UHPLC-MS Phytochemical Profiling and Insight into Bioactivity of Rabelera holostea (Greater Stitchwort) Extract
Abstract
1. Introduction
2. Results
2.1. Phytochemical Profile of R. holostea
2.2. In Vitro Biological Activities of R. holostea
2.3. In Silico Examination of Anti-Inflammatory Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Plant Material and Extract Preparation
4.3. LC/MS Analysis
4.3.1. UHPLC/MS-MS Orbitrap Analysis
4.3.2. UHPLC/(-)HESI-MS2 Quantification of Major Phenolics
4.4. Antioxidant Activity
4.4.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Free-Radical Scavenging Potential
4.4.2. 2,2′-Azinobis-(3-Ethylbenzothiazoline-6-Sulfonic Acid) Diammonium (ABTS) Radical-Cation Scavenging Potential
4.4.3. Inhibition of Lipid Peroxidation
4.4.4. Total Antioxidant Capacity
4.5. Antimicrobial Activity
4.5.1. Tested Microorganisms
4.5.2. Antimicrobial Activity Assays
4.6. Anti-Inflammatory Activity
4.6.1. In Vitro Analysis of Anti-Inflammatory Activity
4.6.2. In Silico Analysis of Anti-Inflammatory Activity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ancheeva, E.; Daletos, G.; Muharini, R.; Lin, W.H.; Teslov, L.; Proksch, P. Flavonoids from Stellaria nemorum and Stellaria holostea. Nat. Prod. Commun. 2015, 10, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:77205026-1 (accessed on 10 September 2022).
- Šilić, Č. Šumske Zeljaste Biljke; Svjetlost: Sarajevo, Yugoslavia, 1987. [Google Scholar]
- Slavokhotova, A.A.; Odintsova, T.I.; Rogozhin, E.A.; Musolyamov, A.K.; Andreev, Y.A.; Grishin, E.V.; Egorov, T.A. Isolation, Molecular Cloning and Antimicrobial Activity of Novel Defensins from Common Chickweed (Stellaria media L.) Seeds. Biochimie 2011, 93, 450–456. [Google Scholar] [CrossRef]
- Oladeji, O.S.; Oyebamiji, A.K. Stellaria media (L.) Vill.—A Plant with Immense Therapeutic Potentials: Phytochemistry and Pharmacology. Heliyon 2020, 6, e04150. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Vasudeva, N.; Sharma, S.K. Quality Assessment and Anti-Obesity Activity of Stellaria media (Linn.) Vill. BMC Complement. Altern. Med. 2012, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Song, J.; Shi, Y.; Wang, C.; Chen, B.; Xie, D.; Jia, X. Anti-Hepatitis B Virus Activity of Chickweed [Stellaria media (L.) Vill.] Extracts in HepG2.2.15 Cells. Molecules 2012, 17, 8633–8646. [Google Scholar] [CrossRef]
- Rogowska, M.; Lenart, M.; Srečec, S.; Ziaja, M.; Parzonko, A.; Bazylko, A. Chemical Composition, Antioxidative and Enzyme Inhibition Activities of Chickweed Herb (Stelaria Media L., Vill.) Ethanolic and Aqueous Extracts. Ind. Crop. Prod. 2017, 97, 448–454. [Google Scholar] [CrossRef]
- Sharples, M.T.; Tripp, E.A. Phylogenetic Relationships Within and Delimitation of the Cosmopolitan Flowering Plant Genus Stellaria L. (Caryophyllaceae): Core Stars and Fallen Stars. Syst. Bot. 2019, 44, 857–876. [Google Scholar] [CrossRef]
- Stojković, D.; Gašić, U.; Drakulić, D.; Zengin, G.; Stevanović, M.; Rajčević, N.; Soković, M. Chemical Profiling, Antimicrobial, Anti-Enzymatic, and Cytotoxic Properties of Phlomis Fruticosa L. J. Pharm. Biomed. Anal. 2021, 195, 113884. [Google Scholar] [CrossRef]
- Geng, P.; Sun, J.; Zhang, M.; Li, X.; Harnly, J.M.; Chen, P. Comprehensive Characterization of C-Glycosyl Flavones in Wheat (Triticum aestivum L.) Germ Using UPLC-PDA-ESI/HRMSn and Mass Defect Filtering. J. Mass Spectrom. 2016, 51, 914–930. [Google Scholar] [CrossRef]
