Novel Janus Kinase Inhibitors in the Treatment of Dermatologic Conditions
Abstract
:1. Introduction
2. JAK/STAT Pathway
3. Janus Kinase Inhibitors
4. Dermatological Conditions Where JAK Inhibitors Are Approved by the FDA or EMA
4.1. Atopic Dermatitis
4.2. Alopecia Areata
4.3. Non-Segmental Vitiligo
4.4. Psoriasis
4.5. JAK Inhibitors in Other Dermatology Conditions
5. Side Effects of Janus Kinase Inhibitors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solimani, F.; Meier, K.; Ghoreschi, K. Emerging Topical and Systemic JAK Inhibitors in Dermatology. Front. Immunol. 2019, 10, 2847. [Google Scholar] [CrossRef]
- Eyerich, K.; Eyerich, S. Immune response patterns in non-communicable inflammatory skin diseases. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, A.S.; Röcken, M.; Ghoreschi, K. Cutaneous immunology: Basics and new concepts. Semin. Immunopathol. 2016, 38, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Shalabi, M.M.K.; Garcia, B.; Coleman, K.; Siller, A., Jr.; Miller, A.; Tyring, S.K. Janus Kinase and Tyrosine Kinase Inhibitors in Dermatology: A Review of Their Utilization, Safety Profile and Future Applications. Skin Ther. Lett. 2022, 27, 4–9. [Google Scholar]
- Shah, R.J.; Banerjee, S.; Raychaudhuri, S.; Raychaudhuri, S.P. JAK-STAT inhibitors in Immune mediated diseases: An Overview. Indian J. Dermatol. Venereol. Leprol. 2023, 89, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.; Cheema, K.S.; Raychaudhuri, S.K.; Raychaudhuri, S.P. Janus kinase-signal transducers and activators of transcription cell signaling in Spondyloarthritis: Rationale and evidence for JAK inhibition. Curr. Opin. Rheumatol. 2021, 33, 348–355. [Google Scholar] [CrossRef]
- Liu, C.; Kieltyka, J.; Fleischmann, R.; Gadina, M.; O’Shea, J.J. A Decade of JAK Inhibitors: What Have We Learned and What May Be the Future? Arthritis Rheumatol. 2021, 73, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Bousoik, E.; Montazeri Aliabadi, H. “Do We Know Jack” About JAK? A Closer Look at JAK/STAT Signaling Pathway. Front. Oncol. 2018, 8, 287. [Google Scholar] [CrossRef]
- Smith, P.; Yao, W.; Shepard, S.; Covington, M.; Lee, J.; Lofland, J.; Naim, A.; Sheth, T.; Parikh, B.; Yeleswaram, S. Developing a JAK Inhibitor for Targeted Local Delivery: Ruxolitinib Cream. Pharmaceutics 2021, 13, 1044. [Google Scholar] [CrossRef] [PubMed]
- Tsiogka, A.; Kyriazopoulou, M.; Kontochristopoulos, G.; Nicolaidou, E.; Stratigos, A.; Rigopoulos, D.; Gregoriou, S. The JAK/STAT Pathway and Its Selective Inhibition in the Treatment of Atopic Dermatitis: A Systematic Review. J. Clin. Med. 2022, 11, 4431. [Google Scholar] [CrossRef]
- Caiazzo, G.; Caiazzo, A.; Napolitano, M.; Megna, M.; Potestio, L.; Fornaro, L.; Parisi, M.; Luciano, M.A.; Ruggiero, A.; Testa, A.; et al. The Use of JAK/STAT Inhibitors in Chronic Inflammatory Disorders. Clin. Med. 2023, 12, 2865. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Yao, Q.; Gu, X.; Shi, Q.; Yuan, X.; Chu, Q.; Bao, Z.; Lu, J.; Li, L. Evolving cognition of the JAK-STAT signaling pathway: Autoimmune disorders and cancer. Signal Transduct. Target Ther. 2023, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Rusiñol, L.; Puig, L. Tyk2 Targeting in Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 3391. [Google Scholar] [CrossRef]
- Liau, N.P.D.; Laktyushin, A.; Morris, R.; Sandow, J.J.; Nicola, N.A.; Kershaw, N.J.; Babon, J.J. Enzymatic Characterization of Wild-Type and Mutant Janus Kinase 1. Cancers 2019, 11, 1701. [Google Scholar] [CrossRef]
- Clark, J.D.; Flanagan, M.E.; Telliez, J.B. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J. Med. Chem. 2014, 57, 5023–5038. [Google Scholar] [CrossRef]
- Dodington, D.W.; Desai, H.R.; Woo, M. JAK/STAT—Emerging Players in Metabolism. Trends Endocrinol. Metab. 2018, 29, 55–65. [Google Scholar] [CrossRef]
- Mahjoor, M.; Mahmoudvand, G.; Farokhi, S.; Shadab, A.; Kashfi, M.; Afkhami, H. Double-edged sword of JAK/STAT signaling pathway in viral infections: Novel insights into virotherapy. Cell Commun. Signal. 2023, 21, 272. [Google Scholar] [CrossRef]
- Hu, Q.; Bian, Q.; Rong, D.; Wang, L.; Song, J.; Huang, H.S.; Zeng, J.; Mei, J.; Wang, P.Y. JAK/STAT pathway: Extracellular signals, diseases, immunity, and therapeutic regimens. Front. Bioeng. Biotechnol. 2023, 11, 1110765. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Luo, F. The Role of JAK/STAT Pathway in Fibrotic Diseases: Molecular and Cellular Mechanisms. Biomolecules 2023, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- Lensing, M.; Jabbari, A. An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. Front. Immunol. 2022, 13, 955035. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.D.; Kuo, F.I.; Smith, P.A. Targeting the Janus Kinase Family in Autoimmune Skin Diseases. Front. Immunol. 2019, 10, 2342. [Google Scholar] [CrossRef]
- American Academy of Dermatology Association. Jak Inhibitors: What Your Dermatologist Wants You to Know. Available online: https://www.aad.org/public/diseases/a-z/jak-inhibitors (accessed on 18 October 2023).
