Bioactive Natural Pigments’ Extraction, Isolation, and Stability in Food Applications
Abstract
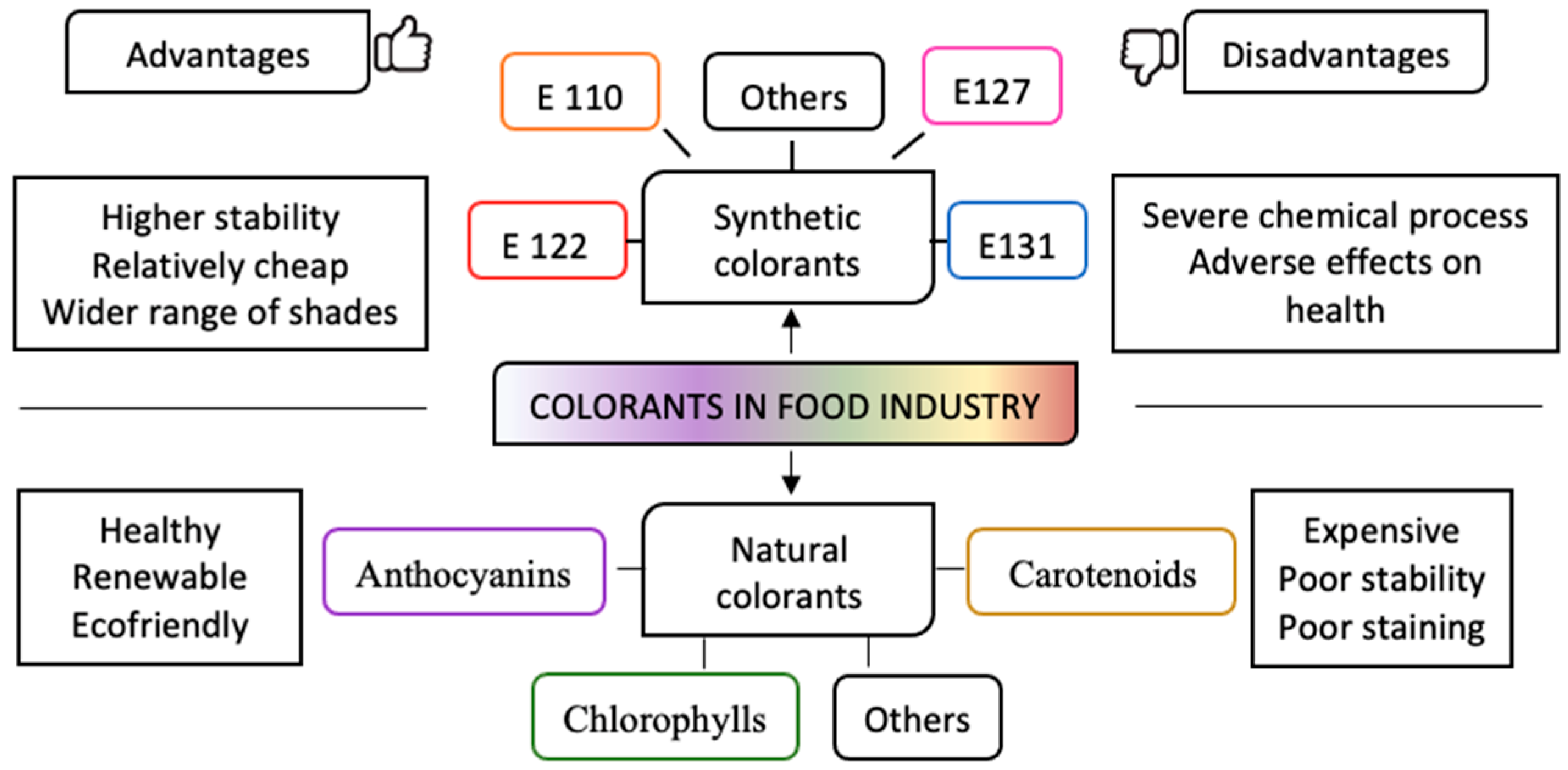
1. Importance of Natural Colorant Application in the Food Industry
2. Molecular Structures of Natural Origin with Colorant Properties
3. Extraction and Purification of Plant-Based Extracts
3.1. Anthocyanins
| Plant Matrix | Extraction Aaproach | Solvent | Extraction Conditions | Anthocyanin Recovery Yield | Ref. |
|---|---|---|---|---|---|
| Purple sweet potatoes (Ipomoea batatas L.) | Conventional solvent extraction | Ethanol 80%; HCl 0.1% (v/v) | T (°C): 60 t (min): 90 | 217.58 mg·(100 g)−1 Cyanidin-3-O-glucoside DW | [105] |
| Ultrasound-assisted extraction | Ethanol 90% (v/v); HCl 0.1% (v/v) | T (°C): 50 t (min): 45 Power (W): 200 | 229.41 mg·(100 g)−1 Cyanidin-3-O-glucoside DW | ||
| Accelerated solvent extraction | Ethanol 80% v/v; HCl 0.1% (v/v) | T (°C): 90 Static time (min): 15 Static cycle: 2 | 244.07 mg·(100 g)−1 Cyanidin-3-O-glucoside DW | ||
| Blackberries (Rubus glaucus Beneth) | Cold extraction | Methanol; C6H8O9 1% | t (h): 72 | 1.478 g·kg−1 Cyanidin-3-O-glucoside | [80] |
| Purple corncob (Zea mays L.) | Conventional solvent extraction | Ethanol 20%, pH of 2 | T (°C): 25, 60, 75, and 90 t (min): 30, 60, 120, and 240 The best extraction conditions (75 °C and 240 min) | Values between 11.567 and 37.127 mg·g−1 of purple corncob Total anthocyanins | [106] |
| Eggplant (Solanum melongena L.) | Heat solvent extraction | Ethanol 50% v/v; orthophosphoric acid 1% | T (°C): 305 t (h): 4 | 62 mg·(100 g)−1 in eggplant peel Total anthocyanins | [91] |
| Haskap berry (Lonicera caerulea L.) pulp | Conventional solvent extraction | Methanol/water 80:20 (v:v); formic acid, 0.02 mL | T (°C): 35 t (min): 20 | 38.3% Total anthocyanins | [107] |
| Supercritical carbon dioxide (scCO2) | Water | The highest total anthocyanin (TA) yield was achieved at 45 MPa, 65 °C, and 5.4 g water to 3.2 g berry pulp paste, 15 min static and 20 min dynamic time | 52.7% Total anthocyanins | ||
| Haskap berry (L. caerulea) pulp | Juice extraction | Water | Two-step press process followed by osmotic treatment | 24.58 mg Cyanidin-3-glucoside/g DW | [108] |
| One press and osmotic treatment | 32.24 mg Cyanidin-3-glucoside/g DW | ||||
| Haskap berry (L. caerulea) pulp | Conventional solvent extraction | Ethanol/water 80:20 (v:v); trifluoroacetic acid 0.1% | Double extraction of 1 h each | 97.9 mg·g−1 ext. Total anthocyanins | [25] |
| Blueberries (Vaccinium sp.), O’Neal variety | Solid–liquid extraction | Ethanol; citric acid 1% | T (°C): 36 T (h): 2 | 879.0 mg·(100 mL)−1 Cyanidin-3-glucoside | [82] |
| Mulberry (Morus alba L.) wine residues | Ultrasonic-assisted enzymatic extraction | Water acidified to a pH of 3.5 Enzyme dosage: 0.22% | T (°C): 52 Power (W): 315 t (min): 94 | 5.98 mg·g−1 Total anthocyanins | [95] |
| Açai (Euterpe oleracea Mart.) | Pressurized Liquid Extraction | Methanol/water 43% | T (°C): 81 200 atm 60 s purge pH: 7.00 50% flushing | 5.76 mg·g−1 açai Total anthocyanins | [97] |
| Blueberry (Vaccinium myrtillus L.) peels | Microwave extractions | Natural deep eutectic solvent (Choline chloride:lactic acid) | T (°C): 60 T (min): 15 | 25.83 mg·g−1 matrix Total anthocyanins | [94] |
| Ultrasound-assisted extractions | Natural deep eutectic solvent (Choline chloride:lactic acid) | 30 min of sonication Power (W): 500 | 21.18 mg·g−1 matrix Total anthocyanins | ||
| Residues of red grape (Vitis vinifera L.) skins | Ohmic heating effect | Water | I. T (°C): 40; t (min): 20 II. T (°C): 40 a 100; t (s): 20 Electric field: 80 and 16 V/cm Frequency (kHz): 25 | 1349 μg·g−1 | [109] |
3.2. Chlorophylls
3.3. Carotenoids
| Plant Matrix | Extraction Approach | Solvent | Conditions | Carotenoid Recovery Yield | Reference |
|---|---|---|---|---|---|
| Pericarp of tamarillo (Cyphomandra betacea Sendt var. roja) | Conventional solvent extraction | n-Hexane/petroleum ether 50:50% | t (h): 48 Absence of light | 0.051 g CT/g pericarp | [132] |
| Tomato (Solanum lycopersicum L.) byproducts | Soxhlet | Ethanol | t (h): 5 | 0.703 mg/g lycopene 0.034 mg/g β-carotene extract | [133] |
| Peach palm (Bactris gasipaes Kunth) fruit peel | Ultrasound-assisted extraction | Soybean oil | T (°C): 48 t (min): 28 Solid–solvent ratio (g/mL): 0.0037 | 151.50 mg/100 g of dry peel Carotenoid content | [126] |
| Enzyme-treated carrot (Daucus carota L.) pomace | Ultrasonication | Flaxseed oil (green solvent) | Cycle: 45% Probe radius: 13 mm Power (W): 750 t (min): 12 | 21.67 ± 0.40 μg/g Total carotenoid content | [128] |
| High-shear dispersion | Flaxseed oil (green solvent) | 20,000 rpm t (min): 12 | 82.66 ± 0.06 μg/g Total carotenoid content | ||
| Passion fruit cortex (Passiflora edulis f. flavicarpa) | Immersion | Ethanol 90%, acidified with citric acid at 0.03% | T (°C): 29 t (h): 2 500 RPM No light | 113.08 ± 8.84 μg of β-carotene/100 g | [130] |
| Thermostatic bath | Ethanol 90%, acidified with citric acid at 0.03% | T (°C): 60 t (h): 24 | 10.34 ± 5.18 μg of β-carotene/100 g | ||
| Soxhlet | Raw material–solvent ratio: 1:40 | t (h): 2 | 1037.99 ± 48.70 μg of β-carotene/100 g | ||
| Cantaloupe melon fruits (Cucumis melo L.) | Ultrasound-assisted extraction | Hexane/acetone 80:20 | Amplitude: 100% t (min): 10 | 124.61 ± 3.82 μg/g | [134] |
| Canistel (Pouteria campechiana Kunth) Baehni.) fruits | Agitation Extraction | n-Hexane Dichloromethane n-hexane/dichloromethane (1:1) Ratios of solvent to sample of 15:1 | T (°C): 40 200 rpm t (min): 30 After 6000 rpm t (min): 10 | 5.17 ± 0.08 g β-carotene equivalent per 100 g dry weight | [135] |
| n-Hexane Dichloromethane n-hexane/dichloromethane (1:1) Ratios of solvent to sample of 30:1 | T (°C): 40 200 rpm t (min): 30 After 6000 rpm t (min): 10 | 3.12 ± 0.01 g β-carotene equivalent per 100 g dry weight | |||
| Carrots (Daucus carota L.) peels | Supercritical CO2 | Ethanol 15.5% | T (°C): 59 p (bar): 349 | 86.1% of carotenoid recovery | [136] |
| Carrot (Daucus carota L.) juice processing waste | Microwave-assisted extraction | Oil (8.06:1 g/g) | Power (W): 165 t (min): 9.39 | 77.48% | [129] |
| Conventional extraction | Oil (20:1 g/g) | T (°C): 65 t (min): 30 and 180 | 50% and 87% of carotenoid recovery | ||
| Mango (Mangifera indica L. var. Sugar) peel | Supercritical fluid extraction | Ethanol 15% w/w | 25.0 MPa T (°C): 60 | 1.9 mg all-trans-β-carotene equivalent g−1 dried mango peel | [131] |
4. Stabilization of Natural Colorant Formulations
5. Application of Colorant Formulations in Food
6. Conclusion and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lakshmi, C. Food Coloring: The Natural Way. Res. J. Chem. Sci. 2014, 4, 2231–2606. [Google Scholar]
- Mathias-Rettig, K.; Ah-Hen, K. El color en los alimentos un criterio de calidad medible. AgroSur 2014, 42, 39–48. [Google Scholar] [CrossRef]
- Lessa Constant, P.B.; Stringheta, P.C.; Sandi, D. Corantes alimentícios. Bol. Ceppa 2002, 20, 203–220. [Google Scholar] [CrossRef]
- European Food Safety Authority. Food Colours. 2013. Available online: https://www.efsa.europa.eu/en/topics/topic/food-colours (accessed on 25 November 2022).
- Sánchez, R. La química del color en los alimentos. Química Viva 2013, 12, 234–246. [Google Scholar]
