Cyclic Glycine-Proline (cGP) Normalises Insulin-Like Growth Factor-1 (IGF-1) Function: Clinical Significance in the Ageing Brain and in Age-Related Neurological Conditions
Abstract
1. Introduction
1.1. Decline in IGF-1 Function with Age and Its Association with Age-Related Conditions
1.2. Regulation of Bioavailablility of IGF-1 in Circulation
2. cGP Is a Bioactive Compound
2.1. Formation of Endogenous cGP
2.2. cGP as a Bioactive Peptide
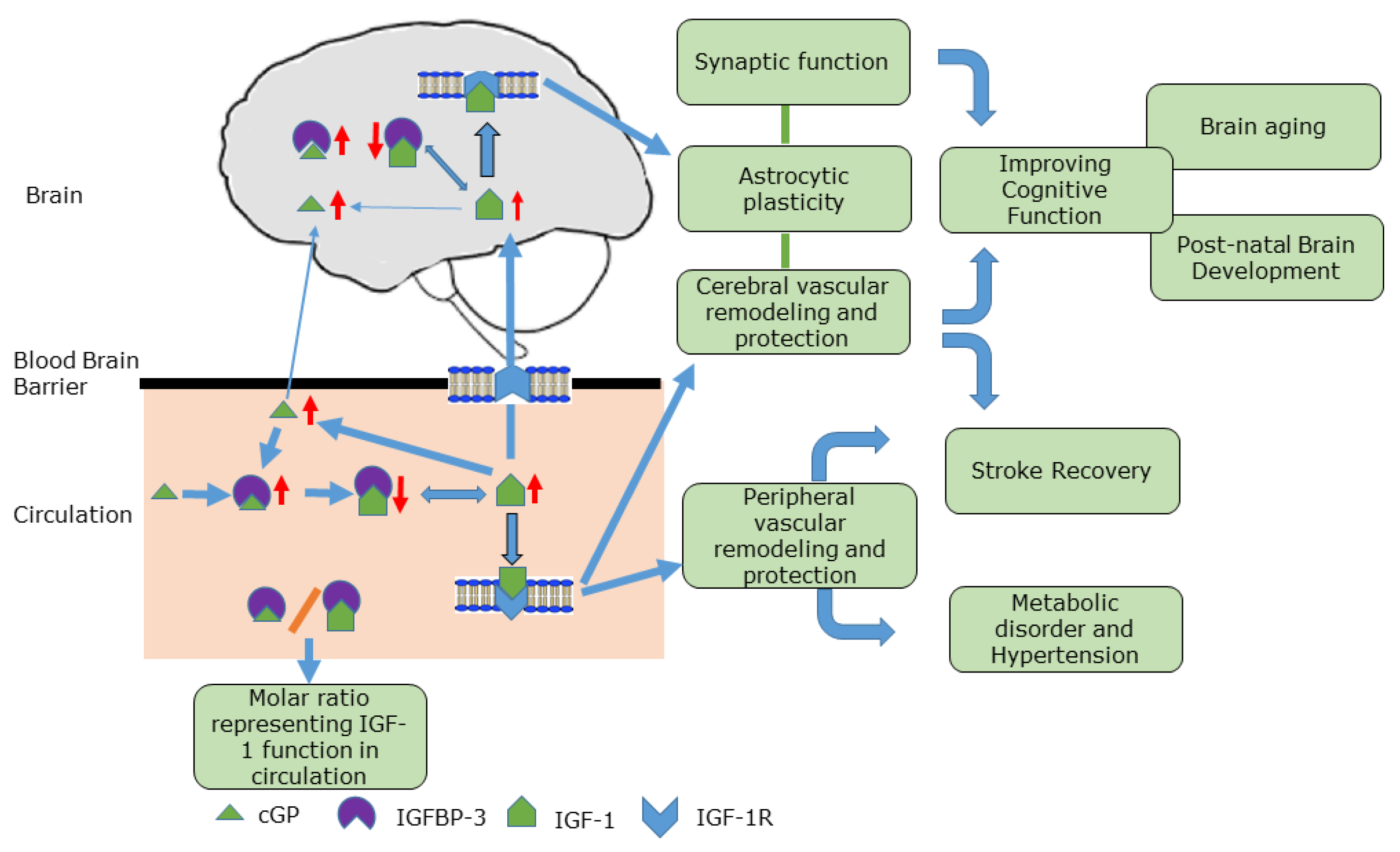
3. Mode of Action of cGP
4. Clinical Relevance
4.1. Hypertension
4.2. Stroke
4.3. Age-Related Cognitive Decline
4.4. Parkinson’s Disease (PD)
4.5. Alzheimer’s Disease (AD)
4.6. A Plasma Biomarker for Age-Related Neurological Diseases
5. Discovery of Natural cGP
6. Limitations of the Review and Future Studies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wennberg, A.M.V.; Hagen, C.E.; Machulda, M.M.; Hollman, J.H.; Roberts, R.O.; Knopman, D.S.; Petersen, R.C.; Mielke, M.M. The association between peripheral total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 and functional and cognitive outcomes in the Mayo Clinic Study of Aging. Neurobiol. Aging 2018, 66, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A.H.; Vahdatpour, C.; Sanfeliu, A.; Tropea, D. The role of Insulin-Like Growth Factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience 2016, 325, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, W.E.; Ramsey, M.; Carter, C.S. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res. Rev. 2005, 4, 195–212. [Google Scholar] [CrossRef]
- Clarke, I.J.; Fletcher, T.P.; Pomares, C.C.; Holmes, J.H.; Dunshea, F.; Thomas, G.B.; Tilbrook, A.J.; Walton, P.E.; Galloway, D.B. Effect of high-protein feed supplements on concentrations of growth hormone (GH), insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 in plasma and on the amounts of GH and messenger RNA for GH in the pituitary glands of adult rams. J. Endocrinol. 1993, 138, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Bennet, L.; Gluckman, P.D.; Gunn, A.J. Insulin-like growth factor-1 and post-ischemic brain injury. Prog. Neurobiol. 2003, 70, 443–462. [Google Scholar] [CrossRef]
- Leng, L.; Xing, Y.; Liang, Y.; Wang, C.; Ma, H. Relationship between circulating insulin-like growth factor-1 and blood pressure in adults: A systematic review and meta-analysis of observational studies. Growth Horm. IGF Res. Off. J. Growth Horm. Res. Soc. Int. IGF Res. Soc. 2021, 60–61, 101416. [Google Scholar] [CrossRef]
- Lopez-Lopez, C.; LeRoith, D.; Torres-Aleman, I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc. Natl. Acad. Sci. USA 2004, 101, 9833–9838. [Google Scholar] [CrossRef]
- Higashi, Y.; Gautam, S.; Delafontaine, P.; Sukhanov, S. IGF-1 and cardiovascular disease. Growth Horm IGF Res 2019, 45, 6–16. [Google Scholar] [CrossRef]
- Junnila, R.K.; List, E.O.; Berryman, D.E.; Murrey, J.W.; Kopchick, J.J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 2013, 9, 366–376. [Google Scholar] [CrossRef]
- Deak, F.; Sonntag, W.E. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 611–625. [Google Scholar] [CrossRef]
- Ungvari, Z.; Csiszar, A. The emerging role of IGF-1 deficiency in cardiovascular aging: Recent advances. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Klempt, N.; Guan, J.; Mallard, C.; Sirimanne, E.; Dragunow, M.; Klempt, M.; Singh, K.; Williams, C.; Nikolics, K. A role for IGF-1 in the rescue of CNS neurons following hypoxic- ischemic injury. Biochem. Biophys. Res. Commun. 1992, 182, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Sierra, C.; Coca, A.; Schiffrin, E.L. Vascular mechanisms in the pathogenesis of stroke. Curr. Hypertens. Rep. 2011, 13, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Maake, C.; Murphy, L.J. Enhanced proteolytic activity directed against the N-terminal of IGF-I in diabetic rats. J. Endocrinol. 1999, 162, 243–250. [Google Scholar] [CrossRef]
