Wu, Y. Highly Efficient Synthesis of Aryl and Heteroaryl Trifluoromethyl Ketones via o-iodobenzoic acid (IBX). Tetrahedron Lett. 2013, 54, 4483–4486. [Google Scholar] [CrossRef]
- Moss, R.A.; Perez, L.A.; Turro, N.J.; Gould, I.R.; Hacker, N.P. Hammett Analysis of Absolute Carbene Addition Rate Constants. Tetrahedron Lett. 1983, 24, 685–688. [Google Scholar] [CrossRef]
- Mueller, P.H.; Rondan, N.G.; Houk, K.N.; Harrison, J.F.; Hooper, D.; Willen, B.H.; Liebman, J.F. Carbene Singlet-Triplet Gaps. Linear Correlations with Substituent Donation. J. Am. Chem. Soc. 1981, 103, 5049–5052. [Google Scholar] [CrossRef]
- Creary, X. Regioselectivity in the Addition of Singlet and Triplet Carbenes to 1,1-Dimethylallene. A Probe for Carbene Multiplicity. J. Am. Chem. Soc. 1980, 102, 1611–1618. [Google Scholar] [CrossRef]
- Wang, J.; Kubicki, J.; Peng, H.; Platz, M.S. Influence of Solvent on Carbene Intersystem Crossing Rates. J. Am. Chem. Soc. 2008, 130, 6604–6609. [Google Scholar] [CrossRef]
- Faisal, M.; Saeed, A.; Hussain, S.; Dar, P.; Larik, F.A. Recent Developments in Synthetic Chemistry and Biological Activities of Pyrazole Derivatives. J. Chem. Sci. 2019, 131, 70. [Google Scholar] [CrossRef]
- Costa, R.F.; Turones, L.C.; Cavalcante, K.V.N.; Rosa Júnior, I.A.; Xavier, C.H.; Rosseto, L.P.; Napolitano, H.B.; Castro, P.F.D.S.; Neto, M.L.F.; Galvão, G.M.; et al. Heterocyclic Compounds: Pharmacology of Pyrazole Analogs from Rational Structural Considerations. Front. Pharmacol. 2021, 12, 666725. [Google Scholar] [CrossRef]
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A Review of the Recent Development in the Synthesis and Biological Evaluations of Pyrazole Derivatives. Biomedicines 2022, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Hussain, S.; Haider, A.; Saeed, A.; Laril, F.A. Assessing the Effectiveness of Oxidative Approaches for the Synthesis of Aldehydes and Ketones from Oxidation of Iodomethyl Group. Chem. Pap. 2019, 73, 1053–1067. [Google Scholar] [CrossRef]
- Ran, F.; Liu, Y.; Zhang, D.; Liu, M.; Zhao, G. Discovery of Novel Pyrazole Derivatives as Potential Anticancer Agents in MCL. Bioorg. Med. Chem. Lett. 2019, 29, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Sony Jacob, K.; Swastika, G. A Battle Against Aids: New Pyrazole Key to an Older Lock-reverse Transcriptase. Int. J. Pharm. Pharm. Sci. 2016, 8, 75–79. [Google Scholar]
- Kumar, S.; Gupta, S.; Rani, V.; Sharma, P. Pyrazole Containing Anti-HIV Agents: An Update. Med. Chem. 2022, 18, 831–846. [Google Scholar] [CrossRef]
- Bekhit, A.A.; Hassan, A.M.; Abd El Razik, H.A.; El-Miligy, M.M.; El-Agroudy, E.J.; Bekhit, A.-D. New Heterocyclic Hybrids of Pyrazole and Its Bioisosteres: Design, Synthesis and Biological Evaluation as Dual Acting Antimalarial-Antileishmanial Agents. Eur. J. Med. Chem. 2015, 94, 30–44. [Google Scholar] [CrossRef]
