Abstract
Radical reactions are powerful in creating carbon–carbon and carbon–heteroatom bonds. Designing one-pot radical reactions with cascade transformations to assemble the cyclic skeletons with two new functional groups is both synthetically and operationally efficient. Summarized in this paper is the recent development of reactions involving radical addition and cyclization of dienes, diynes, enynes, as well as arene-bridged and arene-terminated compounds for the preparation of difunctionalization cyclic compounds. Reactions carried out with radical initiators, transition metal-catalysis, photoredox, and electrochemical conditions are included.
1. Introduction
Synthetic radicals are a topic of current interest due to their feasible radical transformations, such as addition, cyclization, coupling, atom/group transfer, rearrangement and fragmentation, which are powerful in the construction of carbon–carbon bonds, carbon–heteroatom bonds and the formation of diverse ring skeletons [1,2]. The recent developments on photoredox catalysis [3] and electrochemical reactions [4] have sped up the research in this field. Among the board scope of free radical reactions, the radical difunctionalization of alkenes and alkynes has attracted special attention since the substrates are readily available, the reaction process is operationally simple, and two functional groups are introduced to the products in regio- and diastereoselective fashions.
There is a large number of reviews on the radical difunctionalizations [5,6,7,8,9,10,11,12,13,14,15,16]. In a recent paper from our group, we summarized radical addition followed by nucleophilic addition for 1,2- and remote difunctionalizations to introduce X and Y groups to the products (Scheme 1, I) [17]. Presented in this paper is another kind of radical difunctionalization that is initiated with the addition of radical X. followed by radical cyclization and then a second functionalization with Y through coupling or addition to obtain the product (Scheme 1, II).
Scheme 1.
Two kinds of radical difunctionalization reactions.
More information on the radical addition and cyclization-based difunctionalization reactions is shown in Scheme 2. There are three different kinds of substrates: (I) dienes, diynes, and enynes; (II) arene-tethered dienes or enynes; and (III) arene-terminated alkenes and alkynes. The cyclized radical intermediates could have four ways for the second functionalization with Y: (I) coupling with radical Y; (II) metal-catalyzed reaction with Met-Y; (III) oxidation to a cation and then undergoing nucleophilic reaction with Y−; and IV) reduction to an anion and then undergoing electrophilic reaction with Y+. The difunctionalization reactions could be carried out as a one-pot reaction with the following cascade reaction sequence: (1) addition of the initial radical X. to introduce the first functional group; (2) radical cyclization to form the ring; and (3) second functionalization with Y to obtain the product. Compared to the two kinds of reactions shown in Scheme 1, the first one is relatively simple and has been well established. The second one can generate structurally more attractive fused-, bridged-, or spiro-ring systems, but they are more synthetically challenging and under active development. The reactions presented in this paper are organized based on three kinds of starting materials shown in Scheme 2. Reaction-related substrates are also discussed in the last section of the paper.

Scheme 2.
Radical addition and cyclization-based difunctionalizations.
The radical reactions presented in this paper could be conducted using one of the following methods: (1) using radical initiators such as azodiisobutyronitrile (AIBN), t-butyl nitrite (t-BuONO), aryldiazonium tetrafluoroborates; peroxides such as dicumyl peroxide (DCP), di-t-butyl peroxide (DTBP), and t-butyl hydroperoxide (TBHP) and t-butyl peroxybenzoate (TBPB); (2) using single electron transfer (SET) agents such as hypervalent iodine reagents (HIRs), hypervalent bromine reagents (HBrRs), ceric ammonium nitrate (CAN), Mn(OAc)2, and Na2S2O5; (3) under photoredox catalysis such as Ru(bpy)3Cl2 and Ir(ppy)3), [Ir(dtbbpy)(ppy)2]PF6, N-methyl-9-mesityl acridinium (Mes-Acr+), fac-Ir(ppy)3, Na2-Eosin Y; and (3) through electrochemical reactions.
A wide range of functional groups could be incorporated to the products through the difunctionalization reactions, which include halogens (Cl, Br and I), aryl (Ar), alkyl (R), cyano (CN), trifluoromethyl (CF3) or perfluoroalkyl (RF), 2-ethoxy-1,1-difluoro-2-oxoethyl (CF2CO2Et) or 2-ethoxy-1-fluoro-2-oxoethyl (CHFCO2Et), 2-cyanopropan-2-yl (C(CH3)2CN), carbamoyl (CONH2), aryl carbonyl (ArCO), alkyl carbonyl (RCO), hydroxy (OH), carbamoyl oxy (O2CNR2), azido (N3), amino (NR2), aryldiazenyl (Ar-N=N), nitro (NO2), nitroso (NO), sulfonyl (Ts), trifluoromethylthio (CF3S), methylthio (CH3S), arylthio (ArS), phosphorus (PO(OR)2), alkyl silyl (R3Si), aryl silyl (Ar3Si), phenylselanyl (PhSe) and heteroatom-containing groups.
2. Reaction of Dienes and Enynes
Presented in this section are the radical addition and cyclization-initiated difunctionalization reactions of 1,n-dienes and -enynes with a reaction sequence shown in Scheme 3. The common substrates include dienes (I-A), enynes (I-B, I-C, I-H), dienyl amides (I-F), enynyl amides (I-D, I-E, I-G) with the Z as a carbon or heteroatom (Scheme 4). Since there are two unsaturated carbon–carbon bonds in the substrates which are available for the radical addition, the regioselectivity for the initial radical addition is critical. As indicated in Scheme 4, the steric hindrance (I-A to I-D) and conjugation effect of the groups, such as C=O and Ar (I-E to I-H), are the major factors to direct the position for the initial radical addition.

Scheme 3.
General reaction scheme for difunctionalization of dienes and enynes.

Scheme 4.
Diene and enyne compounds with pointed position for the initial radical addition.
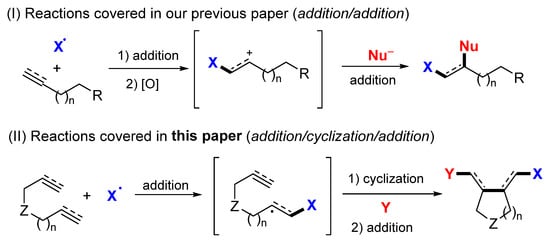
In 2005, Ogawa and coworker reported a near-UV light-mediated radical reaction of dienes, diynes, and enynes for the synthesis of iodoperfluoroalkylated cyclic products. The reactions of dienes, diynes, or enynes and perfluoroalkyl iodides in PhCF3 under the irradiation of xenon lamp afforded products 1 as a mixture of cis/trans isomers in moderate-to-good yields (Scheme 5) [18]. A proposed mechanism indicated that the n-C4F9 radical generated from n-C4F9I under the light adds to diene. The intermediate M-1 undergoes 5-exo cyclization to give alkenyl M-2, which then reacts with n-C4F9I through the iodine atom transfer to give product 1a.
Scheme 5.
Synthesis of iodoperfluoroalkylated products.
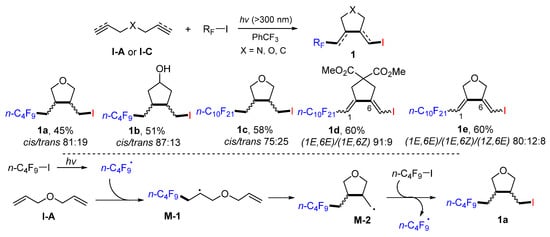
A sun lamp-mediated radical reaction for making azidosulfonylated cyclic products was reported by the Renaud group in 2008. Dienes, diynes, or enynes in dry benzene reacted with benzenesulfonyl azide with radical initiator di-t-butyldiazene to give azidosulfone products 2 in moderate-to-excellent yields (Scheme 6) [19]. This method is good for the formation of tertiary and secondary azides 2a–d, but not for primary azide 2e. The reaction process involves the addition of PhSO2 radical to the less hindered alkene to form intermediate radical M-3, 5-exo cyclization for radical M-4, and N3 radical transfer from PhSO2N3 to give product 2a.
Scheme 6.
Synthesis of azidosulfonylated cyclic products.
1,6-Enynes are the most popular substrates for radical reactions to make difunctionalized five-membered rings. A method for making iodotriflouromethylated N-heterocycles was reported by the Liu group in 2014. The reaction of 1,6-enynes, NaSO2CF3 and I2O5 in CH2Cl2/H2O afforded pyrrolidines products 3 in moderate-to-high yields (Scheme 7) [20]. The CF3 radical generated from NaSO2CF3 through SET of I2O5 adds to the alkenyl group of 1,6-enynes followed by cyclization and the capture of iodine to give products 3. The CF3 radical could be trapped by 2-methyl-2-nitrosopropane (MNP) to form M-5 for ESR detection.

Scheme 7.
Synthesis of iodotriflouromethylated pyrrolidines.
A method for cyclative trifluoromethylation of 1,6-enynes was reported by the Liang group in 2014. The reaction of 1,6-enynes, Togni’s reagent, and TMSCN (or TMSN3) in CH3CN under the catalysis of CuII gave CF3-containing heterocycles 4 and 5 (Scheme 8) [21]. The CF3 radical produced from the Togni’s reagent under the catalysis of CuII adds to the C=C double bond of 1,6-enyne to form the radical intermediate M-6, which is converted to cyclized metal complex M-7 through path a or path b. At the last step, the reaction of M-7 with TMSCN or TMSN3 gives corresponding cyanotrifluoromethylated or azidotrifluoromethylated five-membered ring products 4a or 5a.
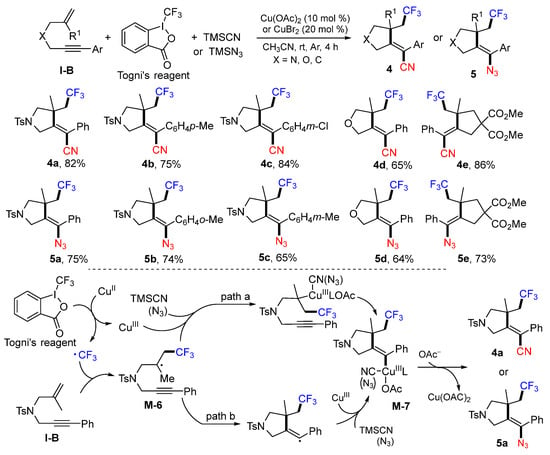
Scheme 8.
Synthesis of cyanotrifluoromethylated or azidotrifluoromethylated heterocycles.
A Togni’s reagent-based synthesis of CF3-substituted spiro 2H-azirines was reported by the Liang group in 2015. The reaction of 1,6-enynes with Togni’s reagent and TMSN3 in the presence of Cu0 powder as a catalyst afforded diastereomeric products 6 in good-to-excellent yields (Scheme 9) [22]. A proposed mechanism suggests that the CF3 radical generated from Togni’s reagent through SET of Cu0 is added to the C=C bond of 1,6-enyne to produce the radical intermediate M-8. Sequential 5-exo cyclization and trapping of the radical M-9 with CuII and TMSN3 give CuII azide complex M-10. Complex M-10 may also be obtained from the formation of complex M-11 and subsequent cyclization. Reductive elimination of M-10 followed by the elimination of N2 from azide M-12 gives alkenyl nitrene M-13. The cyclization of M-14, a resonance structure of alkenyl nitrene M-13, gives the spiroketal products 6 as a pair of diastereomers.
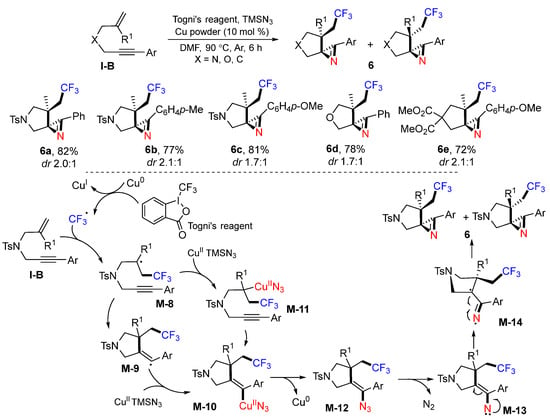
Scheme 9.
Synthesis of CF3-substituted spirocyclic 2H-azirines.
Liang’s lab introduced a method for Pd-catalyzed radical cyclative iododifluoromethylation of 1,6-enynes in 2015. The reaction of 1,6-enynes and ethyl difluoroiodoacetate in dioxane under the catalysis of Pd(PPh3)2Cl2 and bis-[2-(diphenyl-phosphino)phenyl]ether (DPE-Phos) gave iododifluoromethylated heterocycles 7 in good-to-excellent yields (Scheme 10) [23]. The CF2CO2Et radical is generated from ICF2CO2Et through the reduction of Pd0Ln. Radical addition to the C=C double bond of 1,6-enynes followed by the cyclization to PdILnI-activated alkyne group and reductive elimination of the Pd0Ln gives iododifluoromethylated products 7.
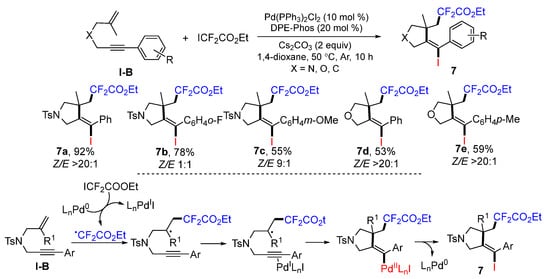
Scheme 10.
Synthesis of iododifluoromethylated heterocycles.
A sulfonyl radical-initiated iodosulfonylation reaction of 1,6-enynes was reported by the Liang group in 2016. The reaction of 1,6-enynes and sulfonyl hydrazide in the presence of I2/TBHP gave five-membered heterocycles 8 in good-to-excellent yields (Scheme 11) [24].
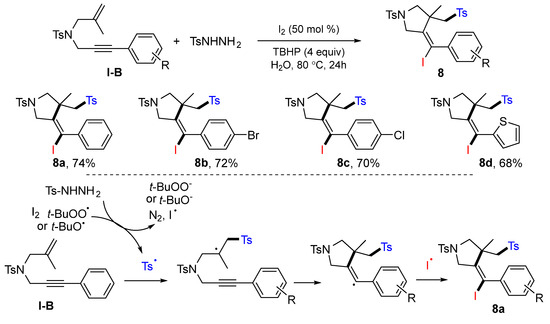
Scheme 11.
Synthesis of iodosulfonylated five-membered heterocycles.
A proposed mechanism indicated that the sulfonyl radical generated from the reaction of sulfonyl hydrazide and TBHP adds to the C=C double bond of 1,6-enyne, followed by the radical cyclization and coupling with iodine radical, to give product 8a.
In 2018, the Liang group introduced radical cyclization of 1,6-enynes for the synthesis of substituted pyrrolidine derivatives. The reaction of 1,6-enynes, ICF2CO2Et in the presence of N-methylpiperidine or borophenylic acids/K2CO3 afforded substituted pyrroles 9 or 10 in moderate-to-good yields (Scheme 12) [25]. The initial CF2CO2Et radical generated from the reaction of ICF2CO2Et adds to the C=C double bond of the 1,6-enyne followed by 5-exo cyclization to give radical intermediate M-15. Radical M-15 abstracts iodo atom from iododifluoromethylation to give product 9a; otherwise, coupling of M-15 with borophenylic acid gives product 10a.

Scheme 12.
Synthesis of functionalized pyrrolidines.
A visible light-mediated radical sulfonylative and azidosulfonylative cyclization of 1,6-enynes for the synthesis of highly functionalized heterocycles was introduced by the Lam group in 2017. The reaction of 1,6-enynes and sulfonyl azides in THF in the presence of a photoactive iridium complex afforded difunctionalized heterocycles 11 or 12 in moderate-to-excellent yields (Scheme 13) [26]. The use of THF as the solvent was critical for the success of the reactions. The reaction mechanism suggests that the sulfonyl radical generated from TsN3 under the visible light catalysis of [Ir(dtbbpy)(ppy)2]PF6 adds to the triple bond of 1,6-enyne, followed by cyclization of the vinyl radical, giving six-membered tertiary radical M-16. Product 11a is then obtained via azidation of M-16 with the arylsulfonyl azide and the sulfonyl radical is regenerated. When R1 is H, addition of the sulfonyl radical happens at the terminal carbon of the triple, followed by cyclization of the vinyl radical to give five-membered ring product 12a.

Scheme 13.
Synthesis of azidosulfonated heterocycles.
The Wu group, in 2017, introduced a reaction of 1,6-enynes with DABCO·(SO2)2 and two equivalents of ArN2BF4 in DCE to give diazosulfonated six-membered heterocycles 13 in moderate-to-good yields (Scheme 14) [27]. Five-membered heterocycles 14a could be obtained using unsubstituted terminal alkynes as the substrates. The reaction mechanism suggests that the initially sulfonyl radicals, generated from the reaction of ArN2BF4 with DABCO·(SO2)2, adds to the C≡C bond of 1,6-enynes to form vinyl radical M-18, followed by 6-exo cyclization and trapping with aryldiazonium cation to give intermediates M-19. The last step SET of arylsulfonyl radical or DABCO·(SO2)2 to radical M-19 gave products 13.
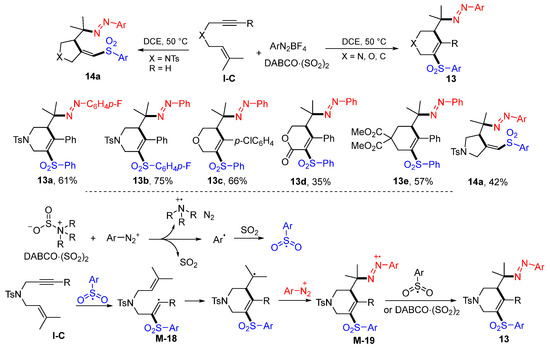
Scheme 14.
Synthesis diazosulfonated heterocycles.
The Xu group, in 2018, introduced a visible light-mediated radical atom transfer radical cyclization (ATRC) of 1,6- and 1,7-enynes for the synthesis of sulfonyl and trifluoromethylthio functionalized vinylsulfones. In the ATRC reactions, two functional groups are from the same reagent. The reaction of enynes and PhSO2SCF3 in the presence of PPh3AuNTf2 and Ru(bpy)3Cl2 under the irradiation of blue LED afforded five- or six-membered vinylsulfones 15 in good yields (Scheme 15) [28]. A proposed mechanism for the reaction of 1,6-enyne indicated that the sulfonyl radical generated from PhSO2SCF3 under photocatalysis of PPh3AuNTf2 and Ru(bpy)3Cl2 adds to the triple bond to form benzyl radical M-20, followed by 6-exo cyclization to give tertiary radical M-21. It then couples CF3S radical to give product 15a. For the reaction of a 1,6-enyne without substitution on the terminal carbon (R1 = H), sulfonyl radical adds to the terminal carbon of alkyne followed by 5-exo cyclization, leading to product 15d. A similar process for the reaction of 1,7-enyne, which has no terminal carbon substitution on alkyne, affords product 15e.