- Cinats, A.; Heck, E.; Robertson, L. Janus Kinase Inhibitors: A Review of Their Emerging Applications in Dermatology. Skin Ther. Lett. 2018, 23, 5–9. [Google Scholar]
- Reich, K.; Kabashima, K.; Peris, K.; Silverberg, J.I.; Eichenfield, L.F.; Bieber, T.; Kaszuba, A.; Kolodsick, J.; Yang, F.E.; Gamalo, M.; et al. Efficacy and Safety of Baricitinib Combined with Topical Corticosteroids for Treatment of Moderate to Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 1333–1343. [Google Scholar] [CrossRef]
- Samuel, C.; Cornman, H.; Kambala, A.; Kwatra, S.G. A Review on the Safety of Using JAK Inhibitors in Dermatology: Clinical and Laboratory Monitoring. Dermatol. Ther. 2023, 13, 729–749. [Google Scholar] [CrossRef]
- Corbella-Bagot, L.; Riquelme-McLoughlin, C.; Morgado-Carrasco, D. Long-Term Safety Profile and Off-Label Use of JAK Inhibitors in Dermatological Disorders. Actas Dermosifiliogr. 2023, 114, 784–801. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Zhang, H.; Guo, Y.; Yao, Z. Update on the Pathogenesis and Therapy of Atopic Dermatitis. Clin. Rev. Allergy Immunol. 2021, 61, 324–338. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Hamann, C.R.; Linneberg, A.; Dantoft, T.M.; Skov, L.; Gislason, G.H.; Wu, J.J.; Egeberg, A. Atopic dermatitis is associated with anxiety, depression, and suicidal ideation, but not with psychiatric hospitalization or suicide. Allergy 2018, 73, 214–220. [Google Scholar] [CrossRef]
- Wang, C.H.; Fu, Y.; Chi, C.C. Association of atopic dermatitis with inflammatory bowel disease: A systematic review and meta-analysis. Dermatol. Sin. 2020, 38, 159–165. [Google Scholar] [CrossRef]
- Mowen, K.A.; Glimcher, L.H. Signaling pathways in Th2 development. Immunol. Rev. 2004, 202, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Kamata, M.; Tada, Y. Optimal Use of Jak Inhibitors and Biologics for Atopic Dermatitis on the Basis of the Current Evidence. JID Innov. 2023, 3, 100195. [Google Scholar] [CrossRef] [PubMed]
- Chovatiya, R.; Paller, A.S. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin. Immunol. 2021, 148, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhang, H.; Chan, L.S. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT 2013, 2, e24137. [Google Scholar] [CrossRef]
- Brauweiler, A.M.; Goleva, E.; Leung, D.Y.M. Th2 cytokines increase Staphylococcus aureus alpha toxin-induced keratinocyte death through the signal transducer and activator of transcription 6 (STAT6). J. Investig. Dermatol. 2014, 134, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Boniface, K.; Bernard, F.X.; Garcia, M.; Gurney, A.L.; Lecron, J.C.; Morel, F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J. Immunol. 2005, 174, 3695–3702. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.; Boguniewicz, M.; Howell, M.D. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 2008, 126, 332–337. [Google Scholar] [CrossRef]
- Gao, L.; Bin, L.; Rafaels, N.M.; Huang, L.; Potee, J.; Ruczinski, I.; Beaty, T.H.; Paller, A.S.; Schneider, L.C.; Gallo, R.; et al. Targeted deep sequencing identifies rare loss-of-function variants in IFNGR1 for risk of atopic dermatitis complicated by eczema herpeticum. J. Allergy Clin. Immunol. 2015, 136, 1591–1600. [Google Scholar] [CrossRef]
- Papp, K.; Szepietowski, J.C.; Kircik, L.; Toth, D.; Eichenfield, L.F.; Forman, S.B.; Kuligowski, M.E.; Kallender, H.; Sun, K.; Ren, H.; et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: Results from two phase 3 studies. J. Am. Acad. Dermatol. 2023, 88, 1008–1016. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Teixeira, H.D.; Simpson, E.L.; Papp, K.A.; Pangan, A.L.; Blauvelt, A.; Thaçi, D.; Chu, C.Y.; Hong, H.C.; Katoh, N.; et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): Results from two replicate double-blind, randomised controlled phase 3 trials. Lancet 2021, 397, 2151–2168, Erratum in Lancet 2021, 397, 2150. [Google Scholar] [CrossRef]
- Blauvelt, A.; Ladizinski, B.; Prajapati, V.H.; Laquer, V.; Fischer, A.; Eisman, S.; Hu, X.; Wu, T.; Calimlim, B.M.; Kaplan, B.; et al. Efficacy and safety of switching from dupilumab to upadacitinib versus continuous upadacitinib in moderate-to-severe atopic dermatitis: Results from an open-label extension of the phase 3, randomized, controlled trial (Heads Up). J. Am. Acad. Dermatol. 2023, 89, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Cohen, S.B.; Winthrop, K.L.; Nash, P.; Irvine, A.D.; Deodhar, A.; Mysler, E.; Tanaka, Y.; Liu, J.; Lacerda, A.P.; et al. Safety profile of upadacitinib over 15,000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open 2023, 9, e002735. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Silverberg, J.I.; Lynde, C.W.; Bieber, T.; Eisman, S.; Zdybski, J.; Gubelin, W.; Simpson, E.L.; Valenzuela, F.; Criado, P.R.; et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: Results from the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN phase 3 trial. J. Am. Acad. Dermatol. 2022, 86, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Silverberg, J.I.; Thyssen, J.P.; Viguier, M.; Thaçi, D.; de Bruin-Weller, M.; Weidinger, S.; Chan, G.; DiBonaventura, M.; Biswas, P.; et al. Efficacy and Safety of Abrocitinib in Patients with Severe and/or Difficult-to-Treat Atopic Dermatitis: A Post Hoc Analysis of the Randomized Phase 3 JADE COMPARE Trial. Am. J. Clin. Dermatol. 2023, 24, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhao, Y.; Zhang, J. Efficacy and safety of abrocitinib and upadacitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: A systematic review and meta-analysis. Heliyon 2023, 9, e16704. [Google Scholar] [CrossRef] [PubMed]
