Establishment of LC-MS/MS Method for Determination of GMDTC in Rat Plasma and Its Application in Preclinical Pharmacokinetics
Abstract
1. Introduction
2. Results
2.1. Method Validation
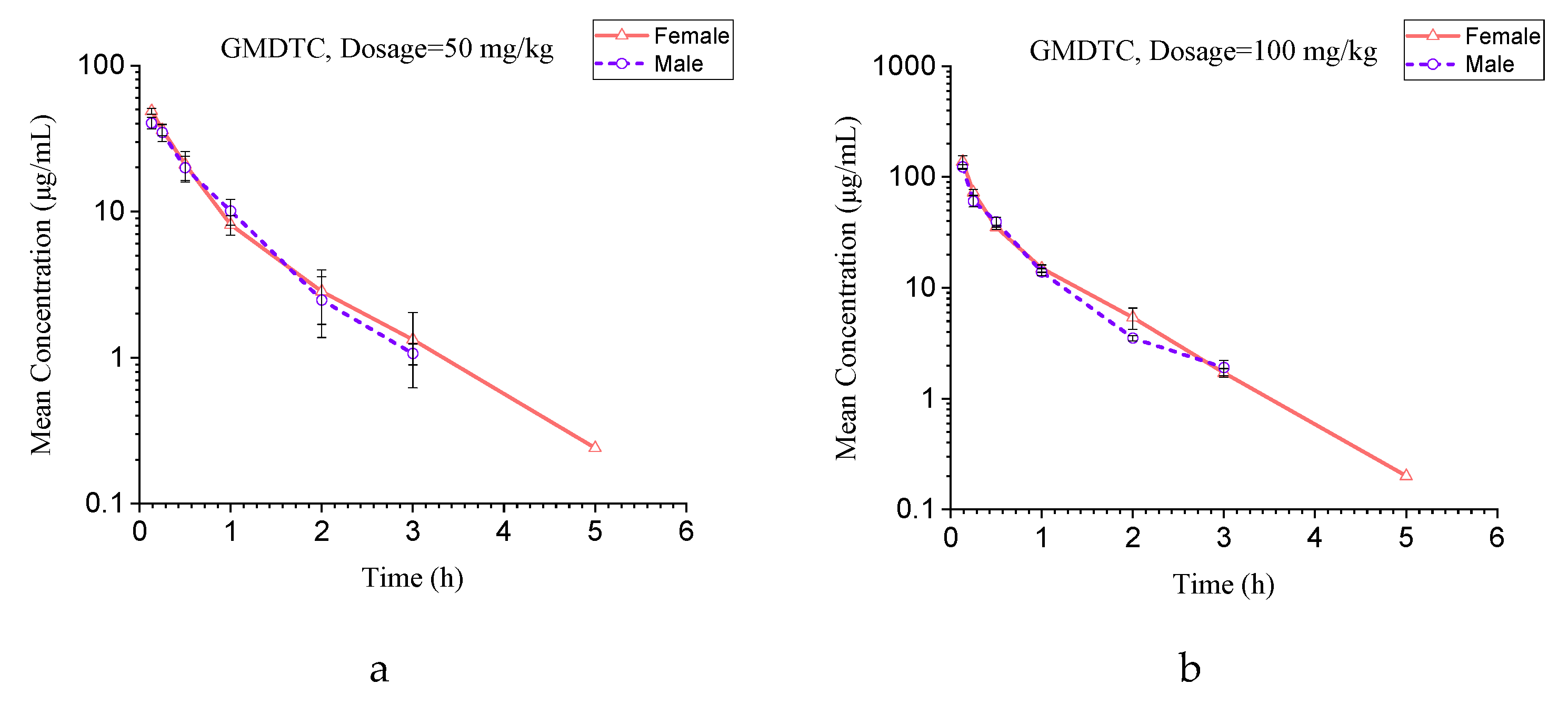
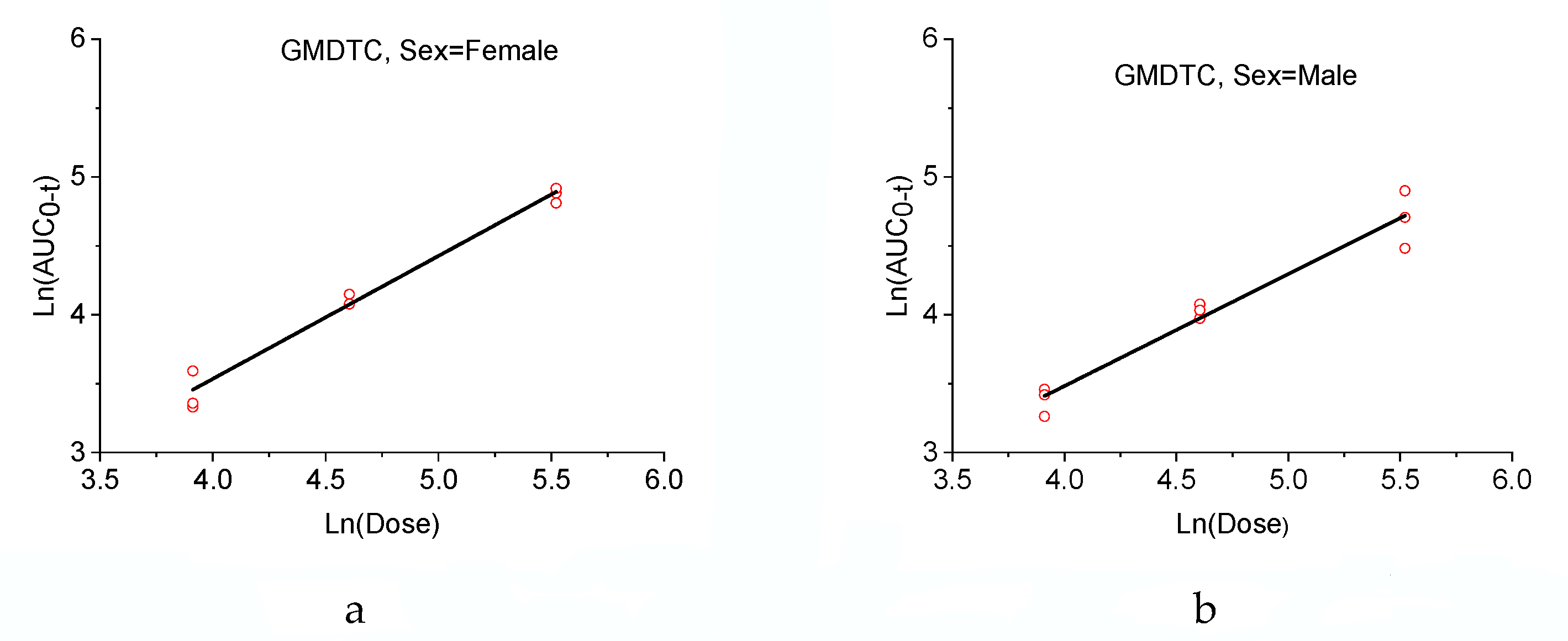
2.2. Pharmacokinetic Application
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Analytical System and Chromatographic Conditions
4.4. Plasma Sample Preparation and Calibration Curves
4.5. Method Validation
4.5.1. Selectivity
4.5.2. Linearity and Lower Limit of Quantification (LLOQ)
4.5.3. Precision and Accuracy
4.5.4. Extraction Recovery and Matrix Effect
4.5.5. Carryover Effect
4.5.6. Dilution Integrity
4.5.7. Stability
4.6. Application to Pharmacokinetic Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Heather, H.R.; Sherri, D.; Suzanne, F. Cadmium: Mitigation strategies to reduce dietary exposure. J Food Sci. 2020, 85, 260–267. [Google Scholar]
- Laura, C.T.; Peláez-Vico, M.A.; Coral, D.; Luisa, M.S.; Romero-Puertas, M.C. Role of nitric oxide in plant responses to heavy metal stress: Exogenous application versus endogenous production. J. Exp. Bot. 2019, 70, 4477–4488. [Google Scholar]
- Mehrdad, R.R.; Mehravar, R.R.; Sohrab, K.; Ali-Akbar, M. Cadmium toxicity and treatment: An update. Caspian J. Intern. Med. 2017, 8, 135–145. [Google Scholar]