Scheme 15.
Synthesis of sulfonyl and trifluoromethylthio functionalized vinylsulfones.
A visible light-mediated ATRC of 1,6-enyne for the preparation of chloroalkyl-substituted cyclic alkenyl sulfones using sulfonyl chlorides as the key reactants was reported by the Zhu group in 2018. The reactions of 1,6-enynes and sulfonyl chlorides in the presence of [Ir(dtbbpy)(ppy)2]PF6 under the irradiation of blue LED gave five- or six-membered chloroalkyl-substituted cyclic alkenyl sulfones 16 or 17 (Scheme 16) [29]. As the reaction mechanism indicated, the sulfonyl radical generated from TsCl under the photoredox of [Ir(dtbbpy)(ppy)2]PF6 adds to the C≡C bond of the 1,6-enyne followed by 5-exo or 6-exo cyclization to form the carbon radicals M-24 or M-25. They are oxidized to carbocations M-26 and M-27 and then react with chlorine anion to form products 16 and 17, respectively.

Scheme 16.
Synthesis of five- and six-membered alkenyl sulfones.
In 2018, the Liu group reported the synthesis of bromotrifluoromethylated five- and six-membered heterocycles. The reaction of 1,6- or 1,7-enynes, NaSO2CF3 and NaBrO3 in DCM/H2O produced products 18 in good yields (Scheme 17) [30]. The CF3 radical, generated from the reaction of NaSO2CF3 and NaBrO3, adds to the terminal carbon of alkene followed by 5-exo or 6-exo cyclization (n = 2) and then Br-atom abstraction to give product 18.
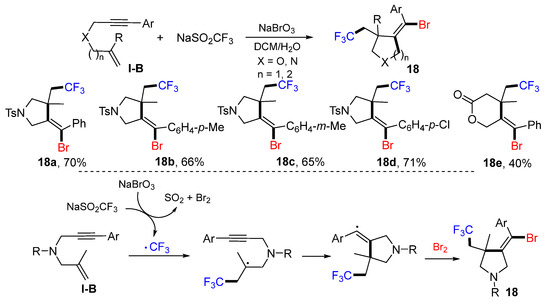
Scheme 17.
Synthesis of bromotrifluoromethylated five- and six-membered heterocycles.
Lin and coworkers reported an electrochemical reaction for the preparation of chlorotrifluoromethylated pyrrolidines in 2018. The reaction was carried out using HOAc-MeCN as solvent at room temperature under electrochemical conditions. The reaction of 1,6-enynes, CF3SO2Na and MgCl2 in the presence of LiClO4 and Mn(OAc)2 gave chlorotrifluoromethylated pyrrolidines 19 in excellent yields (Scheme 18) [31]. The initial CF3 radical generated from the anodically coupled electrolysis adds to the C=C double bond of 1,6-enynes followed by 5-exo cyclization to afford the vinyl radical M-28, which couples with the Cl radical to give product 19.

Scheme 18.
Synthesis of chlorotrifluoromethylated pyrrolidines.
A visible light-promoted reaction of 1,6-enynes for the synthesis of difunctionalized pyrrolidines was introduced by the Wang group in 2020. The reaction of 1,6-enynes, and chalcogens (such as benzenesulfono–selenoate) in acetone at room temperature under the radiation of blue LED afforded products 20 in moderate-to-good yields (Scheme 19) [32]. The reaction mechanism suggests that tosyl and phenylselenyl radicals are generated from Se-phenyl 4-methylbenzenesulfonoselenoate under photo irradiation. The tosyl radical adds to the C=C bond of 1,6-enyne followed by 5-exo cyclization and capture of phenylselenyl radical to give product 20a.
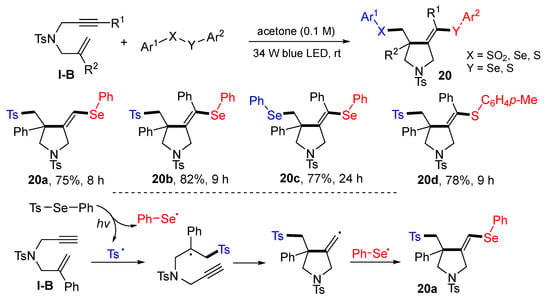
Scheme 19.
Synthesis of functionalized pyrrolidines.
An iodine radical-initiated reaction for the synthesis of difunctionalized N-heterocyclic compounds was reported by the Wang group in 2020. The reactions of 1,6- or 1,7-enynes, TBHP and I2 in CH3CN gave compound 21 in moderate-to-good yields (Scheme 20) [33]. The reaction mechanism suggests that iodide radical, generated from the reaction of I2 with TBHP, adds to the C≡C triple bond of enyne followed by 6-exo cyclization to yield tertiary radical M-29. Addition of hydroxyl radical or t-butylperox radical to M-29 could lead to the formation of product 21a.

Scheme 20.
Synthesis of iodo and hydroxy-functionalized N-heterocyclics.
In 2021, Zhu and co-workers reported the synthesis of iodo- and nitro-functionalized cyclic compounds such aspyrrolidines, tetrahydrofurans, and cyclopentanes. The reaction of 1,6-enynes, t-BuONO, and iodoform in CH3CN under heating gave five-membered heterocycles 22 in moderate-to-excellent yields (Scheme 21) [34]. The reaction mechanism suggests that nitroso radical formed from the homolysis of t-BuONO adds to the C=C bond of the 1,6-enyne followed by 5-exo cyclization, oxidation to cation, and then iodination with CHI3 to give product 22a.
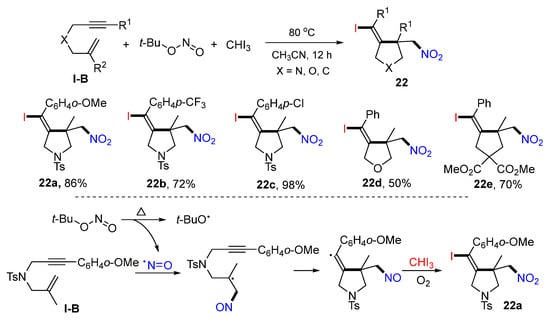
Scheme 21.
Synthesis of iodo- and nitro-functionalized cyclic compounds.
In 2021, Zhu and co-workers reported diarylselenylative cyclization reaction of 1,6-enynes for the synthesis of five-membered heterocycles. The reaction of 1,6-enyne and diaryldiselane in toluene under the radiation of light at room temperature afforded products 23 in moderate to good yields (Scheme 22) [35]. The reaction mechanism shows that the PhSe radical generated via photo homolytic cleavage of PhSeSePh adds to the triple bond of 1,6-enyne followed by 5-exo cyclization to form tertiary carbon radical M-30, which then couples with PhSe radical to give product 23a.
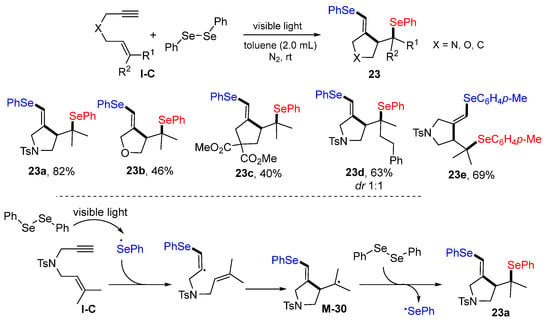
Scheme 22.
Synthesis of diarylselenylated five-membered rings.
The reaction of 1,6-enynes for the synthesis of dihalogenated pyrrolidines was reported by the Tong group in 2021. The reaction of 1,6-enynes, PhI(OAc)2 and lithium halide at room temperature gave product 24 in moderate-to-good yields (Scheme 23) [36]. A suggested mechanism for the reaction with LiCl indicated that the Cl radical generated via a single electron oxidation of LiCl with PhI(OAc)2 adds to the C=C double bond of 1,6-enyne followed by 5-exo cyclization and Cl atom abstraction to give dichloro pyrrolidine 24d.
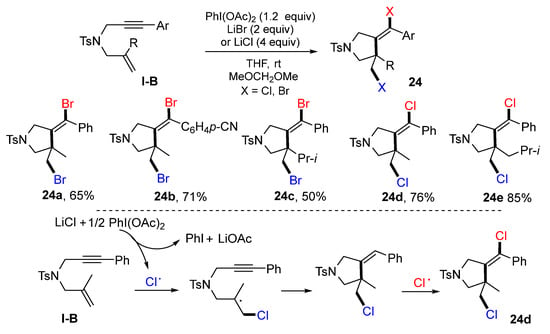
Scheme 23.
Synthesis of dihalogenated pyrrolidines.
In 2021, Li and Tian’s lab reported Fe-catalyzed radical reaction of 1,6-enynes for the synthesis of difunctionalized heterocycles. The reaction of 1,6-enynes, t-butyl nitrite (TBN) and KI or NaBr as materials in CH3CN under the catalysis of FeSO4·7H2O gave products 25 in good-to-excellent yields (Scheme 24) [37]. As shown in the proposed mechanism, NO2 radical produced from TBN adds to the C=C bond of 1,6-enyne followed by 5-exo cyclization to give vinyl radical. This radical intermediate is iodinated through two possible pathways to give target product 25a.
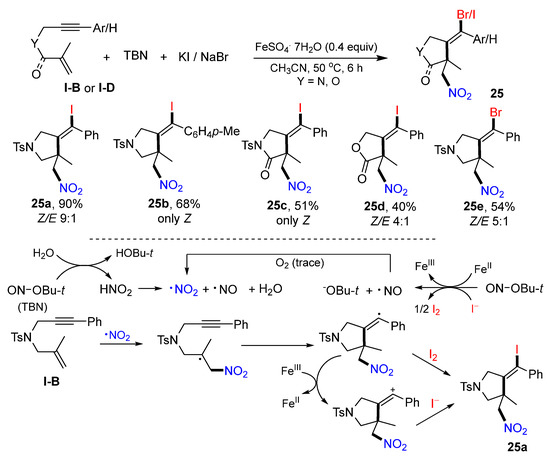
Scheme 24.
Synthesis of nitrohalogenated heterocyclic compounds.
A Cu-catalyzed radical reaction of 1,6-enynes for the synthesis of cyanoalkylsulfonyl-ated pyrrolidines was introduced by He and coworkers in 2021. The reaction of 1,6-enynes, diselenides, DABCO(SO2)2 and cyclic ketone oxime esters in DCE with CuOAc as a catalyst afforded functionalized pyrrolidines 26 in moderate-to-good yields (Scheme 25) [38]. As indicated in the proposed mechanism, cyanoalkylsulfonyl radical generated from the reaction of cyclic ketone oxime esters and DABCO(SO2)2 adds to the C=C double bond of 1,6-enyne followed by 5-exo cyclization and then couples with PhSe radical to give product 26a.

Scheme 25.
Synthesis of cyanoalkylsulfonylated pyrrolidines.
In 2019, Zhu and Hou’s group reported a visible light-mediated radical reaction for the synthesis of chlorotrifluoromethylated and chlorotrichloromethylated pyrrolidines, cyclopentanes and related compounds. The reaction of 1,6-enynes and CF3SO2Cl (or CCl3SO2Cl) in CH2Cl2 using Acr+-Mes or Ir(dtbbpy(ppy)2PF6 as a photocatalyst gave products 27 in good-to-excellent yields (Scheme 26) [39]. A proposed mechanism indicated that CF3 radical generated from CF3SO2Cl via SET adds to the C=C bond of 1,6-enynes, followed by 5-exo cyclization and coupling with Cl radical, to give product 27a.

Scheme 26.
Synthesis of functionalized five-membered rings.
In 2022, Li and Yang reported a visible light-promoted reaction of 1,6-enynes for the synthesis of the iodovinyl- and CF2-functionalized heterocycles. The reaction of 1,6-enynes, ICF2CO2Et under the radiation of blue LED afforded products 28 in good-to-excellent yields (Scheme 27) [40]. The reaction mechanism suggests that CF2CO2Et radical derived from ICF2CO2Et adds to the C=C double bond of 1,6-enyne, followed by 5-exo cyclization and capture of iodine atom from ICF2CO2Et, to give product 28.

Scheme 27.
Synthesis of iodovinyl- and CF2-functionalized heterocycles.
Zhu and co-workers, in 2022, reported a photo synthetic method for making iodo- and sulfonyl-containing cyclic compounds. The reaction of 1,6-enynes, ArSO2Na, and iodoform in CH3CN under visible light irradiation gave products 29 in good-to-excellent yields (Scheme 28) [41]. The reaction mechanism suggests that ArSO2 radical derived from ArSO2Na adds to the C=C double bond of 1,6-enyne, followed by 5-exo cyclization and iodine atom transfer from the complex of ArSO2Na and CHI3, to give product 29a.