- Heller, S.T.; Natarajan, S.R. 1,3-Diketones from Acid Chlorides and Ketones: A Rapid and General One-Pot Synthesis of Pyrazoles. Org. Lett. 2006, 8, 2675–2678. [Google Scholar] [CrossRef]
- Chebib, M.; Johnston, G.A. GABA-Activated Ligand Gated Ion Channels: Medicinal Chemistry and Molecular Biology. J. Med. Chem. 2000, 43, 1427–1447. [Google Scholar] [CrossRef]
- Zhu, S.; Noviello, C.M.; Teng, J.; Walsh, R.M., Jr.; Kim, J.J.; Hibbs, R.E. Structure of a Human Synaptic GABAA Receptor. Nature 2018, 559, 67–72. [Google Scholar] [CrossRef]
- Singh, N.S.; Sharma, R.; Singh, S.K.; Singh, D.K. A Comprehensive Review of Environmental Fate and Degradation of Fipronil and Its Toxic Metabolites. Environ. Res. 2021, 199, 111316. [Google Scholar] [CrossRef]
- Sirisoma, N.S.; Ratra, G.S.; Tomizawa, M.; Casida, J.E. Fipronil-Based Photoaffinity Probe for Drosophila and Human Beta 3 GABA Receptors. Bioorg. Med. Chem. Lett. 2001, 11, 2979–2981. [Google Scholar] [CrossRef]
- Sammelson, R.E.; Casida, J.E. Synthesis of a Tritium-Labeled, Fipronil-Based, Highly Potent, Photoaffinity Probe for the GABA Receptor. J. Org. Chem. 2003, 68, 8075–8079. [Google Scholar] [CrossRef]
- Delfino, J.M.; Schreiber, S.L.; Richards, F.M. Design, Synthesis, and Properties of a Photoactivatable Membrane-Spanning Phospholipidic Probe. J. Am. Chem. Soc. 1993, 115, 3458–3474. [Google Scholar] [CrossRef]
- Weber, T.; Brunner, J. 2-(Tributylstannyl)-4-[3-(trifluoromethyl)-3H-diazirin-3-yl]benzyl Alcohol: A Building Block for Photolabeling and Cross-Linking Reagents of Very High Specific Radioactivity. J. Am. Chem. Soc. 1995, 117, 3084–3095. [Google Scholar] [CrossRef]
- Li, G.; Samadder, P.; Arthur, G.; Bittmana, R. Synthesis and Antiproliferative Properties of a Photoactivatable Analogue of ET-18-OCH3. Tetrahedron 2001, 57, 8925–8932. [Google Scholar] [CrossRef]
- Leroux, F.; Schlosser, M. The “Aryne” Route to Biaryls Featuring Uncommon Substituent Patterns. Angew. Chem. Int. Ed. 2002, 41, 4272–4274. [Google Scholar] [CrossRef]
- Dess, D.B.; Martin, J.C. Readily Accessible 12-I-5 Oxidant for the Conversion of Primary and Secondary Alcohols to Aldehydes and Ketones. J. Org. Chem. 1983, 48, 4155–4156. [Google Scholar] [CrossRef]
- Linderman, R.J.; Graves, D.M. Oxidation of Fluoroalkyl-Substituted Carbinols by the Dess-Martin. J. Org. Chem. 1989, 54, 661–668. [Google Scholar] [CrossRef]
- Kosemura, S.; Emori, H.; Yamamura, S.; Anai, T.; Aizawa, H.; Ohtake, N.; Hasegawa, K. Isolation and Characterization of 4-Chloro-6,7-dimethoxybenzoxazolin-2-one, A New Auxin-inhibiting Benzoxazolinone from Zea mays. Chem. Lett. 1995, 24, 1053–1054. [Google Scholar] [CrossRef]
- Hoshi-Sakoda, M.; Usui, K.; Ishizuka, K.; Kosemura, S.; Yamamura, S.; Hasegawa, K. Structure-Activity Relationships of Benzoxazolinones with Respect to Auxin-Induced Growth and Auxin-Binding Protein. Phytochemistry 1994, 37, 297–300. [Google Scholar] [CrossRef]
