Abstract
Photocatalytic inactivation of pathogens in aqueous waste is gaining increasing attention. Several homogeneous and heterogeneous photocatalytic protocols exist using the Fenton’s reagent and TiO2, respectively. A comprehensive study of homogeneous and heterogeneous photocatalysis on a range of microorganisms will significantly establish the most efficient method. Here, we report a comparative study of TiO2- and Fe+3-based photocatalytic inactivation under UV-A of diverse microorganisms, including Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria, bacterial spores (Bacillus stearothermophilus spores) and viruses (MS2). We also present data on the optimization of TiO2 photocatalysis, including optimal catalyst concentration and H2O2 supplementation. Our results indicate that both photo-Fenton and TiO2 could be successfully applied for the management of microbial loads in liquids. Efficient microorganism inactivation is achieved with homogeneous photocatalysis (7 mg/L Fe+3, 100 mg/L H2O2, UV-A) in a shorter processing time compared to heterogeneous photocatalysis (0.5 g/L TiO2, UV-A), whereas similar or shorter processing is required when heterogenous photocatalysis is performed using microorganism-specific optimized TiO2 concentrations and H2O2 supplementation (100 mg/L); higher H2O2 concentrations further enhance the heterogenous photocatalytic inactivation efficiency. Our study provides a template protocol for the design and further application for large-scale photocatalytic approaches to inactivate pathogens in liquid biomedical waste.
1. Introduction
Photocatalytic oxidation has come to the fore as an alternative and environmentally friendly method for inactivation of hazardous substances and microorganisms in aqueous solutions, with extensions to the treatment of drinking water [1,2], urban and industrial wastewater [3,4,5,6,7,8,9] and more recently to biomedical liquid waste [10,11].
Photocatalytic oxidation, as an advanced oxidation process (AOP), is based on the production of reactive oxygen species (ROS) or free radicals such as superoxide radicals (O2−), hydroperoxyl radicals (HO2.) and hydroxyl radicals (HO˙), which non-selectively attack organic molecules, eventually resulting in the formation of CO2 and inorganic salts [2]. Several photocatalytic approaches have been reported, using catalysts either occurring in the same phase as the reactants (homogeneous) or not (heterogeneous). The use of Fenton’s reagent (Fe+2 and H2O2) and TiO2 in the context of homogeneous and heterogeneous photocatalytic oxidation, respectively, has been extensively studied [12,13,14,15]. In the photo-Fenton process, HO˙ and O2.− are generated during the irradiation of the H2O2 and Fe2+ mixture (Fenton’s reagent) in acid conditions. The addition of oxalic acid to the solution containing Fe+3 leads to the formation of ferrioxalate complexes (ferrioxalate-assisted photo-Fenton process) that under irradiation can also produce oxidative species such as O2.−, HO2˙ and HO˙ radicals. TiO2-based photocatalysis is initiated with the irradiation of TiO2 with a photon of energy equal to or greater than its band gap width, and the formation of photogenerated electron/hole (e−/h+) pairs. In aqueous suspensions the produced holes (h + VB) and electrons (e−CB), can react with surface HO− groups and O2, respectively, leading to the formation of HO˙ and O2.− radicals [8].
Extensive research has been conducted aiming at the development of optimized homogeneous and heterogeneous photocatalytic approaches for the decomposition and detoxification of hazardous organic substances in aqueous solutions, such as antibiotics [13,15,16,17], pesticides [18,19,20] and dyes [21,22,23,24]. Similar studies have been conducted for the evaluation of the photocatalytic inactivation of microorganisms in suspension [25], focusing on endospores, considered to be one of the most resistant targets [12,26,27], viruses [28,29,30], fungi [14], Gram-positive and Gram-negative bacteria [31].
Microorganism inactivation mediated by photo-Fenton and TiO2 photocatalysis is achieved through different pathways depending on the microbe. In general, microorganisms’ photocatalytic inactivation represents the synergistic effect of UV light and oxidative radicals generated by TiO2 or Fe+3 following UV irradiation. UV-A affects microbial proteins and nucleic acids through ROS production. Previous studies aiming to delineate the mechanism of TiO2-based photocatalytic inactivation reported catalyst adsorption on the microbes’ surface. Next, the ROS react with organic molecules on the microbes’ outer surface, such as proteins (porins, proteins involved in oxidative stress response, in transport, and in bacterial metabolism) and polyunsaturated fatty acids [32], and destroy the cell wall and membrane, as evidenced by scanning electron microscopy [12,33]. Interestingly, it has recently been reported that different TiO2 configurations generate different types of ROS, exerting differential bactericidal efficacies [34]. In the case of viruses, ROS attack the phospholipid bilayer and the envelope and/or capsid proteins [35]. In all cases, damage of the microbes’ outer surface allows ROS penetration inside the cell and leakage of cellular contents [33]. Having gained access to the cytoplasm, free radicals promote oxidative stress, attack and decompose cellular enzymes. Oxidative stress induces antioxidant cellular responses to counter the effects; however, enzymes mediating such responses are affected by oxidative agents, resulting in malfunction of antioxidant defense. Moreover, oxidative species attack and destroy nucleic acids, further contributing to microorganism inactivation. Similar effects have been reported in photo-Fenton approaches. Regarding microorganism-specific inactivation mechanisms, in the case of MS2, photo-Fenton inactivation is mainly exerted through oxidant generation in the bulk, in contrast to bacterial inactivation, which is mostly attributed to intracellular oxidative-stress [36].
Previous studies reported that B. stearothermophilus spores are more efficiently inactivated using 0.1 g/L TiO2, resulting in complete inactivation following 90 min treatment [12]. Optimal TiO2 concentration for S. aureus inactivation has been reported to be 0.1 g/L [31]; for MS2 inactivation, previous studies used 1 g/L TiO2, and required irradiation (300 to 420 nm) treatment for 120 min to achieve 0.95-log inactivation [37]. Several studies have been conducted on TiO2-based E. coli inactivation (some of them reviewed in Foster et al. 2011 [33], among other microorganisms). In a study by Khani et al. in 2016, following catalyst concentration titration experiments, 0.5 g/L was determined as the optimal TiO2 concentration for E. coli inactivation under irradiation of 300–420 nm, after a processing time of 150 min [38]. Regarding photo-Fenton approaches, a 9-log inactivation of Bacillus subtilis spores was achieved under UV-A irradiation at 365 nm using 2.5 mM Fe+2 and 100 mM H2O2 [26]. Exposure to 600 W/m2 irradiation for 30 min in the presence of Fe+3 and H2O2 at a 1:1 ratio resulted in a 4-log MS2 reduction [39].