Scheme 28.
Synthesis of iodo- and sulfonyl-containing cyclic compounds.
In 2022, a photo reaction of β-caryophyllene, a 1,5-diene with one alkene in the ring and another one out of the ring, for the synthesis of iodo- and CF2-containing protoilludanes was reported by the Huang group. The reaction of β-caryophyllene and ICF2COR in the presence of 2-bromophenol and base under the irradiation of blue LED afforded functionalized protoilludanes 30 in excellent yields (Scheme 29) [42]. A reaction mechanism suggests that the EDA complex generated from 2-bromophenol and ICF2COR leads to the formation of CF2COR radical. It then selectively adds to C8 of β-caryophyllen, followed by the cyclization and abstraction of iodine atom from ICF2COR to give the product 30.
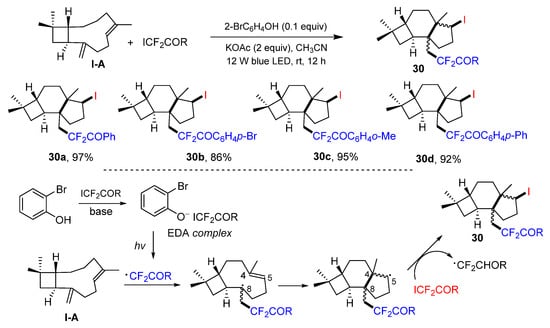
Scheme 29.
Synthesis of iodo- and CF2-containing protoilludanes.
In 2019, the Liu group reported a met-catalyzed reaction of 1,6-enynes or 1,6-enynyl amides for the synthesis of bromotrihalomethylated pyrrolidines. The reaction of 1,6-enynes, and CCl3Br or CBr4 in 1,4-dioxane under the catalysis of [Rh(cod)Cl]2 and DPE-Phos at 100 °C for 12 h gave products 31 in moderate-to-good yields (Scheme 30) [43]. The reaction mechanism suggests that CCl3 radical, generated from CCl3Br under the catalysis of [Rh(cod)Cl]2 and DPE-Phos, adds to the C=C double bond of 1,6-enyne followed by 5-exo cyclization to RhII-LBr activated alkyne and then L-RhI elimination to give product 31a.
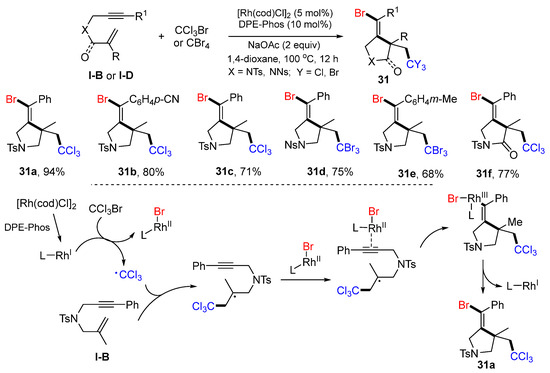
Scheme 30.
Synthesis of bromotrihalomethylated pyrrolidines.
Hou and coworkers, in 2022, reported a Cu-induced radical reaction of 1,6-enynes for the synthesis of functionalized five-membered rings. The reaction of 1,6-enynes, BrCH2CN in the presence of CuI, 1,10-phenanthroline and NaHCO3 in CH3CN afforded products 32 in good yields (Scheme 31) [44]. The reaction mechanism suggests that the CH2CN radical derived from BrCH2CN adds to C=C double bond of 1,6-enyne followed by 5-exo cyclization and bromine atom-transfer to give product 32a.
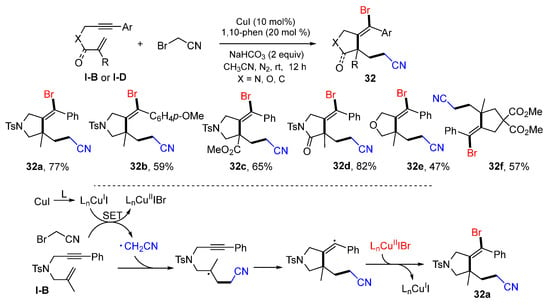
Scheme 31.
Synthesis of functionalized five-membered rings.
1,6-Eneynyl amides are another kind of popular substrates for radical reactions in the synthesis of functionalized 2-pyrrolidones [45]. In 2008, Feray and Bertrand reported an R2Zn-mediated radical reaction of 1,6-eneynyl amides for the synthesis of functionalized pyrrolidin-2-ones. The reaction of 1,6-eneynyl amides and alkyliodides in the presence dialkylzinc at room temperature gave product 33 in high yields as a mixture of E/Z isomers (Scheme 32) [46]. The reaction mechanism suggests that the t-butyl radical, generated from the reaction of t-BuI and R2Zn in the presence of oxygen, selectively adds to the triple bond of amide to form a stabilized vinyl radical, which then undergoes 5-exo cyclization followed by iodine atom transfer from t-BuI to give product 33.
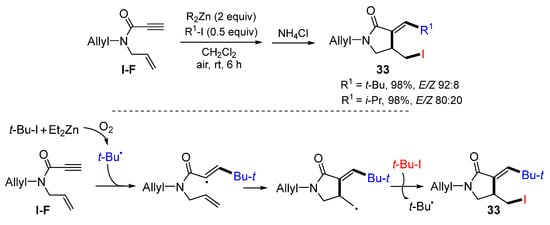
Scheme 32.
Synthesis of functionalized pyrrolidin-2-ones.
Xuan and co-workers introduced a reaction of 6-enynyl amides for the synthesis of substituted 2-pyrrolidinones in 2018. The reaction of 6-enynyl amides, NIS (or NBS), and sulfonyl hydrazide in CH3CN and in the presence TBHP afforded γ-lactams 34 in good to excellent yields (Scheme 33) [47]. The reaction mechanism suggests that sulfonyl radical generated from arylsulfonyl hydrazide adds to the C=C double bond of amide followed by 5-exo cyclization and then coupling with iodine radical to give product 34a.
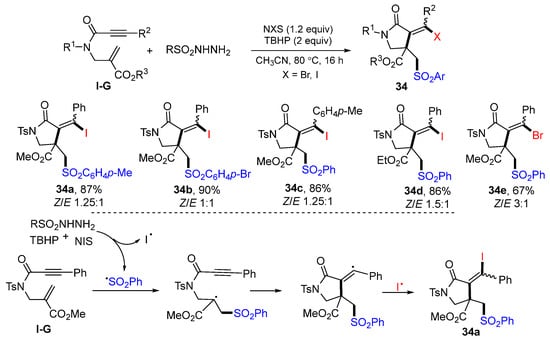
Scheme 33.
Synthesis difunctionalized γ-lactams.
Wei and co-workers reported a protocol of cyclative chloroazidation of 1,6-enynyl amides for the synthesis of substituted 2-pyrrolidinones in 2018. The reaction of 1,6-enynyl amides, TMSN3 and NCS in DCE in the presence of PIDA gave product 35 in moderate yields (Scheme 34) [48]. The reaction mechanism suggests that N3 and Cl radicals were generated from TMSN3 and NCS. The addition of N3 radical to the C=C double bond of amide followed by 5-exo cyclization and coupling with the Cl radical affords product 35a.
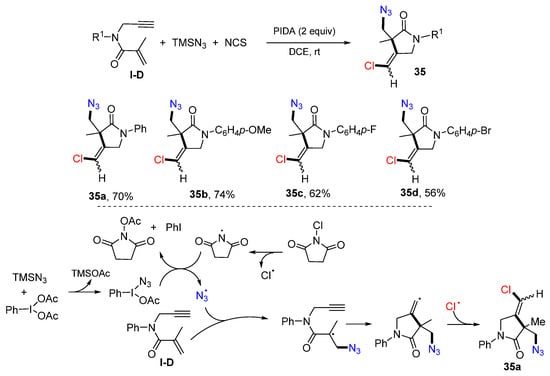
Scheme 34.
Synthesis of difunctionalized pyrrolidin-2-ones.
In 2022, Li and coworkers reported a reaction of 1,6-enynyl amides for the synthesis of γ-lactams. The reaction of 1,6-enynyl amides and sulfonyl hydrazides in H2O at 70 °C for 20 h in the presence of TBHP gave product 36 in moderate-to-good yields (Scheme 35) [49]. The reaction mechanism suggests that PhSO2 radical, generated from the reaction of PhSO2NHNH2 with TBHP and TBAI, adds to the C=C double bond of amide followed by 5-exo cyclization and coupling with iodine radical to give product 36a.

Scheme 35.
Synthesis difunctionalized γ-lactams.
A photoredox ATRC reaction of 1,6-dienyl amides for the synthesis of functionalized pyrrolidin-2-ones was developed by the Miyabe group in 2015. The reaction of 1,6-dienyl amides and iodoalkanes in aqueous media and catalyzed by Ru(bpy)3Cl2·6H2O and (i-Pr)2NEt gave product 37 in fair-to-good yields (Scheme 36) [50]. Other than i-C3F7I, other iodo compounds such ICH2CN and ICH2CF3 are also good radical precursors. The reaction mechanism suggests that the i-C3F7 radical generated from i-PrI via the photoredox process adds to the C=C double bond of amide, followed by 5-exo cyclization and then iodine atom transfer from i-PrI to give product 37a.
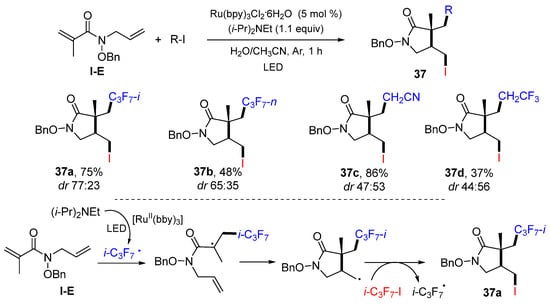
Scheme 36.
Synthesis of difunctionalized pyrrolidin-2-ones.
Li and Wei, in 2021, reported a Cu-catalyzed radical reaction of 1,6-dienyl amides for the synthesis of substituted γ-lactams. The reaction of 1,6-dienyl amides and RSO2NHNH2 in CH3CN in the presence of CuI and TBHP gave product 38 in moderate-to-good yields (Scheme 37) [51]. The reaction mechanism suggests that the sulfonyl radical, generated from the reaction of RSO2NHNH2 with TBHP, adds to the C=C double bond of amide followed by 5-exo cyclization, oxidation to carbocation, and trapping I− anion of CuI to provide iodosulfonylation of product 38a.

Scheme 37.
Synthesis difunctionalized γ-lactams.
A photoredox reaction of carbonyl-containing 1,6-enynes for the synthesis of cyclopentanone derivatives was reported by Zhou, Yu and their coworkers in 2020. The reaction of gem-dialkylthio enynes, cyclobutanone oxime esters, and boronic acids in the presence of Cu(CH3CN)4BF4, dtbbpy and K3PO4 in CH3CN under irradiation of blue LED gave functionalized aryl thienyl sulfide 39 in moderate-to-good yields and with good chemo- and diastereoselectivities (Scheme 38) [52]. The reaction mechanism suggests that γ-cyanoalkyl radical, generated from homolytic α,β-C−C cleavage of N-centered iminyl, which is derived from cyclobutanone oxime esters, adds to the C=C bond of gem-dialkylthio 1,3-enyne followed by 5-exo cyclization, radical rearrangement and fragment of ethylene to give sulfur-centered radical M-31. Radical M-31 reacts with the LCuIIPh complex followed by reductive elimination to give product 39a.
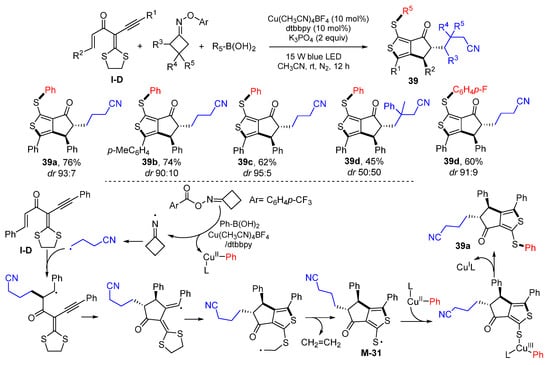
Scheme 38.
Synthesis of aryl thienyl sulfides.
A reaction of 1,6-enynyl with two carbonyl groups for the synthesis of functionalized succinimides was introduced by the Rong group in 2020. The reaction of 1,6-enynyl amides, NBS or NCS, TMSN3, and PIDA in DCM at room temperature for 3–5 min afforded products 40 as E/Z isomers in excellent yields (Scheme 39) [53]. The reaction mechanism suggests that the azide radical, resulting from the reaction of PIDA and TMSN3, adds to alkene moiety of 1,6-enyne, followed by 5-exo cyclization and coupling with the bromine radical from NBS, to give product 40a.
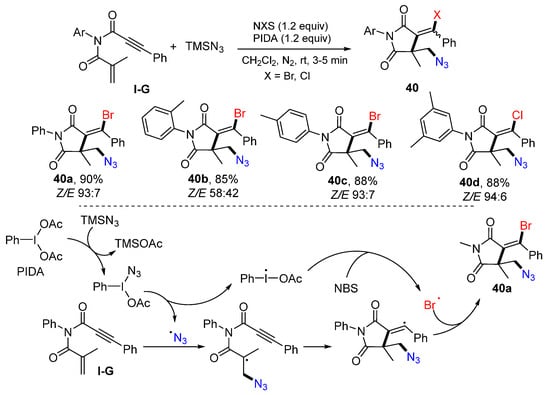
Scheme 39.
Synthesis of functionalized succinimides.
3. Reaction of Arene-Tethered Dienes and Enynes
Presented in this section are the radical addition and cyclization-initiated difunctionalization reactions of arene-bridged 1,n-dienes -diynes, and -enynes with a reaction sequence shown in Scheme 40. It is noteworthy that most substrates found in the literature are enynes but not dienes (like II-J) or diynes (like II-I) (Scheme 41). The enynyl substrates include the most popular 1,7-enynyl amides II-A and other ones containing the carbonyl group (II-B to II-E). Other substrates may contain heteroatom or conjugate groups (such as CN and Ar) at the terminal carbon of the unsaturated bonds (II-F to II-H). Between the two unsaturated carbon–carbon bonds in the substrates, the regioselectivity for the initial radical addition is directed by the steric and the conjugation effects of the substituents. The R1 group on the terminal carbon of alkyne is commonly employed to block the initial radical addition to the alkyne. Substrate II-J is an exception in which the initial radical addition does not go to the conjugated alkene.
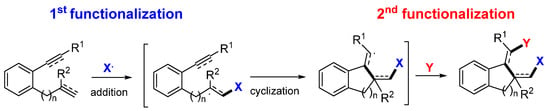
Scheme 40.
General reaction for the difunctionalization of arene-bridged dienes and enynes.
Scheme 41.
Arene-bridged enynes, dienes, and diynes with pointed position for the initial radical addition.
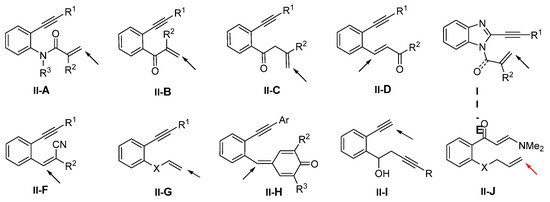
Benzene-tethered 1,7-enynyl amides are popular substrates for radical difunctionalization reactions. In 2014, the Li group introduced a reaction of such substrates for the synthesis of dinitropyrrolo[4,3,2-de]-quinolinones. The reaction of 1,7-enynyl amides and t-BuONO in DMSO afforded product 41 in good-to-excellent yields (Scheme 42) [54]. It was found that the amount of H2O had a significant influence on the reaction. The reaction mechanism suggests that NO2 radical generated in situ from t-BuONO adds to the C=C double bond of amide followed by 6-exo cyclization to form intermediate M-32. The reaction of M-32 with NO or NO2 radical followed by electrophilic addition of NO or NO2 radical to the phenyl ring gave cationic intermediates M-33 and M-34. Cationic radical intermediates M-35 and M-36 were produced through the treatment of the cationic intermediates M-33 and M-34 with NO or NO2 radical and then lead to the formation of product 41a after the redox reaction.
Scheme 42.
Synthesis of dinitropyrrolo[4,3,2-de]-quinolinones.
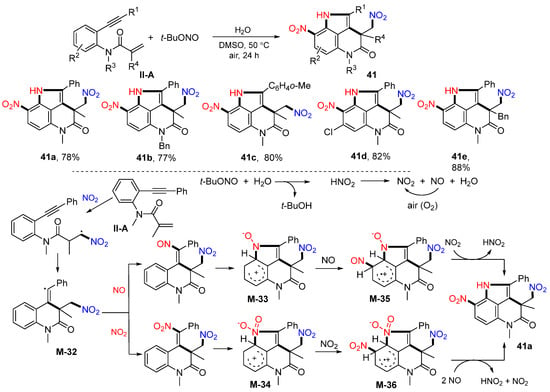
The Wu group, in 2016, introduced a photoredox reaction of benzene-tethered 1,7-enynyl amides for the synthesis of trifluoroethyl-substituted 3,4-dihydroquinolin-2(1H)-ones. The reaction of 1,7-enynyl amides and Togni’s reagent in the presence of NaI and PhCO2H under UV irradiation gave 42 in moderate-to-good yields (Scheme 43) [55]. The proposed mechanism indicated that trifluoromethyl radical derived from the Togni’s reagent adds to the C=C double bond of amide, followed by 6-exo cyclization and oxidation to cation for the reaction with iodide anion, to give product 42.
Scheme 43.
Synthesis of iodotrifluoromethylated 3,4-dihydroquinolin-2(1H)-ones.
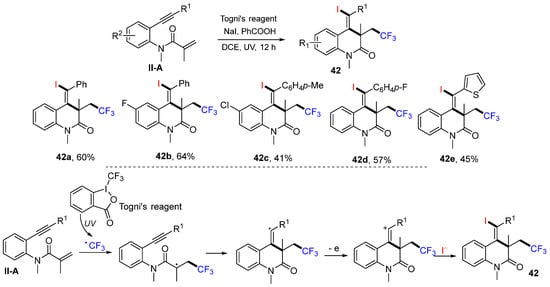
In 2016, the Jiang group reported a reaction of benzene-bridged 1,7-enynyl amides for the synthesis of substituted 3,4-dihydroquinolin-2(1H)-ones. The reaction of 1,7-enynyl amides, TMSN3 and NIS (or NBS and NCS) in the presence of PhI(OAc)2 in CH2Cl2 gave products 43 in good-to-excellent yields (Scheme 44) [56]. A reaction mechanism suggests that N3 radical generated from the reaction of PhI(OAc)2 and TMSN3 adds to the C=C double bond of amide followed by 6-exo cyclization and coupling with iodine radical from NIS to give product 43.
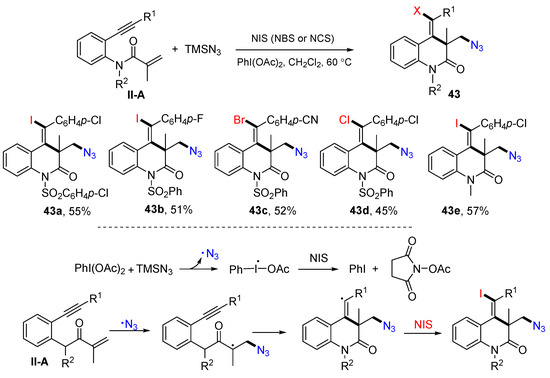
Scheme 44.
Synthesis of 3,4-dihydroquinolin-2(1H)-ones.
A transition metal-mediated radical reaction of benzene-bridged 1,7-enynyl amides for the synthesis of substituted pyrrolo[3,4-c]quinolinones was reported by the Wan group in 2016. The trans-fused products were obtained when using MnIII as a catalyst, whereas cis-products were obtained using CuII as a catalyst. The reactions of amides and TMSN3 in the presence of Mn(OAc)3/NFSI or Cu(ClO4)2/TBPB in CH3CN afforded trans- or cis-fused products 44, respectively, in good-to-excellent yields (Scheme 45) [57]. A reaction mechanism suggests that N3 radical generated from TMSN3 adds to the C=C double bond of amides followed by 6-exo cyclization, releasing of N2, then azido group transfer to afford the desired trans- or cis-fused product 44.
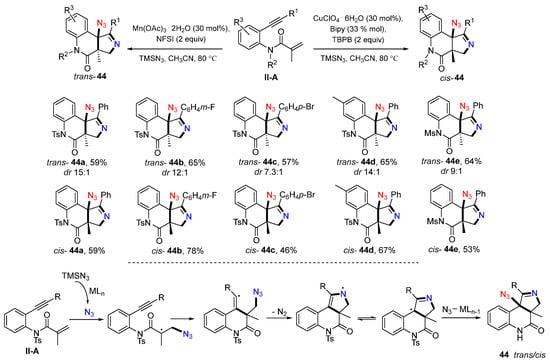
Scheme 45.
Synthesis of azido-substituted pyrrolo[3,4-c]quinolinones.
The Tu group reported a method for the synthesis of densely functionalized 3,4-dihydro-quinolin-2(1H)-ones in 2016. The reaction of benzene-tethered 1,7-enynyl amides, arylsulfonyl hydrazides and NIS (or NBS) in DEC in the presence of TBHP afforded product 45 in good-to-excellent yields (Scheme 46) [58]. The reaction mechanism suggests that the sulfonyl radical derived from sulfonyl hydrazides adds to the C=C double bond of amides, followed by 6-exo cyclization and coupling with iodine radical from NIS, to give product 45.
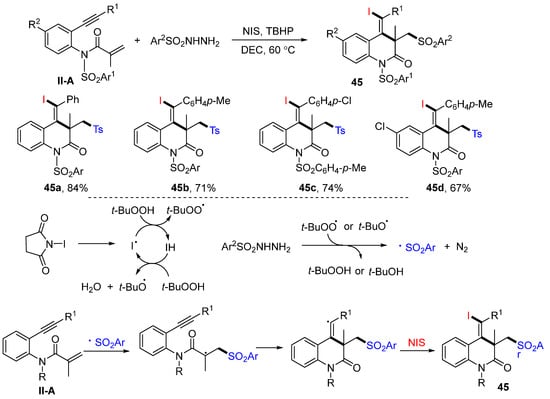
Scheme 46.
Synthesis of functionalized 3,4-dihydroquinolin-2(1H)-ones.
A new method for the synthesis of 3,4-dihydroquinolin-2(1H)-ones was reported by the Guo group in 2017. The reaction of benzene-tethered 1,7-enynyl amides, sulfinic acids and diphenyl diselenides in EtOH-H2O and in the presence of TBHB to give product 46 in moderate-to-excellent yields (Scheme 47) [59]. Carrying out the reaction under micro flow conditions could reduce the reaction time to less than 1 min. The reaction mechanism suggests that the sulfonyl radical, produced from the arylsulfinic acid with the oxidation of TBHP, adds to the C=C double bond of amide followed by 6-exo cyclization and coupling with phenylselenyl radical to give product 46a.
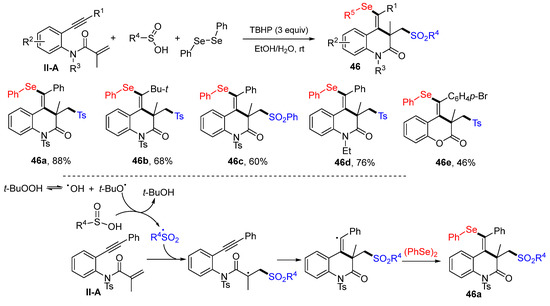
Scheme 47.
Synthesis of functionalized 3,4-dihydroquinolin-2(1H)-ones.
A Cu-catalyzed radical trifluoromethylative spirocyclization reaction of benzene-tethered 1,7-enynyl amides for the synthesis of trifluoromethyl-substituted 1′H-spiro-[azirine-2,4′-quinolin]-2′(3′H)-ones was introduced by the Han group in 2017. The reaction of amides, Togni’s reagent and TMSN3 in DMF and in the presence of CuII catalyst gave product 47 in good-to-excellent yields (Scheme 48) [60]. The reaction mechanism suggests that the CF3 radical from Togni’s reagent adds to the C=C double bond of amides; then, it goes through path a or b to give cyclized CuIII-azido complex M-37, followed by reductive catalyst elimination and denitrogenative cyclization to give product 47.
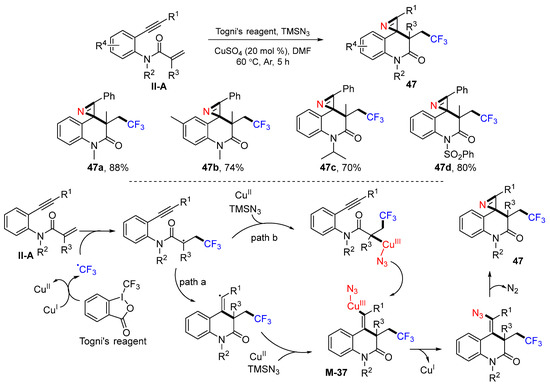
Scheme 48.
Preparation of spiro[2,5]azirinequinolinones.
The Guo group, in 2019, reported two photoredox methods for the synthesis of trifluoroethyl-substituted 3,4-dihydroquinolin-2(1H)-ones. Method 1 is the reaction of 1,7-enynyl amides, CF3SO2Na, NCS (or NBS) using photocatalyst N-methyl-9-mesityl acridinium (Mes-Acr+). Method 2 is the reaction of 1,7-enynyl amides and CF3SO2Cl using photocatalyst fac-Ir(ppy)3. These two methods gave product 48 in moderate-to-excellent yields (Scheme 49) [61]. The proposed reaction mechanism indicated that for method 1, the CF3 radical generated from the CF3SO2Na under the photocatalysis of Mes-Acr+ adds to the C=C bond of amide followed by 6-exo cyclization and coupling with bromo radical from NBS to give product 48d. In method 2, the CF3 radical generated from the CF3SO2Cl under the photocatalysis of fac-Ir(ppy)3 goes through similar addition, cyclization and halogen atom abstraction processes to afford product 48a.

