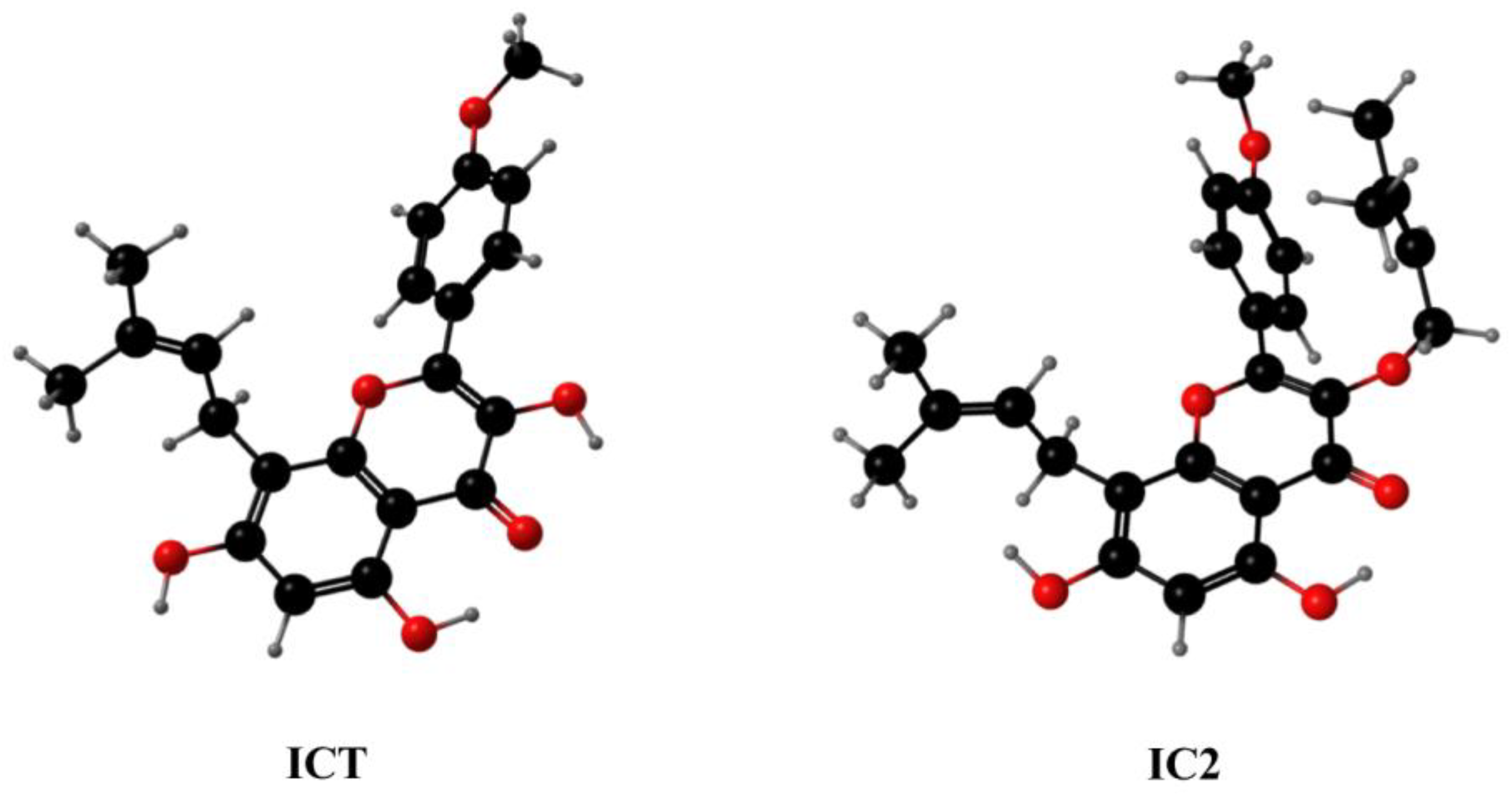
Icaritin Derivative IC2 Induces Cytoprotective Autophagy of Breast Cancer Cells via SCD1 Inhibition
Abstract
1. Introduction
2. Results
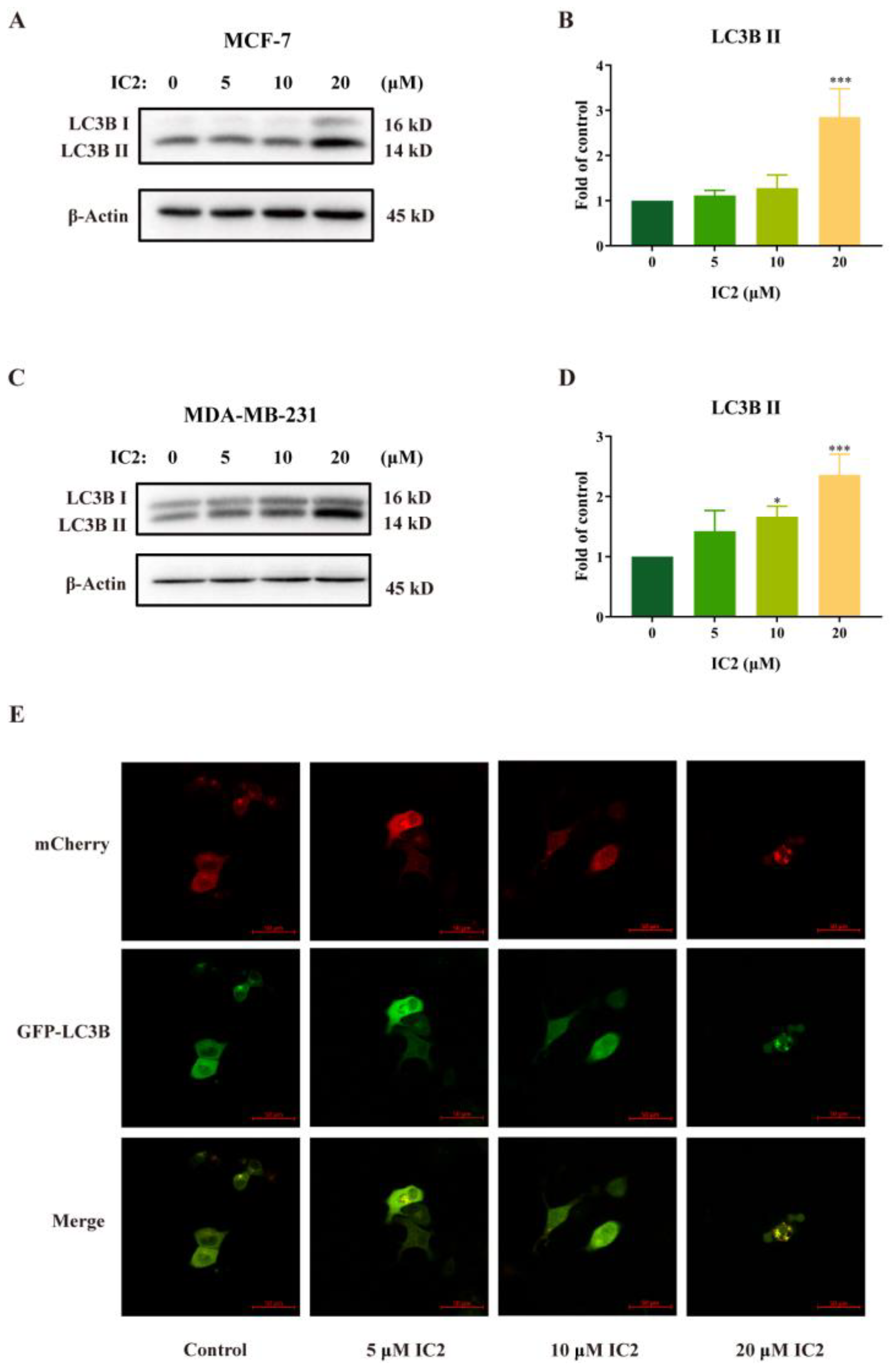
2.1. IC2 Induces Cell Autophagy in Breast Cancer Cells
2.2. IC2 Induces Autophagy by AMPK/mTOR and MAPK Signaling Pathways in MCF-7 Cells
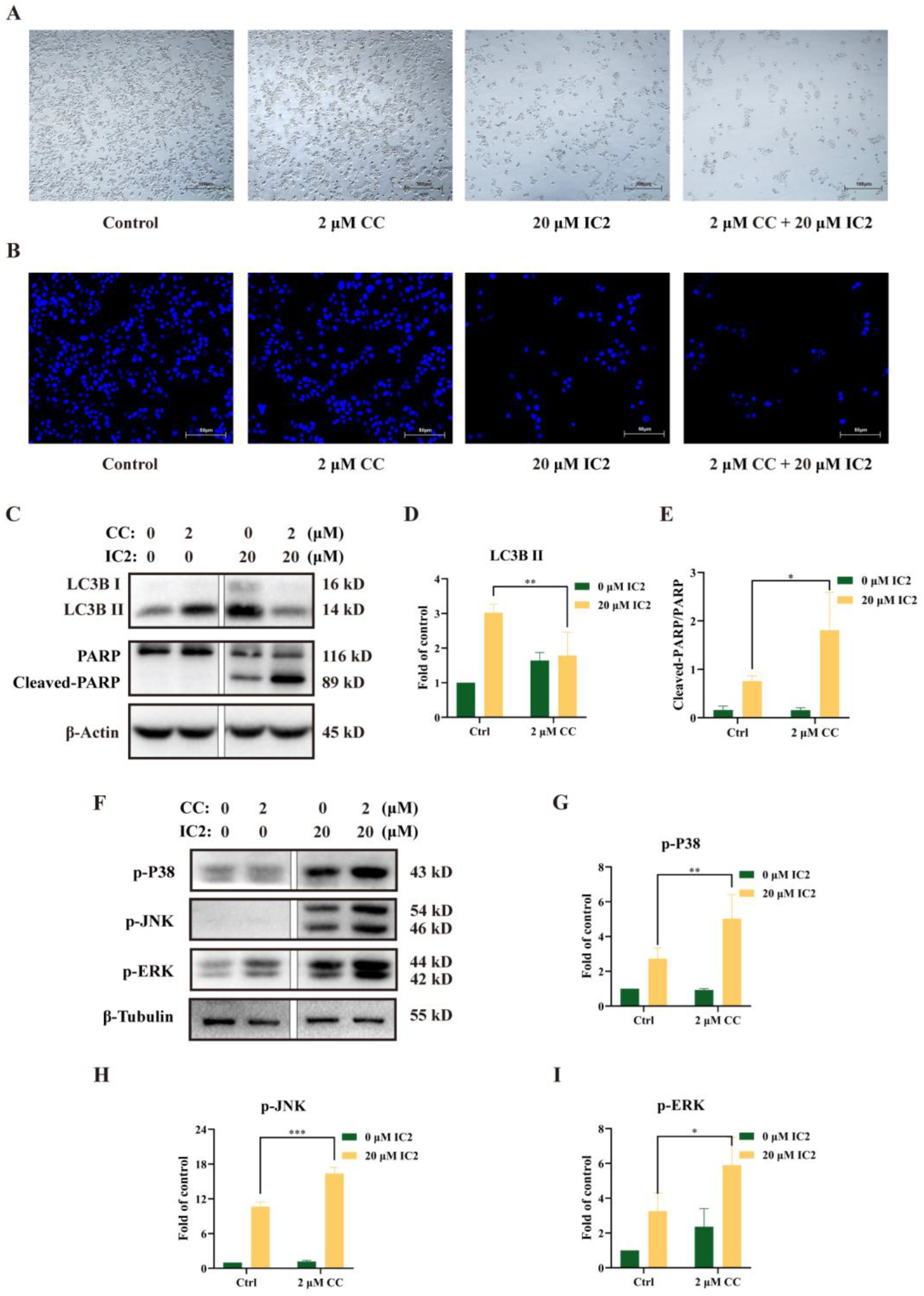
2.3. IC2 Induces Cytoprotective Autophagy and AMPK Is Involved in Regulating the Balance of Autophagy and Apoptosis
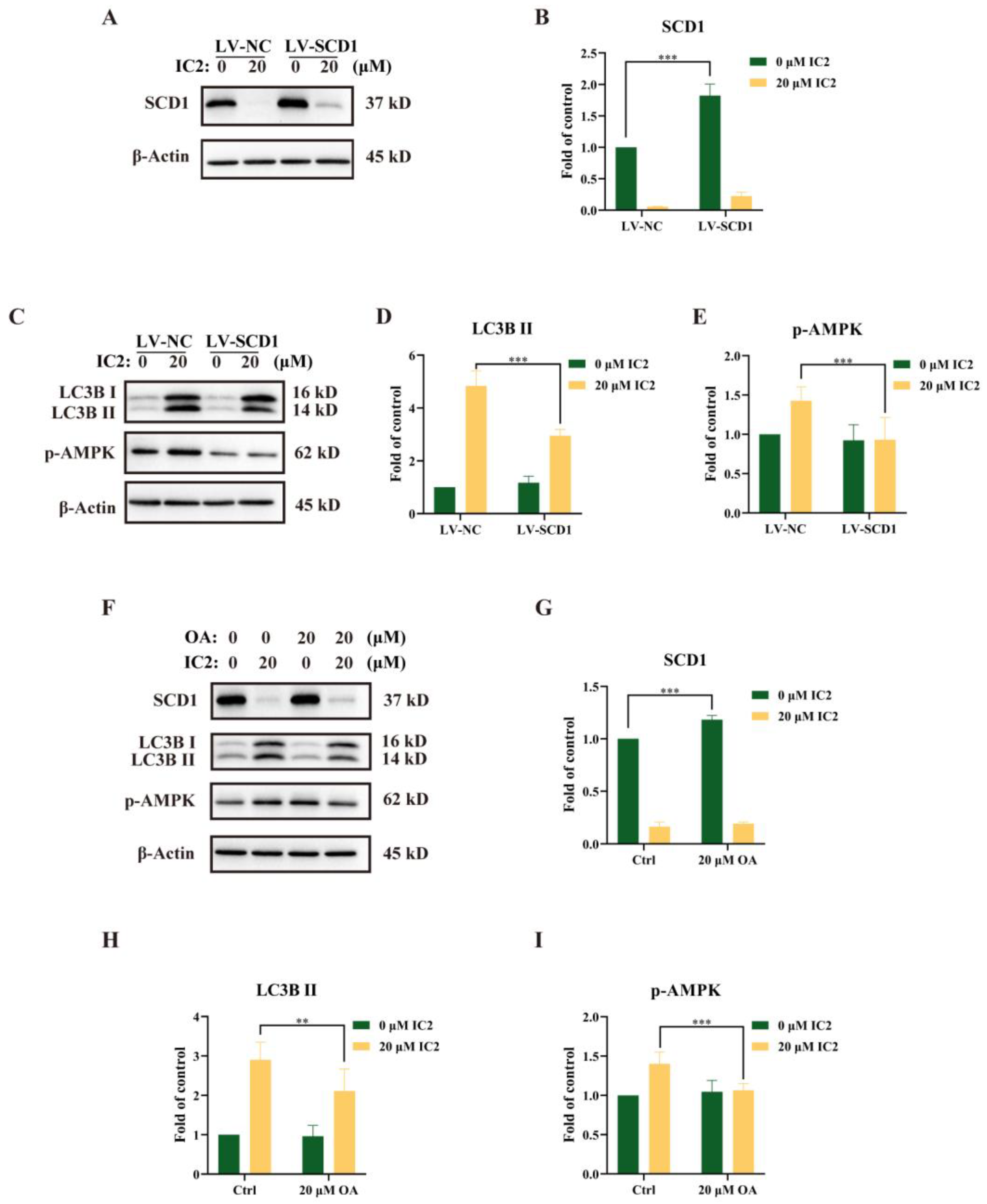
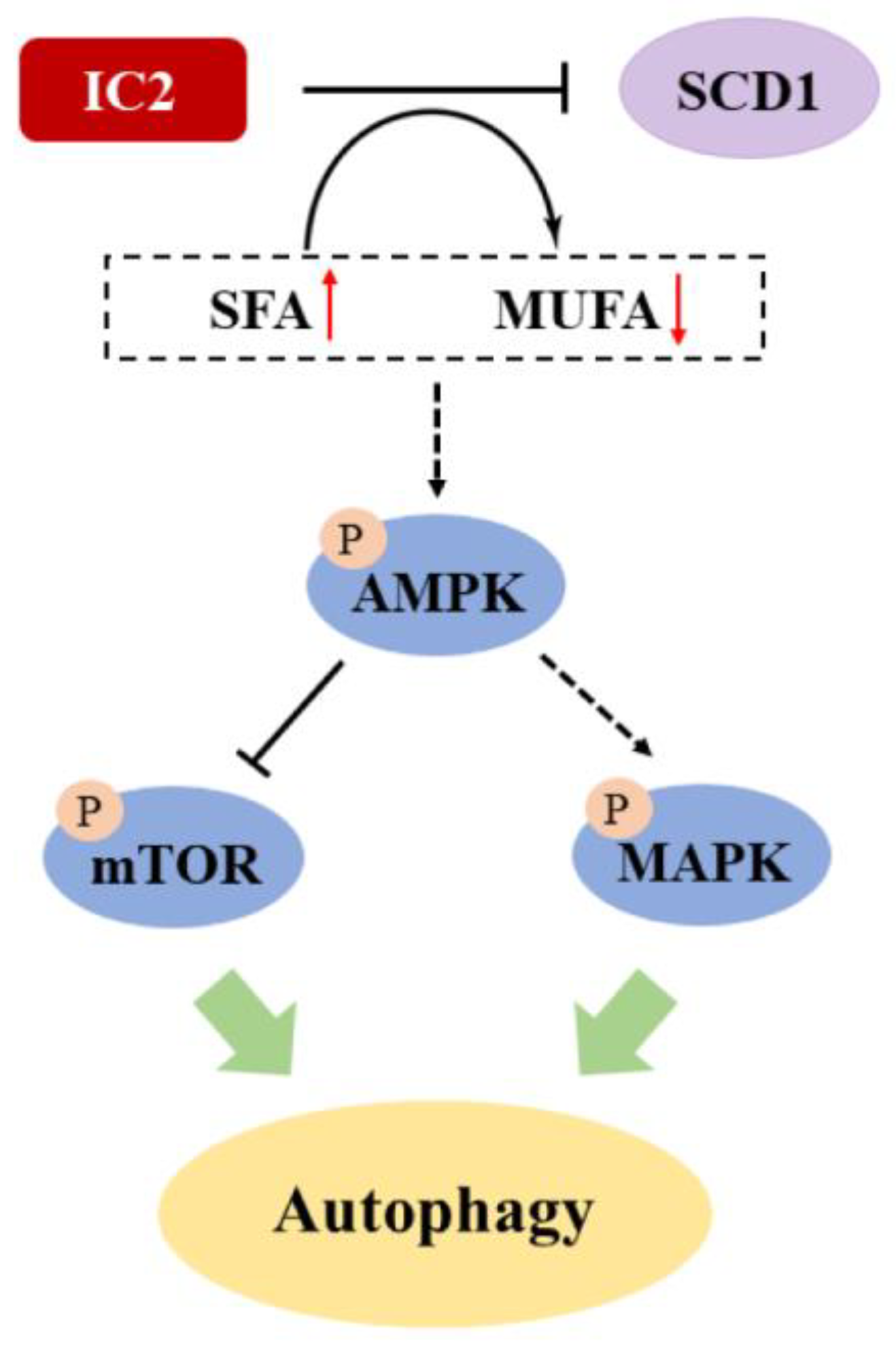
2.4. IC2-Induced Autophagy Depends on the Inhibition of SCD1
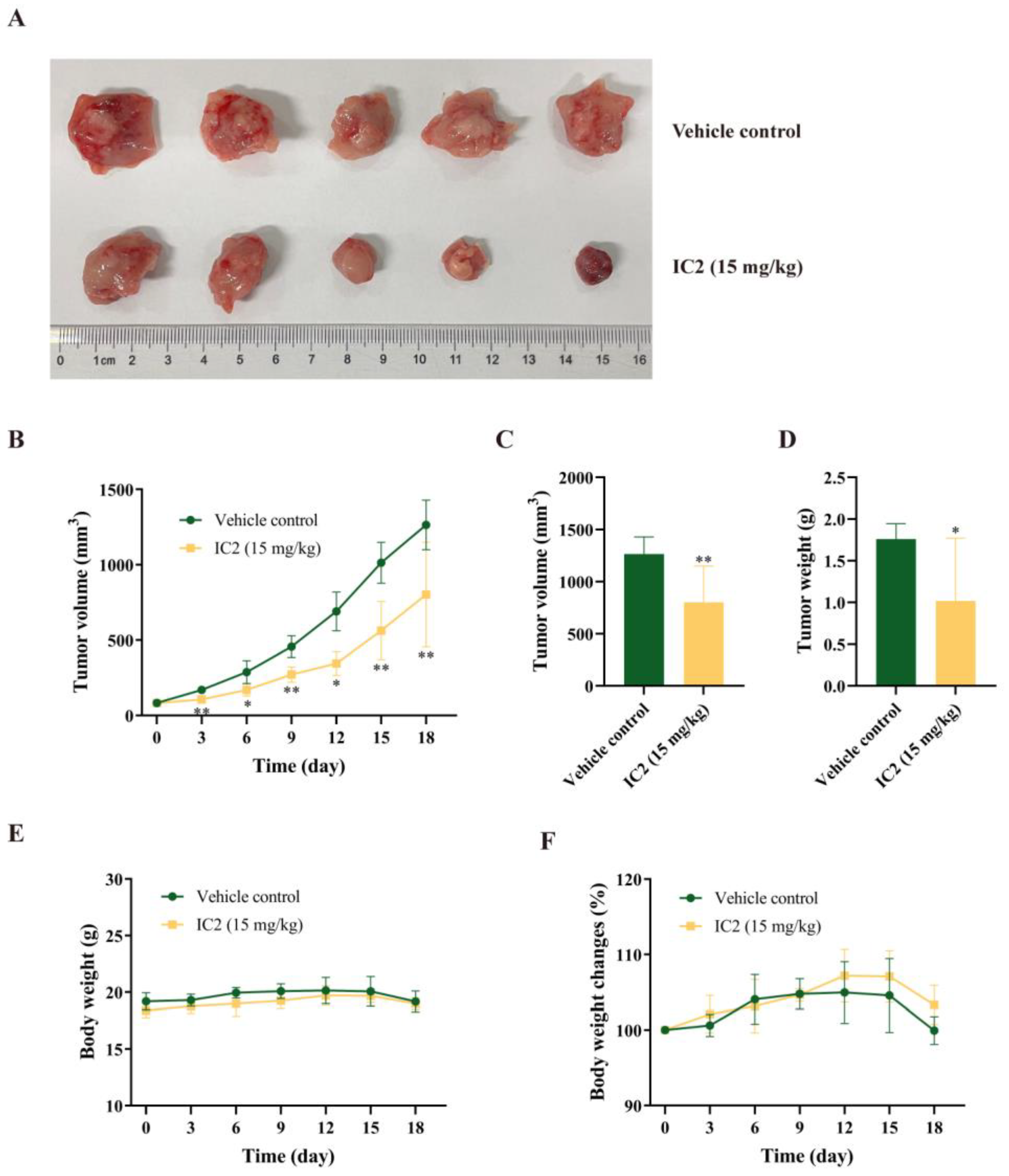
2.5. IC2 Exerts Antitumor Activity in 4T1 Breast Cancer Xenograft Model
3. Discussion
4. Materials and Methods
4.1. Experimental Reagents
4.2. Cell Culture
4.3. Preparation of IC2
4.4. Western Blot
4.5. Autophagic Flux Observation
4.6. DAPI Staining
4.7. Lentiviral Overexpression Model
4.8. Establishment of the 4T1 Breast Cancer Model
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Poudyal, H.; Brown, L. Stearoyl-coa desaturase: A vital checkpoint in the development and progression of obesity. Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Ran, H.; Zhu, Y.; Deng, R.; Zhang, Q.; Liu, X.; Feng, M.; Zhong, J.; Lin, S.; Tong, X.; Su, Q. Stearoyl-coa desaturase-1 promotes colorectal cancer metastasis in response to glucose by suppressing pten. J. Exp. Clin. Cancer Res. 2018, 37, 54. [Google Scholar] [CrossRef] [PubMed]
