Anti-Cancer Peptides: Status and Future Prospects
Abstract
1. Introduction
2. Conformations of ACPs
2.1. ACPs with α-Helical Conformations
2.2. ACPs with β-Sheet Conformations
2.3. Linear, Hybrid, Diastereomeric and Synthetic ACPs
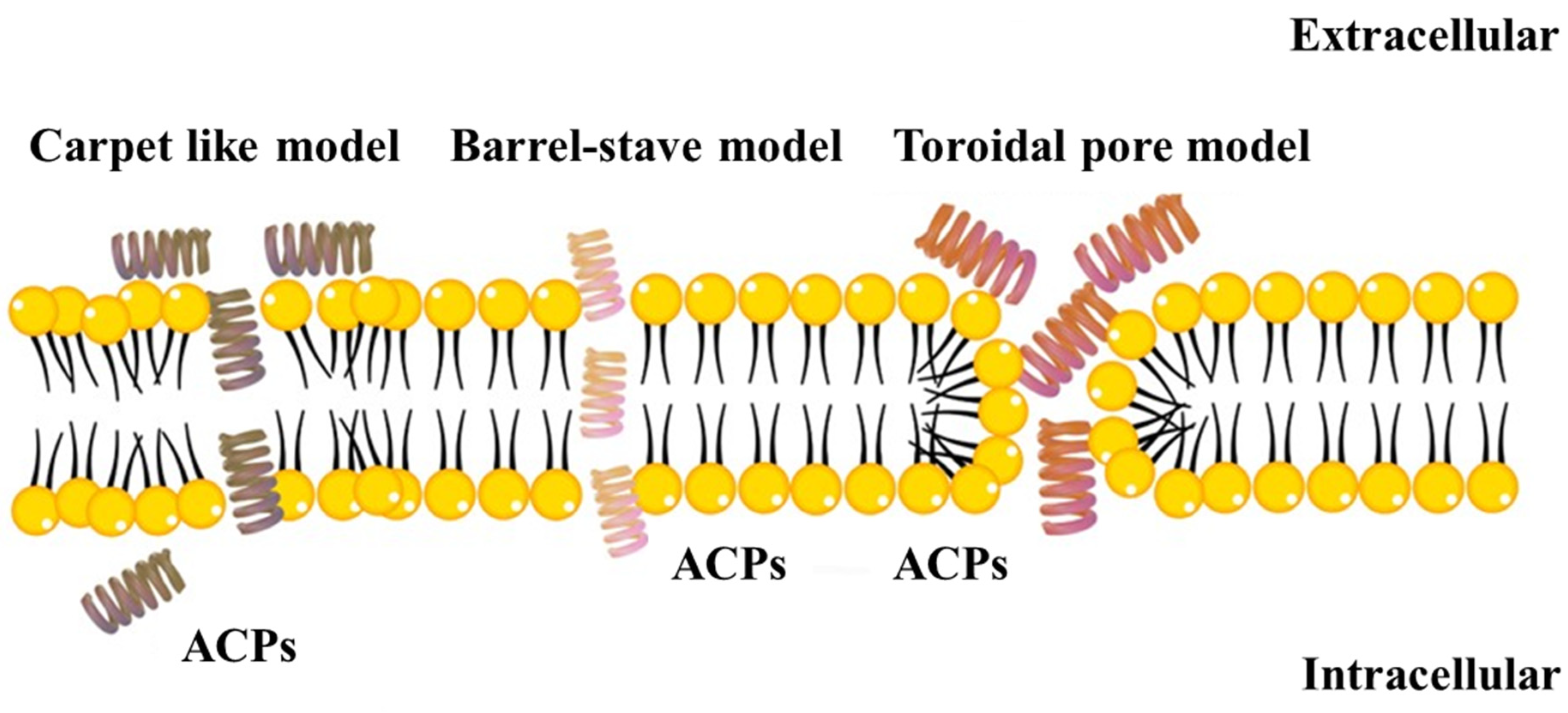
3. Modes of Action of ACPs
3.1. Membrane Interaction Mechanisms
3.1.1. The Carpet Model
3.1.2. The Barrel-Stave Model
3.1.3. The Toroidal Pore Model
3.1.4. Other Minor Models
3.2. Non-Membrane Interactions
4. Effects of Hypoxia, pH and Enzyme Activation on ACPs
5. ACPs as Diagnostic Tools
5.1. Imaging Biosensors Employing ACPs
5.2. Non-Imaging Biosensing Techniques Employing ACPs
6. Synthesis and Modification of ACPs
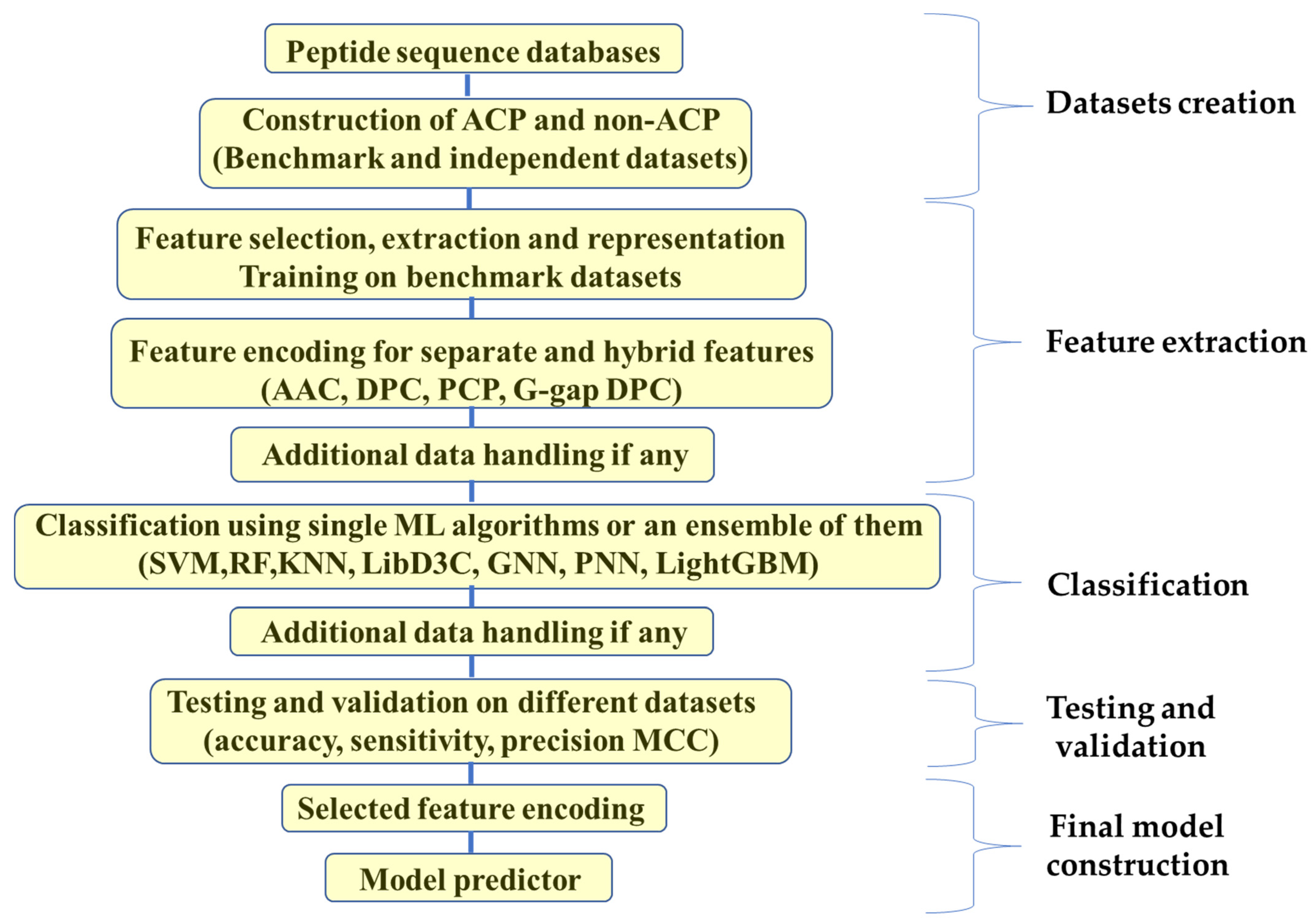
7. Computational Approaches in ACPs Synthesis
7.1. Traditional Machine Learning
7.2. Deep Learning (DL)
7.3. Hybrid Approach and New Methods
8. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bakare, O.O.; Gokul, A.; Wu, R.; Niekerk, L.A.; Klein, A.; Keyster, M. Biomedical Relevance of Novel Anticancer Peptides in the Sensitive Treatment of Cancer. Biomolecules 2021, 11, 1120. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.S.; Weiher, H.; Schmidt-Wolf, I.G.H. Targeting Prostate Cancer with a Combination of WNT Inhibitors and a Bi-Functional Peptide. Anticancer Res. 2017, 37, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Buscaill, P.; Rivas, S. Transcriptional Control of Plant Defence Responses. Curr. Opin. Plant Biol. 2014, 20, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Taveira, G.B.; Carvalho, A.O.; Rodrigues, R.; Trindade, F.G.; da Cunha, M.; Gomes, V.M. Thionin-like Peptide from Capsicum annuum Fruits: Mechanism of Action and Synergism with Fluconazole against Candida Species Applied Microbiology. BMC Microbiol. 2016, 16, 12. [Google Scholar] [CrossRef]
- Banting, F.G.; Best, C.H.; Collip, J.B.; Campbell, W.R.; Fletcher, A.A. Pancreatic Extracts in the Treatment of Diabetes Mellitus. Can. Med. Assoc. J. 1922, 12, 141–146. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic Peptides: Historical Perspectives, Current Development Trends, and Future Directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Fuchs, J.A.; Grisoni, F.; Kossenjans, M.; Hiss, J.A.; Schneider, G. Lipophilicity Prediction of Peptides and Peptide Derivatives by Consensus Machine Learning. MedChemComm 2018, 9, 1538–1546. [Google Scholar] [CrossRef]
- Li, W.; Joshi, M.D.; Singhania, S.; Ramsey, K.H.; Murthy, A.K. Peptide Vaccine: Progress and Challenges. Vaccines 2014, 2, 515–536. [Google Scholar] [CrossRef]
- Verbeke, F.; de Craemer, S.; Debunne, N.; Janssens, Y.; Wynendaele, E.; van de Wiele, C.; de Spiegeleer, B. Peptides as Quorum Sensing Molecules: Measurement Techniques and Obtained Levels in Vitro and in Vivo. Front. Neurosci. 2017, 11, 183. [Google Scholar] [CrossRef]
- Basith, S.; Manavalan, B.; Shin, T.H.; Lee, G. IGHBP: Computational Identification of Growth Hormone Binding Proteins from Sequences Using Extremely Randomised Tree. Comput. Struct. Biotechnol. J. 2018, 16, 412–420. [Google Scholar] [CrossRef]
- Manavalan, B.; Shin, T.H.; Kim, M.O.; Lee, G. AIPpred: Sequence-Based Prediction of Anti-Inflammatory Peptides Using Random Forest. Front. Pharmacol. 2018, 9, 276. [Google Scholar] [CrossRef]
- Tesauro, D.; Accardo, A.; Diaferia, C.; Milano, V.; Guillon, J.; Ronga, L.; Rossi, F. Peptide-Based Drug-Delivery Systems in Biotechnological Applications: Recent Advances and Perspectives. Molecules 2019, 24, 351. [Google Scholar] [CrossRef] [PubMed]
- Manavalan, B.; Basith, S.; Shin, T.H.; Choi, S.; Kim, M.O.; Lee, G. MLACP: Machine-Learning-Based Prediction of Anticancer Peptides. Oncotarget 2017, 8, 77121. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.A.; Freire, J.M.; Pérez-Peinado, C.; Domingues, M.M.; Gaspar, D.; Vale, N.; Gomes, P.; Andreu, D.; Henriques, S.T.; Castanho, M.A.R.B.; et al. New Potent Membrane-Targeting Antibacterial Peptides from Viral Capsid Proteins. Front. Microbiol. 2017, 8, 775. [Google Scholar] [CrossRef]
- Kim, J.S.; Jeong, J.H.; Kim, Y. Design, Characterization, and Antimicrobial Activity of a Novel Antimicrobial Peptide Derived from Bovine Lactophoricin. J. Microbiol. Biotechnol. 2017, 27, 759–767. [Google Scholar] [CrossRef]
- Iikuni, N.; Hahn, B.H.; la Cava, A. Potential for Anti-DNA Immunoglobulin Peptide Therapy in Systemic Lupus Erythematosus. Expert Opin. Biol. Ther. 2009, 9, 201–206. [Google Scholar] [CrossRef]
- McGovern, D.P.; Astle, A.T.; Clavin, S.L.; Newell, F.N. Task-Specific Transfer of Perceptual Learning across Sensory Modalities. Curr. Biol. 2016, 26, R20–R21. [Google Scholar] [CrossRef] [PubMed]
- Basith, S.; Manavalan, B.; Hwan Shin, T.; Lee, G. Machine Intelligence in Peptide Therapeutics: A next-Generation Tool for Rapid Disease Screening. Med. Res. Rev. 2020, 40, 1276–1314. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Cui, F.; Zou, Q.; Zhang, L.; Xu, L. Anticancer Peptides Prediction with Deep Representation Learning Features. Brief. Bioinform. 2021, 22, bbab008. [Google Scholar] [CrossRef]
- Liscano, Y.; Oñate-Garzón, J.; Delgado, J.P. Peptides with Dual Antimicrobial–Anticancer Activity: Strategies to Overcome Peptide Limitations and Rational Design of Anticancer Peptides. Molecules 2020, 25, 4245. [Google Scholar] [CrossRef]
- Iwasaki, T.; Ishibashi, J.; Tanaka, H.; Sato, M.; Asaoka, A.; Taylor, D.M.; Yamakawa, M. Selective Cancer Cell Cytotoxicity of Enantiomeric 9-Mer Peptides Derived from Beetle Defensins Depends on Negatively Charged Phosphatidylserine on the Cell Surface. Peptides 2009, 30, 660–668. [Google Scholar] [CrossRef]
- Brandenburg, L.O.; Merres, J.; Albrecht, L.J.; Varoga, D.; Pufe, T. Antimicrobial Peptides: Multifunctional Drugs for Different Applications. Polymers 2012, 4, 539–560. [Google Scholar] [CrossRef]
- Fernebro, J. Fighting Bacterial Infections—Future Treatment Options. Drug Resist. Updat. 2011, 14, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Zhang, L.; Yu, L.; Huang, J.; Huang, S.; Yao, Y. A Review for Antimicrobial Peptides with Anticancer Properties: Re-Purposing of Potential Anticancer Agents. BIO Integr. 2020, 1, 156–167. [Google Scholar] [CrossRef]
- Rozek, T.; Wegener, K.L.; Bowie, J.H.; Olver, I.N.; Carver, J.A.; Wallace, J.C.; Tyler, M.J. The Antibiotic and Anticancer Active Aurein Peptides from the Australian Bell Frogs Litoria Aurea and Litoria Raniformis: The Solution Structure of Aurein 1.2. Eur. J. Biochem. 2000, 267, 5330–5341. [Google Scholar] [CrossRef]
- Yang, S.; Lee, C.W.; Kim, H.J.; Jung, H.-H.; Kim, J.I.; Shin, S.Y.; Shin, S.-H. Structural Analysis and Mode of Action of BMAP-27, a Cathelicidin-Derived Antimicrobial Peptide. Peptides 2019, 118, 170106. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Ramamoorthy, A. Studies on Anticancer Activities of Antimicrobial Peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 357–375. [Google Scholar] [CrossRef]
- Li, B.; Lyu, P.; Xie, S.; Qin, H.; Pu, W.; Xu, H.; Chen, T.; Shaw, C.; Ge, L.; Kwok, H.F. LFB: A Novel Antimicrobial Brevinin-like Peptide from the Skin Secretion of the Fujian Large Headed Frog, Limnonectes Fujianensi. Biomolecules 2019, 9, 242. [Google Scholar] [CrossRef]
- Ye, J.S.; Zheng, X.J.; Leung, K.W.; Chen, H.M.; Sheu, F.S. Induction of Transient Ion Channel-like Pores in a Cancer Cell by Antibiotic Peptide. J. Biochem. 2004, 136, 255–259. [Google Scholar] [CrossRef]
- Chen, H.M.; Wang, W.; Smith, D.; Chan, S.C. Effects of the Anti-Bacterial Peptide Cecropin B and Its Analogs, Cecropins B-1 and B-2, on Liposomes, Bacteria, and Cancer Cells. Biochim. Biophys. Acta Gen. Subj. 1997, 1336, 171–179. [Google Scholar] [CrossRef]
- Doyle, J.; Brinkworth, C.S.; Wegener, K.L.; Carver, J.A.; Llewellyn, L.E.; Olver, I.N.; Bowie, J.H.; Wabnitz, P.A.; Tyler, M.J. NNOS Inhibition, Antimicrobial and Anticancer Activity of the Amphibian Skin Peptide, Citropin 1.1 and Synthetic Modifications: The Solution Structure of a Modified Citropin 1.1. Eur. J. Biochem. 2003, 270, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, U.; Oledzka, E.; Zgadzaj, A.; Bauer, M.; Sobczak, M. A Novel Delivery System for the Controlled Release~ of Antimicrobial Peptides: Citropin 1.1 and Temporin A. Polymers 2018, 10, 489. [Google Scholar] [CrossRef]
