Stimulus-Responsive Ultrathin Films for Bioapplications: A Concise Review
Abstract
1. Introduction
1.1. Significance of Nanosheets
1.2. Insight into Nanomaterials and Nanostructures Applied to Medicine
1.3. Application of Nanosheets (NSHs) in Nanomedicine
2. Methods of Synthesis of NSHs
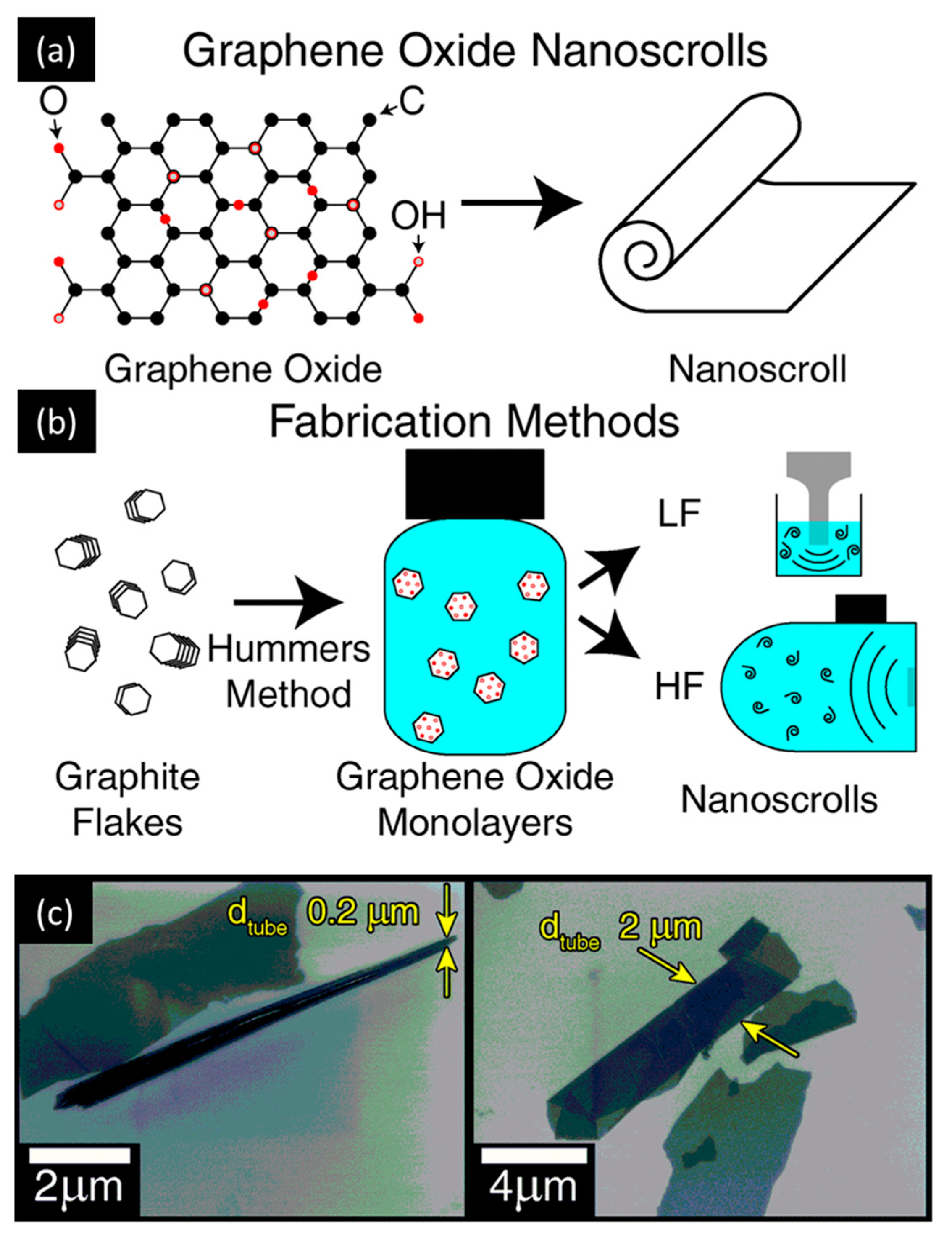
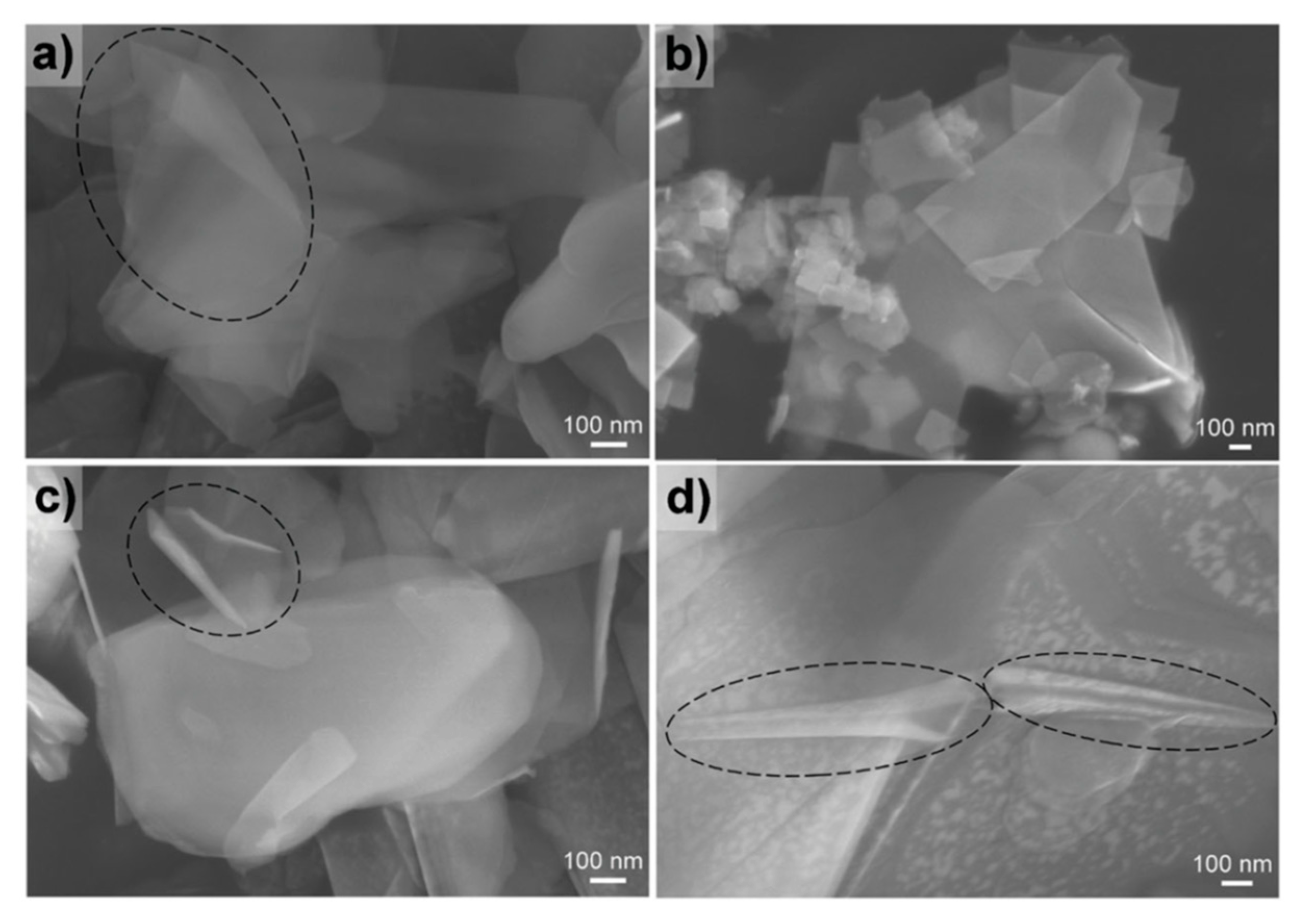
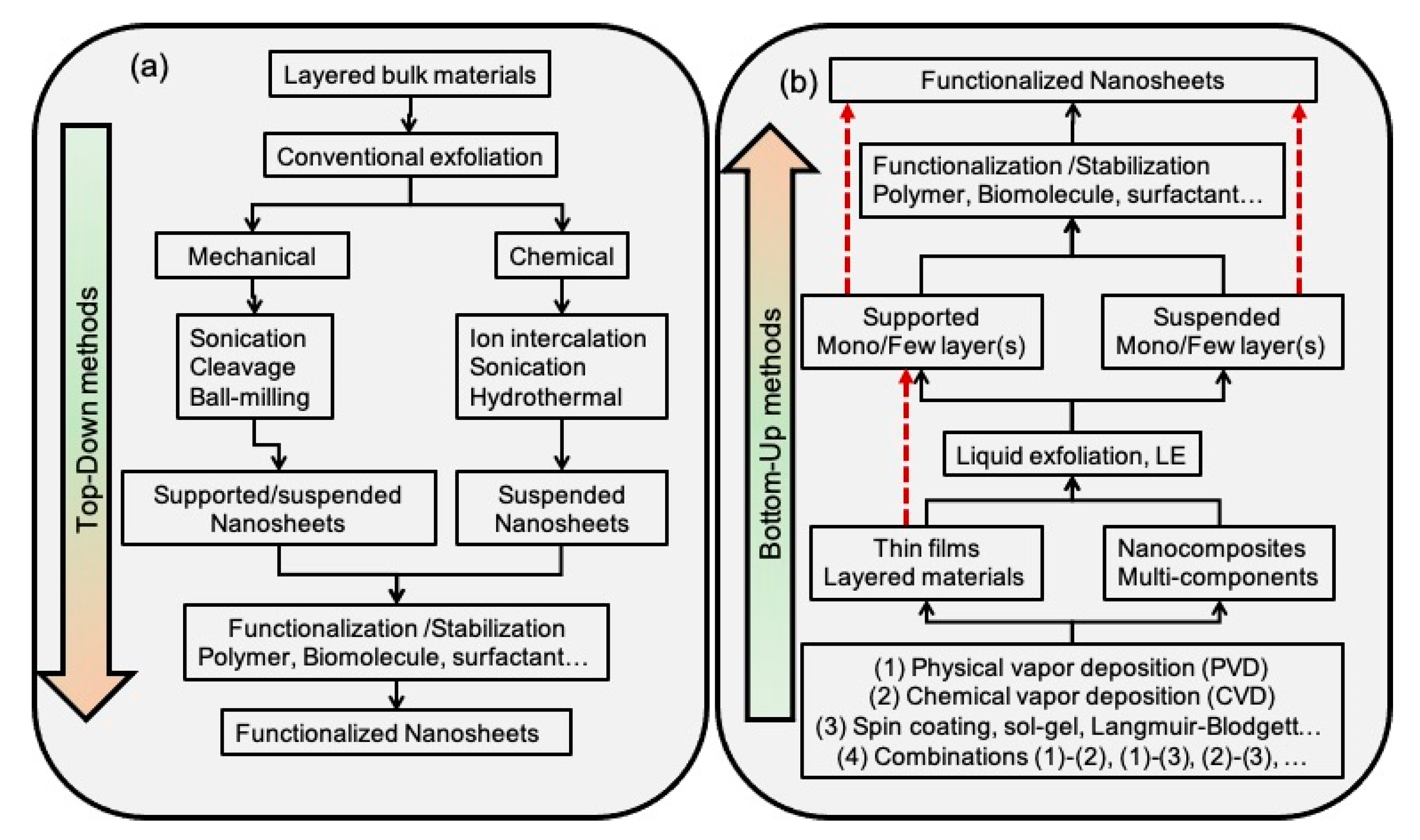
2.1. Top-Down Synthesis
2.2. Bottom-Up Synthesis
2.3. Summary Points
3. Examples of NSHs Produced by Bottom-Up Methods
3.1. Colloidal Smart NSHs for Biomedical Application
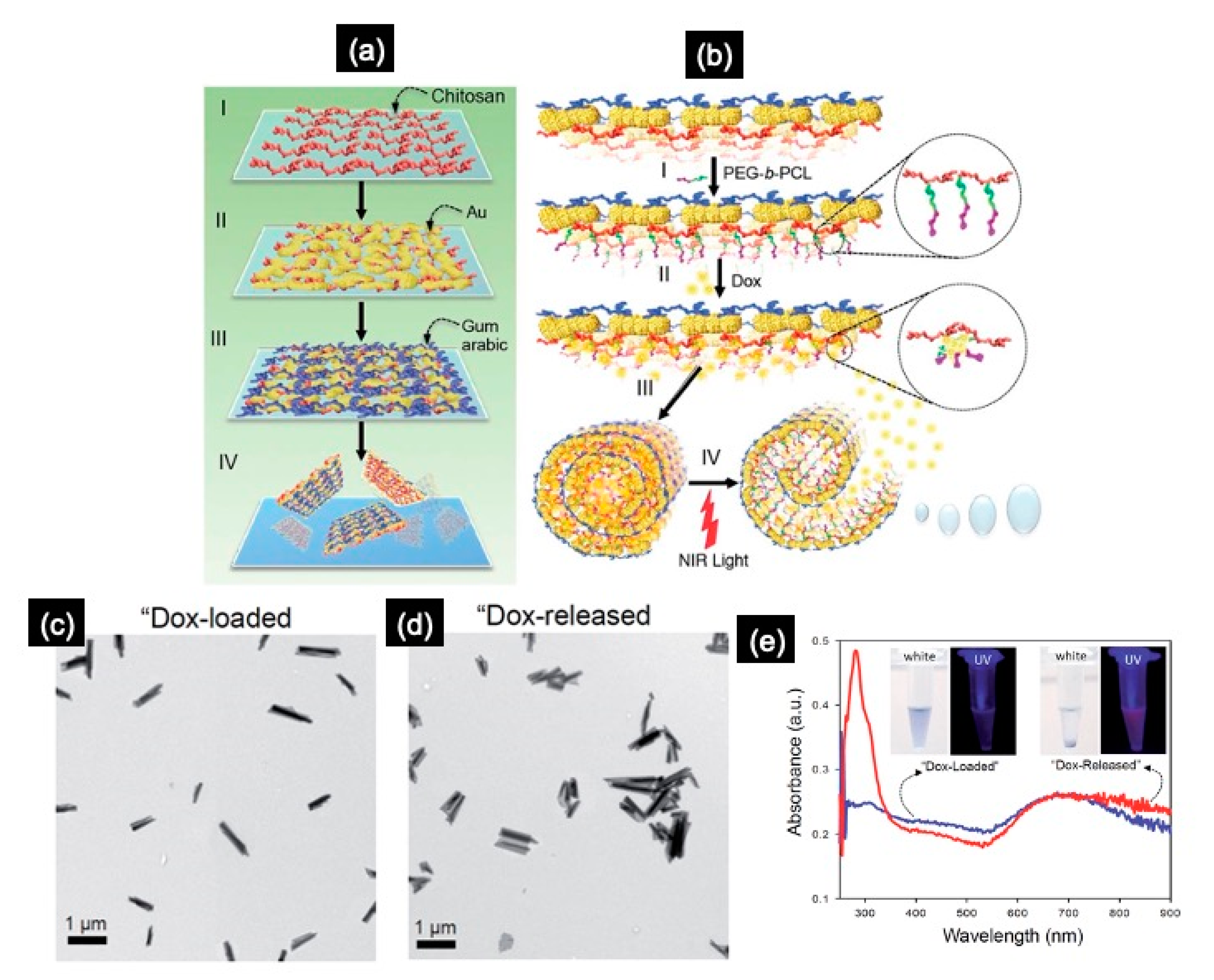
3.1.1. Hybrid NSHs
3.1.2. Janus NSHs
3.2. NSHs for Molecular Sensing Enhancement
3.2.1. Preparations of the NSHs-NPs
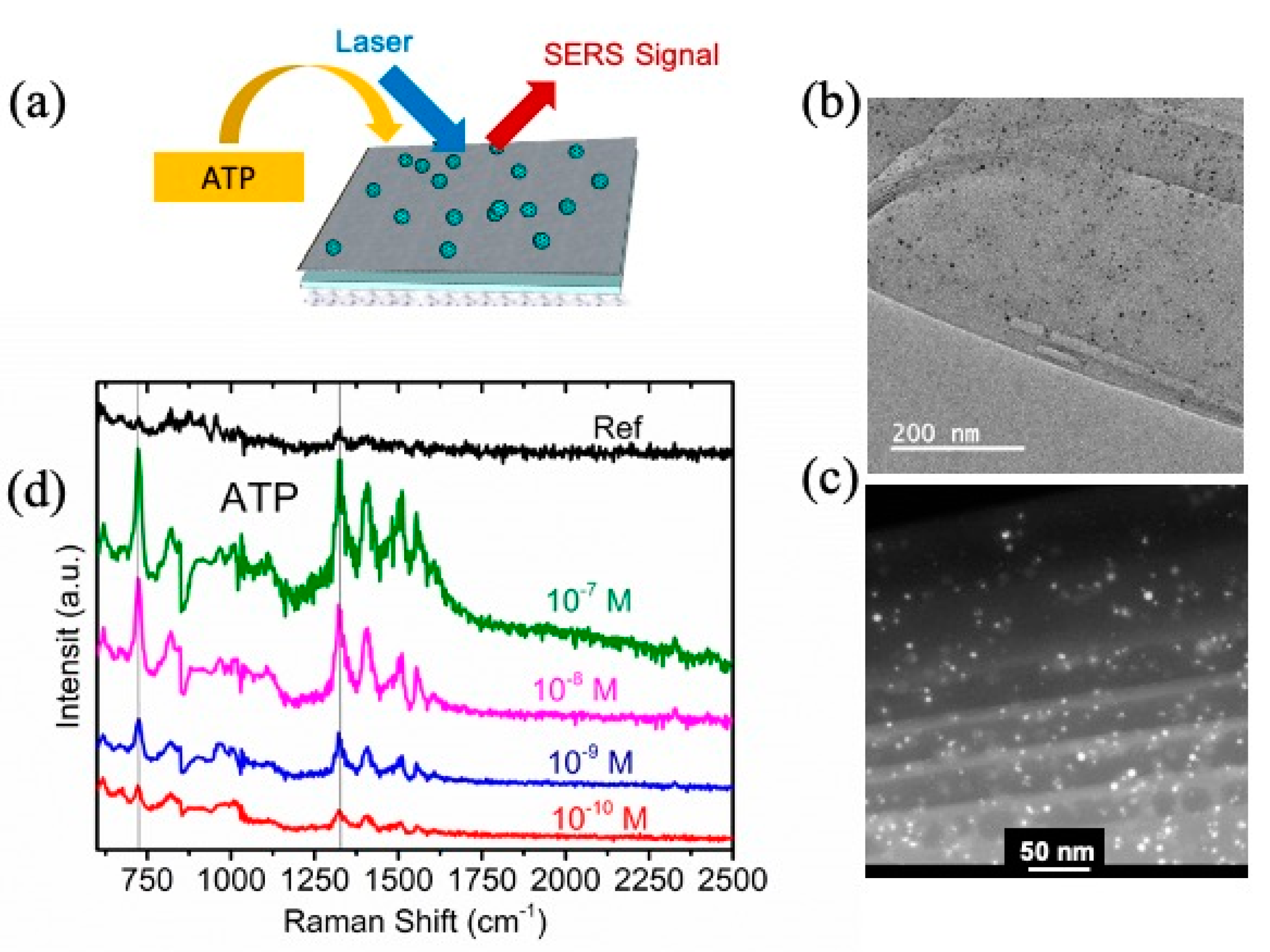
3.2.2. Evaluation of NSHs-NPs for ATP Detection
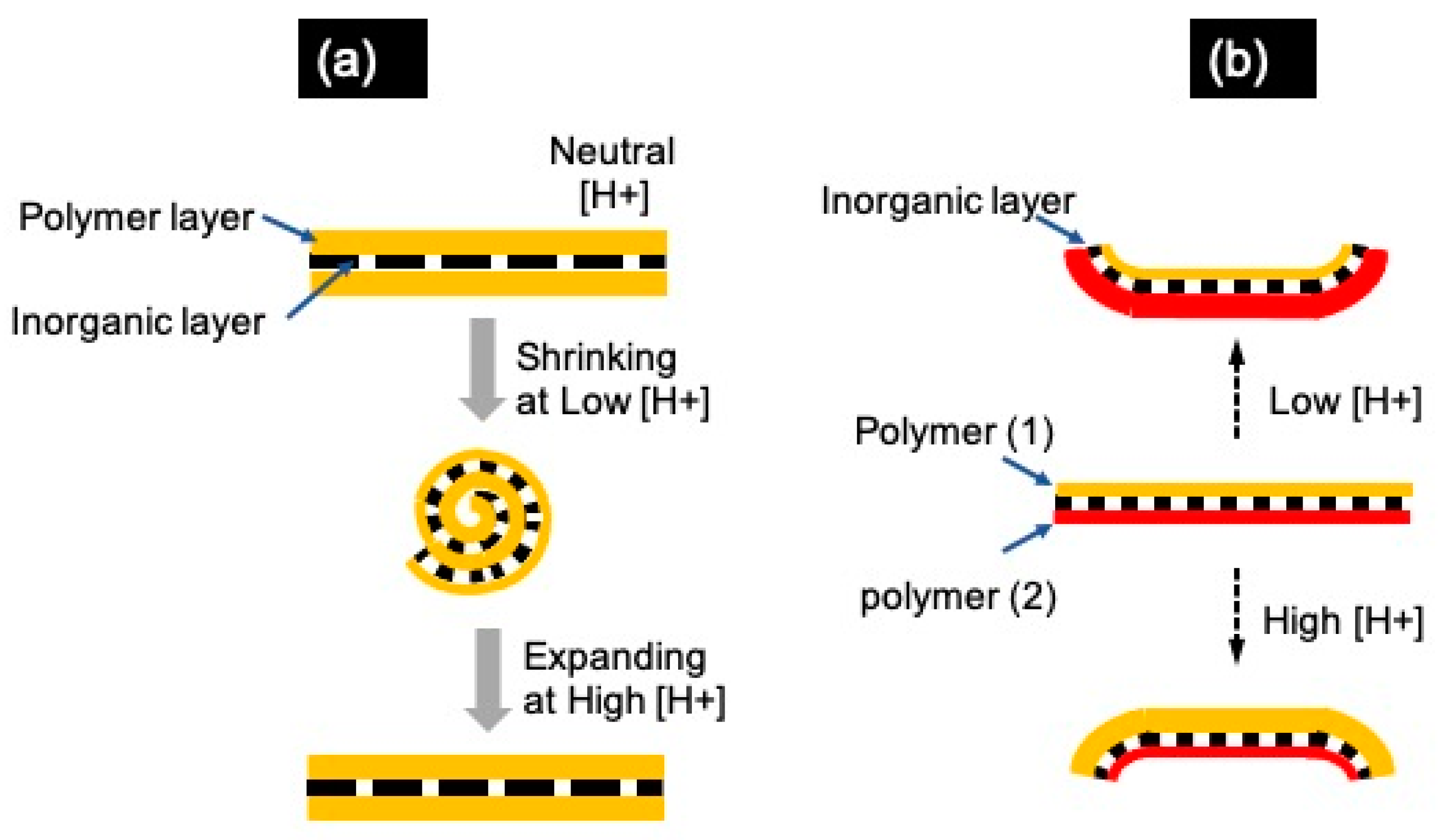
4. Mechanisms behind the Scrolling and Unscrolling of NSHs
4.1. Inorganic NSHs
4.2. Hybrid Inorganic–Organic NSHs and Janus Hybrid NHSs
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, G.; Wang, Y. Nanostructures and Nanomaterials, 2nd ed.; World Scientific Publishing: Singapore, 2011. [Google Scholar]
- Ye, S.; Brown, A.P.; Stammers, A.C.; Thomson, N.H.; Wen, J.; Roach, L.; Bushby, R.J.; Coletta, P.L.; Critchley, C.S.D.; Markham, A.F.; et al. Sub-Nanometer Thick Gold Nanosheets as Highly Efficient Catalysts. Adv. Sci. 2019, 6, 1900911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tan, C.; Yin, Z.; Zhang, H. Hybrid nanostructures based on two-dimensional nanomaterials. Adv. Mater. Process. 2014, 26, 2185–2204. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Benelmekki, M. Two-dimensional nanostructures for biomedical applications. In Frontiers of Nanoscience, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 14, pp. 103–120. [Google Scholar]
- Li, X.; Shan, J.; Zhang, W.; Su, S.; Yuwen, L.; Wang, L. Recent advances in synthesis and biomedical applications of two-dimensional transition metal dichalcogenide nanosheets. Small 2017, 13, 1602660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sunami, Y.; Hashimoto, H. Nanosheet technology towards biomedical application. Nanomaterials 2017, 7, 246. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, C.; Zhang, H.; Wang, L. Two-dimensional graphene analogues for biomedical applications. Chem. Soc. Rev. 2015, 44, 2681–2701. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, C.; Du, D.; Zhu, J.; Lin, Y. Graphene-like two-dimensional layered nanomaterials: Applications in biosensors and nanomedicine. Nanoscale 2015, 7, 14217–14231. [Google Scholar] [CrossRef]