- Igal, R.A. Roles of stearoylcoa desaturase-1 in the regulation of cancer cell growth, survival and tumorigenesis. Cancers 2011, 3, 2462–2477. [Google Scholar] [CrossRef] [PubMed]
- She, K.; Fang, S.; Du, W.; Fan, X.; He, J.; Pan, H.; Huang, L.; He, P.; Huang, J. Scd1 is required for egfr-targeting cancer therapy of lung cancer via re-activation of egfr/pi3k/akt signals. Cancer Cell Int. 2019, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Nashed, M.; Chisholm, J.W.; Igal, R.A. Stearoyl-coa desaturase activity modulates the activation of epidermal growth factor receptor in human lung cancer cells. Exp. Biol. Med. 2012, 237, 1007–1017. [Google Scholar] [CrossRef]
- Lingrand, M.; Lalonde, S.; Jutras-Carignan, A.; Bergeron, K.F.; Rassart, E.; Mounier, C. Scd1 activity promotes cell migration via a pld-mtor pathway in the mda-mb-231 triple-negative breast cancer cell line. Breast Cancer 2020, 27, 594–606. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, S.; Ruzzo, W.L. Spatial modeling of prostate cancer metabolic gene expression reveals extensive heterogeneity and selective vulnerabilities. Sci. Rep. 2020, 10, 3490. [Google Scholar] [CrossRef]
- Melana, J.P.; Mignolli, F.; Stoyanoff, T.; Aguirre, M.V.; Balboa, M.A.; Balsinde, J.; Rodríguez, J.P. The hypoxic microenvironment induces stearoyl-coa desaturase-1 overexpression and lipidomic profile changes in clear cell renal cell carcinoma. Cancers 2021, 13, 2962. [Google Scholar] [CrossRef]
