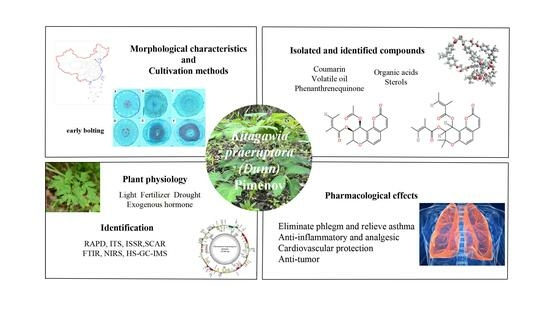
A Review on the Morphology, Cultivation, Identification, Phytochemistry, and Pharmacology of Kitagawia praeruptora (Dunn) Pimenov
Abstract
1. Introduction
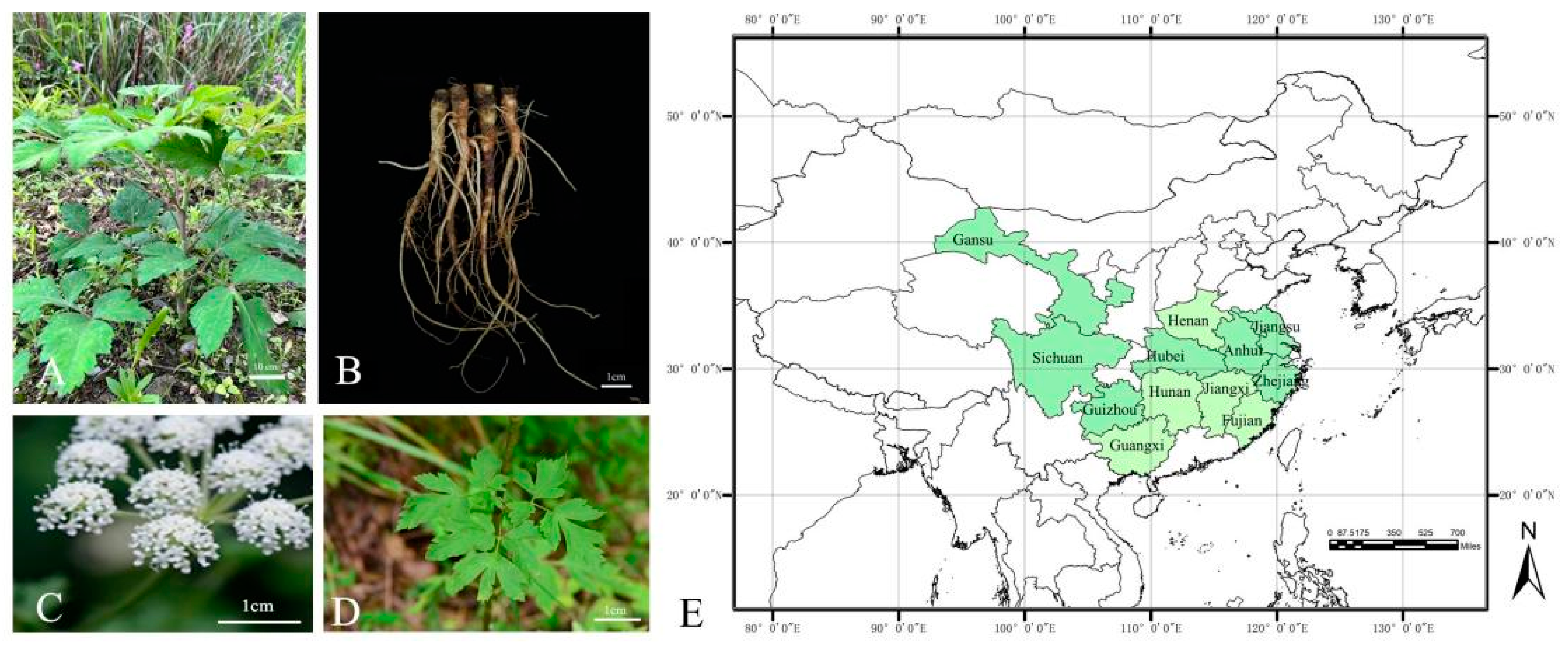
2. Botany
3. Ethnobotany
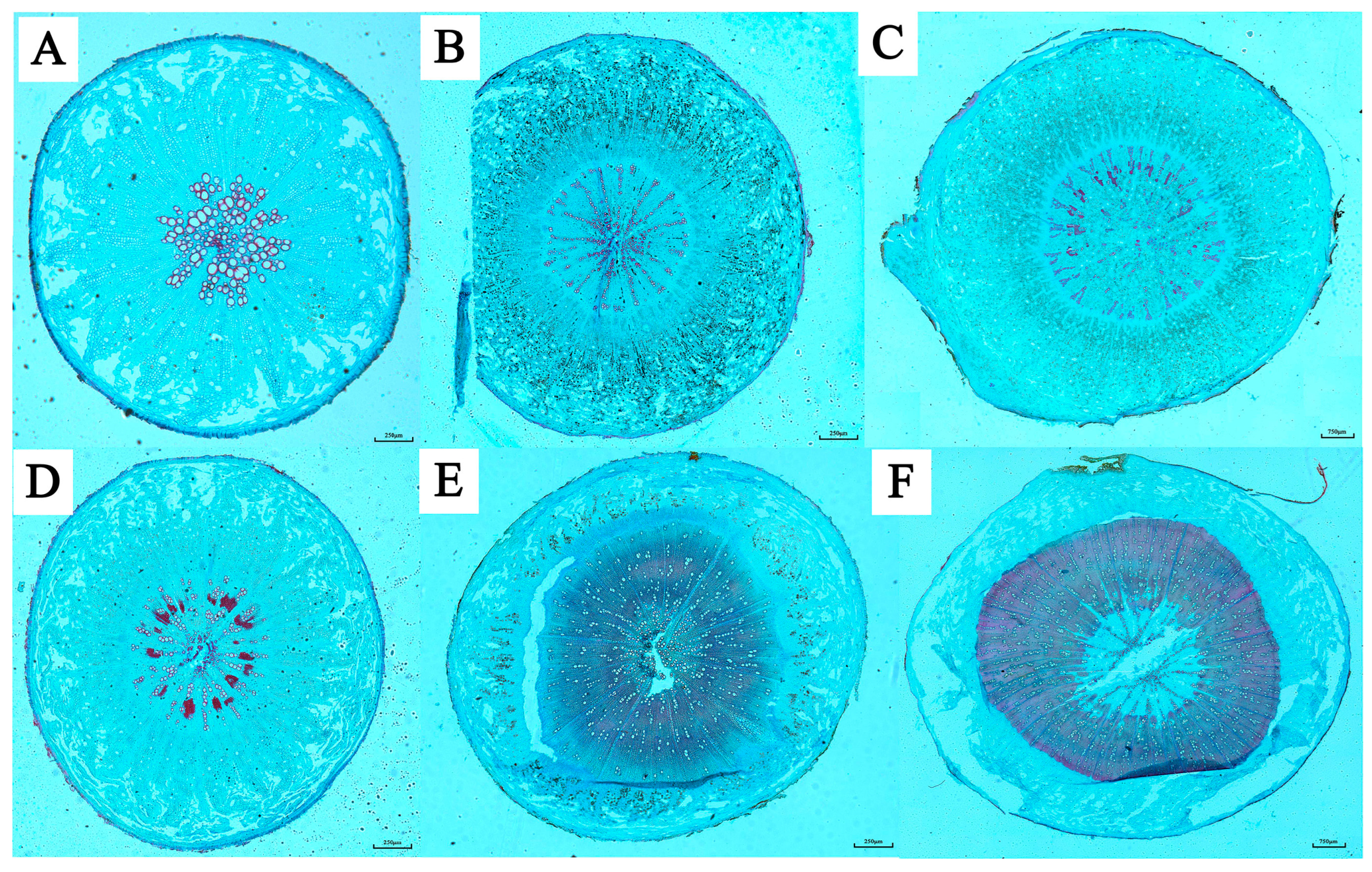
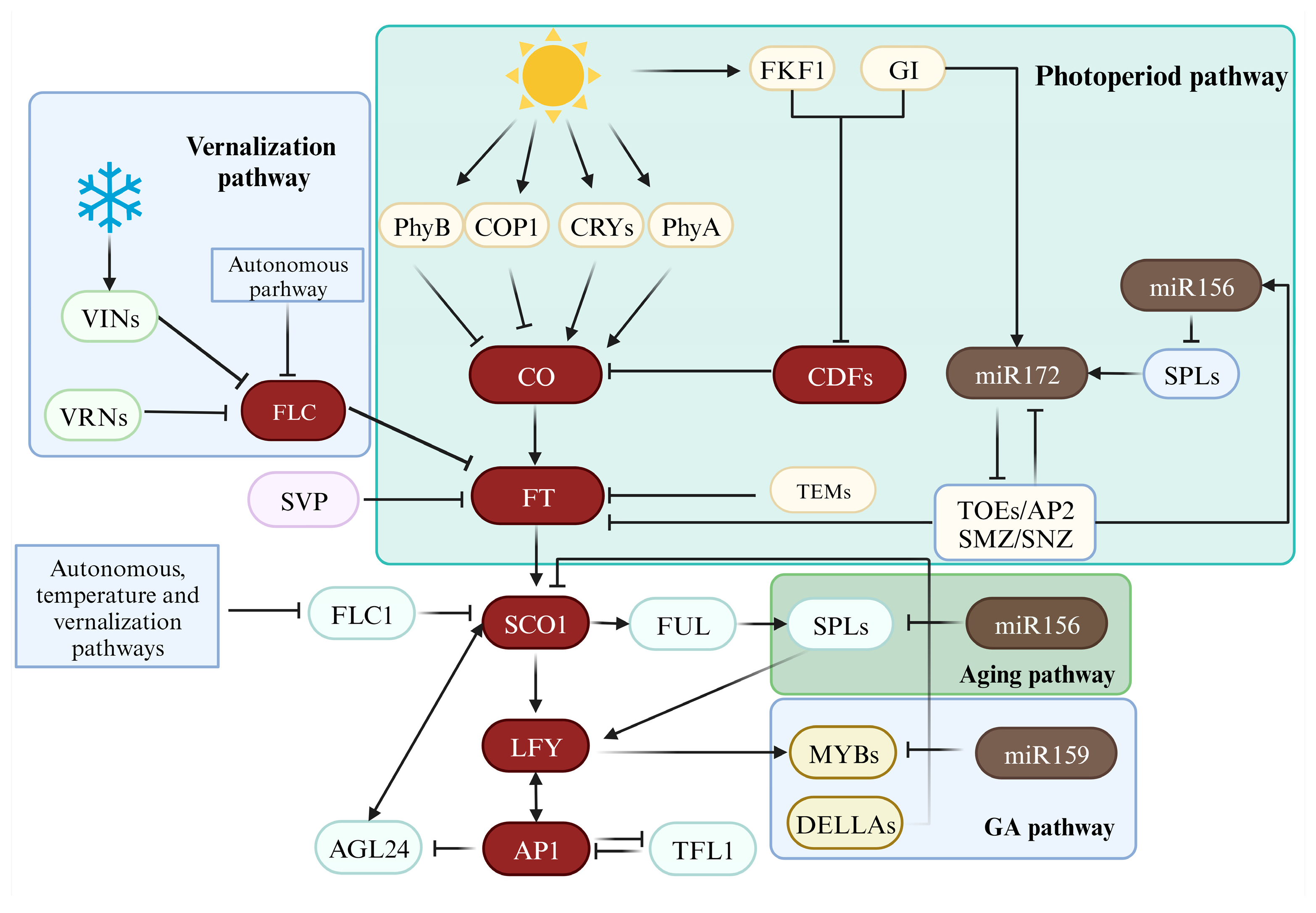
4. Cultivation
5. Identification
6. Phytochemistry
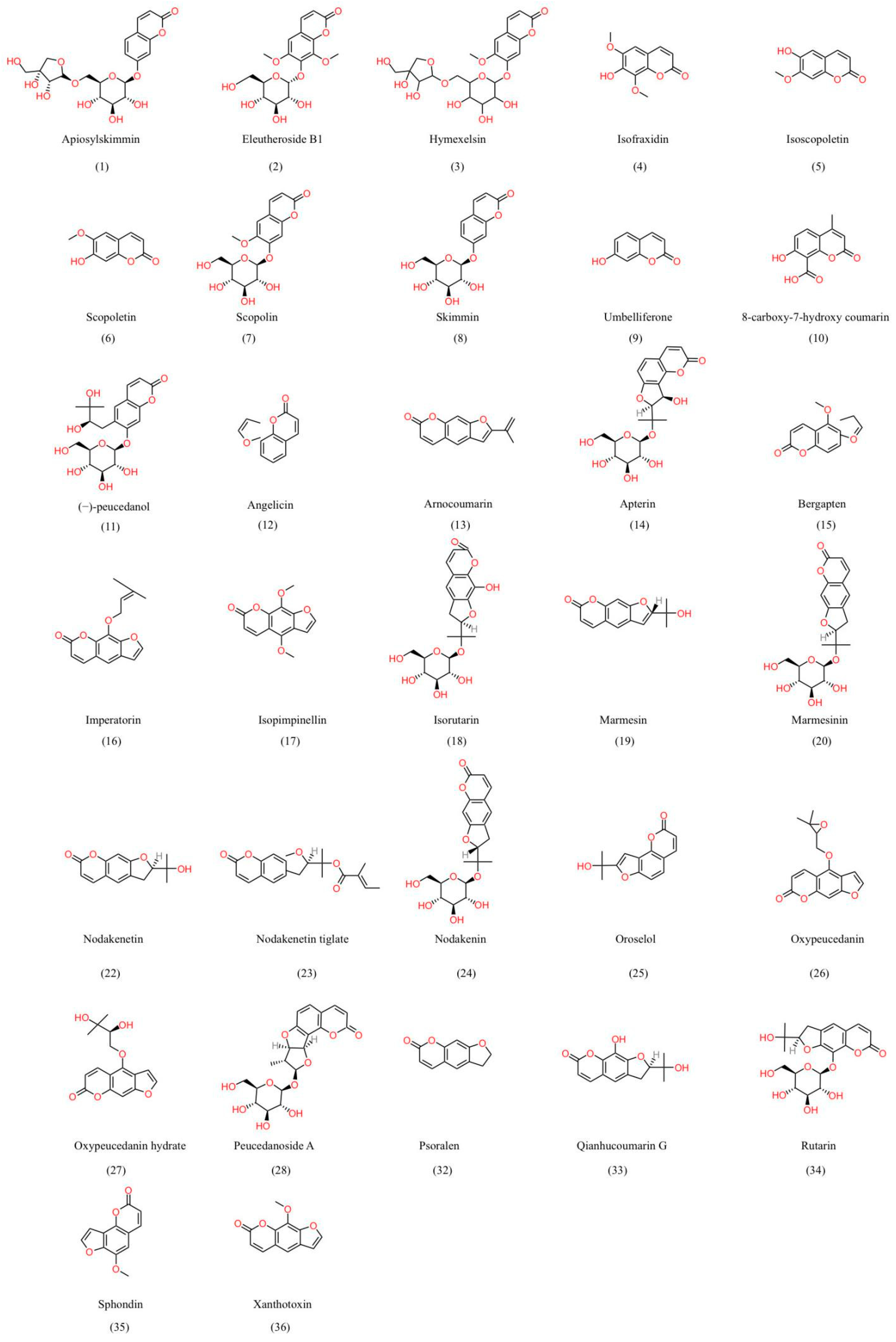
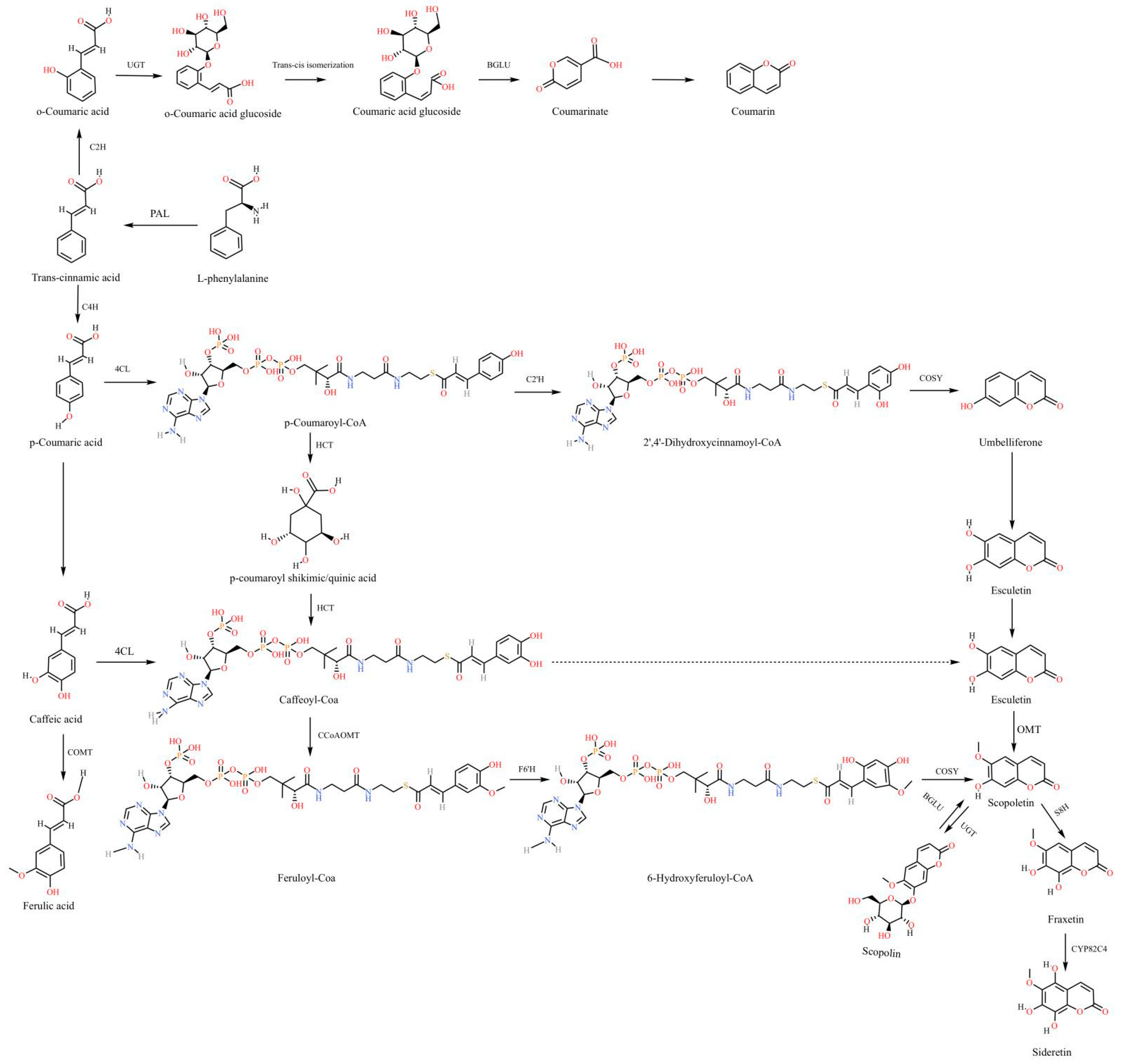
6.1. Coumarins
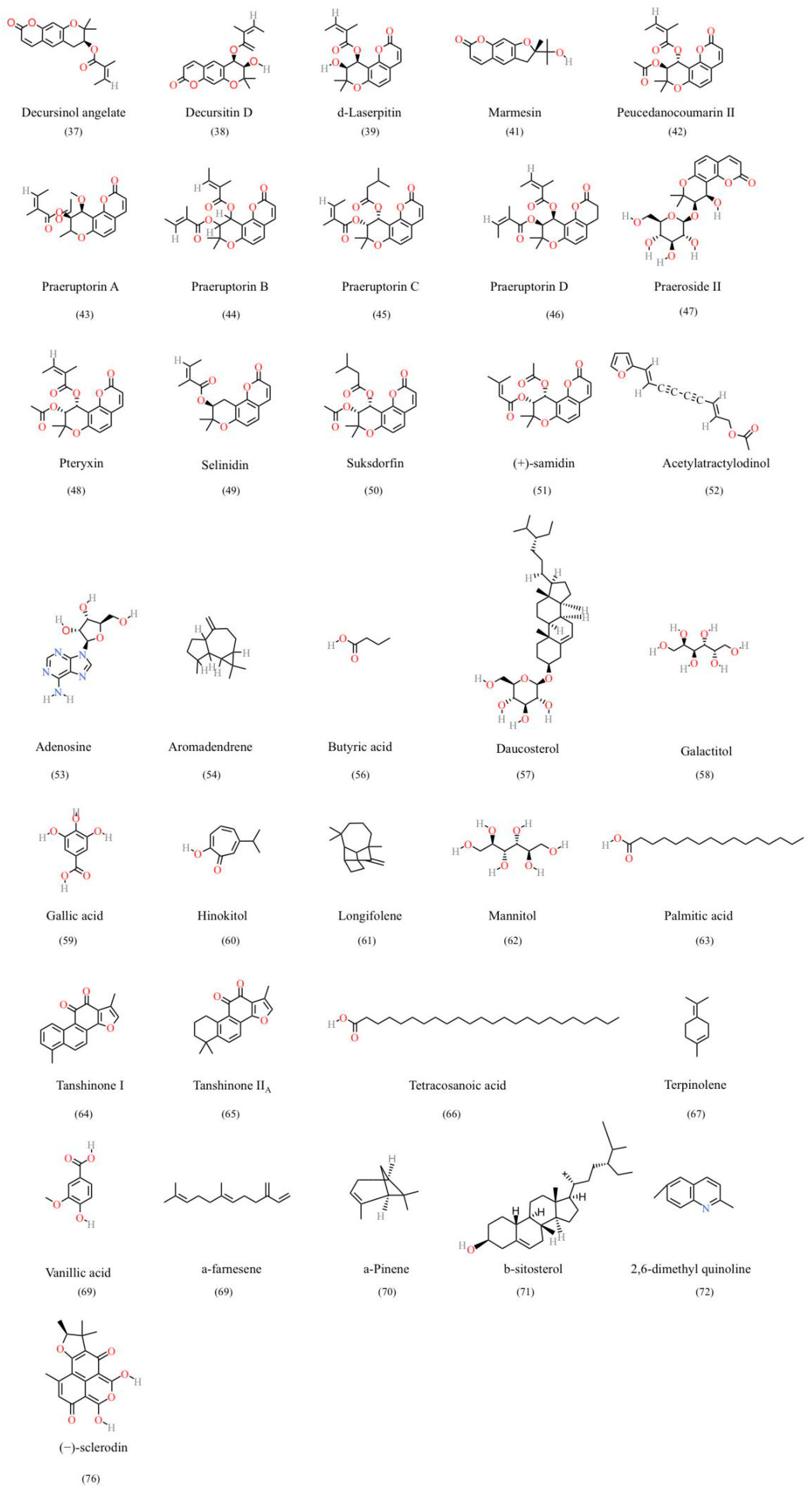
6.2. Other Constituents
| Classification | No. | Chemical Components | Formula | References | Test Methods | CAS Identifiers |
|---|---|---|---|---|---|---|
| Simple coumarin | 1 | Apiosylskimmin | C20H24O12 | [26] | UPLC-HR-Orbitrap-MS | 103529-94-8 |
| 2 | Eleutheroside B1 | C17H20O10 | [93] | NMR/MS | 16845-16-2 | |
| 3 | Hymexelsin | C21H26O13 | [94] | HPLC | 117842-09-8 | |
| 4 | Isofraxidin | C11H10O5 | [95] | 486-21-5 | ||
| 5 | Isoscopoletin | C10H8O4 | [96] | LC-MS | 776-86-3 | |
| 6 | Scopoletin | C10H8O4 | [96] | LC-MS | 92-61-5 | |
| 7 | Scopolin | C16H18O9 | [97] | UPLC-Q-TOF-HRMS | 531-44-2 | |
| 8 | Skimmin | C15H16O8 | [97] | HPLC | 93-39-0 | |
| 9 | Umbelliferone | C9H6O3 | [77] | DMR | 93-35-6 | |
| 10 | 8-carboxy-7-hydroxy coumarin | C11H8O5 | [98] | NMR | 5112-55-0 | |
| 11 | (−)-peucedanol | C15H18O5 | [99] | HPLC/PAD | 28095-18-3 | |
| Furanocoumarin compounds | 12 | Angelicin | C11H6O8 | [80] | HPLC/NMR/MS | 523-50-2 |
| 13 | Arnocoumarin | C14H10O3 | 11037-15-3 | |||
| 14 | Apterin | C20H24O10 | [100] | LC-MS | 53947-89-0 | |
| 15 | Bergapten | C12H8O4 | [101] | HPLC | 484-20-8 | |
| 16 | Imperatorin | C16H14O4 | [102] | IMP | 482-44-0 | |
| 17 | Isopimpinellin | C13H10O5 | [103] | HPLC | 482-27-9 | |
| 18 | Isorutarin | C16H21NO3 | [79] | NMR | 53846-51-8 | |
| 19 | Marmesin | C14H14O4 | [104] | NMR/MS | 13849-08-6 | |
| 20 | Marmesinin | C20H24O9 | [79] | NMR | 27497-13-8 | |
| 21 | Marmesin-11-O-β-D-glucopyranosyl (1→6)-β-D-glucopyranoside | [104] | NMR/MS | |||
| 22 | Nodakenetin | C14H14O4 | [89] | NMR/MS | 495-32-9 | |
| 23 | Nodakenetin tiglate | C19H20O5 | [105] | HPLC | 106974-21-4 | |
| 24 | Nodakenin | C26H34O14 | [106] | UPLC | 495-31-8 | |
| 25 | Oroselol | C14H12O4 | [90] | HPLC | 1891-25-4 | |
| 26 | Oxypeucedanin | C16H14O5 | [91] | NMR | 737-52-0 | |
| 27 | Oxypeucedanin hydrate | C16H16O16 | [91] | NMR | 133164-11-1 | |
| 28 | Peucedanoside A | C20H22O10 | [107] | NMR | 946122-87-8 | |
| 29 | Peucedanoside B | [107] | NMR | |||
| 30 | Praeroside I | [79] | NMR | |||
| 31 | Praeroside VII | [108] | NMR | |||
| 32 | Psoralen | C11H6O3 | 66-97-7 | |||
| 33 | Qianhucoumarin G | [109] | NMR | |||
| 34 | Rutarin | C20H24O10 | [79] | NMR | 20320-81-4 | |
| 35 | Sphondin | C12H8O4 | [110] | HPLC | 483-66-9 | |
| 36 | Xanthotoxin | C12H8O4 | [101] | HPLC | 298-81-7 | |
| Pyranocoumarin compounds | 37 | Decursinol angelate | C19H20O5 | [82] | UHPLC-MS/MS | 130848-06-5 |
| 38 | Decursitin D | C19H20O6 | [111] | HPLC | 245446-61-1 | |
| 39 | d-Laserpitin | C19H20O6 | [112] | UPLC-MS/MS | 134002-17-8 | |
| 40 | Longshengensin A | C21H22O7 | [113] | NMR/MS | ||
| 41 | Marmesin | C14H14O4 | [97] | UPLC-Q-TOF-HRMS | 13849-08-6 | |
| 42 | Peucedanocoumarin II | C21H22O7 | [114] | NMR | 130464-56-1 | |
| 43 | Praeruptorin A | C21H22O | [115] | HPLC/LC–MS/MS | 73069-25-7 | |
| 44 | Praeruptorin B | C24H26O | [73] | HPLC/LC–MS/MS | 81740-07-0 | |
| 45 | Praeruptorin C | C24H28O7 | [116] | HPLC/LC–MS/MS | 72463-77-5 | |
| 46 | Praeruptorin D | C24H26O | [117] | HPLC | ||
| 47 | Praeroside II | C20H24O10 | [118] | UPLC-HR-Orbitrap-MS | 86940-46-7 | |
| 48 | Pteryxin | C21H22O7 | [119] | NMR | 13161-75-6 | |
| 49 | Selinidin | C19H20O5 | [74] | UPLC-MS/MS | 19427-82-8 | |
| 50 | Suksdorfin | C21H24O7 | [120] | HPLC | 53023-17-9 | |
| 51 | (+)-samidin | C21H22O7 | [74] | HPLC | 477-33-8 | |
| Other constituents | 52 | Acetylatractylodinol | C15H12O3 | [111] | NMR | 61582-39-6 |
| 53 | Adenosine | C10H13N5O4 | [121] | NMR | 58-61-7 | |
| 54 | Aromadendrene | C15H24 | [122] | GC/MS | 109119-91-7 | |
| 55 | Baihuaqianhuoside | C6H12O6 | [86] | NMR | 155969-61-2 | |
| 56 | Butyric acid | C4H8O2 | [93] | NMR | 107-92-6 | |
| 57 | Daucosterol | C35H60O6 | [87] | NMR | 74-58-8 | |
| 58 | Galactitol | C6H14O6 | [87] | NMR | 608-66-2 | |
| 59 | Gallic acid | C7H6O5 | [86] | NMR | 149-91-7 | |
| 60 | Hinokitol | C10H12O2 | [123] | GC/MS | 499-44-5 | |
| 61 | Longifolene | C15H24 | [124] | GC/MS | 475-20-7 | |
| 62 | Mannitol | C6H14O6 | [125] | HPLC | 69-65-8 | |
| 63 | Palmitic acid | C16H32O2 | [126] | NMR | 57-10-3 | |
| 64 | Tanshinone I | C18H12O3 | [93] | NMR/MS | 568-73-0 | |
| 65 | Tanshinone IIA | C19H18O3 | [93] | NMR/MS | 568-72-9 | |
| 66 | Tetracosanoic acid | C24H48O2 | [126] | NMR/MS | 302912-17-0 | |
| 67 | Terpinolene | C10H16 | [127] | HS-SPME/GC-MS | 586-62-9 | |
| 68 | Vanillic acid | C8H8O4 | [86] | NMR | 121-34-6 | |
| 69 | α-farnesene | C15H24 | [128] | HS-SPME/GC-MS | 502-61-4 | |
| 70 | α-Pinene | C10H16 | [129] | HS-SPME/GC-MS | 7785-26-4 | |
| 71 | β-sitosterol | C29H50O | [87] | NMR | ||
| 72 | 2,6-dimethyl quinoline | C11H11N | [126] | NMR/MS | 877-43-0 | |
| 73 | 4H-1-benzopyran-4-one,5-hydroxy-6-methoxy-2-phenyl-7-O-α-D-glucuronyl acid | [91] | Reverse-phase silica gel column | |||
| 74 | 4H-1-benzopyran-4-one,5-hydroxy-6-methoxy-2-phenyl-7-O-α-D-glucuronyl methyl ester | [91] | Reverse-phase silica gel column | |||
| 75 | 9,10-dihydrophenanthrinic acid | [130] | NMR | |||
| 76 | (−)-sclerodin | C18H16O6 | [126] | NMR | 104855-18-7 |
7. Pharmacology
7.1. Eliminate Phlegm and Relieve Asthma
7.2. Anti-Inflammatory and Analgesic Properties
7.3. Cardiovascular Protection
7.4. Antineoplastic Activity
7.5. Other Pharmacological Effects
8. Safety and Toxicity
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, R.; Feng, L.; Sun, A.; Kong, L. Preparative isolation, and purification of coumarins from Peucedanum praeruptorum Dunn by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1057, 89–94. [Google Scholar] [CrossRef]
- Sheh, M.L. Peucedanum. In Flora of China; Missouri Botanical Garden Press: St. Louis, MO, USA, 1992; Volume 55, pp. 127–158. [Google Scholar]
- Sheh, M.L.; Watson, M.F. Peucedanum Linnaeus. In Flora of China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2005; Volume 14, pp. 182–192. [Google Scholar]
- Yamazaki, T. Umbelliferae in Japan II. J. Jpn. Bot. 2001, 76, 283–285. [Google Scholar]
- Chang, C.S.; Kim, H.; Chang, K.S. Provisional Checklist of Vascular Plants for the Korea Peninsula Flora (KPF); Designpost: Pajo, Philippines, 2014. [Google Scholar]