- Han, X.; Huang, J.; Lin, H.; Wang, Z.; Li, P.; Chen, Y. 2D ultrathin MXene-based drug-delivery nanoplatform for synergistic photothermal ablation and chemotherapy of cancer. Adv. Healthc. Mater. 2018, 7, e1701394. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Liu, B.; Cheng, H.M. Preparation of 2D material dispersions and their applications. Chem. Soc. Rev. 2018, 47, 6224–6266. [Google Scholar] [CrossRef]
- Tana, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2010, 44, 2713–2731. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lee, J.-H.; Rathnam, C.; Hou, Y.; Choi, J.-W.; Lee, K.-B. Dual-Enhanced Raman Scattering-Based Characterization of Stem Cell Differentiation Using Graphene-Plasmonic Hybrid Nanoarray. Nano Lett. 2019, 19, 8138–8148. [Google Scholar] [CrossRef] [PubMed]
- Khosravani, N.; Ahmadi, V.; Kakanejadifard, A.; Adeli, M. Thermoresponsive and antibacterial two-dimensional polyglycerol-interlocked-polynipam for targeted drug delivery. J. Nanostruct. Chem. 2022, 1–11. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; Sattari, S.; Beyranvand, S.; Faghani, A.; Ludwig, K.; Schwibbert, K.; Böttcher, C.; Haag, R.; Adeli, M. Thermoresponsive Amphiphilic Functionalization of Thermally Reduced Graphene Oxide to Study Graphene/Bacteria Hydrophobic Interactions. Langmuir 2019, 35, 4736–4746. [Google Scholar] [CrossRef]
- Xu, W.; Paidi, S.K.; Qin, Z.; Huang, Q.; Yu, C.-H.; Pagaduan, J.V.; Buehler, M.J.; Barman, I.; Gracias, D.H. Self-Folding Hybrid Graphene Skin for 3D Biosensing. Nano Lett. 2019, 19, 1409–1417. [Google Scholar] [CrossRef]
- Li, J.; Zeng, H.; Zeng, Z.; Zeng, Y.; Xie, T. Promising Graphene-Based Nanomaterials and Their Biomedical Applications and Potential Risks: A Comprehensive Review. ACS Biomater. Sci. Eng. 2021, 7, 5363–5396. [Google Scholar] [CrossRef]
- Tao, L.; Li, Z.; Chen, K.; Zhou, Y.; Li, H.; Wang, X.; Zhan, R.; Hou, X.; Zhao, Y.; Xu, J.; et al. Spontaneously formed plasmonic-MoTe2 hybrid platform for ultrasensitive Raman enhancement. Cell Rep. Phys. Sci. 2021, 2, 100526. [Google Scholar] [CrossRef]
- Krukemeyer, M.G.; Krenn, V.; Huebner, F.; Wagner, W.; Resch, R. History and Possible Uses of Nanomedicine Based on Nanoparticles and Nanotechnological Progress. J. Nanomed. Nanotechnol. 2015, 6, 336. [Google Scholar] [CrossRef]