- Williams, I.C.; Park, M.H.; Tsang, S.; Sperling, S.A.; Manning, C. Cognitive Function and Vascular Risk Factors Among Older African American Adults. J. Immigr. Minor. Health 2018, 20, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Gao, Q.; Nyunt, M.S.; Gong, L.; Lunaria, J.B.; Lim, M.L.; Ling, A.; Lam, C.S.; Richards, A.M.; Ling, L.H.; et al. Vascular Health Indices and Cognitive Domain Function: Singapore Longitudinal Ageing Studies. J. Alzheimers Dis. 2016, 50, 27–40. [Google Scholar] [CrossRef]
- Zhang, R.; Kadar, T.; Sirimanne, E.; MacGibbon, A.; Guan, J. Age-related memory decline is associated with vascular and microglial degeneration in aged rats. Behav. Brain Res. 2012, 235, 210–217. [Google Scholar] [CrossRef]
- Boisclair, Y.R.; Rhoads, R.P.; Ueki, I.; Wang, J.; Ooi, G.T. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: An important but forgotten component of the circulating IGF system. J. Endocrinol. 2001, 170, 63–70. [Google Scholar] [CrossRef]
- Hellstrom, A.; Ley, D.; Hallberg, B.; Lofqvist, C.; Hansen-Pupp, I.; Ramenghi, L.A.; Borg, J.; Smith, L.E.H.; Hard, A.L. IGF-1 as a Drug for Preterm Infants: A Step-Wise Clinical Development. Curr. Pharm. Des. 2017, 23, 5964–5970. [Google Scholar] [CrossRef]
- Adem, A.; Jossan, S.; d’Argy, R.; Gillberg, P.; Nordberg, A.; Winblad, B.; Sara, V.R. Insulin-like growth factor 1 (IGF-1) receptors in the human brain: Quantitative autoradiographic localization. Brain Res. 1989, 503, 299–303. [Google Scholar] [CrossRef]
- Drakenberg, K.; Sara, V.R.; Falkmer, S.; Gammeltoft, S.; Maake, C.; Reinecke, M. Identification of IGF-1 receptors in primitive vertebrates. Regul. Pept. 1993, 43, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Sara, V.R.; Hall, K. Insulin-like growth factors and their binding proteins. Physiol. Rev. 1990, 70, 591–614. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, L.; Oh, Y. Targeting IGF-I, IGFBPs and IGF-I receptor system in cancer: The current and future in breast cancer therapy. Recent Pat. Anti-Canc. 2011, 6, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.A.; Gallaher, B.W.; Ambler, G.R.; Gluckman, P.D.; Breier, B.H. IGF-I and IGF-binding protein-3 in plasma of GH-deficient rats. J. Endocrinol. 1996, 150, 67–76. [Google Scholar] [CrossRef]
- Baxter, R.C. Insulin-like growth factor (IGF)-binding proteins: Interactions with IGFs and intrinsic bioactivities. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E967–E976. [Google Scholar] [CrossRef]
- Kang, D.; Waldvogel, H.; Wang, A.; Fan, D.; Faull, R.; Curtis, M.; Shorten, P.R.; Guan, J. The autocrine regulation of insulin-like growth factor-1 in human brain of Alzheimer’s disease. Psychoneuroendocrinology 2021, 127, 105191. [Google Scholar] [CrossRef]
- Yang, P.; Waldvogel, H.; Turner, C.; Faull, R.; Dragunow, M.; Guan, J. Vascular Remodelling is Impaired in Parkinson Disease. J. Alzheimers Dis. Park. 2017, 7, 2161-0460. [Google Scholar] [CrossRef]
- Tham, A.; Nordberg, A.; Grissom, F.E.; Carlsson-Skwirut, C.; Viitanen, M.; Sara, V.R. Insulin-like growth factors and insulin-like growth factor binding proteins in cerebrospinal fluid and serum of patients with dementia of the Alzheimer type. J. Neural Transm. Park. Dis. Dement. Sect. 1993, 5, 165–176. [Google Scholar] [CrossRef]
- Guan, J.; Gluckman, P.; Yang, P.; Krissansen, G.; Sun, X.; Zhou, Y.; Wen, J.; Phillips, G.; Shorten, P.R.; McMahon, C.D.; et al. Cyclic glycine-proline regulates IGF-1 homeostasis by altering the binding of IGFBP-3 to IGF-1. Sci. Rep. 2014, 4, 4388. [Google Scholar] [CrossRef]
- Yamamoto, H.; Murphy, L.J. Enzymatic conversion of IGF-I to des(1-3)IGF-I in rat serum and tissues: A further potential site of growth hormone regulation of IGF-I action. J. Endocrinol. 1995, 146, 141–148. [Google Scholar] [CrossRef]