- Ferreres, F.; Gil-Izquierdo, A.; Vinholes, J.; Grosso, C.; Valentão, P.; Andrade, P.B. Approach to the Study of C-Glycosyl Flavones Acylated with Aliphatic and Aromatic Acids from Spergularia Rubra by High-Performance Liquid Chromatography-Photodiode Array Detection/Electrospray Ionization Multi-Stage Mass Spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 700–712. [Google Scholar] [CrossRef]
- Sait, S.; Hamri-Zeghichi, S.; Boulekbache-Makhlouf, L.; Madani, K.; Rigou, P.; Brighenti, V.; Pio Prencipe, F.; Benvenuti, S.; Pellati, F. HPLC-UV/DAD and ESI-MSn Analysis of Flavonoids and Antioxidant Activity of an Algerian Medicinal Plant: Paronychia Argentea Lam. J. Pharm. Biomed. Anal. 2015, 111, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Katanić, J.; Boroja, T.; Mihailović, V.; Nikles, S.; Pan, S.-P.; Rosić, G.; Selaković, D.; Joksimović, J.; Mitrović, S.; Bauer, R. In Vitro and In Vivo Assessment of Meadowsweet (Filipendula ulmaria) as Anti-Inflammatory Agent. J. Ethnopharmacol. 2016, 193, 627–636. [Google Scholar] [CrossRef]
- Vecchio, A.J.; Malkowski, M.G. The Structure of NS-398 Bound to Cyclooxygenase-2. J. Struct. Biol. 2011, 176, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Akarasereenont, P.; Thiemermann, C.; Flower, R.J.; Vane, J.R. Selectivity of Nonsteroidal Antiinflammatory Drugs as Inhibitors of Constitutive and Inducible Cyclooxygenase. Proc. Natl. Acad. Sci. USA 1993, 90, 11693–11697. [Google Scholar] [CrossRef] [PubMed]
- Harman, C.A.; Turman, M.V.; Kozak, K.R.; Marnett, L.J.; Smith, W.L.; Garavito, R.M. Structural Basis of Enantioselective Inhibition of Cyclooxygenase-1 by S-α-Substituted Indomethacin Ethanolamides. J. Biol. Chem. 2007, 282, 28096–28105. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic Compounds: Current Industrial Applications, Limitations and Future Challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Bouillant, M.L.; de Arce, F.F.; Favre-Bonvin, J.; Chopin, J.; Zoll, A.; Mathieu, G. Structural Determination of 6-C-Diglycosyl-8-C-Glycosyl-Flavones and 6-C-Glycosyl-8-C-Diglycosylflavones by Mass Spectrometry of Their Permethyl Ethers. Phytochemistry 1984, 23, 2653–2657. [Google Scholar] [CrossRef]
- Mikšátková, P.; Ancheeva, E.; Hejtmánková, K.; Teslov, L.; Lapčík, O. Determination of Flavonoids in Stellaria by High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Anal. Lett. 2014, 47, 2317–2331. [Google Scholar] [CrossRef]
- Garnova, N.; Filippova, A.; Kasatkin, M.; Tikhonova, Y. Biologically Active Substances in the Aboveground Part of Three Stellaria Species. Res. J. Pharm. Technol. 2022, 15, 3153–3158. [Google Scholar] [CrossRef]
- Jakimiuk, K.; Wink, M.; Tomczyk, M. Flavonoids of the Caryophyllaceae; Springer Netherlands: Berlin/Heidelberg, Germany, 2022; Volume 21, ISBN 0123456789. [Google Scholar]
- Miere, F.; Teușdea, A.C.; Laslo, V.; Cavalu, S.; Fritea, L.; Dobjanschi, L.; Zdrinca, M.; Zdrinca, M.; Ganea, M.; Pașc, P.; et al. Evaluation of In Vitro Wound-Healing Potential, Antioxidant Capacity, and Antimicrobial Activity of Stellaria media (L.) Vill. Appl. Sci. 2021, 11, 11526. [Google Scholar] [CrossRef]
