Baicalein Induces G2/M Cell Cycle Arrest Associated with ROS Generation and CHK2 Activation in Highly Invasive Human Ovarian Cancer Cells
Abstract
1. Introduction
2. Results
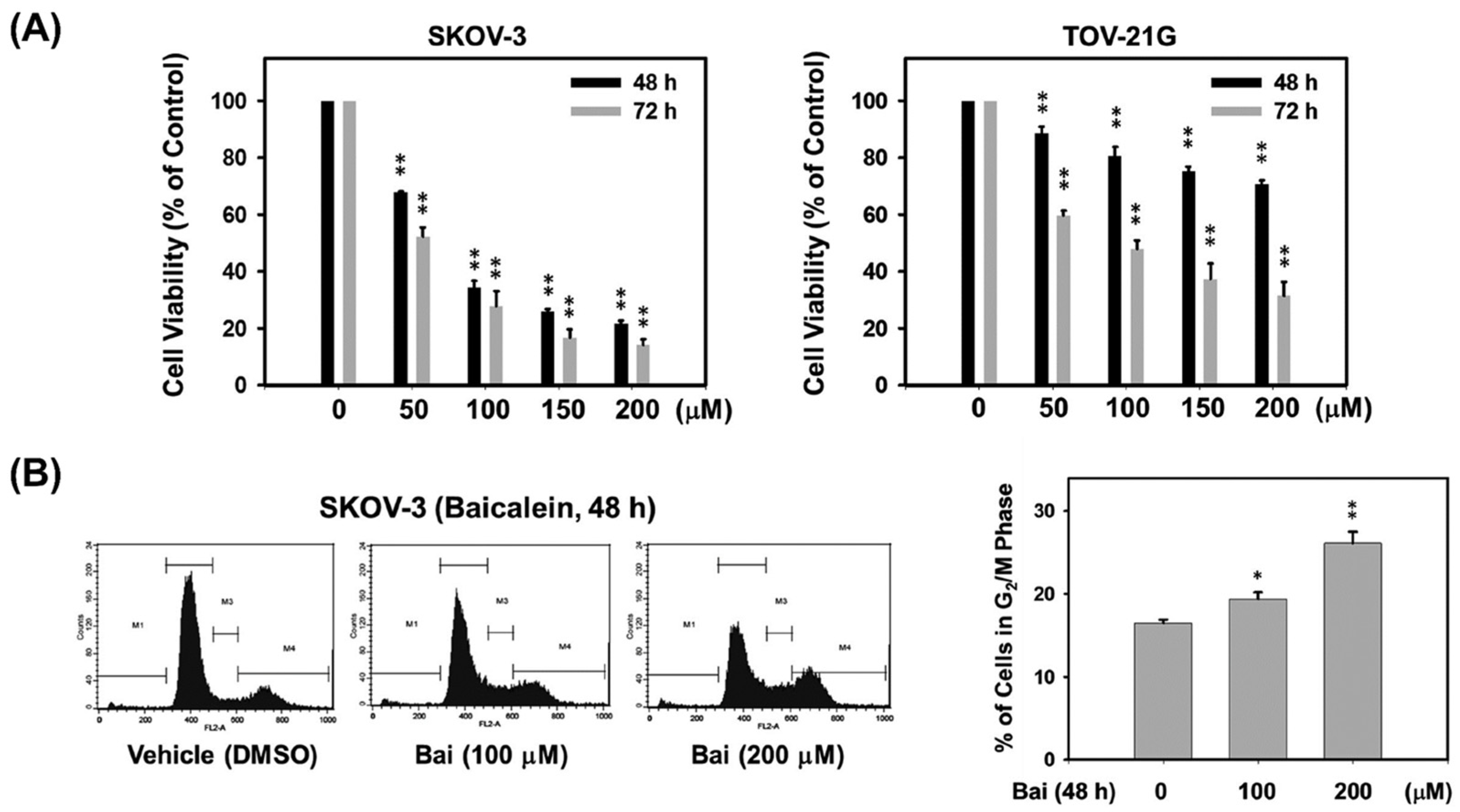
2.1. Baicalein Suppresses Highly Invasive Ovarian Cancer Cell Proliferation by Inducing G2/M Cell Cycle Arrest
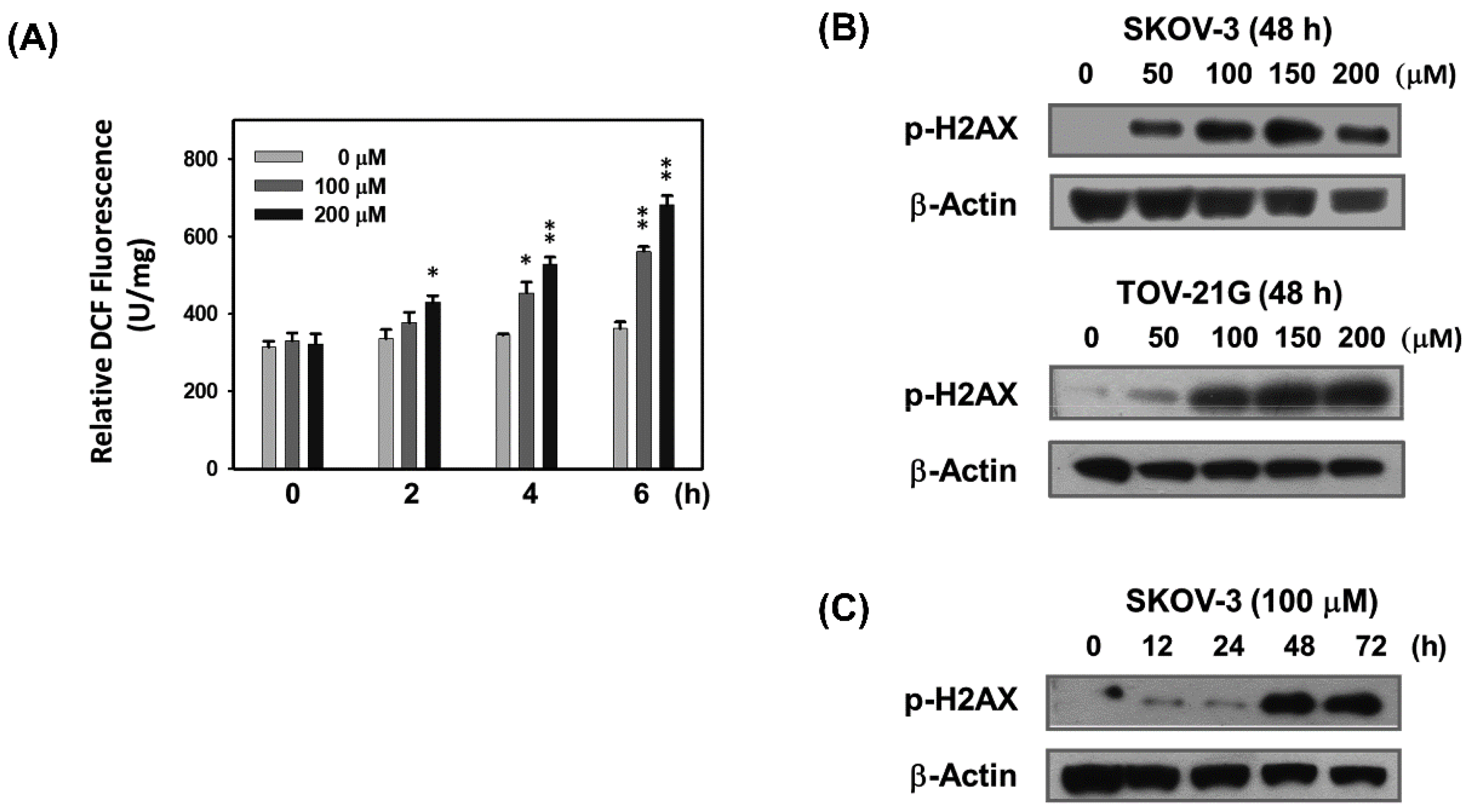
2.2. Baicalein Causes ROS Generation and DNA Breakage in Ovarian Cancer Cells
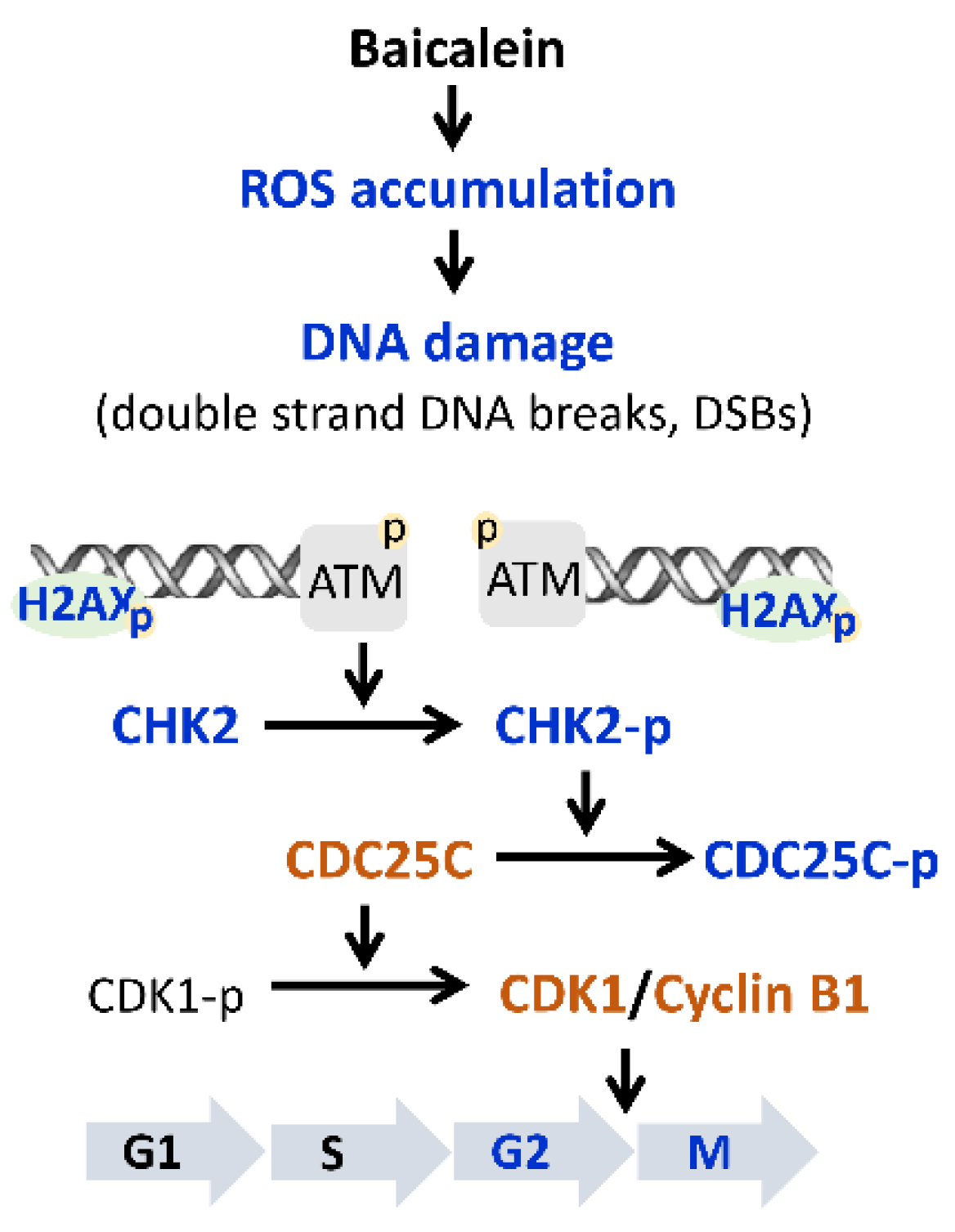
2.3. Baicalein Activates the ATM/CHK2/CDC25C Signaling Pathway in Ovarian Cancer Cells
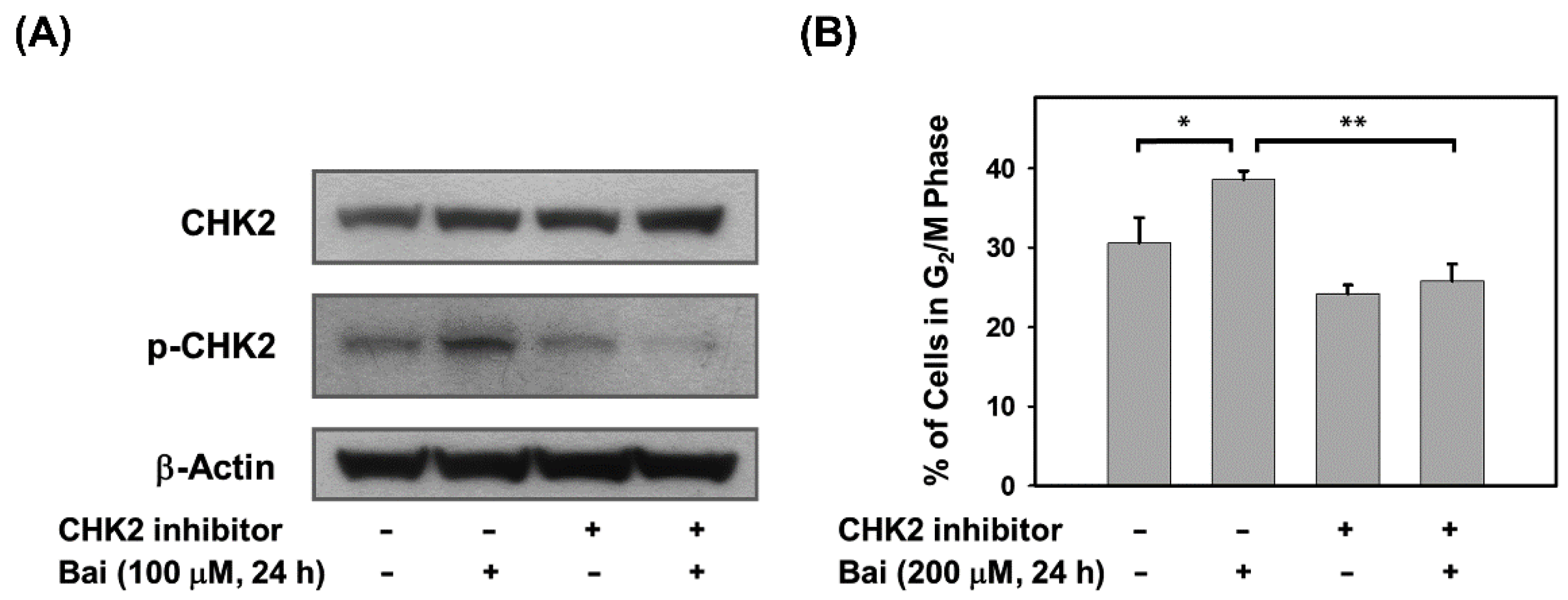
2.4. Blocking of CHK2 Activity Attenuates Baicalein-Induced G2/M Arrest in Ovarian Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Chemicals
4.2. Cell Viability Assay
4.3. Flow Cytometric Analysis
4.4. Determination of ROS Generation
4.5. Immunoblot Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.-L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.-L.; Miller, K.-D.; Fuchs, H.-E.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Lisio, M.-A.; Fu, L.; Goyeneche, A.; Gao, Z.-H.; Telleria, C. High-grade serous ovarian cancer: Basic sciences, clinical and therapeutic standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef] [PubMed]
- Lassus, H.; Leminen, A.; Vayrynen, A.; Cheng, G.; Gustafsson, J.-A.; Isola, J.; Butzow, R. ERBB2 amplification is superior to protein expression status in predicting patient outcome in serous ovarian carcinoma. Gynecol. Oncol. 2004, 92, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kandala, P.