Mechanochemical Degradation of Biopolymers
Abstract
1. Introduction
2. Historical Background
3. Theoretical Background and Instrumentation
3.1. Cavitation
3.1.1. Hydrodynamic Cavitation
3.1.2. Ultrasonic-Triggered Cavitation (Sonocavitation)
- −
- Medical imaging and chemical analysis utilize low-power, high-frequency ultrasound in the so-called extended or diagnostic ultrasound frequency range, typically ranging from 2 to 10 MHz.
- −
- High power and low frequencies, i.e., in the range of 20–1000 kHz, are suitable for cleaning, welding, and sonochemical reactions.
3.2. Grinding
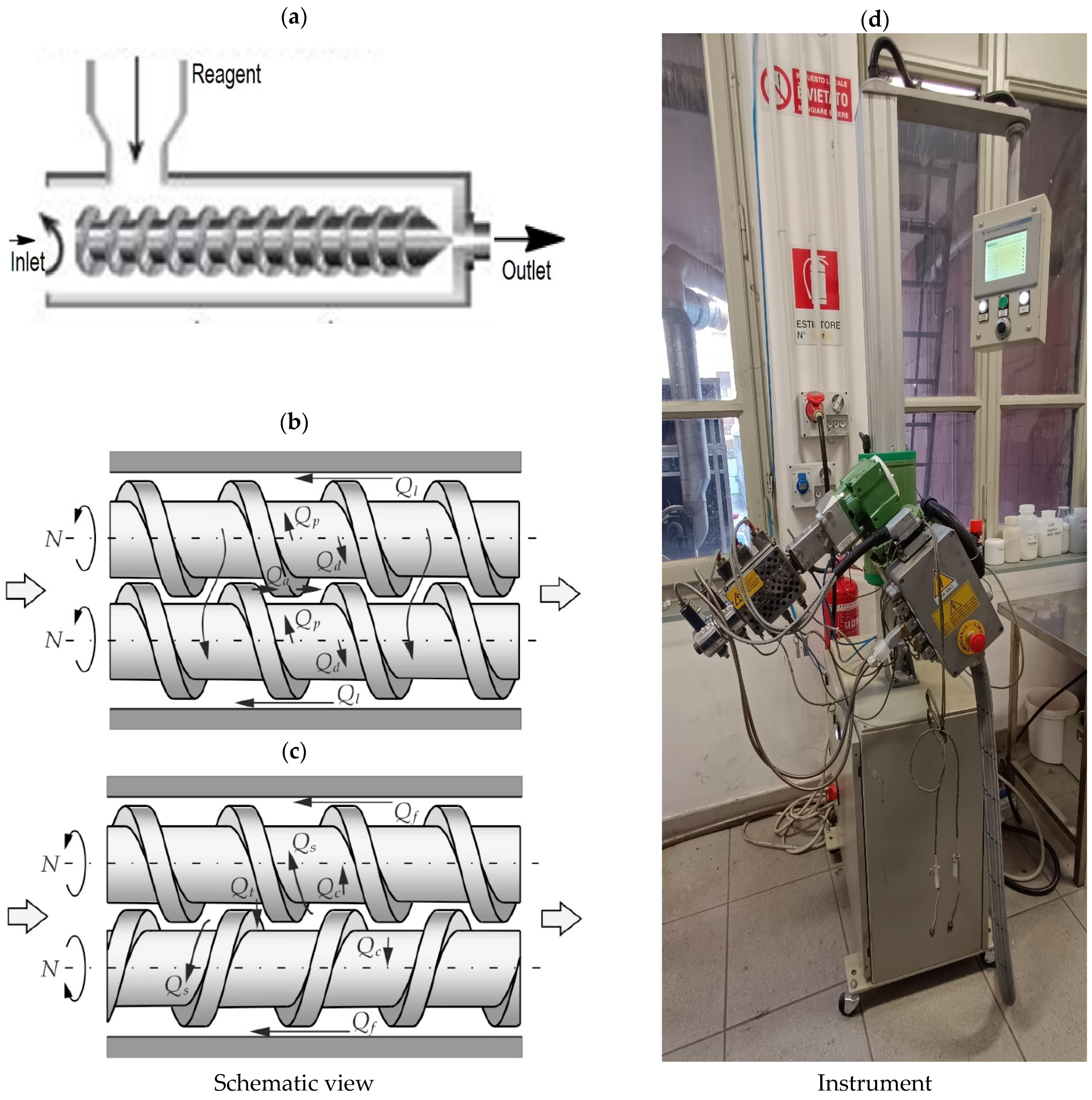
3.3. Extrusion
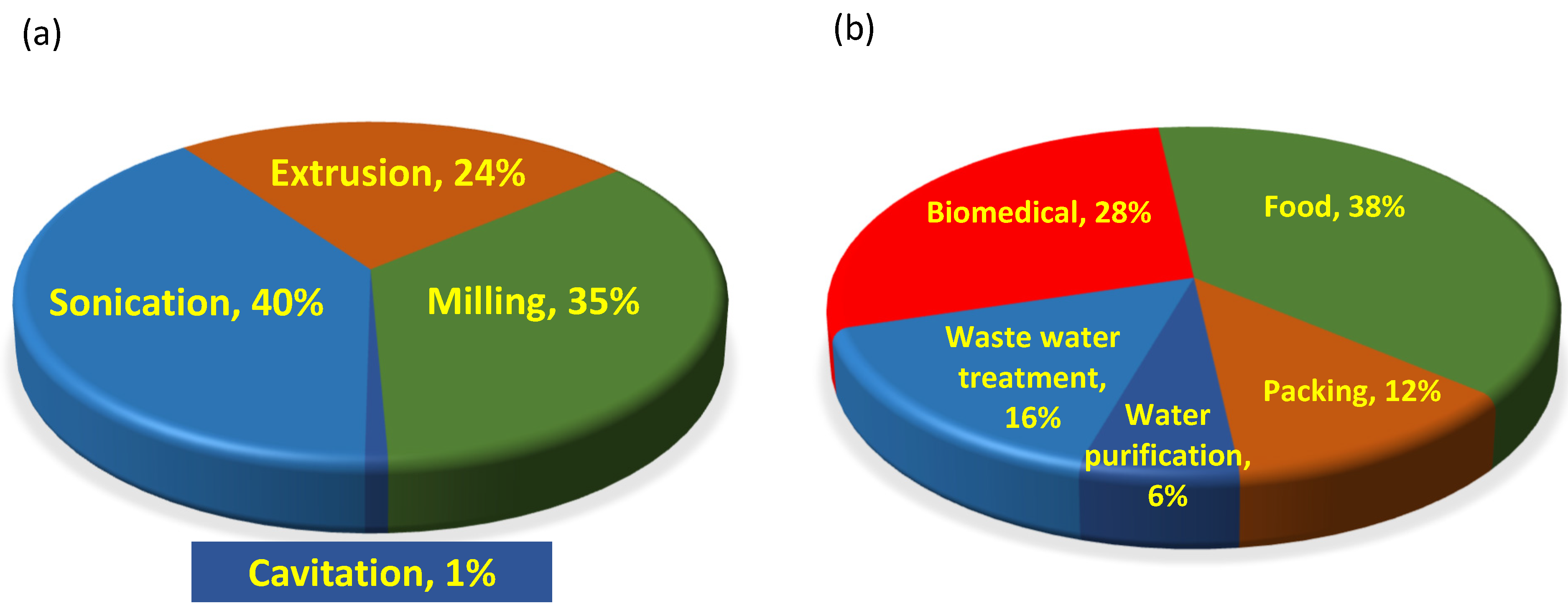
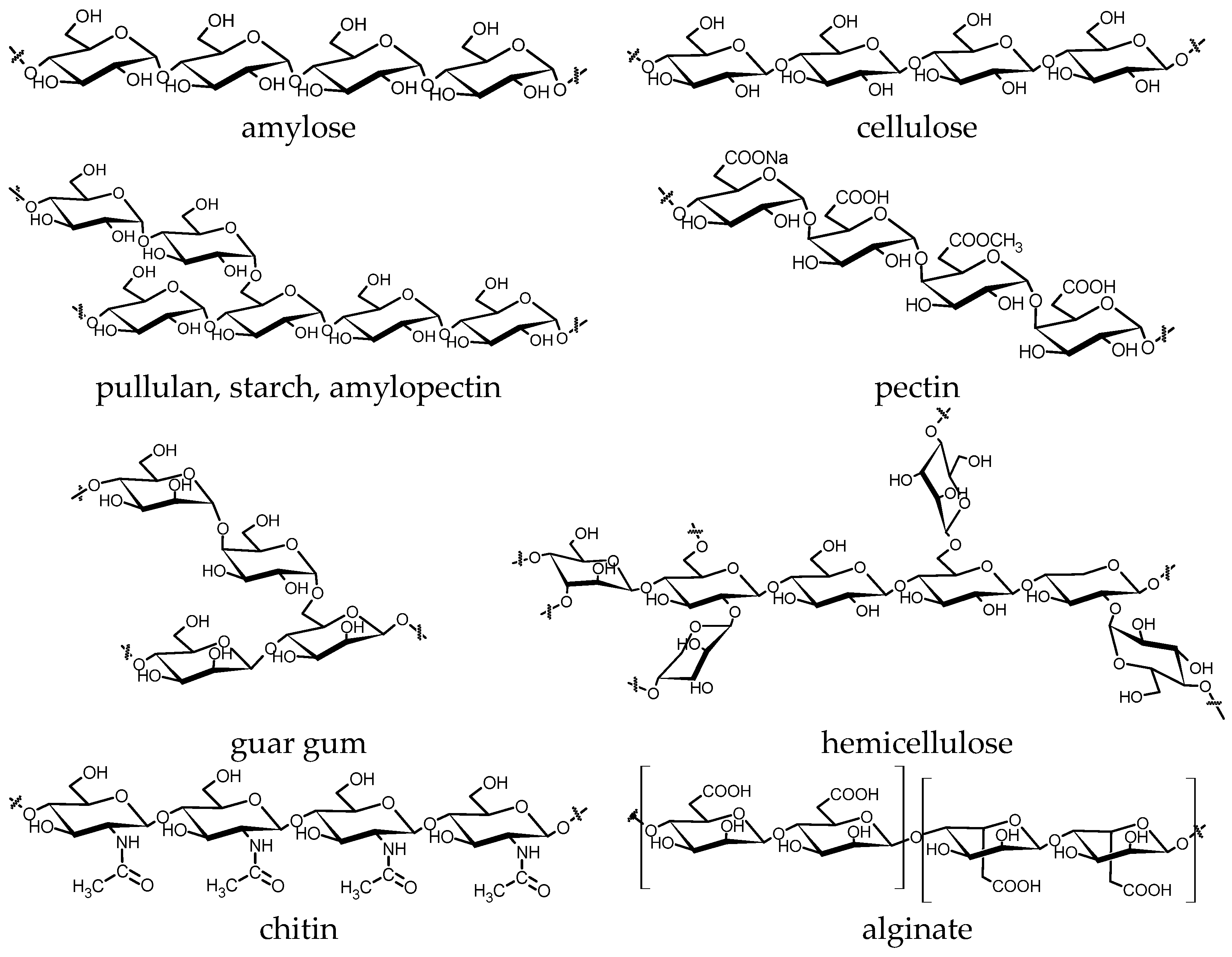
4. Mechanochemical Transformation of Biopolymers
- −
- Identifying new reactivity patterns is necessary due to the inadequacy of current experimental tools that are insufficient for studying force-inhibited reactions in mechanochemistry.
- −
- The development of efficient and accurate methods to determine the activation free energies of mechanistically distinct reactions as a function of applied force, to understand deeply the effect of mechanical stress on the chemical reactivity, and to enable the efficient design of materials with the desired mechanochemical profile.
