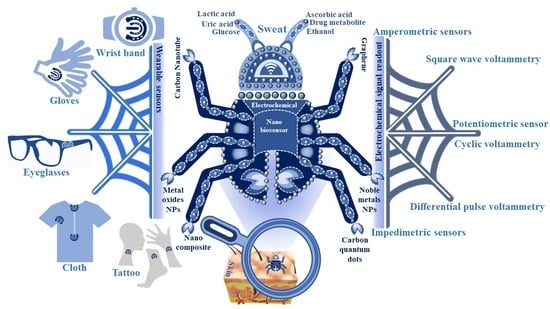
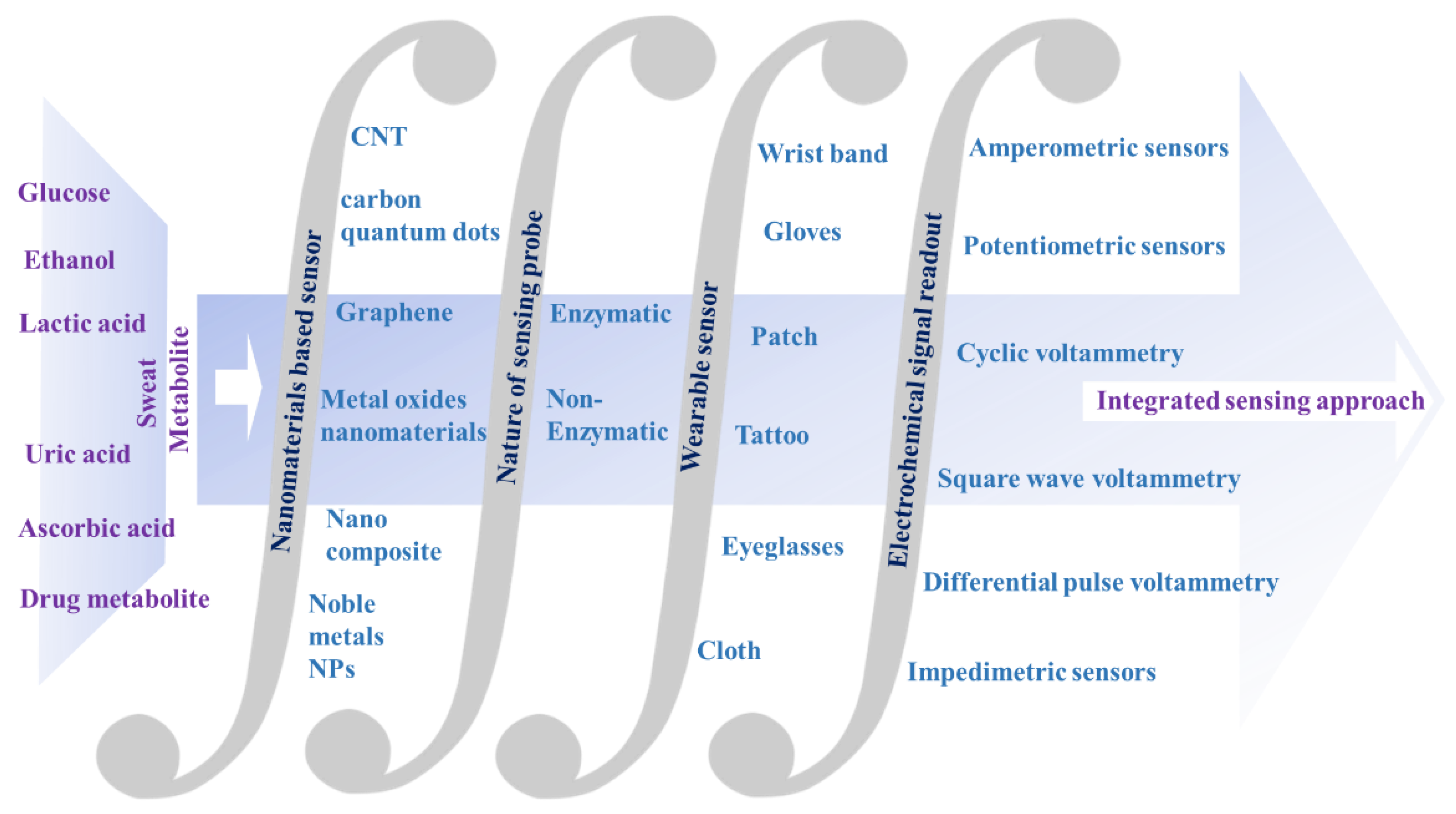
Electrochemical Nanosensors for Sensitization of Sweat Metabolites: From Concept Mapping to Personalized Health Monitoring
Abstract
1. Introduction
2. Analysis of Sweat Metabolites and an Initiative for Early Stage Disease Diagnosis
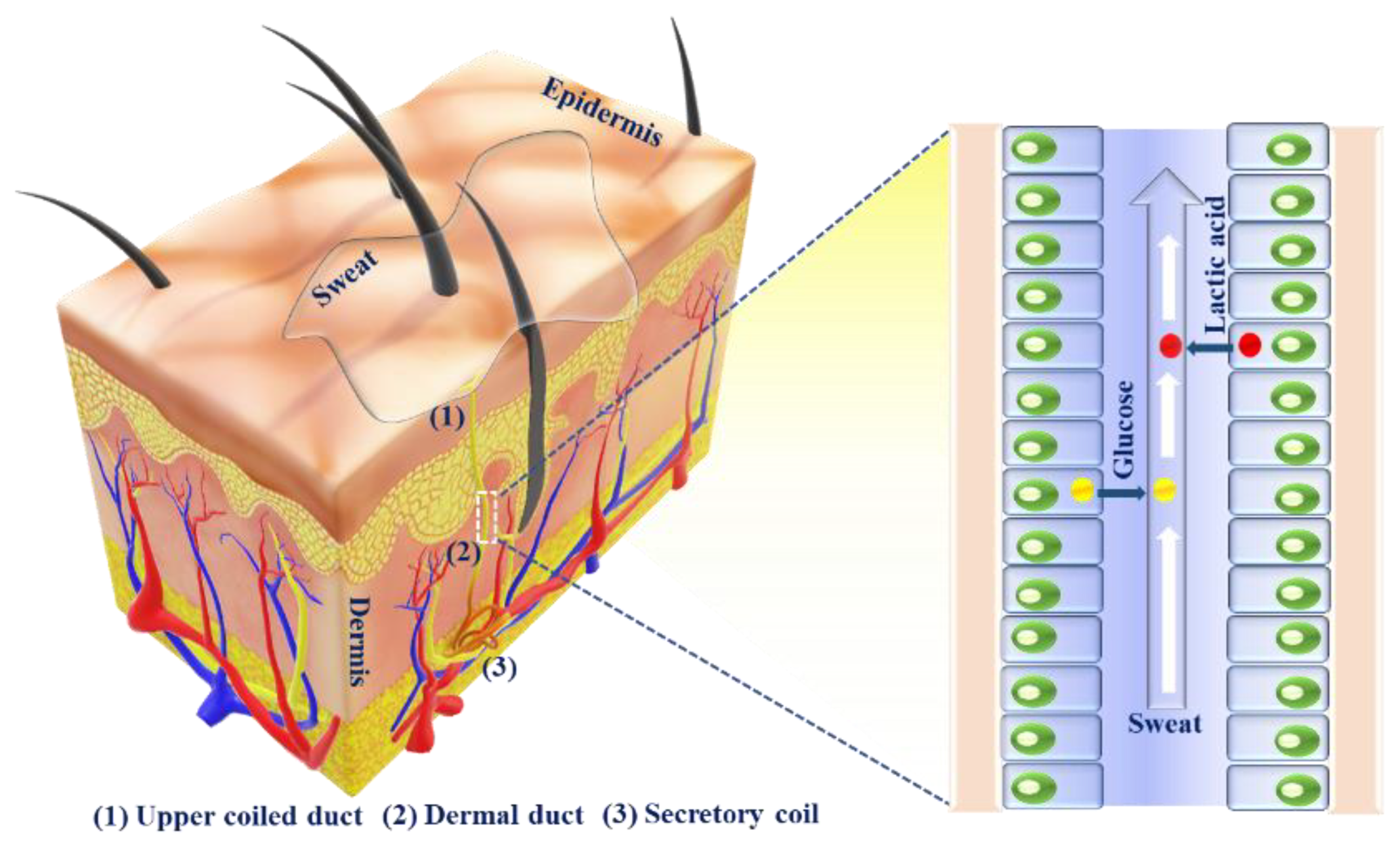
2.1. Sweat Production
2.2. Sweat Metabolites
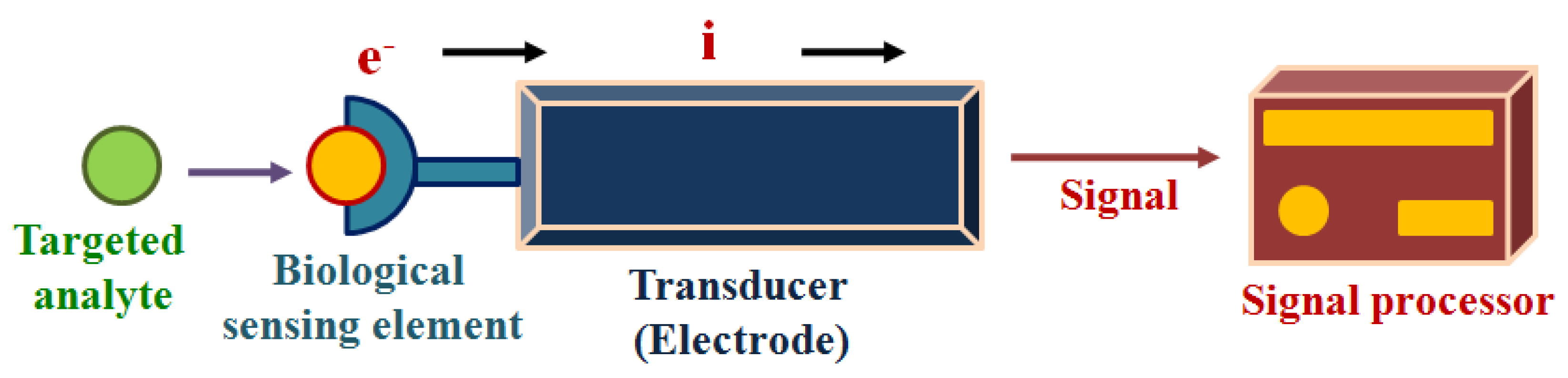
3. Detection of Sweat Metabolites: Initiative in the Context of Electrochemical Sensing Platform
- The availability of the accessible active sites on the electrocatalytic sensor surface for adsorption and interaction of the active sites with the targeted analytes.
- The capability of accessible active sites on the electrocatalytic sensor surface for engaging in multi-step electrocatalytic reaction.
- Rapid charge transfer.
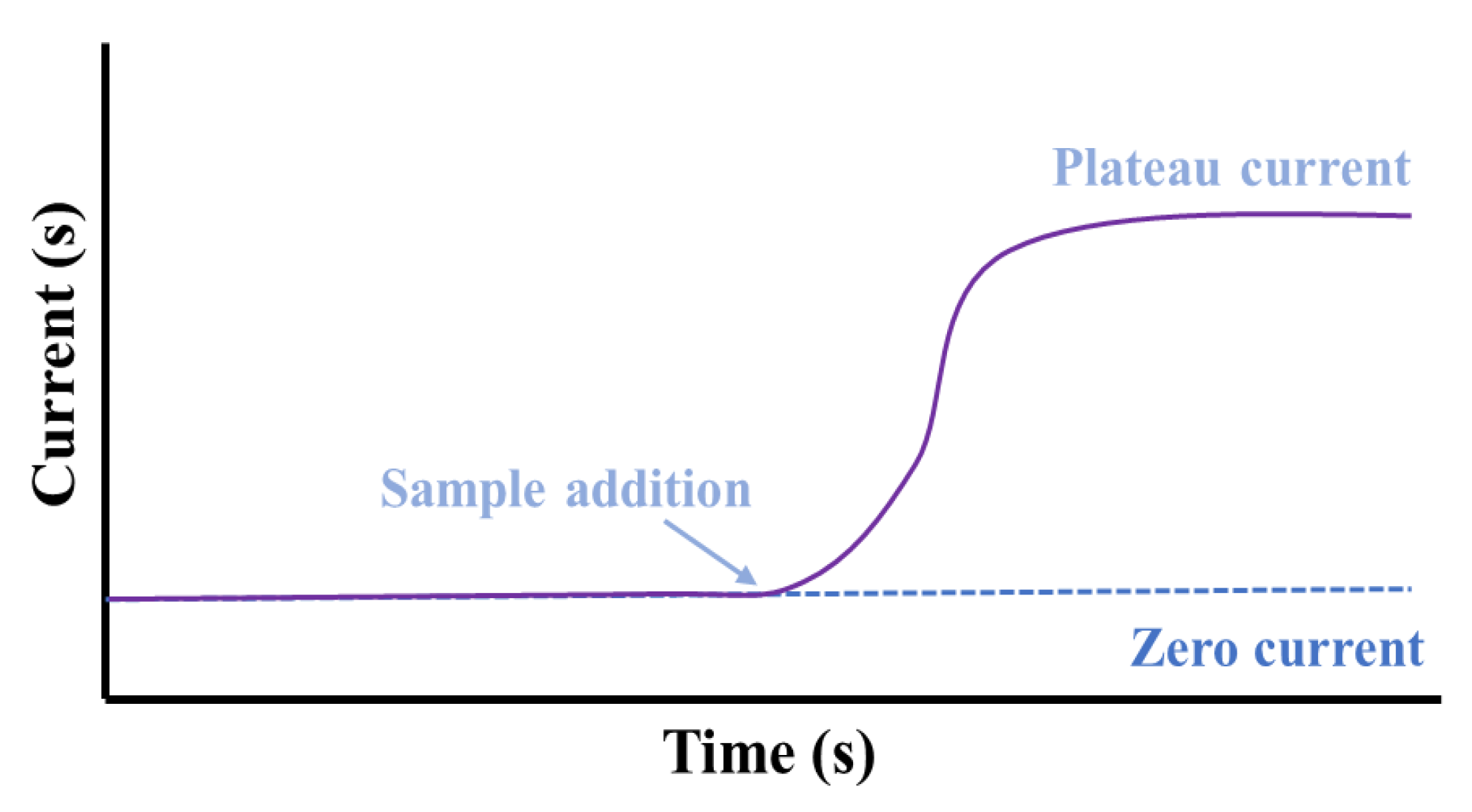
3.1. Amperometric Sensors
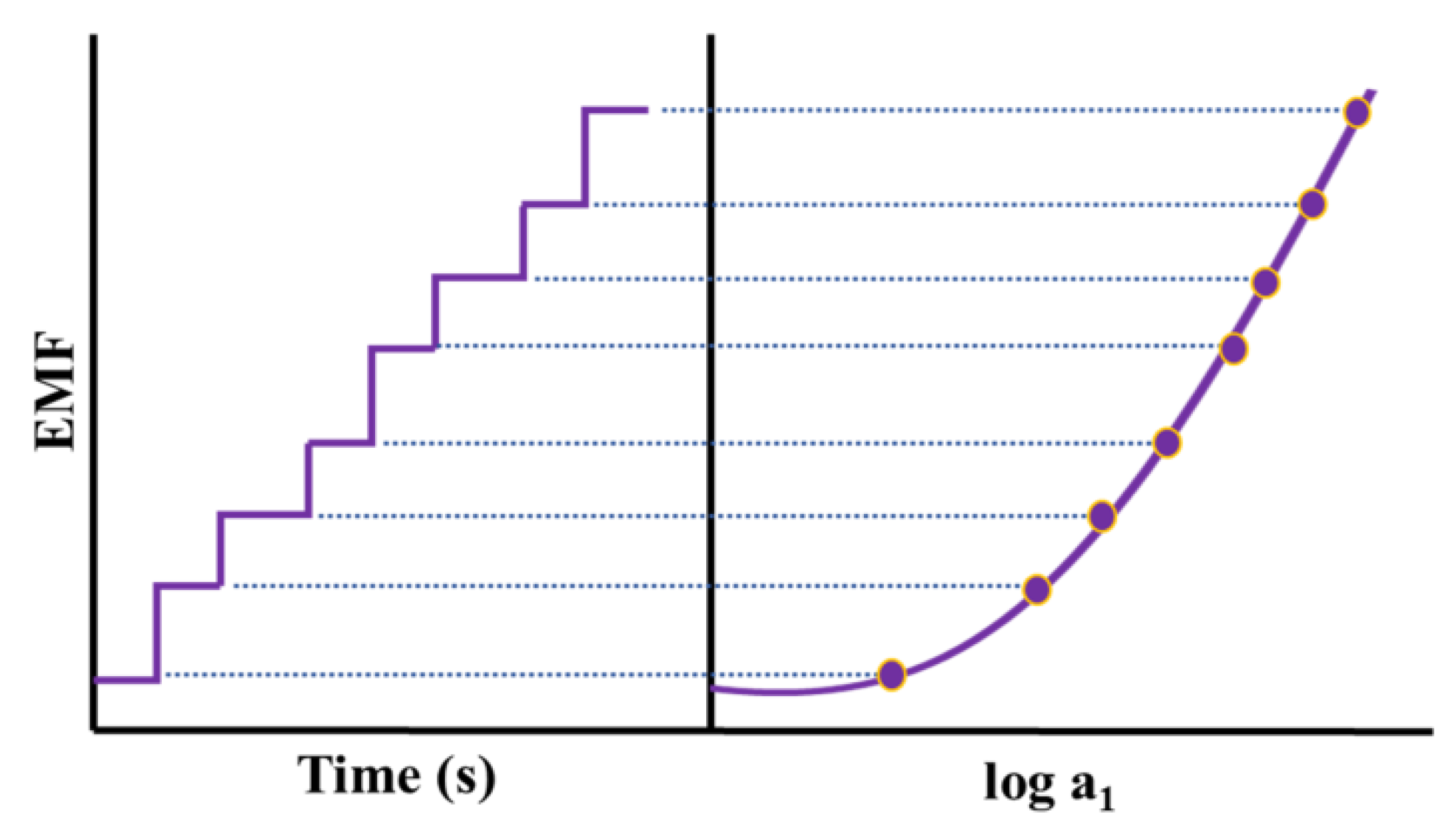
3.2. Potentiometric Sensors
3.3. Voltammetric Sensors
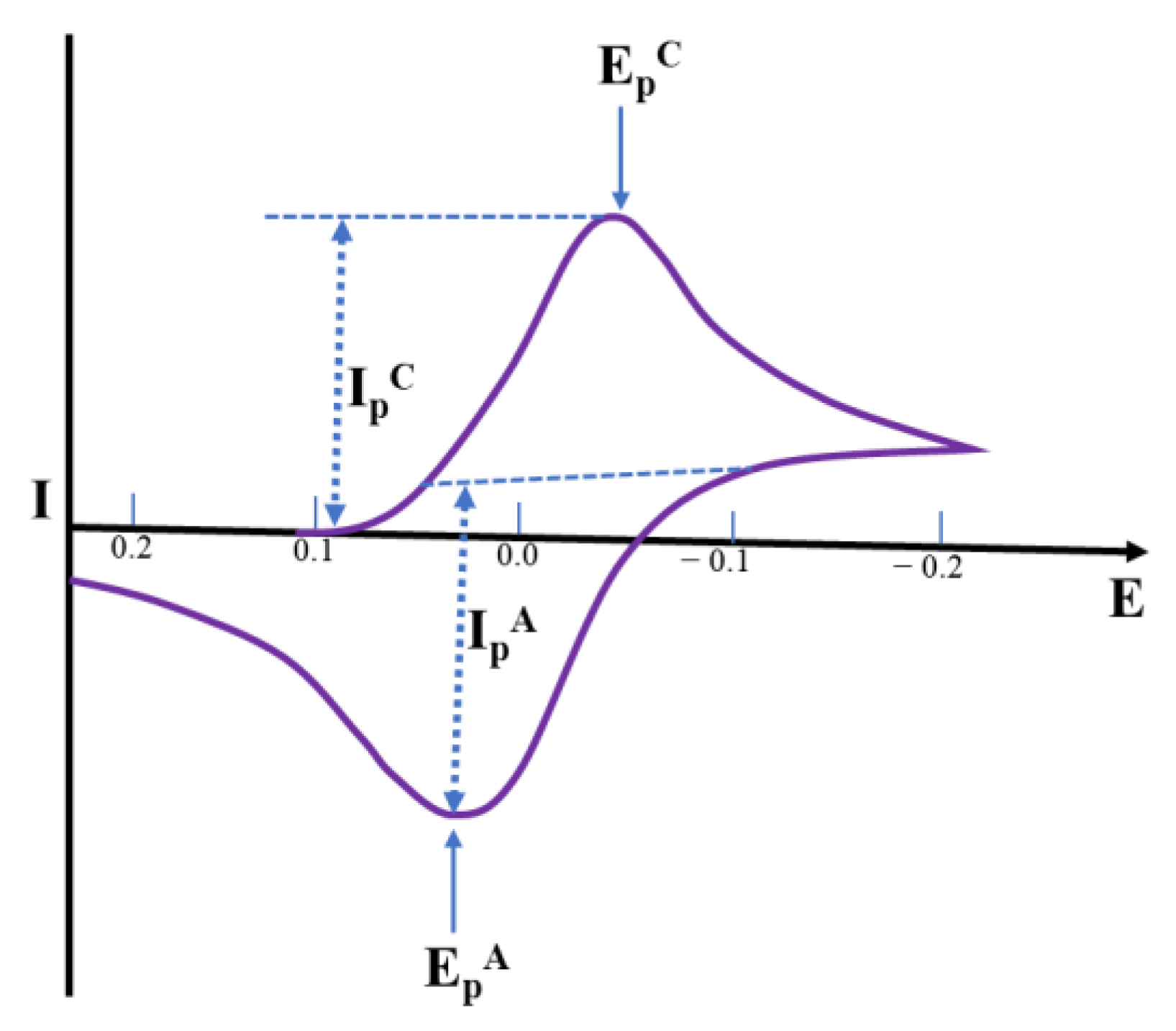
3.3.1. Cyclic Voltammetric (CV) Sensors
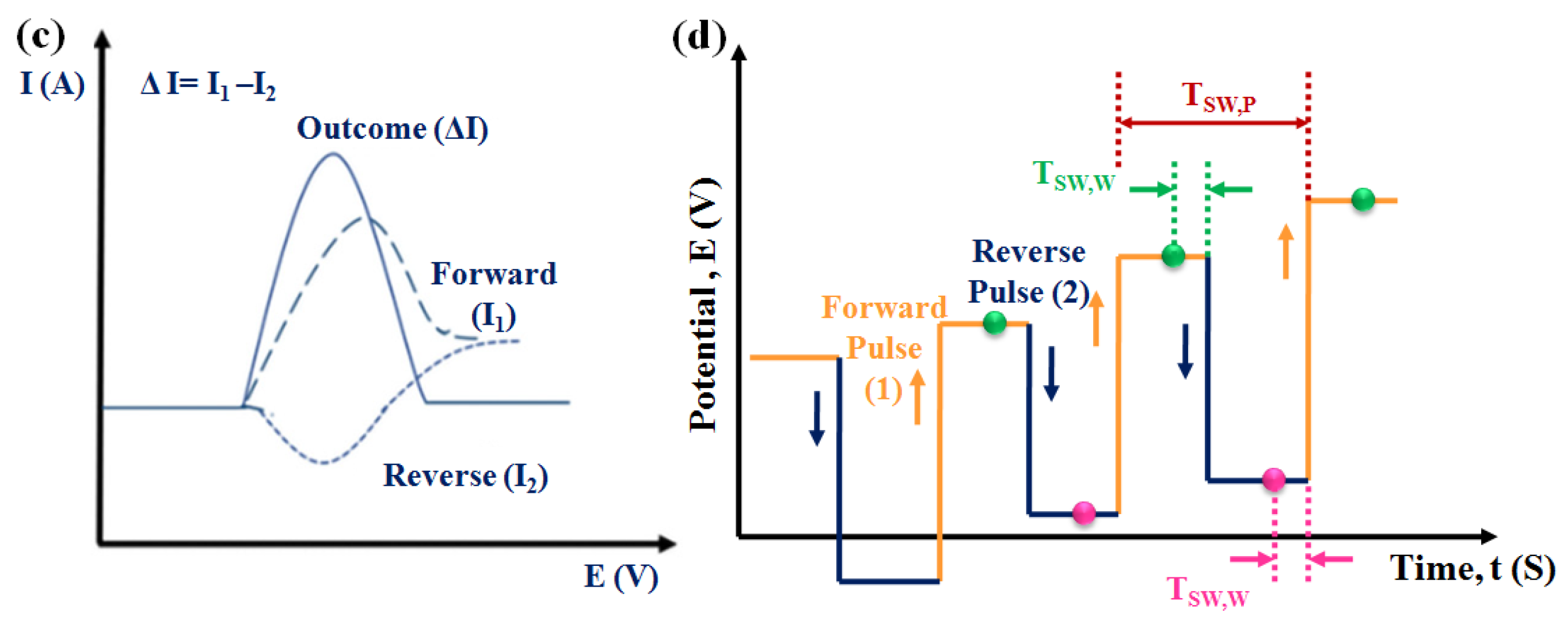
3.3.2. Differential Pulse Voltammetric (DPV) Sensors
3.3.3. Square Wave Voltammetric (SWV) Sensors
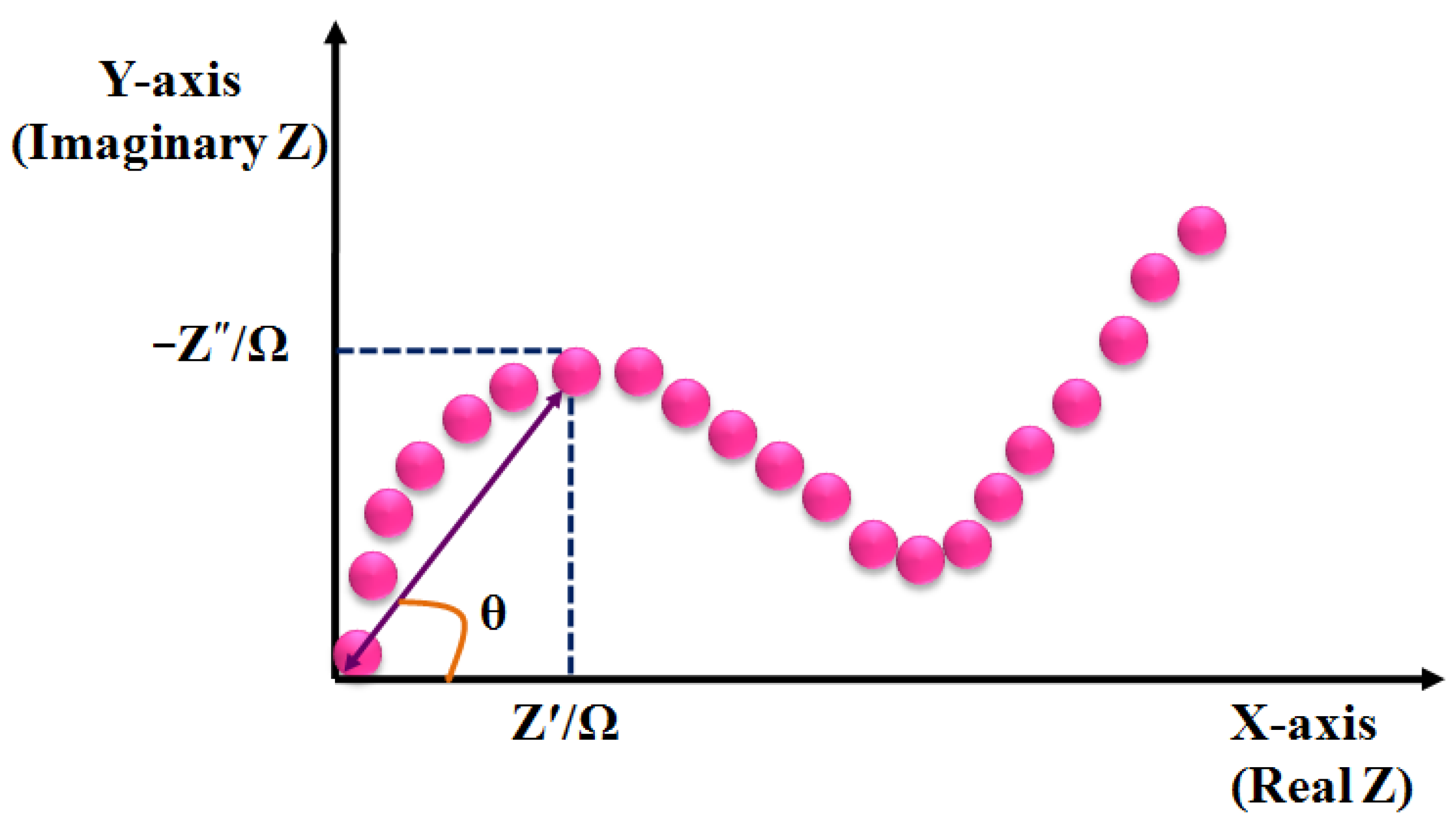
3.4. Impedimetric Sensors
4. Nanomaterials-based Electrochemical Sensing of Sweat Metabolites
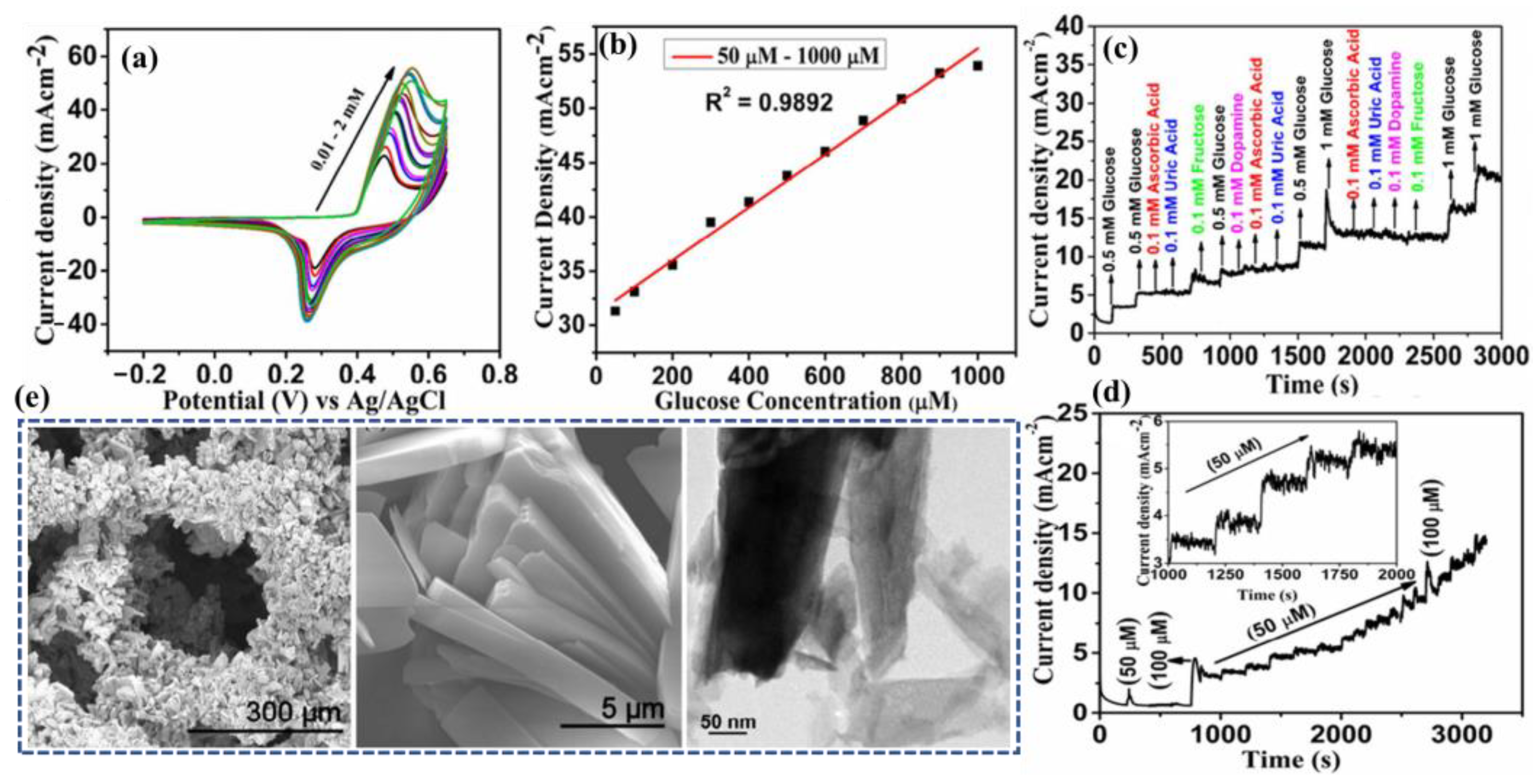
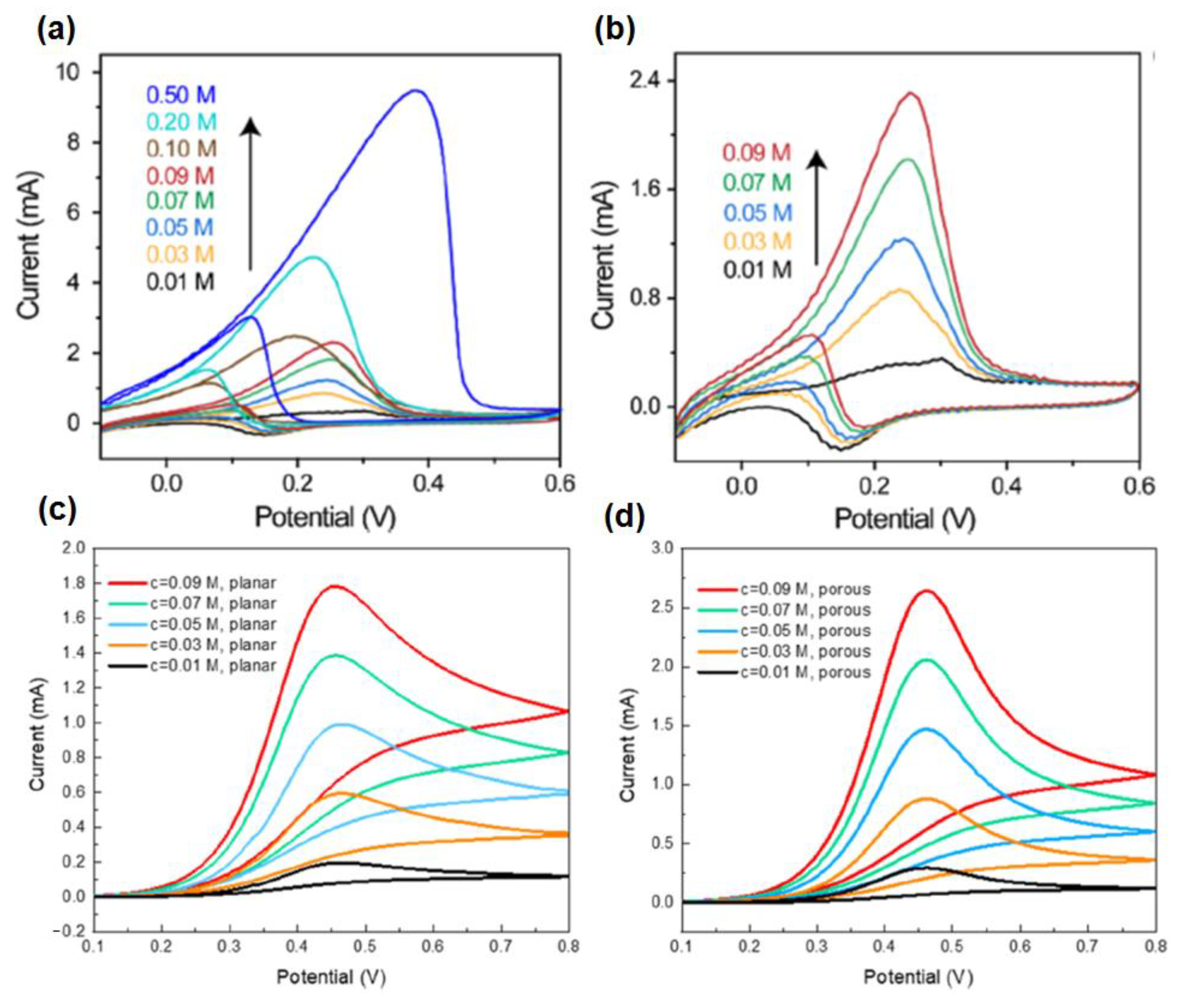
4.1. Glucose @ a Major Biomarker for Diabetes Mellitus

4.2. Urea/Uric Acid @ a Major Biomarker for Renal Dysfunction
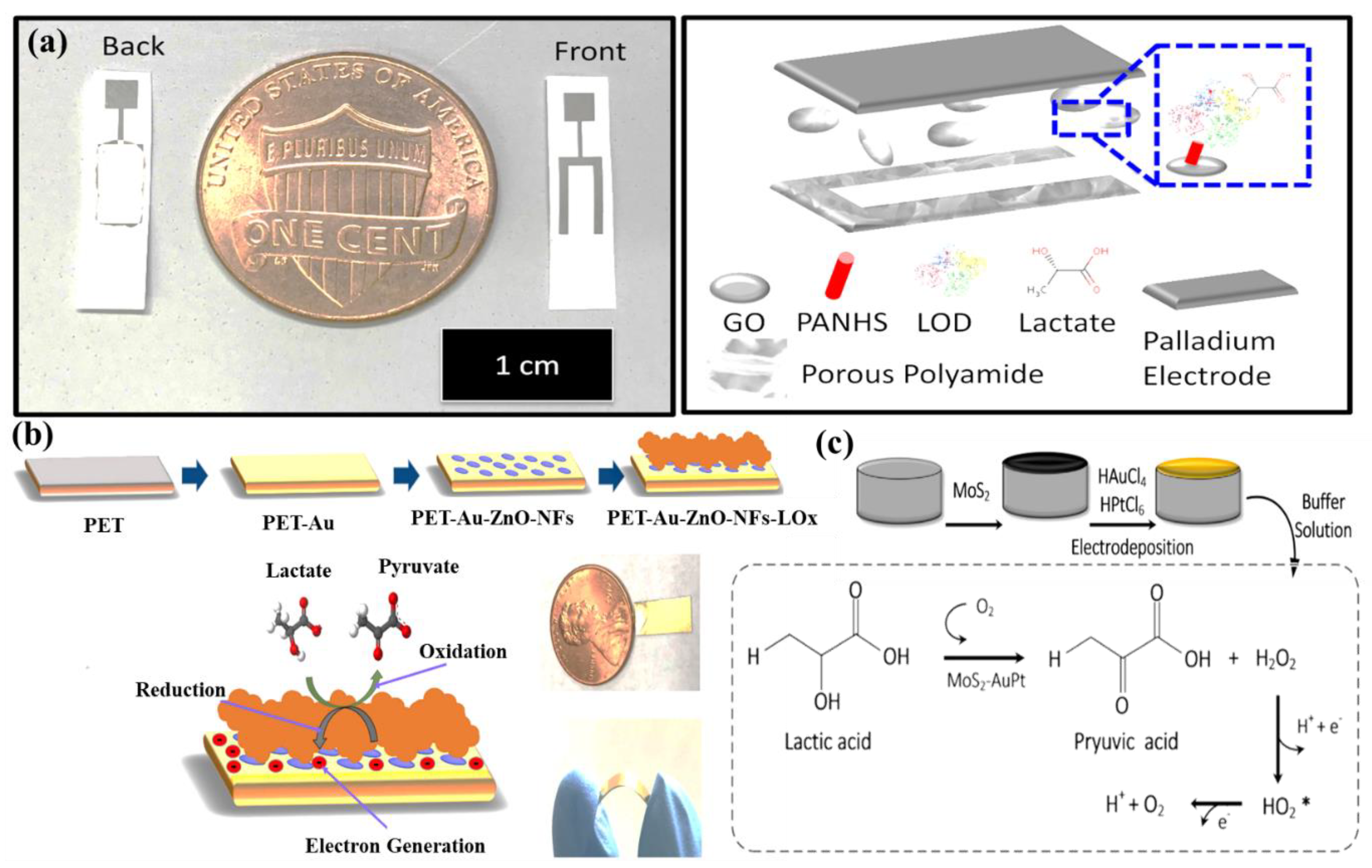
4.3. Lactic Acid/Lactate @ a Major Biomarker for Stress Ischemia
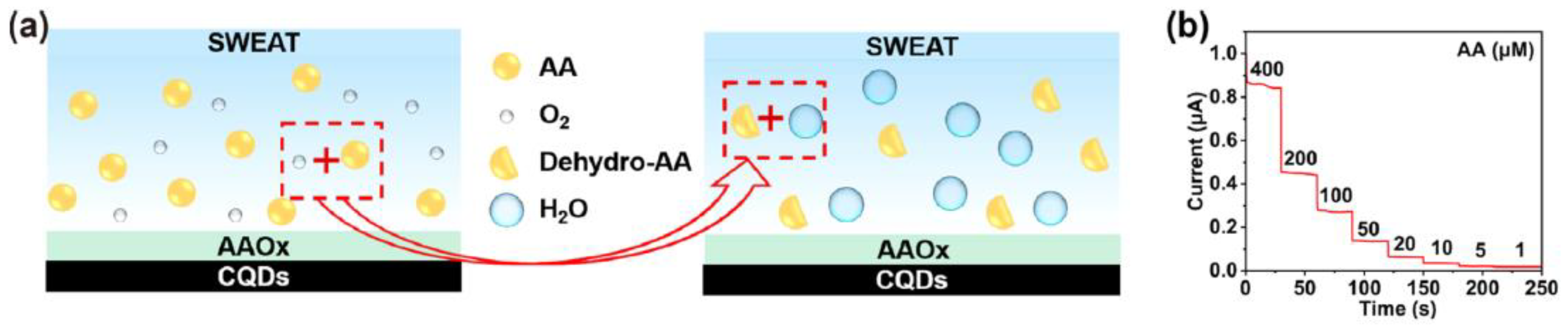
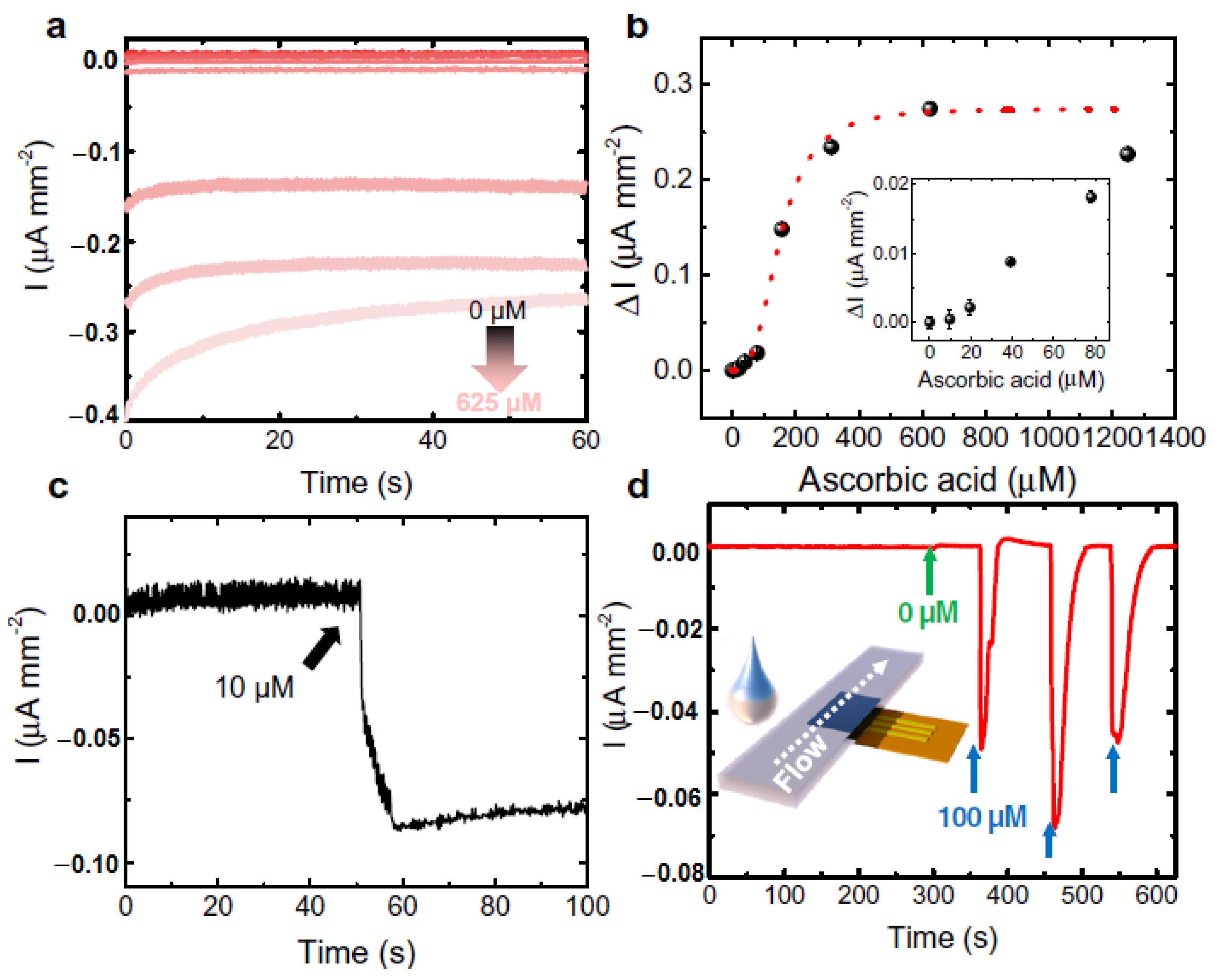
4.4. Ascorbic Acid (AA) @ a Major Biomarker for Kidney Disease
4.5. Ethanol @ aMajor Biomarker for Drunk Driving
| Targeted Sweat Metabolites | Nanosensors | Detection Principle | Sensitivity | Detection Limit | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| Glucose | Hydrogel-based microneedle system | Alteration in the heights and swelling ratios of microneedles and potentiostat | - | - | 8–22 mM | [60] |
| Enokitake mushroom-like standing gold nanowires | Chronoamperometry | 23.72 µA·mM−1·cm−2 | 10 µM | 0–800 µM | [61] | |
| Gold nanostructure | Chronoamperometry CV | - | 7 µM | 25 to 250 µM | [62] | |
| GOx/PtNP/acetic acid treated LIG | Chronoamperometry CV | 4.622 µA/mM | 300 nM | 0.0003–2.1 mM | [63] | |
| Nickel phosphate nano flakes | Chronoamperometry CV | 24.39 mA·mM−1·cm−2 | 97 nM | 10–1000 µM | [64] | |
| TiO2 nanotube arrays@Ti mesh | CV | - | 0.0361 mM | 0.02–0.7 mM | [73] | |
| Urea/Uric acid | alpha nickel hydroxide nanoparticles | Chronoamperometry CV | 2.5 ± 0.08 µA | 2.28 × 10−8 M | - | [67] |
| TiO2 nanotube arrays@Ti mesh | CV | - | 2.0675 mM | 1–70 mM | [73] | |
| Lactic acid | AuPtNPs functionalized MoS2 nanosheet | CV EIS SWV | - | 0.00033 mM | 0.005–3 mM | [70] |
