Recent Trends in the Petasis Reaction: A Review of Novel Catalytic Synthetic Approaches with Applications of the Petasis Reaction
Abstract
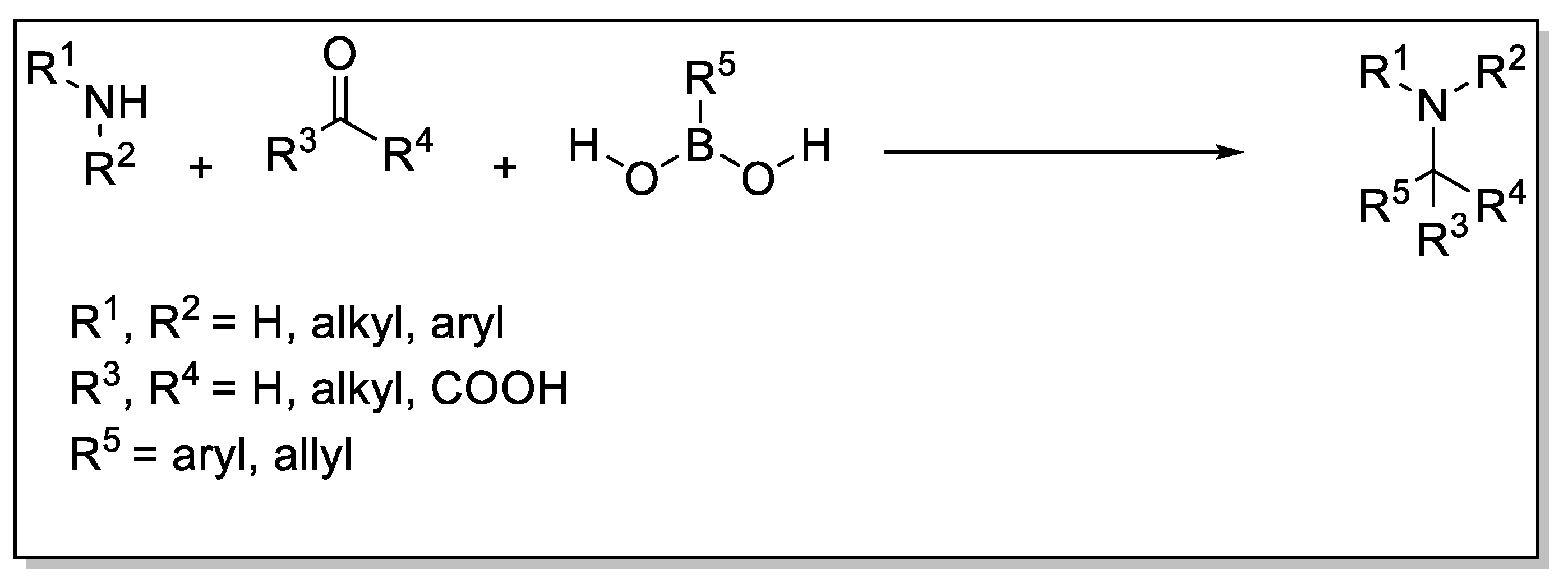
1. Introduction
2. Review of Literature
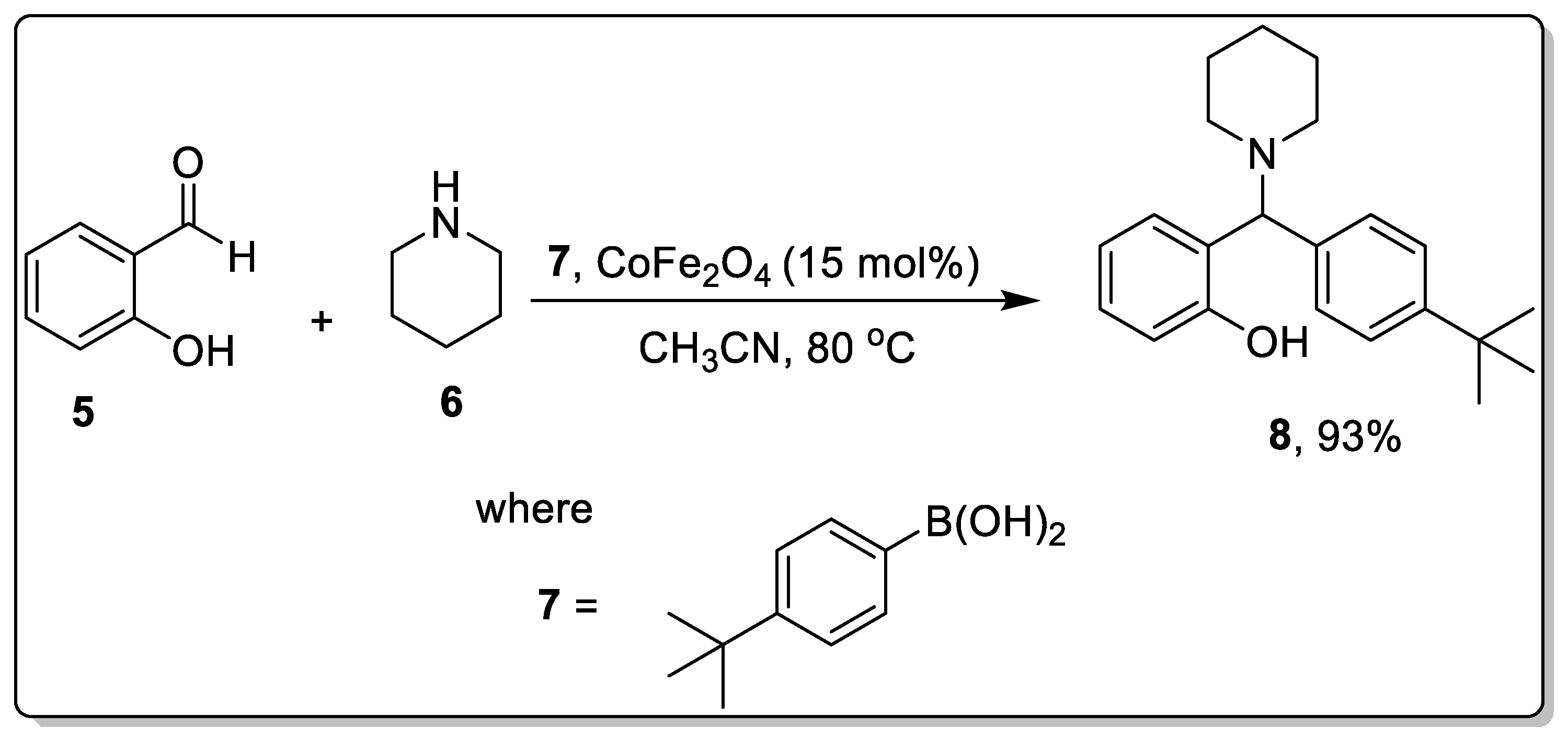
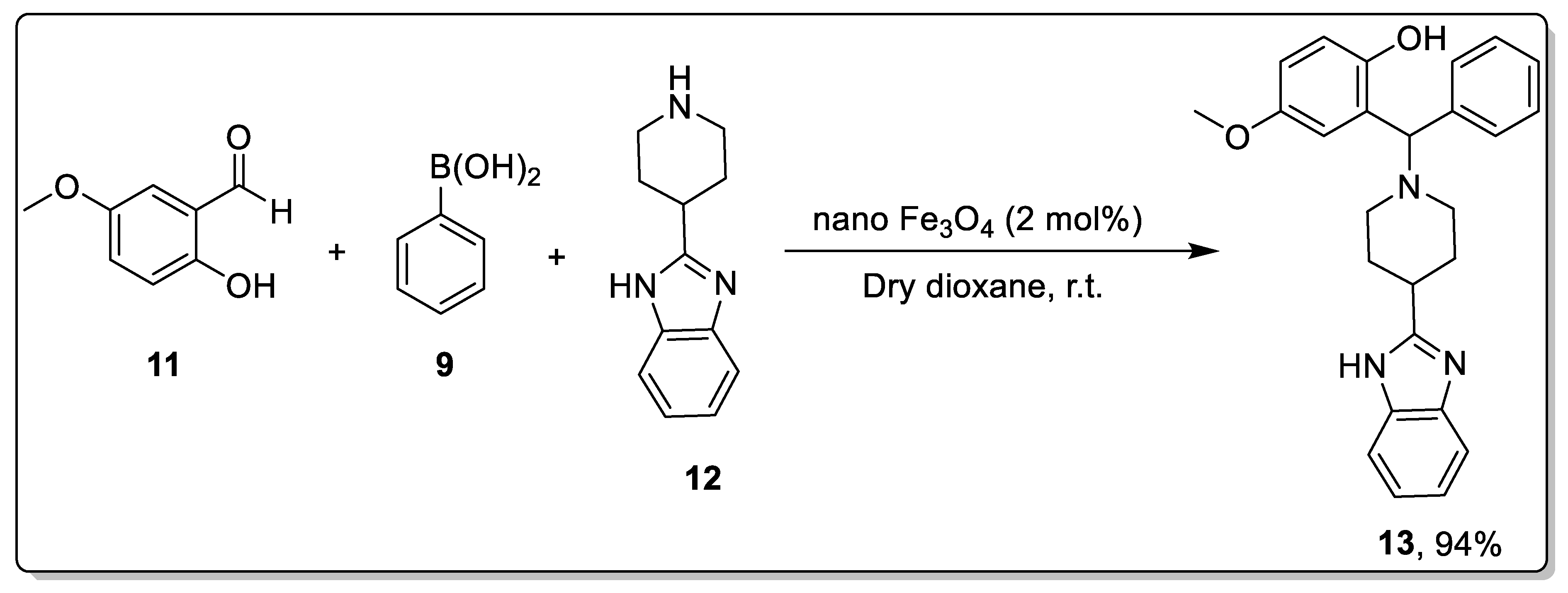
2.1. Petasis Reaction Catalyzed by Nanoparticles
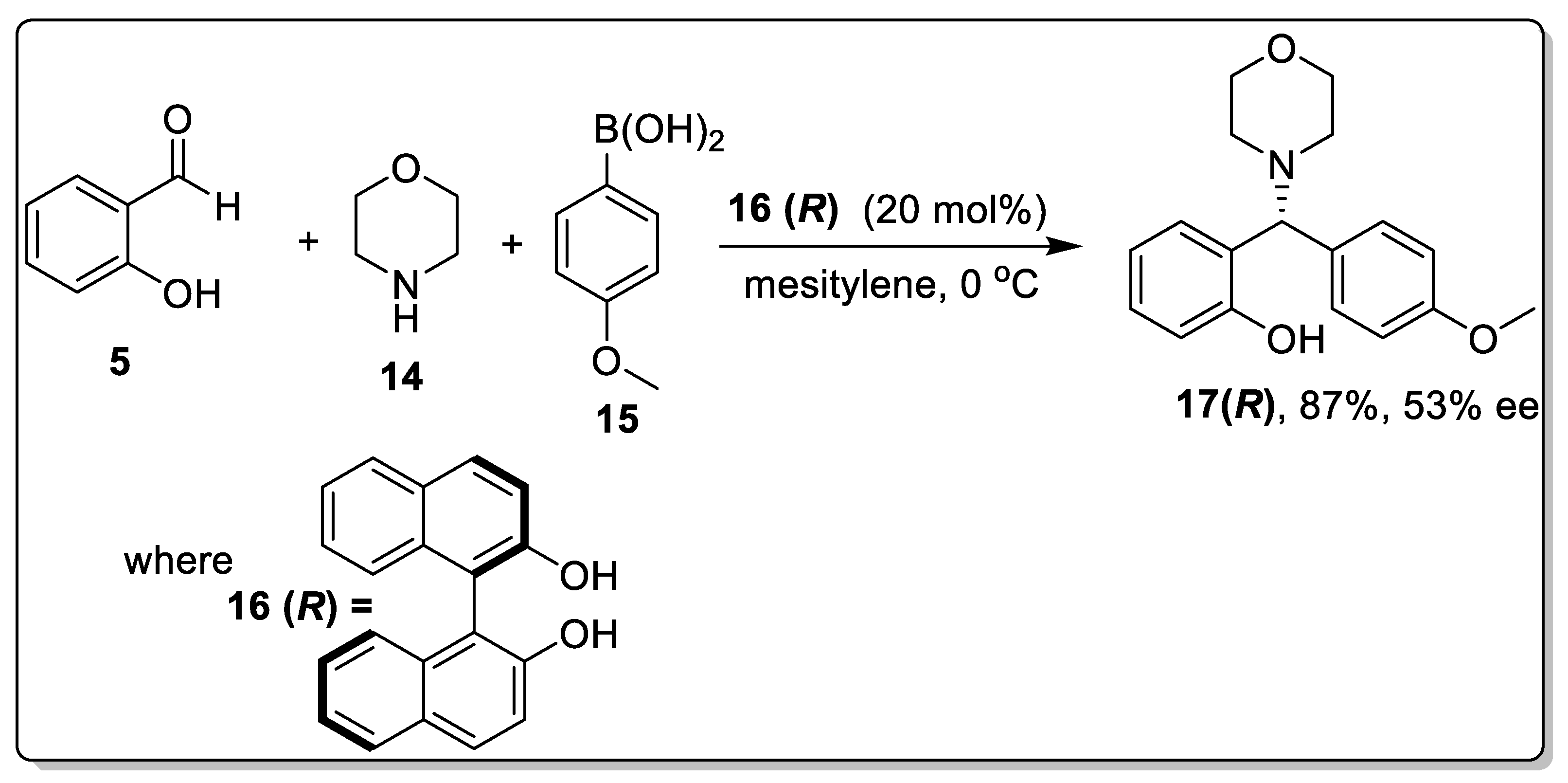
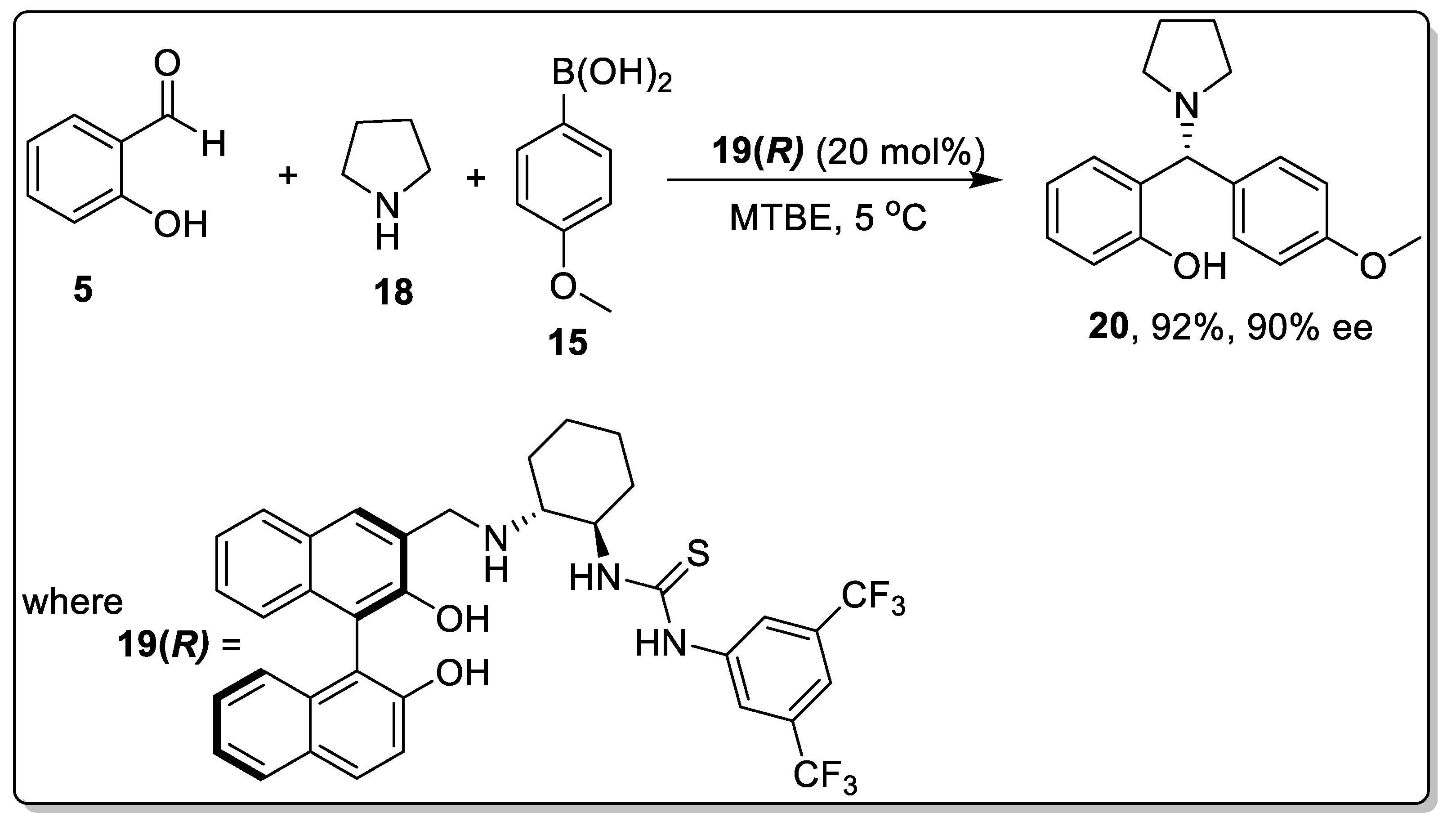
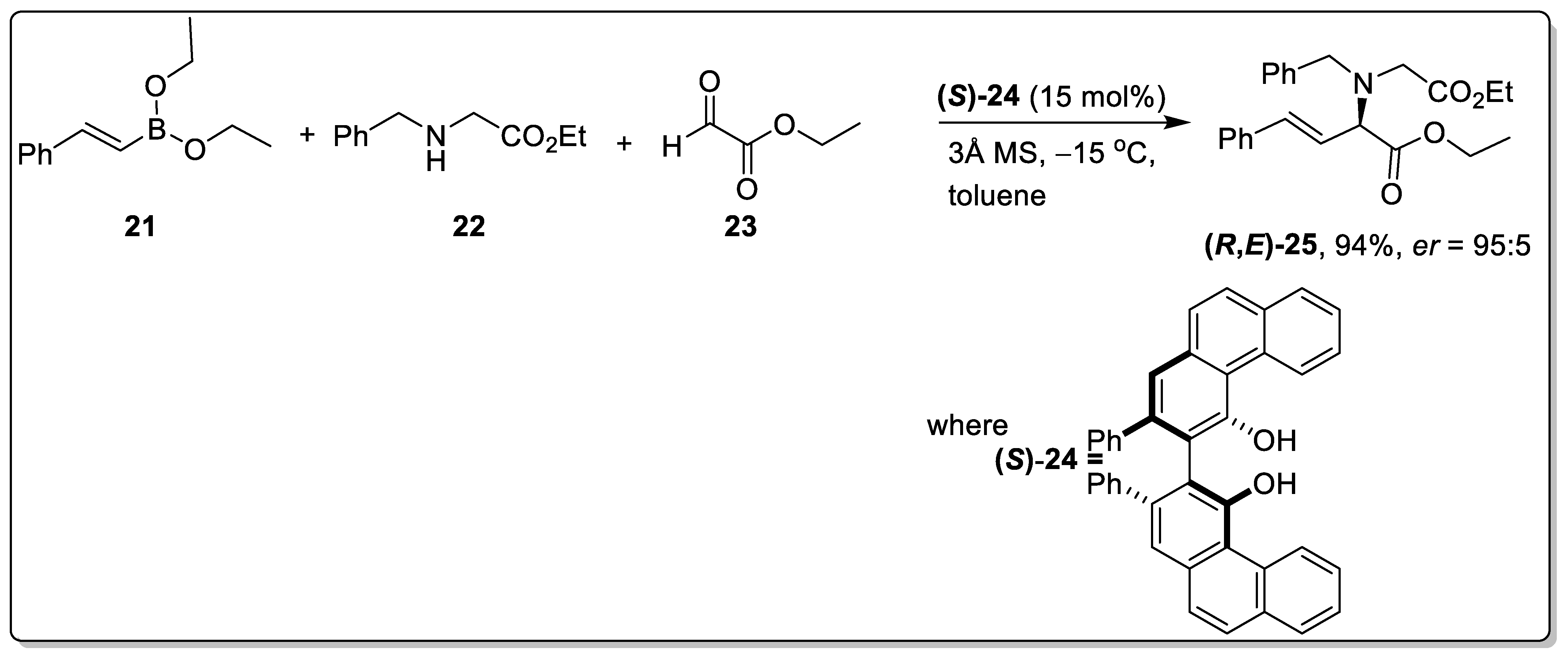
2.2. Petasis Reaction Involving Chiral Catalysts
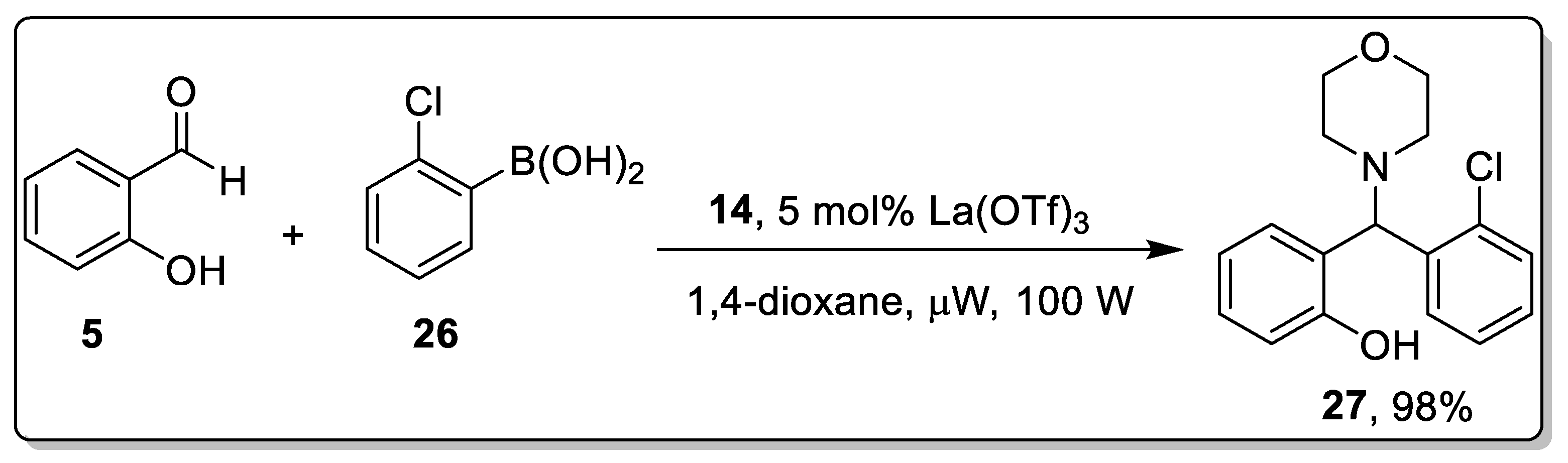
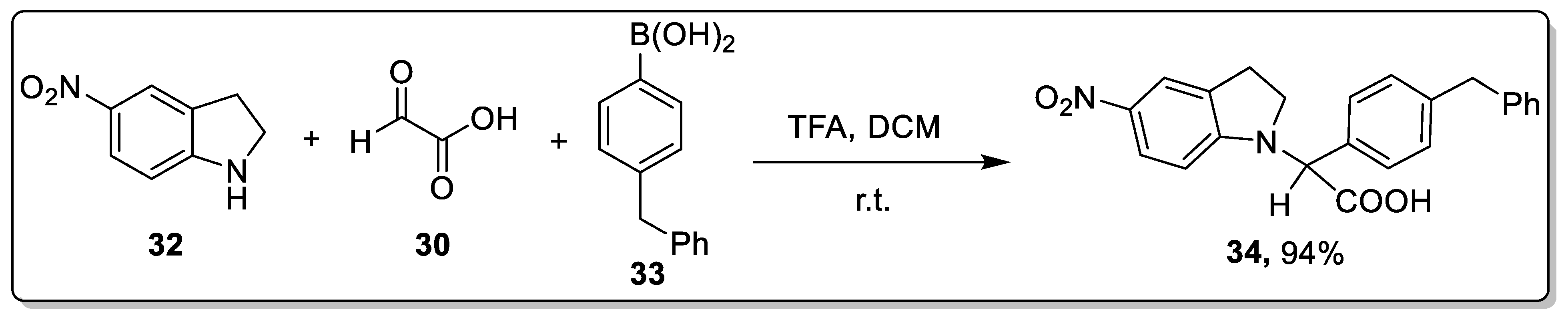
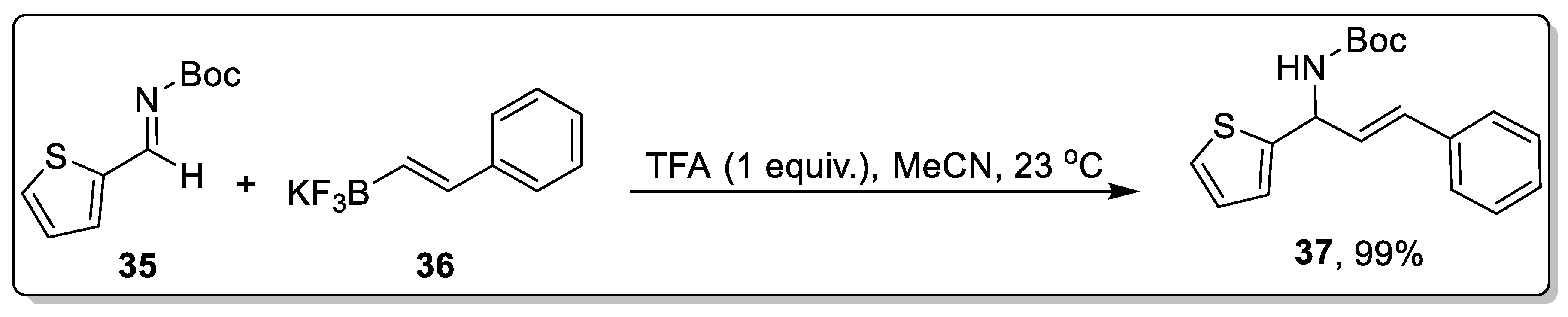
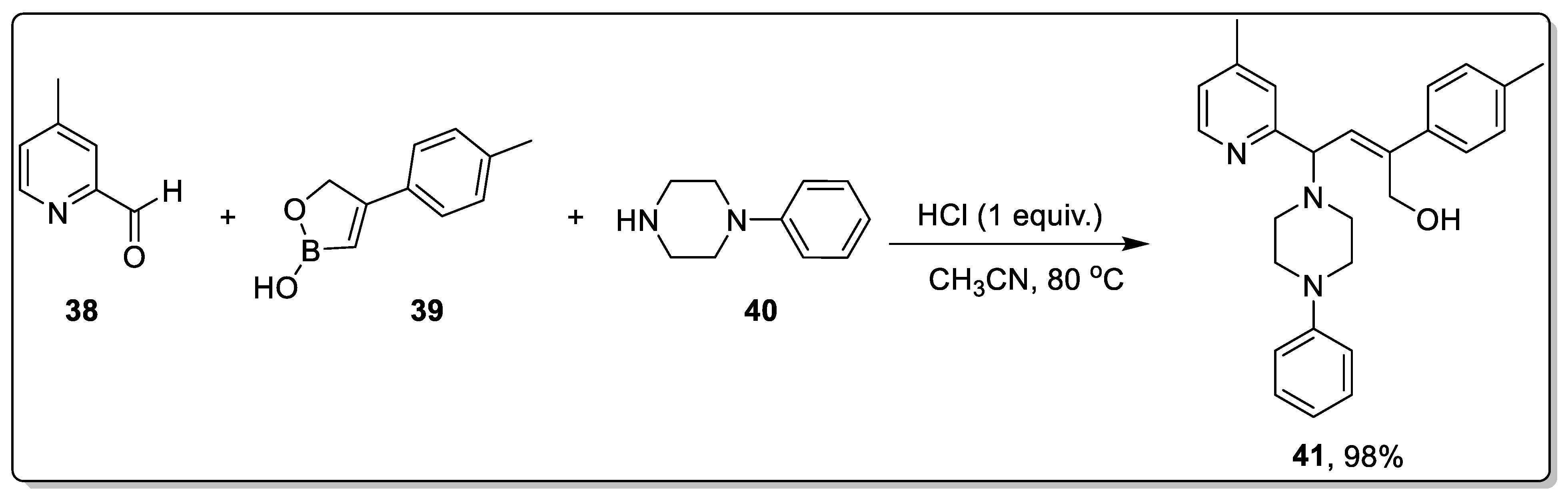
2.3. Acid-Catalyzed Petasis Reaction
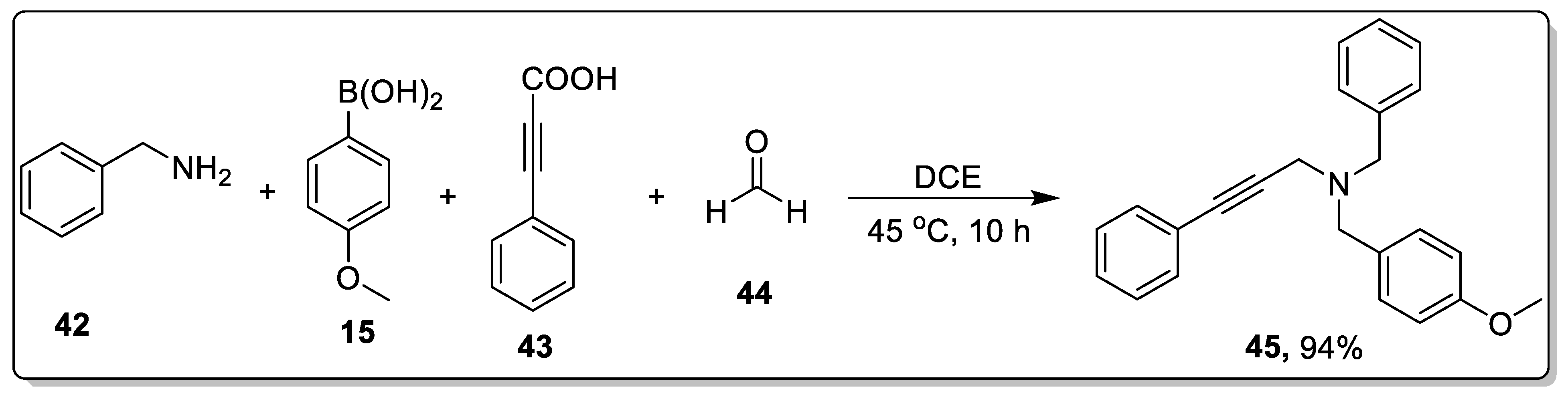
2.4. Catalyst-Free Petasis Reaction
2.5. Solvent-Free Synthesis via Petasis Reaction
2.6. Base-Catalyzed Petasis Reaction
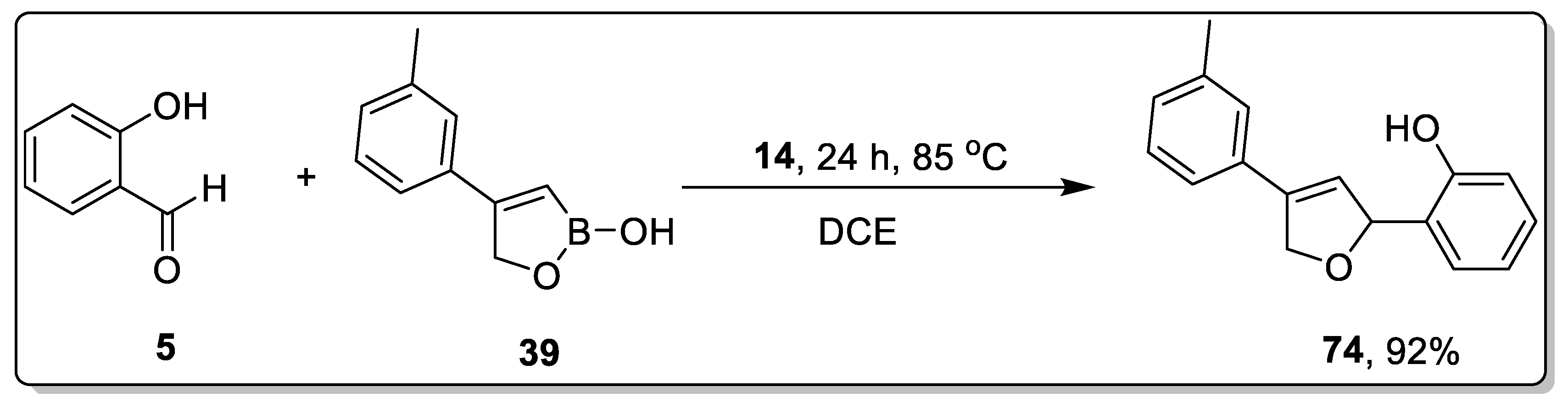
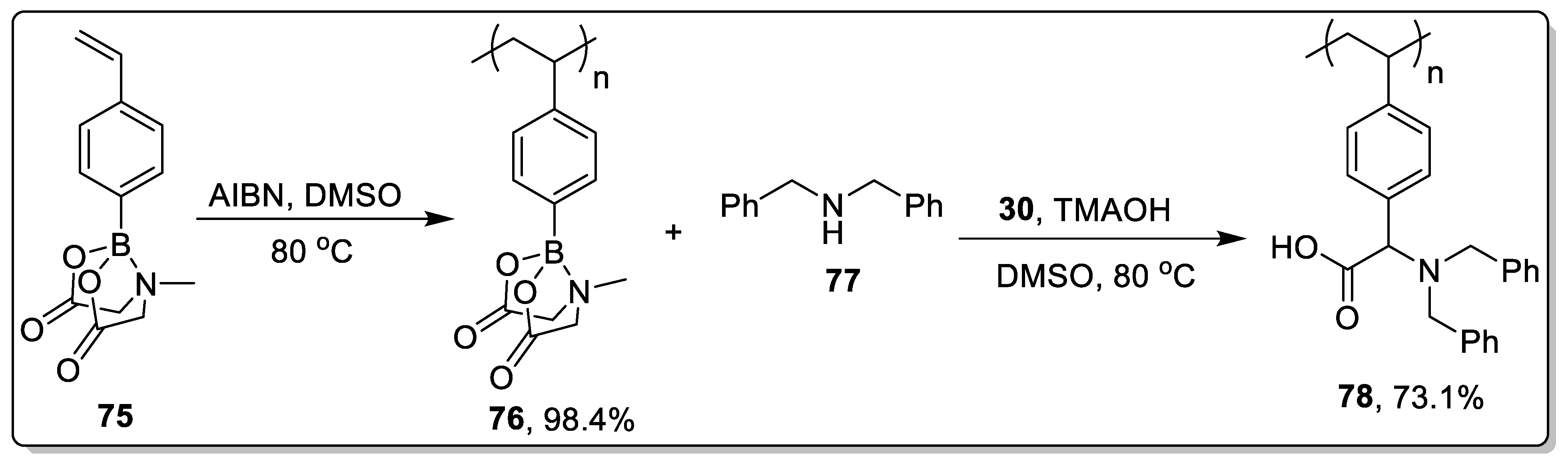
2.7. Miscellaneous Catalysts
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganem, B. Strategies for innovation in multicomponent reaction design. Acc. Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Feng, X. Asymmetric strecker reactions. Chem. Rev. 2011, 111, 6947–6983. [Google Scholar] [CrossRef] [PubMed]
- Hantzsch, A. Condensation product of aldehyde ammonia and ketone-like compounds. Ber. Dtsch. Chem. Ges. 1881, 14, 1637–1638. [Google Scholar] [CrossRef]
- Kappe, C.O. Biologically active dihydropyrimidones of the Biginelli-type a literature survey. Eur. J. Med. Chem. 2000, 35, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Reza Kazemizadeh, A.; Ramazani, A. Synthetic applications of Passerini reaction. Curr. Org. Chem. 2012, 16, 418–450. [Google Scholar] [CrossRef]
- Mushtaq, A.; Zahoor, A.F.; Ahmad, S.; Parveen, B.; Ali, K.G. Novel synthetic methods toward the synthesis of Betti bases: An update. Chem. Pap. 2023, 77, 4751–4795. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Toporova, M.S.; Kodess, M.I.; Ezhikova, M.A.; Isenov, M.L.; Pervova, M.G.; Kravchenko, M.A.; Medvinskiy, I.D.; Skornyakov, S.N.; Rusinov, G.L.; et al. Synthesis, X-ray crystal structure and antimycobacterial activity of enantiomerically pure 1-ethyl-2, 3-dicyano-5-(het) aryl-6-hetaryl-1, 6-dihydropyrazines. Arkivoc 2014, 2014, 247–270. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Slepukhin, P.A.; Kravchenko, M.A.; Skornyakov, S.N.; Kungurov, N.V.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Synthesis, antimycobacterial and antifungal evaluation of some new 1-ethyl-5-(hetero) aryl-6-styryl-1, 6-dihydropyrazine-2, 3-dicarbonitriles. Bioorg. Med. Chem. Lett. 2015, 25, 524–528. [Google Scholar] [CrossRef]
