Low-Valent-Tungsten-Catalyzed Aerobic Oxidative Cross-Dehydrogenative Coupling Reaction
Abstract
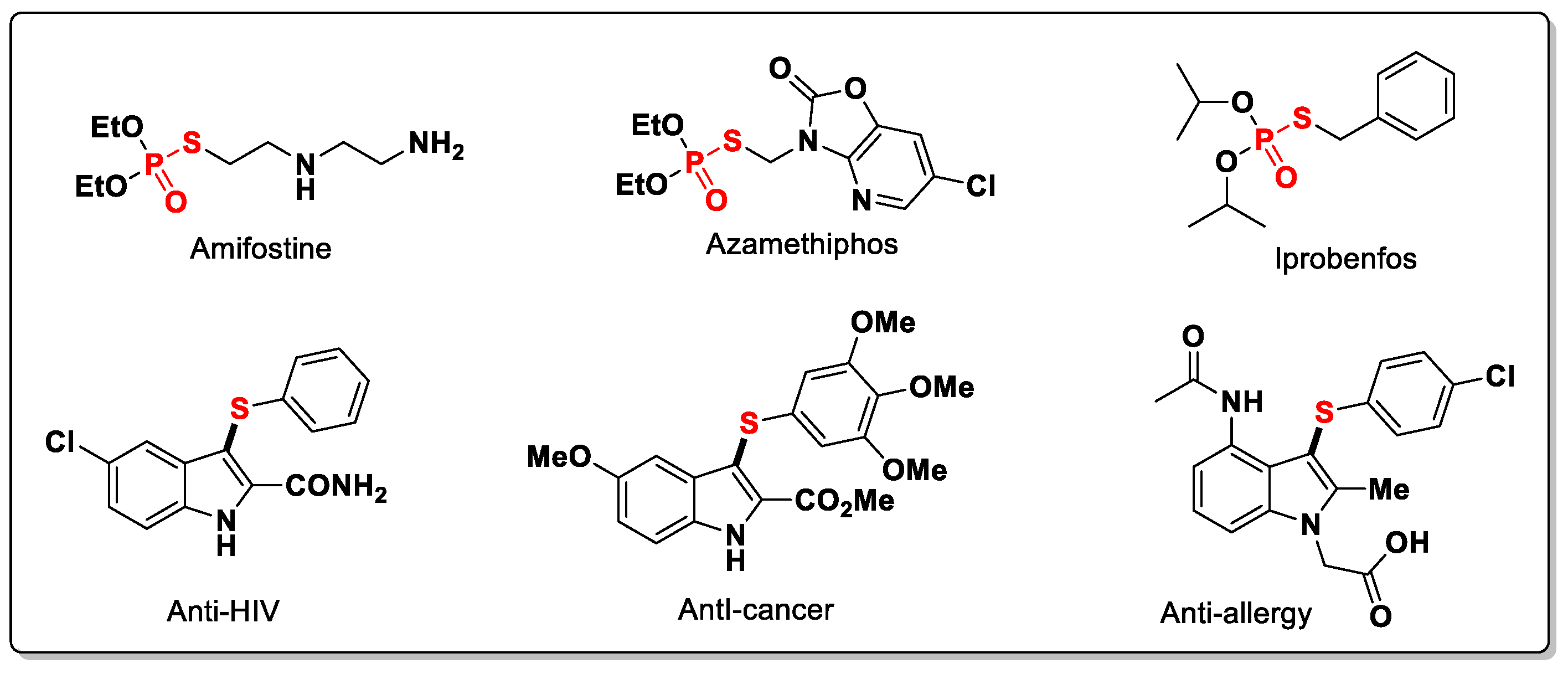
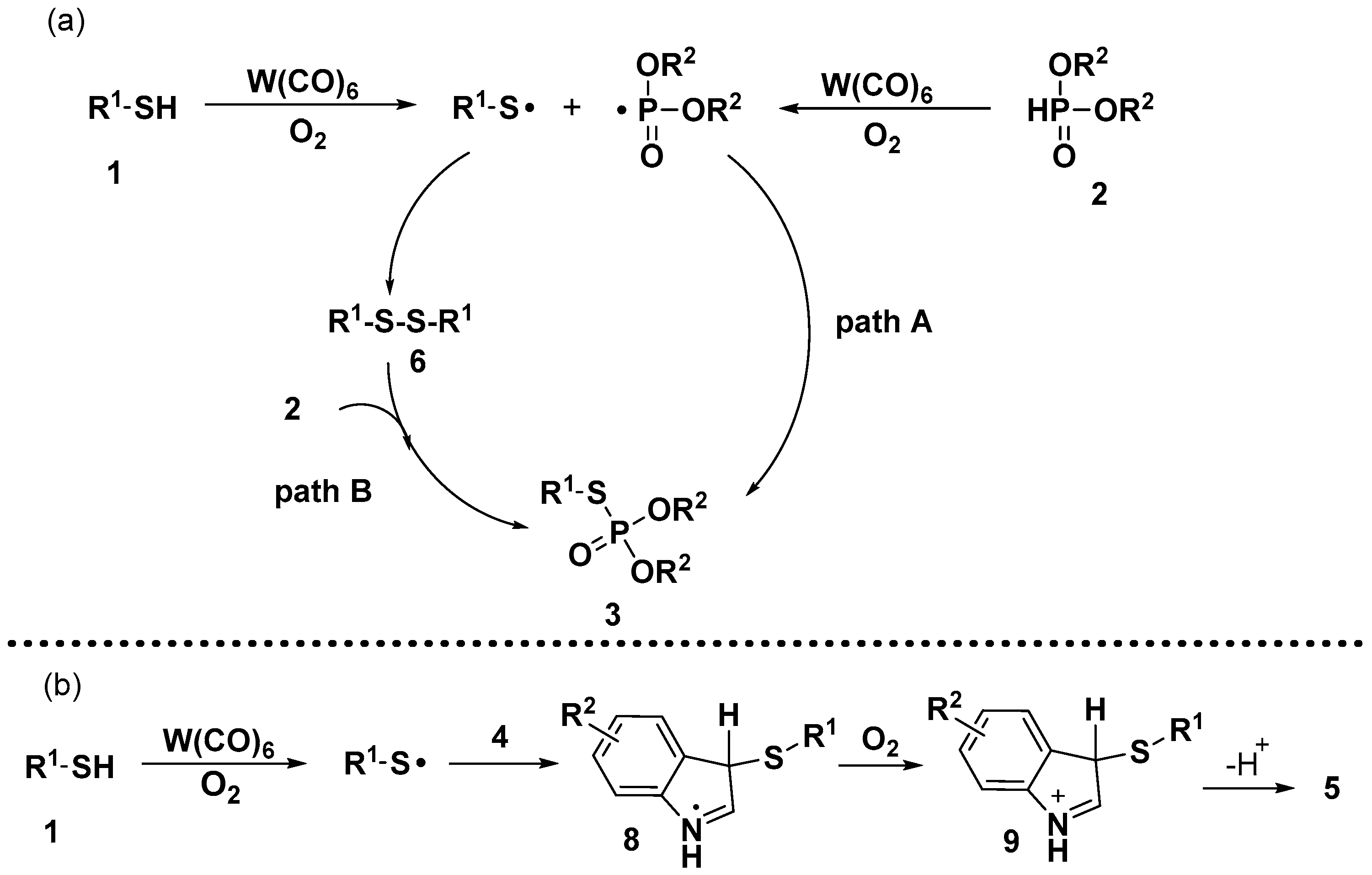
:1. Introduction
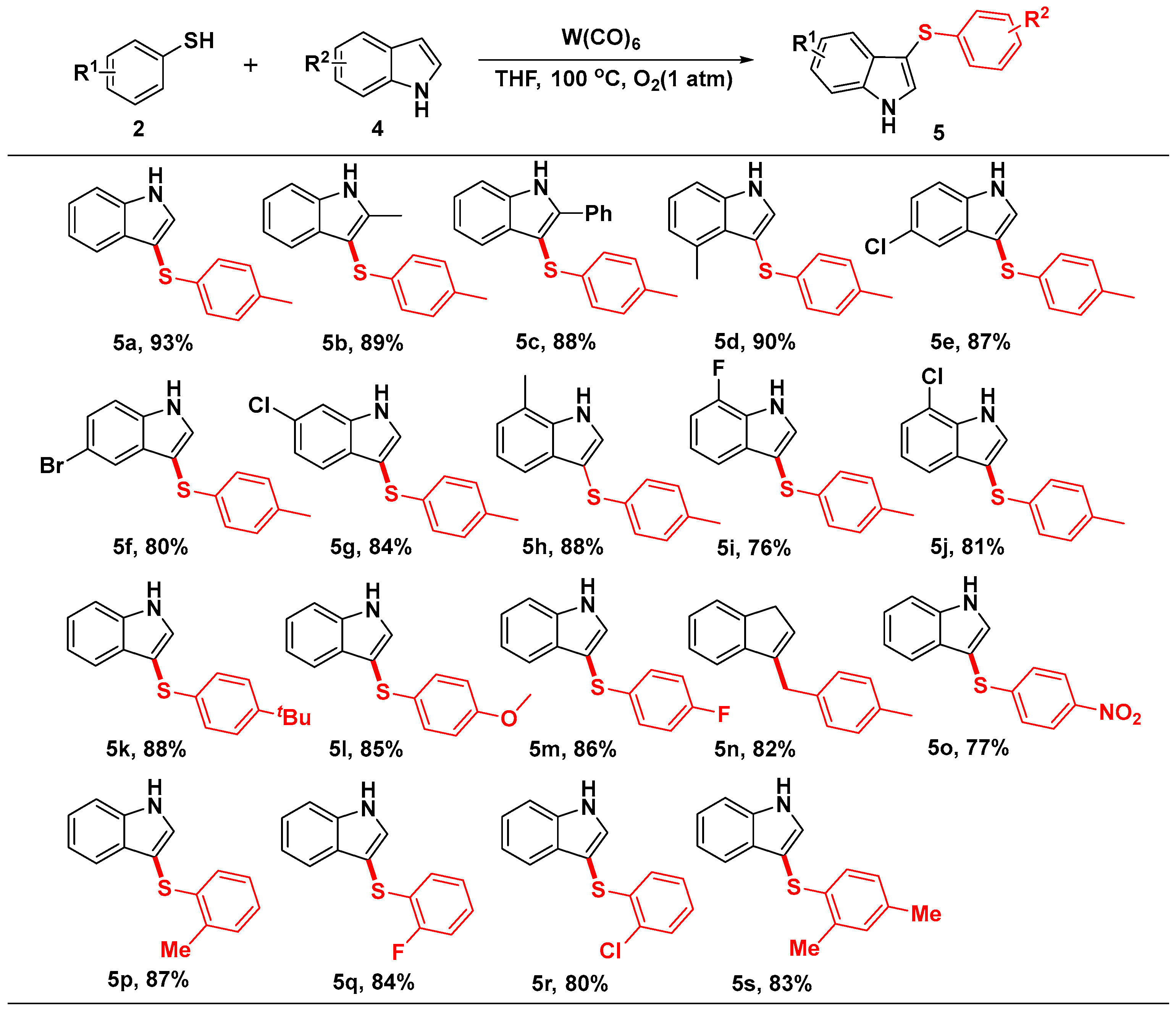
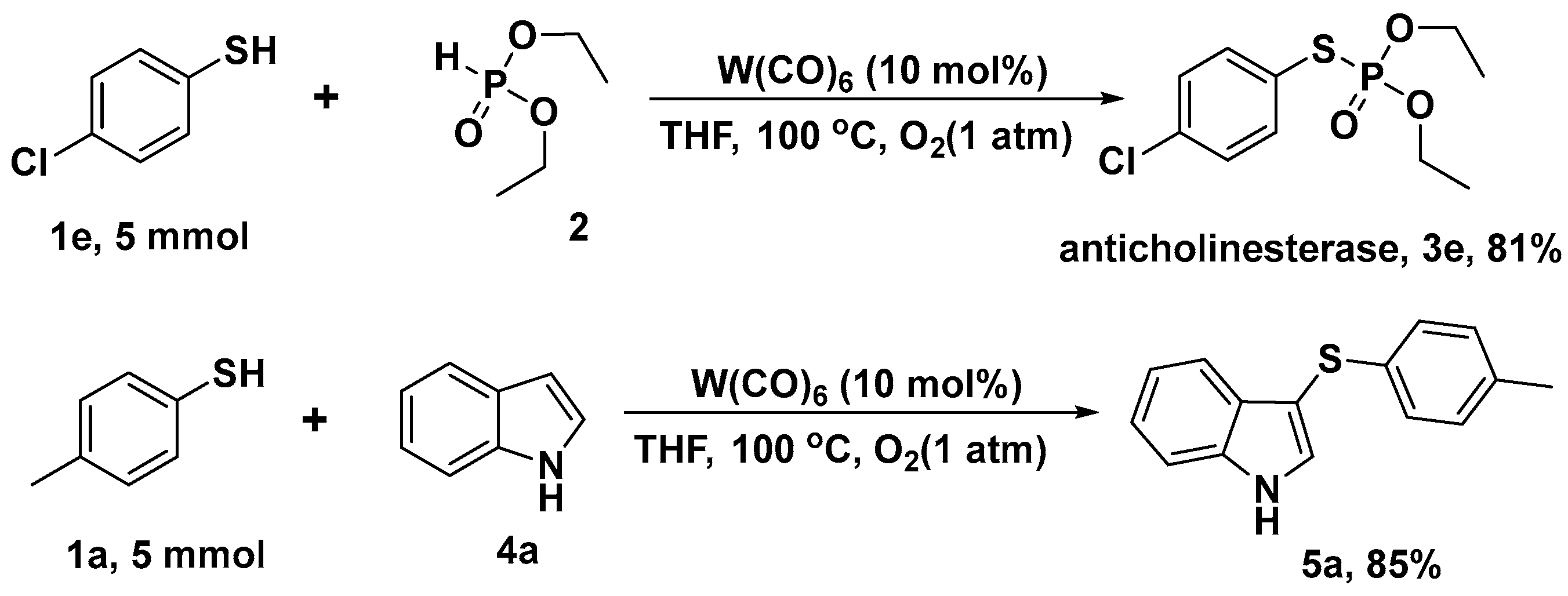
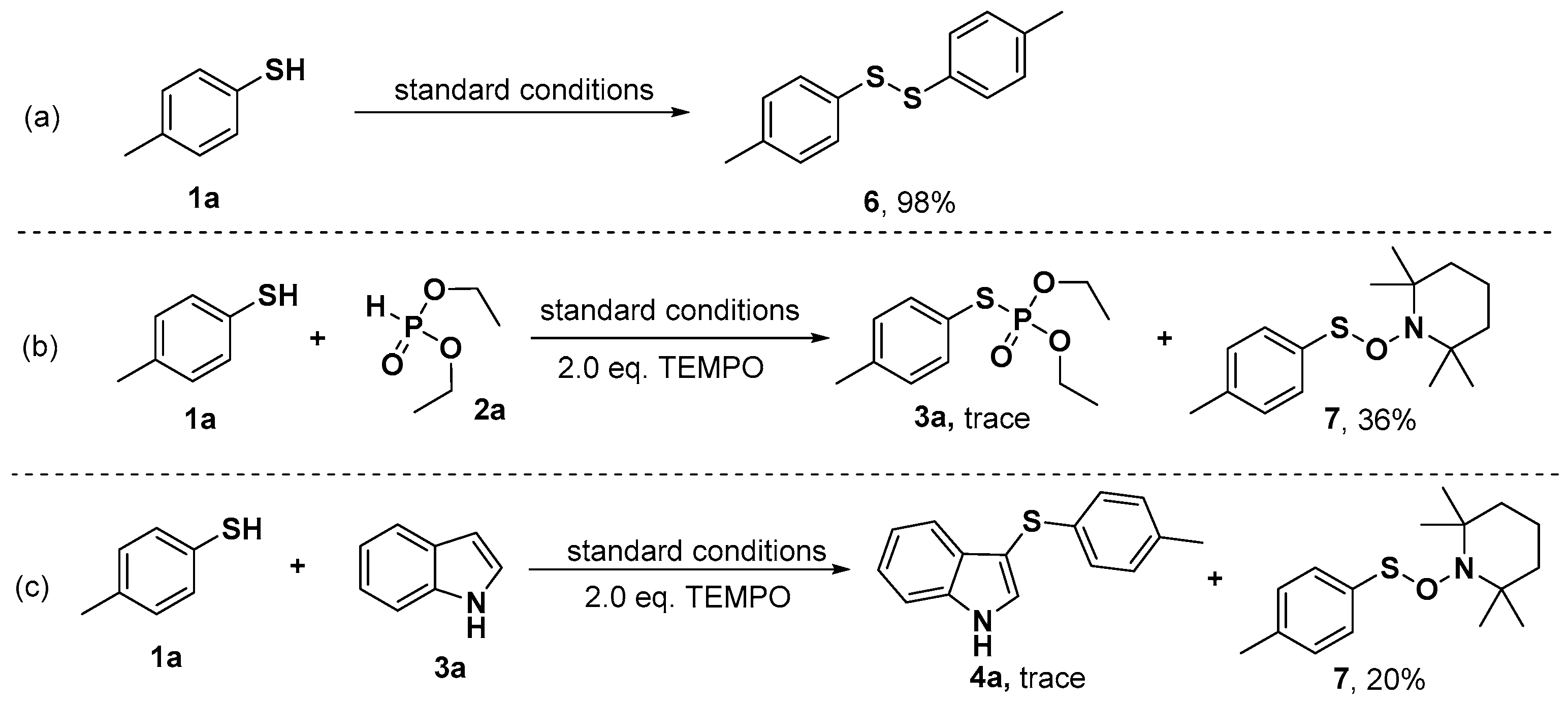
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. General Methods for the Preparation of Thiophosphates
3.3. General Methods for the Preparation of Thiophosphates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef]
- Horsman, G.P.; Zechel, D.L. Phosphonate Biochemistry. Chem. Rev. 2017, 117, 5704–5783. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.V.; Blagoev, B.; Gnad, F.; Macek, B.; Kumar, C.; Mortensen, P.; Mann, M. Global, In Vivo, and Site-Specific Phosphorylation Dynamics in Signaling Networks. Cell 2006, 127, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.S.; Yang, T.; Mishra, S.; Cronin, C.; Chakraborty, S.; Shen, J.-B.; Liang, B.T.; Jacobson, K.A. 5′-Phosphate and 5′-Phosphonate Ester Derivatives of (N)-Methanocarba Adenosine with In Vivo Cardioprotective Activity. J. Med. Chem. 2013, 56, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Noro, M.; Fujita, S.; Wada, T. Stereoselective Synthesis of P-Modified α-Glycosyl Phosphates by the Oxazaphospholidine Approach. Org. Lett. 2013, 15, 5948–5951. [Google Scholar] [CrossRef]