Most of the previous studies focused either on the homogeneous or the heterogeneous inactivation of individual microorganisms, and only few addressed the comparative effectiveness of photocatalytic inactivation on different microorganisms [37,40,41,42]. Currently, there is no study addressing the efficiency of UV-A mediated homogeneous and heterogeneous photocatalysis against different microorganisms under the same experimental set-up. Data generated under different experimental conditions may only provide indirect insights into the effectiveness of photocatalytic oxidation approaches on microorganisms’ inactivation.
Here, we report the comparative effectiveness of TiO2- (heterogeneous) and Fe+3- (photo-Fenton, homogeneous) based photocatalysis under UV-A irradiation against a range of microorganisms including bacterial spores (Bacillus stearothermophilus spores) as a representative of the most resistant to inactivation targets, MS2 bacteriophage as a model of human enteroviruses, Staphylococcus aureus and Escherichia coli as representatives of Gram-positive and Gram-negative bacteria, respectively, at concentrations similar to pathogen titers in biological fluids of patients with bloodstream infections.
We also present data on the optimization of heterogeneous photocatalytic approaches, entailing catalyst concentration and H2O2 supplementation. Our data indicates that both photo-Fenton and TiO2 could be successfully applied for microbe inactivation. Indeed, efficient microorganism inactivation is achieved with homogeneous photocatalysis (7 mg/L Fe+3, 100 mg/L H2O2, UV-A) in a shorter processing time compared to heterogeneous photocatalysis (0.5 g/L TiO2, UV-A), whereas similar or shorter processing is required when heterogenous photocatalysis is performed under microorganism-specific optimized TiO2 concentrations and H2O2 supplementation (100 mg/L or 1000 mg/L).
2. Results
2.1. Comparative Assessment of Homogeneous and Heterogeneous Photocatalysis for the Inactivation of Model Microorganisms
We report the effectiveness of TiO2- and photo-Fenton-based photocatalytic oxidation on the inactivation of microorganisms representative of different microbial groups. These groups of microorganisms are detected in biomedical liquid waste [43] and display different degrees of resistance against conventional inactivation methods, such as chemical treatment (chlorine or ozone) [44] irradiation (UV) [45,46], electro-thermal-deactivation (ETD) [47] and microwaving [48].
To assess microorganism inactivation, we estimated viability reduction (Nt/N0) and inactivation efficiency (I) following treatment with TiO2 (0.5 g/L) or with the photo-Fenton reagent (7 mg/L Fe+3, 100 mg/L H2O2) under UV-A irradiation (Figure S1A,B, Supplementary Materials). We observed a shorter processing time required for microorganism inactivation under homogeneous photocatalysis; indeed, homogenous photocatalysis achieved a 3-log reduction of B. stearothermophilus spores within 60 min of processing and resulted in no bacterial growth after 180 min of treatment. In contrast, the heterogeneous approach achieved a 3-log reduction at 300 min of treatment. Similarly, for MS2 inactivation, no plaque formation was detected in samples collected after 30 min of processing with TiO2, while the same result was achieved following a 5 min treatment with the photo-Fenton reagent. Intriguingly, S. aureus presented a distinct pattern of inactivation, according to which both homogeneous and heterogeneous approaches resulted in no bacterial growth following 30 min of treatment; however, intermediate timepoints suggest a sharper decline of S. aureus viability through heterogeneous photocatalytic oxidation. Based on the existing literature, we speculate that this observation may be associated with: (a) the tendency of S. aureus to aggregate at high concentrations [49,50], rendering it less accessible to the chemical attack by the produced oxidizing agents, and (b) with the absorbance of TiO2 particles on the microorganisms’ surface [51], providing a direct contact between the pathogen and oxidizing agents. E. coli inactivation was only assessed in the case of heterogeneous photocatalysis, since it is highly susceptible to the low pH required for the photo-Fenton reagent. E. coli was the most susceptible among the tested microorganisms, resulting in no growth following 5 min of treatment with TiO2.
2.2. Heterogeneous Photocatalysis Optimization
We next aimed to improve the performance of heterogeneous photocatalytic oxidation. To this end, we tested different catalyst concentrations (0.1 g/L, 0.5 g/L, 1 g/L) and further supplemented the best performing condition with increasing concentrations of H2O2 (100 mg/L, 500 mg/L, 1000 mg/L). Table 1 summarizes heterogeneous photocatalysis optimization results. We noticed that the lower catalyst concentrations (0.1 g/L, 0.5 g/L) were more effective in most of the cases; in particular, the lowest catalyst concentration (0.1 g/L) resulted in optimal inactivation in the cases of B. stearothermophilus spores, S. aureus and MS2; intermediate catalyst concentration (0.5 g/L) performed equally well in the cases of S. aureus and MS2, while it was most efficient in E. coli inactivation. These observations are in line with previous studies suggesting that higher TiO2 concentrations may promote nanoparticle aggregation, resulting in light scattering and thus reduced efficiency [52]. We also noticed that increasing H2O2 concentrations significantly enhance inactivation efficiency. This effect should be attributed to increased hydroxyl radical production, due to H2O2 photolysis. Under optimal heterogeneous photocatalysis, complete inactivation of B. stearothermophilus spores was achieved following 60 min treatment (0.1 g/L TiO2, 1000 mg/L H2O2); S. aureus and MS2 were completely inactivated following 5 min treatment at medium TiO2 (0.5 g/L) and medium to high H2O2 (500 mg/L, 1000 mg/L) concentration, whereas treatment for only 2 min was sufficient to inactivate E. coli at 0.5 g/L TiO2 supplemented with low H2O2 concentration (100 mg/L).

Table 1.
Heterogeneous photocatalysis optimization.
2.3. Comparative Assessment of Homogeneous and Optimized Heteogeneous Photocatalysis for the Inactivation of Model Microorganisms
Next, we compared the efficacy of homogeneous and optimized heterogeneous photocatalysis (Figure 1 and Figure S2). To this end, we plotted viability reduction (Nt/N0) as determined for the homogeneous (7 mg/L Fe3+, 100 mg/L H2O2, pH 3.0, UV-A) and heterogeneous approach. Heterogeneous photocatalysis was performed under microorganism-specific TiO2 concentration optimized conditions, in the presence of 100 mg/L H2O2, similar to the H2O2 concentration used for homogeneous photocatalysis. In addition, data obtained from heterogeneous photocatalysis performed under the before-mentioned optimized conditions, but in the presence of the highest H2O2 concentration tested (1000 mg/L) were also plotted to visualize the effect of increasing H2O2 concentrations on inactivation efficiency (Figure 1 and Figure S2).
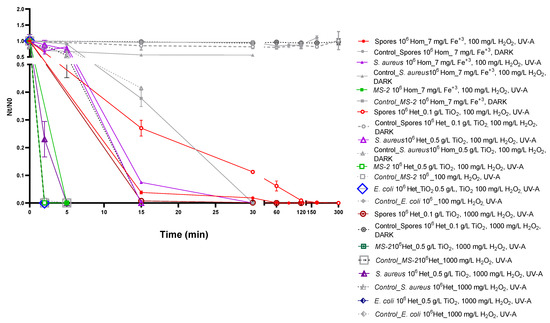
Figure 1.