| Zinc oxide nanoflakes | CV EIS | 11.76 µA/decade/cm2 | 1.26 nM | 10 pM–20 mM | [71] | |
| Graphene oxide (GO) nanosheets | EIS | - | 1 mM | 4–80 mM | [72] | |
| TiO2 nanotube arrays@Ti mesh | CV | - | 0.0131 mM | 0.8–7 mM | [73] | |
| Gold nanopine needles | Chronoamperometry CV | - | 54 µM | - | [62] | |
| Ascorbic acid | Carbon quantum dot electrode based enzymatic sensor | Amperometry | 2.02 nA µM−1 | 12 nM | 1–400 µM | [77] |
| Modified gold microelectrodes with trapped CuO nanoparticles | Amperometry CV | 0.103 V log (µM)−1 of peak shift | 1.97 µM | 10–150 µM | [81] | |
| Nanorod of conductive Ni-MOF | CV | 0.814 µA µM−1 cm−2 | 1 µM | 2–200 µM | [82] | |
| NdNiO3 nanotubes supported on GO flexible electrodes | CV chronomperometry | 0.031 µA µM−1 cm−2 | 3.8 µM | 30–1100 µM | [83] | |
| Laser induced carbonization of graphene oxide filled biomass-derived polymer poly(furfuryl alcohol) (PFA/GO) based non-enzymatic sensor | CV DPV chronomperometry | 0.03748 µA µM−1 cm−2 | 1.0 µM | 50–5000 µM | [84] | |
| Janus carbon nanocomposite | CV EIS DPV | - | 47 pM | 10−12 M–10−6 M. | [85] | |
| Ethanol | Zinc oxide thin film electrodes, surface functionalized with alcohol oxidase enzyme | EIS | 0.2 ± 0.02 µA/mM | 0.01 mg/dL | 0.01–200 mg/dL | [93] |
| Gold nanowire aerogel (Au NW-gel) | CV EIS | - | - | 0.01–0.5 M | [94] | |
| Ethanol metabolite, ethyl glucuronide (EtG) | Gold electrode, surface functionalized with monoclonal antibodies and thiolated charge transfer molecule | SWV | - | 0.1 µg/L | 0.1–100 µg/L | [95] |
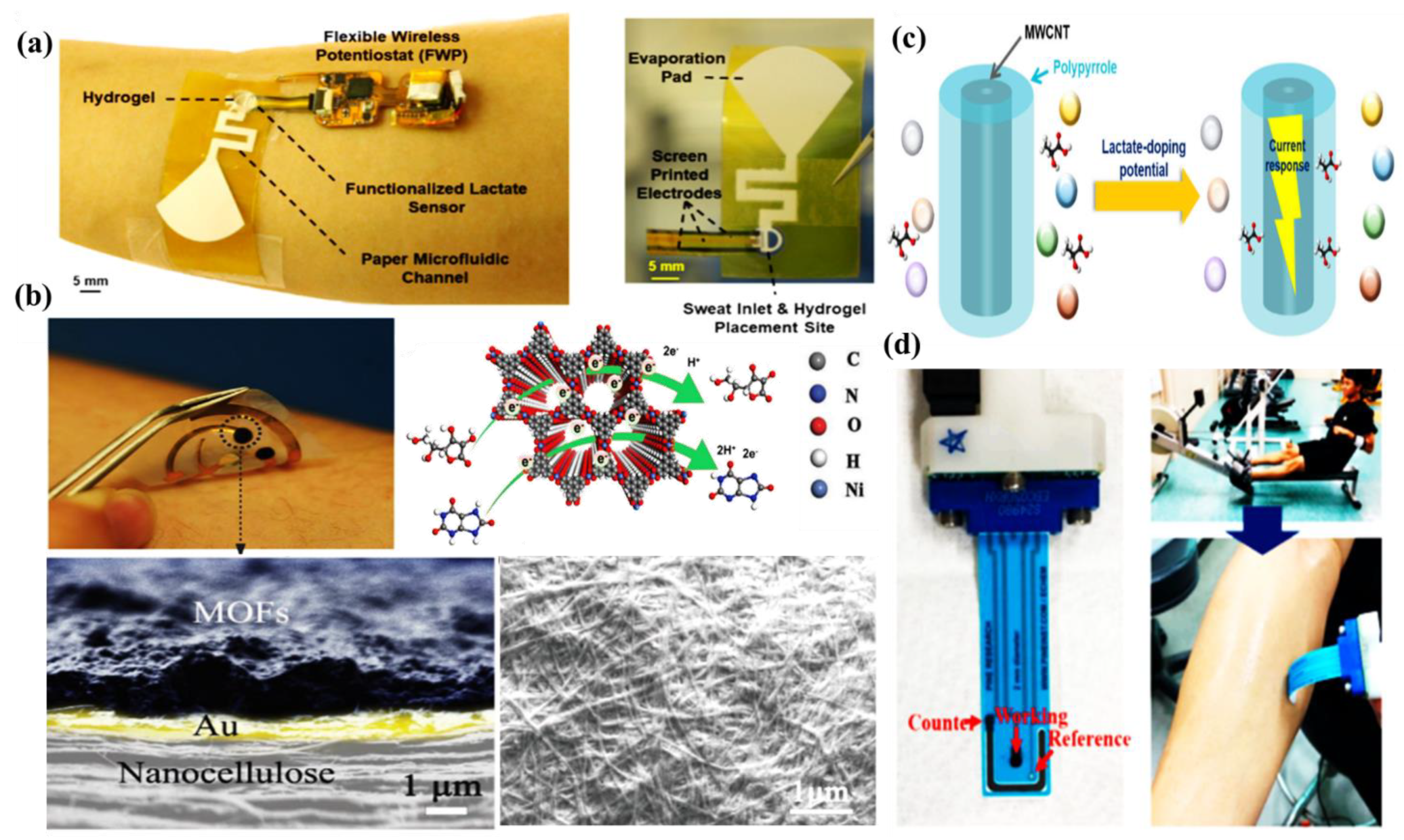
5. Nanomaterials Integrated Wearable Electrochemical Sensors@ Need of the Hour for Personalized Health Monitoring
5.1. Wearable Glasses Sensor
5.2. Wearable Gloves Sensor
5.3. Wearable Patch Sensor
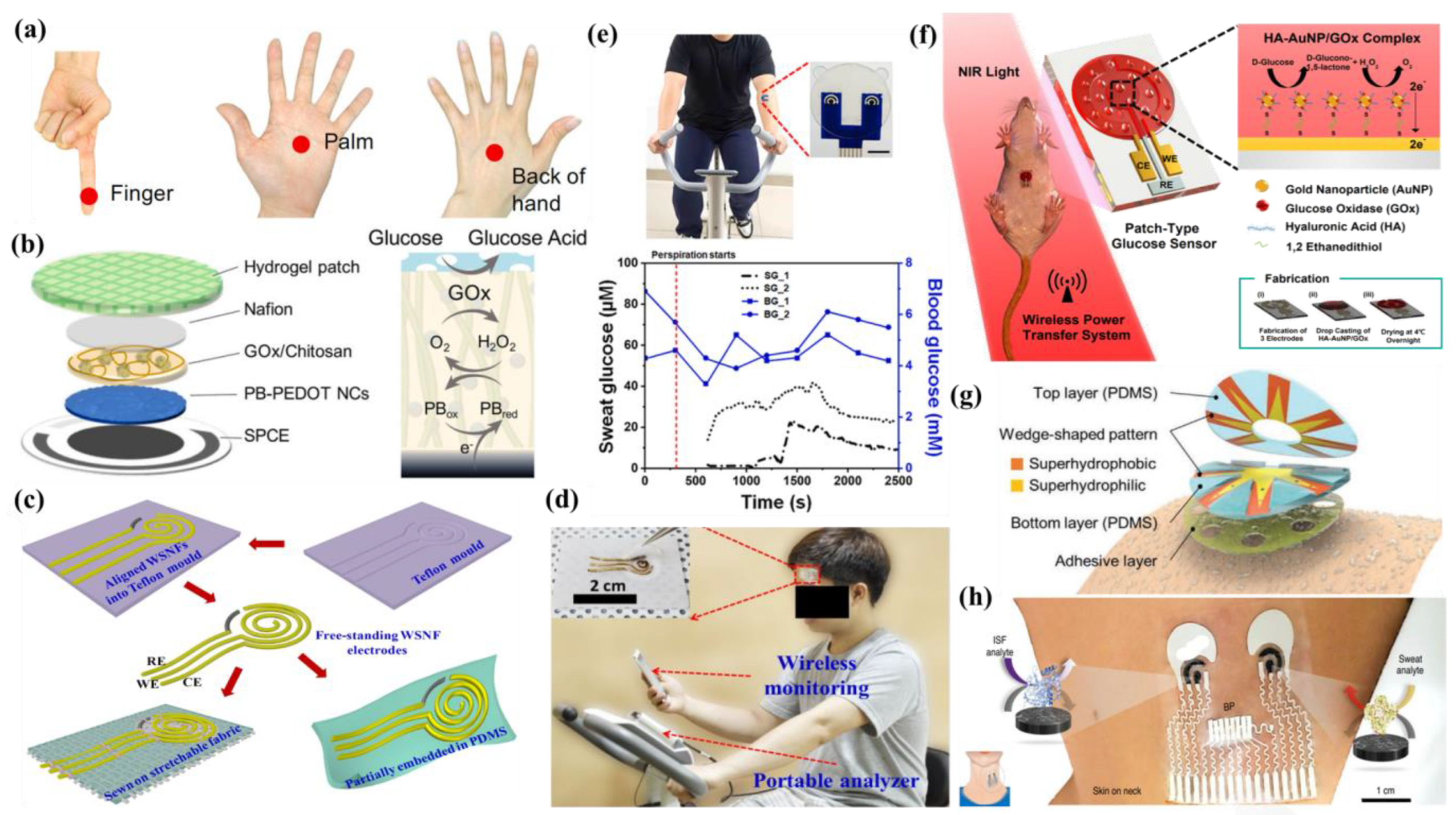
5.4. Wearable Tattoo Sensor
5.5. Wearable Band Sensor

5.6. Others
| Operational Platform | Nano Biosensor | Targeted Sweat Metabolites | Sensitivity | Detection Limit | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| Eye Glasses | Bio-enzyme Gel-Membrane | Lactate | 0.74 µA mM−1 | 0.04 mM | 0–25 mM | [101] |
| Gloves | Carbonaceous nanomaterials | Uric acid and Drug metabolites (Paracetamol, paroxetine and ethinylestradiol) | - | Uric acid: 1.37 µM Paracetamol:0.247 µM Paroxetine: 0.493 µM Ethinylestradiol: 0.935 µM | Uric acid: 1.0–41.0 × 10−6 M Drug metabolites: 1.0–10.0 × 10−6 M | [104] |
| CNT | Lactate | 0.358 µA mM−1 | 2.5 µM | 47.6 µM–1.52 mM | [105] | |
| Enzymatic gold electrode | Ethanol AA | - | - | AA: 0–300 µM | [106] | |
| Patch | Prussian blue-doped poly (3,4-ethylenedioxythiophene nanocomposite (PB-PEDOT NC) electrode | Glucose | - | 4 µM | 6.25 µM to 0.8 mM | [108] |
| Microfluidic sweat patch coupled with AuNPs modified biosensor | Glucose, lactate | Glucose: 24.8 µA mM−1 cm−2 Lactate: 1.0 µA mM−1 cm−2 | Glucose: 7.34 µM Lactate: 1.24 mM | - | [110] | |
| Nanohybrid fiber (WSNF) in which Au nanowrinkles partially cover the rGO/PU composite | Glucose | 140 µA.mM−1 cm−2 | 500 nM | - | [109] | |
