Ag Nanoparticles and Rod-Shaped AgCl Decorated Porous PEDOT as a Bifunctional Material for Hydrogen Evolution Catalyst and Supercapacitor Electrode
Abstract
:1. Introduction
2. Results and Discussion
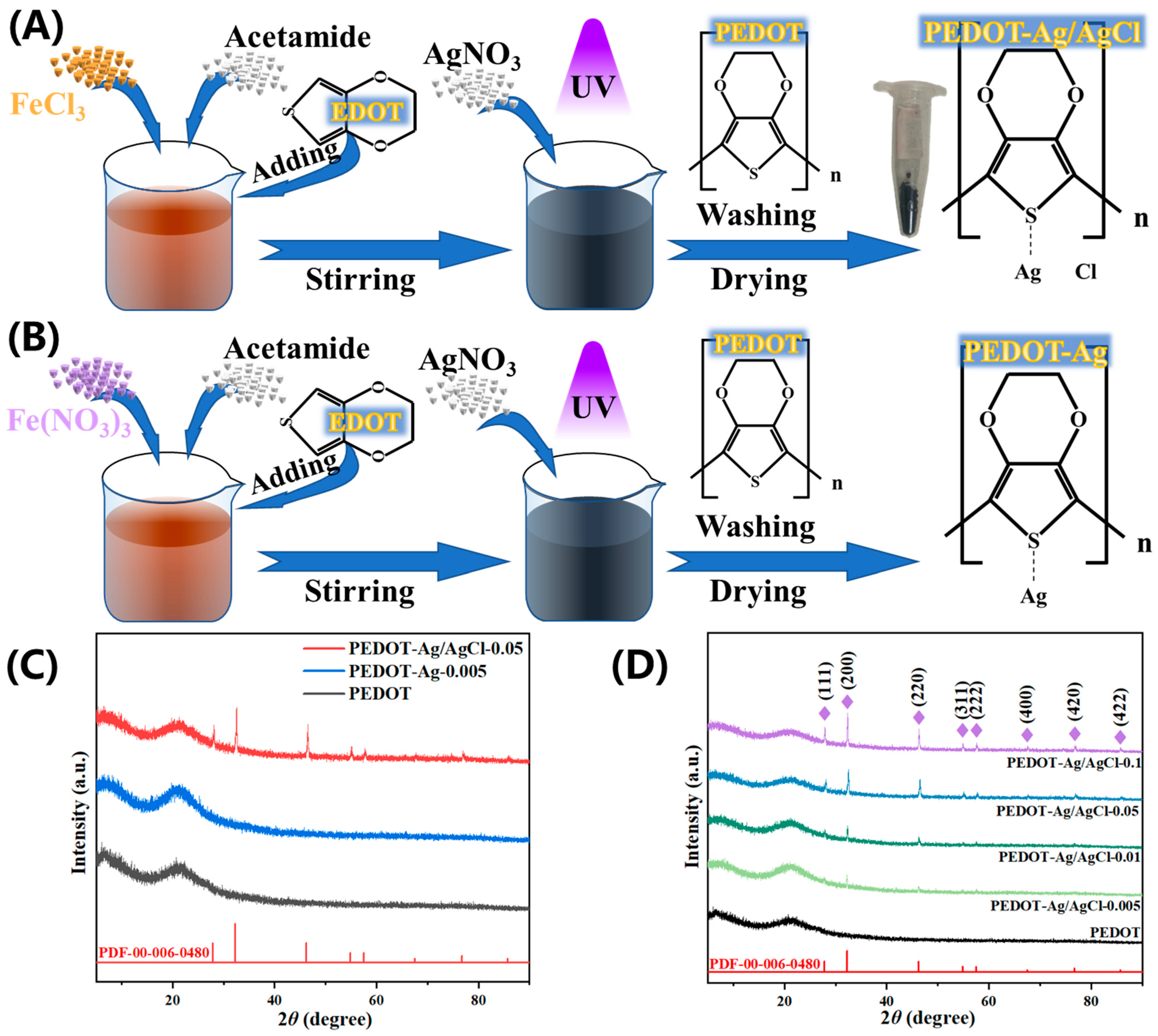
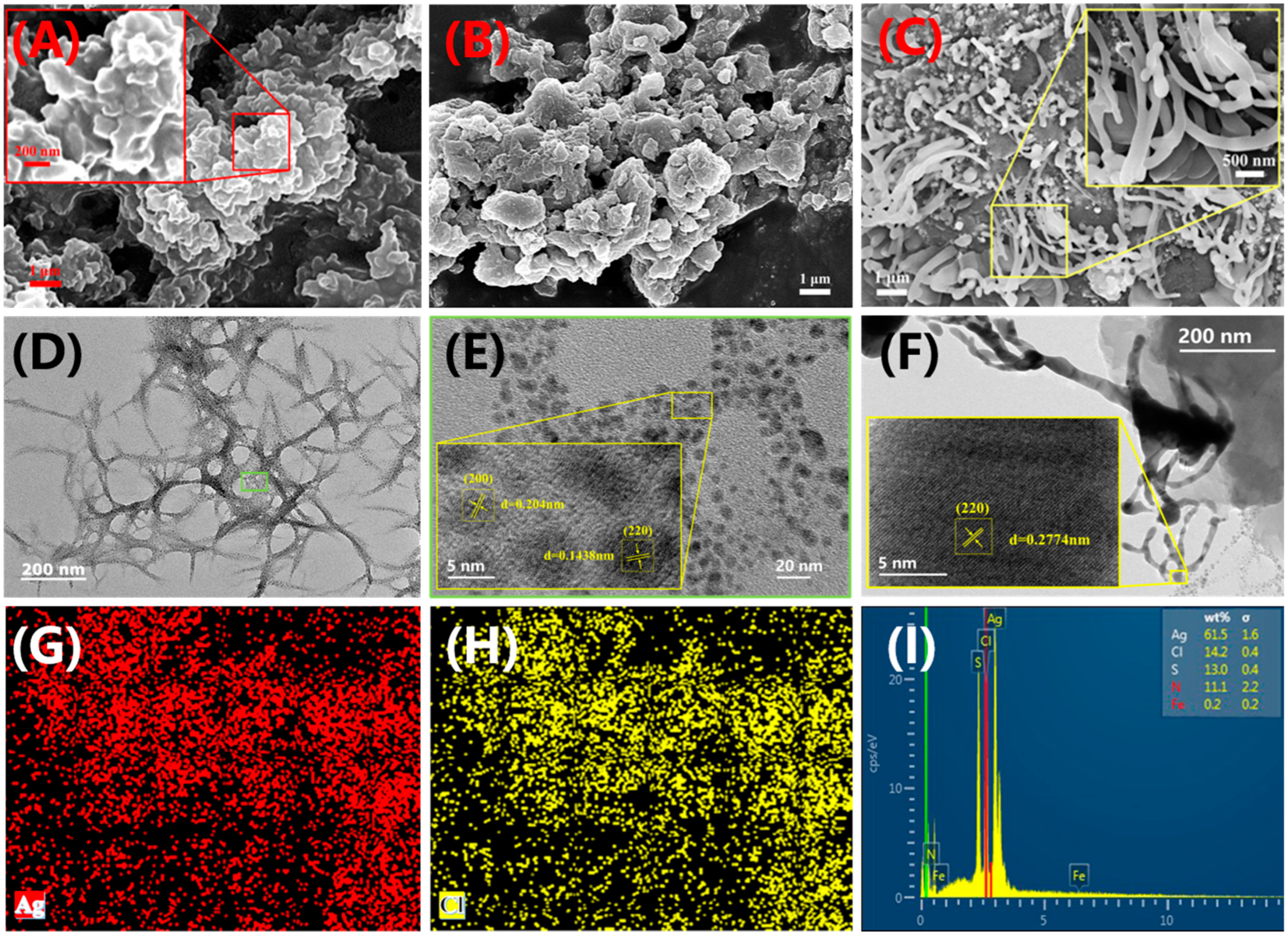
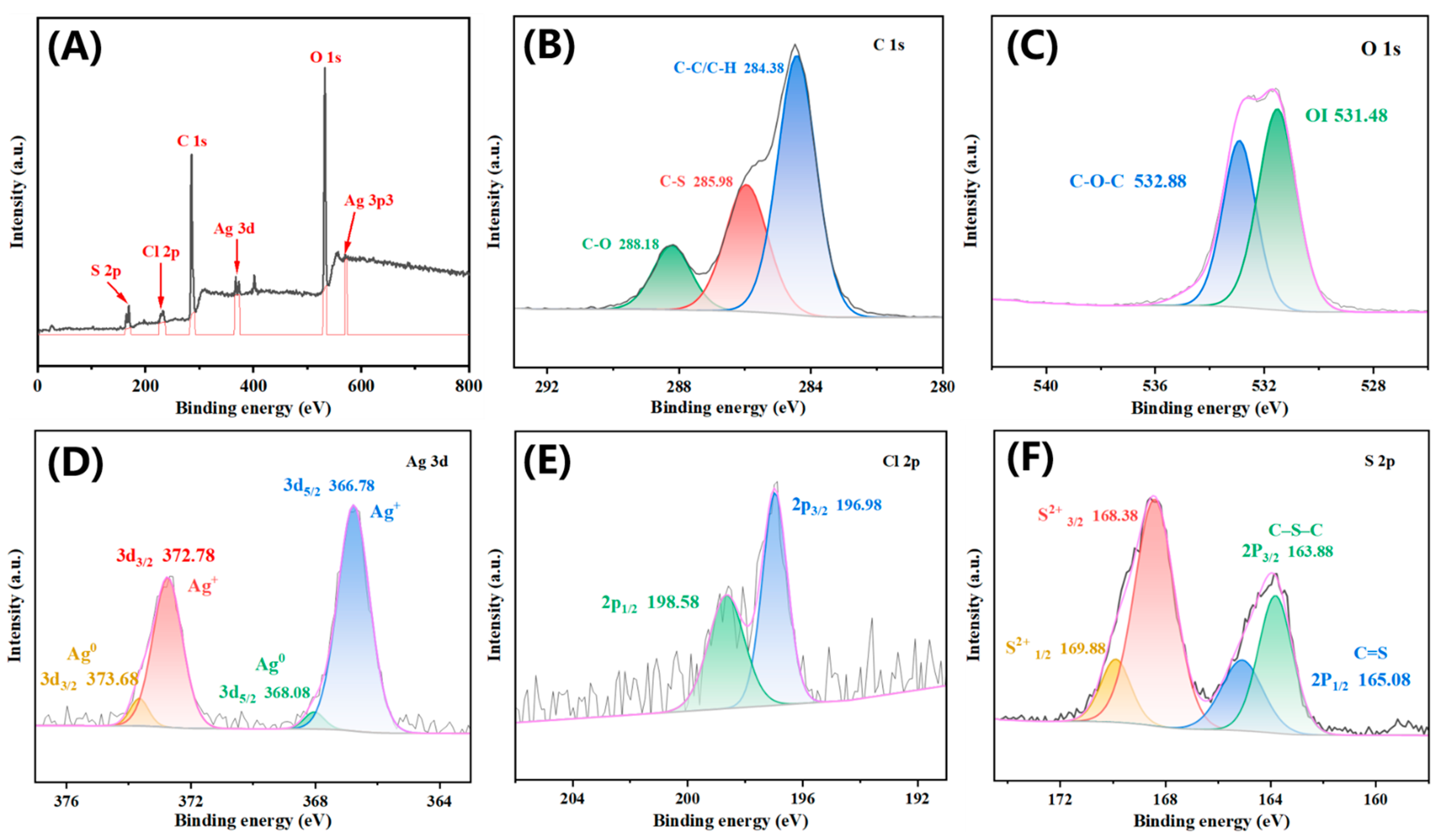
2.1. Synthesis and Characterisation
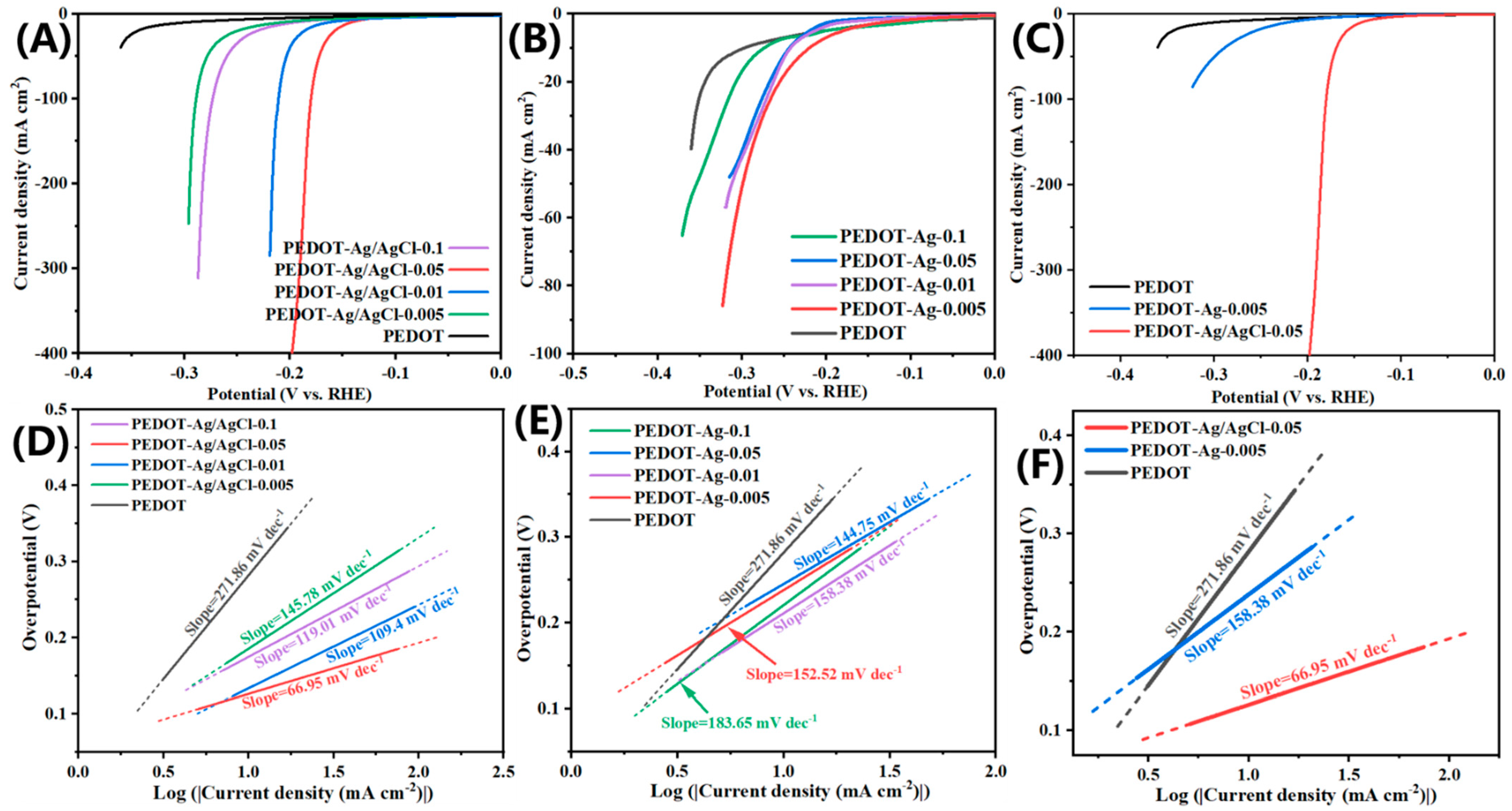
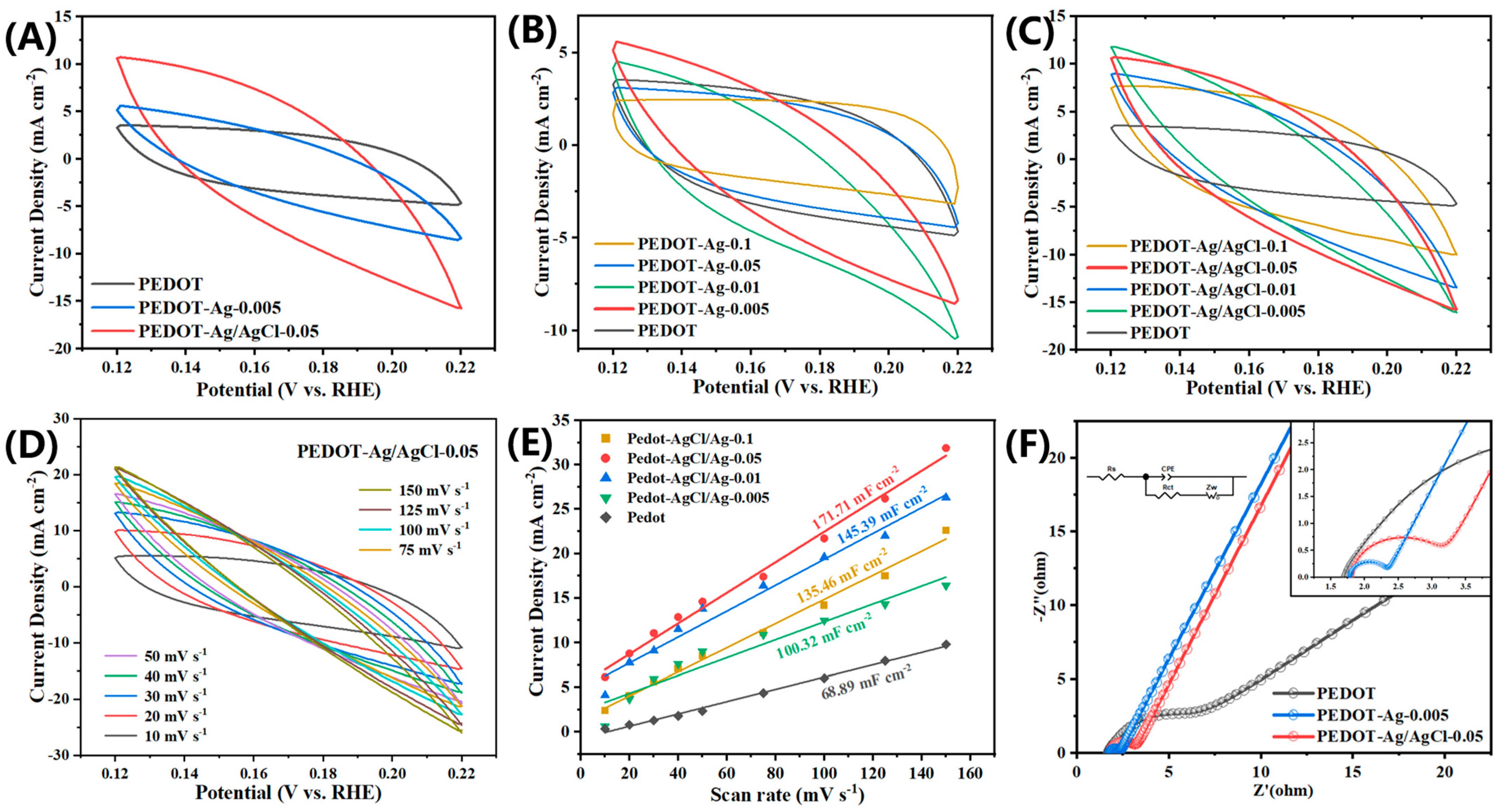
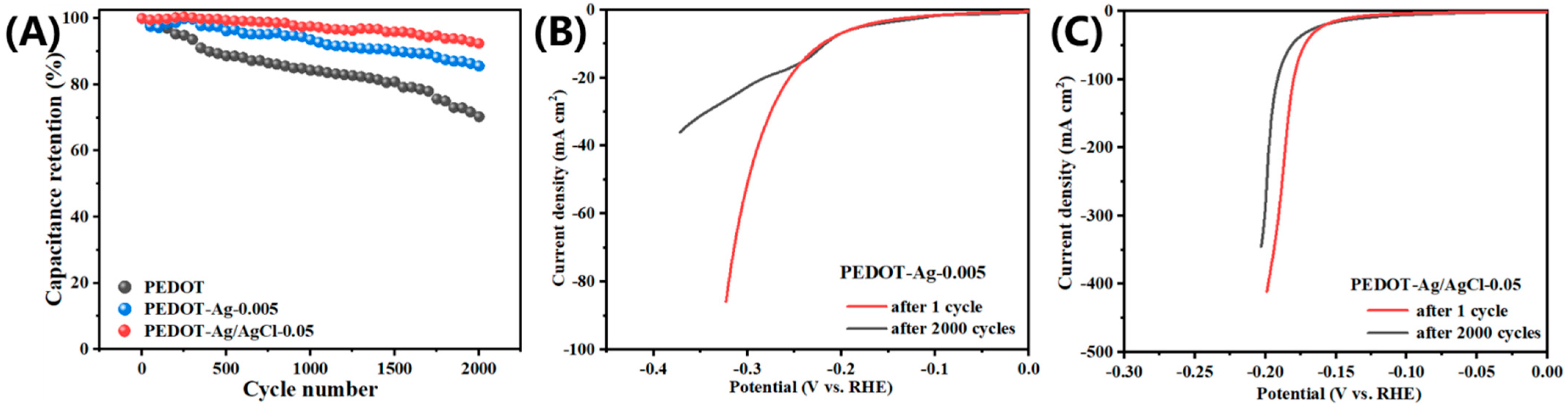
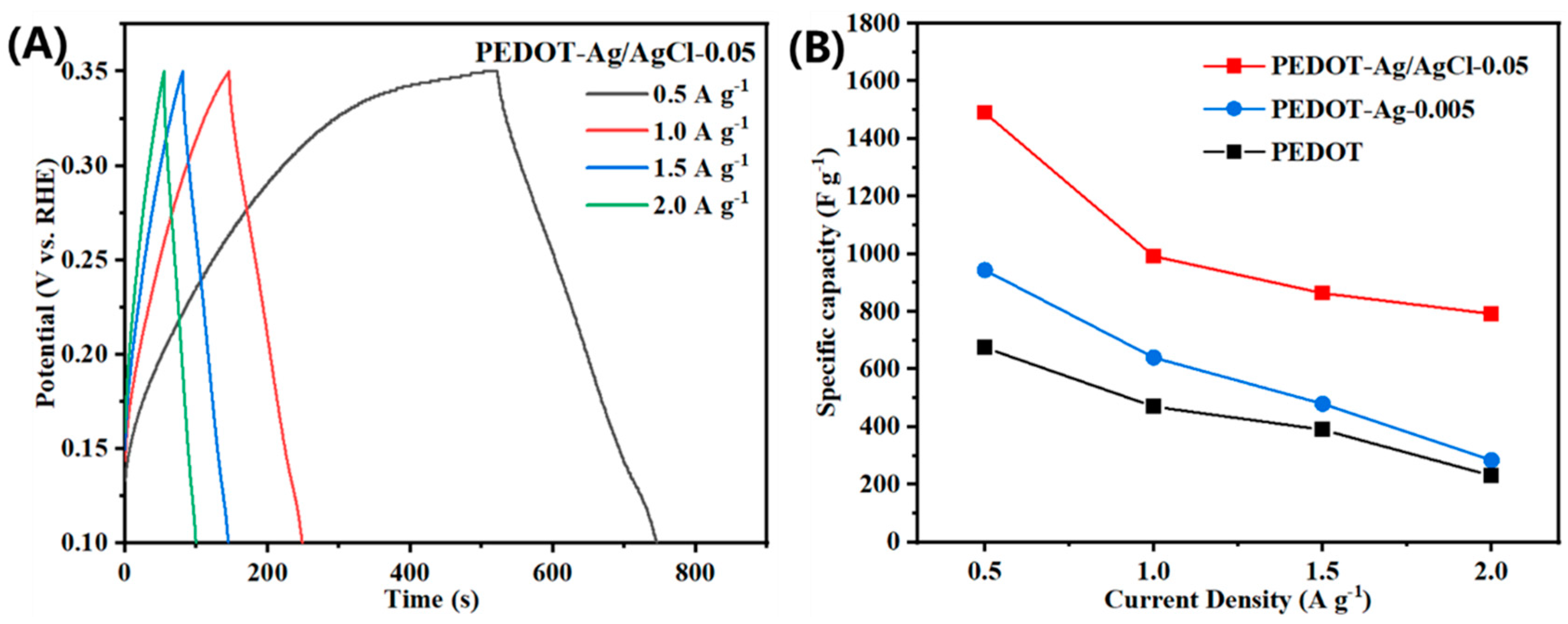
2.2. Electrochemical Properties
3. Experimental Section
3.1. Reagents and Materials
3.2. Preparation of PEDOT
3.3. Preparation of PEDOT-Ag and PEDOT-Ag/AgCl
3.4. Characterization and Electrochemical Measurements
3.5. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, G.; Wang, P.; Li, H.; Hu, B.; Sun, Y.; Huang, R.; Liu, L. Spin-state reconfiguration induced by alternating magnetic field for efficient oxygen evolution reaction. Nat. Commun. 2021, 12, 4827. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent Trends and Perspectives in Electrochemical Water Splitting with an Emphasis on Sulfide, Selenide, and Phosphide Catalysts of Fe, Co, and Ni: A Review. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, P.; Hu, B.; Shen, X.; Liu, C.; Tao, W.; Huang, P.; Liu, L. Spin-related symmetry breaking induced by half-disordered hybridization in BixEr2-xRu2O7 pyrochlores for acidic oxygen evolution. Nat. Commun. 2022, 13, 4106. [Google Scholar] [CrossRef]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Han, S.; Fang, Z.; Xiao, Z.; Lin, S. In Situ Filling of the Oxygen Vacancies with Dual Heteroatoms in Co3O4 for Efficient Overall Water Splitting. Molecules 2023, 28, 4134. [Google Scholar] [CrossRef] [PubMed]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Deng, X.; Mi, Y.; Liu, Y.; Sun, Y.; Cheng, Y.; Wang, W. Co, N co-doped carbon nanosheets coupled with NiCo2O4 as an efficient bifunctional oxygen catalyst for Zn-air batteries. Int. J. Hydrog. Energy 2023, 48, 13452–13459. [Google Scholar] [CrossRef]
