Synthesis and Evaluation of Compound Targeting α7 and β2 Subunits in Nicotinic Acetylcholinergic Receptor
Abstract
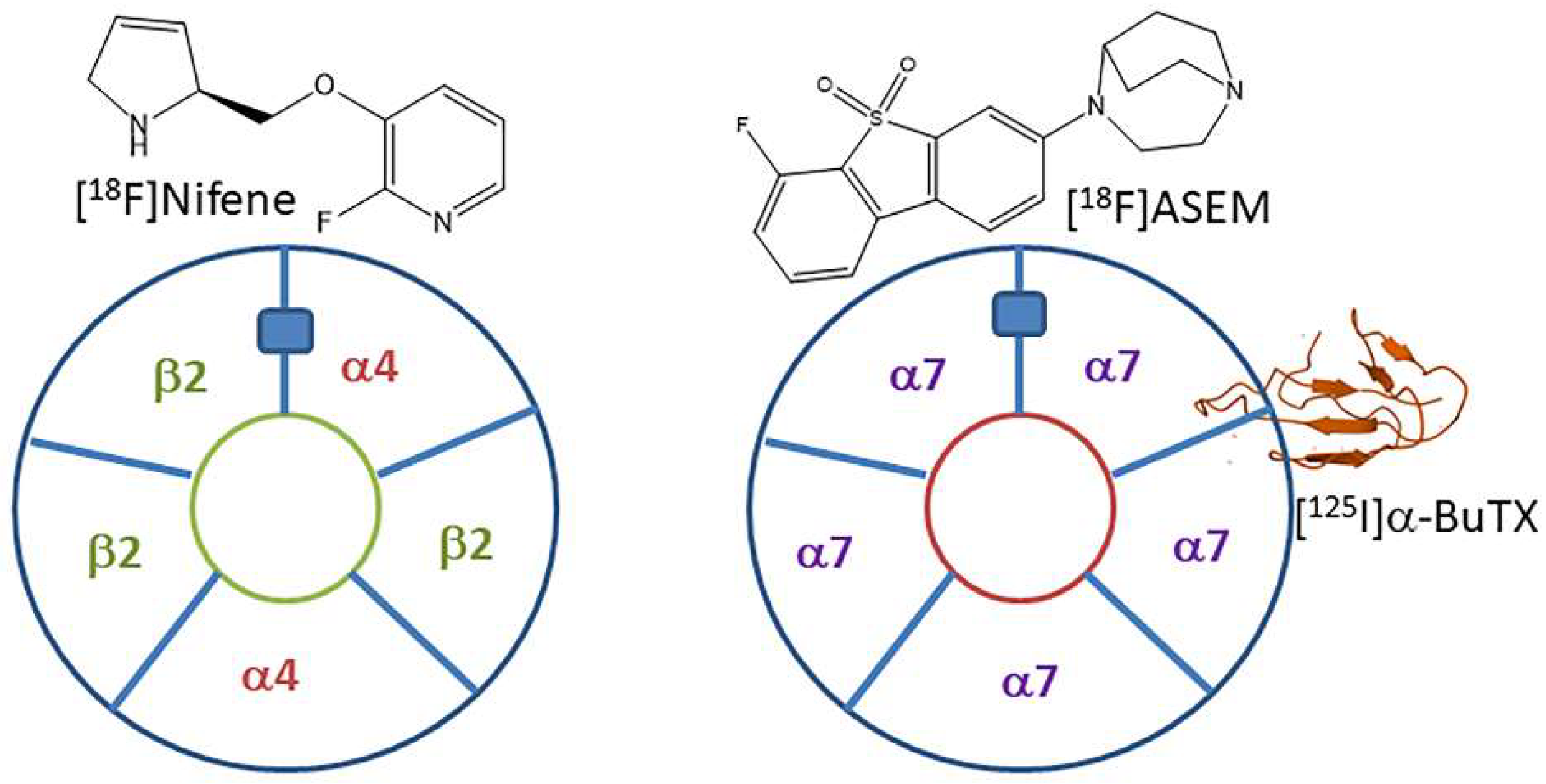
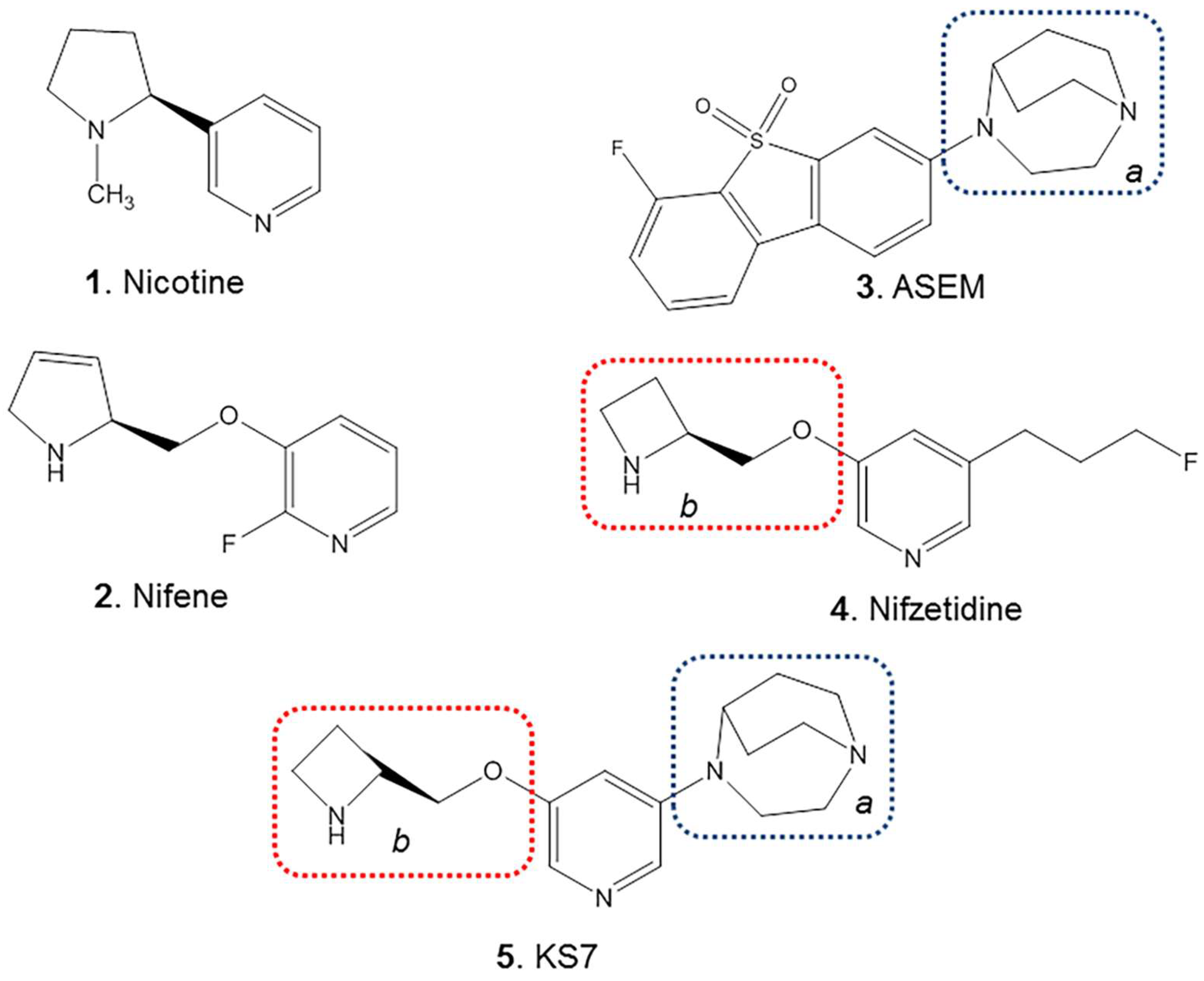
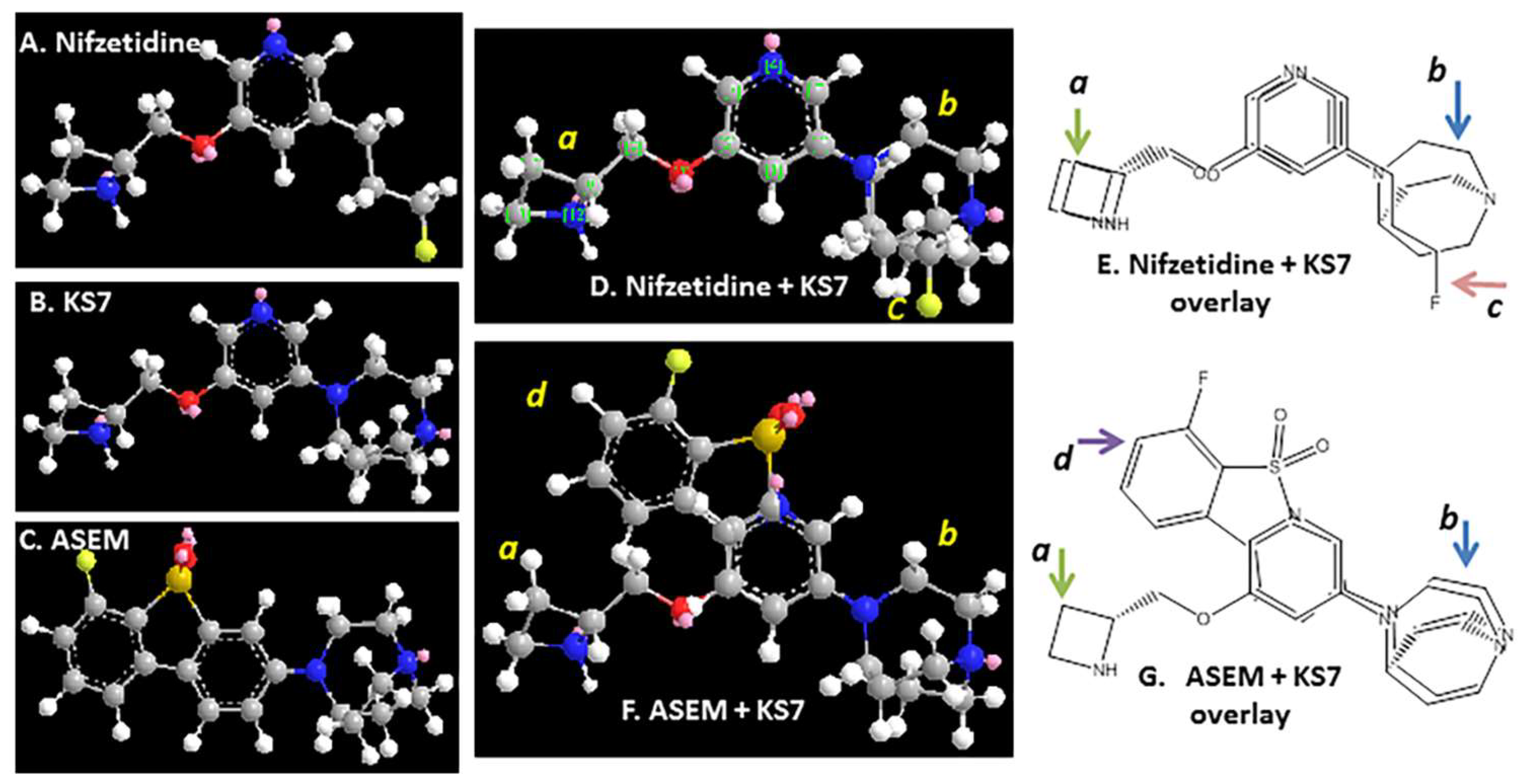
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Animals
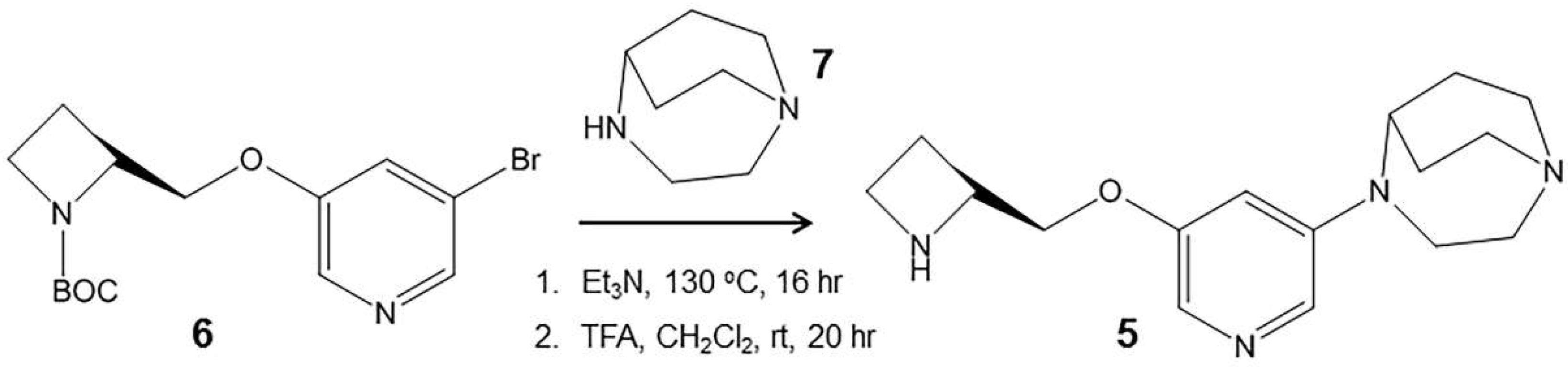
3.3. Synthesis
3.4. In Vitro Autoradiographic Studies α4β2* nAChR—[18F]Nifene
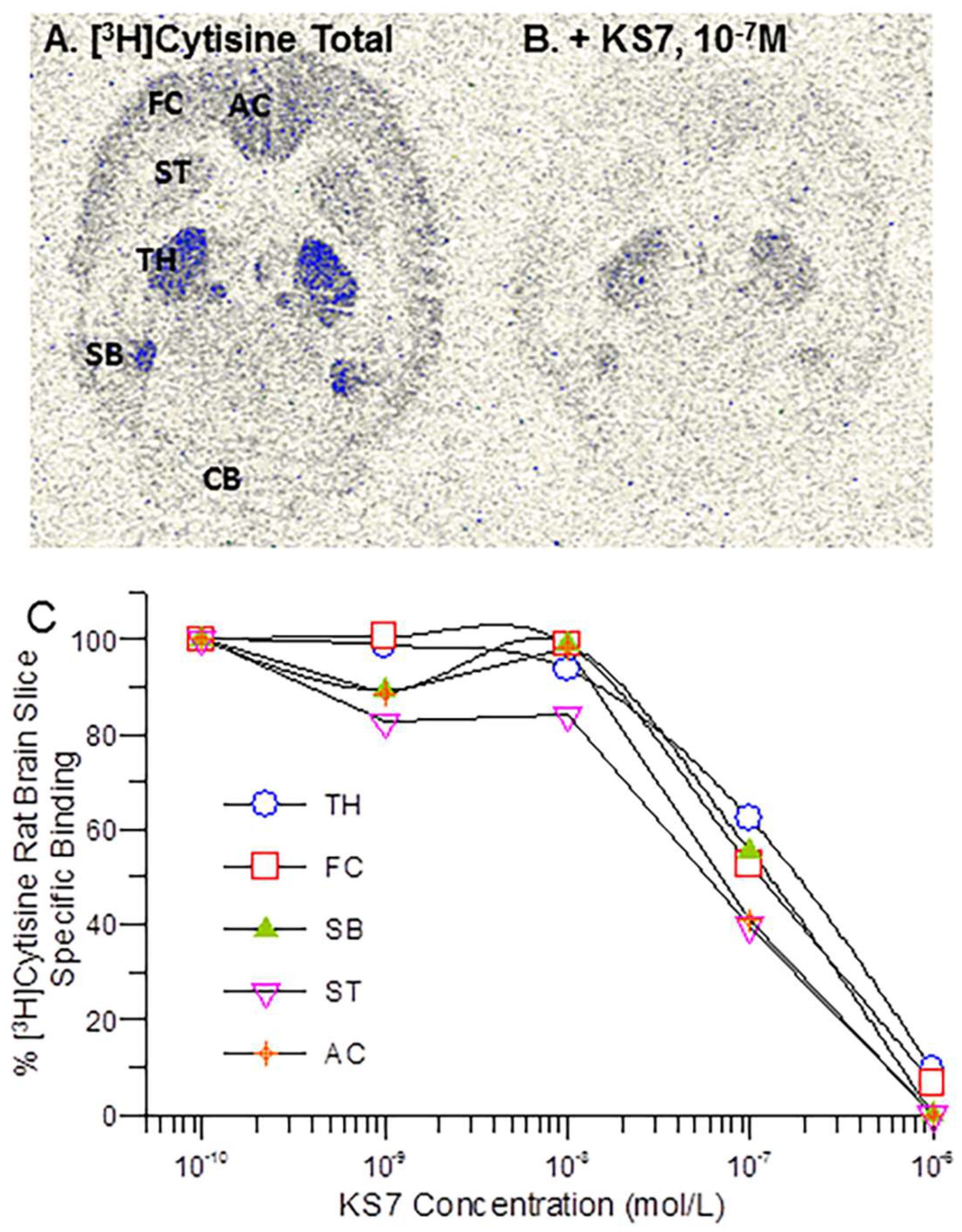
3.5. In Vitro Binding Affinity Studies α4β2* nAChR—[3H]Cytisine
3.6. In Vitro Autoradiographic Studies α7 nAChR—α-[125I] BuTX
3.7. In Vitro Binding Affinity Studies α7 nAChR—[125I]α-Bungarotoxin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valles, A.S.; Barrantes, F.J. Nicotinic acetylcholine receptor dysfunction in addiction and in some neurodegenerative and neuropsychiatric disease. Cells 2023, 12, 2051. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Maskos, U. Role of the nicotinic acetylcholine receptor in Alzheimer’s disease pathology and treatment. Neuropharmacology 2015, 96 Pt B, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Nguyen, G.A.; Danh, T.B.; Sandhu, A.K.; Melkonyan, L.L.; Syed, A.U.; Mukherjee, J. Abnormal [18F]NIFENE binding in transgenic 5xFAD mouse model of Alzheimer’s disease: In vivo PET/CT imaging studies of α4β2* nicotinic acetylcholinergic receptors and in vitro correlations with Aβ plaques. Synapse 2023, 77, e22265. [Google Scholar] [CrossRef] [PubMed]
- Campoy, A.T.; Liang, C.; Ladwa, R.M.; Patel, K.K.; Patel, I.H.; Mukherjee, J. [18F]Nifene PET/CT Imaging in Mice: Improved Methods and Preliminary Studies of α4β2* Nicotinic Acetylcholinergic Receptors in Transgenic A53T Mouse Model of α-Synucleinopathy and Post-Mortem Human Parkinson’s Disease. Molecules 2021, 26, 7360. [Google Scholar] [CrossRef] [PubMed]