- Lyu, Y.B.; Zhao, F.; Qiu, Y.D.; Ding, L.; Qu, Y.L.; Xiong, J.H.; Lu, Y.F.; Ji, S.S.; Wu, B.; Hu, X.J.; et al. Association of cadmium internal exposure with chronic kidney disease in Chinese adults. Zhonghua Yi Xue Za Zhi. 2021, 101, 1921–1928. [Google Scholar] [PubMed]
- Tsai, K.F.; Hsu, P.C.; Kung, C.T.; Lee, C.T.; You, H.L.; Huang, W.T.; Li, S.H.; Cheng, F.J.; Wang, C.C.; Lee, W.C. The Risk Factors of Blood Cadmium Elevation in Chronic Kidney Disease. Int. J. Environ. Res. Public Health 2021, 18, 12337. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Devleesschauwer, B.; Bolger, P.M.; Goodman, E.; Gibb, H.J. Global burden of late-stage chronic kidney disease resulting from dietary exposure to cadmium, 2015. Environ. Res. 2019, 169, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Filippini, T.; Ajsuvakova, O.P.; Aaseth, J.; Gluhcheva, Y.G.; Ivanova, J.M.; Bjørklund, G.; Skalnaya, M.G.; Gatiatulina, E.R.; Popova, E.V.; et al. The role of cadmium in obesity and diabetes. Sci. Total Environ. 2017, 601–602, 741–755. [Google Scholar] [CrossRef]
- Cristina, D.A.; Mariano, G.; Claudia, P.; Ciro, S.; Daniele, G.; Prisco, P.; Andrea, L.; Annamaria, C.; Rosario, P. The environment and male reproduction: The effect of cadmium exposure on reproductive function and its implication in fertility. Reprod. Toxicol. 2017, 73, 105–127. [Google Scholar]