- Wang, C.; Du, X.; Zhang, X. Controlled synthesis of W–Co3S4@Co3O4 as an environmentally friendly and low cost electrocatalyst for overall water splitting. Int. J. Hydrog. Energy 2023, 48, 12739–12752. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Lu, X. Construction of NiFe-Layered Double Hydroxides Arrays as Robust Electrocatalyst for Oxygen Evolution Reaction. Catalysts 2023, 13, 586. [Google Scholar] [CrossRef]
- Xu, X.; Shao, Z.; Jiang, S.P. High-Entropy Materials for Water Electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- Lee, J.; Son, N.; Shin, J.; Pandey, S.; Woo, J.S.; Kang, M. Highly efficient hydrogen evolution reaction performance and long-term stability of spherical Ni100−xFex alloy grown directly on a carbon paper electrode. J. Alloys. Compd. 2021, 869, 159265. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Wang, Y.; Ge, J.; Liu, C.; Xing, W. Enhanced electrocatalytic performance for the hydrogen evolution reaction through surface enrichment of platinum nanoclusters alloying with ruthenium in situ embedded in carbon. Energy Environ. Sci. 2018, 11, 1232–1239. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Zhang, X.; Cheng, C.; Xiao, L.; Zhou, M.; Dong, C.; Liu, H.; Du, X.; Yang, J. A Strongly Coupled Ag(S)@NiO/Nickel Foam Electrode Induced by Laser Direct Writing for Hydrogen Evolution at Ultrahigh Current Densities with Long-Term Durability. Small Methods 2023, 7, 2300461. [Google Scholar] [CrossRef]
- Huo, H.; Tian, H.; Nie, S.; He, Q.; Zhang, Z.; Liu, C. Ordered Ag composite electrode for an efficient alkaline hydrogen evolution reaction. J. Mater. Sci. Mater. El. 2023, 34, 1223. [Google Scholar] [CrossRef]
- Kong, L.J.; Xie, Y.M.; Chen, X.Y.; Xi, C.; Zhang, F.F.; Wang, M.; Shang, L.; Huang, Y.; Du, X.-W.; Kulinich, S.A. Ag-doped Cu nanosheet arrays for efficient hydrogen evolution reaction. Chem. Commun. 2023, 59, 6533–6535. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, L.W.; Wang, J.J. Investigating the Structural Evolution and Catalytic Activity of c-Co/Co3Mo Electrocatalysts for Alkaline Hydrogen Evolution Reaction. Molecules 2023, 28, 6986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power. Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Balasingam, S.K.; Lee, J.S.; Jun, Y. Few-layered MoSe2 nanosheets as an advanced electrode material for supercapacitors. Dalton. Trans. 2015, 44, 15491–15498. [Google Scholar] [CrossRef]