- Papo, N.; Braunstein, A.; Eshhar, Z.; Shai, Y. Suppression of Human Prostate Tumor Growth in Mice by a Cytolytic D-, L-Amino Acid Peptide: Membrane Lysis, Increased Necrosis, and Inhibition of Prostate-Specific Antigen Secretion. Cancer Res. 2004, 64, 5779–5786. [Google Scholar] [CrossRef]
- Won, H.S.; Seo, M.D.; Jung, S.J.; Lee, S.J.; Kang, S.J.; Son, W.S.; Kim, H.J.; Park, T.K.; Park, S.J.; Lee, B.J. Structural Determinants for the Membrane Interaction of Novel Bioactive Undecapeptides Derived from Gaegurin 5. J. Med. Chem. 2006, 49, 4886–4895. [Google Scholar] [CrossRef] [PubMed]
- Won, H.-S.; Kang, S.-J.; Lee, B.-J. Action Mechanism and Structural Requirements of the Antimicrobial Peptides, Gaegurins. Biochim. Biophys. Acta (BBA)—Biomembr. 2009, 1788, 1620–1629. [Google Scholar] [CrossRef]
- Mandke, P.; Vasquez, K.M. Interactions of High Mobility Group Box Protein 1 (HMGB1) with Nucleic Acids: Implications in DNA Repair and Immune Responses. DNA Repair 2019, 83, 102701. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zeng, Z.; Jin, T.; Zhang, H.; Xiong, X.; Gu, L. The Role of High Mobility Group Box 1 in Ischemic Stroke. Front. Cell. Neurosci. 2019, 13, 127. [Google Scholar] [CrossRef]
- PepDraw. Available online: http://www2.tulane.edu/~biochem/WW/PepDraw/index.html (accessed on 21 December 2022).
- Fruitwala, S.; El-Naccache, D.W.; Chang, T.L. Multifaceted Immune Functions of Human Defensins and Underlying Mechanisms. Semin. Cell Dev. Biol. 2019, 88, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, L.; Li, J.; Suresh, A.; Verma, C.; Foo, Y.H.; Yap, E.P.H.; Tan, D.T.H.; Beuerman, R.W. Linear Analogues of Human β-Defensin 3: Concepts for Design of Antimicrobial Peptides with Reduced Cytotoxicity to Mammalian Cells. ChemBioChem 2008, 9, 964–973. [Google Scholar] [CrossRef]
- Hwang, P.M.; Zhou, N.; Shan, X.; Arrowsmith, C.H.; Vogel, H.J. Three-Dimensional Solution Structure of Lactoferricin B, an Antimicrobial Peptide Derived from Bovine Lactoferrin. Biochemistry 1998, 37, 4288–4298. [Google Scholar] [CrossRef]
- Aghazadeh, H.; Memariani, H.; Ranjbar, R.; Pooshang Bagheri, K. The Activity and Action Mechanism of Novel Short Selective LL-37-Derived Anticancer Peptides against Clinical Isolates of Escherichia Coli. Chem. Biol. Drug Des. 2019, 93, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Pinto, I.B.; dos Santos Machado, L.; Meneguetti, B.T.; Nogueira, M.L.; Espínola Carvalho, C.M.; Roel, A.R.; Franco, O.L. Utilization of Antimicrobial Peptides, Analogues and Mimics in Creating Antimicrobial Surfaces and Bio-Materials. Biochem. Eng. J. 2019, 150, 107237. [Google Scholar] [CrossRef]
- Frey, S.; Tamm, L.K. Orientation of Melittin in Phospholipid Bilayers. A Polarized Attenuated Total Reflection Infrared Study. Biophys. J. 1991, 60, 922–930. [Google Scholar] [CrossRef]
- Kim, Y.; Hahm, K.S.; Lee, D.; Lee, M.; Lee, S.H.; Kim, J.; Song, W.; Eom, S.; Park, E.; Yang, S.T.; et al. Antibacterial, Antitumor and Hemolytic Activities of α-Helical Antibiotic Peptide, P18 and Its Analogs. J. Pept. Res. 2001, 58, 504–514. [Google Scholar] [CrossRef]
- Chan, Y.R.; Gallo, R.L. PR-39, a Syndecan-Inducing Antimicrobial Peptide, Binds and Affects P130(Cas). J. Biol. Chem. 1998, 273, 28978–28985. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, R.; Zhang, J.; Chen, F.; Wang, K.-J. A Novel Antimicrobial Peptide Spampcin56–86 from Scylla paramamosain Exerting Rapid Bactericidal and Anti-Biofilm Activity In Vitro and Anti-Infection In Vivo. Int. J. Mol. Sci. 2022, 23, 13316. [Google Scholar] [CrossRef]
- Nakamura, T.; Furunaka, H.; Miyata, T.; Tokunaga, F.; Muta, T.; Iwanaga, S.; Niwa, M.; Takao, T.; Shimonishi, Y. Tachyplesin, a Class of Antimicrobial Peptide from the Hemocytes of the Horseshoe Crab (Tachypleus Tridentatus). Isolation and Chemical Structure. J. Biol. Chem. 1988, 263, 16709–16713. [Google Scholar] [CrossRef]
- Risso, A.; Zanetti, M.; Gennaro, R. Cytotoxicity and Apoptosis Mediated by Two Peptides of Innate Immunity. Cell. Immunol. 1998, 189, 107–115. [Google Scholar] [CrossRef]
- McManus, A.M.; Otvos, L.; Hoffmann, R.; Craik, D.J. Conformational Studies by NMR of the Antimicrobial Peptide, Drosocin, and Its Non-Glycosylated Derivative: Effects of Glycosylation on Solution Conformation. Biochemistry 1999, 38, 705–714. [Google Scholar] [CrossRef]
- Sørensen, O.E.; Follin, P.; Johnsen, A.H.; Calafat, J.; Sandra Tjabringa, G.; Hiemstra, P.S.; Borregaard, N. Human Cathelicidin, HCAP-18, Is Processed to the Antimicrobial Peptide LL-37 by Extracellular Cleavage with Proteinase 3. Blood 2001, 97, 3951–3959. [Google Scholar] [CrossRef]
- Sørensen, O.; Arnljots, K.; Cowland, J.B.; Bainton, D.F.; Borregaard, N. The Human Antibacterial Cathelicidin, HCAP-18, Is Synthesized in Myelocytes and Metamyelocytes and Localized to Specific Granules in Neutrophils. Blood 1997, 90, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.F.; Sandstedt, B.; Sørensen, O.; Weber, G.; Borregaard, N.; Ståhle-Bäckdahl, M. The Human Cationic Antimicrobial Protein (HCAP18), a Peptide Antibiotic, Is Widely Expressed in Human Squamous Epithelia and Colocalizes with Interleukin-6. Infect. Immun. 1999, 67, 2561–2566. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Weinberg, A. Human Antimicrobial Peptides and Cancer. Semin. Cell Dev. Biol. 2019, 88, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Niyonsaba, F.; Iwabuchi, K.; Someya, A.; Hirata, M.; Matsuda, H.; Ogawa, H.; Nagaoka, I. A Cathelicidin Family of Human Antibacterial Peptide LL-37 Induces Mast Cell Chemotaxis. Immunology 2002, 106, 20–26. [Google Scholar] [CrossRef]
- Carretero, M.; Escámez, M.J.; García, M.; Duarte, B.; Holguín, A.; Retamosa, L.; Jorcano, J.L.; del Río, M.; Larcher, F. In Vitro and in Vivo Wound Healing-Promoting Activities of Human Cathelicidin LL-37. J. Investig. Dermatol. 2008, 128, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Steiner, H.; Hultmark, D.; Engström, Å.; Bennich, H.; Boman, H.G. Sequence and Specificity of Two Antibacterial Proteins Involved in Insect Immunity. Nature 1981, 292, 246–248. [Google Scholar] [CrossRef]
- Lee, J.Y.; Boman, A.; Chuanxin, S.; Andersson, M.; Jornvall, H.; Mutt, V.; Boman, H.G. Antibacterial Peptides from Pig Intestine: Isolation of a Mammalian Cecropin. Proc. Natl. Acad. Sci. USA 1989, 86, 9159–9162. [Google Scholar] [CrossRef]
- Boman, H.G.; Hultmark, D. Cell-Free Immunity in Insects. Annu. Rev. Microbiol. 1987, 41, 103–126. [Google Scholar] [CrossRef]
- Chan, S.C.; Yau, W.L.; Wang, W.; Smith, D.K.; Sheu, F.S.; Chen, H.M. Microscopic Observations of the Different Morphological Changes Caused by Anti-Bacterial Peptides on Klebsiella Pneumoniae and HL-60 Leukemia Cells. J. Pept. Sci. 1998, 4, 413–425. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.S.; Bang, Y.J.; Kim, S.J.; Lee, B.J. In Vitro Activities of Native and Designed Peptide Antibiotics against Drug Sensitive and Resistant Tumor Cell Lines. Peptides 2003, 24, 945–953. [Google Scholar] [CrossRef]
- Cruciani, R.A.; Barker, J.L.; Zasloff, M.; Chen, H.C.; Colamonici, O. Antibiotic Magainins Exert Cytolytic Activity against Transformed Cell Lines through Channel Formation. Proc. Natl. Acad. Sci. USA 1991, 88, 3792–3796. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Chamorro, L.; Puertollano, M.A.; Puertollano, E.; de Cienfuegos, G.Á.; de Pablo, M.A. In Vitro Biological Activities of Magainin Alone or in Combination with Nisin. Peptides 2006, 27, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Magainins, a Class of Antimicrobial Peptides from Xenopus Skin: Isolation, Characterization of Two Active Forms, and Partial CDNA Sequence of a Precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.; Zasloff, M. Potential Therapeutic Applications of Magainins and Other Antimicrobial Agents of Animal Origin. In Ciba Foundation Symposium; Wiley: Hoboken, NJ, USA, 1994; Volume 186. [Google Scholar]
- Baker, M.A.; Maloy, W.L.; Zasloff, M.; Jacob, L.S. Anticancer Efficacy of Magainin2 and Analogue Peptides. Cancer Res. 1993, 53, 3052–3057. [Google Scholar]
- Lehmann, J.; Retz, M.; Sidhu, S.S.; Suttmann, H.; Sell, M.; Paulsen, F.; Harder, J.; Unteregger, G.; Stöckle, M. Antitumor Activity of the Antimicrobial Peptide Magainin II against Bladder Cancer Cell Lines. Eur. Urol. 2006, 50, 141–147. [Google Scholar] [CrossRef]
- Park, J.M.; Jung, J.E.; Lee, B.J. Antimicrobial Peptides from the Skin of a Korean Frog, Rana Rugosa. Biochem. Biophys. Res. Commun. 1994, 205, 948–954. [Google Scholar] [CrossRef]
