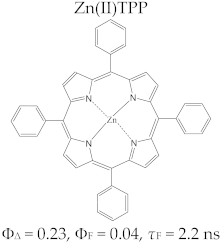
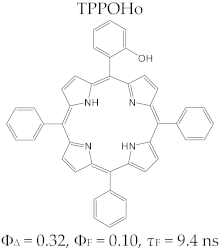
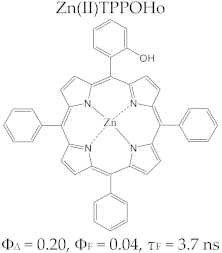
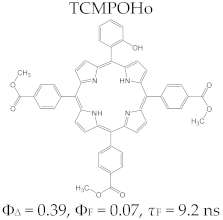
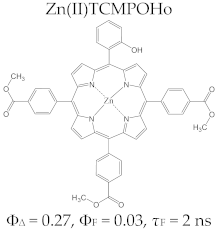
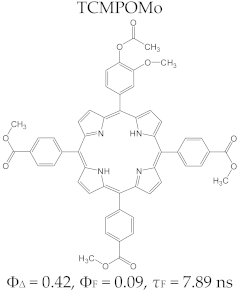
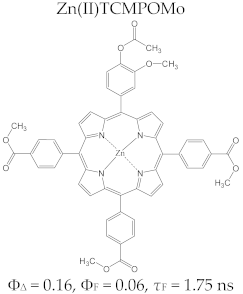
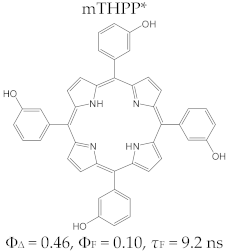
Porphyrin Macrocycles: General Properties and Theranostic Potential
Abstract
1. Introduction
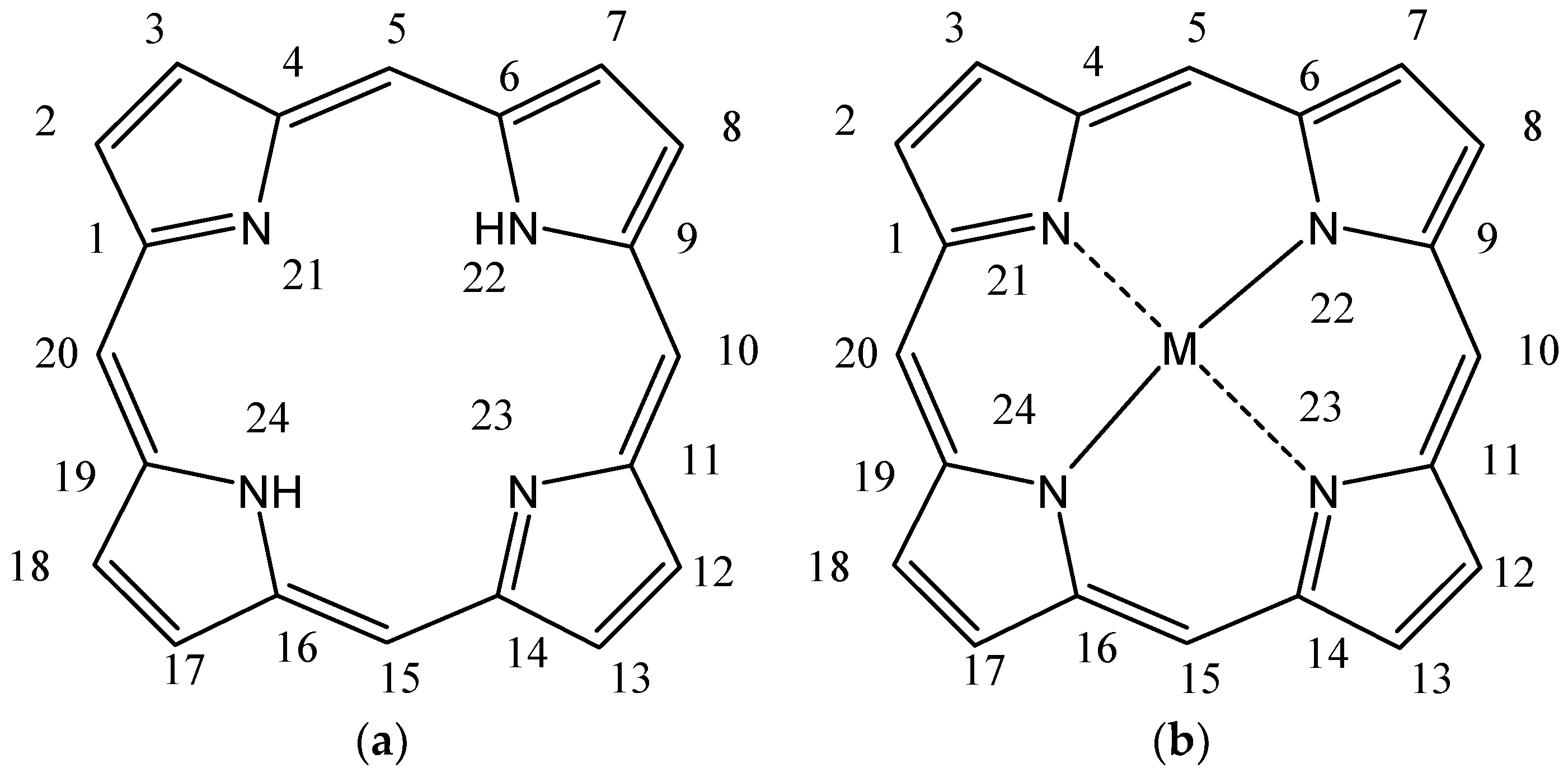
2. General Properties of Porphyrin Macrocycle
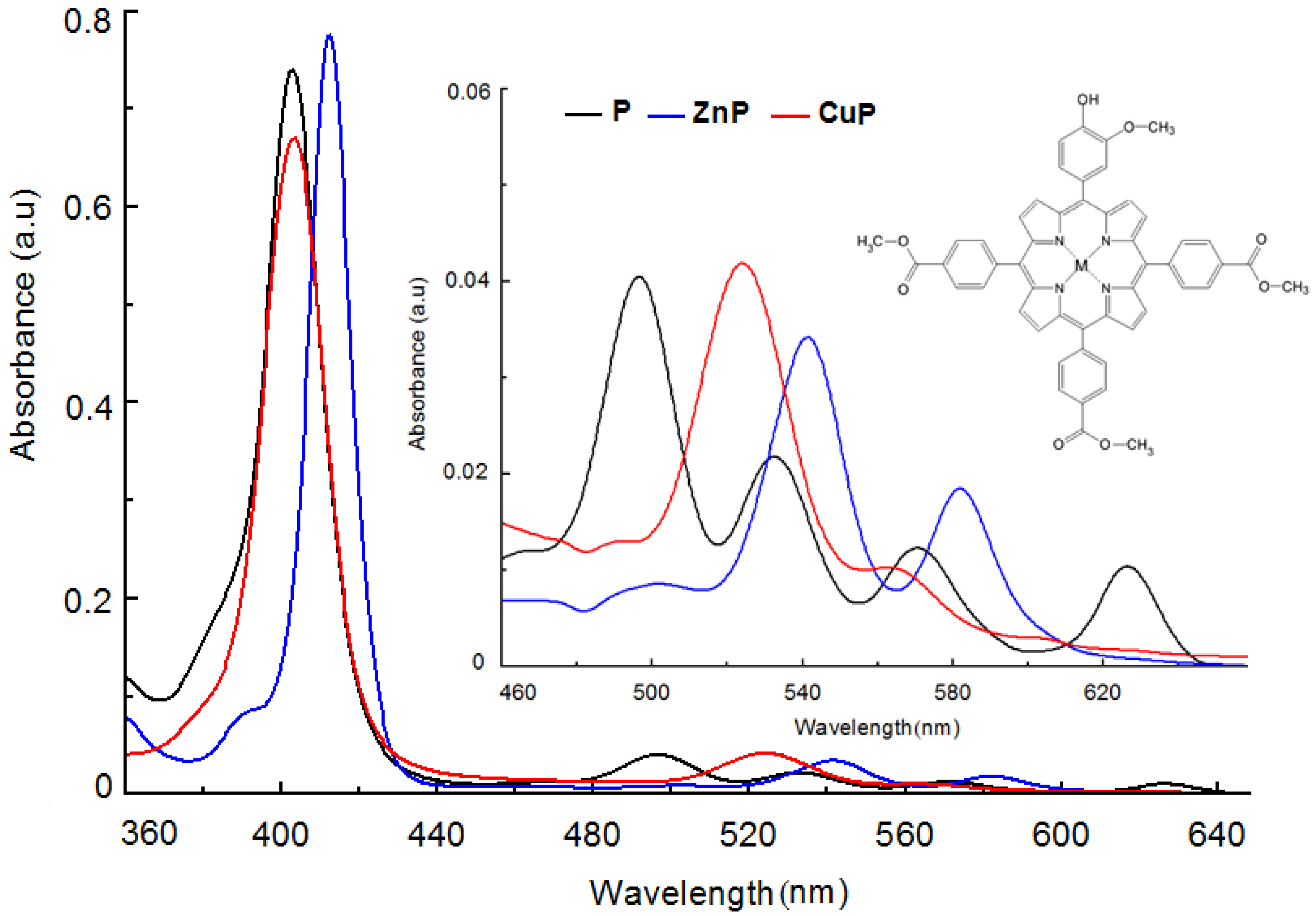
3. Aspects regarding Porphyrin Macrocycles as Photosensitizers
3.1. Short Background regarding to the Porphyrinoid Photosensitizers
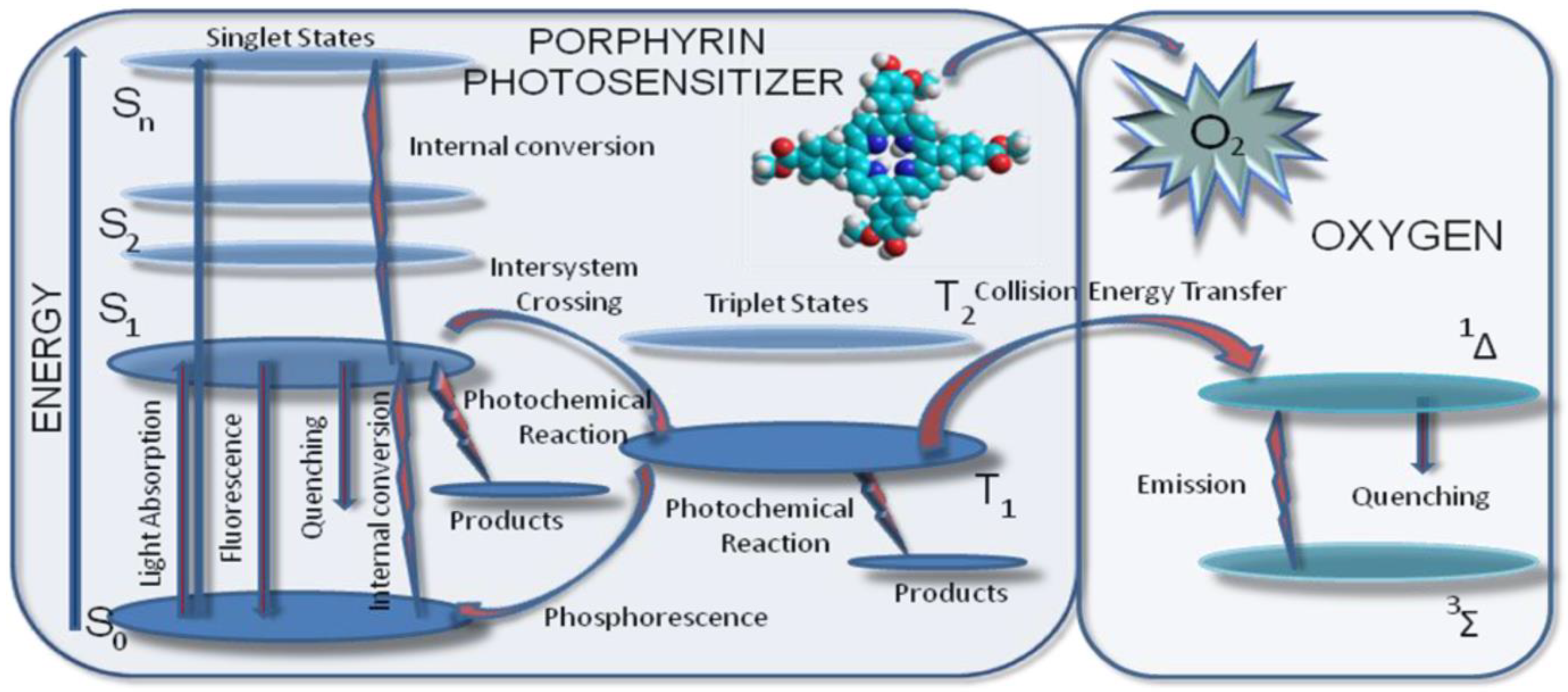
3.2. Basic Photochemical Principles in PDT
3.3. Chemical and Pharmaceutical Aspects Concerning Photosensitizers with Tetrapyrrolic Structures Used in Diagnosis and Antitumor Therapy
- It has a unique, well-defined structure with maximum purity and can be obtained by modern ecological methods;
- It has a structural profile that allows optimal internalization at the tumor level and is described by a well-defined distribution of lipophillic/hydrophilic groups at the periphery of tetrapyrrolic macrocycles;
- It has a well-defined spectral profile with maximum absorption in the therapeutic range (600–850 nm) associated with good efficiency in generating singlet oxygen (ΦΔ ≥ 0.5) and a triplet state with a lifetime in the microsecond range;
- It is soluble in nontoxic solvents accepted for pharmaceutical formulations;
- It is nontoxic in the absence of light;
- It provides rapid clearance from the organism;
- There is no toxic effect with the necessary dose for a therapeutic effect;
- There is an absence of toxicity for the photosensitizer metabolites.