- Kosemura, S.; Emori, H.; Yamamura, S.; Anai, T.; Tomita, K.; Hasegawa, K. Design of Photoaflinity Reagents for Labeling the Auxin Receptor in Maize. Tetrahedron Lett. 1997, 38, 2125–2128. [Google Scholar] [CrossRef]
- Hatanaka, Y.; Hashimoto, M.; Nakayama, H.; Kanaoka, Y. Synthesis of Nitro-Substituted Aryl Diazirines. An Entry to Chromogenic Carbene Precursors for Photoaffinity Labeling. Chem. Pharm. Bull. 1994, 42, 826–831. [Google Scholar] [CrossRef]
- Hatanaka, Y.; Hashimoto, M.; Kurihara, H.; Nakayama, H.; Kanaoka, Y. A Novel Family of Aromatic Diazirines for Photoaffinity Labeling. J. Org. Chem. 1994, 59, 383–387. [Google Scholar] [CrossRef]
- Kohler, M.; Clarenbach, C.F.; Bahler, C.; Brack, T.; Russi, E.W.; Bloch, K.E. Disability and Survival in Duchenne Muscular Dystrophy. J. Neurol. Neurosurg. Psychiatry 2009, 80, 320–325. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Henricson, E.K.; Abresch, R.T.; Han, J.J.; Escolar, D.M.; Florence, J.M.; Duong, T.; Arrieta, A.; Clemens, P.R.; Hoffman, E.P.; et al. Cinrg Investigators. The Cooperative International Neuromuscular Research Group Duchenne Natural History Study—A Longitudinal Investigation in the Era of Glucocorticoid Therapy: Design of Protocol and the Methods Used. Muscle Nerve 2013, 48, 32–54. [Google Scholar] [CrossRef]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and Management of Duchenne Muscular Dystrophy, Part 1: Diagnosis, and Pharmacological and Psychosocial Management. Lancet Neurol. 2010, 9, 77–93. [Google Scholar] [CrossRef]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and Management of Duchenne Muscular Dystrophy, Part 2: Implementation of Multidisciplinary Care. Lancet Neurol. 2010, 9, 177–189. [Google Scholar] [CrossRef]
- Moxley, R.T., III; Pandya, S.; Ciafaloni, E.; Fox, D.J.; Campbell, K. Change in Natural History of Duchenne Muscular Dystrophy with Long-Term Corticosteroid Treatment: Implications for Management. J. Child Neurol. 2010, 25, 1116–1129. [Google Scholar] [CrossRef]
- Guiraud, S.; Roblin, D.; Kay, D.E. The Potential of Utrophin Modulators for the Treatment of Duchenne Muscular Dystrophy. Expert Opin. Orphan. Drugs 2018, 6, 179–192. [Google Scholar] [CrossRef]
- Wilkinson, I.V.L.; Perkins, K.J.; Dugdale, H.; Moir, L.; Vuorinen, A.; Chatzopoulou, M.; Squire, S.E.; Monecke, S.; Lomow, A.; Geese, M.; et al. Chemical Proteomics and Phenotypic Profiling Identifies the Aryl Hydrocarbon Receptor as a Molecular Target of the Utrophin Modulator Ezutromid. Angew. Chem. Int. Ed. 2020, 59, 2420–2428. [Google Scholar] [CrossRef]
- Medvedev, A.; Kopylov, A.; Buneeva, O.; Zgoda, V.; Archakov, A. Affinity-Based Proteomic Profiling: Problems and Achievements. Proteomics 2012, 12, 621–637. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, C.J.; Chen, G.Y.; Yao, S.Q. Cell-Based Proteome Profiling of Potential Dasatinib Targets by Use of Affinity-Based Probes. J. Am. Chem. Soc. 2012, 134, 3001–3014. [Google Scholar] [CrossRef]
