Recent Progress in Advanced Polyester Elastomers for Tissue Engineering and Bioelectronics
Abstract
:1. Introduction
2. Synthetic Pathways and Functionalization of Polyester Elastomers
2.1. Synthetic Pathways of Representative Polyesters
2.1.1. Physically Crosslinked Polyester Elastomers
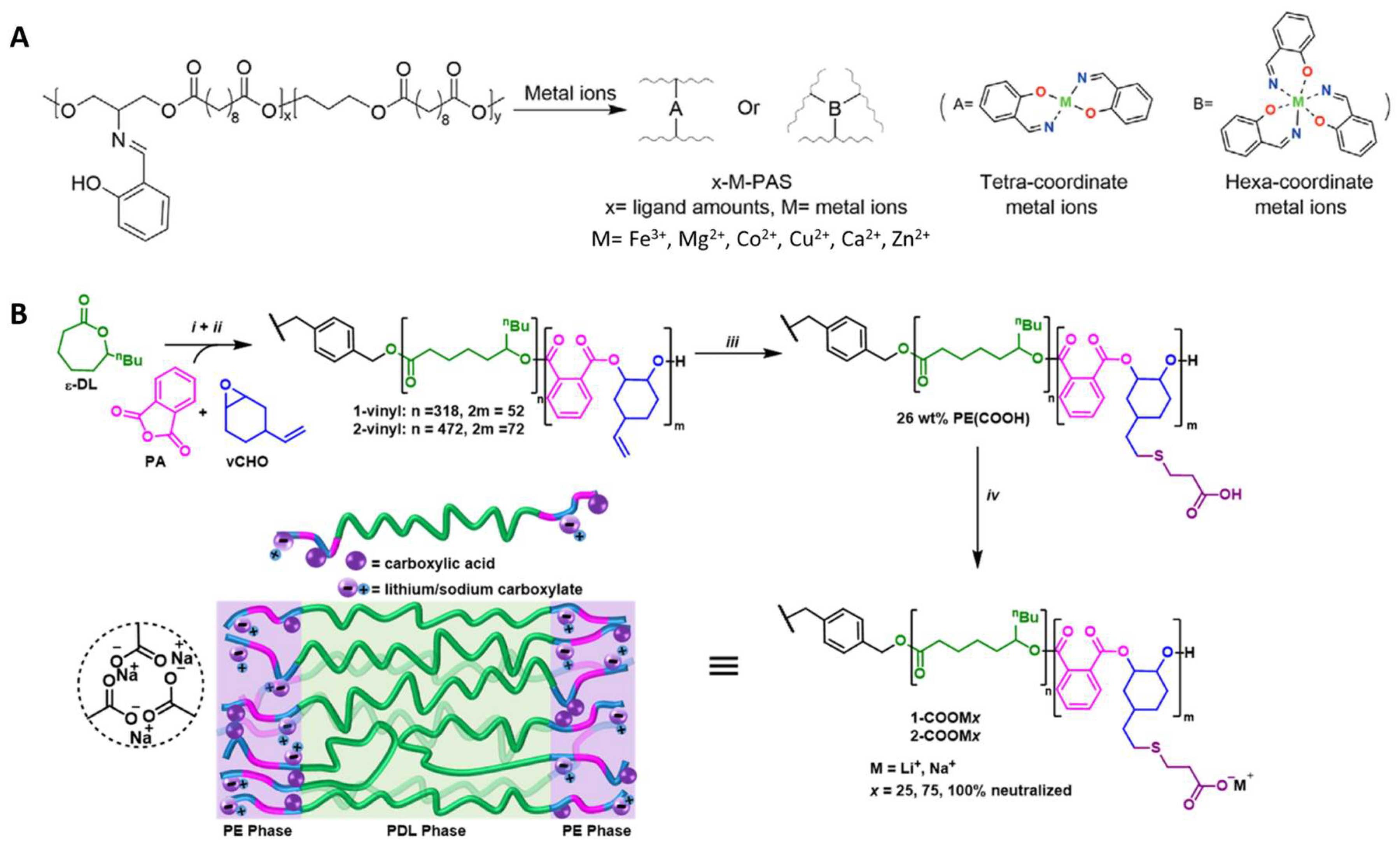
2.1.2. Chemically Crosslinked Polyester Elastomers
2.2. Route of Degradation
2.3. Functionalization of Polyesters
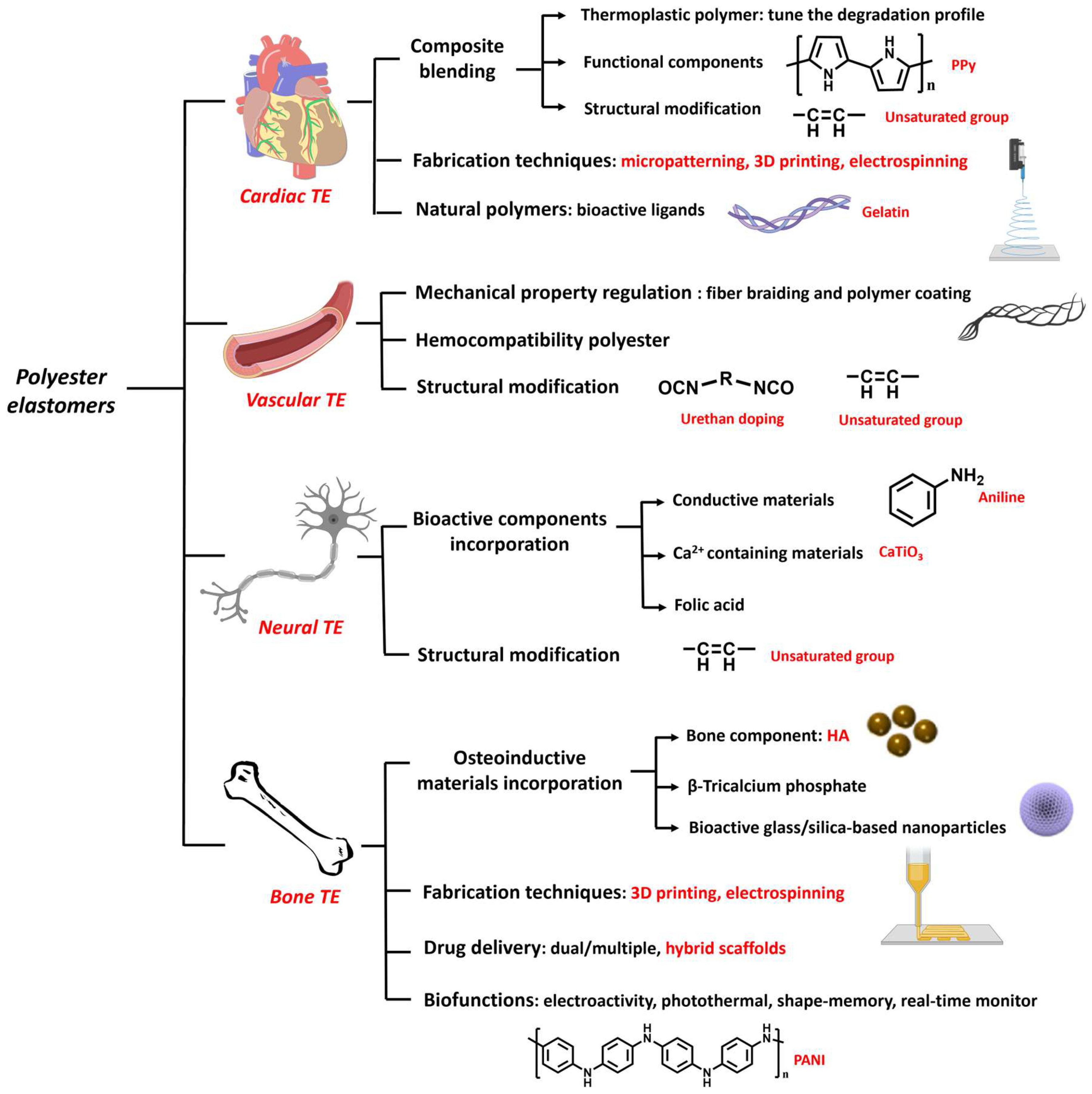
3. Biomedical Applications of Polyester Elastomers
3.1. Cardiac Tissue Engineering

3.2. Vascular Tissue Engineering
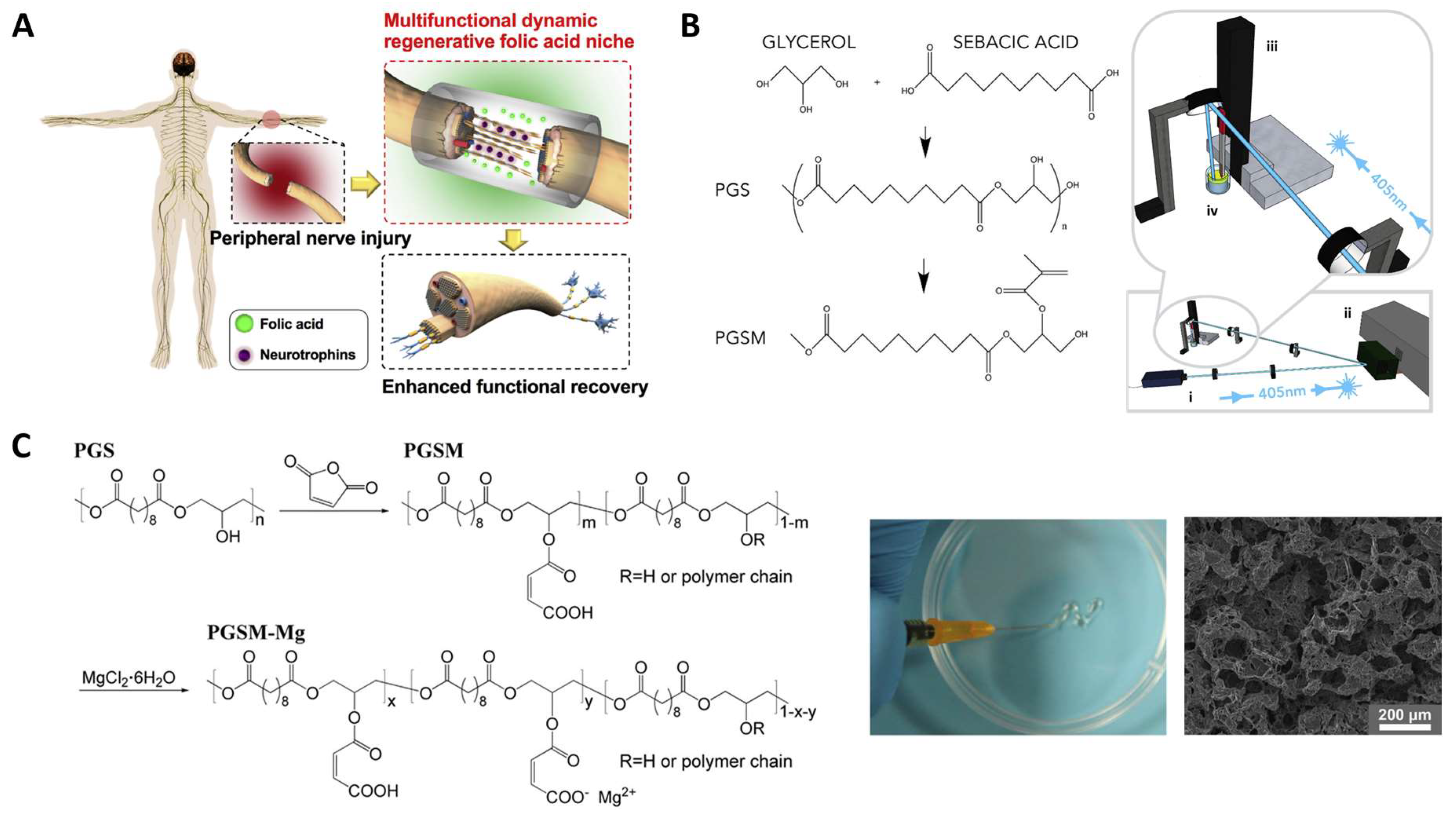
3.3. Neural Tissue Engineering
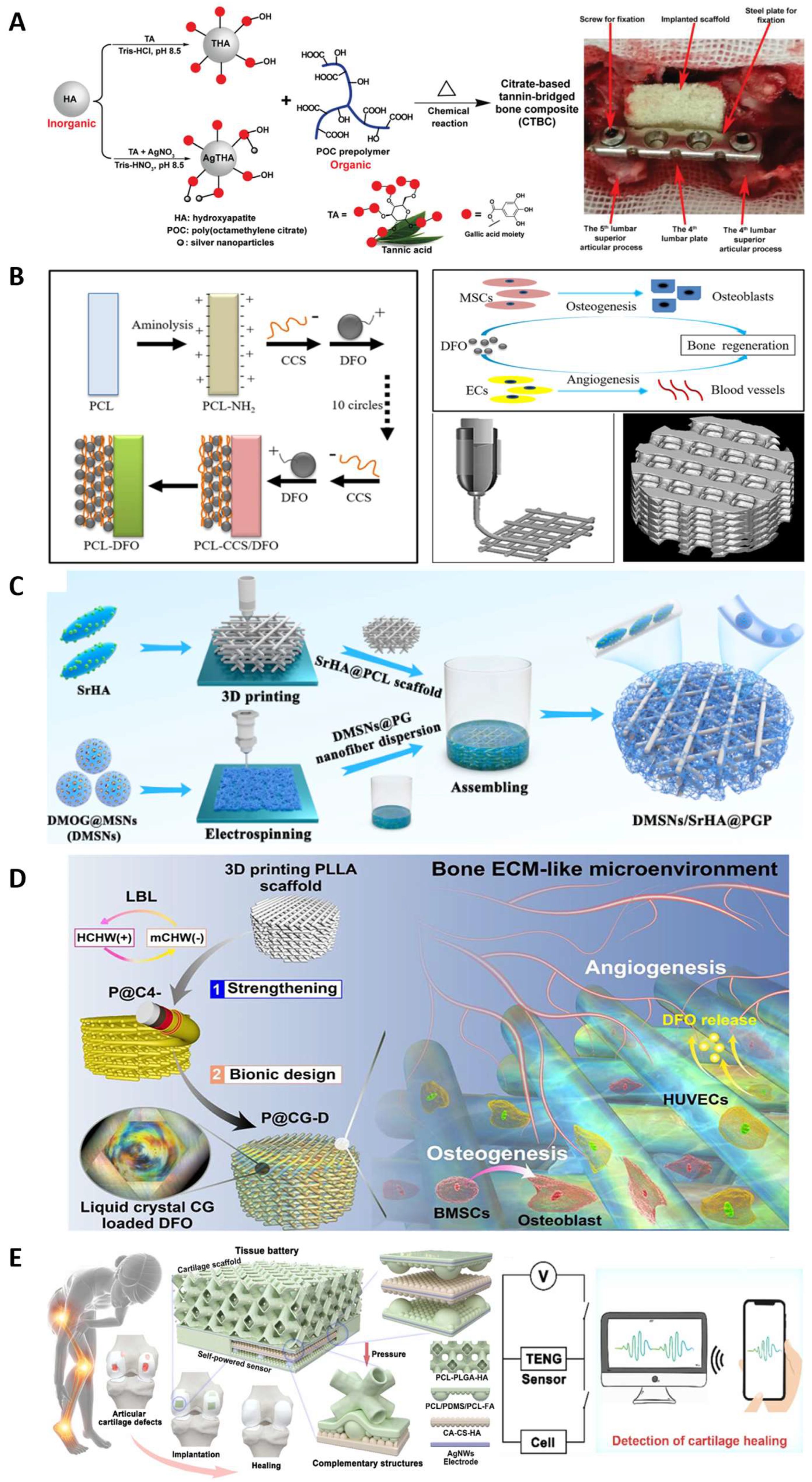
3.4. Bone Tissue Engineering
3.5. Bioelectronics
4. Conclusions and Perspectives
4.1. Conclusions
4.2. Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.; Liang, S.; Thouas, G.A. Elastomeric biomaterials for tissue engineering. Prog. Polym. Sci. 2013, 38, 584–671. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Serrano, M.C.; Chung, E.J.; Ameer, G.A. Advances and Applications of Biodegradable Elastomers in Regenerative Medicine. Adv. Funct. Mater. 2010, 20, 192–208. [Google Scholar] [CrossRef]
- Webb, A.R.; Yang, J.; Ameer, G.A. Biodegradable polyester elastomers in tissue engineering. Expert Opin. Biol. Ther. 2004, 4, 801–812. [Google Scholar] [CrossRef]
- Lai, J.-C.; Jia, X.-Y.; Wang, D.-P.; Deng, Y.-B.; Zheng, P.; Li, C.-H.; Zuo, J.-L.; Bao, Z. Thermodynamically stable whilst kinetically labile coordination bonds lead to strong and tough self-healing polymers. Nat. Commun. 2019, 10, 1164. [Google Scholar] [CrossRef] [PubMed]
- Qiu, A.; Li, P.; Yang, Z.; Yao, Y.; Lee, I.; Ma, J. A Path Beyond Metal and Silicon: Polymer/Nanomaterial Composites for Stretchable Strain Sensors. Adv. Funct. Mater. 2019, 29, 1806306. [Google Scholar] [CrossRef]
- Behl, M.; Razzaq, M.Y.; Lendlein, A. Multifunctional Shape-Memory Polymers. Adv. Mater. 2010, 22, 3388–3410. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Meng, Y.; Shen, C.; Pei, Q. Stiffness Variable Polymers Comprising Phase-Changing Side-Chains: Material Syntheses and Application Explorations. Adv. Mater. 2022, 34, 2109798. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, D.; Cheng, B. Preparation and research of intrinsic self-healing elastomers based on hydrogen and ionic bond. Compos. Sci. Technol. 2020, 193, 108127. [Google Scholar] [CrossRef]
- Ji, X.L.; Jing, J.K.; Jiang, W.; Jiang, B.Z. Tensile modulus of polymer nanocomposites. Polym. Eng. Sci. 2002, 42, 983–993. [Google Scholar] [CrossRef]
- Nguyen, H.K.; Nakajima, K. Evidence of the Transition from a Flexible to Rigid Percolating Network in Polymer Nanocomposites. Macromolecules 2022, 55, 2739–2745. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, K.; Kai, D.; Li, Z.; Loh, X.J. Polyester elastomers for soft tissue engineering. Chem. Soc. Rev. 2018, 47, 4545–4580. [Google Scholar] [CrossRef] [PubMed]
- Jikei, M.; Takeyama, Y.; Yamadoi, Y.; Shinbo, N.; Matsumoto, K.; Motokawa, M.; Ishibashi, K.; Yamamoto, F. Synthesis and properties of Poly(L-lactide)-Poly(ɛ-caprolactone) multiblock copolymers by the self-polycondensation of diblock macromonomers. Polym. J. 2015, 47, 657–665. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Z.; Ou-Yang, W.; Pan, X.; Wang, X.; Huang, P.; Zhang, C.; Kong, D.; Wang, W. 3D printing of implantable elastic PLCL copolymer scaffolds. Soft Matter 2020, 16, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Zada, M.H.; Kumar, A.; Elmalak, O.; Markovitz, E.; Icekson, R.; Domb, A.J. In vitro and in vivo degradation behavior and the long-term performance of biodegradable PLCL balloon implants. Int. J. Pharm. 2020, 574, 118870. [Google Scholar] [CrossRef]
- Lee, J.; Tae, G.; Kim, Y.H.; Park, I.S.; Kim, S.-H.; Kim, S.H. The effect of gelatin incorporation into electrospun poly(l-lactide-co-ɛ-caprolactone) fibers on mechanical properties and cytocompatibility. Biomaterials 2008, 29, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.; Carvalho, A.M.; Fernandes, E.M.; Gomes, M.E.; Reis, R.L.; Bayon, Y.; Zeugolis, D.I. Development and characterisation of cytocompatible polyester substrates with tunable mechanical properties and degradation rate. Acta Biomater. 2021, 121, 303–315. [Google Scholar] [CrossRef]
- Jee, M.H.; Park, J.H.; Choi, S.Y.; Baik, D.H. Effects of Introduction of L-Lactide on Microstructures, Thermal Properties and In vitro Degradation of Poly(glycolide-co-ε-caprolactone) Block Copolymer. Fibers Polym. 2022, 23, 2683–2691. [Google Scholar] [CrossRef]
- Li, Y.; Huang, W.; Cook, W.D.; Chen, Q. A comparative study on poly(xylitol sebacate) and poly(glycerol sebacate): Mechanical properties, biodegradation and cytocompatibility. Biomed. Mater. 2013, 8, 035006. [Google Scholar] [CrossRef]
- Wan, L.; Lu, L.; Zhu, T.; Liu, Z.; Du, R.; Luo, Q.; Xu, Q.; Zhang, Q.; Jia, X. Bulk Erosion Degradation Mechanism for Poly(1,8-octanediol-co-citrate) Elastomer: An In Vivo and In Vitro Investigation. Biomacromolecules 2022, 23, 4268–4281. [Google Scholar] [CrossRef]
- Yang, J.; Webb, A.R.; Ameer, G.A. Novel Citric Acid-Based Biodegradable Elastomers for Tissue Engineering. Adv. Mater. 2004, 16, 511–516. [Google Scholar] [CrossRef]
