Photochromic and Luminescent Properties of a Salt of a Hybrid Molecule Based on C60 Fullerene and Spiropyran—A Promising Approach to the Creation of Anticancer Drugs
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. UV–Vis and Luminescent Studies
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Minkin, V.I. Photo–, Thermo–, Solvato–, and Electrochromic Spiroheterocyclic Compounds. Chem. Rev. 2004, 104, 2751–2776. [Google Scholar] [CrossRef] [PubMed]
- Klajn, R. Spiropyran–based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef] [PubMed]
- Khuzin, A.A.; Tuktarov, A.R.; Barachevsky, V.A.; Valova, T.M.; Tulyabaev, A.R.; Dzhemilev, U.M. Synthesis, photo and acidochromic properties of spiropyran-containing methanofullerenes. RSC Adv. 2020, 10, 15888–15892. [Google Scholar] [CrossRef] [PubMed]
- Nakazumi, H.; Matsumoto, S.; Isagawa, K. Photochromic behavior of spiropyrans in glass thin films formed by the sol–gel method. In Advanced Materials 93, IIA: Biomaterials, Organic and Intelligent Materials, Proceedings of the Third International Union of Materials Research Societies International Conference, Tokyo, Japan, 31 August–4 September 1993; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar] [CrossRef]