- Jessica, M.M.; Ana, C.R.; Victoria, P.; Mary, T. Associations between blood cadmium concentration and kidney function in the U.S. population: Impact of sex, diabetes and hypertension. Environ. Res. 2019, 169, 180–188. [Google Scholar]
- George, K. Cadmium, osteoporosis and calcium metabolism. Biometals 2004, 17, 493–498. [Google Scholar]
- Reyes-Hinojosa, D.; Lozada-Pérez, C.A.; Cuevas, Y.Z.; López-Reyes, A.; Martínez-Nava, G.; Fernández-Torres, J.; Olivos-Meza, A.; Landa-Solis, J.; Gutiérrez-Ruiz, M.C.; Castillo, E.R.D.; et al. Toxicity of cadmium in musculoskeletal diseases. Environ. Toxicol. Pharmacol. 2019, 72, 103219. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A. Cadmium and cancer. Met. Ions Life Sci. 2013, 11, 491–507. [Google Scholar] [PubMed]
- Chen, Q.Y.; DesMarais, T.; Costa, M. Metals and Mechanisms of Carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Cadmium; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2012.
- IARC. Cadmium and Cadmium Compounds. In IARC Summary & Evaluation; IARC: Lyon, France, 1993; Volume 58. Available online: https://inchem.org/documents/iarc/vol58/mono58-2.html (accessed on 15 October 2022).
- International Agency for Research on Cancer (IARC). IARC Cadmium and Cadmium Compounds; WHO Press: Lyon, France, 2012. [Google Scholar]
- WHO. Exposure to Cadmium: A Major Public Health Concern; WHO: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/rest/bitstreams/1257885/retrieve (accessed on 24 January 2023).
