Abstract
For many decades, natural resources have traditionally been employed in skin care. Here, we explored the phytochemical profile of the aqueous and ethanolic leaf extracts of Cupressus arizonica Greene and assessed their antioxidant, antiaging and antibacterial activities in vitro. Liquid chromatography–mass spectrometry (LC-MS/MS) analysis led to the tentative identification of 67 compounds consisting mainly of phenolic and fatty acids, diterpene acids, proanthocyanidins and flavonoid and biflavonoid glycosides. The aqueous extract demonstrated substantial in vitro antioxidant potential at FRAP and DPPH assays and inhibited the four target enzymes (collagenase, elastase, tyrosinase, and hyaluronidase) engaged in skin remodeling and aging with IC50 values close to those of the standard drugs. Moreover, the aqueous extract at 25 mg/mL suppressed biofilm formation by Pseudomonas aeruginosa, a bacterial pathogen causing common skin manifestations, and decreased its swarming and swimming motilities. In conclusion, C. arizonica leaves can be considered a promising candidate for potential application in skin aging.
1. Introduction
Skin represents the largest and most complex part of our body. Skin aging is a complicated biological phenomenon associated with alterations in the physical, structural, and physiological characters of the epidermis and dermis. During aging, the skin suffers from several adverse visible signs including dryness, wrinkle formation, reduction in skin elasticity, decreased wound healing capacity, uneven pigmentation, and discoloration. Skin aging is prompted by several extrinsic and intrinsic factors. The latter include hormonal, genetic, and cellular metabolic factors, while extrinsic factors include chronic exposure to external agents such as chemicals, pollutants, toxins, and predominantly, exposure to sunlight ultraviolet radiation, particularly UV-B, which leads to photoaging and potentially skin cancer. These factors ultimately lead to free radicals or reactive oxygen species (ROS), the generation of which further triggers the phenomenon of oxidative stress (OxS) in the skin [1].
Free radicals or ROS potentially stimulate the oxidation of skin lipids and proteins, leading to DNA damage and cell death that elicit a change in structural skin cell components and destruction of the cell membranes. In addition, an increase in matrix metalloproteinases (MMPs) expression in human skin can be enhanced by excessive ROS and OxS. Such proteolytic enzymes (MMPs) degrade extracellular matrix (ECM) proteins including elastin, collagen and hyaluronic acid [1]. The breakdown of collagen network and elastin fibers by MMP family members collagenase and elastase results in the loss of skin elasticity and appearance of wrinkles and consequently promotes skin aging. Additionally, hyaluronic acid, which is necessary to maintain the moisture of the skin as well as its smoothness and plasticity, is subjected to cleavage by the hyaluronidase, leading to inadequate skin hydration with subsequent dryness and sagging. Furthermore, one of the notable signs of skin aging is the excessive production of melanin, which is regulated by the tyrosinase enzyme. Although melanin has a vital role in the skin against UV-induced damage, its overproduction causes various skin problems such as hyperpigmentation and age spots. Therefore, these previously mentioned enzymes can be considered as potential targets of phytochemical inhibitors, especially those with significant antioxidant capacity, against skin aging and hyperpigmentation [2,3].
Biofilms are complex microbial cells embedded in an extracellular matrix composed of polysaccharides, proteins, extracellular DNA, and lipids. These biofilms provide an extra covering around their aggregated cells; thus, bacteria within the biofilm are more resistant to different groups of antimicrobial agents and even escape host immune reactions, causing many health problems [4]. The reduced sensitivity of biofilm-forming bacteria to antibiotics may be due to the fact that the surface layer of a biofilm is only exposed to lethal levels of antibiotics because of deactivation, adsorption of antibiotics to the biofilm extracellular matrix or other biochemical, physical or mechanical mechanisms that limit the effective penetration of antibiotics into the biofilm substratum [5]. Pseudomonas aeruginosa is a Gram-negative opportunistic bacterium and one of the pathogenic biofilm-producing bacteria that can cause different infections related to the skin. Recently, it has been the focus of intense research owing to its well-known involvement in diseases and increased resistance to antimicrobial agents [6,7]. Various plant extracts have been reported to inhibit P. aeruginosa biofilm production [8,9,10]. Currently, the discovery of natural resources for cosmeceutical and dermatological agents has become a crucial aim to meet consumers’ requirements and avoid the adverse effects of synthetic ingredients [2].
Gymnosperms possess the potential to supply promising candidates for drug discovery, but comprehensive studies have not investigated many plants. Conifers are a class of cone-bearing trees and shrubs belonging to the gymnosperms. The Cupressaceae family is considered the third largest gymnosperm family and one of the most important conifers. It includes 28–33 genera and 130–136 species of evergreen monoecious or dioecious trees and shrubs. Cupressaceae members have ornamental, economic and medicinal importance [11,12].
The genus Cupressus (cypress) is the second largest genus belonging to the family Cupressaceae. It comprises about 25 species of monoecious trees or large shrubs. It is mostly distributed in the northern hemisphere, including Asia, the Mediterranean region, northwest Africa, southern China, and North and Central America. The leaves are opposite decussate, and needle-like on young plants (up to 2 years old) and scale-like on adult trees. The seed cones are terminal and woody, maturing 1–2 years from pollination. Pollen cones are usually terminal and yellow. The seeds are many per scale, small, winged, and flat to angled in shape. Cupressus species have ornamental value because of their pyramidal or columnar crown shape and were used traditionally for the treatment of rheumatoid arthritis, pertussis, and respiratory problems. Cupressus members are well known to produce both volatile (essential oils) and nonvolatile constituents, mainly flavonoids, biflavonoids and diterpenes [12,13,14,15].