- Hydrophobic photosensitizers, which have peripheral substituent hydrophobic functional groups and very reduced solubility in polar solvents, alcohol, or water, at a physiological pH;
- Hydrophilic photosensitizers, which have three or more peripheral functional substituent hydrophilic groups and slight water solubility at physiological pH;
- Amphiphilic photosensitizers, which have in their structure hydrophobic and hydrophilic functional groups and water solubility at physiological pH.
3.3.1. PHOTOFRIN®
3.3.2. FOSCAN®
3.3.3. LASERPHYRIN®
3.3.4. VISUDYNE®
3.3.5. PHOTOCHLOR®
3.3.6. PURLYTIN®
3.3.7. TOOKAD®
3.3.8. LUTRIN®
3.3.9. PHOTOSENS®
4. Strategies Aimed at Improving the Therapeutic Potential of Porphyrin Photosensitizers
4.1. Design and Synthesis of New Unsymmetrical Porphyrins for Theranostics Applications
4.2. Functionalizing of Porphyrin Type Macrocycles with Fragments of Bioactive Molecules
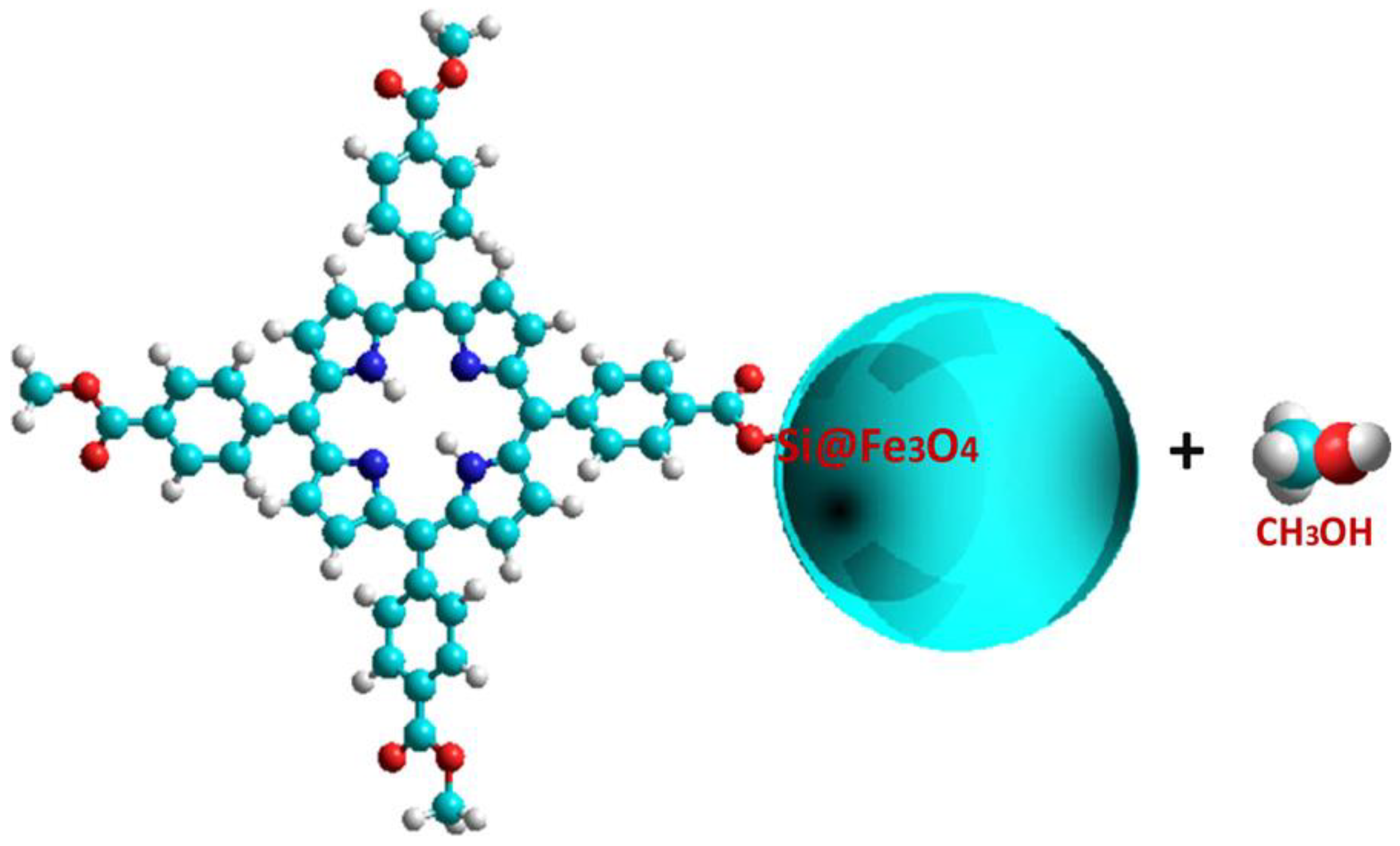
4.3. Functionalizing of Porphyrin with Metal-Based Nanoparticles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TCMPMOHP | 5-(4-hydroxy-3-methoxyphenyl)-10,15,20-tris(4-carboxymethylphenyl)porphyrin |
| Zn(II)TCMPMOHP | 5-(4-hydroxy-3-methoxyphenyl)-10,15,20-tris(4-carboxymethylphenyl)porphyrinatozinc(II) |
| Cu(II)TCMPMOHP | 5-(4-hydroxy-3-methoxyphenyl)-10,15,20-tris(4-carboxymethylphenyl)porphyrinatocopper(II) |
| TMAPMOHp | 5-(4-hydroxy-3-methoxyphenyl)-10,15,20-tris(4-acetoxy-3-methoxyphenyl)porphyrin |
| Zn(II)TMAPMOHp | 5-(4-hydroxy-3-methoxyphenyl)-10,15,20-tris(4-acetoxy-3-methoxyphenyl)porphyrinatozinc(II) |
| Cu(II)TMAPMOHp | 5-(4-hydroxy-3-methoxyphenyl)-10,15,20-tris(4-acetoxy-3-methoxyphenyl)porphyrinatocopper(II) |
| TMAPDOH | 5-(2,4-dihydroxyphenyl)-10,15,20-tris(4-acetoxy-3-methoxyphenyl)porphyrin |
| TCMPOHo | 5-(2-hydroxyphenyl)-10,15,20-tris(4-carboxymethylphenyl)porphyrin |
| Zn(II)TCMPOHo | 5-(2-hydroxyphenyl)-10,15,20-tris(4-carboxymethylphenyl)porphyrinatozinc(II) |
| Cu(II)TCMPOHo | 5-(2-hydroxyphenyl)-10,15,20-tris(4-carboxymethylphenyl)porphyrinatocopper(II) |
| TRMOPP | 5-[(3,4-methylenedioxy)phenyl]-10,15,20-tris(4-carboxymethylphenyl)porphyrin |
| Zn(II)TRMOPP | 5-[(3,4-methylenedioxy)phenyl]-10,15,20-tris(4-carboxymethylphenyl)porphyrinatozinc(II) |
| Cu(II)TRMOPP | 5-[(3,4-methylenedioxy)phenyl]-10,15,20-tris(4-carboxymethylphenyl)porphyrinatocopper(II) |
| TCMPOHp | 5-(4-hydroxyphenyl)-10,15,20-tris(4-carboxymethylphenyl)porphyrin |
| Zn(II)TCMPOHp | 5-(4-hydroxyphenyl)-10,15,20-tris(4-carboxymethylphenyl)porphyrinatozinc(II) |
| Cu(II)TCMPOHp | 5-(4-hydroxyphenyl)-10,15,20-tris(4-carboxymethylphenyl)porphyrinatocopper(II) |
| TCMPOMo | 5-(4-acetoxy-3-methoxyphenyl)-10,15,20-tris(4-carboxymethylphenyl)porphyrin |
| Zn(II)TCMPOMo | 5-(4-acetoxy-3-methoxyphenyl)-10,15,20-tris(4-carboxymethylphenyl)porphyrinatozinc(II) |
| Cu(II)TCMPOMo | 5-(4-acetoxy-3-methoxyphenyl)-10,15,20-tris(4-carboxymethylphenyl)porphyrinatocopper(II) |
| TCMPOHm | 5-(3-hydroxyphenyl)-10,15,20-tris(4-carboxymethylphenyl)porphyrin |
| Zn(II)TCMPOHm | 5-(3-hydroxyphenyl)-10,15,20-tris(4-carboxymethylphenyl)porphyrinatozinc(II) |
| Cu(II)TCMPOHm | 5-(3-hydroxyphenyl)-10,15,20-tris(4-carboxymethylphenyl)porphyrinatocopper(II) |
| TMAPOHm | 5-(3-hydroxyphenyl)-10,15,20-tris(4-acetoxy-3-methoxyphenyl)porphyrin |
| Zn(II)TMAPOHm | 5-(3-hydroxyphenyl)-10,15,20-tris(4-acetoxy-3-methoxyphenyl)porphyrinatozinc(II) |
| Cu(II)TMAPOHm | 5-(3-hydroxyphenyl)-10,15,20-tris(4-acetoxy-3-methoxyphenyl)porphyrinatocopper(II) |
| TMAPOHo | 5-(2-hydroxyphenyl)-10,15,20-tris(4-acetoxy-3-methoxyphenyl)porphyrin |
| Zn(II)TMAPOHo | 5-(2-hydroxyphenyl)-10,15,20-tris(4-acetoxy-3-methoxyphenyl)porphyrinatozinc(II) |
| Cu(II)TMAPOHo | 5-(2-hydroxyphenyl)-10,15,20-tris(4-acetoxy-3-methoxyphenyl)porphyrinatocopper(II) |
| Zn(II)TMAPOHp | 5-(4-hydroxyphenyl)-10,15,20-tris(4-acetoxy-3-methoxyphenyl)porphyrinatozinc(II) |
| Cu(II)TMAPOHp | 5-(4-hydroxyphenyl)-10,15,20-tris(4-acetoxy-3-methoxyphenyl) porphyrinatocopper(II) |
| TCMPDMOH | 5,15-bis-(4-hydroxy-3-methoxyphenyl)-10,20-bis(4-carboxymethylphenyl)porphyrin |
| TPP | 5,10,15,20-tetrakis-phenyl porphyrin |
| Zn(II)TPP | 5,10,15,20-tetrakis-phenyl porphyrinatozinc(II) |
| TPPOHo | 5-(2-hydroxyphenyl)-10,15,20-triphenyl porphyrin |
| Zn(II)TPPOHo | 5-(2-hydroxyphenyl)-10,15,20-triphenyl porphyrinatozinc(II) |
| mTHPP | 5,10,15,20-tetrakis(3-hydroxyphenyl)porphyrin |
| mTHPC | 5,10,15,20-tetrakis(3-hydroxyphenyl)chlorin |
| TCMP | 5,10,15,20-tetrakis(4-carboxymethylphenyl)porphyrin |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Sarbadhikary, P.; George, B.P.; Abrahamse, H. Recentadvances in photosensitizers as multifunctional theranostic agents forimaging-guided photodynamictherapy of cancer. Theranostics 2021, 11, 9054–9088. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef] [PubMed]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- Pandey, R.K.; Zheng, G. Porphyrins as photosensitizers in photodynamic therapy. In The Porphyrin Handbook; Kadish, K.M., Guilard, R., Smith, K.M., Eds.; Academic Press: New York, NY, USA, 2000; Volume 6, pp. 157–230. [Google Scholar]
- Simpson, M.C.; Novikova, I.N. Porphyrins: Electronic structure and ultraviolet/visible absorption spectroscopy. In Fundamentals of Porphyrin Chemistry: A 21st Century Approach; Brothers, P.J., Senge, O.M., Eds.; John Wiley & Sons Ltd.: New Jersey, NJ, USA, 2022; Volume 1, pp. 505–586. [Google Scholar]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as Diagnostic and Therapeutic Agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [PubMed]
- Plekhova, N.; Shevchenko, O.; Korshunova, O.; Stepanyugina, A.; Tananaev, I.; Apanasevich, V. Development of Novel Tetrapyrrole Structure Photosensitizers for Cancer Photodynamic Therapy. Bioengineering 2022, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: From molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A.; Peng, Q.; Moan, J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem. Photobiol. Sci. 2007, 6, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Betz, F. Untersuchung uber die biologische (photodynamische) wirkung des hamatoporphyrins und anderer derivate des blutundgallenfarbstoffs. Dtsch. Arch. Klin. Med. 1913, 112, 476–503. [Google Scholar]
- Policard, A. Etudes sur les aspects offerts par des tumeursexperimentales examinees a la lumiere des Woods. Compt. Rend. Soc. Biol. 1924, 91, 1423–1426. [Google Scholar]
- Battersby, A.R.; Fookes, C.J.; Matcham, G.W.; McDonald, E. Biosynthesis of the pigments of life: Formation of the macrocycle. Nature 1980, 285, 17–21. [Google Scholar] [CrossRef]
- Brothers, P.J.; Gosh, A. Coordination chemistry. In Fundamentals of Porphyrin Chemistry: A 21st Century Approach; Brothers, P.J., Senge, O.M., Eds.; John Wiley & Sons Ltd.: New Jersey, NJ, USA, 2022; Volume 4, pp. 141–146. [Google Scholar]
- Sessler, J.L.; Tomat, E. Transition-metal complexes of expanded porphyrins. Acc. Chem. Res. 2007, 40, 371–379. [Google Scholar] [CrossRef]
- Nelson, J.S.; Roberts, W.G.; Berns, M.W. In vivo studies on the utilization of Mono-l-aspartyl chlorin (NPe6) for photodynamic therapy. Cancer Res. 1987, 47, 4681–4685. [Google Scholar]