- Tinkle, S.; McNeil, S.E.; Mühlebach, S.; Bawa, R.; Borchard, G.; Barenholz, Y.C.; Tamarkin, L.; Desai, N. Nanomedicines: Addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014, 1313, 35–56. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhan, Q.; Yang, J.-C.; Bitla, Y.; Liu, P.; Li, C.-I.; Liu, J.H.; Kumar, V.S.; Arenholz, E.; He, Q.; et al. Enhanced Structural and Magnetic Coupling in a Mesocrystal-Assisted Nanocomposite. ACS Appl. Mater. Interfaces 2016, 8, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Benelmekki, M. Interfacial transformation of amorphous carbon nanofilms upon FeAgSi nanoparticle landing and its colloidal nanoscrolls. ACS Appl. Mater. Interfaces 2016, 8, 33121–33130. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Rozin, M.J.; Tao, A.R. Plasmonic nanocomposites: Polymer-guided strategies for assembling metal nanoparticles. Nanoscale 2013, 5, 5677–5691. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, A.; Brown, S.C.; Bengtsson, N.; Walter, G.A.; Grobmyer, S.R.; Iwakuma, N.; Santra, S.; Scott, E.W.; Moudgil, B.M. Multimodal nanoparticulate bioimaging contrast agents. Methods Mol. Biol. 2010, 624, 67–81. [Google Scholar]
- Koslowska, D.; Foran, P.; MacMahon, P.; Shelly, M.J.; Eustace, S.; OKennedy, R. Molecular and magnetic resonance imaging: The value of immunoliposomes. Adv. Drug. Deliv. Rev. 2009, 61, 1402–1411. [Google Scholar] [CrossRef]
- Keunen, O.; Taxt, T.; Grüner, R.; Lund-Johansen, M.; Tonn, J.-C.; Pavlin, T.; Bjerkvig, R.; Niclou, S.P.; Thorsen, F. Multimodal imaging of gliomas in the context of evolving cellular and molecular therapies. Adv. Drug Deliv. Rev. 2014, 76, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Shah, S.; Chhowalla, M.; Lee, K. Design, Synthesis, and Characterization of Graphene–Nanoparticle Hybrid Materials for Bioapplications. Chem. Rev. 2015, 115, 2483–2531. [Google Scholar] [CrossRef] [PubMed]
- Benelmekki, M.; Gasso, S.; Martinez, L.M. Simultaneous optical and magnetophoretic monitoring of DNA hybridization using superparamagnetic and plasmonic colloids. J. Coll. Surf. Biointefaces 2020, 193, 111126. [Google Scholar] [CrossRef]
- Storhoff, J.; Lucas, A.; Garimella, V.; Bao, Y.P.; Muller, U.R. Homogeneous detection of unamplified genomic DNA sequences based on colorimetric scatter of gold nanoparticle probes. Nat. Biotechnol. 2004, 22, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Vig, K.; Eroglu, E.; Tiwari, P.M.; Soni, S.; Casey, K.; Singh, S.R. Nanomedicine: Future of Diagnostics and Therapeutics In Nanotechnology: Diagnostics and Therapeutics, 1st ed.; Navani, N.K., Sinha, S., Eds.; Studium Press LLC: Houston, TX, USA, 2012; Volume 7. [Google Scholar]