Cupressus arizonica Greene (Arizona cypress) is an evergreen tree (5–25 m in height). Stems are short, 1–2 mm in diameter and four-sided. The leaves are dull greyish green to bright blue green, scale-like, and bear rounded shoots. Pollen cones are 2–5 mm in length, 2–2.5 mm in diameter, and cylindric to four-sided in shape, while seed cones are 10–35 mm in length, spherical to ovoid, and open after maturation. C. arizonica is widely distributed in the southwestern United States and Mexico [13,16].
C. arizonica leaves are rich in volatile oils [17,18], which showed insecticidal [18], antifungal [19] as well as larvicidal activities [20]. To the best of our knowledge, so far, there has been no comprehensive phytochemical investigation on biflavonoids, flavonoids, other phenolic compounds and diterpenoids of the leaves, which motivated us to investigate a comprehensive map of the aqueous and ethanolic leaf extracts using HPLC-MS/MS. This study was also intended to confirm the antibiofilm activity of C. arizonica leaf extracts against a strong biofilm-forming bacterium, Pseudomonas aeruginosa, and investigate the in vitro antioxidant and inhibitory activities of the extracts against the different skin key enzymes, mainly elastase, hyaluronidase, collagenase and tyrosinase.
2. Results
2.1. In Vitro Antioxidant Assays
The in vitro antioxidant activities of the aqueous and ethanolic extracts of C. arizonica leaves using DPPH and FRAP assays are presented in Table 1. Results of the present study showed that the aqueous extract had higher total phenolic (TPC) and total flavonoid (TFC) contents than the ethanolic extract. The TPC ranged from 139.99 ± 0.04 to 88.86 ± 0.08 GA/g extract, and the TFC ranged from 1.54 ± 0.05 to 1.12 ± 0.16 mg quercetin/g extract, respectively (Table 1). In accordance with this finding, the aqueous extract displayed conspicuous antioxidant activity, exhibiting an IC50 of 55.45 ± 0.51 μg/mL in DPPH and 15.1 ± 0.04 mM FeSO4 equivalent/mg sample in FRAP assay, in comparison to quercetin and BHT, respectively (Table 1), whereas the ethanolic extract showed an IC50 of 61.89 ± 0.11 μg/mL in DPPH and 11.56 ± 0.01 mM FeSO4 equivalent/mg sample in FRAP assay, in comparison to quercetin and BHT, respectively (Table 1). Similar results were also described for the ethanolic extract of wood knots from Iran [21].

Table 1.
In vitro antioxidant activities (DPPH, FRAP) and TPC and TFC for the aqueous and ethanolic extracts of C. arizonica leaves.
2.2. In Vitro Anti-Aging Activities
The two extracts were investigated for their in vitro blocking activity against four enzymes involved in skin aging, namely elastase, tyrosinase, collagenase and hyaluronidase (Table 2). The IC50 of aqueous extract that inhibited these enzymes was much less than that of the ethanolic extract. It exhibited stronger inhibition against tyrosinase, elastase, and hyaluronidase, respectively, and showed less inhibition against collagenase compared to the references kojic acid and quercetin.

Table 2.
In vitro enzyme inhibitory activities of the aqueous and ethanolic extracts of C. arizonica leaves.
2.3. Antibacterial Activities
2.3.1. Minimum Inhibitory Concentration
The growth of P. aeruginosa in MH media supplemented with increasing concentrations of each extract was inhibited in a dose-dependent manner by the extract (Figure 1). A significant decrease in the turbidity of the suspension caused by bacterial growth was observed starting from 12.5 mg/mL of both extracts. Comparatively, the bacterial growth in media amended with the aqueous extract was lower than in media containing the ethanolic extract. Notably, the MIC for both extracts was >100 mg/mL, as there was no complete inhibition at the highest concentration tested. Nevertheless, growth was inhibited by 79.83 and 90.71% using the aqueous and ethanolic extracts, respectively (Figure 1). Using the standard antibiotic, ampicillin, the complete inhibition of P. aeruginosa growth was noted at 0.3125 mg/mL.
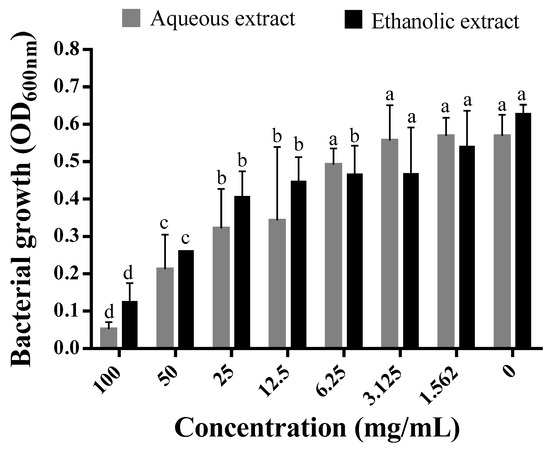
Figure 1.