- Sharma, V.; McKone, H.T.; Markow, P.G. A global perspective on the history, use, and identification of synthetic food dyes. J. Chem. Educ. 2011, 88, 24–28. [Google Scholar] [CrossRef]
- Feketea, G.; Tsabouri, S. Common food colorants and allergic reactions in children: Myth or reality? Food Chem. 2017, 230, 578–588. [Google Scholar] [CrossRef]
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: A randomized, double-blinded, placebo-controlled trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health safety issues of synthetic food colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Masone, D.; Chanforan, C. Study on the interaction of artificial and natural food colorants with human serum albumin: A computational point of view. Comput. Biol. Chem. 2015, 56, 152–158. [Google Scholar] [CrossRef]
- Streit, N.; Queiroz, M.; Jacob, E.; Jacob-Lopes, E.; Queiroz, M. Producción de pigmentos naturales (clorofila-a) en biorrefinerias agroindustriales. Ver. Cienc. Tecnol. 2015, 8, 27–34. [Google Scholar] [CrossRef]
- Ibáñez, F.; Torres, P.; Irigoyen, A. Aditivos Alimentario—Área de Nutrición y Bromatologia; Universidad Pública de Nava rra: Pamplona, Spain, 2003. [Google Scholar]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- The Code of Federal Regulations (CFR). Title 21-Food and Drugs Chapter I—Food and Drug Administration, Department of Health and Human Services Subchapter A-General, Subpart A—General Provisions, Part 70 Color Additive; The Code of Federal Regulations (CFR): Washington, DC, USA, 2023. [Google Scholar]
- The Code of Federal Regulations (CFR). Title 21-Food and Drugs Chapter I—Food and Drug Administration, Department of Health and Human Services Subchapter A—General Subpart A-General, Part 80 Color Additive Certification; The Code of Federal Regulations (CFR): Washington, DC, USA, 2023. [Google Scholar]
- Gonzalez De Mejia, E.; Zhang, Q.; Penta, K.; Eroglu, A.; Lila, M.A. The Colors of Health: Chemistry, Bioactivity, and Market Demand for Colorful Foods and Natural Food Sources of Colorants. Annu. Rev. Food Sci. Technol. 2020, 11, 145–182. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A. Carotenoids and other pigments as natural colorants. Pure Appl. Chem. 2006, 78, 1477–1491. [Google Scholar] [CrossRef]
- Pereira, C.; Dias, M.I.; Pinela, J.; Roriz, C.L.; Ferreira, I.C.F.R.; Barros, L. Betalains. In Nutraceutical and Functional Food Components; Elsevier: Amsterdam, The Netherlands, 2022; pp. 461–507. [Google Scholar]
- El-Refai, A.; Shalaby, M.; El-Gammal, R.; Motawea, E.; Ali, A. Effect of Adding of Moringa and Turmeric as Nutritive Food Colorants on Chemical, Physical and Rheological Properties of Pan Bread. J. Food Dairy Sci. 2021, 12, 225–233. [Google Scholar] [CrossRef]
- Matufi, F.; Choopani, A. Spirulina, food of past, present and future. Health Biotechnol. Biopharma 2020, 3, 1–20. [Google Scholar]
- Manzoor, M.; Singh, J.; Gani, A.; Noor, N. Valorization of natural colors as health-promoting bioactive compounds: Phytochemical profile, extraction techniques, and pharmacological perspectives. Food Chem. 2021, 362, 130141. [Google Scholar] [CrossRef]
- Carpio Jiménez C del Flores, C.S.; Giusti, M. Caracterización de las antocianinas de los frutos de Berberis boliviana Lechler. Rev. Soc. Química Perú 2009, 75, 76–86. [Google Scholar]
- Aguilera-Otíz, M.; Reza-Vargas, M.D.C.; Chew-Madinaveita, R.G.; Meza-Velázquez, J.A. Propiedades funcionales de las antocianinas. Biotecnia 2011, 13, 16–22. [Google Scholar] [CrossRef]
- Molina, A.K.; Vega, E.N.; Pereira, C.; Dias, M.I.; Heleno, S.A.; Rodrigues, P.; Fernandes, I.P.; Barreiro, M.F.; Kostic, M.; Sokovic, M.; et al. Promising antioxidant and antimicrobial food colourants from Lonicera caerulea L. var. Kamtschatica. Antioxidants 2019, 8, 394. [Google Scholar] [CrossRef]
- Vega, E.N.; Molina, A.K.; Pereira, C.; Dias, M.I.; Heleno, S.A.; Rodrigues, P.; Fernandes, I.P.; Barreiro, M.F.; Kostić, M.; Soković, M.; et al. Anthocyanins from Rubus fruticosus L. and Morus nigra L. applied as food colorants: A Natural Alternative. Plants 2021, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.J.; Xavier, M.F.; Quadri, M.G.N.; Quadri, M.B. Antocianinas: Uma breve revisão das características estruturais e da estabilidade. Rev. Bras. Agro Ciências 2007, 13, 291–297. [Google Scholar]
- Ćujić, N.; Savikin, K.; Miloradovic, Z.; Milan, I.; Vajic, U.J.; Karanovic, D.; Grujic-Milanovic, J.; Jovovic, D.; Mihailovic-Stanojevic, N. Characterization of dried chokeberry fruit extract and its chronic eects on blood pressure and oxidative stress in spontaneously hypertensive rats. J. Funct. Foods 2018, 44, 330–339. [Google Scholar] [CrossRef]
- Neves, D.; Valentão, P.; Bernardo, J.; Oliveira, M.C.; Ferreira, J.M.G.; Pereira, D.M.; Andrade, P.B.; Videira, R.A. A new insight on elderberry anthocyanins bioactivity: Modulation of mitochondrial redox chain functionality and cell redox state. J. Funct. Foods 2019, 56, 145–155. [Google Scholar] [CrossRef]
- Fernández, J.; García, L.; Monte, J.; Villar, C.J.; Lombo, F. Functional anthocyanin-rich sausages diminish colorectal cancer in an animal model and reduce pro-inflammatory bacteria in the intestinal microbiota. Genes 2018, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, T.; Li, N.; Wang, X.; Chen, G.; Lyu, X. Bilberry anthocyanin extract promotes intestinal barrier function and inhibits digestive enzyme activity by regulating the gut microbiota in aging rats. Food Funct. 2019, 10, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef]
- Castañeda-Sánchez, A.; Guerrero-Beltrán, J. Pigmentos en frutas y hortalizas rojas: Antocianinas. Temas Sel. Ing. Aliment. 2015, 9, 25–33. [Google Scholar]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Amogne, N.Y.; Ayele, D.W.; Tsigie, Y.A. Recent advances in anthocyanin dyes extracted from plants for dye sensitized solar cell. Mater. Renew. Sustain. Energy 2020, 9, 23. [Google Scholar]
- Cai, D.; Li, X.; Chen, J.; Jiang, X.; Ma, X.; Sun, J.; Tian, L.; Vidyarthi, S.K.; Xu, J.; Pan, Z.; et al. A comprehensive review on innovative and advanced stabilization approaches of anthocyanin by modifying structure and controlling environmental factors. Food Chem. 2022, 366, 130611. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, A.; Díaz, L.; del Rio, J. Evolución de la Fisiología Vegetal en los ultimos 100 años. Eubacteria 2015, 34, 74–82. [Google Scholar]
- Gross, J. Pigments in Vegetables: Chlorophylls and Carotenoids; Springer: New York, NY, USA, 2012. [Google Scholar]
- Schoefs, B. Chlorophyll and carotenoid analysis in food products. Properties of the pigments and methods of analysis. Trends Food Sci. Technol. 2002, 13, 361–371. [Google Scholar] [CrossRef]
- Balder, H.F.; de Vogel, J.; Jansen, M.C.J.F.; Weijenberg, M.P.; Van Den Brandt, P.A.; Westenbrink, S.; Van Der Meer, R.D.; Goldbohm, R.A. Heme and chlorophyll intake and risk of colorectal cancer in the Netherlands cohort study. Cancer Epidemiol. Biomark. Prev. 2006, 15, 717–725. [Google Scholar] [CrossRef]
- Liu, M.H.; Li, Y.F.; Chen, B.H. Preparation of chlorophyll nanoemulsion from pomelo leaves and its inhibition effect on melanoma cells A375. Plants 2021, 10, 1664. [Google Scholar] [CrossRef]