- Gauldie, J.; Hanson, J.M.; Shipolini, R.A.; Vernon, C.A. The Structures of Some Peptides from Bee Venom. Eur. J. Biochem. 1978, 83, 405–410. [Google Scholar] [CrossRef]
- Habermann, E.; Jentsch, J. Sequenzanalyse Des Melittins Aus Den Tryptischen Und Peptischen Spaltstücken. Hoppe Seylers Z. Physiol. Chem. 1967, 348, 37–50. [Google Scholar] [CrossRef]
- Tosteson, M.T.; Tosteson, D.C. The Sting. Melittin Forms Channels in Lipid Bilayers. Biophys. J. 1981, 36, 109–116. [Google Scholar] [CrossRef]
- Tosteson, M.T.; Holmes, S.J.; Razin, M.; Tosteson, D.C. Melittin Lysis of Red Cells. J. Membr. Biol. 1985, 87, 35–44. [Google Scholar] [CrossRef]
- Papo, N.; Shai, Y. Host Defense Peptides as New Weapons in Cancer Treatment. Cell. Mol. Life Sci. 2005, 62, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, F. Cationic Amphiphilic Peptides with Cancer-Selective Toxicity. Eur. J. Pharmacol. 2009, 625, 190–194. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Lichtenstein, A.K.; Ganz, T. Defensins: Antimicrobial and Cytotoxic Peptides of Mammalian Cells. Annu. Rev. Immunol. 1993, 11, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Arceo-Martinez, M.T.; Guzmán-Rodríguez, J.; Palomera-Sánchez, Z.; Ochoa-Zarzosa, A.; López-Meza, J.E. Defensin Γ-Thionin from Capsicum Chinense Induces Apoptosis in the Human Breast Cancer Cell Line MCF-7 and Regulate Histone H3 Epigenetic Modifications. FASEB J. 2018, 32, 804–833. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Dhople, V.; Krukemeyer, A.; Ramamoorthy, A. The Human Beta-Defensin-3, an Antibacterial Peptide with Multiple Biological Functions. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Ganz, T.; Lehrer, R.I. Defensins and Other Endogenous Peptide Antibiotics of Vertebrates. J. Leukoc. Biol. 1995, 58, 128–136. [Google Scholar] [CrossRef]
- Selsted, M.E.; Harwig, S.S.L. Determination of the Disulfide Array in the Human Defensin HNP-2. A Covalently Cyclized Peptide. J. Biol. Chem. 1989, 264, 4003–4007. [Google Scholar] [CrossRef]
- Hill, C.P.; Yee, J.; Selsted, M.E.; Eisenberg, D. Crystal Structure of Defensin HNP-3, an Amphiphilic Dimer: Mechanisms of Membrane Permeabilization. Science (1979) 1991, 251, 1481–1485. [Google Scholar] [CrossRef]
- Ganz, T.; Selsted, M.E.; Szklarek, D.; Harwig, S.S.; Daher, K.; Bainton, D.F.; Lehrer, R.I. Defensins. Natural Peptide Antibiotics of Human Neutrophils. J. Clin. Investig. 1985, 76, 1427–1435. [Google Scholar] [CrossRef]
- Szyk, A.; Wu, Z.; Tucker, K.; Yang, D.; Lu, W.; Lubkowski, J. Crystal Structures of Human α-Defensins HNP4, HD5, and HD6. Protein Sci. 2006, 15, 2749–2760. [Google Scholar] [CrossRef]
- Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K.; Tomita, M. Identification of the Bactericidal Domain of Lactoferrin. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. 1992, 1121, 130–136. [Google Scholar] [CrossRef]
- Eliassen, L.T.; Berge, G.; Leknessund, A.; Wikman, M.; Lindin, I.; Løkke, C.; Ponthan, F.; Johnsen, J.I.; Sveinbjørnsson, B.; Kogner, P.; et al. The Antimicrobial Peptide, Lactoferricin B, Is Cytotoxic to Neuroblastoma Cells in Vitro and Inhibits Xenograft Growth in Vivo. Int. J. Cancer 2006, 119, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Furlong, S.J.; Ridgway, N.D.; Hoskin, D.W. Modulation of Ceramide Metabolism in T-Leukemia Cell Lines Potentiates Apoptosis Induced by the Cationic Antimicrobial Peptide Bovine Lactoferricin. Int. J. Oncol. 2008, 32, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.C.; Watanabe, R.; Koike, Y.; Mitobe, M.; Shimazaki, K.I.; Watanabe, S.; Azuma, I. Apoptosis in Human Leukemic Cells Induced by Lactoferricin, a Bovine Milk Protein-Derived Peptide: Involvement of Reactive Oxygen Species. Biochem. Biophys. Res. Commun. 1997, 237, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Tone Eliassen, L.; Berge, G.; Sveinbjørnsson, B.; Svendsen, J.S.; Vorland, L.H.; Rekdal, Ø. Evidence for a Direct Antitumor Mechanism of Action of Bovine Lactoferricin. Anticancer Res. 2002, 22, 2703–2710. [Google Scholar]
- Mader, J.S.; Salsman, J.; Conrad, D.M.; Hoskin, D.W. Bovine Lactoferricin Selectively Induces Apoptosis in Human Leukemia and Carcinoma Cell Lines. Mol. Cancer Ther. 2005, 4, 612–624. [Google Scholar] [CrossRef]
- Furlong, S.J.; Mader, J.S.; Hoskin, D.W. Lactoferricin-Induced Apoptosis in Estrogen-Nonresponsive MDA-MB-435 Breast Cancer Cells Is Enhanced by C6 Ceramide or Tamoxifen. Oncol. Rep. 2006, 15, 1385–1390. [Google Scholar] [CrossRef]
- Mader, J.S.; Smyth, D.; Marshall, J.; Hoskin, D.W. Bovine Lactoferricin Inhibits Basic Fibroblast Growth Factor- and Vascular Endothelial Growth Factor165-Induced Angiogenesis by Competing for Heparin-like Binding Sites on Endothelial Cells. Am. J. Pathol. 2006, 169, 1753–1766. [Google Scholar] [CrossRef]
- Rao, A.G. Conformation and Antimicrobial Activity of Linear Derivatives of Tachyplesin Lacking Disulfide Bonds. Arch. Biochem. Biophys. 1999, 361, 127–134. [Google Scholar] [CrossRef]
- Ramamoorthy, A.; Thennarasu, S.; Tan, A.; Gottipati, K.; Sreekumar, S.; Heyl, D.L.; An, F.Y.P.; Shelburne, C.E. Deletion of All Cysteines in Tachyplesin I Abolishes Hemolytic Activity and Retains Antimicrobial Activity and Lipopolysaccharide Selective Binding. Biochemistry 2006, 45, 6529–6540. [Google Scholar] [CrossRef]
- Agerberth, B.; Lee, J.-Y.; Bergman, T.; Carlquist, M.; Boman, H.G.; Mutt, V.; Jörnvall, H. Amino Acid Sequence of PR-39: Isolation from Pig Intestine of a New Member of the Family of Proline-arginine-rich Antibacterial Peptides. Eur. J. Biochem. 1991, 202, 849–854. [Google Scholar] [CrossRef]
- Shi, J.; Ross, C.R.; Chengappa, M.M.; Sylte, M.J.; McVey, D.S.; Blecha, F. Antibacterial Activity of a Synthetic Peptide (PR-26) Derived from PR-39, a Proline-Arginine-Rich Neutrophil Antimicrobial Peptide. Antimicrob. Agents Chemother. 1996, 40, 115–121. [Google Scholar] [CrossRef]
- Shin, S.Y.; Lee, M.K.; Kim, K.L.; Hahm, K.S. Structure-Antitumor and Hemolytic Activity Relationships of Synthetic Peptides Derived from Cecropin A-Magainin 2 and Cecropin A-Melittin Hybrid Peptides. J. Pept. Res. 1997, 50, 279–285. [Google Scholar] [CrossRef]
- Shin, S.Y.; Kang, J.H.; Hahm, K.S. Structure-Antibacterial, Antitumor and Hemolytic Activity Relationships of Cecropin A-Magainin 2 and Cecropin A-Melittin Hybrid Peptides. J. Pept. Res. 1999, 53, 82–90. [Google Scholar] [CrossRef]
- Oh, D.; Shin, S.Y.; Kang, J.H.; Hahm, K.S.; Kim, K.L.; Kim, Y. NMR Structural Characterization of Cecropin A(1-8)—Magainin 2(1-12) and Cecropin A(1-8)—Melittin(1-12) Hybrid Peptides. J. Pept. Res. 1999, 53, 578–589. [Google Scholar] [CrossRef]
- Oren, Z.; Hong, J.; Shai, Y. A Repertoire of Novel Antibacterial Diastereomeric Peptides with Selective Cytolytic Activity. J. Biol. Chem. 1997, 272, 14643–14649. [Google Scholar] [CrossRef] [PubMed]
- Papo, N.; Shai, Y. New Lytic Peptides Based on the D,L-Amphipathic Helix Motif Preferentially Kill Tumor Cells Compared to Normal Cells. Biochemistry 2003, 42, 9346–9354. [Google Scholar] [CrossRef] [PubMed]
- Papo, N.; Shahar, M.; Eisenbach, L.; Shai, Y. A Novel Lytic Peptide Composed of DL-Amino Acids Selectively Kills Cancer Cells in Culture and in Mice. J. Biol. Chem. 2003, 278, 21018–21023. [Google Scholar] [CrossRef]
- Papo, N.; Seger, D.; Makovitzki, A.; Kalchenko, V.; Eshhar, Z.; Degani, H.; Shai, Y. Inhibition of Tumor Growth and Elimination of Multiple Metastases in Human Prostate and Breast Xenografts by Systemic Inoculation of a Host Defense-like Lytic Peptide. Cancer Res. 2006, 66, 5371–5378. [Google Scholar] [CrossRef] [PubMed]
- Sloballe, P.W.; Lee Maloy, W.; Myrga, M.L.; Jacob, L.S.; Herlyn, M. Experimental Local Therapy of Human Melanoma with Lytic Magainin Peptides. Int. J. Cancer 1995, 60, 280–284. [Google Scholar] [CrossRef]
- Ohsaki, Y.; Gazdar, A.F.; Chen, H.C.; Johnson, B.E. Antitumor Activity of Magainin Analogues against Human Lung Cancer Cell Lines. Cancer Res. 1992, 52, 3534–3538. [Google Scholar]
- Shin, S.Y.; Kang, J.H.; Jang, S.Y.; Kim, Y.; Kim, K.L.; Hahm, K.S. Effects of the Hinge Region of Cecropin A(1-8)-Magainin 2(1-12), a Synthetic Antimicrobial Peptide, on Liposomes, Bacterial and Tumor Cells. Biochim. Biophys. Acta Biomembr. 2000, 1463, 209–218. [Google Scholar] [CrossRef]