- Natali, M.; Giordani, S. Molecular switches as photocontrollable “smart” receptors. Chem. Soc. Rev. 2012, 41, 4010–4029. [Google Scholar] [CrossRef]
- Hiroyuki, A.; Kenji, S.; Takayuki, Y.; Tohru, T.; Xingguo, L.; Makoto, K. Spiropyran as a Regulator of DNA Hybridization with Reversed Switching Mode to That of Azobenzene. Chem. Lett. 2001, 30, 108–109. [Google Scholar] [CrossRef]
- Ramos–Garcia, R.; Delgado–Macuil, R.; Iturbe–Castillo, D.; de los Santos, E.G.; Corral, F.S. Polarization dependence on the holographic recording in spiropyran–doped polymers. Opt. Quantum Electron. 2003, 35, 641–650. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Salikhov, R.B.; Khuzin, A.A.; Popod’ko, N.R.; Safargalin, I.N.; Mullagaliev, I.N.; Dzhemilev, U.M. Photocontrolled organic field effect transistors based on the fullerene C60 and spiropyran hybrid molecule. RSC Adv. 2019, 9, 7505–7508. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Liu, G.; Tian, J.; Wang, H.; Gong, M.; Liu, S. Reversibly Switching Bilayer Permeability and Release Modules of Photochromic Polymersomes Stabilized by Cooperative Noncovalent Interactions. J. Am. Chem. Soc. 2015, 137, 48–15262. [Google Scholar] [CrossRef]
