Comparison of Ophiopogon japonicus and Liriope spicata var. prolifera from Different Origins Based on Multi-Component Quantification and Anticancer Activity
Abstract
1. Introduction
2. Results and Discussion
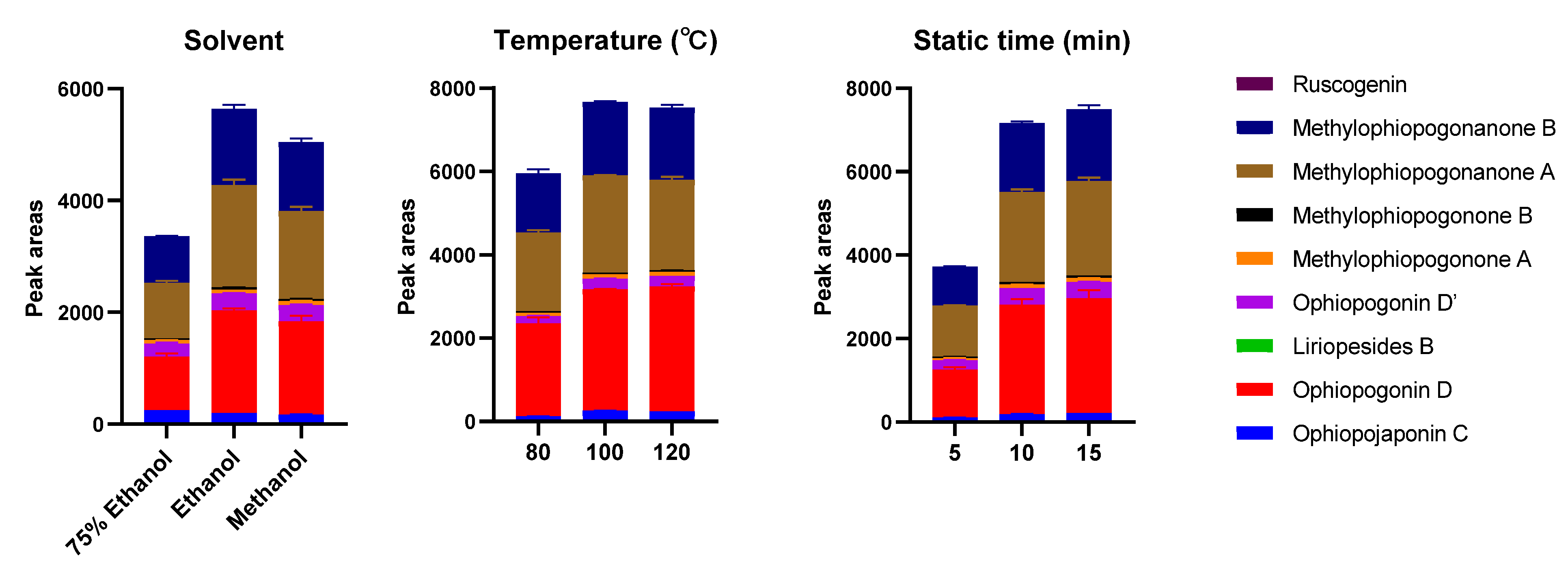
2.1. Optimization of PLE Conditions
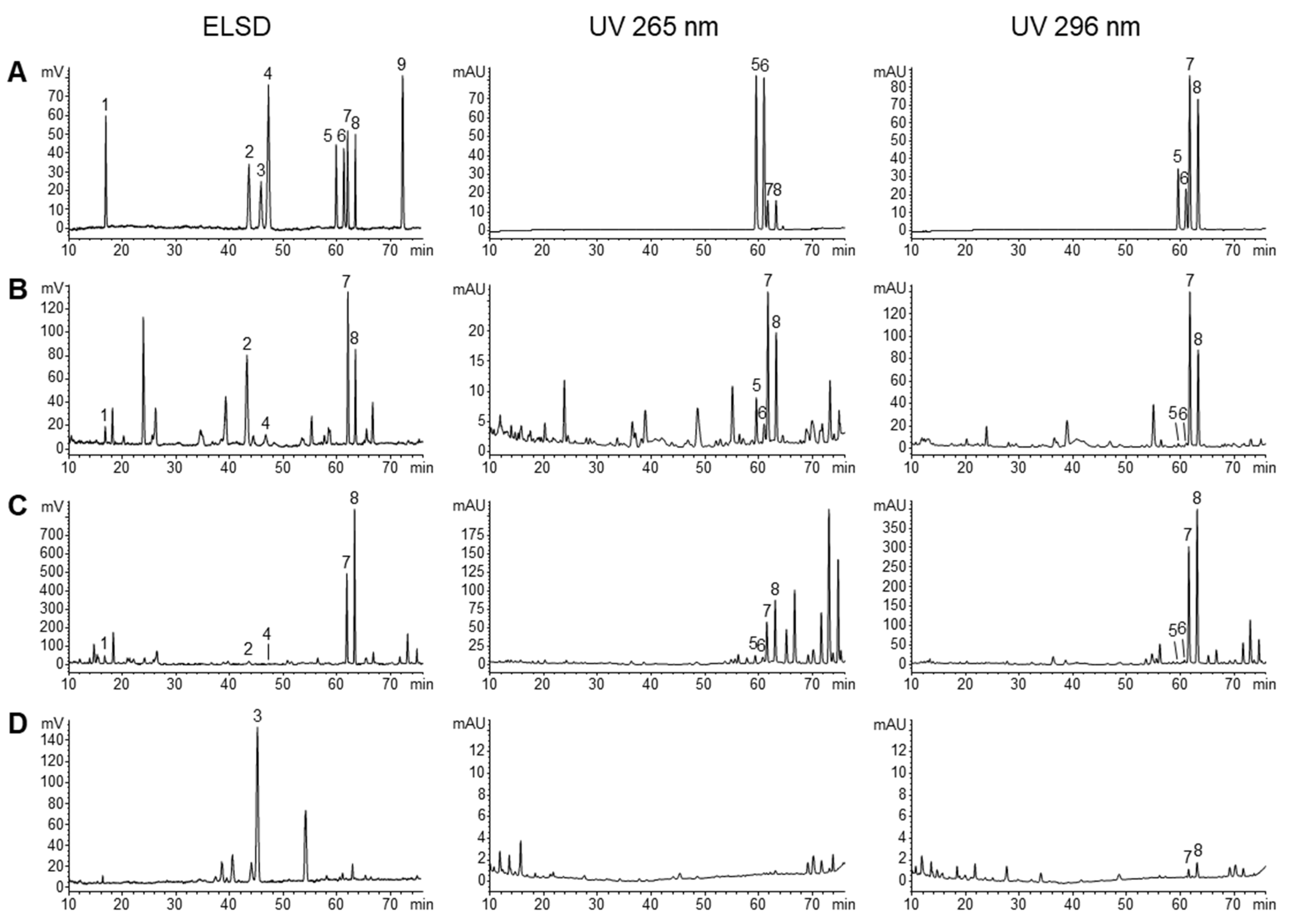
2.2. Validation of the HPLC Method
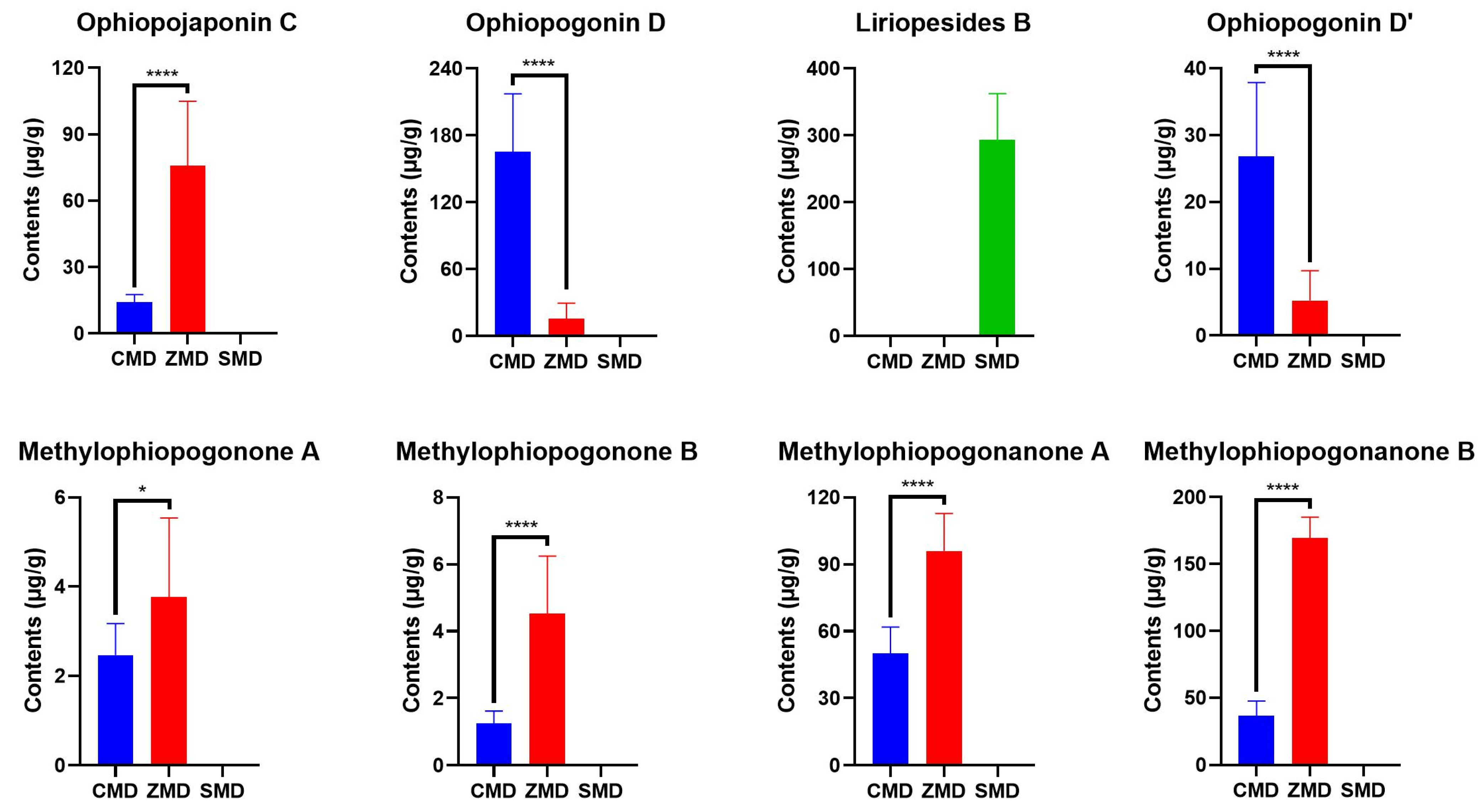
2.3. Quantification of the Investigated Compounds in CMD, ZMD and SMD
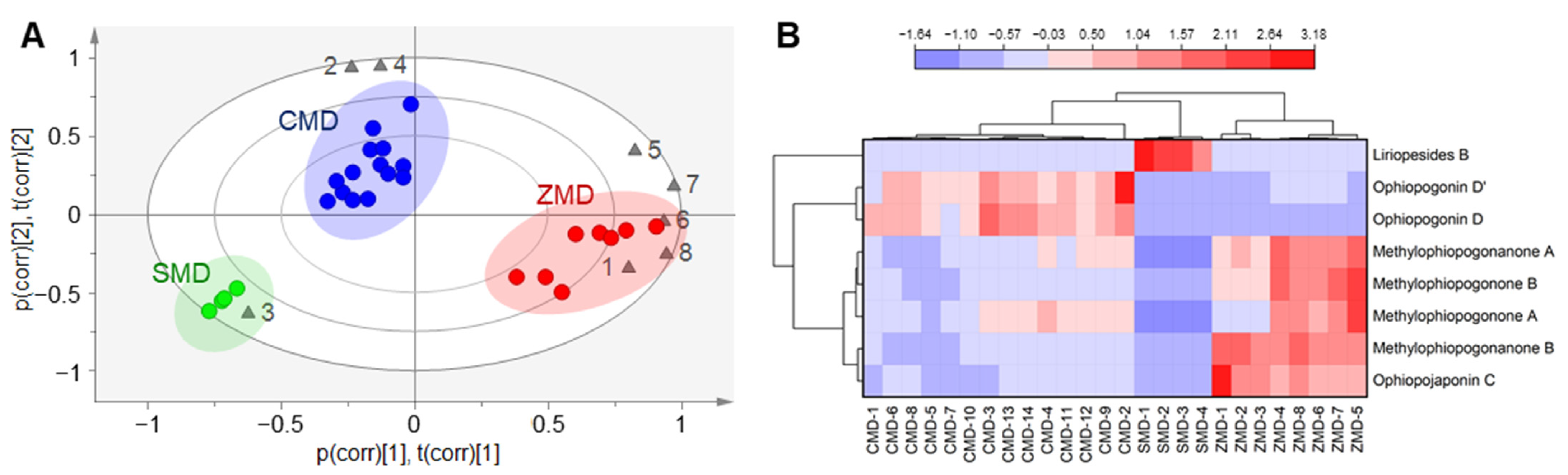
2.4. Comparison of the Chemical Composition of CMD, ZMD and SMD by Multivariate Statistical Analysis
2.5. In Vitro Anticancer Activity of CMD, ZMD and SMD Extracts and Their Components
3. Materials and Methods
3.1. Materials and Reagents
3.2. Sample Preparation
3.3. HPLC-DAD-ELSD Analysis
3.4. Validation of the HPLC Method
3.4.1. Calibration Curves, LOD and LOQ
3.4.2. Precision and Repeatability
3.4.3. Recovery
3.5. Cell Culture
3.6. Cytotoxicity Assay
3.7. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Ophiopogonis Radix. In Chinese Pharmacopoeia of the People’s Republic of China, 2020 ed.; China Medical Science and Technology Press: Beijing, China, 2020; Volume I, pp. 162–163. [Google Scholar]
- Lin, Y.; Zhu, D.; Qi, J.; Qin, M.; Yu, B. Characterization of homoisoflavonoids in different cultivation regions of Ophiopogon japonicus and related antioxidant activity. J. Pharm. Biomed. Anal. 2010, 52, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.L.; Li, A.; Huang, N.; Lu, F.; Hou, D. Antioxidant and immunoregulatory activity of different polysaccharide fractions from tuber of Ophiopogon japonicus. Carbohydr. Polym. 2011, 86, 1273–1280. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, W.-F.; Shen, H.-Y.; Shen, P.-Q.; Zhang, J.; Wang, D.-D.; Xu, H.; Wang, H.; Yan, T.-T.; Wang, L.; et al. Comparison of bioactive components and pharmacological activities of Ophiopogon japonicas extracts from different geographical origins. J. Pharm. Biomed. Anal. 2017, 138, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Sun, Y.; Lin, Y.; Cheng, Z.; Zheng, W.; Yu, B.; Xu, Q. Anti-inflammatory activities of aqueous extract from Radix ophiopogon japonicus and its two constituents. Biol. Pharm. Bull. 2005, 28, 1234–1238. [Google Scholar] [CrossRef]