- Howell, B.A.; Alrubayyi, A. 2-Dopyl-1,4-di(2-dopylpropanoyl)benzene, an effective phosphorus flame retardant. Polym. Degrad. Stab. 2019, 162, 196–200. [Google Scholar] [CrossRef]
- Jones, D.J.; O’Leary, E.M.; O’Sullivan, T.P. Modern Synthetic Approaches to Phosphorus-Sulfur Bond Formation in Organophosphorus Compounds. Adv. Synth. Catal. 2020, 362, 2801–2846. [Google Scholar] [CrossRef]
- Peng, S.K.; Dong, Z.-B. Recent Advances in Sulfur-Centered S-X (X = N, P, O) Bond Formation Catalyzed by Transition Metals. Eur. J. Org. Chem. 2020, 2020, 5488–5495. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Ananikov, V.P. Transition-Metal-Catalyzed C–S, C–Se, and C–Te Bond Formation via Cross-Coupling and Atom-Economic Addition Reactions. Chem. Rev. 2011, 111, 1596–1636. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Lee, C.-F. N-Chlorosuccinimide-promoted synthesis of thiophosphates from thiols and phosphonates under mild conditions. Green Chem. 2014, 16, 357–364. [Google Scholar] [CrossRef]
- Wang, W.-M.; Liu, L.-J.; Yao, L.; Meng, F.-J.; Sun, Y.-M.; Zhao, C.-Q.; Xu, Q.; Han, L.-B. Stereospecific Preparations of P-Stereogenic Phosphonothioates and Phosphonoselenoates. J. Org. Chem. 2016, 81, 6843–6847. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Moon, Y.; Choi, H.; Hong, S. Metal- and oxidant-free S–P(O) bond construction via direct coupling of P(O)H with sulfinic acids. Green Chem. 2017, 19, 1005–1013. [Google Scholar] [CrossRef]