- National Health and Family Planning Commission of the People’s Republic of China. Diagnostis of Occupational Cadmium Poisoning; Standards Press of China: Beijing, China, 2016. Available online: http://www.nhc.gov.cn/ewebeditor/uploadfile/2018/03/20180329135658417.pdf (accessed on 19 October 2022). (In Chinese)
- Tang, X.J.; Zhu, J.Q.; Zhong, Z.Y.; Luo, M.H.; Li, G.X.; Gong, Z.H.; Zhang, C.Z.; Fei, F.; Ruan, X.L.; Zhou, J.L.; et al. Mobilization and removing of cadmium from kidney by GMDTC utilizing renal glucose reabsorption pathway. Toxicol. Appl. Pharmacol. 2016, 305, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Division of Drug Information, Center for Drug Evaluation and Research, Food and Drug Administration, Bioanalytical Method Validation, Guidance for Industry. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm070107.pdf (accessed on 19 October 2022).
- Zheng, Q.Q.; Chen, C.L.; Tang, Q.I.; Huang, S.F. Dithiocarbamate Compounds and Application Thereof. Chinese Patent CN108546242B, 27 April 2018. [Google Scholar]
- Sothern, R.B.; Gruber, S.A. Further commentary: Physiological parameters in laboratory animals and humans. Pharm. Res. 1994, 11, 349–350. [Google Scholar] [CrossRef] [PubMed]
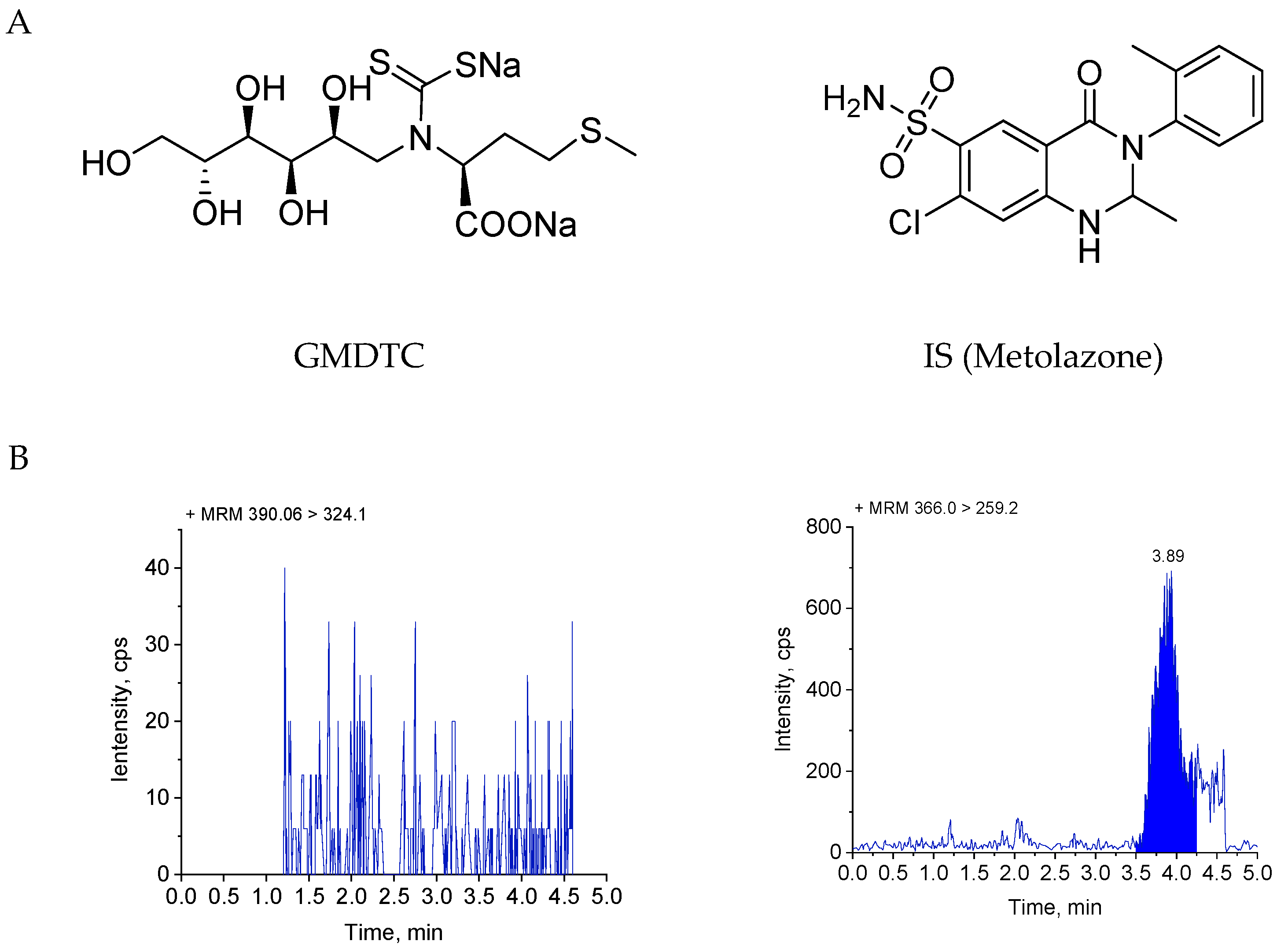
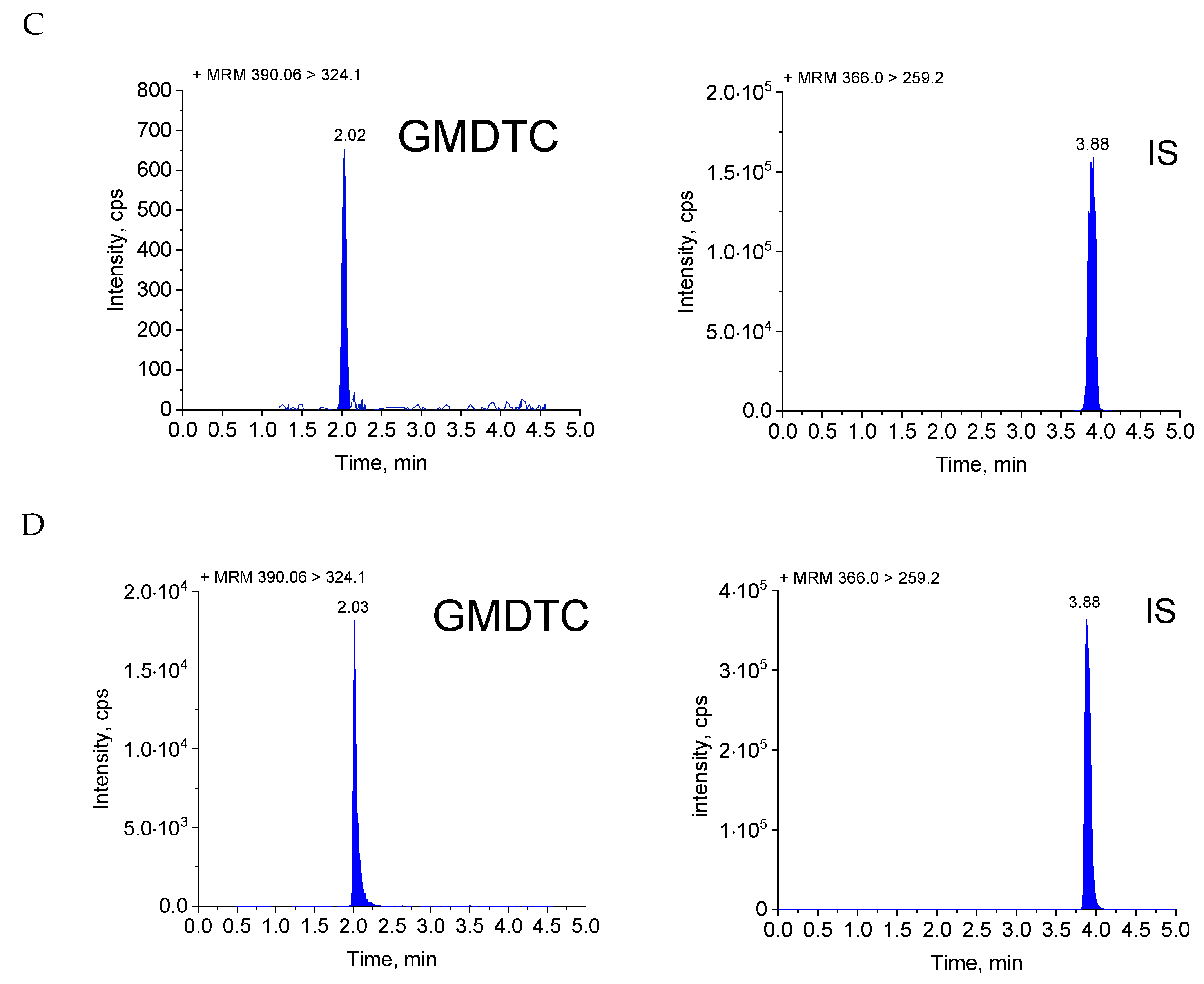
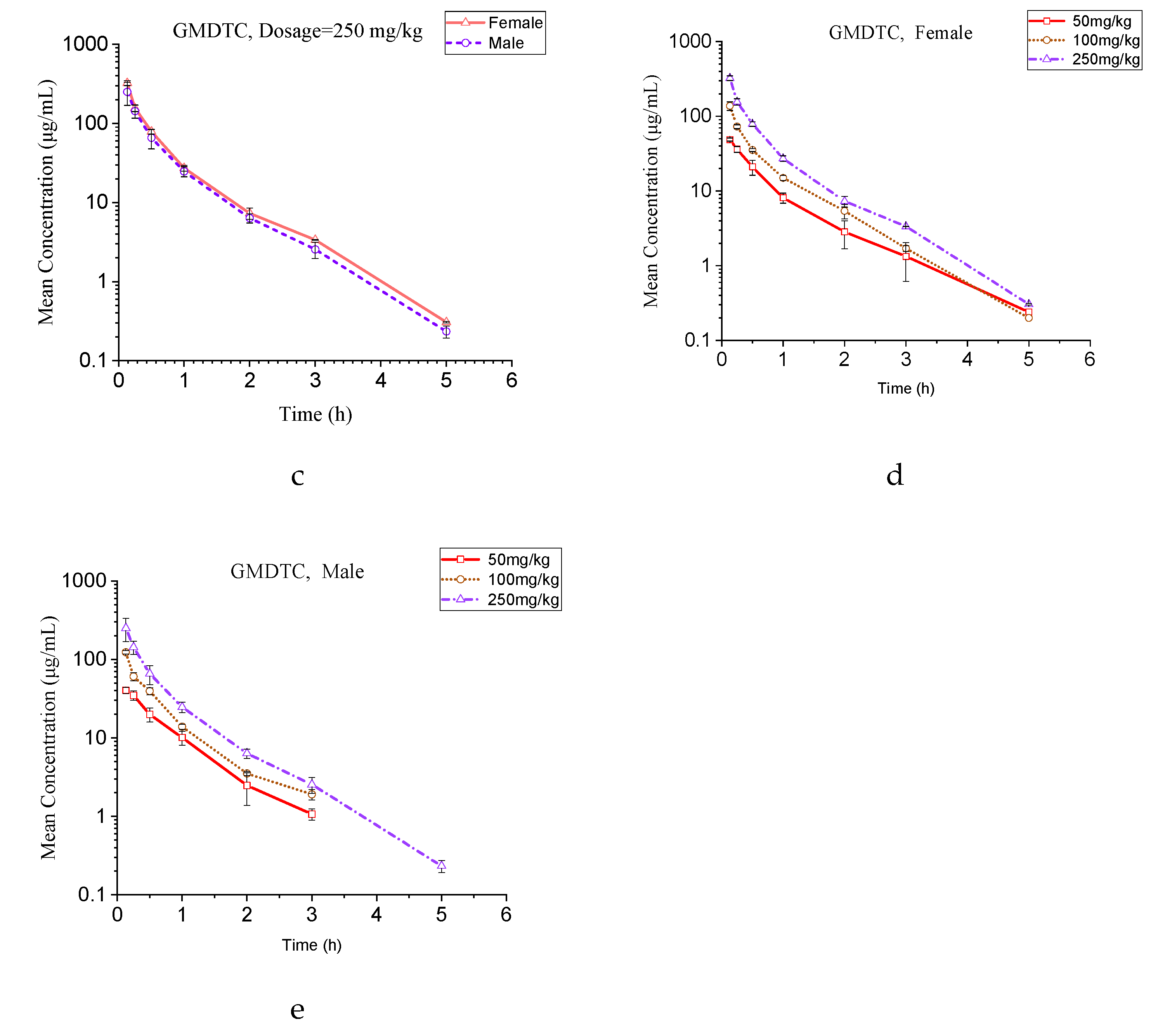
| Nominal Concentration (ng/mL) | Measured Concentration (ng/mL) | Precision (RSD, %) | Accuracy (RE, %) |
|---|---|---|---|
| Intra-day batch (n = 6) | |||
| 50 | 51.20 ± 3.82 | 7.47 | 2.40 |
| 150 | 160.80 ± 8.87 | 5.52 | 7.20 |
| 800 | 859.09 ± 13.61 | 1.64 | 3.64 |
| 4000 | 4196.08 ± 123.90 | 2.95 | 4.90 |
| Inter-day batch (n = 36) | |||
| 50 | 53.93 ± 3.61 | 6.70 | 7.87 |
| 150 | 159.53 ± 11.43 | 7.17 | 6.36 |
| 800 | 855 ± 54.56 | 6.38 | 6.88 |
| 4000 | 4245.33 ± 238.89 | 5.63 | 6.13 |
| Concentration (ng/mL) | Extraction Recovery | Matrix Effect | ||
|---|---|---|---|---|
| Mean (%) | RSD (%) | Mean (%) | RSD (%) | |
| 150 | 105.86 | 3.17 | 1.04 | 4.15 |
| 800 | 108.30 | 4.13 | ||
| 4000 | 104.37 | 2.84 | 0.93 | 2.93 |
| Dilution Times | Nominal Concentration (ng/mL) | Measured Concentration (ng/mL) | Precision (RSD, %) | Accuracy (RE, %) |
