Construction of Smart Biomaterials for Promoting Diabetic Wound Healing
Abstract
1. Introduction
2. Wound Healing
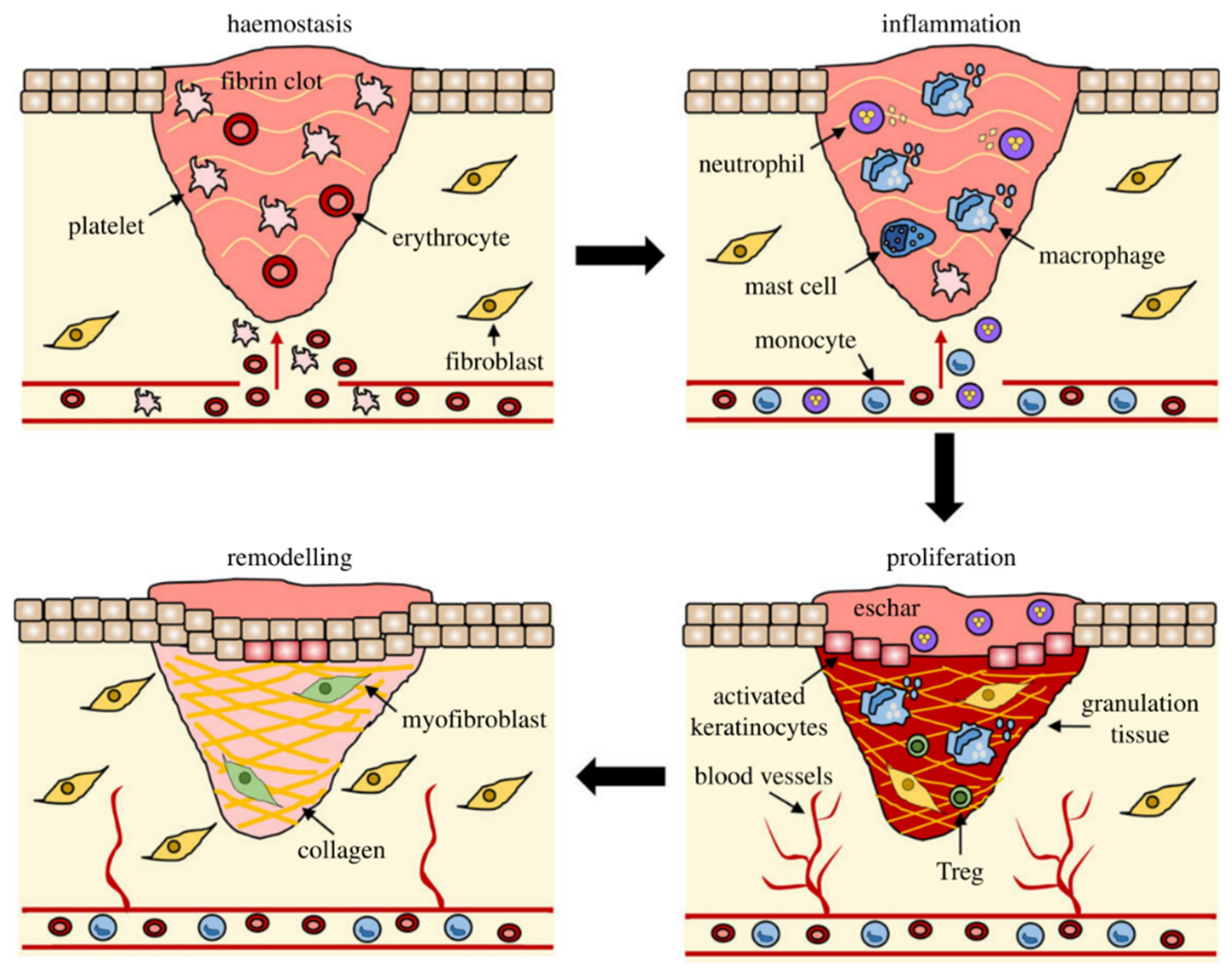
2.1. Normal Wound Healing
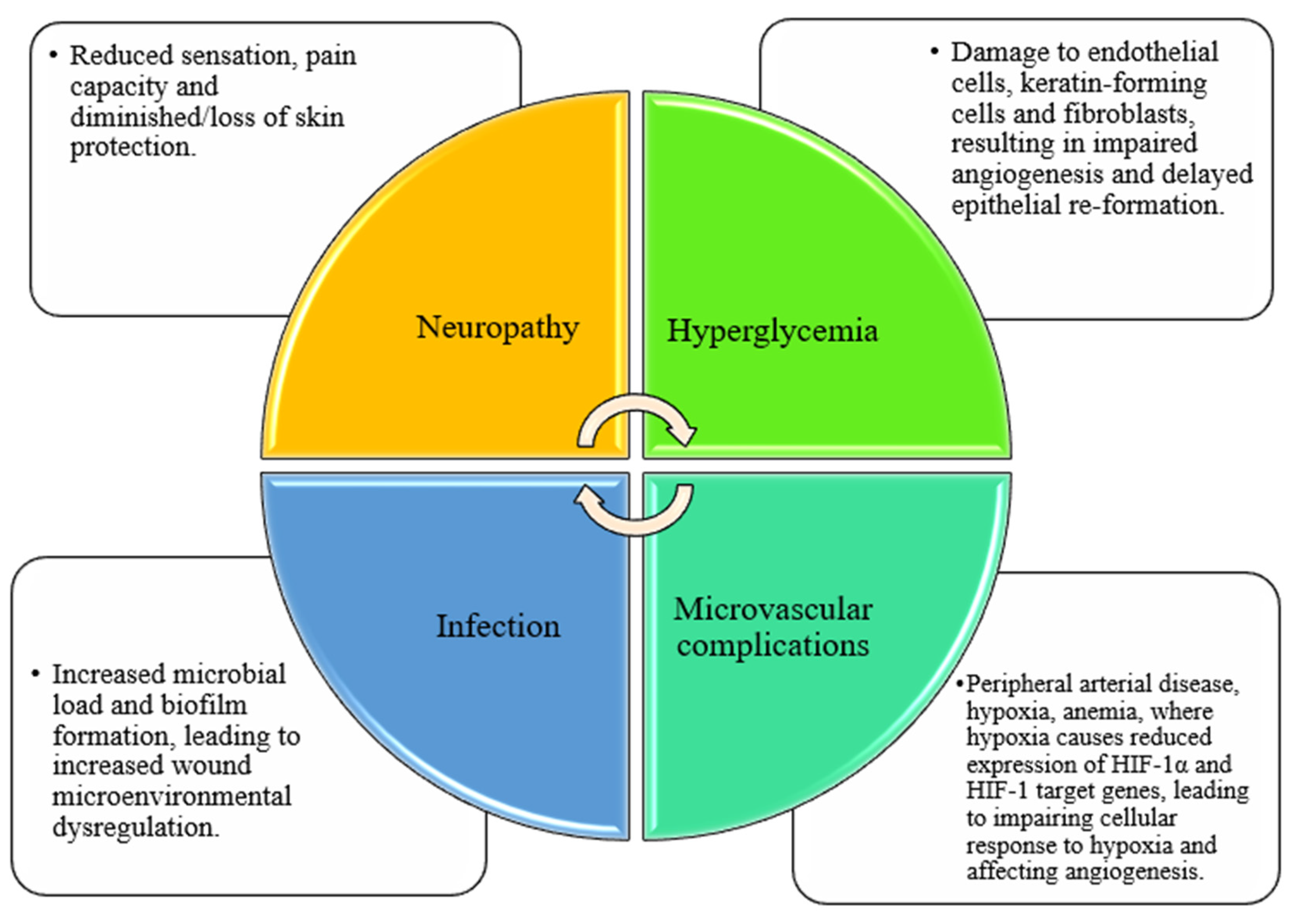
2.2. Diabetic Wound Healing and Related Influencing Factors
2.3. The Limitations of Traditional Dressings for the Treatment of Diabetic Wounds
3. The Scaffolds of New Smart Biomaterials
3.1. Natural Polymers
3.2. Synthetic Polymers
4. The Application of Smart Biomaterials in Diabetic Wounds
4.1. Delivery System for Diabetic Wound Healing
4.1.1. Drugs with Anti-Inflammatory Activity
4.1.2. Bioactive Agency
Exosomes
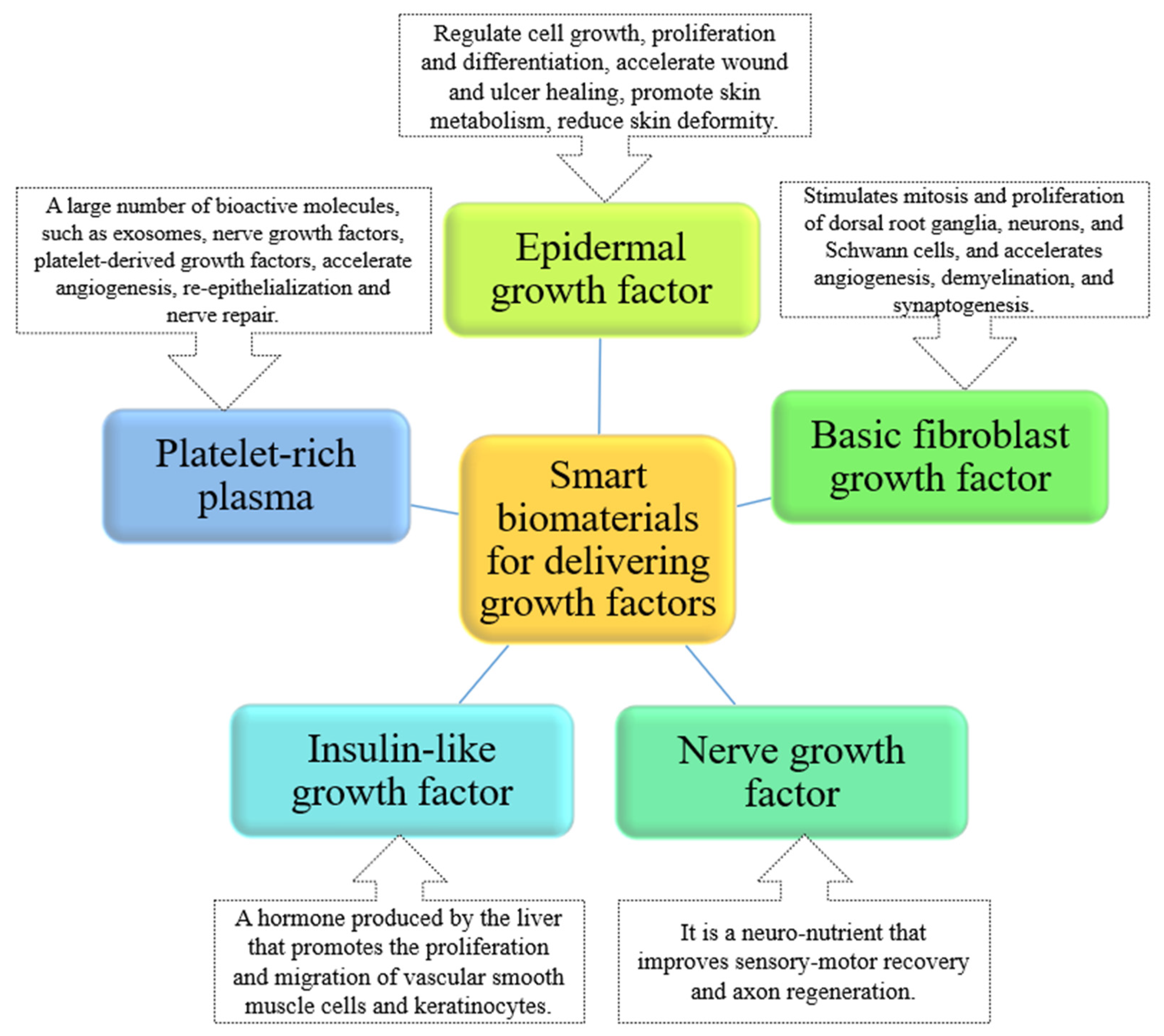
Growth Factors
Probiotics
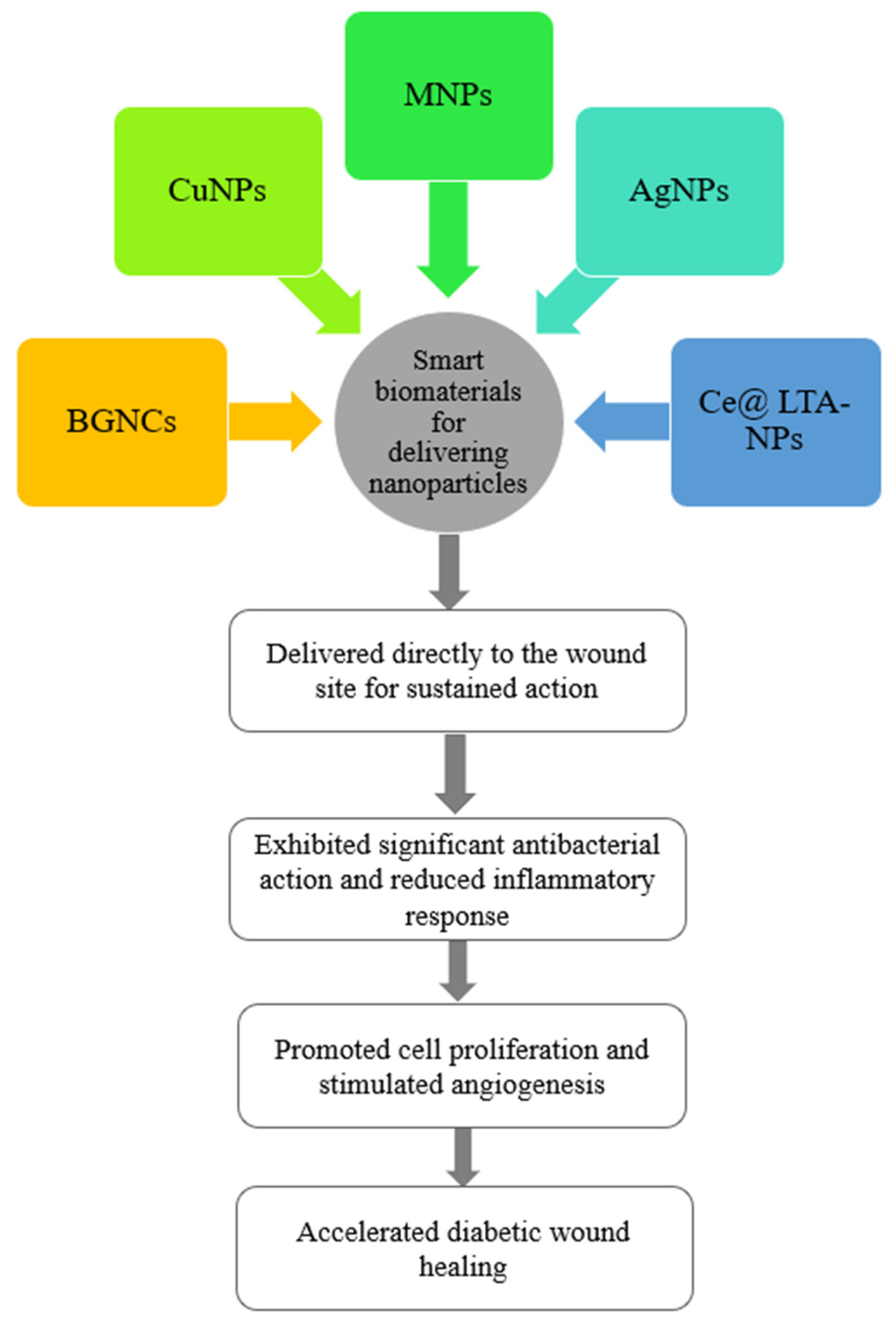
