Phytochemical Analysis and Anticancer Properties of Drimia maritima Bulb Extracts on Colorectal Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Identification of Chemical Constituents of D. maritima Bulb Extract by GC-MS
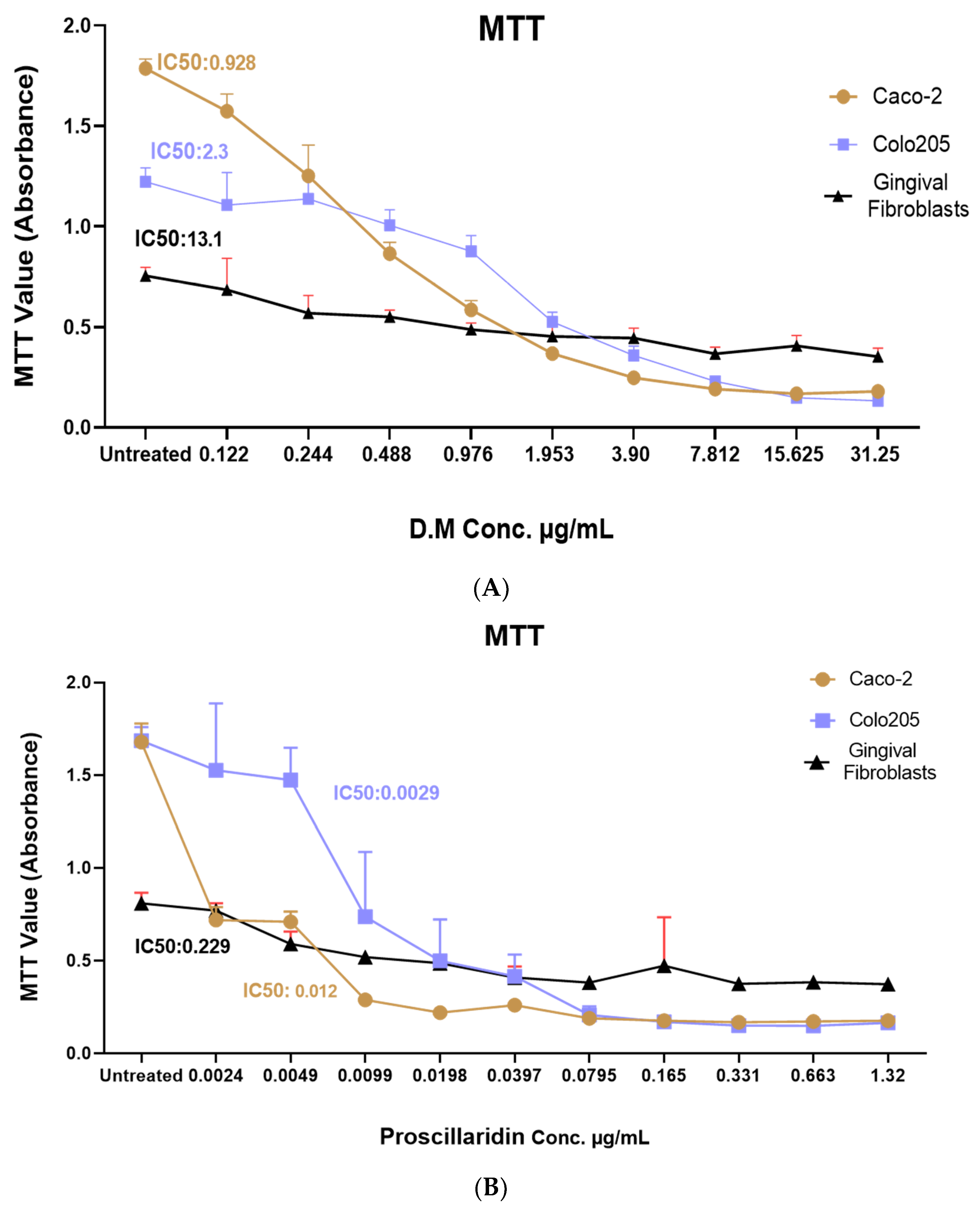
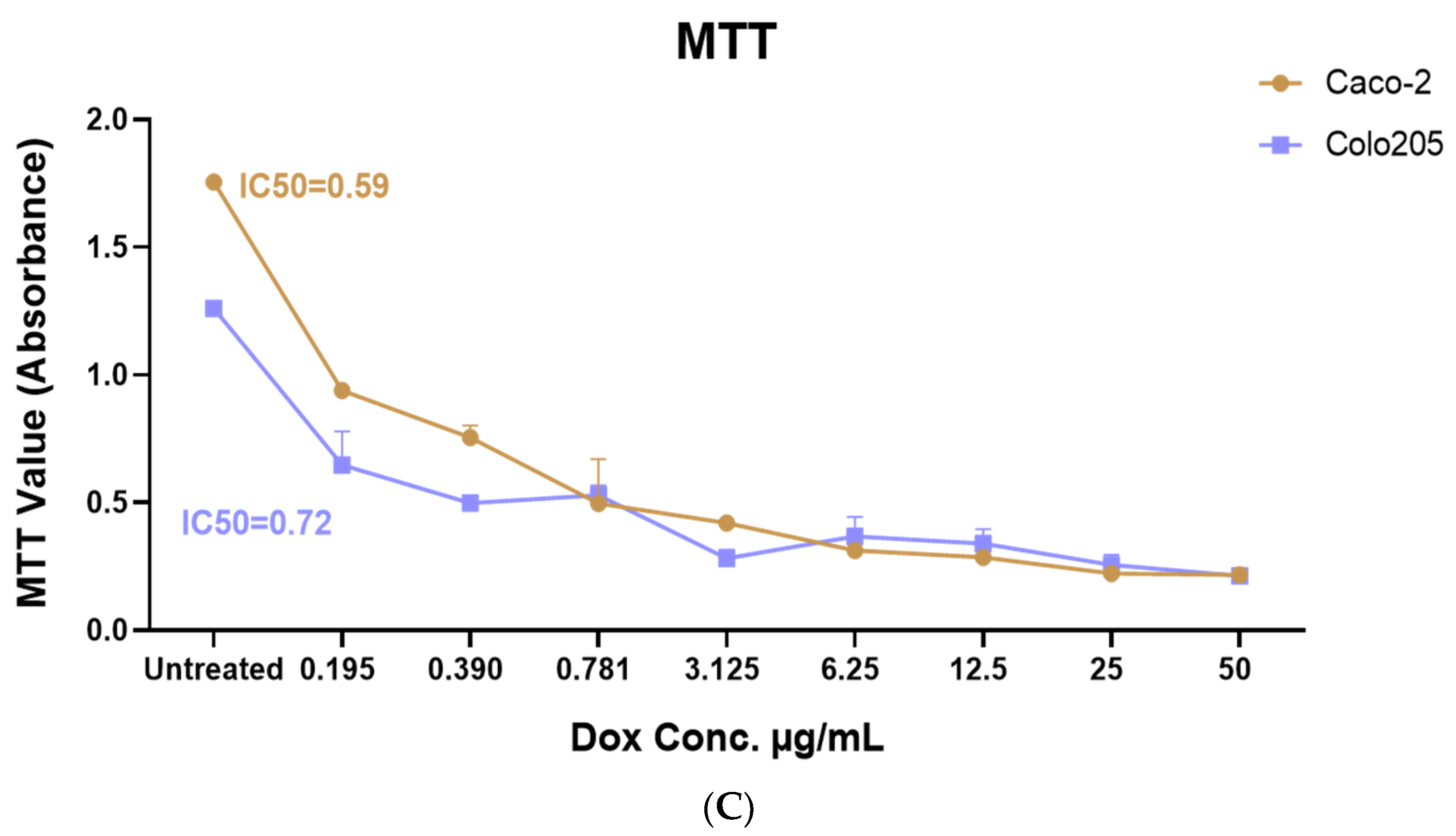
2.2. Drimia maritima Bulb Extract and ProA Can Selectively Inhibit the Proliferation of COLO-205 and Caco-2 Cancer Cells
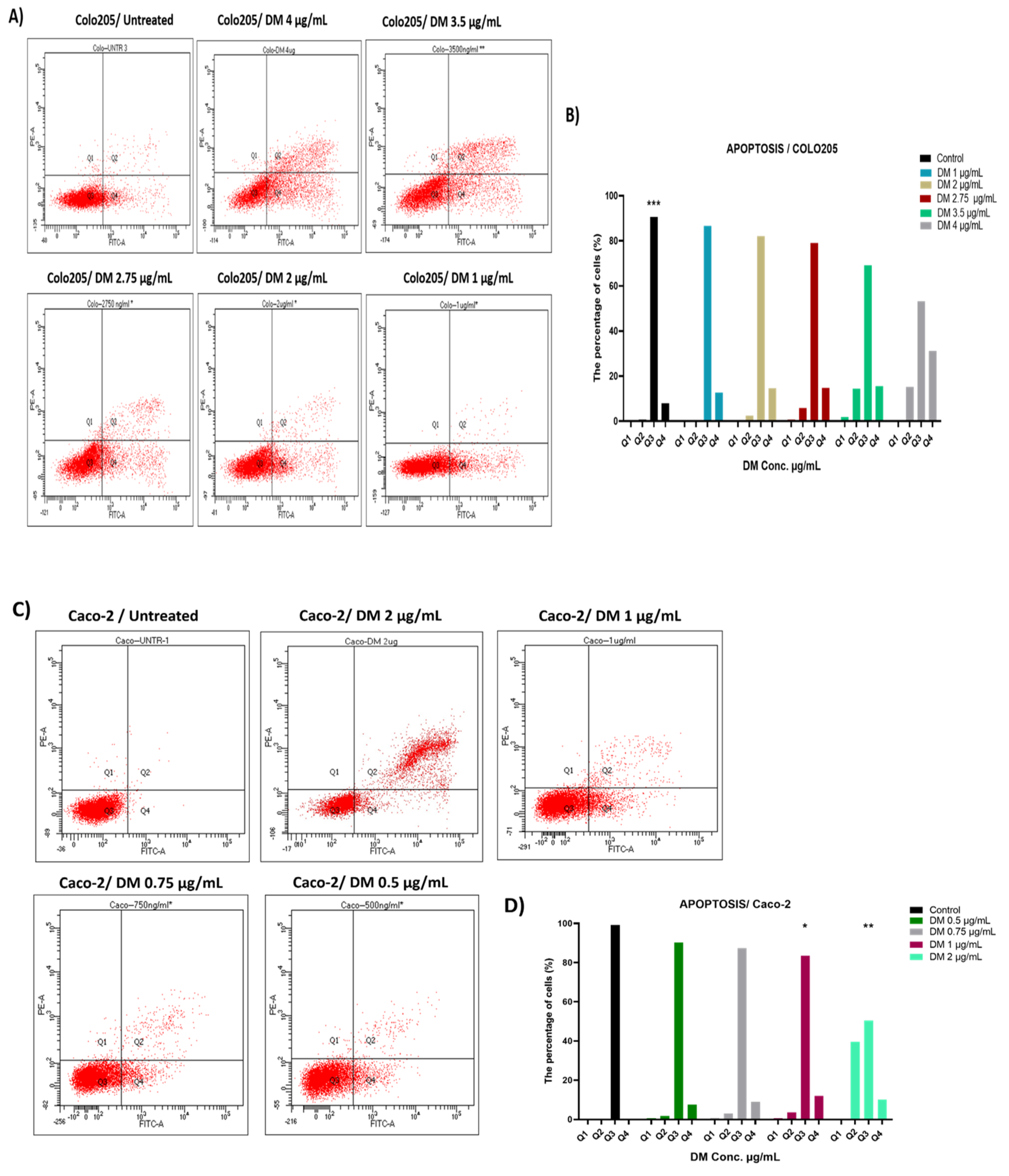
2.3. D. maritima Bulb Extract Can Induce Early and Late Apoptosis in Colon Cancer Cell Lines
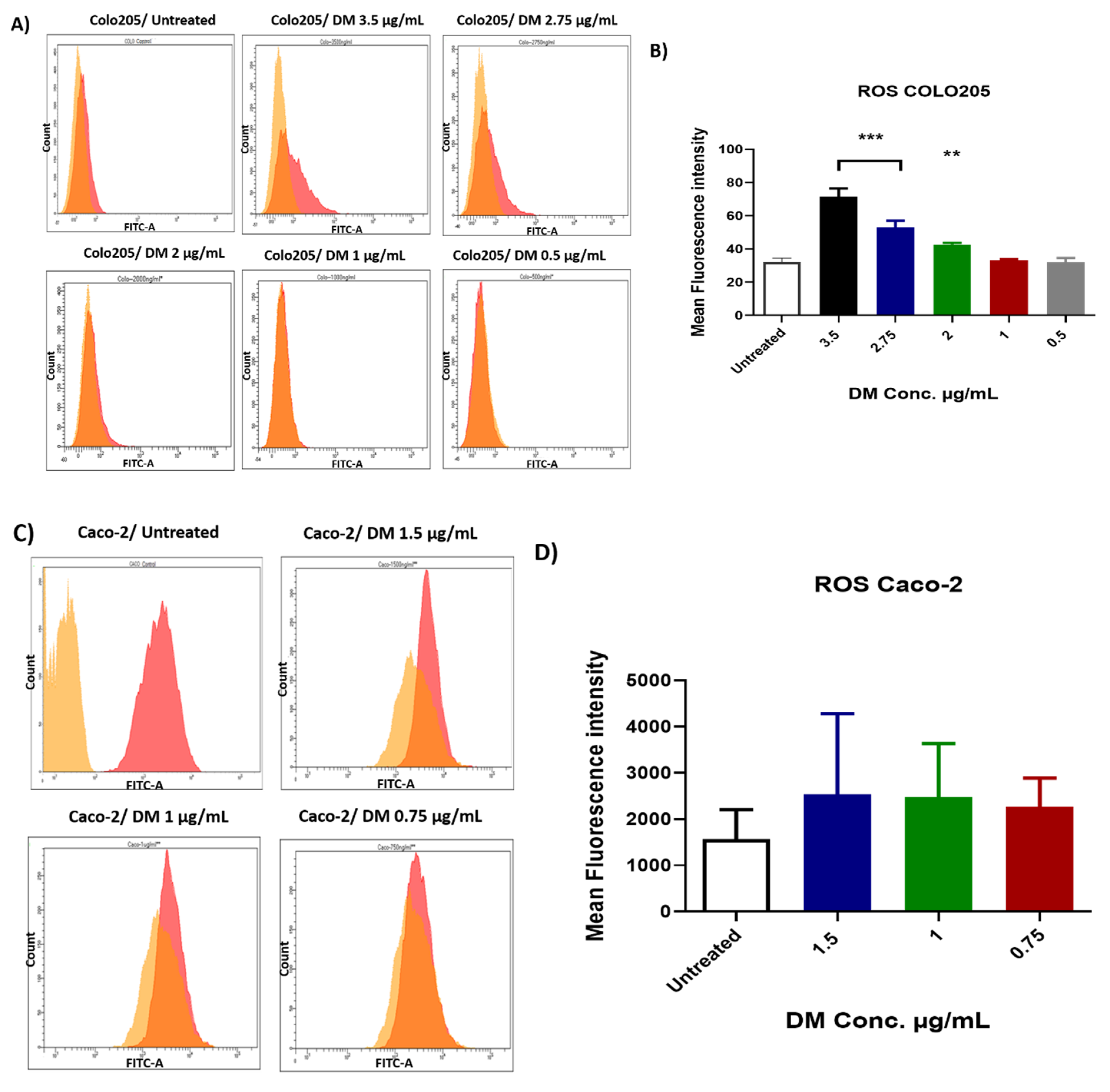
2.4. D. maritima Bulb Extract Induces the Production of ROS in Colon Cancer Cells
2.5. D. maritima Bulb Extract Affects Mitochondrial Membrane Potential (ΔΨm) in Colon Cancer Cells
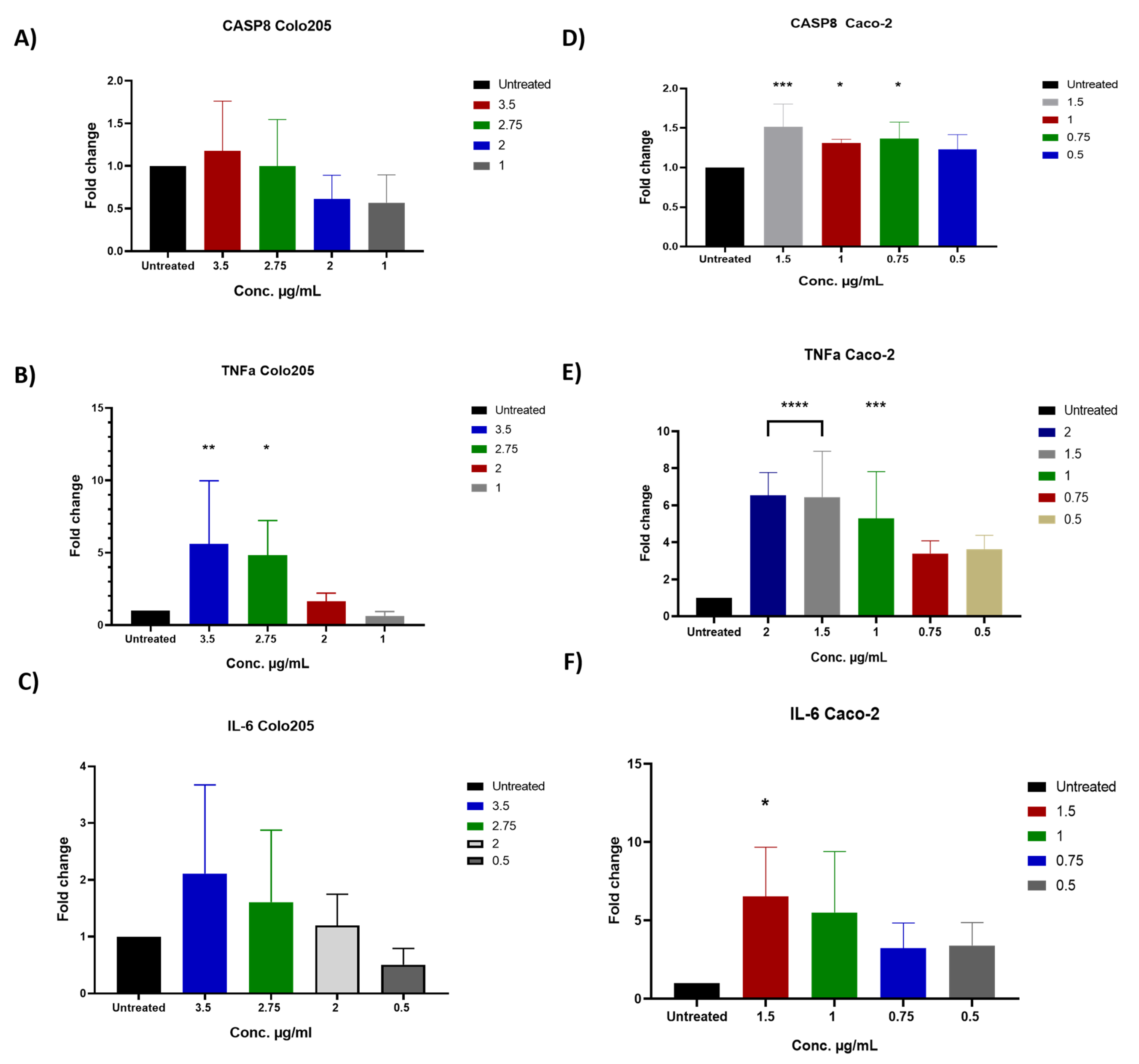
2.6. The Impact of D. maritima Bulb Extract on Gene Expression in Colon Cancer Cell Lines
3. Discussion
4. Materials and Methods
4.1. Plant Collection, Classification, and Extraction
4.2. Identification and Characterization of D. maritima Bulb Extract by Gas Chromatography-Mass Spectrometry (GC-MS)
4.3. Drugs Preparation
4.4. Cell Culture
4.5. Cell Viability and Proliferation Assay
4.6. Apoptosis Assay
4.7. Total Reactive Oxygen Species Measurement
4.8. Mitochondrial Membrane Potential (ΔΨm)
4.9. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Amplification
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Blackburn, E.H.; Collins, K. Telomerase: An RNP enzyme synthesizes DNA. Cold Spring Harb. Perspect. Biol. 2011, 3, a003558. [Google Scholar] [CrossRef]
- Adjiri, A. DNA Mutations May Not Be the Cause of Cancer. Oncol. Ther. 2017, 5, 85–101. [Google Scholar] [CrossRef]