- Huang, G.M.; Jiang, Q.H.; Cai, C.; Qu, M.; Shen, W. Scd1 negatively regulates autophagy-induced cell death in human hepatocellular carcinoma through inactivation of the ampk signaling pathway. Cancer Lett. 2015, 358, 180–190. [Google Scholar] [CrossRef]
- Yang, C.; Jin, Y.Y.; Mei, J.; Hu, D.; Jiao, X.; Che, H.L.; Tang, C.L.; Zhang, Y.; Wu, G.S. Identification of icaritin derivative ic2 as an scd-1 inhibitor with anti-breast cancer properties through induction of cell apoptosis. Cancer Cell. Int. 2022, 22, 202. [Google Scholar] [CrossRef]
- Ascenzi, F.; De Vitis, C.; Maugeri-Saccà, M.; Napoli, C.; Ciliberto, G.; Mancini, R. Scd1, autophagy and cancer: Implications for therapy. J. Exp. Clin. Cancer Res. 2021, 40, 265. [Google Scholar] [CrossRef] [PubMed]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.U. Regulation of the innate immune system by autophagy: Neutrophils, eosinophils, mast cells, nk cells. Cell. Death Differ. 2019, 26, 703–714. [Google Scholar] [CrossRef]
- Sui, X.; Kong, N.; Ye, L.; Han, W.; Zhou, J.; Zhang, Q.; He, C.; Pan, H. P38 and jnk mapk pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014, 344, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Janji, B.; Viry, E.; Moussay, E.; Paggetti, J.; Arakelian, T.; Mgrditchian, T.; Messai, Y.; Noman, M.Z.; Van Moer, K.; Hasmim, M.; et al. The multifaceted role of autophagy in tumor evasion from immune surveillance. Oncotarget 2016, 7, 17591–17607. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.A.; Bayraktar Ekmekcigil, O.; Bagca, B.G.; Avci, C.B.; Sabitaliyevich, U.Y.; Zhenisovna, T.G.; Aras, A.; Farooqi, A.A. Role of autophagy in breast cancer development and progression: Opposite sides of the same coin. Adv. Exp. Med. Biol. 2019, 1152, 65–73. [Google Scholar] [CrossRef]