- −
- The development of quantitative microscopic models of mechanochemistry in sonicated solutions, as only a few publications have reported reactions in sonicated polymer solutions and solids under stress. To date, sonication experiments do not appear to add much to simple qualitative considerations based on molecular geometry in rationalizing the mechanochemical behavior of solids or in selecting monomers to achieve the desired solid-state mechanochemical reaction.
- −
- In fact, the effect of polymer architecture on mechanochemical properties is unknown. The chain dynamics of topologically complex polymers often differ significantly from those of linear analogs.
- −
- New mechanochromic compounds possess a force/velocity profile suitable for precise quantitative analysis, both computationally and experimentally, via single-molecule force spectroscopy. These compounds offer a vast range of customizations through straightforward chemical modifications. Further, their reaction to mechanical stress is reversible and long-lasting and induced by the energy input.
- −
- Laying the fundamentals of accurate models of polymer mechanochemistry, rather than relying on localized reactions, by incorporating macroscopic control parameters, such as stress tensors of solid materials, the formulation of pressure gradients in flow systems, the effect of acoustic power fluxes, and the like. Moreover, the estimation of single-chain forces by bulk rates and product distributions of reactions with well-established microscopic mechanochemical kinetics and mechanisms as a function of macroscopic control parameters.
- −
- The description of mechanochemical reaction rates as a function of excitation time and the quantitation of the accumulation of single-chain forces by local deformation of strained materials. The experiments need accurate activation and standard free energies for the reactions as a function of local reactive and single-chain stress and reliable tools for quantifying reaction rates in solids. The empirical data available so far suggest that mechanochromism offers a potentially convenient method for quantifying reaction progress in loaded solids by studying changes in optical properties.
- −
- A comprehensive analysis of the nature, extent, mechanisms, and macroscopic manifestations of mechanochemical phenomena in valuable technological processes is necessary. Although empirical evidence shows the increasing importance of mechanochemistry in polymers, there remains an unexplored need for a thorough review [173].
4.1. Cavitation
4.2. Grinding
4.3. Extrusion
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández, J.G.; Avila-Ortiz, C.G.; Juaristi, E. Chapter 9.11 Useful Chemical Activation Alternatives in Solvent-Free Organic Reactions, 2nd ed.; Molander, G.A., Knochel, P., Eds.; Elsevier BV: Amsterdam, The Netherlands, 2014; Volume 9, pp. 287–314. [Google Scholar] [CrossRef]
- Frišcic, T. Chemistry through Ball Milling; AccessScience; McGraw-Hill: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Wollenhaupt, M.; Zoloff, M.; Marx, D. Mechanochemistry of Cyclopropane Ring-Opening Reactions. In High Performance Computing in Science and Engineering ’15; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 229–238. [Google Scholar] [CrossRef]
- Wollenhaupt, M.; Krupička, M.; Marx, D. Should the Woodward-Hoffmann Rules Be Applied to Mechanochemical Reactions? ChemPhysChem 2015, 16, 1593–1597. [Google Scholar] [CrossRef]
- Brügner, O.; Walter, M. Temperature and Loading Rate Dependent Rupture Forces from Universal Paths in Mechanochemistry. Phys. Rev. Mater. 2018, 2, 113603. [Google Scholar] [CrossRef]
- Avila-Ortiz, C.G.; Pérez-Venegas, M.; Vargas-Caporali, J.; Juaristi, E. Recent Applications of Mechanochemistry in Enantioselective Synthesis. Tetrahedron Lett. 2019, 60, 1749–1757. [Google Scholar] [CrossRef]
- Staudinger, H.; Dreher, E. Über Hochpolymere Verbindungen, 138. Mitteil. Über das Zerreissen von Faden-Molekülen der Cellulose Beim Vermahlen. Ber. Dtsch. Chem. Ges. 1936, 69, 1091–1098. [Google Scholar] [CrossRef]
- Staudinger, H.; Heuer, W. Über Hochpolymere Verbindungen, 93. Mitteil. Über das Zerreissen der Faden-Moleküle des Polystyrols. Ber. Dtsch. Chem. Ges. 1934, 67, 1159–1164. [Google Scholar] [CrossRef]
- Staudinger, H.; Fritschi, J. Über Isopren und Kautschuk. 5. Mitteil. Über der Hydrierung des Kautschuks und über seine Konstitution. Helv. Chim. Acta 1922, 5, 785–806. [Google Scholar] [CrossRef]
- Busse, W.F. Mastication of Rubber an Oxidation Process. Ind. Eng. Chem. 1932, 24, 140–146. [Google Scholar] [CrossRef]
- Encina, M.V.; Lissi, E.; Sarasúa, M.; Gargallo, L.; Radic, D. Ultrasonic Degradation of Polyvinylpyrrolidone: Effect of Peroxide Linkages. J. Polym. Sci. Polym. Lett. 1980, 18, 757–760. [Google Scholar] [CrossRef]
- Ghanem, M.A.; Basu, A.; Behrou, R.; Boechler, N.; Boydston, A.J.; Craig, S.L.; Lin, Y.; Lynde, B.E.; Nelson, A.; Shen, H.; et al. The Role of Polymer Mechanochemistry in Responsive Materials and Additive Manufacturing. Nat. Rev. Mater. 2020, 6, 84–98. [Google Scholar] [CrossRef]
- Blanksby, S.J.; Ellison, G.B. Bond Dissociation Energies of Organic Molecules. Acc. Chem. Res. 2003, 36, 255–263. [Google Scholar] [CrossRef]
- Darwent, B.D. No. 31, Bond Dissociation Energies in Simple Molecules; National Standard Reference Data Series; National Bureau of Standards: Gaithersburg, MD, USA, 1970; Volume NSRDS 31.
- Kerr, J.A. Bond Dissociation Energies by Kinetic Methods. Chem. Rev. 1966, 66, 465–500. [Google Scholar] [CrossRef]
- Ceretti, D.V.A.; Edeleva, M.; Cardon, L.; D’hooge, D.R. Molecular Pathways for Polymer Degradation during Conventional Processing, Additive Manufacturing, and Mechanical Recycling. Molecules 2023, 28, 2344. [Google Scholar] [CrossRef]
- May, P.A.; Moore, J.S. Polymer Mechanochemistry: Techniques to Generate Molecular Force via Elongational Flows. Chem. Soc. Rev. 2013, 42, 7497–7506. [Google Scholar] [CrossRef]
- Anderson, L.; Boulatov, R. Chapter Three—Polymer Mechanochemistry: A New Frontier for Physical Organic Chemistry. Adv. Phys. Org. Chem. 2018, 52, 87–143. [Google Scholar] [CrossRef]
- Zhurkov, S.N. Kinetic Concept of the Strength of Solids. Int. J. Fract. 1984, 26, 295–307. [Google Scholar] [CrossRef]
- Weertman, J. Rate of Growth of Fatigue Cracks Calculated from the Theory of Infinitesimal Dislocations Distributed on a Plane. Int. J. Fract. 1984, 26, 308–315. [Google Scholar] [CrossRef]
- Alrbaihat, M.; Al-Zeidaneen, F.K.; Abu-Afifeh, Q. Reviews of the Kinetics of Mechanochemistry: Theoretical and Modeling Aspects. Mater. Today Proc. 2022, 65, 3651–3656. [Google Scholar] [CrossRef]
- Basedow, A.M.; Ebert, K.H. Effects of Mechanical Stress on the Reactivity of Polymers: Activation of Acid Hydrolysis of Dextran by Ultrasound. Polym. Bull. 1979, 1, 299–306. [Google Scholar] [CrossRef]
- Butyagin, P.Y.; Streletskii, A.N. The Kinetics and Energy Balance of Mechanochemical Transformations. Phys. Solid State 2005, 47, 856–862. [Google Scholar] [CrossRef]
- Dular, M. Hydrodynamic Cavitation Damage in Water at Elevated Temperatures. In Proceedings of the 16th International Symposium on Transport Phenomena and Dynamics of Rotating Machinery, Honolulu, HI, USA, 10–15 April 2016. [Google Scholar]
- Repinc, S.K.; Bizjan, B.; Budhiraja, V.; Dular, M.; Gostiša, J.; Humar, B.B.; Kaurin, A.; Kržan, A.; Levstek, M.; Arteaga, J.F.M.; et al. Integral Analysis of Hydrodynamic Cavitation Effects on Waste Activated Sludge Characteristics, Potentially Toxic Metals, Microorganisms and Identification of Microplastics. Sci. Total Environ. 2022, 806, 151414. [Google Scholar] [CrossRef]
- Gogate, P.R.; Prajapat, A.L. Depolymerization Using Sonochemical Reactors: A Critical Review. Ultrason. Sonochem. 2015, 27, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.R.S.S.; Padmakumar, A.; Kalita, U.; Samanta, S.; Baral, A.; Singha, N.K.; Ashokkumar, M.; Qiao, G.G. Ultrasonics in Polymer Science: Applications and Challenges. Prog. Mater Sci. 2023, 136, 101113. [Google Scholar] [CrossRef]
- Hoo, D.Y.; Low, Z.L.; Low, D.Y.S.; Tang, S.Y.; Manickam, S.; Tan, K.W.; Ban, Z.H. Ultrasonic Cavitation: An Effective Cleaner and Greener Intensification Technology in the Extraction and Surface Modification of Nanocellulose. Ultrason. Sonochem. 2022, 90, 106176. [Google Scholar] [CrossRef] [PubMed]
- Kawadkar, A.S.; Gogate, P.R. Intensified Depolymerization Using Ultrasound—A Review of Mechanisms, Reactors, Operating Conditions and Applications. Chem. Eng. Process. Process Intensif. 2023, 191, 109446. [Google Scholar] [CrossRef]
- Adeleye, O.O.; Awodiran, S.T.; Ajayi, A.O.; Ogunmoyela, T.F. Influence of Extrusion Cooking on Physicochemical Properties and Starch Digestion Kinetics of Sphenostylis Stenocarpa, Cajanus Cajan, and Vigna Subterranean Grains. PLoS ONE 2020, 15, e0242697. [Google Scholar] [CrossRef] [PubMed]
- van der Sman, R.; van der Goot, A. Hypotheses Concerning Structuring of Extruded Meat Analogs. Curr. Res. Food Sci. 2023, 6, 100510. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Lian, S.; Zou, Y.; Chen, B.; He, Y.; Zheng, M.; Zhao, Y.; Wang, H. Preparation, Multi-Scale Structures, and Functionalities of Acetylated Starch: An Updated Review. Int. J. Biol. Macromol. 2023, 249, 126142. [Google Scholar] [CrossRef]
- Kian-Pour, N.; Yildirim-Yalcin, M.; Kurt, A.; Ozmen, D.; Toker, O.S. A Review on Latest Innovations in Physical Modifications of Galactomannans. Food Hydrocolloid. 2023, 138, 108470. [Google Scholar] [CrossRef]