- Balasingam, S.K.; Thirumurugan, A.; Lee, J.S.; Jun, Y. Amorphous MoSx thin-film-coated carbon fiber paper as a 3D electrode for long cycle life symmetric supercapacitors. Nanoscale 2016, 8, 11787–11791. [Google Scholar] [CrossRef]
- Balasingam, S.K.; Lee, M.; Kim, B.H.; Lee, J.S.; Jun, Y. Freeze-dried MoS2 sponge electrodes for enhanced electrochemical energy storage. Dalton. Trans. 2017, 46, 2122–2218. [Google Scholar] [CrossRef]
- Zheng, S.; Zheng, L.; Zhu, Z.; Chen, J.; Kang, J.; Huang, Z.; Yang, D. MoS2 Nanosheet Arrays Rooted on Hollow rGO Spheres as Bifunctional Hydrogen Evolution Catalyst and Supercapacitor Electrode. Nano-Micro Lett. 2018, 10, 62. [Google Scholar] [CrossRef]
- Epstein, A.J. Electrically Conducting Polymers: Science and Technology. MRS Bull. 1997, 22, 16–23. [Google Scholar] [CrossRef]
- Kirchmeyer, S.; Reuter, K. Scientific importance, properties and growing applications of poly(3,4-ethylenedioxythiophene). J. Mater. Chem. 2005, 15, 2077–2088. [Google Scholar] [CrossRef]
- Anothumakkool, B.; Soni, R.; Bhange, S.N.; Kurungot, S. Novel scalable synthesis of highly conducting and robust PEDOT paper for a high performance flexible solid supercapacitor. Energy Environ. Sci. 2015, 8, 1339–1347. [Google Scholar] [CrossRef]
- Groenendaal, L.; Zotti, G.; Aubert, P.H.; Waybright, S.M.; Reynolds, J.R. Electrochemistry of Poly(3,4-alkylenedioxythiophene) Derivatives. Adv. Mater. 2003, 15, 855–879. [Google Scholar] [CrossRef]
- Ruano, G.; Molina, B.G.; Torras, J.; Alemán, C. Free-Standing, Flexible Nanofeatured Polymeric Films Prepared by Spin-Coating and Anodic Polymerization as Electrodes for Supercapacitors. Molecules 2021, 26, 4345. [Google Scholar] [CrossRef]
- Rajesh, M.; Raj, C.J.; Manikandan, R.; Kim, B.C.; Park, S.Y.; Yu, K.H. A high performance PEDOT/PEDOT symmetric supercapacitor by facile in-situ hydrothermal polymerization of PEDOT nanostructures on flexible carbon fibre cloth electrodes. Mater. Today Energy 2017, 6, 96–104. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Yang, Y.; Zhao, Y.; Yang, W.; Mao, X.; He, X.; Li, S. The preparation and electrochemical properties of PEDOT:PSS/MnO2/PEDOT ternary film and its application in flexible micro-supercapacitor. Electrochim. Acta. 2016, 193, 199–205. [Google Scholar] [CrossRef]