- Donnem, T.; Reynolds, A.R.; Kuczynski, E.A.; Gatter, K.; Vermeulen, P.B.; Kerbel, R.S.; Harris, A.L.; Pezzella, F. Non-angiogenic tumours and their influence on cancer biology. Nat. Rev. Cancer 2018, 18, 323–336. [Google Scholar] [CrossRef] [PubMed]
- van Zijl, F.; Krupitza, G.; Mikulits, W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat. Res. 2011, 728, 23–34. [Google Scholar] [CrossRef]
- Alzaghal, M. Epidemiology of Colorectal Cancer in Jordan, From 2003 to 2012. J. Glob. Oncol. 2018, 4, 32s. [Google Scholar] [CrossRef]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef]
- Habtemariam, S.; Lentini, G. Plant-derived anticancer agents: Lessons from the pharmacology of geniposide and its aglycone, genipin. Biomedicines 2018, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Hrncic, M.K.; Skerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Naghibi, F.; Khalaj, A.; Mosaddegh, M.; Malekmohamadi, M.; Hamzeloo-Moghadam, M. Cytotoxic activity evaluation of some medicinal plants, selected from Iranian traditional medicine Pharmacopoeia to treat cancer and related disorders. J. Ethnopharmacol. 2014, 155, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.; Shaala, L.A.; Alshali, K.Z.; Youssef, D.T. Urgineaglyceride A: A new monoacylglycerol from the Egyptian Drimia maritima bulbs. Nat. Prod. Res. 2014, 28, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Nejatbakhsh, F.; Karegar-Borzi, H.; Amin, G.; Eslaminejad, A.; Hosseini, M.; Bozorgi, M.; Gharabaghi, M.A. Squill Oxymel, a traditional formulation from Drimia Maritima (L.) Stearn, as an add-on treatment in patients with moderate to severe persistent asthma: A pilot, triple-blind, randomized clinical trial. J. Ethnopharmacol. 2017, 196, 186–192. [Google Scholar] [CrossRef]