-K.; Srivastava, S.-K. Activation of checkpoint kinase 2 by 3,3′-diindolylmethane is required for causing G2/M cell cycle arrest in human ovarian cancer cells. Mol. Pharmacol. 2010, 78, 297–309. [Google Scholar] [CrossRef]
- Silwal-Pandit, L.; Langerød, A.; Børresen-Dale, A.-L. TP53 mutations in breast and ovarian cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a026252. [Google Scholar] [CrossRef]
- Srinivas, U.-S.; Tan, B.-W.-Q.; Vellayappan, B.-A.; Jeyasekharan, A.-D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Phan, L.-M.; Rezaeian, A.-H. ATM: Main features, signaling pathways, and its diverse roles in DNA damage response, tumor suppression, and cancer development. Genes 2021, 12, 845. [Google Scholar] [CrossRef]
- Ueno, S.; Sudo, T.; Hirasawa, A. ATM: Functions of ATM kinase and its relevance to hereditary tumors. Int. J. Mol. Sci. 2022, 23, 523. [Google Scholar] [CrossRef]
- Choi, W.; Lee, E.-S. Therapeutic targeting of DNA damage response in cancer. Int. J. Mol. Sci. 2022, 23, 1701. [Google Scholar] [CrossRef]
- Romagnolo, D.-F.; Selmin, O.-I. Flavonoids and cancer prevention: A review of the evidence. J. Nutr. Gerontol. Geriatr. 2012, 31, 206–238. [Google Scholar] [CrossRef]
- Rothwell, J.-A.; Knaze, V.; Zamora-Ros, R. Polyphenols: Dietary assessment and role in the prevention of cancers. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Bie, B.; Sun, J.; Guo, Y.; Li, J.; Jiang, W.; Yang, J.; Huang, C.; Li, Z. Baicalein: A review of its anti-cancer effects and mechanisms in hepatocellular carcinoma. Biomed. Pharmacother. 