- McAdam, B.; Fournet, M.B.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef]
- Iron, R.; Mehdikhani, M.; Naghashzargar, E.; Karbasi, S.; Semnani, D. Effects of nano-bioactive glass on structural, mechanical and bioactivity properties of Poly (3-hydroxybutyrate) electrospun scaffold for bone tissue engineering applications. Mater. Technol. 2019, 34, 540–548. [Google Scholar] [CrossRef]
- Zhuikov, V.A.; Akoulina, E.A.; Chesnokova, D.V.; Wenhao, Y.; Makhina, T.K.; Demyanova, I.V.; Zhuikova, Y.V.; Voinova, V.V.; Belishev, N.V.; Surmenev, R.A.; et al. The Growth of 3T3 Fibroblasts on PHB, PLA and PHB/PLA Blend Films at Different Stages of Their Biodegradation In Vitro. Polymers 2021, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Boesel, L.F.; Meur, S.L.; Thöny-Meyer, L.; Ren, Q. The effect of molecular weight on the material properties of biosynthesized poly(4-hydroxybutyrate). Int. J. Biol. Macromol. 2014, 71, 124–130. [Google Scholar] [CrossRef]
- Ma, H.; Sun, Y.; Tang, Y.; Shen, Y.; Kan, Z.; Li, Q.; Fang, S.; Lu, Y.; Zhou, X.; Li, Z. Robust Electrospun Nanofibers from Chemosynthetic Poly(4-hydroxybutyrate) as Artificial Dural Substitute. Macromol. Biosci. 2021, 21, 2100134. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Chung, A.-L.; Wu, L.-P.; Zhang, X.; Wu, Q.; Chen, J.-C.; Chen, G.-Q. Biosynthesis and Characterization of Polyhydroxyalkanoate Block Copolymer P3HB-b-P4HB. Biomacromolecules 2011, 12, 3166–3173. [Google Scholar] [CrossRef] [PubMed]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Ahmadi, T.; Monshi, A.; Mortazavi, V.; Fathi, M.H.; Sharifi, S.; Kharaziha, M.; Khazdooz, L.; Zarei, A.; Taghian Dehaghani, M. Fabrication and characterization of polycaprolactone fumarate/gelatin-based nanocomposite incorporated with silicon and magnesium co-doped fluorapatite nanoparticles using electrospinning method. Mater. Sci. Eng. C 2020, 106, 110172. [Google Scholar] [CrossRef]
- Jabbari, E.; Wang, S.; Lu, L.; Gruetzmacher, J.A.; Ameenuddin, S.; Hefferan, T.E.; Currier, B.L.; Windebank, A.J.; Yaszemski, M.J. Synthesis, Material Properties, and Biocompatibility of a Novel Self-Cross-Linkable Poly(caprolactone fumarate) as an Injectable Tissue Engineering Scaffold. Biomacromolecules 2005, 6, 2503–2511. [Google Scholar] [CrossRef]
- Sharma, D.; Satapathy, B.K. Mechanical Properties of Aliphatic Polyester-Based Structurally Engineered Composite Patches. Macromol. Symp. 2019, 384, 1800153. [Google Scholar] [CrossRef]
- Gupta, P.K.; Gahtori, R.; Govarthanan, K.; Sharma, V.; Pappuru, S.; Pandit, S.; Mathuriya, A.S.; Dholpuria, S.; Bishi, D.K. Recent trends in biodegradable polyester nanomaterials for cancer therapy. Mater. Sci. Eng. C 2021, 127, 112198. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Zamboulis, A.; Koumentakou, I.; Michailidou, G.; Noordam, M.J.; Bikiaris, D.N. Biocompatible Synthetic Polymers for Tissue Engineering Purposes. Biomacromolecules 2022, 23, 1841–1863. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, B.; Hemavathi, A.B.; Panda, P.K. Poly (ε-caprolactone)-based electrospun nano-featured substrate for tissue engineering applications: A review. Prog. Biomater. 2021, 10, 91–117. [Google Scholar] [CrossRef] [PubMed]
- Toh, H.W.; Toong, D.W.Y.; Ng, J.C.K.; Ow, V.; Lu, S.; Tan, L.P.; Wong, P.E.H.; Venkatraman, S.; Huang, Y.; Ang, H.Y. Polymer blends and polymer composites for cardiovascular implants. Eur. Polym. J. 2021, 146, 110249. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly(glycolic acid) (PGA): A versatile building block expanding high performance and sustainable bioplastic applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- D’Alterio, M.C.; D’Auria, I.; Gaeta, L.; Tedesco, C.; Brenna, S.; Pellecchia, C. Are Well Performing Catalysts for the Ring Opening Polymerization of l-Lactide under Mild Laboratory Conditions Suitable for the Industrial Process? The Case of New Highly Active Zn(II) Catalysts. Macromolecules 2022, 55, 5115–5122. [Google Scholar] [CrossRef]
- Kunduru, K.R.; Hogerat, R.; Ghosal, K.; Shaheen-Mualim, M.; Farah, S. Renewable polyol-based biodegradable polyesters as greener plastics for industrial applications. Chem. Eng. J. 2023, 459, 141211. [Google Scholar] [CrossRef]
- Larrañaga, A.; Lizundia, E. A review on the thermomechanical properties and biodegradation behaviour of polyesters. Eur. Polym. J. 2019, 121, 109296. [Google Scholar] [CrossRef]
- Yang, J.; Webb, A.R.; Pickerill, S.J.; Hageman, G.; Ameer, G.A. Synthesis and evaluation of poly(diol citrate) biodegradable elastomers. Biomaterials 2006, 27, 1889–1898. [Google Scholar] [CrossRef]
- Bettinger, C.J. Biodegradable Elastomers for Tissue Engineering and Cell–Biomaterial Interactions. Macromol. Biosci. 2011, 11, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Bat, E.; Zhang, Z.; Feijen, J.; Grijpma, D.W.; Poot, A.A. Biodegradable elastomers for biomedical applications and regenerative medicine. Regen. Med. 2014, 9, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Feijoo, J.L.; Yang, M.-C. Comparison of abiotic and biotic degradation of PDLLA, PCL and partially miscible PDLLA/PCL blend. Eur. Polym. J. 2013, 49, 706–717. [Google Scholar] [CrossRef]