- Anker, J.; Hall, W.; Lyandres, O.; Shah, N.C.; Zhao, J.; VanDuyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, S.M.; Chandran, V. Exploring the Psoriatic Arthritis Proteome in Search of Novel Biomarkers. Proteomes 2018, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Sze, J.Y.Y.; Ivanov, A.P.; Cass, A.E.G.; Edel, J.B. Single molecule multiplexed nanopore protein screening in human serum using aptamer modified DNA carriers. Nat. Commun. 2017, 8, 1552. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.M.; Mukherji, S. Nanoplasmonic biosensors: Current perspectives. Nanobiosens. Dis. Diagn. 2015, 4, 75–88. [Google Scholar]
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef]
- Holzinger, M.; Goff, A.L.; Cosnier, S. Synergetic Effects of Combined Nanomaterials for Biosensing Applications. Sensors 2017, 17, 1010. [Google Scholar] [CrossRef]
- Chamorro-Garcia, A.; Merkoc, A. Nanobiosensors in diagnostics. Nanobiomedicine 2016, 3, 1849543516663574. [Google Scholar] [CrossRef]
- Vigneshvar, S.C.; Sudhakumari, C.; Senthilkumaran, B.; Prakash, H. Recent advances in biosensor technology for potential applications—An overview. Front. Bioeng. Biotechnol. 2016, 4, 11. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, Q.; Xiao, F.; Duan, H. 2D nanomaterials based electrochemical biosensors for cancer diagnosis. Biosens. Bioelectron. 2017, 89, 136–151. [Google Scholar] [CrossRef]
- Mo, L.; Lia, J.; Liua, Q.; Qiua, L.; Tan, W. Nucleic acid-functionalized transition metal nanosheets for biosensing applications. Biosens. Bioelectron. 2017, 89, 201–211. [Google Scholar] [CrossRef]
- Key, J.; Leary, J.F. Nanoparticles for multimodal in vivo imaging in nanomedicine. J. Nanomed. 2014, 9, 711–726. [Google Scholar]
- Benelmekki, M. Nanomaterials: The Original Product of Nanotechnology, 1st ed.; IOP Concise Physics; Morgan & Claypool: San Rafael, CA, USA, 2019. [Google Scholar]
- Benelmekki, M.; Erbe, A. Nanostructured Thin Films: Fundamentals and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 14, pp. 1–30. [Google Scholar]
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Lombardo, A.; Hasan, T.; Sun, Z.; Colombo, L.; Ferrari, A.C. Production, processing and placement of graphene and two dimensional crystals. Mater. Today 2012, 15, 564. [Google Scholar] [CrossRef]