- Kitahiro, Y.; Koike, A.; Sonoki, A.; Muto, M.; Ozaki, K.; Shibano, M. Anti-inflammatory activities of Ophiopogonis Radix on hydrogen peroxide-induced cellular senescence of normal human dermal fibroblasts. J. Nat. Med. 2018, 72, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yuan, J.; Zhou, L.; Zhu, M.; Shi, Z.; Song, J.; Xu, Q.; Yin, G.; Lv, Y.; Luo, Y.; et al. Regulation of different components from Ophiopogon japonicus on autophagy in human lung adenocarcinoma A549 Cells through PI3K/Akt/mTOR signaling pathway. Biomed. Pharmacother. 2017, 87, 118–126. [Google Scholar] [CrossRef]
- Kou, J.; Yu, B.; Xu, Q. Inhibitory effects of ethanol extract from Radix Ophiopogon japonicus on venous thrombosis linked with its endothelium-protective and anti-adhesive activities. Vasc. Pharmacol. 2005, 43, 157–163. [Google Scholar] [CrossRef]
- Cai, H.-Z.; Tang, Y.-P.; Chen, X.-Y.; Xie, H.-B.; Chen, Q.-Y.; Xu, Z.-L. Ophiopogon japonicas (Linn. f.) Ker-Gawl. extract ameliorates chronic heart failure in rats. Trop. J. Pharm. Res. 2019, 18, 1853–1857. [Google Scholar] [CrossRef]
- Ding, L.; Li, P.; Lau, C.B.S.; Chan, Y.W.; Xu, D.; Fung, K.-P.; Su, W. Mechanistic Studies on the Antidiabetic Activity of a Polysaccharide-rich Extract of Radix Ophiopogonis. Phytother. Res. 2012, 26, 101–105. [Google Scholar] [CrossRef]
- Li, P.-B.; Lin, W.-L.; Wang, Y.-G.; Peng, W.; Cai, X.-Y.; Su, W.-W. Antidiabetic activities of oligosaccharides of Ophiopogonis japonicus in experimental type 2 diabetic rats. Int. J. Biol. Macromol. 2012, 51, 749–755. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Guo, L.-X.; Hu, W.-H.; Peng, Z.-T.; Wang, C.; Chen, Z.-C.; Liu, E.Y.L.; Dong, T.T.X.; Wang, T.-J.; Tsim, K.W.K. Polysaccharide from tuberous roots of Ophiopogon japonicus regulates gut microbiota and its metabolites during alleviation of high-fat diet-induced type-2 diabetes in mice. J. Funct. Foods. 2019, 63, 103593. [Google Scholar] [CrossRef]