Scheme 49.
Synthesis of trifluoromethylated 3,4-dihydroquinolin-2(1H)-ones.
A visible light-induced radical reaction for the synthesis of haloperfluorinated N-heterocycles was reported by the Tang group in 2019. The reaction of 1,6- or 1,7-enynyl amides, perfluoroalkyl iodides/bromides in 1,4-dioxane and in the presence of fac-Ir(ppy)3 and K3PO4 under blue LED irradiation afforded product 49 in good yields and stereoselectivity (Scheme 50) [62]. The reaction mechanism suggests that n-C4F9 radical generated under the photocatalysis with of fac-Ir(ppy)3 adds to the C=C bond of amide, followed by 6-exo cyclization and coupling with iodine radical, to selectively give product 49a as the Z-isomer.
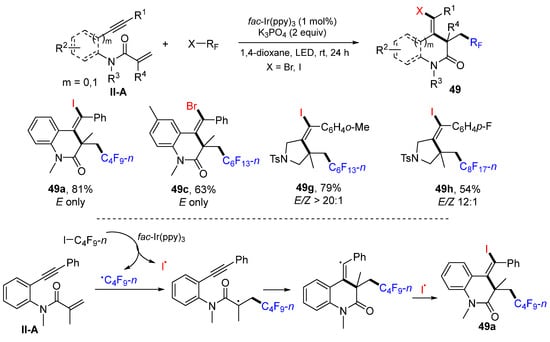
Scheme 50.
Synthesis of haloperfluorinated N-heterocycles.
The Andrade group reported an ultrafast Fe-promoted reaction for the synthesis of 2-quinolinone-fused γ-lactones in 2021. The reaction of benzene-tethered 1,7-enynyl amides and formamide and Fenton’s reagent under microwave irradiation for 10 s gave product 50 in a good overall yield (Scheme 51) [63]. The reaction mechanism suggests that the hydroxyl radical generated from Fenton’s reaction adds to the C=C double bond of amide followed by 6-exo cyclization, coupling with hydroxyl radical, epoxidation, and lactonization to give product 50a.
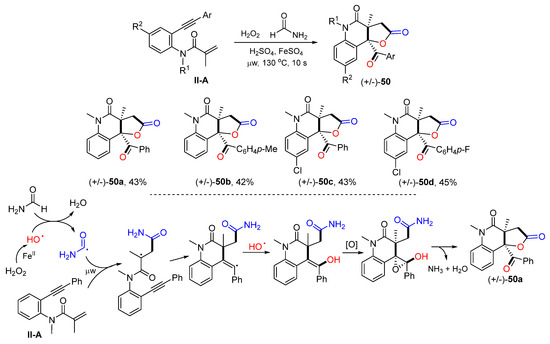
Scheme 51.
Synthesis of 2-quinolinone-fused γ-lactones.
In 2022, Wu, Ying and their coworkers introduced a Pd-catalyzed reaction for the synthesis of perfluoroalkyl and carbonylated 3,4-dihydroquinolin-2(1H)-ones. The reaction of 1,7-enynyl amides, perfluoroalkyl iodides, alcohols and benzene-1,3,5-triyl triformate (TFBen) in PhCF3 and in the presence of PdCl2(Ph3P)2, DPEphos, NIS, and Cs2CO3 gave product 51 in high yields with excellent E/Z selectivity (Scheme 52) [64]. In this reaction, TFBen was used as the CO source and alcohols when making the ester products. A reaction mechanism suggests that the n-C4F9 radical derived from n-C4F9I adds to the C=C double bond of amide followed by 6-exo cyclization, incorporation with the Pd-catalyst, CO insertion, and esterification with MeOH to afford product 51a.
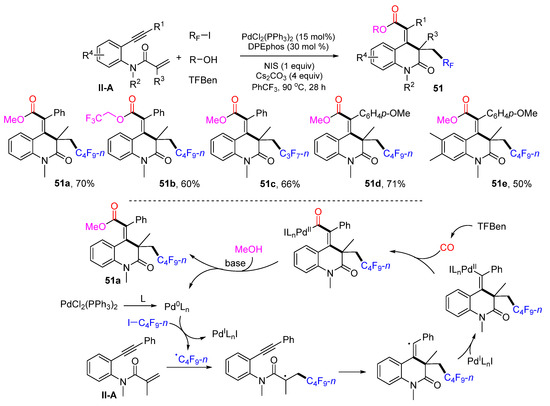
Scheme 52.
Synthesis of perfluoroalkyl and carbonylated 3,4-dihydroquinolin-2(1H)-ones.
Benzene-linked 1,6-eneynyl ethers are a class of good substrates for radical difunctionalization. Li and coworkers reported a reaction of such substrates for the synthesis of dicarbonylated benzofurans in 2015. The reaction of benzene-linked 1,6-eneynyl ethers, 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), t-BuONO and O2 in DMF at 40 °C for 8 h gave product 52 in moderate-to-good yields (Scheme 53) [65]. Two oxygen atoms were introduced to the product from O2 and TEMPO, respectively. t-BuONO is a key reagent which provides NO2 and NO after decomposition of HNO2. The reaction mechanism suggests that the addition of TEMPO to the C=C double bond of ethers followed by 5-exo cyclization, trapping of O2, oxidative cleavage of the N-O bond to release 2,6,6-tetramethyl-1-nitroso-piperidine, and O-O bond cleavage/isomerization to afford product 52a.
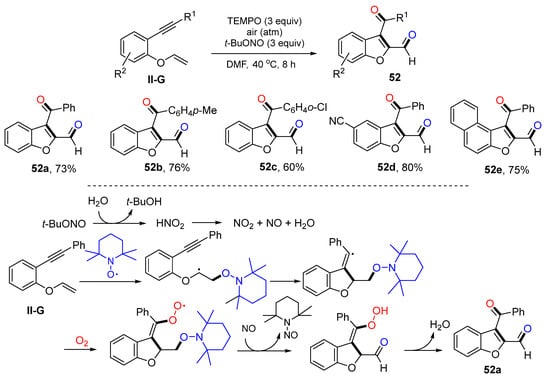
Scheme 53.
Synthesis of dicarbonylated benzofurans.
An Ag-catalyzed reaction of 1,6-eneynyl ethers for the synthesis of sulfonyl-methylated benzofurans was reported by Wu, Jiang and their coworkers in 2017. The reaction of benzene-linked 1,6-eneynyl ethers and sodium sulfinates in CH3CN and in the presence of K2S2O8 and AgNO3 afforded product 53 in moderate-to-good yields (Scheme 54) [66]. The reaction mechanism suggests that the sulfonyl radical generated from the oxidation of PhSO2Na adds to the C=C double bond of ethers followed by 5-exo cyclization, oxidation to cation, nucleophilic addition of H2O, and enol/ketone isomerization to give product 53a.
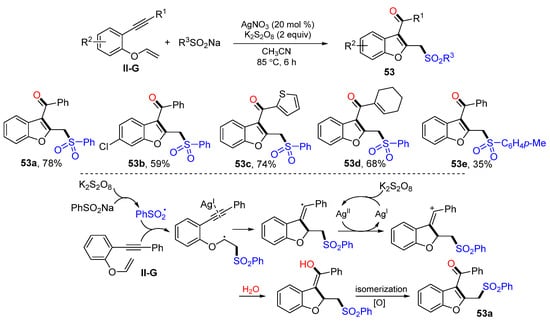
Scheme 54.
Synthesis of carbonyl and sulfonylmethylated benzofurans.
In 2017, Kumar and coworkers reported a visible light-induced reaction for the synthesis of trifluoromethylacylated benzofurans, benzothiophenes, and indoles. The reaction of 1-ethynyl-2-(vinyloxy)-benzenes and CF3SO2Na in CH3CN/H2O using phenanthrene-9,10-dione (PQ) as a photoredox catalyst gave heterocycles 54 in good yields (Scheme 55) [67]. The proposed reaction mechanism suggests that the CF3 radical, generated from CF3SO2Na with photo-activated PQ, adds to the C=C double bond of 1-ethynyl-2-(vinyloxy)-benzenes followed by 5-exo cyclization, electron transfer from PQH radical, H2O addition and deprotonation, resulting in product 54.
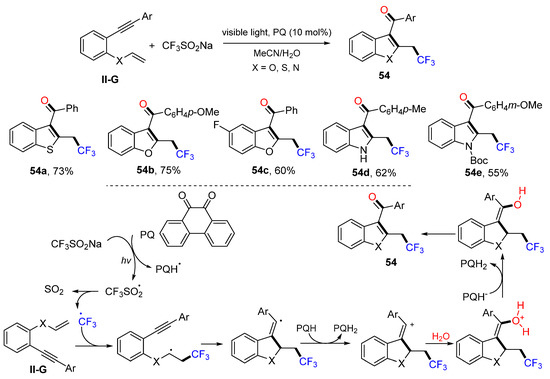
Scheme 55.
Synthesis of trifluoromethylated and acylated heterocycles.
A reaction of 1,6-eneynyl ethers for the synthesis of sulfonylacylated benzofurans was introduced by the Sun group in 2018. The reaction of oxygen-linked 1,6-enynes, DMSO and H2O in the presence of NH4I afforded product 55 in moderate-to-high yields (Scheme 56) [68]. A reaction mechanism suggests that the reaction between DMSO and NH4I produced MeS and OH radicals. Addition of MeS radical to the C=C double bond of ethers followed by 5-exo cyclization, OH radical coupling, axidation of sulfide, and keto-enol tautomerism resulted in product 55a.
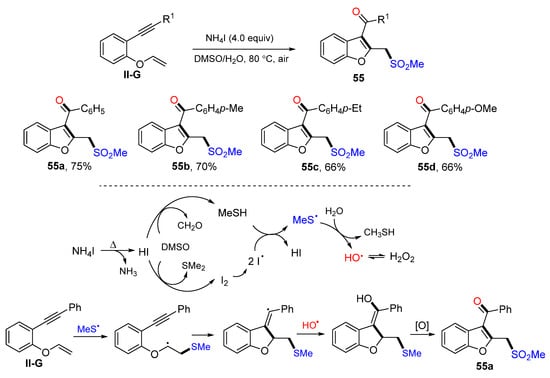
Scheme 56.
Synthesis of difunctionalized benzofurans.
In 2020, the Zhang group introduced a Pd-catalyzed radical oxidative aryldifluoroalkylation of benzene-tethered 1,6-enynes for the synthesis of difluoroalkylated benzofuran, benzothiophene, and indole derivatives. The reaction of 1,6-enynes, ethyl difluoroiodoacetate and arylboronic acids 1,4-dioxane or DCE under the catalysis of PdCl2(PhP3)2 and DPE-phos gave product 56 in moderate-to-good yields (Scheme 57) [69]. The resultant products can be converted into aromatic five-membered rings 57 via Fe(OTf)3-catalyzed isomerization. A reaction mechanism suggests that the CF2CO2Et radical generated from ICF2CO2Et adds to the C=C double bond of 1,6-enyne followed by 5-exo cyclization to form M-38 and then reacts with PdII to form intermediate M-39. Intermediate M-39 could also be generated from M-38 through iodine transfer with ICF2CO2Et and then with Pd0. Coupling M-39 with phenylboronic acid finishes the reaction and gives product 56a.
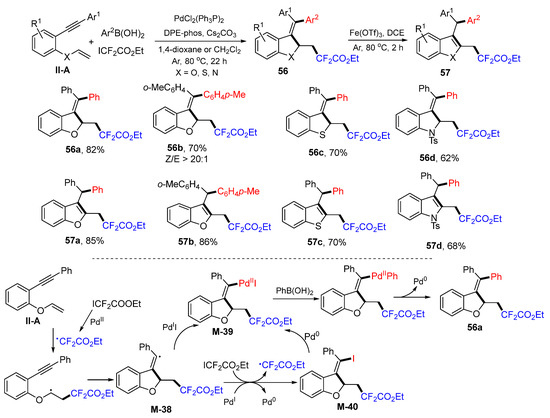
Scheme 57.
Synthesis of aryldifluoroalkylated heterocycles.
A Cu-catalyzed radical reaction of benzene-tethered 1,6-enynes for the synthesis of trifluoroethylated dihydrobenzofurans was reported by the Jiang group in 2019. The reaction of 1,6-enynes, Togni’s reagent, CO2 and amines in DMSO under the catalysis of CuSO4 gave products 58 in good yields (Scheme 58) [70]. The proposed reaction mechanism suggests that the CF3 radical derived from the Togni’s reagent adds to 1,6-enynes followed by 5-exo cyclization to form radical M-41. Then, it might have two pathways to form product 58a. In path a, vinyl radical M-41 is oxidized by CuII to a cation M-42, followed by trapping with carbamate anion to form 58a. Alternatively, in path b, vinyl radical M-41 reacts with CuSO4, CO2, and amine to form carbamato complex M-43, which leads to the formation of product 58a after reductive elimination of the catalyst.
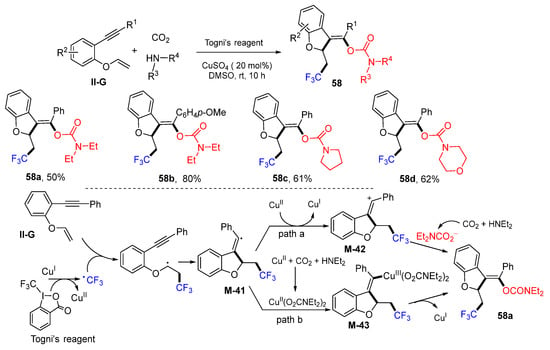
Scheme 58.
Synthesis of trifluoromethyl dihydrobenzofurans.
Gao, Ying and their coworkers reported a Pd-induced radical reaction for the synthesis of difluoroalkyl- and alkenylphosphinyl-functionalized heterocycles in 2021. The reaction of 2-vinyloxy arylalkynes, ICF2CO2Et and diphenylphosphine oxides in DCE under the catalysis of PdCl2(PPh3)2 and Xantphos gave product 59 in good yields and stereoselectivity (Scheme 59) [71]. A reaction mechanism suggests that the CF2CO2Et radical derived from ICF2CO2Et under the catalysis of PdII adds to the C=C double bond of 2-vinyloxy arylalkynes followed by 5-exo cyclization and iodine atom transfer from PdI, through the oxidative addition of Pd0 to vinyl iodide, formation of diphenylphosphine oxide complex, reductive elimination of Pd catalyst to give product 59a.
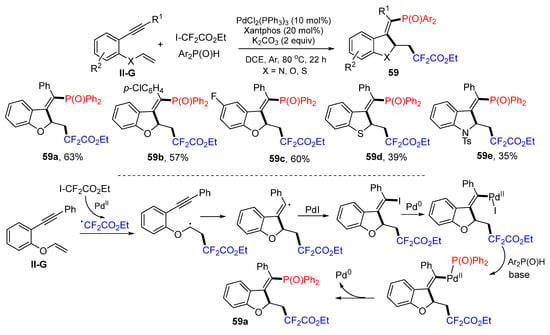
Scheme 59.
Synthesis of aifluoroalkyl and alkenylphosphinylated heterocycles.
Using benzene-tethered and carbonyl-containing 1,6-enynes as a substrate for Cu-catalyzed radical reaction for the construction of cyanotrifluoromethylated 1-indanones was introduced by the Jiang group in 2020. The reaction of benzene-tethered 1,6-enynes, Togni’s reagent and trimethylsilyl cyanide (TMSCN) under the catalysis of Cu(OTf)2 gave product 60 in good yields (Scheme 60) [72]. A reaction mechanism suggests that the trifluoromethyl radical generated from Togni’s reagent under the catalysis of CuII adds to the C=C double bond of 1,6-enyne followed by 5-exo cyclization, formation of CuIII-complex containing CN, and reductive elimination of the Cu-catalyst to give product 60a. By using benzene-tethered 1,7-enynes, the Jiang group extended the reaction scope for the synthesis of cyanotrifluoromethylated (Z)-3,4-dihydronaphthalen-1(2H)-ones 61 (Scheme 61) [73].
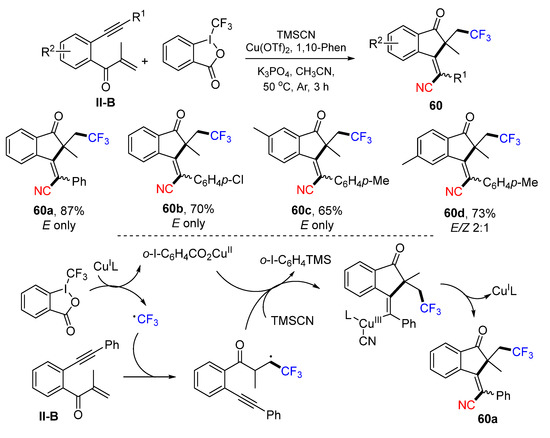
Scheme 60.
Synthesis of cyanotrifluoromethylated 1-indanones.