- Taly, A.; Corringer, P.J.; Guedin, D.; Lestage, P.; Changeux, J.P. Nicotinic receptors: Allosteric transitions and therapeutic targets in the nervous system. Nat. Rev. Drug Discov. 2009, 8, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.N.T.; Abraham, N.; Lewis, R.J. Structure-Function of Neuronal Nicotinic Acetylcholine Receptor Inhibitors Derived from Natural Toxins. Front. Neurosci. 2020, 14, 609005. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, J.; Lao, P.J.; Betthauser, T.J.; Samra, G.K.; Pan, M.L.; Patel, I.H.; Liang, C.; Metherate, R.; Christian, B.T. Human brain imaging of nicotinic acetylcholine α4β2* receptors using [18F]Nifene: Selectivity, functional activity, toxicity, aging effects, gender effects, and extrathalamic pathways. J. Comp. Neurol. 2018, 526, 80–95. [Google Scholar] [CrossRef]
- Sabri, O.; Meyer, P.M.; Gräf, S.; Hesse, S.; Wilke, S.; Becker, G.A.; Rullmann, M.; Patt, M.; Luthardt, J.; Wagenknecht, G.; et al. Cognitive correlates of α4β2 nicotinic acetylcholine receptors in mild Alzheimer’s dementia. Brain 2018, 141, 1840–1854. [Google Scholar] [CrossRef]
- Sultzer, D.L.; Melrose, R.J.; Riskin-Jones, H.; Narvaez, T.A.; Veliz, J.; Ando, T.K.; Juarez, K.O.; Harwood, D.G.; Brody, A.L.; Mandelkern, M.A. Cholinergic Receptor Binding in Alzheimer Disease and Healthy Aging: Assessment In Vivo with Positron Emission Tomography Imaging. Am. J. Geriatr. Psychiatry 2017, 25, 342–353. [Google Scholar] [CrossRef]
- Quik, M.; Bordia, T.; Zhang, D.; Perez, X.A. Nicotine and Nicotinic Receptor Drugs: Potential for Parkinson’s Disease and Drug-Induced Movement Disorders. Int. Rev. Neurobiol. 2015, 124, 247–271. [Google Scholar]
- Perez-Lloret, S.; Barrantes, F.J. Deficits in cholinergic neurotransmission and their clinical correlates in Parkinson’s disease. NPJ Park. Dis. 2016, 2, 16001. [Google Scholar] [CrossRef] [PubMed]
- Pichika, R.; Easwaramoorthy, B.; Collins, D.; Christian, B.T.; Shi, B.; Narayanan, T.K.; Potkin, S.G.; Mukherjee, J. Nicotine α4β2* receptor imaging agents. Part II. Synthesis and biological evaluation of 2-[18F]fluoro-3-[2-(S)-pyrrolinyl-3,4-dehydromethoxy]pyridine (18F-Nifene) in rodents and imaging by PET in non-human primate. Nucl. Med. Biol. 2006, 33, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Bieszczad, K.M.; Kant, R.; Constantinescu, C.C.; Pandey, S.K.; Kawai, H.D.; Metherate, R.; Weinberger, N.M.; Mukherjee, J. Nicotinic acetylcholine receptors in rat forebrain that bind 18F-nifene: Relating PET imaging, autoradiography, and behavior. Synapse 2012, 66, 418–434. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.; Constantinescu, C.C.; Parekh, P.; Pandey, S.K.; Pan, M.L.; Easwaramoorthy, B.; Mukherjee, J. Evaluation of F-nifene binding to α4β2 nicotinic receptors in the rat brain using microPET imaging. EJNMMI Res. 2011, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Lao, P.J.; Betthauser, T.J.; Tudorascu, D.L.; Barnhart, T.E.; Hillmer, A.T.; Stone, C.K.; Mukherjee, J.; Christian, B.T. [18F]Nifene test-retest reproducibility in first-in-human imaging of α4β2* nicotinic acetylcholine receptors. Synapse 2017, 71, e21981. [Google Scholar] [CrossRef]
- Pichika, R.; Kuruvilla, S.A.; Patel, N.; Vu, K.; Sinha, S.; Easwaramoorthy, B.; Narayanan, T.K.; Shi, B.; Christian, B.; Mukherjee, J. Nicotinic α4β2 receptor imaging agents. Part IV. Synthesis and biological evaluation of 3-(2-(S)-3,4-dehydropyrrolinyl methoxy)-5-(3′-18F-fluoropropyl)pyridine (18F-Nifrolene) using PET. Nucl. Med. Biol. 2013, 40, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Pichika, R.; Easwaramoorthy, B.; Christian, B.T.; Shi, B.; Narayanan, T.K.; Collins, D.; Mukherjee, J. Nicotinic α4β2 receptor imaging agents. Part III. Synthesis and biological evaluation of 3-(2-(S)-azetidinylmethoxy)-5-(3′-18F-fluoropropyl)pyridine (18F-nifzetidine). Nucl. Med. Biol. 2011, 38, 1183–1192. [Google Scholar] [CrossRef][Green Version]
- Pandey, S.K.; Pan, S.; Kant, R.; Kuruvilla, S.A.; Pan, M.L.; Mukherjee, J. Synthesis and evaluation of 3-123I-iodo-5-[2-(S)-3-pyrrolinylmethoxy]-pyridine (niodene) as a potential nicotinic α4β2 receptor imaging agent. Bioorg Med. Chem. Lett. 2012, 22, 7610–7614. [Google Scholar] [CrossRef][Green Version]
- Kuruvilla, S.A.; Hillmer, A.T.; Wooten, D.W.; Patel, A.; Christian, B.T.; Mukherjee, J. Synthesis and evaluation of 2-18F-fluoro-5-iodo-3-[2-(S)-3,4-dehydropyrrolinylmethoxy]pyridine (18F-niofene) as a potential imaging agent for nicotinic α4β2 receptors. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 354–364. [Google Scholar]