- Ahmad, S.; Zahoor, A.F.; Naqvi, S.A.R.; Akash, M. Recent trends in ring opening of epoxides with sulfur nucleophiles. Mol. Divers. 2018, 22, 191–205. [Google Scholar] [CrossRef]
- Da Silva, A.R.; Dos Santos, D.A.; Paixão, M.W.; Corrêa, A.G. Stereoselective Multicomponent Reactions in the Synthesis or Transformations of Epoxides and Aziridines. Molecules 2018, 24, 630. [Google Scholar] [CrossRef]
- Aziz, H.; Zahoor, A.F.; Ahmad, S. Pyrazole bearing molecules as bioactive scaffolds: A review. J. Chil. Chem. Soc. 2020, 65, 4746–4753. [Google Scholar] [CrossRef]
- Peng, W.; Michael, G.; Thomas, E.N. Reactivity and Synthetic Applications of Multicomponent Petasis Reactions. Chem. Rev. 2019, 119, 11245–11290. [Google Scholar] [CrossRef]
- Fodor, A.; Hell, Z.; Pirault-Roy, L. Catalytic activity of metal-doped porous materials in the salicylaldehyde Petasis-Borono Mannich reaction. Monatsh. Chem. 2016, 147, 749–753. [Google Scholar] [CrossRef]
- Akhtar, R.; Zahoor, A.F.; Rasool, N.; Ahmad, M.; Ali, K.G. Recent trends in the chemistry of Sandmeyer reaction: A review. Mol. Divers 2022, 26, 1837–1873. [Google Scholar] [CrossRef] [PubMed]
- Bering, L.; Antonchick, A.P. Regioselective metal-free cross-coupling of quinoline N-oxides with boronic acids. Org. Lett. 2015, 17, 3134–3137. [Google Scholar] [CrossRef]
- Abonia, R.; Garay, A.; Castillo, J.C.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Cobo, J.; Butassi, E.; Zacchino, S. Design of two alternative routes for the synthesis of naftifine and analogues as potential antifungal agents. Molecules 2018, 23, 520. [Google Scholar] [CrossRef]
- Petasis, N.A.; Akritopoulou, I. The boronic acid mannich reaction: A new method for the synthesis of geometrically pure allylamines. Tetrahedron Lett. 1993, 34, 583–586. [Google Scholar] [CrossRef]
- Smith, A.B.; Simov, V. Total Synthesis of the marine natural product (−)-clavosolide A. A showcase for the Petasis− Ferrier union/rearrangement tactic. Org. Lett. 2006, 8, 3315–3318. [Google Scholar] [CrossRef]