- Pimenov, M.G. Updated Checklist of Chinese Umbelliferae: Nomenclature, Synonymy, Typification, Distribution. Turczaninowia 2017, 20, 106–239. [Google Scholar] [CrossRef]
- Plunkett, G.M.; Pimenov, M.G.; Reduron, J.P.; Kljuykov, E.V.; van Wyk, B.E.; Ostroumova, T.A.; Henwood, M.J.; Tilney, P.M.; Spalik, K.; Watson, M.F.; et al. Apiaceae. In Flowering Plants. Eudicots: Apiales, Gentianales (Except Rubiaceae); Kadereit, J.W., Bittrich, V., Eds.; The Families and Genera of Vascular Plants; Springer International Publishing: Cham, Switzerland, 2018; Volume 15, pp. 9–206. [Google Scholar]
- Zhou, J.; Wang, W.; Liu, M.; Liu, Z. Molecular authentication of the traditional medicinal plant Peucedanum praeruptorum and its substitutes and adulterants by DNA-barcoding technique. Pharmacogn. Mag. 2014, 10, 385–390. [Google Scholar] [CrossRef]
- Song, Y.; Jing, W.; Yan, R.; Wang, Y. Research progress of the studies on the roots of Peucedanum praeruptorum dunn (Peucedani radix). Pak. J. Pharm Sci. 2015, 28, 71–81. [Google Scholar] [PubMed]
- Shi, T.T.; Zhang, X.B.; Zhang, K.; Guo, L.P.; Huang, L.Q. Study of extracting Peucedanum praeruptorum planted area in Ningguo of Anhui province based on multi-source and multi-phase image. China J. Chin. Mater. Med. 2017, 42, 4362–4367. [Google Scholar] [CrossRef]
- Song, L.Y.; Liu, J.S.; Tan, Y.J.; Zha, G.S. The present situation and prospect of the research on the Chinese Traditional Medicine Peucedanum praeruptorum Dunn. J. Yichun Univ. 2020, 42, 22–25. [Google Scholar] [CrossRef]
- Yang, C.Q.; Wu, Z.B.; Yang, T.J.; Deng, C.F. Herbal Textual Research on Growth Habit and distribution of Producing Area about Qianhu. Lishizhen Med. Mater. Medica Res. 2019, 30, 1471–1473. [Google Scholar] [CrossRef]
- Du, Y.; Zhou, J.; Guo, R. Imitation wild planting technology under forest of Peucedanum praeruptorum in Fangxian county. Heilongjiang Agric. Sci. 2021, 10, 139–141. [Google Scholar] [CrossRef]
- Liu, C.K.; Lei, J.Q.; Jiang, Q.P.; Zhou, D.S.; He, X.J. The complete plastomes of seven Peucedanum plants: Comparative and phylogenetic analyses for the Peucedanum genus. BMC Plant Biol. 2022, 22, 101. [Google Scholar] [CrossRef]
- Liu, J.L.; Wang, S.S.; Li, X.F.; Lu, X.Y.; Li, H.; Hong, C.X. Investigation and Analysis on the Present Situation of Peucedanum praeruptorum Resources in Its Producing Area. Mod. Agric. Sci. Technol. 2021, 1, 89–99. [Google Scholar] [CrossRef]
- Liu, J.S.; Song, L.Y.; Wang, R.Y.; Xu, F.W.; Zhang, C.Z. Analysis of the influencing factors and reduction measures of early extraction of Peucedanum praeruptorum Dunn. South Chin. Agric. 2022, 16, 84–87. [Google Scholar] [CrossRef]
- Qiu, X.X.; Zhang, L.; Yue, J.Y.; Wang, M. Investigation on Factors Affecting Contents of 3 Coumarins in Ningqianhu. J. Chin. Med. Mater. 2016, 39, 713–716. [Google Scholar] [CrossRef]
- Chen, X. Research on biological characteristics and artificial cultivation technology of Ningqian Hu. Anhui Agric. Sci. Bull. 2017, 23, 116–118. [Google Scholar] [CrossRef]
- Zheng, Y.; Jian, Q.P.; Zhao, R.Q.; Chen, Y.L.; Huang, C.; Liu, Z.P.; Xian, Z.Q.; Wan, M.; Jia, F.M.; Wang, G.Q.; et al. Effects of Different Planting Patterns on Yield and Profit of Peucedanum praeruptorum. Tillage Cultiv. 2019, 39, 31–32. [Google Scholar] [CrossRef]
- Liu, S.L.; Wang, X.H.; Gao, Y.G.; Zhao, Y.; Zhang, A.H.; Xu, Y.H.; Zhang, L.X. Transcriptomic analysis identifies differentially expressed genes (DEGs) associated with bolting and flowering in Saposhnikovia divaricata. Chin. J. Nat. Med. 2018, 16, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cui, X.; Jin, L.; Li, M.; Wei, J. Bolting reduces ferulic acid and flavonoid biosynthesis and induces root lignification in Angelica sinensis. Plant Physiol. Biochem. 2022, 170, 171–179. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Xiao, Y.; Zhou, G. Early Bolting, Yield, and Quality of Angelica sinensis (Oliv.) Diels Responses to Intercropping Patterns. Plants 2022, 11, 2950. [Google Scholar] [CrossRef]
- Xie, J.; Tang, X.; Xie, C.; Wang, Y.; Huang, J.; Jin, J.; Liu, H.; Zhong, C.; Zhou, R.; Ren, G.; et al. Comparative analysis of root anatomical structure, chemical components and differentially expressed genes between early bolting and unbolting in Peucedanum praeruptorum Dunn. Genomics 2023, 115, 110557. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Hao, Q.X.; Kang, L.P.; Zhang, Y.; Chen, M.L.; Wang, T.L.; Guo, L.P. Advance in studying early bolting of Umbelliferae medicinal plant. China J. Chin. Mater. Med. 2016, 41, 20–23. [Google Scholar] [CrossRef]
- Xie, J.; Zhen, L.P.; Ren, G.-X.; Tang, X.-Y.; Liu, H.; Zhong, C.; Jin, J.; Huang, J.-H.; Zhang, S.-H. Influence of early bolting on anatomical structure and coumarins of Peucedanum praeruptorum. China J. Chin. Mater. Med. 2020, 45, 4861–4866. [Google Scholar] [CrossRef]
- Luo, L.; Liu, X.; Jin, X.; Liu, Y.; Ma, J.; Zhang, S.; Zhang, D.; Chen, X.; Sheng, L.; Li, Y. Simultaneous determination of skimmin, apiosylskimmin, 7-hydroxycoumarin and 7-hydroxycoumarin glucuronide in rat plasma by liquid chromatography-Orbitrap mass spectrometry and its application to pharmacokinetics. Biomed. Chromatogr. 2022, 36, e5223. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Monakhos, S.G.; Lim, Y.P.; Yi, S.Y. Early Defense Mechanisms of Brassica oleracea in Response to Attack by Xanthomonas campestris pv. campestris. Plants 2021, 10, 2705. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.; Revanna, R.; Pither-Joyce, M.; Shaw, M.; Wright, K.; Thomson, S.; Moya, L.; Lee, R.; Macknight, R.; McCallum, J. Genetic analyses of bolting in bulb onion (Allium cepa L.). Theor. Appl. Genet. 2014, 127, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Rahim, M.A.; Zhao, Y.; Yang, S.; Wang, Z.; Su, H.; Li, L.; Niu, L.; Harun-Ur-Rashid, M.; Yuan, Y.; et al. Comparative Transcriptome Analysis of Early- and Late-Bolting Traits in Chinese Cabbage (Brassica rapa). Front. Genet. 2021, 12, 590830. [Google Scholar] [CrossRef]
- Wang, J.L.; Li, X.X.; Qiu, Y.; Zhang, X.H.; Shen, D.; Wang, H.P.; Sun, Y.Y.; Duan, M.M. Research Advances on Molecular Markers and Regulation Mechanism for the Bolting and Blooming Characteristics of Brassicaceae Vegetables. J. Plant Genet. Res. 2015, 16, 1283–1289. [Google Scholar] [CrossRef]
- Xu, L.; Liu, H.; Kilian, A.; Bhoite, R.; Liu, G.; Si, P.; Wang, J.; Zhou, W.; Yan, G. QTL Mapping Using a High-Density Genetic Map to Identify Candidate Genes Associated with Metribuzin Tolerance in Hexaploid Wheat (Triticum aestivum L.). Front. Plant Sci. 2020, 11, 573439. [Google Scholar] [CrossRef]