- Horti, A.G.; Gao, Y.; Kuwabara, H.; Wang, Y.; Abazyan, S.; Yasuda, R.P.; Tran, T.; Xiao, Y.; Sahibzada, N.; Holt, D.P.; et al. [18F]ASEM, a radiolabeled antagonist for imaging α7-nicotinic acetylcholine receptor (α7-nAChR) with positron emission tomography (PET). J. Nucl. Med. 2014, 55, 672–677. [Google Scholar] [CrossRef]
- Donat, C.K.; Hansen, H.H.; Hansen, H.D.; Mease, R.C.; Horti, A.G.; Pomper, M.G.; L’Estrade, E.T.; Herth, M.M.; Peters, D.; Knudsen, G.M.; et al. In Vitro and In Vivo Characterization of Dibenzothiophene Derivatives [125I]Iodo-ASEM and [18F]ASEM as Radiotracers of Homo- and Heteromeric α7 Nicotinic Acetylcholine Receptors. Molecules 2020, 25, 1425. [Google Scholar] [CrossRef] [PubMed]
- Hawrot, E.; Wilson, P.T.; Gershoni, J.M.; Reese, J.H.; Lentz, T.L. Alpha-bungarotoxin binding to a high molecular weight component from lower vertebrate brain identified on dodecyl sulfate protein-blots. Brain Res. 1986, 373, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Love, R.A.; Stroud, R.M.; Love, R.A.; Stroud, R.M. The crystal structure of a-bungarotoxin at 2.5A resolution: Relation to solution structure and binding to acetylcholine receptor. Protein Eng. 1986, 1, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.A.; Perry, D.C. An Autoradiographic analysis of [125I] α-bungarotoxin binding in rat brain after chronic nicotine exposure. Neurosci. Lett. 2006, 404, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Colloby, S.J.; Perry, E.K.; Pakrasi, S.; Pimlott, S.L.; Wyper, D.J.; McKeith, I.G.; Williams, E.D.; O’Brien, J.T. Nicotinic 123I-5IA-85380 single photon emission computed tomography as a predictor of cognitive progression in Alzheimer’s disease and dementia with Lewy bodies. Am. J. Geriatr. Psychiatry 2010, 18, 86–90. [Google Scholar] [CrossRef]
- Okada, H.; Ouchi, Y.; Ogawa, M.; Futatsubashi, M.; Saito, Y.; Yoshikawa, E.; Terada, T.; Oboshi, Y.; Tsukada, H.; Ueki, T.; et al. Alterations in α4β2 nicotinic receptors in cognitive decline in Alzheimer’s aetiopathology. Brain 2013, 136 Pt 10, 3004–3017. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, J.; Sarazin, M.; Chauvire, V.; Stankoff, B.; Kas, A.; Lacomblez, L.; Peyronneau, M.-A.; Bottlaender, M. Cholinergic changes in aging and Alzheimer’s disease. An [18F]-F-A-85380 exploratory PET study. Alzheimer Dis. Assoc. Disord. 2017, 31, 8–12. [Google Scholar] [CrossRef]