- Smith, A.B.; Basu, K.; Bosanac, T. Total synthesis of (−)-okilactomycin. J. Am. Chem. Soc. 2007, 129, 14872–14874. [Google Scholar] [CrossRef]
- Smith, A.B.; Mesaros, E.F.; Meyer, E.A. Total synthesis of (−)-kendomycin exploiting a Petasis− Ferrier rearrangement/ring-closing olefin metathesis synthetic strategy. J. Am. Chem. 2005, 127, 6948–6949. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B.; Razler, T.M.; Ciavarri, J.P.; Hirose, T.; Ishikawa, T.; Meis, R.M. A second-generation total synthesis of (+)-phorboxazole A. J. Org. Chem. 2008, 73, 1192–1200. [Google Scholar] [CrossRef]
- Frauenlob, R.; Garcia, C.; Bradshaw, G.A.; Burke, H.M.; Bergin, E. A copper-catalyzed Petasis reaction for the synthesis of tertiary amines and amino esters. J. Org. Chem. 2012, 77, 4445–4449. [Google Scholar] [CrossRef] [PubMed]
- Mundal, D.A.; Lutz, K.E.; Thomson, R.J. A direct synthesis of allenes by a traceless Petasis reaction. J. Am. Chem. Soc. 2012, 134, 5782–5785. [Google Scholar] [CrossRef]
- Murafuji, T.; Tasaki, Y.; Fujinaga, M.; Tao, K.; Kamijo, S.; Ishiguro, K. Blue Amino Acids Derived from Azulen-1-ylboronic Acid Pinacol Ester via the Petasis Reaction. Synthesis 2017, 49, 1037–1042. [Google Scholar] [CrossRef]
- Wu, P.; Petersen, M.A.; Cohrt, A.E.; Petersen, R.; Clausen, M.H.; Nielsen, T.E. Reductive cyclization and Petasis-like reaction for the synthesis of functionalized γ-lactams. Eur. J. Org. Chem 2015, 11, 2346–2350. [Google Scholar] [CrossRef]
- Hwang, J.; Borgelt, L.; Wu, P. Multicomponent Petasis reaction for the synthesis of functionalized 2-aminothiophenes and thienodiazepines. ACS Comb. Sci. 2020, 22, 495–499. [Google Scholar] [CrossRef]
- Chihara, K.; Kishikawa, N.; Ohyama, K.; Nakashima, K.; Kuroda, N. Determination of glyoxylic acid in urine by liquid chromatography with fluorescence detection, using a novel derivatization procedure based on the Petasis reaction. Anal. Bioanal. Chem. 2012, 403, 2765–2770. [Google Scholar] [CrossRef][Green Version]
- Churches, Q.I.; Johnson, J.K.; Fifer, N.L.; Hutton, C.A. Anomalies in the stereoselectivity of the Petasis reaction using styrenyl boronic acids. Aust. J. Chem. 2011, 64, 62–67. [Google Scholar] [CrossRef]