- Köhler, K.; Brunner, E.; Guan, X.L.; Boucke, K.; Greber, U.F.; Mohanty, S.; Barth, J.M.; Wenk, M.R.; Hafen, E. A combined proteomic and genetic analysis identifies a role for the lipid desaturase desat1 in starvation-induced autophagy in drosophila. Autophagy 2009, 5, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xiang, J.; Li, Y.; Xia, Y.; Xu, S.; Gao, X.; Qiao, S. Inhibition of stearoyl-coa desaturase 1 potentiates anti-tumor activity of amodiaquine in non-small cell lung cancer. Biol. Pharm. Bull. 2022, 45, 438–445. [Google Scholar] [CrossRef]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, Y.; Yang, H.; Hu, Y.; Li, X. Nanomedicine-boosting icaritin-based immunotherapy of advanced hepatocellular carcinoma. Mil. Med. Res. 2022, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Luo, J.N.H.; Dong, Y.R.; Wang, Z.Z.; Xiao, W.; Zhao, L.G. Biotransformation of the total flavonoid extract of epimedium into icaritin by two thermostable glycosidases from dictyoglomus thermophilum dsm3960. Process Biochem. 2021, 105, 8–18. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, S.Q. Antiosteoporosis effects, pharmacokinetics, and drug delivery systems of icaritin: Advances and prospects. Pharmaceuticals 2022, 15, 397. [Google Scholar] [CrossRef] [PubMed]