- Rosa, D.S.; Bardi, M.A.G.; Machado, L.D.B.; Dias, D.B.; Silva, L.G.A.; Kodama, Y. Starch Plasticized with Glycerol from Biodiesel and Polypropylene Blends. J. Therm. Anal. Calorim. 2010, 102, 181–186. [Google Scholar] [CrossRef]
- Guo, X.; Junna, X.; Wolcott, M.P.; Zhang, J. Mechanochemical Oleation of Lignin through Ball Milling and Properties of Its Blends with Pla. ChemistrySelect 2016, 1, 3449–3454. [Google Scholar] [CrossRef]
- Jubinville, D.; Esmizadeh, E.; Saikrishnan, S.; Tzoganakis, C.; Mekonnen, T. A Comprehensive Review of Global Production and Recycling Methods of Polyolefin (po) Based Products and Their Post-Recycling Applications. Sustain. Mater. Technol. 2020, 25, e00188. [Google Scholar] [CrossRef]
- Mukherjee, A.; Koller, M. Microbial Polyhydroxyalkanoate (PHA) Biopolymers—Intrinsically Natural. Bioengineering 2023, 10, 855. [Google Scholar] [CrossRef]
- Lee, C.H.; Sapuan, S.M.; Ilyas, R.A.; Lee, S.H.; Khalina, A. Development and Processing of PLA, PHA, and Other Biopolymers. In Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 47–63. [Google Scholar] [CrossRef]
- Margetić, D.; Štrukil, V. Recent Advances in Mechanochemical Organic Synthesis. In Organic Synthesis—A Nascent Relook; Nandeshwarappa, B.P., Ed.; IntechOpen Limited: London, UK, 2020. [Google Scholar] [CrossRef]
- Ling, A.R.; Baker, J.L. XCVI.—Halogen derivatives of Quinone. Part III. Derivatives of Quinhydrone. J. Chem. Soc. Trans. 1893, 63, 1314–1327. [Google Scholar] [CrossRef]
- Darrigol, O.; Frisch, U. From Newton’s mechanics to Euler’s equations. Phys. D 2008, 237, 1855–1869. [Google Scholar] [CrossRef]
- Agazzi, E. (Ed.) Scienziati e Tecnologi Dalle Origini al 1875, Vol. 3. Da Ransome, Robert a Žukovskij, Nikolaj Egorovič; A. Mondadori: Milano, Italy, 1975; Volume 3. [Google Scholar]
- Thornycroft, J.I.; Barnaby, S.W. Torpedo-Boat Destroyers. (including Appendix and Plate at Back of Volume). Minutes Proc. Inst. Civ. Eng. 1895, 122, 51–69. [Google Scholar] [CrossRef]
- Richards, W.T.; Loomis, A.L. The Chemical Effects of High Frequency Sound Waves I. A Preliminary Survey. J. Am. Chem. Soc. 1927, 49, 3086–3100. [Google Scholar] [CrossRef]
- Wood, R.W.; Loomis, A.L. XXXVIII. The Physical and Biological Effects of High-Frequency Sound-Waves of Great Intensity. Phil. Mag. J. Sci. 1927, 4, 417–436. [Google Scholar] [CrossRef]
- Suslick, K.S. The Chemical Effects of Ultrasound. Sci. Am. 1989, 260, 80–86. [Google Scholar] [CrossRef]
- Katsaros, T.; Liritzis, I.; Laskaris, N. Identification of Theophrastus’ Pigments Egyptios Yanos and Psimythion from Archaeological Excavations. ArchéoSciences 2010, 34, 69–80. [Google Scholar] [CrossRef]
- Margetić, D.; Štrukil, V. Chapter 1—Practical Considerations in Mechanochemical Organic Synthesis. In Mechanochemical Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–54. [Google Scholar] [CrossRef]
- Bramah, J. Obtaining and Applying Motive Power. British Patent GB179502045A, 1795. [Google Scholar]
- Backus, R.G.; Boshold, R.F.; Johannisson, T.G.; Noble, P.D.; Pfeffer, J.B.; Schiebold, T.A.; Spearman, J.E. Drawing, Extruding, and Upsetting, 4th ed.; Wick, C., Benedict, J.T., Veilleux, R.F., Eds.; Society of Manufacturing Engineers: Dearborn, MI, USA, 1988; Volume 2, pp. 13–20. [Google Scholar]
- Whistler, R.L.; Goatley, J.L. Starch-Polyacrylamide Grafts by Ball Milling. J. Polym. Sci. 1962, 62, S123–S125. [Google Scholar] [CrossRef]
- Harper, J.M.; Clark, J.P. Food extrusion. CRC Crit. Rev. Food Sci. Nutr. 2009, 11, 155–215. [Google Scholar] [CrossRef]
- De Smit, K.; Marien, Y.W.; Van Steenberge, P.H.M.; D’hooge, D.R.; Edeleva, M. Playing with process conditions to increase the industrial sustainability of poly(lactic acid)-based materials. React. Chem. Eng. 2023, 8, 1598–1612. [Google Scholar] [CrossRef]
- Al-Assaf, S.; Gulrez, S.K.H.; Czechowska-Biskup, R.; Wach, R.A.; Rosiak, J.M.; Ulanski, P. Chapter 4 Radiation Modification of Polysaccharides; Al-Assaf, S., Coqueret, X., Dahlan, K.Z.H.M., Sen, M., Ulanski, P., Eds.; International Atomic Energy Agency: Vienna, Austria, 2016; pp. 77–116. [Google Scholar]
- Bychkov, A.; Simonova, V.; Zarubin, V.; Cherepetskaya, E.; Karabutov, A. The Progress in Photoacoustic and Laser Ultrasonic Tomographic Imaging for Biomedicine and Industry: A Review. Appl. Sci. 2018, 8, 1931. [Google Scholar] [CrossRef]
- Ivanov, I.A.; Dub, V.S.; Karabutov, A.A.; Cherepetskaya, E.B.; Bychkov, A.S.; Kudinov, I.A.; Gapeev, A.A.; Krivilyov, M.D.; Simakov, N.N.; Gruzd, S.A.; et al. Effect of Laser-Induced Ultrasound Treatment on Material Structure in Laser Surface Treatment for Selective Laser Melting Applications. Sci. Rep. 2021, 11, 23501. [Google Scholar] [CrossRef] [PubMed]
- Ying, K.-N.; Ni, C.-Y.; Dai, L.-N.; Yuan, L.; Kan, W.-W.; Shen, Z.-H. Multi-Mode Laser-Ultrasound Imaging Using Time-Domain Synthetic Aperture Focusing Technique (t-Saft). Photoacoustics 2022, 27, 100370. [Google Scholar] [CrossRef]
- Hosten, B.; Bacon, C.; Guilliorit, E. Acoustic Wave Generation by Microwaves and Applications to Nondestructive Evaluation. Ultrasonics 2002, 40, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Martina, K.; Trotta, F.; Robaldo, B.; Belliardi, N.; Jicsinszky, L.; Cravotto, G. Efficient Regioselective Functionalizations of Cyclodextrins Carried out under Microwaves or Power Ultrasound. Tetrahedron Lett. 2007, 48, 9185–9189. [Google Scholar] [CrossRef]
- Cravotto, G.; Caporaso, M.; Jicsinszky, L.; Martina, K. Enabling Technologies and Green Processes in Cyclodextrin Chemistry. Beilstein J. Org. Chem. 2016, 12, 278–294. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Yue, Q. Review on Microwave-Matter Interaction Fundamentals and Efficient Microwave-Associated Heating Strategies. Materials 2016, 9, 231. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.V. Photoacoustic Imaging in Biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Cegla, F. Microwaves Trigger Thermo-Acoustic Ultrasound Generation. Adv. Photonics 2020, 2, 030501. [Google Scholar] [CrossRef]
- Lan, L.; Li, Y.; Yang-Tran, T.; Jiang, Y.; Cao, Y.; Cheng, J.-X. Ultraefficient Thermoacoustic Conversion through a Split Ring Resonator. Adv. Photonics 2020, 2, 036006. [Google Scholar] [CrossRef]
- Eisenstein, M. A Measure of Molecular Muscle. Nature 2017, 544, 255–257. [Google Scholar] [CrossRef]
- Marszalek, P.E.; Oberhauser, A.F.; Pang, Y.-P.; Fernandez, J.M. Polysaccharide Elasticity Governed by Chair Boat Transitions of the Glucopyranose Ring. Nature 1998, 396, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Price, G.J.; West, P.J.; Smith, P.F. Control of Polymer Structure Using Power Ultrasound. Ultrason. Sonochem. 1994, 1, S51–S57. [Google Scholar] [CrossRef]
- Garcia-Manyes, S.; Beedle, A.E.M. Steering Chemical Reactions with Force. Nat. Rev. Chem. 2017, 1, 83. [Google Scholar] [CrossRef]
- Vogel, V. Mechanotransduction Involving Multimodular Proteins: Converting Force into Biochemical Signals. Annu. Rev. Biophys. Biomol. Struct. 2006, 35, 459–488. [Google Scholar] [CrossRef]
- Carmichael, A.J.; Riesz, P. Photoinduced Reactions of Anthraquinone Antitumor Agents with Peptides and Nucleic Acid Bases: An Electron Spin Resonance and Spin Trapping Study. Arch. Biochem. Biophys. 1985, 237, 433–444. [Google Scholar] [CrossRef]
- Sohma, J. Mechanochemistry of Polymers. Prog. Polym. Sci. 1989, 14, 451–596. [Google Scholar] [CrossRef]
- Xia, H.; Zhao, Y.; Tong, R. Ultrasound-Mediated Polymeric Micelle Drug Delivery. Adv. Exp. Med. Biol. 2016, 880, 365–384. [Google Scholar] [CrossRef]
- Arciszewski, J.; Auclair, K. Mechanoenzymatic Reactions Involving Polymeric Substrates or Products. ChemSuschem 2022, 15, e202102084. [Google Scholar] [CrossRef]
- Al-Ithawi, W.K.A.; Khasanov, A.F.; Kovalev, I.S.; Nikonov, I.L.; Platonov, V.A.; Kopchuk, D.S.; Santra, S.; Zyryanov, G.V.; Ranu, B.C. Tm-Free and Tm-Catalyzed Mechanosynthesis of Functional Polymers. Polymers 2023, 15, 1853. [Google Scholar] [CrossRef]
- Acciardo, E.; Tabasso, S.; Cravotto, G.; Bensaid, S. Process Intensification Strategies for Lignin Valorization. Chem. Eng. Process. Process Intensif. 2022, 171, 108732. [Google Scholar] [CrossRef]
- Shi, Z.; Li, H.; Li, X. Sonopharmacology: Polymer Mechanochemistry for Drug Activation. Mater. Today 2023, 64, 1–3. [Google Scholar] [CrossRef]
- Gobindlal, K.; Zujovic, Z.; Jaine, J.; Weber, C.C.; Sperry, J. Solvent-Free, Ambient Temperature and Pressure destruction of Perfluorosulfonic Acids under Mechanochemical Conditions: Degradation Intermediates and Fluorine Fate. Environ. Sci. Technol. 2023, 57, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Poulesquen, A.; Vergnes, B.; Cassagnau, P.; Gimenez, J.; Michel, A. Polymerization of E-Caprolactone in a Twin Screw Extruder. Int. Polym. Proc. 2001, 16, 31–38. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Naffakh, M.; Marco, C.; Ellis, G.; Gómez-Fatou, M.A. High-Performance Nanocomposites Based on Polyetherketones. Prog. Mater Sci. 2012, 57, 1106–1190. [Google Scholar] [CrossRef]
- Ghosh, A. Performance Modifying Techniques for Recycled Thermoplastics. Res. Conserv. Recyc. 2021, 175, 105887. [Google Scholar] [CrossRef]
- Cataño, F.A.; Moreno-Serna, V.; Cament, A.; Loyo, C.; Yáñez-S, M.; Ortiz, J.A.; Zapata, P.A. Green Composites Based on Thermoplastic Starch Reinforced with Micro- and Nano-Cellulose by Melt Blending—A Review. Int. J. Biol. Macromol. 2023, 248, 125939. [Google Scholar] [CrossRef]
- Varaprasad, K.; Karthikeyan, C.; Yallapu, M.M.; Sadiku, R. The Significance of Biomacromolecule Alginate for the 3D Printing of Hydrogels for Biomedical Applications. Int. J. Biol. Macromol. 2022, 212, 561–578. [Google Scholar] [CrossRef]