Scheme 61.
Synthesis of cyanotrifluoromethylated (Z)-3,4-dihydronaphthalen-1(2H)-ones.
A Cu-catalyzed radical for the synthesis of cyanoalkyl and ester-functionalized 1-indanones was introduced by the Jiang group in 2021. The reaction of 1,6-enynes, cyclobutanone oxime esters in DCE at 80 °C under the catalysis of CuBr and1,10-Phen gave product 62 in good yields (Scheme 62) [74]. Both functional groups come from cyclic oxime esters. A reaction mechanism suggests that the γ-cyanoalkyl radical, generated from cyclic oxime ester via a SET process with CuILn, adds to the C=C double bond of 1,6-enyne followed by 5-exo cyclization, formation of a CuIII complex containing the ester group, and reductive elimination CuILn to give product 62a.
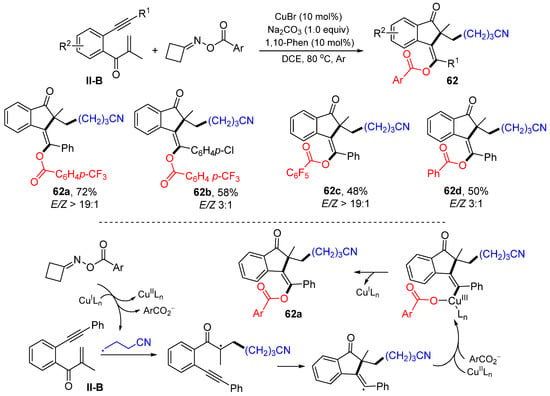
Scheme 62.
Synthesis of cyanoalkyl and ester-functionalized 1-indanones.
A visible light-induced radical reaction of benzene-tethered 1,6-enynes for the synthesis of the thiosulfonylated pyrrolo[1,2-a]benzimidazoles was reported by the Chen group in 2021. The reaction of 1,6-enynes and PhSO2SPh in CH3CN under the photo catalysis of Na2-Eosin Y gave 63 in moderate-to-good yields (Scheme 63) [75]. The reaction mechanism suggests that the sulfonyl radical derived from PhSO2SPh adds to the C=C double bond of 1,6-enynes followed by 5-exo cyclization and coupling with the SPh radical to afford product 63a.
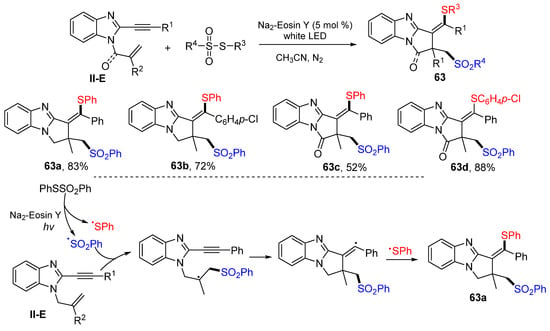
Scheme 63.
Synthesis of the thiosulfonylated pyrrolo-[1,2-a]benzimidazoles.
The Tu and Jiang groups, in 2016, reported a radical reaction of 1,5-enynes for the synthesis of sulfonylated indeno[1,2-d]pyridazines. The reaction of 1,5-enynes, arylsulfonyl hydrazides in CH3CN and in the presence of I2 and TBHP gave products 64 in good yields (Scheme 64) [76]. A reaction mechanism suggests that sulfonylhydrazone, generated from the condensation of 1,5-enynes with the arylsulfonyl hydrazide, reacts with the tosyl radical, which is also derived from arylsulfonyl hydrazide followed by 5-exo cyclization, 1,6-H atom transfer, 6-endo cyclization of the N-radical, and aromatization to give product 64a.

Scheme 64.
Synthesis of disulfonylated indeno[1,2-d]pyridazines.
A Pd-catalyzed radical cyclization of 1,7-enynes for the synthesis of functionalized (E)-3,4-dihydro-naphthalen-1(2H)-ones was reported by Jiang, Tu and their coworkers in 2018. The reaction of 1,7-enynes, sulfinic acids and N-fluorobenzenesulfonimide (NFSI) in THF under the catalysis of [Pd(CH3CN)4](BF4)2 gave 65 in good yields and high stereoselectivity (Scheme 65) [77]. A possible reaction mechanism suggests that 1,7-enynes generate a PdII complex which then reacts with NFSI to form PdIV complex M-44 for following two pathways. Under the reaction conditions for path a, complex M-44 eliminates HBs2N, followed by the addition of R3SO2 radical, 6-exo cyclization, and reductive elimination of Pd catalyst to give fluorosulfonated product 65. Under the reaction conditions for path b, HF is released from complex M-44 followed by the similar reaction process of R3SO2 radical addition, 6-exo cyclization, and reductive elimination of Pd catalyst to give benzenesulfonylated products 66.

Scheme 65.
Synthesis of functionalized 3,4-dihydroquinolin-2(1H)-ones.
Using of benzene-tethered 1,8-dienes for Ir-catalyzed oxidative difluorinative radical cyclization for the preparation of enol and CF2-containing benzoxepines was reported by the Yang group in 2018. The reaction of 1,8-dienes and BrCF2CO2Et in CH2Cl2/H2O under the photoredox catalysis with Ir(dtbbpy)(bpy)2PF6 afforded benzoxepine product 67 in good yields (Scheme 66) [78]. A reaction mechanism suggests that the CF2CO2Et radical, generated from BrCF2CO2Et under the photocatalysis of Ir(dtbbpy)(bpy)2PF6, adds to the C=C double bond of 1,8-dienes followed by 7-exo cyclization, the formation of an iminium ion through the oxidization of [IrIV(dtbbpy)(bpy)2PF6]+, and iminium hydrolysis to give product 67.
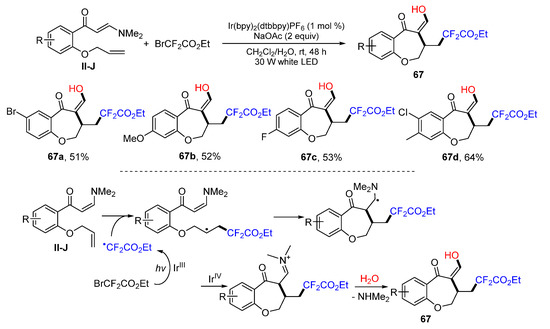
Scheme 66.
Synthesis of enol and CF2-containing benzoxepines.
Using unique benzene-tethered 1,5-enynes, the use of 4-(2-ethynylbenzylidene)cyclohexa-2,5-dien-1-ones for the synthesis of substituted spiroindene compounds was introduced by Yao in 2018. The reaction of 1,5-enynes, TMSN3 and NIS in dioxane in the presence of TBPB gave product 68 in good-to-excellent yields (Scheme 67) [79]. The suggested reaction mechanism indicated that N3 radical derived from TMSN3 adds to the double bond of 1,5-enynes to give cyclohexadienone radical M-45 (path a), which then undergoes 5-exo cyclization to form spirocyclic vinyl intermediates M-46, followed by iodine atom transfer from NIS to selectively give iodo- and azido-functionalized spiroindene products 68a as an E-isomer. Due to the steric hindrance of M-47, cyclization through path b leading to the formation of Z-product 68a’ is unfavorable.

Scheme 67.
Synthesis of iodo- and azido-functionalized spiroindenes.
A metal-catalyzed radical spiroannulation of 1,5-enynes for the synthesis of fluorine-containing (Z)-spiroindenes was reported by Jiang’s group in 2020. The reaction of 1,5-enynes and ICF2CO2Et in DCE at 70 °C under the catalysis of PdCl2 and 9,9-dimethyl-4,5-bis(diphenylphosphino)xanthenes (Xant-Phos) gave iododifluoro-acetylated product 69 in good yields (Scheme 68) [80]. However, the use of BrCF2CO2Et or C4F9I as the fluoroalkylation reagents failed to give the corresponding (Z)-spiroindenes. Another reaction of 1,5-enynes, Togni’s reagent and TMSCN in CH3CN at 50 °C under the catalysis of Cu(OAc)2 and 3,4,7,8-tetramethyl-1,10-phenanthroline (tmphen) gave trifluoromethylated products 70. For the synthesis of 69a, the reaction mechanism suggests that the CF2CO2Et radical derived from ICF2CO2Et adds to the C=C double bond of 1,5-enynes followed by 5-exo spirocyclization, formation of the PdII-I complex, and reductive elimination of Pd catalyst to afford iododifluoroacetylated product 69a. In the synthesis of CF3-functionalized products 70, the CF3 radical derived from Togni’s reagent has a similar spirocyclization mechanism to form cyanotrifluoromethylated spiroindene product 70a. The Tu and Jiang groups extended this reaction in the synthesis of iodosulfonylated spiroindenes, which involves an ionic instead of a radical cyclization [81].

Scheme 68.
Synthesis of functionalized spiroindene compounds.
Using dicyano-substituted benzene-tethered 1,5-enynes for a visible light-driven radical haloazidative cyclization for the synthesis of holoazido-functionalized indenes was accomplished by the Li group in 2020. The reaction of 1,5-enynes, TMSN3, and N-iodo (bromo or chloro) succinimide in DMF under the radiation of LED (380–385 nm) afforded product 71 in moderate-to-good yields (Scheme 69) [82]. The suggested reaction mechanism indicated that the azide radical generated from TMSN3 under the photo conditions adds to the double bond of 1,5-enyne followed by 5-exo cyclization and I-atom transfer from NIS to give product 71a.
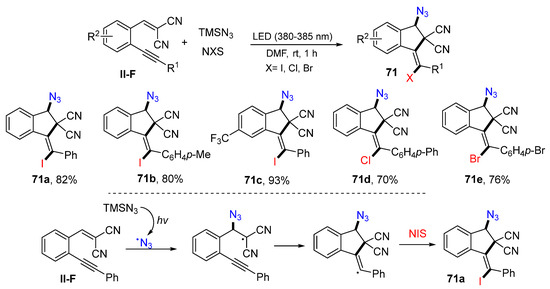
Scheme 69.
Synthesis of holoazido-functionalized indenes.
Using benzene-tethered 1,7-diynes for the synthesis of iododifluoroacetal tetrahydronaphthalen-1-ols was introduced by the Jiang group in 2021. The reaction of 1,7-diynes and ICF2CO2Et under photoredox catalysis with fac-Ir(ppy)3 gave difluoromethyl-containing (1E,2E)-tetrahydronaphthalen-1-ols 72 bearing two exocyclic C=C double bonds as major stereoisomers in good yields (Scheme 70) [83]. A reaction mechanism suggests that the CF2CO2E radical derived from ICF2CO2Et under the photocatalysis adds to the terminal alkyne of 1,7-diyne followed by 6-exo cyclization, SET of DIPEA to form cation, and nucleophilic addition with iodide anion to give (1E,2E)-product 72a as a major isomer.
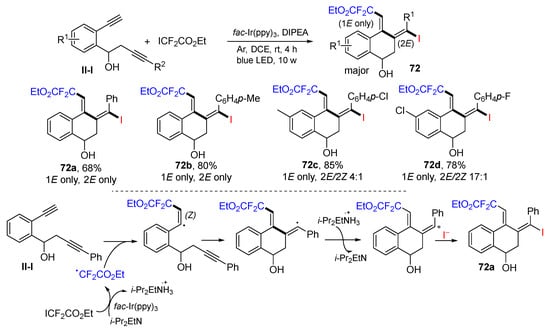
Scheme 70.
Synthesis of iododifluoroacetal tetrahydronaphthalen-1-ols.
4. Reaction of Arene-Terminated Alkenes and Alkynes
Presented in this section are the radical addition and cyclization-initiated difunctionalization reactions of arene-terminated alkenes and alkynes with a reaction sequence shown in Scheme 71. For the class of substrates shown in Scheme 72, the initial radical addition happens at the alkene or alkyne groups instead of the arene. Sequential radical cyclization leads to the formation of spiro- or fused-ring compounds. The only exception is the reaction of substrate III-E. The radical is added to the benzyne ring (via the benzyne intermediate). Among the general substrates, the reactions of alkynes III-A (arylpropiolamides if Y is NR) for making spiro compounds are much more popular than those of substrates III-B to III-E for making fused cyclic products.

Scheme 71.
General reaction scheme for the difunctionalization of arene-terminated alkenes and alkynes.
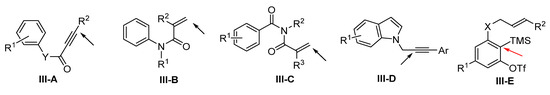
Scheme 72.
Aryl-terminated alkenes and alkynes with the pointed position for the initial radical addition.
There are several reports on the reaction of arylpropiolamides for the synthesis of 3-functionalized azaspiro[4,5]trienones. In 2014, Li and co-workers reported a radical spirocyclization reaction of arylpropiolamides for the synthesis of 3-acylated azaspiro[4,5]trienones. The reaction of alkynyl amides and aldehydes in the presence of TBHP gave product 73 in good-to-excellent yields (Scheme 73) [84]. The reaction mechanism suggests that the carbonyl radical generated from aldehyde adds to alkyne followed by ipso-carbocyclization, coupling with OH radical and oxidation of OH group to give 3-acylspiro[4,5]trienone 73a. In 2014, Li’s group also reported a Cu-catalyzed radical spirocyclization of aryl alkynyl amides for the synthesis of azaspiro[4,5]trienones. The reaction of arylpropiolamides and cyclic ethers in t-BuOAc under the catalysis of CuII and TBHP gave product 74 in good yields (Scheme 74) [85].
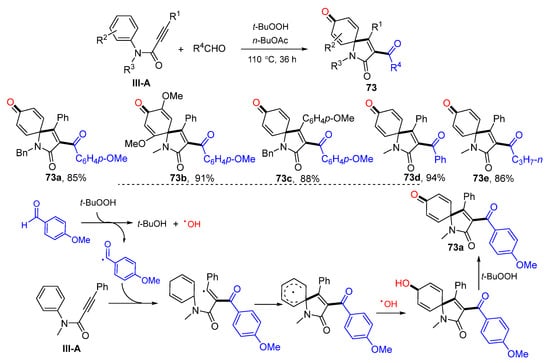
Scheme 73.
Synthesis of 3-acyl azaspiro[4,5]trienones.
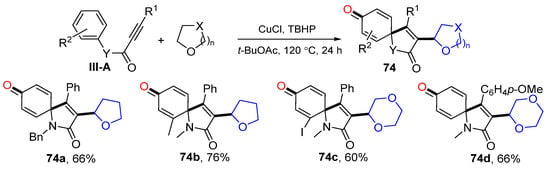
Scheme 74.
Synthesis of substituted azaspiro[4,5]trienones.
A Cu-catalyzed radical spirocyclization of arylpropiolamides for the synthesis of 3-triflouromrthylated azaspiro[4,5]trienones was reported by the Liang group in 2015. The reaction of alkynyl amides and NaSO2CF3 (Langlois’ reagent) in CH3CN in the presence of TBHP, MnO2 and CuCl gave product 75 in good-to-excellent yields (Scheme 75) [86]. The reaction mechanism suggests that the CF3 radical derived from the Langlois’ reagent adds to the C≡C triple bond followed by ipso-carbocyclization, coupling with the t-BuOO radical, and elimination of t-BuOH to give product 75a.
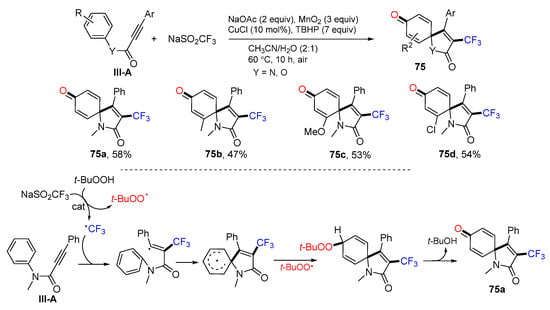
Scheme 75.
Synthesis of 3-trifluoromethyl azaspiro[4,5]trienones.
In 2015, the Wang group introduced an Ag-catalyzed radical spirocyclization of arylpropiolamides for the construction of 3-arylthiolated azaspiro[4,5]trienones. The reaction of alkynyl amides, thiophenols and H2O in 1,4-dioxane under the catalysis AgI gave product 76 in moderate-to-good yields (Scheme 76) [87]. A proposed reaction mechanism suggests that the thiyl radical produced from thiophenol adds to the carbon triple bond of arylpropiolamides followed by the ipso-carboncyclization, SET to form carbocation, nucleophilic addition of H2O, and oxidization of OH to give product 76.