- Boonyasuppayakorn, S.; Reichert, E.D.; Manzano, M.; Nagarajan, K.; Padmanabhan, R. Amodiaquine, an antimalarial drug, inhibits dengue virus type 2 replication and infectivity. Antivir. Res. 2014, 106, 125–134. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, S.Y.; Park, C.Y.; Kim, Y.T.; Kim, B.J.; Song, Y.J.; Kim, B.G.; Kim, Y.B.; Cho, C.H.; Kim, J.H.; et al. A multicentre, randomised, open-label parallel-group Phase 2b study of belotecan versus topotecan for recurrent ovarian cancer. Brit. J. Cancer 2021, 124, 375–382. [Google Scholar] [CrossRef]
- Petasis, N.A.; Boral, S. One-step three-component reaction among organoboronic acids, amines and salicylaldehydes. Tetrahedron Lett. 2001, 42, 539–542. [Google Scholar] [CrossRef]
- Kulkarni, A.M.; Pandit, K.S.; Chavan, P.V.; Desai, U.V.; Wadgaonkar, P.P. Cobalt ferrite nanoparticles: A magnetically separable and reusable catalyst for Petasis-Borono–Mannich reaction. RSC Adv. 2015, 5, 70586–70594. [Google Scholar] [CrossRef]
- Rafiee, F.; Hosseinvand, S. CuII Immobolized on the amidinoglycine functionalized magnetic graphene oxide promoted the alkyl aminophenols synthesis. Iran. J. Sci. Technol. Trans. Sci. 2021, 45, 503–514. [Google Scholar] [CrossRef]
- Chacko, P.; Shivashankar, K. Synthesis of aminomethylphenol derivatives via magnetic nano Fe3O4 catalyzed one pot Petasis borono-Mannich reaction. J. Chem. Sci. 2018, 130, 154. [Google Scholar] [CrossRef]
- Han, W.Y.; Zuo, J.; Zhang, X.M.; Yuan, W.C. Enantioselective Petasis reaction among salicylaldehydes, amines, and organoboronic acids catalyzed by BINOL. Tetrahedron 2013, 69, 537–541. [Google Scholar] [CrossRef]
- Han, W.Y.; Zuo, J.; Zhang, X.M.; Yuan, W.C. Enantioselective Organocatalytic Three-Component Petasis Reaction among Salicylaldehydes, Amines, and Organoboronic Acids. Org. Lett. 2012, 14, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Beauseigneur, A.; Martel, A.; Dhal, R.; Laurent, M.; Dujardin, G. Access to α-substituted amino acid derivatives via 1, 3-dipolar cycloaddition of α-amino ester derived nitrones. J. Org. Chem. 2010, 75, 611–620. [Google Scholar] [CrossRef]
- Lou, S.; Schaus, S.E. Asymmetric Petasis reactions catalyzed by chiral biphenols. J. Am. Chem. Soc. 2008, 130, 6922–6923. [Google Scholar] [CrossRef]
- Reddy, B.N.; Rani, C.R.; Reddy, S.M.; Pathak, M. An efficient and green La(OTf)3 catalyzed Petasis borono–Mannich reaction for the synthesis of tertiary amines. Res. Chem. Intermed. 2016, 42, 7533–7549. [Google Scholar] [CrossRef]