- Thyssen, J.P.; Werfel, T.; Barbarot, S.; Hunter, H.J.A.; Pierce, E.; Sun, L.; Cirri, L.; Buchanan, A.S.; Lu, N.; Wollenberg, A. Maintained improvement in physician- and patient-reported outcomes with baricitinib in adults with moderate-to-severe atopic dermatitis who were treated for up to 104 weeks in a randomized trial. J. Dermatolog. Treat. 2023, 34, 2190430. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Papp, K.; Forman, S.; Han, G.; Waibel, J.; Rueda, M.J.; Sun, L.; Chen, Y.F.; Goldblum, O.; Pierce, E.; et al. The contribution of itch and skin severity improvements to the Dermatology Life Quality Index in patients with atopic dermatitis in baricitinib phase III trials. Br. J. Dermatol. 2022, 186, 1047–1049. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Simpson, E.L.; Wollenberg, A.; Bissonnette, R.; Kabashima, K.; DeLozier, A.M.; Sun, L.; Cardillo, T.; Nunes, F.P.; Reich, K. Long-term Efficacy of Baricitinib in Adults with Moderate to Severe Atopic Dermatitis Who Were Treatment Responders or Partial Responders: An Extension Study of 2 Randomized Clinical Trials. JAMA Dermatol. 2021, 157, 691–699. [Google Scholar] [CrossRef]
- Taylor, P.C.; Bieber, T.; Alten, R.; Witte, T.; Galloway, J.; Deberdt, W.; Issa, M.; Haladyj, E.; De La Torre, I.; Grond, S.; et al. Baricitinib Safety for Events of Special Interest in Populations at Risk: Analysis from Randomised Trial Data Across Rheumatologic and Dermatologic Indications. Adv. Ther. 2023, 40, 1867–1883. [Google Scholar] [CrossRef]
- Strazzulla, L.C.; Wang, E.H.C.; Avila, L.; Lo Sicco, K.; Brinster, N.; Christiano, A.M.; Shapiro, J. Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J. Am. Acad. Dermatol. 2018, 78, 1–12. [Google Scholar] [CrossRef]
- Liu, M.; Gao, Y.; Yuan, Y.; Yang, K.; Shen, C.; Wang, J.; Tian, J. Janus Kinase Inhibitors for Alopecia Areata: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2320351. [Google Scholar] [CrossRef]
- Al-Dhubaibi, M.S.; Alsenaid, A.; Alhetheli, G.; Abd Elneam, A.I. Trichoscopy pattern in alopecia areata: A systematic review and meta-analysis. Skin Res. Technol. 2023, 29, e13378. [Google Scholar] [CrossRef]
- Gilhar, A.; Etzioni, A.; Paus, R. Alopecia areata. N. Engl. J. Med. 2012, 366, 1515–1525. [Google Scholar] [CrossRef]
- Zhou, C.; Li, X.; Wang, C.; Zhang, J. Alopecia Areata: An Update on Etiopathogenesis, Diagnosis, and Management. Clin. Rev. Allergy Immunol. 2021, 61, 403–423. [Google Scholar] [CrossRef]
- Faria, S.; Freitas, E.; Torres, T. Efficacy and safety of baricitinib in patients with alopecia areata: Evidence to date. Drugs Context 2023, 12, 2023-6-2. [Google Scholar] [CrossRef]
- Olayinka, J.J.T.; Richmond, J.M. Immunopathogenesis of alopecia areata. Curr. Res. Immunol. 2021, 2, 7–11. [Google Scholar] [CrossRef]
- Ito, T.; Kageyama, R.; Nakazawa, S.; Honda, T. Understanding the significance of cytokines and chemokines in the pathogenesis of alopecia areata. Exp. Dermatol. 2020, 29, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ito, N.; Saatoff, M.; Hashizume, H.; Fukamizu, H.; Nickoloff, B.J.; Takigawa, M.; Paus, R. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J. Investig. Dermatol. 2008, 128, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://news.yale.edu/2023/06/26/fda-approves-second-yale-researched-treatment-alopecia-areata (accessed on 19 October 2023).
- King, B.; Ohyama, M.; Kwon, O.; Zlotogorski, A.; Ko, J.; Mesinkovska, N.A.; Hordinsky, M.; Dutronc, Y.; Wu, W.S.; McCollam, J.; et al. Two Phase 3 Trials of Baricitinib for Alopecia Areata. N. Engl. J. Med. 2022, 386, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Ritlecitinib: First Approval. Drugs 2023, 83, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- King, B.; Zhang, X.; Harcha, W.G.; Szepietowski, J.C.; Shapiro, J.; Lynde, C.; Mesinkovska, N.A.; Zwillich, S.H.; Napatalung, L.; Wajsbrot, D.; et al. Efficacy and safety of ritlecitinib in adults and adolescents with alopecia areata: A randomised, double-blind, multicentre, phase 2b-3 trial. Lancet 2023, 401, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Ezzedine, K.; Eleftheriadou, V.; Whitton, M.; van Geel, N. Vitiligo. Lancet 2015, 386, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Radi, G.; Simonetti, O.; Diotallevi, F.; Campanati, A.; Brisigotti, V.; Molinelli, E.; Offidani, A. How can I take care of you? The dermatologist meets patients’ needs during the COVID-19 pandemic. Dermatol. Ther. 2020, 33, e13740. [Google Scholar] [CrossRef]
- Iwanowski, T.; Szlązak, P.; Zabłotna, M.; Olszewska, B.; Sokołowska-Wojdyło, M. Translation, cross-cultural adaptation and validation of the vitiligo-specific health-related quality of life instrument (VitiQoL) into Polish. Postepy Dermatol. Alergol. 2021, 38, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Diotallevi, F.; Gioacchini, H.; De Simoni, E.; Marani, A.; Candelora, M.; Paolinelli, M.; Molinelli, E.; Offidani, A.; Simonetti, O. Vitiligo, from Pathogenesis to Therapeutic Advances: State of the Art. Int. J. Mol. Sci. 2023, 24, 4910. [Google Scholar] [CrossRef]
- Tanemura, A. Understanding of Pathomechanisms and Clinical Practice for Vitiligo. Ann. Dermatol. 2023, 35, 333–341. [Google Scholar] [CrossRef]
- Lopez, J.A.; Susanto, O.; Jenkins, M.R.; Lukoyanova, N.; Sutton, V.R.; Law, R.H.; Johnston, A.; Bird, C.H.; Bird, P.I.; Whisstock, J.C.; et al. Perforin forms transient pores on the target cell plasma membrane to facilitate rapid access of granzymes during killer cell attack. Blood 2013, 121, 2659–2668. [Google Scholar] [CrossRef]
- Abdallah, M.; El-Mofty, M.; Anbar, T.; Rasheed, H.; Esmat, S.; Al-Tawdy, A.; Fawzy, M.M.; Abdel-Halim, D.; Hegazy, R.; Gawdat, H.; et al. CXCL-10 and Interleukin-6 are reliable serum markers for vitiligo activity: A multicenter cross-sectional study. Pigment Cell Melanoma Res. 2018, 31, 330–336. [Google Scholar] [CrossRef]
- Regazzetti, C.; Joly, F.; Marty, C.; Rivier, M.; Mehul, B.; Reiniche, P.; Mounier, C.; Rival, Y.; Piwnica, D.; Cavalie, M.; et al. Transcriptional Analysis of Vitiligo Skin Reveals the Alteration of WNT Pathway: A Promising Target for Repigmenting Vitiligo Patients. J. Investig. Dermatol. 2015, 135, 3105–3114. [Google Scholar] [CrossRef]
- Relke, N.; Gooderham, M. The Use of Janus Kinase Inhibitors in Vitiligo: A Review of the Literature. J. Cutan Med. Surg. 2019, 23, 298–306. [Google Scholar] [CrossRef]
- Yang, L.; Wei, Y.; Sun, Y.; Shi, W.; Yang, J.; Zhu, L.; Li, M. Interferon-gamma Inhibits Melanogenesis and Induces Apoptosis in Melanocytes: A Pivotal Role of CD8+ Cytotoxic T Lymphocytes in Vitiligo. Acta Derm. Venereol. 2015, 95, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older (accessed on 25 October 2023).