| MIPs-AgNWs biosensor | Lactate | - | 0.22 µM | - | [111] | |
| glucose oxidase coated microneedle-type glucose sensor | Glucose | - | millimolar scale | - | [112] | |
| Au/PDMS film coated with Ni–Co MOF nanosheet | Glucose | 205.1 µA mM−1 cm−2 | 4.25 µM | 20–790 µM | [113] | |
| (Ni-Co MOF/Ag/rGO/ PU) fiber-based wearable electrochemical sensor | Glucose | 425.9 µA. mM−1 cm−2 | 3.28 µM | 10 µM−0.66 mM | [114] | |
| Anti-EtG monoclonal antibodies-based assay | Ethyl glucuronide (EtG), an ethanol metabolite | - | - | 10–10,000 ng/L | [115] | |
| Poly(3,4-ethylenedioxythiophene): poly(4-styrenesulfonate) (PEDOT:PSS) and Pt nanoparticles-based enzymatic sensor | Glucose | 325.99 ± 0.8 µA mM−1 cm−2 | 10.3 µM | 0–2.5 mM | [116] | |
| HA-AuNP/GOx complex | Glucose | 12.37 µA⋅dL/mg⋅cm2 | 0.5 mg/dL | - | [117] | |
| PB-LOx electrode | Lactate | −641 ± 21 nA mM−1 | 32.6 µM | - | [118] | |
| Thin hydrogel micropatch with PtNPs deposited MWCNT-modified Au electrode | Lactate | 1.88 ± 0.24 µA | 0.12 ± 0.02 mM | 0−4 mM | [119] | |
| GOx/Lox-based Cactus-spine-inspired patch | Glucose, Lactate | - | 250 µM | - | [120] | |
| Three-dimensional paper-based microfluidic electrochemical integrated device (3D-PMED) | Glucose | 35.7 µA mM−1 cm−2 | 5 mM | - | [45] | |
| Cellulose/-cyclodextrin nanofiber patch | Glucose | 5.08 µA mM−1 | 93.5 µM | 0–1 mM | [121] | |
| Reduced graphene oxide (rGO)-based nanostructured composite | Glucose | 48 µA mM−1 cm−2 | 5 µM | 0–2.4 mM | [122] | |
| Enzyme immobilized Styrene-ethylene-butylene-styrene block copolymer (SEBS)-based stretchable material | Glucose, Alcohol, Lactate, Caffeine | - | - | - | [123] | |
| Nanoporous gold combined with fully stretchable capillary microfluidics | Glucose | 253.4 µA cm−2 mM−1 | - | 0.01–1 mM | [128] | |
| Tattoo | Ascorbate oxidase (AAOx) on flexible printable tattoo electrodes | Vitamin C | - | - | 0–1000 µM | [80] |
| Band | NiCo2O4-based micro-supercapacitors | Glucose, Lactate | Glucose: 0.5 µA/µM Lactate: 0.0075 µA/µM | Glucose: 10 µM | Glucose: 10–200 µM Lactate: 5–25 mM | [135] |
| Au nano-dendrites-based enzymatic sensor | Drug metabolite (levodopa) | PBS: 15 nA/µM Sweat: 1.7 nA/µM | Sweat: 1.25 µM | - | [133] | |
| Au nano-dendrites-based enzymatic sensor | Nicotine | PBS: 4.3 nA/µM Sweat: 4.4 nA/µM | Sweat: 1.6 µM | 0–20 µM | [134] | |
| Au/thiolate self-assembled monolayers-based enzymatic sensor | Glucose | 126 ± 14 nA/mM | 301 ± 2 nM | 0.1–0.6 mM | [139] | |
| Conductive threads and zinc-oxide nano wires(ZnO NWs) based enzymatic sensor | Lactate | - | 3.61 mM | 0–25 mM | [140] | |
| Wearable cloth-based enzymatic sensor integrated with MWCNT and Prussian blue | Glucose, Lactate | Glucose: 105.93 µA mM−1cm−2 Lactate: 2.28 µA mM−1 cm−2 | Glucose: 4.95 µM Lactate: 0.25 mM | Glucose: 0.05–1 mM | [141] | |
| Self-pumping Janus textile bands based on electrospinning hydrophobic polyurethane (PU) nanofiber array | Glucose, Lactate | Glucose: 8 nA/µM Lactate: 67 nA/µM | - | Glucose: 0–200 µM Lactate: 0–25 mM | [142] | |