- Atherton, F.; Todd, A. Studies on phosphorylation. Part III. Further observations on the reaction of phosphites with polyhalogen compounds in presence of bases and its application to the phosphorylation of alcohols. J. Chem. Soc. 1947, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Shen, R.; Xu, Q.; Goto, M.; Zhao, Y.; Han, L.-B. Stereospecific Coupling of H-Phosphinates and Secondary Phosphine Oxides with Amines and Alcohols: A General Method for the Preparation of Optically Active Organophosphorus Acid Derivatives. J. Org. Chem. 2010, 75, 3890–3892. [Google Scholar] [CrossRef] [PubMed]
- Li, W.S.; Chen, T.; Saga, Y.; Han, L.-B. Chloroform-based Atherton-Todd-type reactions of alcohols and thiols with secondary phosphine oxides generating phosphinothioates and phosphinates. RSC Adv. 2015, 5, 71544–71546. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Gusarova, N.K.; Volkov, P.A.; Ivanova, N.I.; Khrapova, K.O. First Examples of the Atherton-Todd-Like Reaction in the Absence of Bases. Heteroat. Chem. 2016, 27, 44–47. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, T.; Li, S.; Shimada, S.; Han, L.B. Efficient Pd-Catalyzed Dehydrogenative Coupling of P(O)H with RS-H: A Precise Construction of P(O)–S Bonds. J. Am. Chem. Soc. 2016, 138, 5825–5828. [Google Scholar] [CrossRef]