- Chen, M.-H.; Chen, X.-J.; Wang, M.; Lin, L.-G.; Wang, Y.-T. Ophiopogon japonicus–A phytochemical, ethnomedicinal and pharmacological review. J. Ethnopharmacol. 2016, 181, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-G.; Liu, Q.-Y.; Ye, Y. Naturally occurring homoisoflavonoids and their pharmacological activities. Planta Med. 2014, 80, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Tong, W.; Wang, S.; Li, J.; Zheng, J.; Fan, X.; Liu, L. Comparison of the chemical consituents and immunomodulatory activity of ophiopogonis radix from two different producing areas. J. Pharm. Biomed. Anal. 2017, 134, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dong, Z.; Wu, H.; Ding, W.; Zhao, M.; Shi, Q.; Wang, Q. Comparative studies on Ophiopogonis and Liriopes based on the determination of 11 bioactive components using LC-MS/MS and Hierarchical clustering analysis. Food Res. Int. 2014, 57, 15–25. [Google Scholar] [CrossRef]
- Luo, H.; Ming, L.S.; Tong, T.T.; Tang, Y.; Yang, J.; Shen, L.; Cui, H.; Yang, A.; Huang, H. Chemical Comparison of Ophiopogonis radix and Liriopes radix Based on Quantitative Analysis of Multiple Components by HPLC Coupled with Electrospray Ionization Tandem Triple Quadrupole Mass Spectrometry. J. AOAC Int. 2020, 103, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Lin, Z.; Xu, B.; Wang, Y.; Shi, X.; Qiao, Y.; Zhang, J. Metabolomics data fusion between near infrared spectroscopy and high-resolution mass spectrometry: A synergetic approach to boost performance or induce confusion. Talanta 2018, 189, 641–648. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, X.; Godjevac, D.; Bueno, P.C.P.; Salome Abarca, L.F.; Jang, Y.P.; Wang, M.; Choi, Y.H. Metabolic Profiling of Saponin-Rich Ophiopogon japonicus Roots Based on 1H NMR and HPTLC Platforms. Planta Med. 2019, 85, 917–924. [Google Scholar] [CrossRef]
- Hua, X.; Hong, H.-J.; Zhang, D.-Y.; Liu, Q.; Leong, F.; Yang, Q.; Hu, Y.-J.; Chen, X.-J. Rapid Screening of Lipase Inhibitors from Ophiopogonis Radix Using High-Performance Thin Layer Chromatography by Two Step Gradient Elution Combined with Bioautographic Method. Molecules 2022, 27, 1155. [Google Scholar] [CrossRef]