- Singhal, P.; Small, W.; Cosgriff-Hernandez, E.; Maitland, D.J.; Wilson, T.S. Low density biodegradable shape memory polyurethane foams for embolic biomedical applications. Acta Biomater. 2014, 10, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Thouas, G.A.; Chen, Q.-Z. Biodegradable soft elastomers: Synthesis/properties of materials and fabrication of scaffolds. RSC Adv. 2012, 2, 8229–8242. [Google Scholar] [CrossRef]
- Ding, X.; Wang, Y. Weak bond-based injectable and stimuli responsive hydrogels for biomedical applications. J. Mater. Chem. B 2017, 5, 887–906. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Miller, P.G.; Ding, X.; Stowell, C.E.T.; Kelly, K.M.; Wang, Y. Chelation Crosslinking of Biodegradable Elastomers. Adv. Mater. 2020, 32, 2003761. [Google Scholar] [CrossRef]
- Wang, M.; Xu, P.; Lei, B. Engineering multifunctional bioactive citrate-based biomaterials for tissue engineering. Bioact. Mater. 2023, 19, 511–537. [Google Scholar] [CrossRef]
- Vogt, L.; Ruther, F.; Salehi, S.; Boccaccini, A.R. Poly(Glycerol Sebacate) in Biomedical Applications—A Review of the Recent Literature. Adv. Healthc. Mater. 2021, 10, 2002026. [Google Scholar] [CrossRef]
- Huyer, L.D.; Bannerman, A.D.; Wang, Y.; Savoji, H.; Knee-Walden, E.J.; Brissenden, A.; Yee, B.; Shoaib, M.; Bobicki, E.; Amsden, B.G.; et al. Tunable Bioelastomers: One-Pot Synthesis of Unsaturated Polyester Bioelastomer with Controllable Material Curing for Microscale Designs (Adv. Healthcare Mater. 16/2019). Adv. Healthc. Mater. 2019, 8, 1970064. [Google Scholar] [CrossRef]
- Zou, W.; Dong, J.; Luo, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: From Old Chemistry to Modern Day Innovations. Adv. Mater. 2017, 29, 1606100. [Google Scholar] [CrossRef]
- Choi, Y.S.; Hsueh, Y.-Y.; Koo, J.; Yang, Q.; Avila, R.; Hu, B.; Xie, Z.; Lee, G.; Ning, Z.; Liu, C.; et al. Stretchable, dynamic covalent polymers for soft, long-lived bioresorbable electronic stimulators designed to facilitate neuromuscular regeneration. Nat. Commun. 2020, 11, 5990. [Google Scholar] [CrossRef]
- Chen, S.; Sun, L.; Zhou, X.; Guo, Y.; Song, J.; Qian, S.; Liu, Z.; Guan, Q.; Jeffries, E.M.; Liu, W.; et al. Mechanically and biologically skin-like elastomers for bio-integrated electronics. Nat. Commun. 2020, 11, 1107. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, L.; Yang, Q.; Huang, S.; Shi, H.; Long, Q.; Qian, B.; Liu, Z.; Guan, Q.; Liu, M.; et al. Self-healing polyurethane-elastomer with mechanical tunability for multiple biomedical applications in vivo. Nat. Commun. 2021, 12, 4395. [Google Scholar] [CrossRef] [PubMed]
- Filippidi, E.; Cristiani, T.R.; Eisenbach, C.D.; Waite, J.H.; Israelachvili, J.N.; Ahn, B.K.; Valentine, M.T. Toughening elastomers using mussel-inspired iron-catechol complexes. Science 2017, 358, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Khare, E.; Holten-Andersen, N.; Buehler, M.J. Transition-metal coordinate bonds for bioinspired macromolecules with tunable mechanical properties. Nat. Rev. Mater. 2021, 6, 421–436. [Google Scholar] [CrossRef]
- Zheng, N.; Xu, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: A Molecular Platform for Designing Functions beyond Chemical Recycling and Self-Healing. Chem. Rev. 2021, 121, 1716–1745. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liang, J.; Wang, Z.; Qin, J.; Zhang, Q.; Zhu, S.; Zhang, K.; Zhu, H. Tough, Recyclable, and Degradable Elastomers for Potential Biomedical Applications. Adv. Mater. 2023, 35, 2210092. [Google Scholar] [CrossRef] [PubMed]
- Gregory, G.L.; Williams, C.K. Exploiting Sodium Coordination in Alternating Monomer Sequences to Toughen Degradable Block Polyester Thermoplastic Elastomers. Macromolecules 2022, 55, 2290–2299. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, Y.; Nan, Y.; Okuro, K.; Aida, T. Mechanically robust, readily repairable polymers via tailored noncovalent cross-linking. Science 2018, 359, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Ihsan, A.B.; Akasaki, T.; Sato, K.; Haque, M.A.; Nakajima, T.; Gong, J.P. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef]
- Kushner, A.M.; Vossler, J.D.; Williams, G.A.; Guan, Z. A Biomimetic Modular Polymer with Tough and Adaptive Properties. J. Am. Chem. Soc. 2009, 131, 8766–8768. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, B.; Zhang, L.; Hu, G.-H. Progress in bio-inspired sacrificial bonds in artificial polymeric materials. Chem. Soc. Rev. 2017, 46, 6301–6329. [Google Scholar] [CrossRef]
- Rufaihah, A.J.; Yasa, I.C.; Ramanujam, V.S.; Arularasu, S.C.; Kofidis, T.; Guler, M.O.; Tekinay, A.B. Angiogenic peptide nanofibers repair cardiac tissue defect after myocardial infarction. Acta Biomater. 2017, 58, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Bouten, C.V.C.; Dankers, P.Y.W.; Driessen-Mol, A.; Pedron, S.; Brizard, A.M.A.; Baaijens, F.P.T. Substrates for cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Schickel, M.E.; Stevenson, M.D.; Sarang-Sieminski, A.L.; Gooch, K.J.; Ghadiali, S.N.; Hart, R.T. Fibers in the Extracellular Matrix Enable Long-Range Stress Transmission between Cells. Biophys. J. 2013, 104, 1410–1418. [Google Scholar] [CrossRef]
- Roshanbinfar, K.; Vogt, L.; Ruther, F.; Roether, J.A.; Boccaccini, A.R.; Engel, F.B. Nanofibrous Composite with Tailorable Electrical and Mechanical Properties for Cardiac Tissue Engineering. Adv. Funct. Mater. 2020, 30, 1908612. [Google Scholar] [CrossRef]