- Dongye, Z.; Wu, X.; Wen, Y.; Ding, X.; Wang, C.; Zhao, T.; Li, J.; Wu, Y. Icaritin and intratumoral injection of cpg treatment synergistically promote t cell infiltration and antitumor immune response in mice. Int. Immunopharmacol. 2022, 111, 109093. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Zhang, W.; Yan, X. Anti-inflammatory and immunoregulatory effects of icariin and icaritin. Biomed. Pharmacother. 2022, 151, 113180. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, Z.; Shi, J. Pharmacological effects of icariin. Adv. Pharmacol. 2020, 87, 179–203. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, X.; Meng, J.; Wang, Z.Y. An anticancer agent icaritin induces sustained activation of the extracellular signal-regulated kinase (erk) pathway and inhibits growth of breast cancer cells. Eur. J. Pharmacol. 2011, 658, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Qin, L.; Sheng, H.; Wang, X.L.; Wang, Y.X.; Yeung, D.K.; Griffith, J.F.; Yao, X.S.; Xie, X.H.; Li, Z.R.; et al. A novel semisynthesized small molecule icaritin reduces incidence of steroid-associated osteonecrosis with inhibition of both thrombosis and lipid-deposition in a dose-dependent manner. Bone 2009, 44, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.K.; Li, Q.; Ming Xu, J.; Liang, J.; Cheng, Y.; Fan, Y.; Jiang, J.; Ye, H.; Tao, H.; Li, L.; et al. Icaritin-induced immunomodulatory efficacy in advanced hepatitis b virus-related hepatocellular carcinoma: Immunodynamic biomarkers and overall survival. Cancer Sci. 2020, 111, 4218–4231. [Google Scholar] [CrossRef]