- Ye, G.; Ye, M.; Guo, D.; Huang, C. Determination of homoisoflavonoids in Ophiopogon japonicus by RP-HPLC. Chromatographia 2005, 61, 121–125. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Yang, W.; Zhang, S.; Wang, F.; Tang, Z. Chemical Fingerprinting of Wild Germplasm Resource of Ophiopogon japonicus from Sichuan Basin, China by RP-HPLC Coupled with Hierarchical Cluster Analysis. Anal. Lett. 2010, 43, 2411–2423. [Google Scholar] [CrossRef]
- Li, X.-E.; Wang, Y.-X.; Sun, P.; Liao, D.-Q. Determination of saponin content in hang maidong and chuan maidong via HPLC-ELSD analysis. J. Anal. Methods Chem. 2016, 7214607. [Google Scholar] [CrossRef]
- Liu, C.-H.; Li, M.; Feng, Y.-Q.; Hu, Y.-J.; Yu, B.-Y.; Qi, J. Determination of ruscogenin in ophiopogonis radix by high-performance liquid chromatography-evaporative light scattering detector coupled with hierarchical clustering analysis. Pharmacogn. Mag. 2016, 12, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.-G.; Kang, C.-Z.; Kang, L.-P.; Yang, J.; Wang, S.; He, Y.-L.; Deng, A.-P.; Wang, H.-Y.; Huang, L.-Q.; Guo, L.-P. Structural characterization and discrimination of Ophiopogon japonicas (Liliaceae) from different geographical origins based on metabolite profiling analysis. J. Pharm. Biomed. Anal. 2020, 185, 113212. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Lee, S.-G.; Sethi, G.; Ahn, K.S. Ophiopogonin D, a steroidal glycoside abrogates STAT3 signaling cascade and exhibits anti-cancer activity by causing GSH/GSSG imbalance in lung carcinoma. Cancers 2018, 10, 427. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, H.; Zhu, M.; Song, W.; Wang, J.; Wu, C.; Kong, Y.; Guo, J.; Li, N.; Liu, J.; et al. Ophiopogonin D’, a natural product from Radix ophiopogonis, induces in vitro and in vivo RIPK1-dependent and caspase-independent apoptotic death in androgen-independent human prostate cancer cells. Front. Pharmacol. 2018, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wu, C.; Zhu, M.; Song, W.; Wang, H.; Wang, J.; Guo, J.; Li, N.; Liu, J.; Li, Y.; et al. Ophiopogonin D’ induces RIPK1-dependent necroptosis in androgen-dependent LNCaP prostate cancer cells. Int. J. Oncol. 2020, 56, 439–447. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Department of Health, Hong Kong Special Administrative Region, the People’s Republic of China. Radix Ophiopogonis. In Hong Kong Chinese Materis Medica Standards; Government of the Hong Kong Special Administrative Region, the People’s Republic of China: Hong Kong, China, 2010; Volume 3, pp. 165–174. [Google Scholar]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Sun, Y.; Zhao, H.; Guo, Z.; Yu, B. Liriopesides B inhibited cell growth and decreased CA125 level in human ovarian cancer A2780 cells. Nat. Prod. Res. 2017, 31, 2198–2202. [Google Scholar] [CrossRef]
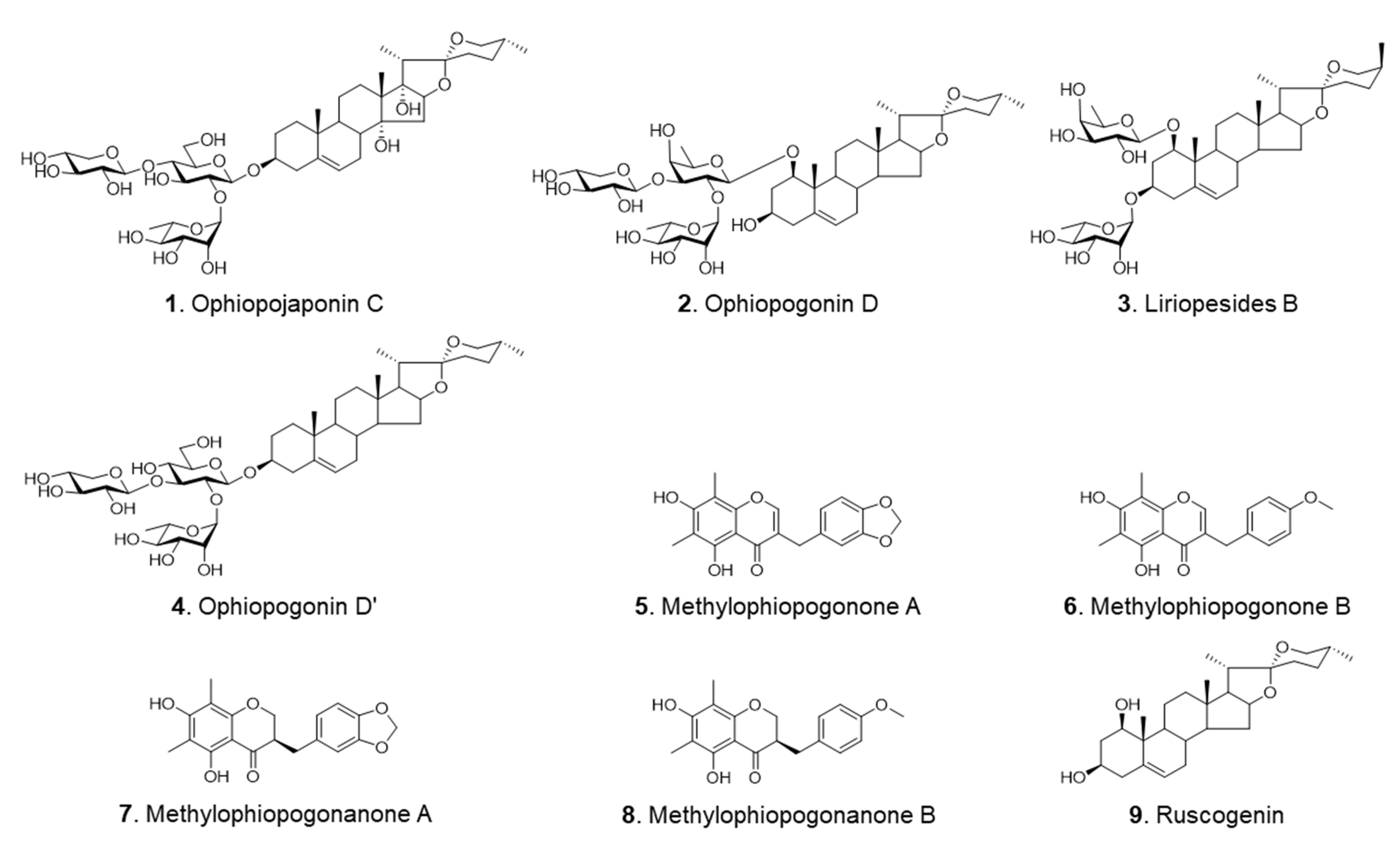

| Analyte | Calibration Curve | LOD (μg/mL) | LOQ (μg/mL) | ||
|---|---|---|---|---|---|
| Regressive Equation | Test Range (μg/mL) | R2 | |||
| Ophiopojaponin C | y = 12.53x − 141.06 | 24.20–121.10 | 0.9992 | 12.10 | 24.20 |
| Ophiopogonin D | y = 16.71x − 333.27 | 50.70–182.00 | 0.9998 | 25.30 | 50.70 |
| Liriopesides B | y = 18.02x − 630.93 | 52.00–182.00 | 0.9982 | 26.00 | 52.00 |
| Ophiopogonin D’ | y = 18.99x − 597.45 | 49.00–247.00 | 0.9930 | 24.90 | 49.00 |
| Methylophiopogonone A | y = 39.79x − 64.29 | 11.90–59.40 | 1.0000 | 0.08 | 0.24 |
| Methylophiopogonone B | y = 40.59x − 55.02 | 11.20–56.10 | 0.9999 | 0.08 | 0.23 |
| Methylophiopogonanone A | y = 36.77x − 58.73 | 12.30–61.70 | 0.9999 | 0.08 | 0.23 |
| Methylophiopogonanone B | y = 32.27x − 51.54 | 11.90–59.40 | 1.0000 | 0.07 | 0.22 |
| Ruscogenin | y = 24.294x − 438.83 | 24.90–124.00 | 0.9987 | 12.40 | 24.90 |
| Analyte | Precision | Repeatability | Accuracy | ||||
|---|---|---|---|---|---|---|---|
| Intra-Day RSD, % (n = 6) | Inter-Day RSD, % (n = 6) | Low RSD, % (n = 3) | Medium RSD, % (n = 3) | High RSD, % (n = 3) | Recovery, % | RSD, % (n = 3) | |
| Ophiopojaponin C | 0.17 | 1.45 | 0.67 | 1.37 | 3.41 | 103.9 | 0.35 |
| Ophiopogonin D | 0.43 | 0.60 | 3.56 | 2.10 | 2.37 | 105.3 | 0.68 |
| Liriopesides B | 0.28 | 1.64 | - * | - | - | 99.7 | 0.63 |
| Ophiopogonin D’ | 0.95 | 0.70 | 4.04 | 1.48 | 0.63 | 102.0 | 0.45 |
| Methylophiopogonone A | 0.27 | 0.40 | 3.67 | 1.01 | 1.77 | 94.4 | 0.21 |
| Methylophiopogonone B | 0.29 | 0.88 | 2.82 | 2.19 | 3.26 | 99.2 | 0.88 |
| Methylophiopogonanone A | 0.30 | 1.22 | 4.01 | 0.95 | 0.33 | 96.5 | 0.40 |