2017, 93, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Deepa, P.; Kim, P.-M.; Park, S.-J.; Kim, S. Baicalein as a potent neuroprotective agent: A review. Biomed. Pharmacother. 2017, 95, 1021–1032. [Google Scholar] [CrossRef]
- Liu, H.; Dong, Y.; Gao, Y.; Du, Z.; Wang, Y.; Cheng, P.; Chen, A.; Huang, H. The fascinating effects of baicalein on cancer: A review. Int. J. Mol. Sci. 2016, 17, 1681. [Google Scholar] [CrossRef]
- Zhao, X.; Qu, J.; Liu, X.; Wang, J.; Ma, X.; Zhao, X.; Yang, Q.; Yan, W.; Zhao, Z.; Hui, Y.; et al. Baicalein suppress EMT of breast cancer by mediating tumor-associated macrophages polarization. Am. J. Cancer Res. 2018, 8, 1528–1540. [Google Scholar] [PubMed]
- Zhang, Y.; Song, L.; Cai, L.; Wei, R.; Hu, H.; Jin, W. Effects of baicalein on apoptosis, cell cycle arrest, migration and invasion of osteosarcoma cells. Food Chem. Toxicol. 2013, 53, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Park, C.; Han, M.-H.; Hong, S.-H.; Kim, G.-Y.; Hong, S.-H.; Kim, N.-D.; Choi, Y.-H. Baicalein induces caspase-dependent apoptosis associated with the generation of ROS and the activation of AMPK in human lung carcinoma A549 cells. Drug Dev. Res. 2016, 77, 73–86. [Google Scholar] [CrossRef]
- Choi, E.-O.; Park, C.; Hwang, H.-J.; Hong, S.-H.; Kim, G.-Y.; Cho, E.-J.; Kim, W.-J.; Choi, Y.-H. Baicalein induces apoptosis via ROS-dependent activation of caspases in human bladder cancer 5637 cells. Int. J. Oncol. 2016, 49, 1009–1018. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Yang, C.-X.; Zhang, L.; Yang, C.-Y.; Xu, X.-Q. Baicalein, as a prooxidant, triggers mitochondrial apoptosis in MCF-7 human breast cancer cells through mobilization of intracellular copper and reactive oxygen species generation. Onco Targets Ther. 2019, 12, 10749–10761. [Google Scholar] [CrossRef]
- Moloney, J.-N.; Cotter, T.-G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Kopustinskiene, D.-M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Chou, D.-S.; Lee, J.-J.; Hsiao, G.; Hsieh, C.-Y.; Tsai, Y.-J.; Chen, T.-F.; Sheu, J.-R. Baicalein induction of hydroxyl radical formation via 12-lipoxygenase in human platelets: An ESR study. J. Agric. Food Chem. 2007, 55, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Furuno, K.; Akasako, T.; Sugihara, N. The contribution of the pyrogallol moiety to the superoxide radical scavenging activity of flavonoids. Biol. Pharm. Bull. 2002, 25, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Chen, A.-Y.; Ye, X.; Luo, H.; Rankin, G.-O.; Chen, Y.-C. Inhibitory effect of baicalin and baicalein on ovarian cancer cells. Int. J. Mol. Sci. 2013, 14, 6012–6025. [Google Scholar] [CrossRef]
- Qi, Z.; Yin, F.; Lu, L.; Shen, L.; Qi, S.; Lan, L.; Luo, L.; Yin, Z. Baicalein reduces lipopolysaccharide-induced inflammation via suppressing JAK/STATs activation and ROS production. Inflamm. Res. 2013, 62, 845–855. [Google Scholar] [CrossRef]