| Others | ZIF-8/GO composite integrated with tyrosinase enzyme | Levodopa (L-dopa) | - | 0.45 µM | 1–95 µM | [93] |
| Glucose oxidase/chitosan/AuNPs-based enzymatic sensor | Glucose | 1.27 µA cm−2 mM−1 | 24 µM | 0.1–1 mM | [143] | |
| SWCNT-based enzymatic sensor | Glucose, Lactate | Glucose: 345.5 nA mM−1 cm−2 Lactate: 3169 nA mM−1 cm−2 | - | Glucose: 0–200 µM Lactate: 5–25 mM | [144] | |
| Chitosan/single walled carbon nanotubes-based enzymatic sensor | Glucose, Lactate | Glucose: 2.054 nA/µM Lactate: 25 nA/mM | - | Glucose: 0–300 µM Lactate: 5–25 mM | [147] | |
| Carbon nanotubes (CNT) -ethylene-vinyl acetate copolymer (EVA) film coupled with GOx/HRP integrated enzymatic sensor | Glucose | 270 ± 10 µA mM−1 cm−2 | Upto 1.0 mM | 3 µM | [148] | |
| carbon nanotubes (CNTs) and gold nanotubes (Au NTs)-based molecularly imprinted sensor | Urea | - | 1–100 mM | 0.1 mM | [149] | |
| NiCu(OOH)/polystyrene (PS) electrode with carbon nanotube | Urea | 10.72 µAmM−1 cm−2 | 2.00–30.00 mM | 4.67 µM | [150] | |
| Nanotextured electrode-based enzymatic sensor | AA | 2.0 nA µM−1 | 1000–5000 µM | ≈4 µM | [151] | |
| CNT-Au nanosheets-CoWO4 based stretchable sensor | Glucose | 10.89 µA mM−1 cm−2 | 1.3 µM | 0–0.3 mM | [152] | |
| PANI and single-walled CNT-based enzymatic sensor | Glucose | - | 0.1 mM | 0.1–50 mM | [49] | |
| Electrospun carbon nanofibers (CNFs)-based wearable sensor | Uric acid | - | - | 10–400 µM | [153] | |
| G. xylinum derived microbial nanocellulose-based wearable skin-like sensor | Uric acid | 0.18 A.M−1 | 1.8 µM | 0–70 µM | [154] |
6. Conclusions
7. Outlook and Future Prospects
- (i)
- Sensitivity and specificity: Due to the presence of sweat metabolites at the trace level, ultrasensitive sensors with preconcentration techniques are obligatory. There is a plethora of literature on non-enzymatic sweat sensing. However, in most cases, they suffer from selectivity issues. Interfering agents and non-specific binding may cause signal alteration, producing a false-negative or -positive response. This indicates that sensor specificity is one of the most crucial parameters, which requires further improvement by improvising judiciously designed nanomaterials with enhanced surface areas and improved electrocatalytic activity.
- (ii)
- Simultaneous analysis of multiple sweat analytes: The currently developed sensors are mostly aligned towards monitoring limited numbers of sweat metabolites. Therefore, wearable sensors may be fabricated in such a way that they should be capable enough for simultaneous monitoring of multiple analytes in sweat, as this strategy offers cost-effective, and less time and sample-consuming responses. In this regard, a microfluidics strategy may be integrated for fast in situ detection of the targeted biomarkers present in sweat specimens. This will offer a comprehensive understanding of the wearer’s health status.