- Kumar, P.R.; George, P. Antidiabetic effect of Sauropus androgynus L. leaves in alloxan induced diabetic mice. J. Pure Appl. Microbiol. 2015, 9, 2565–2570. [Google Scholar]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Green natural colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Elvira-Torales, L.I.; García-Alonso, J.; Periago-Castón, M.J. Nutritional importance of carotenoids and their effect on liver health: A review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Vicario, I.M.; Heredia, F.J. Provitamin A carotenoids and ascorbic acid contents of the different types of orange juices marketed in Spain. Food Chem. 2007, 101, 177–184. [Google Scholar] [CrossRef]
- Raju, M.; Varakumar, S.; Lakshminarayana, R.; Krishnakantha, T.P.; Baskaran, V. Carotenoid composition and vitamin A activity of medicinally important green leafy vegetables. Food Chem. 2007, 101, 1598–1605. [Google Scholar] [CrossRef]
- Böhm, F.; Tinkler, J.H.; Truscott, T.G. Carotenoids protect against cell membrane damage by the nitrogen dioxide radical. Nat. Med. 1995, 1, 98–99. [Google Scholar] [CrossRef]
- Naguib, Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T.; Mochida, K.; Kozuka, M.; Ito, Y.; Fujiwara, Y.; Hashimoto, K.; Enjo, F.; Ogata, M.; Nobukuni, Y.; Tokuda, H.; et al. Cancer chemopreventive activity of carotenoids in the fruits of red paprika Capsicum annuum L. Cancer Lett. 2001, 172, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Benson, J.; Curtin, K.; Ma, K.N.; Schaeffer, D.; Potter, J.D. Carotenoids and colon cancer. Am. J. Clin. Nutr. 2000, 71, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Snodderly, D.M. Evidence for protection against degeneration by carotenoids and antioxidant. Am. J. Clin. Nutr. 1995, 62, S1448–S1461. [Google Scholar] [CrossRef]
- Trumbo, P.R.; Ellwood, K.C. Lutein and zeaxanthin intakes and risk of age-related macular degeneration and cataracts: An evaluation using the Food and Drug Administration’s evidence-based review system for health claims. Am. J. Clin. Nutr. 2006, 84, 971–974. [Google Scholar] [CrossRef]
- Tavani, A.; la Vecchia, C. β-Carotene and risk of coronary heart disease. A review of observational and intervention studies. Biomed. Pharmacother. 1999, 53, 409–416. [Google Scholar] [CrossRef]
- Montesano, D.; Blasi, F.; Cossignani, L. Lycopene and cardiovascular Disease: An overview. Ann. Short Rep. 2019, 2, 2–4. [Google Scholar]
- Rowles, J.L.; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377. [Google Scholar] [CrossRef]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and other nutrients from haematococcus pluvialis—Multifunctional applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.M.-M.; Vicario, I.M.; Heredia, F.J. Estabilidad de los pigmentos carotenoides en los alimentos. Arch. Latinoam. Nutr. 2004, 54, 209–215. [Google Scholar]
- Yang, Y.; Zhang J liang Zhou, Q. Targets and mechanisms of dietary anthocyanins to combat hyperglycemia and hyperuricemia: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1119–1143. [Google Scholar] [CrossRef]
- Yan, F.; Dai, G.; Zheng, X. Mulberry anthocyanin extract ameliorates insulin resistance by regulating PI3K/AKT pathway in HepG2 cells and db/db mice. J. Nutr. Biochem. 2016, 36, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.S.; Lakshmi, M.J.; Sharavana, G.; Sathaiah, G.; Sreerama, Y.N.; Baskaran, V. Lactucaxanthin-a potential anti-diabetic carotenoid from lettuce (Lactuca sativa) inhibits α-amylase and α-glucosidase activity in vitro and in diabetic rats. Food Funct. 2017, 8, 1124–1131. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Teixeira, D.; De Freitas, V.; Mateus, N.; Calhau, C. Blueberry anthocyanins and pyruvic acid adducts: Anticancer properties in breast cancer cell lines. Phytother. Res. 2010, 24, 1862–1869. [Google Scholar] [CrossRef]
- Hui, C.; Bin, Y.; Xiaoping, Y.; Long, Y.; Chunye, C.; Mantian, M.; Wenhua, L. Anticancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and in vivo. Nutr. Cancer 2010, 62, 1128–1136. [Google Scholar] [CrossRef]
- Jing, P.; Bomser, J.A.; Schwartz, S.J.; He, J.; Magnuson, B.A.; Giusti, M.M. Structure-function relationships of anthocyanins from various anthocyanin-rich extracts on the inhibition of colon cancer cell growth. J. Agric. Food Chem. 2008, 56, 9391–9398. [Google Scholar] [CrossRef]
- El-Sayed, W.M.; Hussin, W.A.; Mahmoud, A.A.; Alfredan, M.A. The conyza triloba extracts with high chlorophyll content and free radical scavenging activity had anticancer activity in cell lines. Biomed. Res. Int. 2013, 2013, 945638. [Google Scholar] [CrossRef]
- Campestrini, L.H.; Melo, P.S.; Peres, L.E.P.; Calhelha, R.C.; Ferreira, I.C.F.R.; Alencar, S.M. A new variety of purple tomato as a rich source of bioactive carotenoids and its potential health benefits. Heliyon 2019, 5, e02831. [Google Scholar] [CrossRef]
- Bell, D.R.; Gochenaur, K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J. Appl. Physiol. 2006, 100, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; Di Stefano, G.; González-Paramás, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Noordin, L.; Wan Mohamad Noor, W.N.I.; Safuan, S.; Wan Ahmad, W.A.N. Therapeutic effects of anthocyanin-rich Hibiscus sabdariffa L. extract on body mass index, lipid profile and fatty liver in obese-hypercholesterolaemic rat model. Int. J. Basic Clin. Pharmacol. 2019, 9, 1. [Google Scholar] [CrossRef]
- Aizawa, K.; Inakuma, T. Dietary capsanthin, the main carotenoid in paprika (Capsicum annuum), alters plasma high-density lipoprotein-cholesterol levels and hepatic gene expression in rats. Br. J. Nutr. 2009, 102, 1760–1766. [Google Scholar] [CrossRef]
- Miyake, S.; Takahashi, N.; Sasaki, M.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Vision preservation during retinal inflammation by anthocyanin-rich bilberry extract: Cellular and molecular mechanism. Lab. Investig. 2012, 92, 102–109. [Google Scholar] [CrossRef]
- Paik, S.S.; Jeong, E.; Jung, S.W.; Ha, T.J.; Kang, S.; Sim, S.; Jeon, J.H.; Chun, M.H.; Kim, I.B. Anthocyanins from the seed coat of black soybean reduce retinal degeneration induced by N-methyl-N-nitrosourea. Exp. Eye Res 2012, 97, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bendokas, V.; Šarkinas, A.; Jasinauskienë, D.; Anisimovienë-Haimi, S.; Stanys, V.; Šikšnianas, T. Antimicrobial activity of berries extracts of four Ribes species, their phenolic content and anthocyanin composition. Folia Hortic. 2018, 30, 249–257. [Google Scholar] [CrossRef]
- Chen, H.; Yu, W.; Chen, G.; Meng, S.; Xiang, Z.; He, N. Antinociceptive and antibacterial properties of anthocyanins and flavonols from fruits of black and non-black mulberries. Molecules 2018, 23, 4. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Effect of Fruiting Body Maturity Stage on Chemical Composition and Antimicrobial Activity of Lactarius sp. Mushrooms. J. Agric. Food Chem. 2007, 55, 8766–8771. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Rodrigues, M.; Santos, A.O.; Alves, G.; Silva, L.R. Antioxidant status, antidiabetic properties and effects on Caco-2 cells of colored and non-colored enriched extracts of sweet cherry fruits. Nutrients 2018, 10, 1688. [Google Scholar] [CrossRef]