- Sun, J.-G.; Weng, W.-Z.; Li, P.; Zhang, B. Dimethyl sulfoxide as a mild oxidant in S–P(O) bond construction: Simple and metal-free approaches to phosphinothioates. Green Chem. 2017, 19, 1128–1133. [Google Scholar] [CrossRef]
- Song, S.; Zhang, Y.; Yeerlan, A.; Zhu, B.; Liu, J.; Jiao, N. Cs2CO3-Catalyzed Aerobic Oxidative Cross-Dehydrogenative Coupling of Thiols with Phosphonates and Arenes. Angew. Chem. Int. Ed. 2017, 56, 2487–2491. [Google Scholar] [CrossRef]
- Huang, H.; Ash, J.; Kang, J.Y. Base-controlled Fe(Pc)-catalyzed aerobic oxidation of thiols for the synthesis of S-S and S-P(O) bonds. Org. Biomol. Chem. 2018, 16, 4236–4242. [Google Scholar] [CrossRef]
- Xue, J.-W.; Zeng, M.; Zhang, S.; Chen, Z.; Yin, G. Lewis Acid Promoted Aerobic Oxidative Coupling of Thiols with Phosphonates by Simple Nickel(II) Catalyst: Substrate Scope and Mechanistic Studies. J. Org. Chem. 2019, 84, 4179–4190. [Google Scholar] [CrossRef]
- Zhang, H.; Zhan, Z.; Lin, Y.; Shi, Y.; Li, G.; Wang, Q.; Deng, Y.; Hai, L.; Wu, Y. Visible light photoredox catalyzed thiophosphate synthesis using methylene blue as a promoter. Org. Chem. Front. 2018, 5, 1416–1422. [Google Scholar] [CrossRef]