- Rosmarin, D.; Passeron, T.; Pandya, A.G.; Grimes, P.; Harris, J.E.; Desai, S.R.; Lebwohl, M.; Ruer-Mulard, M.; Seneschal, J.; Wolkerstorfer, A.; et al. Two Phase 3, Randomized, Controlled Trials of Ruxolitinib Cream for Vitiligo. N. Engl. J. Med. 2022, 387, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Dand, N.; Mahil, S.K.; Capon, F.; Smith, C.H.; Simpson, M.A.; Barker, J.N. Psoriasis and Genetics. Acta Derm. Venereol. 2020, 100, adv00030. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Psoriasis; World Health Organization: Geneva, Switzerland, 2016; Available online: http://www.who.int/iris/handle/10665/204417 (accessed on 15 October 2023).
- Liang, Y.; Sarkar, M.K.; Tsoi, L.C.; Gudjonsson, J.E. Psoriasis: A mixed autoimmune and autoinflammatory disease. Curr. Opin. Immunol. 2017, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C.; De Pità, O.; Girolomoni, G. Resident skin cells in psoriasis: A special look at the pathogenetic functions of keratinocytes. Clin. Dermatol. 2007, 25, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Afzali, B.; Lombardi, G.; Lechler, R.I.; Lord, G.M. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin. Exp. Immunol. 2007, 148, 32–46. [Google Scholar] [CrossRef]
- Boutet, M.A.; Nerviani, A.; Gallo Afflitto, G.; Pitzalis, C. Role of the IL-23/IL-17 Axis in Psoriasis and Psoriatic Arthritis: The Clinical Importance of Its Divergence in Skin and Joints. Int. J. Mol. Sci. 2018, 19, 530. [Google Scholar] [CrossRef]
- Zheng, Y.; Danilenko, D.M.; Valdez, P.; Kasman, I.; Eastham-Anderson, J.; Wu, J.; Ouyang, W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007, 445, 648–651. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Camporeale, A.; Poli, V. IL-6, IL-17 and STAT3: A holy trinity in auto-immunity? Front. Biosci. Landmark Ed. 2012, 17, 2306–2326. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Gooderham, M.; Warren, R.B.; Papp, K.A.; Strober, B.; Thaçi, D.; Morita, A.; Szepietowski, J.C.; Imafuku, S.; Colston, E.; et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J. Am. Acad. Dermatol. 2023, 88, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis-and (accessed on 25 October 2023).
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Charles-Schoeman, C.; Fleischmann, R.M.; Mysler, E.; Greenwalda, M.; Wanga, C.; Chen, A.S.; Connel, C.A.; Woolcott, J.; Menon, S.; Chen, Y.; et al. POS0239 risk of venous thromboembolic events in patients with rheumatoid arthritis aged ≥ 50 years with ≥ 1 cardiovascular risk factor: Results from a phase 3b/4 randomised study of tofacitinib vs tumour necrosis factor inhibitors. Ann. Rheum. Dis. 2022, 81, 358–359. [Google Scholar] [CrossRef]
- Buch, M.H.; Charles-Schoeman, C.; Curtis, J.; Daugados, M.; Bhatt, D.L.; Giles, J.T.; Ytterberg, S.R.; Koch, G.G.; Vranic, I.; Wu, J.; et al. POS0237 major adverse cardiovascular events, malignancies and venous thromboembolism by baseline cardiovascular risk: A post hoc analysis of oral surveillance. Ann. Rheum. Dis. 2022, 81, 356–357. [Google Scholar] [CrossRef]
- Schneeweiss, M.C.; Kim, S.C.; Wyss, R.; Jin, Y.; Chin, K.; Merola, J.F.; Mostaghimi, A.; Silverberg, J.I.; Schneeweiss, S. Incidence of Venous Thromboembolism in Patients with Dermatologist-Diagnosed Chronic Inflammatory Skin Diseases. JAMA Dermatol. 2021, 157, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Sinclair, R.; Forman, S.; Wollenberg, A.; Aschoff, R.; Cork, M.; Bieber, T.; Thyssen, J.P.; Yosipovitch, G.; Flohr, C.; et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet 2020, 396, 255–266. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Simpson, E.L.; Thyssen, J.P.; Gooderham, M.; Chan, G.; Feeney, C.; Biswas, P.; Valdez, H.; DiBonaventura, M.; Nduaka, C.; et al. Efficacy and Safety of Abrocitinib in Patients with Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 863–873. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Thaçi, D.; Pangan, A.L.; Hong, H.C.; Papp, K.A.; Reich, K.; Beck, L.A.; Mohamed, M.F.; Othman, A.A.; Anderson, J.K.; et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2020, 145, 877–884. [Google Scholar] [CrossRef]