- Xu, J.-Y.; Xiong, Y.-Y.; Lu, X.-T.; Yang, Y.-J. Regulation of Type 2 Immunity in Myocardial Infarction. Front. Immunol. 2019, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.; Kraus, X.; Tallawi, M.; Scharfe, B.; El Fray, M.; Aifantis, K.E.; Boccaccini, A.R.; Göken, M. Dynamic mechanical characterization of poly(glycerol sebacate)/poly(butylene succinate-butylene dilinoleate) blends for cardiac tissue engineering by flat punch nanoindentation. Mater. Lett. 2018, 221, 115–118. [Google Scholar] [CrossRef]
- Bannerman, A.D.; Huyer, L.D.; Montgomery, M.; Zhao, N.; Velikonja, C.; Bender, T.P.; Radisic, M. Biomaterial Adhesive Patches: Elastic Biomaterial Scaffold with Spatially Varying Adhesive Design (Adv. Biosys. 8/2020). Adv. Biosyst. 2020, 4, 2070081. [Google Scholar] [CrossRef]
- Liang, H.; Mirinejad, M.S.; Asefnejad, A.; Baharifar, H.; Li, X.; Saber-Samandari, S.; Toghraie, D.; Khandan, A. Fabrication of tragacanthin gum-carboxymethyl chitosan bio-nanocomposite wound dressing with silver-titanium nanoparticles using freeze-drying method. Mater. Chem. Phys. 2022, 279, 125770. [Google Scholar] [CrossRef]
- Sander, M.M.; Ferreira, C.A. Synthesis and characterization of a conductive and self-healing composite. Synth. Met. 2018, 243, 58–66. [Google Scholar] [CrossRef]
- Radisic, M.; Huyer, L.D.; Montgomery, M. Highly Elastic and Moldable Polyester Biomaterial for Cardiac Tissue Engineering Applications. Patent US20170298175, 19 October 2017. [Google Scholar]
- Langer, R.S.; Karp, J.M.; Nunes-Pereira, M.J.M.; Ouyang, B.; Ferreira, L.D.S.; Sarkar, D. Urethane-Crosslinked Biodegradable Elastomers, Compositions and Manufacture of an Elastomeric Crosslinked Polyester Material. Patent US20210230343, 29 July 2021. [Google Scholar]
- Hu, T.; Wu, Y.; Zhao, X.; Wang, L.; Bi, L.; Ma, P.X.; Guo, B. Micropatterned, electroactive, and biodegradable poly(glycerol sebacate)-aniline trimer elastomer for cardiac tissue engineering. Chem. Eng. J. 2019, 366, 208–222. [Google Scholar] [CrossRef]
- Ruther, F.; Zimmermann, A.; Engel, F.B.; Boccaccini, A.R. Improvement of the Layer Adhesion of Composite Cardiac Patches. Adv. Eng. Mater. 2020, 22, 1900986. [Google Scholar] [CrossRef]
- Ezazi, N.Z.; Ajdary, R.; Correia, A.; Mäkilä, E.; Salonen, J.; Kemell, M.; Hirvonen, J.; Rojas, O.J.; Ruskoaho, H.J.; Santos, H.A. Fabrication and Characterization of Drug-Loaded Conductive Poly(glycerol sebacate)/Nanoparticle-Based Composite Patch for Myocardial Infarction Applications. ACS Appl. Mater. Interfaces 2020, 12, 6899–6909. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lei, D.; Huang, S.; Yang, Q.; Song, B.; Guo, Y.; Shen, A.; Yuan, Z.; Li, S.; Qing, F.-L.; et al. Elastic 3D-Printed Hybrid Polymeric Scaffold Improves Cardiac Remodeling after Myocardial Infarction. Adv. Healthc. Mater. 2019, 8, 1900065. [Google Scholar] [CrossRef] [PubMed]
- Abu Ammar, A.; Abu Much, A. Flexible Biodegradable Microneedles Containing Polyester. Patent WO2023194999, 12 October 2023. [Google Scholar]
- Heath, D.E.; Cooper, S.L. The development of polymeric biomaterials inspired by the extracellular matrix. J. Biomater. Sci. Polym. Ed. 2017, 28, 1051–1069. [Google Scholar] [CrossRef] [PubMed]
- García, A.J. Get a grip: Integrins in cell–biomaterial interactions. Biomaterials 2005, 26, 7525–7529. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-Y.; Chen, Y.-C.; Lin, T.-J.; Hsu, Y.-C.; Lin, C.-Y.; Yuan, R.-H.; Yu, J.; Teng, M.-S.; Hirtz, M.; Chen, M.H.-C.; et al. Vapor-Based Multicomponent Coatings for Antifouling and Biofunctional Synergic Modifications. Adv. Funct. Mater. 2014, 24, 2281–2287. [Google Scholar] [CrossRef]
- Karimi, F.; O’Connor, A.J.; Qiao, G.G.; Heath, D.E. Multivalent Ligands: Integrin Clustering Matters: A Review of Biomaterials Functionalized with Multivalent Integrin-Binding Ligands to Improve Cell Adhesion, Migration, Differentiation, Angiogenesis, and Biomedical Device Integration (Adv. Healthcare Mater. 12/2018). Adv. Healthc. Mater. 2018, 7, 1870048. [Google Scholar]
- Hamada, T.; Dubois, J.L.N.; Bellamy, V.; Pidial, L.; Hagège, A.; Pereira, M.N.; Menasché, P. In vitro controlled release of extracellular vesicles for cardiac repair from poly(glycerol sebacate) acrylate-based polymers. Acta Biomater. 2020, 115, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Nemati, S.; Kim, S.-j.; Shin, Y.M.; Shin, H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Tan, R.P.; Yang, N.; Rnjak-Kovacina, J.; Wise, S.G. Bioengineering artificial blood vessels from natural materials. Trends Biotechnol. 2022, 40, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Tang, F.; Jiang, S.; Khamis, H.; Bongers, A.; Whitelock, J.M.; Lord, M.S.; Rnjak-Kovacina, J. A Biomimetic Approach toward Enhancing Angiogenesis: Recombinantly Expressed Domain V of Human Perlecan Is a Bioactive Molecule That Promotes Angiogenesis and Vascularization of Implanted Biomaterials. Adv. Sci. 2020, 7, 2000900. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Zhang, C.; Cheng, M.; Huang, J.; Liu, Q.; Yuan, G.; Lin, K.; Yu, H. Challenges and strategies for in situ endothelialization and long-term lumen patency of vascular grafts. Bioact. Mater. 2021, 6, 1791–1809. [Google Scholar] [CrossRef] [PubMed]