- Chen, G.; Wang, T.; Zhang, J.; Liu, P.; Sun, H.; Zhuang, X.; Chen, M.; Feng, X. Accelerated Hydrogen Evolution Kinetics on NiFe-Layered Double Hydroxide Electrocatalysts by Tailoring Water Dissociation Active Sites. Adv. Mater. 2018, 30, 1706279. [Google Scholar] [CrossRef]
- Behrooz, N.; Ghaffarinejad, A.; Sadeghi, N. Ag/Cu nano alloy as an electrocatalyst for hydrogen production. J. Electroanal. Chem. 2016, 782, 1–8. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Xue, B.; Zhang, J.; Xu, Z.; Wang, L.; Gao, X.; Luo, F.; Li, F. Bifunctional Water Splitting Performance of NiFe LDH Improved by Pd2+ Doping. ChemElectroChem 2023, 10, e202201025. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, B.; Ding, Z.; Li, Y.; Ma, D.; Li, M.; Sun, Y.; Wang, C.; Liu, Y.; Sun, X. Enhancement of power conversion efficiency of Ag-substituted Cu2ZnSn(S,Se)4 solar cells via tuning Cu2+/(Cu++Cu2+) percentage in precursor solution. Sol. Energy Mater. Sol. Cells 2023, 261, 112502. [Google Scholar] [CrossRef]
- Dikmen, Z.; Dikmen, G.; Bütün, V. Fluorophore-assisted green fabrication of flexible and cost-effective Ag nanoparticles decorated PVA nanofibers for SERS based trace detection. J. Photoch. Photobio. A 2023, 445, 115074. [Google Scholar] [CrossRef]
- Hu, Z.; Xin, Y.; Fu, Q. Ultrahigh sensitivity and wide strain range of porous pressure sensor based on binary conductive fillers by in-situ polymerization. J. Polym. Res. 2021, 28, 134. [Google Scholar] [CrossRef]
- Selvaganesh, S.V.; Mathiyarasu, J.; Phani, K.L.N.; Yegnaraman, V. Chemical Synthesis of PEDOT–Au Nanocomposite. Nanoscale. Res. Lett. 2007, 2, 546. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, J.; Dong, W.; Chen, H.; Huang, X.; Sun, B.; Chen, L. Temperature dependent conductivity of vapor-phase polymerized PEDOT films. Synthetic. Met. 2013, 176, 86–91. [Google Scholar] [CrossRef]
- Zhang, L.; Jamal, R.; Zhao, Q.; Wang, M.; Abdiryim, T. Preparation of PEDOT/GO, PEDOT/MnO2, and PEDOT/GO/MnO2 nanocomposites and their application in catalytic degradation of methylene blue. Nanoscale. Res. Lett. 2015, 10, 148. [Google Scholar] [CrossRef]