- Rätzsch, M.; Arnold, M.; Borsig, E.; Bucka, H.; Reichelt, N. Radical Reactions on Polypropylene in the Solid State. Prog. Polym. Sci. 2002, 27, 1195–1282. [Google Scholar] [CrossRef]
- Yan, J.; Bockstaller, M.R.; Matyjaszewski, K. Brush-Modified Materials: Control of Molecular Architecture, Assembly Behavior, Properties and Applications. Prog. Polym. Sci. 2020, 100, 101180. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B. Numerical Investigation of the Cavitation Characteristics in Venturi Tubes: The Role of Converging and Diverging Sections. Appl. Sci. 2023, 13, 7476. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, F.; Yao, Z.; Cai, Z.; Ma, X.; Li, Z.; Gao, Z. Optimization of Flow and Mixing in a Venturi Tube Mixer with a Two-Step Method Using Numerical Simulation. Processes 2023, 11, 1083. [Google Scholar] [CrossRef]
- Lifshitz, E.M.; Landau, L.D. Fluid Mechanics, 2nd ed.; Pergamon Press: Oxford, UK, 1987; Volume 6. [Google Scholar]
- Cintas, P.; Cravotto, G.; Barge, A.; Martina, K. Interplay between Mechanochemistry and Sonochemistry. Top. Curr. Chem. 2014, 369, 239–284. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. Harnessing Mechanochemical Effects with Ultrasound-Induced Reactions. Chem. Sci. 2012, 3, 295–307. [Google Scholar] [CrossRef]
- Cravotto, G.; Martina, K.; Moran, M.J.; Cintas, P. Chapter Fourteen—Sonomechanochemistry. In Nontraditional Activation Methods in Green and Sustainable Applications: Microwaves; Ultrasounds; Photo-, Electro- and Mechanochemistry and High Hydrostatic Pressure; Advances in Green and Sustainable Chemistry; Török, B., Schäfer, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 467–510. [Google Scholar] [CrossRef]
- Rokhina, E.V.; Lens, P.; Virkutyte, J. Low-Frequency Ultrasound in Biotechnology: State of the Art. Trends Biotechnol. 2009, 27, 298–306. [Google Scholar] [CrossRef]
- Mason, T.J. Developments in Ultrasound-Non-Medical. Prog. Biophys. Mol. Biol. 2007, 93, 166–175. [Google Scholar] [CrossRef]
- Zourob, M.; Hawkes, J.J.; Coakley, W.T.; Brown, B.J.T.; Fielden, P.R.; McDonnell, M.B.; Goddard, N.J. Optical Leaky Waveguide Sensor for Detection of Bacteria with Ultrasound Attractor Force. Anal. Chem. 2005, 77, 6163–6168. [Google Scholar] [CrossRef]
- Cordero-Rando, M.d.M.; de Cisneros, J.L.H.-H.; Blanco, E.; Naranjo-Rodríguez, I. The Sonogel-Carbon Electrode As a Sol-Gel Graphite-Based Electrode. Anal. Chem. 2002, 74, 2423–2427. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Kim, H.-S.; Lee, S. Incorporation of ultrasonic cell disintegration into a membrane bioreactor for zero sludge production. Process Biochem. 2004, 39, 1923–1929. [Google Scholar] [CrossRef]
- Feng, X.; Lei, H.; Deng, J.; Yu, Q.; Li, H. Physical and chemical characteristics of waste activated sludge treated ultrasonically. Chem. Eng. Process. Process Intensif. 2009, 48, 187–194. [Google Scholar] [CrossRef]
- Robinson, T.M.; Jicsinszky, L.; Karginov, A.V.; Karginov, V.A. Inhibition of Clostridium Perfringens Epsilon Toxin by Β-Cyclodextrin derivatives. Int. J. Pharm. 2017, 531, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S. Mechanochemistry and Sonochemistry: Concluding Remarks. Faraday Discuss. 2014, 170, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Tuziuti, T.; Iida, Y.; Mitome, H. Theoretical Study of the Ambient-Pressure Dependence of Sonochemical Reactions. J. Chem. Phys. 2003, 119, 346–356. [Google Scholar] [CrossRef]
- Gutierrez, M.; Henglein, A.; Dohrmann, J.K. Hydrogen atom reactions in the sonolysis of aqueous solutions. J. Phys. Chem. 1987, 91, 6687–6690. [Google Scholar] [CrossRef]
- Ziembowicz, S.; Kida, M.; Koszelnik, P. Sonochemical Formation of Hydrogen Peroxide. Proceedings 2018, 2, 188. [Google Scholar] [CrossRef]
- Kohno, M.; Mokudai, T.; Ozawa, T.; Niwano, Y. Free radical formation from sonolysis of water in the presence of different gases. J. Clin. Biochem. Nutr. 2011, 49, 96–101. [Google Scholar] [CrossRef]
- Liu, P.; Wu, Z.; Fang, Z.; Cravotto, G. Sonolytic degradation kinetics and mechanisms of antibiotics in water and cow milk. Ultrason. Sonochem. 2023, 99, 106518. [Google Scholar] [CrossRef]
- Mišík, V.; Riesz, P. Nitric Oxide Formation by Ultrasound in Aqueous Solutions. J. Phys. Chem. 1996, 100, 17986–17994. [Google Scholar] [CrossRef]
- Pereira, T.C.; Flores, E.M.M.; Abramova, A.V.; Verdini, F.; Gaudino, E.C.; Bucciol, F.; Cravotto, G. Simultaneous Hydrodynamic Cavitation and Glow Plasma Discharge for the Degradation of Metronidazole in Drinking Water. Ultrason. Sonochem. 2023, 95, 106388. [Google Scholar] [CrossRef]
- Qureishy, T.; Løyland, S.; Jørgensen, S.J.; Færgestad, E.M.; Norby, T.; Uggerud, E. Mechanisms for sonochemical oxidation of nitrogen. Phys. Chem. Chem. Phys. 2022, 24, 15357–15364. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Z.; Manzoli, M.; Jicsinszky, L.; Cavalli, R.; Battaglia, L.; Cravotto, G. Medium-High Frequency Sonication Dominates Spherical-SiO2 Nanoparticle Size. Ultrason. Sonochem. 2022, 90, 106181. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Z.; Manzoli, M.; Jicsinszky, L.; Cavalli, R.; Battaglia, L.; Cravotto, G. Medium-High Frequency Sonication Dominates Spherical-SiO2 Nanoparticle Size. SSRN Electr. J. 2022, 4180121. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hemar, Y.; Ashokkumar, M.; Paturel, S.; Lewis, G.D. Inactivation of Bacteria and Yeast Using High-Frequency Ultrasound Treatment. Water Res. 2014, 60, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Tuziuti, T.; Kozuka, T.; Towata, A.; Iida, Y. Relationship between the Bubble Temperature and Main Oxidant Created inside an Air Bubble under Ultrasound. J. Chem. Phys. 2007, 127, 154502. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K. Numerical Simulations for Sonochemistry. Ultrason. Sonochem. 2021, 78, 105728. [Google Scholar] [CrossRef]
- Didenko, Y.T.; Suslick, K.S. The Energy Efficiency of Formation of Photons, Radicals and Ions during Single-Bubble Cavitation. Nature 2002, 418, 394–397. [Google Scholar] [CrossRef]
- Stricker, L.; Dollet, B.; Rivas, D.F.; Lohse, D. Interacting Bubble Clouds and Their Sonochemical Production. J. Acoust. Soc. Am. 2013, 134, 1854–1862. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, L.; Wu, Y.; Chen, W. The Role of the Bubble-Bubble Interaction on Radial Pulsations of Bubbles. Ultrason. Sonochem. 2021, 73, 105535. [Google Scholar] [CrossRef] [PubMed]
- Kruszelnicka, W.; Hlosta, J.; Diviš, J.; Gierz, Ł. Study of the Relationships between Multi-Hole, Multi-Disc Mill Performance Parameters and Comminution Indicators. Sustainability 2021, 13, 8260. [Google Scholar] [CrossRef]
- Lomovskiy, I.; Bychkov, A.; Lomovsky, O.; Skripkina, T. Mechanochemical and Size Reduction Machines for Biorefining. Molecules 2020, 25, 5345. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, J.; Wretland, A.; Berglund, J. Abrasive Water Jet Milling as An Efficient Manufacturing Method for Superalloy Gas Turbine Components. J. Manuf. Mater. Process. 2022, 6, 124. [Google Scholar] [CrossRef]
- Nied, R. Chapter 5 Rotor Impact Mills. In Handbook of Powder Technology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 12, pp. 229–249. [Google Scholar] [CrossRef]
- Burgio, N.; Iasonna, A.; Magini, M.; Martelli, S.; Padella, F. Mechanical Alloying of the Fe-Zr System. Correlation between Input Energy and End Products. Il Nuovo Cimento D 1991, 13, 459–476. [Google Scholar] [CrossRef]
- Chattopadhyay, P.P.; Manna, I.; Talapatra, S.; Pabi, S.K. A Mathematical Analysis of Milling Mechanics in a Planetary Ball Mill. Mater. Chem. Phys. 2001, 68, 85–94. [Google Scholar] [CrossRef]
- Magini, M.; Iasonna, A.; Padella, F. Ball Milling: An Experimental Support to the Energy Transfer Evaluated by the Collision Model. Scr. Mater. 1996, 34, 13–19. [Google Scholar] [CrossRef]
- Margetić, D.; Štrukil, V. Experiments for Introduction of Mechanochemistry in the Undergraduate Curriculum. In Mechanochemical Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 2016; pp. 351–360. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Zhao, J.; Sun, W.; Zhang, Y.; Qiao, J.; Xing, G.; Wang, X. Model Study of Mechanicochemical Degradation in a Planetary Ball Mill. Sustainability 2023, 15, 1353. [Google Scholar] [CrossRef]
- Schilz, J. Internal Kinematics of Tumbler and Planetary Ball Mills: A Mathematical Model for the Parameter Setting. Mater. Trans. JIM 1998, 39, 1152–1157. [Google Scholar] [CrossRef][Green Version]
- Le Brun, P.; Froyen, L.; Delaey, L. The Modelling of the Mechanical Alloying Process in a Planetary Ball Mill: Comparison between Theory and In-Situ Observations. Mater. Sci. Eng. A 1993, 161, 75–82. [Google Scholar] [CrossRef]
- Broseghini, M.; Gelisio, L.; D’incau, M.; Ricardo, C.L.A.; Pugno, N.M.; Scardi, P. Modeling of the Planetary Ball-Milling Process: The Case Study of Ceramic Powders. J. Eur. Ceram. Soc. 2016, 36, 2205–2212. [Google Scholar] [CrossRef]
- Maurice, D.R.; Courtney, T.H. The Physics of Mechanical Alloying: A First Report. Metall. Trans. A 1990, 21, 289–303. [Google Scholar] [CrossRef]
- Kalnitsky, V.S.; Rusakov, O.V.; Sergeenkov, V.E. A Planetary Mill Modelling in Chaotic Mode. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1155, 012089. [Google Scholar] [CrossRef]
- Kwade, A. Mill Selection and Process Optimization Using a Physical Grinding Model. Int. J. Miner. Process. 2004, 74, S93–S101. [Google Scholar] [CrossRef]
- Burmeister, C.F.; Kwade, A. Process Engineering with Planetary Ball Mills. Chem. Soc. Rev. 2013, 42, 7660–7667. [Google Scholar] [CrossRef] [PubMed]
- Kakuk, G.; Zsoldos, I.; Csanády, Á.; Oldal, I. Contributions to the Modelling of the Milling Process in a Planetary Ball Mill. Rev. Adv. Mater. Sci. 2009, 22, 21–38. [Google Scholar]
