An Update on the Therapeutic Anticancer Potential of Ocimum sanctum L.: “Elixir of Life”
Abstract
1. Introduction
- Ocimum americanum L.: The leaves of the Ocimum americanum plant have eugenol, pinene, myrcene, camphene, sabinene, bicyclogermacrene, bisabolene, bisabolene, 1,8-cineole, limonene, fenchone, linalool oxide, linalool, borneol, camphor, 4-terpineol, and cis-piperitol [11]. Ocimum americanum is native to the equatorial regions of Africa, the Indian subcontinent, China, and Southeast Asia. It has since become naturalized in Queensland, Christmas Island, and other parts of tropical Australia. People have used it to treat coughs, ulcers, tuberculosis, haemorrhoids, stomach pains, and problems with the eyes and ears. It has also been used to treat stomach aches, diarrhoea, diabetes, and constipation [9,12,13,14].
- Ocimum basilicum L., also referred to as sweet basil, is a plant that is indigenous to the Indian subcontinent, Southeast Asia, Russia, Ukraine, Cameroon, Africa, Guinea, Mali, Mexico, Central America, South America, and a variety of islands in the ocean. It is also cultivated in a number of countries around the world. Patients who suffer from cardiovascular disease, diabetes, chronic pain, fever, vomiting, diarrhoea, and other conditions are given this as a preventative measure in addition to a treatment for their illness. In addition to having properties that make it an effective sedative, it can also be used to treat skin infections, bites from snakes, and stings from insects [15,16,17,18]. Compounds such as pinene, myrcene, 4-hexen-1-ol acetate, 4-eucalyptol, cis-linaloloxide, 1,6-octadien-3-ol,3,7-dimethyl, methyl ethyl cyclopentene, l-menthol, l-(-)-menthol, and estragole were obtained from Ocimum basilicum leaves through other compounds that can be found in the leaves include N-cyano-3-methylbut-2-enamine, formic acid, cyclohexyl ester, eugenol, cyclohexyl phenyl hydrazide, citral, 4-methyl-1-(1-methylethyl) cyclohexane, phenol 2,3,5-trimethyl, copaene, cis-7,10,13,16-docos [19,20].
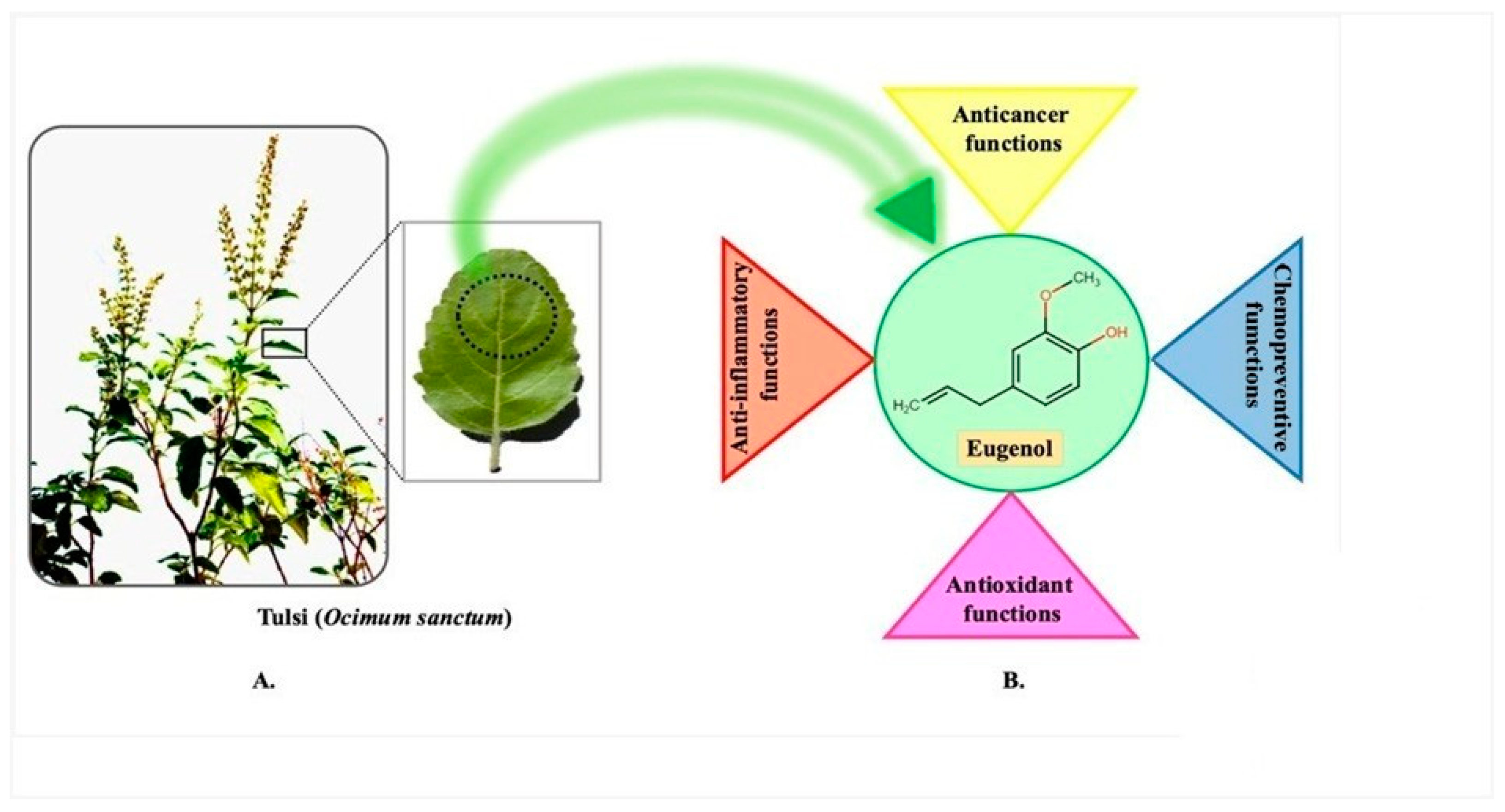
- Ocimum tenuiflorum L. (Ocimum sanctum), also known as Holy basil or tulsi, has been naturalized in Kenya, Fiji, French Polynesia, the West Indies, and Venezuela. It is originally from China, the Indian Subcontinent, Southeast Asia, New Guinea, and Queensland, Australia. In traditional medicine, it is employed to manage and treat a variety of conditions, including headaches, fevers, coughs, the common cold, influenza, sore throats, colic pain, asthma, diarrhoea, digestive disorders, bronchitis, influenza, insomnia, arthritis, and malaria fever. In addition, it can be used as a treatment for scorpion stings and snakebites [21,22,23]. The chemical compounds eugenol, cyclohexane, 1,2,4-triethene, and caryophyllene can be found in the extracts that are made from the leaves. Compounds such as the following have been utilized: benzenemethenamine; N,N-,4-tetramethyl-; 10-heptadecen-8-ynoic acid; cyclopentane, cyclopropylidene-; Z,Z-4,16-octadecadien-1-ol acetate; benzenemethenamine; and 3′,8,8′-Trimethoxy-3-piperidyl-2,2′-binaphthalene-1,1′,4,4′-tetrone.
- Ocimum gratissimum: The Ocimum gratissimum plant is indigenous to India, China, Nigeria, and both Australia and New Zealand. Diabetes, infections of the upper respiratory tract, pneumonia, epilepsy, fever, convulsions, diarrhoea, headaches, and flu are just some of the conditions that the leaves, stems, roots, and flowers of this plant are used to treat and prevent [24,25,26,27,28,29,30,31]. Compounds such as eugenol, methyl eugenol, cis-ocimene, trans-ocimene, pinene, camphor, germacrene-D, trans-caryophyllene, farnesene, l-bisabolene, thymol, methyl chavicol, linalool, limonene, and methyl eugenol can be found in the extract of the Ocimum gratissimum plant.
- Ocimum kilimandscharicum: The camphor basil is a plant that is native to India, Thailand, Ethiopia, Tanzania, Kenya, Uganda, and Sudan. It is also cultivated in Uganda and Kenya. Cough, cold, measles, abdominal pain, measles, diarrhoea, and diarrhoea are some of the conditions that it is used to treat. In addition to these uses, it is also put to use as an insect repellent and for the control of pests in storage.
- Ocimum campechianum Mill., (the Amazonian basil) can be found all over the Americas. Any portion of the plant, such as the fruits, seeds, flowers, leaves, bark, roots, and so on, can be used to produce plant-based natural constituents, meaning that any part of the plant may have active ingredients. The mixtures of secondary products found in plants are often what give plant materials their positive therapeutic properties. This idea is congruent with the idea that a particular plant’s combination of secondary metabolites has therapeutic properties that are specific to that plant’s species or group of species [32]. Tulsi has a variety of chemical components, including carvacrol, caryophyllene, elemene, eugenol, linalool, rosmarinic acid, oleanolic acid, germacrene, and ursolic acid (Table 1). Tulsi is thought to have stimulating and diuretic properties [33]. The leaves of medicinal herbs can also be used to produce volatile and fixed oils. Daily intake of its leaves and products is said to prevent ailments, increase health, lifespan, and happiness. The nutritional ingredients of tulsi are presented in Table 2. Several scientific studies have examined the potent therapeutic potential of tulsi, including numerous experiments on humans and animals. These studies have revealed that tulsi has a variety of therapeutic benefits, including anti-inflammatory, anti-pyretic, anti-allergic, anti-asthmatic, anti-tussive, anti-ulcer, anti-emetic, anti-spasmodic, mosquito repellent, anti-diarrhoeal, antioxidant, anti-stress, hepato-protective, cardio-protective, neuro-protective, anti-hypercholesterolemic, antidiabetic, anti-coagulant, adaptogenic, anti-thyroid, anti-cataract, anti-carcinogenic, radioprotective, anti-hypertensive, analgesic, diuretic, antifertility, anti-ulcer, anti-leukodermal, anti-microbial (including antiviral, antibacterial, antifungal, anti-protozoal, anti-malarial, anti-helminthic), anti-arthritic, anti-toxic, wound heal effect, immunomodulatory, CNS depressant, memory enhancement activities [34].