- (iii)
- Circumvention of sensor calibration: Most of the potentiometry-based wearable chemical sweat sensors necessitate calibration procedures, conditioning solutions storage, and other cumbersome procedures. Moreover, the on-field application of these devices entails a stable response over a wide range of temperatures, sunlight, sweat compositions, and other issues that might alter the sensing response. These inconsistencies are important due to the extremely low concentrations of the targeted metabolites present in sweat. Herein, interfering species could further lessen the sensing performance of the fabricated sensor. In this context, for large-scale production, wearable devices should be self-calibrated, as the sensitivity is largely affected by various environmental factors, such as temperature, humidity, sweat collection spots, etc. Therefore, calibration-free or factory-calibrated sensors are gaining much more commercial acceptance.
- (iv)
- Circumvention of multistep sensing: Traditional mechanized biosensors adopt multistep sensing mechanisms for the detection of the analytes at ultralow levels. Moreover, sample pre-treatments are also necessary to improve the selectivity of the sensor. However, these kinds of techniques are not compatible with wearable devices, as in these platforms, sweat specimens must be collected directly from the skin to produce an in situ signal. Hence, newly developed wearable electrochemical sensors for highly sensitive and selective sweat metabolite sensing, which are stable over various environmental conditions, must avoid multistep sensing.
- (v)
- Direct contact with sweat: Difficulties can also arise from the processes of fabrication, and materials, wherein various components must be encapsulated. For instance, chemical sensors should remain in direct contact with sweat, although supportive electronics must be entirely wrapped and should be separated from any biofluid or moisture contact. The contamination of sweat from the exterior of the skin or from the skin adjacent can hardly be eliminated after sweat secretion and in skin contact, which has a huge impact on data correctness. Furthermore, plain washing of the skin surface cannot alleviate bacterial contamination on the skin and may create substantial faults. One possible solution may be the isolation of sweat from the skin surface by coating it with a barricading layer of an oil-based substance on the skin surface.
- (vi)
- Sampling with improved rates of sweat: At present, hard work or iontophoresis is the major approach to stimulate the generation of sweat. However, sweat secretion rates will not exceed 20 nL/min/gL, even after exhaustive exercise. Locally stimulated sweating via an iontophoresis method is more suitable for infants, aged persons, etc., for obtaining enough sweat specimens in inactive conditions in comparison to the condition of exercise. However, controlling the current density of the device should be properly carried out, as repetitive application of an iontophoretic current at the same spot may be harmful to skin. Therefore, the development of ultrasensitive sensors with better sensitivity with a low volume of sweat will be highly promising.
- (vii)
- Scale-up manufacturing of the sensors: A technological emphasis is also required for scale-up manufacturing of the sensors. Commercial-scale manufacturing not only requires a highly feasible fabrication strategy, but should also be relatively cost-effective.
- (viii)
- Reproducibility and durability: In most wearable devices, adhesives are used, but these last only for a few days due to several factors, such as skin oils, irritation, baths, etc. Additionally, in most cases, there occurs consumption or degradation of the chemical probes used in sensors over time. Hence, long-term reusability and durability of the sensors in the complex biological matrices are of immediate need that are reliant on the fabrication of advanced materials and subsequent manufacturing.
- (ix)
- Difficulty in detection due to the complexity of the human body: Keeping in mind the complications of the human body, skin-interfaced flexible systems may identify different biomolecular levels and vital signs, representing a fruitful solution for precisely forecasting and recognizing more explicit health circumstances. Research in these commands will have a noteworthy impact on personalized healthcare.
- (x)
- Better accuracy in sensing response: The judicious designing of more new electrode materials is of tremendous importance to obtain more accurate signal responses in the presence of harsh environmental factors (e.g., high temperature, high humidity, variation in pH, etc.) and to avoid motion falsification during movement.
- (xi)
- Biocompatibility: One crucial parameter of wearable sweat sensing is the development of biocompatible sensors. As the wearable sensors remain tightly in contact with the skin for a long period of time, the sensor material should be biocompatible in nature along with its intrinsic sensing characteristics to avoid skin allergies or other skin-related issues after long-term uses of the wearable sensors. In most of the cases, researchers are focused toward the development of sensors with improved flexibility, stretchability or conductivity, sacrificing the biocompatibility of sensor materials. Hence, considerable research attention should be paid to the development of biocompatible sensor material.
- (xii)
- Mechanical and operational stability for wearable sensors: Mechanical and operational stability of the developed sensors is of paramount importance to obtain a reliable response signal for their applicability during longer operational time periods. Therefore, special attention should be levied on the issues of surface biofouling, enzymatic stability, etc., for longer operational stability.
- (xiii)
- Enhancement of user comfort: Further research attention may be levied to increase the user comfort of wearable devices. To solve the issue, development of soft flexible electronics may be taken into consideration to suit the skin contours and to enhance the wearers’ comfort with the device mainly with the interface of the skin and wearable electronics.
- (xiv)
- Requirement of continuous power supply: Equal challenges also exist in technological interventions for continuously powering wearable chemical sensors. Most wearable devices exploit commercial batteries despite their weight, bulk, and mechanical properties. However, flexible batteries have some potential in this context, but at a significant added price. Research examples of wearable flexible batteries are of interest, but most of them have mediocre performance. In this context, battery-free strategies, based on wireless power transfer using near-field communication (NFC) technologies, are much more advantageous, but need adjacent propinquity (up to ∼1 m) to a transmission antenna for the interminable acquisition of data. Eventually, an efficient combination of various subsystems is required in wearable sweat sensors, which can overcome this technological hurdle.
- (xv)
- Validation: After meeting the above unmet criteria and obtaining confirmatory data from centralized lab tests, the sensing performance of the developed sensors in terms of accuracy and precision should be clinically validated against gold standard LC-MS methods for widespread commercial applications, as slight positive or negative signal predictions will lead to serious life-threatening issues.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S.W.; Gaitonde, S.; Begtrup, G.; Katchman, B.A. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 2019, 37, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Brothers, M.C.; DeBrosse, M.; Grigsby, C.C.; Naik, R.R.; Hussain, S.M.; Heikenfeld, J.; Kim, S.S. Achievements and challenges for real-time sensing of analytes in sweat within wearable platforms. Acc. Chem. Res. 2019, 52, 297–306. [Google Scholar] [CrossRef]
- Han, S.-T.; Peng, H.; Sun, Q.; Venkatesh, S.; Chung, K.-S.; Lau, S.C.; Zhou, Y.; Roy, V.A.L. An Overview of the Development of Flexible Sensors. Adv. Mater. 2017, 29, e1700375. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jeang, W.J.; Ghaffari, R.; Rogers, J.A. Wearable sensors for biochemical sweat analysis. Annu. Rev. Anal. Chem. 2019, 12, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Windmiller, J.R.; Wang, J. Wearable Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2013, 25, 29–46. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Sempionatto, J.R.; Barfidokht, A.; Mishra, R.K.; Campbell, A.S.; Hubble, L.J.; Wang, J. Wearable Bioelectronics: Enzyme-Based Body-Worn Electronic Devices. Acc. Chem. Res. 2018, 51, 2820–2828. [Google Scholar] [CrossRef]
- Ghaffari, R.; Yang, D.S.; Kim, J.; Mansour, A.; Wright, J.A., Jr.; Model, J.B.; Wright, D.E.; Rogers, J.A.; Ray, T.R. State of Sweat: Emerging Wearable Systems for Real-Time, Non-invasive Sweat Sensing and Analytics. ACS Sens. 2021, 6, 2787–2801. [Google Scholar] [CrossRef]
- Xu, J.; Fang, Y.; Chen, J. Wearable Biosensors for Non-Invasive Sweat Diagnostics. Biosensors 2021, 11, 245. [Google Scholar] [CrossRef]
- Legner, C.; Kalwa, U.; Patel, V.; Chesmore, A.; Pandey, S. Sweat sensing in the smart wearables era: Towards integrative, multifunctional and body-compliant perspiration analysis. Sens. Actuator A Phys. 2019, 296, 200–221. [Google Scholar] [CrossRef]
- Costa, N.G.; Antunes, J.C.; Paleo, A.J.; Rocha, A.M. A Review on Flexible Electrochemical Biosensors to Monitor Alcohol in Sweat. Biosensors 2022, 12, 252. [Google Scholar] [CrossRef]
- Singh, A.; Ahmed, A.; Sharma, A.; Arya, S. Graphene and Its Derivatives: Synthesis and Application in the Electrochemical Detection of Analytes in Sweat. Biosensors 2022, 12, 910. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Zhou, X.; Yuan, M.; Qu, D.; Zheng, Y.; Vishinkin, R.; Khatib, M.; Wu, W.; Haick, H. Disease Detection with Molecular Biomarkers: From Chemistry of Body Fluids to Nature-Inspired Chemical Sensors. Chem. Rev. 2019, 119, 11761–11817. [Google Scholar] [CrossRef]
- Heikenfeld, J. Non-invasive analyte access and sensing through eccrine sweat: Challenges and outlook circa 2016. Electroanalysis 2016, 28, 1242–1249. [Google Scholar] [CrossRef]
- Mena-Bravo, A.; Luque de Castro, M.D. Sweat: A sample with limited present applications and promising future in metabolomics. J. Pharm. Biomed. Anal. 2014, 90, 139–147. [Google Scholar] [CrossRef]
- Sonner, Z.; Wilder, E.; Heikenfeld, J.; Kasting, G.; Beyette, F.; Swaile, D.; Sherman, F.; Joyce, J.; Hagen, J.; Kelley-Loughnane, N.; et al. The Microfluidics of the Eccrine Sweat Gland, Including Biomarker Partitioning, Transport, and Biosensing Implications. Biomicrofluidics 2015, 9, 031301. [Google Scholar] [CrossRef]
- Qiao, L.; Benzigar, M.; Subramony, A.; Lovell, N.; Liu, G. Advances in Sweat Wearables: Sample Extraction, Real-Time Biosensing, and Flexible Platforms. ACS Appl. Mater. Interfaces 2020, 12, 34337–34361. [Google Scholar] [CrossRef]
- Serag, A.; Shakkour, Z.; Halboup, A.M.; Kobeissy, F.; Farag, M.A. Sweat metabolome and proteome: Recent trends in analytical advances and potential biological functions. J. Proteom. 2021, 246, 104310. [Google Scholar] [CrossRef]
- Tousoulis, D.; Antoniades, C.; Tountas, C.; Bosinakou, E.; Kotsopoulou, M.; Toutouzas, P.; Stefanadis, C. Vitamin C Affects Thrombosis/Fibrinolysis System and Reactive Hyperemia in Patients with Type 2 Diabetes and Coronary Artery Disease. Diabetes care 2003, 26, 10. [Google Scholar] [CrossRef]
- Yang, H.; Du, L.; Zhang, Z. Potential biomarkers in septic shock besides lactate. Exp. Biol. Med. 2020, 245, 1066–1072. [Google Scholar] [CrossRef]
- Tofler, G.H.; Stec, J.J.; Stubbe, I.; Beadle, J.; Feng, D.; Lipinska, I.; Taylor, A. The Effect of Vitamin C Supplementation on Coagulability and Lipid Levels in Healthy Male Subjects. Thromb. Res. 2000, 100, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Gamella, M.; Campuzano, S.; Manso, J.; Rivera, G.G.; Lopez-Colino, F.; Reviejo, A.J.; Pingarrón, J.M. A novel non-invasive electrochemical biosensing device for in situ determination of the alcohol content in blood by monitoring ethanol in sweat. Anal. Chim. Acta 2014, 806, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, S.; Karim, S.; Akram, M.R.; Khan, A.K.M.; Zia, A.A.; Siddiqi, R.; Murtaza, G. Recent Developments in Sweat Analysis and Its Applications. Int. J. Anal. Chem. 2015, 7, 164974. [Google Scholar] [CrossRef] [PubMed]
- Brasiera, N.; Eckstein, J. Sweat as a Source of Next-Generation Digital Biomarkers. Digit Biomark 2019, 3, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.S.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.-H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef]
- Al-Tamer, Y.Y.; Hadi, E.A.; Al-Badrani, I.e.I. Sweat urea, uric acid and creatinine concentrations in uraemic patients. Urol. Res. 1997, 25, 337–340. [Google Scholar] [CrossRef]
- Yuta, S.; Daisuke, N.; Yasuyuki, S.; Toshinobu, R.; Hidehiko, I.; Kotaro, M.; Masato, S.; Takatomo, W.; Takeo, N.; Morio, M.; et al. A novel device for detecting anaerobic threshold using sweat lactate during exercise. Sci. Rep. 2021, 11, 4929. [Google Scholar]
- Ming, L.; Yang, W.; Dong, L.; Rui, Y.; Jing, X.; Hao, H. A stable sandwich-type amperometric biosensor based on poly(3,4-ethylenedioxythiophene)–single walled carbon nanotubes/ascorbate oxidase/nafion films for detection of L-ascorbic acid. Sens. Actuators B Chem. 2011, 159, 277–285. [Google Scholar]