Homogeneous (7 mg/L Fe3+, 100 mg/L H2O2, pH 3.0, UV-A, solid symbols) and heterogeneous photocatalysis optimized for microorganism-dependent TiO2 concentration (no fill symbols) supplemented with either 100 mg/L H2O2 (continuous lines) or 1000 mg/L H2O2 (dashed lines) for the inactivation of 106 cfu/mL Bacillus stearothermophilus spores (red), 106 pfu/mL MS2 (green), 106 cfu/mL Staphylococcus aureus (purple) and 106 cfu/mL Escherichia coli (blue). The graph depicts viability reduction (Nt/N0) of treated samples and corresponding controls (grey symbols and lines) in relation to processing time. Zero values in the Y axis represent cases for which no microorganism growth was detected (0 cfu/mL or pfu/mL). Error bars correspond to standard errors from triplicates. The legend shows experimental conditions for processed samples. Escherichia coli is highly susceptible at pH 3 required for homogeneous photocatalysis, and thus not included in these experiments.
Our data shows that under the same H2O2 concentration (100 mg/L), both approaches require a processing time of 180 min for B. stearothermophilus spores’ inactivation. Similarly, for MS2, the optimized heterogeneous photocatalysis performed equally well compared to the homogeneous approach, allowing complete inactivation after 5 min of treatment. On the other hand, photocatalytic inactivation of S. aureus was achieved within a 2-fold shorter processing time (15 min) under the optimized heterogeneous approach in the presence of 100 mg/L H2O2, highlighting the microorganism-specific differences of the tested approaches in terms of efficiency.
Interestingly, further reduction of the required processing time was observed, when heterogeneous photocatalysis was performed using higher H2O2 concentration (1000 mg/L). Indeed, under these conditions, a 3-fold reduction of the processing time for inactivation of B. stearothermophilus spores was observed (from 180 min for homogeneous to 60 min for optimized heterogeneous photocatalysis). Similarly, a reduction of processing time by a factor of 6 (from 30 min for homogeneous to 5 min for optimized heterogeneous photocatalysis) was observed for the inactivation of S. aureus; in the case of MS2, higher H2O2 supplementation resulted in complete inactivation after 5 min of treatment. Similarly, H2O2 supplementation in heterogeneous photocatalysis further reduced the processing time required for E. coli inactivation to 2 min compared to 5 min for the originally tested, non-optimized conditions.
A detailed optimization of homogeneous photocatalysis was not considered in this study, due to the initial observation that homogeneous outperforms heterogenous photocatalysis. However, we did assess the effects of different Fe+3 concentrations (7 mg/L, 14 mg/L) on the inactivation of the most resistant microorganisms, namely B. stearothermophilus spores and S. aureus. Our data indicate that the Fe+3 concentrations tested deliver similar results. In addition, we assessed the effect of increasing H2O2 concentration (100 mg/L, 500 mg/L, 1000 mg/L) on S. aureus inactivation, verifying that, similar to heterogeneous photocatalysis, supplementation with increasing H2O2 concentration improves inactivation efficiency (data not shown). Interestingly, optimized homogeneous conditions achieved complete inactivation of S. aureus following 5 min of treatment, similar to the optimized heterogeneous photocatalysis (Figure 1, Table 1), further supporting that both photocatalysis approaches are efficient for microorganism inactivation.
3. Discussion
We have conducted a comparative study to assess the effectiveness of photo-Fenton- and TiO2-based photocatalysis under UV-A irradiation on the inactivation of a range of microorganisms in suspension, in a laboratory scale. Several studies on microorganism inactivation through photocatalytic oxidation approaches have been reported (reviewed by Venkata Laxma Reddy et al. 2017 [53] and by Bono et al. 2021 [35] for TiO2-based methods). Heterogeneity in terms of experimental procedures such as catalyst configuration and concentration, irradiation source (e.g., artificial, solar), irradiation wavelength and intensity, photocatalytic set-up configuration, operational pH (especially for Fenton and Fenton-like approaches) and microorganism strains, as well as their initial concentrations tested, hampers a direct comparison of the published results. Even in studies where most of the tested conditions were similar (e.g., catalyst configuration and concentration, microbial strains and concentration), variation associated with the utilized irradiation does not allow direct comparison of inactivation efficiency among previous studies or with the present study. We aimed to comparatively assess the effectiveness of different photocatalytic approaches on different microorganisms’ inactivation. This necessitates testing under the same experimental set-up, not previously conducted for TiO2- and Fenton-based approaches against the microorganisms tested in this study. Our study has been performed in a laboratory scale using individual microorganisms and is, to our knowledge, the first study that comparatively assesses the before-mentioned photocatalytic approaches on the inactivation of the studied microorganisms under the same experimental conditions.
We tested a range of model microorganisms, representative of different microbial groups (bacterial spores—Bacillus stearothermophilus spores, viruses—MS2, as a surrogate of human enteroviruses, Gram-positive—Staphylococcus aureus and Gram-negative -Escherichia coli, bacteria). Differential resistance to inactivation following photocatalytic oxidation was observed, displaying the same pattern irrespectively of the photocatalytic method utilized. The following classification in descending order was detected; B. stearothermophilus spores > S. aureus > MS2 > E. coli. This classification is in tandem with previous studies comparing the efficiency of microorganisms’ photocatalytic inactivation under the same experimental conditions, highlighting the high resistance of bacterial spores [54]. Similarly, MS2 has been reported as more resitant to photocatalytic inactivation compared to E. coli [37,55,56], whereas contradictory results have been reported on the resistance of Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria. In particular, and in line with our data, the Gram-positive S. aureus has been reported to be more resistant compared to the Gram-negative E. coli [57,58,59,60], in contrast to other studies reporting the opposite [40,41,61].
The observed differential resistance to photocatalytic inactivation may be related to the size, structure and chemical composition of the tested microorganisms. Previous studies focusing on TiO2-based photocatalytic inactivation, reported that the catalyst is absorbed on the surface of the microbes. Produced reactive oxygen species attack and destroy the microbes’ outer surface [12,33], allowing ROS penetration inside the cell and leakage of cellular contents, followed by cell lysis and complete mineralization of the microorganism [33]. Structural elements affect the rate of oxidative attack. In this regard, spores, consisting of a thick, multilayer coat, are expected to be more resistant to photocatalytic inactivation [33]. Similarly, differences in the cell wall structure between Gram-positive and Gram-negative bacteria may contribute to their differential susceptibility to photocatalytic inactivation. In addition, species-specific differences related to oxidative stress responses, such as production of catalase by S. aureus, may contribute to the observed differential susceptibilities to photocatalytic oxidation. In addition, differences related to nucleic acid repair mechanisms, which are lacking in MS2, may explain this microorganism’s high susceptibility, possibly associated with higher vulnerability to nucleic acid damage through ROS [62].