| Methylophiopogonanone B | 0.30 | 1.23 | 2.96 | 3.63 | 3.17 | 95.0 | 0.22 |
| Ruscogenin | 0.44 | 3.93 | - | - | - | 96.1 | 4.21 |
| Samples | 1 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | TS 2 | TF 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CMD-1 | 11.26 | 164.12 | - 4 | 14.64 | 1.58 | 0.97 | 36.92 | 34.45 | - | 190.02 | 73.92 |
| CMD-2 | 20.01 | 212.38 | - | 60.15 | 3.20 | 1.79 | 73.69 | 63.01 | - | 292.54 | 141.69 |
| CMD-3 | 11.35 | 266.85 | - | 34.72 | 3.16 | 1.60 | 47.08 | 41.11 | - | 312.92 | 92.95 |
| CMD-4 | 20.06 | 183.02 | - | 22.58 | 3.36 | 1.58 | 60.49 | 42.75 | - | 225.66 | 108.18 |
| CMD-5 | 10.92 | 98.37 | - | 19.75 | 1.27 | 0.49 | 35.29 | 18.31 | - | 129.04 | 55.36 |
| CMD-6 | 13.10 | 162.45 | - | 25.95 | 2.33 | 1.19 | 36.17 | 27.23 | - | 201.50 | 66.92 |
| CMD-7 | 11.15 | 88.41 | - | 18.96 | 1.96 | 0.81 | 42.94 | 26.71 | - | 118.52 | 72.42 |
| CMD-8 | 16.64 | 154.7 | - | 24.23 | 1.58 | 0.93 | 33.06 | 22.85 | - | 195.57 | 58.42 |
| CMD-9 | 16.17 | 158.19 | - | 30.96 | 2.44 | 1.41 | 56.17 | 40.61 | - | 205.32 | 100.63 |
| CMD-10 | 9.07 | 99.10 | - | 17.95 | 2.02 | 1.09 | 51.81 | 38.81 | - | 126.12 | 93.73 |
| CMD-11 | 14.66 | 154.16 | - | 23.54 | 2.91 | 1.48 | 54.10 | 40.24 | - | 192.36 | 98.73 |
| CMD-12 | 14.88 | 127.67 | - | 23.36 | 3.25 | 1.46 | 63.14 | 41.93 | - | 165.91 | 109.78 |
| CMD-13 | 13.83 | 224.59 | - | 29.10 | 2.52 | 1.26 | 54.95 | 36.89 | - | 267.52 | 95.62 |
| CMD-14 | 16.93 | 217.14 | - | 29.79 | 3.00 | 1.49 | 53.21 | 38.41 | - | 263.86 | 96.11 |
| Mean | 14.29 | 165.08 | / | 26.83 | 2.47 | 1.25 | 49.93 | 36.67 | / | 206.20 | 90.32 |
| ZMD-1 | 140.92 | - | - | - | 1.65 | 2.69 | 72.46 | 181.12 | - | 140.92 | 257.92 |
| ZMD-2 | 84.08 | - | - | - | 1.95 | 2.70 | 87.64 | 194.18 | - | 84.08 | 286.47 |
| ZMD-3 | 85.11 | - | - | - | 1.80 | 2.48 | 73.29 | 163.42 | - | 85.11 | 240.99 |
| ZMD-4 | 63.64 | 33.37 | - | 8.73 | 4.92 | 5.72 | 114.82 | 171.26 | - | 105.74 | 296.72 |
| ZMD-5 | 53.97 | 21.13 | - | 6.45 | 6.45 | 6.96 | 113.95 | 163.82 | - | 81.55 | 291.18 |
| ZMD-6 | 52.61 | 23.68 | - | 8.90 | 4.09 | 5.09 | 100.59 | 144.14 | - | 85.19 | 253.91 |