- Sung, K.; Jahyun, K.; Jangyeol, Y.; Aurelie, H.F.; Boram, L.; Seongbin, J.; Shu, L.; Jungli, C.; Yong, S.O.; Geumbee, L.; et al. Soft, skin-interfaced microfluidic systems with integrated enzymatic assays for measuring the concentration of ammonia and ethanol in sweat. Lab on A Chip 2020, 20, 84–92. [Google Scholar]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Turner, A. Biosensors: Then and now. Trends Biotechnol. 2013, 31, 119–120. [Google Scholar] [CrossRef]
- Bej, S.; Ghosh, M.; Das, R.; Banerjee, P. Evaluation of nanomaterials-grafted enzymes for application in contaminants degradation: Need of the hour with proposed IoT synchronized nanosensor fit sustainable clean water technology in en masse. J. Indian Chem. Soc. 2022, 99, 100429. [Google Scholar] [CrossRef]
- Charoenkitamorn, K.; Yakoh, A.; Jampasa, S.; Chaiyo, S.; Chailapakul, O. Electrochemical and optical biosensors for biological sensing applications. Science 2020, 46, 245. [Google Scholar] [CrossRef]
- Song, S.; Wang, K.; Yan, L.; Brouzgou, A.; Zhang, Y.; Wang, Y.; Tsiakaras, P. Ceria promoted Pd/C catalysts for glucose electrooxidation in alkaline media. Appl. Catal. B Environm. 2015, 176–177, 233–239. [Google Scholar] [CrossRef]
- Yue, X.; Xu, F.; Zhang, L.; Ren, G.; Sheng, H.; Wang, J.; Wang, K.; Yu, L.; Wang, J.; Li, G.; et al. Simple, Skin-Attachable, and Multifunctional Colorimetric Sweat Sensor. ACS Sens. 2022, 7, 2198–2208. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Yan, B. A fluorescent wearable platform for sweat Cl− analysis and logic smart-device fabrication based on color adjustable lanthanide MOFs. J. Mater. Chem. C 2018, 6, 1863–1869. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Karman, C.; Vilà, N.; Despas, C.; Walcarius, A. Indirect amperometric detection of non-redox ions using a ferrocene-functionalized and oriented mesoporous silica thin film electrode. Electrochim. Acta 2017, 228, 659–666. [Google Scholar] [CrossRef]
- Ikariyama, Y.; Heineman, W.R. Polypyrrole electrode as a detector for electroinactive anions by flow injection analysis. Anal. Chem. 1986, 58, 1803–1806. [Google Scholar] [CrossRef]
- Mariaulle, P.; Sinapi, F.; Lamberts, L.; Walcarius, A. Application of electrodes modified with ion-exchange polymers for the amperometric detection of non-redox cations and anions in combination to ion chromatography. Electrochim. Acta 2001, 46, 3543–3553. [Google Scholar] [CrossRef]
- Amine, A.; Mohammadi, H. Encyclopedia of Analytical Science, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 70–79. [Google Scholar]
- Saha, T.; Songkakul, T.; Knisely, C.T.; Yokus, M.A.; Daniele, M.A.; Dickey, M.D.; Bozkurt, A.; Velev, O.D. Wireless Wearable Electrochemical Sensing Platform with Zero Power Osmotic Sweat Extraction for Continuous Lactate Monitoring. ACS Sens. 2022, 7, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yi, J.; Wang, T.; Feng, Y.; Wang, J.; Yu, J.; Zhang, F.; Jiang, Z.; Lv, Z.; Li, H.; et al. Wet-Adhesive On-Skin Sensors Based on Metal-Organic Frameworks for Wireless Monitoring of Metabolites in Sweat. Adv. Mater. 2022, 34, 2201768. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Lim, H.; Lee, H.N.; Park, Y.M.; Park, J.S.; Kim, H.J. Selective Nonenzymatic Amperometric Detection of Lactic Acid in Human Sweat Utilizing a Multi-Walled Carbon Nanotube (MWCNT)-Polypyrrole Core-Shell Nanowire. Biosensors 2020, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Liang, B.; Tu, T.; Wei, J.; Fang, L.; Ye, X. Three-dimensional paper-based microfluidic electrochemical integrated devices (3D-PMED) for wearable electrochemical glucose detection. RSC Adv. 2019, 9, 5674. [Google Scholar] [CrossRef]
- Kahlert, H. Potentiometry; Springer: Berlin/Heidelberg, Germany, 2010; pp. 237–256. [Google Scholar]
- Bakker, E.; Pretsch, E. Potentiometric sensors for trace-level analysis. Trends Anal. Chem. 2005, 24, 3. [Google Scholar] [CrossRef]
- Diaz, M.T.; Qin, Y. Potentiometry, Chapter 18a, Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2006; pp. 625–659. [Google Scholar]
- Mou, L.; Xia, Y.; Jiang, X. Epidermal Sensor for Potentiometric Analysis of Metabolite and Electrolyte. Anal. Chem. 2021, 93, 11525–11531. [Google Scholar] [CrossRef]
- Fischer, O.; Fischerová, E. Voltammetric Methods for the Investigation of Chemical Kinetics; Springer: Berlin/Heidelberg, Germany, 1995; pp. 159–249. [Google Scholar]
- Evans, D.H.; O’Connell, K.M.; Petersen, R.A.; Kelly, M.J. Cyclic Voltammetry. J. Chem. Educ. 1983, 60, 4–290. [Google Scholar] [CrossRef]
- Mabboi, G.A. An Introduction to Cyclic Voltammetry. J. Chem. Educ. 1983, 60, 607–702. [Google Scholar]
- Royal Society of Chemistry. Enzymes. Chem. Biol. 2004. [Google Scholar]
- Uslu, B.; Ozkan, S. Electroanalytical Methods for the Determination of Pharmaceuticals: A Review of Recent Trends and Developments. Anal. Lett. 2011, 44, 2644–2702. [Google Scholar] [CrossRef]
- Hamann, C.H.; Hamnett, A.; Vielstich, W. Electrochemistry, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Skoog, D.; West, D. Fundamentos de Química Analítica; Ediciones Paraninfo: Madrid, Spain, 2005; Volume 80. [Google Scholar]
- Das, R.; Pal, R.; Bej, S.; Mondal, M.; Kundu, K.; Banerjee, P. Recent progress in 0D optical nanoprobes for applications in the sensing of (bio)analytes with the prospect of global health monitoring and detailed mechanistic insights. Mater. Adv. 2022, 3, 4421–4459. [Google Scholar] [CrossRef]
- Lee, H.; Choi, K.T.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol 2016, 11, 566–572. [Google Scholar] [CrossRef]
- Munjea, R.D.; Muthukumar, S.; Prasad, S. Lancet-free and label-free diagnostics of glucose in sweat using Zinc Oxide based flexible bioelectronics. Sens. Actuators B Chem. 2017, 238, 482–490. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Yang, X.; Shi, Z.; Li, J.; Xue, L.; Liu, S.; Lei, Y. A responsive hydrogel-based microneedle system for minimally invasive glucose monitoring. Smart Mater. Med. 2023, 4, 69–77. [Google Scholar] [CrossRef]
- Zhai, Q.; Gong, S.; Wang, Y.; Lyu, Q.; Liu, Y.; Ling, Y.; Wang, J.; Simon, G.P.; Cheng, W. Enokitake Mushroom-like Standing Gold Nanowires toward Wearable Non-invasive Bimodal Glucose and Strain Sensing. ACS Appl. Mater. Interfaces 2019, 11, 9724–9729. [Google Scholar] [CrossRef]
- Yu, M.; Li, Y.T.; Hu, Y.; Tang, L.; Yang, F.; Lv, W.; Zhang, Z.; Zhang, G. Gold nanostructure-programmed flexible electrochemical biosensor for detection of glucose and lactate in sweat. J. Electroanal. Chem. 2021, 882, 115029. [Google Scholar] [CrossRef]
- Yoon, H.; Nah, J.; Kim, H.; Ko, S.; Sharifuzzaman, M.; Barman, S.C.; Xuan, X.; Kim, J.; Park, J.Y. A chemically modified laser-induced porous graphene based flexible and ultrasensitive electrochemical biosensor for sweat glucose detection. Sens. Actuators B Chem. 2020, 311, 127866. [Google Scholar] [CrossRef]
- Padmanathan, N.; Shao, H.; Razeeb, K.M. Multifunctional nickel phosphate nano/micro flakes 3D electrode for electrochemical energy storage, non-enzymatic glucose and sweat pH sensors. ACS Appl. Mater. Interfaces 2018, 10, 8599–8610. [Google Scholar] [CrossRef]
- Abbas, Y.; Ali, S.; Basharat, M.; Zou, W.; Yang, F.; Liu, W.; Zhang, S.; Wu, Z.; Akhtar, N.; Wu, D. Doped Carbon Nanoparticle−Ionic Liquid Composites as Heteroatom Electrochemical Sensors for Uric Acid. ACS Appl. Nano Mater. 2020, 3, 11383–11390. [Google Scholar] [CrossRef]
- Tripathi, A.; Harris, K.D.; Elias, A.L. Peroxidase-Like Behavior of Ni Thin Films Deposited by Glancing Angle Deposition for Enzyme-Free Uric Acid Sensing. ACS Omega 2020, 5, 9123–9130. [Google Scholar] [CrossRef]
- Azeredo, N.F.B.; Gonçalves, J.M.; Rossini, P.O.; Araki, K.; Wang, J.; Angnes, L. Uric acid electrochemical sensing in biofluids based on Ni/Zn hydroxide nanocatalyst. Microchim. Acta 2020, 187, 379. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.Y.; Qin, Y.; Fan, W.T.; Yan, L.P.; Chen, M.; Liu, L.Y.; Huang, W.H. A three-dimensional electrochemical biosensor integrated with hydrogel for cells culture and lactate release monitoring. J. Electroanal. Chem. 2022, 915, 116338. [Google Scholar] [CrossRef]
- Zaryanov, N.V.; Nikitina, V.N.; Karpova, E.V.; Karyakina, E.E.; Karyakin, A.A. Non-enzymatic sensor for lactate detection in human sweat. Anal. Chem. 2017, 89, 11198–11202. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Cao, L.; Qin, H.; Wei, S.; Gu, M.; Zhao, F.; Chen, Z. Non-enzymatic lactic acid sensor based on AuPtNPs functionalized MoS2 nanosheet as electrode modified materials. J. Electroanal. Chem. 2021, 903, 115806. [Google Scholar] [CrossRef]
- Alam, F.; Jalal, A.H.; Forouzanfar, S.; Karabiyik, M.; Baboukani, A.R.; Pala, N. Flexible and Linker-Free Enzymatic Sensors Based on Zinc Oxide Nanoflakes for Non-invasive L-Lactate Sensing in Sweat. IEEE sens. J. 2020, 20, 10–15. [Google Scholar] [CrossRef]
- Lin, K.C.; Muthukumar, S.; Prasad, S. Flex-GO (Flexible graphene oxide) sensor for electrochemical monitoring lactate in low-volume passive perspired human sweat. Talanta 2020, 214, 120810. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, P.; Chen, T.; Lv, Q.; Gao, L.; Liu, B.; Duan, J.; Wuc, Z.; Li, J. Construction of flexible and wearable 3D TiO2 NTs@Ti mesh for physiological detection based on sweat. JCIS Open 2021, 2, 100007. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Nejad, F.G.; Beitollahi, H.; Jahani, S.; Rezapour, M.; Larijani, B. Highly sensitive voltammetric sensor for determination of ascorbic acid using graphite screen printed electrode modified with ZnO/Al2O3 nanocomposite. Int. J. Electrochem. Sci. 2017, 12, 3231–3240. [Google Scholar] [CrossRef]
- Hu, G.K.; Ge, L.; Li, Y.Y.; Mukhtar, M.; Shen, B.; Yang, D.H.; Li, J.G. Carbon dots derived from flax straw for highly sensitive and selective detections of cobalt, chromium, and ascorbic acid. J. Colloid Interface Sci. 2020, 579, 96–108. [Google Scholar] [CrossRef]
- Matthew, G.; Peter, E. Dietary Vitamin C in Human Health. Adv. Food Nutr. Res. 2018, 83, 281–310. [Google Scholar]
- Wei, J.; Zhang, X.; Mugo, S.M.; Zhang, Q. A Portable Sweat Sensor Based on Carbon Quantum Dots for Multiplex Detection of Cardiovascular Health Biomarkers. Anal. Chem. 2022, 94, 12772–12780. [Google Scholar] [CrossRef]
- Dai, H.; Yang, C.; Tong, Y.; Xu, G.; Ma, X.; Lin, Y.; Chen, G. Label-free electrochemiluminescent immunosensor for α-fetoprotein: Performance of Nafion–carbon nanodots nanocomposite films as antibody carriers. Chem. Commun. 2012, 48, 3055–3057. [Google Scholar] [CrossRef]
- Li, K.; Xu, J.; Arsalan, M.; Cheng, N.; Sheng, Q.; Zheng, J.; Cao, W.; Yue, T. Nitrogen Doped Carbon Dots Derived from Natural Seeds and Their Application for Electrochemical Sensing. J. Electrochem. Soc. 2019, 166, B56. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Khorshed, A.A.; Ahmed, A.; Loyola, A.N.D.; Silva, E.; Barfidokht, A.; Yin, L.; Goud, K.Y.; Mohamed, M.A.; Bailey, E.; et al. Epidermal enzymatic biosensors for sweat vitamin C: Toward personalized nutrition. ACS Sens. 2020, 5, 1804–1813. [Google Scholar] [CrossRef]
- Ibarlucea, B.; Pérez Roig, A.; Belyaev, D.; Baraban, L.; Cuniberti, G. Electrochemical detection of ascorbic acid in artificial sweat using a flexible alginate/CuO-modified electrode. Microchim. Acta 2020, 187, 520. [Google Scholar]
- Wang, L.; Pan, L.; Han, X.; Ha, M.N.; Li, K.; Yu, H.; Zhang, Q.; Li, Y.; Hou, C.; Wang, H. A portable ascorbic acid in sweat analysis system based on highly crystalline conductive nickel-based metal-organic framework (Ni-MOF). J. Colloid Interface Sci. 2022, 616, 326–337. [Google Scholar] [CrossRef]
- Rossato, J.H.H.; Oliveira, M.E.; Lopes, B.V.; Gallo, B.B.; Rosa, A.B.L.; Piva, E.; Barba, D.; Rosei, F.; Carren~o, N.L.V.; Escote, M.T. A Flexible Electrochemical Biosensor Based on NdNiO3 Nanotubes for Ascorbic Acid Detection. ACS Appl. Nano Mater. 2022, 5, 3394–3405. [Google Scholar] [CrossRef]
- Loguercio, L.F.; Thesing, A.; Noremberg, B.d.S.; Lopes, B.V.; Maron, G.K.; Machado, G.; Pope, M.A.; Carreno, N.L.V. Direct Laser Writing of Poly(furfuryl Alcohol)/Graphene Oxide Electrodes for Electrochemical Determination of Ascorbic Acid. ChemElectroChem 2022, 9, e202200334. [Google Scholar]
- Suriyaprakash, J.; Shan, L.; Gupta, N.; Wang, H.; Wu, L. Janus 2D-carbon nanocomposite-based ascorbic acid sensing device: Experimental and theoretical approaches. Compos. B. Eng. 2022, 245, 110233. [Google Scholar] [CrossRef]
- Rehm, J.; Room, R.; Graham, K.; Monteiro, M.; Gmel, G.; Sempos, C.T. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: An overview. Addiction 2003, 98, 1209–1228. [Google Scholar] [CrossRef]
- Rehm, J.; Room, R.; Monteiro, M.; Gmel, G.; Graham, K.; Rehn, N.; Sempos, C.T.; Jernigan, D. Alcohol as a risk factor for global burden of disease. Eur. Addict Res. 2003, 9, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Hammer, J.H.P.M.; Spiker, D.A. Global Status Report on Alcohol and Health, Global Status Report on Alcohol. World Health Organization. 2018. Available online: https://www.who.int/publications-detail-redirect/9789241565639 (accessed on 5 November 2022).
- Apte, M.V.; Pirola, R.C.; Wilson, J.S. Pancreas: Alcoholic Pancreatitis: It’s the Alcohol, Stupid. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Gjerde, H.; Gopalan, S.S.; Normann, P.T. Alcohol, drugs, and road traffic crashes in India: A systematic review. Traffic Inj. Prev. 2012, 13, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.dw.com/en/why-alcohol-consumption-is-on-the-rise-in-india/a-63296901 (accessed on 5 November 2022).