Our data highlights efficient microorganism inactivation with both photo-Fenton and TiO2 in a relatively short processing time. Efficient microorganism inactivation is achieved with homogeneous photocatalysis (7 mg/L Fe+3, 100 mg/L H2O2, UV-A) in a shorter processing time compared to the heterogeneous approach (0.5 g/L TiO2, UV-A). Similar or shorter processing time is required when heterogenous photocatalysis is performed under microorganism-specific optimized TiO2 concentrations and H2O2 supplementation (100 mg/L or 1000 mg/L). Even though a detailed optimization of homogeneous photocatalysis conditions was not within the scope of this study, we did observe enhanced inactivation efficiency when homogeneous photocatalysis was performed using higher H2O2 concentrations.
Collectively, our data indicate that both homogeneous and heterogeneous photocatalysis are efficient for microorganism inactivation in suspension; following optimization and H2O2 supplementation, heterogeneous photocatalysis performance is significantly improved, resulting in a similar or better inactivation efficiency compared to the homogeneous approach. In addition, further improvement of homogeneous photocatalysis efficiency may be achieved through additional H2O2 supplementation, in an H2O2 concentration-dependent manner, as indicated by our experimentation on S. aureus.
Thus, both photo-Fenton and TiO2 can be successfully applied for the management of microbial loads in liquids. Consideration of additional approach-specific aspects would further enable the selection of the most suitable method. These include the low toxicity and negligible biological effects of TiO2, its efficient photoactivity, resistance against photocorrosion (high chemical stability) and acids [2,63], and the possibility to reuse this catalyst, reducing the total operational costs. Moreover, optimization of the TiO2 efficiency may be achieved in a cost-efficient way by supplementation with H2O2. On the other hand, the photo-Fenton approach requires a much cheaper catalyst and delivers efficient inactivation within relatively low treatment time; however, this approach results in the production of sludge, making difficult the isolation and re-use of the catalyst. In addition, operational costs associated with pH adjustment and sludge removal before final disposal should be also considered [64]. As an alternative, the application of a hybrid photocatalytic model has been proposed and reported to be up to 1.5 times more efficient than the individual processes [65].
This work is considered a template for follow-up studies on large scale liquid biomedical waste for identification of the best performing conditions. Biomedical waste produced by hospitals and biochemical laboratories harbor hazardous, toxic substances and pathogenic microorganisms and require efficient inactivation prior to final disposal. Efficient biomedical waste management is necessary in terms of protecting the environment and ensuring public health, and currently represents one of the biggest challenges. The application of advanced oxidation processes for the inactivation of hazardous chemicals [11] and microorganisms [66] in medical liquid waste is emerging as a promising, efficient, sustainable and environmentally friendly approach.
4. Materials and Methods
4.1. Microorganisms and Culture Conditions
All experiments were conducted using model microorganisms; Bacillus stearothermophilus (ATCC 7953, spores), MS2 bacteriophage (ATCC 15597-Β1), Staphylococcus aureus (ATCC 6538, Gram-positive bacteria) (kindly provided by Emeritus Professor Minas Arsenakis, Laboratory of General Microbiology, Department of Genetics Development and Molecular Biology, School of Biology, Aristotle University of Thessaloniki, Greece, 54124) and Escherichia coli (XL1-blue, Gram-negative bacteria). MS2 presents similarities in terms of size, shape and genetic material type (RNA) with human enteroviruses, and thus was included in our study as a non-pathogenic simulator of these pathogens.
Microorganisms were propagated in Tryptic Soy Broth/Agar-TSB (casein peptone 17 g/ L, soya peptone 3 g/ L, K2HPO4 2.5 g/ L, NaCl 5 g/ L, glucose 2.5 g/L, for Bacillus stearothermophilus) and Luria-Bertani-LB (10 g/L tryptone, 5 g/L yeast extract and 5 g/L NaCl, for S. aureus and E.coli). In the case of E. coli, LB was supplemented with 12 μg/mL tetracycline (LB-tet). LB was used for propagation of the MS2 host E. coli Top10F’ strain. Agar plates of the before mentioned media, containing 16 g/L of bacteriological agar, were used for microorganisms’ quantification. The top agar method was applied in order to assure equal distribution of the plated microbes. eTop agar of each media, contained 7 g/L of agar.
Bacillus stearothermophilus spores were initially purchased, impregnated on paper strips (ATCC 7953, 106 cfu/strip) and processed as previously described [12] for the preparation of endospore working stocks. MS2 stocks containing 2 × 1011 plaque forming units (pfu) were used for the preparation of MS2 working stocks. MS2 was propagated through infection of an early log phase E. coli Top10F’ liquid culture in LB-tet. To this end, 100 mL of LB-tet media were inoculated with a single E. coli Top10F’ colony from a fresh LB-tet agar Petri dish. The culture was incubated at 37 °C under constant agitation, until OD600 reached a value of 0.15. Simultaneously, 2 μL of the phage stock were added. The culture was left at rest for 5 min in the incubator to allow bacterial infection and was then incubated for 20 h at 37 °C, under constant agitation. After centrifugation (20 min, 3.500× g at 4 °C), the phage-containing supernatant was filtered through a 0.2 nm syringe filter and stored at 4 °C. Typical yields of the procedure ranged between 2 to 5 × 1011 pfu mL−1. The exact titer of the B. stearothermophilus and MS2 preparations was determined by colony counting of plated serial dilutions. To this end, B. stearothermophilus spores were activated by boiling for 5 min before plating, while MS2 samples were incubated with a fresh host E. coli Top10F’ culture before plating. Stock E. coli and S. aureus vials containing 1 mL of corresponding o/n cultures supplemented with 10% glycerol were used for setting 50 mL liquid cultures.
4.2. Chemical Reagents
Heterogeneous photocatalysis was conducted with TiO2 P25 (Degussa, anatase/rutile = 7/3, SBET (Brunauer–Emmett–Teller specific surface area) = 55 ± 15 m2/g, particle size/diameter = 21 nm, CAS No 13463-67-7). For the homogeneous photocatalysis process, the photo-Fenton (Fe3+/H2O2/UV-A) reagent (FeCl3, Chem-Lab, Cat No CL00.0910.1000) was used in acid conditions (pH 3) [12]. All other culture media and chemical reagents used for microorganism growth and buffer preparation were purchased from Applichem.