- Acharya, S.; Das, B.; Thupakula, U.; Ariga, K.; Sarma, D.D.; Israelachvili, J.; Golan, Y. A bottom-up approach toward fabrication of ultrathin PbS sheets. Nano Lett. 2013, 13, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Ban, T.; Nakagawa, T.; Ohya, Y. Bottom-up synthesis of titanate nanosheets in aqueous sols and their morphology change by the addition of organic ligands and dialysis. Cryst. Growth Des. 2015, 15, 1801–1807. [Google Scholar] [CrossRef]
- Li, L.H.; Chen, Y.; Behan, G.; Zhang, H.; Petravicc, M.; Glushenkov, A.M. Large-scale mechanical peeling of boron nitride nanosheets by low-energy ball milling. J. Mater. Chem. 2011, 21, 11862–11866. [Google Scholar] [CrossRef]
- Li, X.; Hao, X.; Zhao, M.; Wu, Y.; Yang, J.; Tian, Y.; Qian, G. Exfoliation of Hexagonal Boron Nitride by Molten Hydroxides. Adv. Mater. 2013, 25, 2200–2204. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, X.; Wang, X.; Bando, Y.; Golberg, D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Pacilé, D.; Meyer, J.C.; Girit, Ç.Ö.; Zettl, A. The two-dimensional phase of boron nitride: Few-atomic-layer sheets and suspended membranes. Appl. Phys. Lett. 2008, 92, 133107. [Google Scholar] [CrossRef]
- Lee, C.; Li, Q.; Kalb, W.; Liu, X.-Z.; Berger, H.; Carpick, R.W.; Hone, J. Frictional Characteristics of Atomically Thin Sheets. Science 2010, 328, 76–80. [Google Scholar] [CrossRef]
- Janot, R.; Guérard, D. Ball-milling: The behavior of graphite as a function of the dispersal media. Carbon 2002, 40, 2887–2896. [Google Scholar] [CrossRef]
- Antisari, M.V.; Montone, A.; Jovic, N.; Piscopiello, E.; Alvani, C. Low energy pure shear milling: A method for the preparation of graphite nano-sheets. Scr. Mater. 2006, 55, 1047–1050. [Google Scholar] [CrossRef]
- Zhao, W.; Fang, M.; Wu, F.; Wu, H.; Wang, L.; Chen, G. Preparation of graphene by exfoliation of graphite using wet ball milling. J. Mater. Chem. 2010, 20, 5817–5819. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Zhao, J.; Liu, F. Mechanical properties of graphene oxides. Nanoscale 2012, 4, 5910–5916. [Google Scholar] [CrossRef]
- Amadei, C.A.; Stein, I.Y.; Silverberg, G.J.; Wardle, B.L.; Vecitis, C.D. Fabrication and morphology tuning of graphene oxide nanoscrolls. Nanoscale 2016, 8, 6783. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Lee, D.; Lee, B.; Park, K.H.; Ryu, H.J.; Jeon, S.; Hong, S.H. Scalable Exfoliation Process for Highly Soluble Boron Nitride Nanoplatelets by Hydroxide-Assisted Ball Milling. Nano Lett. 2015, 15, 1238–1244. [Google Scholar] [CrossRef]