- Kumarasamy, Y.; Cox, P.J.; Jaspars, M.; Nahar, L.; Sarker, S.D. Screening Seeds of Scottish Plants for Antibacterial Activity. J. Ethnopharmacol. 2002, 83, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the Anti-Inflammatory and Antioxidant Activities of Luteolin, Kaempferol, Apigenin and Quercetin. South Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 72, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Mićović, T.; Katanić Stanković, J.S.; Bauer, R.; Nöst, X.; Marković, Z.; Milenković, D.; Jakovljević, V.; Tomović, M.; Bradić, J.; Stešević, D.; et al. In Vitro, In Vivo and In Silico Evaluation of the Anti-Inflammatory Potential of Hyssopus officinalis L. subsp aristatus (Godr.) Nyman (Lamiaceae). J. Ethnopharmacol. 2022, 293, 115201. [Google Scholar] [CrossRef]
- Aboulaghras, S.; Sahib, N.; Bakrim, S.; Benali, T.; Charfi, S.; Guaouguaou, F.E.; El Omari, N.; Gallo, M.; Montesano, D.; Zengin, G.; et al. Health Benefits and Pharmacological Aspects of Chrysoeriol. Pharmaceuticals 2022, 15, 973. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.; Barreiro, M.; Ferreira, I. Hydroxycinnamic Acids and Their Derivatives: Cosmeceutical Significance, Challenges and Future Perspectives, a Review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Bellik, Y.; Boukraâ, L.; Alzahrani, H.A.; Bakhotmah, B.A.; Abdellah, F.; Hammoudi, S.M.; Iguer-Ouada, M. Molecular Mechanism Underlying Anti-Inflammatory and Anti-Allergic Activities of Phytochemicals: An Update. Molecules 2012, 18, 322–353. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic Acid-Human Pharmacokinetics and Health Benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic Profiles of Leaves, Grapes and Wine of Grapevine Variety Vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef]
- Budzianowski, J.; Pakulski, G. Two C, O-Glycosylflavones from Stellaria media L. Planta Med. 1991, 57, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, M.V.; Vodoslavskyi, V.M.; Obodianskyi, M.A. Research of Phenolic Compounds of Ruta graveolens L. and Stellaria media (L.) Vill. Asian J. Pharm. Clin. Res. 2018, 11, 152–156. [Google Scholar] [CrossRef]
- Jakimiuk, K.; Strawa, J.W.; Granica, S.; Locatelli, M.; Tartaglia, A.; Tomczyk, M. Determination of Flavonoids in Selected Scleranthus Species and Their Anti-Collagenase and Antioxidant Potential. Molecules 2022, 27, 2015. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.P.; Matos, R.P.; Silva, S.T.; Andrade, P.B.; Ferreres, F.; Gil-Izquierdo, A.; Meireles, S.; Brandão, T.M.; Valentão, P. A New Iced Tea Base Herbal Beverage with Spergularia rubra Extract: Metabolic Profile Stability and in vitro Enzyme Inhibition. J. Agric. Food Chem. 2013, 61, 8650–8656. [Google Scholar] [CrossRef] [PubMed]
- Zheleva-Dimitrova, D.; Zengin, G.; Balabanova, V.; Voynikov, Y.; Lozanov, V.; Lazarova, I.; Gevrenova, R. Chemical Characterization with in vitro Biological Activities of Gypsophila Species. J. Pharm. Biomed. Anal. 2018, 155, 56–69. [Google Scholar] [CrossRef]
- Banjanac, T.; Dragićević, M.; Šiler, B.; Gašić, U.; Bohanec, B.; Nestorović Živković, J.; Trifunović, S.; Mišić, D. Chemodiversity of Two Closely Related Tetraploid Centaurium Species and Their Hexaploid Hybrid: Metabolomic Search for High-Resolution Taxonomic Classifiers. Phytochemistry 2017, 140, 27–44. [Google Scholar] [CrossRef]
- Kumarasamy, Y.; Byres, M.; Cox, P.J.; Jaspars, M.; Nahar, L.; Sarker, S.D. Screening Seeds of Some Scottish Plants for Free Radical Scavenging Activity. Phyther. Res. 2007, 21, 615–621. [Google Scholar] [CrossRef]