- Ocimum Sanctum (Ocimum tenuiflorum L.)Multiple pharmacological effects of Ocimum sanctum have been documented and described in the literature. It has been shown by Rahman et al. [35] that Ocimum sanctum has antimicrobial activity against many different types of bacteria. These include Staphylococci sp., E. coli, Shigella sp., S. aureus, Enterobacteria sp., P. aernginosa, S. Typhi, Staphylococci sp., C. albicans, and K. pneumonia [35]. Ocimum sanctum extract was found to be a potent anti-tuberculosis agent in an in vitro study conducted by Farivar et al. [36]. Several different fungi, including A. solani, C. guillermondii, C. capsici, Curvularia sp., F. solani, H. oryzae, and A. flavus, were found to be effectively combated by Ocimum sanctum’s aqueous, hexane, chloroform, and n-butanol extracts and essential oil, as reported by Khan et al. [37]. Significant antioxidant activity was observed in vitro and in vivo when Ocimum sanctum was extracted using methanol, hydroalcoholic, and aqueous solvents, as reported by Kelm et al. [38]. Oral consumption of Ocimum sanctum significantly protects liver and aortic tissue from hypercholesterolemia-induced peroxidative damage, as reported by Geetha et al. [39]. Blood sugar levels in streptozotocin-induced and glucose-fed perglycaemic diabetic rats were significantly lowered after oral administration of Ocimum sanctum extract, as documented by Siva et al. Gholap and Kar found similar results, namely that Ocimum sanctum decreased cortisol and glucose levels in the blood and exhibited elicited antiperoxidative activity in their study [40,41]. Researchers Aruna et al. found that the incidences of squamous cell carcinoma and hematoma in experimental rats were significantly reduced when they were given Ocimum sanctum leaves [42]. Ocimum sanctum aqueous leaf extract significantly reduced hydroxyl (OH) radical-induced deoxyribose degeneration, according to research by Ganasoundari et al. [43]. Additionally, they demonstrated that WR2721 and Ocimum sanctum’s synergistic activity produced a more potent effect against OH radical activity than either agent alone [43]. Using an excision model, Shetty et al. tested the effects of an aqueous leaf extract from Ocimum sanctum on tumour necrosis factor-alpha (TNF-Alfa) in laboratory rats. The rate of epithelization and wound contraction was shown to be significantly increased by the Ocimum sanctum extract, indicating a significant wound healing effect. The oil of Ocimum sanctum demonstrated significant antiulcer activity in a study of aspirin, indomethacin, alcohol (ethanol 50%), histamine, reserpine, serotonin, and stress-induced ulcers in rats [44]. Laboratory rats’ humoral immune responses were found to be altered after exposure to a steam-distilled leave extract of Ocimum sanctum, as reported by Mediratta et al. [45]. This may be because of mediators released during hypersensitivity reactions, tissue responses to these mediators, or both [46]. Ocimum sanctum’s immunomodulatory effect was also demonstrated by Godhwani et al. in a separate experiment using widal and sheep erythrocyte agglutination tests. Ocimum sanctum has been shown to be effective in the treatment of asthma and related conditions, as demonstrated by Sridevi et al. [47]. Ocimum sanctum was reported to have the potential to stabilize mast cells, suppress IgE, and inhibit the release of inflammatory mediators, suggesting it is responsible for these effects [48]. According to research done by Ravindran et al. [49], the Ocimum sanctum has been found to have anti-stress properties due to the fact that it helps restore normal levels of neurotransmitters in the body after being exposed to noise stress. Researchers in the past demonstrated that the essential oil of Ocimum sanctum possessed powerful anti-helminthic activity by using Caenorhabditis elegans as a model organism. Experiments were performed on various extracts of Ocimum sanctum stem and leaves (n = 132) to test for anticonvulsant activity. The experiments used the maximal electroshock model as the experimental design, and phenytoin was used as the standard. It was discovered that extracts of the leaf and stem made with ethanol and chloroform produced significant preventive effects against toxic convulsions induced by trans-corneal electroshock. These convulsions were caused by toxic electric shocks delivered through the cornea. Ocimum sanctum has a strong cardioprotective effect against myocardial agents, as has been demonstrated in animal studies [50]. Antivenomous effects of Ocimum sanctum have been studied and found to be effective against venomous dog, snake, scorpion, and insect bites [50].Ocimum sanctum, as discovered by Sood et al., significantly shielded isoproterenol-induced myocardial necrosis in experimental rats by boosting endogenous antioxidants’ activity [51]. Furthermore, Ocimum sanctum L. alcoholic extract was found to improve scopolamine-induced amnesia and age-related memory loss in mice. Step-down latency (SDL) and acetylcholine esterase inhibition were both significantly enhanced by Ocimum sanctum. This approach might be helpful for patients suffering from dementia, Alzheimer’s disease, and other types of cognitive impairment [52].
2. Phytochemical Composition
2.1. Eugenol
2.2. Caryophyllene
2.3. Ursolic Acid (UrsA)
2.4. Rosmarinic Acid (RA)
2.5. Apigenin
2.6. Carvacrol
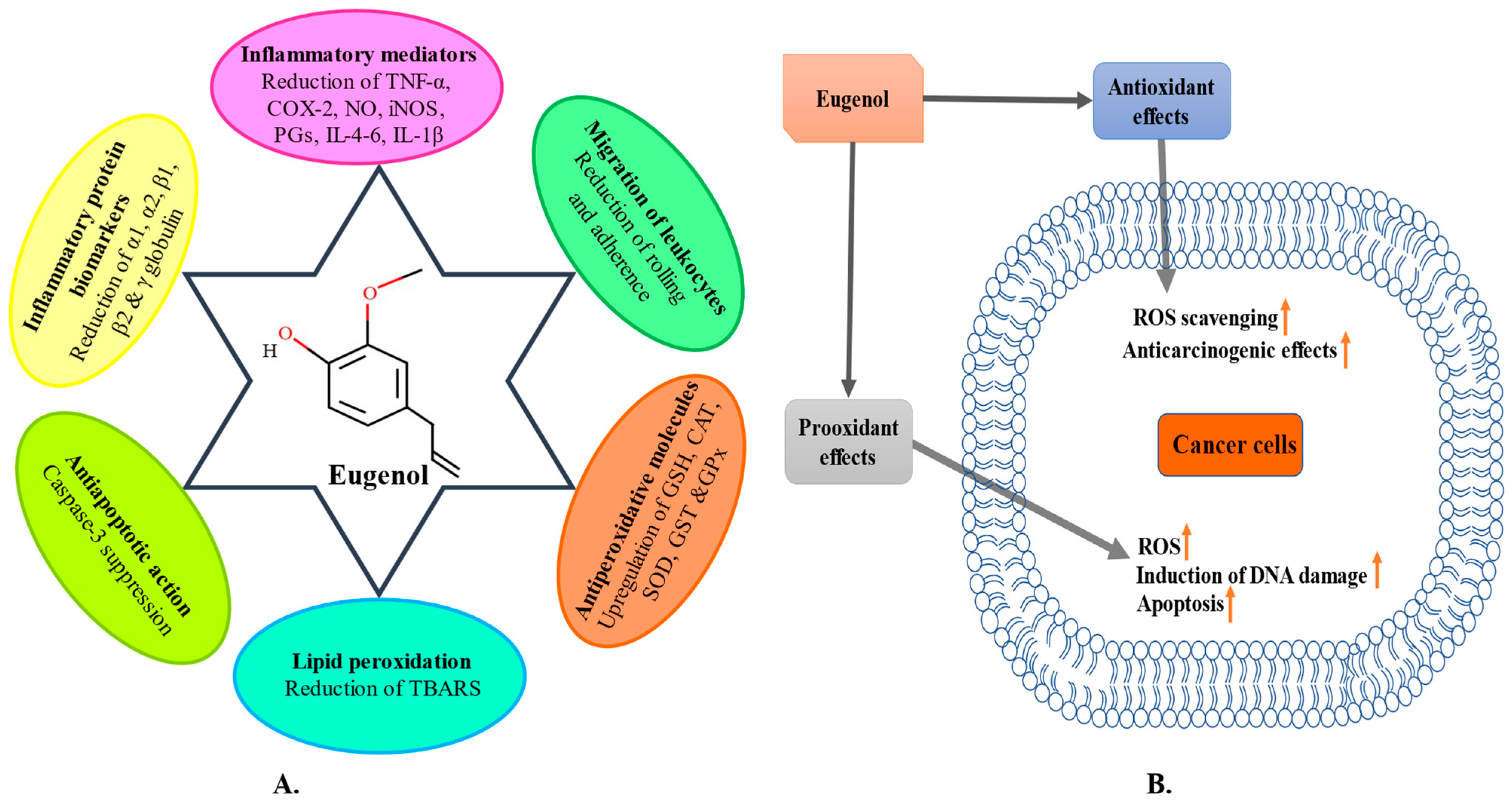
3. Anti-Inflammatory Potential
4. Antioxidant Potential
5. Anticancer Potential
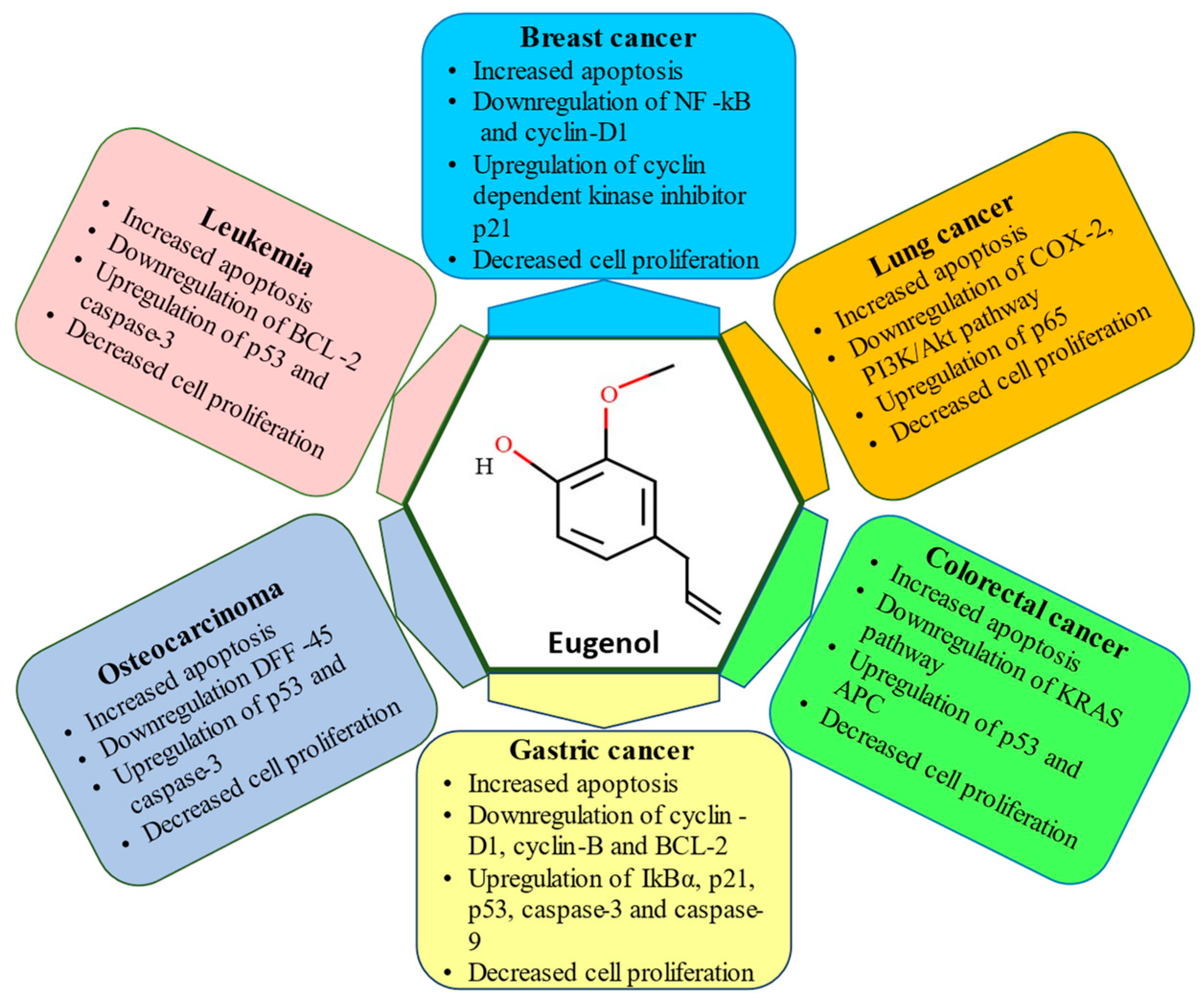
6. Skin Cancer
7. Lung Cancer
8. Breast Cancer
9. Gastric Cancer
10. Osteosarcoma
11. Colorectal Cancer
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheek, D.M.; Naxerova, K. Mapping the long road to cancer. Cell 2022, 185, 939–940. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, N.; Cohen, M.M. The Clinical Efficacy and Safety of Tulsi in Humans: A Systematic Review of the Literature. Evid.-Based Complement. Altern. Med. 2017, 2017, 9217567. [Google Scholar] [CrossRef] [PubMed]
- Flegkas, A.; MilosevićIfantis, T.; Barda, C.; Samara, P.; Tsitsilonis, O.; Skaltsa, H. Antiproliferative activity of (−)-rabdosiin isolated from Ocimum sanctum L. Medicines 2019, 6, 37. [Google Scholar] [CrossRef]
- Banerjee, A.; Pavane, M.S.; Banu, L.H.; Gopikar, A.S.R.; Elizabeth, K.R.; Pathak, S. Traditional medicine for aging-related disorders: Implications for drug discovery. In Stem Cells and Aging; Elsevier: Amsterdam, The Netherlands, 2021; pp. 281–297. [Google Scholar]
- Zaidi, K.U.; Shah, F.; Parmar, R.; Thawani, V. Anticandidal synergistic activity of Ocimum sanctum and fluconazole of azole resistance strains of clinical isolates. J. Mycol. Med. 2018, 28, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.; Bishayee, A. Ocimum sanctum Linn. (Tulsi): An Ethnomedicinal Plant for the Prevention and Treatment of Cancer. Anticancer Drugs 2013, 24, 659–666. [Google Scholar] [CrossRef]
- Enegide, C.; Charles C, O. Ocimum Species: Ethnomedicinal Uses, Phytochemistry and Pharmacological Importance. Int. J. Curr. Res. Physiol. Pharmacol. 2021, 1–12. [Google Scholar] [CrossRef]
- Sharma, M.; Kishore, K.; Gupta, S.K.; Joshi, S.; Arya, D.S. Cardioprotective potential of Ocimum sanctum in isoproterenol induced myocardial infarction in rats. Mol. Cell Biochem. 2001, 225, 75–83. [Google Scholar] [CrossRef]
- Harsha, M.; Mohan Kumar, K.; Kagathur, S.; Amberkar, V. Effect of Ocimum Sanctum Extract on Leukemic Cell Lines:A Preliminary in-Vitro Study. J. Oral Maxillofac. Pathol. 2020, 24, 93. [Google Scholar] [CrossRef]
- Hussain, A.; Brahmbhatt, K.; Priyani, A.; Ahmed, M.; Rizvi, T.A.; Sharma, C. Eugenol Enhances the Chemotherapeutic Potential of Gemcitabine and Induces Anticarcinogenic and Anti-Inflammatory Activity in Human Cervical Cancer Cells. Cancer Biother. Radiopharm. 2011, 26, 519–527. [Google Scholar] [CrossRef]
- Vandana, S.P.; Suresh, R.N. Evaluation of Cardioprotective Activity of Ginkgo Biloba and Ocimum Sanctum in Rodents. Altern. Med. Rev. 2009, 14, 161. [Google Scholar]
- Shukla, S.T.; Kulkarni, V.H.; Habbu, P.V.; Jagadeesh, K.S.; Patil, B.S.; Smita, D.M. Hepatoprotective and antioxidant activities of crude fractions of endophytic fungi of Ocimum sanctum Linn. in rats. Orient. Pharm. Exp. Med. 2012, 12, 81–91. [Google Scholar] [CrossRef]