- Real, C.; Gotor, F.J. Effects of the Speed Ratio on the Efficiency of Planetary Mills. Heliyon 2019, 5, e01227. [Google Scholar] [CrossRef]
- Blanc, N.; Mayer-Laigle, C.; Frank, X.; Radjai, F.; Delenne, J.-Y. Evolution of Grinding Energy and Particle Size during Dry Ball-Milling of Silica Sand. Powder Technol. 2020, 376, 661–667. [Google Scholar] [CrossRef]
- Sacher, E.; Engel, P.A.; Bayer, R.G. Mechanochemical Aspects of Repetitive Impacts and Sliding on Polymers. J. Appl. Polym. Sci. 1979, 24, 1503–1514. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Caporaso, M.; Gaudino, E.C.; Giovannoli, C.; Cravotto, G. Synthesis of Randomly Substituted Anionic Cyclodextrins in Ball Milling. Molecules 2017, 22, 485. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Calsolaro, F.; Martina, K.; Bucciol, F.; Manzoli, M.; Cravotto, G. Reaction of Oxiranes with Cyclodextrins under High-Energy Ball-Milling Conditions. Beilstein J. Org. Chem. 2019, 15, 1448–1459. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Bucciol, F.; Manzoli, M.; Cravotto, G. Comparative Studies of Mechanochemically Synthesized Insoluble Beta-Cyclodextrin Polymers. Curr. Org. Chem. 2021, 25, 1923–1936. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Caporaso, M.; Tuza, K.; Martina, K.; Gaudino, E.C.; Cravotto, G. Nucleophilic Substitutions of 6I-O-Monotosyl-β-cyclodextrin in a Planetary Ball Mill. ACS Sustain. Chem. Eng. 2016, 4, 919–929. [Google Scholar] [CrossRef]
- Takacs, L.; Mchenry, J.S. Temperature of the Milling Balls in Shaker and Planetary Mills. J. Mater. Sci. 2006, 41, 5246–5249. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of Mechanochemistry: From Nanoparticles to Technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Zhurkov, S.N.; Vettegren, V.; Korsukov, V.E.; Novak, I.I. Infrared Spectroscopic Study of the Chemical Bonds in Stressed Polymers. Fiz. Tverd. Tela 1969, 11, 290–295. [Google Scholar]
- Zhurkov, S.N.; Suchkov, V.; Novak, I.I.; Zosin, L. Molecular Orientation and the Tensile Strength of Polystyrene. Polym. Mech. 1969, 5, 531–534. [Google Scholar] [CrossRef]
- Crist, B.; Ratner, M.A.; Brower, A.L.; Sabin, J.R. Ab Initio Calculations of the Deformation of Polyethylene. J. Appl. Phys. 1969, 50, 6047–6051. [Google Scholar] [CrossRef]
- Lazarou, Y.G.; Prosmitis, A.V.; Papadimitriou, V.C.; Papagiannakopoulos, P. Theoretical Calculation of Bond Dissociation Energies and Enthalpies of Formation for Halogenated Molecules. J. Phys. Chem. A 2001, 105, 6729–6742. [Google Scholar] [CrossRef]
- Peterson, G.I.; Ko, W.; Hwang, Y.-J.; Choi, T.-L. Mechanochemical Degradation of Amorphous Polymers with Ball-Mill Grinding: Influence of the Glass Transition Temperature. Macromolecules 2020, 53, 7795–7802. [Google Scholar] [CrossRef]
- Wu, D.; Tu, M.; Wang, Z.; Wu, C.; Yu, C.; Battino, M.; El-Seedi, H.R.; Du, M. Biological and Conventional Food Processing Modifications on Food Proteins: Structure, Functionality, and Bioactivity. Biotechnol. Adv. 2020, 40, 107491. [Google Scholar] [CrossRef]
- Oberg, E.; Jones, F.D.; Horton, H.L.; Ryffel, H.H. Machinery’s Handbook, 26th ed.; Mccauley, C.J., Heald, R., Hussain, M.I., Eds.; Industrial Press, Inc.: New York, NY, USA, 2000; p. 2640. [Google Scholar]
- Wick, C. Tool and Manufacturing Engineers Handbook (Vol 2: Forming), 4th ed.; Wick, C., Ed.; Society of Manufacturing Engineers: Dearborn, MI, USA, 1984; Volume 2. [Google Scholar]
- Zambrano, Y.; Contardo, I.; Moreno, M.C.; Bouchon, P. Effect of Extrusion Temperature and Feed Moisture Content on the Microstructural Properties of Rice-Flour Pellets and Their Impact on the Expanded Product. Foods 2022, 11, 198. [Google Scholar] [CrossRef]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Bigg, D.M.; Smith, E.G.; Epstein, M.M.; Fiorentino, R.J. Warm hydrostatic extrusion of polyethylene. Polym. Eng. Sci 1978, 18, 908–916. [Google Scholar] [CrossRef]
- Keleb, E.; Vermeire, A.; Vervaet, C.; Remon, J. Cold extrusion as a continuous single-step granulation and tabletting process. Eur. J. Pharm. Biopharm. 2001, 52, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Chadha, D.; Young, O.; Otter, D.; Kam, R. Physical analysis of friction cooked RTE snacks. Innov. Food Sci. Emerg. Technol. 2021, 69, 102643. [Google Scholar] [CrossRef]
- Sousa, J.; Teixeira, P.F.; Hilliou, L.; Covas, J.A. Experimental Validation of a Micro-Extrusion Set-Up with In-Line Rheometry for the Production and Monitoring of Filaments for 3D-Printing. Micromachines 2023, 14, 1496. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.W.; Chan, W.L. A review on the state-of-the-art microforming technologies. Int. J. Adv. Manuf. Technol. 2012, 67, 2411–2437. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Grillo, G.; Manzoli, M.; Tabasso, S.; Maccagnan, S.; Cravotto, G. Mechanochemical Applications of Reactive Extrusion from Organic Synthesis to Catalytic and Active Materials. Molecules 2022, 27, 449. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, A.; Wilczyński, K. Modeling of Twin Screw Extrusion of Polymeric Materials. Polymers 2022, 14, 274. [Google Scholar] [CrossRef]
- Raquez, J.; Narayan, R.; Dubois, P. Recent Advances in Reactive Extrusion Processing of Biodegradable Polymer-Based Compositions. Macromol. Mater. Eng. 2008, 293, 447–470. [Google Scholar] [CrossRef]
- Edeleva, M.; De Smit, K.; Debrie, S.; Verberckmoes, A.; Marien, Y.W.; D’hooge, D.R. Molecular scale-driven upgrading of extrusion technology for sustainable polymer processing and recycling. Curr. Opin. Green Sustain. Chem. 2023, 43, 100848. [Google Scholar] [CrossRef]
- Main, P.; Petersmann, S.; Wild, N.; Feuchter, M.; Duretek, I.; Edeleva, M.; Ragaert, P.; Cardon, L.; Lucyshyn, T. Impact of Multiple Reprocessing on Properties of Polyhydroxybutyrate and Polypropylene. Polymers 2023, 15, 4126. [Google Scholar] [CrossRef] [PubMed]
- Velghe, I.; Buffel, B.; Vandeginste, V.; Thielemans, W.; Desplentere, F. Review on the Degradation of Poly(lactic acid) during Melt Processing. Polymers 2023, 15, 2047. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Halley, P.J.; Gilbert, R.G. Mechanism of Degradation of Starch, a Highly Branched Polymer, during Extrusion. Macromolecules 2010, 43, 2855–2864. [Google Scholar] [CrossRef]
- Emin, M.A.; Schuchmann, H.P. A mechanistic approach to analyze extrusion processing of biopolymers by numerical, rheological, and optical methods. Trends Food Sci. Technol. 2017, 60, 88–95. [Google Scholar] [CrossRef]
- Guiao, K.S.; Gupta, A.; Tzoganakis, C.; Mekonnen, T.H. Reactive extrusion as a sustainable alternative for the processing and valorization of biomass components. J. Clean. Prod. 2022, 355, 131840. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Sutera, F.; Gulino, E.; Morreale, M. Degradation and Recycling of Films Based on Biodegradable Polymers: A Short Review. Polymers 2019, 11, 651. [Google Scholar] [CrossRef]
- Montano-Herrera, L.; Pratt, S.; Arcos-Hernandez, M.V.; Halley, P.J.; Lant, P.A.; Werker, A.; Laycock, B. In-line monitoring of thermal degradation of PHA during melt-processing by Near-Infrared spectroscopy. New Biotechnol. 2014, 31, 357–363. [Google Scholar] [CrossRef]
- Vergnes, B.; Berzin, F. Modeling of Reactive Systems in Twin-Screw Extrusion: Challenges and Applications. C. R. Chim. 2006, 9, 1409–1418. [Google Scholar] [CrossRef]
- De Smit, K.; Wieme, T.; Marien, Y.W.; Van Steenberge, P.H.M.; D’hooge, D.R.; Edeleva, M. Multi-scale reactive extrusion modelling approaches to design polymer synthesis, modification and mechanical recycling. React. Chem. Eng. 2022, 7, 245–263. [Google Scholar] [CrossRef]
- Sun, D.; Zhu, X.; Gao, M. 3D Numerical Simulation of Reactive Extrusion Processes for Preparing PP/TiO2 Nanocomposites in a Corotating Twin Screw Extruder. Materials 2019, 12, 671. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, F.; Santana Pérez, O.; Maspoch, M. Kinetics of the Thermal Degradation of Poly(lactic acid) and Polyamide Bioblends. Polymers 2021, 13, 3996. [Google Scholar] [CrossRef] [PubMed]
- Berzin, F.; Vergnes, B.; Delamare, L. Rheological Behavior of Controlled-Rheology Polypropylenes Obtained by Peroxide-Promoted Degradation during Extrusion: Comparison between Homopolymer and Copolymer. J. Appl. Polym. Sci. 2001, 80, 1243–1252. [Google Scholar] [CrossRef]
- Li, M.; Tang, L.; Xue, F.; Landers, R. Numerical Simulation of Ram Extrusion Process for Ceramic Materials. In Proceedings of the 22nd Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, Austin, TX, USA, 8–10 August 2011; Volume 333, p. 02011. [Google Scholar] [CrossRef]
- Akbulatov, S.; Boulatov, R. Experimental Polymer Mechanochemistry and Its Interpretational Frameworks. ChemPhysChem 2017, 18, 1422–1450. [Google Scholar] [CrossRef] [PubMed]
- Abella, L.L.; Yamamoto, K.; Fukuda, K.; Nanbu, S.; Oikawa, N.; Morita, K.; Matsumoto, T. Ab Initio Calculations for the Reaction Paths of Levoglucosan: An Intermediate of Cellulose Pyrolysis. Mem. Fac. Eng. Kyushu Univ. 2006, 66, 147–168. [Google Scholar]
- Turner, J.A.; Adrianov, T.; Zakaria, M.A.; Taylor, M.S. Effects of Configuration and Substitution on C-H Bond Dissociation Enthalpies in Carbohydrate derivatives: A Systematic Computational Study. J. Org. Chem. 2021, 87, 1421–1433. [Google Scholar] [CrossRef]
- Lee, B.; Niu, Z.; Wang, J.; Slebodnick, C.; Craig, S.L. Relative Mechanical Strengths of Weak Bonds in Sonochemical Polymer Mechanochemistry. J. Am. Chem. Soc. 2015, 137, 10826–10832. [Google Scholar] [CrossRef]