- Katanić Stanković, J.S.; Srećković, N.; Mišić, D.; Gašić, U.; Imbimbo, P.; Monti, D.M.; Mihailović, V. Bioactivity, Biocompatibility and Phytochemical Assessment of Lilac Sage, Salvia verticillata L. (Lamiaceae)—A Plant Rich in Rosmarinic Acid. Ind. Crop. Prod. 2020, 143, 111932. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Hsu, C.-K.; Chiang, B.-H.; Chen, Y.-S.; Yang, J.-H.; Liu, C.-L. Improving the Antioxidant Activity of Buckwheat (Fagopyrum tataricm Gaertn) Sprout with Trace Element Water. Food Chem. 2008, 108, 633–641. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre Plate-Based Antibacterial Assay Incorporating Resazurin as an Indicator of Cell Growth, and Its Application in the In Vitro Antibacterial Screening of Phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32. [Google Scholar]
- NCCLS. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Second Edition, Document M27-A2; NCCLS: Wayne, PA, USA, 2002; ISBN 1-56238-469-4. [Google Scholar]
- NCCLS. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard. NCCLS Document M38-A; NCCLS: Wayne, PA, USA, 2002; ISBN 1-56238-470-8. [Google Scholar]
- Srećković, N.; Mišić, D.; Gašić, U.; Matić, S.L.; Katanić Stanković, J.S.; Mihailović, N.; Monti, D.M.; Elia, L.D.; Mihailović, V. Meadow Sage (Salvia pratensis L.): A Neglected Sage Species with Valuable Phenolic Compounds and Biological Potential. Ind. Crop. Prod. 2022, 189, 115841. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Grozdeva, M.; Hess, S.; Hüll, M.; Danesch, U.; Bodensieck, A.; Bauer, R. Petasites hybridus Extracts In Vitro Inhibit COX-2 and PGE2 Release by Direct Interaction with the Enzyme and by Preventing P42/44 MAP Kinase Activation in Rat Primary Microglial Cells. Planta Med. 2005, 71, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.1; Wallingford Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Biovia Dassault Systèmes. Discovery Studio Modeling Environment; Biovia Dassault Systèmes: San Diego, CA, USA, 2017. [Google Scholar]
- Zhang, Y.; Forli, S.; Omelchenko, A.; Sanner, M.F. AutoGridFR: Improvements on AutoDock Affinity Maps and Associated Software Tools. J. Comput. Chem. 2019, 40, 2882–2886. [Google Scholar] [CrossRef] [PubMed]

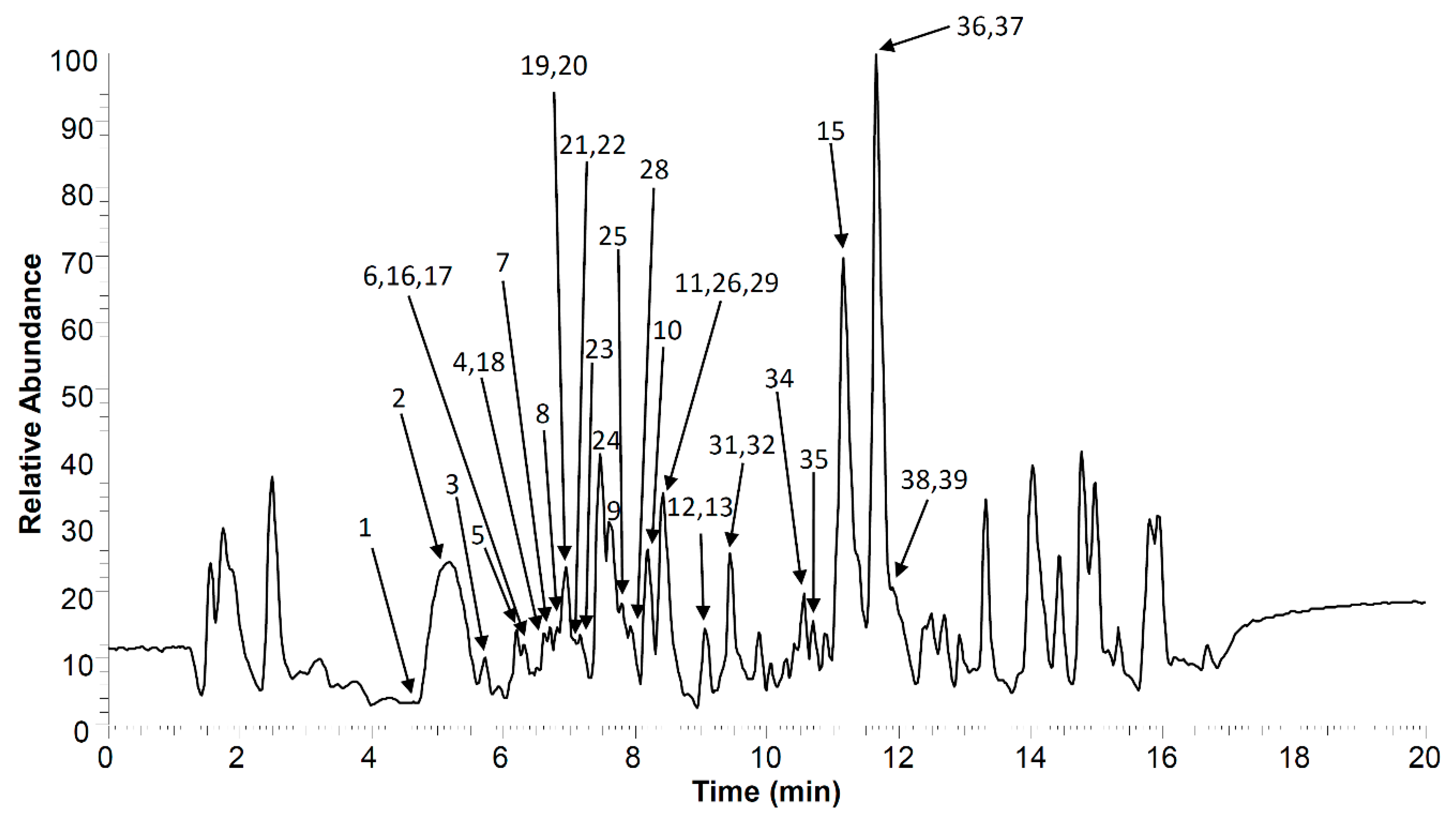

| No | Compound Name | tR, min | Molecular Formula, [M–H]− | Calculated Mass, [M–H]− | Exact Mass, [M–H]− | Δ mDa | MS2 Fragments, (% Base Peak) | MS3 Fragments, (% Base Peak) | MS4 Fragments, (% Base Peak) |
|---|---|---|---|---|---|---|---|---|---|
| Hydroxybenzoic acids | |||||||||
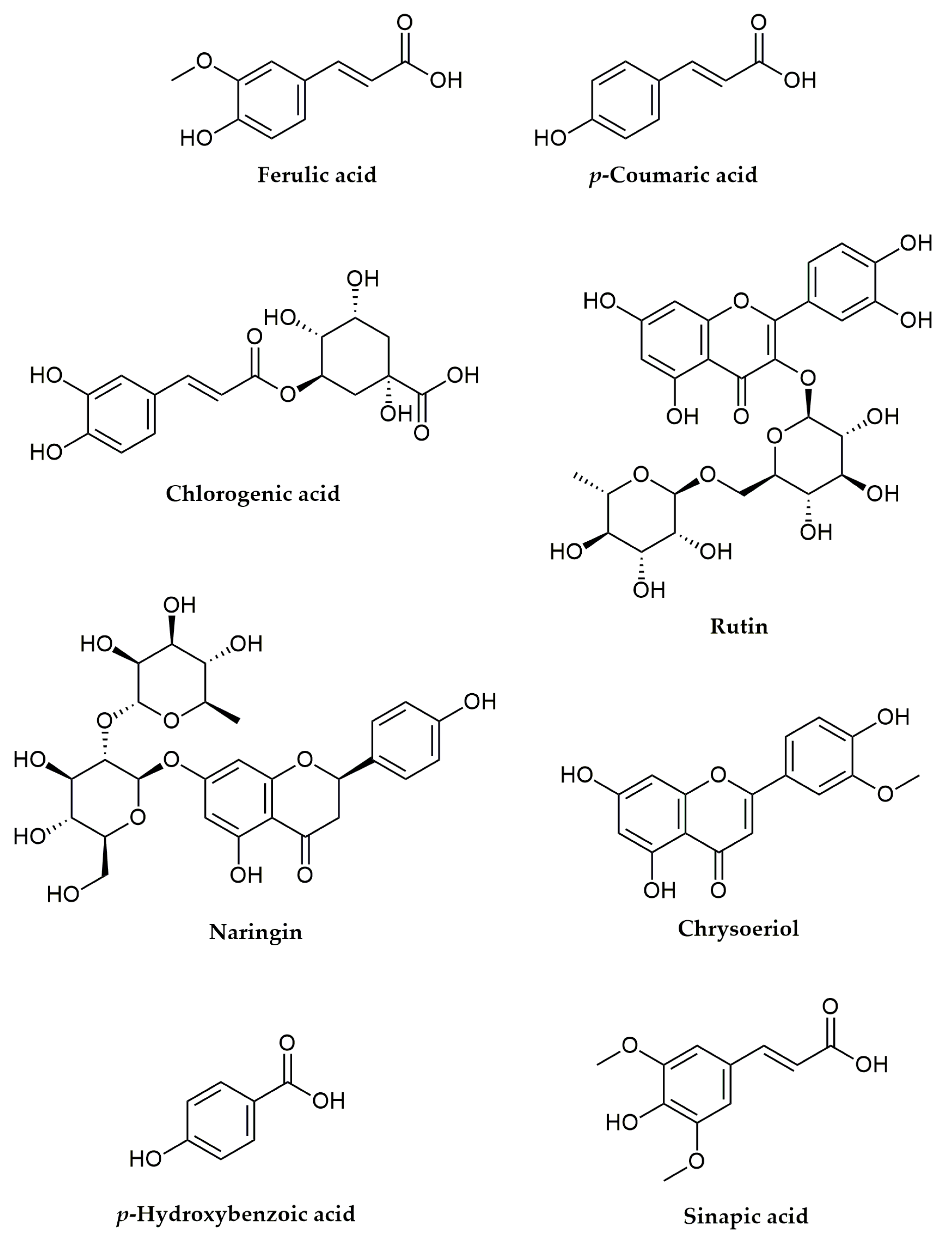
| 1 | Gallic acida | 4.69 | C7H5O5− | 169.01425 | 169.01217 | 2.08 | 69(5), 84(7), 123(8), 124(7), 125(100), 126(8), 127(3) | 53(58), 81(100), 83(6), 97(85), 98(21), 125(9) | ND |
| 2 | Dihydroxybenzoic acid hexoside I | 5.65 | C13H15O9− | 315.07216 | 315.06992 | 2.23 | 108(8), 109(12), 152(50), 153(100), 163(9), 165(12), 268(8) | 109(100), 123(3) | ND |
| 3 | Dihydroxybenzoic acid hexoside II | 5.97 | C13H15O9− | 315.07216 | 315.07002 | 2.14 | 109(9), 135(3), 151(4), 153(100), 154(6) | 109(100), 123(6) | 53(18), 81(100) |
| 4 | p-Hydroxybenzoic acida | 6.70 | C7H5O3− | 137.02442 | 137.02354 | 0.88 | 93(100) | ND | ND |
| Hydrocinnamic acids | |||||||||
| 5 | Coumaroylquinic acid I | 6.20 | C16H17O8− | 337.09289 | 337.09048 | 2.42 | 119(21), 145(100), 163(56), 219(20), 277(50), 293(17), 319(35) | 117(100), 145(3) | ND |
| 6 | Coumaric acid dihexoside | 6.52 | C21H27O13− | 487.14572 | 487.14282 | 2.90 | 145(7), 163(100), 187(20), 221(4), 323(14), 397(5), 427(8) | 119(100) | ND |