Effect of the different concentrations of aqueous and ethanolic extracts of C. arizonica leaves on bacterial growth rate of P. aeruginosa. Different letters in superscript indicate a statistical difference at p < 0.05.
2.3.2. Effect of C. arizonica Leaf Extracts on Biofilm Production
The biofilm production by P. aeruginosa in MH media supplemented with 1/8 and 1/4 MICs of each extract was estimated using the crystal violet assay. At 1/8 MIC, corresponding to 12.5 mg/mL for both extracts, no significant decreases were noted as compared to extract-free MH media. However, when the dose was increased to 1/4 MIC (25 mg/mL), the amount of produced biofilm was significantly reduced by the two extracts. These decreases reached 78.8 and 57.9% using the aqueous and ethanolic extracts, respectively (Figure 2).
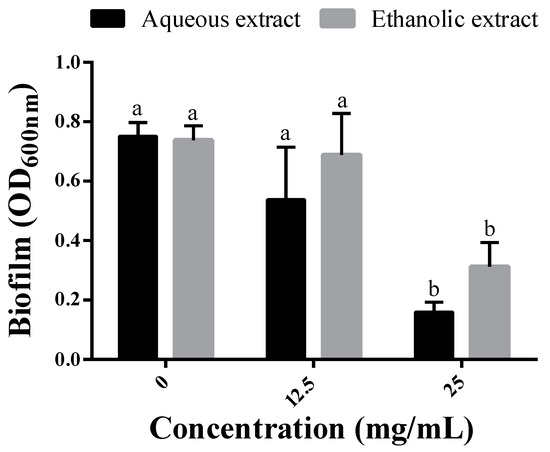
Figure 2.
Biofilm production by P. aeruginosa grown in the absence (0 mg/mL) and presence of the extracts at 12.5 mg/mL (1/8 MIC) and 25 mg/mL (1/4 MIC). Different letters in superscript indicate a statistical difference at p < 0.05.
2.3.3. Effect of C. arizonica Leaf Extracts on the Swimming and Swarming Motilities
The swarming and swimming motilities of P. aeruginosa on agar plates supplemented with 1/8 and 1/4 MICs were monitored as they are crucial mechanisms allowing pathogens to move across surfaces during infections. As shown in Figure 3a, the aqueous extract was more potent than the ethanolic extract in inhibiting the swarming motility of the bacterium (in a dose-dependent manner by up to 70.8%). However, the ethanolic extract showed no significant effect at the two concentrations tested. As for the swimming assay, the aqueous extract was active at only 1/4 MIC (25 mg/mL) with 69.56% inhibition, while the ethanolic extract exhibited no inhibitory effect on the swimming ability of the tested bacterium (Figure 3b).
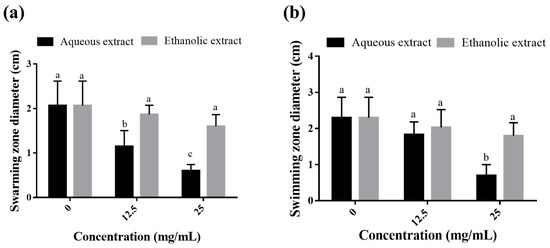
Figure 3.
Effect of sub-MICs of C. arizonica leaf extracts on the swarming (a) and swimming (b) motilities of P. aeruginosa on agar plates. Different letters in superscript indicate a statistical difference at p < 0.05.
2.4. Phytochemical Profiling
The aqueous and ethanolic extracts of C. arizonica leaves were analyzed by HPLC-PDA-MS/MS. Altogether, 67 secondary metabolites belonging to different chemical classes were annotated. The tentative identification of compounds was based on their MS and MS2 spectral data and retention times, as illustrated in Table 3. They included organic acids and their derivatives, phenolic acids, and their glycosides, diterpene acids, procyanidins and flavonoid glycosides such as quercetin, kaempferol and isorhamnetin-related glycosides as well as biflavonoids. Significant differences were detected from both extracts, where the aqueous extract was rich in phenolic acids and proanthocyanidins, while the ethanolic extract was rich in biflavonoids and fatty acids (Figure 4).

Table 3.
Annotated metabolites by HPLC-MS/MS analyses of aqueous and ethanolic extracts from C. arizonica leaves.
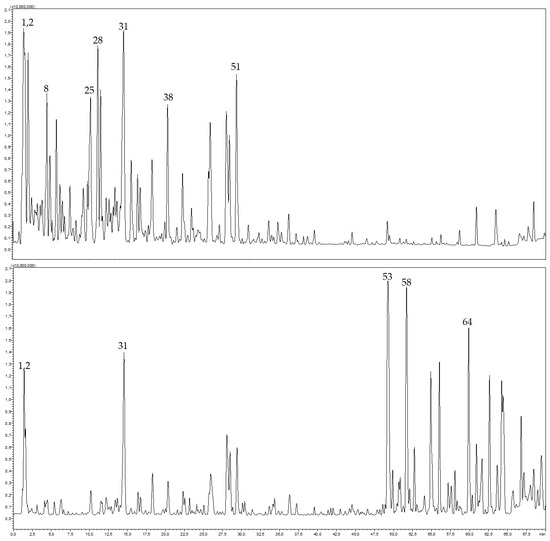
Figure 4.