- Horvathova, E.; Navarova, J.; Galova, E.; Sevcovicova, A.; Chodakova, L.; Snahnicanova, Z.; Melusova, M.; Kozics, K.; Slamenova, D. Assessment of Antioxidative, Chelating, and DNA-Protective Effects of Selected Essential Oil Components (Eugenol, Carvacrol, Thymol, Borneol, Eucalyptol) of Plants and Intact Rosmarinus Officinalis Oil. J Agric Food Chem. 2014, 62, 6632–6639. [Google Scholar] [CrossRef]
- Manikandan, P.; Murugan, R.S.; Abbas, H.; Abraham, S.K.; Nagini, S. Ocimum Sanctum Linn. (Holy Basil) Ethanolic Leaf Extract Protects Against 7,12-Dimethylbenz[a]Anthracene-Induced Genotoxicity, Oxidative Stress, and Imbalance in Xenobiotic-Metabolizing Enzymes. J. Med. Food 2007, 10, 495–502. [Google Scholar] [CrossRef]
- Das, S.K.; Vasudevan, D.M. Tulsi: The Indian holy power plant. Nat. Prod. Radiance 2006, 5, 279–283. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Mateen, S.; Rehman, T.; Shahzad, S.; Naeem, S.S.; Faizy, A.F.; Khan, A.Q.; Khan, M.S.; Husain, F.M.; Moin, S. Anti-oxidant and anti-inflammatory effects of cinnamaldehyde and eugenol on mononuclear cells of rheumatoid arthritis patients. Eur. J. Pharmacol. 2019, 852, 14–24. [Google Scholar] [CrossRef]
- Zari, A.T.; Zari, T.A.; Hakeem, K.R. Anticancer Properties of Eugenol: A Review. Molecules 2021, 26, 7407. [Google Scholar] [CrossRef]
- Chenni, M.; el Abed, D.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Comparative study of essential oils extracted from Egyptian basil leaves (Ocimum basilicum L.) using hydro-distillation and solvent-free microwave extraction. Molecules 2016, 21, 113. [Google Scholar] [CrossRef]
- Al-Trad, B.; Alkhateeb, H.; Alsmadi, W.; Al-Zoubi, M. Eugenol ameliorates insulin resistance, oxidative stress and inflammation in high fat-diet/streptozotocin-induced diabetic rat. Life Sci. 2018, 216, 183–188. [Google Scholar] [CrossRef]
- da Silva Bruckmann, F.D.S.; Viana, A.R.; Lopes, L.Q.S.; Santos, R.C.V.; Muller, E.I.; Mortari, S.R.; Rhoden, C.R.B. Synthesis, characterization, and biological activity evaluation of magnetite-functionalized eugenol. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1459–1472. [Google Scholar] [CrossRef]
- Yi, J.-L.; Shi, S.; Shen, Y.-L.; Wang, L.; Chen, H.-Y.; Zhu, J.; Ding, Y. Myricetin and methyl eugenol combination enhances the anticancer activity, cell cycle arrest and apoptosis induction of cis-platin against HeLa cervical cancer cell lines. Int. J. Clin. Exp. Pathol. 2015, 8, 1116–1127. [Google Scholar]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Raja, M.R.C.; Srinivasan, V.; Selvaraj, S.; Mahapatra, S.K. Versatile and synergistic potential of eugenol: A review. Pharm. Anal. Acta 2015, 6, 367–372. [Google Scholar]
- Khalil, A.A.; ur Rahman, U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential oil eugenol: Sources, extraction techniques and nutraceutical perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef]
- El-SaberBatiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; Abdel-Daim, M.M.; Prasad Devkota, H. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- B Aggarwal, B.; Prasad, S.; Reuter, S.; Kannappan, R.; R Yadav, V.; Park, B.; Hye, K.J.; Gupta, S.; Phromnoi, K.; Sundaram, C.; et al. Identification of novel anti-inflammatory agents from Ayurvedic medicine for prevention of chronic diseases: “reverse pharmacology” and “bedside to bench” approach. Curr. Drug Targets 2011, 12, 1595–1653. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M. Eugenol—From the remote Maluku Islands to the international market place: A review of a remarkable and versatile molecule. Molecules 2012, 17, 6953–6981. [Google Scholar] [CrossRef]
- Yadav, M.K.; Chae, S.W.; Im, G.J.; Chung, J.W.; Song, J.J. Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. PLoS ONE 2015, 10, e0119564. [Google Scholar] [CrossRef]
- Pattanayak, P.; Behera, P.; Das, D.; Panda, S. Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharm. Rev. 2010, 4, 95. [Google Scholar] [CrossRef]
- Falagas, M.E.; Bliziotis, I.A. Pandrug-resistant Gram-negative bacteria: The dawn of the post-antibiotic era? Int. J. Antimicrob. Agents 2007, 29, 630–636. [Google Scholar] [CrossRef]
- Wangcharoen, W.; Wallaya, M. Antioxidant capacity and phenolic content of chilies. Agric. Nat. Resour. 2007, 41, 561–569. [Google Scholar]
- Rahman, S.; Islam, R.; Alam, K.; Hena, M.A.; Jamal, M. Ocimum Sanctum L.: A Review of Phytochemical and Pharmacological Profile. Am. J. Drug Discov. Dev. 2011. [Google Scholar] [CrossRef]
- Farivar, T.; Fard, A.; Zahedani, S.; Naderi, M.; Moud, B. Anti Tuberculosis Effect of Ocimum Sanctum Extracts in in Vitro and Macrophage Culture. J. Med. Sci. 2006, 6, 348–351. [Google Scholar] [CrossRef]
- Khan, A.; Ahmad, A.; Manzoor, N.; Khan, L.A. Antifungal Activities of Ocimum Sanctum Essential Oil and Its Lead Molecules. Nat Prod Commun. 2010, 5, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Kelm, M.A.; Nair, M.G.; Strasburg, G.M.; DeWitt, D.L. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine 2000, 7, 7–13. [Google Scholar] [CrossRef]
- Geetha; Kedlaya, R.; Vasudevan, D.M. Inhibition of Lipid Peroxidation by Botanical Extracts of Ocimum Sanctum: In Vivo and in Vitro Studies. Life Sci. 2004, 76, 21–28. [Google Scholar] [CrossRef]
- Siva, M.; Shanmugam, K.R.; Shanmugam, B.; Venkata Subbaiah, G.; Ravi, S.; Sathyavelu Reddy, K.; Mallikarjuna, K. Ocimum Sanctum: A Review on the Pharmacological Properties. Int. J. Basic Clin. Pharmacol. 2016, 558–565. [Google Scholar] [CrossRef]
- Gholap, S.; Kar, A. Hypoglycaemic Effects of Some Plant Extracts Are Possibly Mediated through Inhibition in Corticosteroid Concentration. Pharmazie 2004, 59, 876–878. [Google Scholar]
- Aruna, K.; Sivaramakrishnan, V.M. Anticarcinogenic Effects of Some Indian Plant Products. Food Chem. Toxicol. 1992, 30, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Ganasoundari, A.; Uma Devi, P.; Rao, B.S.S. Enhancement of Bone Marrow Radioprotection and Reduction of WR-2721 Toxicity by Ocimum Sanctum. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1998, 397, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.M.; Abdelmohsen, U.R.; Ayoub, A.T.; Salem, M.A.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Metabolic Profiling, Histopathological Anti-Ulcer Study, Molecular Docking and Molecular Dynamics of Ursolic Acid Isolated from Ocimum Forskolei Benth. (Family Lamiaceae). South Afr. J. Bot. 2020, 131, 311–319. [Google Scholar] [CrossRef]
- Mediratta, P.K.; Sharma, K.K.; Singh, S. Evaluation of Immunomodulatory Potential of Ocimum Sanctum Seed Oil and Its Possible Mechanism of Action. J. Ethnopharmacol. 2002, 80, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, M. Evaluation of In Vitro Anticancer Activity of Ocimum Basilicum, Alhagi Maurorum, Calendula Officinalis and Their Parasite Cuscuta Campestris. PLoS ONE 2014, 9, e116049. [Google Scholar] [CrossRef]
- Sridevi, M.; Kalaiarasi, P.; Pugalendi, K. Antihyperlipidemic Activity of Alcoholic Leaf Extract of Solanum Surattense in Streptozotocin-Diabetic Rats. Asian Pac. J. Trop. Biomed. 2011, 1, S276–S280. [Google Scholar] [CrossRef]
- Abdullah, M.L.; Hafez, M.M.; Al-Hoshani, A.; Al-Shabanah, O. Anti-Metastatic and Anti-Proliferative Activity of Eugenol against Triple Negative and HER2 Positive Breast Cancer Cells. BMC Complement. Altern. Med. 2018, 18, 321. [Google Scholar] [CrossRef]
- Ravindran, R.; Devi, R.S.; Samson, J.; Senthilvelan, M. Noise-Stress-Induced Brain Neurotransmitter Changes and the Effect of Ocimum Sanctum (Linn) Treatment in Albino Rats. J. Pharmacol. Sci. 2005, 98, 354–360. [Google Scholar] [CrossRef]
- Manikandan, P.; Vinothini, G.; Vidya Priyadarsini, R.; Prathiba, D.; Nagini, S. Eugenol Inhibits Cell Proliferation via NF-ΚB Suppression in a Rat Model of Gastric Carcinogenesis Induced by MNNG. Investig. New Drugs 2011, 29, 110–117. [Google Scholar] [CrossRef]
- Sood, S.; Narang, D.; Thomas, M.K.; Gupta, Y.K.; Maulik, S.K. Effect of Ocimum Sanctum Linn. on Cardiac Changes in Rats Subjected to Chronic Restraint Stress. J. Ethnopharmacol. 2006, 108, 423–427. [Google Scholar] [CrossRef]
- Chniguir, A.; Saguem, M.H.; El-Benna, J.; Bachoual, R. Eugenol inhibits neutrophil myeloperoxidase in vitro and atten ates LPS-induced lung inflammation in mice. Europe PMC 2022. Preprint. [Google Scholar]