- Reich, K.; Teixeira, H.D.; de Bruin-Weller, M.; Bieber, T.; Soong, W.; Kabashima, K.; Werfel, T.; Zeng, J.; Huang, X.; Hu, X.; et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): Results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2021, 397, 2169–2181. [Google Scholar] [CrossRef]
- Simpson, E.L.; Papp, K.A.; Blauvelt, A.; Chu, C.Y.; Hong, H.C.; Katoh, N.; Calimlim, B.M.; Thyssen, J.P.; Chiou, A.S.; Bissonnette, R.; et al. Efficacy and Safety of Upadacitinib in Patients with Moderate to Severe Atopic Dermatitis: Analysis of Follow-Up Data from the Measure Up 1 and Measure Up 2 Randomized Clinical Trials. JAMA Dermatol. 2022, 158, 404–413. [Google Scholar] [CrossRef] [PubMed]


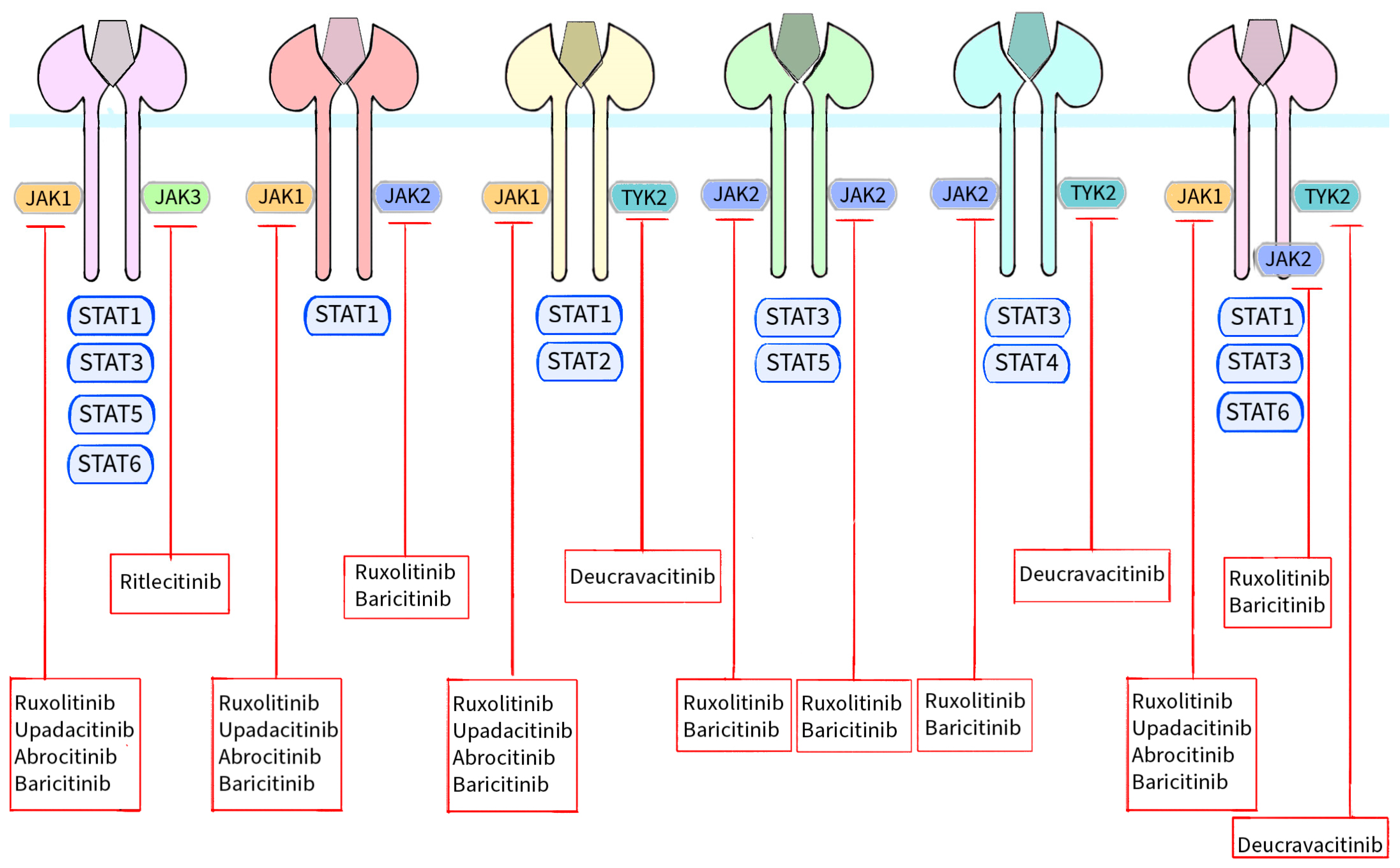
| Kinases | Cytokines or Hormones |
|---|---|
| JAK1, JAK3 | IL-2, IL-4, IL-7, IL-9, IL-15, IL-21, TSLP |
| JAK1, JAK2 | IFNγ, IL-27, IL-31, IL-35 |
| JAK1, TYK2 | IFNα, IFNβ, IFNκ, IFNω, IFNε, IFNλ, IL-10, IL-19, IL-20, IL-22, IL-24, IL-26 |
| JAK2, JAK2 | EPO, TPO, G-CSF, GM-CSF, GH, Leptin, IL-3, IL-5 |
| JAK2, TYK2 | IL-12, IL-23 |
| JAK1, JAK2, TYK2 | OSM, LIF, IL-6, IL-11, IL-13 |
| JAK Inhibitors | Generation | Target | Form | Route of Administration | FDA Approved Dermatological Condition | EMA Approved Dermatological Condition |
|---|---|---|---|---|---|---|
| Ruxolitinib | 1st | JAK1, JAK2 | Cream 1.5% | Topical | Atopic dermatitis (mild to moderate) Vitiligo (non-segmental) | - |
| Upadicitinib | 2nd | JAK1 | Tablets 15 mg and 30 mg | Oral | Atopic dermatitis (moderate to severe) | Atopic dermatitis (moderate to severe) |
| Abrocitinib | 2nd | JAK1 | Tablets 100 mg and 200 mg | Oral | Atopic dermatitis (moderate to severe) | Atopic dermatitis (moderate to severe) |
| Baricitinib | 1st | JAK1, JAK2 | Tablets 2 mg and 4 mg | Oral | Alopecia areata | Alopecia areata Atopic dermatitis (moderate to severe) |
| Deucravacitinib | 2nd | TYK2 | Tablets 6 mg | Oral | Psoriasis | Psoriasis |
| Ritlecitinib | 2nd | JAK3 | Tablets 50 mg | Oral | Alopecia areata | Alopecia areata |
| Cytokine | Importance in Atopic Dermatitis | Janus Kinase That Transmits Signal to the Cell Nucleus |
|---|---|---|
| IL-4 | Inhibition of gene expression for filaggrin, loricrin, involucrin and lipid components of the skin barrier Pruritus Modulation of gene expression of cathelicidin and β-defensins | JAK1, JAK3 |
| IL-5 | Eosinophilia activator | - |
| IL-13 | Inhibition of gene expression for filaggrin, loricrin, involucrin and lipid components of the skin barrier Pruritus Modulation of gene expression of cathelicidin and β-defensins | JAK1, JAK2, TYK2 |
| IL-31 | Pruritus | JAK1, JAK2 |
| Janus Inhibitor | Age of Group | Target | Administration | Phase | Study Number | Sponsor |
|---|---|---|---|---|---|---|
| Active Clinical Trials | ||||||
| Ruxolitinib | ≥12 yo–<18 yo | JAK1, JAK2 | Topical | Phase 3 | NCT05456529 | Incyte Corporation |
| Ruxolitinib | 2 yo–11 yo | JAK1, JAK2 | Topical | Phase 3 | NCT04921969 | Incyte Corporation |
| Upadacitinib | 2 yo–12 yo | JAK1 | Oral | Phase 1 | NCT03646604 | AbbVie |
| Upadacitinib | 12 yo–64 yo | JAK1 | Oral | Phase 3 | NCT05601882 | AbbVie |
| Upadacitinib | 12 yo–75 yo | JAK1 | Oral | Phase 3 | NCT03569293 | AbbVie |
| Upadacitinib | 12 yo–75 yo | JAK1 | Oral | Phase 3 | NCT03607422 | AbbVie |
| Upadacitinib | 12 yo–75 yo | JAK1 | Oral | Phase 3 | NCT03568318 | AbbVie |
| Upadacitinib | 18 yo–64 yo | JAK1 | Oral | Phase 4 | NCT05507580 | AbbVie |
| Upadacitinib | ≥18 yo | JAK1 | Oral | - | NCT05989932 | SIDeMaST |
| Abrocitinib | ≥12 yo | JAK1 | Oral | Phase 3 | NCT03422822 | Pfizer |
| Abrocitinib | ≥18 yo | JAK1 | Oral | - | NCT05250115 | Pfizer |
| Abrocitinib | ≥12 yo | JAK1 | Oral | - | NCT05391061 | Pfizer |
| Abrocitinib | ≥0 yo | JAK1 | Oral | - | NCT05721937 | Pfizer |
| Abrocitinib | ≥18 yo | JAK1 | Oral | - | NCT05689151 | Pfizer |
| Abrocitinib | ≥18 yo | JAK1 | Oral | Phase 4 | NCT05602207 | Innovaderm Research Inc. |
| Tofacitinib | ≥18 yo | JAK1, JAK3 | Topical | Phase 2 | NCT05487963 | CAGE Bio Inc. |
| Tofacitinib | 12 yo–50 yo (patients with Down Syndrome) | JAK1, JAK3 | Oral | Phase 2 | NCT04246372 | University of Colorado, Denver |
| Baricitinib | 2 yo–17 yo | JAK1, JAK2 | Oral | Phase 3 | NCT03952559 | Eli Lilly and Company |