- Jeong, M.; Park, J.; Kwon, S. Molecular Switches and Motors Powered by Orthogonal Stimuli. Eur. J. Org. Chem. 2020, 47, 7254–7283. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Khuzin, A.A.; Dzhemilev, U.M. Light–controlled molecular switches based on carbon clusters. Synthesis, properties and application prospects. Russ. Chem. Rev. 2017, 86, 474–509. [Google Scholar] [CrossRef]
- Benedetto, A.; Ballone, P. Room–Temperature Ionic Liquids and Biomembranes: Setting the Stage for Applications in Pharmacology, Biomedicine, and Bionanotechnology. Langmuir 2018, 34, 9579–9597. [Google Scholar] [CrossRef]
- Kumari, P.; Pillai, V.V.S.; Benedetto, A. Mechanisms of action of ionic liquids on living cells: The state of the art. Biophys. Rev. 2020, 12, 1187–1215. [Google Scholar] [CrossRef]
- Dzhemileva, L.U.; D’yakonov, V.A.; Seitkalieva, M.M.; Kulikovskaya, N.S.; Egorova, K.S.; Ananikov, V.P. A large–scale study of ionic liquids employed in chemistry and energy research to reveal cytotoxicity mechanisms and to develop a safe design guide. Green Chem. 2021, 23, 6414–6430. [Google Scholar] [CrossRef]