| 7 | Coumaroylquinic acid II | 6.81 | C16H17O8− | 337.09289 | 337.09043 | 2.47 | 117(6), 119(16), 145(100), 146(8), 163(58), 277(52), 291(6) | 117(100) | ND |
| 8 | 5-O-Caffeoylquinic acid (Chlorogenic acid)a | 6.95 | C16H17O9− | 353.08781 | 353.08502 | 2.79 | 179(3), 191(100), 192(6) | 85(100), 87(19), 111(33), 127(83), 171(24), 173(57) | 53(100) |
| 9 | Caffeic acida | 7.76 | C9H7O4− | 179.03498 | 179.03400 | 0.98 | 89(23), 133(24), 134(12), 135(100), 136(14), 143(17), 161(18) | 78(7), 91(27), 93(6), 106(19), 107(100) | ND |
| 10 | Verbascoside | 8.12 | C29H35O15− | 623.19814 | 623.19537 | 2.78 | 315(3), 461(100), 462(14) | 135(66), 143(6), 161(13), 297(16), 315(100) | 119(11), 135(100), 143(4), 161(3), 179(3) |
| 11 | p-Coumaric acida | 8.57 | C9H7O3− | 163.04007 | 163.03920 | 0.87 | 119(100), 120(8), 121(5), 131(6), 133(5), 135(6), 136(4) | 91(100), 92(11), 168(9) | ND |
| 12 | Sinapic acida | 9.02 | C11H11O5− | 223.06120 | 223.05900 | 2.20 | 164(18), 179(31), 208(100) | 149(13), 164(100), 193(9) | 135(34), 149(100) |
| 13 | Ferulic acida | 9.14 | C10H9O4− | 193.05063 | 193.04956 | 1.08 | 111(57), 134(34), 147(100), 148(10), 149(95), 150(10), 178(71) | 57(4), 85(6), 99(4), 103(100), 119(3), 129(41) | 59(100) |
| 14 | Rosmarinic acid | 9.22 | C18H15O8− | 359.07517 | 359.07724 | −2.07 | 161(100), 297(63), 313(40), 341(26), 197(23) | 133(100) | ND |
| 15 | Coumaric acid methyl ester | 11.32 | C10H9O3− | 177.05572 | 177.05467 | 1.05 | 117(13), 118(43), 119(3), 145(100), 146(9), 162(36), 177(8) | 83(3), 117(100) | ND |
| Flavonoid C-glycosides | |||||||||
| 16 | Luteolin 6-C-pentoside-8-C-(6”-hexosyl)-hexoside | 6.45 | C32H37O20− | 741.18249 | 741.18096 | 1.53 | 369(22), 399(34), 429(11), 441(11), 459(100), 460(19), 489(38) | 369(100), 381(4), 399(88), 423(3), 441(30) | 298(31), 312(4), 313(40), 341(100) |
| 17 | Apigenin 6-C-hexoside-8-C-(6”-hexosyl)-hexoside | 6.52 | C33H39O20− | 755.19814 | 755.19914 | −1.00 | 353(71), 354(14), 383(42), 473(100), 474(19), 635(34), 665(23) | 353(100), 354(5), 383(31), 455(5) | 282(3), 297(53), 307(3), 325(100), 326(3) |
| 18 | Apigenin 6-C-(6”-hexosyl)-hexoside 8-C-pentoside I | 6.70 | C32H37O19− | 725.18758 | 725.18392 | 3.66 | 353(64), 383(60), 443(100), 444(19), 635(34), 665(31), 707(15) | 353(100), 354(6), 365(5), 383(55), 384(3), 425(18) | 233(3), 297(54), 325(100), 335(3) |
| 19 | Luteolin 8-C-(6”-hexosyl)-hexoside | 6.95 | C27H29O16− | 609.14024 | 609.14037 | −0.14 | 297(6), 327(100), 328(12), 357(100), 358(14), 369(12), 393(7) | 133(3), 191(3), 255(4), 284(20), 299(100), 300(11) | 213(55), 231(27), 240(39), 255(100), 257(26) |
| 20 | Apigenin 6-C-(6”-hexosyl)-hexoside 8-C-pentoside II | 6.98 | C32H37O19− | 725.19345 | 725.19138 | 2.07 | 353(47), 383(30), 443(100), 444(19), 473(38), 527(16), 635(18) | 353(100), 354(5), 383(26), 425(3) | 282(3), 297(49), 325(100), 326(3) |
| 21 | Apigenin 6,8-di-C-hexoside | 7.11 | C27H29O15− | 593.15119 | 593.14703 | 4.16 | 353(44), 354(10), 383(23), 473(100), 474(20), 503(30), 575(9) | 353(100), 354(4), 383(16) | 282(3), 297(53), 325(100) |
| 22 | Luteolin 6-C-pentoside-8-C-hexoside | 7.18 | C26H27O15− | 579.12967 | 579.13175 | −2.08 | 369(21), 399(30), 459(53), 489(100), 490(20), 519(19), 561(14) | 369(100), 370(5), 399(67), 411(4), 429(17), 471(12) | 298(28), 312(5), 313(33), 341(100) |