- Sara, V.R.; Carlsson-Skwirut, C.; Drakenberg, K.; Giacobini, M.B.; Håkansson, L.; Mirmiran, M.; Nordberg, A.; Olson, L.; Reinecke, M.; Ståhlbom, P.A.; et al. The biological role of truncated Insulin-like Growth Factor-1 and the tripeptide GPE in the central nervous system. Ann. N. Y. Acad. Sci. 1993, 692, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, J.P.; Gerard, A. Role of insulin-like growth factor binding proteins in limitation of IGF-1 degradation into the N-methyl-D-aspartate receptor antagonist GPE: Evidence from gonadotrophin-releasing hormone secretion in vitro at two developmental stages. Brain Res. 1999, 847, 147–152. [Google Scholar] [CrossRef]
- Guan, J.; Harris, P.; Brimble, M.; Lei, Y.; Lu, J.; Yang, Y.; Gunn, A.J. The role for IGF-1-derived small neuropeptides as a therapeutic target for neurological disorders. Expert Opin. Ther. Targets 2015, 19, 785–793. [Google Scholar] [CrossRef]
- Guan, J.; Thomas, G.B.; Lin, H.; Mathai, S.; Bachelor, D.C.; George, S.; Gluckman, P.D. Neuroprotective effects of the N-terminal tripeptide of insulin-like growth factor-1, glycine-proline-glutamate (GPE) following intravenous infusion in hypoxic-ischemic adult rats. Neuropharmacology 2004, 47, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.M.; Batchelor, D.C.; Thomas, G.B.; Wen, J.Y.; Rafiee, M.; Lin, H.; Guan, J. Central penetration and stability of N-terminal tripeptide of insulin-like growth factor-I, glycine-proline-glutamate in adult rat. Neuropeptides 2005, 39, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Krishnamurthi, R.; Harris, P.; Barber, P.A.; Guan, J. Plasma cyclic glycine proline/IGF-1 ratio predicts clinical outcome and recovery in stroke patients. Ann. Clin. Transl. Neurol. 2019, 6, 669–677. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Boyko, S.S.; Akparov, V.; Ostrovskaya, R.U.; Skoldinov, S.P.; Rozantsev, G.G.; Voronina, T.A.; Zherdev, V.P.; Seredenin, S.B. Identification of a novel endogenous memory facilitating cyclic dipeptide cyclo-prolylglycine in rat brain. FEBS Lett. 1996, 391, 149–152. [Google Scholar] [CrossRef]
- Povarnina, P.Y.; Kolyasnikova, K.N.; Nikolaev, S.V.; Antipova, T.A.; Gudasheva, T.A. Neuropeptide Cycloprolylglycine Exhibits Neuroprotective Activity after Systemic Administration to Rats with Modeled Incomplete Global Ischemia and in In Vitro Modeled Glutamate Neurotoxicity. Bull. Exp. Biol. Med. 2016, 160, 653–655. [Google Scholar] [CrossRef]
- Ostrovskaya, R.U.; Mirsoev, T.K.; Romanova, G.A.; Gudasheva, T.A.; Kravchenko, E.V.; Trofimov, C.C.; Voronina, T.A.; Seredenin, S.B. Proline-containing dipeptide GVS-111 retains nootropic activity after oral administration. Bull. Exp. Biol. Med. 2001, 132, 959–962. [Google Scholar] [CrossRef]
- Ostrovskaya, R.U.; Romanova, G.A.; Barskov, I.V.; Shanina, E.V.; Gudasheva, T.A.; Victorov, I.V.; Voronina, T.A.; Seredenin, S.B. Memory restoring and neuroprotective effects of the proline-containing dipeptide, GVS-111, in a photochemical stroke model. Behav. Pharm. 1999, 10, 549–553. [Google Scholar] [CrossRef]
- Guan, J.; Gluckman, P.D. IGF-1 derived small neuropeptides and analogues: A novel strategy for the development of pharmaceuticals for neurological conditions. Br. J. Pharm. 2009, 157, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Mathai, S.; Harris, P.; Wen, J.Y.; Zhang, R.; Brimble, M.; Gluckman, P. Peripheral administration of a novel diketopiperazine, NNZ 2591, prevents brain injury and improves somatosensory-motor function following hypoxia-ischemia in adult rats. Neuropharmacology 2007, 53, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Namihira, M.; Yamamoto, S.; Numata, N.; Hyodo, K. Oral administration of cyclic glycyl-proline facilitates task learning in a rat stroke model. Behav. Brain Res. 2022, 417, 113561. [Google Scholar] [CrossRef] [PubMed]