- Piotrovsky, L.B.; Kiselev, O.I. Fullerenes and Viruses. Fuller. Nanotub. Carbon Nanostruct. 2005, 12, 397–403. [Google Scholar] [CrossRef]
- Jafvert, C.T.; Kulkarni, P.P. Buckminsterfullerene’s (C60) Octanol−Water Partition Coefficient (Kow) and Aqueous Solubility. Environ. Sci. Technol. 2008, 42, 5945–5950. [Google Scholar] [CrossRef]
- Piotrovskiy, L.B.; Litasova, E.V.; Dumpis, M.A.; Yakovleva, E.E.; Dravolina, O.A.; Bespalov, A.Y. Enhanced brain penetration of hexamethonium in complexes with derivatives of fullerene C60. Dokl. Biochem. Biophys. 2016, 468, 173–175. [Google Scholar] [CrossRef]
- Fagan, A.; Bartkowski, M.; Giordani, S. Spiropyran–Based Drug Delivery Systems. Front. Chem. 2021, 9, 720087. [Google Scholar] [CrossRef]
- Mandal, M.; Banik, D.; Karak, A.; Manna, S.K.; Mahapatra, A.K. Spiropyran–Merocyanine Based Photochromic Fluorescent Probes: Design, Synthesis, and Applications. ACS Omega 2022, 7, 36988–37007. [Google Scholar] [CrossRef]