- Fu, L.; Chen, G.; Jiang, N.; Yu, J.; Lin, C.-T.; Yu, A. In-situ growth of metal nanoparticles on boron nitride nanosheets as highly efficient catalysts. J. Mater. Chem. A 2016, 4, 19107–19115. [Google Scholar] [CrossRef]
- Miranti, R.; Qayyum, M.-S.; Sharma, A.; Einarsrud, M.-A.; Mestres, N.; Benelmekki, M. Spectroscopic study of partially oxidized BN nanoscrolls induced by low-frequency ultrasonic irradiation. App. Surf. Sci. 2020, 515, 146055. [Google Scholar] [CrossRef]
- Ausländer, S.; Ausländer, D.; Fussenegger, M. Synthetic biology- the synthesis of biology. Angew. Chem. Int. Ed. 2017, 56, 6396–6419. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, G.; Yan, H.; Wang, J.; Yu, Y.; Hu, X.; Wu, X.; Mei, Y. Fabrication and stimuli-responsive behavior of flexible micro-scrolls. Soft Matter 2012, 8, 7103. [Google Scholar] [CrossRef]
- Kim, J.-H.; Bohra, M.; Cassidy, C.; Singh, V.; Sowwan, M. Smart composite nanosheets with adaptive optical properties. ACS Appl. Mater. Interfaces 2014, 6, 13339–13343. [Google Scholar] [CrossRef]
- Walther, A.; Muller, A.H.E. Janus particles: Synthesis, self-assembly, physical properties, and applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Andre, X.; Drechsler, M.; Abetz, V.; Müller, A.H.E. Janus discs. J. Am. Chem. Soc. 2007, 129, 6187–6198. [Google Scholar] [CrossRef]
- Kai, S.; Ashaduzzaman, M.; Uemura, S.; Kunitake, M. Composite polymer materials consisting of nanofilms formed by click reaction between polymers at an oil–water interface. Chem. Lett. 2011, 40, 270–272. [Google Scholar] [CrossRef]
- Yang, H.L.; Liang, F.X.; Wang, X.; Chen, Y.; Zhang, C.L.; Wang, Q.; Qu, X.Z.; Li, J.; Wu, D.C.; Yang, Z.Z. Responsive Janus composite nanosheets. Macromolecules 2013, 46, 2754–2759. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Liang, F.X.; Zhang, G.L.; Ji, X.; Wang, Q.; Song, X.; Yang, Z. Dually responsive Janus composite nanosheets. Macromolecules 2015, 48, 3598–3602. [Google Scholar] [CrossRef]
- Kirillova, A.; Stoychev, G.; Ionov, L.; Eichhorn, K.J.; Malanin, M.; Synytska, A. Platelet Janus particles with hairy polymer shells for multifunctional materials. ACS Appl. Mater. Interfaces 2014, 6, 13106–13114. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, F.; Yang, H.; Zhang, C.; Wang, Q.; Qu, X.; Li, J.; Cai, Y.; Qiu, D.; Yang, Z. Janus nanosheets of polymer-inorganic layered composites. Macromolecules 2012, 45, 1460–1467. [Google Scholar] [CrossRef]