- Won, S.J.; Eschweiler, J.D.; Majmudar, J.D.; Chong, F.S.; Hwang, S.Y.; Ruotolo, B.T.; Martin, B.R. Affinity-Based Selectivity Profiling of an In-Class Selective Competitive Inhibitor of Acyl Protein Thioesterase 2. ACS Med. Chem. Lett. 2016, 8, 215–220. [Google Scholar] [CrossRef]
- Chancellor, D.R.; Davies, K.E.; De Moor, O.; Dorgan, C.R.; Johnson, P.D.; Lambert, A.G.; Lawrence, D.; Lecci, C.; Maillol, C.; Middleton, P.J.; et al. Discovery of 2-Arylbenzoxazoles as Upregulators of Utrophin Production for the Treatment of Duchenne Muscular Dystrophy. J. Med. Chem. 2011, 54, 3241–3250. [Google Scholar] [CrossRef]
- Rudzinski, D.M.; Kelly, C.B.; Leadbeater, N.E. A Weinreb Amide Approach to the Synthesis of Trifluoromethylketones. Chem. Commun. 2012, 48, 9610–9612. [Google Scholar] [CrossRef]
- Hill, J.R.; Robertson, A.A.B. Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis. J. Med. Chem. 2018, 61, 6945–6963. [Google Scholar] [CrossRef]
- Chen, Z.; Ku, T.C.; Seley-Radtke, K.L. Thiophene-Expanded Guanosine Analogues of Gemcitabine. Bioorg. Med. Chem. Lett. 2015, 25, 4274–4276. [Google Scholar] [CrossRef]
- Pillai, A.D.; Rathod, P.D.; Xavier, F.P.; Padh, H.; Sudarsanam, V.; Vasu, K.K. Tetra Substituted Thiophenes as Anti-Inflammatory Agents: Exploitation of Analogue-Based Drug Design. Bioorg. Med. Chem. 2005, 13, 6685–6692. [Google Scholar] [CrossRef]
- Amr, A.-G.; Sherif, M.H.; Assy, M.G.; Al-Omar, M.A.; Ragab, I. Antiarrhythmic, Serotonin Antagonist and Antianxiety Activities of Novel Substituted Thiophene Derivatives Synthesized from 2-Amino-4,5,6,7-Tetrahydro-N-Phenylbenzo[b]thiophene-3-Carboxamide. Eur. J. Med. Chem. 2010, 45, 5935–5942. [Google Scholar] [CrossRef]
- Alomar, K.; Landreau, A.; Allain, M.; Bouet, G.; Larcher, G. Synthesis, Structure and Antifungal Activity of Thiophene-2,3-Dicarboxaldehyde Bis(thiosemicarbazone) and Nickel(II), Copper(II) and Cadmium(II) Complexes: Unsymmetrical Coordination Mode of Nickel Complex. J. Inorg. Biochem. 2013, 126, 76–83. [Google Scholar] [CrossRef]
- Emmitte, K.A.; Andrews, C.W.; Badiang, J.G.; Davis-Ward, R.G.; Dickson, H.D.; Drewry, D.H.; Emerson, H.K.; Epperly, A.H.; Hassler, D.F.; Knick, V.B.; et al. Discovery of Thiophene Inhibitors of Polo-Like Kinase. Bioorg. Med. Chem. Lett. 2009, 19, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Park, H.; Bauer, L.; Ryu, J.C.; Yoon, S.O. Thiophene-Pyrazolourea Derivatives as Potent, Orally Bioavailable, and Isoform-Selective JNK3 Inhibitors. ACS Med. Chem. Lett. 2020, 12, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Vanparijs, O.; Hermans, L.; Thienpont, D. Anthelmintic Activity of Flubendazole against Trichinella Spiralis in Rats. Vet. Parasitol. 1979, 5, 237–242. [Google Scholar] [CrossRef]