- Lou, P.; Zhao, J.; Kim, J.S.; Shen, S.; Del Carpio, D.P.; Song, X.; Jin, M.; Vreugdenhil, D.; Wang, X.; Koornneef, M.; et al. Quantitative trait loci for flowering time and morphological traits in multiple populations of Brassica rapa. J. Exp. Bot. 2007, 58, 4005–4016. [Google Scholar] [CrossRef]
- Zhao, J.; Kulkarni, V.; Liu, N.; Del Carpio, D.P.; Bucher, J.; Bonnema, G. BrFLC2 (FLOWERING LOCUS C) as a candidate gene for a vernalization response QTL in Brassica rapa. J. Exp. Bot. 2010, 61, 1817–1825. [Google Scholar] [CrossRef]
- Kawanabe, T.; Fujimoto, R. Inflorescence abnormalities occur with overexpression of Arabidopsis lyrata FT in the fwa mutant of Arabidopsis thaliana. Plant Sci. 2011, 181, 496–503. [Google Scholar] [CrossRef]
- Miao, X.Y.; Qu, H.P.; Han, Y.L.; He, C.F.; Qiu, D.W.; Cheng, Z.W. The protein elicitor Hrip1 enhances resistance to insects and early bolting and flowering in Arabidopsis thaliana. PLoS ONE 2019, 14, e0216082. [Google Scholar] [CrossRef] [PubMed]
- Hui, N.N.; Wang, L.; Li, J.P.; Qian, T.Y. Effects of 8 exogenous hormones on bolting and yield of Angelica sinensis. Gansu Agric. Sci. Tech. 2015, 12, 27–30. [Google Scholar] [CrossRef]
- Hou, K.; Chen, J.W.; Zhai, J.Y.; Shen, H.; Chen, L.; Wu, W. Effects of Plant Growth Regulators on the Growth and Development, Yield and Quality of Angelica dahurica. China J. Chin. Mater. Med. 2013, 38, 2082–2085. [Google Scholar]
- Sun, P.X.; Zhang, L.P.; Wu, D.; Zhang, W.; Huang, C.; Cheng, S.H.; Zhu, G.P.; Liu, P.W. Effects of GA3 and MeJA treatment on growth and physiological indexes of Chinese cabbage seedlings. Chin. Cucur. Veg. 2023, 36, 56–63. [Google Scholar] [CrossRef]
- Song, C.; Li, X.; Jia, B.; Liu, L.; Ou, J.; Han, B. De novo Transcriptome Sequencing Coupled with Co-expression Analysis Reveal the Transcriptional Regulation of Key Genes Involved in the Formation of Active Ingredients in Peucedanum praeruptorum Dunn Under Bolting Period. Front. Genet. 2021, 12, 683037. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Li, X.; Jia, B.; Liu, L.; Wei, P.; Manzoor, M.A.; Wang, F.; Li, B.Y.; Wang, G.; Chen, C.; et al. Comparative Transcriptomics Unveil the Crucial Genes Involved in Coumarin Biosynthesis in Peucedanum praeruptorum Dunn. Front. Plant Sci. 2022, 13, 899819. [Google Scholar] [CrossRef]
- Miao, X.S.; Cui, Q.Y.; Wang, Z.Y.; Liu, X.N.; Zhao, A.B.; Qiao, Y.J.; Wu, Z.S. Advance in quality assessment of Chinese materia medica using microscopic and morphological methods. Chin. J. Nat Med. 2017, 15, 653–663. [Google Scholar] [CrossRef]
- Shi, S.M.; Pan, M.J.; Wang, J.; Chen, C.Q. Application of molecular identification techniques in Chinese materia medica. J. Chin. Med. Mater. 2016, 47, 3121–3126. [Google Scholar] [CrossRef]
- Zhou, Y. Identification of Radix Peucedani and Its Adulterant Radix Peucedani Terebinthaceum. Chin. Pharm. 2008, 17, 62–63. [Google Scholar]
- Qi, Z.C.; Pi, E.; Zhang, X.D.; Möller, M.; Jiang, B.; Lu, H.F. Ontogenesis of D-type stomata, and cork-warts on the leaf epidermis of Camellia japonica (Theaceae) and functional assessment. Flora 2017, 228, 24–30. [Google Scholar] [CrossRef]
- Huang, D.L.; Xu, Y.Q.; Chen, X.K. Rapid identification of Pencedani praeruptorum and its counterfeit Saposhmikoviae divaricata by second derivative spectroscopy. J. ShaoGuan Univ. 2009, 30, 50–53. [Google Scholar] [CrossRef]
- Wang, F.; Jia, B.; Song, X.; Dai, J.; Li, X.; Gao, H.; Pan, H.; Yan, H.; Han, B. Rapid Identification of Peucedanum praeruptorum Dunn and Its Adulterants by Hand-Held Near-Infrared Spectroscopy. J. AOAC Int. 2022, 105, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, Y.; Cen, Y.; Yang, D.; Qi, Z.; Hou, Z.; Han, S.; Cai, Z.; Liu, K. Bivariate Correlation Analysis of the Chemometric Profiles of Chinese Wild Salvia miltiorrhiza Based on UPLC-Qqq-MS and Antioxidant Activities. Molecules 2018, 23, 538. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Luo, Y.; Chen, B.; Zheng, C.; Du, W.; Shi, X.; Guo, Z. Determination of Characteristic Volatile Component Fingerprint of Peucedanum praeruptorum Dunn at Different Harvest Periods Based on HS-GC-IMS. J. AOAC Int. 2023, 106, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Pan, J.; Li, X.; Li, J.; Liu, H.; Hou, Z.; Yang, Z.; Xu, L. Genome-wide identification of U-box gene family and expression analysis under abiotic stresses in Salvia miltiorrhiza. Biocell 2022, 46, 1751. [Google Scholar] [CrossRef]
- Choo, B.K.; Moon, B.C.; Ji, Y.; Kim, B.B.; Choi, G.; Yoon, T.; Kim, H.K. Development of SCAR markers for the discrimination of three species of medicinal plants, Angelica decursiva (Peucedanum decursivum), Peucedanum praeruptorum and Anthricus sylvestris, based on the internal transcribed spacer (ITS) sequence and random amplified polymorphic DNA (RAPD). Biol. Pharm. Bull. 2009, 32, 24–30. [Google Scholar] [CrossRef]
- Yang, Q.; Jiang, Y.; Wang, Y.; Han, R.; Liang, Z.; He, Q.; Jia, Q. SSR Loci Analysis in Transcriptome and Molecular Marker Development in Polygonatum sibiricum. Biomed. Res. 2022, 2022, 4237913. [Google Scholar] [CrossRef]
- Liu, T.S.; Lin, L.M.; Xiao, B.M.; Pan, Q.P.; Zhou, X.J. Identification of the Commercial Medicinal Materials “Qian-Hu” (Radix Peucedani) and Probing their Genetic Relations by RAPD Technique. China Assoc. Chin. Med. 2006, 24, 1838–1840. [Google Scholar] [CrossRef]
- Liu, Y.M.; Yi, Z.; Xiong, Y.X.; Xiu, Y.; Chen, K.L. Research on Genetic diversity of Peucedanum praeruptorum with ISSR markers method. Lishizhen Med. Mater. Medica Res. 2014, 25, 1982–1984. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.C.; Huang, Y.B.; Xiao, H.W.; Wu, J.J.; Qi, Z.C.; Wei, Y.K. Conservation Genomics of Wild Red Sage (Salvia miltiorrhiza) and Its Endangered Relatives in China: Population Structure and Interspecific Relationships Revealed From 2b-RAD Data. Front. Genet. 2021, 12, 688323. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.Y.; Jin, X.J.; Zhang, S.L.; Yang, J.W.; Fei, S.P.; Huang, Y.S.; Liu, Y.; Qi, Z.C.; Li, P. Smilax weniae, a New Species of Smilacaceae from Limestone Areas Bordering Guizhou and Guangxi, China. Plants 2022, 11, 1032. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.C.; Yu, Y.; Liu, X.; Pais, A.; Ranney, T.; Whetten, R.; Xiang, Q.Y. Phylogenomics of polyploid Fothergilla (Hamamelidaceae) by RAD-tag based GBS-Insights into species origin and effects of software pipelines. J. Syst. Evol. 2015, 53, 432–447. [Google Scholar] [CrossRef]
- Hou, D.Y.; Song, J.Y.; Yang, P.; Zhou, H.; Xin, T.Y.; Yao, H. Identification of Peucedanum praeruptorum and Peucedanum decursivum and Their Counterfeit Products Based on ITS2 Sequence. China J. Chin. Mater. Med. 2014, 39, 4186–4190. [Google Scholar] [CrossRef]