4.1.3. Antibacterial Nanoparticles
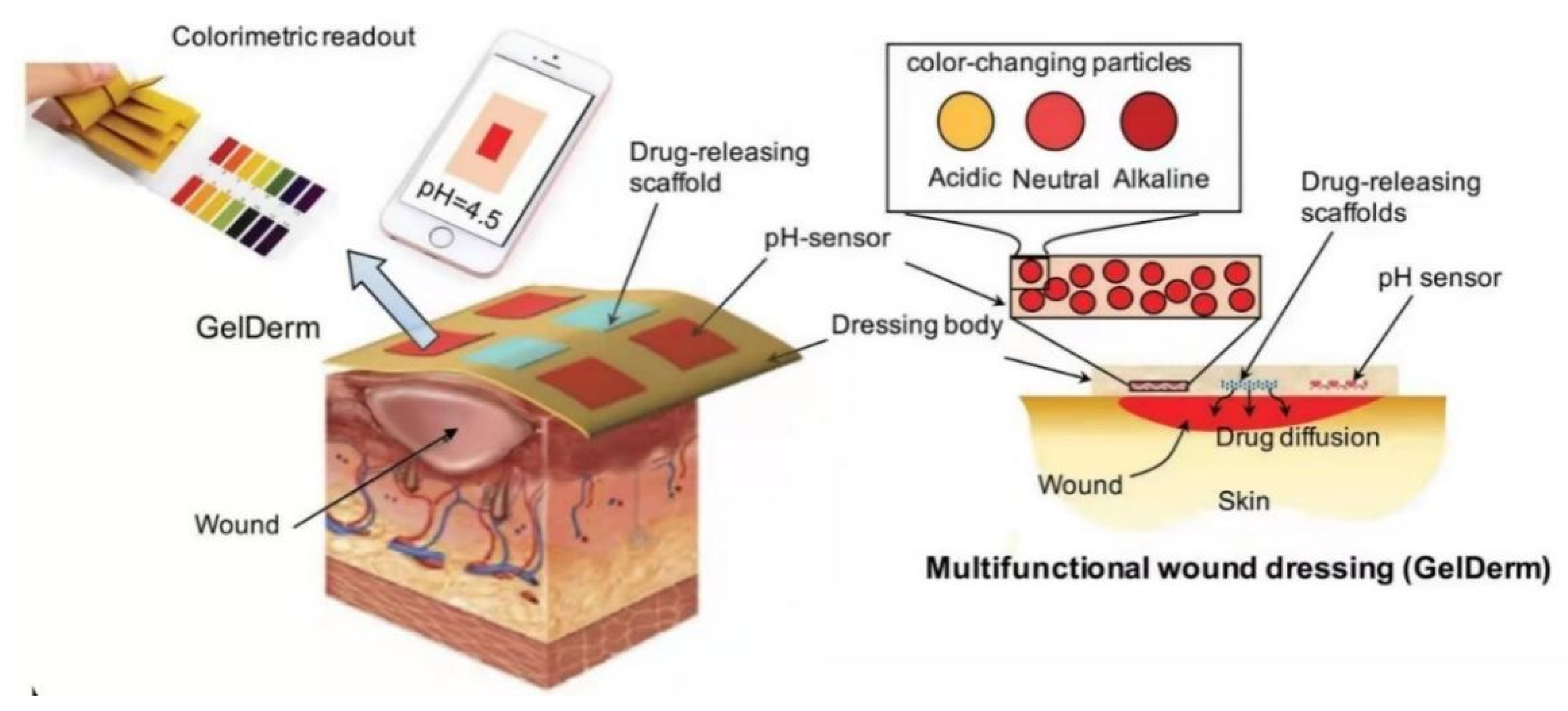
4.2. Stimulus-Response System for Diabetic Wound Healing
4.3. Other Types of Systems for Diabetic Wound Healing
5. The Advantages of Smart Biomaterials
- ✧
- As the scaffolds of intelligent material, natural, and synthetic polymers, it can serve a similar function as the extracellular matrix component, with good biocompatibility, biodegradability, mechanical stability, self-healing, injectable resistance, adhesion, and antimicrobial properties. Furthermore, these can cover wounds of irregular shape and maintain a moderately moist environment;
- ✧
- Smart biomaterials can act as a delivery system for the topical application of drugs to wounds, reducing irritation and drug resistance, delivering bioactive agency containing exosomes, and growth factors. Such a delivery system can overcome their short half-life and rapid clearance, effectively exerting their effects of inhibiting excessive oxidative stress and inflammatory responses, promoting the conversion from the inflammatory stage to the proliferative and remodeling stage, and thus accelerating wound recovery;
- ✧
- Furthermore, smart biomaterials also respond to the wound microenvironment and monitor diabetic wounds, sensing changes in the wound microenvironment in real-time. Integration with electronic platforms can help medical staff to better manage chronic wounds and provide data that feed into clinical decision-making.