- Lou, Z.; Huang, B.; Wang, P.; Wang, Z.; Qin, X.; Zhang, X.; Cheng, H.; Zhenga, Z.; Daib, Y. The synthesis of the near-spherical AgCl crystal for visible light photocatalytic applications. Dalton Trans. 2011, 40, 4104–4110. [Google Scholar] [CrossRef]
- Ashok, K.D.; Palanichamy, V.; Roopan, S.M. Photocatalytic action of AgCl nanoparticles and its antibacterial activity. J. Photoch. Photobio. B 2014, 138, 302–306. [Google Scholar] [CrossRef]
- Han, C.; Ge, L.; Chen, C.; Li, Y.; Zhao, Z.; Xiao, X.; Li, Z.; Zhang, J. Site-selected synthesis of novel Ag@AgCl nanoframes with efficient visible light induced photocatalytic activity. J. Mater. Chem. A 2014, 2, 12594–12600. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Lou, Z.; Zhang, X.; Qin, X.; Dai, Y.; Zheng, Z.; Wang, X. Synthesis of Highly Efficient Ag@AgCl Plasmonic Photocatalysts with Various Structures. Chem. Eur. J. 2010, 16, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Liu, Y.; Tong, Z.; Zhu, Y.; Cao, K.; Chen, W.; Zhao, D.; Yu, H. Micro-interfacial polymerization of porous PEDOT for printable electronic devices. EcoMat. 2022, 5, e12288. [Google Scholar] [CrossRef]
- Nocchetti, M.; Donnadio, A.; Ambrogi, V.; Andreani, P.; Bastianini, M.; Pietrella, D.; Latterini, L. Ag/AgCl nanoparticle decorated layered double hydroxides: Synthesis, characterization and antimicrobial properties. J. Mater. Chem. B 2013, 1, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Noh, H.; Lee, J.; Nah, I.W.; Cho, W.I.; Kim, H.T. In situ coating of Poly(3,4-ethylenedioxythiophene) on sulfur cathode for high performance lithium-sulfur batteries. J. Power. Sources 2016, 332, 72–78. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Xu, Y.; Guo, J.; Zhang, S.; Lu, Y. A Ti3C2TX@PEDOT composite for electrode materials of supercapacitors. J. Electroanal. Chem. 2021, 881, 114958. [Google Scholar] [CrossRef]
- Che, Y.; Zhang, H.; Abdiryim, T.; Jamal, R.; Kadir, A.; Helil, Z.; Liu, H. Ultraviolet photodetectors based on TiO2 nanorod arrays/PEDOT-type conducting polymers. Opt. Mater. 2021, 122, 111805. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, K.; Gerard Moloney, M.; Qiu, J. Strain sensing metacomposites of polyaniline/silver nanoparticles/carbon foam. Compos. Part A-Appl. S. 2021, 144, 106351. [Google Scholar] [CrossRef]