4.3. Photocatalytic Inactivation
Photocatalytic oxidation of microorganisms was performed in a bench-scale photocatalytic reactor (Figure 2), equipped with five parallel UV-A lamps as a light source (TLD 8 W/08, Phillips, emitting light with a spectral peak centered on 365 nm, 30 cm long, connected to a voltage stabilizer). Experiments were performed in 6-well plates under constant stirring (400 rpm) by a magnetic stirrer, in a final volume of 10 mL. The reaction plates were placed at 10 cm from the irradiation source. The intensity of the incident irradiation received by the treated samples at this distance was measured using a Photometer/Radiometer PMA 2100 (Solar Light Co., Glenside, PY, USA) equipped with a UV-A detector and determined to be 4.59 mW cm−2. Microorganisms were added at a final concentration of 106 colony forming units per milliliter (cfu/mL) or 106 plaque forming units/milliliter (pfu/mL), similar to the levels of pathogen titers in biological fluids of patients with bloodstream infections, representing the highest possible concentrations in biomedical liquid waste [67,68]. E. coli and S. aureus liquid cultures (50 mL) at the exponential growth phase (OD600 = 0.5, corresponding to approximately 4 × 108 cells/mL) were centrifuged (20 min, 2.500 g, 4 °C), washed twice with sterile Phosphate Buffered Saline (PBS) and resuspended in an equal volume of PBS (50 mL). To ensure desired microorganism concentration (initial concentration of 106 cells/mL) in the subsequent photocatalytic reactions, 25 μL of the bacterial suspension was used per 10 mL photocatalysis reactions. Volumes required for 106 cfu/mL or pfu/mL of B. stearothermophilus spores and MS2, respectively, were calculated based on their previously determined titters.
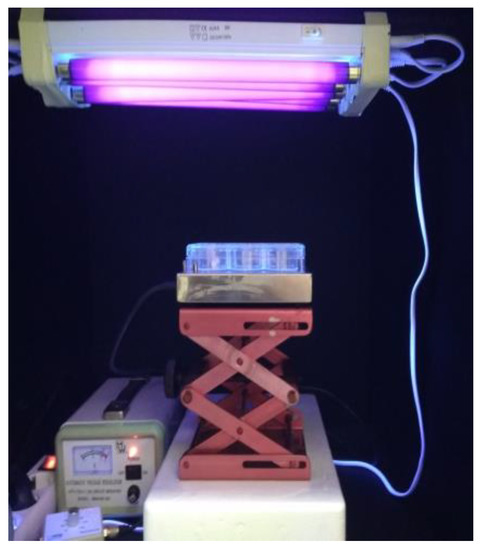
Figure 2.
Configuration of the photocatalytic set-up. The photograph depicts the bench-scale photocatalytic reactor used in this study. UV-A lamps connected to a voltage stabilizer and emitting at a peak of 365 nm were used as a source of irradiation. Photocatalysis was performed in 6-well plates placed at a distance of 10 cm from the light source. A magnetic stirrer was used to ensure constant stirring of the treated samples.
Heterogeneous photocatalysis was performed in PBS, using 0.5 g/L TiO2. Homogeneous photocatalysis was performed in H2O pH 3, using 7 mg/L FeCl3 and 100 mg/L H2O2.
At different time points (0, 2, 5, 15, 30, 60, 90, 120, 180, 300 min, depending on the microorganism), samples (10–1000 μL) were collected and kept on ice and in dark until plated on appropriate nutrient media using the double layer agar method to determine microorganism inactivation. Specifically, for B. stearothermophilus spores, samples were boiled for 5 min to activate endospore germination, then mixed with 3 mL TSB top agar equilibrated at 60 °C and overlayed on TSB agar plates. Plates were incubated o/n at 60 °C, and the formed colonies were counted. For MS2, 10 μL from each sample were mixed by pipetting with 50 μL of a mid-log phase culture (OD600 = 0.5 − 0.8) of the E. coli Top10F’ host and incubated for 5 min at room temperature to allow bacterial infection. Then, samples were mixed with 3 mL LB-top agar equilibrated at 42 °C and overlayed on LB agar plates. Plates were incubated at 37 °C; the viable phage content (pfu) was quantified by counting the plaques formed on the bacteria lawn. For S. aureus and E. coli, collected samples were mixed with 3 mL LB top agar equilibrated at 42 °C, vortexed and spread on agar plates of the appropriate nutrient media (LB and LB-tet, respectively). Plates were incubated o/n at 37 °C, and the formed colonies were counted.
Negative control experiments were performed in the absence of a catalyst or in the presence of a catalyst, but without illumination. Each experimental procedure was performed in triplicates. For heterogeneous photocatalysis optimization, different TiO2 concentrations were tested; 0.1 g/L, 0.5 g/L and 1 g/L. The best-performing TiO2 concentration was then used in experiments supplemented with increasing concentrations of H2O2 (100 mg/L, 500 mg/L, 1000 mg/L).
4.4. Estimation of Photocatalytic Inactivation Efficiency
Microorganism viability reduction in relation to processing time was measured based on the ratio Nt/N0; Nt corresponds to the cfu/mL or pfu/mL determined through colony or plaque counting for each microorganism at the tested time point; N0 corresponds to the initial cfu/mL or pfu/mL (time point 0). We plotted Nt/N0 ratios relative to the processing time per microorganism and condition for each sample and corresponding controls. In addition, we estimated the inactivation efficiency (I) for each sample and time point, based on the colonies or plaques (cfu/mL or pfu/mL) detected in the tested sample (s), relative to zero time (Nts/N0s) and relative to corresponding controls I (Ntc/N0c). The ratio was used. For easier graphical representation the ratio was multiplied by 105 and expressed as log10 values; I .
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28031199/s1, Figure S1: Homogeneous and heterogeneous photocatalytic inactivation of tested microorganisms; Figure S2: Inactivation efficiency of homogeneous and optimized heterogeneous photocatalysis.
Author Contributions
Conceptualization, I.P. (Ioannis Poulios), D.D., K.X., A.A. and T.S.; methodology, I.P. (Ioannis Paspaltsis), S.T., C.B., E.K.; investigation, I.P. (Ioannis Paspaltsis), E.K., S.S., C.B., S.T.; writing—original draft preparation, E.K., S.S., I.P. (Ioannis Poulios); writing—review and editing, T.S., K.X., D.D., M.A.; funding acquisition, T.S., A.A., I.P. (Ioannis Poulios). All authors have read and agreed to the published version of the manuscript.
Funding
This research has been co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH-CREATE-INNOVATE (project code: Τ1ΕΔΚ—02678).
Data Availability Statement
Not applicable.