- Eliassen, L.T.; Haug, B.E.; Berge, G.; Rekdal, Ø. Enhanced Antitumour Activity of 15-Residue Bovine Lactoferricin Derivatives Containing Bulky Aromatic Amino Acids and Lipophilic N-Terminal Modifications. J. Pept. Sci. 2003, 9, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Strøm, M.B.; Mekonnen, S.M.; Svendsen, J.S.; Rekdal, Ø. The Effects of Shortening Lactoferrin Derived Peptides against Tumour Cells, Bacteria and Normal Human Cells. J. Pept. Sci. 2004, 10, 37–46. [Google Scholar] [CrossRef]
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin: A Lactoferrin-Derived Peptide with Antimicrobial, Antiviral, Antitumor and Immunological Properties. Cell. Mol. Life Sci. 2005, 62, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Ellerby, H.M.; Arap, W.; Ellerby, L.M.; Kain, R.; Andrusiak, R.; del Rio, G.; Krajewski, S.; Lombardo, C.R.; Rao, R.; Ruoslahti, E.; et al. Anti-Cancer Activity of Targeted pro-Apoptotic Peptides. Nat. Med. 1999, 5, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Droin, N.; Hendra, J.B.; Ducoroy, P.; Solary, E. Human Defensins as Cancer Biomarkers and Antitumour Molecules. J. Proteom. 2009, 72, 918–927. [Google Scholar] [CrossRef]
- Baxter, A.A.; Lay, F.T.; Poon, I.K.H.; Kvansakul, M.; Hulett, M.D. Tumor Cell Membrane-Targeting Cationic Antimicrobial Peptides: Novel Insights into Mechanisms of Action and Therapeutic Prospects. Cell. Mol. Life Sci. 2017, 74, 3809–3825. [Google Scholar] [CrossRef]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with Dual Antimicrobial and Anticancer Activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Raman, K.; Kuberan, B. Chemical Tumor Biology of Heparan Sulfate Proteoglycans. Curr. Chem. Biol. 2010, 4, 20–31. [Google Scholar] [CrossRef]
- Chan, S.C.; Hui, L.; Chen, H.M. Enhancement of the Cytolytic Effect of Anti-Bacterial Cecropin by the Microvilli of Cancer Cells. Anticancer Res. 1998, 18, 4467–4474. [Google Scholar] [PubMed]
- Kunda, N.K. Antimicrobial Peptides as Novel Therapeutics for Non-Small Cell Lung Cancer. Drug Discov. Today 2020, 25, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, U.; Sobczak, M.; Oledzka, E. Current State of a Dual Behaviour of Antimicrobial Peptides—Therapeutic Agents and Promising Delivery Vectors. Chem. Biol. Drug Des. 2017, 90, 1079–1093. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, N.; Nagaraj, R. Interaction of Antimicrobial Peptides with Biological and Model Membranes: Structural and Charge Requirements for Activity. Biochim. Biophys. Acta Biomembr. 1999, 1462, 29–54. [Google Scholar] [CrossRef] [PubMed]
- Ebenhan, T.; Gheysens, O.; Kruger, H.G.; Zeevaart, J.R.; Sathekge, M.M. Antimicrobial Peptides: Their Role as Infection-Selective Tracers for Molecular Imaging. Biomed. Res. Int. 2014, 2014, 867381. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, A.; Tornesello, A.L.; Tornesello, M.L.; Buonaguro, F.M. Cell Penetrating Peptides as Molecular Carriers for Anti-Cancer Agents. Molecules 2018, 23, 295. [Google Scholar] [CrossRef]
- Järvå, M.; Lay, F.T.; Phan, T.K.; Humble, C.; Poon, I.K.H.; Bleackley, M.R.; Anderson, M.A.; Hulett, M.D.; Kvansakul, M. X-ray Structure of a Carpet-like Antimicrobial Defensin-Phospholipid Membrane Disruption Complex. Nat. Commun. 2018, 9, 1962. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. Molecular Recognition between Membrane-Spanning Polypeptides. Trends Biochem. Sci. 1995, 20, 460–464. [Google Scholar] [CrossRef]
- Epand, R.M.; Shai, Y.; Segrest, J.P.; Anantharamiah, G.M. Mechanisms for the Modulation of Membrane Bilayer Properties by Amphipathic Helical Peptides. Biopolymers 1995, 37, 319–338. [Google Scholar] [CrossRef]
- Gaspar, D.; Salomé Veiga, A.; Castanho, M.A.R.B. From Antimicrobial to Anticancer Peptides. A Review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef]
- Mader, J.S.; Richardson, A.; Salsman, J.; Top, D.; de Antueno, R.; Duncan, R.; Hoskin, D.W. Bovine Lactoferricin Causes Apoptosis in Jurkat T-Leukemia Cells by Sequential Permeabilization of the Cell Membrane and Targeting of Mitochondria. Exp. Cell Res. 2007, 313, 2634–2650. [Google Scholar] [CrossRef]
- Oren, Z.; Shai, Y. Mode of Action of Linear Amphipathic α-Helical Antimicrobial Peptides. Biopolymers 1998, 47, 451–463. [Google Scholar] [CrossRef]
- Last, N.B.; Schlamadinger, D.E.; Miranker, A.D. A Common Landscape for Membraneactive Peptides. Protein Sci. 2013, 22, 870–882. [Google Scholar] [CrossRef]
- Henzler Wildman, K.A.; Lee, D.K.; Ramamoorthy, A. Mechanism of Lipid Bilayer Disruption by the Human Antimicrobial Peptide, LL-37. Biochemistry 2003, 42, 6545–6558. [Google Scholar] [CrossRef]
- Okumura, K.; Itoh, A.; Isogai, E.; Hirose, K.; Hosokawa, Y.; Abiko, Y.; Shibata, T.; Hirata, M.; Isogai, H. C-Terminal Domain of Human CAP18 Antimicrobial Peptide Induces Apoptosis in Oral Squamous Cell Carcinoma SAS-H1 Cells. Cancer Lett. 2004, 212, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Mader, J.S.; Mookherjee, N.; Hancock, R.E.W.; Bleackley, R.C. The Human Host Defense Peptide LL-37 Induces Apoptosis in a Calpain- and Apoptosis-Inducing Factor-Dependent Manner Involving Bax Activity. Mol. Cancer Res. 2009, 7, 689–702. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial Peptides as Anticancer Agents: Functional Properties and Biological Activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.A.; Markovic-Lipkovski, J.; Klatt, T.; Gamper, J.; Schwarz, G.; Beck, H.; Deeg, M.; Kalbacher, H.; Widmann, S.; Wessels, J.T.; et al. Human α-Defensins HNPs-1, -2, and -3 in Renal Cell Carcinoma: Influences on Tumor Cell Proliferation. Am. J. Pathol. 2002, 160, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Kagan, B.L.; Selsted, M.E.; Ganz, T.; Lehrer, R.I. Antimicrobial Defensin Peptides Form Voltage-Dependent Ion-Permeable Channels in Planar Lipid Bilayer Membranes. Proc. Natl. Acad. Sci. USA 1990, 87, 210–214. [Google Scholar] [CrossRef]
- Gera, J.F.; Lichtenstein, A. Human Neutrophil Peptide Defensins Induce Single Strand DNA Breaks in Target Cells. Cell. Immunol. 1991, 138, 108–120. [Google Scholar] [CrossRef]
- Chavakis, T.; Cines, D.B.; Rhee, J.-S.; Liang, O.D.; Schubert, U.; Hammes, H.-P.; Higazi, A.A.-R.; Nawroth, P.P.; Preissner, K.T.; Bdeir, K. Regulation of Neovascularization by Human Neutrophil Peptides (A-defensins): A Link between Inflammation and Angiogenesis. FASEB J. 2004, 18, 1306–1308. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.; Ganz, T.; Selsted, M.E.; Lehrer, R.I. In Vitro Tumor Cell Cytolysis Mediated by Peptide Defensins of Human and Rabbit Granulocytes. Blood 1986, 68, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Abiko, Y.; Kurashige, Y.; Takeshima, M.; Yamazaki, M.; Kusano, K.; Saitoh, M.; Nakashima, K.; Inoue, T.; Kaku, T. Effect of Defensin Peptides on Eukaryotic Cells: Primary Epithelial Cells, Fibroblasts and Squamous Cell Carcinoma Cell Lines. J. Dermatol. Sci. 2004, 36, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.K.; Ganz, T.; Nguyen, T.-M.; Selsted, M.E.; Lehrer, R.I. Mechanism of Target Cytolysis by Peptide Defensins. Target Cell Metabolic Activities, Possibly Involving Endocytosis, Are Crucial for Expression of Cytotoxicity. J. Immunol. 1988, 140, 2686–2694. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Chapple, D.S. Peptide Antibiotics. Antimicrob. Agents Chemother. 1999, 43, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.V. Melittin Resistance: A Counterselection for Ras Transformation. Oncogene 1992, 7, 193–201. [Google Scholar] [PubMed]
- Sharma, S.V. Melittin-Induced Hyperactivation of Phospholipase A2 Activity and Calcium Influx in Ras-Transformed Cells. Oncogene 1993, 8, 939–947. [Google Scholar]
- Saini, S.S.; Chopra, A.K.; Peterson, J.W. Melittin Activates Endogenous Phospholipase D during Cytolysis of Human Monocytic Leukemia Cells. Toxicon 1999, 37, 1605–1619. [Google Scholar] [CrossRef]
- Teixeira, V.; Feio, M.J.; Bastos, M. Role of Lipids in the Interaction of Antimicrobial Peptides with Membranes. Prog. Lipid Res. 2012, 51, 149–177. [Google Scholar]
- Chen, J.; Xu, X.M.; Underhill, C.B.; Yang, S.; Wang, L.; Chen, Y.; Hong, S.; Creswell, K.; Zhang, L. Tachyplesin Activates the Classic Complement Pathway to Kill Tumor Cells. Cancer Res. 2005, 65, 4614–4622. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.L.; Wang, Y.Y.; Liang, Y.; Li, Q.F. Effects of Tachyplesin and N-Sodium Butyrate on Proliferation and Gene Expression of Human Gastric Adenocarcinoma Cell Line BGC-823. World J. Gastroenterol. 2006, 12, 1694–1698. [Google Scholar] [CrossRef]