- He, Q.; Yin, Y.; Pan, X.; Wu, Y.; Li, X. Albendazole-induced autophagy blockade contributes to elevated apoptosis in cholangiocarcinoma cells through ampk/mtor activation. Toxicol. Appl. Pharmacol. 2022, 454, 116214. [Google Scholar] [CrossRef]
- Que, T.; Ren, B.; Fan, Y.; Liu, T.; Hou, T.; Dan, W.; Liu, B.; Wei, Y.; Lei, Y.; Zeng, J.; et al. Capsaicin inhibits the migration, invasion and emt of renal cancer cells by inducing ampk/mtor-mediated autophagy. Chem. Biol. Interact. 2022, 366, 110043. [Google Scholar] [CrossRef]
- Zhou, Y.; Ming, J.; Li, Y.; Deng, M.; Chen, Q.; Ma, Y.; Chen, Z.; Zhang, Y.; Liu, S. Ligustilide attenuates nitric oxide-induced apoptosis in rat chondrocytes and cartilage degradation via inhibiting jnk and p38 mapk pathways. J. Cell. Mol. Med. 2019, 23, 3357–3368. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, D. Ginsenoside rg5 induces g2/m phase arrest, apoptosis and autophagy via regulating ros-mediated mapk pathways against human gastric cancer. Biochem. Pharmacol. 2019, 168, 285–304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Guo, Y.; Li, S.; Han, R.; Ying, J.; Zhu, H.; Wang, Y.; Yin, L.; Han, Y.; Sun, L.; et al. A novel anti-cancer agent icaritin suppresses hepatocellular carcinoma initiation and malignant growth through the il-6/jak2/stat3 pathway. Oncotarget 2015, 6, 31927–31943. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Luo, C.; Yan, M.; Zhao, G.; Ma, L.; Gao, W. Treatment for liver cancer: From sorafenib to natural products. Eur. J. Med. Chem. 2021, 224, 113690. [Google Scholar] [CrossRef]