6. Conclusions, Challenges, and Outlook
- ✧
- Diabetic wound restoration is a dynamic and sequential process, with each stage closely related to ensuring tissue regeneration. However, most studies examine only one or two stages, instead of the entire process of wound closure;
- ✧
- Most of the reported bioactive dressings are largely dependent on the activity of loaded biologic agents to enhance diabetic wound repair and skin reconstruction, and there is a relative lack of focus on the inherent pro-healing properties of biomaterials;
- ✧
- Loaded drugs with anti-inflammatory activity and bioactive agency show explosive release at the early stage, potentially reducing bioavailability and causing damage to skin tissue.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, D.; Zhang, Y.; Bowers, D.T.; Liu, W.; Ma, M. Functional Hydrogels for Diabetic Wound Management. APL Bioeng. 2021, 5, 031503. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.; Mandal, B.B. Silk Biomaterials in Wound Healing and Skin Regeneration Therapeutics: From Bench to Bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Everett, E.; Mathioudakis, N. Update on Management of Diabetic Foot Ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, H.; Bai, Y.; Wang, Y.; Tao, L.; Wei, Y.; Fan, Y.; Guo, X.; Liu, H. Improving Chronic Diabetic Wound Healing through an Injectable and Self-Healing Hydrogel with Platelet-Rich Plasma Release. ACS Appl. Mater. Interfaces 2020, 12, 55659–55674. [Google Scholar] [CrossRef]
- Bandyk, D.F. The Diabetic Foot: Pathophysiology, Evaluation, and Treatment. Semin. Vasc. Surg. 2018, 31, 43–48. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Holl, J.; Kowalewski, C.; Zimek, Z.; Fiedor, P.; Kaminski, A.; Oldak, T.; Moniuszko, M.; Eljaszewicz, A. Chronic Diabetic Wounds and Their Treatment with Skin Substitutes. Cells 2021, 10, 655. [Google Scholar] [CrossRef]
- Boniakowski, A.E.; Kimball, A.S.; Jacobs, B.N.; Kunkel, S.L.; Gallagher, K.A. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J. Immunol. 2017, 199, 17–24. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Yang, R.; Wang, J.; Chen, X.; Shi, Y.; Xie, J. Epidermal Stem Cells in Wound Healing and Regeneration. Stem Cells Int. 2020, 2020, 9148310. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Z.; Zhao, M.; Liu, G.; Wu, J. Advances of Hydrogel Dressings in Diabetic Wounds. Biomater. Sci. 2021, 9, 1530–1546. [Google Scholar] [CrossRef] [PubMed]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [PubMed]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in Wound Healing and Skin Tissue Engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zheng, J.; Wang, Y.; Zhang, W.; Hu, D. Emerging Progress on the Mechanism and Technology in Wound Repair. Biomed. Pharmacother. 2019, 117, 109191. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Han, K.; Dong, K.; Zheng, C.; Zhang, Y.; Long, Q.; Lu, T. Potential Applications of Nanomaterials and Technology for Diabetic Wound Healing. Int. J. Nanomed. 2020, 15, 9717–9743. [Google Scholar] [CrossRef]
- Karppinen, S.M.; Heljasvaara, R.; Gullberg, D.; Tasanen, K.; Pihlajaniemi, T. Toward Understanding Scarless Skin Wound Healing and Pathological Scarring [Version 1; Peer Review: 2 Approved]. F1000Research 2019, 8, 787. [Google Scholar] [CrossRef]
- Rousselle, P.; Braye, F.; Dayan, G. Re-Epithelialization of Adult Skin Wounds: Cellular Mechanisms and Therapeutic Strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef]
- Blanco-Fernandez, B.; Castaño, O.; Mateos-Timoneda, M.Á.; Engel, E.; Pérez-Amodio, S. Nanotechnology Approaches in Chronic Wound Healing. Adv. Wound Care 2021, 10, 234–256. [Google Scholar] [CrossRef]
- Burgess, J.L.; Wyant, W.A.; Abujamra, B.A.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Fui, L.W.; Lok, M.P.W.; Govindasamy, V.; Yong, T.K.; Lek, T.K.; Das, A.K. Understanding the Multifaceted Mechanisms of Diabetic Wound Healing and Therapeutic Application of Stem Cells Conditioned Medium in the Healing Process. J. Tissue Eng. Regen. Med. 2019, 13, 2218–2233. [Google Scholar] [CrossRef] [PubMed]
- Den Dekker, A.; Davis, F.M.; Kunkel, S.L.; Gallagher, K.A. Targeting Epigenetic Mechanisms in Diabetic Wound Healing. Transl. Res. 2019, 204, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Healthc. Mater. 2019, 8, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxidative Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent Advancements in Biopolymer and Metal Nanoparticle-Based Materials in Diabetic Wound Healing Management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef]
- Davis, F.M.; Kimball, A.; Boniakowski, A.; Gallagher, K. Dysfunctional Wound Healing in Diabetic Foot Ulcers: New Crossroads. Curr. Diabetes Rep. 2018, 18, 2. [Google Scholar] [CrossRef]
- Aitcheson, S.M.; Frentiu, F.D.; Hurn, S.E.; Edwards, K.; Murray, R.Z. Skin Wound Healing: Normal Macrophage Function and Macrophage Dysfunction in Diabetic Wounds. Molecules 2021, 26, 4917. [Google Scholar] [CrossRef]