Acknowledgments
S.S. is an MSc student at School of Biology, Aristotle University of Thessaloniki, Greece, “Applications in Biology”, direction “Applied Genetics and Biodiagnostics”. T.S.’s contribution is dedicated in memory of all common adventures with Vasilis Pagonis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fanourakis, S.K.; Peña-Bahamonde, J.; Bandara, P.C.; Rodrigues, D.F. Nano-Based Adsorbent and Photocatalyst Use for Pharmaceutical Contaminant Removal during Indirect Potable Water Reuse. NPJ Clean Water 2020, 3, 1. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Babalola, S.O.; Onwudiwe, D.C. Photocatalytic Inactivation as a Method of Elimination of E. Coli from Drinking Water. Appl. Sci. 2021, 11, 1313. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
- Poulopoulos, S.G.; Yerkinova, A.; Ulykbanova, G.; Inglezakis, V.J. Photocatalytic Treatment of Organic Pollutants in a Synthetic Wastewater Using UV Light and Combinations of TiO2, H2O2 and Fe(III). PLoS ONE 2019, 14, 1–20. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic Activity Improvement and Application of UV-TiO2 Photocatalysis in Textile Wastewater Treatment: A Review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Hodaifa, G.; Agabo García, C.; Borja, R. Study of Catalysts’ Influence on Photocatalysis/Photodegradation of Olive Oil Mill Wastewater. Determination of the Optimum Working Conditions. Catalysts 2020, 10, 554. [Google Scholar] [CrossRef]
- Murcia Mesa, J.J.; Hernández Niño, J.S.; González, W.; Rojas, H.; Hidalgo, M.C.; Navío, J.A. Photocatalytic Treatment of Stained Wastewater Coming from Handicraft Factories. A Case Study at the Pilot Plant Level. Water (Basel) 2021, 13, 2705. [Google Scholar] [CrossRef]
- Antonopoulou, M. Homogeneous and Heterogeneous Photocatalysis for the Treatment of Pharmaceutical Industry Wastewaters: A Review. Toxics 2022, 10, 539. [Google Scholar] [CrossRef]
- Sagadevan, S.; Fatimah, I.; Egbosiuba, T.C.; Alshahateet, S.F.; Lett, J.A.; Weldegebrieal, G.K.; Le, M.-V.; Johan, M.R. Photocatalytic Efficiency of Titanium Dioxide for Dyes and Heavy Metals Removal from Wastewater. Bull. Chem. React. Eng. Catal. 2022, 17, 430–450. [Google Scholar] [CrossRef]
- Mehta, M.; Chopra, L.; Manikanika. Applications of Nano Photocatalysts in the Degradation of Biomedical Waste: A Short Review. Mater. Today Proc. 2022, 68, 695–700. [Google Scholar] [CrossRef]
- Tsoumachidou, S.; Berberidou, C.; Kitsiou, V.; Poulios, I. Photocatalytic Oxidation of Simulated and Real Hazardous Medical Wastewater: Decolorization, Mineralization and Toxicity Evaluation. J. Chem. Technol. Biotechnol. 2021, 96, 3207–3215. [Google Scholar] [CrossRef]
- Berberidou, C.; Paspaltsis, I.; Pavlidou, E.; Sklaviadis, T.; Poulios, I. Heterogenous Photocatalytic Inactivation of B. Stearothermophilus Endospores in Aqueous Suspensions under Artificial and Solar Irradiation. Appl. Catal. B 2012, 125, 375–382. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic Degradation of Amoxicillin, Ampicillin and Cloxacillin Antibiotics in Aqueous Solution Using UV/TiO2 and UV/H2O2/TiO2 Photocatalysis. Desalination 2010, 252, 46–52. [Google Scholar] [CrossRef]
- Soboleva, N.M.; Saprykina, M.N.; Kosinova, V.N.; Nosonovich, A.A.; Goncharuk, V.V. Inactivation of Candida Albicans in the Photo-Fenton System. J. Water Chem. Technol. 2012, 34, 69–102. [Google Scholar] [CrossRef]
- Wang, Q.; Pang, W.; Mao, Y.; Sun, Q.; Zhang, P.; Ke, Q.; Dai, C.; Zhao, M.; Yu, H. Study of the Degradation of Trimethoprim Using Photo-Fenton Oxidation Technology. Water (Basel) 2019, 11, 207. [Google Scholar] [CrossRef]
- Fazilati, M. Photocatalytic Degradation of Amoxicillin, Cephalexin, and Tetracycline from Aqueous Solution: Comparison of Efficiency in the Usage of TiO2, ZnO, or GO-Fe3O4 Nanoparticles. Desalination Water Treat. 2019, 169, 222–231. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Giraldo-Aguirre, A.L.; Silva-Agredo, J.; Flórez-Acosta, O.A.; Torres-Palma, R.A. Removal of Antibiotic Cloxacillin by Means of Electrochemical Oxidation, TiO2 Photocatalysis, and Photo-Fenton Processes: Analysis of Degradation Pathways and Effect of the Water Matrix on the Elimination of Antimicrobial Activity. Environ. Sci. Pollut. Res. 2017, 24, 6339–6352. [Google Scholar] [CrossRef]
- Massoud, A.; Derbalah, A.; El-Mehasseb, I.; Allah, M.S.; Ahmed, M.S.; Albrakati, A.; Elmahallawy, E.K. Photocatalytic Detoxification of Some Insecticides in Aqueous Media Using TiO2 Nanocatalyst. Int. J. Environ. Res. Public Health 2021, 18, 9278. [Google Scholar] [CrossRef]
- Berberidou, C.; Kitsiou, V.; Lambropoulou, D.A.; Michailidou, D.; Kouras, A.; Poulios, I. Decomposition and Detoxification of the Insecticide Thiacloprid by TiO 2 -mediated Photocatalysis: Kinetics, Intermediate Products and Transformation Pathways. J. Chem. Technol. Biotechnol. 2019, 94, 2475–2486. [Google Scholar] [CrossRef]
- Midik Ertosun, F.; Cellat, K.; Eren, O.; Gül, Ş.; Kuşvuran, E.; Şen, F. Comparison of Nanoscale Zero-Valent Iron, Fenton, and Photo-Fenton Processes for Degradation of Pesticide 2,4-Dichlorophenoxyacetic Acid in Aqueous Solution. SN Appl. Sci. 2019, 1, 1491. [Google Scholar] [CrossRef]
- Creţescu, I.; Lutic, D. Advanced Removal of Crystal Violet Dye from Aqueous Solutions by Photocatalysis Using Commercial Products Containing Titanium Dioxide. Comptes Rendus. Chim. 2022, 25, 39–50. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jellali, S.; Hamed, N.B.H.; el Jery, A.; Khezami, L.; Assadi, A.A.; Amrane, A. Photocatalytic Treatment of Wastewater Containing Simultaneous Organic and Inorganic Pollution: Competition and Operating Parameters Effects. Catalysts 2021, 11, 855. [Google Scholar] [CrossRef]