- Jyani, G.; Prinja, S.; Ambekar, A.; Bahuguna, P.; Kumar, R. Health impact and economic burden of alcohol consumption in India. Int. J. Drug Policy 2019, 69, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Fan, C.; Xu, T.; Su, L.; Zhang, X. An electrochemical wearable sensor for levodopa quantification in sweat based on a metal–Organic framework/graphene oxide composite with integrated enzymes. Sens. Actuators B Chem. 2022, 359, 131586. [Google Scholar] [CrossRef]
- Guan, S.; Xu, B.; Yang, Y.; Zhu, X.; Chen, R.; Ye, D.; Liao, Q. Gold Nanowire Aerogel-Based Biosensor for Highly Sensitive Ethanol Detection in Simulated Sweat. ACS Appl. Nano Mater. 2022, 5, 11091–11099. [Google Scholar] [CrossRef]
- Kinnamon, D.; Muthukumar, S.; Selvam, A.P.; Prasad, S. Portable Chronic Alcohol Consumption Monitor in Human Sweat through Square-Wave Voltammetry. SLAS Technol. 2018, 23, 144–153. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol Metabolism. Clin. Liv. Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Ghaffari, R.; Rogers, J.A.; Ray, T.R. Recent progress, challenges, and opportunities for wearable biochemical sensors for sweat analysis. Sens. Actuators B Chem. 2021, 332, 129447. [Google Scholar] [CrossRef]
- Qiao, Y.; Qiao, L.; Chen, Z.; Liu, B.; Gao, L.; Zhang, L. Wearable Sensor for Continuous Sweat Biomarker Monitoring. Chemosensors 2022, 10, 273. [Google Scholar] [CrossRef]
- Michael, C.; Giuseppino, F.; Norbert, R. Wearable flexible sweat sensors for healthcare monitoring: A review. J. R. Soc. Interface 2019, 16, 20190217. [Google Scholar]
- Zhang, L.; Liu, J.; Fu, Z.; Qi, L. A Wearable Biosensor Based on Bienzyme Gel-Membrane for Sweat Lactate Monitoring by Mounting on Eyeglasses. J. Nanosci. Nanotechnol. 2020, 20, 1495–1503. [Google Scholar] [CrossRef]
- Walid, Y.; Al-Ani, Z.; Gray, R. Silicone impression materials and latex gloves. Is interaction fact or fallacy? Dent. Update 2012, 39, 39–42. [Google Scholar] [CrossRef]
- Demolder, C.; Molina, A.; Hammond, F.L.; Yeo, W.-H. Recent advances in wearable biosensing gloves and sensory feedback biosystems for enhancing rehabilitation, prostheses, healthcare, and virtual reality. Biosens. Bioelectron. 2021, 190, 113443. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Machado, S.A.S.; Oliveira, O.N., Jr. Wearable glove-embedded sensors for therapeutic drug monitoring in sweat for personalized medicine. Chem. Eng. J. 2022, 435, 135047. [Google Scholar] [CrossRef]
- Luo, X.; Shi, W.; Yu, H.; Xie, Z.; Li, K.; Cui, Y. Wearable Carbon Nanotube-Based Biosensors on Gloves for Lactate. Sensors 2018, 18, 3398. [Google Scholar] [CrossRef]
- Bariya, M.; Li, L.; Ghattamaneni, R.; Ahn, C.H.; Nyein, H.Y.Y.; Tai, L.-C.; Javey, A. Glove-based sensors for multimodal monitoring of natural sweat. Sci. Adv. 2020, 6, eabb8308. [Google Scholar] [CrossRef]
- Manasa, G.; Mascarenhas, R.J.; Shetti, N.P.; Malode, S.J.; Mishra, A.; Basu, S.; Aminabhavi, T.M. Skin Patchable Sensor Surveillance for Continuous Glucose Monitoring. ACS Appl. Bio Mater. 2022, 5, 945–970. [Google Scholar] [CrossRef]
- Lin, P.H.; Sheu, S.C.; Chen, C.W.; Huang, S.C.; Li, B.R. Wearable hydrogel patch with non-invasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef]
- Toi, P.T.; Trung, T.Q.; Dang, T.M.L.; Bae, C.W.; Lee, N.E. Highly Electrocatalytic, Durable, and Stretchable Nanohybrid Fiber for On-body Sweat Glucose Detection. ACS Appl. Mater. Interfaces 2019, 11, 10707–10717. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Hui, J.; Zhou, L.; Lin, B.; Sun, H.; Bai, Y.; Zhao, J.; Mao, H. A low-cost and simple-fabricated epidermal sweat patch based on “cut-and-paste” manufacture. Sens. Actuators B Chem. 2022, 368, 132184. [Google Scholar] [CrossRef]
- Zhanga, Q.; Jianga, D.; Xu, C.; Ge, Y.; Liua, X.; Wei, Q.; Huanga, L.; Rena, X.; Wangd, C.; Wanga, Y. Wearable electrochemical biosensor based on molecularly imprinted Ag nanowires for non-invasive monitoring lactate in human sweat. Sens. Actuators B Chem. 2020, 320, 128325. [Google Scholar] [CrossRef]
- Kil, H.J.; Kim, S.R.; Park, J.W. A Self-Charging Supercapacitor for a Patch-Type Glucose Sensor. ACS Appl. Mater. Interfaces 2022, 14, 3838–3848. [Google Scholar] [CrossRef]
- Shu, Y.; Shang, Z.; Su, T.; Zhang, S.; Lu, Q.; Xu, Q.; Hu, X. A highly flexible Ni–Co MOF nanosheet coated Au/PDMS film based wearable electrochemical sensor for continuous human sweat glucose monitoring. Analyst 2022, 147, 1440–1448. [Google Scholar] [CrossRef]
- Shu, Y.; Su, T.; Lu, Q.; Shang, Z.; Xu, Q.; Hu, X. Highly Stretchable Wearable Electrochemical Sensor Based on Ni-Co MOF Nanosheet-Decorated Ag/rGO/PU Fiber for Continuous Sweat Glucose Detection. Anal. Chem. 2021, 93, 16222–16230. [Google Scholar] [CrossRef]
- Kinnamon, D.; Lin, K.-C.; Sankhala, D.; Muthukumar, S.; Prasad, S. AWARE: A Wearable Awareness with Real-time Exposure, for monitoring alcohol consumption impact through Ethyl Glucuronide detection. Alcohol 2019, 81, 93–99. [Google Scholar]
- Li, T.; Liang, B.; Ye, Z.; Zhang, L.; Xu, S.; Tu, T.; Zhang, Y.; Cai, Y.; Zhang, B.; Fang, L.; et al. An integrated and conductive hydrogel-paper patch for simultaneous sensing of Chemical-Electrophysiological signals. Biosens. Bioelectron. 2022, 198, 113855. [Google Scholar] [CrossRef]
- Kim, S.K.; Jeon, C.; Lee, G.H.; Koo, J.; Cho, S.H.; Han, S.; Shin, M.H.; Sim, J.Y.; Hahn, S.K. Hyaluronate-Gold Nanoparticle/Glucose Oxidase Complex for Highly Sensitive Wireless Non-invasive Glucose Sensors. ACS Appl. Mater. Interfaces 2019, 11, 37347–37356. [Google Scholar]
- Xuan, X.; Rafols, C.P.; Chen, C.; Cuartero, M.; Crespo, G.A. Lactate Biosensing for Reliable On-Body Sweat Analysis. ACS Sens. 2021, 6, 2763–2771. [Google Scholar] [CrossRef]
- Lin, S.; Wang, B.; Zhao, Y.; Shih, R.; Cheng, X.; Yu, W.; Hojaiji, H.; Lin, H.; Hoffman, C.; Ly, D.; et al. Natural Perspiration Sampling and in Situ Electrochemical Analysis with Hydrogel Micropatches for User-Identifiable and Wireless Chemo/Biosensing. ACS Sens. 2020, 5, 93–102. [Google Scholar]
- Son, J.; Bae, G.Y.; Lee, S.; Lee, G.; Kim, S.W.; Kim, D.; Chung, S.; Cho, K. Cactus-Spine-Inspired Sweat-Collecting Patch for Fast and Continuous Monitoring of Sweat. Adv. Mater. 2021, 33, 2102740. [Google Scholar] [CrossRef]
- Kim, K.O.; Kima, G.J.; Kim, J.H. A cellulose/b-cyclodextrin nanofiber patch as a wearable epidermal glucose sensor. RSC Adv. 2019, 9, 22790. [Google Scholar] [CrossRef]
- Xuan, X.; Yoon, H.S.; Park, J.Y. A Wearable Electrochemical Glucose Sensor based on Simple and Low-Cost Fabrication Supported Micro-Patterned Reduced Graphene Oxide Nanocomposite Electrode on Flexible Substrate. Biosens. Bioelectron. 2018, 109, 75–82. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lin, M.; Yin, L.; lapaz, E.D.; Pei, K.; Sonsa-ard, T.; Silva, A.N.L.; Khorshed, A.A.; Zhang, F.; Tostado, N.; et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng 2021, 5, 737–748. [Google Scholar] [CrossRef]
- Shang, K.; Wang, S.; Chen, S.; Wang, X. Sensitivity Detection of Uric Acid and Creatinine in Human Urine Based on Nanoporous Gold. Biosensors 2022, 12, 588. [Google Scholar] [CrossRef]
- Chen, S.; Shang, K.; Gao, X.; Wang, X. The development of NAD+-dependent dehydrogenase screen-printed biosensor based on enzyme and nanoporous gold co-catalytic strategy. Biosens. Bioelectron. 2022, 211, 114376. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Wang, S.; Ma, H.; Shen, Y.; Wang, X. A Multifunctional Electrochemical Sensor for the Simultaneous Detection of Ascorbic Acid, Dopamine, Uric Acid, and Nitrite. J. AOAC Int. 2021, 104, 860–866. [Google Scholar] [CrossRef]
- Sondhi, P.; Lingden, D.; Bhattarai, J.K.; Demchenko, A.V.; Stine, K.J. Applications of Nanoporous Gold in Therapy, Drug Delivery, and Diagnostics. Metals 2023, 13, 78. [Google Scholar] [CrossRef]