- Mauzerall, D. Porphyrins, Chlorophyll, and Photosynthesis; Springer: Berlin/Heidelberg, Germany, 1977; pp. 117–124. [Google Scholar] [CrossRef]
- Armstrong, D.; Stratton, R.D. Oxidative Stress and Antioxidant Protection: The Science of Free Radical Biology and Disease; John Wiley &Sons: Hoboken, NJ, USA, 2016; pp. 415–470. [Google Scholar]
- Nantes, I.L.; Crespilho, F.N.; Mugnol, K.C.U.; Araujo-Chaves, J.C.; Nascimento, O.R.; Pinto, S.M.S. Magnetic circular dichroism applied in the study of symmetry and functional properties of porphyrinoids. In Circular Dichroism: Theory and Spectroscopy, 1st ed.; David, S.R., Ed.; Nova Science Publishers: New York, NY, USA, 2010; pp. 321–344. [Google Scholar]
- Van Santen, R. Catalysis in perspective: Historic review. In Catalysis: From Principles to Applications, 1st ed.; Beller, M., Renken, A., Van Santen, R., Eds.; Wiley-VCH: Weinheim, Germany, 2012; pp. 3–19. [Google Scholar]
- Gouterman, M. Optical spectra and electronic structure of porphyrins and related rings. In The Porphyrins; Dolphin, D., Ed.; Academic Press: New York, NY, USA, 1978; Volume 3, pp. 11–87. [Google Scholar]
- Gouterman, M.; Wagniere, G.H.; Snyder, L.C. Spectra of porphyrins: Part II. Four orbital model. J. Mol. Spectrosc. 1963, 11, 108–127. [Google Scholar] [CrossRef]
- Milgrom, L.R. What porphyrins are and what they do. In The Colours of Life. An Introduction to the Chemistry of Porphyrins and Related Compounds; Oxford University Press: Oxford, UK, 1997; Volume 1, pp. 1–85. [Google Scholar]
- Boscencu, R.; Socoteanu, R.P.; Manda, G.; Radulea, N.; Anastasescu, M.; Gama, A.; Ferreira Machado, I.; Vieira Ferreira, L.F. New A3B porphyrins as potenţial candidates for theranostic. Synthesis and photochemical behaviour. Dyes Pigments 2019, 160, 410–417. [Google Scholar] [CrossRef]
- Dar, U.A.; Shah, S.A. UV–visible and fluorescence spectroscopic assessment of meso-tetrakis-(4-halophenyl) porphyrin; H2TXPP (X=F, Cl, Br, I) in THF and THF-water system: Effect of pH and aggregation behaviour. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118570. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xia, R.; Li, C.; Li, Y.; Sun, T.; Xie, Z.; Jing, X. Nanoscale aggregates of porphyrins: Red-shifted absorption, enhanced absorbance and phototherapeutic activity. Mater. Chem. Front. 2021, 5, 8333–8340. [Google Scholar] [CrossRef]
- Dabrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyzioł, A.; Arnaut, L.G. Engineering of relevant photodynamic processes through structural modifications of metallotetrapyrrolic photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Meshkov, I.N.; Bulach, V.; Gorbunova, Y.G.; Gostev, F.E.; Nadtochenko, V.A.; Tsivadze, A.Y.; Hosseini, M.W. Tuning photochemical properties of phosphorus (V) porphyrin photosensitizers. Chem. Commun. 2017, 53, 9918–9921. [Google Scholar] [CrossRef]
- Horváth, O.; Valicsek, Z.; Fodor, M.A.; Major, M.M.; Imran, M.; Grampp, G.; Wankmüller, A. Visible light-driven photophysics and photochemistry of water-soluble metalloporphyrins. Coord. Chem. Rev. 2016, 325, 59–66. [Google Scholar] [CrossRef]
- Vieira Ferreira, L.F.; Ferreira Machado, I.L. Surface photochemistry: Organic molecules within nanocavities of calixarenes. Curr. Drug Discov. Technol. 2007, 4, 229–245. [Google Scholar] [CrossRef]
- Strickler, S.J.; Berg, R.A. Relationship between Absorption Intensity and Fluorescence Lifetime of Molecules. J. Chem. Phys. 1962, 37, 814–822. [Google Scholar] [CrossRef]
- Harriman, A.; Hosie, R.J. Luminescence of porphyrins and metalloporphyrins. Fluorescence of substituted tetraphenylporphyrins. J. Photochem. 1981, 15, 163–167. [Google Scholar] [CrossRef]
- Nifiatis, F.; Athas, J.C.K.; Gunaratne, D.; Gurung, Y.; Monette, K.; Shivokevich, P.J. Substituent effects of porphyrin on singlet oxygen generation quantum yields. Open Spectrosc. J. 2011, 5, 1–12. [Google Scholar] [CrossRef]
- Harriman, A. Luminescence of porphyrins and metalloporphyrins, Zinc(II), nickel(II) and manganese(II) porphyrins. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1980, 76, 1978–1985. [Google Scholar] [CrossRef]
- Chen, C.Y.; Sun, E.; Fan, D. Synthesis and physicochemical properties of metallobacteriochlorins. Inorg. Chem. 2012, 51, 9443–9464. [Google Scholar] [CrossRef] [PubMed]
- Von Tapiener, H.; Jesionek, A. TherapeutischeVersuchemitfluoreszierendenStoffen. Münchener Med. Wochenschr. 1903, 50, 2042–2051. [Google Scholar]
- Silver, H. Psoriasis vulgaris treated with hemathoporphyrin. Arch Dermatol. Physiol. 1937, 36, 1118–1119. [Google Scholar]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar] [PubMed]
- Dahlman, A.; Wile, A.G.; Burns, R.G.; Johnson, F.; Berns, M.W. Laser photoradiation therapy of cancer. Cancer Res. 1982, 43, 430–434. [Google Scholar]
- Diezel, W.; Meffert, H.; Sonnichsen, N. Stability of nystatin in an o/w-cream. Dermatol. Monatsschr. 1980, 166, 75–79. [Google Scholar]
- Dougherty, T.J.; Henderson, B.; Schwartz, S.; Winkelman, J.W.; Lipson, R.L. Historical perspective Photodynamic therapy. In Basic Principles and Clinical Application; Henderson, B.W., Dougherty, T.J., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1992; pp. 1–15. [Google Scholar]
- Dougherty, T.J. Hematoporphyrin as a photosensitizer of tumors. Photochem. Photobiol. 1983, 38, 377–379. [Google Scholar] [CrossRef]
- Dougherty, T.J. Photodynamic therapy. Photochem.Photobiol. 1993, 58, 895–900. [Google Scholar] [CrossRef]
- Jori, G. Tumour photosensitizers: Approaches to enhance the selectivity and efficiency of photodynamic therapy. J. Photochem. Photobiol B Biol. 1996, 36, 87–93. [Google Scholar] [CrossRef]
- Detty, M.R.; Gibson, S.L.; Wagner, S.J. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J. Med. Chem. 2004, 47, 3897–3915. [Google Scholar] [CrossRef] [PubMed]
- Boyle, R.B.; Dolphin, D. Structure and biodistribution relationships of photodynamic sensitizers. Photochem. Photobiol. 1996, 64, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Kudinova, N.V.; Berezov, T.T. Photodynamic therapy of cancer: Search for ideal photosensitizer. Biochem. Suppl. Ser. B Biomed. Chem. 2010, 4, 95–103. [Google Scholar] [CrossRef]
- Myrzakhmetov, B.; Arnoux, P.; Acherar, S.; Vanderesse, R.; Frochot, C. Folic acid conjugates with photosensitizers for cancer targeting in photodynamic therapy: Synthesis and photophysical properties. Bioorg. Med. Chem. 2017, 25, 1–10. [Google Scholar]
- Clark, P.A.; Alahmad, A.J.; Qian, T.; Zhang, R.R.; Wilson, H.K.; Weichert, J.P.; Palecek, S.P.; Kuo, J.S.; Shusta, E.V. Analysis of cancer-targeting alkylphosphocholine analog permeability characteristics using a human induced pluripotent stem cell blood-brain barrier model. Mol. Pharm. 2016, 13, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.R.; Swanson, K.I.; Hall, L.T.; Weichert, J.P.; Kuo, J.S. Diapeutic cancer-targeting alkylphosphocholine analogs may advance management of brain malignancies. CNS Oncol. 2016, 5, 223–231. [Google Scholar] [CrossRef]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in photosensitizers and light delivery forphotodynamic therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef]