- Han, B.X.; Yuan, Y.; Huang, L.Q.; Zhao, Q.; Tan, L.L.; Song, X.W.; He, X.M.; Xu, T.; Liu, F.; Wang, J. Specific PCR Identification between Peucedanum praeruptorum and Angelica decursiva and Identification between Them and Adulterant Using DNA Barcode. Pharmacogn. Mag. 2017, 13, 38–45. [Google Scholar] [CrossRef]
- Coissac, E.; Hollingsworth, P.M.; Lavergne, S.; Taberlet, P. From barcodes to genomes: Extending the concept of DNA barcoding. Mol. Ecol. 2016, 25, 1423–1428. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Han, P.; Liu, H.; Gong, J.; Zhou, W.; Shi, B.; Liu, A.; Xu, L. Genome-wide investigation of bHLH genes and expression analysis under different biotic and abiotic stresses in Helianthus annuus L. Int. J. Biol. Macromol. 2021, 189, 72–83. [Google Scholar] [CrossRef]
- Li, Y.; Geng, M.; Xu, Z.; Wang, Q.; Li, L.; Xu, M.; Li, M. The complete plastome of Peucedanum praeruptorum (Apiaceae). Mitochondrial DNA B Resour. 2019, 4, 3612–3613. [Google Scholar] [CrossRef]
- Chu, S.; Chen, L.; Xie, H.; Xie, J.; Zhao, Y.; Tong, Z.; Xu, R.; Peng, H. Comparative analysis and chemical profiling of different forms of Peucedani radix. J. Pharmaceut. Biomed. 2020, 189, 113410. [Google Scholar] [CrossRef]
- Wang, F.Y.; You, H.Q.; Guo, Y.H.; Wei, Y.K.; Xia, P.G.; Yang, Z.Q.; Ren, M.; Guo, H.; Han, R.L.; Yang, D.F. Essential oils from three kinds of fingered citrons and their antibacterial activities. Ind. Crop. Prod. 2020, 147, 112172. [Google Scholar] [CrossRef]
- Chen, F.Y.; Tu, L.F.; Liu, D.P.; Ma, J.; Luo, Y.M. Chemical constituents from the roots of Peucedanum praeruptorum Dunn and their chemotaxonomic significance. Biochem. Syst. Ecol. 2021, 99, 104355. [Google Scholar] [CrossRef]
- Li, X.Y.; Zu, Y.Y.; Ning, W.; Tang, M.X.; Gong, C.; Niu, S.L.; Hua, H.M. A new xanthyletin-type coumarin from the roots of Peucedanum praeruptorum. J. Asian Nat. Prod. Res. 2020, 22, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.D.; Kim, J.Y.; Ryu, H.W.; Kim, J.H.; Han, S.I.; Ra, J.E.; Seo, K.H.; Jang, K.C.; Lee, J.H. Identification and characterisation of coumarins from the roots of Angelica dahurica and their inhibitory effects against cholinesterase. J. Funct. Foods 2013, 5, 1421–1431. [Google Scholar] [CrossRef]
- Brown, S.A. Biosynthesis of Coumarin and Herniarin in Lavender. Science 1962, 137, 977–978. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yue, Y.D.; Tang, F.; Guo, X.F. Coumarins from the leaves of Bambusa pervariabilis McClure. J. Asian Nat. Prod. Res. 2010, 12, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Chu, S.S.; Zhang, L.; Xie, J.; Dai, M.; Wu, X.; Peng, H.S. Tissue-Specific Metabolite Profiling on the Different Parts of Bolting and Unbolting Peucedanum praeruptorum Dunn (Qianhu) by Laser Microdissection Combined with UPLC-Q/TOF⁻MS and HPLC⁻DAD. Molecules 2019, 24, 1439. [Google Scholar] [CrossRef]
- Lee, S.O.; Choi, S.Z.; Lee, J.H.; Chung, S.H.; Park, S.H.; Kang, H.C.; Yang, E.Y.; Cho, H.J.; Lee, K.R. Antidiabetic coumarin and cyclitol compounds from Peucedanum japonicum. Arch. Pharm. Res. 2004, 27, 1207–1210. [Google Scholar] [CrossRef]
- Song, Y.L.; Jing, W.H.; Du, G.; Yang, F.Q.; Yan, R.; Wang, Y.T. Qualitative analysis and enantiospecific determination of angular type pyranocoumarins in Peucedani Radix using achiral and chiral liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2014, 1338, 24–37. [Google Scholar] [CrossRef]
- Song, Y.L.; Jing, W.H.; Yang, F.Q.; Shi, Z.; Yao, M.C.; Yan, R.; Wang, Y.T. Simultaneously enantiospecific determination of (+)-trans-khellactone, (+/−)-praeruptorin A, (+/−)-praeruptorin B, (+)-praeruptorin E, and their metabolites, (+/−)-cis-khellactone, in rat plasma using online solid phase extraction-chiral LC-MS/MS. J. Pharmaceut. Biomed. 2014, 88, 269–277. [Google Scholar] [CrossRef]
- Shehzad, O.; Khan, S.; Ha, I.J.; Park, Y.; Tosun, A.; Kim, Y.S. Application of stepwise gradients in counter-current chromatography: A rapid and economical strategy for the one-step separation of eight coumarins from Seseli resinosum. J. Chromatogr. A 2013, 1310, 66–73. [Google Scholar] [CrossRef]
- Shimizu, B. 2-Oxoglutarate-dependent dioxygenases in the biosynthesis of simple coumarins. Front. Plant Sci. 2014, 5, 549. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Wu, F.; Yan, Q.; Zhang, J.Y. Research progress on plant coumarin biosynthesis pathway and genes encoding the key enzymes. Acta Prataculturae Sin. 2022, 31, 217–228. [Google Scholar] [CrossRef]
- Wang, R.X.; Song, J.; Sun, B.; Yan, X.; Zhang, W.Z.; Zhao, C. Research Progress of Function and Biosynthesis of Coumarins. China Biotechnol. 2022, 42, 79–90. [Google Scholar]
- Fernández-Peña, L.; Matos, M.J.; López, E. Recent Advances in Biologically Active Coumarins from Marine Sources: Synthesis and Evaluation. Mar. Drugs 2022, 21, 37. [Google Scholar] [CrossRef]
- Okuyama, T.; Takata, M.; Shibata, S. Structures of Linear Furano- and Simple-Coumarin Glycosides of Bai-Hua Qian-Hu. Planta Med. 1989, 55, 64–67. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Z.; Cai, Y.; Dou, X.; Liang, Y.; Zhang, W.; Wu, G.; Ye, J. A novel strategy integrating gas phase fractionation with staggered mass range and LC-MS/MS molecular network for comprehensive metabolites profiling of Gui Ling Ji in rats. J. Pharm. Biomed. 2023, 222, 115092. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Kobayashi, A.; Kajiyama, S.; Kawazu, K.; Kanzaki, H.; Kim, C.M. Antimicrobial constituents of Angelica dahurica roots. Phytochemistry 1997, 44, 887–889. [Google Scholar] [CrossRef]
- Kim, S.J.; Ko, S.M.; Choi, E.J.; Ham, S.H.; Kwon, Y.D.; Lee, Y.B.; Cho, H.Y. Simultaneous Determination of Decursin, Decursinol Angelate, Nodakenin, and Decursinol of Angelica gigas Nakai in Human Plasma by UHPLC-MS/MS: Application to Pharmacokinetic Study. Molecules 2018, 23, 1019. [Google Scholar] [CrossRef]
- Kimura, Y.; Okuda, H. Histamine-Release Effectors from Angelica dahurica var. dahurica Root. J. Nat. Prod. 1997, 60, 249–251. [Google Scholar] [CrossRef]
- Song, Y.L.; Jing, W.H.; Chen, Y.G.; Yuan, Y.F.; Yan, R.; Wang, Y.T. 1H nuclear magnetic resonance based-metabolomic characterization of Peucedani Radix and simultaneous determination of praeruptorin A and praeruptorin B. J. Pharmaceut. Biomed. 2014, 93, 86–94. [Google Scholar] [CrossRef]
- Piplani, P.; Jain, A.; Devi, D.; Anjali; Sharma, A.; Silakari, P. Design, synthesis and pharmacological evaluation of some novel indanone derivatives as acetylcholinesterase inhibitors for the management of cognitive dysfunction. Bioorg. Med. Chem. 2018, 26, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.Y.; Li, Y.; Pei, Y.H.; Yu, R.M.; Min, Z.D.; Zhu, T.R. Isolation and structure elucidation of BaiHuaQianHuoside and PD-C-I from Peucedanum praeruptorum. Acta Pharm. Sin. 1994, 29, 276–280. [Google Scholar] [CrossRef]