- Hiroshi, S.; Shoko, I.; Tomohiko, M.; Kazue, S.; Chiyako, S.; Taisei, K.; Shigemi, T.; Hideki, N.; Yurika, M.; Atsuko, O.; et al. Comparative Study of Biological Activity of Three Commercial Products of Sasa senanensis Rehder Leaf Extract. In Vivo 2012, 26, 259–264. [Google Scholar]
- Rizk, E.M.; El-Kady, A.T.; El-Bialy, A.R. Charactrization of carotenoids (lyco-red) extracted from tomato peels and its uses as natural colorants and antioxidants of ice cream. Ann. Agric. Sci. 2014, 59, 53–61. [Google Scholar] [CrossRef]
- Chantaro, P.; Devahastin, S.; Chiewchan, N. Production of antioxidant high dietary fiber powder from carrot peels. LWT—Food Sci. Technol. 2008, 41, 1987–1994. [Google Scholar] [CrossRef]
- Ramírez, M.; Rojas-Aguilar, N.Y.; Correa-Higuera, L.J. Obtención de un colorante natural alimentario de mora de Castilla (Rubus glaucus benth). Cienc. Desarro. 2006, 2, 115–130. [Google Scholar]
- Kechinski, C.P.; Guimarães, P.V.R.; Noreña, C.P.Z.; Tessaro, I.C.; Marczak, L.D.F. Degradation Kinetics of Anthocyanin in Blueberry Juice during Thermal Treatment. J. Food Sci. 2010, 75, 173–176. [Google Scholar] [CrossRef]
- Zapata, L.; Heredia, A.; Quinteros, C.; Melleret, A.; Clemente, G.; Cárcel, J. Optimization of cranberry anthocyanin extraction. Sci. Teach. Technol. 2014, 25, 166–192. [Google Scholar]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Favaro, M.M.A. Extração, Estabilidade e Quantificação de Antocianinas de Frutas Típicas Brasileiras para Aplicação Industrial como Corantes. Master’s Thesis, State University of Campinas, Institute of Chemistry, Campinas, Brazil, 2008. [Google Scholar]
- Tait, M.A.; Hik, D.S. Is dimethylsulfoxide a reliable solvent for extracting chlorophyll under field conditions? Photosynth. Res. 2003, 78, 87–91. [Google Scholar] [CrossRef]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef]
- Schwartz, S.J.; von Elbe, J.H. High Performance Liquid Chromatography of Plant Pigments—A Review. J. Liq. Chromatogr. 1982, 5, 43–73. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Jimenez, A.R.; Paredes-Lopez, O. Natural Pigments: Carotenoids, Anthocyanins, and Betalains—Characteristics, Biosynthesis, Processing, and Stability. Crit. Rev. Food Sci. Nutr. 2010, 40, 173–289. [Google Scholar] [CrossRef] [PubMed]
- Schiozer, A.L.; Barata, L.E.S. Estabilidade de Corantes e Pigmentos de Origem Vegetal Stability of Natural Pigments and Dyes. Rev. Fitos 2013, 3, 6–24. [Google Scholar] [CrossRef]
- Silva, S.; Morais, R.M.; Costa, E.M.; Pintado, M.E.; Calhau, C. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2015, 57, 3072–3083. [Google Scholar] [CrossRef] [PubMed]
- Heras, I.; Alvis, A.; Arrazola, G. Optimizacion del proceso de extraccion de antocianinas y evaluacion de la capacidad antioxidante de berenjena (Solana melonera L.). Inf. Tecnol. 2013, 24, 93–102. [Google Scholar] [CrossRef]
- Puertas, M.; Ríos, Y.; Rojano, B. Determinación de antocianinas mediante extracción asistida por radiación de microondas en frijol (Phaseolus vulgaris L.) de alto consumo en Antioquia. Rev. Cuba. Plantas Med. 2013, 18, 288–297. [Google Scholar]
- Flores, E. Extracción de antioxidantes de las bayas del sauco (Sambucus nigra L. subsp. peruviana) con ultrasonido, microondas, enzimas y maceración para la obtención de zumos funcionales. Inf. Tecnol. 2017, 28, 121–132. [Google Scholar] [CrossRef]
- Grillo, G.; Gunjević, V.; Radošević, K.; Redovniković, I.R.; Cravotto, G. Deep eutectic solvents and nonconventional technologies for blueberry-peel extraction: Kinetics, anthocyanin stability, and antiproliferative activity. Antioxidants 2020, 9, 1069. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, G.; Khan, M.A.; Yan, Z.; Beta, T. Ultrasonic-assisted enzymatic extraction and identification of anthocyanin components from mulberry wine residues. Food Chem 2020, 323, 126714. [Google Scholar] [CrossRef]
- Garcia-Mendoza, M.D.P.; Espinosa-Pardo, F.A.; Baseggio, A.M.; Barbero, G.F.; Maróstica Junior, M.R.; Rostagno, M.A.; Martínez, J. Extraction of phenolic compounds and anthocyanins from juçara (Euterpe edulis Mart.) residues using pressurized liquids and supercritical fluids. J. Supercrit. Fluids 2017, 119, 9–16. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Carrera, C.; Palma, M.; Álvarez, J.A.; Ayuso, J.; Barbero, G.F. Extraction of anthocyanins and total phenolic compounds from açai (euterpe oleracea mart.) using an experimental design methodology. Part 1: Pressurized liquid extraction. Agronomy 2020, 10, 183. [Google Scholar] [CrossRef]
- Swer, T.L.; Mukhim, C.; Bashir, K.; Chauhan, K. Optimization of enzyme aided extraction of anthocyanins from Prunus nepalensis L. LWT—Food Sci. Technol. 2018, 91, 382–390. [Google Scholar] [CrossRef]
- Türkyılmaz, M.; Hamzaoğlu, F.; Özkan, M. Effects of sucrose and copigment sources on the major anthocyanins isolated from sour cherries. Food Chem. 2019, 281, 242–250. [Google Scholar] [CrossRef]
- Lima, Á.S.; Oliveira BS de Shabudin, S.V.; Almeida, M.; Freire, M.G.; Bica, K. Purification of anthocyanins from grape pomace by centrifugal partition chromatography. J. Mol. Liq. 2021, 326, 115324. [Google Scholar] [CrossRef]
- Schwarz, M.; Hillebrand, S.; Habben, S.; Degenhardt, A.; Winterhalter, P. Application of high-speed countercurrent chromatography to the large-scale isolation of anthocyanins. Biochem. Eng. J. 2003, 14, 179–189. [Google Scholar] [CrossRef]
- Ferreiro-González, M.; Carrera, C.; Ruiz-Rodríguez, A.; Barbero, G.F.; Ayuso, J.; Palma, M.; Barroso, C.G. A new solid phase extraction for the determination of anthocyanins in grapes. Molecules 2014, 19, 21398–21410. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, S.L.; Petropoulos, S.A.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Fernandes, Â.; Leme, C.M.M.; Alexopoulos, A.; Santos-Buelga, C.; Ferreira, I.C.F.R.; et al. Phenolic composition and cell-based biological activities of ten coloured potato peels (Solanum tuberosum L.). Food Chem. 2021, 363, 130360. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Inês Dias, M.; Calhelha, R.C.; Alves, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Development of new bilberry (Vaccinium myrtillus L.) based snacks: Nutritional, chemical and bioactive features. Food Chem. 2021, 334, 127511. [Google Scholar] [CrossRef]
- Cai, Z.; Qu, Z.; Lan, Y.; Zhao, S.; Ma, X.; Wan, Q.; Jing, P.; Li, P. Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes. Food Chem. 2016, 197, 266–272. [Google Scholar] [CrossRef]
- Gorriti Gutierrez, A.; Arroyo Acevedo, J.; Negron Ballarte, L.; Jurado Teixeira, B.; Purizaca Llajaruna, H.; Santiagoaquise, I. Antocianinas, fenoles totales y actividad antioxidante de las corontas del maíz morado (Zea mays L.): Método de extracción. Bol. Latinoam. Caribe Plantas Med. Aromát. 2009, 8, 509–518. [Google Scholar]
- Jiao, G.; Kermanshahi pour, A. Extraction of anthocyanins from haskap berry pulp using supercritical carbon dioxide: Influence of co-solvent composition and pretreatment. LWT 2018, 98, 237–244. [Google Scholar] [CrossRef]
- Khattab, R.; Ghanem, A.; Brooks, M.S.L. Quality of dried haskap berries (Lonicera caerulea L.) as affected by prior juice extraction, osmotic treatment, and drying conditions. Dry. Technol. 2016, 35, 375–391. [Google Scholar] [CrossRef]