HPLC-MS profile of aqueous extract (top) and ethanolic extract (bottom) from C. arizonica leaves.
3. Discussion
Oxidative stress is considered as the main cause of skin aging through various mechanisms. Thus, the search for plants that inhibit oxidative stress-induced skin aging can be an excellent approach to delay this process. Phytochemicals such as polyphenolics are well-known for their potential antioxidant capacity. Consequently, antioxidant-synthesizing plants are promising candidates to prevent the skin-aging process. Hence, in this study, we conducted an evaluation of the in vitro antioxidant and antiaging properties of aqueous and ethanolic extracts of C. arizonica leaves, along with an HPLC-MS/MS analysis and assessment of their antibacterial and antibiofilm activities.
Comparatively, the aqueous extract had higher polyphenolic and flavonoid contents in addition to higher antioxidant potential than the ethanolic extract. The phytochemical profiles of both the aqueous and ethanolic extracts from C. arizonica leaves were investigated using the LC-MS/MS analysis, indicating that the higher antioxidant activity of the aqueous extract was most probably due to the higher contents of flavonoids, phenolic acids and proanthocyanidins predominating in the extract, which are powerful antioxidants through their free radical scavenging and metal ion chelation capacities [22]. For instance, the presence of phenolic acid derivatives remarkably enhanced the antioxidant activity, as demonstrated in many other phenolic acid-containing herbs [23]. Additionally, proanthocyanidins (monomers, dimers and trimers) found in the bark extract revealed exceptional antioxidant capacity [24]. Furthermore, the previously reported antioxidant activities of quercetin, kaempferol and isorhamnetin-related glycosides effectively confirmed their crucial role in the antioxidant activity of the extracts [25,26].
We revealed that the aqueous and ethanolic extracts from C. arizonica leaves are rich sources of antioxidant compounds (Table 3) that can behave synergistically against target skin-aging enzymes and accordingly improve the skin aging process. Our results are in good agreement with the reported data concerning the antiaging activity of these compounds. In particular, plants producing chlorogenic acid, catechins, and quercetin glucoside exhibited considerable in vitro antiaging activity through inhibitory effects against the previously mentioned enzymes [27,28]. Also, previous investigations on plants containing quercetin glucoside, kaempferol rutinoside and isorhamnetin glycosides demonstrated that they possess significantly anti-collagenase, anti-hyaluronidase and anti-elastase activities [29].
The Gram-negative bacterium P. aeruginosa, a common pathogen, is a major cause of persistent skin infections owing to its ability to form biofilms, where the bacteria are aggregated and enclosed in a self-made extracellular matrix. Most of the currently used antimicrobial agents may reduce the number of bacteria in biofilms, but they cannot completely eliminate the biofilms because of their intrinsic antibiotic tolerance and resistance, hence allowing infections to relapse [4]. Thus, the present study also aimed to confirm the antibiofilm potential of aqueous and ethanolic extracts of C. arizonica as well as their effects on swimming and swarming motilities of P. aeruginosa using two sub-inhibitory concentrations (1/4 and 1/8 MICs). The aqueous extract at the dose of 25 mg/mL (1/4 MIC) significantly inhibited biofilm production and decreased the swimming and swarming motilities of P. aeruginosa. This could be useful in weakening bacterial pathogenicity by destroying biofilm and reducing the spread of infections. Similar findings were described before for extracts rich in polyphenols, such as those from Salix tetrasperma flowers and bark [30], Ximenia americana leaf [30,31] and Lavandula coronopifolia aerial parts [32].
4. Material and Methods
4.1. Plant Material and Extraction
C. arizonica fresh leaves were collected from El-Orman Botanical Garden, Giza, Egypt in April 2021. A voucher sample was maintained at the botanical herbarium of Pharmacognosy Department, Faculty of Pharmacy, Zagazig University under accession number ZU-Ph-Cog-020100. The plant material was air-dried, ground, and then subjected to ultrasound-assisted extraction (UAE) using water and ethyl alcohol (10 g × 400 mL for each extraction) for 15 min under the following extraction parameters: 20 Hz, 5 °C, 30% sound wave amplification, and a pulse of 10 s. The aqueous and ethanolic extracts were filtered using glass wool, then centrifuged for 7 min at 6000 rpm. The combined filtrates (for each extract) were evaporated under reduced pressure using a Buchi rotavapor R-300 (Flawil, Switzerland), then freeze-dried to yield fine dried powders (1.14 g and 0.80 g, respectively).
4.2. In Vitro Assays
The antioxidant activity was estimated using two colorimetric assays, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) [33] and ferric reducing antioxidant power (FRAP) assays [34], following the previously reported protocol [35]. Total polyphenolic content (TPC) and total flavonoids content (TFC) were evaluated as previously reported [35]. Detailed methods are provided in the Supplementary Materials.