- Henglin, M.; Stein, G.; Hushcha, P.V.; Snoek, J.; Wiltschko, A.B.; Cheng, S. Machine Learning Approaches in Cardiovascular Imaging. Circ. Cardiovasc. Imaging 2017, 10, e005614. [Google Scholar] [CrossRef]
- Zhang, K.; Mikos, A.G.; Reis, R.L.; Zhang, X. Translation of biomaterials from bench to clinic. Bioact. Mater. 2022, 18, 337–338. [Google Scholar] [CrossRef]
- Sharma, U.; Concagh, D.; Core, L.; Kuang, Y.; You, C.; Pham, Q.; Zugates, G.; Busold, R.; Webber, S.; Merlo, J.; et al. The development of bioresorbable composite polymeric implants with high mechanical strength. Nat. Mater. 2018, 17, 96–103. [Google Scholar] [CrossRef]
- Ubachs, R.L.J.M. Medical Stent with Post Intervention Increasing Diameter. Patent WO2023110554, 22 June 2023. [Google Scholar]
- Fu, J.; Ding, X.; Stowell, C.E.T.; Wu, Y.-L.; Wang, Y. Slow degrading poly(glycerol sebacate) derivatives improve vascular graft remodeling in a rat carotid artery interposition model. Biomaterials 2020, 257, 120251. [Google Scholar] [CrossRef]
- Wu, W.; Allen, R.A.; Wang, Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat. Med. 2012, 18, 1148–1153. [Google Scholar] [CrossRef]
- Savoji, H.; Huyer, L.D.; Mohammadi, M.H.; Lai, B.F.L.; Rafatian, N.; Bannerman, D.; Shoaib, M.; Bobicki, E.R.; Ramachandran, A.; Radisic, M. 3D Printing of Vascular Tubes Using Bioelastomer Prepolymers by Freeform Reversible Embedding. ACS Biomater. Sci. Eng. 2020, 6, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, D.; Allen, J.; Hoshi, R.; Yang, J.; Lui, K.; Ameer, G. Hemocompatibility evaluation of poly(diol citrate) in vitro for vascular tissue engineering. J. Biomed. Mater. Res. Part A 2007, 82 Pt A, 907–916. [Google Scholar] [CrossRef]
- Gregory, E.K.; Webb, A.; Vercammen, J.M.; Kelly, M.E.; Akar, B.; van Lith, R.; Bahnson, E.M.; Jiang, W.; Ameer, G.A.; Kibbe, M.R. Inhibiting intimal hyperplasia in prosthetic vascular grafts via immobilized all-trans retinoic acid. J. Control. Release 2018, 274, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Motlagh, D.; Allen, J.B.; Webb, A.R.; Kibbe, M.R.; Aalami, O.; Kapadia, M.; Carroll, T.J.; Ameer, G.A. Modulating Expanded Polytetrafluoroethylene Vascular Graft Host Response via Citric Acid-Based Biodegradable Elastomers. Adv. Mater. 2006, 18, 1493–1498. [Google Scholar] [CrossRef]
- van Lith, R.; Gregory, E.K.; Yang, J.; Kibbe, M.R.; Ameer, G.A. Engineering biodegradable polyester elastomers with antioxidant properties to attenuate oxidative stress in tissues. Biomaterials 2014, 35, 8113–8122. [Google Scholar] [CrossRef] [PubMed]
- Dey, J.; Xu, H.; Nguyen, K.T.; Yang, J. Crosslinked urethane doped polyester biphasic scaffolds: Potential for in vivo vascular tissue engineering. J. Biomed. Mater. Res. Part A 2010, 95 Pt A, 361–370. [Google Scholar] [CrossRef]
- Gyawali, D.; Tran, R.T.; Guleserian, K.J.; Tang, L.; Yang, J. Citric-Acid-Derived Photo-Cross-Linked Biodegradable Elastomers. J. Biomater. Sci. Polym. Ed. 2010, 21, 1761–1782. [Google Scholar] [CrossRef]
- Montgomery, M.; Huyer, L.D.; Bannerman, D.; Mohammadi, M.H.; Conant, G.; Radisic, M. Method for the Fabrication of Elastomeric Polyester Scaffolds for Tissue Engineering and Minimally Invasive Delivery. ACS Biomater. Sci. Eng. 2018, 4, 3691–3703. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, X.; Li, Z.; Zhang, L.; Sun, W.; Li, L.; Tang, F. A new process for customized patient-specific aortic stent graft using 3D printing technique. Med. Eng. Phys. 2020, 77, 80–87. [Google Scholar] [CrossRef]
- Soares, S.; von Boxberg, Y.; Nothias, F. Repair strategies for traumatic spinal cord injury, with special emphasis on novel biomaterial-based approaches. Rev. Neurol. 2020, 176, 252–260. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, L.; Guo, B.; Shao, Y.; Ma, P.X. Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering. Biomaterials 2016, 87, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Kharazi, A.Z.; Dini, G.; Naser, R. Fabrication and evaluation of a nerve guidance conduit capable of Ca2+ ion release to accelerate axon extension in peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2018, 106, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Kehoe, S.; Adhi, S.K.; Ajithkumar, T.G.; Moane, S.; O’Shea, H.; Boyd, D. Composition–structure–property (Zn2+ and Ca2+ ion release) evaluation of Si–Na–Ca–Zn–Ce glasses: Potential components for nerve guidance conduits. Mater. Sci. Eng. C 2011, 31, 669–676. [Google Scholar] [CrossRef]
- Li, G.; Xiao, Q.; McNaughton, R.; Han, L.; Zhang, L.; Wang, Y.; Yang, Y. Nanoengineered porous chitosan/CaTiO3 hybrid scaffolds for accelerating Schwann cells growth in peripheral nerve regeneration. Colloids Surf. B Biointerfaces 2017, 158, 57–67. [Google Scholar] [CrossRef]
- Ghafaralahi, S.; Ebrahimian-Hosseinabadi, M.; Zargar Kharazi, A. Poly(glycerol-sebacate)/poly(caprolactone)/graphene nanocomposites for nerve tissue engineering. J. Bioact. Compat. Polym. 2018, 33, 529–542. [Google Scholar] [CrossRef]
- Kim, G.B.; Chen, Y.; Kang, W.; Guo, J.; Payne, R.; Li, H.; Wei, Q.; Baker, J.; Dong, C.; Zhang, S.; et al. The critical chemical and mechanical regulation of folic acid on neural engineering. Biomaterials 2018, 178, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Harding, A.J.; Albadawi, E.; Boissonade, F.M.; Haycock, J.W.; Claeyssens, F. Additive manufactured biodegradable poly(glycerol sebacate methacrylate) nerve guidance conduits. Acta Biomater. 2018, 78, 48–63. [Google Scholar] [CrossRef]