| 23 | Apigenin 8-C-(6”-hexosyl)-hexoside | 7.27 | C27H29O15− | 593.15119 | 593.14849 | 2.71 | 246(3), 283(11), 311(100), 312(10), 341(19), 353(4), 473(7) | 283(100), 284(10) | 163(88), 211(30), 224(28), 239(100), 283(50) |
| 24 | Apigenin 6-C-hexoside 8-C-pentoside | 7.46 | C26H27O14− | 563.14063 | 563.13740 | 3.23 | 353(29), 383(22), 443(100), 444(21), 473(59), 474(14), 545(11) | 353(100), 354(13), 383(23), 384(3), 425(3) | 297(47), 298(4), 323(3), 325(100) |
| 25 | Luteolin 8-C-hexoside | 7.78 | C21H19O11− | 447.09329 | 447.09015 | 3.13 | 172(3), 327(100), 328(8), 357(48), 358(3), 369(3), 429(10) | 284(9), 298(3), 299(100), 300(7) | 199(33), 213(65), 231(33), 240(46), 255(100) |
| 26 | Chrysoeriol (3′-Methyl luteolin) 6-C-hexoside | 8.36 | C22H21O11− | 461.10894 | 461.10556 | 3.37 | 341(100), 342(8), 371(16), 443(3) | 298(100), 313(29), 326(5) | 253(49), 255(38), 269(88), 270(100), 298(94) |
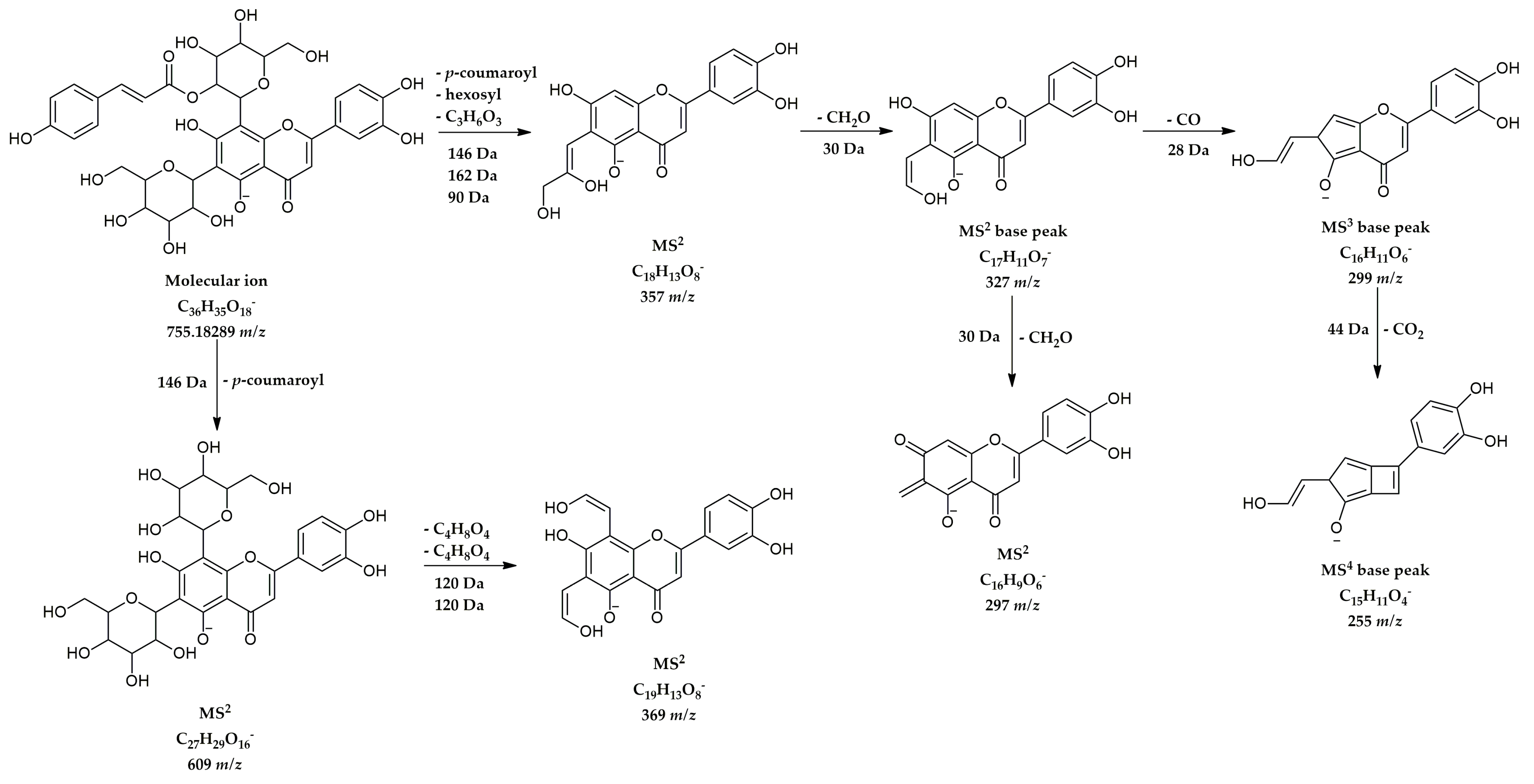
| 27 | Luteolin 6-C-hexoside-8-C-(2”-coumaroyl)-hexoside | 8.57 | C36H35O18− | 755.18289 | 755.18424 | −1.35 | 297(10), 327(100), 328(14), 357(67), 358(11), 369(10), 609(12) | 255(3), 284(18), 285(3), 298(5), 299(100), 300(9) | 213(62), 227(43), 240(46), 255(100), 257(40) |
| Flavonoid O-glycosides | |||||||||
| 28 | Quercetin 3-O-(6”-rhamnosyl)-hexoside (Rutin)a | 8.00 | C27H29O16− | 609.14611 | 609.14506 | 1.05 | 255(5), 271(8), 285(5), 300(41), 301(100), 302(17), 343(8) | 151(66), 179(100), 229(6), 256(13), 272(15), 273(19) | 151(100) |
| 29 | Quercetin 3-O-galactoside (Hyperoside)a | 8.50 | C21H19O12− | 463.08233 | 463.08276 | −0.44 | 299(3), 300(22), 301(100), 302(15) | 151(81), 179(100), 255(12), 257(14), 271(19), 273(18) | 151(100) |
| 30 | Naringin 7-O-(2”-rhamnosyl)-hexoside (Naringin)a | 8.85 | C27H31O14− | 579.16606 | 579.16645 | −0.39 | 235(13), 271(48), 272(7), 313(17), 357(5), 459(100), 460(17) | 151(22), 235(68), 271(49), 339(28), 357(100), 441(23) | 125(14), 151(79), 168(24), 169(17), 339(100) |
| 31 | Jaceosidin 7-O-hexoside (Jaceoside) | 8.99 | C23H23O12− | 491.11363 | 491.11550 | −1.87 | 314(13), 328(15), 329(58), 330(8), 343(9), 476(100), 477(23) | 313(38), 314(99), 315(33), 343(100), 357(10), 461(63) | 315(32), 328(100), 329(4) |
| 32 | Jaceosidin 7-O-hexuronide | 9.08 | C23H21O13− | 505.09877 | 505.09558 | 3.18 | 175(4), 315(4), 329(100), 330(15) | 299(4), 314(100), 315(9) | 285(9), 299(100) |
| Flavonoid aglycones | |||||||||
| 33 | Catechina | 7.22 | C15H13O6− | 289.07176 | 289.06838 | 3.38 | 179(12), 203(12), 205(37), 231(7), 245(100), 246(16), 247(8) | 161(18), 187(24), 188(14), 203(100), 227(25), 230(5) | 161(53), 173(15), 175(100), 185(35), 188(77) |
| 34 | Eriodictyol a | 10.63 | C15H11O6− | 287.05024 | 287.05213 | −1.90 | 151(100), 152(8), 199(8), 241(9), 253(5), 257(8), 269(4) | 65(4), 83(5), 107(100) | 65(100) |
| 35 | Luteolin a | 10.71 | C15H9O6− | 285.04046 | 285.03692 | 3.54 | 151(37), 175(79), 199(80), 217(65), 241(100), 243(53), 285(45) | 197(81), 198(100), 199(51), 212(18), 213(39), 226(31) | ND |
| 36 | Naringenin a | 11.57 | C15H11O5− | 271.06120 | 271.05761 | 3.59 | 107(5), 149(8), 151(100), 152(7), 177(21), 225(19), 227(5) | 65(4), 83(5), 107(100) | 65(100) |
| 37 | Apigenin a | 11.61 | C15H9O5− | 269.04950 | 269.04810 | 1.39 | 149(38), 151(100), 181(18), 201(27), 225(93), 227(20), 269(34) | 83(3), 107(100) | 63(12), 65(100) |
| 38 | Kaempferol a | 11.78 | C15H9O6− | 285.04046 | 285.03693 | 3.53 | 229(15), 241(16), 255(67), 256(100), 257(45), 284(19), 285(46) | 211(36), 212(71), 227(100), 228(51), 229(20), 256(17) | ND |