- Rad, J.K.; Balzade, Z.; Mahdavian, A.R. Spiropyran–based advanced photoswitchable materials: A fascinating pathway to the future stimuli–responsive devices. J. Photochem. Photobiol. C 2022, 51, 100487–100552. [Google Scholar] [CrossRef]
- Galimov, D.I.; Tuktarov, A.R.; Sabirov, D.S.; Khuzin, A.A.; Dzhemilev, U.M. Reversible luminescence switching of a photochromic fullerene [60]–containing spiropyran. J. Photochem. Photobiol. A 2019, 375, 64–70. [Google Scholar] [CrossRef]
- Khuzin, A.A.; Galimov, D.I.; Tulyabaev, A.R.; Khuzina, L.L. Synthesis, Photochromic and Luminescent Properties of Ammonium Salts of Spiropyrans. Molecules 2022, 27, 8492. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.R.; Li, S.; Önfelt, B.; Andréasson, J. Light–induced cytotoxicity of a photochromic spiropyran. Chem. Commun. 2011, 47, 11020–11022. [Google Scholar] [CrossRef] [PubMed]
- Prato, M.; Maggini, M.; Giacometti, C.; Scorrano, G.; Sandona, G.; Farniac, G. Synthesis and electrochemical properties of substituted fulleropyrrolidines. Tetrahedron 1996, 52, 5221–5234. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Khuzin, A.A.; Tulyabaev, A.R.; Venidictova, O.V.; Valova, T.M.; Barachevsky, V.A.; Khalilov, L.M.; Dzhemilev, U.M. Synthesis, structure and photochromic properties of hybrid molecules based on fullerene C60 and spiropyrans. RSC Adv. 2016, 6, 71151–71155. [Google Scholar] [CrossRef]
- Andreev, S.; Purgina, D.; Bashkatova, E.; Garshev, A.; Maerle, A.; Andreev, I.; Osipova, N.; Shershakova, N.; Khaitov, M. Study of Fullerene Aqueous Dispersion Prepared by Novel Dialysis Method: Simple Way to Fullerene Aqueous Solution. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 792–800. [Google Scholar] [CrossRef]
- Kyzyma, O.; Bashmakova, N.; Gorshkov, Y.; Ivankov, O.; Mikheev, I.; Kuzmenko, M.; Kutovyy, S.; Nikolaienko, T. Interaction between the plant alkaloid berberine and fullerene C70: Experimental and quantum–chemical study. J. Mol. Liq. 2019, 278, 452–459. [Google Scholar] [CrossRef]
- Mikheev, I.V.; Pirogova, M.O.; Usoltseva, L.O.; Uzhel, A.S.; Bolotnik, T.A.; Kareev, I.E.; Bubnov, V.P.; Lukonina, N.S.; Volkov, D.S.; Goryunkov, A.A.; et al. Green and rapid preparation of long–term stable aqueous dispersions of fullerenes and endohedral fullerenes: The pros and cons of an ultrasonic probe. Ultrason. Sonochem. 2021, 73, 105533–105543. [Google Scholar] [CrossRef]