- Coughlin, J.M.; Rubin, L.H.; Du, Y.; Rowe, S.P.; Crawford, J.L.; Rosenthal, H.B.; Frey, S.M.; Marshall, E.S.; Shinehouse, L.K.; Chen, A.; et al. High availability of the a-7 nicotinic acetylcholine receptor in brains of individuals with mild cognitive impairment: A pilot study using 18F-ASEM PET. J. Nucl. Med. 2020, 61, 423–426. [Google Scholar] [CrossRef]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; Tredici, K.D. Stages of the pathologic process in Alzheimer’s disease age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Shen, J.; Steffensen, S.; Wu, J. Functional α7β2 nicotinic acetylcholine receptors expressed in hippocampal interneurons exhibit high sensitivity to pathological level of amyloid β peptides. BMC Neurosci. 2012, 13, 155. [Google Scholar] [CrossRef]
- Moretti, M.; Zoli, M.; George, A.A.; Lukas, R.J.; Pistillo, F.; Maskos, U.; Whiteaker, P.; Gotti, C. The novel α7β2-nicotinic acetylcholine receptor subtype is expressed in mouse and human basal forebrain: Biochemical and pharmacological characterization. Mol. Pharmacol. 2014, 86, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, Q.; Tang, P.; Mikkelsen, J.D.; Shen, J.; Whiteaker, P.; Yakel, J.L. Heteromeric α7β2 Nicotinic Acetylcholine Receptors in the Brain. Trends Pharmacol. Sci. 2016, 37, 562–574. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Zwart, R.; Ursu, D.; Jensen, M.M.; Pinborg, L.H.; Gilmour, G.; Wu, J.; Sher, E.; Mikkelsen, J.D. α7 and β2 Nicotinic Acetylcholine Receptor Subunits Form Heteromeric Receptor Complexes that Are Expressed in the Human Cortex and Display Distinct Pharmacological Properties. PLoS ONE 2015, 10, e0130572. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Murray, T. The role of a recently discovered alpha-7 nicotinic acetylcholine receptor on amyloid-beta pathology in Alzheimer’s disease. Alzheimer’s Dement. 2017, 13 (Suppl. S7), P1502–P1503. [Google Scholar] [CrossRef]