| 39 | Chrysoeriol a | 11.82 | C16H11O6− | 299.05024 | 299.05222 | −1.98 | 284(100), 285(13) | 256(100), 284(6) | 211(17), 212(16), 227(100), 228(33), 239(15) |
| Phenolic Compound | tR, min | Linearity Equations (A + BX) × 105 | Correlation R2 | LOD, µg/mL | LOQ, µg/mL | Parent Ion, m/z | Product Ion, m/z (Collision Energy, eV) | Content (mg/kg d.e.) |
|---|---|---|---|---|---|---|---|---|
| Gallic acid | 2.14 | Y = −0.23 + 5.19X | 0.9905 | 0.12 | 0.41 | 169.032 | 79.11 (31); 125.04 (16) | 1.04 |
| Chlorogenic acid | 4.99 | Y = −0.48 + 34.94X | 0.9923 | 0.13 | 0.43 | 353.103 | 191.28 (25) | 46.35 |
| p-Hydroxybenzoic acid | 5.23 | Y = −0.25 + 2.70X | 0.9916 | 0.21 | 0.70 | 137.057 | 93.19 (19); 108.33 (22) | 6.54 |
| Catechin | 5.46 | Y = −0.11 + 3.23X | 0.9937 | 0.08 | 0.26 | 289.050 | 245.10 (16); 123.08 (34) | 0.47 |
| Caffeic acid | 5.51 | Y = −1.18 + 55.82X | 0.9917 | 0.11 | 0.38 | 179.004 | 134.00 (13); 135.00 (16) | 2.09 |
| Rutin | 6.04 | Y = 0.63 + 38.76X | 0.9939 | 0.14 | 0.46 | 609.197 | 299.98 (42); 301.20 (32) | 3.41 |
| p-Coumaric acid | 6.15 | Y = −0.41 + 36.64X | 0.9923 | 0.10 | 0.33 | 163.031 | 93.12 (39); 119.09 (16) | 81.18 |
| Hyperoside | 6.40 | Y = 0.63 + 60.91X | 0.9927 | 0.10 | 0.32 | 463.002 | 271.01 (44); 300.02 (29) | 0.84 |
| Ferulic acid | 6.55 | Y = 0.08 + 10.07X | 0.9978 | 0.04 | 0.13 | 193.057 | 134.00 (18); 178.00 (15) | 42.98 |
| Sinapic acid | 6.68 | Y = −0.02 + 0.69X | 0.9941 | 0.09 | 0.31 | 223.082 | 149.21 (36) | 4.20 |
| Naringin | 6.84 | Y = 0.02 + 1.41X | 0.9915 | 0.11 | 0.35 | 579.241 | 271.36 (33); 151.42 (43) | 1.16 |
| Eriodictyol | 8.12 | Y = −0.87 + 38.38X | 0.9974 | 0.06 | 0.19 | 286.974 | 150.93 (19); 135.02 (22) | 0.34 |
| Luteolin | 8.21 | Y = −2.09 + 54.69X | 0.9977 | 0.05 | 0.17 | 285.035 | 151.03 (18); 133.06 (36) | 0.35 |
| Naringenin | 8.88 | Y = −0.54 + 36.66X | 0.9990 | 0.04 | 0.12 | 271.036 | 151.01 (20); 107.07 (26) | 0.15 |
| Apigenin | 8.89 | Y = −1.16 + 45.10X | 0.9973 | 0.06 | 0.20 | 269.032 | 151.00 (26); 117.07 (43) | 0.18 |
| Kaempferol | 8.91 | Y = −0.08 + 2.64X | 0.9913 | 0.12 | 0.39 | 285.074 | 211.00 (32); 227.00 (32) | 0.86 |
| Chrysoeriol | 9.15 | Y = −0.40 + 7.19X | 0.9946 | 0.07 | 0.24 | 298.933 | 210.89 (43); 159.17 (26) | 3.83 |
| Sample and Standards | IC50 Values (μg/mL) | Total Antioxidant Capacity (mg AAE/g) | ||
|---|---|---|---|---|
| DPPH· Scavenging Activity | ABTS·+ Scavenging Activity | Inhibition of Lipid Peroxidation | ||
| R. holostea extract | 246.7 ± 6.8 c | 420.4 ± 9.3 b | 570.4 ± 9.9 | 192.0 ± 3.9 |
| CA | 2.97 ± 0.31 a | 12.16 ± 2.04 a | - | - |
| QU | 1.41 ± 0.19 a | 8.37 ± 1.12 a | - | - |
| BHT | 13.61 ± 1.74 b | 26.09 ± 2.84 a | 3.92 ± 0.76 | - |
| Bacteria (ATCC and Isolates) | MIC Values | Fungi | MIC Values | ||
|---|---|---|---|---|---|
| R. holostea Extract | Chloramphenicol | R. holostea Extract | Ketoconazole | ||
| Micrococcus lysodeikticus ATCC 4698, G+ | 10 × 103 | 2.5 | Fusarium oxysporum FSB 91 | 1250 | 0.31 |
| Enterococcus faecalis ATCC 29212, G+ | 10 × 103 | 10 | Trichoderma longibrachiatum FSB 13 | 20 × 103 | 1.25 |
| Enterococcus faecalis FSB 24, G+ | 5 × 103 | 2.5 | Phialophora fastigiata FSB 81 | 5 × 103 | 10 |
| Bacillus mycoides FSB 1, G+ | 20 × 103 | 10 | Alternaria alternata FSB 51 | 10 × 103 | 5 |
| Escherichia coli ATCC 25922, G- | 10 × 103 | 10 | Penicillium verrucosum FSB 21 | 10 × 103 | 2.5 |