- Van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic photodynamic therapy: Basic principles, current clinical status and future directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Tanaka, M.; Kataoka, H.; Mabuchi, M.; Sakuma, S.; Takahashi, S.; Tujii, R.; Akashi, H.; Ohi, H.; Yano, S.; Morita, A.; et al. Anticancer effects of novel photodynamic therapy with glycoconjugated chlorin for gastric and colon cancer. Anticancer Res. 2011, 31, 763–769. [Google Scholar]
- Lapa, C.; Schreder, M.; Schirbel, A.; Samnick, S.; Kortum, K.M.; Hermann, H.; Kropf, S.; Einsele, H.; Buck, A.K.; Wester, H.J. [68Ga] Penixafor-PET/CT for imaging of chemokine receptor CXCR4 expression in multiple myeloma–Comparison to [18F] FDG and laboratory values. Theranostics 2017, 7, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Broughton, L.J.; Giuntini, F.; Savoie, H.; Bryden, F.; Boyle, R.W.; Maraveyas, A.; Madden, L.A. Duramycin-porphyrin conjugates for targeting of tumour cells using photodynamic therapy. J. Photochem. Photobiol. B. 2016, 163, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Li, Z.; Xu, W.; Zhou, C.; Yang, L.; Zhang, Q.; Li, L.; Liu, F.; Han, L.; Ge, Y. Porphyrin-containing polyaspartamide gadolinium complexes as potential magnetic resonance imaging contrast agents. Int. J. Pharm. 2011, 407, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Mokwena, M.G.; Kruger, C.A.; Ivan, M.; Heidi, A. A review of nanoparticle photosensitizer drug delivery uptake systems for photodynamic treatment of lung cancers. Photodiagn. Photodyn. Ther. 2018, 22, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Fakayode, O.J.; Kruger, C.A.; Songca, S.P.; Abrahamse, H.; Oluwafemi, O.S. Photodynamic therapy evaluation of methoxypolyethylene glycol-thiol-SPIONs-gold-meso-tetrakis(4-hydroxyphenyl) porphyrin conjugate against breast cancer cells. Mater. Sci. Eng. C 2018, 92, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Penon, O.; Marín, M.J.; Russell, D.A.; Pérez-García, L. Water soluble, multifunctional antibody-porphyrin gold nanoparticles for targeted photodynamic therapy. J. Colloid Interface Sci. 2017, 496, 100–110. [Google Scholar] [CrossRef]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Baron, E.D.; Suggs, A.K. Introduction to photobiology. Dermatol. Clin. 2014, 32, 255–266. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Photodynamic therapy: Novel third-generation photosensitizers one step closer? Br. J. Pharmacol. 2008, 154, 1–3. [Google Scholar] [CrossRef]
- Wayne, C.E.; Wayne, R.P. Photochemical principles. In Photochemistry, of Oxford Chemistry Primers, Oxford Science; Oxford University Press: Oxford, UK, 1996; Volume 39, pp. 11–12. [Google Scholar]
- Schweitzer, C.; Schmidt, R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 2003, 103, 1685–1757. [Google Scholar] [CrossRef]
- Gilbert, A.; Baggott, J. Essentials of Molecular Photochemistry; Blackwell Scientific: Oxford, UK, 1991; Chapter 4; pp. 92–99. [Google Scholar]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one–Photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.N.; Filipe, P.; Morlière, P.; Mazière, J.C.; Freitas, J.P.; de Castro, C.J.L.; Santus, R. Photodynamic therapies: Principles and present medical ap-plications. Bio Med. Mater. Eng. 2006, 16, 147–154. [Google Scholar]
- Nyman, E.S.; Hynninen, P.H. Research advances in the use of tetrapyrrolic photosensitisers for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2004, 73, 1–28. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Dougherty, T.J. Basic principles of photodynamic therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Bonnett, R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 1995, 24, 19–33. [Google Scholar] [CrossRef]
- Sharman, W.M.; Allen, C.M.; van Lier, J.E. Photodynamic therapeutics: Basic principles and clinical applications. Drug Discov. Today 1999, 4, 507–517. [Google Scholar] [CrossRef]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef]
- Hirth, A.; Michelsen, U.; Wohrle, D. PhotodynamischeTumortherapie. Chem. Unserer Zeit 1999, 33, 84–94. [Google Scholar] [CrossRef]
- Sandland, J.; Malatesti, N.; Boyle, R. Porphyrins and related macrocycles: Combining photosensitization with radio- or optical-imaging for next generation theranostic agents. Photodiagn. Photodyn. Ther. 2018, 23, 281–294. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Cuenca, R.E.; Downie, G.H.; Camnitz, P.; Brodish, B.; Sibata, C.H. Clinical photodynamic therapy of head and neck cancers–A review of applications and outcomes. Photodiagn. Photodyn. Ther. 2005, 2, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. A review of progress in clinical photodynamic therapy. Technol. Cancer Res. Treat. 2005, 4, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.; Moghissi, K.; Downie, G.; Dixon, K. Photodynamic therapy (PDT) for lung cancer. Photodiagn. Photodyn. Ther. 2011, 8, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.M.; Mac Robert, A.J.; Mosse, C.A.; Periera, B.; Bown, S.G.; Keshtgar, M.R.S. Photodynamic therapy: Inception to application in breast cancer. Breast 2017, 31, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Stegh, A.H. Toward personalized cancer nanomedicine–past, present, and future. Integr. Biol. 2012, 5, 48–65. [Google Scholar] [CrossRef]
- Pineiro, M.; Pereira, M.M.; d’ Rocha Gonsalves, A.M.; Arnaut, L.G.; Formosinho, S.J. Singlet oxygen quantum yields from halogenated chlorins: Potential new photodynamic therapy agents. J. Photochem. Photobiol. A Chem. 2001, 138, 147–157. [Google Scholar] [CrossRef]
- Spagnul, C.; Turner, L.C.; Boyle, R.W. Immobilized photosensitizers for antimicrobial applications. J. Photochem. Photobiol. B Biol. 2015, 150, 11–30. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Caruso, E.; Tettamanti, G.; Banfi, S.; Barbieri, P. Photoinduced antibacterial activity of two dicationic 5,15-diarylporphyrins. J. Photochem. Photobiol. B Biol. 2013, 127, 123–132. [Google Scholar] [CrossRef]
- Moritz, M.N.O.; Gonçalves, J.L.S.; Linares, I.A.P.; Perussi, J.R.; de Oliveira, K.T. Semi-synthesis and PDT activities of a new amphiphilic chlorin derivative. Photodiagn. Photodyn. Ther. 2017, 17, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Sahin, T.; Liu, M.; Lindsey, J.S.; Bocian, D.F.; Holten, D. Photophysical comparisons of PEGylated porphyrins, chlorins and bacteriochlorins. New J. Chem. 2016, 40, 9648–9656. [Google Scholar] [CrossRef]
- Pisarek, S.; Maximova, K.; Gryko, D. Strategies toward the synthesis of amphiphilic porphyrins. Tetrahedron 2014, 38, 6685–6715. [Google Scholar] [CrossRef]
- Liu, M.; Chen, C.Y.; Mandal, A.K.; Chandrashaker, V.; Evans-Storms, R.B.; Pitner, J.B.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Bioconjugatable, PEGylated Hydroporphyrins for Photochemistry and Photomedicine. Narrow-Band, Red-Emitting Chlorins. New J. Chem. 2016, 40, 7721–7740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jiang, J.; Liu, M.; Taniguchi, M.; Mandal, A.K.; Evans-Storms, R.B.; Pitner, J.B.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Bioconjugatable, PEGylated Hydroporphyrins for Photochemistry and Photomedicine. Narrow-Band, Near-Infrared-Emitting Bacteriochlorins. New J. Chem. 2016, 40, 7750–7767. [Google Scholar] [CrossRef]