- Stöter, M.; Gödrich, S.; Feicht, P.; Rosenfeldt, S.; Thurn, H.; Neubauer, J.W.; Seuss, M.; Lindner, P.; Kalo, H.; Moller, M.; et al. Controlled exfoliation of layered silicate heterostructures into bilayers and their conversion into giant Janus platelets. Angew. Chem. Int. Ed. 2016, 55, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wang, Q.; Ling, C.L.F.X.; Li, X.Z.; Quand, J.L.; Yang, Z.Z. pH responsive Janus polymeric nanosheets. Chin. Chem. Lett. 2015, 26, 657–661. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lu, T.-M. Bio-inspired Janus composite nanoscroll for on-demand tumor targeting. RSC Adv. 2016, 6, 17179. [Google Scholar] [CrossRef]
- Fang, H.; Yin, H.J.; Lv, M.Y.; Xu, H.J.; Zhao, Y.M.; Zhang, X.; Wu, Z.L.; Liu, L.; Tan, T.W. Approach for determination of ATP:ADP molar ratio in mixed solution by surface-enhanced Raman scattering. Biosens. Bioelectron. 2015, 69, 71–76. [Google Scholar] [CrossRef]
- Manzetti, S.; Vasilache, D.; Francesco, E. Emerging carbon-based nanosensor devices: Structures, functions and applications. Adv. Manuf. 2015, 3, 63–71. [Google Scholar] [CrossRef]
- Shi, X.; Cheng, Y.; Pugno, N.M.; Gao, H. A translational nanoactuator based on carbon nanoscrolls on substrates. Appl. Phys. Lett. 2010, 96, 053115. [Google Scholar] [CrossRef]
- Karimi, H.; Ahmadi, M.T.; Khosrowabadi, E.; Rahmani, R.; Saeidimanesh, M.; Ismail, R.; Naghib, S.D.; Akbari, E. Analytical prediction of liquid-gated graphene nanoscroll biosensor performance. RSC Adv. 2014, 4, 16153–16162. [Google Scholar] [CrossRef]
- Turchanin, A.; Beyer, A.; Nottbohm, C.; Zhang, X.; Stosch, R.; Sologubenko, A.; Mayer, J.; Hinze, P.; Weimann, T.; Gölzhäuser, A. One nanometer thin carbon nanosheets with tunable conductivity and stiffness. Adv. Mater. 2009, 21, 1233–1237. [Google Scholar] [CrossRef]
- Kim, J.-H.; Benelmekki, M. Emerging Applications of Nanoparticles and Architecture Nanostructures; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 18; pp. 553–574. [Google Scholar]
- Chen, P.; Shen, A.; Zhou, X.; Hu, J. Bio-Raman spectroscopy: A potential clinical analytical method assisting in disease diagnosis. Anal. Methods 2011, 3, 1257–1269. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; DeSouza, M.L.; Souza, K.S.; Dos-Santos, D.P.; Andradec, G.F.S.; Temperini, M.L.A. Critical assessment of enhancement factor measurements in surface-enhanced Raman scattering on different substrates. Phys. Chem. Chem. Phys. 2015, 17, 21294–21301. [Google Scholar] [CrossRef] [PubMed]