- Obeidat, M.; Sharab, A. Antimicrobial and anticancer activities of extracts from Urginea maritima fruits. Afr. J. Tradit. Complement. Altern. Med. 2018, 15, 74–84. [Google Scholar] [CrossRef]
- Rhimi, W.; Salem, I.B.; Camarda, A.; Saidi, M.; Boulila, A.; Otranto, D.; Cafarchia, C. Chemical characterization and acaricidal activity of Drimia maritima (L) bulbs and Dittrichia viscosa leaves against Dermanyssus gallinae. Vet. Parasitol. 2019, 268, 61–66. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Burman, R.; Mansour, A.; Turki, Z.; Boulos, L.; Gullbo, J.; Goransson, U. The traditional medical uses and cytotoxic activities of sixty-one Egyptian plants: Discovery of an active cardiac glycoside from Urginea maritima. J. Ethnopharmacol. 2013, 145, 746–757. [Google Scholar] [CrossRef]
- Laka, K.; Mapheto, K.B.F.; Mbita, Z. Selective in vitro cytotoxicity effect of Drimia calcarata bulb extracts against p53 mutant HT-29 and p53 wild-type Caco-2 colorectal cancer cells through STAT5B regulation. Toxicol. Rep. 2021, 8, 1265–1279. [Google Scholar] [CrossRef] [PubMed]
- Berges, R.; Denicolai, E.; Tchoghandjian, A.; Baeza-Kallee, N.; Honore, S.; Figarella-Branger, D.; Braguer, D. Proscillaridin A exerts anti-tumor effects through GSK3beta activation and alteration of microtubule dynamics in glioblastoma. Cell Death Dis. 2018, 9, 984. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Z.; Fan, X.; Duan, F.; Jiang, Z.; Pan, H.; Luo, L.; Zhou, Y.; Li, Y.; Yao, Y.J.; Yao, X.J.; et al. Proscillaridin A induces apoptosis and suppresses non-small-cell lung cancer tumor growth via calcium-induced DR4 upregulation. Cell Death Dis. 2018, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- Hamzeloo-Moghadam, M.; Aghaei, M.; Abdolmohammadi, M.H.; Khalaj, A.; Fallahian, F. Cytotoxic effect of Drimia maritima bulb extract and induction of mitochondrial apoptotic signaling in human breast cancer cells, MCF-7 and MDA-MB-468. OncoTargets Ther. 2018, 11, 7669–7677. [Google Scholar] [CrossRef]
- Kaczanowski, S. Apoptosis: Its origin, history, maintenance and the medical implications for cancer and aging. Phys. Biol. 2016, 13, 031001. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Banerjee, V.; Czinn, S.; Blanchard, T. Increased reactive oxygen species levels cause ER stress and cytotoxicity in andrographolide treated colon cancer cells. Oncotarget 2017, 8, 26142–26153. [Google Scholar] [CrossRef]
- Kuczler, M.D.; Olseen, A.M.; Pienta, K.J.; Amend, S.R. ROS-induced cell cycle arrest as a mechanism of resistance in polyaneuploid cancer cells (PACCs). Prog. Biophys. Mol. Biol. 2021, 165, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, A.; Guler, E.M. Curcumin induce DNA damage and apoptosis through generation of reactive oxygen species and reducing mitochondrial membrane potential in melanoma cancer cells. Cell. Mol. Biol. 2017, 63, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Qiu, W.; Dudgeon, C.; Liu, H.; Huang, C.; Zambetti, G.P.; Yu, J.; Zhang, L. PUMA is directly activated by NF-kappaB and contributes to TNF-alpha-induced apoptosis. Cell Death Differ. 2009, 16, 1192–1202. [Google Scholar] [CrossRef]