| Baricitinib | 18 yo–75 yo | JAK1, JAK2 | Oral | - | NCT05969730 | Mazandaran University of Medical Sciences |
| Completed clinical trials | ||||||
| Ruxolitinib | ≥2 yo–17 yo | JAK1, JAK2 | Topical | Phase 1 | NCT03257644 | Incyte Corporation |
| Ruxolitinib | 12 yo–65 yo | JAK1, JAK2 | Topical | Phase 1 | NCT03920852 | Incyte Corporation |
| Ruxolitinib | 2 yo–11 yo | JAK1, JAK2 | Topical | Phase 1 | NCT05034822 | Incyte Corporation |
| Ruxolitinib | 18 yo–70 yo | JAK1, JAK2 | Topical | Phase 2 | NCT03011892 | Incyte Corporation |
| Ruxolitinib | 18 yo–65 yo | JAK1, JAK2 | Topical | Phase 2 | NCT04839380 | Incyte Corporation |
| Ruxolitinib | ≥12 yo | JAK1, JAK2 | Topical | Phase 3 | NCT03745638 | Incyte Corporation |
| Ruxolitinib | ≥12 yo–17 yo | JAK1, JAK2 | Topical | Phase 3 | NCT03745651 | Incyte Corporation |
| Tofacitinib | 18 yo–60 yo | JAK1, JAK3 | Oral | Phase 2 | NCT02001181 | Pfizer |
| Upadacitinib | 18 yo–75 yo | JAK1 | Oral | Phase 2 | NCT02925117 | AbbVie |
| Upadacitinib | 12 yo–75 yo | JAK1 | Oral | Phase 2 | NCT03661138 | AbbVie |
| Upadacitinib | 18 yo–75 yo | JAK1 | Oral | Phase 3 | NCT04195698 | AbbVie |
| Upadacitinib | 18 yo–75 yo | JAK1 | Oral | Phase 3 | NCT03738397 | AbbVie |
| Abrocitinib | ≥18 yo | JAK1 | Oral | Phase 3 | NCT04345367 | Pfizer |
| Baricitinib | ≥18 yo | JAK1, JAK2 | Oral | Phase 2 | NCT02576938 | Eli Lilly and Company |
| Baricitinib | ≥18 yo | JAK1, JAK2 | Oral | Phase 3 | NCT03334422 | Eli Lilly and Company |
| Baricitinib | ≥18 yo | JAK1, JAK2 | Oral | Phase 3 | NCT03435081 | Eli Lilly and Company |
| Baricitinib | ≥18 yo | JAK1, JAK2 | Oral | Phase 3 | NCT03334396 | Eli Lilly and Company |
| Baricitinib | ≥18 yo | JAK1, JAK2 | Oral | Phase 3 | NCT03733301 | Eli Lilly and Company |
| Baricitinib | ≥18 yo | JAK1, JAK2 | Oral | Phase 3 | NCT03428100 | Eli Lilly and Company |
| Delgocitinib | ≥2 yo | JAK1, JAK2, JAK3, TYK2 | Topical | Phase 1 | NCT03826901 | LEO Pharma |
| Delgocitinib | ≥18 yo | JAK1, JAK2, JAK3, TYK2 | Topical | Phase 2 | NCT03725722 | LEO Pharma |
| Jaktinib | 18 yo–65 yo | JAK1, JAK2, JAK3, TYK2 | Oral | Phase 2 | NCT04539639 | Suzhou Zelgen Biopharmaceuticals Co., Ltd. |
| Janus Inhibitor | Age of Group | Target | Administration | Phase | Study Number | Sponsor |
|---|---|---|---|---|---|---|
| Active Clinical Trials | ||||||
| PF-06651600 | ≥12 yo | JAK3 | Oral | Phase 3 | NCT04006457 | Pfizer |
| Baricitinib | 18 yo–70 yo | JAK1, JAK2 | Oral | Phase 3 | NCT03899259 | Eli Lilly and Company |
| Baricitinib | 18 yo–70 | JAK1, JAK2 | Oral | Phase 2/3 | NCT03570749 | Eli Lilly and Company |
| Jaktinib | 18 yo–65 yo | JAK1, JAK2, JAK3 | Topical | Phase 1/2 | NCT04445363 | Suzhou Zelgen Biopharmaceuticals Co., Ltd. |
| Jaktinib | 18 yo–65 yo | JAK1, JAK2, JAK3 | Oral | Phase 3 | NCT05255237 | Suzhou Zelgen Biopharmaceuticals Co., Ltd. |
| Tofacitinib | 12 yo–50 yo (patients with Down Syndrome) | JAK1, JAK3 | Oral | Phase 2 | NCT04246372 | University of Colorado, Denver |
| Upadacitinib | 12 yo–63 yo | JAK1 | Oral | Phase 3 | NCT06012240 | AbbVie |
| Completed clinical trials | ||||||
| Delgocitinib | ≥18 yo | JAK1, JAK2, JAK3, TYK2 | Topical | Phase 2 | NCT05332366 | LEO Pharma |
| Jaktinib | ≥12 yo | JAK1, JAK2, JAK3 | Oral | Phase 2 | NCT04034134 | Suzhou Zelgen Biopharmaceuticals Co., Ltd. |
| Ruxolitinib | 18 yo–75 yo | JAK1, JAK2 | Oral | Phase 2 | NCT01950780 | Columbia University |
| Tofacitinib | 18 yo–65 yo | JAK1, JAK3 | Oral | Phase 2 | NCT02299297 | Columbia University |
| Tofacitinib | ≥18 yo | JAK1, JAK3 | Oral | Phase 2 | NCT02812342 | Yale University |
| Tofacitinib | 18 yo–90 yo | JAK1, JAK3 | Oral | Phase 2 | NCT02197455 | Yale University |
| Tofacitinib | 18 yo–60 yo | JAK1, JAK3 | Oral | Phase 4 | NCT03800979 | Institute of Dermatology, Thailand |
| Tofacitinib | ≥18 yo | JAK1, JAK3 | Oral | - | NCT02312882 | Stanford University |
| PF-06700841 | ≥18 yo | JAK1, TYK2 | Oral | Phase 2 | NCT05076006 | Emma Guttman |
| Janus Inhibitor | Age of Group | Target | Administration | Phase | Study Number | Sponsor |
|---|---|---|---|---|---|---|
| Active Clinical Trials | ||||||
| Baricitinib | ≥12 yo | JAK1, JAK2 | Oral | - | NCT05950542 | Assiut University |