- Petrocelli, G.; Farabegoli, F.; Valerii, M.C.; Giovannini, C.; Sardo, A.; Spisni, E. Molecules Present in Plant Essential Oils for Prevention and Treatment of Colorectal Cancer (CRC). Molecules 2021, 26, 885. [Google Scholar] [CrossRef]
- Manaharan, T.; Thirugnanasampandan, R.; Jayakumar, R.; Kanthimathi, M.S.; Ramya, G.; Ramnath, M.G. Purified essential oil from Ocimum sanctum Linn. triggers the apoptotic mechanism in human breast cancer cells. Pharmacogn. Mag. 2016, 12 (Suppl. S3), 327–331. [Google Scholar] [CrossRef]
- Gurav, T.P.; Dholakia, B.B.; Giri, A.P. A glance at the chemodiversity of Ocimum species: Trends, implications, and strategies for the quality and yield improvement of essential oil. Phytochem. Rev. 2022, 21, 879–913. [Google Scholar] [CrossRef] [PubMed]
- Rengarajan, T.; Nandakumar, N.; Rajendran, P.; Haribabu, L.; Nishigaki, I.; Balasubramanian, M.P. D-pinitol promotes apoptosis in MCF-7 cells via induction of p53 and Bax and inhibition of Bcl-2 and NF-κB. Asian Pac. J. Cancer Prev. 2014, 15, 1757–1762. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, Thyme, and Other Plant Sources: Health and Potential Uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Anusmitha, K.M.; Aruna, M.; Job, J.T.; Narayanankutty, A.; Pb, B.; Rajagopal, R.; Alfarhan, A.; Barcelo, D. Phytochemical analysis, antioxidant, anti-inflammatory, anti-genotoxic, and anticancer activities of different Ocimum plant extracts prepared by ultrasound-assisted method. Physiol. Mol. Plant Pathol. 2021, 117, 101746. [Google Scholar] [CrossRef]
- Carvalho, R.P.R.; Lima, G.D. de A.; Ribeiro, F.C.D.; Ervilha, L.O.G.; Oliveira, E.L.; Viana, A.G.A.; Machado-Neves, M. Eugenol Reduces Serum Testosterone Levels and Sperm Viability in Adult Wistar Rats. Reprod. Toxicol. 2022, 113, 110–119. [Google Scholar] [CrossRef]
- Anjum, N.F.; Shanmugarajan, D.; Shivaraju, V.K.; Faizan, S.; Naishima, N.L.; Kumar, B.P.; Javid, S.; Purohit, M.N. Novel derivatives of eugenol as potent anti-inflammatory agents via PPARγ agonism: Rational design, synthesis, analysis, PPARγ protein binding assay and computational studies. RSC Adv. 2022, 12, 16966–16978. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.F.M.; Monte, F.J.Q.; de Lemos, T.L.G.; do Nascimento, P.G.G.; de Medeiros Costa, A.K.; de Paiva, L.M.M. Eugenol derivatives: Synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J. 2018, 12, 34. [Google Scholar] [CrossRef]
- Gemma, A.; Takenaka, K.; Hosoya, Y.; Matuda, K.; Seike, M.; Kurimoto, F.; Ono, Y.; Uematsu, K.; Takeda, Y.; Hibino, S.; et al. Altered expression of several genes in highly metastatic subpopulations of a human pulmonary adenocarcinoma cell line. Eur. J. Cancer 2001, 37, 1554–1561. [Google Scholar] [CrossRef]
- Vora, U.; Vyas, V.K.; Wal, P.; Saxena, B. Effects of eugenol on the behavioral and pathological progression in the MPTP-induced Parkinson’s disease mouse model. Drug Discov. Ther. 2022, 16, 154–163. [Google Scholar] [CrossRef]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological properties and health benefits of eugenol: A comprehensive review. Oxidative Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef]
- Legault, J.; Pichette, A. Potentiating Effect of β-Caryophyllene on Anticancer Activity of α-Humulene, Isocaryophyllene and Paclitaxel. J. Pharm. Pharmacol. 2010, 59, 1643–1647. [Google Scholar] [CrossRef]
- Alma, M.H.; Mavi, A.; Yildirim, A.; Digrak, M.; Hirata, T. Screening Chemical Composition and in Vitro Antioxidant and Antimicrobial Activities of the Essential Oils from Origanum Syriacum L. Growing in Turkey. Biol. Pharm. Bull. 2003, 26, 1725–1729. [Google Scholar] [CrossRef]
- Al-Fatlawi, A.A.; Ahmad, A. Cytotoxicity and Pro-Apoptotic Activity of Carvacrol on Human Breast Cancer Cell Line MCF-7. World J. Pharm. Sci. 2014, 2, 1134–1415. [Google Scholar]
- Yehya, A.H.; Asif, M.; Majid, A.M.A.; Oon, C.E. Complementary effects of Orthosiphon stamineus standardized ethanolic extract and rosmarinic acid in combination with gemcitabine on pancreatic cancer. Biomed. J. 2020, 44, 694–708. [Google Scholar] [CrossRef]
- Kim, S.S.; Oh, O.J.; Min, H.Y.; Park, E.J.; Kim, Y.; Park, H.J.; Han, Y.N.; Lee, S.K. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264. 7 cells. Life Sci. 2003, 73, 337–348. [Google Scholar] [CrossRef]
- Mari, A.; Mani, G.; Nagabhishek, S.N.; Balaraman, G.; Subramanian, N.; Mirza, F.B.; Sundaram, J.; Thiruvengadam, D. Carvacrol Promotes Cell Cycle Arrest and Apoptosis through PI3K/AKT Signaling Pathway in MCF-7 Breast Cancer Cells. Chin. J. Integr. Med. 2021, 27, 680–687. [Google Scholar] [CrossRef]
- Xu, Z.; Han, X.; Ou, D.; Liu, T.; Li, Z.; Jiang, G.; Liu, J.; Zhang, J. Targeting PI3K/AKT/MTOR-Mediated Autophagy for Tumor Therapy. Appl. Microbiol. Biotechnol. 2020, 104, 575–587. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Mahmood, T.; Kanwal, S.; Ali, B.; Khalil, A.T.; Shah, A.; Alam, M.M.; Badshah, H. Ursolic acid a promising candidate in the therapeutics of breast cancer: Current status and future implications. Biomed. Pharmacother. 2018, 108, 752–756. [Google Scholar] [CrossRef]
- Nunes, S.R.R.P.; Madureira, A.R.; Campos, D.; Sarmento, B.; Gomes, A.M.; Pintado, M.M.; Reis, F. Therapeutic and Nutraceutical Potential of Rosmarinic Acid—Cytoprotective Properties and Pharmacokinetic Profile. Crit. Rev. Food Sci. Nutr. 2015, 57, 1799–1806. [Google Scholar] [CrossRef]
- Magalhães, D.B.; Castro, I.; Lopes-Rodrigues, V.; Pereira, J.M.; Barros, L.; Ferreira, I.C.F.R.; Xavier, C.P.R.; Vasconcelos, M.H. Melissa officinalis L. ethanolic extract inhibits the growth of a lung cancer cell line by interfering with the cell cycle and inducing apoptosis. Food Funct. 2018, 9, 3134–3142. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Alagawany, M.; El-Hack, M.E.A.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic acid: Modes of action, medicinal values and health benefits. Anim. Health Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, L.; Jin, D.; Xin, Y.; Tian, L.; Wang, T.; Zhao, D.; Wang, Z.; Wang, J. Rosmarinic Acid and Related Dietary Supplements: Potential Applications in the Prevention and Treatment of Cancer. Biomolecules 2022, 12, 1410. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Tang, H.; Pan, Y.; Hu, B.; Huang, G. Rosmarinic acid inhibits cell proliferation, migration, and invasion and induces apoptosis in human glioma cells. Int. J. Mol. Med. 2021, 47, 67. [Google Scholar] [CrossRef]
- Utispan, K.; Niyomtham, N.; Yingyongnarongkul, B.; Koontongkaew, S. Ethanolic extract of Ocimum sanctum leaves reduced invasion and matrix metalloproteinase activity of head and neck cancer cell lines. Asian Pac. J. Cancer Prev. 2020, 21, 363. [Google Scholar] [CrossRef]
- Elansary, H.O.; Mahmoud, E.A. In vitro antioxidant and antiproliferative activities of six international basil cultivars. Nat. Prod. Res. 2015, 29, 2149–2154. [Google Scholar] [CrossRef]
- Tai, J.; Cheung, S.; Wu, M.; Hasman, D. Antiproliferation effect of Rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomedicine 2012, 19, 436–443. [Google Scholar] [CrossRef]
- Scheckel, K.A.; Degner, S.C.; Romagnolo, D.F. Rosmarinic acid antagonizes activator protein-1–dependent activation of cyclooxygenase-2 expression in human cancer and nonmalignant cell lines. J. Nutr. 2008, 138, 2098–2105. [Google Scholar] [CrossRef]
- Han, Y.H.; Kee, J.Y.; Hong, S.H. Rosmarinic acid activates AMPK to inhibit metastasis of colorectal cancer. Front. Pharmacol. 2018, 9, 68. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, J.; Yang, Y.; Wang, X.; Chen, G.; Shi, A.; Lu, Y.; Jia, S.; Kang, X.; Lu, L. Rosmarinic acid exerts an anticancer effect on osteosarcoma cells by inhibiting DJ-1 via regulation of the PTEN-PI3K-Akt signaling pathway. Phytomedicine 2020, 68, 153186. [Google Scholar] [CrossRef]
- Yang, K.; Shen, Z.; Zou, Y.; Gao, K. Rosmarinic acid inhibits migration, invasion, and p38/AP-1 signaling via miR-1225-5p in colorectal cancer cells. J. Recept. Signal Transduct. 2021, 41, 284–293. [Google Scholar] [CrossRef]
- Han, Y.; Ma, L.; Zhao, L.E.; Feng, W.; Zheng, X. Rosmarinic inhibits cell proliferation, invasion and migration via up-regulating miR-506 and suppressing MMP2/16 expression in pancreatic cancer. Biomed. Pharmacother. 2019, 115, 108878. [Google Scholar] [CrossRef]
- Jung, C.H.I.Y.; Kim, S.-Y.; Lee, C. Carvacrol Targets AXL to Inhibit Cell Proliferation and Migration in Non-Small Cell Lung Cancer Cells. Anticancer Res. 2018, 38, 279. [Google Scholar]