- Sun, L.; Wang, M.; Chen, S.; Sun, B.; Guo, Y.; He, C.; Mo, X.; Zhu, B.; You, Z. Molecularly engineered metal-based bioactive soft materials–Neuroactive magnesium ion/polymer hybrids. Acta Biomater. 2019, 85, 310–319. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [PubMed]
- Habibovic, P.; de Groot, K. Osteoinductive biomaterials—Properties and relevance in bone repair. J. Tissue Eng. Regen. Med. 2007, 1, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Habibovic, P.; Bao, C.; Hu, J.; van Blitterswijk, C.A.; Yuan, H.; Chen, W.; Xu, H.H.K. The homing of bone marrow MSCs to non-osseous sites for ectopic bone formation induced by osteoinductive calcium phosphate. Biomaterials 2013, 34, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium Phosphate-Based Osteoinductive Materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—Past, Present, and Future in Bone Regeneration. Bone Tissue Regen. Insights 2016, 7, BTRI.S36138. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, L.; Wang, Y.; Chen, X.; Zhang, P. Improved Cell Adhesion and Osteogenesis of op-HA/PLGA Composite by Poly(dopamine)-Assisted Immobilization of Collagen Mimetic Peptide and Osteogenic Growth Peptide. ACS Appl. Mater. Interfaces 2016, 8, 26559–26569. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Xie, D.; Guo, J.; Mehdizadeh, M.R.; Tran, R.T.; Chen, R.; Sun, D.; Qian, G.; Jin, D.; Bai, X.; Yang, J. Development of injectable citrate-based bioadhesive bone implants. J. Mater. Chem. B 2015, 3, 387–398. [Google Scholar] [CrossRef]
- Salihu, R.; Razak, S.I.A.; Zawawi, N.A.; Kadir, M.R.A.; Ismail, N.I.; Jusoh, N.; Mohamad, M.R.; Hasraf Mat Nayan, N. Citric acid: A green cross-linker of biomaterials for biomedical applications. Eur. Polym. J. 2021, 146, 110271. [Google Scholar] [CrossRef]
- Guo, Y.; Tran, R.T.; Xie, D.; Wang, Y.; Nguyen, D.Y.; Gerhard, E.; Guo, J.; Tang, J.; Zhang, Z.; Bai, X.; et al. Citrate-based biphasic scaffolds for the repair of large segmental bone defects. J. Biomed. Mater. Res. Part A 2015, 103, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Y.; Niu, W.; Chen, M.; Xue, Y.; Ge, J.; Ma, P.X.; Lei, B. Biodegradable Multifunctional Bioactive Glass-Based Nanocomposite Elastomers with Controlled Biomineralization Activity, Real-Time Bioimaging Tracking, and Decreased Inflammatory Response. ACS Appl. Mater. Interfaces 2018, 10, 17722–17731. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.H.; Hosseinzadeh, B.; Kazemi, H.; Kiani, M.A.; Hajati, S. Facile Synthesis of Mixed Metal–Organic Frameworks: Electrode Materials for Supercapacitors with Excellent Areal Capacitance and Operational Stability. ACS Appl. Mater. Interfaces 2018, 10, 23063–23073. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tian, X.; Xie, D.; Rahn, K.; Gerhard, E.; Kuzma, M.L.; Zhou, D.; Dong, C.; Bai, X.; Lu, Z.; et al. Citrate-Based Tannin-Bridged Bone Composites for Lumbar Fusion. Adv. Funct. Mater. 2020, 30, 2002438. [Google Scholar] [CrossRef]
- Tan, X.; Gerhard, E.; Wang, Y.; Tran, R.T.; Xu, H.; Yan, S.; Rizk, E.B.; Armstrong, A.D.; Zhou, Y.; Du, J.; et al. Development of Biodegradable Osteopromotive Citrate-Based Bone Putty. Small 2022, 18, 2203003. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 2019, 190–191, 97–110. [Google Scholar] [CrossRef]
- Ha, Y.; Ma, X.; Li, S.; Li, T.; Li, Z.; Qian, Y.; Shafiq, M.; Wang, J.; Zhou, X.; He, C. Bone Microenvironment-Mimetic Scaffolds with Hierarchical Microstructure for Enhanced Vascularization and Bone Regeneration. Adv. Funct. Mater. 2022, 32, 2200011. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Zhang, C.; Wu, Z.; Liu, J. Recent Developments of Biomaterials for Additive Manufacturing of Bone Scaffolds. Adv. Healthc. Mater. 2020, 9, 2000724. [Google Scholar] [CrossRef]
- Vanaei, S.; Parizi, M.S.; Vanaei, S.; Salemizadehparizi, F.; Vanaei, H.R. An Overview on Materials and Techniques in 3D Bioprinting Toward Biomedical Application. Eng. Regen. 2021, 2, 1–18. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, H.; Wang, Z. Preparation of Polyester Resin for Bone Tissue Engineering Scaffold. Patent CN116262815, 15 December 2021. [Google Scholar]
- Wang, S.; Li, W.; Wang, Y. Preparation of Photocurable Resin for Three-Dimensional Printing and Bone Tissue Engineering. Patent CN113402870, 17 September 2021. [Google Scholar]
- Zhou, X.; Qian, Y.; Chen, L.; Li, T.; Sun, X.; Ma, X.; Wang, J.; He, C. Flowerbed-Inspired Biomimetic Scaffold with Rapid Internal Tissue Infiltration and Vascularization Capacity for Bone Repair. ACS Nano 2023, 17, 5140–5156. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, L.; Chen, J.; Li, Y.; Wen, W.; Lu, L.; Li, L.; Li, H.; Liu, M.; Zhou, C.; et al. Bone ECM-like 3D Printing Scaffold with Liquid Crystalline and Viscoelastic Microenvironment for Bone Regeneration. ACS Nano 2022, 16, 21020–21035. [Google Scholar] [CrossRef]