- Singh-Mallah, G.; Singh, K.; McMahon, C.D.; Harris, P.; Brimble, M.A.; Thorstensen, E.; Guan, J. Maternally Administered Cyclic Glycine-Proline Increases Insulin-Like Growth Factor-1 Bioavailability and Novelty Recognition in Developing Offspring. Endocrinology 2016, 157, 3130–3139. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Alamri, Y.; Liu, K.; MacAskill, M.; Harris, P.; Brimble, M.; Dalrymple-Alford, J.; Prickett, T.; Menzies, O.; Laurenson, A.; et al. Supplementation of Blackcurrant Anthocyanins Increased Cyclic Glycine-Proline in the Cerebrospinal Fluid of Parkinson Patients: Potential Treatment to Improve Insulin-Like Growth Factor-1 Function. Nutrients 2018, 10, 714. [Google Scholar] [CrossRef]
- Li, F.; Liu, K.; Wang, A.; Harris, P.W.R.; Vickers, M.H.; Guan, J. Cyclic glycine-proline administration normalizes high-fat diet-induced synaptophysin expression in obese rats. Neuropeptides 2019, 76, 101935. [Google Scholar] [CrossRef]
- Guan, J.; Zhang, R.; Dale-Gandar, L.; Hodgkinson, S.; Vickers, M. NNZ-2591, a novel diketopiperazine, prevented scopolamine-induced acute memory impairment in the adult rat. Behav. Brain Res. 2010, 210, 7. [Google Scholar] [CrossRef]
- Krishnamurthi, R.; Mathai, S.; Kim, H.; Zhang, R.; Guan, J. A novel diketopiperazine improves functional recovery given after the onset of 6-OHDA induced motor deficit in rats. Br. J. Pharmacol. 2008, 156, 662–672. [Google Scholar]
- Berry-Kravis, E.; Horrigan, J.P.; Tartaglia, N.; Hagerman, R.; Kolevzon, A.; Erickson, C.A.; Hatti, S.; Snape, M.; Yaroshinsky, A.; Stoms, G.; et al. A Double-Blind, Randomized, Placebo-Controlled Clinical Study of Trofinetide in the Treatment of Fragile X Syndrome. Pediatr. Neurol. 2020, 110, 30–41. [Google Scholar] [CrossRef]
- Darwish, M.; Youakim, J.M.; Harlick, J.; DeKarske, D.; Stankovic, S. A Phase 1, Open-Label Study to Evaluate the Effects of Food and Evening Dosing on the Pharmacokinetics of Oral Trofinetide in Healthy Adult Subjects. Clin. Drug Investig. 2022, 42, 513–524. [Google Scholar] [CrossRef]
- Neul, J.L.; Percy, A.K.; Benke, T.A.; Berry-Kravis, E.M.; Glaze, D.G.; Peters, S.U.; Jones, N.E.; Youakim, J.M. Design and outcome measures of LAVENDER, a phase 3 study of trofinetide for Rett syndrome. Contemp. Clin. Trials 2022, 114, 106704. [Google Scholar] [CrossRef] [PubMed]
- Oosterholt, S.P.; Horrigan, J.; Jones, N.; Glass, L.; Della Pasqua, O. Population pharmacokinetics of NNZ-2566 in healthy subjects. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 109S, S98–S107. [Google Scholar] [CrossRef] [PubMed]
- Glaze, D.G.; Neul, J.L.; Percy, A.; Feyma, T.; Beisang, A.; Yaroshinsky, A.; Stoms, G.; Zuchero, D.; Horrigan, J.; Glass, L.; et al. A Double-Blind, Randomized, Placebo-Controlled Clinical Study of Trofinetide in the Treatment of Rett Syndrome. Pediatr. Neurol. 2017, 76, 37–46. [Google Scholar] [CrossRef]
- Glaze, D.G.; Neul, J.L.; Kaufmann, W.E.; Berry-Kravis, E.; Condon, S.; Stoms, G.; Oosterholt, S.; Della Pasqua, O.; Glass, L.; Jones, N.E.; et al. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology 2019, 92, e1912–e1925. [Google Scholar] [CrossRef] [PubMed]
- Sharonova, I.N.; Bukanova, Y.V.; Gudasheva, T.A.; Skrebitsky, V.G. Effect of Endogenous Neuropeptide Cycloprolylglycine on GABAA Receptors in Cerebellar Purkinje Cells. Bull. Exp. Biol. Med. 2019, 167, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Gudasheva, T.A.; Grigoriev, V.V.; Koliasnikova, K.N.; Zamoyski, V.L.; Seredenin, S.B. Neuropeptide cycloprolylglycine is an endogenous positive modulator of AMPA receptors. Doklady. Biochem. Biophys. 2016, 471, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Saura, J.; Curatolo, L.; Williams, C.E.; Gatti, S.; Benatti, L.; Peeters, C.; Guan, J.; Dragunow, M.; Post, C.; Faull, R.L.; et al. Neuroprotective effects of Gly-Pro-Glu, the N-terminal tripeptide of IGF-1, in the hippocampus in vitro. Neuroreport 1999, 10, 161–164. [Google Scholar] [CrossRef]