- Ouyang, G.L.; Li, Q.F.; Peng, X.X.; Liu, Q.R.; Hong, S.G. Effects of Tachyplesin on Proliferation and Differentiation of Human Hepatocellular Carcinoma SMMC-7721 Cells. World J. Gastroenterol. 2002, 8, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, T.; Fujimoto, Y.; Ikuta, K.; Saito, H.; Ohhira, M.; Ono, M.; Kohgo, Y. Proline Rich Antimicrobial Peptide, PR-39 Gene Transduction Altered Invasive Activity and Actin Structure in Human Hepatocellular Carcinoma Cells. Br. J. Cancer 1999, 81, 393–403. [Google Scholar] [CrossRef]
- Tanaka, K.; Fujimoto, Y.; Suzuki, M.; Suzuki, Y.; Ohtake, T.; Saito, H.; Kohgo, Y. PI3-Kinase P85α Is a Target Molecule of Proline-Rich Antimicrobial Peptide to Suppress Proliferation of Ras-Transformed Cells. Jpn. J. Cancer Res. 2001, 92, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.R.; Zanetti, M.; Gennaro, R.; Gallo, R.L. Anti-Microbial Activity and Cell Binding Are Controled by Sequence Determinants in the Anti-Microbial Peptide PR-39. J. Investig. Dermatol. 2001, 116, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Gao, Y.; Qi, Y.; Chen, L.; Ma, Y.; Li, Y. Peptide-Based Cancer Therapy: Opportunity and Challenge. Cancer Lett. 2014, 351, 13–22. [Google Scholar] [CrossRef]
- Wu, X.; Huang, H.; Wang, C.; Lin, S.; Huang, Y.; Wang, Y.; Liang, G.; Yan, Q.; Xiao, J.; Wu, J.; et al. Identification of a Novel Peptide That Blocks Basic Fibroblast Growth Factor-Mediated Cell Proliferation. Oncotarget 2013, 4, 1819–1828. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.; Li, T.; Li, Y.; Wang, R.; He, D.; Luo, W.; Li, X.; Wu, X. Screening a Phage Display Library for a Novel FGF8b-Binding Peptide with Anti-Tumor Effect on Prostate Cancer. Exp. Cell Res. 2013, 319, 1156–1164. [Google Scholar] [CrossRef]
- Lee, E.; Koskimaki, J.E.; Pandey, N.B.; Popel, A.S. Inhibition of Lymphangiogenesis and Angiogenesis in Breast Tumor Xenografts and Lymph Nodes by a Peptide Derived from Transmembrane Protein 45A. Neoplasia 2013, 15, 112–124. [Google Scholar] [CrossRef]
- Deslouches, B.; Peter Di, Y. Antimicrobial Peptides with Selective Antitumor Mechanisms: Prospect for Anticancer Applications. Oncotarget 2017, 8, 46635–46651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.N.; Liu, N. Effect of Bovine Lactoferricin on DNA Methyltransferase 1 Levels in Jurkat T-Leukemia Cells. J. Dairy Sci. 2010, 93, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Okumura, K.; Isogai, H.; Isogai, E. The Human Cathelicidin Antimicrobial Peptide LL-37 and Mimics Are Potential Anticancer Drugs. Front. Oncol. 2015, 5, 144. [Google Scholar] [CrossRef]
- Wu, W.K.K.; Sung, J.J.Y.; To, K.F.; Yu, L.; Li, H.T.; Li, Z.J.; Chu, K.M.; Yu, J.; Cho, C.H. The Host Defense Peptide LL-37 Activates the Tumor-Suppressing Bone Morphogenetic Protein Signaling via Inhibition of Proteasome in Gastric Cancer Cells. J. Cell. Physiol. 2010, 223, 178–186. [Google Scholar] [CrossRef]
- Sveinbjørnsson, B.; Camilio, K.A.; Haug, B.E.; Rekdal, Ø. LTX-315: A First-in-Class Oncolytic Peptide That Reprograms the Tumor Microenvironment. Future Med. Chem. 2017, 9, 1339–1344. [Google Scholar] [CrossRef]
- Xie, W.; Mondragón, L.; Mauseth, B.; Wang, Y.; Pol, J.; Lévesque, S.; Zhou, H.; Yamazaki, T.; Eksteen, J.J.; Zitvogel, L.; et al. Tumor Lysis with LTX-401 Creates Anticancer Immunity. Oncoimmunology 2019, 8, e1594555. [Google Scholar] [CrossRef]
- Zhou, H.; Forveille, S.; Sauvat, A.; Yamazaki, T.; Senovilla, L.; Ma, Y.; Liu, P.; Yang, H.; Bezu, L.; Müller, K.; et al. The Oncolytic Peptide LTX-315 Triggers Immunogenic Cell Death. Cell Death Dis. 2016, 7, e2134. [Google Scholar] [CrossRef]
- Zweytick, D. LTX-315—A Promising Novel Antitumor Peptide and Immunotherapeutic Agent. Cell Stress 2019, 3, 328. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Hiraoka, M.; Kizaka-Kondoh, S. Antitumor Effect of TAT-Oxygen-Dependent Degradation-Caspase-3 Fusion Protein Specifically Stabilized and Activated in Hypoxic Tumor Cells. Cancer Res. 2002, 62, 2013–2018. [Google Scholar]
- DeBerardinis, R.J.; Chandel, N.S. We Need to Talk about the Warburg Effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef]
- Varum, S.; Rodrigues, A.S.; Moura, M.B.; Momcilovic, O.; Easley IV, C.A.; Ramalho-Santos, J.; van Houten, B.; Schatten, G. Energy Metabolism in Human Pluripotent Stem Cells and Their Differentiated Counterparts. PLoS ONE 2011, 6, e20914. [Google Scholar] [CrossRef]
- Xie, J.; Wu, H.; Dai, C.; Pan, Q.; Ding, Z.; Hu, D.; Ji, B.; Luo, Y.; Hu, X. Beyond Warburg Effect—Dual Metabolic Nature of Cancer Cells. Sci. Rep. 2014, 4, 4927. [Google Scholar] [CrossRef]
- Chiche, J.; Brahimi-Horn, M.C.; Pouysségur, J. Tumour Hypoxia Induces a Metabolic Shift Causing Acidosis: A Common Feature in Cancer. J. Cell. Mol. Med. 2010, 14, 771–794. [Google Scholar] [CrossRef]
- Švastová, E.; Hulíková, A.; Rafajová, M.; Zat’Ovičová, M.; Gibadulinová, A.; Casini, A.; Cecchi, A.; Scozzafava, A.; Supuran, C.T.; Pastorek, J.; et al. Hypoxia Activates the Capacity of Tumor-Associated Carbonic Anhydrase IX to Acidify Extracellular PH. FEBS Lett. 2004, 577, 439–445. [Google Scholar] [CrossRef]
- Ye, Z.; Yue, L.; Shi, J.; Shao, M.; Wu, T. Role of IDO and TDO in Cancers and Related Diseases and the Therapeutic Implications. J. Cancer 2019, 10, 2771–2782. [Google Scholar] [CrossRef]
- Reshkin, S.J.; Cardone, R.A.; Harguindey, S. Na+-H+ Exchanger, PH Regulation and Cancer. Recent Pat. Anticancer Drug Discov. 2012, 8, 85–99. [Google Scholar] [CrossRef]
- Juang, V.; Chang, C.H.; Wang, C.S.; Wang, H.E.; Lo, Y.L. PH-Responsive PEG-Shedding and Targeting Peptide-Modified Nanoparticles for Dual-Delivery of Irinotecan and MicroRNA to Enhance Tumor-Specific Therapy. Small 2019, 15, 1903296. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Hou, Y.; Chen, X.; Zhang, P.; Kang, M.; Jin, Q.; Ji, J.; Gao, M. Metformin-Induced Stromal Depletion to Enhance the Penetration of Gemcitabine-Loaded Magnetic Nanoparticles for Pancreatic Cancer Targeted Therapy. J. Am. Chem. Soc. 2020, 142, 4944–4954. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.F.; Rath, P.; Rothschild, K.J.; Engelman, D.M. Spontaneous, PH-Dependent Membrane Insertion of a Transbilayer α-Helix. Biochemistry 1997, 36, 15177–15192. [Google Scholar] [CrossRef]
- Reshetnyak, Y.K.; Andreev, O.A.; Lehnert, U.; Engelman, D.M. Translocation of Molecules into Cells by PH-Dependent Insertion of a Transmembrane Helix. Proc. Natl. Acad. Sci. USA 2006, 103, 6460–6465. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Olson, E.S.; Nguyen, Q.T.; Roy, M.; Jennings, P.A.; Tsien, R.Y. Tumor Imaging by Means of Proteolytic Activation of Cell-Penetrating Peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 17867–17872. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E.; Duza, T.; Zhang, L. Vascular Homing Peptides with Cell-Penetrating Properties. Curr. Pharm. Des. 2005, 11, 3655–3660. [Google Scholar] [CrossRef] [PubMed]
- Lingasamy, P.; Teesalu, T. Homing Peptides for Cancer Therapy. Adv. Exp. Med. Biol. 2021, 1295, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Desale, K.; Kuche, K.; Jain, S. Cell-Penetrating Peptides (CPPs): An Overview of Applications for Improving the Potential of Nanotherapeutics. Biomater. Sci. 2021, 9, 1153–1188. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.K.; Stahl, P.; Hensel, A.; Knauer, S.; Hirschhäuser, C.; Schmuck, C. Cancer-Cell-Specific Drug Delivery by a Tumor-Homing CPP-Gossypol Conjugate Employing a Tracelessly Cleavable Linker. Chem.—Eur. J. 2020, 26, 3010–3015. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. Tumor Penetrating Peptides for Improved Drug Delivery. Adv. Drug Deliv. Rev. 2017, 110–111, 3–12. [Google Scholar] [CrossRef]
- Petrenko, V.A.; Gillespie, J.W. Paradigm Shift in Bacteriophage-Mediated Delivery of Anticancer Drugs: From Targeted ‘Magic Bullets’ to Self-Navigated ‘Magic Missiles. ’ Expert Opin. Drug Deliv. 2017, 14, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Luo, G.; Giannelli, S.; Szeto, H.H. Mitochondria-Targeted Peptide Prevents Mitochondrial Depolarization and Apoptosis Induced by Tert-Butyl Hydroperoxide in Neuronal Cell Lines. Biochem. Pharmacol. 2005, 70, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Szeto, H.H.; Schiller, P.W.; Zhao, K.; Luo, G. Fluorescent Dyes Alter Intracellular Targeting and Function of Cell-penetrating Tetrapeptides. FASEB J. 2005, 19, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.; Mather, S.J.; Piaggio, G.; Malviya, G.; Dierckx, R.A. Molecular Imaging of Inflammation/Infection: Nuclear Medicine and Optical Imaging Agents and Methods. Chem. Rev. 2010, 110, 3112–3145. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Waser, B. Concomitant Expression of Several Peptide Receptors in Neuroendocrine Tumours: Molecular Basis for in Vivo Multireceptor Tumour Targeting. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Xie, J.; Chen, X. Peptides and Peptide Hormones for Molecular Imaging and Disease Diagnosis. Chem. Rev. 2010, 110, 3087–3111. [Google Scholar] [CrossRef] [PubMed]