- Kong, L.Y.; Pei, Y.H.; Li, X.; Zhu, T.R.; Ao, S.C. Isolation, and structure elucidation of QianHuCoumarin A. Acta Pharm. Sin. 1993, 28, 432–436. [Google Scholar] [CrossRef]
- Chen, Z.X.; Huang, B.S.; She, Q.L.; Zeng, G.F. The chemical constituents of Bai-Hua-Qian-Hu, the root of Peucedanum Praeruptorum Dunn. (Umbelliferae)—Four new coumarins. Acta. Pharmacol. Sin. 1979, 14, 486–496. [Google Scholar] [CrossRef]
- Asahara, T.; Sakakibara, I.; Okuyama, T.; Shibata, S. Studies on Coumarins of a Chinese Drug “Qian-Hu” V. Coumarin-glycosides from “Zi-Hua Qian-Hu”1. Planta Med. 1984, 50, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, C.; Okuyama, T.; Shibata, S.; Iitaka, Y. Studies on Coumarins of a Chinese Drug “Qian-Hu”; VI1, Coumarins of Angelica edulis. Planta Med. 1984, 50, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiao, Y.Q.; Taniguchi, M.; Baba, K. Studies on chemical constituents in roots of Peucedanum praeruptorum V. China J. Chin. Mater. Med. 2012, 37, 3573–3576. [Google Scholar] [CrossRef]
- Yu, N.J.; Liu, S.J.; Liang, Y.M.; Fang, C.W.; Xie, L.L.; Ma, K.; Li, W.J. A Comparison of Chemical Components of Volatile Oils from the Decocting Pieces of Peucedanum praeruptorum in Different Regions. J. Anhui Colg. 2007, 26, 44–45. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, Y.Q.; Taniguchi, M.; Baba, K. Studies on chemical constituents in roots of Peucedanum praeruptorum III. China J. Chin. Mater. Med. 2009, 34, 1005–1006. [Google Scholar] [CrossRef]
- Ishii, H.; Okada, Y.; Baba, M.; Okuyama, T. Studies of coumarins from the Chinese drug Qianhu, XXVII: Structure of a new simple coumarin glycoside from Bai-Hua Qianhu, Peucedanum praeruptorum. Chem. Pharm. Bull. 2008, 56, 1349–1351. [Google Scholar] [CrossRef]
- Yamazaki, T.; Tokiwa, T. Isofraxidin, a coumarin component from Acanthopanax senticosus, inhibits matrix metalloproteinase-7 expression and cell invasion of human hepatoma cells. Biol. Pharm. Bull. 2010, 33, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zhang, Y.; Wang, G.; Ji, M.; Wang, B.; He, G.; Wang, Q.; Bai, F.; Xu, K.; Yuan, D.; et al. Conduction of a chemical structure-guided metabolic phenotype analysis method targeting phenylpropane pathway via LC-MS: Ginkgo biloba and soybean as examples. Food Chem. 2022, 390, 133155. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.H.; Zhao, X.J.; Chen, X.; Zhang, Y.H.; Wang, C.Q.; Zhao, Q.Y.; Jiao, B.N. Ultra-performance liquid chromatography-quadrupole-time-of-flight high-resolution mass spectrometry method for rapid screening of bioactive compounds in lemon fruits. Food Ferment. Ind. 2021, 47, 222–230. [Google Scholar] [CrossRef]
- Rodríguez-Domínguez, J.; Kirsch, G. Zirconyl chloride: A useful catalyst in the pechmann coumarin synthesis. Synth. Stuttg. 2006, 2006, 1895–1897. [Google Scholar] [CrossRef]
- Seo, U.M.; Zhao, B.T.; Kim, Y.H.; Kang, J.S.; Son, J.K.; Woo, M.H.; Woo, M. Simultaneous analysis of seven marker compounds from Saposhnikoviae Radix, Glehniae Radix and Peucedani Japonici Radix by HPLC/PDA. Arch. Pharm. Res. 2016, 39, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N. Coumarins of Lovage Roots (Levisticum officinale W.D.J.Koch): LC-MS Profile, Quantification, and Stability during Postharvest Storage. Metabolites 2023, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, S.; Si, J.; Zhao, Y.; Zhao, M.; Ji, E. Shashen-Maidong Decoction inhibited cancer growth under intermittent hypoxia conditions by suppressing oxidative stress and inflammation. J. Ethnopharmacol. 2022, 299, 115654. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, L.; Shi, X.; Mei, W.; Huang, Z.; Zhang, L.; Li, X.; Xu, B.; Zhang, L.; Wang, P. Imperatorin alleviated NLR family pyrin domain-containing 3 inflammasome cascade-induced synovial fibrosis and synovitis in rats with knee osteoarthritis. Bioengineered 2021, 12, 12954–12964. [Google Scholar] [CrossRef]
- Li, G.; Xiang, S.; Pan, Y.; Long, X.; Cheng, Y.; Han, L.; Zhao, X. Effects of Cold-Pressing and Hydrodistillation on the Active Non-volatile Components in Lemon Essential Oil and the Effects of the Resulting Oils on Aging-Related Oxidative Stress in Mice. Front. Nutr. 2021, 8, 689094. [Google Scholar] [CrossRef]
- Wang, K.; Nie, Z.X.; Sun, Y.P.; Liu, S.Z.; Wang, G.K. A Study on Chemical Components of Peucedanum praeruptorum Dunn. Anhui Med. J. 2018, 37, 83–85. [Google Scholar] [CrossRef]
- Liu, R.; Sun, Q.; Shi, Y.; Kong, L. Isolation and purification of coumarin compounds from the root of Peucedanum decursivum (Miq.) Maxim by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1076, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, M.; Sun, L.; Jiang, X.; Zhao, M.; Zhao, C. Rapid characterization of the chemical constituents of Sanhua decoction by UHPLC coupled with Fourier transform ion cyclotron resonance mass spectrometry. Rsc. Adv. 2020, 10, 26109–26119. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Okada, Y.; Okuyama, T.; Tu, P. 1H and 13C NMR assignments for two new angular furanocoumarin glycosides from Peucedanum praeruptorum. Magn. Reson. Chem. 2007, 45, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.T.; Okada, Y.; Ma, T.J.; Okuyama, T.; Tu, P.F. Two new coumarin glycosides from Peucedanum praeruptorum. J. Asian Nat. Prod. Res. 2008, 10, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.Y.; Li, Y.; Min, Z.D.; Li, X.; Zhu, T.R. Coumarins from Peucedanum praeruptorum. Phytochemistry 1996, 41, 1423–1426. [Google Scholar] [CrossRef]
- Nitao, J.K.; Berhow, M.; Duval, S.M.; Weisleder, D.; Vaughn, S.F.; Zangerl, A.; Berenbaum, M.R. Characterization of furanocoumarin metabolites in parsnip webworm, Depressaria pastinacella. J. Chem. Ecol. 2003, 29, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiao, Y.Q.; Taniguchi, M.; Baba, K. Studies on chemical constituents in roots of Peucedanum praeruptorum. China J. Chin. Mater. Med. 2005, 30, 675–676. [Google Scholar] [CrossRef]
- Lee, J.H.; Mei, H.C.; Kuo, I.C.; Lee, T.H.; Chen, Y.H.; Lee, C.K. Characterizing Tyrosinase Modulators from the Roots of Angelica keiskei Using Tyrosinase Inhibition Assay and UPLC-MS/MS as the Combinatorial Novel Approach. Molecules 2019, 24, 3297. [Google Scholar] [CrossRef]
- Huang, P.; Zheng, X.Z.; Xi, Z.M.; Zhong, X.Q. Isolation and structure elucidation of longshengensin A from Peucedanum longshengense. Acta Pharmacol. Sin. 1997, 32, 62–64. [Google Scholar] [CrossRef]
- Takata, M.; Shibata, S.; Okuyama, T. Structures of Angular Pyranocoumarins of Bai-Hua Qian-Hu, the Root of Peucedanum praeruptorum1. Planta Med. 1990, 56, 307–311. [Google Scholar] [CrossRef]