- Rath, P.C.; Aggarwal, B.B. TNF-induced signaling in apoptosis. J. Clin. Immunol. 1999, 19, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Du, F.; Wang, X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell 2008, 133, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.R.T.; Aissa, A.F.; Ribeiro, D.L.; Hernandes, L.C.; Machado, C.S.; Bianchi, M.L.P.; Sampaio, S.V.; Antunes, L.M.G. The toxin BjussuLAAO-II induces oxidative stress and DNA damage, upregulates the inflammatory cytokine genes TNF and IL6, and downregulates the apoptotic-related genes BAX, BCL2 and RELA in human Caco-2 cells. Int. J. Biol. Macromol. 2018, 109, 212–219. [Google Scholar] [CrossRef]
- Yaseen, M.M.; Abuharfeil, N.M.; Darmani, H. The impact of MDSCs on the efficacy of preventive and therapeutic HIV vaccines. Cell. Immunol. 2021, 369, 104440. [Google Scholar] [CrossRef]
- Knittel, D.N.; Stintzing, F.C.; Kammerer, D.R. Simultaneous determination of bufadienolides and phenolic compounds in sea squill (Drimia maritima (L.) Stearn) by HPLC-DAD-MS n as a means to differentiate individual plant parts and developmental stages. Anal. Bioanal. Chem. 2014, 406, 6035–6050. [Google Scholar] [CrossRef]
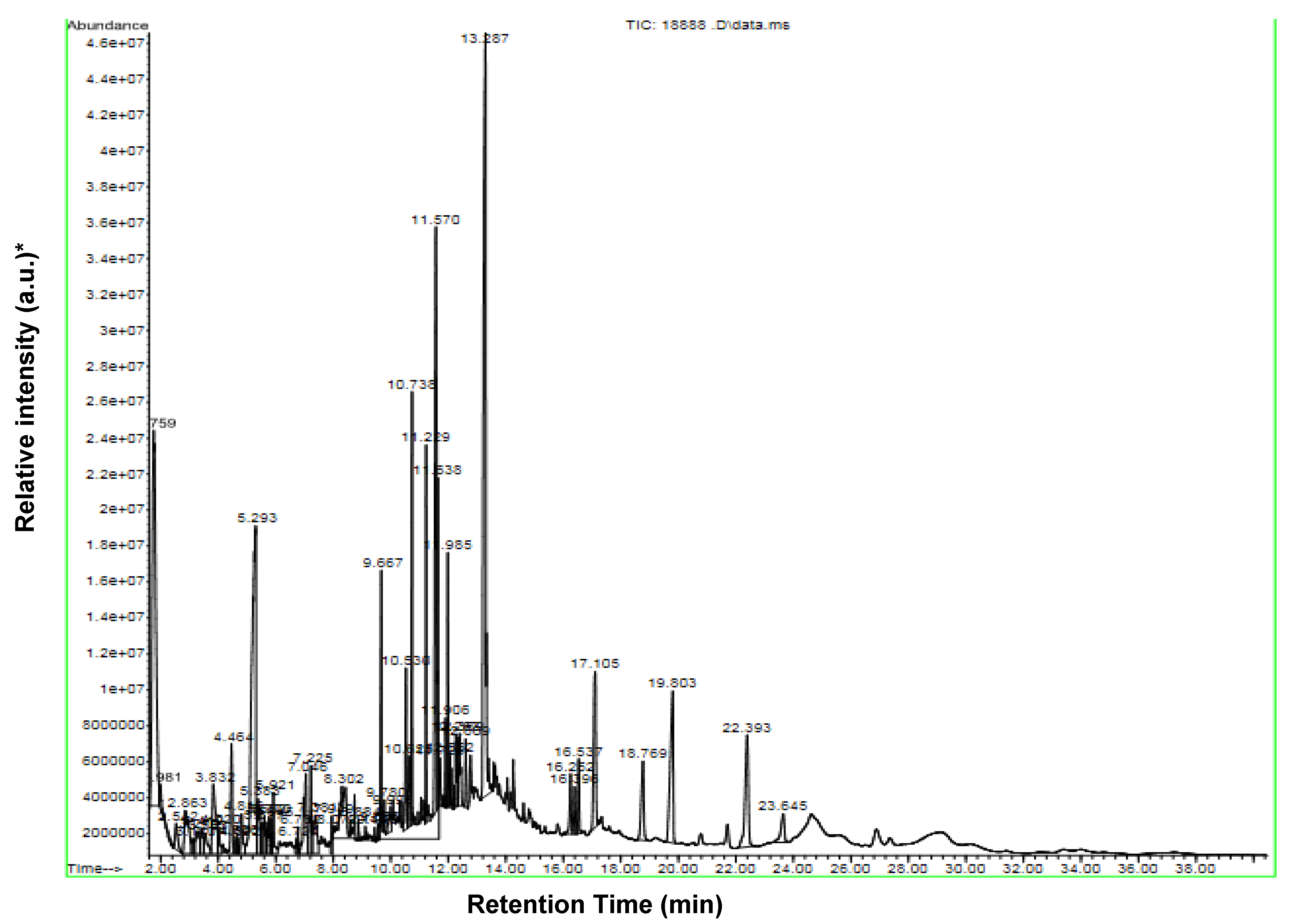

| No. | Component | Cas # | Content (%) |
|---|---|---|---|
| 1 | 2,3-Butanediol | 513-85-9 | 10.454 |
| 2 | Furancarboxaldehyde | 98-01-1 | 0.28 |
| 3 | Isobutanoic acid | 79-31-2 | 0.544 |
| 4 | 2-Furancarboxaldehyde, 5-methyl | 620-02-0 | 1.389 |
| 5 | Erythritol | 149-32-6 | 0.267 |
| 6 | Oxetane, 3,3-dimethyl | 6921-35-3 | 0.626 |
| 7 | Limonene | 138-86-3 | 0.449 |
| 8 | Methyl-3-furanthiol | 28588-74-1 | 0.523 |
| 9 | 2H-Pyrazole-3-carbohydrazide | 9-64-26275 | 1.82 |