4.3. Enzymatic Activities
4.3.1. Collagenase Inhibition
The assay was conducted as previously described [27] with slight modifications using Tricine buffer (50 mM, consisting of 400 mM NaCl and 10 mM CaCl2, pH 7.5). Collagenase enzyme, from Clostridium histolyticum (ChC—EC.3.4.23.3), was dissolved in the buffer to yield an enzyme initial concentration of 0.8 unit/mL. A 2 mM solution of the synthetic substrate N-[3-(2-furyl) acryloyl] -Leu-Gly-Pro-Ala (FALGPA) was prepared by dissolving in Tricine buffer. Serial diluted samples were incubated for 15 min with the enzyme in Tricine buffer. Then, the substrate was added to each serial solution. A microplate reader was used to measure the absorbance at 490 nm. Quercetin was used as positive control. Triplicates were established for each reaction, and the collagenase inhibition (%) was calculated as follows: Inhibition (%) = [(A control − A sample/A control) × 100].
A sample: absorbance value of the collagenase activity from samples or positive control (the enzyme activity in presence of the extracts or positive control), A control: absorbance value of the collagenase activity from negative control (the enzyme activity in absence of the extracts).
4.3.2. Tyrosinase Inhibition
The tyrosinase inhibition assay was carried out using L-DOPA as substrate and mushroom tyrosinase according to the previously reported method [27]. The reaction consisted of 15 μL of mushroom tyrosinase enzyme (2500 U/mL), 15 μL of samples to be tested (100–6.25 μg/mL), 100 μL of L-DOPA substrate (5 mM), and 100 μL of phosphate buffer (0.05 M, pH of 6.5). After the addition of L-DOPA, the reaction mixture was observed at 475 nm for dopachrome formation using a microplate reader. Kojic acid was used as positive control, and each reaction was performed in triplicate. The tyrosinase enzyme inhibition (%) was calculated using the following formula: Inhibition (%) = [(A control − A sample/A control) × 100]
A sample: absorbance value of the tyrosinase enzyme activity from samples or positive control.
A control: absorbance value of the tyrosinase enzyme activity from negative control (solution without samples).
4.3.3. Elastase Inhibition
This assay was conducted according to a previous study [27]. A stock solution (3.33 mg/mL) of Porcine pancreatic elastase enzyme was prepared in sterile water, while a 1.6 mM solution of N-Succinyl-Ala-Ala-Ala-p-nitroanilide (AAAPVN) was prepared in buffer and used as a substrate. Test samples (100 to 6.25 μg/mL) were incubated with the enzyme for 15 min. Afterwards, AAAPVN substrate was added to initiate the enzyme reaction. The final reaction mixture had a total volume of 250 μL, consisting of 0.8 mM AAAPVN, 1 μg/mL PE, 25 μg test sample, and the buffer vehicle. A microplate reader was used to check the absorbance at 400 nm. Kojic acid was used as positive control, and each reaction was performed in triplicate. Elastase enzyme inhibition (%) was calculated as follows: Inhibition (%) = [(A control − A sample/A control) × 100]
A sample: absorbance value of the elastase activity from samples or positive control.
A control: absorbance value of the elastase activity from negative control (solution without samples).
4.3.4. Hyaluronidase Inhibition
Hyaluronidase inhibition assay was performed as previously reported [27] using hyaluronidase enzyme (1.5 mg/mL) and hyaluronic acid (1 mg/mL in 0.1 M acetate buffer; pH 3.5) as a substrate. The reaction mixture contained 25 μL of 12.5 mM CaCl2, 12.5 μL of each test sample (100–6.25 μg/mL), 1.5 mg/mL of hyaluronidase enzyme, and 100 μL of substrate hyaluronic acid. Next, the reaction mixture was put in a water bath at 100 °C for 3 min, and then, 25 μL of KBO2 (0.8 M) was added to all tubes. After cooling the tubes to room temperature, 800 μL DMAB (4 g DMAB in 40 mL acetic acid and 5 mL 10 N HCl) was added to the tubes, followed by incubation for 20 min, then the contents of the tubes were transferred to respective wells in a 96-well microplate. The absorbance was read at 600 nm, and each reaction was carried out in triplicate. Kojic acid was used as a positive control. Hyaluronidase enzyme inhibition (%) was calculated as follows: Inhibition (%) = [(A control − A sample/A control) × 100]
A sample: absorbance value of the hyaluronidase activity from samples or positive control.
A control: absorbance value of the hyaluronidase activity from negative control (solution without samples).
4.4. Antibacterial Activities
4.4.1. Determination of the Minimum Inhibitory Concentration (MIC)
The MIC of C. arizonica aqueous and ethanolic leaf extracts against Pseudomonas aeruginosa was determined by the broth microdilution assay in a 96-well microtiter microplate [36,37]. First, the samples were dissolved in Muller–Hinton (MH) broth with 5% of dimethyl sulfoxide (DMSO) to obtain a final concentration of 100 mg/mL. Next, the extracts were sterilized using 0.22 μm sterile syringe filters and two-fold serially diluted into the plate’s wells in triplicate with final extract concentrations ranging from 1.562 to 100 mg/mL. After completing the volume to 200 μL per well by MH medium, fresh overnight suspensions of P. aeruginosa adjusted to a turbidity of OD600nm = 0.6 were introduced into each well (2 μL per well). Wells with no bacterial inoculation were established as negative controls. In addition, media without extract amendment were used as growth controls, and ampicillin (10–0.078125 mg/mL) was established as the reference antibiotic. The plate was incubated at 37 °C under shaking at 150 rpm under aerobic conditions. After 18 h incubation, the bacterial growth was visually and spectrophotometrically checked. The lowest concentration of the extract that inhibited the visible growth of the bacterium was defined as the MIC.