|---|---|---|---|---|
| 10 | 6000 | 6753.99 ± 137.86 | 2.04 | 12.40 |
| 100 | 60,000 | 66,991.62 ± 1070.94 | 1.60 | 11.65 |
| 500 | 80,000 | 83,227.13 ± 2514.19 | 3.02 | 4.03 |
| Stability Studies | Nominal Concentration (ng/mL) | Measured Concentration (ng/mL) | Precision (RSD, %) | Accuracy (RE, %) |
|---|---|---|---|---|
| Short-term stability a | 150 | 156.03 ± 1.18 | 0.76 | 4.02 |
| 4000 | 4042.76 ± 81.92 | 2.02 | 1.07 | |
| Long-term stability b | 150 | 154.41 ± 8.58 | 5.56 | 2.94 |
| 4000 | 3958.67± 73.22 | 1.85 | −1.03 | |
| Freeze–thaw stability c | 150 | 167.98 ± 1.67 | 1.00 | 11.98 |
| 4000 | 4476.71 ± 122.19 | 2.73 | 11.92 | |
| Post-preparative stability d | 150 | 137.78 ± 6.61 | 4.80 | −8.14 |
| 4000 | 3628.76 ± 69.53 | 1.92 | −9.28 |
| Parameters | Units | 50 mg/kg | 100 mg/kg | 250 mg/kg | |||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | ||
| t1/2 | (h) | 0.55 ± 0.04 | 0.65 ± 0.06 | 0.54 ± 0.03 | 0.65 ± 0.04 | 0.62 ± 0.01 | 0.64 ± 0.02 |
| Tmax | (h) | 0.133 ± 0.00 | 0.133 ± 0.00 | 0.133 ± 0.00 | 0.133 ± 0.00 | 0.133 ± 0.00 | 0.133 ± 0.00 |