| 10 | Butanedioic acid, monomethyl ester | 3878-55-5 | 0.379 |
| 11 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl | 28564-83-2 | 1.333 |
| 12 | (E,5S)-3,5-dimethylhept-3-en-1-yne | 997029-22-6 | 0.219 |
| 13 | 2-Furancarboxaldehyde, 5-(chloromethyl)( | 1623-88-7 | 0.142 |
| 14 | 4H-Pyran-4-one, 3,5-dihydroxy-2-methyl | 1073-96-7 | 0.666 |
| 15 | 5-Hydroxymethylfurfural | 67-47-0 | 9.857 |
| 16 | α-D-Glucopyranoside, O-α-d-glucopyranosyl-(1.fwdarw.3)-β-d-fructofuranosyl | 597-12-6 | 0.843 |
| 17 | α-D-Glucopyranoside, O-α-d-glucopyranosyl-(1.fwdarw.3)-β-d-fructofuranosyl | 597-12-6 | 0.482 |
| 18 | 3-Methoxybenzyl alcohol | 6971-51-3 | 0.538 |
| 19 | 5-Acetoxymethyl-2-furaldehyde | 10551-58-3 | 0.449 |
| 20 | 5-Hydroxymethylfurfural | 67-47-0 | 0.335 |
| 21 | Glutaric acid, 2-naphthyl tridecyl ester | 998725-55-8 | 0.56 |
| 22 | 8-Oxabicyclo[5.1.0]oct-5-en-2-ol, 1,4,4-trimethyl | 58795-43-0 | 0.14 |
| 23 | Cycloheptasiloxane, tetradecamethyl | 107-50-6 | 0.16 |
| 24 | Ethyl hydrogen succinate | 1070-34-4 | 1.679 |
| 25 | Phenol, 2,4-bis(1,1-dimethylethyl) | 96-76-4 | 1.029 |
| 26 | β-D-Glucopyranose, 1,6-anhydro | 498-07-7 | 1.072 |
| 27 | α-D-Glucopyranoside, O-α-d-glucopyranosyl-(1.fwdarw.3)-β-d-fructofuranosyl | 597-12-6 | 0.217 |
| 28 | 6Methoxy-2-amido-5,6-dihydrothiazolo[2,3-c]-1,2,4-triazole | 997204-67-5 | 3.361 |
| 29 | Myristic acid | 544-63-8 | 0.155 |
| 30 | Ethyl hydrogen succinate | 1070-34-4 | 0.339 |
| 31 | Hexadecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | 761-35-3 | 0.067 |
| 32 | Palmitoleic acid | 373-49-9 | 0.063 |
| 33 | n-Hexadecanoic acid | 57-10-3 | 0.105 |
| 34 | Hexadecanoic acid, ethyl ester | 628-97-7 | 8.057 |