- Davidse, L.C.; Flach, W. Interaction of Thiabendazole with Fungal Tubulin. Biochim. Biophys. Acta 1978, 543, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Ladd, D.L.; Harrsch, P.B.; Kruse, L.I. Synthesis and Tubulin Binding of 4′-(l-Azi-2,2,2-trifluoroethyI)oncodazole, a Photolabile Analogue of Oncodazole. J. Org. Chem. 1988, 53, 417–420. [Google Scholar] [CrossRef]
- Liao, X.; Liu, L.; Tan, Y.; Jiang, G.; Fang, H.; Xiong, Y.; Duan, X.; Jiang, G.; Wang, J. Synthesis of Ruthenium Complexes Functionalized with Benzothiophene and Their Antibacterial Activity against Staphylococcus aureus. Dalton Trans. 2021, 50, 5607–5616. [Google Scholar] [CrossRef]
- Sweidan, K.; Engelmann, J.; Rayyan, W.A.; Sabbah, D.; Zarga, M.A.; Sabbah, D.; Al-Qirim, T.; Al-Hiari, Y.; Sheikha, G.A.; Shattat, G. Synthesis and Preliminary Biological Evaluation of New Heterocyclic Carboxamide Models. Lett. Drug Des. Discov. 2015, 12, 417–429. [Google Scholar] [CrossRef]
- Martorana, A.; Gentile, C.; Perricone, U.; Piccionello, A.P.; Bartolotta, R.; Terenzi, A.; Pace, A.; Mingoia, F.; Almerico, A.M.; Lauria, A. Synthesis, Antiproliferative Activity, and in Silico Insights of New 3-Benzoylamino-Benzo[b]thiophene Derivatives. Eur. J. Med. Chem. 2015, 90, 537–546. [Google Scholar] [CrossRef]
- Fakhr, I.M.; Radwan, M.A.; el-Batran, S.; Abd el-Salam, O.M.; el-Shenawy, S.M. Synthesis and Pharmacological Evaluation of 2-Substituted Benzo[b]thiophenes as Anti-inflammatory and Analgesic Agents. Eur. J. Med. Chem. 2009, 44, 1718–1725. [Google Scholar] [CrossRef]
- Wang, J.; Sheridan, R.S. A Singlet Aryl-CF3 Carbene: 2-Benzothienyl(trifluoromethyl)carbene and Interconversion with a Strained Cyclic Allene. Org. Lett. 2007, 9, 3177–3180. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Sharma, A.; Mao, R.; Jiang, N.; Dun, B.; She, J.X. Derivatives Containing Both Coumarin and Benzimidazole Potently Induce Caspase-Dependent Apoptosis of Cancer Cells through Inhibition of PI3K-AKT-mTOR Signaling. Anticancer Drugs 2015, 26, 667–677. [Google Scholar] [CrossRef]
- Yeung, K.S.; Meanwell, N.A.; Qiu, Z.; Hernandez, D.; Zhang, S.; McPhee, F.; Weinheimer, S.; Clark, J.M.; Janc, J.W. Structure-Activity Relationship Studies of a Bisbenzimidazole-Based, Zn2+ -Dependent Inhibitor of HCV NS3 Serine Protease. Bioorg. Med. Chem. Lett. 2001, 11, 2355–2359. [Google Scholar] [CrossRef]
- Beaulieu, P.L.; Bousquet, Y.; Gauthier, J.; Gillard, J.; Marquis, M.; McKercher, G.; Pellerin, C.; Valois, S.; Kukolj, G. Non-Nucleoside Benzimidazole-Based Allosteric Inhibitors of the Hepatitis C Virus NS5B Polymerase: Inhibition of Subgenomic Hepatitis C Virus RNA Replicons in Huh-7 Cells. J. Med. Chem. 2004, 47, 6884–6892. [Google Scholar] [CrossRef]