| ZMD-7 | 59.69 | 27.45 | - | 10.07 | 4.56 | 5.90 | 96.42 | 157.31 | - | 97.21 | 264.19 |
| ZMD-8 | 67.45 | 20.77 | - | 7.79 | 4.73 | 4.71 | 108.17 | 179.85 | - | 96.01 | 297.46 |
| Mean | 75.93 | 25.28 | / | 8.39 | 3.77 | 4.53 | 95.92 | 169.39 | / | 96.98 | 273.61 |
| SMD-1 | - | - | 377.44 | - | + 5 | + | + | + | - | 377.44 | / |
| SMD-2 | - | - | 301.65 | - | + | + | + | + | - | 301.65 | / |
| SMD-3 | - | - | 284.67 | - | - | - | + | + | - | 284.67 | / |
| SMD-4 | - | - | 208.55 | - | - | - | + | + | - | 208.55 | / |
| Mean | / | / | 293.08 | / | / | / | / | / | / | 293.08 | / |
| Analyte | IC50 (Mean ± SD, n = 3) |
|---|---|
| CMD extract | 35.8 ± 0.5 μg/mL (0.967 ± 0.012 mg crude drug/mL) |
| ZMD extract | 64.5 ± 17.7 μg/mL (0.892 ± 0.245 mg crude drug/mL) |
| SMD extract | 119.9 ± 25.6 μg/mL (6.251 ± 1.335 mg crude drug/mL) |
| Ophiopojaponin C | >50 μM |
| Ophiopogonin D | >50 μM |
| Ophiopogonin D’ | 0.89 ± 0.64 μM |
| Methylophiopogonone A | 2.61 ± 2.07 μM |
| Methylophiopogonone B | 8.25 ± 3.31 μM |
| Methylophiopogonanone A | 3.98 ± 2.38 μM |
| Methylophiopogonanone B | 3.25 ± 3.46 μM |
| No. | Code | Species | Origin |
|---|---|---|---|
| 1 | CMD-1 | Ophiopogon japonicus | Laoma, Santai, Sichuan, China |
| 2 | CMD-2 | Ophiopogon japonicus | Laoma, Santai, Sichuan, China |
| 3 | CMD-3 | Ophiopogon japonicus | Laoma, Santai, Sichuan, China |
| 4 | CMD-4 | Ophiopogon japonicus | Laoma, Santai, Sichuan, China |
| 5 | CMD-5 | Ophiopogon japonicus | Laoma, Santai, Sichuan, China |
| 6 | CMD-6 | Ophiopogon japonicus | Laoma, Santai, Sichuan, China |
| 7 | CMD-7 | Ophiopogon japonicus | Xinde, Santai, Sichuan, China |
| 8 | CMD-8 | Ophiopogon japonicus | Zhengsheng, Santai, Sichuan, China |
| 9 | CMD-9 | Ophiopogon japonicus | Huanyuan, Santai, Sichuan, China |
| 10 | CMD-10 | Ophiopogon japonicus | Laoma, Santai, Sichuan, China |
| 11 | CMD-11 | Ophiopogon japonicus | Xinde, Santai, Sichuan, China |
| 12 | CMD-12 | Ophiopogon japonicus | Laoma, Santai, Sichuan, China |
| 13 | CMD-13 | Ophiopogon japonicus | Guangming, Santai, Sichuan, China |
| 14 | CMD-14 | Ophiopogon japonicus | Lingxing, Santai, Sichuan, China |