- Ding, X.; Li, L.; Wang, Y.; Chen, J.; Huang, Y.; Xu, K. Design of guanidinium ionic liquid-based microwave-assisted extraction for the efficient extraction of Praeruptorin A from Radix peucedani. J. Sep. Sci. 2014, 37, 3539–3547. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Chen, G.; Liu, H. Simultaneous Quantification of Three Pyranocoumarins of Peucedanum praeruptorum in Rat Plasma by Liquid Chromatography-Tandem Mass Spectrometry: Application to Pharmacokinetic Study. J. Chromatogr. Sci. 2015, 53, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Yue, W.; Du, X.; Ren, L.; Li, Q. Pharmacokinetics and Tissue Distribution Study of Praeruptorin D from Radix Peucedani in Rats by High-Performance Liquid Chromatography (HPLC). IJMS 2012, 13, 9129–9141. [Google Scholar] [CrossRef] [PubMed]
- Hisamoto, M.; Kikuzaki, H.; Ohigashi, H.; Nakatani, N. Antioxidant compounds from the leaves of Peucedanum japonicum thunb. J. Agric. Food Chem. 2003, 51, 5255–5261. [Google Scholar] [CrossRef] [PubMed]
- Nugara, R.N.; Inafuku, M.; Takara, K.; Iwasaki, H.; Oku, H. Pteryxin: A coumarin in Peucedanum japonicum Thunb leaves exerts antiobesity activity through modulation of adipogenic gene network. Nutrition 2014, 30, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Willette, R.E.; Soine, T.O. Coumarins i. isolation, purification, and structure determination of pteryxin and suksdorfin. J. Pharm. 2010, 51, 149–156. [Google Scholar] [CrossRef]
- Jacobson, K.A.; van Galen, P.J.; Williams, M. Adenosine receptors: Pharmacology, structure-activity relationships, and therapeutic potential. J. Med. Chem. 1992, 35, 407–422. [Google Scholar] [CrossRef]
- Giuliani, C.; Pieraccini, G.; Santilli, C.; Tani, C.; Bottoni, M.; Schiff, S.; Fico, G.; Papini, A.; Falsini, S. Anatomical Investigation and GC/MS Analysis of ‘Coco de Mer’, Lodoicea maldivica (Arecaceae). Chem. Biodivers. 2020, 17, e2000707. [Google Scholar] [CrossRef]
- Nakanishi, K. Tetsuo Nozoe’s “Autograph Books by Chemists 1953–1994”: An essay. Chem. Rec. 2013, 13, 343–352. [Google Scholar] [CrossRef]
- Szmigielski, R.; Cieslak, M.; Rudziński, K.J.; Maciejewska, B. Identification of volatiles from Pinus silvestris attractive for Monochamus galloprovincialis using a SPME-GC/MS platform. Environ. Sci. Pollut. Res. Int. 2011, 19, 2860–2869. [Google Scholar] [CrossRef][Green Version]
- Nolan, S.J.; Thornton, J.; Murray, C.S.; Dwyer, T. Inhaled mannitol for cystic fibrosis. Cochrane Database Syst. Rev. 2015, CD008649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiao, Y.Q.; Taniguchi, M.; Baba, K. Studies on chemical constituents in roots of Peucedanum praeruptorum II. China J. Chin. Mater. Med. 2006, 31, 1333–1334. [Google Scholar] [CrossRef]
- de Sousa, D.B.; da Silva, G.S.; Serrano, L.A.L.; Martins, M.V.V.; Rodrigues, T.H.S.; Lima, M.A.S.; Zocolo, G.J. Metabolomic Profile of Volatile Organic Compounds from Leaves of Cashew Clones by HS-SPME/GC-MS for the Identification of Candidates for Anthracnose Resistance Markers. J. Chem. Ecol. 2023, 49, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Z.; Yu, H.T.; Yu, X.Y.; Zhu, L.J.; Yu, Z.F. Comparison of volatile compounds in Chrysanthemum nankingense during storage based on HS-SPME-GC-MS and E-nose. J. Food Meas. Charact. 2023, 17, 3134–3148. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef]
- Zhang, C.; Li, L.; Xiao, Y.Q.; Li, W.; Yin, X.J.; Tian, G.F. A new phenanthraquinone from the roots of Peucedanum praeruptorum. Chin. Chem. Lett. 2010, 21, 816–817. [Google Scholar] [CrossRef]
- Guan, F.L.; Jin, W.B.; Zhang, X.H.; Zhao, N.C. The Effects of Praeruptorin A on the Contraction of isolated Tracheal Smooth Muscle Previously Induced by High K and Acetylcholine. J. Chin. Med. Univ. 1994, 023, 549–552. [Google Scholar]
- Xiong, Y.Y.; Wang, J.S.; Wu, F.H.; Li, J.; Zhou, L.; Kong, L.Y. Effects of (±)-praeruptorin A on airway inflammation, airway hyperresponsiveness and NF-κB signaling pathway in a mouse model of allergic airway disease. Eur. J. Pharmacol. 2012, 683, 316–324. [Google Scholar] [CrossRef]
- Xiong, Y.Y.; Wang, J.S.; Wu, F.H.; Li, J.; Zhou, L.; Kong, L.Y. The effects of (±)-Praeruptorin A on airway inflammation, remodeling and transforming growth factor-β1/Smad signaling pathway in a murine model of allergic asthma. Int. Immunopharmacol. 2012, 14, 392–400. [Google Scholar] [CrossRef]
- Bayrami, G.; Boskabady, M.H.; Iranshahi, M.; Gholamnezhad, Z. Relaxant effects of asafoetida extract and its constituent umbelliprenin on guinea-pig tracheal smooth muscle. Chin. J. Integr. Med. 2013. online ahead of publishing. [Google Scholar] [CrossRef]
- Wang, D.C.; Zhang, X.Z.; Feng, L. Antioxidative Effects of Total Coumarins from Peucedanum praeruptorum Dunn invitro. Her. Med. 2008, 27, 899–901. [Google Scholar] [CrossRef]
- Ozaki, H.; Nishidono, Y.; Fujii, A.; Okuyama, T.; Nakamura, K.; Maesako, T.; Shirako, S.; Nakatake, R.; Tanaka, K.; Ikeya, Y.; et al. Identification of Anti-Inflammatory Compounds from Peucedanum praeruptorum Roots by Using Nitric Oxide-Producing Rat Hepatocytes Stimulated by Interleukin 1β. Molecules 2023, 28, 5076. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Epifano, F.; Genovese, S.; Marcotullio, M.C.; Sosa, S.; Tubaro, A. Antiinflammatory activity of coumarins from Ligusticum lucidum Mill. subsp. cuneifolium (Guss.) Tammaro (Apiaceae). Phytother. Res. 2010, 24, 1697–1699. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.J.; Ci, W.; Wang, G.F.; Zhang, J.Y.; Wu, S.Y.; Xu, W.; Jin, H.; Zhu, Z.G.; Zhang, J.J.; Pang, J.X.; et al. Praeruptorin A inhibits lipopolysaccharide-induced inflammatory response in murine macrophages through inhibition of NF-κB pathway activation. Phytother. Res. 2011, 25, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, X.B.; Yang, Q.; Zhang, K.; Zhang, N.; Guo, Y.Y.; Feng, B.; Zhao, M.G.; Wu, Y.M. The neuroprotective effect of praeruptorin C against NMDA-induced apoptosis through down-regulating of GluN2B-containing NMDA receptors. Toxicol Vitro 2013, 27, 908–914. [Google Scholar] [CrossRef]
- Yu, P.J.; Jin, H.; Zhang, J.Y.; Wang, G.F.; Li, J.R.; Zhu, Z.G.; Tian, Y.X.; Wu, S.Y.; Xu, W.; Zhang, J.J.; et al. Pyranocoumarins Isolated from Peucedanum praeruptorum Dunn Suppress Lipopolysaccharide-Induced Inflammatory Response in Murine Macrophages Through Inhibition of NF-κB and STAT3 Activation. Inflammation 2012, 35, 967–977. [Google Scholar] [CrossRef]
- Chang, T.H.; Liu, X.Y.; Zhang, X.H.; Wang, H.L. Effects of dl-praeruptorin A on interleukin-6 level and Fas, bax, bcl-2 protein expression in ischemia-reperfusion myocardium. Acta Pharmacol. Sin. 2002, 23, 769–774. [Google Scholar]