- Shen, J.; Li, Q.-W.; Zhang, X.-Y.; Wang, X.; Li, G.-Z.; Li, W.-Z.; Yang, S.-D.; Yang, B. Tf2O/DMSO-Promoted P−O and P−S Bond Formation: A Scalable Synthesis of Multifarious Organophosphinates and Thiophosphates. Org. Lett. 2021, 23, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Nuth, M.; Guan, H.; Zhukovskaya, N.; Saw, Y.L.; Ricciardi, R.P. Design of Potent Poxvirus Inhibitors of the Heterodimeric Processivity Factor Required for Viral Replication. J. Med. Chem. 2013, 56, 3235–3246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Z.; Chen, Q.; Yang, G.-F. A review on recent developments of indole-containing antiviral agents. Eur. J. Med. Chem. 2015, 89, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.S.; Subba Reddy, B.V.; Reddy, Y.J.; Praneeth, K. Iron(III) Chloride: A Versatile Catalyst for the Practical Synthesis of 3-Sulfenylindoles. Synthesis 2009, 9, 1520–1524. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Yang, S.; An, Y.; Wu, W.; Jiang, H. Palladium-Catalyzed Oxidative Sulfenylation of Indoles and Related Electron-Rich Heteroarenes with Aryl Boronic Acids and Elemental Sulfur. J. Org. Chem. 2016, 81, 7771–7783. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Liu, S.; Feng, H.; Benassi, E.; Qian, B. Iodine/Manganese Catalyzed Sulfenylation of Indole via Dehydrogenative Oxidative Coupling in Anisole. Adv. Synth. Catal. 2020, 362, 2666–2671. [Google Scholar] [CrossRef]