- Pereira, R.N.; Coelho, M.I.; Genisheva, Z.; Fernandes, J.M.; Vicente, A.A.; Pintado, M.E.; Teixeira, J.A. Using Ohmic Heating effect on grape skins as a pretreatment for anthocyanins extraction. Food Bioprod. Process. 2020, 124, 320–328. [Google Scholar] [CrossRef]
- Maestrin, A.P.J.; Neri, C.R.; de Oliveira, K.T.; Iamamoto, O.A.S.E.Y. Extração e purificação de clorofila a, da alga spirulina maxima: Um experimento para os cursos de química. Quim. Nova 2009, 32, 1670–1672. [Google Scholar] [CrossRef]
- Barbieri Junior, É.; Pereyra Rossiello, R.O.; Frota Morenz, M.J.; Cristiane Ribeiro, R. Comparação de métodos diretos de extração e quantificação dos teores de clorofilasem folhas do capim-Tifton 85. Ciência Rural. 2010, 40, 633–636. [Google Scholar] [CrossRef]
- Smith, K.M.; Goff, D.A.; Simpson, D.J. Meso Substitution of Chlorophyll Derivatives: Direct Route for Transformation of Bacteriopheophorbides d into Bacteriopheophorbides c. J. Am. Chem. Soc. 1985, 107, 4946–4954. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- De, K.I.; Correia, O.; de Andrade, J.A.M.; Nascimento, R.B.T.; Da Silva, E.C. Comparação de métodos de extração de clorofila em folhas de Clitoria fairchildiana (Fabaceae) e Gossypium sp. (Malvaceae). In Proceedings of the 64th National Congress of Botany, Belo Horizonte, Brazil, 10–15 November 2013. [Google Scholar]
- Müller, A.H.; Gough, S.P.; Bollivar, D.W.; Meldal, M.; Willows, R.; Hansson, M. Methods for the preparation of chlorophyllide a: An intermediate of the chlorophyll biosynthetic pathway. Anal. Biochem. 2011, 419, 271–276. [Google Scholar] [CrossRef]
- Santos, R.P.; Ferreira Da Cruz, A.C.; Iarema, L.; Kuki, K.N.; Campos Otoni, W. Protocolo para extração de pigmentos foliares em porta-enxertos de videira micropropagados. Rev. Ceres 2008, 55, 356–364. [Google Scholar]
- Scopel, W.; Zimmer Barbosa, J.; Luis Vieira, M. Extração de pigmentos foliares em plantas de canola. Unoesc Ciência—ACET 2011, 2, 87–94. [Google Scholar]
- Barnes, D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of dmso for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Molina, A.K.; Gomes, L.C.; Prieto, M.A.; Ferreira, I.C.F.R.; Pereira, C.; Dias, M.I.; Barros, L. Extraction of chlorophylls from Daucus carota L. and Solanum lycopersicum var. cerasiforme crop by-products. Food Chem. Adv. 2022, 1, 100048. [Google Scholar] [CrossRef]
- Kwang, H.C.; Lee, H.J.; Koo, S.Y.; Song, D.G.; Lee, D.U.; Pan, C.H. Optimization of pressurized liquid extraction of carotenoids and chlorophylls from chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 793–797. [Google Scholar]
- Singh, A.K.; Rana, H.K.; Pandey, A.K. Analysis of chlorophylls. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 635–650. [Google Scholar]
- Georgiopoulou, I.; Tzima, S.; Pappa, G.D.; Louli, V.; Magoulas, K.; Voutsas, E. Experimental design and optimization of recovering bioactive compounds from chlorella vulgaris through conventional extraction. Molecules 2022, 27, 29. [Google Scholar] [CrossRef] [PubMed]
- Ferreira da Cruz, A.C.; Pereira Santos, R.; Iarema, L.; Goulart Fernandes, K.R.; Naomi Kuki, K.; Finamore Araújo, R.; Campos Otoni, W. Métodos Comparativos na Extração de Pigmentos Foliares de Três Híbridos de Bixa orellana L. Rev. Bras. Biociênc. 2007, 5, 777–779. [Google Scholar]
- Meléndez Martínez, A.J. Carotenoides en Agroalimentación y Salud; Editorial Terracota, SA de CV: Mexico City, Mexico, 2017; pp. 574–608. [Google Scholar]
- Pawliszyn Janusz Bayona, J.M.; Lord, H.L.; Le, X.C.; Mondello, L. Comprehensive Sampling and Sample Preparation: Analytical Techniques for Scientists; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Ordoñez-Santos, L.E.; Martínez-Girón, J.; Rodríguez-Rodríguez, D.X. Extraction of total carotenoids from peach palm fruit (Bactris gasipaes) peel by means of ultrasound application and vegetable oil. Dyna 2019, 86, 91–96. [Google Scholar]
- Cheng, S.-H.; Khoo, H.E.; Kong, K.W.; Prasad, K.N.; Galanakis, C.M. Extraction of carotenoids and applications. In Carotenoids: Properties, Processing and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 259–288. [Google Scholar]
- Tiwari, S.; Upadhyay, N.; Singh, A.K.; Meena, G.S.; Arora, S. Organic solvent-free extraction of carotenoids from carrot bio-waste and its physico-chemical properties. J. Food Sci. Technol. 2019, 56, 4678–4687. [Google Scholar] [CrossRef]
- Elik, A.; Yanık, D.K.; Göğüş, F. Microwave-assisted extraction of carotenoids from carrot juice processing waste using flaxseed oil as a solvent. LWT 2020, 123, 109100. [Google Scholar] [CrossRef]
- Tarazona-Díaz, M.; Becerra, N.; Piedra, J.; Beltrán, R. Obtención de un colorante a partir de corteza de maracuyá con el uso de técnicas convencionales de extracción. Rev. UDCA Actual. Divulg. Cient. 2020, 23. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.D.P.; Gutiérrez, L.F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.E. Valorisation of mango peel: Proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Durán, M.G.; Alvarez, M.J.M. Evaluación de algunas mezclas de solventes en la extracción de carotenoides del pericarpio de tamarillo (Cyphomandra betacea Sendt). Cienc. Tecnol. Aliment. 2000, 3, 34–38. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.C.; Lourenço, S.C.; Duarte, D.F.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of tomato (Solanum lycopersicum L.) pomace ethanolic extract by spray drying: Optimization of process conditions. Appl. Sci. 2019, 9, 612. [Google Scholar] [CrossRef]
- Benmeziane, A.; Boulekbache-Makhlouf, L.; Mapelli-Brahm, P.; Khodja, N.K.; Remini, H.; Madani, K.; Meléndez-Martínez, A.J. Extraction of carotenoids from cantaloupe waste and determination of its mineral composition. Food Res. Int. 2018, 111, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.B.T.; Nguyen, H.V.H. The quality of natural pigment isolated from Canistel fruits (Pouteria campechiana (Kunth) Baehni) grown in Vietnam as affected by extraction solvents, pH and cooking temperatures. J. Food Meas. Charact. 2022, 16, 2676–2684. [Google Scholar] [CrossRef]
- De, M.; Lima, A.; Charalampopoulos, D.; Chatzifragkou, A. Optimisation and modelling of supercritical CO2 extraction process of carotenoids from carrot peels. J. Supercrit. Fluids 2017, 133, 94–102. [Google Scholar]
- Benucci, I.; Lombardelli, C.; Mazzocchi, C.; Esti, M. Natural colorants from vegetable food waste: Recovery, regulatory aspects, and stability-A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2715–2737. [Google Scholar] [CrossRef]
- Choudhury, N.; Meghwal, M.; Das, K. Microencapsulation: An overview on concepts, methods, properties and applications in foods. Food Front. 2021, 2, 426–442. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural colorants from plant pigments and their encapsulation: An emerging window for the food industry. LWT 2022, 153, 112527. [Google Scholar] [CrossRef]
- Saénz, C.; Tapia, S.; Chávez, J.; Robert, P. Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