- Sara, V.R.; Carlsson-Sdwirut, C.; Bergman, T.; Jornvall, H.; Roberts, P.J.; Crawford, M.; Hakansson, L.N.; Civalero, I.; Nordberg, A. Indentification of Gly-Pre-Glu(GPE), the aminoterminal tripeptide of insulin-like growth factor 1 which is truncted in brain, as a novel neuroaction peptide. Biochem. Biophys. Res. Commun. 1989, 165, 766–771. [Google Scholar] [CrossRef]
- Guan, J.; Bennet, L.; Gluckman, P.D.; Gunn, A.J. Treatment in animal models. Endocr. Dev. 2005, 9, 31–43. [Google Scholar]
- Svedin, P.; Guan, J.; Mathai, S.; Zhang, R.; Wang, X.; Gustavsson, M.; Hagberg, H.; Mallard, C. Delayed peripheral administration of a GPE analogue induces astrogliosis and angiogenesis and reduces inflammation and brain injury following hypoxia-ischemia in the neonatal rat. Dev. Neurosci. 2007, 29, 393–402. [Google Scholar] [CrossRef]
- Shapira, S.; Mathai, S.; Zhang, R.; Guan, J. Delayed peripheral administration of the N-terminal tripeptide of IGF-1 (GPE) reduces brain damage following microsphere induced embolic damage in young adult and aged rats. Neurosci. Lett. 2009, 454, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.M.A.; Shorten, P.R.; Wake, G.C.; Guan, J. Modeling the effect of insulin-like growth factor-1 on human cell growth. Math. Biosci. 2015, 259, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Kizza, J.; Lewington, S.; Mappin-Kasirer, B.; Turnbull, I.; Guo, Y.; Bian, Z.; Chen, Y.; Yang, L.; Chen, Z.; Clarke, R. Cardiovascular risk factors and Parkinson’s disease in 500,000 Chinese adults. Ann. Clin. Transl. Neurol. 2019, 6, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Fu, Z.; Le, W. Exercise and Parkinson’s disease. Int. Rev. Neurobiol. 2019, 147, 45–74. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Moeini, M.; Li, B.; Thorin, É.; Lesage, F. Hypertension accelerates cerebral tissue PO(2) disruption in Alzheimer’s disease. Neurosci. Lett. 2020, 715, 134626. [Google Scholar] [CrossRef] [PubMed]
- Morovic, S.; Budincevic, H.; Govori, V.; Demarin, V. Possibilities of Dementia Prevention—It is Never Too Early to Start. J. Med. Life 2019, 12, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Solis, E., Jr.; Hascup, K.N.; Hascup, E.R. Alzheimer’s Disease: The Link Between Amyloid-β and Neurovascular Dysfunction. J. Alzheimer’s Dis. JAD 2020, 76, 1179–1198. [Google Scholar] [CrossRef]
- Srikanthan, K.; Feyh, A.; Visweshwar, H.; Shapiro, J.I.; Sodhi, K. Systematic Review of Metabolic Syndrome Biomarkers: A Panel for Early Detection, Management, and Risk Stratification in the West Virginian Population. Int. J. Med. Sci. 2016, 13, 25–38. [Google Scholar] [CrossRef]
- Binoux, M. The IGF system in metabolism regulation. Diabete Metab. 1995, 21, 330–337. [Google Scholar]
- Karmali, R.; Dalovisio, A.; Borgia, J.A.; Venugopal, P.; Kim, B.W.; Grant-Szymanski, K.; Hari, P.; Lazarus, H. All in the family: Clueing into the link between metabolic syndrome and hematologic malignancies. Blood Rev. 2015, 29, 71–80. [Google Scholar] [CrossRef]
- Savastano, S.; Di Somma, C.; Pizza, G.; De Rosa, A.; Nedi, V.; Rossi, A.; Orio, F.; Lombardi, G.; Colao, A.; Tarantino, G. Liver-spleen axis, insulin-like growth factor-(IGF)-I axis and fat mass in overweight/obese females. J. Transl. Med. 2011, 16, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Schutte, A.E.; Volpe, M.; Tocci, G.; Conti, E. Revisiting the relationship between blood pressure and insulin-like growth factor-1. Hypertension 2014, 63, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Singh-Mallah, G.; Liu, K.; Thorstensen, E.; Shorten, P.; Mitchell, E.A.; Taylor, R.; Harris, P.; Brimble, M.; Thompson, J.M.D.; et al. The role for cyclic Glycine-Proline, a biological regulator of insulin-like growth factor-1 in pregnancy-related obesity and weight changes. J. Biol. Regul. Homeost Agents 2018, 32, 465–478. [Google Scholar]