- Lou, P.; Liu, S.; Wang, Y.; Pan, C.; Xu, X.; Zhao, M.; Liao, G.; Yang, G.; Yuan, Y.; Li, L.; et al. Injectable Self-Assembling Peptide Nanofiber Hydrogel as a Bioactive 3D Platform to Promote Chronic Wound Tissue Regeneration. Acta Biomater. 2021, 135, 100–112. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lee, H.C.; Chen, C.L.; Kuo, M.C.; Ramachandran, S.; Chen, R.F.; Kuo, Y.R. The Effects of Silver-Releasing Foam Dressings on Diabetic Foot Ulcer Healing. J. Clin. Med. 2021, 10, 1495. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, J.H.; Wang, S.H.; Ko, T.H.; Tseng, G.C. Evaluation of Silver-Containing Activated Carbon Fiber for Wound Healing Study: In Vitro and in Vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 2288–2296. [Google Scholar] [CrossRef]
- Mude, L.; Sanapalli, B.K.R.; Anoop Narayanan, V.; Singh, S.K.; Karri, V.V.S.R. Overview of in Situ Gelling Injectable Hydrogels for Diabetic Wounds. Drug Dev. Res. 2021, 82, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Gianino, E.; Miller, C.; Gilmore, J. Smart Wound Dressings for Diabetic Chronic Wounds. Bioengineering 2018, 5, 51. [Google Scholar] [CrossRef]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; An, S.J.; Jeong, S.I.; Gwon, H.J.; Lim, Y.M.; Nho, Y.C. Chestnut Honey Impregnated Carboxymethyl Cellulose Hydrogel for Diabetic Ulcer Healing. Polymers 2017, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, Y.; Shu, F.; Zhou, R.; Bao, B.; Xiao, S.; Li, K.; Lin, Q.; Zhu, L.; Xia, Z. In Situ-Formed Adhesive Hyaluronic Acid Hydrogel with Prolonged Amnion-Derived Conditioned Medium Release for Diabetic Wound Repair. Carbohydr. Polym. 2022, 276, 118752. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Sohail, M.; Khan, S.A.; Kousar, M. Improved Drug Delivery and Accelerated Diabetic Wound Healing by Chondroitin Sulfate Grafted Alginate-Based Thermoreversible Hydrogels. Mater. Sci. Eng. C 2021, 126, 112169. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, S.; Kanda, N.; Hirasawa, Y.; Noda, K.; Matsuura, Y.; Suzuki, S.; Kawai, K. The Utility of Silk-Elastin Hydrogel as a New Material for Wound Healing. Plast. Reconstr. Surg. Glob. Open 2018, 6, 6–11. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Wang, S.; Dong, C.; Zhang, X.; Zhang, R.; Yang, L. Dextran and Peptide-Based PH-Sensitive Hydrogel Boosts Healing Process in Multidrug-Resistant Bacteria-Infected Wounds. Carbohydr. Polym. 2022, 278, 118994. [Google Scholar] [CrossRef] [PubMed]
- Certelli, A.; Valente, P.; Uccelli, A.; Grosso, A.; Di Maggio, N.; D’Amico, R.; Briquez, P.S.; Hubbell, J.A.; Wolff, T.; Gürke, L.; et al. Robust Angiogenesis and Arteriogenesis in the Skin of Diabetic Mice by Transient Delivery of Engineered VEGF and PDGF-BB Proteins in Fibrin Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 552. [Google Scholar] [CrossRef]
- Rezvanian, M.; Mohd Amin, M.C.I.; Ng, S.F. Development and Physicochemical Characterization of Alginate Composite Film Loaded with Simvastatin as a Potential Wound Dressing. Carbohydr. Polym. 2016, 137, 295–304. [Google Scholar] [CrossRef]
- Qian, B.; Li, J.; Guo, K.; Guo, N.; Zhong, A.; Yang, J.; Wang, J.; Xiao, P.; Sun, J.; Xiong, L. Antioxidant Biocompatible Composite Collagen Dressing for Diabetic Wound Healing in Rat Model. Regen. Biomater. 2021, 8, rbab003. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver Nanoparticle Impregnated Chitosan-PEG Hydrogel Enhances Wound Healing in Diabetes Induced Rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Elshaarani, T.; Yu, H.; Wang, L.; Feng, J.; Li, C.; Zhou, W.; Khan, A.; Usman, M.; Amin, B.U.; Khan, R. Chitosan Reinforced Hydrogels with Swelling-Shrinking Behaviors in Response to Glucose Concentration. Int. J. Biol. Macromol. 2020, 161, 109–121. [Google Scholar] [CrossRef]
- Choi, M.; Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Yoo, J.W. Chitosan-Based Nitric Oxide-Releasing Dressing for Anti-Biofilm and in Vivo Healing Activities in MRSA Biofilm-Infected Wounds. Int. J. Biol. Macromol. 2020, 142, 680–692. [Google Scholar] [CrossRef]
- Hasan, N.; Lee, J.; Ahn, H.J.; Hwang, W.R.; Bahar, M.A.; Habibie, H.; Amir, M.N.; Lallo, S.; Son, H.J.; Yoo, J.W. Nitric Oxide-releasing Bacterial Cellulose/Chitosan Crosslinked Hydrogels for the Treatment of Polymicrobial Wound Infections. Pharmaceutics 2022, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic Acid—Based Wound Dressings: A Review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Bhardwaj, R.; Arora, R.; Singh, A.; Mukherjee, M.; Rajput, S.K. Acceleration of Wound Healing in Diabetic Rats through Poly Dimethylaminoethyl Acrylate-Hyaluronic Acid Polymeric Hydrogel Impregnated with a Didymocarpus Pedicellatus Plant Extract. ACS Omega 2020, 5, 24239–24246. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Song, L.; Sun, B.; Chu, D.; Yang, L.; Li, M.; Li, H.; Dai, Y.; Yu, Z.; Guo, J. Modulation of Macrophages by a Paeoniflorin-Loaded Hyaluronic Acid-Based Hydrogel Promotes Diabetic Wound Healing. Mater. Today Bio 2021, 12, 100139. [Google Scholar] [CrossRef] [PubMed]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/Hyaluronic Acid/Alginate and an Assorted Polymers Loaded with Honey, Plant, and Marine Compounds for Progressive Wound Healing—Know-How. Int. J. Biol. Macromol. 2021, 186, 656–685. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, W.; Lei, Y.; Gaucher, C.; Pei, S.; Zhang, J.; Xia, X. Edaravone-Loaded Alginate-Based Nanocomposite Hydrogel Accelerated Chronic Wound Healing in Diabetic Mice. Mar. Drugs 2019, 17, 285. [Google Scholar] [CrossRef] [PubMed]