| Klebsiella pneumoniae ATCC 70063, G- | 10 × 103 | 10 | Penicillium canescens FSB 24 | 5 × 103 | 1.25 |
| Pseudomonas aeruginosa ATCC 10145, G- | >20 × 103 | 10 | Aspergillus glaucus FSB 32 | 20 × 103 | 2.5 |
| Azobacter chroococcum FSB 14, G- | 5 × 103 | 5 | Aspergillus brasiliensis FSB 31 | 20 × 103 | 0.62 |
| Complexes | ∆Gbind(kcal/mol) | Ki (µM) | FIE (kcal/mol) | vdW + Hbond + Desolv Energy (kcal/mol) | Electrostatic Energy (kcal/mol) | FTIE (kcal/mol) | TFE (kcal/mol) | USE (kcal/mol) |
|---|---|---|---|---|---|---|---|---|
| COX-1-IND | −9.64 | 0.08 | −11.31 | −10.25 | −1.06 | −0.53 | +1.49 | −0.71 |
| COX-1-p-CA | −5.46 | 99.17 | −6.77 | −5.89 | −0.88 | +0.05 | +1.19 | −0.06 |
| COX-1-FA | −6.00 | 40.21 | −6.89 | −6.05 | −0.83 | −0.84 | +1.49 | −0.24 |
| COX-1-CA | −10.03 | 0.04 | −9.75 | −8.79 | −0.96 | −4.93 | +3.28 | −1.37 |
| COX-1-p-HBA | −4.94 | 239.52 | −5.84 | −5.59 | −0.25 | −0.01 | +0.89 | −0.01 |
| COX-1-SIN | −6.32 | 23.24 | −7.57 | −6.95 | −0.62 | −1.12 | +1.79 | −0.58 |
| COX-1-CHR | −9.36 | 0.14 | −9.62 | −9.49 | −0.13 | −1.63 | +1.49 | −0.40 |
| COX-1-NAR | −4.66 | 382.65 | −4.37 | −4.31 | −0.07 | −6.55 | +4.18 | −2.09 |
| COX-1-RU | −6.41 | 20.04 | −8.16 | −7.99 | −0.17 | −5.20 | +4.77 | −2.17 |
| Complexes | ∆Gbind (kcal/mol) | Ki (µM) | FIE (kcal/mol) | vdW + Hbond + Desolv Energy (kcal/mol) | Electrostatic Energy (kcal/mol) | FTIE (kcal/mol) | TFE (kcal/mol) | USE (kcal/mol) |
|---|---|---|---|---|---|---|---|---|
| COX-2-NS398 | −8.26 | 0.88 | −9.75 | −9.08 | −0.67 | −0.94 | +1.49 | −0.93 |
| COX-2-p-CA | −5.08 | 188.97 | −6.28 | −5.40 | −0.88 | −0.06 | +1.19 | −0.06 |
| COX-2-FA | −5.96 | 42.42 | −6.90 | −6.20 | −0.70 | −0.80 | +1.49 | −0.24 |
| COX-2-CA | −10.93 | 0.01 | −11.52 | −11.04 | −0.49 | −4.06 | +3.28 | −1.37 |
| COX-2-p-HBA | −4.52 | 488.92 | −5.41 | −4.50 | −0.91 | −0.01 | +0.89 | −0.01 |
| COX-2-SIN | −6.21 | 28.03 | −7.47 | −6.77 | −0.70 | −1.11 | +1.79 | −0.58 |
| COX-2-CHR | −9.51 | 0.12 | −9.79 | −9.73 | −0.06 | −1.61 | +1.49 | −0.40 |
| COX-2-NAR | −5.53 | 88.88 | −6.57 | −6.62 | +0.04 | −5.23 | +4.18 | −2.10 |
| COX-2-RU | −4.13 | 937.71 | −6.74 | −6.72 | −0.02 | −4.36 | +4.77 | −2.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katanić Stanković, J.S.; Đorović Jovanović, J.; Mišić, D.; Gašić, U.; Nikles, S.; Marković, Z.; Bauer, R. UHPLC-MS Phytochemical Profiling and Insight into Bioactivity of Rabelera holostea (Greater Stitchwort) Extract. Molecules 2023, 28, 1274. https://doi.org/10.3390/molecules28031274
Katanić Stanković JS, Đorović Jovanović J, Mišić D, Gašić U, Nikles S, Marković Z, Bauer R. UHPLC-MS Phytochemical Profiling and Insight into Bioactivity of Rabelera holostea (Greater Stitchwort) Extract. Molecules. 2023; 28(3):1274. https://doi.org/10.3390/molecules28031274
Chicago/Turabian StyleKatanić Stanković, Jelena S., Jelena Đorović Jovanović, Danijela Mišić, Uroš Gašić, Stefanie Nikles, Zoran Marković, and Rudolf Bauer. 2023. "UHPLC-MS Phytochemical Profiling and Insight into Bioactivity of Rabelera holostea (Greater Stitchwort) Extract" Molecules 28, no. 3: 1274. https://doi.org/10.3390/molecules28031274
APA StyleKatanić Stanković, J. S., Đorović Jovanović, J., Mišić, D., Gašić, U., Nikles, S., Marković, Z., & Bauer, R. (2023). UHPLC-MS Phytochemical Profiling and Insight into Bioactivity of Rabelera holostea (Greater Stitchwort) Extract. Molecules, 28(3), 1274. https://doi.org/10.3390/molecules28031274