- Nayak, C.A.; Rastogi, N.K. Effect of selected additives on microencapsulation of anthocyanin by spray drying. Dry. Technol. 2010, 28, 1396–1404. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.M.; Ortega-Regules, A.E.; Lozada-Ramírez, J.D.; Pérez-Pérez, M.C.I.; Vernon-Carter, E.J.; Welti-Chanes, J. Color and chemical stability of spray-dried blueberry extract using mesquite gum as wall material. J. Food Compos. Anal. 2011, 24, 889–894. [Google Scholar] [CrossRef]
- Idham, Z.; Muhamad, I.I.; Sarmidi, M.R. Degradation kinetics and color stability of spray-dried encapsulated anthocyanins from Hibiscus sabdariffa L. J. Food Process. Eng. 2012, 35, 522–542. [Google Scholar] [CrossRef]
- Kandasamy, S.; Naveen, R. A review on the encapsulation of bioactive components using spray-drying and freeze-drying techniques. J. Food Process. Eng. 2022, 45, e14059. [Google Scholar] [CrossRef]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of grape (Vitis labrusca var. Bordo) skin phenolic extract using gum Arabic, polydextrose, and partially hydrolyzed guar gum as encapsulating agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Parashar, D.P.; Raychaiudhuri, U.; Chakraborty, R. Studying the effect of different drying methods on phenolic content, antioxidant activity, color and antimicrobial activity in Assam tea (Camellia assamica). J. Plant Biochem. Biotechnol. 2022, 31, 615–624. [Google Scholar] [CrossRef]
- Ferreira, D.S.; Faria, A.F.; Grosso, C.R.F.; Mercadante, A.Z. Encapsulation of Blackberry Anthocyanins by Thermal Gelation of Curdlan. J. Braz. Chem. Soc. 2009, 20, 1908–1915. [Google Scholar] [CrossRef]
- Avendaño-Romero, G.C.; López-Malo, A.; Palou, E. Propiedades del alginato y aplicaciones en alimentos. Prop. Alginato Apl. Aliment. 2013, 7, 87–96. [Google Scholar]
- Ferreira, D.S. Compostos Bioativos em amora-Preta e Encapsulação do Seu Extrato Antociânico por Gelificação Térmica com Curdlana. Master Thesis, State University of Campinas, Campinas, Brazil, 2008. [Google Scholar]
- Heidari, F.; Jafari Mahdi, S.; Mohammad Ziaiifar, A.; Malekjani, N. Stability and release mechanisms of double emulsions loaded with bioactive compounds; a critical review. Adv. Colloid Interface Sci. 2022, 299, 102567. [Google Scholar] [CrossRef] [PubMed]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Forney, C.F.; Lim, L.T.; Xu, W.; Xu, G. Coencapsulation of polyphenols and anthocyanins from blueberry pomace by double emulsion stabilized by whey proteins: Effect of Homogenization parameters. Molecules 2018, 23, 2525. [Google Scholar] [CrossRef]
- Petito, N.D.L.; Devens, J.M.; Falcão, D.Q.; Dantas, F.M.L.; Passos, T.S.; Araujo, K.G.L. Nanoencapsulation of Red Bell Pepper Carotenoids: Comparison of Encapsulating Agents in an Emulsion Based System. Colorants 2022, 1, 132–148. [Google Scholar] [CrossRef]
- Bamidele, O.P.; Emmambux, M.N. Encapsulation of bioactive compounds by “extrusion” technologies: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3100–3118. [Google Scholar] [CrossRef]
- Rigi, M.; Ojagh, S.M.; Alishahi, A.; Hasani, S. Characterizing and Developing the Antioxidant and Antimicrobial Properties of the Nano-Encapsulated Bioactive Compounds of Spirulina platensis in Liposome. J. Aquat. Food Prod. Technol. 2022, 31, 591–598. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F.; Chen, L. Encapsulation of anthocyanin in liposomes using supercritical carbon dioxide: Effects of anthocyanin and sterol concentrations. J. Funct. Foods 2017, 34, 159–167. [Google Scholar] [CrossRef]
- Lima, P.M.; Dacanal, G.C.; Pinho, L.S.; Pérez-Córdoba, L.J.; Thomazini, M.; Moraes, I.C.F.; Favaro-Trindade, C.S. Production of a rich-carotenoid colorant from pumpkin peels using oil-in-water emulsion followed by spray drying. Food Res. Int. 2021, 148, 110627. [Google Scholar] [CrossRef] [PubMed]
- Konar, N.; Durmaz, Y.; Genc Polat, D.; Mert, B. Optimization of spray drying for Chlorella vulgaris by using RSM methodology and maltodextrin. J. Food Process. Preserv. 2022, 46, e16594. [Google Scholar] [CrossRef]
- Mussi, L.P.; Pereira, N.R. Storage stability of freeze-dried powder of jambolan (Syzygium cumini (L.) fruits at different degrees of maturity and packages. Braz. J. Food Technol. 2022, 25. [Google Scholar] [CrossRef]
- Ying, L.; Jinfeng, B.; Qinqin, C.; Xuan, L.; Wu, X.; Gou, M. Effects of ultrasound, heat, ascorbic acid and CaCl2 treatments on color enhancement and flavor changes of freeze-dried carrots during the storage period. Food Chem. 2022, 373, 131526. [Google Scholar]
- Sozzi, A.; Zambon, M.; Mazza, G.; Salvatori, D. Fluidized bed drying of blackberry wastes: Drying kinetics, particle characterization and nutritional value of the obtained granular solids. Powder Technol. 2021, 385, 37–49. [Google Scholar] [CrossRef]
- Haas, K.; Dohnal, T.; Andreu, P.; Zehetner, E.; Kiesslich, A.; Volkert, M.; Fryer, P.; Jaeger, H. Particle engineering for improved stability and handling properties of carrot concentrate powders using fluidized bed granulation and agglomeration. Powder Technol. 2020, 370, 104–115. [Google Scholar] [CrossRef]
- Barathiraja, R.; Thirumal, P.; Saraswathy, G.; Rahamathullah, I. Drying of turkey berry in a fluidized bed dryer with mild steel, copper and aluminum inert materials: Kinetics and quality determination. J. Mech. Sci. Technol. 2021, 35, 2707–2717. [Google Scholar] [CrossRef]
- de Moura, S.C.S.R.; Schettini, G.N.; Garcia, A.O.; Gallina, D.A.; Alvim, I.D.; Hubinger, M.D. Stability of Hibiscus Extract Encapsulated by Ionic Gelation Incorporated in Yogurt. Food Bioprocess Technol. 2019, 12, 1500–1515. [Google Scholar] [CrossRef]
- Tekin, İ.; Ersus, S. Electrically assisted ionic gelling encapsulation of enzymatically extracted zinc-chlorophyll derivatives from stinging nettle (Urtica urens L.). J. Food Process. Eng. 2021, 44, e13743. [Google Scholar] [CrossRef]
- Gharanjig, H.; Gharanjig, K.; Farzi, G.; Hosseinnezhad, M.; Jafari, S.M. Novel complex coacervates based on Zedo gum, cress seed gum and gelatin for loading of natural anthocyanins. Int. J. Biol. Macromol. 2020, 164, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Marfil, P.H.M.; Paulo, B.B.; Alvim, I.D.; Nicoletti, V.R. Production and characterization of palm oil microcapsules obtained by complex coacervation in gelatin/gum Arabic. J. Food Process. Eng. 2018, 41, e12673. [Google Scholar] [CrossRef]
- Agarry, I.E.; Wang, Z.; Cai, T.; Kan, J.; Chen, K. Chlorophyll encapsulation by complex coacervation and vibration nozzle technology: Characterization and stability study. Innov. Food Sci. Emerg. Technol. 2022, 78, 103017. [Google Scholar] [CrossRef]
- Guldiken, B.; Gibis, M.; Boyacioglu, D.; Capanoglu, E.; Weiss, J. Impact of liposomal encapsulation on degradation of anthocyanins of black carrot extract by adding ascorbic acid. Food Funct. 2017, 8, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Xiong, Y.; Peng, S.; McClements, D.J.; Zou, L.; Liang, R.; Liu, W. Improving norbixin dispersibility and stability by liposomal encapsulation using the pH-driven method. J. Sci. Food Agric. 2022, 102, 2070–2079. [Google Scholar] [CrossRef]
- Fan, L.; Wu, Q.; Chu, M. Near infrared fluorescent chlorophyll nanoscale liposomes for sentinel lymph node mapping. Int. J. Nanomed. 2012, 7, 3071–3080. [Google Scholar]