- Li, F.; Liu, K.; Gray, C.; Harris, P.; Reynolds, C.M.; Vickers, M.H.; Guan, J. Cyclic glycine-proline normalizes systolic blood pressure in high-fat diet-induced obese male rats. Nutr. Metab. Cardiovasc. Dis. 2019, 30, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Turin, T.C.; Okamura, T.; Afzal, A.R.; Rumana, N.; Watanabe, M.; Higashiyama, A.; Nakao, Y.; Nakai, M.; Takegami, M.; Nishimura, K.; et al. Hypertension and lifetime risk of stroke. J. Hypertens. 2016, 34, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.W.; Dams Ramos, C.M.; Matin, N.; Dorrance, A.M. The effects of hypertension on the cerebral circulation. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1598–H1614. [Google Scholar] [CrossRef]
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [CrossRef]
- Rost, N.S.; Bottle, A.; Lee, J.M.; Randall, M.; Middleton, S.; Shaw, L.; Thijs, V.; Rinkel, G.J.; Hemmen, T.M. Stroke Severity Is a Crucial Predictor of Outcome: An International Prospective Validation Study. J. Am. Heart Assoc. 2016, 5, e002433. [Google Scholar] [CrossRef]
- Schwab, S.; Spranger, M.; Krempien, S.; Hacke, W.; Bettendorf, M. Plasma insulin-like growth factor I and IGF binding protein 3 levels in patients with acute cerebral ischemic injury. Stroke 1997, 28, 1744–1748. [Google Scholar] [CrossRef]
- Aberg, D.; Jood, K.; Blomstrand, C.; Jern, C.; Nilsson, M.; Isgaard, J.; Aberg, N.D. Serum IGF-I levels correlate to improvement of functional outcome after ischemic stroke. J. Clin. Endocrinol. Metab. 2011, 96, E1055–E1064. [Google Scholar] [CrossRef]
- Dong, X.; Chang, G.; Ji, X.F.; Tao, D.B.; Wang, Y.X. The relationship between serum insulin-like growth factor I levels and ischemic stroke risk. PloS ONE 2014, 9, e94845. [Google Scholar] [CrossRef] [PubMed]
- Renner, O.; Tsimpas, A.; Kostin, S.; Valable, S.; Petit, E.; Schaper, W.; Marti, H.H. Time- and cell type-specific induction of platelet-derived growth factor receptor-beta during cerebral ischemia. Brain Res. Mol. Brain Res. 2003, 113, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Raz, N.; Rodrigue, K.M.; Kennedy, K.M.; Acker, J.D. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology 2007, 21, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Badran, A.; Hollocks, M.J.; Brookes, R.L.; Morris, R.G.; Markus, H.S. Framingham vascular age is associated with worse cognitive performance in the middle-aged and elderly. Neuropsychol. Dev. Cogn. Sect. B Aging Neuropsychol. Cogn. 2019, 26, 531–540. [Google Scholar] [CrossRef]
- Aleman, A.; Torres-Aleman, I. Circulating insulin-like growth factor I and cognitive function: Neuromodulation throughout the lifespan. Prog. Neurobiol. 2009, 89, 256–265. [Google Scholar] [CrossRef]
- Muller, A.P.; Fernandez, A.M.; Haas, C.; Zimmer, E.; Portela, L.V.; Torres-Aleman, I. Reduced brain insulin-like growth factor I function during aging. Mol. Cell Neurosci. 2012, 49, 9–12. [Google Scholar] [CrossRef]
- Okereke, O.; Kang, J.H.; Ma, J.; Hankinson, S.E.; Pollak, M.N.; Grodstein, F. Plasma IGF-I levels and cognitive performance in older women. Neurobiol. Aging 2007, 28, 135–142. [Google Scholar] [CrossRef]
- Okereke, O.I.; Kang, J.H.; Ma, J.; Gaziano, J.M.; Grodstein, F. Midlife plasma insulin-like growth factor I and cognitive function in older men. J. Clin. Endocrinol. Metab. 2006, 91, 4306–4312. [Google Scholar] [CrossRef]
- Fan, D.; Pitcher, T.; Dalrymple-Alford, J.; MacAskill, M.; Anderson, T.; Guan, J. Changes of plasma cGP/IGF-1 molar ratio with age is associated with cognitive status of Parkinson disease. Alzheimer’s Dement. 2020, 12, e12025. [Google Scholar] [CrossRef]