| Cmax | (μg/mL) | 40.40 ± 3.72 | 48.78 ± 2.30 | 123.32± 6.25 | 138.05 ± 17.98 | 250.77 ± 81.50 | 324.36 ± 22.68 |
| AUC0-t | (h·μg/mL) | 29.48 ± 2.97 | 31.01 ± 4.63 | 56.20 ± 2.88 | 61.95 ± 2.49 | 111.14 ± 22.90 | 130.53 ± 7.05 |
| AUC0-∞ | (h·μg/mL) | 30.19 ± 3.18 | 31.60 ± 4.35 | 57.31 ± 2.88 | 63.12 ± 3.33 | 111.36 ± 22.86 | 131.69 ± 8.25 |
| Vd | (L/kg) | 1.31 ± 0.09 | 1.48 ± 0.10 | 1.36 ± 0.11 | 1.49 ± 0.15 | 2.08 ± 0.46 | 1.76 ± 0.12 |
| Vss | (L/kg) | 1.14 ± 0.10 | 1.13 ± 0.03 | 1.00 ± 0.07 | 0.99 ± 0.08 | 1.32 ± 0.49 | 1.03 ± 0.10 |
| Cl | (L/h/kg) | 1.67 ± 0.19 | 1.60 ± 0.20 | 1.75 ± 0.09 | 1.59 ± 0.09 | 2.31 ± 0.48 | 1.90 ± 0.12 |
| MRT0-t | (h) | 0.61 ± 0.07 | 0.65 ± 0.15 | 0.51 ± 0.01 | 0.56 ± 0.05 | 0.55 ± 0.09 | 0.51 ± 0.06 |
| MRT0-∞ | (h) | 0.69 ± 0.09 | 0.72 ± 0.12 | 0.57 ± 0.02 | 0.62 ± 0.03 | 0.56 ± 0.09 | 0.54 ± 0.03 |
| Analytes | Q1 (Da) | Q3 (Da) | DP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|
| GMDTC | 390.06 | 324.1 | 90 | 25 | 10 |