- Harel, M.; Kasher, R.; Nicolas, A.; Guss, J.M.; Balass, M.; Fridkin, M.; Smit, A.B.; Brejc, K.; Sixma, T.K.; Katchalski-Katzir, E.; et al. The binding site of acetylcholine receptor as visualized in the X-ray structure of a complex between alpha-bungarotoxin and a mimotope peptide. Neuron 2001, 32, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Schrimpf, M.R.; Sippy, K.B.; Briggs, C.A.; Anderson, D.J.; Li, T.; Ji, J.; Frost, J.M.; Surowy, C.S.; Bunnelle, W.H.; Gopalakrishnan, M.; et al. SAR of α7 nicotinic receptor agonists derived from tilorone: Exploration of a novel nicotinic pharmacophore. Bioorg. Med. Chem. Lett. 2012, 22, 1633–1638. [Google Scholar] [CrossRef]
- Easwaramoorthy, B.; Pichika, R.; Collins, D.; Potkin, S.G.; Leslie, F.M.; Mukherjee, J. Effect of acetylcholinesterase inhibitors on nicotinic α4β2 receptor PET radiotracer, 18F-Nifene: A measure of acetylcholine competition. Synapse 2007, 61, 29–36. [Google Scholar] [CrossRef]
- Wong, D.F.; Kuwabara, H.; Pomper, M.; Holt, D.P.; Brasic, J.R.; George, N.; Frolov, B.; Willis, W.; Gao, Y.; Valentine, H.; et al. Human brain imaging of α7 nAChR with [18F]ASEM: A new PET radiotracer for neuropsychiatry and determination of drug occupancy. Mol. Imaging Biol. 2014, 16, 730–738. [Google Scholar] [CrossRef]
- Azam, L.; Winzer-Serhan, U.; Leslie, F.M. Co-expression of α7 and β2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience 2003, 119, 965–977. [Google Scholar] [CrossRef]
- Meeker, R.B.; Michels, K.M.; Libber, M.T.; Hayward, J.N. Characteristics and distribution of high- and low-affinity alpha bungarotoxin binding sites in the rat hypothalamus. J. Neurosci. 1986, 6, 1866–1875. [Google Scholar] [CrossRef]
- Dickinson, J.A.; Kew, J.N.C.; Wonnacott, S. Presynaptic α7- and β2-containing nicotinic acetylcholine receptors modulate excitatotry amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol. Pharmacol. 