- Barros, N.R.; Ahadian, S.; Tebon, P.; Rudge, M.V.C.; Barbosa, A.M.P.; Herculano, R.D. Highly Absorptive Dressing Composed of Natural Latex Loaded with Alginate for Exudate Control and Healing of Diabetic Wounds. Mater. Sci. Eng. C 2021, 119, 111589. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Sigen, A.; Gao, Y.; Guo, L.; Creagh-Flynn, J.; Zhou, D.; Greiser, U.; Dong, Y.; Wang, F.; Tai, H.; et al. A Hybrid Injectable Hydrogel from Hyperbranched PEG Macromer as a Stem Cell Delivery and Retention Platform for Diabetic Wound Healing. Acta Biomater. 2018, 75, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef] [PubMed]
- Man, Z.; Sidi, L.; Xubo, Y.; Jin, Z.; Xin, H. An in Situ Catechol Functionalized ε-Polylysine/Polyacrylamide Hydrogel Formed by Hydrogen Bonding Recombination with High Mechanical Property for Hemostasis. Int. J. Biol. Macromol. 2021, 191, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Shao, C.; Cui, C.; Xu, F.; Lei, J.; Yang, J. Autonomous Self-Healing Silk Fibroin Injectable Hydrogels Formed via Surfactant-Free Hydrophobic Association. ACS Appl. Mater. Interfaces 2020, 12, 1628–1639. [Google Scholar] [CrossRef]
- Murakawa, K.; King, D.R.; Sun, T.; Guo, H.; Kurokawa, T.; Gong, J.P. Polyelectrolyte Complexation via Viscoelastic Phase Separation Results in Tough and Self-Recovering Porous Hydrogels. J. Mater. Chem. B 2019, 7, 5296–5305. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.N.; Zeng, H.; Ji, X.; Xie, T.; Yan, X.; Wu, Z.L.; Huang, F. Reversible Ion-Conducting Switch in a Novel Single-Ion Supramolecular Hydrogel Enabled by Photoresponsive Host–Guest Molecular Recognition. Adv. Mater. 2019, 31, 1807328. [Google Scholar] [CrossRef]
- Pentlavalli, S.; Chambers, P.; Sathy, B.N.; O’Doherty, M.; Chalanqui, M.; Kelly, D.J.; Haut-Donahue, T.; McCarthy, H.O.; Dunne, N.J. Simple Radical Polymerization of Poly(Alginate-Graft-N-Isopropylacrylamide) Injectable Thermoresponsive Hydrogel with the Potential for Localized and Sustained Delivery of Stem Cells and Bioactive Molecules. Macromol. Biosci. 2017, 17, 1700118. [Google Scholar] [CrossRef]
- Wang, K.; Dong, R.; Tang, J.; Li, H.; Dang, J.; Zhang, Z.; Yu, Z.; Guo, B.; Yi, C. Exosomes Laden Self-Healing Injectable Hydrogel Enhances Diabetic Wound Healing via Regulating Macrophage Polarization to Accelerate Angiogenesis. Chem. Eng. J. 2022, 430, 132664. [Google Scholar] [CrossRef]
- Schanuel, F.S.; Raggio Santos, K.S.; Monte-Alto-Costa, A.; de Oliveira, M.G. Combined Nitric Oxide-Releasing Poly(Vinyl Alcohol) Film/F127 Hydrogel for Accelerating Wound Healing. Colloids Surf. B Biointerfaces 2015, 130, 182–191. [Google Scholar] [CrossRef]
- Khan, A.H.; Cook, J.K.; Wortmann, W.J.; Kersker, N.D.; Rao, A.; Pojman, J.A.; Melvin, A.T. Synthesis and Characterization of Thiol-Acrylate Hydrogels Using a Base-Catalyzed Michael Addition for 3D Cell Culture Applications. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2020, 108, 2294–2307. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; Yu, F.; Zhao, Y.X.; Mo, X.M.; Pan, J.F. In Situ Forming Hydrogel of Natural Polysaccharides through Schiff Base Reaction for Soft Tissue Adhesive and Hemostasis. Int. J. Biol. Macromol. 2020, 147, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Lake, R.; Park, S.; Edwards, S.; Jones, C.; Jeong, K.J. Injectable Macroporous Hydrogel Formed by Enzymatic Cross-Linking of Gelatin Microgels. ACS Appl. Bio Mater. 2018, 1, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Ghobril, C.; Grinstaff, M.W. The Chemistry and Engineering of Polymeric Hydrogel Adhesives for Wound Closure: A Tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, X.; Yu, J.; Chen, X.; Wang, R.; Zhang, M.; Zhang, Q.; Zhang, Y.; Wang, S.; Cheng, Y. Bioactive Skin-Mimicking Hydrogel Band-Aids for Diabetic Wound Healing and Infectious Skin Incision Treatment. Bioact. Mater. 2021, 6, 3962–3975. [Google Scholar] [CrossRef]
- Obuobi, S.; Voo, Z.X.; Low, M.W.; Czarny, B.; Selvarajan, V.; Ibrahim, N.L.; Yang, Y.Y.; Lai, P.; Ee, R. Phenylboronic Acid Functionalized Polycarbonate Hydrogels for Controlled Release of Polymyxin B in Pseudomonas aeruginosa Infected Burn Wounds. Adv. Healthc. Mater. 2018, 7, 1701388. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, X.; Zhang, R.; Zhang, K.; Li, Y.; Xu, F.J. Self-Assembled Herbal Medicine Encapsulated by an Oxidation-Sensitive Supramolecular Hydrogel for Chronic Wound Treatment. ACS Appl. Mater. Interfaces 2020, 12, 56898–56907. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, L.; Liu, P.; Yu, T.; Lin, C.; Yan, C.; Hu, Y.; Zhou, W.; Sun, Y.; Panayi, A.C.; et al. All-in-One: Multifunctional Hydrogel Accelerates Oxidative Diabetic Wound Healing through Timed-Release of Exosome and Fibroblast Growth Factor. Small 2021, 18, 2104229. [Google Scholar] [CrossRef]