- Lee, B.; Niu, Z.; Craig, S.L. The Mechanical Strength of a Mechanical Bond: Sonochemical Polymer Mechanochemistry of Poly(catenane) Copolymers. Angew. Chem. Int. Ed. 2016, 55, 13086–13089. [Google Scholar] [CrossRef]
- Tureček, F. N-Cα Bond Dissociation Energies and Kinetics in Amide and Peptide Radicals. Is the Dissociation a Non-Ergodic Process? J. Am. Chem. Soc. 2003, 125, 5954–5963. [Google Scholar] [CrossRef]
- Schüth, F.; Rinaldi, R.; Meine, N.; Käldström, M.; Hilgert, J.; Rechulski, M.D.K. Mechanocatalytic Depolymerization of Cellulose and Raw Biomass and Downstream Processing of the Products. Catal. Today 2014, 234, 24–30. [Google Scholar] [CrossRef]
- Nomura, S.; Sato, S.; Erata, T. DFT approach to the pathway of conformational changes of cellulose C6-hydroxymethyl group with simple cellotetraose model involving the mechanism of mercerization process. Chem. Phys. Lett. 2020, 742, 137154. [Google Scholar] [CrossRef]
- Loerbroks, C.; Rinaldi, R.; Thiel, W. The Electronic Nature of the 1,4mbox-beta-Glycosidic Bond and Its Chemical Environment: DFT Insights into Cellulose Chemistry. Chem. Eur. J. 2013, 19, 16282–16294. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, W.; Gou, S.; Ye, Z.; Feng, M.; Lai, N.; Liang, L. Synthesis and Evaluation of Novel Water-Soluble Copolymers Based on Acrylamide and Modular Β-Cyclodextrin. Carbohydr. Polym. 2013, 96, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hasjim, J.; Xie, F.; Halley, P.J.; Gilbert, R.G. Shear degradation of molecular, crystalline, and granular structures of starch during extrusion. Starch/Stärke 2013, 66, 595–605. [Google Scholar] [CrossRef]
- Barbee, M.H.; Wang, J.; Kouznetsova, T.; Lu, M.; Craig, S.L. Mechanochemical Ring-Opening of Allylic Epoxides. Macromolecules 2019, 52, 6234–6240. [Google Scholar] [CrossRef]
- Wick, C.R.; Topraksal, E.; Smith, D.M.; Smith, A.-S. Evaluating the predictive character of the method of constrained geometries simulate external force with density functional theory. Forces Mech. 2022, 9, 100143. [Google Scholar] [CrossRef]
- Chen, N.; Pilla, S. A comprehensive review on transforming lignocellulosic materials into biocarbon and its utilization for composites applications. Compos. Part C Open Access 2022, 7, 100225. [Google Scholar] [CrossRef]
- Kumar, V.; Chakraborty, P.; Janghu, P.; Umesh, M.; Sarojini, S.; Pasrija, R.; Kaur, K.; Lakkaboyana, S.K.; Sugumar, V.; Nandhagopal, M.; et al. Potential of banana based cellulose materials for advanced applications: A review on properties and technical challenges. Carbohydr. Polym. Technol. Appl. 2023, 6, 100366. [Google Scholar] [CrossRef]
- Rananavare, A.P.; Lee, J. Hydrophobic cotton fabric synthesized via dispersion polymerization from poly(glycidyl methacrylate) nanoparticles for self-cleaning applications. Prog. Org. Coat. 2022, 170, 107006. [Google Scholar] [CrossRef]
- Ginni, G.; Kavitha, S.; Yukesh Kannah, R.; Bhatia, S.K.; Adish Kumar, S.; Rajkumar, M.; Kumar, G.; Pugazhendhi, A.; Chi, N.T.L.; Rajesh Banu, J. Valorization of agricultural residues: Different biorefinery routes. J. Environ. Chem. Eng. 2022, 9, 105435. [Google Scholar] [CrossRef]
- Rocky, M.M.H.; Rahman, I.M.M.; Biswas, F.B.; Rahman, S.; Endo, M.; Wong, K.H.; Mashio, A.S.; Hasegawa, H. Cellulose-based materials for scavenging toxic and precious metals from water and wastewater: A review. Chem. Eng. J. 2023, 472, 144677. [Google Scholar] [CrossRef]
- Calderón, B.A.; McCaughey, M.S.; Thompson, C.W.; Barinelli, V.L.; Sobkowicz, M.J. Evaluating the Influence of Specific Mechanical Energy on Biopolymer Blends Prepared via High-Speed Reactive Extrusion. ACS Appl. Polym. Mater. 2019, 1, 1410–1419. [Google Scholar] [CrossRef]
- Castéran, F.; Delage, K.; Hascoët, N.; Ammar, A.; Chinesta, F.; Cassagnau, P. Data-Driven Modelling of Polyethylene Recycling under High-Temperature Extrusion. Polymers 2022, 14, 800. [Google Scholar] [CrossRef]
- Striegel, A.M. Influence of Anomeric Configuration on Mechanochemical Degradation of Polysaccharides: Cellulose versus Amylose. Biomacromolecules 2007, 8, 3944–3949. [Google Scholar] [CrossRef] [PubMed]
- Striegel, A.M. Influence of Chain Architecture on the Mechanochemical Degradation of Macromolecules. J. Biochem. Bioph. Meth. 2003, 56, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Lipatova, I.M.; Losev, N.V.; Makarova, L.I. The Influence of the Combined Impact of Shear Stress and Cavitation on the Structure and Sorption Properties of Chitin. Carbohyd. Polym. 2019, 209, 320–327. [Google Scholar] [CrossRef]
- Matsuyama, T.; Menhofer, H.; Heusinger, H. Esr Spin Trap Investigations on Aqueous Glucose Solutions Irradiated by Ultrasound and Gamma-Rays. Int. J. Radiat. Appl. Instrum. Part C 1988, 32, 735–739. [Google Scholar] [CrossRef]
- Robo, M.T.; Collias, D.; Zimmerman, P.M. Interplay between Applied Force and Radical Attack in the Mechanochemical Chain Scission of Poly(acrylic Acid). J. Phys. Chem. A 2022, 126, 521–528. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Rokita, B.; Ulanski, P.; Rosiak, J.M. Radiation-Induced and Sonochemical Degradation of Chitosan As a Way to Increase Its Fat-Binding Capacity. Nucl. Instrum. Methods Phys. Res. Sect. B 2005, 236, 383–390. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Rokita, B.; Lotfy, S.; Ulanski, P.; Rosiak, J.M. Degradation of Chitosan and Starch by 360-Khz Ultrasound. Carbohyd. Polym. 2005, 60, 175–184. [Google Scholar] [CrossRef]
- Ulanski, P.; Sonntag, C.V. Oh-Radical-Induced Chain Scission of Chitosan in the Absence and Presence of Dioxygen. J. Chem. Soc. Perkin Trans. 2000, 2, 2022–2028. [Google Scholar] [CrossRef]
- Portenlänger, G.; Heusinger, H. Polymer Formation from Aqueous Solutions of A-D-Glucose by Ultrasound and Λ-Rays. Ultrason. Sonochem. 1994, 1, S125–S129. [Google Scholar] [CrossRef]
- Rassokhin, D.N.; Kovalev, G.V.; Bugaenko, L.T. Comparative Study of the Sonolysis and Radiolysis of Carboxymethylcellulose in Aqueous Solution. Mendeleev Commun. 1992, 2, 103–105. [Google Scholar] [CrossRef]
- Ehrlich, H.; Koutsoukos, P.G.; Demadis, K.D.; Pokrovsky, O.S. Principles of Demineralization: Modern Strategies for the Isolation of Organic Frameworks: Part II. Decalcification. Micron 2009, 40, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Striegel, A.M. Observations Regarding On-Column, Flow-Induced Degradation during Sec Analysis. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 3105–3114. [Google Scholar] [CrossRef]
- Huang, D.; Wang, J.; Song, C.; Zhao, Y. Ultrasound-Responsive Matters for Biomedical Applications. Innovation 2023, 4, 100421. [Google Scholar] [CrossRef]
- Zuo, J.Y.; Knoerzer, K.; Mawson, R.; Kentish, S.; Ashokkumar, M. The Pasting Properties of Sonicated Waxy Rice Starch Suspensions. Ultrason. Sonochem. 2009, 16, 462–468. [Google Scholar] [CrossRef]
- Schmidt-Naake, G.; Drache, M.; Weber, M. Combination of Mechanochemical Degradation of Polymers with Controlled Free-Radical Polymerization. Macromol. Chem. Phys. 2002, 203, 2232–2238. [Google Scholar] [CrossRef]
- Lauberte, L.; Telysheva, G.; Cravotto, G.; Andersone, A.; Janceva, S.; Dizhbite, T.; Arshanitsa, A.; Jurkjane, V.; Vevere, L.; Grillo, G.; et al. Lignin—Derived Antioxidants As Value-Added Products Obtained under Cavitation Treatments of the Wheat Straw Processing for Sugar Production. J. Clean. Prod. 2021, 303, 126369. [Google Scholar] [CrossRef]
- Sun, D.; Sun, S.-C.; Wang, B.; Sun, S.-F.; Shi, Q.; Zheng, L.; Wang, S.-F.; Liu, S.-J.; Li, M.-F.; Cao, X.-F.; et al. Effect of Various Pretreatments on Improving Cellulose Enzymatic Digestibility of Tobacco Stalk and the Structural Features of Co-Produced Hemicelluloses. Bioresour. Technol. 2020, 297, 122471. [Google Scholar] [CrossRef] [PubMed]
- Amirjalayer, S.; Fuchs, H.; Marx, D. understanding the Mechanocatalytic Conversion of Biomass: A Low-Energy One-Step Reaction Mechanism by Applying Mechanical Force. Angew. Chem. Int. Ed. 2019, 58, 5232–5235. [Google Scholar] [CrossRef] [PubMed]
- Hick, S.M.; Griebel, C.; Restrepo, D.T.; Truitt, J.H.; Buker, E.J.; Bylda, C.; Blair, R.G. Mechanocatalysis for Biomass-derived Chemicals and Fuels. ACS Sym. Ser. 2010, 12, 468–474. [Google Scholar] [CrossRef]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, Y.; Teng, C.; Quan, H.; Yang, X.; Li, H.; Li, X.; Yan, L. Fast and Selective Degradation of Biomass for Xylose, Glucose and Lignin under Mild Conditions. Molecules 2023, 28, 3306. [Google Scholar] [CrossRef] [PubMed]
- Zhurkov, S.N.; Novak, I.I.; Levin, B.Y.; Savitskii, A.V.; Vettegren, V.I. Relation between Polymer Strength and Molecular Orientation. Polym. Sci. USSR 1965, 7, 1331–1336. [Google Scholar] [CrossRef]
- L’vov, K.M.; Gasymov, O.K.; Mamedov, S.V. The Mechanical Degradation of Silk Fibroin at 77 K. Polym. Sci. USSR 1984, 26, 2127–2132. [Google Scholar] [CrossRef]
- Dubinskaya, A.M. Free Radicals Formed during the Mechanical Degradation of Polypeptides. Polym. Sci. USSR 1984, 26, 1861–1871. [Google Scholar] [CrossRef]
- Berlin, A.A.; Dubinskaya, A.M. Studies in the Mechanochemistry of Polymers—X. Initiation of Polymerization by Radicals Formed during the Ultrasonic Degradation of Macromolecules. Polym. Sci. USSR 1962, 3, 345–351. [Google Scholar] [CrossRef]
- Alinezhad, P.; Staji, H.; Sani, R.N. Comparison of Three Methods Including Temperature, H2O2/Ascorbic Acid/Sonication, and Nitrous Acid Treatments for Overcoming the Inhibitory Effect of Heparin on DNA Amplification in Realtime-PCR. Int. J. Biol. Macromol. 2022, 209, 1298–1306. [Google Scholar] [CrossRef]