- Radziuk, D.; Moehwald, H. Prospects for plasmonic hot spots in single molecule SERS towards the chemical imaging of live cells. Phys. Chem. Chem. Phys. 2015, 17, 21072–21093. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhou, H.; Zhang, H.; Zhang, Y.; Wang, G.; Zhao, H. 3D Fe3O4@Au@Ag nanoflowers assembled magnetoplasmonic chains for in situ SERS monitoring of plasmon-assisted catalytic reactions. J. Mater. Chem. A 2016, 4, 8866–8874. [Google Scholar] [CrossRef]
- Huang, M.; Cavallo, F.; Liu, F.; Lagally, M.G. Nanomechanical architecture of semiconductor nanomembranes. Nanoscale 2011, 3, 96–120. [Google Scholar] [CrossRef]
- Perim, E.; Galvao, D.S. The structure and dynamics of boron nitride nanoscrolls. Nanotechnology 2009, 20, 335702. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, T.; Gracia-Espino, E.; Barzegar, H.R.; Jia, X.; Nitze, F.; Hu, G.; Nordblad, P.; Tai, C.-W.; Wagberg, T. Formation of nitrogen-doped graphene nanoscrolls by adsorption of magnetic γ-Fe2O3 nanoparticles. Nat. Commun. 2013, 4, 2319. [Google Scholar] [CrossRef]
- Wang, W.; Gai, Y.; Xiao, D.; Zhao, Y. A facile and general approach for production of nanoscrolls with high-yield from two-dimensional nanosheets. Sci. Rep. 2018, 8, 15262. [Google Scholar] [CrossRef]
- Reis, R.L.; Neves, N.M.; Mano, J.F.; Gomes, M.E.; Marques, A.P.; Azevedo, H.S. Natural-Based Polymers for Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2008; pp. 129–154. [Google Scholar]
- Yao, K.; Li, J.; Yao, F.; Yin, Y. Chitosan-Based Hydrogels: Functions and Applications; CRC Press: Boca Raton, FL, USA, 2012; pp. 235–338. [Google Scholar]
- Kim, J.-H.; Bohra, M.; Singh, V.D.; Galea, A.D.; Grammatikopoulos, P.; Sowwan, M.I. Controllable and Reversible pH-Responsive Rollable 2D Nanostructures 2019. U.S. Patent 10279540B2, 25 October 2017. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benelmekki, M.; Kim, J.-H. Stimulus-Responsive Ultrathin Films for Bioapplications: A Concise Review. Molecules 2023, 28, 1020. https://doi.org/10.3390/molecules28031020
Benelmekki M, Kim J-H. Stimulus-Responsive Ultrathin Films for Bioapplications: A Concise Review. Molecules. 2023; 28(3):1020. https://doi.org/10.3390/molecules28031020
Chicago/Turabian StyleBenelmekki, Maria, and Jeong-Hwan Kim. 2023. "Stimulus-Responsive Ultrathin Films for Bioapplications: A Concise Review" Molecules 28, no. 3: 1020. https://doi.org/10.3390/molecules28031020
APA StyleBenelmekki, M., & Kim, J.-H. (2023). Stimulus-Responsive Ultrathin Films for Bioapplications: A Concise Review. Molecules, 28(3), 1020. https://doi.org/10.3390/molecules28031020