- Wang, A. Plasma Cyclic Glycine-Proline/Insulin-Like Growth Factor-1 Molar Ratio Is Associated with Cognitive Function in Elder People. Ph.D. Thesis, University of Auckland, Auckland, New Zealand, 2019. [Google Scholar]
- Guan, J.; Pavlovic, D.; Dalkie, N.; Waldvogel, H.J.; O’ Carroll, S.J.; Green, C.R.; Nicholson, L.F. Vascular Degeneration in Parkinson’s Disease. Brain Pathol. 2012, 16, 1750–3639. [Google Scholar] [CrossRef]
- Yang, P.; Pavlovic, D.; Waldvogel, H.; Dragunow, M.; Synek, B.; Turner, C.; Faull, R.; Guan, J. String Vessel Formation is Increased in the Brain of Parkinson Disease. J. Park. Dis. 2015, 5, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Reid, W.G.; Hely, M.A.; Morris, J.G.; Loy, C.; Halliday, G.M. Dementia in Parkinson’s disease: A 20-year neuropsychological study (Sydney Multicentre Study). J. Neurol. Neurosurg. Psychiatry 2011, 82, 1033–1037. [Google Scholar] [CrossRef]
- Wood, K.-L.; Myall, D.J.; Livingston, L.; Pitcher, T.L.; MacAskill, M.R.; Geurtsen, G.; Anderson, T.J.; Dalrymple-Alford, J.C. Different PD-MCI criteria and risk of dementia in Parkinson’s disease: Four year longitudinal study. Npj Park. Dis. 2016, 2, 15027. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, J.; Boel, J.A.; de Bie, R.M.A.; Geskus, R.B.; Schmand, B.A.; Dalrymple-Alford, J.C.; Marras, C.; Adler, C.H.; Goldman, J.G.; Troster, A.I.; et al. Mild cognitive impairment as a risk factor for Parkinson’s disease dementia. Mov. Disord. 2017, 32, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Pellecchia, M.T.; Santangelo, G.; Picillo, M.; Pivonello, R.; Longo, K.; Pivonello, C.; Vitale, C.; Amboni, M.; De Rosa, A.; Moccia, M.; et al. Insulin-like growth factor-1 predicts cognitive functions at 2-year follow-up in early, drug-naive Parkinson’s disease. Eur. J. Neurol. 2014, 21, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Dore, S.; Kar, S.; Rowe, W.; Quirion, R. Distribution and levels of [125I]IGF-I, [125I]IGF-II and [125I]insulin receptor binding sites in the hippocampus of aged memory-unimpaired and -impaired rats. Neuroscience 1997, 80, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Picillo, M.; Erro, R.; Santangelo, G.; Pivonello, R.; Longo, K.; Pivonello, C.; Vitale, C.; Amboni, M.; Moccia, M.; Colao, A.; et al. Insulin-like growth factor-1 and progression of motor symptoms in early, drug-naïve Parkinson’s disease. J. Neurol. 2013, 260, 1724–1730. [Google Scholar] [CrossRef]
- Godau, J.; Knauel, K.; Weber, K.; Brockmann, K.; Maetzler, W.; Binder, G.; Berg, D. Serum insulinlike growth factor 1 as possible marker for risk and early diagnosis of Parkinson disease. Arch. Neurol. 2011, 68, 925–931. [Google Scholar] [CrossRef]
- Godau, J.; Herfurth, M.; Kattner, B.; Gasser, T.; Berg, D. Increased serum insulin-like growth factor 1 in early idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2010, 81, 536–538. [Google Scholar] [CrossRef]
- Cortes-Canteli, M.; Iadecola, C. Alzheimer’s Disease and Vascular Aging: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 942–951. [Google Scholar] [CrossRef]
- Carro, E.; Torres-Aleman, I. Serum insulin-like growth factor I in brain function. Keio J. Med. 2006, 55, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Procter, T.V.; Williams, A.; Montagne, A. Interplay between Brain Pericytes and Endothelial Cells in Dementia. Am. J. Pathol. 2021, 191, 1917–1931. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, L.Y.; Venneri, A.; Farkas, E.; Evans, P.C.; Marzo, A.; Frangi, A.F. Vascular dysfunction in the pathogenesis of Alzheimer’s disease—A review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol. Dis. 2015, 82, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Uemura, K.; Asada, M.; Maesako, M.; Akiyama, H.; Shimohama, S.; Takahashi, R.; Kinoshita, A. The participation of insulin-like growth factor-binding protein 3 released by astrocytes in the pathology of Alzheimer’s disease. Mol. Brain 2015, 8, 82. [Google Scholar] [CrossRef]