- Rose, N.G.; Blaskovich, M.A.; Wong, A.; Lajoie, G.A. Synthesis of enantiomerically enriched β,γ-unsaturated-α-amino acids. Tetrahedron 2001, 57, 1497–1507. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.H. Lewis acid promoted highly diastereoselective Petasis borono-Mannich reaction: Efficient synthesis of optically active β,γ-unsaturated α-amino acids. Org. Lett. 2012, 14, 2062–2065. [Google Scholar] [CrossRef]
- Zhang, J.; Yun, F.; Xie, R.; Cheng, C.; Chen, G.; Li, J.; Tang, P.; Yuan, Q. Petasis three-component reaction accelerated by trifluoroacetic acid: Synthesis of indoline-derived glycines. Tetrahedron Lett. 2016, 57, 3916–3919. [Google Scholar] [CrossRef]
- Quach, T.D.; Batey, R.A. Copper (II)-catalyzed ether synthesis from aliphatic alcohols and potassium organotrifluoroborate salts. Org. Lett. 2003, 5, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- Carrera, D.E. The acid promoted Petasis reaction of organotrifluoroborates with imines and enamines. Chem. Commun. 2017, 53, 11185–11188. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Lee, H.Y. Au (I)-catalyzed cyclization of epoxyalkynes to allylic alcohol containing spiroketals and application to the total synthesis of (−)-alotaketal A. Org. Lett. 2020, 22, 4073–4077. [Google Scholar] [CrossRef] [PubMed]
- Jeso, V.; Micalizio, G.C. Total synthesis of lehualide B by allylic alcohol− alkyne reductive cross-coupling. J. Am. Chem. Soc. 2010, 132, 11422–11424. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Duan, Y.; Li, H.; Zhao, B.; Yang, J. An efficient HCl promoted Petasis reaction of 2-pyridinecarbaldehydes, amines and 1, 2-oxborol-2 (5H)-ols. Tetrahedron Lett. 2018, 59, 2502–2505. [Google Scholar] [CrossRef]
- Lo, V.K.Y.; Liu, Y.; Wong, M.K.; Che, C.M. Gold (III) salen complex-catalyzed synthesis of propargylamines via a three-component coupling reaction. Org. Lett. 2006, 8, 1529–1532. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Jia, H.; Sun, Z. Mild and Catalyst-free Petasis/decarboxylative domino reaction: Chemoselective synthesis of N-benzyl propargylamines. J. Org. Chem. 2014, 79, 11812–11818. [Google Scholar] [CrossRef]
- Nimmagadda, S.K.; Zhang, Z.; Antilla, J.C. Asymmetric one-pot synthesis of 1,3-oxazolidines and 1,3-oxazinanes via hemiaminal intermediates. Org. Lett. 2014, 16, 4098–4101. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, L.; Wang, J.; Song, G. Direct synthesis of N-substituted 1,3-oxazolidines via a hetero-domino Petasis borono-Mannich reaction of 1,2-amino alcohols, formaldehyde, and organoboronic acids. Chem. Heterocycl. Compd. 2019, 55, 648–653. [Google Scholar] [CrossRef]