- Chen, T.Y.; Wen, T.K.; Dai, N.T.; Hsu, S.H. Cryogel/Hydrogel Biomaterials and Acupuncture Combined to Promote Diabetic Skin Wound Healing through Immunomodulation. Biomaterials 2021, 269, 120608. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, G.; Hong, W.; Zhang, Y.; Xu, B.; Song, G.; Liu, T.; Hong, C.; Ruan, L. Rapid Gelation of Oxidized Hyaluronic Acid and Succinyl Chitosan for Integration with Insulin-Loaded Micelles and Epidermal Growth Factor on Diabetic Wound Healing. Mater. Sci. Eng. C 2020, 117, 111273. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Wu, Y.; Zhao, Y.; Chen, H.; Yuan, Y.; Xu, K.; Zhang, H.; Lu, Y.; Wang, J. Heparin-Poloxamer Thermosensitive Hydrogel Loaded with BFGF and NGF Enhances Peripheral Nerve Regeneration in Diabetic Rats. Biomaterials 2018, 168, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef] [PubMed]
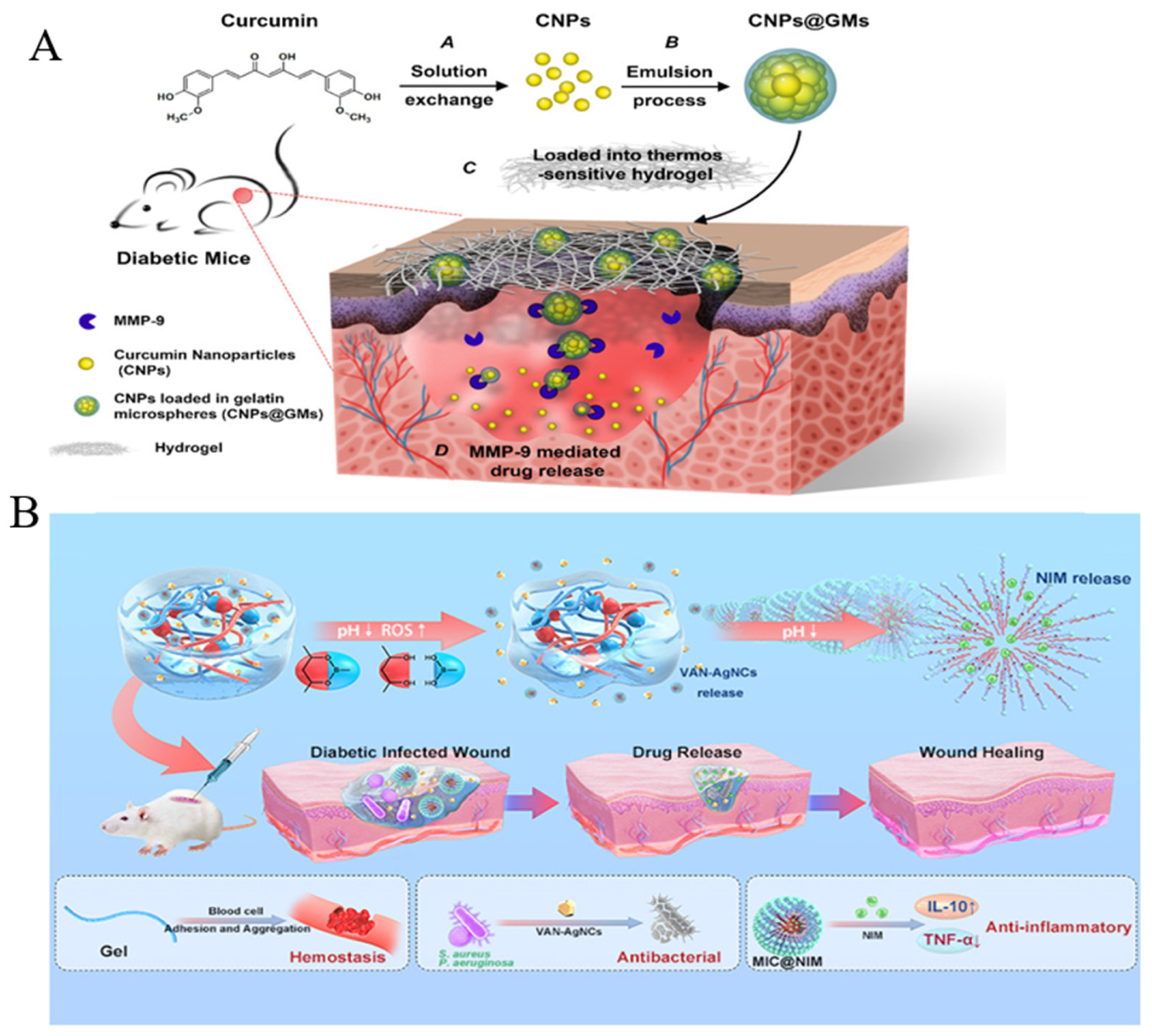
- Liu, J.; Chen, Z.; Wang, J.; Li, R.; Li, T.; Chang, M.; Yan, F.; Wang, Y. Encapsulation of Curcumin Nanoparticles with MMP9-Responsive and Thermos-Sensitive Hydrogel Improves Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 16315–16326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Long, L.; Yang, L.; Fu, D.; Hu, C.; Kong, Q.; Wang, Y. Inflammation-Responsive Drug-Loaded Hydrogels with Sequential Hemostasis, Antibacterial, and Anti-Inflammatory Behavior for Chronically Infected Diabetic Wound Treatment. ACS Appl. Mater. Interfaces 2021, 13, 33584–33599. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
- Henriques-Antunes, H.; Cardoso, R.M.S.; Zonari, A.; Correia, J.; Leal, E.C.; Jiménez-Balsa, A.; Lino, M.M.; Barradas, A.; Kostic, I.; Gomes, C.; et al. The Kinetics of Small Extracellular Vesicle Delivery Impacts Skin Tissue Regeneration. ACS Nano 2019, 13, 8694–8707. [Google Scholar] [CrossRef]
- Shiekh, P.A.; Singh, A.; Kumar, A. Exosome Laden Oxygen Releasing Antioxidant and Antibacterial Cryogel Wound Dressing OxOBand Alleviate Diabetic and Infectious Wound Healing. Biomaterials 2020, 249, 120020. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, W. The Fabrication of a Highly e Ffi Cient Self-Healing Hydrogel from Natural Biopolymers Loaded with Exosomes for the Synergistic Promotion of Severe Wound Healing. Biomater. Sci. 2020, 8, 313–324. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.M.; Han, S.H.; Ko, E.A.; Yoon, D.S.; Park, I.K.; Shin, H.C.; Park, K.H.; Lee, J.W. Development of Stabilized Dual Growth Factor-Loaded Hyaluronate Collagen Dressing Matrix. J. Tissue Eng. 2021, 12, 1999750. [Google Scholar] [CrossRef]
- Lin, M.J.; Lu, M.C.; Chan, Y.C.; Huang, Y.F.; Chang, H.Y. An Insulin-like Growth Factor-1 Conjugated Bombyx Mori Silk Fibroin Film for Diabeticwound Healing: Fabrication, Physicochemical Property Characterization, and Dosage Optimization in Vitro and in Vivo. Pharmaceutics 2021, 13, 1459. [Google Scholar] [CrossRef]
- Mohtashami, M.; Mohamadi, M.; Azimi-Nezhad, M.; Saeidi, J.; Nia, F.F.; Ghasemi, A. Lactobacillus Bulgaricus and Lactobacillus Plantarum Improve Diabetic Wound Healing through Modulating Inflammatory Factors. Biotechnol. Appl. Biochem. 2021, 68, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, H.; Wang, J.; Yao, M.; Peng, Y.; Liu, T.; Li, Z.; Luo, G.; Deng, J. Engineering Bacteria-Activated Multifunctionalized Hydrogel for Promoting Diabetic Wound Healing. Adv. Funct. Mater. 2021, 31, 2105749. [Google Scholar] [CrossRef]
- Lu, Y.F.; Deng, J.; Wang, J.; Luo, G.X. Effects and Mechanism of Lactococcus Lactis Thermo-Sensitive Hydrogel on the Wound Healing of Full-Thickness Skin Defects in Diabetic Mice. Zhonghua Shao Shang Za Zhi 2020, 36, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Badhwar, R.; Mangla, B.; Neupane, Y.R.; Khanna, K.; Popli, H. Quercetin Loaded Silver Nanoparticles in Hydrogel Matrices for Diabetic Wound Healing. Nanotechnology 2021, 32, 505102. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Chen, J.; Guo, J.; Yuan, M.; Wan, G.; Yan, C.; Li, W.; Machens, H.-G.; Rinkevich, Y.; et al. Calcium Ion Cross-Linked Sodium Alginate Hydrogels Containing Deferoxamine and Copper Nanoparticles for Diabetic Wound Healing. Int. J. Biol. Macromol. 2022, 202, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Qian, K.; Chen, J.; Yifeng, E.; Shi, Y.; Li, H.; Zhao, L. A Thermoreversible Antibacterial Zeolite-Based Nanoparticles Loaded Hydrogel Promotes Diabetic Wound Healing via Detrimental Factor Neutralization and ROS Scavenging. J. Nanobiotechnol. 2021, 19, 414. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Chen, M.; Xi, Y.; Cheng, W.; Mao, C.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; et al. Efficient Angiogenesis-Based Diabetic Wound Healing/Skin Reconstruction through Bioactive Antibacterial Adhesive Ultraviolet Shielding Nanodressing with Exosome Release. ACS Nano 2019, 13, 10279–10293. [Google Scholar] [CrossRef]
- Guo, C.; Wu, Y.; Li, W.; Wang, Y.; Kong, Q. Development of a Microenvironment-Responsive Hydrogel Promoting Chronically Infected Diabetic Wound Healing through Sequential Hemostatic, Antibacterial, and Angiogenic Activities. ACS Appl. Mater. Interfaces 2022, 14, 30480–30492. [Google Scholar] [CrossRef]