- Patel, S.K.; Patel, S.G.; Patel, G.V. Degradation of Reactive Dye in Aqueous Solution by Fenton, Photo-Fenton Process and Combination Process with Activated Charcoal and TiO2. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2020, 90, 579–591. [Google Scholar] [CrossRef]
- Suhan, M.B.K.; Mahtab, S.M.T.; Aziz, W.; Akter, S.; Islam, M.S. Sudan Black B Dye Degradation in Aqueous Solution by Fenton Oxidation Process: Kinetics and Cost Analysis. Case Stud. Chem. Environ. Eng. 2021, 4, 100126. [Google Scholar] [CrossRef]
- Elgohary, E.A.; Mohamed, Y.M.A.; el Nazer, H.A.; Baaloudj, O.; Alyami, M.S.S.; el Jery, A.; Assadi, A.A.; Amrane, A. A Review of the Use of Semiconductors as Catalysts in the Photocatalytic Inactivation of Microorganisms. Catalysts 2021, 11, 1498. [Google Scholar] [CrossRef]
- Bandala, E.R.; Perez, R.; Lee, A.E.V.; Sanchez-Salas, J.L.; Quiroz, A.M.; Mendez-Rojas, M.A. Bacillus Subtilis spore inactivation in water using photo-assisted Fenton reaction. Sustain. Environ. Res. 2011, 21, 285–290. [Google Scholar]
- Lee, S.-H.; Pumprueg, S.; Moudgil, B.; Sigmund, W. Inactivation of Bacterial Endospores by Photocatalytic Nanocomposites. Colloids Surf. B Biointerfaces 2005, 40, 93–98. [Google Scholar] [CrossRef]
- Kim, C.; Choi, M.; Jang, J. Nitrogen-Doped SiO2/TiO2 Core/Shell Nanoparticles as Highly Efficient Visible Light Photocatalyst. Catal. Commun. 2010, 11, 378–382. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Asadzadeh-Khaneghah, S.; Feizpoor, S.; Rouhi, A. Review on Heterogeneous Photocatalytic Disinfection of Waterborne, Airborne, and Foodborne Viruses: Can We Win against Pathogenic Viruses? J. Colloid. Interface Sci. 2020, 580, 503–514. [Google Scholar] [CrossRef]
- Lanrewaju, A.A.; Enitan-Folami, A.M.; Sabiu, S.; Swalaha, F.M. A Review on Disinfection Methods for Inactivation of Waterborne Viruses. Front Microbiol. 2022, 13, 1–19. [Google Scholar] [CrossRef]
- Ripolles-Avila, C.; Martinez-Garcia, M.; Hascoët, A.-S.; Rodríguez-Jerez, J.J. Bactericidal Efficacy of UV Activated TiO 2 Nanoparticles against Gram-Positive and Gram-Negative Bacteria on Suspension. CyTA—J. Food 2019, 17, 408–418. [Google Scholar] [CrossRef]
- Carré, G.; Hamon, E.; Ennahar, S.; Estner, M.; Lett, M.-C.; Horvatovich, P.; Gies, J.-P.; Keller, V.; Keller, N.; Andre, P. TiO 2 Photocatalysis Damages Lipids and Proteins in Escherichia Coli. Appl. Environ. Microbiol. 2014, 80, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic Disinfection Using Titanium Dioxide: Spectrum and Mechanism of Antimicrobial Activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.-S.; Lee, J.-I.; Kang, D.-H. TiO2-Based Photocatalyst Generated Reactive Oxygen Species Cause Cell Membrane Disruption of Staphylococcus Aureus and Escherichia Coli O157:H7. Food Microbiol. 2023, 109, 104119. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Punta, C.; Candiani, G. Effect of UV Irradiation and TiO2-Photocatalysis on Airborne Bacteria and Viruses: An Overview. Materials 2021, 14, 1075. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S. Analogies and Differences among Bacterial and Viral Disinfection by the Photo-Fenton Process at Neutral PH: A Mini Review. Environ. Sci. Pollut. Res. 2018, 25, 27676–27692. [Google Scholar] [CrossRef]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Different Inactivation Behaviors of MS2 Phage and Escherichia Coli in TiO 2 Photocatalytic Disinfection. Appl. Environ. Microbiol. 2005, 71, 270–275. [Google Scholar] [CrossRef]
- Khani, M.; Amin, N.A.S.; Hosseini, S.N.; Heidarrezaei, M. Kinetics Study of the Photocatalytic Inactivation of Escherichia Coli. Int. J. Nano Biomater. 2016, 6, 139–150. [Google Scholar] [CrossRef]
- Giannakis, S.; Liu, S.; Carratalà, A.; Rtimi, S.; Talebi Amiri, M.; Bensimon, M.; Pulgarin, C. Iron Oxide-Mediated Semiconductor Photocatalysis vs. Heterogeneous Photo-Fenton Treatment of Viruses in Wastewater. Impact of the Oxide Particle Size. J. Hazard. Mater. 2017, 339, 223–231. [Google Scholar] [CrossRef]
- Rodrigues-Silva, C.; Miranda, S.M.; Lopes, F.V.S.; Silva, M.; Dezotti, M.; Silva, A.M.T.; Faria, J.L.; Boaventura, R.A.R.; Vilar, V.J.P.; Pinto, E. Bacteria and Fungi Inactivation by Photocatalysis under UVA Irradiation: Liquid and Gas Phase. Environ. Sci. Pollut. Res. 2017, 24, 6372–6381. [Google Scholar] [CrossRef]
- Hitkova, H.; Stoyanova, A.; Ivanova, N.; Sredkova, M.; Popova, V.; Iordanova, R.; Bachvarova-Nedelcheva, A. Study of antibacterial activity of nonhydrolytic synthesized TiO2 against E. coli, P. aeruginosa and S. aureus . Beilstein J. Nanotechnol. 2012, 4, 9–17. [Google Scholar]
- Armon, R.; Narkis, N.; Neeman, I. Photocatalytic Inactivation of Different Bacteria and Bacteriophages in Drinking Water at Different TiO2 Concentration With or Without Exposure to O2. J. Adv. Oxid. Technol. 2017, 3, 145–150. [Google Scholar] [CrossRef]
- Chartier, Y. Safe Management of Wastes from Health-Care Activities; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Hong, J.; Zhan, S.; Yu, Z.; Hong, J.; Qi, C. Life-Cycle Environmental and Economic Assessment of Medical Waste Treatment. J. Clean Prod. 2018, 174, 65–73. [Google Scholar] [CrossRef]