- Singh, P.; Mishra, S.K.; Noel, S.; Sharma, S.; Rath, S.K. Acute exposure of apigenin induces hepatotoxicity in Swiss mice. PLoS ONE 2012, 7, e31964. [Google Scholar] [CrossRef]
- Kaur, P.; Shukla, S.; Gupta, S. Plant flavonoid apigenin inactivates Akt to trigger apoptosis in human prostate cancer: An in vitro and in vivo study. Carcinogenesis 2008, 29, 2210–2217. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Song, Y.U.; Yao, J.; Huang, K.; Zhu, X. Apigenin suppresses colorectal cancer cell proliferation, migration and invasion via inhibition of the Wnt/β-catenin signaling pathway. Oncol. Lett. 2016, 11, 3075–3080. [Google Scholar] [CrossRef]
- Hu, W.J.; Liu, J.; Zhong, L.K.; Wang, J. Apigenin enhances the antitumor effects of cetuximab in nasopharyngeal carcinoma by inhibiting EGFR signaling. Biomed. Pharmacother. 2018, 102, 681–688. [Google Scholar] [CrossRef]
- Cao, H.-H.; Chu, J.-H.; Kwan, H.Y.; Su, T.; Yu, H.; Cheng, B.C.-Y.; Fu, X.-Q.; Guo, H.; Li, T.; Tse, A.K.-W.; et al. Inhibition of the STAT3 signaling pathway contributes to apigenin-mediated anti-metastatic effect in melanoma. Sci. Rep. 2016, 6, 21731. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.; Gilardini Montani, M.S.; Santarelli, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Apigenin, by activating p53 and inhibiting STAT3, modulates the balance between pro-apoptotic and pro-survival pathways to induce PEL cell death. J. Exp. Clin. Cancer Res. 2017, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Park, S.; Bazer, F.W.; Song, G. Apigenin reduces survival of choriocarcinoma cells by inducing apoptosis via the PI3K/AKT and ERK1/2 MAPK pathways. J. Cell Physiol. 2016, 231, 2690–2699. [Google Scholar] [CrossRef]
- Shao, H.; Jing, K.; Mahmoud, E.; Huang, H.; Fang, X.; Yu, C. Apigenin Sensitizes Colon Cancer Cells to Antitumor Activity of ABT-263. Mol. Cancer Ther. 2013, 12, 2640–2650. [Google Scholar] [CrossRef]
- Liu, R.; Ji, P.; Liu, B.; Qiao, H.; Wang, X.; Zhou, L.; Deng, T.; Ba, Y. Apigenin enhances the cisplatin cytotoxic effect through p53-modulated apoptosis. Oncol. Lett. 2016, 13, 1024–1030. [Google Scholar] [CrossRef]
- Seo, H.S.; Sikder, M.A.; Lee, H.J.; Ryu, J.; Lee, C.J. Apigenin inhibits tumor necrosis factor-α-induced production and gene expression of mucin through regulating nuclear factor-kappa B signaling pathway in airway epithelial cells. Biomol. Ther. 2014, 22, 525–531. [Google Scholar] [CrossRef]
- Yang, C.; Song, J.; Hwang, S.; Choi, J.; Song, G.; Lim, W. Apigenin enhances apoptosis induction by 5-fluorouracil through regulation of thymidylate synthase in colorectal cancer cells. Redox Biol. 2021, 47, 102144. [Google Scholar] [CrossRef]
- Shukla, S.; Shankar, E.; Fu, P.; MacLennan, G.T.; Gupta, S. Suppression of NF-κB and NF-κB-regulated gene expression by apigenin through IκBα and IKK pathway in TRAMP mice. PLoS ONE 2015, 10, e0138710. [Google Scholar] [CrossRef]
- Shukla, S.; Fu, P.; Gupta, S. Apigenin Induces Apoptosis by Targeting Inhibitor of Apoptosis Proteins and Ku70-Bax Interaction in Prostate Cancer. Apoptosis 2014, 19, 883–894. [Google Scholar] [CrossRef]
- Yang, J.; Pi, C.; Wang, G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed. Pharmacother. 2018, 103, 699–707. [Google Scholar] [CrossRef]
- Tseng, T.H.; Chien, M.H.; Lin, W.L.; Wen, Y.C.; Chow, J.M.; Chen, C.K.; Kuo, T.; Lee, W.J. Inhibition of MDA-MB-231 breast cancer cell proliferation and tumor growth by apigenin through induction of G2/M arrest and histone H3 acetylation-mediated p21WAF1/CIP1 expression. Environ. Toxicol. 2017, 32, 434–444. [Google Scholar] [CrossRef]
- Meng, S.; Zhu, Y.; Li, J.-F.; Wang, X.; Liang, Z.; Li, S.-Q.; Xu, X.; Chen, H.; Liu, B.; Zheng, X.-Y.; et al. Apigenin inhibits renal cell carcinoma cell proliferation. Oncotarget 2017, 8, 19834–19842. [Google Scholar] [CrossRef]
- Bauer, D.; Redmon, N.; Mazzio, E.; Soliman, K.F. Apigenin inhibits TNFα/IL-1α-induced CCL2 release through IKBK-epsilon signaling in MDA-MB-231 human breast cancer cells. PLoS ONE 2017, 12, e0175558. [Google Scholar] [CrossRef]
- Liu, L.Z.; Fang, J.; Zhou, Q.; Hu, X.; Shi, X.; Jiang, B.H. Apigenin inhibits expression of vascular endothelial growth factor and angiogenesis in human lung cancer cells: Implication of chemoprevention of lung cancer. Mol. Pharmacol. 2005, 68, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Afaq, F.; Mukhtar, H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene 2002, 21, 3727–3738. [Google Scholar] [CrossRef]
- dos Santos, C.P.; Pinto, J.A.O.; dos Santos, C.A.; Cruz, E.M.O.; de Fátima Arrigoni-Blank, M.; Andrade, T.M.; de Alexandria Santos, D.; Alves, P.B.; Blank, A.F. Harvest Time and Geographical Origin Affect the Essential Oil of Lippia Gracilis Schauer. Ind. Crops Prod. 2016, 79, 205–210. [Google Scholar] [CrossRef]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Baygar, T.; Ozgul Onal, M.; Ozturk, F. Comparison of Ultrastructural Changes and the Anticarcinogenic Effects of Thymol and Carvacrol on Ovarian Cancer Cells: Which Is More Effective? UltrastructPathol 2020, 44, 193–202. [Google Scholar] [CrossRef]
- Koparal, A.T.; Zeytinoğlu, M. Effects of Carvacrol on a Human Non-Small Cell Lung Cancer (NSCLC) Cell Line, A549. In Animal Cell Technology: Basic & Applied Aspects; Springer: Dordrecht, The Netherlands, 2003; pp. 207–211. [Google Scholar]
- Ozkan, A.; Erdogan, A. A Comparative Study of the Antioxidant/Prooxidant Effects of Carvacrol and Thymol at Various Concentrations on Membrane and DNA of Parental and Drug Resistant H1299 Cells. Nat. Prod. Commun. 2012, 7, 1934578X1200701. [Google Scholar] [CrossRef]
- Özkan, A.; Erdogan, A. A Comparative Evaluation of Antioxidant and Anticancer Activity of Essential Oil from Origanum Onites (Lamiaceae) and Its Two Major Phenolic Components. Turk. J. Biol. 2011. [Google Scholar] [CrossRef]
- Sanchez, A.; Tripathy, D.; Yin, X.; Luo, J.; Martinez, J.; Grammas, P. Pigment Epithelium-Derived Factor (PEDF) Protects Cortical Neurons in Vitro from Oxidant Injury by Activation of Extracellular Signal-Regulated Kinase (ERK) 1/2 and Induction of Bcl-2. Neurosci. Res. 2012, 72, 1–8. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Moreno, F.J.; Bermúdez, J.M.; Aucejo, S.; Cameán, A.M. Cytotoxicity and Morphological Effects Induced by Carvacrol and Thymol on the Human Cell Line Caco-2. Food Chem. Toxicol. 2014, 64, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Calibasi Kocal, G.; Pakdemirli, A. Antiproliferative Effects of Carvacrol on Neuroblastoma Cells. J. Dr. Behcet Child. Hosp. 2020. [Google Scholar] [CrossRef]
- Fan, K.; Li, X.; Cao, Y.; Qi, H.; Li, L.; Zhang, Q.; Sun, H. Carvacrol Inhibits Proliferation and Induces Apoptosis in Human Colon Cancer Cells. Anticancer Drugs 2015, 26, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Jamali, T.; Kavoosi, G.; Safavi, M.; Ardestani, S.K. In-Vitro Evaluation of Apoptotic Effect of OEO and Thymol in 2D and 3D Cell Cultures and the Study of Their Interaction Mode with DNA. Sci. Rep. 2018, 8, 15787. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, L.; Wu, Y.; Zhang, Y. Carvacrol Affects Breast Cancer Cells through TRPM7 Mediated Cell Cycle Regulation. Life Sci. 2021, 266, 118894. [Google Scholar] [CrossRef] [PubMed]
- Tayarani-Najaran, Z.; Akaberi, M.; Hassanzadeh, B.; Shirazi, N.; Asili, J.; Al-Najjar, H.; Sahebkar, A.; Emami, S.A. Analysis of the Essential Oils of Five Artemisia Species and Evaluation of Their Cytotoxic and Proapoptotic Effects. Mini-Rev. Med. Chem. 2019, 19, 902–912. [Google Scholar] [CrossRef]
- Baranauskaite, J.; Kubiliene, A.; Marksa, M.; Petrikaite, V.; Vitkevičius, K.; Baranauskas, A.; Bernatoniene, J. The Influence of Different Oregano Species on the Antioxidant Activity Determined Using HPLC Postcolumn DPPH Method and Anticancer Activity of Carvacrol and Rosmarinic Acid. Biomed. Res. Int. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Arunasree, K.M. Anti-Proliferative Effects of Carvacrol on a Human Metastatic Breast Cancer Cell Line, MDA-MB 231. Phytomedicine 2010, 17, 581–588. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Pan, J. Immunopathogenic Mechanisms of Rheumatoid Arthritis and the Use of Anti-Inflammatory Drugs. Intractable Rare Dis. Res. 2021, 10, 154–164. [Google Scholar] [CrossRef]