Scheme 76.
Synthesis of 3-arylthiolated azaspiro[4,5]trienones.
A TEMPO-mediated radical nitrative spirocyclization of arylpropiolamides for the preparation of 2-nitrated azaspiro[4,5]trienones was introduced by Li’s group in 2015. The reaction was carried out using arene-terminaled 1,5-enynes and t-BuONO in EtOAc in the presence of O2 and TEMPO to give nitrated spiro compound 77 in moderate-to-good yields (Scheme 77) [88]. A reaction mechanism suggests that NO2 generated from the oxidization of NO adds to the carbon triple bond of arylpropiolamide followed by ipso-carbocyclization, TEMPO oxidation to form cation, nucleophilic addition of H2O, and oxidization to give product 77a.
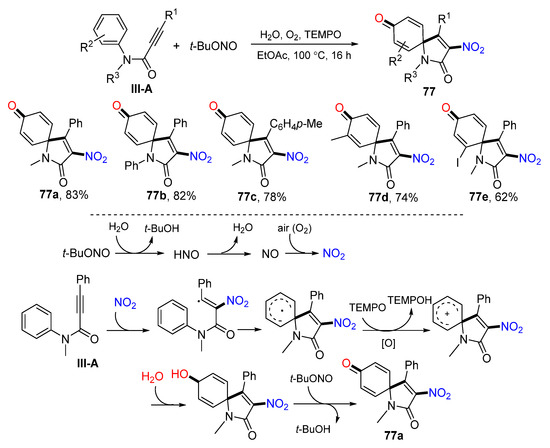
Scheme 77.
Synthesis of 3-nitralated azaspiro[4,5]trienones.
In 2015, Wang and co-workers developed an oxidative radical spirocyclization reaction of arylpropiolamides for the preparation of 3-sulfonated azaspiro[4,5]trienones. The reaction of arylpropiolamides and sulfonylhydrazide in the presence of TBHP and I2O5 afforded product 78 in moderate-to-good yields (Scheme 78) [89]. The reaction mechanism suggests that the sulfonyl radical derived from sulfonylhydrazide adds to the carbon triple bond of amides followed by ipso-cyclization, SET to form cyclohexadienyl cation, nucleophilic addition of H2O, and finally oxidation with TBHP to give product 78.
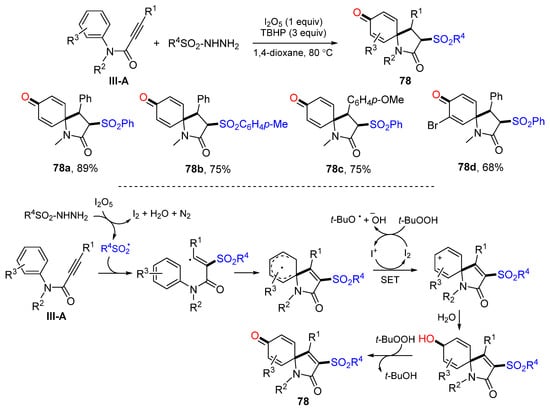
Scheme 78.
Synthesis of 3-sulfonated azaspiro[4,5]trienones.
A new method for radical spirocyclization of arylpropiolamides to synthesize 3-sulfonated azaspiro[4,5]trienones was reported by Liu’s group in 2016. The reaction of amides and AgSCF3 in CH3CN in the presence of K2S2O8 and TBHP gave product 79 in excellent yields (Scheme 79) [90]. A proposed reaction mechanism suggests that the CF3S radical derived from AgSCF3 adds to the carbon double bond of amides, followed by ipso-carbocyclization, coupling with t-butylperoxy radical, and elimination of t-BuOH to give product 79a.
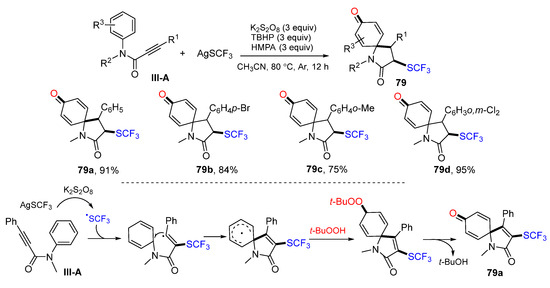
Scheme 79.
Synthesis of SCF3-substituted azaspiro[4,5]trienones.
Other than the reactions of arylpropiolamides for making the spiro compounds described above, the reactions of N-phenylacrylamides have also been developed for making fused-cyclic products. In 2022, Zhang and co-workers reported a Co-promoted reaction for the synthesis of bromoarylthiolated heterocyclic compounds. The reaction of N-arylacrylamides and disulfides in CH3CN in the presence of CoBr2 and (NH4)2S2O8 gave functionalized product 80 in good-to-excellent yields (Scheme 80) [91]. The reaction mechanism suggests that bromine and PhS radicals for the difunctionalization are generated from the reaction of CoBr2 and PhSSPh. The PhS radical adds to the terminal carbon of the double bond of amides, followed by cyclization and bromo radical coupling to give product 80a.
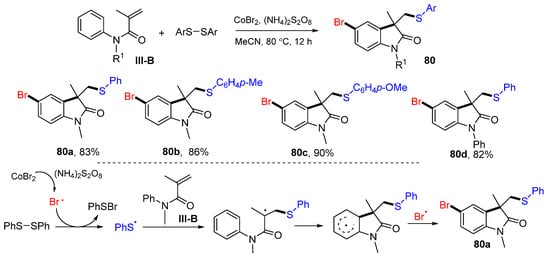
Scheme 80.
Preparation of cyclopentanes.
The reaction of methacryloyl benzamides could result in six-membered ring-fused products. This work was reported by Tang, Chen and their co-workers in 2016 in the development of a Cu-catalyzed radical reaction for the synthesis of dicyanoisoproylated isoquinolinediones. The reaction of methacryloyl benzamides and AIBN in dioxane in the presence of CuI, KF, and K3PO4 gave product 81 in good-to-excellent yields (Scheme 81) [92]. The reaction mechanism suggests that homolytic cleavage of AIBN gives two CNMe2C radicals. One of them adds to the carbon double bond of amides, followed by 6-exo cyclization to the benzene ring, selectively trapping the second CNMe2C radical under the assistance of CuI, and final step aromatization to give isoquinoline-1,3(2H,4H)-dione 81a.

Scheme 81.
Synthesis of dicyanoisoproylated isoquinolinediones.
The reaction of N-propargylindoles could result in the formation of products with a core of 9H-pyrrolo[1,2-a]indol-9-one. In 2022, Du and coworkers developed photoredox radical cyclization of N-propargylindoles for the synthesis of 2-substituted 9H-pyrrolo-[1,2-a]indol-9-ones. The photo reaction of N-propargylindoles and cyclic ethers in MeCN at 80 °C in the presence TBHP and dual catalysts Cu(OAc)2 and Eosin Y give product 82 in moderate yields (Scheme 82) [93]. The proposed mechanism suggests that a THF radical, generated from the reaction of THF with TBHP and the catalysts, adds to the carbon triple bonds of N-propargylindoles followed by 5-exo cyclization to give intermediate M-48. Intermediate M-48 could have three paths to give product 82a, (1) M-48 couples with t-BuOO radical and then oxidation; (2) M-48 traps O2 then reacts with TBHP and CuI catalyst; (3) M-48 oxidized to cation through SET process and then oxidized OH to C=O.
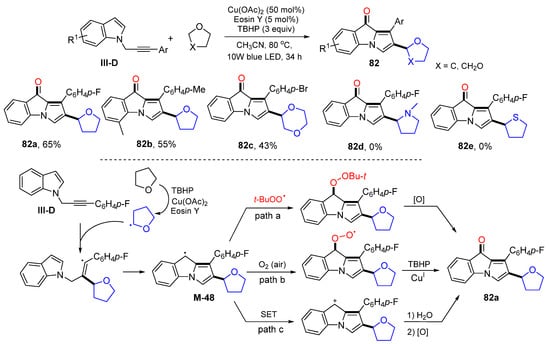
Scheme 82.
Synthesis of 2-substituted 9H-pyrrolo[1,2-a]indol-9-ones.
Other than the addition of an initial radical to the alkene or alkyne group on the side chain presented in previous cases, a radical could add to benzene if the ring is converted to a benzyne. In 2021, the Studer group reported such a reaction in the synthesis of substituted five-membered heterocycles. The reaction of arenes bearing 1,2-TMS and OTs groups with TEMPO in the presence of CsF and 18-crown-6 ether gave product 83 in moderate yields (Scheme 83) [94]. A proposed reaction mechanism suggests that arene is first converted to benzyne with the treatment of CsF and then reacts with TMPO radical followed by 5-exo cyclization and coupling with the second TEMPO to give product 83a.

Scheme 83.
Synthesis of diTEMPO-substituted benzofuran and analogs heterocycles.
5. Reaction of Other Alkene and Alkyne Compounds
Presented in this section are the radical addition-initiated difunctionalizations of alkene- and alkyne-related compounds that cannot be fit in the previous sessions in terms of substrates or reaction mechanism. As shown in Scheme 84, substrates IV-A to IV-C are 1,n-eneallenes; the cyano group in enenitrile IV-D is responsible for the second functionalization; arene-terminated enyne IV-E has a preexisting MeO group on the benzene ring which will be converted to a new functional group during the reaction; arene-terminated IV-F has a leaving group X which will be displaced by a new group at the step of second functionalization. Since the reactions of these substrates are not the major focus of this paper, only selected examples are highlighted.
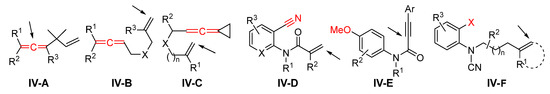
Scheme 84.
Other alkene and alkyne compounds with the pointed position for the initial radical addition.
An early example of radical difunctionalization of eneallenes was reported by the Hatem group in 1995 for the synthesis of bromo- and tosyl-functionalized cyclopantenes. The reaction of eneallenes and tosyl bromide in benzene using AIBN as a radical initiator gave product 84 (Scheme 85) [95]. A proposed reaction mechanism suggests that the tosyl radical generated from TsBr adds the central carbon of allene, followed by 5-exo cyclization and coupling with bromine radical, to give product 84a. Addition of tosyl radical to alkene instead of allene could be possible. However, since no expected product 84a’ was isolated, path b is less favorable than path a.

Scheme 85.
Preparation of tosyl-substituted cyclopentanes.
A later example for the reaction of eneallenes was reported by the Ma group in 2012. It is a Zn-catalyzed radical cyclization for the synthesis of iodoperfluoroalkylated five-membered rings. The reaction of eneallenes and RFI in CH2Cl2 in the presence of Zn powder and HOAc gave product 85 in moderate-to-good yields (Scheme 86) [96]. It is worth mentioning that the two diastereomers of the product 85 could be converted into 3-(1-enylidene)heterocyclopentanes 86 through the TBAF-promoted dehydroiodination reaction. A mechanism for the racial reaction suggests that the perfluoroalkyl radical generated from RFI adds to the alkene carbon of eneallenes followed by 5-exo cyclization and coupling with the iodine radical from RFI to give product 85.

Scheme 86.
Synthesis of iodoperfluoroalkyl substituted five-membered rings.
A more recent example of eneallene reaction was reported by the Shi group in 2021. It is a visible light-induced radical reaction of ene-vinylidenecyclopropanes (ene-VDCP) for the synthesis of iodoperfluoro-alkylated N-heterocycles. The reaction of ene-VDCP, ICF2CO2Et or ICF2CF2CF2CF3 in 1,4-dioxane under the blue LED photocatalysis with fac-Ir(ppy)3 gave 87 in good yields and stereoselectivity (Scheme 87) [97]. The reaction mechanism suggests that the CF2CO2Et radical, generated from ICF2CO2Et under the photolysis, adds to the terminal carbon of alkene followed by 5-exo cyclization, cyclopropane ring-opening, and extraction of iodine atom from ICF2CO2Et to give the final product 87a.

Scheme 87.
Synthesis of iodoperfluoroalkylated N-heterocycles.
An interesting example of using the cyano group as a radical acceptor for the difunctionalization reaction was reported by the Li group in 2015. It is a Cu-catalyzed radical cyclization of arene-tethered enenitrile for the synthesis of substituted quinoline-2,4(1H,3H)-diones. The reaction of o-cyanoarylacrylamide and diphenyl-phosphine oxide in CH3CN in the presence of CuBr2 and Mg(NO3)2·6H2O gave phosphinylated quinoline-2,4(1H,3H)-diones 88 in good-to-excellent yields (Scheme 88) [98]. The reaction mechanism suggests that the Ph2P(O) radical derived from Ph2P(O)H under CuII catalysis adds to the C=C double bond of amide followed by 6-exo cyclization to the CN group and hydrolysis with H2O to give final product 88a.

Scheme 88.
Synthesis of phosphinylated quinoline-2,4(1H,3H)-diones.
In 2016, the Li group also reported a decarboxylative radical reaction of o-cyanoarylacrylamides for the preparation of carbonylated quinoline-2,4(1H,3H)-diones. The reaction of o-cyanoarylacrylamide and α-keto acids in acetone-H2O at 120 °C under the catalysis of AgNO3 and (NH4)2S2O8 gave product 89 in good yields (Scheme 89) [99].
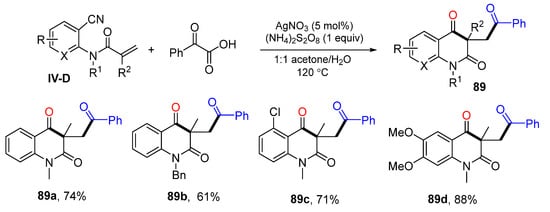
Scheme 89.
Synthesis of phosphinylated quinoline-2,4(1H,3H)-diones.
Having a MeO group on the benzene ring is a useful synthetic approach to assist radical cyclization and for dearomatization. In 2017, Li and co-workers developed a Ni-promoted radical spirocyclization of N-(p-methoxyaryl)propiolamides for the synthesis of 3-substituted azaspiro[4,5]trienones. The reaction of amides and α-bromo esters in DMF in the presence of Ni(acac)2, 1,2-bis(diphenylphosphino)ethane (dppe), TBHP and K2HPO4 gave product 90 in moderate yields (Scheme 90) [100]. A proposed mechanism suggests that alkyl radical derived from α-bromo esters adds to the triple bond of amide followed by ipso-carbocyclization, oxidation with TBHP to form oxonium cation, and a final step of demethylation to give product 90a. The MeO group on the aromatic ring is critical for the radical cyclization and formation of the carbonyl group through diaromatization. The product generated from this method is similar to that presented in Scheme 73, in which there is no preexisting MeO group on the benzene ring.
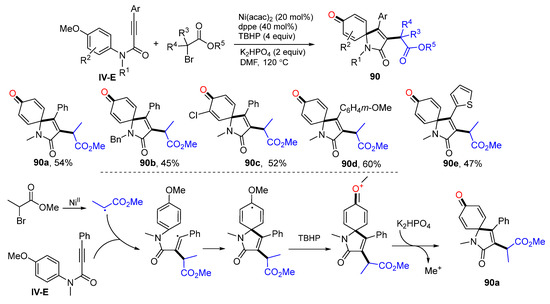
Scheme 90.
Synthesis of 3-alkyl azaspiro[4,5]trienones.
Using a similar synthetic strategy and the alkyne substrate, in 2018, Liu and co-workers reported a visible light-mediated radical spirocyclization of N-(p-methoxyaryl)-propiolamides for the synthesis of 3-acylspiroc (Scheme 91) [101]. The photo reaction of alkynes and benzoyl chloride in CH3CN in the presence of IrIII(ppy)3 and 2,6-lutidine gave product 91 in good-to-excellent yields.