- Wang, W.J.; Lu, H.Q.; Sun, L.M.; Zhong, P.; Sun, G. Effects of praeruptorin C on blood pressure and expression of phospholamban in spontaneously hypertensive rats. Phytomedicine 2014, 21, 195–198. [Google Scholar] [CrossRef]
- Sun, L.; Rao, M.R.; Liu, P.Q. Effects of praeruptorin C on cardiac dysfunction, myocardial compliance, and collagen content in renovascular hypertensive rats. Acta Pharm. Sin. 1997, 32, 578–582. [Google Scholar] [CrossRef]
- Rao, M.R.; Liu, W.B.; Liu, P.Q. Effects of praeruptorin C on vascular hypertrophy, [Ca2+] i, collagen content and NO in renovascular and spontaneously hypertensive rats. J. Chin. Pharm. Sci. 2002, 11, 111–114. [Google Scholar] [CrossRef]
- Rao, M.R.; Sun, L.; Zhang, X.W. Effects of praeruptorum coumarin on heart hemodynamics, myocardial compliance, and collagen content in heart hypertrophy rats. Chin. J. Pharmacol. Toxicol. 2002, 16, 265–269. [Google Scholar] [CrossRef]
- Joa, H.; Vogl, S.; Atanasov, A.G.; Zehl, M.; Nakel, T.; Fakhrudin, N.; Heiss, E.H.; Picker, P.; Urban, E.; Wawrosch, C.; et al. Identification of ostruthin from Peucedanum ostruthium rhizomes as an inhibitor of vascular smooth muscle cell proliferation. J. Nat. Prod. 2011, 74, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Yue, W.; Li, Q. Chemopreventive Effects of Peucedanum praeruptorum Dunn and Its Major Constituents on SGC7901 Gastric Cancer Cells. Molecules 2010, 15, 8060–8071. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Lin, C.L.; Chiou, H.L.; Yang, S.F.; Lin, C.Y.; Liu, C.J.; Hsieh, Y.H. Praeruptorin A Inhibits Human Cervical Cancer Cell Growth and Invasion by Suppressing MMP-2 Expression and ERK1/2 Signaling. Int. J. Mol Sci. 2017, 19, 10. [Google Scholar] [CrossRef]
- Lin, C.L.; Hung, T.W.; Ying, T.H.; Lin, C.J.; Hsieh, Y.H.; Chen, C.M. Praeruptorin B Mitigates the Metastatic Ability of Human Renal Carcinoma Cells through Targeting CTSC and CTSV Expression. IJMS 2020, 21, 2919. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Okuyama, T.; Takata, M.; Shibata, S.; Tokuda, H.; Takayasu, J.; Hasegawa, T.; Nishino, A.; Ueyama, H.; Iwashima, A. Studies on the anti-tumor-promoting activity of naturally occurring substances. IV. Pd-II [(+) anomalin, (+) praeruptorin B], a seselin-type coumarin, inhibits the promotion of skin tumor formation by 12-O-tetradecanoylphorbol-13-acetate in 7,12-dimethylbenz[a]anthracene-initiated mice. Carcinogenesis 1990, 11, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Shen, H.T.; Lin, Y.A.; Yu, Y.L.; Chen, Y.S.; Liu, C.J.; Hsieh, Y.H. Antiproliferative and Antimetastatic Effects of Praeruptorin C on Human Non–Small Cell Lung Cancer through Inactivating ERK/CTSD Signalling Pathways. Molecules 2020, 25, 1625. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Bi, H.; Jin, J.; Niu, L.; Cai, D.; Deng, R.; Li, Y.; Wang, Y.; Huang, M. Effects of praeruptorin A and praeruptorin C, a racemate isolated from Peucedanum praeruptorum, on MRP2 through the CAR pathway. Planta Med. 2013, 79, 1641–1647. [Google Scholar] [CrossRef]
- Yu, P.J.; Li, J.R.; Zhu, Z.G.; Kong, H.Y.; Jin, H.; Zhang, J.Y.; Tian, Y.X.; Li, Z.H.; Wu, X.Y.; Zhang, J.J.; et al. Praeruptorin D and E attenuate lipopolysaccharide/hydrochloric acid induced acute lung injury in mice. Eur. J. Pharmacol. 2013, 710, 39–48. [Google Scholar] [CrossRef]
- Liu, X.; Chin, J.F.; Qu, X.; Bi, H.; Liu, Y.; Yu, Z.; Zhai, Z.; Qin, A.; Zhang, B.; Dai, M. The Beneficial Effect of Praeruptorin C on Osteoporotic Bone in Ovariectomized Mice via Suppression of Osteoclast Formation and Bone Resorption. Front. Pharmacol. 2017, 8, 627. [Google Scholar] [CrossRef]
- Han, S.; Li, C.; Huang, J.; Wei, F.; Zhang, Y.; Wang, S. Cell membrane chromatography coupled with UHPLC-ESI-MS/MS method to screen target components from Peucedanum praeruptorum Dunn acting on α1A adrenergic receptor. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1011, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, Y.; Huang, W.; He, L.; Lin, Z.; Zhou, J.; He, Q. Toxic effects of terpinolene on Microcystis aeruginosa: Physiological, metabolism, gene transcription, and growth effects. Sci. Total Environ. 2020, 719, 137376. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.I.; Lee, J.H.; Lee, Y.C.; Kim, K.S. Antiplasmodial and cytotoxic activity of coumarin derivatives from dried roots of Angelica gigas Nakai in vitro. Immunopharmacol. Immunotoxicol. 2011, 33, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, F.; Boskabady, M.H. A review of the relaxant effect of various medicinal plants on tracheal smooth muscle, their possible mechanism(s) and potency. J. Ethnopharmacol. 2015, 175, 528–548. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kam, K.W.; Zhou, J.J.; Yan, W.Y.; Chen, M.; Wu, S.; Wong, T.M. Effects of heat shock protein 70 activation by metabolic inhibition preconditioning or kappa-opioid receptor stimulation on Ca2+ homeostasis in rat ventricular myocytes subjected to ischemic insults. J. Pharmacol. Exp. Ther. 2004, 310, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, F.; Wang, S.; Xiao, H.; Wang, J.; Li, X.; Yang, H. Endothelium-dependent vasorelaxant effects of praeruptorin a in isolated rat thoracic aorta. Bioengineered 2022, 13, 10038–10046. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.G.; Donnet, C.; Bogaev, R.C.; Blatt, R.J.; McKinney, C.E.; Day, K.H.; Berr, S.S.; Jones, L.R.; Moorman, J.R.; Sweadner, K.J.; et al. Hypertrophy, increased ejection fraction, and reduced Na-K-ATPase activity in phospholemman-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1982–H1988. [Google Scholar] [CrossRef]
- Wang, S.F.; Chen, P.H.; Jiang, W.; Wu, L.; Chen, L.H.; Fan, X.H.; Wang, Y.; Cheng, Y.Y. Identification of the effective constituents for anti-inflammatory activity of Ju-Zhi-Jiang-Tang, an ancient traditional Chinese medicine formula. J. Chromatogr. A. 2014, 1348, 105–124. [Google Scholar] [CrossRef]
- Jung, J.W.; Kim, N.J.; Yun, H.; Han, Y.T. Recent Advances in Synthesis of 4-Arylcoumarins. Molecules 2018, 23, 2417. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, N.; Sui, Z.; Huang, C.; Zeng, Z.; Kong, L. The Molecular and Structural Basis of O-methylation Reaction in Coumarin Biosynthesis in Peucedanum praeruptorum Dunn. Int. J. Mol. Sci. 2019, 20, 1533. [Google Scholar] [CrossRef]
- Kong, L.Y.; Wu, X.L.; Min, Z.D. Semi-synthesis of derivatives with C-3’ and C-4’trans-configuration from (+)-praeruptorin A. Acta Pharm. Sin. 2003, 38, 358–363. [Google Scholar] [CrossRef]
- Ban, X.X.; Jiang, A.W.; Du, P.S.; Liu, Y.N. Study on Acute Toxicity of Methanol Extract from Peucedanum praeruptorum Dunn (Pd) in Mice. J. Tradit. Chin. Med. 2015, 21, 49–51. [Google Scholar] [CrossRef]