- Mchedlov–Petrossyan, N.O. Fullerenes in Liquid Media: An Unsettling Intrusion into the Solution Chemistry. Chem. Rev. 2013, 113, 5149–5193. [Google Scholar] [CrossRef]

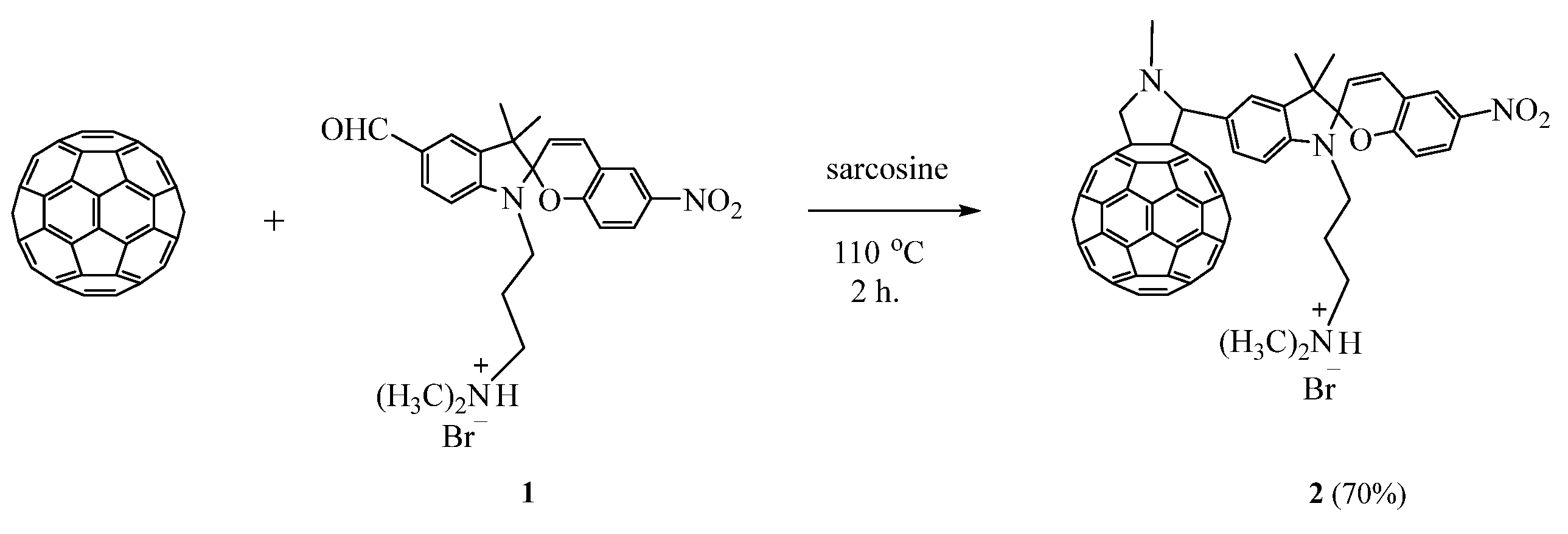
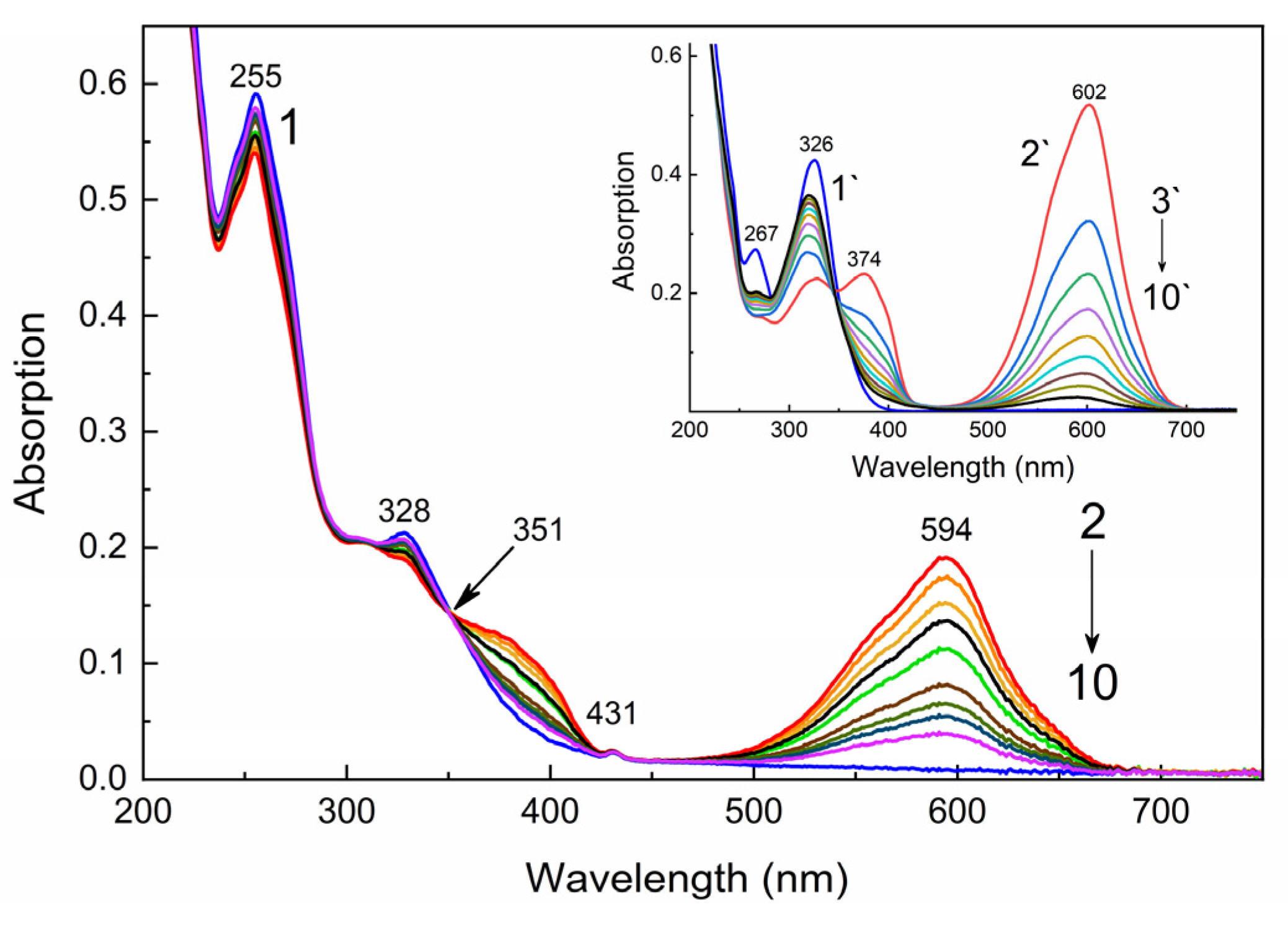
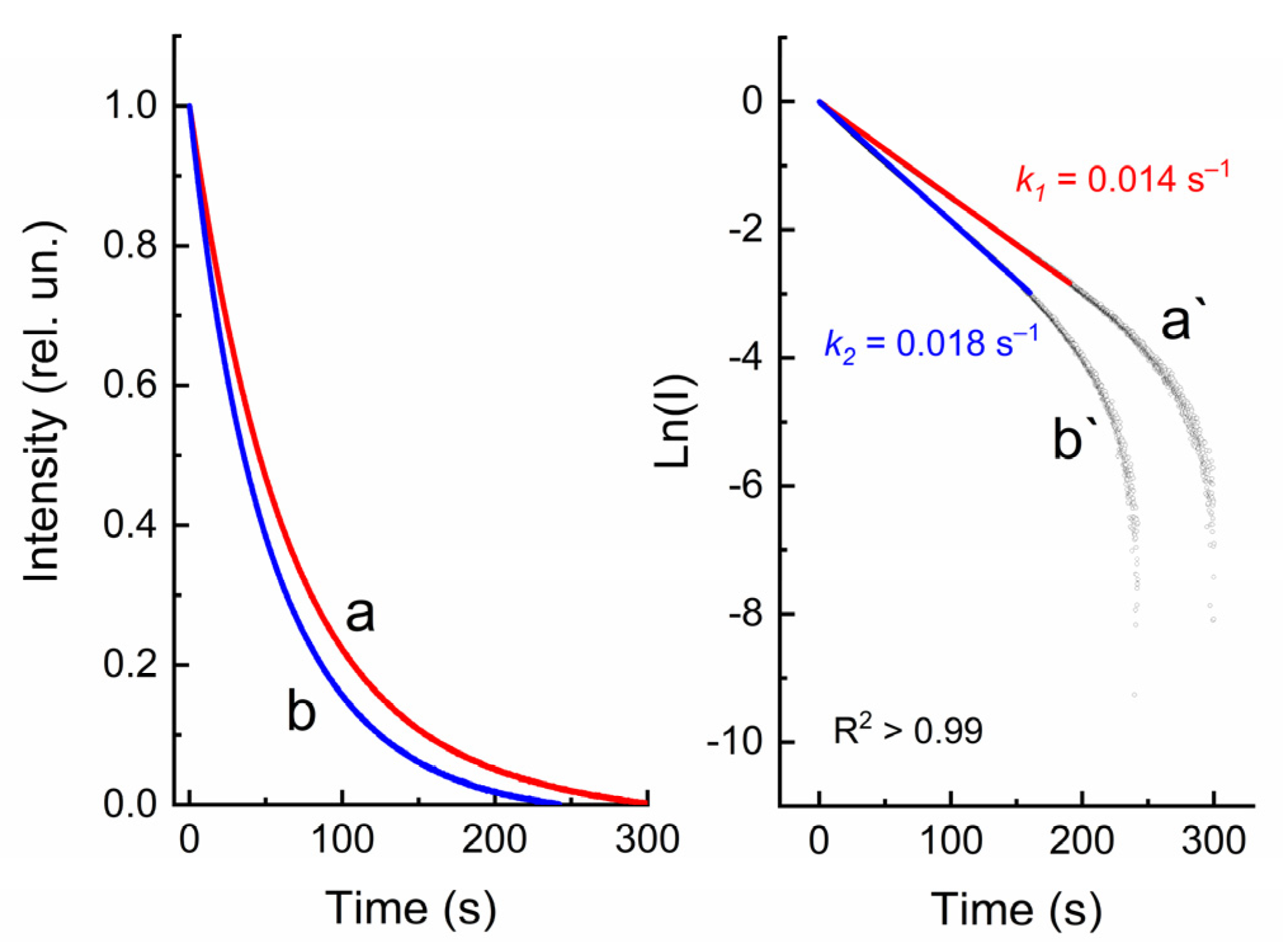
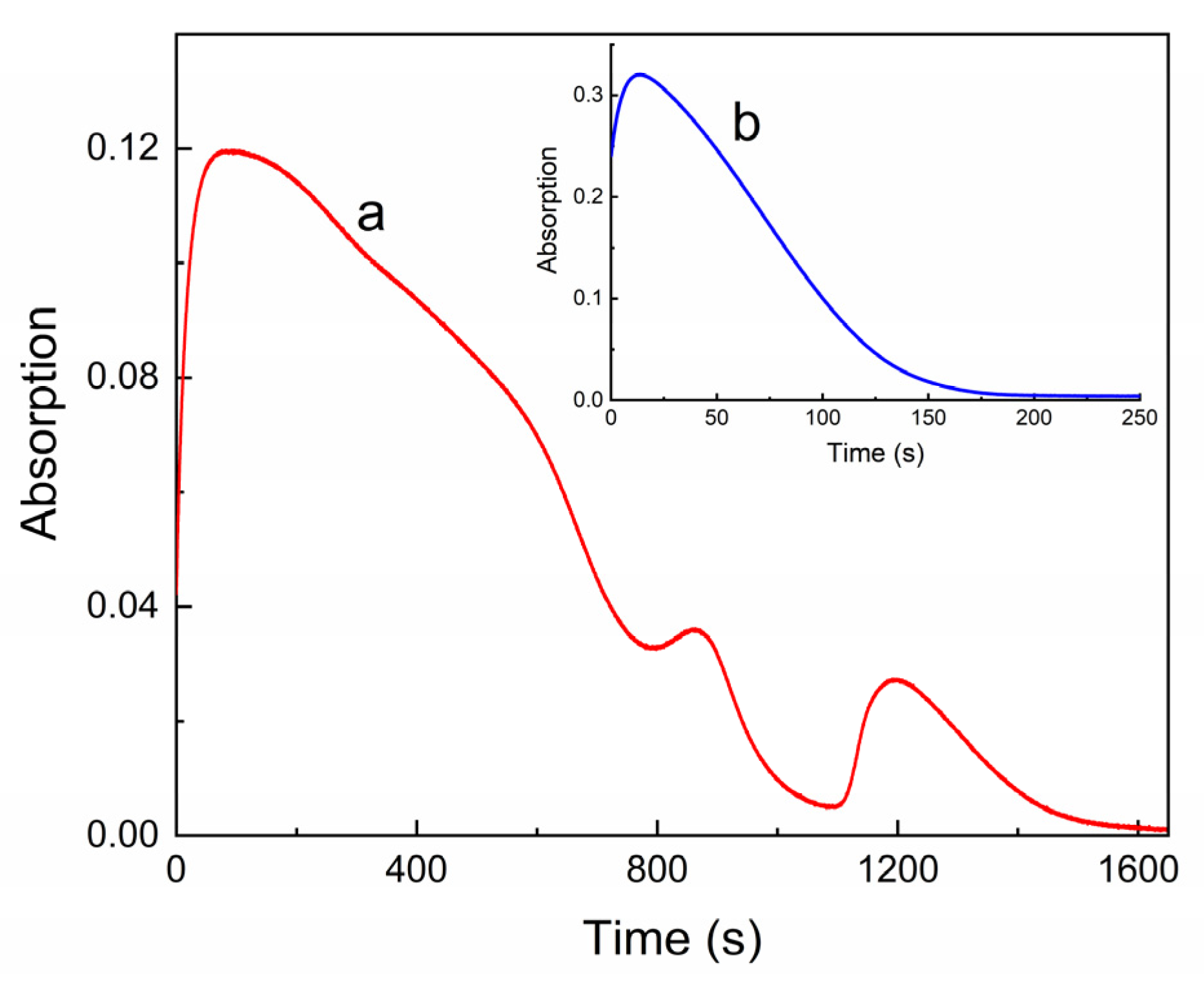
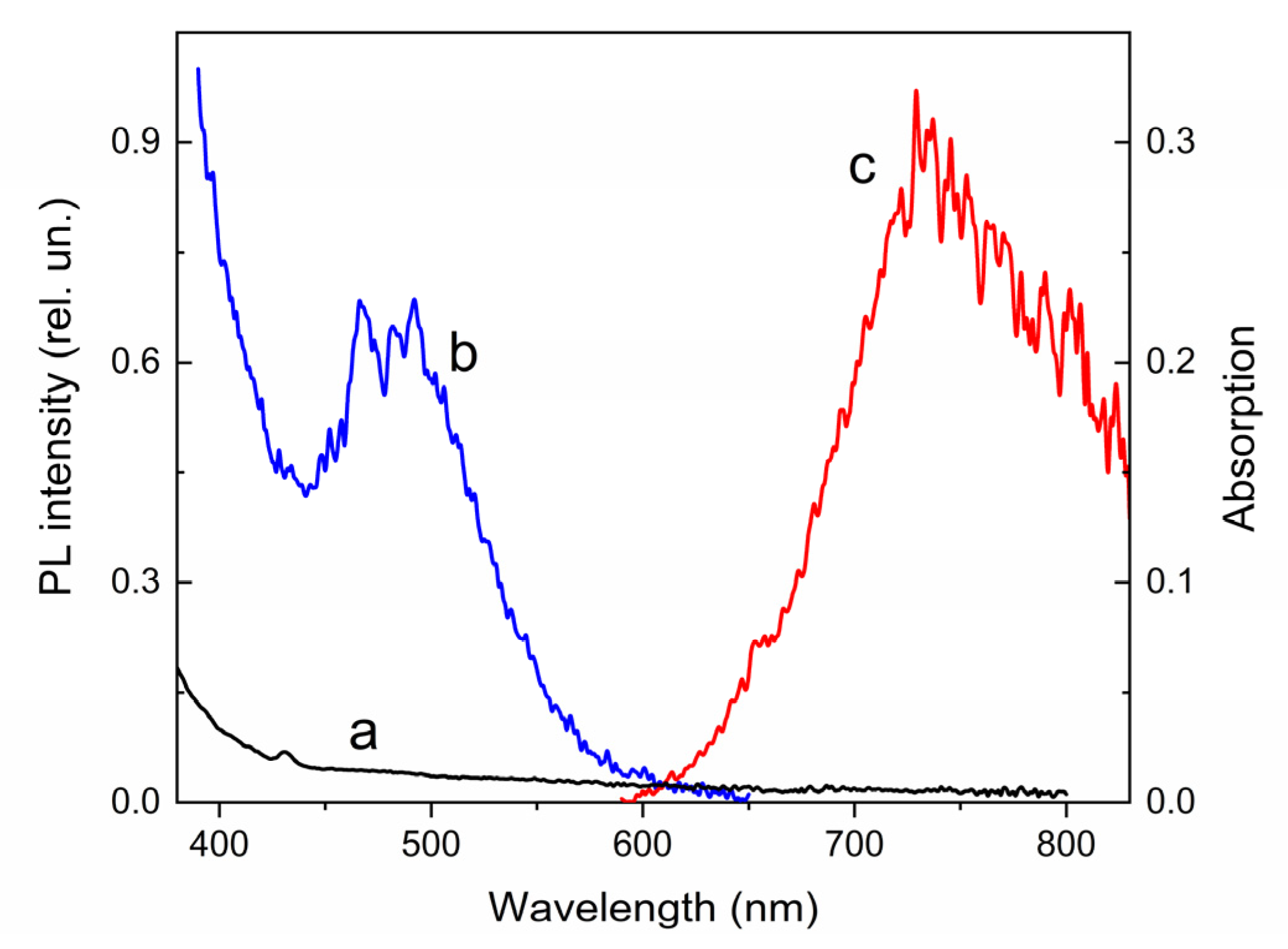
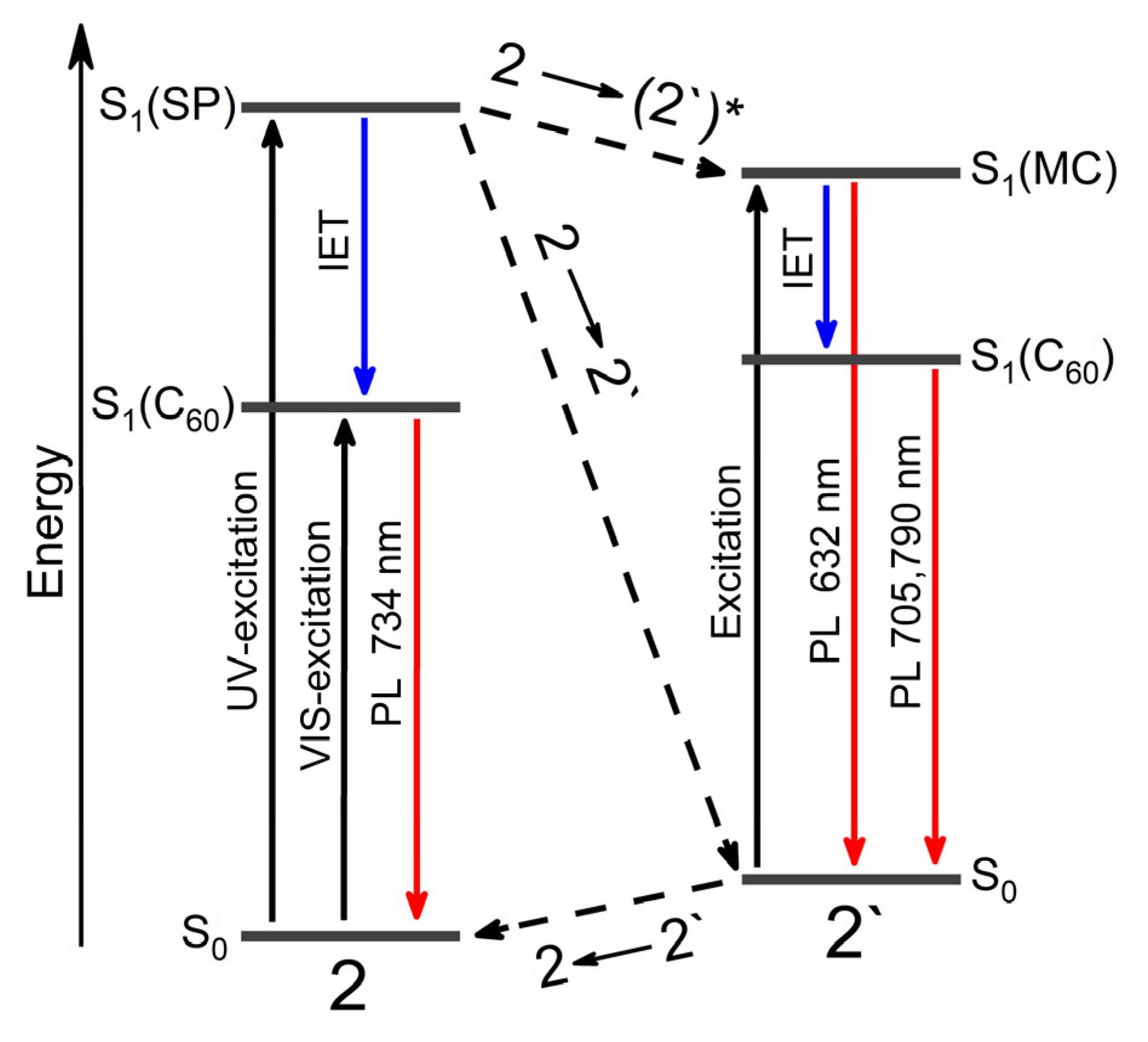
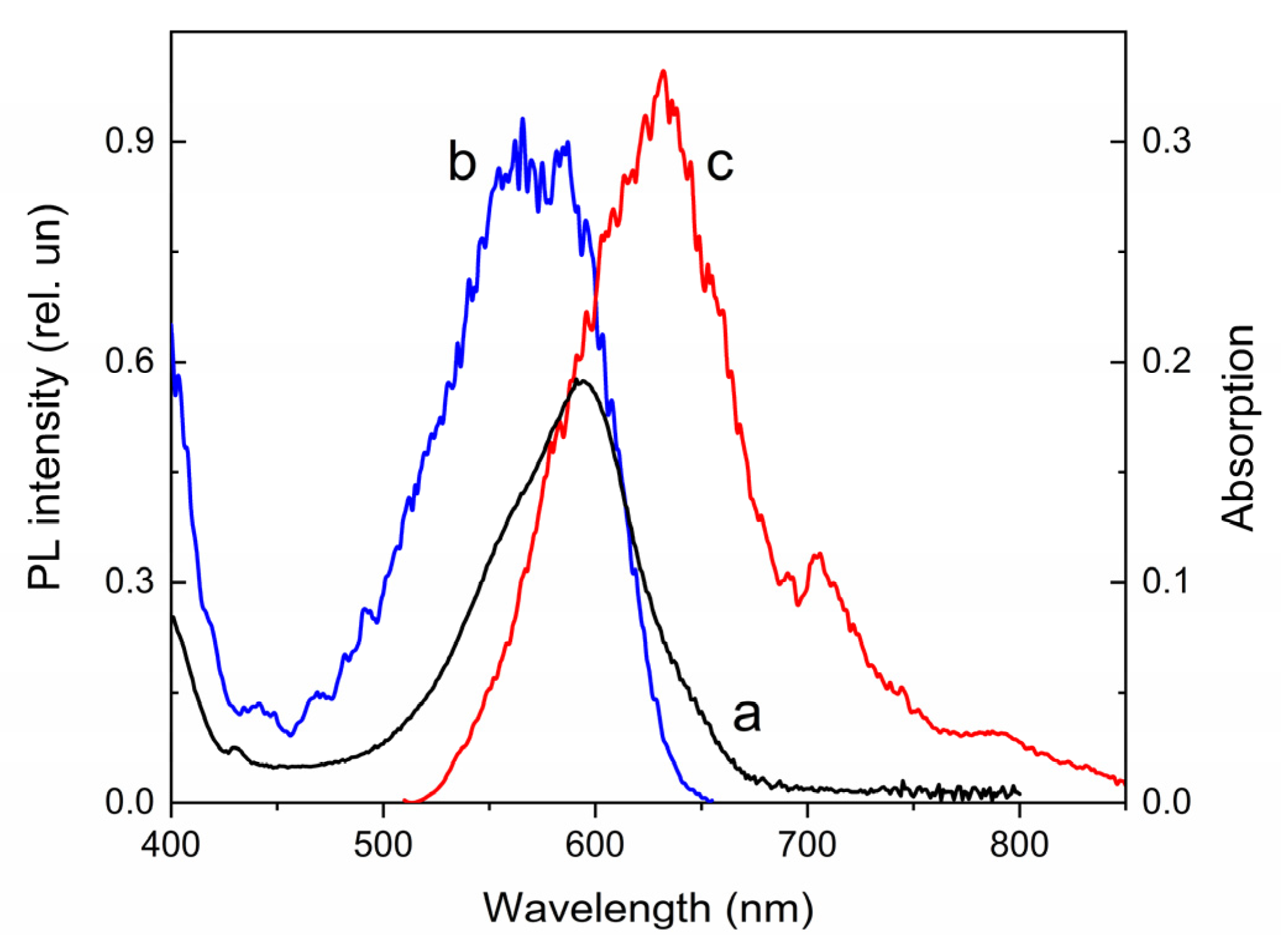
| Compound | λabs (nm) 1 (ε, M−1·cm−1) | k1 (s−1) 2 | k2 (s−1) 3 | τ1/2 (s) 4 | S (rel.un.) 5 | λPL (nm) | λexc (nm) | Rel.un. | τ (ns) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 267 (23,000), 324 (38,500) | 0.028 | 0.031 | 64 | 1.30 | – | – | – | – |
| 1′ | 374 (19,400), 602 (49,800) | 645 | 392, 578 | 4.7 | 2.7 (640 nm) | ||||
| 2 | 255 (59,000), 307 (20,500) 328 (21,300), 431 (2480) | 0.014 | 0.018 | >700 | 0.87 | 734 | 480 | 1.0 | 2.5 (720 nm) |
| 2′ | 381 (11,790), 594 (18,500) | 632, 705, 790 | 574 | 7.4 | 3.1 (640 nm), 2.5 (720 nm) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuzin, A.A.; Galimov, D.I.; Khuzina, L.L. Photochromic