- Roy, A.; Magdaong, N.C.M.; Jing, H.; Rong, J.; Diers, J.R.; Kang, H.S.; Niedzwiedzki, D.M.; Taniguchi, M.; Kirmaier, C.; Lindsey, J.S.; et al. Balancing Panchromatic Absorption and Multistep Charge Separation in a Compact Molecular Architecture. J. Phys. Chem. A 2022, 126, 9353–9365. [Google Scholar] [CrossRef]
- Allison, R.R.; Sibata, C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagn. Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.-I.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Monro, S.; Colón, L.K.; Yin, H.; Roque, J.; Konda, P.; Gujar, S.; Thummel, R.; Lilge, L.; Cameron, G.C.; McFarland, A.S. Ttransition metal complexes and photodynamic therapy from a tumor-centered approach: Challenges, opportunities, and highlights from the development of TLD. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, J.; Fukami, S.; Ichikawa, M.; Mohamed, A.; Kohno, M. Intraoperative photodiagnosis for malignant glioma using photosensitizer talaporfin sodium. Front. Surg. 2019, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Mota, H.C.; Sibata, C.H. Clinical PD/PDT in North America: An historical review. Photodiagn. Photodyn. Ther. 2004, 1, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Yang, V.X.; Muller, P.J.; Herman, P.; Wilson, B.C. A multispectral fluorescence imaging system: Design and initial clinical tests in intra-operative Photofrin photodynamic therapy of brain tumors. Lasers Surg. Med. 2003, 32, 224–232. [Google Scholar] [CrossRef]
- Bogaards, A.; Varma, A.; Zhang, K.; Zach, D.; Bisland, S.K.; Moriyama, E.H.; Lilge, L.; Muller, P.J.; Wilson, B.C. Fluorescence image guided brain tumour resection with adjuvant metronomic photodynamic therapy: Preclinical model and technology development. Photochem. Photobiol. Sci. 2005, 4, 438–442. [Google Scholar] [CrossRef]
- Bogaards, A.; Varma, A.; Collens, S.P.; Lin, A.H.; Giles, A.; Yang, V.X.D.; Bilbao, J.M.; Lilge, L.D.; Muller, P.J.; Wilson, B.C. Increased brain tumor resection using fluorescence image guidance in a preclinical model. Lasers Surg. Med. 2004, 35, 181–190. [Google Scholar] [CrossRef]
- Olivo, M.; Wilson, B.C. Mapping ALA-induced PPIX fluorescence in normal brain and brain tumour using confocal fluorescence microscopy. Int. J. Oncol. 2004, 25, 37–45. [Google Scholar] [CrossRef]
- Hage, R.; Ferreira, J.; Bagnato, V.S.; Vollet-Filho, J.D.; Plapler, H. Pharmacokinetics of photogem using fluorescence spectroscopy in dimethylhydrazine-inducedmurine colorectal carcinoma. Int. J. Photoenergy 2012, 20, 1–8. [Google Scholar] [CrossRef]
- Trindade, F.Z.; Pavarina, A.C.; Ribeiro, A.P.D.; Bagnato, V.S.; Vergani, C.E.; Souza Costa, C.A. Toxicity of photodynamic therapy with LED associated to Photogem®: An in vivo study. Lasers Med. Sci. 2012, 27, 403–411. [Google Scholar] [CrossRef]
- Benes, J.; Pouckova, P.; Zeman, J.; Zadinova, M.; Sunka, P.; Lukes, P.; Kolarova, H. Effects of tandem shock waves combined with photosan and cytostatics on the growth of tumours. Folia Biol. 2011, 57, 255–260. [Google Scholar]
- Bonnett, R.; Martinez, G. Photobleaching of photosensitisers used in photodynamic therapy. Tetrahedron 2001, 57, 9513–9547. [Google Scholar] [CrossRef]
- Lovell, J.F.; Liu, T.W.B.; Chen, J.; Zheng, G. Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.; Ritsch-Marte, M.; Kostron, H. mTHPC-mediated photodynamic diagnosis of malignant brain tumors. Photochem. Photobiol. 2001, 74, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Kostron, H.; Rossler, K. Surgical intervention in patients with malignant glioma. Wien Med. Wochenschr. 2006, 156, 338–341. [Google Scholar] [CrossRef]
- Yow, C.M.N.; Chen, J.Y.; Mak, N.K.; Cheung, N.H.; Leung, A.W.N. Cellular uptake, subcellular localization and photodamaging effect of Temoporfin (Mthpc) in nasopharyngeal carcinoma cells: Comparison with hematoporphyrin derivative. Cancer Lett. 2000, 157, 123–131. [Google Scholar] [CrossRef]
- Ronn, A.M.; Nouri, M.; Lofgren, L.A.; Steinberg, B.M.; Westerborn, A.; Windal, T.; Shikowitz, M.J. Human tissue levels and plasma pharmacokinetics of temoporfin (Foscan®, mTHPC). Lasers Med. Sci. 1996, 11, 267–272. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Gaio, E.; Scheglmann, D.; Reddi, E.; Moret, F. Uptake and photo-toxicity of Foscan®, Foslip® and Fospeg® in multicellular tumor spheroids. J. Photochem. Photobiol. B Biol. 2016, 161, 244–252. [Google Scholar] [CrossRef]
- Compagnin, C.; Moret, F.; Celotti, L.; Miotto, G.; Woodhams, J.H.; MacRobert, A.J.; Scheglmann, D.; Iratni, S.; Reddi, E. Meta-tetra (hydroxyphenyl)chlorin-loaded liposomes sterically stabilised with poly (ethylene glycol) of different lenght and density: Characterisation, in vitro cellular uptake and phototoxicity. Photochem. Photobiol. Sci. 2011, 10, 1751–1759. [Google Scholar] [CrossRef]
- Kiesselich, T.; Berlanda., J.; Plaetzer., K.; Krammer., B.; Berr., F. Comparative characterisation of the efficiency and cellular pharmacokinetics of Foscan® and Foslip® based photodynamic treatment in human biliary tract cancer cell lines. Photochem. Photobiol. Sci. 2007, 6, 619–627. [Google Scholar] [CrossRef]
- Bovis, M.J.; Woodhams, J.H.; Loizidou, M.; Scheglmann, D.; Bown, S.G.; MacRobert, A.J. Improved in vivo delivery of m-THPC via pegylated liposomes for use in photodynamic therapy. J. Control. Release 2012, 30, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Reshetov, V.; Lassalle, H.P.; François, A.; Dumas, D.; Hupont, S.; Gräfe, S.; Filipe, V.; Jiskoot, W.; Guillemin, F.; Zorin, V.; et al. Photodynamic therapy with conventional and PEGylated liposomal formulations of m-THPC (temoporfin): Comparison of treatment efficacy and distribution characteristics in vivo. Int. J. Nanomed. 2013, 8, 3817–3831. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Samplonius, D.F.; de Visscher, S.; Roodenburg, J.L.; Helfrich, W.; Witjes, M.J. Photochemical internalization (PCI)-mediated enhancement of bleomycin cytotoxicity by liposomal mTHPC formulations in human head and neck cancer cells. Lasers Surg. Med. 2014, 46, 650–658. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.; Dobhal, M.P.; Gryshuk, A.; Morgan, J.; Dougherty, T.J.; Oseroff, A.; Pandey, R.K. Methyl pyropheophorbide-a analogues: Potential fluorescent probes for the peripheral-type benzodiazepine receptor. Effect of central metal in photosensitizing efficacy. J. Med. Chem. 2005, 48, 3692–3695. [Google Scholar] [CrossRef]
- Spikes, J.D.; Bommer, J.C. Photosensitizing properties of Mono-l-aspartyl chlorin e6 (NPe6): A candidate sensitizer for the photodynamic therapy of tumors. J. Photochem. Photobiol. B. 1993, 17, 135–143. [Google Scholar] [CrossRef]
- Figueiredo, T.L.C.; Dolphin, D. Mesoarylporphyrins as dienophiles in diels-alder reactions: A novel approach to the synthesis of chlorins, bacteriochlorins, and naphthoporphyrins. Org. Chem. 1998, 11, 297–300. [Google Scholar]