- Eberle, A.N.; Mild, G. Receptor-Mediated Tumor Targeting with Radiopeptides Part 1. General Principles and Methods. J. Recept. Signal Transduct. 2009, 29, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Ferro-Flores, G.; de Ramirez, M.F.; Melendez-Alafort, L.; Santos-Cuevas, C.L. Peptides for In Vivo Target-Specific Cancer Imaging. Mini-Rev. Med. Chem. 2010, 10, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Krohn, K.A. The Physical Chemistry of Ligand-Receptor Binding Identifies Some Limitations to the Analysis of Receptor Images. Nucl. Med. Biol. 2001, 28, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Breeman, W.A.P.; Hofland, L.J.; de Jong, M.; Bernard, B.F.; Srinivasan, A.; Kwekkeboom, D.J.; Visser, T.J.; Krenning, E.P. Evaluation of Radiolabelled Bombesin Analogues for Receptor-Targeted Scintigraphy and Radiotherapy. Int. J. Cancer 1999, 81, 658–663. [Google Scholar] [CrossRef]
- Koopmans, K.P.; Glaudemans, A.W.J.M. Rationale for the Use of Radiolabelled Peptides in Diagnosis and Therapy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 4–10. [Google Scholar] [CrossRef]
- Wang, W.; Ma, Z.; Zhu, S.; Wan, H.; Yue, J.; Ma, H.; Ma, R.; Yang, Q.; Wang, Z.; Li, Q.; et al. Molecular Cancer Imaging in the Second Near-Infrared Window Using a Renal-Excreted NIR-II Fluorophore-Peptide Probe. Adv. Mater. 2018, 30, 1900566. [Google Scholar] [CrossRef]
- Su, Z.; Shen, H.; Wang, H.; Wang, J.; Li, J.; Nienhaus, G.U.; Shang, L.; Wei, G. Motif-Designed Peptide Nanofibers Decorated with Graphene Quantum Dots for Simultaneous Targeting and Imaging of Tumor Cells. Adv. Funct. Mater. 2015, 25, 5472–5478. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, D.; Wang, H.; Li, J.; Nienhaus, G.U.; Su, Z.; Wei, G.; Shang, L. Supramolecular Self-Assembly Bioinspired Synthesis of Luminescent Gold Nanocluster-Embedded Peptide Nanofibers for Temperature Sensing and Cellular Imaging. Bioconjug. Chem. 2017, 28, 2224–2229. [Google Scholar] [CrossRef]
- Reshetnyak, Y.K.; Yao, L.; Zheng, S.; Kuznetsov, S.; Engelman, D.M.; Andreev, O.A. Measuring Tumor Aggressiveness and Targeting Metastatic Lesions with Fluorescent PHLIP. Mol. Imaging Biol. 2011, 13, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.E.; Hensley, H.; Robinson, M.K.; Thévenin, D. Therapeutic Efficacy of a Family of PHLIP-MMAF Conjugates in Cancer Cells and Mouse Models. Mol. Pharm. 2017, 14, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Dharmaratne, N.U.; Kaplan, A.R.; Glazer, P.M. Targeting the Hypoxic and Acidic Tumor Microenvironment with PH-Sensitive Peptides. Cells 2021, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Westby, M.; Souberbielle, B.E.; Szawlowski, P.W.S.; Kemp, G.; Hay, P.; Dalgleish, A.G. Optimisation of a Peptide-Based Indirect ELISA for the Detection of Antibody in the Serum of HIV-1 Seropositive Patients. J. Immunol. Methods 1997, 200, 79–88. [Google Scholar] [CrossRef]
- Aydin, S. A Short History, Principles, and Types of ELISA, and Our Laboratory Experience with Peptide/Protein Analyses Using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Angenendt, P. Progress in Protein and Antibody Microarray Technology. Drug Discov. Today 2005, 10, 503–511. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, R.; Giuffrida, M.C.; Spoto, G. Peptide Nucleic Acid-Based Biosensors for Cancer Diagnosis. Molecules 2017, 22, 1951. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Ma, J.; Kong, D.; Zhang, Z.; Khan, A.; Yi, C.; Hu, K.; Yi, Y.; Li, J. One Step Electrochemical Detection for Matrix Metalloproteinase 2 Based on Anodic Stripping of Silver Nanoparticles Mediated by Host-Guest Interactions. Sens. Actuators B Chem. 2021, 330, 129379. [Google Scholar] [CrossRef]
- Xi, X.; Wen, M.; Song, S.; Zhu, J.; Wen, W.; Zhang, X.; Wang, S. A H2O2-Free Electrochemical Peptide Biosensor Based on Au@Pt Bimetallic Nanorods for Highly Sensitive Sensing of Matrix Metalloproteinase 2. Chem. Commun. 2020, 56, 6039–6042. [Google Scholar] [CrossRef]
- Gogotsi, Y. Nanomaterials Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Wang, J. Nanomaterial-Based Electrochemical Biosensors. Analyst 2005, 130, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Datta, M.; Malhotra, B.D. Prospects of Nanomaterials in Biosensors. Anal. Lett. 2008, 41, 159–209. [Google Scholar] [CrossRef]
- Puiu, M.; Bala, C. Building Switchable Peptide-Architectures on Gold/Composite Surfaces: New Perspectives in Electrochemical Bioassays. Curr. Opin. Electrochem. 2018, 12, 13–20. [Google Scholar] [CrossRef]
- Farshchi, F.; Hasanzadeh, M. Microfluidic Biosensing of Circulating Tumor Cells (CTCs): Recent Progress and Challenges in Efficient Diagnosis of Cancer. Biomed. Pharmacother. 2021, 134, 111153. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.R.; Oddo, A. Fmoc Solid-Phase Peptide Synthesis. In Peptide Antibodies: Methods and Protocols; Houen, G., Ed.; Humana Press: New York, NY, USA, 2015; Volume 1348, pp. 33–50. ISBN 978-1-4939-2999-3. [Google Scholar]
- Jad, Y.E.; Kumar, A.; El-Faham, A.; de La Torre, B.G.; Albericio, F. Green Transformation of Solid-Phase Peptide Synthesis. ACS Sustain. Chem. Eng. 2019, 7, 3671–3683. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Pedersen, S.W.; Armishaw, C.J.; Strømgaard, K. Synthesis of Peptides Using Tert-Butyloxycarbonyl (Boc) as the α-Amino Protection Group. Methods Mol. Biol. 2013, 1047, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Atherton, E.; Fox, H.; Harkiss, D.; Logan, C.J.; Sheppard, R.C.; Williams, B.J. A Mild Procedure for Solid Phase Peptide Synthesis: Use of Fluorenylmethoxycarbonylamino-acids. J. Chem. Soc. Chem. Commun. 1978, 537–539. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; de la Torre, B.G.; Albericio, F. Liquid-Phase Peptide Synthesis (LPPS): A Third Wave for the Preparation of Peptides. Chem. Rev. 2022, 122, 13516–13546. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Liu, D.; Yang, Y. Anti-Cancer Peptides: Classification, Mechanism of Action, Reconstruction and Modification. Open Biol. 2020, 10, 200004. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mant, C.T.; Farmer, S.W.; Hancock, R.E.W.; Vasil, M.L.; Hodges, R.S. Rational Design of α-Helical Antimicrobial Peptides with Enhanced Activities and Specificity/Therapeutic Index. J. Biol. Chem. 2005, 280, 12316–12329. [Google Scholar] [CrossRef] [PubMed]
- Avrahami, D.; Shai, Y. Conjugation of a Magainin Analogue with Lipophilic Acids Controls Hydrophobicity, Solution Assembly, and Cell Selectivity. Biochemistry 2002, 41, 2254–2263. [Google Scholar] [CrossRef]
- Bhonsle, J.B.; Clark, T.; Bartolotti, L.; Hicks, R.P. A Brief Overview of Antimicrobial Peptides Containing Unnatural Amino Acids and Ligand-Based Approaches for Peptide Ligands. Curr. Top. Med. Chem. 2013, 13, 3205–3224. [Google Scholar] [CrossRef]
- Russell, A.L.; Kennedy, A.M.; Spuches, A.M.; Gibson, W.S.; Venugopal, D.; Klapper, D.; Srouji, A.H.; Bhonsle, J.B.; Hicks, R.P. Determining the Effect of the Incorporation of Unnatural Amino Acids into Antimicrobial Peptides on the Interactions with Zwitterionic and Anionic Membrane Model Systems. Chem. Phys. Lipids 2011, 164, 740–758. [Google Scholar] [CrossRef]
- Hicks, R.P.; Russell, A.L. Application of Unnatural Amino Acids to the de Novo Design of Selective Antibiotic Peptides. In Unnatural Amino Acids; Springer: Berlin/Heidelberg, Germany, 2012; pp. 135–167. [Google Scholar]
- Venugopal, D.; Klapper, D.; Srouji, A.H.; Bhonsle, J.B.; Borschel, R.; Mueller, A.; Russell, A.L.; Williams, B.C.; Hicks, R.P. Novel Antimicrobial Peptides That Exhibit Activity against Select Agents and Other Drug Resistant Bacteria. Bioorg. Med. Chem. 2010, 18, 5137–5147. [Google Scholar] [CrossRef]