- Novais, C.; Molina, A.K.; Abreu, R.M.V.; Santo-Buelga, C.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Natural Food Colorants and Preservatives: A Review, a Demand, and a Challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Kitts, D.D.; Tomiuk, S. Studies on mitigating lipid oxidation reactions in a value-added dairy product using a standardized cranberry extract. Agriculture 2013, 3, 236–252. [Google Scholar] [CrossRef]
- de Mejia, E.G.; Dia, V.P.; West, L.; West, M.; Singh, V.; Wang, Z.; Allen, C. Temperature Dependency of Shelf and Thermal Stabilities of Anthocyanins from Corn Distillers’ Dried Grains with Solubles in Different Ethanol Extracts and a Commercially Available Beverage. J. Agric. Food Chem. 2015, 63, 10032–10041. [Google Scholar] [CrossRef]
- Pineda-Vadillo, C.; Nau, F.; Guerin-Dubiard, C.; Jardin, J.; Lechevalier, V.; Sanz-Buenhombre, M.; Guadarrama, A.; Tóth, T.; Csavajda, É.; Hingyi, H.; et al. The food matrix affects the anthocyanin profile of fortified egg and dairy matrices during processing and in vitro digestion. Food Chem. 2016, 214, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Lehto, S.; Buchweitz, M.; Klimm, A.; Straßburger, R.; Bechtold, C.; Ulberth, F. Comparison of food colour regulations in the EU and the US: A review of current provisions. Food Addit. Contam. Part A 2017, 34, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Mateus, N.; de Freitas, V. Anthocyanins as Food Colorants. In Anthocyanins: Biosynthesis, Functions, and Applications; Springer: New York, NY, USA, 2008; pp. 284–304. [Google Scholar]
- Janiszewska-Turak, E.; Pisarska, A.; Królczyk, J.B.; Naturalne, B. Natural food pigments application in food products. Nauka Przyr. Technol. 2016, 10, 51. [Google Scholar] [CrossRef]
- Jurić, S.; Jurić, M.; Król-Kilińska, Ż.; Vlahoviček-Kahlina, K.; Vinceković, M.; Dragović-Uzelac, V.; Donsì, F. Sources, stability, encapsulation and application of natural pigments in foods. Food Rev. Int. 2020, 38, 1735–1790. [Google Scholar] [CrossRef]
- Obón, J.M.; Castellar, M.R.; Alacid, M.; Fernández-López, J.A. Production of a red-purple food colorant from Opuntia stricta fruits by spray drying and its application in food model systems. J. Food Eng. 2009, 90, 471–479. [Google Scholar] [CrossRef]
- Rizk, E.M.; Bedier, S.H.; El_Gendy, M.A. Utilization of carotenoid pigments extracted from tomato peel as natural antioxidants and colorants in sunflower oil and spaghetti. J. Agric. Res. 2014, 92, 309–321. [Google Scholar] [CrossRef]
- Girón, J.M.; Martínez, J.A.; García Hurtado, L.; Cuaran, J.D.; Ocampo, Y.A. Pigmentos vegetales y compuestos naturales aplicados en productos cárnicos como colorantes y/o antioxidantes: Revisión. Inventum 2016, 11, 51–62. [Google Scholar] [CrossRef]
- Pinzón-Zárate, L.X.; Hleap-Zapata, J.I.; Ordóñez-Santos, L.E. Análisis de los parámetros de color en salchichas frankfurt adicionadas con extracto oleoso de residuos de chontaduro (Bactris Gasipaes). Inf. Tecnol. 2015, 26, 45–54. [Google Scholar] [CrossRef]
- Backes, E.; Leichtweis, M.G.; Pereira, C.; Prieto, M.A.; Genena, A.K.; Barreiro, M.F.; Ferreira, I.C.F.R. Ficus carica L. and Prunus spinosa L. extracts as new anthocyanin-based food colorants: A thorough study in confectionery products. Food Chem. 2020, 333, 127457. [Google Scholar] [CrossRef]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; Crizel-Cardozo, M.M.; Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of palm oil by complex coacervation for application in food systems. Food Chem. 2017, 220, 59–66. [Google Scholar] [CrossRef]
- Rutz, J.K.; Zambiazi, R.C.; Borges, C.D.; Crizel-Cardozo, M.M.; Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of purple Brazilian cherry juice in xanthan, tara gums and xanthan-tara hydrogel matrixes. Carbohydr. Polym. 2013, 98, 1256–1265. [Google Scholar] [CrossRef] [PubMed]

| Health-Promoting Effects | Matrix | Chemical Compound | Reference |
|---|---|---|---|
| Combat hyperglycemia and hyperuricemia | Cherries (Prunus avium L.) and purple sweet potato (Ipomoea batatas L.) | Anthocyanins | [59] |
| Mulberry (Morus alba L.) | [60] | ||
| Star gooseberry (Sauropus androgynus L.) | Chlorophylls | [42] | |
| Lettuce (Lactuca sativa) | Carotenoids | [61] | |
| Anticancer | Blueberry (Vaccinium myrtillus) | Anthocyanins | [62] |
| Black rice (Oryza sativa L. indica) | [63] | ||
| Chokeberry (Aronia meloncarpa E.), elderberry (Sambucus nigra L.), bilberry (Vaccinium myrtillus L.), grape (Vitis Vinifera L.), purple carrot (Daucus dacota L.), purple corn (Zea mays L.), and red radish (Raphanus sati Vus L.) | [64] | ||
| Conyza trilova | Chlorophylls | [65] | |
| Pomelo (Citrus grandis) | [41] | ||
| Purple tomato (Solanum lycopersicum L. cv Micro-Tom) | Carotenoids | [66] | |
| Cardiovascular disease | Elderberry (S. nigra), bilberry (V. myrtillus), and chokeberry (A. melanocarpa) | Anthocyanins | [67] |
| Strawberry (Fragaria × ananassa) var. Alba | [68] | ||
| Roselle (Hibiscus sabdariffa L.) | [69] | ||
| Paprika (Capsicum annuum) | Carotenoids | [70] | |
| Visual health | Bilberry (Vaccinium myrtillus L.) | Anthocyanins | [71] |
| Seed coat of black soybean (Glycine max L.) | [72] | ||
| Antimicrobial | Ribes species, several cultivars (Ben Tirran, Lūšiai, Čiornyj negus, Corona’, Au Gs-5, and Jonkher van Tets) | Anthocyanins | [73] |
| Mulberry (Morus nigra L.) and non-black mulberry (Morus mongolica and Morus alba L. ‘Zhenzhubai’) | [74] | ||
| Mushrooms (Lactarius deliciosus (L.) Gray and Lactarius piperatus (L.) Pers) | Carotenoids | [75] | |
| Antioxidant properties | Haskap (Lonicera careulea L.) | Anthocyanins | [25] |
| Sweet cherry fruits (Prunus avium Linnaeus (L.)) | [76] | ||
| Broad-leaf bamboo (Sasa senanensis) | Chlorophylls | [77] | |
| Tomato (Solanum lycopersicum L.) | Carotenoids | [78] | |
| Carrot (Daucus carota L.) peels | [79] |
| Plant Matrix | Extraction Approach | Solvent | Conditions | Chlorophyll Recovery Yield | Reference |
|---|---|---|---|---|---|
| Sheets of Tifton 85 grass (Cynodon spp.) | Maceration | Dimethyl sulfoxide (DMSO) | Volume: 20 mL Eight evaluations of 12 h/12 h T (°C): 23–26 Humidity: 40–75%. | Chlorophyll a: 316 ± 2.93 µmol·m−2 Chlorophyll b: 66 ± 1.41 µmol·m−2 | [111] |
| N,N Dimethylformamide | Volume: 20 mL Eight evaluations of 12 h/12 h T (°C): 23–26 Humidity: 40–75%. | Chlorophyll a: 297 ± 3.58 µmol·m−2 Chlorophyll b: 85 ± 2.03 µmol·m−2 | |||
| 80% acetone | Volume: 20 mL Eight evaluations of 12 h/12 h T (°C): 23–26 Humidity: 40–75%. | Chlorophyll a: 250 ± 2.65 µmol·m−2 Chlorophyll b: 111 ± 1.50 µmol·m−2 | |||
| Absolute ethanol | Volume: 20 mL Eight evaluations of 12 h/12 h T (°C): 23–26 Humidity: 40–75%. | Chlorophyll a: 259 ± 2.84 µmol·m−2 Chlorophyll b: 84 ± 2.25 µmol·m−2 | |||
| Sheets of canola (Brassica napus L. var oleifera) | Maceration | 80% acetone | Conventional extraction | Chlorophyll a: 0.87 mg·g−1 Chlorophyll b: 0.39 mg·g−1 | [117] |
| No maceration | 80% acetone | t (h): 24 Cold camera, no light | Chlorophyll a: 0.98 mg·g−1 Chlorophyll b: 0.38 mg·g−1 | ||
| Carrot (Daucus carota L.) and tomato (Solanum lycopersicum var. cerasiforme), aerial parts | Maceration | Ethanol/water 90/10 v/v | t (min): 60 and 120 | Best: ethanol, 120 min Chlorophyll a: 2.46 ± 0.06 µg·g−1 Chlorophyll b: 28.5 ± 0.2 µg·g−1 | [119] |