- Klangsin, P.; Harding, A.K. Medical Waste Treatment and Disposal Methods Used by Hospitals in Oregon, Washington, and Idaho. J. Air Waste Manag. Assoc. 1998, 48, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Windfeld, E.S.; Brooks, M.S.-L. Medical Waste Management—A Review. J. Environ. Manag. 2015, 163, 98–108. [Google Scholar] [CrossRef]
- Ormsby, A.A. Electro-Thermal Deactivation: One Approach to Comprehensive Medical Waste Management. Med. Waste Anal. 1993, 2, 15–16. [Google Scholar]
- Lee, B.-K.; Ellenbecker, M.J.; Moure-Ersaso, R. Alternatives for Treatment and Disposal Cost Reduction of Regulated Medical Wastes. Waste Manag. 2004, 24, 143–151. [Google Scholar] [CrossRef]
- Trunk, T.; Khalil, H.S.; Leo, J.C. Bacterial Autoaggregation. AIMS Microbiol. 2018, 4, 140–164. [Google Scholar] [CrossRef]
- Haaber, J.; Cohn, M.T.; Frees, D.; Andersen, T.J.; Ingmer, H. Planktonic Aggregates of Staphylococcus Aureus Protect against Common Antibiotics. PLoS ONE 2012, 7, e41075. [Google Scholar] [CrossRef]
- Gogniat, G.; Thyssen, M.; Denis, M.; Pulgarin, C.; Dukan, S. The Bactericidal Effect of TiO2 Photocatalysis Involves Adsorption onto Catalyst and the Loss of Membrane Integrity. FEMS Microbiol Lett. 2006, 258, 18–24. [Google Scholar] [CrossRef]
- Adán, C.; Magnet, A.; Fenoy, S.; Pablos, C.; del Águila, C.; Marugán, J. Concomitant Inactivation of Acanthamoeba Spp. and Escherichia Coli Using Suspended and Immobilized TiO2. Water Res. 2018, 144, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Laxma Reddy, P.V.; Kavitha, B.; Kumar Reddy, P.A.; Kim, K.-H. TiO 2 -Based Photocatalytic Disinfection of Microbes in Aqueous Media: A Review. Environ. Res. 2017, 154, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Lonnen, J.; Kilvington, S.; Kehoe, S.C.; Al-Touati, F.; McGuigan, K.G. Solar and Photocatalytic Disinfection of Protozoan, Fungal and Bacterial Microbes in Drinking Water. Water Res. 2005, 39, 877–883. [Google Scholar] [CrossRef]
- Tsai, T.-M.; Chang, H.-H.; Chang, K.-C.; Liu, Y.-L.; Tseng, C.-C. A Comparative Study of the Bactericidal Effect of Photocatalytic Oxidation by TiO2 on Antibiotic-Resistant and Antibiotic-Sensitive Bacteria. J. Chem. Technol. Biotechnol. 2010, 85, 1642–1653. [Google Scholar] [CrossRef]
- Rao, G.; Brastad, K.S.; Zhang, Q.; Robinson, R.; He, Z.; Li, Y. Enhanced Disinfection of Escherichia Coli and Bacteriophage MS2 in Water Using a Copper and Silver Loaded Titanium Dioxide Nanowire Membrane. Front Environ. Sci. Eng. 2016, 10, 11. [Google Scholar] [CrossRef]
- Bonnet, M.; Massard, C.; Veisseire, P.; Camares, O.; Awitor, K.O. Environmental Toxicity and Antimicrobial Efficiency of Titanium Dioxide Nanoparticles in Suspension. J. Biomater. Nanobiotechnol. 2015, 06, 213–224. [Google Scholar] [CrossRef]
- Gupta, K.; Singh, R.P.; Pandey, A.; Pandey, A. Photocatalytic Antibacterial Performance of TiO 2 and Ag-Doped TiO 2 against S. Aureus . P. Aeruginosa and E. Coli. Beilstein J. Nanotechnol. 2013, 4, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Kühn, K.P.; Chaberny, I.F.; Massholder, K.; Stickler, M.; Benz, V.W.; Sonntag, H.-G.; Erdinger, L. Disinfection of Surfaces by Photocatalytic Oxidation with Titanium Dioxide and UVA Light. Chemosphere 2003, 53, 71–77. [Google Scholar] [CrossRef]
- Mitoraj, D.; Jańczyk, A.; Strus, M.; Kisch, H.; Stochel, G.; Heczko, P.B.; Macyk, W. Visible Light Inactivation of Bacteria and Fungi by Modified Titanium Dioxide. Photochem. Photobiol. Sci. 2007, 6, 642–648. [Google Scholar] [CrossRef]
- Dunlop, P.S.M.; Sheeran, C.P.; Byrne, J.A.; McMahon, M.A.S.; Boyle, M.A.; McGuigan, K.G. Inactivation of Clinically Relevant Pathogens by Photocatalytic Coatings. J. Photochem. Photobiol. A Chem. 2010, 216, 303–310. [Google Scholar] [CrossRef]
- Kokkinos, P.; Venieri, D.; Mantzavinos, D. Advanced Oxidation Processes for Water and Wastewater Viral Disinfection. A Systematic Review. Food Environ. Virol. 2021, 13, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO 2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Kosma, C.; Albanis, T.; Konstantinou, I. An Overview of Homogeneous and Heterogeneous Photocatalysis Applications for the Removal of Pharmaceutical Compounds from Real or Synthetic Hospital Wastewaters under Lab or Pilot Scale. Sci. Total Environ. 2021, 765, 144163. [Google Scholar] [CrossRef] [PubMed]
- Adish Kumar, S.; Sree lekshmi, G.S.; Rajesh Banu, J.; Tae Yeom, I. Synergistic Degradation of Hospital Wastewater by Solar/TiO2/Fe2+/H2O2 Process. Water Qual. Res. J. 2014, 49, 223–233. [Google Scholar] [CrossRef]
- Aranciaga Pajuelo, R.B.; Vargas López, J.P.; Castañeda Olivera, C.A.; Jave Nakayo, J.L.; Benites Alfaro, E.G.; Cabrera Carranza, C.F. Inactivation of Antibiotic Resistant Bacteria in Hospital Wastewater by TiO2/H2O2 Photocatalysis. Chem. Eng. Trans. 2021, 86, 853–858. [Google Scholar] [CrossRef]
- Bacconi, A.; Richmond, G.S.; Baroldi, M.A.; Laffler, T.G.; Blyn, L.B.; Carolan, H.E.; Frinder, M.R.; Toleno, D.M.; Metzgar, D.; Gutierrez, J.R.; et al. Improved Sensitivity for Molecular Detection of Bacterial and Candida Infections in Blood. J. Clin. Microbiol. 2014, 52, 3164–3174. [Google Scholar] [CrossRef] [PubMed]
- Maaroufi, Y.; Heymans, C.; de Bruyne, J.-M.; Duchateau, V.; Rodriguez-Villalobos, H.; Aoun, M.; Crokaert, F. Rapid Detection of Candida Albicans in Clinical Blood Samples by Using a TaqMan-Based PCR Assay. J. Clin. Microbiol. 2003, 41, 3293–3298. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).