Scheme 91.
Synthesis of 3-acyl azaspiro[4,5]trienones.
Scheme 92 shows another example of the reaction of N-(p-methoxyaryl)-propiolamides developed by Liu’s group also for the synthesis of 3-acylspiro[4,5]trienones [102].The photoredox reaction of alkynes, acyl oxime esters, H2O under the catalysis of Ir(ppy)3 gave product 92 in good yields.
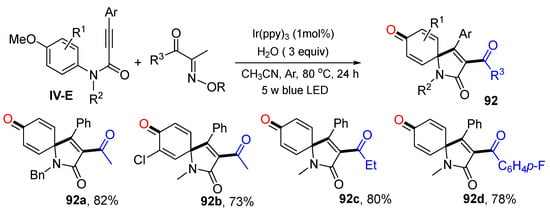
Scheme 92.
Synthesis of 3-acyl azaspiro[4,5]trienones.
The last example in this section is the reaction of arene-terminated alkene, which has a leaving group X on the aromatic ring. Liao and coworkers employed this substrate in the synthesis of functionalized benzosultams. The reaction of N-(2-haloaryl)cyanamide, bromodifluoroalkyl reagents and Na2S2O5 in DMF and H2O at 80 °C afforded product 93 in good yields (Scheme 93) [103]. A proposed reaction mechanism suggests that the CF2CO2Et radical derived from BrCF2CO2Et SO2 adds to the carbon double bond of amide followed through 5-exo cyclization to the CN group, capture of SO2 (generated from Na2S2O5) to form sulfonyl radicals, cyclization to the benzene ring at the carbon with iodine, and a last step of deiodo aromatization to give product 93a.
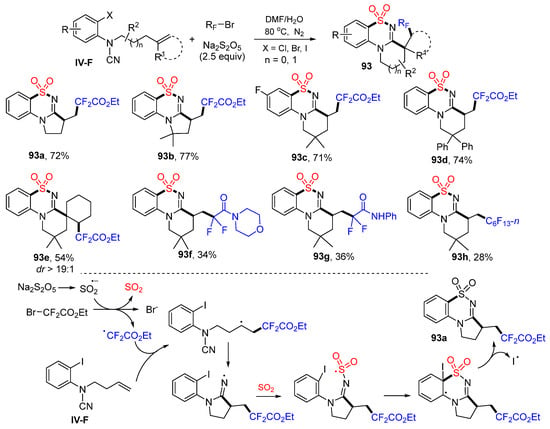
Scheme 93.
Synthesis of functionalized benzosultams.
6. Conclusions
Radical reactions are powerful and versatile synthetic methods for making carbon–carbon and carbon–heteroatom bonds. Designing one-pot and cascade radical transformations to make cyclic ring skeletons are highly efficient and operationally straightforward methods. Summarized in this article are the radical addition followed by cyclization reactions to make difunctionalized cyclic molecules. The second functionalization could be achieved through radical coupling, transition metal-assisted reaction, and nucleophilic or electrophilic substitution reactions, which significantly broaden the scope of difunctionalization reactions. Reactions of substrates such as dienes, diynes, and enynes, as well as of their arene-bridged and terminated analogs, are presented. In addition to conventional radical reactions using radical initiators or under transition metal-catalysis, the recent development of photoredox and electrochemical reactions have enhanced the scope of the radical difunctionalizations. In addition to the difunctionalization of unsaturated carbons such as alkenes and alkynes, we expect to see more development on difunctionalization reactions involving other functional groups, such as CN and N3. We also expect to see more applications in the synthesis of biologically significant molecules and natural products.
Author Contributions
S.Z. literature search and revision of the text and schemes. H.Y. literature search and initial manuscript writing. W.Z. revision and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chatgilialoglu, C.; Studer, A. (Eds.) Encyclopedia of Radicals in Chemistry, Biology and Materials; Wiley: Chichester, UK, 2012. [Google Scholar]
- Zarf, S.Z. Radical Reactions in Organic Synthesis; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible light photoredox catalysis with transition metal complexes: Applications in organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef]
- Yan, M.; Kawamata, Y.; Baran, P.S. Synthetic organic electrochemical methods since 2000: On the verge of a renaissance. Chem. Rev. 2017, 117, 13230–13319. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-C.; Xiao, Y.-T.; Yang, Y.-Z.; Song, R.-J.; Li, J.-H. Recent advances in silver-mediated radical difunctionalization of alkenes. Chem. Cat. Chem. 2020, 12, 5312–5329. [Google Scholar] [CrossRef]
- Ge, Y.; Tian, Y.; Wu, J.; Yan, Q.; Zheng, L.; Ren, Y.; Zhao, J.; Li, Z. Iron-promoted free radical cascade difunctionalization of unsaturated benzamides with silanes. Chem. Commun. 2020, 56, 12656–12659. [Google Scholar] [CrossRef]
- Siu, J.C.; Fu, N.K.; Lin, S. Catalyzing electrosynthesis: A homogeneous electrocatalytic approach to reaction discovery. Acc. Chem. Res. 2020, 53, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Fang, G.C.; Gu, Q.S.; Liu, X.Y. Recent advances in copper-catalysed radical-involved asymmetric 1,2-difunctionalization of alkenes. Chem. Soc. Rev. 2020, 49, 32–48. [Google Scholar] [CrossRef]
- Jiang, H.; Studer, A. Intermolecular radical carboamination of alkenes. Chem. Soc. Rev. 2020, 49, 1790–1811. [Google Scholar] [CrossRef]
- Lan, X.-W.; Wang, N.-X.; Xing, Y. Recent advances in radical difunctionalization of simple alkenes. Eur. J. Org. Chem. 2017, 2017, 5821–5851. [Google Scholar] [CrossRef]
- Bao, X.Z.; Li, J.; Jiang, W.; Huo, C.D. Radical-mediated difunctionalization of styrenes. Synthesis 2019, 51, 4507–4530. [Google Scholar] [CrossRef]
- Lin, J.; Song, R.J.; Hu, M.; Li, J.H. Recent advances in the intermolecular oxidative difunctionalization of alkenes. Chem. Rec. 2019, 19, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tang, G.; Zhao, Y.F. Recent progress toward organophosphorus compounds based on phosphorus-centered radical difunctionalizations. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 589–596. [Google Scholar] [CrossRef]
- Koike, T.; Akita, M. A versatile strategy for difunctionalization of carbon–carbon multiple bonds by photoredox catalysis. Org. Chem. Front. 2016, 3, 1345–1349. [Google Scholar] [CrossRef]
- Besset, T.; Poisson, T.; Pannecoucke, X. Direct vicinal difunctionalization of alkynes: An efficient approach towards the synthesis of highly functionalized fluorinated alkenes. Eur. J. Org. Chem. 2015, 2015, 2765–2789. [Google Scholar] [CrossRef]
- Coppola, G.A.; Pillitteri, S.; Van der Eycken, E.V.; You, S.-L.; Sharma, U.K. Multicomponent reactions and photo/electrochemistry join forces: Atom economy meets energy efficiency. Chem. Soc. Rev. 2022, 51, 2313–2382. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Hu, W.; Zhang, W. Difunctionalization of alkenes and alkynes via intermolecular radical and nucleophilic additions. Molecules 2021, 26, 105. [Google Scholar] [CrossRef]
- Tsuchii, K.; Ueta, Y.; Kamada, N.; Einaga, Y.; Nomoto, A.; Ogawa, A. A facile photoinduced iodoperfluoroalkylation of dienes, diynes, and enynes with perfluoroalkyl iodides via selective radical cyclization. Tetrahedron Lett. 2005, 46, 7275–7278. [Google Scholar] [CrossRef]
- Mantrand, N.; Renaud, P. Azidosulfonylation of alkenes, dienes, and enynes. Tetrahedron 2008, 64, 11860–11864. [Google Scholar] [CrossRef]
- Zhang, L.-Z.; Li, Z.-J.; Liu, Z.-Q. A free-radical cascade trifluoromethylation/cyclization of N-arylmethacrylamides and enynes with sodium trifluoromethanesulfinate and iodine pentoxide. Org. Lett. 2014, 16, 3688–3691. [Google Scholar] [CrossRef]
- He, Y.-T.; Li, L.-H.; Zhou, Z.-Z.; Hua, H.-L.; Qiu, Y.-F.; Liu, X.-Y.; Liang, Y.-M. Copper-catalyzed three-component cyanotrifluoromethylation/azidotrifluoromethylation and carbocyclization of 1,6-enynes. Org. Lett. 2014, 16, 3896–3899. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-T.; Wang, Q.; Zhao, J.-H.; Wang, X.-Z.; Qiu, Y.-F.; Yang, Y.-C.; Hu, J.-Y.; Liu, X.-Y.; Liang, Y.-M. Copper-catalyzed cascade cyclization for the synthesis of trifluoromethyl- substituted spiro-2H-azirines from 1,6-enynes. Adv. Synth. Catal. 2015, 357, 3069–3075. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; He, Y.-T.; Zhang, L.-L.; Wu, X.-X.; Liu, X.-Y.; Liang, Y.-M. Palladium-catalyzed radical cascade iododifluoromethylation/cyclization of 1,6-enynes with ethyl difluoroiodoacetate. Org. Lett. 2015, 17, 4280–4283. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhou, Z.-Z.; He, Y.-T.; Li, L.-H.; Ma, J.-W.; Qiu, Y.-F.; Zhou, P.-X.; Liu, X.-Y.; Xu, P.-F.; Liang, Y.-M. Iodine-promoted radical cyclization in water: A selective reaction of 1,6-enynes with sulfonyl hydrazides. J. Org. Chem. 2016, 81, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, C.-T.; Qiu, Y.-F.; Zhu, X.-Y.; Han, Y.-P.; Xia, Y.; Li, X.-S.; Liang, Y.-M. Base promoted direct difunctionalization/cascade cyclization of 1,6-enynes. Chem. Commun. 2018, 54, 5334–5337. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Pathigoolla, A.; Lowe, G.; Walsh, D.A.; Cooper, M.; Lewis, W.; Lam, H.W. Sulfonylative and azidosulfonylative cyclizations by visible-light-photosensitization of sulfonyl azides in THF. Chem. Eur. J. 2017, 23, 17598–17605. [Google Scholar] [CrossRef]
- An, Y.-Y.; Wu, J. Synthesis of tetrahydropyridine derivatives through a reaction of 1,6-enynes, sulfur dioxide, and aryldiazonium tetrafluoroborates. Org. Lett. 2017, 19, 6028–6031. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, Z.; Tung, C.-H.; Xu, Z. Atom transfer radical addition to alkynes and enynes: A versatile Gold/photoredox approach to thio-functionalized vinylsulfones. ACS Catalysis. 2018, 8, 8237–8243. [Google Scholar] [CrossRef]
- Hou, H.; Li, H.-X.; Xu, Y.; Song, C.-Q.; Wang, C.-L.; Shi, Y.-C.; Han, Y.; Yan, C.-G.; Zhu, S.-Q. Visible-light-mediated chlorosulfonylative cyclizations of 1,6-enynes. Adv. Synth. Catal. 2018, 360, 4325–4329. [Google Scholar] [CrossRef]
- Shang, X.-J.; Liu, D.; Liu, Z.-Q. A NaSO2CF3/NaBrO3-mediated bromotrifluoromethylation of enyne via free-radical cascade processes. Org. Chem. Front. 2018, 5, 2856–2859. [Google Scholar] [CrossRef]
- Ye, K.-Y.; Song, Z.-D.; Sauer, G.S.; Harenberg, J.H.; Fu, N.-K.; Lin, S. Synthesis of chlorotrifluoromethylated pyrrolidines by electrocatalytic radical ene-yne cyclization. Chem. Eur. J. 2018, 24, 12274–12279. [Google Scholar] [CrossRef]
- Mutra, M.R.; Kudale, V.S.; Li, J.; Tsai, W.-H.; Wang, J.-J. Alkene versus alkyne reactivity in unactivated 1,6-enynes: Regio- and chemoselective radical cyclization with chalcogens under metal- and oxidant-free conditions. Green Chem. 2020, 22, 2288–2300. [Google Scholar] [CrossRef]
- Mutra, M.R.; Dhandabani, G.K.; Li, J.; Wang, J.-J. Regio- and chemoselective synthesis of nitrogencontaining heterocycles via the oxidative cascade cyclization of unactivated 1,n-enynes. Chem. Commun. 2020, 56, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.Q.; Cheng, Q.; Yang, H.B.; Chen, X.Y.; Han, Y.; Yan, C.G.; Shi, Y.C.; Hou, H. Three-component radical iodonitrosylative cyclization of 1,6-enynes under metal-free conditions. Org. Lett. 2021, 23, 5044–5048. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Sun, Y.; Pan, Y.J.; Yu, H.G.; Han, Y.; Shi, Y.C.; Yan, C.G.; Zhu, S.Q. Visible-light mediated diarylselenylative cyclization of 1,6-enynes. J. Org. Chem. 2021, 86, 1273–1280. [Google Scholar] [CrossRef]
- Dong, M.; Wang, D.F.; Tong, X.F. PhI(OAc)2-mediated dihalogenative cyclization of 1,6-enyne with lithium halide. Org. Lett. 2021, 23, 3588–3592. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.M.; Ge, Y.X.; Yan, Q.Q.; Tian, Y.F.; Wu, J.L.; Yang, Y.J.; Li, L.J.; Li, Z.J. Iron-mediated radical nitrohalogenation reactions of enynes with tert-butyl nitrite. Synlett. 2021, 32, 1192–1196. [Google Scholar] [CrossRef]
- He, F.-S.; Yao, Y.; Tang, Z.; Xie, W.; Wu, J. Copper-catalyzed regio- and chemoselective selenosulfonylation of 1,6-enynes from sulfur dioxide. Org. Chem. Front. 2021, 8, 6119–6124. [Google Scholar] [CrossRef]
- Hou, H.; Tang, D.; Li, H.; Xu, Y.; Yan, C.-G.; Shi, Y.-C.; Chen, X.-Y.; Zhu, S. Visible-light-driven chlorotrifluoromethylative and chlorotrichloromethylative cyclizations of enynes. J. Org. Chem. 2019, 84, 7509–7517. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, R.Y.; Zhou, P.S.; Wu, J.L.; Li, W.S.; Wang, C.Y.; Li, H.; Li, D.J.; Yang, J.H. Visible-light-driven base-promoted radical cascade difluoroalkylation-cyclization-iodination of 1,6-enynes with ethyl difluoroiodoacetate. Eur. J. Org. Chem. 2022, 2022, e202101395. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, F.R.; Chen, X.Y.; Han, Y.; Yan, C.G.; Shi, Y.C.; Hou, H.; Zhu, S.Q. Visible-light-mediated three-component radical iodosulfonylative cyclization of enynes. Org. Lett. 2022, 24, 2515–2519. [Google Scholar] [CrossRef]
- Fang, J.; Yang, T.; An, M.; Liu, Y.L.; Liu, Y.; Yang, Y.; Zhou, J.; Cao, T.; Ren, Q.; Huang, H. Design, synthesis and biological evaluation of difluoroalkylated protoilludanes obtained by a practical radical cascade difluoroalkylation-cyclization reaction. Tetrahedron Lett. 2022, 89, 153594. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Zhao, J.; Liu, G. Rhodium-catalyzed cascade radical cyclization of 1, 6-enynes with Br-CX3: Access to bromine-containing trihalomethylated pyrrolidines. Asian J. Org. Chem. 2019, 8, 2249–2258. [Google Scholar] [CrossRef]
- Zhu, S.; Pan, Y.; Sun, Y.; Hong, X.; Chen, X.; Han, Y.; Yan, C.; Shi, Y.; Hou, H. Copper-catalyzed bromo-cyanomethylative cyclization of enynes. J. Org. Chem. 2022, 87, 4455–4459. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-L.; Hu, S.-J.; Xu, Q.; Zheng, H.; Wei, W.-T. Radical cyclization of 1,n-enynes and 1,n-dienes for the synthesis of 2-pyrrolidone. Chem. Asian J. 2021, 16, 3068–3081. [Google Scholar] [CrossRef]
- Feray, L.; Bertrand, M.P. Dialkylzinc-mediated atom transfer sequential radical addition cyclization. Eur. J. Org. Chem. 2008, 2018, 3164–3170. [Google Scholar] [CrossRef]
- Cao, X.; Cheng, X.; Xuan, J. Arylsulfonyl radical triggered 1,6-enyne cyclization: Synthesis of γ-lactams containing alkenyl C−X bonds. Org. Lett. 2018, 20, 449–452. [Google Scholar] [CrossRef]
- Ying, W.-W.; Chen, W.-T.; Bao, W.-H.; Gao, L.-H.; Wang, X.-Y.; Chen, G.-P.; Ge, G.-P.; Wei, W.-T. Room temperature, metal-free, radical chloroazidation of 1,6-enynes. Synlett 2018, 29, 1664–1668. [Google Scholar] [CrossRef]
- Yu, X.-C.; Zheng, Y.-N.; Zhang, J.-H.; Zhang, J.; Liu, F.-L.; Tang, K.; Li, T.; Wei, W.-T. Metal-catalyst-free radical cyclization of 1,6-enynes for the selective and switchable synthesis of lactams in water. ACS Sustainable Chem. Eng. 2022, 10, 6057–6062. [Google Scholar] [CrossRef]
- Yoshioka, E.; Kohtani, S.; Tanaka, E.; Hata, Y.; Miyabe, H. Carbon radical addition- cyclization reaction induced by ruthenium-photocatalyst under visible light irradiation. Tetrahedron 2015, 71, 773–781. [Google Scholar] [CrossRef]