| 15 | ZMD-1 | Ophiopogon japonicus | Fuhai, Cixi, Zhejiang, China |
| 16 | ZMD-2 | Ophiopogon japonicus | Fuhai, Cixi, Zhejiang, China |
| 17 | ZMD-3 | Ophiopogon japonicus | Fuhai, Cixi, Zhejiang, China |
| 18 | ZMD-4 | Ophiopogon japonicus | Shengshan, Cixi, Zhejiang, China |
| 19 | ZMD-5 | Ophiopogon japonicus | Shengshan, Cixi, Zhejiang, China |
| 20 | ZMD-6 | Ophiopogon japonicus | Shengshan, Cixi, Zhejiang, China |
| 21 | ZMD-7 | Ophiopogon japonicus | Shengshan, Cixi, Zhejiang, China |
| 22 | ZMD-8 | Ophiopogon japonicus | Shengshan, Cixi, Zhejiang, China |
| 23 | SMD-1 | Liriope spicata var. prolifera | Hubei, China |
| 24 | SMD-2 | Liriope spicata var. prolifera | Hubei, China |
| 25 | SMD-3 | Liriope spicata var. prolifera | Hubei, China |
| 26 | SMD-4 | Liriope spicata var. prolifera | Hubei, China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.-H.; Leong, F.; Gao, S.-J.; Chen, X.; Lu, J.-J.; Lin, L.-G.; Wang, Y.; Chen, X.-J. Comparison of Ophiopogon japonicus and Liriope spicata var. prolifera from Different Origins Based on Multi-Component Quantification and Anticancer Activity. Molecules 2023, 28, 1045. https://doi.org/10.3390/molecules28031045
Chen M-H, Leong F, Gao S-J, Chen X, Lu J-J, Lin L-G, Wang Y, Chen X-J. Comparison of Ophiopogon japonicus and Liriope spicata var. prolifera from Different Origins Based on Multi-Component Quantification and Anticancer Activity. Molecules. 2023; 28(3):1045. https://doi.org/10.3390/molecules28031045
Chicago/Turabian StyleChen, Min-Hui, Fong Leong, Si-Jia Gao, Xin Chen, Jin-Jian Lu, Li-Gen Lin, Yitao Wang, and Xiao-Jia Chen. 2023. "Comparison of Ophiopogon japonicus and Liriope spicata var. prolifera from Different Origins Based on Multi-Component Quantification and Anticancer Activity" Molecules 28, no. 3: 1045. https://doi.org/10.3390/molecules28031045
APA StyleChen, M.-H., Leong, F., Gao, S.-J., Chen, X., Lu, J.-J., Lin, L.-G., Wang, Y., & Chen, X.-J. (2023). Comparison of Ophiopogon japonicus and Liriope spicata var. prolifera from Different Origins Based on Multi-Component Quantification and Anticancer Activity. Molecules, 28(3), 1045. https://doi.org/10.3390/molecules28031045