| IS | 366.0 | 259.2 | 75 | 30 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Zhong, Z.-Y.; Gao, Y.-T.; Ren, X.-F.; Liu, H.-Y.; Tang, X.-J. Establishment of LC-MS/MS Method for Determination of GMDTC in Rat Plasma and Its Application in Preclinical Pharmacokinetics. Molecules 2023, 28, 1191. https://doi.org/10.3390/molecules28031191
Hu W, Zhong Z-Y, Gao Y-T, Ren X-F, Liu H-Y, Tang X-J. Establishment of LC-MS/MS Method for Determination of GMDTC in Rat Plasma and Its Application in Preclinical Pharmacokinetics. Molecules. 2023; 28(3):1191. https://doi.org/10.3390/molecules28031191
Chicago/Turabian StyleHu, Wei, Zhi-Yong Zhong, Yu-Ting Gao, Xue-Feng Ren, Hai-Yang Liu, and Xiao-Jiang Tang. 2023. "Establishment of LC-MS/MS Method for Determination of GMDTC in Rat Plasma and Its Application in Preclinical Pharmacokinetics" Molecules 28, no. 3: 1191. https://doi.org/10.3390/molecules28031191
APA StyleHu, W., Zhong, Z.-Y., Gao, Y.-T., Ren, X.-F., Liu, H.-Y., & Tang, X.-J. (2023). Establishment of LC-MS/MS Method for Determination of GMDTC in Rat Plasma and Its Application in Preclinical Pharmacokinetics. Molecules, 28(3), 1191. https://doi.org/10.3390/molecules28031191