| Ritlecitinib | ≥18 yo | JAK3 | Oral | Phase 3 | NCT06072183 | Pfizer |
| Ritlecitinib | ≥12 yo | JAK3 | Oral | Phase 3 | NCT05583526 | Pfizer |
| Ruxolitinib | 12 yo–99 yo | JAK1, JAK2 | Topical | Phase 2 | NCT05247489 | Incyte Corporation |
| Ruxolitinib | ≥18 yo | JAK1, JAK2 | Topical | Phase 2 | NCT05750823 | Incyte Corporation |
| Tofacitinib | 12 yo–50 yo (patients with Down Syndrome) | JAK1, JAK3 | Oral | Phase 2 | NCT04246372 | University of Colorado, Denver |
| Completed clinical trials | ||||||
| Baricitinib | 18 yo–75 yo | JAK1, JAK2 | Oral | Phase 2 | NCT04822584 | University Hospital, Bordeaux |
| Ruxolitinib | ≥18 yo | JAK1, JAK2 | Topical | Phase 2 | NCT04896385 | Incyte Corporation |
| Ruxolitinib | 18 yo–75 yo | JAK1, JAK2 | Topical | Phase 2 | NCT03099304 | Incyte Corporation |
| Ruxolitinib | ≥12 yo | JAK1, JAK2 | Topical | Phase 3 | NCT04057573 | Incyte Corporation |
| Ruxolitinib | ≥12 yo | JAK1, JAK2 | Topical | Phase 3 | NCT04530344 | Incyte Corporation |
| Ruxolitinib | ≥12 yo | JAK1, JAK2 | Topical | Phase 3 | NCT04052425 | Incyte Corporation |
| Upadacitinib | 18 yo–65 yo | JAK1 | Oral | Phase 2 | NCT04927975 | AbbVie |
| Janus Inhibitor | Age of Group | Target | Administration | Phase | Study Number | Sponsor |
|---|---|---|---|---|---|---|
| Active Clinical Trials | ||||||
| Deucravacitinib | ≥18 yo | TYK2 | Oral | Phase 4 | NCT05478499 | Bristol-Myers Squibb |
| Deucravacitinib | 18 yo–75 yo | TYK2 | Oral | Phase 4 | NCT05858645 | University of California, San Francisco |
| Deucravacitinib | ≥18 yo | TYK2 | Oral | - | NCT06104644 | Bristol-Myers Squibb |
| Jaktinib | 18 yo–65 yo | JAK1, JAK2, JAK3 | Oral | Phase 2 | NCT04612699 | Suzhou Zelgen Biopharmaceuticals Co., Ltd. |
| Tofacitinib | 12 yo–50 yo (patients with Down Syndrome) | JAK1, JAK3 | Oral | Phase 2 | NCT04246372 | University of Colorado, Denver |
| Completed clinical trials | ||||||
| Baricitinib | ≥18 yo | JAK1, JAK2 | Oral | Phase 2 | NCT01490632 | Eli Lilly and Company |
| Ruxolitinib | 18 yo–65 yo | JAK1, JAK2 | Oral | Phase 2 | NCT00617994 | Incyte Corporation |
| Ruxolitinib | 18 yo–75 yo | JAK1, JAK2 | Topical | Phase 2 | NCT00820950 | Incyte Corporation |
| Ruxolitinib | 18 yo–75 yo | JAK1, JAK2 | Topical | Phase 2 | NCT00778700 | Incyte Corporation |
| Tofacitinib | 18 yo–65 yo | JAK1, JAK3 | Oral | Phase 1 | NCT01736696 | Pfizer |
| Tofacitinib | ≥18 yo | JAK1, JAK3 | Topical | Phase 2 | NCT01831466 | Pfizer |
| Tofacitinib | ≥18 yo | JAK1, JAK3 | Oral | Phase 2 | NCT01710046 | Pfizer |
| Tofacitinib | ≥18 yo | JAK1, JAK3 | Oral | Phase 3 | NCT01882439 | Pfizer |
| PF-06826647 | 18 yo–55 yo | TYK2 | Oral | Phase 1 | NCT03210961 | Pfizer |
| PF-06263276 | ≥18 yo | JAK1, JAK2, JAK3, TYK2 | Topical | Phase 1 | NCT02193815 | Pfizer |
| PF-06700841 | 18 yo–75 yo | JAK1, TYK2 | Oral | Phase 2 | NCT02969018 | Pfizer |
| CP-690,550 | 18 yo–65 yo | JAK1, JAK2, JAK3 | Oral | Phase 2 | NCT00678561 | Pfizer |
| CP-690-550 | 18 yo–99 yo | JAK1, JAK2, JAK3 | Oral | Phase 2 | NCT01246583 | Pfizer |
| CP-690,550 | ≥18 yo | JAK1, JAK2, JAK3 | Oral | Phase 3 | NCT01815424 | Pfizer |
| CP-690,550 | ≥18 yo | JAK1, JAK2, JAK3 | Oral | Phase 3 | NCT01309737 | Pfizer |
| CP-690,550 | ≥18 yo | JAK1, JAK2, JAK3 | Oral | Phase 3 | NCT01276639 | Pfizer |
| CP-690,550 | ≥18 yo | JAK1, JAK2, JAK3 | Oral | Phase 3 | NCT01186744 | Pfizer |
| CP-690,550 | ≥20 yo | JAK1, JAK2, JAK3 | Oral | Phase 3 | NCT01519089 | Pfizer |
| Dermatological Condition | JAK Inhibitor | Target | Administration | Status | Phase | Study Number | Sponsor |
|---|---|---|---|---|---|---|---|
| Hidradenitis suppurativa | Tofacitinib | JAK1, JAK3 | Oral | Active | Phase 2 | NCT04246372 | University of Colorado, Denver |
| Upadacitinib | JAK1 | Oral | Active | Phase 3 | NCT05889182 | AbbVie | |
| Deucravacitinib | TYK2 | Oral | Active | Phase 2 | NCT05997277 | Beth Israel Deaconess Medical Center | |
| Upadacitinib | JAK1 | Oral | Completed | Phase 2 | NCT04430855 | AbbVie | |
| INCB054707 | JAK1 | Oral | Completed | Phase 2 | NCT03607487 | Incyte Corporation | |
| Chronic hand eczema | Ruxolitinib | JAK1, JAK2 | Topical | Active | Phase 2 | NCT05906628 | Incyte Corporation |