- Shao, W.; Chen, Y.R.; Xie, F.; Zhang, H.; Wang, H.T.; Chang, N. Facile construction of a ZIF-67/AgCl/Ag heterojunction via chemical etching and surface ion exchange strategy for enhanced visible light driven photocatalysis. RSC Adv. 2020, 10, 38174–38183. [Google Scholar] [CrossRef]
- Greczynski, G.; Kugler, T.; Keil, M.; Osikowicz, W.; Fahlman, M.; Salaneck, W.R. Photoelectron spectroscopy of thin films of PEDOT–PSS conjugated polymer blend: A mini-review and some new results. J. Electron. Spectro. 2001, 121, 1–17. [Google Scholar] [CrossRef]
- Fu, W.C.; Hsieh, Y.T.; Wu, T.Y.; Sun, I.W. Electrochemical Preparation of Porous Poly(3,4-ethylenedioxythiophene) Electrodes from Room Temperature Ionic Liquids for Supercapacitors. J. Electrochem. Soc. 2016, 163, G61. [Google Scholar] [CrossRef]
- Fu, Q.; Han, J.; Wang, X.; Xu, P.; Yao, T.; Zhong, J.; Zhong, W.; Liu, S.; Gao, T.; Zhang, Z.; et al. 2D Transition Metal Dichalcogenides: Design, Modulation, and Challenges in Electrocatalysis. Adv. Mater. 2021, 33, 1907818. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Akshaya, K.B.; Sudhakar, Y.N.; Vijayan, A.; Varghese, A. Amorphous Ru-Pi nanoclusters decorated on PEDOT modified carbon fibre paper as a highly efficient electrocatalyst for oxygen evolution reaction. Mater. Chem. Phys. 2021, 267, 124650. [Google Scholar] [CrossRef]
- Chamani, S.; Sadeghi, E.; Unal, U.; Peighambardoust, N.S.; Aydemir, U. Tuning Electrochemical Hydrogen-Evolution Activity of CoMoO4 through Zn Incorporation. Catalysts 2023, 13, 798. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Shaikh, S.F.; Ubaidullah, M.; Ghanem, M.A.; Mane, R.S. Self-grown one-dimensional nickel sulfo-selenide nanostructured electrocatalysts for water splitting reactions. Int. J. Hydrog. Energy 2020, 45, 15904–15914. [Google Scholar] [CrossRef]
- Pataniya, P.M.; Sumesh, C.K. Enhanced electrocatalytic hydrogen evolution reaction by injection of photogenerated electrons in Ag/WS2 nanohybrids. Appl. Surf. Sci. 2021, 563, 150323. [Google Scholar] [CrossRef]