- Yue, O.; Wang, X.; Hou, M.; Zheng, M.; Hao, D.; Bai, Z.; Zou, X.; Cui, B.; Liu, C.; Liu, X. Smart nanoengineered electronic-scaffolds based on triboelectric nanogenerators as tissue batteries for integrated cartilage therapy. Nano Energy 2023, 107, 108158. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, M.; Gu, P.; Tong, L.; Wang, P.; Zhu, J.; Xu, Y.; Lu, G.; Luo, E.; Liang, J.; et al. Covalently Grafted Biomimetic Matrix Reconstructs the Regenerative Microenvironment of the Porous Gradient Polycaprolactone Scaffold to Accelerate Bone Remodeling. Small 2023, 19, 2206960. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Peng, Z.; Song, P.; Chen, L.; Xu, X.; Li, H.; Tang, P.; Wang, Y.; Su, Z.; Kong, Q.; et al. 3D printing of personalized polylactic acid scaffold laden with GelMA/autologous auricle cartilage to promote ear reconstruction. Bio-Des. Manuf. 2023, 6, 451–463. [Google Scholar] [CrossRef]
- Wang, S.; Luo, B.; Bai, B.; Wang, Q.; Chen, H.; Tan, X.; Tang, Z.; Shen, S.; Zhou, H.; You, Z.; et al. 3D Printed Chondrogenic Functionalized PGS Bioactive Scaffold for Cartilage Regeneration. Adv. Healthc. Mater. 2023, 12, 2301006. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wei, H.; Zhang, X.; Li, Y.; Huang, L.; Wa, Q.; Luo, Y. 3D printed hydrogel/wesselsite-PCL composite scaffold with structural change from core/shell fibers to microchannels for enhanced bone regeneration. Compos. Part B Eng. 2022, 246, 110264. [Google Scholar] [CrossRef]
- Yan, H.; Wang, C.; Zhang, Q.; Yu, P.; Xiao, Y.; Wang, C.; Zhang, P.; Hou, G. Conductive Polyaniline Particles Regulating In Vitro Hydrolytic Degradation and Erosion of Hydroxyapatite/Poly(lactide-co-glycolide) Porous Scaffolds for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2023, 9, 1541–1557. [Google Scholar] [CrossRef]
- Xuan, H.; Hu, H.; Geng, C.; Song, J.; Shen, Y.; Lei, D.; Guan, Q.; Zhao, S.; You, Z. Biofunctionalized chondrogenic shape-memory ternary scaffolds for efficient cell-free cartilage regeneration. Acta Biomater. 2020, 105, 97–110. [Google Scholar] [CrossRef]
- Huang, Z.; Li, J.; Chen, X.; Yang, Q.; Zeng, X.; Bai, R.; Wang, L. Photothermal Sensitive 3D Printed Biodegradable Polyester Scaffolds with Polydopamine Coating for Bone Tissue Engineering. Polymers 2023, 15, 381. [Google Scholar] [CrossRef]
- Wang, H.; Tian, J.; Jiang, Y.; Liu, S.; Zheng, J.; Li, N.; Wang, G.; Dong, F.; Chen, J.; Xie, Y.; et al. A 3D biomimetic optoelectronic scaffold repairs cranial defects. Sci. Adv. 2023, 9, eabq7750. [Google Scholar] [CrossRef]
- Lim, H.-R.; Kim, H.S.; Qazi, R.; Kwon, Y.-T.; Jeong, J.-W.; Yeo, W.-H. Advanced Soft Materials, Sensor Integrations, and Applications of Wearable Flexible Hybrid Electronics in Healthcare, Energy, and Environment. Adv. Mater. 2020, 32, 1901924. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jiang, L.; Shi, R.; Zhang, L. Synthesis, preparation, in vitro degradation, and application of novel degradable bioelastomers—A review. Prog. Polym. Sci. 2012, 37, 715–765. [Google Scholar] [CrossRef]
- Lim, H.-R.; Kim, H.S.; Qazi, R.; Kwon, Y.-T.; Jeong, J.-W.; Yeo, W.-H. Wearable Flexible Hybrid Electronics: Advanced Soft Materials, Sensor Integrations, and Applications of Wearable Flexible Hybrid Electronics in Healthcare, Energy, and Environment (Adv. Mater. 15/2020). Adv. Mater. 2020, 32, 2070116. [Google Scholar] [CrossRef]
- Singh, R.; Bathaei, M.J.; Istif, E.; Beker, L. A Review of Bioresorbable Implantable Medical Devices: Materials, Fabrication, and Implementation. Adv. Healthc. Mater. 2020, 9, 2000790. [Google Scholar] [CrossRef] [PubMed]
- Feiner, R.; Dvir, T. Tissue–electronics interfaces: From implantable devices to engineered tissues. Nat. Rev. Mater. 2017, 3, 17076. [Google Scholar] [CrossRef]
- Boutry, C.M.; Beker, L.; Kaizawa, Y.; Vassos, C.; Tran, H.; Hinckley, A.C.; Pfattner, R.; Niu, S.; Li, J.; Claverie, J.; et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 2019, 3, 47–57. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, S.-R.; Kil, H.-J.; Kim, Y.-C.; Park, J.-W. Highly Conformable, Transparent Electrodes for Epidermal Electronics. Nano Lett. 2018, 18, 4531–4540. [Google Scholar] [CrossRef]
- Tadayyon, G.; Krukiewicz, K.; Britton, J.; Larrañaga, A.; Vallejo-Giraldo, C.; Fernandez-Yague, M.; Guo, Y.; Orpella-Aceret, G.; Li, L.; Poudel, A.; et al. In vitro analysis of a physiological strain sensor formulated from a PEDOT:PSS functionalized carbon nanotube-poly(glycerol sebacate urethane) composite. Mater. Sci. Eng. C 2021, 121, 111857. [Google Scholar] [CrossRef]
- Zhang, L.; Kumar, K.S.; He, H.; Cai, C.J.; He, X.; Gao, H.; Yue, S.; Li, C.; Seet, R.C.-S.; Ren, H.; et al. Fully organic compliant dry electrodes self-adhesive to skin for long-term motion-robust epidermal biopotential monitoring. Nat. Commun. 2020, 11, 4683. [Google Scholar] [CrossRef]
- Chu, X.; Wang, R.; Zhao, H.; Kuang, M.; Yan, J.; Wang, B.; Ma, H.; Cui, M.; Zhang, X. Cross-Links–Entanglements Integrated Networks Contributing to Highly Resilient, Soft, and Self-Adhesive Elastomers with Low Hysteresis for Green Wearable Electronics. ACS Appl. Mater. Interfaces 2022, 14, 16631–16640. [Google Scholar] [CrossRef]