- Hu, S.; Wang, P.; Chen, Y.; Kuang, G.; Wang, C.; Luo, J.; Chen, S. In Vitro Investigation of the Anticancer Activity of Peucedanum praeruptorum Dunn Extract on HepG2 Human Hepatoma Cells. J. Food Qual. 2023, 2023, 9010344. [Google Scholar] [CrossRef]
- Zhu, W.J.; Liu, Y.; Cao, Y.N.; Peng, L.X.; Yan, Z.Y.; Zhao, G. Insights into Health-Promoting Effects of Plant MicroRNAs: A Review. J. Agric. Food Chem. 2021, 69, 14372–14386. [Google Scholar] [CrossRef] [PubMed]
- Villani, V.; Di Marco, G.; Iacovelli, F.; Pietrucci, D.; Canini, A.; Gismondi, A. Profile and potential bioactivity of the miRNome and metabolome expressed in Malva sylvestris L. leaf and flower. BMC Plant Biol. 2023, 23, 439. [Google Scholar] [CrossRef]
- Gismondi, A.; Di Marco, G.; Canini, A. Detection of plant microRNAs in honey. PLoS ONE 2017, 12, e0172981. [Google Scholar] [CrossRef]
- Samad, A.F.A.; Kamaroddin, M.F.; Sajad, M. Cross-Kingdom Regulation by Plant microRNAs Provides Novel Insight into Gene Regulation. Adv. Nutr. 2021, 12, 197–211. [Google Scholar] [CrossRef]
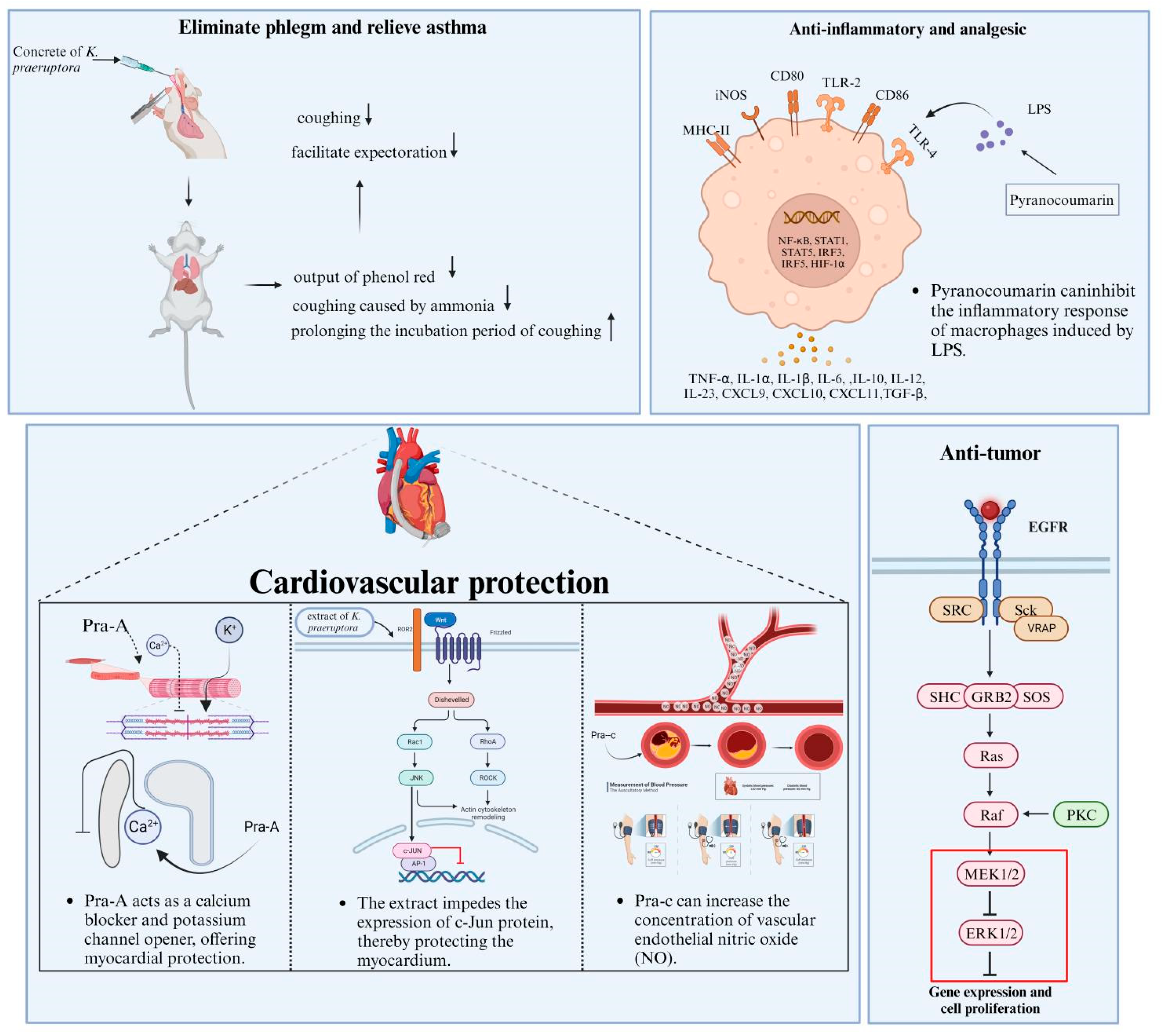
| Pharmacological Activity | Compounds/Extracts | Material/Model | Treatment | Results | References |
|---|---|---|---|---|---|
| Eliminate phlegm and relieve asthma | Praeruptorin A | Rabbits/trachea extricated and immersed in a nutritive solution containing a mixed gas | In vivo 30 µmol/L 0.5 h | ↓ Ca+ and PDC | [131] |
| Mice/chronic asthma model | In vivo 30, 60, 120 mg/kg 5 d | ↓ (IL)-4, IL-5, IL-13, LTC4 and (Ig) E, eotaxin protein and mRNA expression | [132] | ||
| Mice/chronic asthma model | In vivo 30, 60, 120 mg/kg 56 d | ↓ IL-4 and IL-13 in BALF, IgE, TGF-β1 and pSmad2/3 expression | [133] | ||
| Honey-roasted K. praeruptora pieces | Mice/histamine phosphate asthma model | In vivo 2, 4, 8 g/kg 3 d | ↑ Secretion of phenol red | [130] | |
| Coumarin constituent | Rabbits | In vivo 2, 5, 10 mg/mL | ↓ KCl and acetylcholine level | [134] | |
| Anti-inflammatory and analgesic | TCP | Mice | In vivo 31.3, 62.5, 125, 250, 500 µg/mL | ↓ MDA | [135] |
| Praeruptorin A, B, and E | Rat hepatocytes | In vivo 1 nmol/L | ↓ NO synthase expression ↓ mRNAs encoding | [136] | |
| Praeruptorin A | Croton oil-induced ear dermatitis in mice | In vivo 0.3 μmol/cm2 | ↓ 22–43% oedema reduction | [137] | |
| Macrophage cells | In vitro 6.25, 12.5, 25 mg/mL 24 h | ↓ NO, IL-1β and TNF-α, mRNA expressions, iNOSand TNF-α | [138] | ||
| Mice/chronic asthma model | In vivo 30, 60, 120 mg/kg 5 d | ↓ (IL)-4, IL-5, IL-13, LTC4 and (Ig) E, eotaxin protein and mRNA expression | [132] | ||
| Mice/chronic asthma model | In vivo 30, 60, 120 mg/kg 56 d | ↓ IL-4 and IL-13 in BALF, IgE, TGF-β1 and pSmad2/3 expression | [133] | ||
| Praeruptorin C | Cortical neuron cells | In vitro 0, 0.1, 1, 10 µmol/L | ↓ NMDA and Bcl-2 | [139] | |
| Praeruptorin C, D, and E | Macrophage cells | In vitro 4, 8, 16 μg/mL 18 h | ↓ NO, IL-6 and TNF-α, mRNA and r κB-α protein expression | [140] | |
| Cardiovascular protection | Praeruptorin A | Open-chest anesthetized rats | In vivo 2 mg/mL 18 h | ↓ IL-6, Fas, bax and bcl-2 level | [141] |
| Praeruptorin C | Spontaneously hypertensive rats | In vivo 20 mg/kg 48 d | ↓ PLB mRNA expression ↑ HMI and LVMI | [142] | |
| Renovascular hypertensive rats/2K1C-RHR | In vivo 20 mg/kg 63 d | ↑CF/HWW, CO/HWW, LVSP, -dp/d tmax value ↓ LVEDP and T value | [143] | ||
| Renovascular and spontaneously hypertensive rats | In vivo 20 mg/kg 63 d | ↑ Ca2+ and NO level | [144] | ||
| Cortical neuron cells | In vitro 0, 0.1, 1, 10 µmol/L | ↓ NMDA and Bcl-2 | [140] | ||
| Praeruptorin A, B, C, and D | Heart hypertrophy rats/2K1C-RHR | In vivo 30 mg/kg 63 d | ↑-dp/d tmax value ↓ LVEDP and HYP level | [145] | |
| Ostruthin | Vascular smooth muscle cells | In vitro 1, 3, 10, 30 µmol/L 20 h | ↓ VSMC proliferation concentration | [146] | |
| Anti-tumor effect | Methanolic extract | SGC7901 gastric cancer cells | In vitro 50, 100, 200, 300 µg/mL 24 h | ↑ LDH ↓ Cell proliferation | [147] |
| Praeruptorin A | HeLa and SiHa cells | In vitro 0, 10, 20, 30, 40, 50 µmol/L 24 h | ↓ MMP-2, cyclin D1, Skp2, ERK1/2 ↑ TIMP-2, Rb, p16, p21, p27, | [148] | |
| Praeruptorin B | Renal cell carcinoma | In vitro 0, 10, 20, 30, 40, 50 µmol/L 24 h | ↓ EGFR, MEK, ERK, CTSC, CTSV, mRNA and protein expression | [149] | |
| HeLa cells and C3H10T1/2 cells | In vitro 0, 10, 20, 30, 40, 50 µmol/L 140 d | ↓ Tumor promotion | [150] | ||
| Praeruptorin C | Human lung cancer cell lines | In vitro 0, 10, 20,30 µmol/L 24 h | ↓ cyclin D, cathepsin D, ERK1/2 ↑ p21 protein, | [151] | |
| Praeruptorin A and C | HepG2 Cells | In vitro 10, 25, 50, 100, 200 µmol/L 18 and 24 h | ↓ mRNA and protein expression | [152] | |
| Praeruptorin D and E | Mice/acute lung injury | In vivo 0, 20, 40, 80 mg/kg 1 h | ↓ MPO, IκB-α and p65 protein translocation | [153] | |
| Other pharmacological effects | Praeruptorin C | Bone marrow Monocyte/macrophage (BMM) cells | In vitro 0, 5, 10, 20 µmol/L 48 h | ↓ kappa B and c-Jun N-terminal kinase/mitogen-activated protein kinase | [154] |
| Praeruptorin A, B, and C | HEK293 cell line | In vitro 1 mg/mL 1 h | Identified with α1A adrenergic receptor activity | [155] | |
| Terpinolene | Harmful algal blooms (HABs) | In vivo 0.551, 0.881, 1.079, 1.233, 1.470 mmol/L 4 d | ↓ NR and GS activities ↑ COX II, ABC transporter, CaBPs expression | [156] | |
| Marmesinin | Hloroquine-sensitive strain of P. falciparum (D10) | In vitro 0.1, 1, 5, 25, and 100 µmol/L 24 h | ↓ Antiplasmodial D10 ↑ Cytotoxicity SK-OV-3 | [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Ding, L.; Wang, R.; Liang, Z. A Review on the Morphology, Cultivation, Identification, Phytochemistry, and Pharmacology of Kitagawia praeruptora (Dunn) Pimenov. Molecules 2023, 28, 8153. https://doi.org/10.3390/molecules28248153
Wang Q, Ding L, Wang R, Liang Z. A Review on the Morphology, Cultivation, Identification, Phytochemistry, and Pharmacology of Kitagawia praeruptora (Dunn) Pimenov. Molecules. 2023; 28(24):8153. https://doi.org/10.3390/molecules28248153
Chicago/Turabian StyleWang, Qi, Lulu Ding, Ruihong Wang, and Zongsuo Liang. 2023. "A Review on the Morphology, Cultivation, Identification, Phytochemistry, and Pharmacology of Kitagawia praeruptora (Dunn) Pimenov" Molecules 28, no. 24: 8153. https://doi.org/10.3390/molecules28248153
APA StyleWang, Q., Ding, L., Wang, R., & Liang, Z. (2023). A Review on the Morphology, Cultivation, Identification, Phytochemistry, and Pharmacology of Kitagawia praeruptora (Dunn) Pimenov. Molecules, 28(24), 8153. https://doi.org/10.3390/molecules28248153