- Liu, D.; Li, B.; Chen, J.; Gridnev, I.D.; Yan, D.; Zhang, W. Ni-catalyzed asymmetric hydrogenation of N-aryl imino esters for the efficient synthesis of chiral α-aryl glycines. Nat. Commun. 2020, 11, 5935. [Google Scholar] [CrossRef] [PubMed]
- Nanda, K.K.; Trotter, B.W. Diastereoselective Petasis Mannich reactions accelerated by hexafluoroisopropanol: A pyrrolidine-derived arylglycine synthesis. Tetrahedron Lett. 2005, 46, 2025–2028. [Google Scholar] [CrossRef]
- Tabassum, S.; Zahoor, A.F.; Ahmad, S.; Noreen, R.; Khan, S.G.; Ahmad, H. Cross-coupling reactions towards the synthesis of natural products. Mol. Divers 2022, 26, 647–689. [Google Scholar] [CrossRef]
- Theoclitou, M.E.; Robinson, L.A. Novel facile synthesis of 2,2,4 substituted 1,2-dihydroquinolines via a modified Skraup reaction. Tetrahedron Lett. 2002, 43, 3907–3910. [Google Scholar] [CrossRef]
- Chang, Y.M.; Lee, S.H.; Nam, M.H.; Cho, M.Y.; Park, Y.S.; Yoon, C.M. Petasis reaction of activated quinoline and isoquinoline with various boronic acids. Tetrahedron Lett. 2005, 46, 3053–3056. [Google Scholar] [CrossRef]
- Wilkes, J.S. Properties of ionic liquid solvents for catalysis. J. Mol. Catal. A Chem. 2004, 214, 11–17. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.S.; Lakshmi, P.N. Ionic liquid accelerated Petasis reaction: A green protocol for the synthesis of alkylaminophenols. J. Mol. Catal. A Chem. 2007, 274, 101–104. [Google Scholar] [CrossRef]
- Shahzadi, I.; Zahoor, A.F.; Rasul, A.; Rasool, N.; Raza, Z.; Faisal, S.; Parveen, B.; Kamal, S.; Zia-ur-Rehman, M.; Zahid, F.M. Synthesis, anticancer, and computational studies of 1, 3, 4-oxadiazole-purine derivatives. J. Heterocycl. Chem. 2020, 57, 2782–2794. [Google Scholar] [CrossRef]
- Ameen, M.A.; Motamed, S.M.; Abdel-latif, F.F. Highly efficient one-pot synthesis of dihydropyran heterocycles. Chin. Chem. Lett. 2014, 25, 212–214. [Google Scholar] [CrossRef]
- Li, H.; Cui, C.X.; Zhang, G.H.; Li, X.Q.; Yang, J. Regioselective synthesis of functionalized dihydropyrones via the Petasis reaction. J. Org. Chem. 2019, 85, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Azizi, N.; Farhadi, E. Straightforward and rapid Petasis multicomponent reactions in deep eutectic solvent. Curr. Res. Green Sustain. Chem. 2020, 4, 100220. [Google Scholar] [CrossRef]