- Blant, S.A.; Ballini, J.P.; van den Bergh, H.; Fontolliet, C.; Wagničres, G.; Monnier, P. Time-dependent biodistribution of tetra(m-hydroxyphenyl) chlorin and benzoporphyrin derivatives monoacid ring A in the hamster model; comparative fluorescence microscopy study. Photochem. Photobiol. 2000, 71, 333–340. [Google Scholar] [CrossRef]
- Levy, J.G.; Jones, C.A.; Pilson, L.A. The preclinical and clinical development and potential application of benzoporphyrin derivative. Int. Photodyn. 1994, 1, 3–5. [Google Scholar]
- Aveline, B.M.; Hasan, T.; Redmond, R.W. The effects of aggregation, protein binding and cellular incorporation on the photophysical properties of benzoporphyrin derivative monoacid ring A (BPDMA). J. Photochem. Photobiol. B Biol. 1995, 30, 161–169. [Google Scholar] [CrossRef]
- Stables, G.I.; Ash, D.V. Photodynamic therapy. Cancer Treat Rev. 1995, 21, 311–323. [Google Scholar] [CrossRef]
- Huggett, M.T.; Jermyn, M.; Gillams, A.; Illing, R.; Mosse, S.; Novelli, M. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br. J. Cancer 2014, 110, 1698–1704. [Google Scholar] [CrossRef]
- Zhong, W.; Celli, J.P.; Rizvi, I.; Mai, Z.; Spring, B.Q.; Yun, S.H.; Hasan, T. In vivo highresolution fluorescence microendoscopy for ovarian cancer detection and treatment monitoring. Br. J. Cancer. 2009, 101, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.K.; Bellnier, D.A.; Smith, K.M.; Dougherty, T.J. Chlor and porphyrin derivatives as potential photosensitizers in photodynamic therapy. Photochem. Photobiol. 1991, 53, 65–72. [Google Scholar] [CrossRef]
- Pandey, R.K.; Sumlin, A.B.; Constantine, S.; Aoudia, M.; Potter, W.R.; Belinier, D.A.; Henderson, B.W.; Roders, M.A.; Smith, K.M.; Dougherty, T.J. Alkyl ether analogs of chlorophyll-a derivatives, PartSynthesis, photophysical properties and photodynamic efficacy. Photochem. Photobiol. 1996, 64, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Marchal, S.; Francois, A.; Dumas, D.; Guillemin, F.; Bezdetnaya, L. Relationship between subcellular localization of Foscan and caspase activation in photosensitized MCF-7 cells. Br. J. Cancer 2007, 96, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Bellnier, D.A.; Greco, W.R.; Loewen, G.M.; Nava, H.; Oseroff, A.; Pandey, R.K.; Dougherty, T.J. Population pharmacokinetics of the photodynamic therapy agent 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a in cancer patients. Cancer Res. 2003, 63, 1806–1813. [Google Scholar] [PubMed]
- Bellnier, D.A.; Greco, W.R.; Nave, H.; Loewen, G.M.; Oseroff, A.R.; Dougherty, T.J. Mild skin photosensitivity in cancer patients following injection of Photochlor (2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a; HPPH) for photodynamic therapy. Cancer Chemother. Pharmacol. 2006, 57, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Slansky, G.; Li, A.; Dobhal, M.P.; Goswami, L.; Graham, A.; Chen, Y.; Kanter, P.; Alberico, R.A.; Spernyak, J.; Morgan, J.; et al. Chlorophyll-a analogues conjugated with aminobenzyl-DTPA as potential bifunctional agents for magnetic resonance imaging and photodynamic therapy. Bioconjug. Chem. 2005, 16, 32–42. [Google Scholar]
- Morgan, A.R.; Garbo, G.M.; Keck, R.; Selman, S.H. New photosensitizers for photodynamic therapy: Combined effect of metallopurpurin derivatives and light on transplantable bladder tumors. Cancer Res. 1988, 48, 194–198. [Google Scholar]
- Pogue, B.W.; Redmond, R.W.; Trivedi, N.; Hasan, T. Photophysical properties of tin ethyl etiopurpurin I (SnET2) and tinoctaethylbenzochlorin (SnOEBC) in solution and bound to albumin. Photochem. Photobiol. 1998, 68, 809–815. [Google Scholar] [CrossRef]
- Kawczyk-Krupka, A.; Wawrzyniec, K.; Musiol, S.K.; Potempa, M.; Bugaj, A.M.; Sieron, A. Treatment of localized prostate cancer using WST-09 and WST-11 mediated vascular targeted photodynamic therapy-a review. Photodiagn. Photodyn. Ther. 2015, 12, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Azzouzi, A.R.; Lebdai, S.; Benzaghou, F.; Stief, C. Vascular-targeted photodynamic therapy with TOOKAD(R) soluble in localized prostate cancer: Standardization of the procedure. World J. Urol. 2015, 33, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Azzouzi, A.R.; Vincendeau, S.; Barret, E.; Cicco, A.; Kleinclauss, F.; van der Poel, H.G. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): An open-label, phase 3, randomised controlled trial. Lancet Oncol. 2017, 18, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Betrouni, N.; Boukris, S.; Benzaghou, F. Vascular targeted photodynamic therapy with TOOKAD(R) soluble (WST11) in localized prostate cancer: Efficiency of automatic pre-treatment planning. Lasers Med. Sci. 2017, 32, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Sessler, J.L.; Hemmi, G.; Mody, T.D.; Murai, T.; Burrell, A.; Young, S.W. Texaphyrins: Synthesis and applications. Acc. Chem. Res. 1994, 27, 43–50. [Google Scholar] [CrossRef]
- Patel, H.; Mick, R.; Finlay, J.; Zhu, T.C.; Rickter, E.; Cengel, K.A. Motexafin lutetium-photodynamic therapy of prostate cancer: Short- and long-term effects on prostate-specific antigen. Clin. Cancer Res. 2008, 14, 4869–4876. [Google Scholar] [CrossRef]
- Bradley, K.A.; Pollack, I.F.; Reid, J.M.; Adamson, P.C.; Ames, M.M.; Vezina, G. Motexafin gadolinium and involved field radiation therapy for intrinsic pontine glioma of childhood: A Children’s oncology group phase I study. Neuro-Oncology 2008, 10, 752–758. [Google Scholar] [CrossRef]
- Bradley, K.A.; Zhou, T.; McNall-Knapp, R.Y.; Jakacki, R.I.; Levy, A.S.; Vezina, G. Motexafin-gadolinium and involved field radiation therapy for intrinsic pontine glioma of childhood: A children’s oncology group phase 2 study. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 55–60. [Google Scholar] [CrossRef]
- Thomas, S.R.; Khuntia, D. Motexafin gadolinium: A promising radiation sensitizer in brain metastasis. Expert Opin. Drug Discov. 2011, 6, 195–203. [Google Scholar] [CrossRef]
- Weersink, R.A.; Bogaards, A.; Gertner, M.; Davidson, S.R.H.; Zhang, K.; Netchev, G.; Trachtenberg, J.; Wilson, B.C. Techniques for delivery and monitoring of TOOKAD (WST09)-mediated photodynamic therapy of the prostate: Clinical experience and praticalities. J. Photochem. Photobiol. B 2005, 79, 211–222. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, C.; Longo, J.P.F.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Wu, Z.M.; Magetic, D.; Zhang, L.J.; Chen, Z.L. Antitumor effects evaluation of a novel porphyrin derivative in photodynamic therapy. Tumor Biol. 2015, 36, 9685–9692. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.Y.; Wang, X.R.; Gao, Y.H.; Zhang, X.H.; Zhang, L.J.; Song, C.H. Synthesis, photophysical properties and biological evaluation of β- alkylamino porphyrin for photodynamic therapy. Bioorg. Med. Chem. 2016, 24, 6040–6047. [Google Scholar] [CrossRef]