and Luminescent Properties of a Salt of a Hybrid Molecule Based on C60 Fullerene and Spiropyran—A Promising Approach to the Creation of Anticancer Drugs. Molecules 2023, 28, 1107. https://doi.org/10.3390/molecules28031107
Khuzin AA, Galimov DI, Khuzina LL. Photochromic and Luminescent Properties of a Salt of a Hybrid Molecule Based on C60 Fullerene and Spiropyran—A Promising Approach to the Creation of Anticancer Drugs. Molecules. 2023; 28(3):1107. https://doi.org/10.3390/molecules28031107
Chicago/Turabian StyleKhuzin, Artur A., Dim I. Galimov, and Liliya L. Khuzina. 2023. "Photochromic and Luminescent Properties of a Salt of a Hybrid Molecule Based on C60 Fullerene and Spiropyran—A Promising Approach to the Creation of Anticancer Drugs" Molecules 28, no. 3: 1107. https://doi.org/10.3390/molecules28031107
APA StyleKhuzin, A. A., Galimov, D. I., & Khuzina, L. L. (2023). Photochromic and Luminescent Properties of a Salt of a Hybrid Molecule Based on C60 Fullerene and Spiropyran—A Promising Approach to the Creation of Anticancer Drugs. Molecules, 28(3), 1107. https://doi.org/10.3390/molecules28031107