- Bermas, B.L. Non-steroidal anti inflammatory drugs, glucocorticoids and disease modifying anti-rheumatic drugs for the management of rheumatoid arthritis before and during pregnancy. Curr. Opin. Rheumatol. 2014, 26, 334–340. [Google Scholar] [CrossRef]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid. Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef]
- Obrenovich, M.; Li, Y.; Tayahi, M.; Reddy, V.P. Polyphenols and Small Phenolic Acids as Cellular Metabolic Regulators. Curr. Issues Mol. Biol. 2022, 44, 4152–4166. [Google Scholar] [CrossRef]
- das Chagas Pereira de Andrade, F.; Mendes, A.N. Computational analysis of eugenol inhibitory activity in lipoxygenase and cyclooxygenase pathways. Sci. Rep. 2020, 10, 16204. [Google Scholar] [CrossRef] [PubMed]
- Hui, Q.; Ammeter, E.; Liu, S.; Yang, R.; Lu, P.; Lahaye, L.; Yang, C. Eugenol attenuates inflammatory response and enhances barrier function during lipopolysaccharide-induced inflammation in the porcine intestinal epithelial cells. J. Anim. Sci. 2020, 98, skaa245. [Google Scholar] [CrossRef] [PubMed]
- Csikós, E.; Csekő, K.; Kemény, Á.; Draskóczi, L.; Kereskai, L.; Kocsis, B.; Böszörményi, A.; Helyes, Z.; Horváth, G. Pinus sylvestris L. and Syzygiumaromaticum (L.) Merr. & L. M. Perry Essential Oils Inhibit Endotoxin-Induced Airway Hyperreactivity despite Aggravated Inflammatory Mechanisms in Mice. Molecules 2022, 27, 3868. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ali, A. Pharmacological Perspectives of Eugenol in Modern Context. Natural Pharmaceuticals and Green Microbial Technology; Apple Academic Press: Palm Bay, FL, USA, 2021; pp. 31–47. ISBN 1-00-300322-2. [Google Scholar]
- Chen, S.; Wu, X.; Tang, S.; Yin, J.; Song, Z.; He, X.; Yin, Y. Eugenol Alleviates Dextran Sulfate Sodium-Induced Colitis Independent of Intestinal Microbiota in Mice. J. Agric. Food Chem. 2021, 69, 10506–10514. [Google Scholar] [CrossRef]
- Estevão-Silva, C.F.; Kummer, R.; Fachini-Queiroz, F.C.; Grespan, R.; De Melo, G.A.N.; Baroni, S.; Cuman, R.K.N.; Bersani-Amado, C.A. Anethole and eugenol reduce in vitro and in vivo leukocyte migration induced by fMLP, LTB4, and carrageenan. J. Nat. Med. 2014, 68, 567–575. [Google Scholar] [CrossRef]
- Bittencourt-Mernak, M.I.; Pinheiro, N.M.; da Silva, R.C.; Ponci, V.; Banzato, R.; Pinheiro, A.J.; Olivo, C.R.; Tibério, I.F.L.C.; Neto, L.G.L.; Fernanda, P.R.; et al. Effects of Eugenol and Dehydrodieugenol B from Nectandraleucantha against Lipopolysaccharide (LPS)-Induced Experimental Acute Lung Inflammation. J. Nat. Prod. 2021, 84, 2282–2294. [Google Scholar] [CrossRef]
- Zin, W.A.; Silva, A.G.L.S.; Magalhães, C.B.; Carvalho, G.M.C.; Riva, D.R.; Lima, C.C.; Leal-Cardoso, J.H.; Takiya, C.M.; Valença, S.S.; Saldiva, P.H.N.; et al. Eugenol attenuates pulmonary damage induced by diesel exhaust particles. J. Appl. Physiol. 2012, 112, 911–917. [Google Scholar] [CrossRef]
- Wang, M.; Dai, T.; Li, S.; Wang, W. Eugenol suppresses the proliferation and invasion of TNF-α-induced fibroblast-like synoviocytes via regulating NF-κB and COX-2. Biochem. Biophys. Res. Commun. 2022, 612, 63–69. [Google Scholar] [CrossRef]
- Pan, C.; Dong, Z. Antiasthmatic effects of eugenol in a mouse model of allergic asthma by regulation of vitamin D3 upregulated protein 1/NF-κB pathway. Inflammation 2015, 38, 1385–1393. [Google Scholar] [CrossRef]
- Balkrishna, A.; Solleti, S.K.; Singh, H.; Tomer, M.; Sharma, N.; Varshney, A. Calcio-herbal formulation, Divya-Swasari-Ras, alleviates chronic inflammation and suppresses airway remodelling in mouse model of allergic asthma by modulating pro-inflammatory cytokine response. Biomed. Pharmacother. 2020, 126, 110063. [Google Scholar] [CrossRef]
- Abd El Motteleb, D.M.; Selim, S.A.; Mohamed, A.M. Differential Effects of Eugenol against Hepatic Inflammation and Overall Damage Induced by Ischemia/Re-Perfusion Injury. J. Immunotoxicol. 2014, 11, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.P.R.; Ribeiro, F.C.D.; Lima, T.I.; Ervilha, L.O.G.; de Oliveira, E.L.; Faustino, A.D.O.; Lima, G.D.D.A.; Machado-Neves, M. High doses of eugenol cause structural and functional damage to the rat liver. Life Sci. 2022, 304, 120696. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Zhang, J.; Sun, K.; Li, Q.; Kuang, B.; Hou, S.; Gong, N. Methyl eugenol attenuates liver ischemia reperfusion injury via activating PI3K/Akt signaling. Int. Immunopharmacol. 2021, 99, 108023. [Google Scholar] [CrossRef]
- Li, H.; Yuan, W.; Tian, Y.; Tian, F.; Wang, Y.; Sun, X.; Gong, Y. Eugenol alleviated nonalcoholic fatty liver disease in rat via a gut-brain-liver axis involving glucagon-like Peptide-1. Arch. Biochem. Biophys. 2022, 725, 109269. [Google Scholar] [CrossRef]
- Fangjun, L.; Zhijia, Y. Tumor suppressive roles of eugenol in human lung cancer cells. Thorac. Cancer 2018, 9, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Supriyanto, E. Antiproliferative and molecular mechanism of eugenol-induced apoptosis in cancer cells. Molecules. 2012, 17, 6290–6304. [Google Scholar] [CrossRef] [PubMed]
- Magesh, V.; Lee, J.C.; Ahn, K.S.; Lee, H.J.; Lee, H.J.; Lee, E.O.; Shim, B.S.; Jung, H.J.; Kim, J.S.; Kim, D.K.; et al. Ocimum sanctum induces apoptosis in A549 lung cancer cells and suppresses the in vivo growth of Lewis lung carcinoma cells. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 1385–1391. [Google Scholar]
- Fumarola, C.; Bonelli, M.A.; Petronini, P.G.; Alfieri, R.R. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem. Pharmacol. 2014, 90, 197–207. [Google Scholar] [CrossRef]
- He, Y.; Zhou, X.; Jiang, S.; Zhang, Z.; Cao, B.; Liu, J.; Zeng, Y.; Zhao, J.; Mao, X. TRIM25 activates AKT/mTOR by inhibiting PTEN via K63-linked polyubiquitination in non-small cell lung cancer. Acta Pharmacol. Sin. 2022, 43, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Vidhya, N.; Devaraj, S.N. Induction of Apoptosis by Eugenol in Human Breast Cancer Cells; NISCAIR-CSIR: New Delhi, India, 2011. [Google Scholar]
- Sharma, U.K.; Sharma, A.K.; Gupta, A.; Kumar, R.; Pandey, A.; Pandey, A.K. Pharmacological activities of cinnamaldehyde and eugenol: Antioxidant, cytotoxic and anti-leishmanial studies. Cell Mol. Biol. 2017, 63, 73–78. [Google Scholar] [CrossRef]
- Al-Sharif, I.; Remmal, A.; Aboussekhra, A. Eugenol triggers apoptosis in breast cancer cells through E2F1/survivin down-regulation. BMC Cancer 2013, 13, 600. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Kim, M.M. Eugenol with antioxidant activity inhibits MMP-9 related to metastasis in human fibrosarcoma cells. Food Chem. Toxicol. 2013, 55, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Majeed, H.; Antoniou, J.; Fang, Z. Apoptotic effects of eugenol-loaded nanoemulsions in human colon and liver cancer cell lines. Asian Pac. J. Cancer Prev. 2014, 15, 9159–9164. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, C.B.; Riva, D.R.; DePaula, L.J.; Brando-Lima, A.; Koatz, V.L.G.; Leal-Cardoso, J.H.; Zin, W.A.; Faffe, D.S. In vivo anti-inflammatory action of eugenol on lipopolysaccharide-induced lung injury. J. Appl. Physiol. 2010, 108, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Lu, Y.; Ma, C. Anti-inflammatory effects of eugenol on lipopolysaccharide-induced inflammatory reaction in acute lung injury via regulating inflammation and redox status. Int. Immunopharmacol. 2015, 26, 265–271. [Google Scholar] [CrossRef]
- Mahapatra, S.K.; Bhattacharjee, S.; Chakraborty, S.P.; Majumdar, S.; Roy, S. Alteration of immune functions and Th1/Th2 cytokine balance in nicotine-induced murine macrophages: Immunomodulatory role of eugenol and N-acetylcysteine. Int. Immunopharmacol. 2011, 11, 485–495. [Google Scholar] [CrossRef]
- Koh, T.; Murakami, Y.; Tanaka, S.; Machino, M.; Sakagami, H. Re-evaluation of anti-inflammatory potential of eugenol in IL-1β-stimulated gingival fibroblast and pulp cells. In Vivo 2013, 27, 269–273. [Google Scholar]