- Sang, P.; Chen, Z.; Zou, J.; Zhang, Y. K2CO3 promoted direct sulfenylation of indoles: A facile approach towards 3-sulfenylindoles. Green Chem. 2013, 15, 2096–2100. [Google Scholar] [CrossRef]
- Hazarika, S.; Barman, P. Visible-Light Cercosporin Catalyzed Sulfenylation of Electron-Rich Compounds with Thiols under Transition-Metal-Free Conditions. ChemistrySelect 2020, 5, 11583–11589. [Google Scholar] [CrossRef]
- Yuan, W.; Huang, J.; Xu, X.; Wang, L.; Tang, X.-Y. B(C6F5)3-Catalyzed Electron Donor–Acceptor Complex-Mediated Aerobic Sulfenylation of Indoles under Visible-Light Conditions. Org. Lett. 2021, 23, 7139–7143. [Google Scholar] [CrossRef]
- Huang, Q.; Peng, X.; Li, H.; He, H.; Liu, L. Visible-Light-Induced, Graphene Oxide-Promoted C3-Chalcogenylation of Indoles Strategy under Transition-Metal-Free Conditions. Molecules 2022, 27, 772–777. [Google Scholar] [CrossRef]
- Wang, P.; Tang, S.; Huang, P.P.; Lei, A. Electrocatalytic Oxidant-Free Dehydrogenative C−H/S−H Cross-Coupling. Angew. Chem. Int. Ed. 2017, 56, 3009–3013. [Google Scholar] [CrossRef]
- Cai, X.; Shen, Y.; Li, W.; Zhan, W.; Zhang, F.; Xu, C.; Song, H. Low-Valent Tungsten-Catalyzed Controllable Oxidative Dehydrogenative Coupling of Anilines. Org. Lett. 2023, 25, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, J.; Tan, C.; Wu, W.; Jiang, H. DDQ-mediated regioselective C-S bond formation: Efficient access to allylic sulfides. Org. Chem. Front. 2018, 5, 3158–3162. [Google Scholar] [CrossRef]