- Chen, J.J.; Hong, G.; Gao, L.J.; Liu, T.J.; Cao, W.J. In vitro and in vivo antitumor activity of a novel porphyrin-based photosensitizer for photodynamic therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Nakai, M.; Maeda, T.; Mashima, T.; Yano, S.; Sakuma, S.; Otake, E. Synthesis and photodynamic properties of glucopyanoside conjugated indium (III) porphyrins as a bifunctionalagent. J. Porphyr. Phthalocyanies 2013, 17, 1173–1182. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Xodo, L.E.; Cogoi, S.; Rapozzi, V. Photosensitizers binding to nucleic acids as anticancer agents. Future Med. Chem. 2016, 8, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Boscencu, R.; Manda, G.; Radulea, N.; Socoteanu, R.P.; Ceafalan, L.C.; Neagoe, I.V.; Ferreira Machado, I.; Basaga, S.H.; Vieira Ferreira, L.F. Studies on the synthesis, photophysical and biological evaluation of some unsymmetrical meso-tetrasubstituted phenyl porphyrins. Molecules 2017, 22, 1815. [Google Scholar] [CrossRef]
- Boscencu, R.; Manda, G.; Socoteanu, R.P.; Hinescu, M.E.; Neagoe, I.V.; Olariu, L.; Dumitriu, B. Porphyrin Derivative for Theranostic Use. Patent Application No. 2017 01030/2017; published in RO-BOPI 8, 30 August 2018. [Google Scholar]
- Ferreira, L.F.V.; Ferreira, D.P.; Oliveira, A.S.; Boscencu, R.; Socoteanu, R.; Ilie, M.; Constantin, C.; Neagu, M. Synthesis, photophysical and cytotoxicity evaluation of A3B type mesoporphyrinic compounds. Dyes Pigments 2012, 95, 296–303. [Google Scholar] [CrossRef]
- Boscencu, R.; Socoteanu, R.P.; Nacea, V.; Constantin, C.; Manda, G.; Neagu, M.; Ilie, M.; Baconi, D.L.; Oliveira, A.S.; Vieira Ferreira, L.F. Asymmetrically Substituted Tetrapyrrolic Compound, Obtaining Process and Cell Level Biological Evaluation. Patent No. 123419 B1, 30 March 2012. [Google Scholar]
- Socoteanu, R.; Boscencu, R.; Nacea, V.; Constantin, C.; Manda, G.; Neagu, M.; Ilie, M.; Oliveira, A.S.; Vieira Ferreira, L.F. Biofunctionalized Porphyrinic Compound. Patent No. 125018 B1, 30 April 2015. [Google Scholar]
- Boscencu, R.; Socoteanu, R.; Constantin, C.; Neagu, M.; Manda, G.; Nacea, V.; Ilie, M.; Gird, C.E.; Oliveira, A.S.; Vieira Ferreira, L.F. Asymmetric Substituted Porphyrinic Compound as A Photosensitizing Agent and Process for Its Preparation. Patent No. 126761B1, 30 December 2014. [Google Scholar]
- Socoteanu, R.; Boscencu, R.; Nacea, V.; Sousa Oliveira, A.; Vieira Ferreira, L.F. Microwave assisted synthesis of unsymmetrical tetrapyrrolic compounds. Rev. Chim. 2008, 59, 969–972. [Google Scholar] [CrossRef]
- Boscencu, R.; Socoteanu, R.; Vasiliu, G.; Nacea, V. Synthesis under solvent free conditions of some unsymmetrically substituted porphyrinic compounds. Rev. Chim. 2014, 65, 888–891. [Google Scholar]
- Boscencu, R.; Manda, G.; Socoteanu, R.P.; Hinescu, M.E.; Radulea, N.; Neagoe, I.V.; Vieira Ferreira, L.F. Tetrapyrrolic Compound with Theranostic Applications and Obtaining Process. Patent No. 131946, 29 March 2019. [Google Scholar]
- Socoteanu, R.; Manda, G.; Boscencu, R.; Vasiliu, G.; Oliveira, A.S. Synthesis, Spectral Analysis and Preliminary in Vitro Evaluation of Some Tetrapyrrolic Complexes with 3d Metal Ions. Molecules 2015, 20, 15488–15499. [Google Scholar] [CrossRef] [PubMed]
- Boscencu, R. Unsymmetrical Mesoporphyrinic Complexes of Copper(II) and Zinc(II). Microwave-Assisted Synthesis, Spectral Characterization and Cytotoxicity Evaluation. Molecules 2011, 16, 5604–5617. [Google Scholar] [CrossRef]
- Boscencu, R. Microwave Synthesis under Solvent-Free Conditions and Spectral Studies of Some Mesoporphyrinic Complexes. Molecules 2012, 17, 5592–5603. [Google Scholar] [CrossRef]
- Boscencu, R.; Oliveira, A.S.; Ferreira, D.P.; Vieira Ferreira, L.F. Synthesis and spectral evaluation of some unsymmetrical mesoporphyrinic complexes. Int. J. Mol. Sci. 2012, 13, 8112–8125. [Google Scholar] [CrossRef]
- Boscencu, R.; Licsandru, D.; Socoteanu, R.; Oliveira, A.S.; Vieira Ferreira, L.F. Synthesis and characterization of some mesoporphyrinic compounds unsymetricaly substituted. Rev. Chim. 2007, 58, 498–502. [Google Scholar]
- Boscencu, R.; Socoteanu, R.; Ilie, M.; Oliveira, A.S.; Constantin, C.; Vieira Ferreira, L.F. Synthesis, spectraland biological evaluation of some mesoporphyrinic complexes of Zn(II). Rev. Chim. 2009, 60, 1006–1011. [Google Scholar]
- Boscencu, R.; Ilie, M.; Socoteanu, R.; Oliveira, A.S.; Constantin, C.; Neagu, M.; Manda, M.; Vieira Ferreira, L.F. Microwave Synthesis, Basic Spectral and Biological Evaluation of Some Copper (II) Mesoporphyrinic Complexes. Molecules 2010, 15, 3731–3743. [Google Scholar] [CrossRef]
- Boscencu, R.; Socoteanu, R.; Oliveira, A.S.; Vieira Ferreira, L.F.; Nacea, V.; Patrinoiu, G. Synthesis and characterization of some unsymmetrically-substituted mesoporphyrinic mono-hydroxyphenyl complexes of Copper(II). Pol. J. Chem. 2008, 82, 509–522. [Google Scholar]
- Boscencu, R.; Socoteanu, R.; Oliveira, A.S.; Vieira Ferreira, L.F. Studies on Zn(II) monohydroxyphenylmesoporphyrinic complexes. Synthesis and characterization. J. Serb. Chem. Soc. 2008, 73, 713–726. [Google Scholar] [CrossRef]
- Vasiliu, G.; Boscencu, R.; Socoteanu, R.; Nacea, V. Complex combinations of some transition metals with new unsymmetrical porphyrins. Rev. Chim. 2014, 65, 998–1001. [Google Scholar]
- Oliveira, A.S.; Licsandru, D.; Boscencu, R.; Socoteanu, R.; Nacea, V.; Vieira Ferreira, L.F. A Singlet Oxygen Photogeneration and luminescence study of unsymmetrically-substituted meso-porphyrinic compounds. Int. J. Photoenergy 2009, 2009, 10. [Google Scholar] [CrossRef]
- Bonnett, R.; Charlesworth, P.; Djelal, B.; Foley, S.; McGarvey, D.J.; Truscott, T. Photophysical properties of 5,10,15, 20–tetrakis (m-hydroxyphenyl)porphyrin (m-THPP), 5,10,15,20–tetrakis (m-hydroxyphenyl)chlorin (m-THPC) and 5,10,15,20–tetrakis (m-hydroxyphenyl)bacteriochlorin (m-THPBC): A comparative study. J. Chem. Soc. Perkin Trans. 2 1999, 2, 325–328. [Google Scholar] [CrossRef]
- Dobre, M.; Boscencu, R.; Neagoe, I.V.; Surcel, M.; Milanesi, E.; Manda, G. Insight into the web of stress responses triggered at gene expression level by porphyrin-PDT in HT29 human colon carcinoma cells. Pharmaceutics 2021, 13, 1032. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.D.; Silva, J.; Fonseca, S.M.; Arranja, C.T.; Urbano, A.M.; Sobral, A. Photophysical characterization and in vitro phototoxicity evaluation of 5,10,15,20-tetra(quinolin-2-yl) porphyrin as a potential sensitizer forphotodynamic therapy. Molecules 2016, 21, 439. [Google Scholar] [CrossRef]
- Jinadasa, R.G.W.; Zhou, Z.H.; Vicente, M.G.; Smith, K.M. Syntheses and cellular investigations of diaspartateandaspartate-lysine chlorin e6 conjugates. Org. Biomol. Chem. 2016, 14, 1049–1064. [Google Scholar] [CrossRef]
- Gushchina, O.I.; Larkina, E.A.; Nikolskaya, T.A.; Mironov, A.F. Synthesis of aminoderivatives of chlorin e6 and investigation of their biological activity. J. Photochem. Photobiol. B 2015, 153, 76–81. [Google Scholar] [CrossRef]
- Narumi, A.; Tsuji, T.; Shinohara, K.; Yamazaki, H.; Kikuchi, M.; Kawaguchi, S. Maltotriose-conjugation to a fluorinated chlorin derivative generatinga PDT photosensitizer with improved water solubility. Org. Biomol. Chem. 2016, 14, 3608–3613. [Google Scholar] [CrossRef]