| 35 | Heptadecanoic acid | 506-12-7 | 0.159 |
| 36 | Octadecanoic acid | 57-11-4 | 0.417 |
| 37 | Octadecenoic acid (Z) 9 | 112-80-1 | 1.578 |
| 38 | Octadecanoic acid | 57-11-4 | 0.73 |
| 39 | Hexadecanamide | 629-54-9 | 2.309 |
| 40 | Stearic acid, 2-hydroxy-1-methylpropyl ester | 14251-39-9 | 1.497 |
| 41 | 9-Octadecenamide, (Z) | 301-02-0 | 8.142 |
| 42 | Octadecanamide | 124-26-5 | 1.513 |
| 43 | (Z)-(S)-Octadec-9-en-11-olide | 997490-85-5 | 3.409 |
| 44 | n-Propyl 9-octadecenoate | 997641-34-3 | 0.439 |
| 45 | Stearic acid, 2-hydroxy-1-methylpropyl ester | 14251-39-9 | 1.569 |
| 46 | Bis(2-ethylhexyl) phthalate | 117-81-7 | 0.499 |
| 47 | Elaidamide | 301-02-0 | 0.475 |
| 48 | Ethyl iso-allocholate | 112-84-5 | 1.244 |
| 49 | 13-Docosenamide, (Z) | 112-84-5 | 0.545 |
| 50 | Campesterol | 474-62-4 | 13.874 |
| 51 | 6,12-dimethoxy-8-methyl-5,8,9,13-tetrahydro-7H-cyclohept[b]anthracene-5,13-dione | 997713-65-8 | 0.723 |
| 52 | Stigmasterol | 83-48-7 | 0.491 |
| 53 | β-Sitosterol | 83-46-5 | 0.735 |
| 54 | Bufa-20,22-dienolide, 14-hydroxy-3-oxo-, (5β) | 4029-65-6 | 2.642 |
| 55 | Proscillaridin | 466-06-8 | 4.956 |
| 56 | 4-(2,4-Dimethyl-phenyl)-1,7-dimethyl-4-azatricyclo[5.2.1.0(2,6)]decane-3,5,8-trione | 997597-94-2 | 2.78 |
| 57 | Bufa-20,22-dienolide, 3-(acetyloxy)-14,15-epoxy-5-hydroxy-, (3β,5β,15β) | 4029-68-9 | 0.766 |
| Cell Line | Organism | Tissue | Morphology | Culture Properties | Mutant Gene | Chemoresistance |
|---|---|---|---|---|---|---|
| COLO-205 (ATCC® CCL-222™) * | Homo sapiens, human | Colon | Epithelial | Mixed; adherent and suspension | APC, BRAF SMAD4, TP53 | Cisplatin |