- Di, J.Q.; Wang, H.J.; Cui, Z.S.; Hu, J.Y.; Zhang, Z.H. Catalyst-free Synthesis of Aminomethylphenol Derivatives in Cyclopentyl Methyl Ether via Petasis Borono-Mannich Reaction. Curr. Org. Syn. 2021, 18, 294–300. [Google Scholar] [CrossRef]
- Singh, M.S.; Chowdhury, S. Recent developments in solvent-free multicomponent reactions: A perfect synergy for eco-compatible organic synthesis. RSC Adv. 2012, 2, 4547–4592. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Lasemi, Z.; Oloub, M.; Pooryousef, M. A green protocol for the one-pot multicomponent Petasis boronic Mannich reaction using ball milling. J. Iran. Chem. Soc. 2017, 14, 347–355. [Google Scholar] [CrossRef]
- Nun, P.; Martinez, J.; Lamaty, F. Microwave-assisted neat procedure for the Petasis reaction. Synthesis 2010, 12, 2063–2068. [Google Scholar] [CrossRef]
- Parhi, A.K.; Zhang, Y.; Saionz, K.W.; Pradhan, P.; Kaul, M.; Trivedi, K.; Daniel, S.P.; LaVoie, E.J. Antibacterial activity of quinoxalines, quinazolines, and 1,5-naphthyridines. Bioorg. Med. Chem. Lett. 2013, 23, 4968–4974. [Google Scholar] [CrossRef]
- Shekhar, A.C.; Rao, P.S.; Narsaiah, B.; Allanki, A.D.; Sijwali, P.S. Emergence of pyrido quinoxalines as new family of antimalarial agents. Eur. J. Med. Chem. 2014, 77, 280–287. [Google Scholar] [CrossRef]
- Xu, H.; Fan, L.L. Synthesis and antifungal activities of novel 5,6-dihydro-indolo [1,2-a] quinoxaline derivatives. Eur. J. Med. Chem. 2011, 46, 1919–1925. [Google Scholar] [CrossRef]
- Soleymani, M.; Chegeni, M. The Chemistry and Applications of the Quinoxaline Compounds. Curr. Org. Chem. 2019, 23, 1789–1827. [Google Scholar] [CrossRef]
- Ayaz, M.; Dietrich, J.; Hulme, C. A novel route to synthesize libraries of quinoxalines via Petasis methodology in two synthetic operations. Tetrahedron Lett. 2011, 52, 4821–4823. [Google Scholar] [CrossRef][Green Version]
- Eom, D.; Kang, D.; Lee, P.H. Synthesis of 2-alkyl-and aryl-3-ethoxycarbonyl-2,5-dihydrofurans through gold-catalyzed intramolecular hydroalkoxylation. J. Org. Chem. 2010, 75, 7447–7450. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.X.; Li, H.; Yang, X.J.; Yang, J.; Li, X.Q. One-pot synthesis of functionalized 2, 5-dihydrofurans via an amine-promoted Petasis borono-Mannich Reaction. Org. Lett. 2013, 15, 5944–5947. [Google Scholar] [CrossRef] [PubMed]