- Narwal, V.; Deswal, R.; Batra, B.; Kalra, V.; Hooda, R.; Sharma, M.; Rana, J.S. Cholesterol Biosensors: A Review. Steroids 2019, 143, 6–17. [Google Scholar] [CrossRef]
- Milla, P.; Dosio, F.; Cattel, L. PEGylation of Proteins and Liposomes: A Powerful and Flexible Strategy to Improve the Drug Delivery. Curr. Drug Metab. 2012, 13, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.M.; Mantovani, G.; Wang, X.; Haddleton, D.M.; Brayden, D.J. Advances in PEGylation of Important Biotech Molecules: Delivery Aspects. Expert Opin. Drug Deliv. 2008, 5, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.; Dutta, S.; Camara, A.; Chandran, A.; Koller, A.; Watson, B.G.; Sengupta, R.; Ysselstein, D.; Montenegro, P.; Cannon, J. Alpha-Synuclein Is a Target of Fic-Mediated Adenylylation/AMPylation: Possible Implications for Parkinson’s Disease. J. Mol. Biol. 2019, 431, 2266–2282. [Google Scholar] [CrossRef] [PubMed]
- Wan-Cheng Li, D. Editorial (Thematic Issue: Roles of Sumoylation and Phosphorylation in Normal Physiology and Human Diseases). Curr. Mol. Med. 2016, 16, 857–858. [Google Scholar]
- Pocheć, E.; Lityńska, A.; Bubka, M.; Amoresano, A.; Casbarra, A. Characterization of the Oligosaccharide Component of A3β1 Integrin from Human Bladder Carcinoma Cell Line T24 and Its Role in Adhesion and Migration. Eur. J. Cell Biol. 2006, 85, 47–57. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Hsu, J.-T.; Chung, P.-H.; Cheng, W.T.-K.; Jiang, Y.-N.; Ju, Y.-T. Site-Specific N-Glycosylation of Caprine Lysostaphin Restricts Its Bacteriolytic Activity toward Staphylococcus Aureus. Anim. Biotechnol. 2013, 24, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Aicart-Ramos, C.; Valero, R.A.; Rodriguez-Crespo, I. Protein Palmitoylation and Subcellular Trafficking. Biochim. Biophys. Acta (BBA)-Biomembr. 2011, 1808, 2981–2994. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Zhang, P.; Ma, J.; Jian, S.; Zhang, Q.; Liu, B.; Liang, S.; Liu, M.; Zeng, Y.; Liu, Z. The Roles of Fatty-Acid Modification in the Activity of the Anticancer Peptide R-Lycosin-I. Mol. Pharm. 2018, 15, 4612–4620. [Google Scholar] [CrossRef]
- Lee, A.C.-L.; Harris, J.L.; Khanna, K.K.; Hong, J.-H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef] [PubMed]
- Ballester, P.J.; Mitchell, J.B.O. A Machine Learning Approach to Predicting Protein–Ligand Binding Affinity with Applications to Molecular Docking. Bioinformatics 2010, 26, 1169–1175. [Google Scholar] [CrossRef]
- Manavalan, B.; Shin, T.H.; Kim, M.O.; Lee, G. PIP-EL: A New Ensemble Learning Method for Improved Proinflammatory Peptide Predictions. Front. Immunol. 2018, 9, 1783. [Google Scholar] [CrossRef]
- Tallorin, L.; Wang, J.; Kim, W.E.; Sahu, S.; Kosa, N.M.; Yang, P.; Thompson, M.; Gilson, M.K.; Frazier, P.I.; Burkart, M.D. Discovering de Novo Peptide Substrates for Enzymes Using Machine Learning. Nat. Commun. 2018, 9, 5253. [Google Scholar] [CrossRef]
- Agrawal, P.; Bhagat, D.; Mahalwal, M.; Sharma, N.; Raghava, G.P.S. AntiCP 2.0: An Updated Model for Predicting Anticancer Peptides. Brief. Bioinform. 2021, 22, bbaa153. [Google Scholar] [CrossRef]
- Yi, H.-C.; You, Z.-H.; Zhou, X.; Cheng, L.; Li, X.; Jiang, T.-H.; Chen, Z.-H. ACP-DL: A Deep Learning Long Short-Term Memory Model to Predict Anticancer Peptides Using High-Efficiency Feature Representation. Mol. Ther.-Nucleic Acids 2019, 17, 1–9. [Google Scholar] [CrossRef]
- Jiang, T.; Gradus, J.L.; Rosellini, A.J. Supervised Machine Learning: A Brief Primer. Behav. Ther. 2020, 51, 675–687. [Google Scholar] [CrossRef]
- Kawashima, S.; Pokarowski, P.; Pokarowska, M.; Kolinski, A.; Katayama, T.; Kanehisa, M. AAindex: Amino Acid Index Database, Progress Report 2008. Nucleic Acids Res. 2007, 36, D202–D205. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.-W.; Ho, S.-Y. Computational Identification of Ubiquitylation Sites from Protein Sequences. BMC Bioinform. 2008, 9, 310. [Google Scholar] [CrossRef]
- Rao, B.; Zhou, C.; Zhang, G.; Su, R.; Wei, L. ACPred-Fuse: Fusing Multi-View Information Improves the Prediction of Anticancer Peptides. Brief. Bioinform. 2020, 21, 1846–1855. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-Y.; Chen, S.-A.; Hung, H.-Y.; Ou, Y.-Y. Incorporating Distant Sequence Features and Radial Basis Function Networks to Identify Ubiquitin Conjugation Sites. PLoS ONE 2011, 6, e17331. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, P.; Li, F.; Leier, A.; Marquez-Lago, T.T.; Wang, Y.; Webb, G.I.; Smith, A.I.; Daly, R.J.; Chou, K.-C. IFeature: A Python Package and Web Server for Features Extraction and Selection from Protein and Peptide Sequences. Bioinformatics 2018, 34, 2499–2502. [Google Scholar] [CrossRef]
- Sandberg, M.; Eriksson, L.; Jonsson, J.; Sjöström, M.; Wold, S. New Chemical Descriptors Relevant for the Design of Biologically Active Peptides. A Multivariate Characterization of 87 Amino Acids. J. Med. Chem. 1998, 41, 2481–2491. [Google Scholar] [CrossRef]
- Tyagi, A.; Kapoor, P.; Kumar, R.; Chaudhary, K.; Gautam, A.; Raghava, G.P.S. In Silico Models for Designing and Discovering Novel Anticancer Peptides. Sci. Rep. 2013, 3, 2984. [Google Scholar] [CrossRef] [PubMed]
- Hajisharifi, Z.; Piryaiee, M.; Beigi, M.M.; Behbahani, M.; Mohabatkar, H. Predicting Anticancer Peptides with Chou′ s Pseudo Amino Acid Composition and Investigating Their Mutagenicity via Ames Test. J. Theor. Biol. 2014, 341, 34–40. [Google Scholar] [CrossRef]
- Noble, W.S. What Is a Support Vector Machine? Nat. Biotechnol. 2006, 24, 1565–1567. [Google Scholar] [CrossRef]
- Keller, J.M.; Gray, M.R.; Givens, J.A. A Fuzzy K-Nearest Neighbor Algorithm. IEEE Trans. Syst. Man Cybern. 1985, SMC-15, 580–585. [Google Scholar]
- Lakshminarayanan, B.; Pritzel, A.; Blundell, C. Simple and Scalable Predictive Uncertainty Estimation Using Deep Ensembles. In Proceedings of the Conference on Advances in Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; Volume 30. [Google Scholar]
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.-Y. LightGBM: A Highly Efficient Gradient Boosting Decision Tree. In Proceedings of the Conference on Advances in Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; Guyon, I., Luxburg, U., von Bengio, S., Wallach, H., Fergus, R., Vishwanathan, S., Garnett, R., Eds.; Curran Associates, Inc.: Red Hook, NY, USA, 2017; Volume 30, pp. 3149–3157. [Google Scholar]
- Pal, M. Random Forest Classifier for Remote Sensing Classification. Int. J. Remote Sens. 2005, 26, 217–222. [Google Scholar] [CrossRef]
- Chou, K.-C. Some Remarks on Protein Attribute Prediction and Pseudo Amino Acid Composition. J. Theor. Biol. 2011, 273, 236–247. [Google Scholar] [CrossRef]
- Chou, K.-C.; Shen, H.-B. Cell-PLoc: A Package of Web Servers for Predicting Subcellular Localization of Proteins in Various Organisms. Nat. Protoc. 2008, 3, 153–162. [Google Scholar] [CrossRef]
- Chou, K.-C.; Shen, H.-B. Recent Progress in Protein Subcellular Location Prediction. Anal. Biochem. 2007, 370, 1–16. [Google Scholar] [CrossRef]
- Thi Phan, L.; Woo Park, H.; Pitti, T.; Madhavan, T.; Jeon, Y.-J.; Manavalan, B. MLACP 2.0: An Updated Machine Learning Tool for Anticancer Peptide Prediction. Comput. Struct. Biotechnol. J. 2022, 20, 4473–4480. [Google Scholar] [CrossRef] [PubMed]
- Boopathi, V.; Subramaniyam, S.; Malik, A.; Lee, G.; Manavalan, B.; Yang, D.-C. MACPpred: A Support Vector Machine-Based Meta-Predictor for Identification of Anticancer Peptides. Int. J. Mol. Sci. 2019, 20, 1964. [Google Scholar] [CrossRef]
- Zhao, T.; Hu, Y.; Zang, T. DRACP: A Novel Method for Identification of Anticancer Peptides. BMC Bioinform. 2020, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, W.; Wang, D.; Wang, S.; Li, Q. Prediction of Anticancer Peptides Using a Low-Dimensional Feature Model. Front. Bioeng. Biotechnol. 2020, 8, 892. [Google Scholar] [CrossRef]
- Xu, D.; Wu, Y.; Cheng, Z.; Yang, J.; Ding, Y. ACHP: A Web Server for Predicting Anti-Cancer Peptide and Anti-Hypertensive Peptide. Int. J. Pept. Res. Ther. 2021, 27, 1933–1944. [Google Scholar] [CrossRef]
- Vijayakumar, S.; PTV, L. ACPP: A Web Server for Prediction and Design of Anti-Cancer Peptides. Int. J. Pept. Res. Ther. 2015, 21, 99–106. [Google Scholar] [CrossRef]
- Chen, W.; Ding, H.; Feng, P.; Lin, H.; Chou, K.-C. IACP: A Sequence-Based Tool for Identifying Anticancer Peptides. Oncotarget 2016, 7, 16895. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Hayat, M.; Iqbal, M.; Jan, M.A. IACP-GAEnsC: Evolutionary Genetic Algorithm Based Ensemble Classification of Anticancer Peptides by Utilizing Hybrid Feature Space. Artif. Intell. Med. 2017, 79, 62–70. [Google Scholar] [CrossRef]