4.4.2. Biofilm Inhibition Using Crystal Violet Assay
The effects of the sub-MIC concentrations of C. arizonica aqueous and ethanolic leaf extracts on biofilm formation by P. aeruginosa were determined using the crystal violet colorimetric assay [30,31]. Briefly, the extracts at the doses of 1/8 MIC and 1/4 MIC were prepared in MH broth, filtered using 0.22 μm syringe filters, inoculated, and incubated as described above in the MIC assay. After incubation, the bacterial suspensions were discarded, and the wells were gently washed thrice with a phosphate-buffered saline (PBS) solution to remove nonadherent bacterial cells. Thereafter, adherent bacteria were stained by a 1% crystal violet (CV) solution (200 μL per well) and incubated for 15 min at room temperature. Next, CV solutions were discarded, and wells were washed with sterile distilled water to remove excess dye. The plate was air-dried, and the attached biofilm was solubilized by adding 200 μL of 95% ethanol into each well. The amount of biofilm was estimated spectrophotometrically at OD600nm using a multimode plate reader. Media without the extracts’ supplementation were used as positive controls for biofilm production.
4.4.3. Swimming and Swarming Motility Assessments on Plates
C. arizonica aqueous and ethanolic leaf extracts at 1/4 and 1/8 sub-MIC concentrations was evaluated on the swimming and swarming motilities of P. aeruginosa [30]. The swimming (0.5% NaCl, 1% tryptone, and 0.3% agar) and swarming (LB medium, 0.6% agar) plate media [38] were prepared and autoclaved. When the media were relatively cool (<50 °C), they were supplemented with the extract filtered beforehand to obtain final doses of 1/8 and 1/4 MIC. The plates were left to dry under a laminar flow hood. Next, 10 μL of a fresh suspension of P. aeruginosa adjusted to OD600nm = 0.6 were deposited at the middle of agar plates and incubated at 37 °C for 24 h. Media without extracts were established as controls. The motility zone diameters were measured in cm.
4.5. HPLC-PDA-MS/MS Analysis
The phytochemical analysis was performed using HPLC-PDA-MS/MS. A SHIMADZU LC MS 8050 (Shimadzu, Japan) LC system was utilized alongside a triple quadruple spectrometer with an ESI source. A C18 reversed-phase column (Zorbax Eclipse XDB-C18, rapid resolution, 4.6 × 150 mm, 3.5 µm, Agilent, Santa Clara, CA, USA) was used for the separation process. Water and acetonitrile (ACN) (0.1% formic acid each) gradients were employed from 5 % to 30 % ACN over 60 min with a flow rate of 1 mL/min. The injection of samples was automatically performed using autosampler SIL-40C xs. LC solution software (Shimadzu, Japan) was used to operate the instrument. The MS operated in the negative mode.
4.6. Statistical Analysis
Each experiment was carried out three times throughout the study, and the results were provided as means ± standard deviation (SD). The Tukey’s post hoc test was performed to process for significant differences between the group means using IBM SPSS software. It was deemed statistically significant when p ˂ 0.05.
5. Conclusions
In this study, the phytochemical characterization of the aqueous and ethanolic leaf extracts of C. arizonica revealed 67 secondary metabolites belonging mainly to flavonoids, biflavonoids, phenolic acids and proanthocyanins. The aqueous extract displayed substantial in vitro antioxidant activity and appreciable inhibitory activity against the four crucial enzymes (collagenase, elastase, tyrosinase, and hyaluronidase) involved in skin aging. Moreover, the extract exhibited promising antibiofilm activity against P. aeruginosa and impaired its motility. To summarize, this wide range of activities (antioxidant and antibacterial activities) of C. arizonica extracts could be due to their high polyphenolic contents, which confer their promising dermato-cosmeceutical properties. Therefore, C. arizonica leaf extract could be regarded as a prospective candidate for further investigation as an agent to be utilized against skin aging and its associated symptoms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28031036/s1.