- Hirashima, S.; Suzuki, T.; Ishida, T.; Noji, S.; Yata, S.; Ando, I.; Komatsu, M.; Ikeda, S.; Hashimoto, H. Benzimidazole Derivatives Bearing Substituted Biphenyls as Hepatitis C Virus NS5B RNA-Dependent RNA Polymerase Inhibitors: Structure-Activity Relationship Studies and Identification of a Potent and Highly Selective Inhibitor JTK-109. J. Med. Chem. 2006, 49, 4721–4736. [Google Scholar] [CrossRef]
- Ishida, T.; Suzuki, T.; Hirashima, S.; Mizutani, K.; Yoshida, A.; Ando, I.; Ikeda, S.; Adachi, T.; Hashimoto, H. Benzimidazole Inhibitors of Hepatitis C Virus NS5B Polymerase: Identification of 2-[(4-Diarylmethoxy)phenyl]-Benzimidazole. Bioorg. Med. Chem. Lett. 2006, 16, 1859–1863. [Google Scholar] [CrossRef]
- Hunter, P. The Inflammation Theory of Disease. EMBO Rep. 2012, 13, 968–970. [Google Scholar] [CrossRef]
- Liu, C.H.; Abrams, N.D.; Carrick, D.M.; Chander, P.; Dwyer, J.; Hamlet, M.R.J.; Macchiarini, F.; PrabhuDas, M.; Shen, G.L.; Tandon, P.; et al. Biomarkers of Chronic Inflammation in Disease Development and Prevention: Challenges and Opportunities. Nat. Immunol. 2017, 18, 1175–1180. [Google Scholar] [CrossRef]
- Taşdemir, E.; Atmaca, M.; Yıldırım, Y.; Bilgin, H.M.; Demirtaş, B.; Obay, B.D.; Kelle, M.; Oflazoğlu, H.D. Influence of Coumarin and Some coumarin Derivatives on Serum Lipid Profiles in Carbontetrachloride-Exposed Rats. Hum. Exp. Toxicol. 2017, 36, 295–301. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Orhan, I.E.; Cordell, G.A.; Nabavi, S.M.; Budzyńska, B. Implication of Coumarins towards Central Nervous System Disorders. Pharmacol. Res. 2016, 103, 188–203. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Liu, T.; Sun, J.; Wang, X.-J. Synthesis and Application of Coumarin Fluorescence Probes. RSC Adv. 2020, 10, 10826–10847. [Google Scholar] [CrossRef]
- Balewski, Ł.; Szulta, S.; Jalińska, A.; Kornicka, A. A Mini-Review: Recent Advances in Coumarin-Metal Complexes with Biological Properties. Front. Chem. 2021, 9, 781779. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Zhang, Q. Fluorous Aryldiazirine Photoaffinity Labeling Reagents. Org. Lett. 2009, 11, 4882–4885. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Huang, W.; Zhang, Q. Isotope-Coded, Fluorous Photoaffinity Labeling Reagents. Chem. Commun. 2012, 48, 3339–3341. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, A.L.; Taunton, J. Target Identification by Diazirine Photo-Cross-linking and Click Chemistry. Curr. Protoc. Chem. Biol. 2009, 1, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Tomohiro, T.; Yamamoto, A.; Tatsumi, Y.; Hatanaka, Y. [3-(Trifluoromethyl)-3H-Diazirin-3-yl]coumarin as a Carbene-Generating Photocross-Linker with Masked Fluorogenic Beacon. Chem. Commun. 2013, 49, 11551–11553. [Google Scholar] [CrossRef]
- Shen, Y.; Xie, Q.; Norberg, M.; Sausville, E.; Vande Woude, G.; Wenkert, D. Geldanamycin Derivative Inhibition of HGF/SF-Mediated Met Tyrosine Kinase Receptor-Dependent Urokinase-Plasminogen Activation. Bioorg. Med. Chem. 2005, 13, 4960–4971. [Google Scholar] [CrossRef]