2008, 74, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Morales-Perez, C.L.; Noviello, C.M.; Hibbs, R.E. X-ray structure of the human α4β2 nicotinic receptor. Nature 2016, 538, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Crestey, F.; Jensen, A.A.; Soerensen, C.; Magnus, C.B.; Andreasen, J.T.; Peters, G.H.J.; Kristensen, J.L. Dual Nicotinic Acetylcholine Receptor α4β2 Antagonists/α7 Agonists: Synthesis, Docking Studies, and Pharmacological Evaluation of Tetrahydroisoquinolines and Tetrahydroisoquinolinium Salts. J. Med. Chem. 2018, 61, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Chellappan, S.K.; Xiao, Y.; Tueckmantel, W.; Kellar, K.J.; Kozikowski, A.P. Synthesis and pharmacologica evaluation of novel 9- and 10-substituted cytisine derivatives. Nicotinic ligands of enhanced subtype selectivity. J. Med. Chem. 2006, 49, 2673–2676. [Google Scholar] [CrossRef] [PubMed]
- Campello, H.R.; Del Villar, S.G.; Honraedt, A.; Minguez, T.; Oliveria, A.S.F.; Ranaghan, K.E.; Shoemark, D.K.; Bermudez, I.; Gotti, C.; Sessions, R.B.; et al. Unlocking nicotinic selectivity via direct C-H functionalization of (−)-cytisine. Chem 2018, 4, 1710–1725. [Google Scholar] [CrossRef]
- Whiting, P.; Lindstrom, J. Pharmacological properties of immune-isolated neuronal nicotinic receptors. J. Neurosci. 1986, 6, 3061–3069. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Mease, R.C.; Olson, T.T.; Kellar, K.J.; Dannals, R.F.; Pomper, M.G.; Horti, A.G. [125I]Iodo-ASEM, a specific in vivo radioligand for α7-nAChR. Nucl. Med. Biol. 2015, 42, 488–493. [Google Scholar] [CrossRef]
- Gao, Y.; Kellar, K.J.; Yasuda, R.P.; Tran, T.; Xiao, Y.; Dannals, R.F.; Horti, A.G. Derivatives of dibenzothiophene for PET imaging of α7 nicotinic acetylcholine receptors. J. Med. Chem. 2013, 56, 7574–7589. [Google Scholar] [CrossRef]
- Das, M.K.; Mukherjee, J. Radiosynthesis of [F-18]fluoxetine as a potential radiotracer of serotonin reuptake-sites. Int. J. Appl. Radiat. Isot. 1993, 44, 835–842. [Google Scholar] [CrossRef]
- Mukherjee, J.; Shi, B.; Christian, B.T.; Chattopadhyay, S.; Narayanan, T.K. 11C-Fallypride: Radiosynthesis and preliminary evaluation of novel dopamine D2/D3 receptor PET radiotracer in nonhuman primate brain. Bioorganic Med. Chem. 2004, 12, 95–102. [Google Scholar] [CrossRef]