Author Contributions
N.T. (Nora Tawfeek) and E.F. annotated the chemical composition and wrote the manuscript. M.A.O. and W.B.B. performed the in vitro assays. I.M. evaluated the antibacterial activities and wrote the manuscript. N.T. (Noamane Taarji), M.F.M. and M.S. revised the manuscript. M.S. designed the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study is contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.M.; Ayoub, I.M.; Mostafa, N.M.; El Hassab, M.A.; Eldehna, W.M.; Al-Rashood, S.T.; Eldahshan, O.A. Gc/Ms Profiling, Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase and Anti-Hyaluronidase Activities of a Stenocarpus Sinuatus Leaves Extract. Plants 2022, 2022, 918. [Google Scholar] [CrossRef]
- Mayuree, K.; Lourith, N. Plants and Natural Products for the Treatment of Skin Hyperpigmentation—A Review. Planta Med. 2018, 84, 988–1006. [Google Scholar]
- Oana, C.; Tolker-Nielsen, T. Tolerance and Resistance of Pseudomonas Aeruginosa Biofilms to Antimicrobial Agents—How P. Aeruginosa Can Escape Antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar]
- Mulcahy, L.R.; Isabella, V.M.; Lewis, K. Pseudomonas Aeruginosa Biofilms in Disease. Microb. Ecol. 2014, 68, 1–12. [Google Scholar] [CrossRef]
- Wu, D.C.; Chan, W.W.; Metelitsa, A.I.; Fiorillo, L.; Lin, A.N. Pseudomonas Skin Infection. Am. J. Clin. Dermatol. 2011, 12, 157–169. [Google Scholar] [CrossRef]
- Al-Dahmoshi, H.; Al-Obaidi, R.D.; Al-Khafaji, N. Pseudomonas Aeruginosa: Diseases, Biofilm and Antibiotic Resistance. In Pseudomonas Aeruginosa-Biofilm Formation, Infections and Treatments; IntechOpen: London, UK, 2020. [Google Scholar]
- Dolatabadi, S.; Moghadam, H.N.; Mahdavi-Ourtakand, M. Evaluating the Anti-Biofilm and Antibacterial Effects of Juglans Regia L. Extracts against Clinical Isolates of Pseudomonas aeruginosa. Microb. Pathog. 2018, 118, 285–289. [Google Scholar] [CrossRef]
- Mashhady, M.A.; Abkhoo, J.; Jahani, S.; Abyar, S.; Khosravani, F. Inhibitory Effects of Plant Extracts on Pseudomonas Aeruginosa Biofilm Formation. Int. J. Infect. 2016, 3, e38199. [Google Scholar] [CrossRef]
- Zameer, F.; Rukmangada, M.S.; Chauhan, J.B.; Khanum, S.A.; Kumar, P.; Devi, A.T.; Prasad, N.; Dhananjaya, B.L. Evaluation of Adhesive and Anti-Adhesive Properties of Pseudomonas Aeruginosa Biofilms and Their Inhibition by Herbal Plants. Iran. J. Microbiol. 2016, 8, 108. [Google Scholar]
- Schulz, C.; Knopf, P.; Stützel, T.H. Identification Key to the Cypress Family (Cupressaceae). Feddes Repert. Z. Für Bot. Taxon. Und Geobot. 2005, 116, 96–146. [Google Scholar] [CrossRef]
- Homayoun, F. The Genus Cupressus L. Mythology to Biotechnology with Emphasis on Mediterranean Cypress (Cupressus Sempervirens L.). Hortic. Rev. 2020, 47, 213–287. [Google Scholar]
- Bartel, J.A. Cupressaceae Cypress Family. J. Ariz.-Nev. Acad. Sci. 1994, 27, 195–200. [Google Scholar]
- Frezza, C.; De Vita, D.; Sciubba, F.; Toniolo, C.; Tomassini, L.; Nicoletti, M.; Franceschin, M.; Guiso, M.; Bianco, A.; Serafini, M.; et al. There Is Not Only Cupressus Sempervirens L.: A Review on the Phytochemistry and Bioactivities of the Other Cupressus L. Species. Appl. Sci. 2022, 12, 7353. [Google Scholar] [CrossRef]
- Khan, M.F.; Ahamad, T.; Rawat, P. Biomedicinal and Chemical Profile of Cupressus Sempervirens: A Mini Review. Insights Biomed. 2017, 2, 16. [Google Scholar]
- Griffith, M.P.; Bartel, S.C. A Cypress (Cupressus Arizonica, Cupressaceae) in Jeff Davis County, Texas? SIDA Contrib. Bot. 2002, 20, 585–592. [Google Scholar]
- Shafaie, F.; Aramideh, S.; Valizadegan, O.; Safaralizadeh, M.H.; Pesyan, N.N. Gc/Ms Analysis of the Essential Oils of Cupressus Arizonica Greene, Juniperus Communis L. And Mentha Longifolia L. Bull. Chem. Soc. Ethiop. 2019, 33, 389–400. [Google Scholar] [CrossRef]
- Mannai, Y.; Dhahri, S.; Jama, M.L.B.; Hamrouni, L. Insecticidal Activity of Essential Oils of Cupressus Arizonica Greene and C. Sempervirens L. On Tortrix Viridana (Lepidotera, Tortricidae). World J. Biol. Biotechnol. 2021, 6. [Google Scholar] [CrossRef]
- Khouaja, W.; Oliveira, R.; Raïes, A.; Dias, A.C.P. Antifungal Activity of the Essential Oils from Cupressus Arizonica Var. Arizonica and Var. Glabra. Ind. Crops Prod. 2015, 77, 614–623. [Google Scholar] [CrossRef]
- Sedaghat, M.M.; Dehkordi, A.S.; Khanavi, M.; Abai, M.R.; Mohtarami, F.; Vatandoost, H. Chemical Composition and Larvicidal Activity of Essential Oil of Cupressus Arizonica El Greene against Malaria Vector Anopheles Stephensi Liston (Diptera: Culicidae). Pharmacogn. Res. 2011, 3, 135–139. [Google Scholar]