- Li, C.; Liao, M.; He, Z.; Chen, Y.; Mai, J.; Dong, R.; Chen, J.; Chen, L. Metal-Free Oxidative Cross-Dehydrogenative Coupling of Alkenes with Thiophenols. ChemistrySelect 2023, 8, e202300845. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Li, X.; Gao, Y.; Tang, G.; Zhao, Y. Phosphorothiolation of Aryl Boronic Acids Using P(O)H Compounds and Elemental Sulfur. Org. Lett. 2016, 18, 1266–1269. [Google Scholar] [CrossRef]
- Xia, M.; Cheng, J. Catalyst- and oxidant-free coupling of disulfides with H-phosphine oxide: Construction of P–S bond leading to thiophosphinates. Tetrahedron Lett. 2016, 57, 4702–4704. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Y.-C.; Ding, W.-Z.; Zhu, T.; Liu, S.-Z.; Ren, X.; Zou, L.-H. Copper-Catalyzed Regioselective Sulfenylation of Indoles with Arylsulfonyl Chlorides. Asian J. Org. Chem. 2016, 5, 625–628. [Google Scholar] [CrossRef]


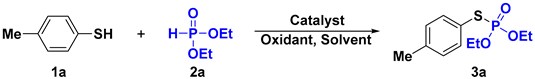 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Oxidant | Solvent | Yield (%) b |
| 1 | W(CO)6 | BQ | MeCN | 29 |
| 2 | W(CO)3(CH3CN)3 | BQ | MeCN | 21 |
| 3 | W(COD)2(CO)4 | BQ | MeCN | 23 |
| 4 | W(CO)6 | BQ | DMSO | 30 |
| 5 | W(CO)6 | BQ | DMF | trace |
| 6 | W(CO)6 | BQ | DMA | trace |
| 7 | W(CO)6 | BQ | DCE | 32 |
| 8 c | W(CO)6 | BQ | Dioxane | trace |