- Ito, M.; Murakami, K.; Yoshino, M. Antioxidant action of eugenol compounds: Role of metal ion in the inhibition of lipid peroxidation. Food Chem. Toxicol. 2005, 43, 461–466. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of eugenol: A structure–activity relationship study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Duque-Soto, C.; Borrás-Linares, I.; Quirantes-Piné, R.; Falcó, I.; Sánchez, G.; Segura-Carretero, A.; Lozano-Sánchez, J. Potential Antioxidant and Antiviral Activities of Hydroethanolic Extracts of Selected Lamiaceae Species. Foods 2022, 11, 1862. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, E.; Costa, F.; Azevedo, A.; Barroso, P.; de Assis, E.; Paulino, L.; Silva, B.; Silva, A.; Souza, A.; Silva, J. Eugenol influences the expression of messenger RNAs for superoxide dismutase and glutathione peroxidase 1 in bovine secondary follicles cultured in vitro. Zygote 2021, 29, 301–306. [Google Scholar] [CrossRef]
- Wani, M.R.; Maheshwari, N.; Shadab, G. Eugenol attenuates TiO2 nanoparticles-induced oxidative damage, biochemical toxicity and DNA damage in Wistar rats: An in vivo study. Environ. Sci. Pollut. Res. 2021, 28, 22664–22678. [Google Scholar] [CrossRef]
- Ahlawat, S.; Kumar, P.; Mohan, H.; Goyal, S.; Sharma, K.K. Inflammatory bowel disease: Tri-directional relationship between microbiota, immune system and intestinal epithelium. Crit. Rev. Microbiol. 2021, 47, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases. Int. J. Mol. Med. 2021, 47, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Okamura, M.; Ueno, T.; Tanaka, S.; Murata, Y.; Kobayashi, H.; Miyamoto, A.; Abe, M.; Fukuda, N. Increased expression of acyl-CoA oxidase 2 in the kidney with plasma phytanic acid and altered gut microbiota in spontaneously hypertensive rats. Hypertens. Res. 2021, 44, 651–661. [Google Scholar] [CrossRef]
- Ghanta, S.; Bhaumik, C.; Manna, M.S. Process development for isolation of dietary eugenol from leaves of basil (Ocimum sanctum) in combination of optimization of process variables and modeling by artificial neural network. J. Indian Chem. Soc. 2022, 99, 100280. [Google Scholar] [CrossRef]
- Hobani, Y.H.; Mohan, S.; Shaheen, E.; Abdelhaleem, A.; Ahmad, F.; Bhatia, S.; Abou-Elhamd, A.S. Gastroprotective effect of low dose Eugenol in experimental rats against ethanol induced toxicity: Involvement of antiinflammatory and antioxidant mechanism. J. Ethnopharmacol. 2022, 289, 115055. [Google Scholar] [CrossRef]
- Tham, E.H.; Dyjack, N.; Kim, B.E.; Rios, C.; Seibold, M.A.; Leung, D.Y.; Goleva, E. Expression and function of the ectopic olfactory receptor OR10G7 in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2018, 143, 1838–1848.e4. [Google Scholar] [CrossRef]
- Makuch, E.; Nowak, A.; Günther, A.; Pełech, R.; Kucharski, Ł.; Duchnik, W.; Klimowicz, A. Enhancement of the antioxidant and skin permeation properties of eugenol by the esterification of eugenol to new derivatives. AMB Express 2020, 10, 187. [Google Scholar] [CrossRef]
- Pradhan, D.; Biswasroy, P.; Haldar, J.; Cheruvanachari, P.; Dubey, D.; Rai, V.K.; Kar, B.; Kar, D.M.; Rath, G.; Ghosh, G. A comprehensive review on phytochemistry, molecular pharmacology, clinical and translational outfit of Ocimum sanctum L. South Afr. J. Bot. 2022, 150, 342–360. [Google Scholar] [CrossRef]
- Kim, D.Y.; Won, K.J.; Hwang, D.I.; Park, S.; Kim, B.; Lee, H.M. Chemical composition, antioxidant and anti-melanogenic activities of essential oils from Chrysanthemum boreale Makino at different harvesting stages. Chem. Biodivers. 2018, 15, e1700506. [Google Scholar] [CrossRef]
- Perez-Roses, R.; Risco, E.; Vila, R.; Penalver, P.; Canigueral, S. Biological and nonbiological antioxidant activity of some essential oils. J. Agric. Food Chem. 2016, 64, 4716–4724. [Google Scholar] [CrossRef]
- Ramos, T.; Santos, E.; Ventura, M.; Pina, J.; Cavalheiro, A.; Fiorucci, A.; Silva, M. Eugenol and TBHQ antioxidant actions in commercial biodiesel obtained by soybean oil and animal fat. Fuel 2020, 286, 119374. [Google Scholar] [CrossRef]
- Sharma, U.K.; Sharma, A.K.; Pandey, A.K. Medicinal attributes of major phenylpropanoids present in cinnamon. BMC Complement. Altern. Med. 2016, 16, 156. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Cedrowski, J.; Grebowski, J.; Litwinienko, G. Antioxidant Activity of Edible Isothiocyanates. Lipid Oxidation in Food and Biological Systems; Springer: Berlin/Heidelberg, Germany, 2022; pp. 277–303. [Google Scholar] [CrossRef]
- Perna, S.; Alawadhi, H.; Riva, A.; Allegrini, P.; Petrangolini, G.; Gasparri, C.; Alalwan, T.A.; Rondanelli, M. In Vitro and In Vivo Anticancer Activity of Basil (Ocimum spp.): Current Insights and Future Prospects. Cancers 2022, 14, 2375. [Google Scholar] [CrossRef]
- Brito, L.D.; Araujo CD, S.; Cavalcante DG, S.M.; Gomes, A.S.; Zocoler, M.A.; Yoshihara, E.; Job, A.E.; Kerche, L.E. In vivo assessment of antioxidant, antigenotoxic, and antimutagenic effects of bark ethanolic extract from Spondias purpurea L. J. Toxicol. Environ. Health Part A 2022, 85, 336–352. [Google Scholar] [CrossRef]
- Jang, J.Y.; Sung, B.; Kim, N.D. Role of Induced Programmed Cell Death in the Chemopreventive Potential of Apigenin. Int. J. Mol. Sci. 2022, 23, 3757. [Google Scholar] [CrossRef]
- Nag, S.; Singh, N.; Kumaria, S. Phytochemicals as Antibacterial Agents: Current Status and Future Perspective. Altern. Antibiot. 2022, 35–55. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Tait, L.; Shekhar, M.; Palomino, E.; Hogan, V.; Piechocki, M.P.; Funasaka, T.; Raz, A. Inhibition of breast tumor growth and angiogenesis by a medicinal herb: Ocimum gratissimum. Int. J. Cancer 2007, 121, 884–894. [Google Scholar] [CrossRef]
- Khanna, N.; Bhatia, J. Antinociceptive action of Ocimum sanctum (Tulsi) in mice: Possible mechanisms involved. J. Ethnopharmacol. 2003, 88, 293–296. [Google Scholar] [CrossRef]
- Koeduka, T.; Fridman, E.; Gang, D.R.; Vassão, D.G.; Jackson, B.L.; Kish, C.M.; Orlova, I.; Spassova, S.M.; Lewis, N.G.; Noel, J.P.; et al. Eugenol and isoeugenol, characteristic aromatic constituents of spices, are biosynthesized via reduction of a coniferyl alcohol ester. Proc. Natl. Acad. Sci. USA 2006, 103, 10128–10133. [Google Scholar] [CrossRef]
- Singh, N.; Verma, P.; Pandey, B.R.; Bhalla, M. Therapeutic potential of Ocimum sanctum in prevention and treatment of cancer and exposure to radiation: An overview. Int. J. Pharm. Sci. Drug Res. 2012, 4, 97–104. [Google Scholar]
- Venkatachalam, G.; Muthusamy, A. Phytochemical evaluation and anticancer activity of Ocimum sanctum L.—A review. J. Pharm. Res. 2018, 12, 917. [Google Scholar]
- Selvi, M.T.; Thirugnanasampandan, R.; Sundarammal, S. Antioxidant and cytotoxic activities of essential oil of Ocimum canum Sims. from India. J. Saudi Chem. Soc. 2015, 19, 97–100. [Google Scholar] [CrossRef]
- Baliga, M.S.; Jimmy, R.; Thilakchand, K.R.; Sunitha, V.; Bhat, N.R.; Saldanha, E.; Rao, S.; Rao, P.; Arora, R.; Palatty, P.L. Ocimum Sanctum L. (Holy Basil or Tulsi) and Its Phytochemicals in the Prevention and Treatment of Cancer. Nutr. Cancer 2013, 65 (Suppl. S1), 26–35. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mondhe, D.; Wani, Z.A.; Pal, H.C.; Mandal, M. Effect of honey and eugenol on Ehrlich ascites and solid carcinoma. J. Biomed. Biotechnol. 2010, 2010, 989163. [Google Scholar] [CrossRef]
- Sukumaran, K.; Unnikrishnan, M.C.; Kuttan, R. Inhibition of tumour promotion in mice by eugenol. Indian J. Physiol. Pharmacol. 1994, 38, 306. [Google Scholar]
- Pal, D.; Banerjee, S.; Mukherjee, S.; Roy, A.; Panda, C.K.; Das, S. Eugenol restricts DMBA croton oil induced skin carcinogenesis in mice: Downregulation of c-Myc and H-ras, and activation of p53 dependent apoptotic pathway. J. Dermatol. Sci. 2010, 59, 31–39. [Google Scholar] [CrossRef]
- Kaur, G.; Athar, M.; Alam, M.S. Eugenol precludes cutaneous chemical carcinogenesis in mouse by preventing oxidative stress and inflammation and by inducing apoptosis. Mol. Carcinog. 2009, 49, 290–301. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef]
- Bachiega, T.F.; de Sousa, J.P.B.; Bastos, J.K.; Sforcin, J.M. Clove and eugenol in noncytotoxic concentrations exert immunomodulatory/anti-inflammatory action on cytokine production by murine macrophages. J. Pharm. Pharmacol. 2012, 64, 610–616. [Google Scholar] [CrossRef]
- Barghi, B.; Shokoohi, M.; Khaki, A.A.; Khaki, A.; Moghimian, M.; Soltani, M. Eugenol improves tissue damage and oxidative stress in adult female rats after ovarian torsion/detorsion. J. Obstet. Gynaecol. 2021, 41, 933–938. [Google Scholar] [CrossRef]
- Ghodousi-Dehnavi, E.; Hosseini, R.H.; Arjmand, M.; Nasri, S.; Zamani, Z. A Metabolomic Investigation of Eugenol on Colorectal Cancer Cell Line HT-29 by Modifying the Expression of APC, p53, and KRAS Genes. Evid. -Based Complement. Altern. Med. 2021, 2021, 1448206. [Google Scholar] [CrossRef]