- Tseng, T.C.; Hsieh, F.Y.; Theato, P.; Wei, Y.; Hsu, S.H. Glucose-Sensitive Self-Healing Hydrogel as Sacrificial Materials to Fabricate Vascularized Constructs. Biomaterials 2017, 133, 20–28. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, W.N.; Xu, P.; Fu, X.; Yu, X.; Chen, L.; Leng, F.; Yu, C.; Yang, Z. Glucose-Responsive Multifunctional Metal–Organic Drug-Loaded Hydrogel for Diabetic Wound Healing. Acta Biomater. 2022, 140, 206–218. [Google Scholar] [CrossRef]
- Mirani, B.; Pagan, E.; Currie, B.; Siddiqui, M.A.; Hosseinzadeh, R.; Mostafalu, P.; Zhang, Y.S.; Ghahary, A.; Akbari, M. An Advanced Multifunctional Hydrogel-Based Dressing for Wound Monitoring and Drug Delivery. Adv. Healthc. Mater. 2017, 6, 1700718. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Bai, M.; Zhu, Y.; Liu, X.; Tian, S.; Long, Y.; Ma, Y.; Wen, C.; Li, Q.; Yang, J.; et al. Pro-Healing Zwitterionic Skin Sensor Enables Multi-Indicator Distinction and Continuous Real-Time Monitoring. Adv. Funct. Mater. 2021, 31, 2106406. [Google Scholar] [CrossRef]
- Cui, R.; Zhang, L.; Ou, R.; Xu, Y.; Xu, L.; Zhan, X.Y.; Li, D. Polysaccharide-Based Hydrogels for Wound Dressing: Design Considerations and Clinical Applications. Front. Bioeng. Biotechnol. 2022, 10, 845735. [Google Scholar] [CrossRef] [PubMed]
| Wound Dressing | Advantages | Limitations |
|---|---|---|
| Gauze | Low cost and readily available. | Require frequent changing, and particulates may remain on the wound bed upon removal and reinjure the wound. |
| Foam | Absorbs more exudates from the wound and allows gaseous exchange. The porous structure gives cushioning for the wound and provides a moist environment. | Requires secondary dressing and not suitable for dry wounds, having poor stability. Difficult to remove from the wound surface. Easy bacterial invasion and infection. Unpleasant odor. |
| Films | They retained a damp wound bed and used it as a secondary dressing with a debriding agent. Transparency of the film can easily monitor the wound. | These dressings should not use for highly exudating wounds and neuropathic ulcers. |
| Hydrocolloids | Through autolysis, these dressings facilitate wound debridement. No secondary dressing is required. Easily removable from the wounds. It causes no pain on application. | The dressing is not useful for dry wounds and requires frequent dressing in case of high exudates. Macerates healthy skin. |
| Iodine dressings | Antiseptic. Moderately adsorbent. | Some data have shown iodine solutions to be toxic to fibroblasts and keratinocytes. Allergy to iodine, wound discoloration. There is no evidence to support a beneficial effect. |
| Silver dressings | Improves wound hygiene and has antibacterial, antifungal, and antibacterial properties. | It may cause silver staining on the wound. No proven evidence for wound healing. |
| Polymer | Synthetic Method | Characteristics of Synthetic Dressings | Reference |
|---|---|---|---|
| Imidazolium alkyl urea reinforced polyurethane (PMI)+ Tannins (TA) | Hydrogen bonding and hydrophobicity | Good mechanical properties, underwater adhesion, and organ hemostasis | [65] |
| Phenylboronic acid-functionalized polycarbonate + Polyethylene glycol (PEG) | Good biocompatibility, mechanical properties, and antibacterial properties | [66] | |
| Ferrocene (Fc) + β-cyclodextrins (CD) | Mutual recognition of host and guest | Good biocompatibility, stability, and anti-inflammatory properties | [67] |
| Carboxyl-terminated aniline tetramer + chitosan | Amidation reaction | Good self-healing ability, injectable, adhesive, biodegradable, biocompatible, and antibacterial | [59] |
| Hyperbranched polyethylene glycol (HP-PEG) + Sulfated hyaluronic acid (HA-SH) | Michael addition reaction | Suitable mechanical stability, injectable, no swelling, and stain resistance | [52] |
| Hyaluronic acid grafted with hydrazide (HAh) + Hyaluronic acid grafted with an aldehyde (HAaq) | Schiff base reaction | Injectable, tight biological adhesion, and efficient self-healing | [68] |
| Ethylene glycol chitosan + Ethylene glycol chitosan (DF-PU) | High porosity, strong liquid absorption, instant self-healing, and injectable | [69] | |
| Oxidized hyaluronic acid (OHA) + Succinyl chitosan (SCS) | Low cytotoxicity, good biocompatibility, and pH response | [70] | |
| Poloxamer 407 + Heparin | Condensation reaction | Good biocompatibility, thermal sensitivity, high porosity, and protection of growth factors | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Yuan, W.; Chen, J.; Wu, L.-P.; You, T. Construction of Smart Biomaterials for Promoting Diabetic Wound Healing. Molecules 2023, 28, 1110. https://doi.org/10.3390/molecules28031110
Huang C, Yuan W, Chen J, Wu L-P, You T. Construction of Smart Biomaterials for Promoting Diabetic Wound Healing. Molecules. 2023; 28(3):1110. https://doi.org/10.3390/molecules28031110
Chicago/Turabian StyleHuang, Chan, Weiyan Yuan, Jun Chen, Lin-Ping Wu, and Tianhui You. 2023. "Construction of Smart Biomaterials for Promoting Diabetic Wound Healing" Molecules 28, no. 3: 1110. https://doi.org/10.3390/molecules28031110
APA StyleHuang, C., Yuan, W., Chen, J., Wu, L.-P., & You, T. (2023). Construction of Smart Biomaterials for Promoting Diabetic Wound Healing. Molecules, 28(3), 1110. https://doi.org/10.3390/molecules28031110