- Nguyen, G.A.H.; Liang, C.; Mukherjee, J. [124I]IBETA, a new Aβ amyloid plaque PET imaging agent for Alzheimer’s disease. Molecules 2022, 27, 4552. [Google Scholar] [CrossRef] [PubMed]



| Brain Region | [3H]Cytisine, α4β2* nAChRs | [125I]α-BuTX, α7* nAChRs | α7/α4β2 Ratio |
|---|---|---|---|
| Thalamus | 1.34 × 10−7 | - | - |
| Frontal Cortex | 1.09 × 10−7 | 9.90 × 10−6 | 91 |
| Anterior Cingulate | 4.95 × 10−8 | 1.26 × 10−5 | 254 |
| Subiculum | 1.72 × 10−7 | - | - |
| Striatum | 8.69 × 10−8 | - | - |
| Hippocampus | - | 3.32 × 10−5 | - |
| Inferior Colliculus | - | 4.68 × 10−5 | - |
| Drug | α4β2*, nM | α7*, nM | α7/α4β2 Ratio | α4β2/α7 Ratio |
|---|---|---|---|---|
| Nicotine | 6.93 a | 223 c | 32 | 0.03 |
| 1.68 b | 133 | |||
| Nifene | 1.07 a | 169 c | 158 | 0.007 |
| 0.50 b | 338 | |||
| Cytisine | 1.51 d | 691 e | 544 e | 0.002 |
| 1.27 e | ||||
| α-Iodobungarotoxin | >1000 f | 0.50 g | 0.0005 | 2000 |
| IodoASEM | 1707 h | 0.5 h | 0.0003 | 3414 h |
| ASEM | 562 i | 0.37 i | 0.0007 | 1370 i |
| KS7 | 109 j | 9900 j | 91 j | 0.01 j |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, K.; Ngo, A.; Keerthisinghe, O.V.; Patel, K.K.; Liang, C.; Mukherjee, J. Synthesis and Evaluation of Compound Targeting α7 and β2 Subunits in Nicotinic Acetylcholinergic Receptor. Molecules 2023, 28, 8128. https://doi.org/10.3390/molecules28248128
Singh K, Ngo A, Keerthisinghe OV, Patel KK, Liang C, Mukherjee J. Synthesis and Evaluation of Compound Targeting α7 and β2 Subunits in Nicotinic Acetylcholinergic Receptor. Molecules. 2023; 28(24):8128. https://doi.org/10.3390/molecules28248128
Chicago/Turabian StyleSingh, Karanveer, Allyson Ngo, Oshini V. Keerthisinghe, Krystal K. Patel, Christopher Liang, and Jogeshwar Mukherjee. 2023. "Synthesis and Evaluation of Compound Targeting α7 and β2 Subunits in Nicotinic Acetylcholinergic Receptor" Molecules 28, no. 24: 8128. https://doi.org/10.3390/molecules28248128
APA StyleSingh, K., Ngo, A., Keerthisinghe, O. V., Patel, K. K., Liang, C., & Mukherjee, J. (2023). Synthesis and Evaluation of Compound Targeting α7 and β2 Subunits in Nicotinic Acetylcholinergic Receptor. Molecules, 28(24), 8128. https://doi.org/10.3390/molecules28248128