- Mehrotra, N. Herbs that heal: Nature’s pharmacy endowed remedies for better health. Ann. Phytomed. Int. J. 2021, 10, 6–22. [Google Scholar] [CrossRef]
- Slameňová, D.; Horváthová, E.; Wsólová, L.; Šramková, M.; Navarová, J. Investigation of anti-oxidative, cytotoxic, DNA-damaging and DNA-protective effects of plant volatiles eugenol and borneol in human-derived HepG2, Caco-2 and VH10 cell lines. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2009, 677, 46–52. [Google Scholar] [CrossRef]
- Pisano, M.; Pagnan, G.; Loi, M.; Mura, M.E.; Tilocca, M.G.; Palmieri, G.; Fabbri, D.; Dettori, M.A.; Delogu, G.; Ponzoni, M.; et al. Antiproliferative and pro-apoptotic activity of eugenol-related biphenyls on malignant melanoma cells. Mol. Cancer 2007, 6, 8. [Google Scholar] [CrossRef]
- Miyazawa, M.; Hisama, M. Suppression of chemical mutagen-induced SOS response by alkylphenols from clove (Syzygiumaromaticum) in the Salmonella typhimurium TA1535/pSK1002 umu test. J. Agric. Food Chem. 2001, 49, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.; Fawzy, M.A.; Hintzsche, H.; Nikaido, T.; Dandekar, T.; Othman, E.M. Eugenol exerts apoptotic effect and modulates the sensitivity of HeLa cells to cisplatin and radiation. Molecules 2019, 24, 3979. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, Z.; Zeng, J.; Chen, L.; Wu, Q.; Mo, J.; Zhang, G.; Song, L.; Xu, W.; Zhang, S.; et al. Eugenol inhibits non-small cell lung cancer by repressing expression of NF-κB-regulated TRIM59. Phytother. Res. 2019, 33, 1562–1569. [Google Scholar] [CrossRef]
- Choudhury, P.; Barua, A.; Roy, A.; Pattanayak, R.; Bhattacharyya, M.; Saha, P. Eugenol Emerges as an Elixir by Targeting β-Catenin, the Central Cancer Stem Cell Regulator in Lung Carcinogenesis: An in Vivo and in Vitro Rationale. Food Funct. 2021, 12, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Yapanto, A.M. Potential of Vicenin-2 in Chitosan Encapsulated Basil (Ocimum sanctum Linn.) Leaf Extract as A Therapeutic Alternative Medicine for Non-small cell carcinoma lung cancer. Int. J. Health Sci. 2022, 2, 13–22. [Google Scholar] [CrossRef]
- Rasul, H.O.; Aziz, B.K.; Ghafour, D.D.; Kivrak, A. In Silico Molecular Docking and Dynamic Simulation of Eugenol Compounds against Breast Cancer. J. Mol. Model 2022, 28, 17. [Google Scholar] [CrossRef]
- AlMotwaa, S.M. Coupling Ifosfamide to nanoemulsion-based clove oil enhances its toxicity on malignant breast cancer and cervical cancer cells. Pharmacia 2021, 68, 779–787. [Google Scholar] [CrossRef]
- Abdullah, M.L.; Al-Shabanah, O.; Hassan, Z.K.; Hafez, M.M. Eugenol-Induced Autophagy and Apoptosis in Breast Cancer Cells via PI3K/AKT/FOXO3a Pathway Inhibition. Int. J. Mol. Sci. 2021, 22, 9243. [Google Scholar] [CrossRef]
- Veiga GL, D.; Silva RD, M.D.; Pereira, E.C.; Azzalis, L.A.; Alves BD, C.A.; Gehrke FD, S.; Gascón, T.M.; Fonseca, F.L.A. The role of Survivin as a biomarker and potential prognostic factor for breast cancer. Rev. Assoc. Med. Bras. 2019, 65, 893–901. [Google Scholar] [CrossRef]
- Albini, A.; Tosetti, F.; Li, V.W.; Noonan, D.M.; Li, W.W. Cancer prevention by targeting angiogenesis. Nat. Rev. Clin. Oncol. 2012, 9, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Park, J.H.; Kim, G.C.; Park, B.S.; Gil, Y.G.; Kim, C.H. The mechanism of apoptosis induced by eugenol in human osteosarcoma cells. J. Korean Assoc. Oral Maxillofac. Surg. 2007, 33, 20–27. [Google Scholar]
- Begum, S.N.; Ray, A.S.; Rahaman, C.H. A comprehensive and systematic review on potential anticancer activities of eugenol: From pre-clinical evidence to molecular mechanisms of action. Phytomedicine 2022, 154456. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mazumdar, A.; Mondhe, D.; Mandal, M. Apoptotic effect of eugenol in human colon cancer cell lines. Cell Biol. Int. 2011, 35, 607–615. [Google Scholar] [CrossRef]
- Wang, S.F.; Chen, S.; Tseng, L.M.; Lee, H.C. Role of the mitochondrial stress response in human cancer progression. Exp. Biol. Med. 2020, 245, 861–878. [Google Scholar] [CrossRef] [PubMed]

| S.No. | Compound Name | IUPAC Name | Chemical Formula | PubChem CID |
|---|---|---|---|---|
| 1. | Eugenol | 2-Methoxy-4-prop-2-enylphenol | C10H12O2 | 3314 |
| 2. | Ursolic acid | (1S,2R,4aS,6aR,6aS,6bR,8aR,10S,12aR,14bS)-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydro-1H-picene-4a-carboxylic acid | C30H48O3 | 64945 |
| 3. | Apigenin | 5,7-Dihydroxy-2-(4-hydroxyphenyl)chromen-4-one | C15H10O5 | 5280443 |
| 4. | Caryophyllene | (1R,4E,9S)-4,11,11-trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene | C15H24 | 5281515 |
| 5. | Carvacrol | 2-Methyl-5-propan-2-ylphenol | C10H14O | 10364 |
| 6. | Cirsimaritin | 5-Hydroxy-2-(4-hydroxyphenyl)-6,7-dimethoxychromen-4-one | C17H14O6 | 188323 |
| 7. | Estragole | 1-Methoxy-4-prop-2-enylbenzene | C10H12O | 8815 |
| 8. | Linalool | 3,7-Dimethylocta-1,6-dien-3-ol | C10H18O | 6549 |
| 9. | Oleanolic acid | (4aS,6aR,6aS,6bR,8aR,10S,12aR,14bS)-10-hydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid | C30H48O3 | 10494 |
| 10. | Rosemarinic acid | 3-(3,4-Dihydroxyphenyl)-2-[I-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxypropanoic acid | C18H16O8 | 5315615 |
| S. No. | Nutritional Components | Contents (per 100 g) |
|---|---|---|
| 1. | Protein | 3.15 g |
| 2. | Carbohydrate | 2.65 g |
| 3. | Fat | 0.64 g |
| 4. | Calcium | 177 mg |
| 5. | Vitamin C | 18 mg |
| 6. | β-Carotene | 3140 µg |
| 7. | Copper | 0.385 mg |
| 8. | Iron | 3.17 mg |
| 9. | Magnesium | 64 mg |
| 10. | Phosphorus | 56 mg |
| 11. | Zinc | 0.81 mg |
| 12. | Sodium | 4 mg |
| 13. | Potassium | 295 mg |
| Bioactive Properties | Molecular Mechanism | References |
|---|---|---|
| Chemopreventive activity | Reduces MMP-2 and phosphate-Akt expression in a human lung cancer cell line | [140,141,142,143,144] |
| EEOS’ potential use as a chemopreventive for lung cancer | ||
| Chemopreventive effects of eugenol on stomach cancer caused by N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) | ||
| Anticancer activity | Inhibits the COX-2 gene in human colon HT-29 cell lines | [38,46,48,69,141,145,146,147,148,149] |
| Apoptosis in MCF-7 human breast cancer cells and gastric cancer AGS cells | ||
| Diminished oxidation of DNA | ||
| Inhibits the action of matrix metalloproteinase (MMP-9) | ||
| Prevents the synthesis of prostaglandin-E2 | ||
| Triggers cell apoptosis | ||
| Target surviving/E2F1 pathways | ||
| Inhibits ERK pathways/proteins | ||
| Anti-inflammatory Activity | Suppression of chemotaxis of neutrophils and macrophages | [69,150,151,152,153] |
| Prevents the expression of inflammatory cytokines | ||
| Inhibitory impact on prostaglandin production | ||
| Negatively regulates TNF-α | ||
| Inhibits COX-2 activity | ||
| Inhibits NF-kappaB pathways | ||
| Antioxidant activity | Suppressive effect on lipid peroxidation | [148,154,155,156,157,158] |
| Hinders ROS and RNS production | ||
| Suppressive effects on hexanal oxidation | ||
| Inhibitory effects on copper-dependent LDL oxidation | ||
| Negatively regulates iron-mediated lipid peroxidation | ||
| Inhibits nonenzymatic peroxidation in liver mitochondria | ||
| Prevents the emergence of illnesses caused by oxidative stress |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.R.; Alotaibi, B.S.; Althafar, Z.M.; Mujamammi, A.H.; Jameela, J. An Update on the Therapeutic Anticancer Potential of Ocimum sanctum L.: “Elixir of Life”. Molecules 2023, 28, 1193. https://doi.org/10.3390/molecules28031193
Hasan MR, Alotaibi BS, Althafar ZM, Mujamammi AH, Jameela J. An Update on the Therapeutic Anticancer Potential of Ocimum sanctum L.: “Elixir of Life”. Molecules. 2023; 28(3):1193. https://doi.org/10.3390/molecules28031193
Chicago/Turabian StyleHasan, Mohammad Raghibul, Bader Saud Alotaibi, Ziyad Mohammed Althafar, Ahmed Hussain Mujamammi, and Jafar Jameela. 2023. "An Update on the Therapeutic Anticancer Potential of Ocimum sanctum L.: “Elixir of Life”" Molecules 28, no. 3: 1193. https://doi.org/10.3390/molecules28031193
APA StyleHasan, M. R., Alotaibi, B. S., Althafar, Z. M., Mujamammi, A. H., & Jameela, J. (2023). An Update on the Therapeutic Anticancer Potential of Ocimum sanctum L.: “Elixir of Life”. Molecules, 28(3), 1193. https://doi.org/10.3390/molecules28031193