| Ultrasound-assisted | Hexane | Power: 100, 200, and 400 W T (min): 5 | Best: ethanol, 400 w Chlorophyll a: 107.7 ± 0.2 µg·g−1 Chlorophyll b: 99.6 ± 0.1 µg·g−1 | ||
| Microalgae (Chlorella vulgaris) | Maceration | Ethanol/water 90/10 v/v | T (°C): 30–60; t (h): 6–24 Solvent-to-biomass ratio: 20–90 mLsolv/gbiom | Chlorophyll total: 53.47 mg·g−1 extr | [122] |
| Three hybrids, crosses between urucum (Bixa orellana L.) | Incubation Maceration | DMSO 80% acetone | T (°C): 25–65 t (h): 24, 48, and 72 | DMSO for chlorophylls a and b > acetone 80% Acetone maximum point: 65 °C in 48 h | [123] |
| Chokecherry (Prunus virginiana) Alpines strawberry (Fragaria vesca) Sunflower (Helianthus annuus) Two graminoids (Andropogon gerardii, big bluestem; Cymbopogon citrates, lemongrass) | Maceration | DMSO 80% acetone | T (°C): 25, 30, and 40 | Chl DMSO < acetone extraction for C. citrates Extraction efficiency was not influenced by temperature. The species may need to be macerated to extraction using DMSO | [85] |
| R. capsulatus CB1200 cultured in Tween 80, supplemented with growth medium | Maceration | Diethyl ether/ethanol (1:1) | Repeatedly washed with 20% ethanol | Chlorophyll a: 7 mg·L−1 | [115] |
| Chlorella vulgaris (KMCC C-024) | Maceration (MAC) | Ethanol 90% | t (h): 6 | Chlorophyll a: 4.26 ± 0.53 mg·g−1 sample Chlorophyll b: 2.58 ± 0.09 mg·g−1 sample | [120] |
| Soxhlet (SOX) | Ethanol 90% | t (h): 2 | Chlorophyll a: 3.32 ± 0.30 mg·g−1 sample Chlorophyll b: 3.45 ± 0.28 mg·g−1 sample | ||
| Ultrasound- assisted extraction (UAE) | Ethanol 90% | t (h): 2 | Chlorophyll a: 5.12 ± 0.29 mg·g−1 sample Chlorophyll b: 3.71 ± 0.41 mg·g−1 sample | ||
| Pressurized liquid extraction (PLE) | Ethanol 90% | t (min): 8, 19, and 30 T (°C): 50, 105, and 160 | Chlorophyll a: 9.63 ± 0.65 mg·g−1 sample Chlorophyll b: 5.77 ± 0.68 mg·g−1 sample | ||
| Leaf pigments of two grapevine rootstock varieties (Vitis vinifera × Vitis rotundifolia and Vitis riparia) | Maceration | DMSO saturated with calcium carbonate | t (h): 24 and 48 | Chlorophyll a: DMSO has been shown to be as efficient as that with 80% acetone Chlorophyll b: DMSO > acetone 80% for V. vinifera × V. rotundifolia | [116] |
| Acetone 80% | t (h): 24 and 48 | ||||
| Clitoria fairchildiana (Fabaceae) and Gossypium sp. (Malvaceae) | Maceration | Ethyl alcohol 95% | Room temperature for maceration and refrigeration for 48 h for conventional | Clitoria fairchildiana Maceration > conventional Gossypium sp. Without differentiation | [114] |
| Encapsulation Method | Particle Size (μm) | Advantages | Disadvantages | Vegetable Source | Reference |
|---|---|---|---|---|---|
| Spray drying | 10–100 | Low process cost, fast, versatile, and the possibility of large-scale production in a continuous mode. High encapsulation efficiency and relatively good storage stability. | Degradation of temperature-sensitive compounds, non-uniform particulates, and small–moderate batch yields. |  Haskap (Lonicera careulea L.) Haskap (Lonicera careulea L.) | [25] |
 Pumpkin (C. moschata) peels Pumpkin (C. moschata) peels | [156] | ||||
 Chlorella vulgaris Chlorella vulgaris | [157] | ||||
| Freeze-drying | 20–5000 | Possibility of encapsulating thermosensitive substances unstable in an aqueous solution. | Long times as well as high cost and energy. Low stability and sensitivity to oxidation. |  Jambolan (Syzygium cumini L.) Jambolan (Syzygium cumini L.) | [158] |
 Carrot (Daucus carota L. cv. Heitianwucun) Carrot (Daucus carota L. cv. Heitianwucun) | [159] | ||||
 Camellia sinensis var. assamica Camellia sinensis var. assamica | [146] | ||||
| Fluidized bed | 20–200 | Low cost, specific capsule size distribution, low product porosity, and smooth as well as uniform drying method. | Drying sticky material is quite difficult. There is a possibility of fine product loss; chances of electrostatic build-up may be high. |  Blackberry (Rubus fruticosus L.) residue Blackberry (Rubus fruticosus L.) residue | [160] |
 Carrot (Daucus carota L.) Carrot (Daucus carota L.) | [161] | ||||
 Turkey berries (Solanum torvum Swartz) Turkey berries (Solanum torvum Swartz) | [162] | ||||
| Emulsion polymerization | 0.1–3 | Micro–nanocapsules with a narrow size distribution. | Difficult to control the capsule formation (polymerization). |  Blueberry (Vaccinium augustifolium Ait.) pomace Blueberry (Vaccinium augustifolium Ait.) pomace | [151] |
 Ripe red bell peppers (Capsicum annum L.) Ripe red bell peppers (Capsicum annum L.) | [152] | ||||
| Ionic gelation | - | Low cost and does not require advanced equipment, high temperatures, and organic solvents. | Laboratory scale: capsules have a high porosity that favors intensive bursting. |  Hibiscus (Hibiscus sabdariffa L.) Hibiscus (Hibiscus sabdariffa L.) | [163] |
 Stinging nettle (Urtica urens L.) Stinging nettle (Urtica urens L.) | [164] | ||||
| Thermal gelation | - | Uses gentle conditions, simple method. | Large gel porosity, low encapsulation efficiency. |  Blackberry fruits (Rubus spp.) Blackberry fruits (Rubus spp.) | [147] |
| Phase separation (coacervation) | 10–800 | Ambient temperature, protection against oxidation and volatility, and the adapted release of active compounds. | High cost, complex, use of toxic chemicals, difficult to control particle size, and very sensitive to pH as well as ionic strength. |  Blue barberry (Berberis integerrima Bunge) Blue barberry (Berberis integerrima Bunge) | [165] |
 Commercial palm oil Commercial palm oil | [166] | ||||
 Fresh spinach (Spinacia oleracea) Fresh spinach (Spinacia oleracea) | [167] | ||||
| Liposome entrapment | 0.1–1 | Can encapsulate aqueous or liposoluble material. Increased adsorption and bioavailability. Non-toxic and non-immunogenic. | Mainly used at the laboratory scale, unstable, expensive, and low encapsulation efficiency. |  Black carrot Black carrot | [168] |
 Annatto seeds (A-750-WS) Annatto seeds (A-750-WS) | [169] | ||||
 Leaves of Chimonanthus salicifolius S.Y.Hu Leaves of Chimonanthus salicifolius S.Y.Hu | [170] |
 Anthocyanins;
Anthocyanins;  carotenoids; and
carotenoids; and  chlorophylls.
chlorophylls.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, A.K.; Corrêa, R.C.G.; Prieto, M.A.; Pereira, C.; Barros, L. Bioactive Natural Pigments’ Extraction, Isolation, and Stability in Food Applications. Molecules 2023, 28, 1200. https://doi.org/10.3390/molecules28031200
Molina AK, Corrêa RCG, Prieto MA, Pereira C, Barros L. Bioactive Natural Pigments’ Extraction, Isolation, and Stability in Food Applications. Molecules. 2023; 28(3):1200. https://doi.org/10.3390/molecules28031200
Chicago/Turabian StyleMolina, Adriana K., Rúbia C. G. Corrêa, Miguel A. Prieto, Carla Pereira, and Lillian Barros. 2023. "Bioactive Natural Pigments’ Extraction, Isolation, and Stability in Food Applications" Molecules 28, no. 3: 1200. https://doi.org/10.3390/molecules28031200
APA StyleMolina, A. K., Corrêa, R. C. G., Prieto, M. A., Pereira, C., & Barros, L. (2023). Bioactive Natural Pigments’ Extraction, Isolation, and Stability in Food Applications. Molecules, 28(3), 1200. https://doi.org/10.3390/molecules28031200