- Bauri, K.; Nandi, M.; De, P. Amino acid-derived stimuli-responsive polymers and their applications. Polym. Chem. 2018, 9, 1257–1287. [Google Scholar] [CrossRef]
- Takahashi, R.; Kakuchi, R. Rational optimization of the Petasis three-component reaction as a feasible elementary reaction in polymer chemistry. Macromol. Chem. Phys. 2021, 222, 2000347. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug. Deliver. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Reddy, S.R.S.; Reddy, B.R.P.; Reddy, P.V.G. Chitosan: Highly efficient, green, and reusable biopolymer catalyst for the synthesis of alkylaminophenols via Petasis borono–Mannich reaction. Tetrahedron Lett. 2015, 56, 4984–4989. [Google Scholar] [CrossRef]
- Vytla, D.; Emmadi, J.; Velayuthaperumal, R.; Shaw, P.; Cavallaro, C.L.; Mathur, A.; Roy, A. Visible-light enabled one-pot three-component Petasis reaction for synthesis of a-substituted secondary sulfonamides/amides/hydrazides. Tetrahedron Lett. 2022, 106, 154055. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, S.; Munawar, S.; Ahmad, S.; Mansha, A.; Zahoor, A.F.; Irfan, A.; Irfan, A.; Kotwica-Mojzych, K.; Soroka, M.; Głowacka, M.; et al. Recent Trends in the Petasis Reaction: A Review of Novel Catalytic Synthetic Approaches with Applications of the Petasis Reaction. Molecules 2023, 28, 8032. https://doi.org/10.3390/molecules28248032
Saeed S, Munawar S, Ahmad S, Mansha A, Zahoor AF, Irfan A, Irfan A, Kotwica-Mojzych K, Soroka M, Głowacka M, et al. Recent Trends in the Petasis Reaction: A Review of Novel Catalytic Synthetic Approaches with Applications of the Petasis Reaction. Molecules. 2023; 28(24):8032. https://doi.org/10.3390/molecules28248032
Chicago/Turabian StyleSaeed, Sadaf, Saba Munawar, Sajjad Ahmad, Asim Mansha, Ameer Fawad Zahoor, Ali Irfan, Ahmad Irfan, Katarzyna Kotwica-Mojzych, Malgorzata Soroka, Mariola Głowacka, and et al. 2023. "Recent Trends in the Petasis Reaction: A Review of Novel Catalytic Synthetic Approaches with Applications of the Petasis Reaction" Molecules 28, no. 24: 8032. https://doi.org/10.3390/molecules28248032
APA StyleSaeed, S., Munawar, S., Ahmad, S., Mansha, A., Zahoor, A. F., Irfan, A., Irfan, A., Kotwica-Mojzych, K., Soroka, M., Głowacka, M., & Mojzych, M. (2023). Recent Trends in the Petasis Reaction: A Review of Novel Catalytic Synthetic Approaches with Applications of the Petasis Reaction. Molecules, 28(24), 8032. https://doi.org/10.3390/molecules28248032