- Roe, S.M.; Prodromou, C.; O’Brien, R.; Ladbury, J.E.; Piper, P.W.; Pearl, L.H. Structural Basis for Inhibition of the Hsp90 Molecular Chaperone by the Antitumor Antibiotics Radicicol and Geldanamycin. J. Med. Chem. 1999, 42, 260–266. [Google Scholar] [CrossRef]
- Hotta, Y.; Kaneko, T.; Hayashi, R.; Yamamoto, A.; Morimoto, S.; Chiba, J.; Tomohiro, T. Photoinduced Electron Transfer-Regulated Protein Labeling with a Coumarin-Based Multifunctional Photocrosslinker. Chem. Asian. J. 2019, 14, 398–402. [Google Scholar] [CrossRef]
- Alterio, V.; Di Fiore, A.; D’Ambrosio, K.; Supuran, C.T.; De Simone, G. Multiple Binding Modes of Iinhibitors to Carbonic Anhydrases: How to Design Specific Drugs Targeting 15 Different Isoforms? Chem. Rev. 2012, 112, 4421–4468. [Google Scholar] [CrossRef]
- Bergmann, F.; Rimon, S.; Segal, R. Effect of pH on the activity of eel esterase towards different substrates. Biochem. J. 1958, 68, 493–499. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin Biosynthesis and Its Role in Plant Development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Negård, M.; Uhlig, S.; Kauserud, H.; Andersen, T.; Høiland, K.; Vrålstad, T. Links between Genetic Groups, Indole Alkaloid Profiles and Ecology within the Grass-Parasitic Claviceps purpurea Species Complex. Toxins 2015, 7, 1431–1456. [Google Scholar] [CrossRef]
- Netz, N.; Opatz, T. Marine Indole Alkaloids. Mar. Drugs 2015, 13, 4814–4914. [Google Scholar] [CrossRef]
- Chen, X.; Zhan, P.; Li, D.; De Clercq, E.; Liu, X. Recent Advances in DAPYs and Related Analogues as HIV-1 NNRTIs. Curr. Med. Chem. 2011, 18, 359–376. [Google Scholar] [CrossRef]
- Goyal, D.; Kaur, A.; Goyal, B. Benzofuran and Indole: Promising Scaffolds for Drug Development in Alzheimer’s Disease. ChemMedChem 2018, 13, 1275–1299. [Google Scholar] [CrossRef]
- Murai, Y.; Masuda, K.; Sakihama, Y.; Hashidoko, Y.; Hatanaka, Y.; Hashimoto, M. Comprehensive Synthesis of Photoreactive (3-Trifluoromethyl)diazirinyl Indole Derivatives from 5- and 6- Trifluoroacetylindoles for Photoaffinity Labeling. J. Org. Chem. 2012, 77, 8581–8587. [Google Scholar] [CrossRef]
- Wartmann, T.; Lindel, T. L-Phototryptophan. Eur. J. Org. Chem. 2013, 2013, 1649–1652. [Google Scholar] [CrossRef]
- Eto, H.; Eguchi, C.; Kagawa, T. Production of L-Tryptophan from γ-Glutamaldehydic Acid. Bull. Chem. Soc. Jpn. 1989, 62, 961–963. [Google Scholar] [CrossRef]
- Baran, P.S.; Hafensteiner, B.D.; Ambhaikar, N.B.; Guerrero, C.A.; Gallagher, J.D. Enantioselective Total Synthesis of Avrainvillamide and the Stephacidins. J. Am. Chem. Soc. 2006, 128, 8678–8693. [Google Scholar] [CrossRef]
- Ma, J.; Yin, W.; Zhou, H.; Liao, X.; Cook, J.M. General Approach to the Total Synthesis of 9-Methoxy-Substituted Indole Alkaloids: Synthesis of Mitragynine, as well as 9-Methoxygeissoschizol and 9-Methoxy-N(b)-Methylgeissoschizol. J. Org. Chem. 2009, 74, 264–273. [Google Scholar] [CrossRef]