- Cheng, J.; North, B.J.; Zhang, T.; Dai, X.; Tao, K.; Guo, J.; Wei, W. The emerging roles of protein homeostasis-governing pathways in Alzheimer’s disease. Aging Cell 2018, 17, e12801. [Google Scholar] [CrossRef]
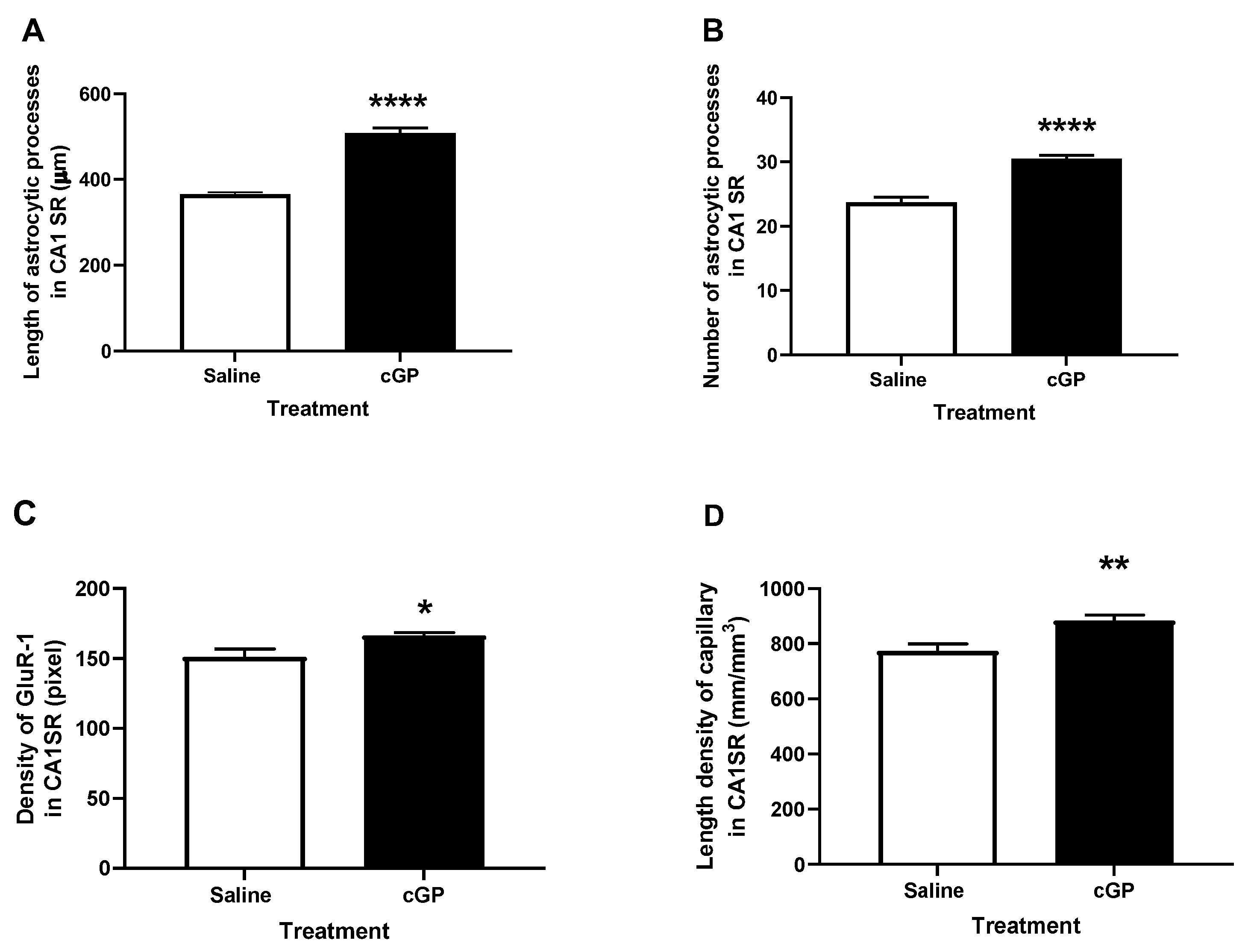
- Singh-Mallah, G.; Ardalan, M.; Kang, D.; Singh, K.; McMahon, C.D.; Mallard, C.; Guan, J. Administration of cyclic glycine-proline during infancy improves adult spatial memory, astrocyte plasticity, vascularization and GluR-1 expression in rats. Nutr. Neurosci. 2021, 25, 10. [Google Scholar] [CrossRef]
- Doiron, M.; Langlois, M.; Dupre, N.; Simard, M. The influence of vascular risk factors on cognitive function in early Parkinson’s disease. Int. J. Geriatr. Psychiatry 2018, 33, 288–297. [Google Scholar] [CrossRef]
- Lo Coco, D.; Lopez, G.; Corrao, S. Cognitive impairment and stroke in elderly patients. Vasc. Health Risk. Manag. 2016, 12, 105–116. [Google Scholar] [CrossRef]
- Picillo, M.; Pivonello, R.; Santangelo, G.; Pivonello, C.; Savastano, R.; Auriemma, R.; Amboni, M.; Scannapieco, S.; Pierro, A.; Colao, A.; et al. Serum IGF-1 is associated with cognitive functions in early, drug-naive Parkinson’s disease. PloS ONE 2017, 12, e0186508. [Google Scholar] [CrossRef]
- Alamri, Y. The use of dietary supplements and perceived quality of life in patients with Parkinson’s disease. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2018, 56, 137–138. [Google Scholar] [CrossRef]

| Dependent Variable (Confounders) | cGP (ng/mL) | IGF-1 (ng/mL) | IGFBP-3 (ng/mL) | |
|---|---|---|---|---|
| UPDRS (Age and MoCA) | B | −0.01 | 0.01 | 0.004 |
| P | 0.98 | 0.60 | 0.02 | |
| 95%CI | −0.50, 0.48 | −0.03, 0.05 | 0.001, 0.007 | |
| UPDRS (Age and Global Z) | B | 0.20 | 0.004 | 0.004 |
| P | 0.38 | 0.85 | 0.02 | |
| 95%CI | −0.25, 0.66 | −0.04, 0.05 | 0.001, 0.007 | |
| UPDRS (Age and LMD) | B | 0.26 | 0.01 | 0.004 |
| P | 0.27 | 0.61 | 0.01 | |
| 95%CI | −0.21, 0.72 | −0.03, 0.06 | 0.001, 0.01 |
| Before | After | t-Test | p | ||
|---|---|---|---|---|---|
| Total HADS | Mean Score | 9.5 | 5.4 | t9 = 3.45 | 0.007 |
| SD | 6.8 | 3.9 | |||
| Anxiety Subscale | Mean Score | 5.4 | 2.5 | t9 = 2.69 | 0.025 |
| SD | 3.6 | 1.8 | |||
| Depression Subscale | Mean Score | 4.1 | 2.9 | t9 = 2.03 | 0.074 |
| SD | 3.5 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, J.; Li, F.; Kang, D.; Anderson, T.; Pitcher, T.; Dalrymple-Alford, J.; Shorten, P.; Singh-Mallah, G. Cyclic Glycine-Proline (cGP) Normalises Insulin-Like Growth Factor-1 (IGF-1) Function: Clinical Significance in the Ageing Brain and in Age-Related Neurological Conditions. Molecules 2023, 28, 1021. https://doi.org/10.3390/molecules28031021
Guan J, Li F, Kang D, Anderson T, Pitcher T, Dalrymple-Alford J, Shorten P, Singh-Mallah G. Cyclic Glycine-Proline (cGP) Normalises Insulin-Like Growth Factor-1 (IGF-1) Function: Clinical Significance in the Ageing Brain and in Age-Related Neurological Conditions. Molecules. 2023; 28(3):1021. https://doi.org/10.3390/molecules28031021
Chicago/Turabian StyleGuan, Jian, Fengxia Li, Dali Kang, Tim Anderson, Toni Pitcher, John Dalrymple-Alford, Paul Shorten, and Gagandeep Singh-Mallah. 2023. "Cyclic Glycine-Proline (cGP) Normalises Insulin-Like Growth Factor-1 (IGF-1) Function: Clinical Significance in the Ageing Brain and in Age-Related Neurological Conditions" Molecules 28, no. 3: 1021. https://doi.org/10.3390/molecules28031021
APA StyleGuan, J., Li, F., Kang, D., Anderson, T., Pitcher, T., Dalrymple-Alford, J., Shorten, P., & Singh-Mallah, G. (2023). Cyclic Glycine-Proline (cGP) Normalises Insulin-Like Growth Factor-1 (IGF-1) Function: Clinical Significance in the Ageing Brain and in Age-Related Neurological Conditions. Molecules, 28(3), 1021. https://doi.org/10.3390/molecules28031021