| 9 | W(CO)6 | BQ | Toluene | 24 |
| 10 | W(CO)6 | BQ | THF | 64 |
| 11 | W(CO)6 | DDQ | THF | 72 |
| 12 | W(CO)6 | NQ | THF | 61 |
| 13 | W(CO)6 | TBHP | THF | 10 |
| 14 | W(CO)6 | K2S2O8 | THF | 16 |
| 15 | W(CO)6 | air | THF | 70 |
| 16 | W(CO)6 | O2 | THF | 73 |
| 17 | W(CO)6 | O2 | THF | 50 |
| 18 d | W(CO)6 | O2 | THF | 90 |
| 19 e | W(CO)6 | O2 | THF | 85 |
| 20 | - | O2 | THF | 10 |
| 21 | W(CO)6 | N2 | THF | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Chen, Y.; Ye, F.; Chen, J.; Zheng, J. Low-Valent-Tungsten-Catalyzed Aerobic Oxidative Cross-Dehydrogenative Coupling Reaction. Molecules 2023, 28, 8071. https://doi.org/10.3390/molecules28248071
Li C, Chen Y, Ye F, Chen J, Zheng J. Low-Valent-Tungsten-Catalyzed Aerobic Oxidative Cross-Dehydrogenative Coupling Reaction. Molecules. 2023; 28(24):8071. https://doi.org/10.3390/molecules28248071
Chicago/Turabian StyleLi, Chunsheng, Yaoyang Chen, Feihua Ye, Junhua Chen, and Jia Zheng. 2023. "Low-Valent-Tungsten-Catalyzed Aerobic Oxidative Cross-Dehydrogenative Coupling Reaction" Molecules 28, no. 24: 8071. https://doi.org/10.3390/molecules28248071
APA StyleLi, C., Chen, Y., Ye, F., Chen, J., & Zheng, J. (2023). Low-Valent-Tungsten-Catalyzed Aerobic Oxidative Cross-Dehydrogenative Coupling Reaction. Molecules, 28(24), 8071. https://doi.org/10.3390/molecules28248071