- Safavi, A.; Kazemi, S.H.; Kazemi, H. Electrocatalytic Behaviors of Silver-Palladium Nanoalloys Modified Carbon Ionic Liquid Electrode towards Hydrogen Evolution Reaction. Fuel 2014, 118, 156–162. [Google Scholar] [CrossRef]
- Andrabi, R.; Pragati, F.; Basu, M.; Pande, S. Decoration of Carbon Nitride Surface with Bimetallic Nanoparticles (Ag/Pt, Ag/Pd, and Ag/Au) via Galvanic Exchange for Hydrogen Evolution Reaction. J. Phys. Chem. C 2017, 121, 19548–19558. [Google Scholar]
- Zhao, T.; Gao, J.; Wu, J.; He, P.; Li, Y.; Yao, J. Highly Active Cobalt/Tungsten Carbide@N-Doped Porous Carbon Nanomaterials Derived from Metal-Organic Frameworks as Bifunctional Catalysts for Overall Water Splitting. Energy Technol. 2019, 7, 1800969. [Google Scholar] [CrossRef]
- Doan, T.L.L.; Tran, D.T.; Nguyen, D.C.; Kim, D.H.; Kim, N.H.; Lee, J.H. Rational Engineering CoxOy Nanosheets via Phosphorous and Sulfur Dual-Coupling for Enhancing Water Splitting and Zn–Air Battery. Adv. Funct. Mater. 2021, 31, 2007822. [Google Scholar] [CrossRef]
- Du, Q.; Zhao, R.; Chen, X.; Liu, L.; Zhang, S.; Guo, T.; Du, J.; Li, J. Synthesis of Ultrathin and Grid-Structural Carbon Nanosheets Coupled with Mo2C for Electrocatalytic Hydrogen Production. Chem. Asian. J. 2021, 16, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; He, J.; Sahoo, S.; Luo, Z.; Zhong, W.; Chen, S.Y.; Guild, C.; Jafari, T.; Dutta, B.; Cetegen, S.A.; et al. Reduced Graphene Oxide Supported Nickel–Manganese–Cobalt Spinel Ternary Oxide Nanocomposites and Their Chemically Converted Sulfide Nanocomposites as Efficient Electrocatalysts for Alkaline Water Splitting. ACS Catal. 2017, 7, 819–832. [Google Scholar] [CrossRef]
- Alobaid, A.; Wang, C.; Adomaitis, R.A. Mechanism and Kinetics of HER and OER on NiFe LDH Films in an Alkaline Electrolyte. J. Electrochem. Soc. 2018, 165, J3395. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Jebakumar Immanuel Edison, T.N.; Sundramoorthy, A.K.; Karthik, N.; Sangaraju, S.; Choi, S.T.; Lee, Y.R. Biowaste-Derived Heteroatom-Doped Porous Carbon as a Sustainable Electrocatalyst for Hydrogen Evolution Reaction. Catalysts 2023, 13, 542. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sust. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Wang, H.; Diao, Y.; Lu, Y.; Yang, H.; Zhou, Q.; Chrulski, K.; D’arcy, J.M. Energy storing bricks for stationary PEDOT supercapacitors. Nat. Commun. 2020, 11, 3882. [Google Scholar] [CrossRef]