- Wu, S.P.; Wang, D.K.; Kang, Q.Q.; Ge, G.P.; Zheng, H.X.; Zhu, M.L.; Li, T.; Zhang, J.Q.; Wei, W.T. Sulfonyl radical triggered selective iodosulfonylation and bicyclization of 1,6-dienes. Chem. Commun. 2021, 57, 8288–8291. [Google Scholar] [CrossRef]
- Lou, J.; Ma, J.; Xu, B.-H.; Zhou, Y.-G.; Yu, Z. Photoinduced, copper-catalyzed three-component annulation of gem-dialkylthio enynes. Org. Lett. 2020, 22, 5202–5206. [Google Scholar] [CrossRef]
- Gu, Y.; Dai, L.; Mao, K.M.; Zhang, J.H.; Wang, C.; Zhao, L.M.; Rong, L.C. Time-economical radical cascade cyclization/haloazidation of 1,6-enynes: Construction of highly functional succinimide derivatives. Org. Lett. 2020, 22, 2956–2960. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.-L.; Song, R.-J.; Qian, P.-C.; Li, J.-H. Cascade nitration/cyclization of 1,7-enynes with t-BuONO and H2O: One-pot self-assembly of pyrrolo [4,3,2-de]quinolinones. Angew. Chem. 2014, 126, 9163–9166. [Google Scholar] [CrossRef]
- An, Y.-Y.; Kuang, Y.-Y.; Wu, J. Synthesis of trifluoromethylated 3,4-dihydro- quinolin- 2(1H)-ones via a photo-induced radical cyclization of benzene-tethered 1,7-enynes with Togni reagent. Org. Chem. Front. 2016, 3, 994–998. [Google Scholar] [CrossRef]
- Wang, A.-F.; Zhu, Y.-L.; Wang, S.-L.; Hao, W.-J.; Li, G.; Tu, S.-J.; Jiang, B. Metal-free radical haloazidation of benzene-tethered 1,7-enynes leading to polyfunctionalized 3,4-dihydro- quinolin-2(1H)-ones. J. Org. Chem. 2016, 81, 1099–1105. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Hu, Y.-C.; Wang, H.-L.; Li, X.-C.; Wan, B.-S. Transition-metal controlled diastereodivergent radical cyclization/azidation cascade of 1,7-enynes. J. Org. Chem. 2016, 81, 4412–4420. [Google Scholar] [CrossRef]
- Zhu, Y.-L.; Jiang, B.; Hao, W.-J.; Wang, A.-F.; Qiu, J.-K.; Wei, P.; Wang, D.-C.; Li, G.; Tu, S.-J. A new cascade halosulfonylation of 1,7-enynes toward 3,4-dihydroquinolin-2(1H)-ones via sulfonyl radical-triggered addition/6-exo-dig cyclization. Chem. Commun. 2016, 52, 1907–1910. [Google Scholar] [CrossRef]
- Qiu, J.-K.; Shan, C.; Wang, D.-C.; Wei, P.; Jiang, B.; Tu, S.-J.; Li, G.; Guo, K. Metal-free radical-triggered selenosulfonation of 1,7-enynes for the rapid synthesis of 3,4-dihydro- quinolin-2(1H)-ones in batch and flow. Adv. Synth. Catal. 2017, 359, 4332–4339. [Google Scholar] [CrossRef]
- Meng, Q.; Chen, F.; Yu, W.; Han, B. Copper-catalyzed cascade cyclization of 1,7-enynes toward trifluoromethyl-substituted 1′H-spiro[azirine-2,4′-quinolin]-2′(3′H)-ones. Org. Lett. 2017, 19, 5186–5189. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, M.-W.; Di, Z.-C.; Cui, Y.-S.; Zhuang, K.-Q.; Qin, L.-Z.; Fang, Z.; Qiu, J.-K.; Li, G.; Guo, K. Photoredox-catalzyed halo-trifluoromethylation of 1,7-enynes for synthesis of 3,4-dihydroquinolin-2(1H)-ones. Adv. Synth. Catal. 2019, 361, 1835–1845. [Google Scholar] [CrossRef]
- Wang, S.-W.; Yu, J.; Zhou, Q.-Y.; Chen, S.-Y.; Xu, Z.-H.; Tang, S. Visible-light-induced atom transfer radical addition and cyclization of perfluoroalkyl halides with 1,n-Enynes. ACS Sustain. Chem. Eng. 2019, 7, 10154–10162. [Google Scholar] [CrossRef]
- Sacchelli, B.A.L.; Rocha, B.C.; Andrade, L.H. Cascade reactions assisted by microwave irradiation: Ultrafast construction of 2-quinolinone-fused γ-lactones from N-(o-ethynylaryl)- acrylamides and formamide. Org. Lett. 2021, 23, 5071–5075. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Wang, J.-S.; Ying, J.; Wu, X.-F. Palladium-catalyzed cascade carbonylative synthesis of perfluoroalkyl and carbonyl-containing 3,4-dihydroquinolin- 2(1H)-one derivatives. Org. Lett. 2022, 24, 8843–8847. [Google Scholar] [CrossRef]
- Hu, M.; Song, R.-J.; Li, J.-H. Metal-free radical 5-exo-dig cyclizations of phenol-linked 1,6-enynes for the synthesis of carbonylated benzofurans. Angew. Chem. Int. Ed. 2015, 54, 608–612. [Google Scholar] [CrossRef]
- Wu, W.-Q.; Yi, S.-J.; Huang, W.; Luo, D.; Jiang, H.-F. Ag-catalyzed oxidative cyclization reaction of 1,6-enynes and sodium sulfinate: Access to sulfonylated benzofurans. Org. Lett. 2017, 19, 2825–2828. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Verma, A.; Kadu, R.; Kumar, S. Visible-light-induced oxidant and metal-free dehydrogenative cascade trifluoromethylation and oxidation of 1,6-enynes with water. Chem. Sci. 2017, 8, 6633–6644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cheng, S.-J.; Cai, Z.-Q.; Liu, P.; Sun, P.-P. Radical addition cascade cyclization of 1,6-enynes with DMSO to access methylsulfonylated and carbonylated benzofurans under transition-metal-free conditions. J. Org. Chem. 2018, 83, 9344–9352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-B.; Wang, C.; Cui, M.-C.; Du, M.-S.; Li, W.-W.; Jia, Z.-X.; Zhao, Q. Synthesis of difluoroalkylated benzofuran, benzothiophene, and indole derivatives via palladium-catalyzed cascade difluoroalkylation and arylation of 1,6-enynes. Org. Lett. 2020, 22, 1149–1154. [Google Scholar] [CrossRef]
- Wang, L.; Qi, C.-R.; Cheng, R.-X.; Liu, H.-J.; Xiong, W.-F.; Jiang, H.-F. Direct access to trifluoromethyl-substituted carbamates from carbon dioxide via copper-catalyzed cascade cyclization of enynes. Org Lett. 2019, 21, 7386–7389. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, G.; Zhao, N.; Zhang, S.; Zhang, M.; Wang, L.; Li, Z.; Ying, J.; Gao, X. Palladium- catalyzed cascade difluoroalkylation and phosphinoylation of 2-vinyloxy arylalkynes: Selective synthesis of difluoroalkyl-containing tetrasubstituted alkenylphosphine oxides. J. Org. Chem. 2021, 86, 10105–10117. [Google Scholar] [CrossRef]
- Zhang, T.-S.; Hao, W.-J.; Cai, P.-J.; Li, G.-G.; Tu, S.-J.; Jiang, B. Copper-catalyzed annulation-cyanotrifluoromethylation of 1,6-enynes toward 1-indanones via a radical process. Front. Chem. 2020, 8, 234. [Google Scholar] [CrossRef]
- Ji, W.Z.; Shi, H.N.; Hao, W.J.; Wei, P.; Tu, S.J.; Jiang, B. Generation of stereodefined (Z)-3,4-dihydro- naphthalen-1(2H)-ones via copper-catalyzed annulation- cyano- trifluoromethylation of 1,7-enynes. Tetrahedron Lett. 2021, 64, 152722. [Google Scholar] [CrossRef]
- Wang, S.-C.; Shen, Y.-T.; Zhang, T.-S.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Cyclic oxime esters as deconstructive bifunctional reagents for cyanoalkyl esterification of 1,6-enynes. J. Org. Chem. 2021, 86, 15488–15497. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, N.-N.; Xu, Y.-L.; Chen, Y.-Y. Visible-light-induced radical cascade reaction of 1-allyl-2-ethynylbenzoimidazoles with thiosulfonates to assemble thiosulfonylated pyrrolo [1,2-a]benzimidazoles. J. Org. Chem. 2021, 86, 16882–16891. [Google Scholar] [CrossRef]
- Jiang, B.; Wu, Y.-N.; Chen, Z.-Z.; Wen, X.; Hao, W.-J.; Wang, S.-L.; Tu, S.-J. Synthesis of sulfonylated anti-indeno [1,2-d]pyridazines via oxidative three-component 1,5-enyne- bicyclization. Tetrahedron Lett. 2016, 57, 4246–4249. [Google Scholar] [CrossRef]
- Zhu, Y.-L.; Zhu, C.-F.; Zhou, P.; Hao, W.-J.; Wang, D.-C.; Tu, S.-J.; Jiang, B. Pd(II)-catalyzed carbonyl-directing activation of alkenes: Selective fluorosulfonylation and aminosulfonylation of 1,7-enynes. J. Org. Chem. 2018, 83, 9641–9653. [Google Scholar] [CrossRef]
- Xiang, H.-Y.; Zhao, Q.-L.; Xia, P.-J.; Xiao, J.-A.; Ye, Z.-P.; Xie, X.; Sheng, H.; Chen, X.-Q.; Yang, H. Visible-light-induced external radical-triggered annulation to access CF2-containing benzoxepine derivatives. Org. Lett. 2018, 20, 1363–1366. [Google Scholar] [CrossRef]
- Pan, R.; Hu, L.-Y.; Han, C.-H.; Lin, A.-J.; Yao, H.-Q. Cascade radical 1,6-addition/ cyclization of para-quinone methides: Leading to spiro [4.5]deca-6,9-dien-8-ones. Org. Lett. 2018, 20, 1974–1977. [Google Scholar] [CrossRef]
- Zuo, H.-D.; Cui, C.-C.; Guo, C.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Metal-catalyzed spiroannulation- fluoromethylfunctionaliztions of 1,5-enynes for the synthesis of stereodefined (Z)-spiroindenes. Chem. Asian J. 2020, 15, 4070–4078. [Google Scholar] [CrossRef]
- Zuo, H.-D.; Hao, W.-J.; Zhu, C.-F.; Guo, C.; Tu, S.-J.; Jiang, B. Electrochemical annulation-iodosulfonylation of para-quinone methides (p-QMs) containing 1,5-Enyne to access (E)-Spiroindenes. Org. Lett. 2020, 22, 4471–4477. [Google Scholar] [CrossRef]
- Zou, L.; Wang, L.; Sun, L.; Xie, X.F.; Li, P.H. Photo-driven haloazidation cyclization of 1,5-enynes having cyano groups with TMSN3 and NIS/NCS/NBS under metal-free conditions. Chem. Comm. 2020, 56, 7933–7936. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Wu, D.; Lin, N.; Liu, Y.-P.; Wang, L.; Zhu, X.-T.; Hao, W.-J.; Wang, S.-L.; Jiang, B. Kharasch-type photocyclization of 1,7-diynes for the stereospecific synthesis of tetrahydronaphthalen-1-ols. Tetrahedron Lett. 2021, 85, 153485. [Google Scholar] [CrossRef]
- Ouyang, X.-H.; Song, R.-J.; Li, Y.; Liu, B.; Li, J.-H. Metal-free oxidative ipso-carboacylation of alkynes: Synthesis of 3-acylspiro [4,5]trienones from N-arylpropiolamides and aldehydes. J. Org. Chem. 2014, 79, 4582–4589. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.-T.; Song, R.-J.; Ouyang, X.-H.; Li, Y.; Li, H.-B.; Li, J.-H. Copper-catalyzed oxidative ipso-carboalkylation of activated alkynes with ethers leading to 3-etherified azaspiro [4.5]trienones. Org. Chem. Front. 2014, 1, 484–489. [Google Scholar] [CrossRef]
- Hua, H.-L.; He, Y.-T.; Qiu, Y.-F.; Li, Y.-X.; Song, B.; Gao, P.; Song, X.-R.; Guo, D.-H.; Liu, X.-Y.; Liang, Y.-M. Copper-catalyzed difunctionalization of activated alkynes by radical oxidation-tandem cyclization/dearomatization to synthesize 3-trifluoromethyl spiro-[4.5] trienones. Chem. Eur. J. 2015, 21, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.-H.; Wei, W.; Yang, D.-S.; Zhang, J.-M.; Xu, Z.-H.; Wen, J.-W.; Wang, H. Silver-catalyzed direct spirocyclization of alkynes with thiophenols: A simple and facile approach to 3-thioazaspiro [4,5]trienones. RSC Adv. 2015, 5, 84657–84661. [Google Scholar] [CrossRef]
- Yang, X.-H.; Ouyang, X.-H.; Wei, W.-T.; Song, R.-J.; Li, J.-H. Nitrative spirocyclization mediated by TEMPO: Synthesis of nitrated spirocycles from N-arylpropiolamides, tert-butyl nitrite and water. Adv. Synth. Catal. 2015, 357, 1161–1166. [Google Scholar] [CrossRef]
- Wen, J.-W.; Wei, W.; Xue, S.-N.; Yang, D.-S.; Lou, Y.; Gao, C.-Y.; Wang, H. Metal-free oxidative spirocyclization of alkynes with sulfonylhydrazides leading to 3-sulfonated azaspiro-[4,5]trienones. J. Org. Chem. 2015, 80, 4966–4972. [Google Scholar] [CrossRef]
- Jin, D.-P.; Gao, P.; Chen, D.-Q.; Chen, S.; Wang, J.; Liu, X.-Y.; Liang, Y.-M. AgSCF3-mediated oxidative trifluoromethythiolation of alkynes with dearomatization to synthesize SCF3- substituted spiro [4,5]trienones. Org. Lett. 2016, 18, 3486–3489. [Google Scholar] [CrossRef]
- Cheng, F.; Bai, X.; Sun, Q.W.; Zhu, G.F.; Dong, Y.X.; Yang, Y.Y.; Gao, X.L.; Guo, B.; Tang, L.; Zhang, J.Q. Cobalt-promoted synthesis of sulfurated oxindoles via radical annulation of N-arylacrylamides with disulfides. Org. Biomol. Chem. 2022, 20, 6423–6431. [Google Scholar] [CrossRef]
- Tang, S.; Deng, Y.-L.; Li, J.; Wang, W.-X.; Wang, Y.-C.; Li, Z.-Z.; Yuan, L.; Chen, S.-L.; Sheng, R.-L. Aerobic oxidative cyclization of benzamides via meta-selective C–H tert-alkylation: Rapid Entry to 7-alkylated isoquinolinediones. Chem. Commun. 2016, 52, 4470–4473. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Ning, Z.; Zhang, X.; Fu, Y.; Du, Z. Copper- and visible-light-catalyzed cascade radical cyclization of N-propargylindoles with cyclic ethers. J. Org. Chem. 2022. [Google Scholar] [CrossRef]
- Scherübl, M.; Daniliuc, C.G.; Studer, A. Arynes as Radical Acceptors: TEMPO-mediated cascades comprising addition, cyclization and trapping. Angew. Chem. Int. Ed. 2021, 60, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Gueddari, F.E.; Grimaldi, J.R.; Hatem, J.M. Tosyl mediated radical cyclization of allylallenes. Tetrahedron Lett. 1995, 36, 6685–6688. [Google Scholar] [CrossRef]
- Zeng, R.; Fu, C.-L.; Ma, S.-M. Formal alkylation of allenes through highly selective radical cyclizations of allene-enes. Angew. Chem. Int. Ed. 2012, 51, 3888–3891. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zhang, X.; Shi, M. Visible-light mediated cascade cyclization of ene-vinylidenecyclo- propanes: Access to fluorinated heterocyclic compounds. Org. Chem. Front. 2021, 8, 3796–3801. [Google Scholar] [CrossRef]
- Li, Y.-M.; Wang, S.-S.; Yu, F.-C.; Shen, Y.-H.; Chang, K.-J. Copper-catalyzed cascade addition/cyclization: Highly efficient access to phosphonylated quinoline-2,4(1H,3H)- diones. Org. Biomol. Chem. 2015, 13, 5376–5380. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Fu, H.; Shen, Y.; Sun, M.; Li, Y.-M. Oxidative radical addition/cyclization cascade for the construction of carbonyl-containing quinoline-2,4(1H,3H)-diones. J. Org. Chem. 2016, 81, 2920–2929. [Google Scholar] [CrossRef]
- Li, M.; Song, R.-J.; Li, J.-H. Nickel-promoted oxidative ipso-annulation of N-(p-methoxyaryl)- propiolamides with α-carbonyl alkyl bromides. Chin. J. Chem. 2017, 35, 299–302. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.-L.; Zhou, C.-S.; Xiong, B.-Q.; Zhang, P.-L.; Yang, C.-A.; Tang, K.-W. Visible-light-mediated ipso-carboacylation of alkynes: Synthesis of 3-acylspiro-[4,5]trienones from N-(p-methoxyaryl)propiolamides and acyl chlorides. J. Org. Chem. 2018, 83, 2210–2218. [Google Scholar] [CrossRef]
- Chen, P.; Xie, J.; Chen, Z.; Xiong, B.-Q.; Liu, Y.; Yang, C.-A.; Tang, K.-W. Visible-light-mediated nitrogen-centered radical strategy: Preparation of 3-acylated spiro [4,5]trienones. Adv. Synth. Catal. 2021, 363, 4440–4446. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Li, C.-J.; Wei, Z.-L.; Liao, W.-W. Multicomponent cyclization with an inorganic sulfur dioxide surrogate: Straightforward construction of difluorinated benzosultams. Org. Lett. 2022, 24, 9112–9117. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).