- Zou, M.; Zhao, P.; Huo, S.; Göstl, R.; Herrmann, A. Activation of Antibiotic-Grafted Polymer Brushes by Ultrasound. ACS Macro Lett. 2022, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Zhao, P.; Fan, J.; Göstl, R.; Herrmann, A. Microgels As Drug Carriers for Sonopharmacology. J. Polym. Sci. 2022, 60, 1864–1870. [Google Scholar] [CrossRef]
- Yildiz, D.; Göstl, R.; Herrmann, A. Sonopharmacology: Controlling Pharmacotherapy and Diagnosis by Ultrasound-Induced Polymer Mechanochemistry. Chem. Sci. 2022, 13, 13708–13719. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Mikami, R. Redox Chemistry of Selenols and Diselenides as Potential Manipulators for Structural Maturation of Peptides and Proteins. Met. Res. 2022, 2, MR202206. [Google Scholar] [CrossRef]
- Shchedrina, V.A.; Novoselov, S.V.; Malinouski, M.Y.; Gladyshev, V.N. Identification and characterization of a selenoprotein family containing a diselenide bond in a redox motif. Proc. Natl. Acad. Sci. USA 2007, 104, 13919–13924. [Google Scholar] [CrossRef]
- Wu, Q.; Yuan, Y.; Chen, F.; Sun, C.; Xu, H.; Chen, Y. Diselenide-Linked Polymers under Sonication. ACS Macro Lett. 2020, 9, 1547–1551. [Google Scholar] [CrossRef]
- Sugita, H.; Lu, Y.; Aoki, D.; Otsuka, H.; Mikami, K. Theoretical and Experimental Investigations of Stable Arylfluorene-Based Radical-Type Mechanophores. Chem. Eur. J. 2023, 29, e202203249. [Google Scholar] [CrossRef]
- Bhangu, S.K.; Ashokkumar, M. Theory of Sonochemistry. Top. Curr. Chem. 2016, 374, 56. [Google Scholar] [CrossRef]
- Joyce, E.; Phull, S.S.; Lorimer, J.P.; Mason, T.J. The Development and Evaluation of Ultrasound for the Treatment of Bacterial Suspensions. A Study of Frequency, Power and Sonication Time on Cultured Bacillus Species. Ultrason. Sonochem. 2003, 10, 315–318. [Google Scholar] [CrossRef]
- Jyothi, A.N. Starch Graft Copolymers: Novel Applications in Industry. Compos. Interfaces 2010, 17, 165–174. [Google Scholar] [CrossRef]
- Riesz, P.; Kondo, T. Free Radical Formation Induced by Ultrasound and Its Biological Implications. Free Radic. Biol. Med. 1992, 13, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Meine, N.; Rinaldi, R.; Schüth, F. Solvent-Free Catalytic Depolymerization of Cellulose to Water-Soluble Oligosaccharides. ChemSusChem 2012, 5, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Ott, R.L. Mechanism of the Mechanical Degradation of Cellulose. J. Polym. Sci. A 1964, 2, 973–982. [Google Scholar] [CrossRef]
- Abagyan, G.V.; Butyagin, P.Y. Electron Spin Resonance Study of the Mechanical Degradation of Polysaccharides. Polym. Sci. USSR 1965, 7, 1563–1569. [Google Scholar] [CrossRef]
- Abagyan, G.V.; Butyagin, P.Y. Mechanochemical Initiation of Free-Radical Reactions in Polysaccharides. Polym. Sci. USSR 1984, 26, 1466–1474. [Google Scholar] [CrossRef]
- Watanabe, J.; Nakano, S. Chemically Modified Starch and Its Use. (Part 2). Yuki Gosei Kagaku Kyokaishi/J. Synth. Org. Chem. Jpn. 1965, 23, 526–530. [Google Scholar] [CrossRef]
- Tomasik, P.; Schilling, C.H. Chemical Modification of Starch. Adv. Carbohydr. Chem. 2004, 59, 175–403. [Google Scholar] [CrossRef]
- Wang, N.; Wang, B.; Wan, Y.; Gao, B.; Rajput, V.D. Alginate-based composites as novel soil conditioners for sustainable applications in agriculture: A critical review. J. Environ. Manag. 2023, 348, 119133. [Google Scholar] [CrossRef]
- Sukhlaaied, W.; Riyajan, S.-A. Synthesis and properties of carrageenan grafted copolymer with poly(vinyl alcohol). Carbohydr. Polym. 2013, 98, 677–685. [Google Scholar] [CrossRef]
- Carr, M.E.; Kim, S.; Yoon, K.J.; Stanley, K.D. Graft Polymerization of Cationic Methacrylate, Acrylamide, and Acrylonitrile Monomers onto Starch by Reactive Extrusion. Cereal Chem. 1992, 69, 70–75. [Google Scholar]
- Patel, A.R.; Patel, M.R.; Patel, K.C.; Patel, R.D. Processibility of starch-graft-polyacrylonitrile copolymers. Angew. Makromol. Chem. 1985, 136, 135–145. [Google Scholar] [CrossRef]
- Hoeger, I.C.; Nair, S.S.; Ragauskas, A.J.; Deng, Y.; Rojas, O.J.; Zhu, J.Y. Mechanical Deconstruction of Lignocellulose Cell Walls and Their Enzymatic Saccharification. Cellulose 2013, 20, 807–818. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhu, J.Y.; Gleisner, R.; Kuster, T.A.; Baxa, U.; Mcneil, S.E. Morphological Development of Cellulose Fibrils of a Bleached Eucalyptus Pulp by Mechanical Fibrillation. Cellulose 2012, 19, 1631–1643. [Google Scholar] [CrossRef]
- Qin, Y.; Qiu, X.; Zhu, J.Y. Understanding Longitudinal Wood Fiber Ultra-Structure for Producing Cellulose Nanofibrils Using Disk Milling with Diluted Acid Prehydrolysis. Sci. Rep. 2016, 6, 35602. [Google Scholar] [CrossRef]
- Wang, W.; Sabo, R.C.; Mozuch, M.D.; Kersten, P.; Zhu, J.Y.; Jin, Y. Physical and Mechanical Properties of Cellulose Nanofibril Films from Bleached Eucalyptus Pulp by Endoglucanase Treatment and Microfluidization. J. Polym. Environ. 2015, 23, 551–558. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D.; Hedenqvist, M.; Ankerfors, M.; Lindström, T. Highly Transparent Films from Carboxymethylated Microfibrillated Cellulose: The Effect of Multiple Homogenization Steps on Key Properties. J. Appl. Polym. Sci. 2010, 119, 2652–2660. [Google Scholar] [CrossRef]
- Dabral, S.; Wotruba, H.; Hernández, J.G.; Bolm, C. Mechanochemical Oxidation and Cleavage of Lignin Β-O-4 Model Compounds and Lignin. ACS Sust. Chem. Eng. 2018, 6, 3242–3254. [Google Scholar] [CrossRef]
- Brittain, A.D.; Chrisandina, N.J.; Cooper, R.E.; Buchanan, M.; Cort, J.R.; Olarte, M.V.; Sievers, C. Quenching of Reactive Intermediates during Mechanochemical Depolymerization of Lignin. Catal. Today 2018, 302, 180–189. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Z.; Wang, X.; Ma, S.; Sun, B.; Wang, F. Mechanochemical Effects on the Structural Properties of Wheat Starch during Vibration Ball Milling of Wheat Endosperm. Int. J. Biol. Macromol. 2022, 206, 306–312. [Google Scholar] [CrossRef]
- Geib, S.M.; Tien, M.; Hoover, K. Identification of Proteins Involved in Lignocellulose Degradation Using in Gel Zymogram Analysis Combined with Mass Spectroscopy-Based Peptide Analysis of Gut Proteins from Larval Asian Longhorned Beetles, Anoplophora Glabripennis. Insect Sci. 2010, 17, 253–264. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Kleine, T.; Buendia, J.; Bolm, C. Mechanochemical Degradation of Lignin and Wood by Solvent-Free Grinding in a Reactive Medium. Green Chem. 2013, 15, 160–166. [Google Scholar] [CrossRef]
- Dabral, S.; Mottweiler, J.; Rinesch, T.; Bolm, C. Base-Catalysed Cleavage of Lignin Β-O-4 Model Compounds in Dimethyl Carbonate. ACS. Sym. Ser. 2015, 17, 4908–4912. [Google Scholar] [CrossRef]
- Schneider, L.; Haverinen, J.; Jaakkola, M.; Lassi, U. Effective Saccharification of Lignocellulosic Barley Straw by Mechanocatalytical Pretreatment Using Potassium Pyrosulfate As a Catalyst. Bioresour. Technol. 2017, 234, 1–7. [Google Scholar] [CrossRef][Green Version]
- Käldström, M.; Meine, N.; Farés, C.; Rinaldi, R.; Schüth, F. Fractionation of ‘Water-Soluble Lignocellulose into Sugars and Sulfur-Free Lignins. Green Chem. 2014, 16, 2454–2462. [Google Scholar] [CrossRef]
- Käldström, M.; Meine, N.; Farés, C.; Schüth, F.; Rinaldi, R. Deciphering “water-Soluble Lignocellulose” Obtained by Mechanocatalysis: New Insights into the Chemical Processes Leading to Deep Depolymerization. Green Chem. 2014, 16, 3528–3538. [Google Scholar] [CrossRef]
- Loustau-Cazalet, C.; Sambusiti, C.; Buche, P.; Solhy, A.; Bilal, E.; Larzek, M.; Barakat, A. Innovative Deconstruction of Biomass Induced by Dry Chemo-Mechanical Activation: Impact on Enzymatic Hydrolysis and Energy Efficiency. ACS Sust. Chem. Eng. 2016, 4, 2689–2697. [Google Scholar] [CrossRef]
- Carrasquillo-Flores, R.; Käldström, M.; Schüth, F.; Dumesic, J.A.; Rinaldi, R. Mechanocatalytic Depolymerization of Dry (ligno)cellulose As an Entry Process for High-Yield Production of Furfurals. ACS Catal. 2013, 3, 993–997. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Li, C.; Li, X.; Kumrungsee, T.; Zhai, X.; Zhou, Z.; Cao, R. The modification of buckwheat polyphenols by different pretreatments and complexation, and its application in oat flour model. Food Biosci. 2023, 56, 103133. [Google Scholar] [CrossRef]
- Ma, Q.; Yu, Y.; Zhou, Z.; Wang, L.; Cao, R. Effects of different treatments on composition, physicochemical and biological properties of soluble dietary fiber in buckwheat bran. Food Biosci. 2023, 53, 102517. [Google Scholar] [CrossRef]
- Zielińska, D.; Siwińska-Ciesielczyk, K.; Bula, K.; Jesionowski, T.; Borysiak, S. TiO2/nanocellulose Hybrids As Functional Additives for Advanced Polypropylene Nanocomposites. Ind. Crops Prod. 2022, 176, 114314. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, J.; Gao, W.; Yang, Q.; Chen, L.; Yang, L.; Sun, Q.; Zhang, H. Effects of Rice Straw Structure on Chaetoglobosin a Production by Chaetomium Globosum Cgmcc 6882. Int. J. Biol. Macromol. 2020, 150, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, A.G.; Tissot, H.; Tissot, P.; Alfonso, D.; Gurny, R.; Doelker, E. Influence of Hot-Melt Extrusion and Compression Molding on Polymer Structure Organization, Investigated by Differential Scanning Calorimetry. J. Appl. Polym. Sci. 2001, 81, 3124–3132. [Google Scholar] [CrossRef]
- Kaleda, A.; Talvistu, K.; Tamm, M.; Viirma, M.; Rosend, J.; Tanilas, K.; Kriisa, M.; Part, N.; Tammik, M.-L. Impact of Fermentation and Phytase Treatment of Pea-Oat Protein Blend on Physicochemical, Sensory, and Nutritional Properties of Extruded Meat Analogs. Foods 2020, 9, 1059. [Google Scholar] [CrossRef] [PubMed]
- Šárka, E.; Sluková, M.; Henke, S. Changes in Phenolics during Cooking Extrusion: A Review. Foods 2021, 10, 2100. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N.; Ray, C.A. Control of Maillard Reactions in Foods: Strategies and Chemical Mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef]