- Hu, J.; Wu, X.; Yang, C.; Rashid, K.; Ma, C.; Hu, M.; Ding, Q.; Jiang, H. Anticancer effect of icaritin on prostate cancer via regulating mir-381-3p and its target gene ube2c. Cancer Med. 2019, 8, 7833–7845. [Google Scholar] [CrossRef] [PubMed]
- Niklaus, N.J.; Tokarchuk, I.; Zbinden, M.; Schläfli, A.M.; Maycotte, P.; Tschan, M.P. The multifaceted functions of autophagy in breast cancer development and treatment. Cells 2021, 10, 1447. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.C.; Wu, Y.; Gao, X.; Qiao, L.; Yang, Y.; Li, F.; Zou, J.; Wang, Y.H.; Zhang, S.Y.; Li, C.L.; et al. The antitumor effects of icaritin against breast cancer is related to estrogen receptors. Curr. Mol. Med. 2021, 21, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell. Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef]
- Zhang, C.; Sui, X.; Jiang, Y.; Wang, X.; Wang, S. Antitumor effects of icaritin and the molecular mechanisms. Discov. Med. 2020, 29, 5–16. [Google Scholar]
- Igal, R.A. Stearoyl-coa desaturase-1: A novel key player in the mechanisms of cell proliferation, programmed cell death and transformation to cancer. Carcinogenesis 2010, 31, 1509–1515. [Google Scholar] [CrossRef]
- Ono, A.; Sano, O.; Kazetani, K.I.; Muraki, T.; Imamura, K.; Sumi, H.; Matsui, J.; Iwata, H. Feedback activation of ampk-mediated autophagy acceleration is a key resistance mechanism against scd1 inhibitor-induced cell growth inhibition. PLoS ONE 2017, 12, e0181243. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Ao, H.; Fu, J.; Guo, Y.; Han, M.; Yan, X.; Chen, X.; Wang, X. A comparative study on the in vitro and in vivo antitumor efficacy of icaritin and hydrous icaritin nanorods. Drug Deliv. 