| Catalysts | Electrolyte | J (mA·cm−2) | Overpotential (mV) | Tafel Slope (mV·dec−1) | Ref. |
|---|---|---|---|---|---|
| CFP/PEDOT/Ru-Pi | 1.0 M PBS | 10 | 350 | - | [54] |
| Co0.5Zn0.5MoO4 | 1.0 M KOH | 10 | 201 | 162.7 | [55] |
| NiSSe | 1.0 M KOH | 10 | 154 | 125 | [56] |
| Ag(10)WS2 | 0.5 M H2SO4 | 10 | 170 | 40 | [57] |
| Ni95Fe5/CP | 1.0 M KOH | 20 | 130 | 95.4 | [11] |
| Ag-Pd nanoalloy | 0.5 M H2SO4 | 10 | 270 | 156 | [58] |
| C3N4/AgPd | 0.5 M H2SO4 | 10 | 308 | 120 | [59] |
| PEDOT-Ag-0.005 | 1.0 M KOH | 20 | 255 | 158.38 | This work |
| PEDOT-Ag/AgCl-0.05 | 1.0 M KOH | 20 | 157 | 66.95 | This work |
| Catalysts | Electrolyte | Rs (Ω cm2) | Rct (Ω cm2) |
|---|---|---|---|
| PEDOT | 1.0 M KOH | 1.69 | 0.6 |
| PEDOT-Ag-0.005 | 1.0 M KOH | 1.80 | 1.7 |
| PEDOT-Ag/AgCl-0.05 | 1.0 M KOH | 1.78 | 5.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, H.; Shu, L.; Li, Z.; Bai, J.; Wen, Y.; Zhu, L.; Geng, Y.; Qin, H. Ag Nanoparticles and Rod-Shaped AgCl Decorated Porous PEDOT as a Bifunctional Material for Hydrogen Evolution Catalyst and Supercapacitor Electrode. Molecules 2023, 28, 8063. https://doi.org/10.3390/molecules28248063
Zhang C, Wang H, Shu L, Li Z, Bai J, Wen Y, Zhu L, Geng Y, Qin H. Ag Nanoparticles and Rod-Shaped AgCl Decorated Porous PEDOT as a Bifunctional Material for Hydrogen Evolution Catalyst and Supercapacitor Electrode. Molecules. 2023; 28(24):8063. https://doi.org/10.3390/molecules28248063
Chicago/Turabian StyleZhang, Chunyong, Haoyu Wang, Li Shu, Zhe Li, Jirong Bai, Yinpin Wen, Lin Zhu, Yin Geng, and Hengfei Qin. 2023. "Ag Nanoparticles and Rod-Shaped AgCl Decorated Porous PEDOT as a Bifunctional Material for Hydrogen Evolution Catalyst and Supercapacitor Electrode" Molecules 28, no. 24: 8063. https://doi.org/10.3390/molecules28248063
APA StyleZhang, C., Wang, H., Shu, L., Li, Z., Bai, J., Wen, Y., Zhu, L., Geng, Y., & Qin, H. (2023). Ag Nanoparticles and Rod-Shaped AgCl Decorated Porous PEDOT as a Bifunctional Material for Hydrogen Evolution Catalyst and Supercapacitor Electrode. Molecules, 28(24), 8063. https://doi.org/10.3390/molecules28248063