- Tavares, M.; da Silva, M.R.M.; de Oliveira de Siqueira, L.B.; Rodrigues, R.A.S.; Bodjolle-D’Almeida, L.; Dos Santos, E.P.; Ricci-Júnior, E. Trends in Insect Repellent Formulations: A Review. Int. J. Pharm. 2018, 539, 190–209. [Google Scholar] [CrossRef]
- Ho, C.-T.; Riha, W.E., III. Formation of Maillard Aromas during Extrusion Cooking; O’Brien, J., Nursten, H.E., Crabbe, M.J.C., Ames, J.M., Eds.; Elsevier (Woodhead Publishing): Amsterdam, The Netherlands, 2005; pp. 187–192. [Google Scholar] [CrossRef]
- Sasanam, S.; Thumthanaruk, B.; Wijuntamook, S.; Rattananupap, V.; Vatanyoopaisarn, S.; Puttanlek, C.; Uttapap, D.; Mussatto, S.I.; Rungsardthong, V. Extrusion of process flavorings from methionine and dextrose using modified starch as a carrier. PLoS ONE 2023, 18, e0269857. [Google Scholar] [CrossRef]
- Yaylayan, V.A.; Fichtali, J.; van de Voort, F.R. Production of Maillard reaction flavour precursors by extrusion processing. Food Res. Int. 1992, 25, 175–180. [Google Scholar] [CrossRef]
- Seethamraju, K.; Bhattacharya, M.; Vaidya, U.; Fulcher, R. Rheology and Morphology of Starch Synthetic-Polymer Blends. Rheol. Acta 1994, 33, 553–567. [Google Scholar] [CrossRef]
- Incarnato, L.; Scarfato, P.; Scatteia, L.; Acierno, D. Rheological Behavior of New Melt Compounded Copolyamide Nanocomposites. Polymer 2004, 45, 3487–3496. [Google Scholar] [CrossRef]
- Lin, H.; Isayev, A.I. Ultrasonic Treatment of Polypropylene, Polyamide 6, and Their Blends. J. Appl. Polym. Sci. 2006, 102, 2643–2653. [Google Scholar] [CrossRef]
- Maresca, D.; Payen, T.; Lee-Gosselin, A.; Ling, B.; Malounda, D.; Demené, C.; Tanter, M.; Shapiro, M.G. Acoustic biomolecules enhance hemodynamic functional ultrasound imaging of neural activity. NeuroImage 2020, 209, 116467. [Google Scholar] [CrossRef]
- Pal, A.K.; Misra, M.; Mohanty, A.K. Silane Treated Starch Dispersed PBAT/PHBV-Based Composites: Improved Barrier Performance for Single-Use Plastic Alternatives. Int. J. Biol. Macromol. 2023, 229, 1009–1022. [Google Scholar] [CrossRef]
- Schmid, E.-M.; Farahnaky, A.; Adhikari, B.; Torley, P.J. High moisture extrusion cooking of meat analogs: A review of mechanisms of protein texturization. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4573–4609. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, H. High moisture food extrusion. Int. J. Food Sci. Tech. 1999, 34, 195–207. [Google Scholar] [CrossRef]
- Zhang, G.; Ni, C.; Ding, Y.; Zhou, H.; Caizhi, O.; Wang, Q.; Wang, J.; Cheng, J. Effects of Low Moisture Extrusion on the Structural and Physicochemical Properties of Adlay (coix Lacryma-Jobi L.) Starch-Based Polymers. Process Biochem. 2020, 96, 30–37. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, H.; Liu, Y.; Zou, X.; Shi, J.; Zhao, Y.; Ye, Y.; Yu, Y.; Guo, J. Preparation of Calcium Alginate/polyethylene Glycol Acrylate Double Network Fiber with Excellent Properties by Dynamic Molding Method. Carbohyd. Polym. 2019, 226, 115277. [Google Scholar] [CrossRef]
- Mencel, K.; Kelar, K.; Jurkowski, B. Influence of Shear Stress on the Structures and Properties of Polyamide 6/montmorillonite Nanocomposites. Polim. W 2009, 54, 361–369. [Google Scholar] [CrossRef]
- You, X.D.; Li, L.; Gao, J.P.; Yu, J.G.; Zhao, Z.X. Biodegradable Extruded Starch Blends. J. Appl. Polym. Sci. 2003, 88, 627–635. [Google Scholar] [CrossRef]
- Pushpadass, H.A.; Kumar, A.; Jackson, D.S.; Wehling, R.L.; Dumais, J.J.; Hanna, M.A. Macromolecular Changes in Extruded Starch-Films Plasticized with Glycerol, Water and Stearic Acid. Starch-Starke 2009, 61, 256–266. [Google Scholar] [CrossRef]
- Dimonie, D.; Petrache, M.; Damian, C.; Anton, L.; Musat, M.; Dima, S.-O.; Jinescu, C.; Maria, R. New Evidences on the Process Sensitivity of Some Renewable Blends Based on Starch Considering Their Melt Rheological Properties. Int. J. Polym. Sci. 2016, 2016, 7873120. [Google Scholar] [CrossRef]
- Koller, M. Biodegradable and Biocompatible Polyhydroxy-Alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef] [PubMed]
- Farhat, W.; Venditti, R.; Mignard, N.; Taha, M.; Becquart, F.; Ayoub, A. Polysaccharides and Lignin Based Hydrogels with Potential Pharmaceutical Use As a Drug Delivery System Produced by a Reactive Extrusion Process. Int. J. Biol. Macromol. 2017, 104, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Lejaeghere, K.; Bihlmayer, G.; Bjorkman, T.; Blaha, P.; Blugel, S.; Blum, V.; Caliste, D.; Castelli, I.E.; Clark, S.J.; Corso, A.D.; et al. Reproducibility in Density Functional Theory Calculations of Solids. Science 2016, 351, aad3000. [Google Scholar] [CrossRef]
- Demina, T.S.; Akopova, T.A.; Vladimirov, L.V.; Zelenetskii, A.N.; Markvicheva, E.A.; Grandfils, C. Polylactide-Based Microspheres Prepared Using Solid-State Copolymerized Chitosan and D,L-lactide. Mater. Sci. Eng. C 2016, 59, 333–338. [Google Scholar] [CrossRef]
- Tesfaye, M.; Patwa, R.; Dhar, P.; Katiyar, V. Nanosilk-Grafted Poly(lactic Acid) Films: Influence of Cross-Linking on Rheology and Thermal Stability. ACS Omega 2017, 2, 7071–7084. [Google Scholar] [CrossRef]
- Akato, K.M.; Nguyen, N.A.; Rajan, K.; Harper, D.P.; Naskar, A.K. A Tough and Sustainable Fiber-Forming Material from Lignin and Waste Poly(ethylene Terephthalate). RSC Adv. 2019, 9, 31202–31211. [Google Scholar] [CrossRef]




| Bond | Aliphatic | Non-Aliphatic | Ref. |
|---|---|---|---|
| N-H | 314 | [14] | |
| O-H | 428 | [14] | |
| S-H | 344 | 314 | [15] |
| Se-H | 305 | [14] | |
| C-C | 284–368 | 410 | [16] |
| C=C | 615 | [16] | |
| C-H | 381–410 | 427–435 | [16] |
| C-O | 350–389 | 381–410 | [16] |
| C=O | 749 | [14] | |
| CO-O | 395–414 | 368–381 | [15] |
| C-N | 293–343 | 460 | [16] |
| C=N | 615 | [16] | |
| O-O | 498 | [14] | |
| C-S | 699 | [14] | |
| S-S | 429 | [14] | |
| C-Se | 582 | [14] | |
| Se-Se | 333 | [14] |
| Transformation | Frequency [kHz] | Power [W] | Sonication Time [min] |
|---|---|---|---|
| Transesterification for biofuel production | 20–48 | 120–1200 | 10–140 |
| Vegetable oil emulsification for biofuel production | 40 | 700–1200 | 120 |
| Peroxidase-catalyzed degradation of phenol | 423 | 5.5 | 20–60 |
| Protease-catalyzed oxidation of untanned leather waste | 40 | N/A | 120 |
| Cellulase-catalyzed degradation of distillery wastewater | 22.5 | 120 | 30–120 |
| Immunosensor B. subtilis var. niger | 1900–3000 | N/A | 3 a |
| Laccase-catalyzed decolorization | 150–850 | 42–120 | 60–540 |
| Tyrosinase-laccase immobilization | 20–40 | 600 | >10 b |
| Increase in dehydrogenate activity of waste-activated sludge | 35 | 80 | 10 |
| Anaerobic digestion of waste sludge | 20–40 | 600 | 60 c |
| Draining waste sludge | 20 | 300–1000 | 15–480 d |
| Reaction Type | Reaction | Reference |
|---|---|---|
| Dissociation of molecules | O2 → 2O• N2 → 2N• N2O + O• → 2NO• | [99,100,101] [99] |
| Hydroxy radical formation | H2O → •H + •OH •H + O2 → •OH + O | [102] |
| Peroxide and peroxy radical formation | O + H2O → H2O2 •H + O2 → HO2• O + •OH → •OOH •OH + H2O → H2O2• | [103] [102] [101] [103] |
| Radical transfer | N2 + •OH → N2O + H• •O• + N2O → 2NO• CO32− + •OH → •CO3− + OH− HCO32− + •OH → •CO3− + H2O | [104] [104] [105] [105] |
| Formation of the hydrated electron | •H + OH− ⇌ H2O + eaq- | [100] |
| Absorption of the hydrated electron | eaq- + H2O → OH− + H• eaq- + H+ → •H eaq- + O2 → 2O• eaq- + N2 → 2N• | [100] [100] [106] [106] |
| Recombination of radicals | •H + •H → H2 •H + •OH → H2O •OOH + H• → H2O2 | [100,101] [107,108] [103] |
| Extrusion Type | Operating Temperature | Typical Subjects | Characteristics and Utilization | Ref. |
|---|---|---|---|---|
| Hot | 350–2000 °C | Metals (alloys), glass, foods (e.g., orange peel) | High machinery and maintenance costs, tearing, blistering of product, and material engineering | [148] |
| Hot cooking | 50–200 °C | Food | Cooking, denaturation, and texturizing macromolecules | [149] |
| Hot melt | 30–150 °C | Organic and inorganic chemicals, plastics, food | Chemical reaction, polymer, pharmaceutical post-processing, and microencapsulation | [150] |
| Warm | 100–1000 °C | Metals, plastics, food | Polymer and food post-processing, alloy production with special purity characteristics | [148,151] |
| Cold | 20–50 °C | Metals, plastics, pharmaceutical ingredients | High pressure, fast extrusion speed, minimal oxidation, high-stability products, rough surface, polymers, and pharmaceutical post-processing | [148,152] |
| Friction | 200–400 °C | Plastics, foods (chips, flakes, etc.) | Energy-efficient, no preheating of feed materials, polymers, and food post-processing | [148,153] |
| Micro | 0–200 °C | Metals, polymers, foods | Uniform small particle distribution (<1 mm), 3D printing, and microelectronic lithography | [148,154,155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jicsinszky, L.; Bucciol, F.; Chaji, S.; Cravotto, G. Mechanochemical Degradation of Biopolymers. Molecules 2023, 28, 8031. https://doi.org/10.3390/molecules28248031
Jicsinszky L, Bucciol F, Chaji S, Cravotto G. Mechanochemical Degradation of Biopolymers. Molecules. 2023; 28(24):8031. https://doi.org/10.3390/molecules28248031
Chicago/Turabian StyleJicsinszky, László, Fabio Bucciol, Salah Chaji, and Giancarlo Cravotto. 2023. "Mechanochemical Degradation of Biopolymers" Molecules 28, no. 24: 8031. https://doi.org/10.3390/molecules28248031
APA StyleJicsinszky, L., Bucciol, F., Chaji, S., & Cravotto, G. (2023). Mechanochemical Degradation of Biopolymers. Molecules, 28(24), 8031. https://doi.org/10.3390/molecules28248031