2020, 27, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, X.; Wang, S.; Jiao, Z.; Sun, T.; Liu, T.; Song, K. Characterization and anti-tumor bioactivity of astragalus polysaccharides by immunomodulation. Int. J. Biol. Macromol. 2020, 145, 985–997. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-X.; Jin, Y.-Y.; Wang, J.; Zhao, Z.-C.; Xue, K.-W.; Xiong, H.; Che, H.-L.; Ge, Y.-J.; Wu, G.-S. Icaritin Derivative IC2 Induces Cytoprotective Autophagy of Breast Cancer Cells via SCD1 Inhibition. Molecules 2023, 28, 1109. https://doi.org/10.3390/molecules28031109
Wang Y-X, Jin Y-Y, Wang J, Zhao Z-C, Xue K-W, Xiong H, Che H-L, Ge Y-J, Wu G-S. Icaritin Derivative IC2 Induces Cytoprotective Autophagy of Breast Cancer Cells via SCD1 Inhibition. Molecules. 2023; 28(3):1109. https://doi.org/10.3390/molecules28031109
Chicago/Turabian StyleWang, Yi-Xuan, Yi-Yuan Jin, Jie Wang, Zi-Cheng Zhao, Ke-Wen Xue, He Xiong, Hui-Lian Che, Yun-Jun Ge, and Guo-Sheng Wu. 2023. "Icaritin Derivative IC2 Induces Cytoprotective Autophagy of Breast Cancer Cells via SCD1 Inhibition" Molecules 28, no. 3: 1109. https://doi.org/10.3390/molecules28031109
APA StyleWang, Y.-X., Jin, Y.-Y., Wang, J., Zhao, Z.-C., Xue, K.-W., Xiong, H., Che, H.-L., Ge, Y.-J., & Wu, G.-S. (2023). Icaritin Derivative IC2 Induces Cytoprotective Autophagy of Breast Cancer Cells via SCD1 Inhibition. Molecules, 28(3), 1109. https://doi.org/10.3390/molecules28031109