- Abdulkhani, A.; Sedaghat, A.; Alizadeh, P.; Tabil, L.G. Extraction of Bioactive Moieties of Cupressus Arizonica. Can. Biosyst. Eng. 2020, 62, 8.1–8.10. [Google Scholar]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Świerczek-Zięba, G. Structure and Antioxidant Activity of Polyphenols Derived from Propolis. Molecules 2013, 19, 78–101. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Chandrasekara, A. Hydroxycinnamates and Their in Vitro and in Vivo Antioxidant Activities. Phytochem. Rev. 2010, 9, 147–170. [Google Scholar] [CrossRef]
- Sobeh, M.; Mahmoud, M.F.; Abdelfattah, M.A.; Cheng, H.; El-Shazly, A.M.; Wink, M. A Proanthocyanidin-Rich Extract from Cassia Abbreviata Exhibits Antioxidant and Hepatoprotective Activities in Vivo. J. Ethnopharmacol. 2018, 213, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and Anti-Inflammatory Activities of Quercetin and Its Derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Silva dos Santos, J.; Goncalves Cirino, J.P.; de Oliveira Carvalho, P.; Ortega, M.M. The Pharmacological Action of Kaempferol in Central Nervous System Diseases: A Review. Front. Pharmacol. 2021, 11, 565700. [Google Scholar] [CrossRef] [PubMed]
- Elgamal, A.M.; El Raey, M.A.; Gaara, A.; Abdelfattah, M.A.; Sobeh, M. Phytochemical Profiling and Anti-Aging Activities of Euphorbia Retusa Extract: In Silico and in Vitro Studies. Arab. J. Chem. 2021, 14, 103159. [Google Scholar] [CrossRef]
- Abdelfattah, M.A.; Dmirieh, M.; Bakrim, W.B.; Mouhtady, O.; Ghareeb, M.A.; Wink, M.; Sobeh, M. Antioxidant and Anti-Aging Effects of Warburgia Salutaris Bark Aqueous Extract: Evidences from in Silico, in Vitro and in Vivo Studies. J. Ethnopharmacol. 2022, 292, 115187. [Google Scholar] [CrossRef]
- Szewczyk, K.; Pietrzak, W.; Klimek, K.; Miazga-Karska, M.; Firlej, A.; Flisiński, M.; Grzywa-Celińska, A. Flavonoid and Phenolic Acids Content and in Vitro Study of the Potential Anti-Aging Properties of Eutrema Japonicum (Miq.) Koidz Cultivated in Wasabi Farm Poland. Int. J. Mol. Sci. 2021, 22, 6219. [Google Scholar] [CrossRef]
- Mostafa, I.; Abbas, H.A.; Ashour, M.L.; Yasri, A.; El-Shazly, A.M.; Wink, M.; Sobeh, M. Polyphenols from Salix Tetrasperma Impair Virulence and Inhibit Quorum Sensing of Pseudomonas aeruginosa. Molecules 2020, 25, 1341. [Google Scholar] [CrossRef]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Benjelloun, S.; Allaoui, A.; Biskri, L. Rhizospheric Phosphate Solubilizing Bacillus Atrophaeus Gqjk17 S8 Increases Quinoa Seedling, Withstands Heavy Metals, and Mitigates Salt Stress. Sustainability 2021, 13, 3307. [Google Scholar] [CrossRef]
- Emam, M.; Abdel-Haleem, D.R.; Salem, M.M.; Abdel-Hafez, L.J.M.; Latif, R.R.A.; Farag, S.M.; Sobeh, M.; El Raey, M.A. Phytochemical Profiling of Lavandula Coronopifolia Poir. Aerial Parts Extract and Its Larvicidal, Antibacterial, and Antibiofilm Activity against Pseudomonas Aeruginosa. Molecules 2021, 26, 1710. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Du, B.; Xu, B. Oxygen Radical Absorbance Capacity (Orac) and Ferric Reducing Antioxidant Power (Frap) of Β-Glucans from Different Sources with Various Molecular Weight. Bioact. Carbohydr. Diet. Fibre 2014, 3, 11–16. [Google Scholar] [CrossRef]
- Ghareeb, M.A.; Mohamed, T.; Saad, A.M.; Refahy, L.A.G.; Sobeh, M.; Wink, M. Hplc-Dad-Esi-Ms/Ms Analysis of Fruits from Firmiana Simplex (L.) and Evaluation of Their Antioxidant and Antigenotoxic Properties. J. Pharm. Pharmacol. 2018, 70, 133–142. [Google Scholar] [CrossRef]
- Abbas, H.A.; Elsherbini, A.M.; Shaldam, M.A. Repurposing Metformin as a Quorum Sensing Inhibitor in Pseudomonas Aeruginosa. Afr. Health Sci. 2017, 17, 808–819. [Google Scholar] [CrossRef]
- Bakrim, W.B.; Nurcahyanti, A.D.R.; Dmirieh, M.; Mahdi, I.; Elgamal, A.M.; El Raey, M.A.; Wink, M.; Sobeh, M. Phytochemical Profiling of the Leaf Extract of Ximenia Americana Var. Caffra and Its Antioxidant, Antibacterial, and Antiaging Activities in Vitro and in Caenorhabditis Elegans: A Cosmeceutical and Dermatological Approach. Oxidative Med. Cell. Longev. 2022, 2022, 3486257. [Google Scholar] [CrossRef]
- Yeung, A.T.; Torfs, E.C.; Jamshidi, F.; Bains, M.; Wiegand, I.; Hancock, R.E.; Overhage, J. Swarming of Pseudomonas Aeruginosa Is Controlled by a Broad Spectrum of Transcriptional Regulators, Including Metr. J. Bacteriol. 2009, 191, 5592–5602. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).