| Caco-2 (ATCC® HTB-37™) * | Homo sapiens, human | Colon | Epithelial | Adherent | APC, SMAD4 TP53 | 5-fluorouracil |
| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| CASP8 | CTG CTG GGG ATG GCC ACT GTG | TCG CCT CGA GGA CAT CGC TCT C |
| TNF-a | GTC AAC CTC CTC TCT GCC AT | CCA AAG TAG ACC TGC CCA GA |
| IL-6 | TTC CAA AGA TGT AGC CGC CC | ACC AGG CAA GTC TCC TCA TT |
| GAPDH | CCT GTT CGA CAG TCA GCC G | CGA CCA AAT CCG TTG ACT CC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Abdallat, K.; Obeidat, M.; Ababneh, N.A.; Zalloum, S.; Al Hadidi, S.; Al-Abdallat, Y.; Zihlif, M.; Awidi, A. Phytochemical Analysis and Anticancer Properties of Drimia maritima Bulb Extracts on Colorectal Cancer Cells. Molecules 2023, 28, 1215. https://doi.org/10.3390/molecules28031215
Al-Abdallat K, Obeidat M, Ababneh NA, Zalloum S, Al Hadidi S, Al-Abdallat Y, Zihlif M, Awidi A. Phytochemical Analysis and Anticancer Properties of Drimia maritima Bulb Extracts on Colorectal Cancer Cells. Molecules. 2023; 28(3):1215. https://doi.org/10.3390/molecules28031215
Chicago/Turabian StyleAl-Abdallat, Khairallah, Maher Obeidat, Nidaa A. Ababneh, Suzan Zalloum, Sabal Al Hadidi, Yahya Al-Abdallat, Malek Zihlif, and Abdalla Awidi. 2023. "Phytochemical Analysis and Anticancer Properties of Drimia maritima Bulb Extracts on Colorectal Cancer Cells" Molecules 28, no. 3: 1215. https://doi.org/10.3390/molecules28031215
APA StyleAl-Abdallat, K., Obeidat, M., Ababneh, N. A., Zalloum, S., Al Hadidi, S., Al-Abdallat, Y., Zihlif, M., & Awidi, A. (2023). Phytochemical Analysis and Anticancer Properties of Drimia maritima Bulb Extracts on Colorectal Cancer Cells. Molecules, 28(3), 1215. https://doi.org/10.3390/molecules28031215