- Pereira, P.; Rizvi, W.; Bhupathiraju, N.; Berisha, N.; Fernandes, R.; Tomé, J.; Drain, C.M. Carbon-1 versus Carbon-3 linkage of d-Galactose to porphyrins: Synthesis, uptake, and photodynamic efficiency. Bioconjug. Chem. 2018, 29, 306–315. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Li, Y.; Liu, Q.; Chen, J.-L.; Zhang, H.; Lac, D.; Zhang, H.; Ferrara, K.W.; Hogiu, W.S.; Lam, K.S.; et al. Novel theranosticnanoporphyrins for photodynamic diagnosis and trimodal therapy for bladder cancer. Biomaterials 2016, 104, 339–351. [Google Scholar] [CrossRef]
- Kim, B.H.; Shin, K.; Kwon, S.G.; Jang, Y.; Lee, H.S.; Lee, H.; Jun, S.W.; Lee, I.; Han, S.Y.; Yim, Y.H.; et al. Sizing by weighing: Characterizing sizes of ultrasmall-sized iron oxide nanocrystals using MALDI-TOF mass spectrometry. J. Am. Chem. Soc. 2013, 135, 2407–2410. [Google Scholar] [CrossRef] [PubMed]
- Vieira Ferreira, L.F.; Ferreira Machado, I.; Gama, A.; Socoteanu, R.P.; Boscencu, R.; Manda, G.; Calhelha, R.C.; Ferreira, I.C.F. RPhotochemical/Photocytotoxicity studies of new tetrapyrrolic structures as potential candidates for cancer theranostics. Curr. Drug Discov. Technol. 2020, 17, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Vieira Ferreira, L.F.; Ferreira Machado, I.; Gama, A.; Lochte, F.; Socoteanu, R.P.; Boscencu, R. Surface photochemical studies of nano-hybrids of A3B porphyrins and Fe3O4 silica-coated nanoparticles. J. Photochem. Photobiol. A Chem. 2020, 387, 1–8. [Google Scholar] [CrossRef]
- Li, S.; Shen, X.; Xu, Q.-H.; Cao, Y. Gold nanorod enhanced conjugated polymer/photosensitizer composite nanoparticles for simultaneous two-photon excitation fluorescence imaging and photodynamic therapy. Nanoscale 2019, 11, 19551–19560. [Google Scholar] [CrossRef]
- Nowostawska, M.; Corr, S.A.; Byrne, S.J.; Conroy, J.; Volkov, Y.; Gun’ko, Y.K. Porphyrin-magnetite nanoconjugates for biological imaging. J. Nanobiotechnol. 2011, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]

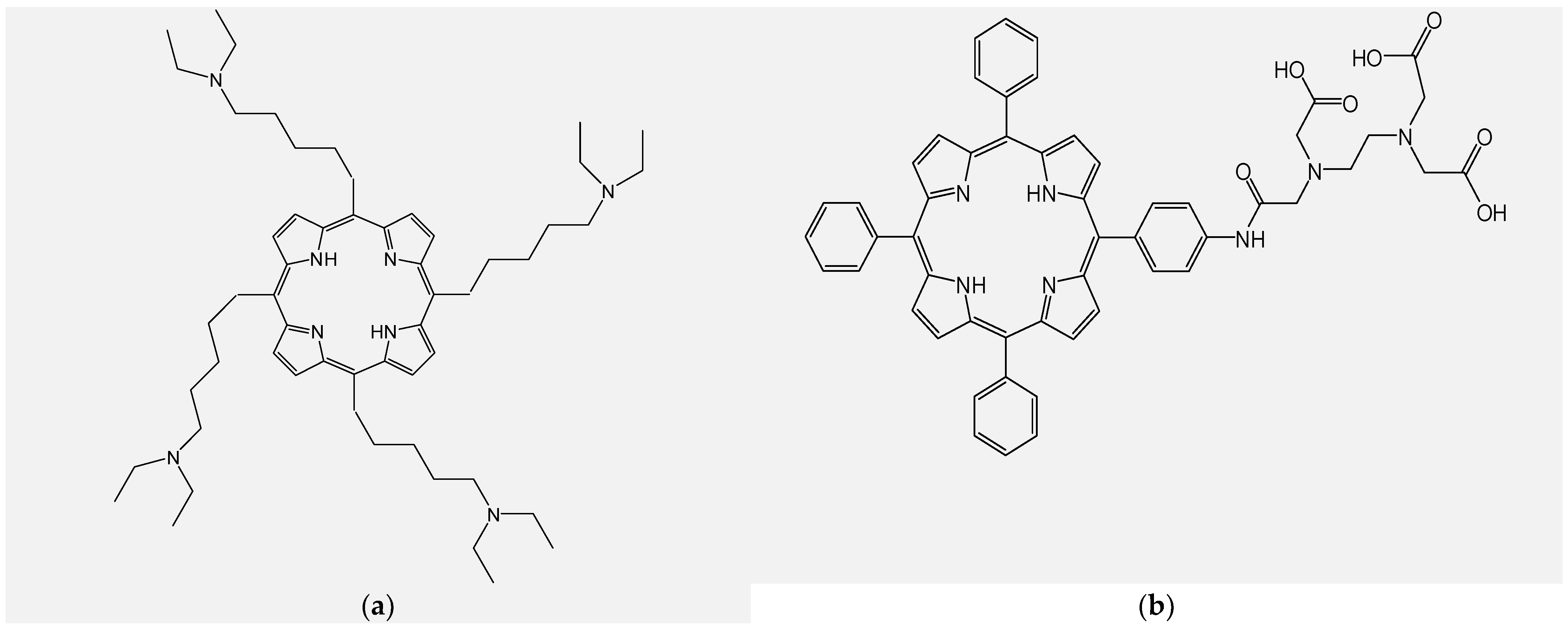
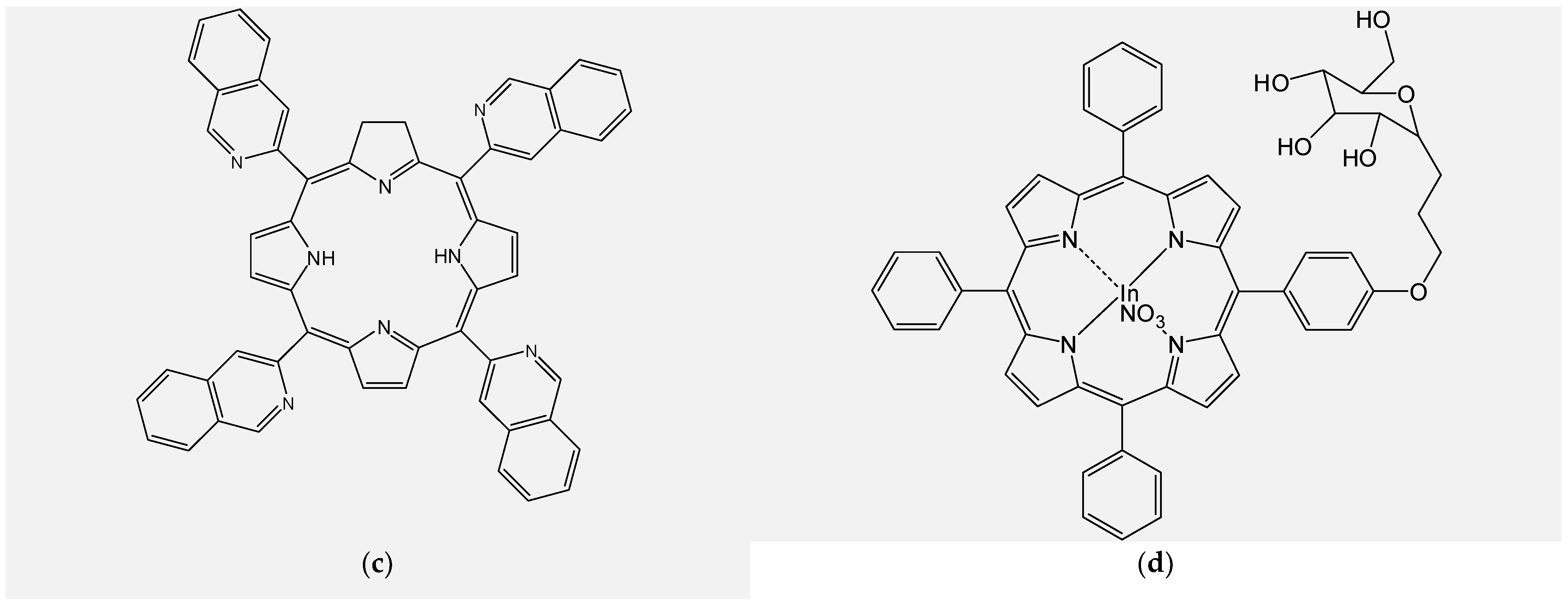
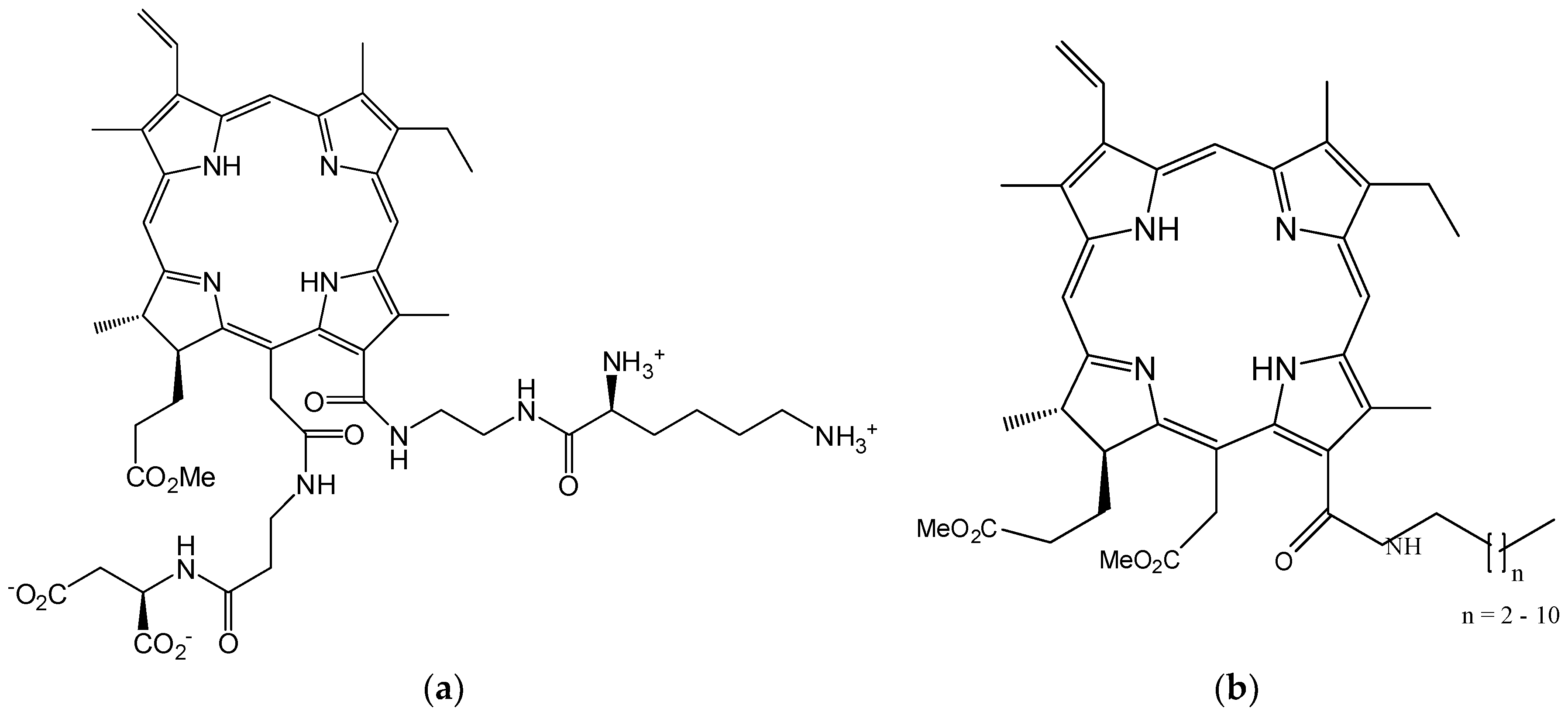
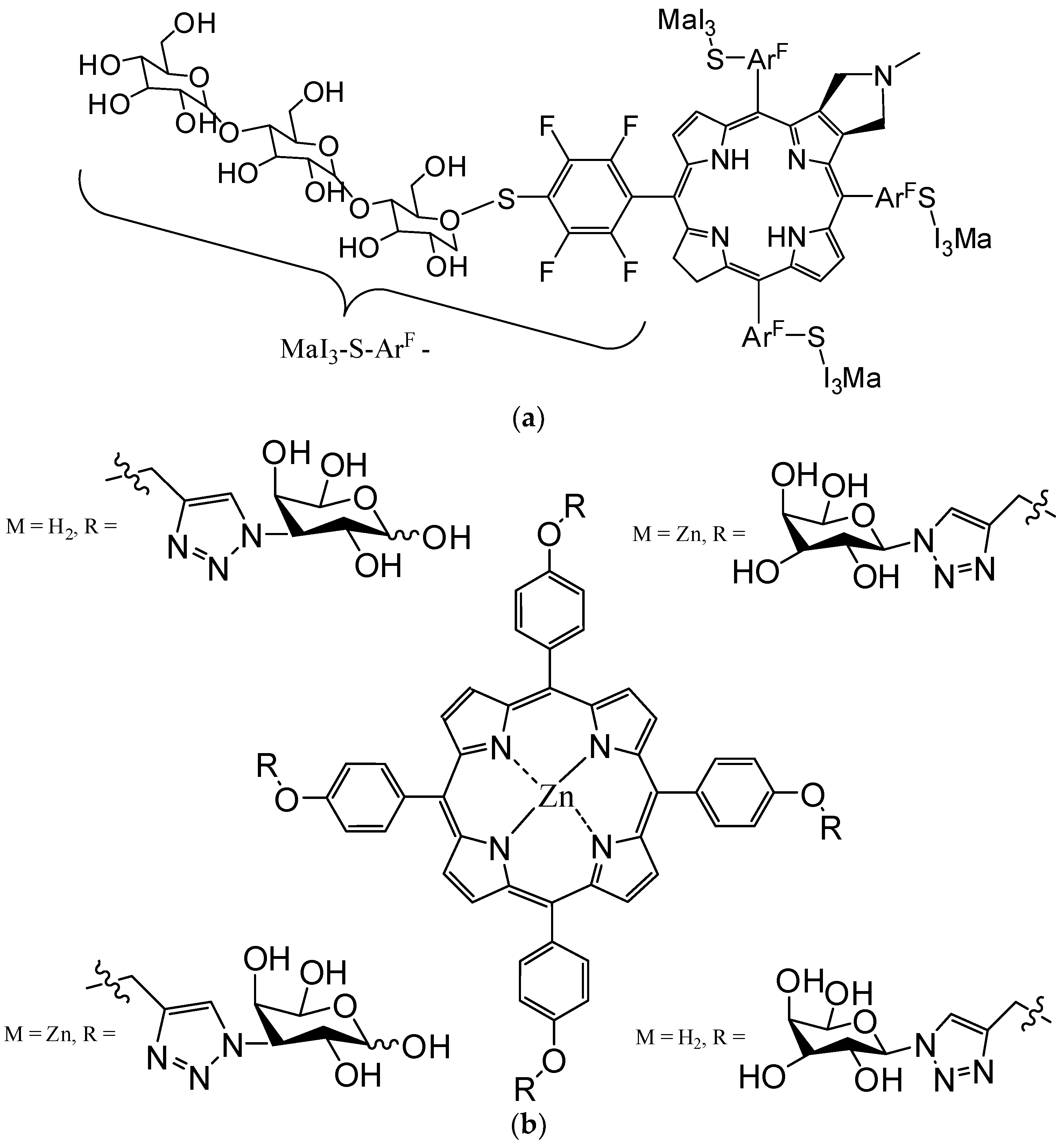
| Photosensitizer | Active Substance, Activation Wavelength λ (nm) | Clinical Approval |
|---|---|---|
| PHOTOFRIN® (Axcan Pharma Inc., Mont-Saint-Hilaire, QC, Canada) | 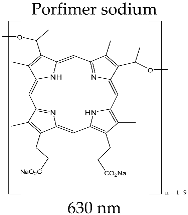 | Theranostic for tumor formations in lung, brain, cervix; therapy for bladder and esophagus cancer; in clinical trial for brain cancer diagnosis |
| FOSCAN® (Biolitec Pharma Ltd., Jena, Germany) | 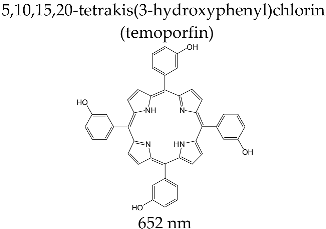 | Photodynamic therapy for carcinoma with squamous cells at late stage in head and throat level; diagnosis of brain, bladder, and ovarian tumors; in clinical studies for therapy for breast, pancreas, prostate, lung, stomach, and skin cancer |
| LASERPHYRIN® (Meiji Seika Pharma Co., Ltd., Tokyo, Japan) | 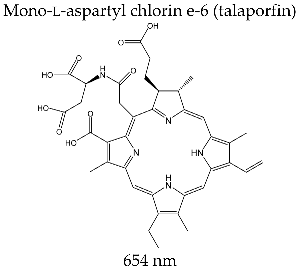 | Photodynamic therapy for lung and brain cancer; intraoperative photodiagnosis of malignant brain formations; clinical studies for treatment of colorectal and liver neoplasms |
| VISUDYNE® (Novartis Pharmaceuticals, East Hanover, NJ, USA) |  | Photodynamic therapy for macular exudative degeneration with subfoveal choroidal neovascularisation of melanoma and psoriasis; clinical trial for diagnosis of ovarian tumor formation |
| PHOTOCHLOR® (Roswell Park Cancer Institute, Buffalo, NY, USA) | 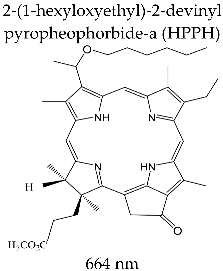 | Clinical trials for therapy (first and second stage) of cervical intraepithelial neoplasia, esophagus tumor, skin, lung, and oral cavity cancer; clinical trials of marker potential in identifying different types of cancer |
| PURLYTIN® (Miravant Medica Technologies, Santa Barbara, CA, USA) | 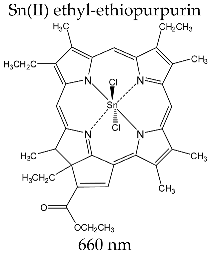 | Clinical trials for photodynamic therapy of breast adenocarcinoma, Kaposi’s sarcoma, prostate cancer, cerebral metastasis, and psoriasis |
| TOOKAD® (WST09) (Negma Lerads/Steba Biotech, Toussous le Noble, France) | 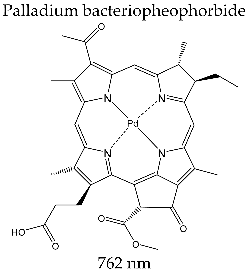 | Stage II/III clinical trials targeting photodynamic therapy for prostate cancer |
| LUTRIN® (Pharmacyclics Inc., Sunnyvale, CA, USA) |  | Clinical trials for prostate, breast, cervical, and brain cancer, melanoma, and Kaposi’s sarcoma |
| PHOTOSENS® (SSC NIOPIK, Moscow, Russia) | 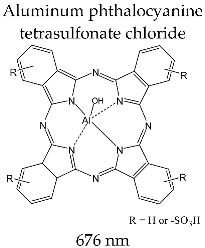 | Clinical trials for gastric, oral, skin and breast cancer |
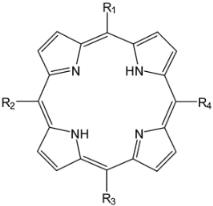  | |||
| A3B type meso-substituted porphyrins | R1 | R2 = R3 = R4 | Ref. |
| TCMPMOHp M(II)TCMPMOHp | 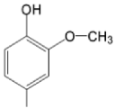 | 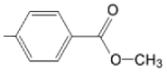 | [31] |
| TMAPMOHp M(II)TMAPMOHp | 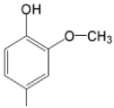 |  | [160] |
| TMAPDOH | 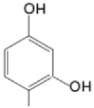 | 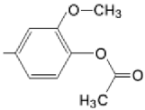 | [161] |
| TCMPOHo M(II)TCMPOHo |  | 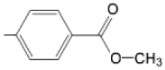 | [162] |
| TRMOPP M(II)TRMOPP | 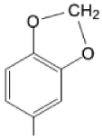 |  | [163,172] |
| TCMPOHp M(II)TCMPOHp | 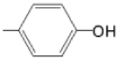 | 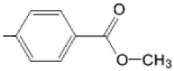 | [164,170] |
| TCMPOMo M(II)TCMPOMo | 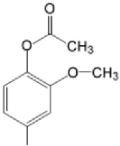 | 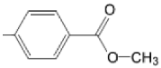 | [165,172] |
| TCMPOHm M(II)TCMPOHm | 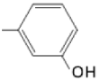 | 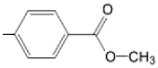 | [166,175] |
| TMAPOHm M(II)TMAPOHm | 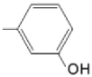 | 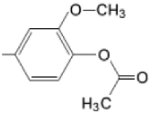 | [167,178] |
| TMAPOHo M(II)TMAPOHo | 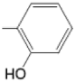 |  | [167,178] |
| M(II)TMAPOHp | 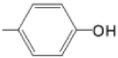 | 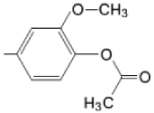 | [171] |
| A2B2 type meso-substituted porphyrins | R1 = R3 | R2 = R4 | |
| TCMPDMOH | 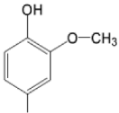 | 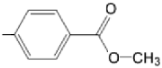 | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boscencu, R.; Radulea, N.; Manda, G.; Machado, I.F.; Socoteanu, R.P.; Lupuliasa, D.; Burloiu, A.M.; Mihai, D.P.; Ferreira, L.F.V. Porphyrin Macrocycles: General Properties and Theranostic Potential. Molecules 2023, 28, 1149. https://doi.org/10.3390/molecules28031149
Boscencu R, Radulea N, Manda G, Machado IF, Socoteanu RP, Lupuliasa D, Burloiu AM, Mihai DP, Ferreira LFV. Porphyrin Macrocycles: General Properties and Theranostic Potential. Molecules. 2023; 28(3):1149. https://doi.org/10.3390/molecules28031149
Chicago/Turabian StyleBoscencu, Rica, Natalia Radulea, Gina Manda, Isabel Ferreira Machado, Radu Petre Socoteanu, Dumitru Lupuliasa, Andreea Mihaela Burloiu, Dragos Paul Mihai, and Luis Filipe Vieira Ferreira. 2023. "Porphyrin Macrocycles: General Properties and Theranostic Potential" Molecules 28, no. 3: 1149. https://doi.org/10.3390/molecules28031149
APA StyleBoscencu, R., Radulea, N., Manda, G., Machado, I. F., Socoteanu, R. P., Lupuliasa, D., Burloiu, A. M., Mihai, D. P., & Ferreira, L. F. V. (2023). Porphyrin Macrocycles: General Properties and Theranostic Potential. Molecules, 28(3), 1149. https://doi.org/10.3390/molecules28031149