- Oh, M.-C.; Piao, M.J.; Fernando, P.-M.; Han, X.; Madduma Hewage, S.-R.; Park, J.-E.; Ko, M.-S.; Jung, U.; Kim, I.-G.; Hyun, J.-W. Baicalein protects human skin cells against ultraviolet B-induced oxidative stress. Biomol. Ther. 2016, 24, 616–622. [Google Scholar] [CrossRef]
- Jeong, J.-Y.; Cha, H.-J.; Choi, E.-O.; Kim, C.-H.; Kim, G.-Y.; Yoo, Y.-H.; Hwang, H.-J.; Park, H.-T.; Yoon, H.-M.; Choi, Y.-H. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of baicalein against oxidative stress-induced DNA damage and apoptosis in HEI193 Schwann cells. Int. J. Med. Sci. 2019, 16, 145–155. [Google Scholar] [CrossRef]
- Park, C.; Choi, E.-O.; Kim, G.-Y.; Hwang, H.-J.; Kim, B.-W.; Yoo, Y.-H.; Park, H.-T.; Choi, Y.-H. Protective effect of baicalein on oxidative stress-induced DNA damage and apoptosis in RT4-D6P2T Schwann cells. Int. J. Med. Sci. 2019, 16, 8–16. [Google Scholar] [CrossRef]
- Guo, A.-M.; Liu, X.; Al-Wahab, Z.; Maddippati, K.-R.; Ali-Fehmi, R.; Scicli, A.-G.; Munkarah, A.-R. Role of 12-lipoxygenase in regulation of ovarian cancer cell proliferation and survival. Cancer Chemother. Pharmacol. 2011, 68, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yan, G.; Lei, J.; Chen, Y.; Wang, T.; Gong, J.; Zhou, Y.; Zhao, H.; Chen, H.; Zhou, Y.; et al. The SP1-12LOX axis promotes chemoresistance and metastasis of ovarian cancer. Mol. Med. 2020, 26, 39. [Google Scholar] [CrossRef] [PubMed]
- Crane, E.-K.; Kwan, S.-Y.; Izaguirre, D.-I.; Tsang, Y.-T.; Mullany, L.-K.; Zu, Z.; Richards, J.-S.; Gershenson, D.-M.; Wong, K.-K. Nutlin-3a: A potential therapeutic opportunity for TP53 wild-type ovarian carcinomas. PLoS ONE 2015, 10, e0135101. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.-C.; Wu, K.; Lin, Y.-Y.; Kuo, H.-P.; Kao, M.-C.; Wang, V.; Hsu, S.-C.; Lee, S.-L. Dual down-regulation of EGFR and ErbB2 by berberine contributes to suppression of migration and invasion of human ovarian cancer cells. Environ. Toxicol. 2021, 36, 737–747. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, T.-C.; Shao, W.-S.; Hsu, S.-C.; Lee, S.-L.; Kao, M.-C.; Wang, V. Baicalein Induces G2/M Cell Cycle Arrest Associated with ROS Generation and CHK2 Activation in Highly Invasive Human Ovarian Cancer Cells. Molecules 2023, 28, 1039. https://doi.org/10.3390/molecules28031039
Chuang T-C, Shao W-S, Hsu S-C, Lee S-L, Kao M-C, Wang V. Baicalein Induces G2/M Cell Cycle Arrest Associated with ROS Generation and CHK2 Activation in Highly Invasive Human Ovarian Cancer Cells. Molecules. 2023; 28(3):1039. https://doi.org/10.3390/molecules28031039
Chicago/Turabian StyleChuang, Tzu-Chao, Wei-Syun Shao, Shih-Chung Hsu, Shou-Lun Lee, Ming-Ching Kao, and Vinchi Wang. 2023. "Baicalein Induces G2/M Cell Cycle Arrest Associated with ROS Generation and CHK2 Activation in Highly Invasive Human Ovarian Cancer Cells" Molecules 28, no. 3: 1039. https://doi.org/10.3390/molecules28031039
APA StyleChuang, T.-C., Shao, W.-S., Hsu, S.-C., Lee, S.-L., Kao, M.-C., & Wang, V. (2023). Baicalein Induces G2/M Cell Cycle Arrest Associated with ROS Generation and CHK2 Activation in Highly Invasive Human Ovarian Cancer Cells. Molecules, 28(3), 1039. https://doi.org/10.3390/molecules28031039