- Blaser, G.; Sanderson, J.M.; Batsanov, A.S.; Howard, J.A.K. The Facile Synthesis of a Series of Tryptophan Derivatives. Tetrahedron Lett. 2008, 49, 2795–2798. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hatanaka, Y.; Nabeta, K. Effective Synthesis of a Carbon-linked Diazirinyl Fatty Acid Derivative via Reduction of the Carbonyl Group to Methylene with Triethylsilane and Trifluoroacetic Acid. Heterocycles 2003, 59, 395–398. [Google Scholar] [CrossRef]
- Collot, V.; Schmitt, M.; Marwah, P.; Bourguignon, J.-J. Regiospecific Functionalization of Indole-2-carboxylates and Diastereoselective Preparation of the Corresponding Indolines. Heterocycles 1999, 51, 2823–2847. [Google Scholar] [CrossRef]
- Leonard, N.J.; Greenfield, J.C.; Schmitz, R.Y.; Skoog, F. Photoaffinity-labeled Auxins: Synthesis and Biological Activity. Plant Physiol. 1975, 55, 1057–1061. [Google Scholar] [CrossRef] [PubMed]

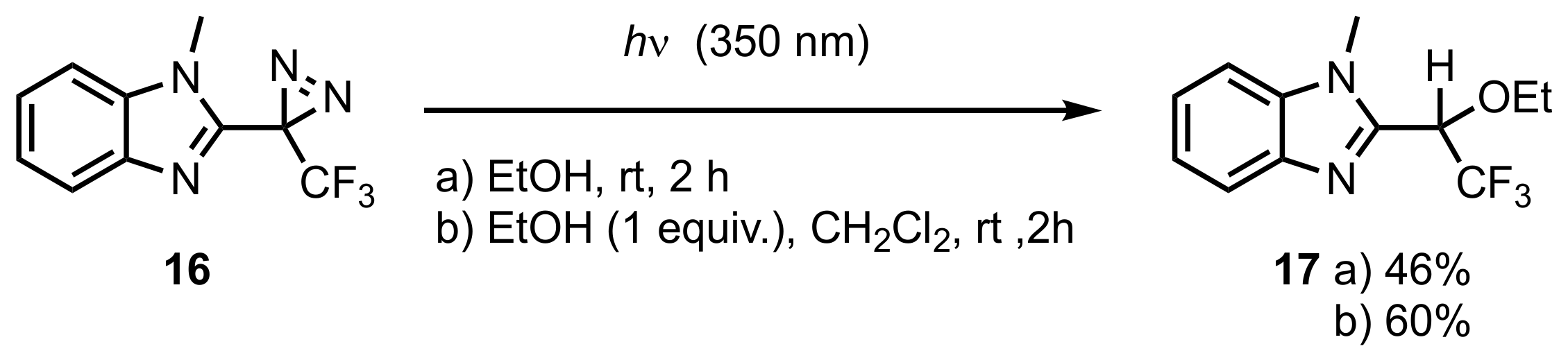
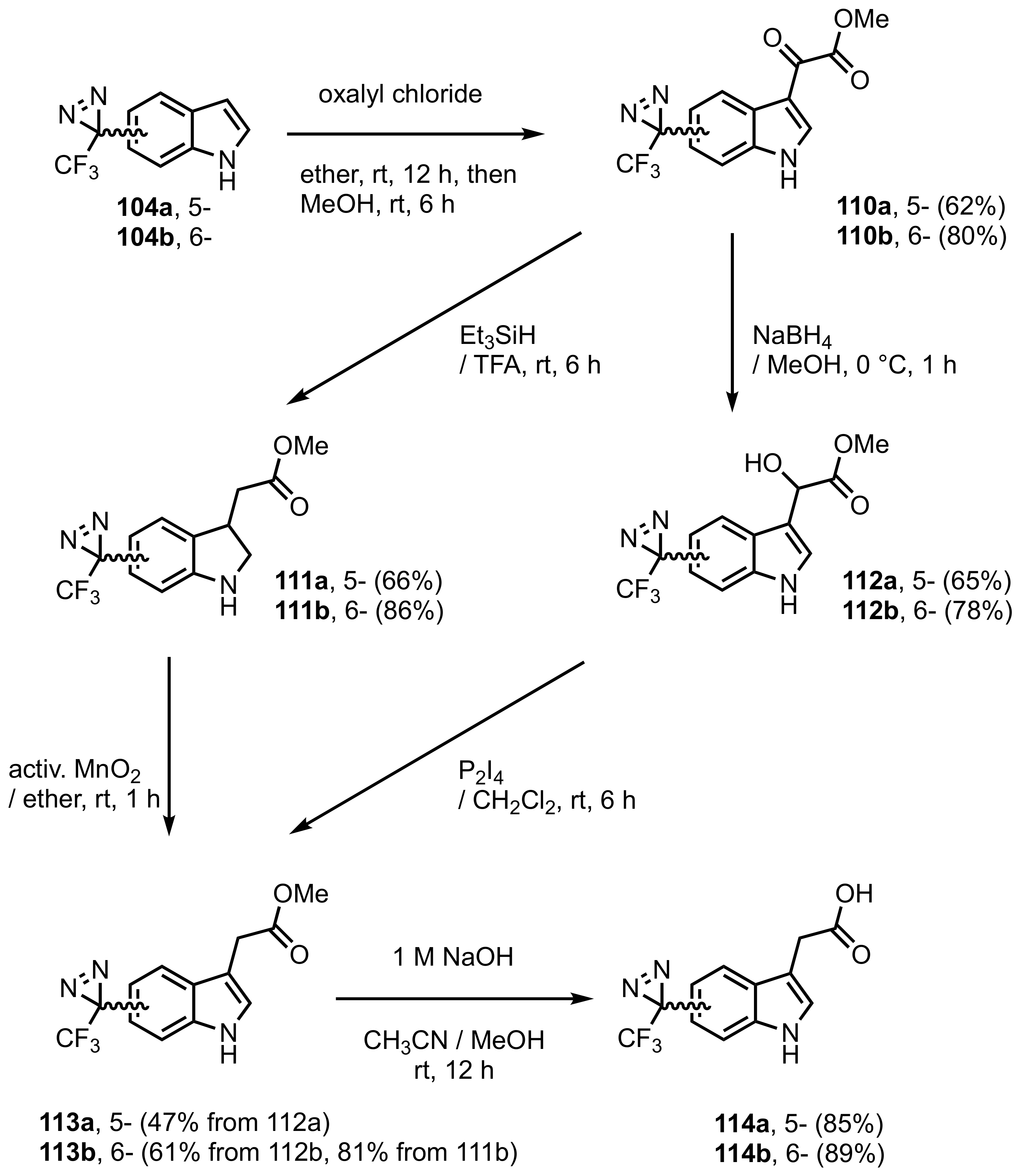
 | |||
|---|---|---|---|
| Duration of Ambient Light Exposure (Days) | Amounts of Unreacted Compound Yield (%) | ||
| 11 | 12 | 13 | |
| 0 | 100 | 100 | 100 |
| 4 | 97.1 | 99 | 87.7 |
| 7 | 95.2 | 98 | 78.1 |
| 14 | 90.1 | 95.2 | 58.1 |
| 18 | 87.7 | 94.3 | 49 |
| 26 | 82.6 | 92.6 | 35 |
| 31 | 79.4 | 90.1 | 26.8 |
| Compound | Aqueous Solubility (mM) | |
|---|---|---|
| pH 7.4 | pH 5.0 | |
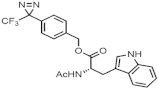 | <0.02 | <0.02 |
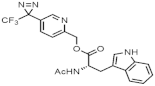 | 4.09 ± 0.19 | 4.29 ± 0.14 |
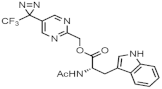 | 133 ± 0.5 | 131 ± 1.7 |
 | 11.3 ± 0.55 | 14.1 ± 0.43 |
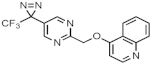
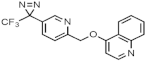 | 374 ± 2.7 | 422 ± 4.9 |
 | ≥1000 | ≥1000 |
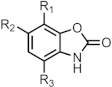 | |||
|---|---|---|---|
| Compound | R1 | R2 | R3 |
| 28 | H | OMe | H |
| 29 | OMe | OMe | H |
| 30 | OMe | OMe | Cl |
| 31 | H | OCH2CHMe2 | H |
| 32 | H | Ac | H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murai, Y.; Hashimoto, M. Heteroaromatic Diazirines Are Essential Building Blocks for Material and Medicinal Chemistry. Molecules 2023, 28, 1408. https://doi.org/10.3390/molecules28031408
Murai Y, Hashimoto M. Heteroaromatic Diazirines Are Essential Building Blocks for Material and Medicinal Chemistry. Molecules. 2023; 28(3):1408. https://doi.org/10.3390/molecules28031408
Chicago/Turabian StyleMurai, Yuta, and Makoto Hashimoto. 2023. "Heteroaromatic Diazirines Are Essential Building Blocks for Material and Medicinal Chemistry" Molecules 28, no. 3: 1408. https://doi.org/10.3390/molecules28031408
APA StyleMurai, Y., & Hashimoto, M. (2023). Heteroaromatic Diazirines Are Essential Building Blocks for Material and Medicinal Chemistry. Molecules, 28(3), 1408. https://doi.org/10.3390/molecules28031408