| Delgocitinib | JAK1, JAK2, JAK3, TYK2 | Topical | Completed | Phase 2 | NCT03683719 | LEO Pharma | |
| Diffuse cutaneous systemic scleroderma | Tofacitinib | JAK1, JAK3 | Oral | Active | Phase 2 | NCT06044844 | Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh |
| Tofacitinib | JAK1, JAK3 | Oral | Completed | Phase 1/2 | NCT03274076 | University of Michigan | |
| Granuloma Annulare | AC-1101 | JAK1, JAK3 | Topical | Active | Phase 1 | NCT05580042 | TWi Biotechnology, Inc. |
| Abrocitinib | JAK1 | Oral | Active | Phase 2 | NCT05650736 | William Damsky | |
| Tofacitinib | JAK1, JAK3 | Oral | Completed | Phase 1 | NCT03910543 | Yale University | |
| Dermatomyositis | Tofacitinib | JAK1, JAK3 | Oral | Completed | Phase 1 | NCT03002649 | Johns Hopkins University |
| Baricitinib | JAK1, JAK3 | Oral | Active | Phase 3 | NCT04972760 | Assistance Publique—Hôpitaux de Paris | |
| Baricitinib | JAK1, JAK3 | Oral | Active | Phase 2 | NCT05524311 | Assistance Publique—Hôpitaux de Paris | |
| Brepocitinib | JAK1, TYK2 | Oral | Active | Phase 3 | NCT05437263 | Priovant Therapeutics, Inc. | |
| Baricitinib | JAK1, JAK2 | Oral | Completed | Phase 2 | NCT05188521 | Aaron R. Mangold | |
| Lupus erythematosus | Deucravacitinib | TYK2 | Topical | Active | Phase 3 | NCT05620407 | Bristol-Myers Squibb |
| Deucravactinib | TYK2 | Topical | Active | Phase 3 | NCT05617677 | Bristol-Myers Squibb | |
| Upadacitinib | JAK1 | Oral | Active | Phase 3 | NCT05843643 | AbbVie | |
| Tofacitinib | JAK3, JAK1 | Oral | Active | Phase 1 | NCT05048238 | National Institute of Allergy and Infectious Diseases (NIAID) | |
| Tofacitinib | JAK3, JAK1 | Oral | Completed | Phase 1 | NCT02535689 | National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) | |
| Tofacitinib | JAK3, JAK1 | Oral | Completed | Phase 2 | NCT03288324 | Children’s Hospital Medical Center, Cincinnati | |
| Delgocitinib | JAK1, JAK2, JAK3, TYK2 | Topical | Completed | Phase 2 | NCT03958955 | LEO Pharma | |
| Baricitinib | JAK1, JAK2 | Oral | Completed | Phase 2 | NCT02708095 | Eli Lilly and Company | |
| Baricitinib | JAK1, JAK2 | Oral | Completed | Phase 3 | NCT03616912 | Eli Lilly and Company | |
| Baricitinib | JAK1, JAK2 | Oral | Completed | Phase 3 | NCT03843125 | Eli Lilly and Company | |
| Baricitinib | JAK1, JAK2 | Oral | Completed | Phase 3 | NCT03616964 | Eli Lilly and Company |
| Selected Side Effects after the Treatment of Dermatological Conditions of Oral Janus Kinase Inhibitors | |
| Infections | Upper respiratory infections |
| Nasopharyngitis | |
| Herpes Simplex reactivation | |
| Herpes Zoster reactivation | |
| Urinary tract infections | |
| Serious infection | |
| Gastrointestinal disorders | Nausea |
| Diarrhea | |
| Neurological disorders | Headache |
| Dizziness | |
| Skin side effects | Acne |
| Itching | |
| Folliculitis | |
| Laboratory abnormalities | Elevated creatine phosphokinase levels |
| Increased levels of cholesterol and low- and high-density lipoproteins | |
| Neutropenia | |
| Thrombocytosis | |
| Venous thromboembolism | |
| Tumors | |
| Selected side effects after the treatment of dermatological conditions of topical Janus kinase inhibitors | |
| Neutropenia | |
| Oral herpes | |
| Application site pain | |
| Application site pruritus | |
| Skin bacterial infection | |
| Alopecia | |
| Application site erythema | |
| Skin papilloma | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryguła, I.; Pikiewicz, W.; Kaminiów, K. Novel Janus Kinase Inhibitors in the Treatment of Dermatologic Conditions. Molecules 2023, 28, 8064. https://doi.org/10.3390/molecules28248064
Ryguła I, Pikiewicz W, Kaminiów K. Novel Janus Kinase Inhibitors in the Treatment of Dermatologic Conditions. Molecules. 2023; 28(24):8064. https://doi.org/10.3390/molecules28248064
Chicago/Turabian StyleRyguła, Izabella, Wojciech Pikiewicz, and Konrad Kaminiów. 2023. "Novel Janus Kinase Inhibitors in the Treatment of Dermatologic Conditions" Molecules 28, no. 24: 8064. https://doi.org/10.3390/molecules28248064
APA StyleRyguła, I., Pikiewicz, W., & Kaminiów, K. (2023). Novel Janus Kinase Inhibitors in the Treatment of Dermatologic Conditions. Molecules, 28(24), 8064. https://doi.org/10.3390/molecules28248064