- Bae, C.W.; Toi, P.T.; Kim, B.Y.; Lee, W.I.; Lee, H.B.; Hanif, A.; Lee, E.H.; Lee, N.-E. Fully Stretchable Capillary Microfluidics Integrated Nanoporous Gold Electrochemical Sensor for Wearable Continuous Glucose Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 14567–14575. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-Based Non-invasive Glucose Monitoring: A Proof-of-Concept Study. Anal. Chem. 2015, 87, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Bandodkar, A.J.; Valdes-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical Tattoo Biosensors for Real-Time Non-invasive Lactate Monitoring in Human Perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeerapan, I.; Imani, S.; Cho, T.N.; Bandodkar, A.J.; Cinti, S.; Mercier, P.P.; Wang, J. Non-invasive Alcohol Monitoring Using a Wearable Tattoo-based Iontophoretic-Biosensing System. ACS Sens. 2016, 1, 1011–1019. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jia, W.; Wang, J. Tattoo-Based Wearable Electrochemical Devices: A Review. Electroanalysis 2015, 27, 1–12. [Google Scholar] [CrossRef]
- Tai, L.-C.; Liaw, T.S.; Lin, Y.; Nyein, H.Y.Y.; Bariya, M.; Ji, W.; Hettick, M.; Zhao, C.; Zhao, J.; Hou, L.; et al. A Wearable Sweat Band for Non-invasive Levodopa Monitoring. Nano Lett. 2019, 19, 6346–6351. [Google Scholar] [CrossRef]
- Tai, L.-C.; Ahn, C.H.; Nyein, H.Y.Y.; Ji, W.; Bariya, M.; Lin, Y.; Li, L.; Javey, A. Nicotine Monitoring with Wearable Sweat Band. ACS Sens. 2020, 5, 1831–1837. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, K.; Chen, D.; Shen, G. Wearable sweat monitoring system with integrated micro-supercapacitors. Nano Energy 2019, 58, 624–632. [Google Scholar] [CrossRef]
- Tsunoda, M.; Hirayama, M.; Tsuda, T.; Ohno, K. Non-invasive monitoring of plasma l-dopa concentrations using sweat samples in Parkinson’s disease. Clin. Chim. Acta 2015, 442, 52–55. [Google Scholar] [CrossRef]
- Beaglehole, R.; Bates, C.; Youdan, B.; Bonita, R. Nicotine without Smoke: Fighting the Tobacco Epidemic with Harm Reduction. Lancet 2019, 394, 718–720. [Google Scholar] [CrossRef]
- Duncan, A.; Heyer, M.P.; Ishikawa, M.; Caligiuri, S.P.B.; Liu, X.-A.; Chen, Z.; Micioni Di Bonaventura, M.V.; Elayouby, K.S.; Ables, J.L.; Howe, W.M.; et al. Habenular TCF7L2 Links Nicotine Addiction to Diabetes. Nature 2019, 574, 372–377. [Google Scholar] [CrossRef]
- Piper, A.; Mansson, I.O.; Khaliliazar, S.; Landin, R.; Hamedi, M.M. A disposable, wearable, flexible, stitched textile electrochemical biosensing platform. Biosens. Bioelectron. 2021, 194, 113604. [Google Scholar] [CrossRef]
- R72 Zhao, C.; Li, X.; Wu, Q.; Liu, X. A thread-based wearable sweat nanobiosensor. Biosens. Bioelectron. 2021, 188, 113270. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, Y.; Zhang, C. A sample-to-answer, wearable cloth-based electrochemical sensor (WCECS) for point-of-care detection of glucose in sweat. Sens. Actuators B Chem. 2021, 343, 130131. [Google Scholar] [CrossRef]
- He, X.; Yang, S.; Pei, Q.; Song, Y.; Liu, C.; Xu, T.; Zhang, X. Integrated Smart Janus Textile Bands for Self-pumping Sweat Sampling and Analysis. ACS Sens. 2020, 5, 1548–1554. [Google Scholar] [CrossRef]
- Mirzajani, H.; Abbasiasl, T.; Mirlou, F.; Istif, E.; Bathaei, M.J.; Dağ, Ç.; Deyneli, O.; Yazıcı, D.; Beker, L. An ultra-compact and wireless tag for battery-free sweat glucose monitoring. Biosens. Bioelectron. 2022, 213, 114450. [Google Scholar] [CrossRef]
- Hao, J.; Zhu, Z.; Hu, C.; Liu, Z. Photosensitive-Stamp-Inspired Scalable Fabrication Strategy of Wearable Sensing Arrays for Non-invasive Real-Time Sweat analysis. Anal. Chem. 2022, 94, 4547–4555. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced Carbon for Flexible and Wearable Electronics. Adv. Mater. 2019, 31, 1801072. [Google Scholar] [CrossRef]
- Yu, L.; Shearer, C.; Shapter, J. Recent Development of Carbon Nanotube Transparent Conductive Films. Chem. Rev. 2016, 116, 13413–13453. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Li, G.; Yan, D.; Liu, C.; Xu, T.; Zhang, X. Ultra-Small Wearable Flexible Biosensor for Continuous Sweat Analysis. ACS Sens. 2022, 7, 3102–3107. [Google Scholar] [CrossRef]
- Xia, H.-Q.; Tang, H.; Zhou, B.; Li, Y.; Zhang, X.; Shi, Z.; Deng, L.; Song, R.; Li, L.; Zhang, Z.; et al. Mediator-free electron-transfer on patternable hierarchical meso/macro porous bienzyme interface for highly-sensitive sweat glucose and surface electromyography monitoring. Sens. Actuators B Chem. 2020, 312, 127962. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Liu, R.; Qin, Y.; Qiu, Q.-F.; Chen, Z.; Cheng, S.-B.; Huang, W.-H. Flexible Electrochemical Urea Sensor Based on Surface Molecularly Imprinted Nanotubes for Detection of Human Sweat. Anal. Chem. 2018, 90, 13081–13087. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Sim, M.; Oh, T.-S.; Yoon, Y.S.; Kim, D.-J. Flexible Electrochemical Sensor Based on NiCu(OOH) for Monitoring Urea in Human Sweat. J. Electrochem. Soc. 2021, 168, 117510. [Google Scholar] [CrossRef]
- Zhao, J.; Nyein, H.Y.Y.; Hou, L.; Lin, Y.; Bariya, M.; Ahn, C.H.; Ji, W.; Fan, Z.; Javey, A. A Wearable Nutrition Tracker. Adv. Mater. 2020, 33, 2006444. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Hong, S.Y.; Jeong, Y.R.; Yun, J.; Park, H.; Jin, S.W.; Lee, G.; Oh, J.H.; Lee, H.; Lee, S.-S.; et al. A Skin-Attachable, Stretchable Electrochemical Sweat Sensor for Glucose and pH Detection. ACS Appl. Mater. Interfaces 2018, 10, 13729–13740. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, M.; Li, J.; Liu, L.; Yu, J.; Li, Z.; Ding, B. Wearable biosensor for sensitive detection of uric acid in artificial sweat enabled by a fiber structured sensing interface. Nano Energy 2021, 85, 106031. [Google Scholar] [CrossRef]
- Silvaa, R.R.; Raymundo-Pereiraa, P.A.; Camposb, A.M.; Wilsona, D.; Otonic, C.G.; Barudd, H.S.; Costae, C.A.R.; Domeneguettif, R.R.; Balogha, D.T.; Ribeirof, S.J.L.; et al. Microbial nanocellulose adherent to human skin used in electrochemical sensors to detect metal ions and biomarkers in sweat. Talanta 2020, 218, 121153. [Google Scholar] [CrossRef]





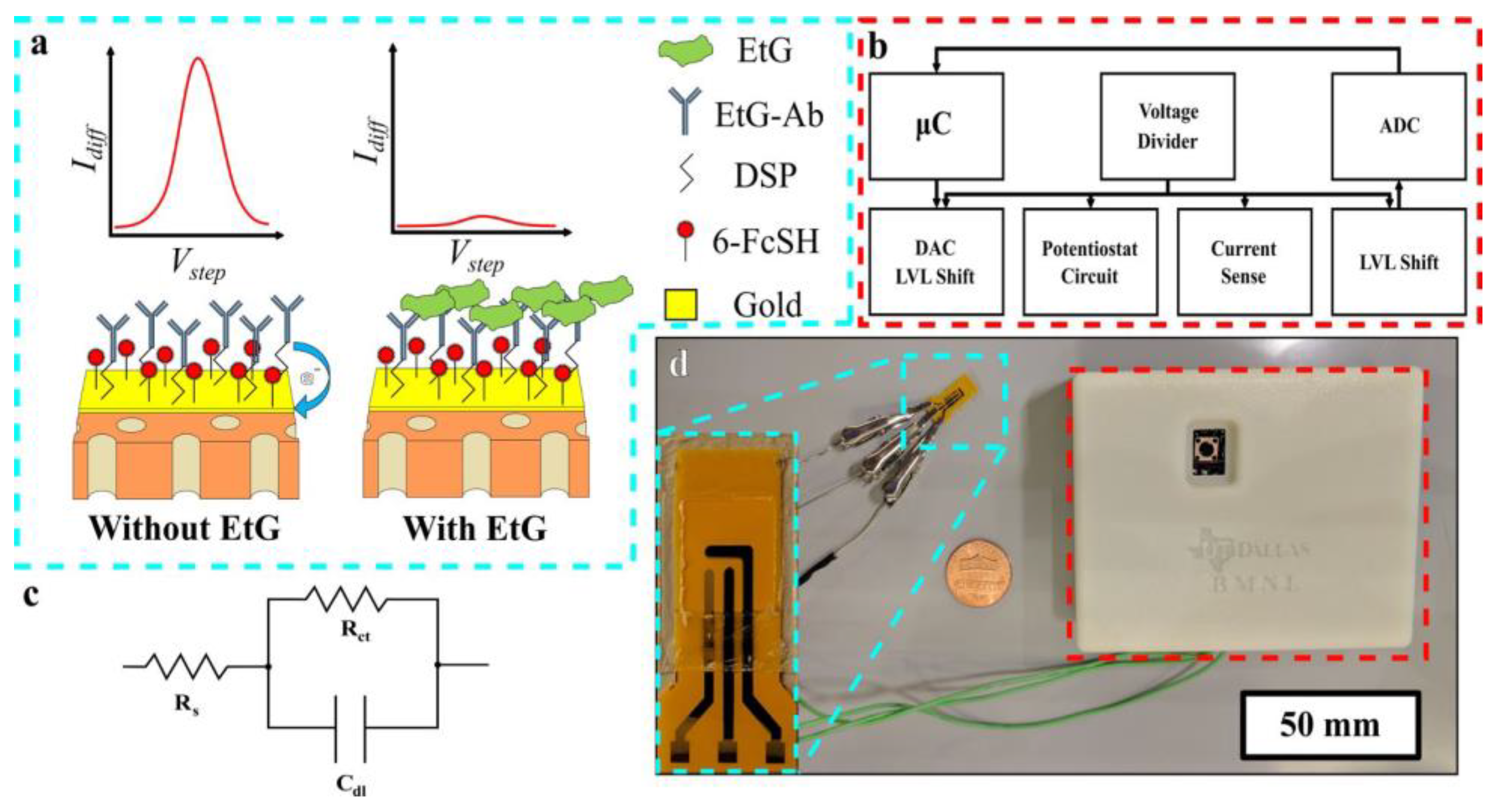
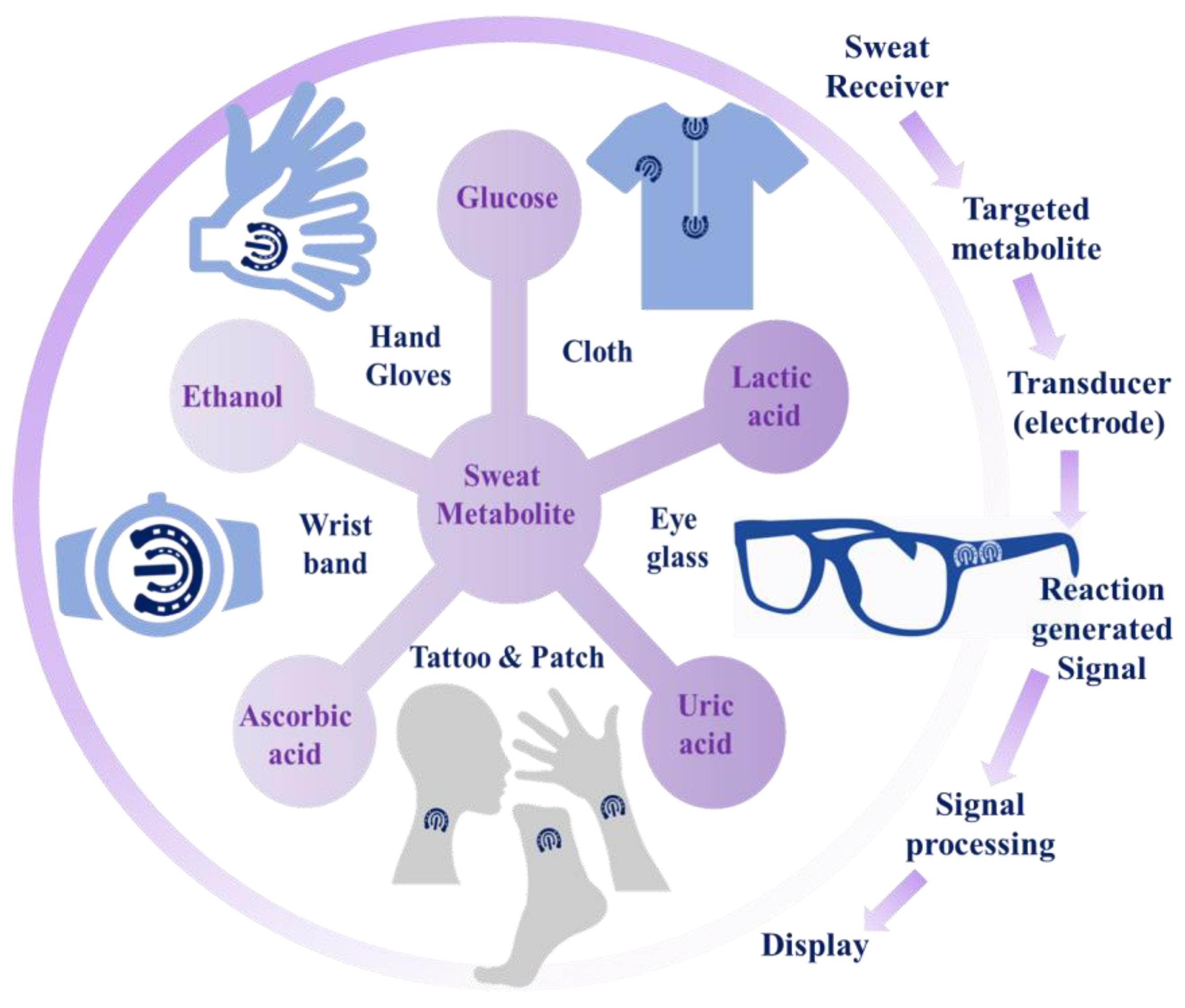

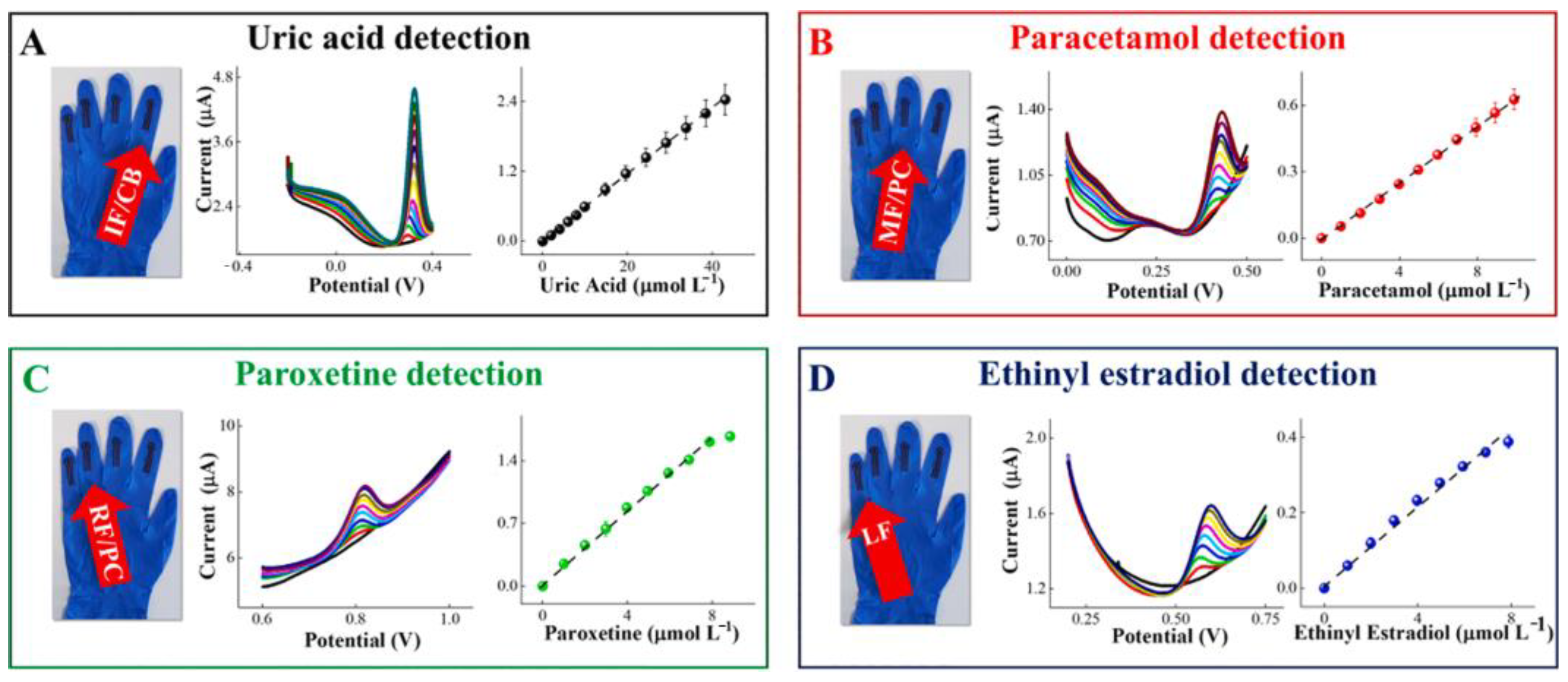
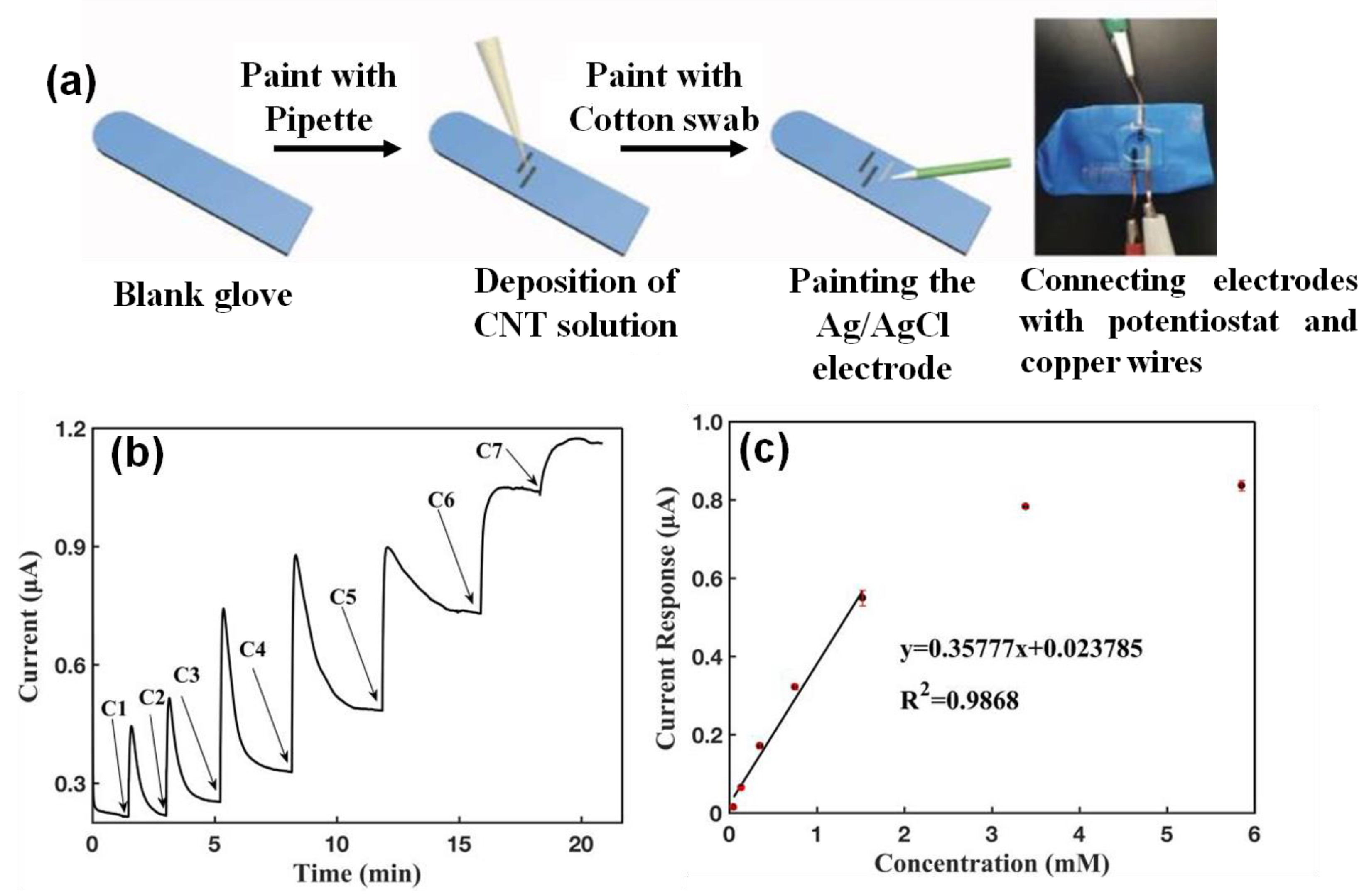





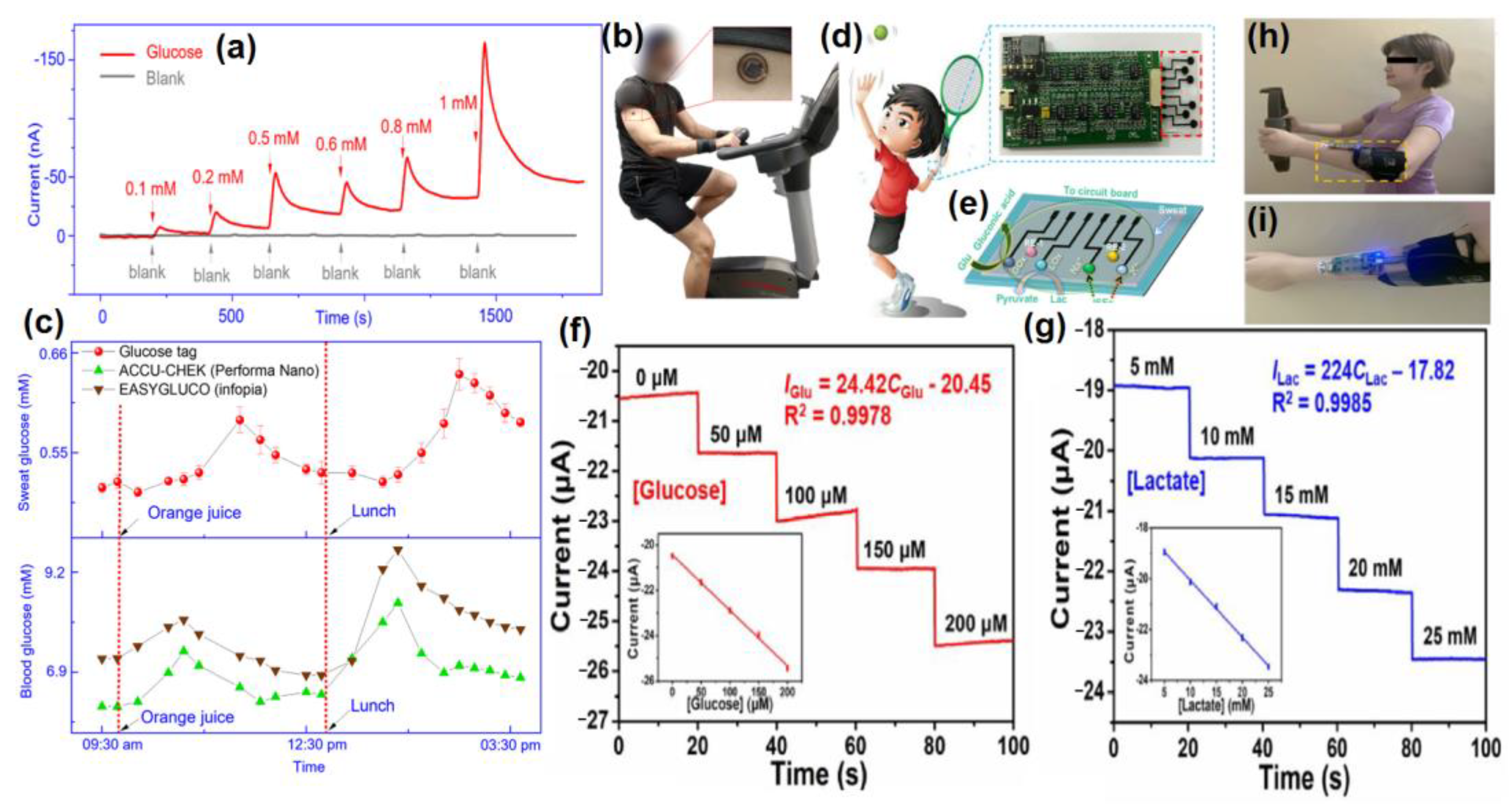

| Sweat Metabolites | Relative Content | Related Health Condition | Ref. |
|---|---|---|---|
| Glucose | 10–200 µM | Diabetes | [25] |
| Urea/uric acid | 2–10 mM | Renal dysfunction, Gout | [26] |
| Lactic acid | 5–20 mM | Stress Ischemia, Cystic Fibrosis | [27] |
| Ascorbic acid | 10–50 µM | Kidney Disease, Thrombosis, Cancer | [28] |
| Ethanol | 2.5–22.5 mM | Alcoholism, Diabetes, Drunk driving | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, R.; Nag, S.; Banerjee, P. Electrochemical Nanosensors for Sensitization of Sweat Metabolites: From Concept Mapping to Personalized Health Monitoring. Molecules 2023, 28, 1259. https://doi.org/10.3390/molecules28031259
Das R, Nag S, Banerjee P. Electrochemical Nanosensors for Sensitization of Sweat Metabolites: From Concept Mapping to Personalized Health Monitoring. Molecules. 2023; 28(3):1259. https://doi.org/10.3390/molecules28031259
Chicago/Turabian StyleDas, Riyanka, Somrita Nag, and Priyabrata Banerjee. 2023. "Electrochemical Nanosensors for Sensitization of Sweat Metabolites: From Concept Mapping to Personalized Health Monitoring" Molecules 28, no. 3: 1259. https://doi.org/10.3390/molecules28031259
APA StyleDas, R., Nag, S., & Banerjee, P. (2023). Electrochemical Nanosensors for Sensitization of Sweat Metabolites: From Concept Mapping to Personalized Health Monitoring. Molecules, 28(3), 1259. https://doi.org/10.3390/molecules28031259