- Schaduangrat, N.; Nantasenamat, C.; Prachayasittikul, V.; Shoombuatong, W. ACPred: A Computational Tool for the Prediction and Analysis of Anticancer Peptides. Molecules 2019, 24, 1973. [Google Scholar] [CrossRef]
- Wei, L.; Zhou, C.; Chen, H.; Song, J.; Su, R. ACPred-FL: A Sequence-Based Predictor Using Effective Feature Representation to Improve the Prediction of Anti-Cancer Peptides. Bioinformatics 2018, 34, 4007–4016. [Google Scholar] [CrossRef]
- Kabir, M.; Arif, M.; Ahmad, S.; Ali, Z.; Swati, Z.N.K.; Yu, D.-J. Intelligent Computational Method for Discrimination of Anticancer Peptides by Incorporating Sequential and Evolutionary Profiles Information. Chemom. Intell. Lab. Syst. 2018, 182, 158–165. [Google Scholar] [CrossRef]
- Yu, L.; Jing, R.; Liu, F.; Luo, J.; Li, Y. DeepACP: A Novel Computational Approach for Accurate Identification of Anticancer Peptides by Deep Learning Algorithm. Mol. Ther.-Nucleic Acids 2020, 22, 862–870. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, Y.; Cui, L.; Su, R.; Wei, L. Learning Embedding Features Based on Multisense-Scaled Attention Architecture to Improve the Predictive Performance of Anticancer Peptides. Bioinformatics 2021, 37, 4684–4693. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Gao, R.; Zhang, Y.; de Marinis, Y. PTPD: Predicting Therapeutic Peptides by Deep Learning and Word2vec. BMC Bioinform. 2019, 20, 456. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Muhammod, R.; Khan, Z.H.; Adilina, S.; Sharma, A.; Shatabda, S.; Dehzangi, A. ACP-MHCNN: An Accurate Multi-Headed Deep-Convolutional Neural Network to Predict Anticancer Peptides. Sci. Rep. 2021, 11, 23676. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, J.; Zhao, H.; Li, H.; Wang, J. CL-ACP: A Parallel Combination of CNN and LSTM Anticancer Peptide Recognition Model. BMC Bioinform. 2021, 22, 512. [Google Scholar] [CrossRef]
- Sun, M.; Yang, S.; Hu, X.; Zhou, Y. ACPNet: A Deep Learning Network to Identify Anticancer Peptides by Hybrid Sequence Information. Molecules 2022, 27, 1544. [Google Scholar] [CrossRef]
- Chen, X.-G.; Zhang, W.; Yang, X.; Li, C.; Chen, H. ACP-DA: Improving the Prediction of Anticancer Peptides Using Data Augmentation. Front. Genet. 2021, 12, 698477. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.; Zhang, L.; Zhang, G. Acp-Gcn: The Identification of Anticancer Peptides Based on Graph Convolution Networks. IEEE Access 2020, 8, 176005–176011. [Google Scholar] [CrossRef]
- Chen, J.; Cheong, H.H.; Siu, S.W.I. XDeep-AcPEP: Deep Learning Method for Anticancer Peptide Activity Prediction Based on Convolutional Neural Network and Multitask Learning. J. Chem. Inf. Model. 2021, 61, 3789–3803. [Google Scholar] [CrossRef] [PubMed]


| Peptide | Source | Primary Amino Acid Sequence a | Class | Net Charge b | Anticancer Mechanism | Reference |
|---|---|---|---|---|---|---|
| Aurein 1.2 | Litoria raniformis | GLFDIIKKIAESF | α-Helix | +1 | Barrel-stave pore mechanism | [25] |
| BMAP-27 | Bos taurus | GRFKRFRKKFKKLFKKLSPVIPLLHL | α-Helix | +10 | Membranolytic | [26] |
| BMAP-28 | Bos taurus | GGLRSLGRKILRAWKKYGPIIVPIIRI | α-Helix | +7 | Membranolytic | [27] |
| Brevinin | Limnonectes fujianensis frog | KLKNFAKGVAQSLLNKASCKLSGQC | Mixed α-Helix, β-sheet and random coil | +5 | Lysosomal death pathway and autophagy-like cell death through depolarizing the transmembrane potential of cancer cells | [28] |
| Cecropin A | Silk moth Hyalophora cecropia | KWKLFKKIEKVGQNIRDGIIKAGPAVAVVGQATQIAK | α-Helix | +7 | Membranolytic Apoptosis inducer | [29] |
| Cecropin B | Silk moth Hyalophora cecropia | KWKVFKKIEKMGRNIRNGIVKAGPAIAVLGEAKAL | α-Helix | +8 | Tumor growth inhibition using pore formation and apoptosis | [30] |
| Citropin 1.1 | Litoria citropa frog | GLFDVIKKVASVIGGL | α-Helix | +2 | Carpet model of membrane disruption | [31,32] |
| D-K6L9 | Synthetic | LKLLKKLLKKLLKLL | α-Helix | +3 | Reduce neovascularization through cell membrane depolarization | [33] |
| Gaegurins | Rana rugose frog | Gaegurin 5: FLGALFKVASKVLPSVKCAITKKC | α-Helix | +4 | Destruction of cell membranes through a carpet-like model and/or barrel-stave model | [34,35] |
| Gaegurin 6: FLPLLAGLAANFLPTIICFISYKC | ||||||
| HMGB1 | Homo sapiens | GRRRRSVQWCAVSQPEATKCFQWQRNMRKVRGPPVSCIKRDSPIQCIQA | α-Helix | +9 | Immature dendritic cells activation and tumor-specific cytotoxic generation | [36,37,38] |
| HNP-1, HNP-2 and HNP-3 | Homo sapiens | HNP-1: ACYCRIPACIAGERRYGTCIYQGRLWAFCC | β-Sheet | +3 | Membranolytic Antiangiogenic c Cytolytic activity | [39] |
| HNP-2: CYCRIPACIAGERRYGTCIYQGRLWAFCC | ||||||
| HNP-3: DCYCRIPACIAGERRYGTCIYQGRLWAFCC | ||||||
| hBD3 | Homo sapiens | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK | Mixed | +11 | Binding to the phosphatidylinositol 4,5-bisphosphate | [40] |
| LfcinB * | Mammalian lactoferrin | FKC1RRWQWRMKKLGAPSITC1VRRAF | β-Sheet | +8 | Membranolytic Apoptosis inducer Antiangiogenic | [41] |
| LL-37 * | Homo sapiens | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | α-Helix | +6 | Toroidal pore formation | [42] |
| Magainin 2 * | Xenopus laevis frog | GIGKFLHSAKKFGKAFVGEIMNS | α-Helix | +3 | Formation of pores on cell membranes Apoptosis | [43] |
| Melittin * | Venom of the European honeybee Apis mellifera | GIGAVLKVLTTGLPALISWIKRKRQQ | α-Helix | +6 | Destabilizes the membrane through the barrel stave mechanism PLA2 d activator PLD e activator | [44] |
| P18 | Synthetic hybrid | KWKLFKKIPKFLHLAKKF-NH2 | α-Helix | +7 | Membranolytic | [38,45] |
| PR-39 | Porcine small intestine and neutrophils | RRRPRPPYLPRPRPPPFFPPRLPPRIPPGFPPRFPPRFP | Linear | +11 | Induces syndecan-1 expression | [46,47] |
| Tachyplesin I * | Tachypleus tridentatus crab | KWC1FRVC2YRGIC2YRRC1R | β-Sheet | +6 | Binds hyaluronan and activates complement (C1q) Antiangiogenic c Induces cancer cell differentiation | [48] |
| Benchmark Dataset | Independent Dataset | Features | Classifier | Accuracy (%) | MCC | Reference | |
|---|---|---|---|---|---|---|---|
| ACPP | SA_TRAIN | Balanced randomly generated peptides SA_IND | Protein-relatedness measures, including compositional, centroidal and distributional measures of amino acid residues | SVM | 96 | 0.92 | [257] |
| iACP | Hajisharifi et al. [243] | Balanced 300 peptides | One gap DPC | SVM | 92.67 | 0.85 | [258] |
| iACP-GAEnsC | Hajisharifi et al. [243] | NA | Pseudo g-Gap DPC | Ensemble method (SVM/RF/PNN/KNN/GRNN) | 96.45 | 0.91 | [259] |
| Amphiphilic pseudo amino acid composition | |||||||
| Reduce amino acid alphabet composition | |||||||
| ACPred | Hajisharifi et al. [243] | Balanced 205 peptides | AAC | SVM/RF | 95.61 | 0.91 | [260] |
| DPC | |||||||
| PCP | |||||||
| Pseudo AAC | |||||||
| Amphiphilic pseudo AAC | |||||||
| ACPred-FL | balanced dataset ACP500 | balanced dataset ACP164 | Composition–Transition–Distribution | SVM | 91.4 | 0.835 | [261] |
| AAC | |||||||
| G-gap DPC | |||||||
| Adaptive skip DPC | |||||||
| BP Features | |||||||
| Overlapping Property Features | |||||||
| Twenty-One-Bit Features | |||||||
| Target ACP | Hajisharifi et al. [243] | Balanced 205 peptides | Composite protein sequence representation | SVM/KNN/RF | 98.78 | 0.97 | [262] |
| Split AAC | |||||||
| Pseudo position-specific scoring matrix |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaly, G.; Tallima, H.; Dabbish, E.; Badr ElDin, N.; Abd El-Rahman, M.K.; Ibrahim, M.A.A.; Shoeib, T. Anti-Cancer Peptides: Status and Future Prospects. Molecules 2023, 28, 1148. https://doi.org/10.3390/molecules28031148
Ghaly G, Tallima H, Dabbish E, Badr ElDin N, Abd El-Rahman MK, Ibrahim MAA, Shoeib T. Anti-Cancer Peptides: Status and Future Prospects. Molecules. 2023; 28(3):1148. https://doi.org/10.3390/molecules28031148
Chicago/Turabian StyleGhaly, Gehane, Hatem Tallima, Eslam Dabbish, Norhan Badr ElDin, Mohamed K. Abd El-Rahman, Mahmoud A. A. Ibrahim, and Tamer Shoeib. 2023. "Anti-Cancer Peptides: Status and Future Prospects" Molecules 28, no. 3: 1148. https://doi.org/10.3390/molecules28031148
APA StyleGhaly, G., Tallima, H., Dabbish, E., Badr ElDin, N., Abd El-Rahman, M. K., Ibrahim, M. A. A., & Shoeib, T. (2023). Anti-Cancer Peptides: Status and Future Prospects. Molecules, 28(3), 1148. https://doi.org/10.3390/molecules28031148