- Tran, H.; Feig, V.R.; Liu, K.; Wu, H.-C.; Chen, R.; Xu, J.; Deisseroth, K.; Bao, Z. Stretchable and Fully Degradable Semiconductors for Transient Electronics. ACS Cent. Sci. 2019, 5, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Sencadas, V.; Tawk, C.; Alici, G. Environmentally Friendly and Biodegradable Ultrasensitive Piezoresistive Sensors for Wearable Electronics Applications. ACS Appl. Mater. Interfaces 2020, 12, 8761–8772. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-W.; Lee, C.H.; Cheng, H.; Jeong, J.-W.; Kang, S.-K.; Kim, J.-H.; Shin, J.; Yang, J.; Liu, Z.; Ameer, G.A.; et al. Biodegradable Elastomers and Silicon Nanomembranes/Nanoribbons for Stretchable, Transient Electronics, and Biosensors. Nano Lett. 2015, 15, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Chi, X.; Feng, Z.; Yang, C.; Gao, R.; Li, S.; Zhang, C.; Chen, X.; Huang, P.; et al. Biomimetic integration of tough polymer elastomer with conductive hydrogel for highly stretchable, flexible electronic. Nano Energy 2022, 92, 106735. [Google Scholar] [CrossRef]
- Pu, W.; Fu, D.; Wang, Z.; Gan, X.; Lu, X.; Yang, L.; Xia, H. Realizing Crack Diagnosing and Self-Healing by Electricity with a Dynamic Crosslinked Flexible Polyurethane Composite. Adv. Sci. 2018, 5, 1800101. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.S.; Wan, P.; Yang, H.; Shah, S.A.A.; Chen, X. Healable Transparent Electronic Devices. Adv. Funct. Mater. 2017, 27, 1606339. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, J.; Jiang, C.; Liu, Z.; Sun, L.; Chen, S.; Xuan, H.; Lei, D.; Guan, Q.; Ye, X.; et al. Peptidoglycan-inspired autonomous ultrafast self-healing bio-friendly elastomers for bio-integrated electronics. Natl. Sci. Rev. 2021, 8, nwaa154. [Google Scholar] [CrossRef]
- Jia, L.; Jiang, J.; Xiang, T.; Zhou, S. Multifunctional Biomimetic Microstructured Surfaces for Healthcare Applications. Adv. Mater. Interfaces 2022, 9, 2201270. [Google Scholar] [CrossRef]
- Wen, X.; Sun, S.; Wu, P. Dynamic wrinkling of a hydrogel–elastomer hybrid microtube enables blood vessel-like hydraulic pressure sensing and flow regulation. Mater. Horiz. 2020, 7, 2150–2157. [Google Scholar] [CrossRef]
- Yuk, H.; Zhang, T.; Parada, G.A.; Liu, X.; Zhao, X. Skin-inspired hydrogel–elastomer hybrids with robust interfaces and functional microstructures. Nat. Commun. 2016, 7, 12028. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, C.; Yang, M.; Pan, Y.; Yin, Q.; Tang, J.; Qi, H.J.; Suo, Z. Printing Hydrogels and Elastomers in Arbitrary Sequence with Strong Adhesion. Adv. Funct. Mater. 2019, 29, 1901721. [Google Scholar] [CrossRef]
- Liu, T.; Liu, M.; Dou, S.; Sun, J.; Cong, Z.; Jiang, C.; Du, C.; Pu, X.; Hu, W.; Wang, Z.L. Triboelectric-Nanogenerator-Based Soft Energy-Harvesting Skin Enabled by Toughly Bonded Elastomer/Hydrogel Hybrids. ACS Nano 2018, 12, 2818–2826. [Google Scholar] [CrossRef]
- Ma, C.; Tian, X.; Kim, J.P.; Xie, D.; Ao, X.; Shan, D.; Lin, Q.; Hudock, M.R.; Bai, X.; Yang, J. Citrate-based materials fuel human stem cells by metabonegenic regulation. Proc. Natl. Acad. Sci. USA 2018, 115, E11741–E11750. [Google Scholar] [CrossRef] [PubMed]


| Materials | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation (%) | Degradation | Ref. |
|---|---|---|---|---|---|
| PLCL | 19.6–95 | 17.2–26.6 | 388–1974 | 19% in 15 weeks in vivo 10% in 26 weeks, 50% in 52 weeks in vitro | [13,14,15,16] |
| PGCL | 110–292.98 | 0.28–8 | 100–168 | 20–40% in 40 days in vitro | [17,18] |
| PGS | 0.05–1.5 | 0.4–1.5 | 100–500 | 13% after 35 days in vitro | [19] |
| POC | 0.42−16.4 | 0.35−6.1 | 100–265 | 20% after 28 days in vivo 100% after 15−68 weeks in vitro | [20,21] |
| P3HB | 74.45−3500 | 1.3−554 | 3.8−26 | <10% in 6 weeks in vivo | [22,23,24] |
| P4HB | 0.1−670 | 2.3−70 | 10−1450 | 2–12 months in vivo | [25,26,27,28] |
| PCLF | 3–7 | 0.5–17 | 230−800 | — | [29,30,31] |
| Applications | Backbone Polyester Materials | Function of Elastomer | Other Functional Materials | Ref. | |
|---|---|---|---|---|---|
| Cardiac tissue engineering | Cardiac repair patch | PGS | elastic | PBS-DLA | [69] |
| PGS | conductive film | PPy | [72] | ||
| POMaC | adhesive | dopamine | [70] | ||
| PICO | injectable | [75] | |||
| PGS-co-aniline | conductive | [75] | |||
| PCL, PGS | 3D printing | [78] | |||
| PGS | conductive | PPy, collagen | [76] | ||
| PGSA-g-EG | injectable | [84] | |||
| Vascular tissue engineering | Vessel treatment requiring expanding | PGA, PGCL | elastic | [91] | |
| vascular implants | PGS-palmitic acid | elastic | [93] | ||
| POC | antithrombus and endothelialization | ePTFE, atRA | [97] | ||
| POMaC | elastic | [102] | |||
| cardiovascular tissue regeneration | PITCO | 3D printing | [95] | ||
| Nerve tissue engineering | Nerve repair | PGS | conductive | CaTiO3 | [108] |
| PCL, PGS | conductive | graphene nanosheets | [111] | ||
| Folic acid-doped CUPE | regulation of cells | [112] | |||
| Methacrylated PGS | elastic | [113] | |||
| PGS-maleate | injectable | Mg2+ | [114] | ||
| Bone tissue engineering | Bone tissue regeneration | PCS | elastic | silica nanoparticles | [127] |
| bioactive glass | [128] | ||||
| Lumbar fusion | POC | elastic | HA, TA, Ag NPs | [129] | |
| Bone putty | BPLP-Ser | intrinsically fluorescent elastic scaffold | HA | [130] | |
| Vascularized bone regeneration | PCL | long-term scaffold | Strontium-HA, DMOG-silica nanoparticles | [137] | |
| channeled scaffold | sacrificial hydrogel | [132,143] | |||
| Bone regeneration | PLGA | conductive scaffold | Sulfonic acid-doped PANI, HA | [144] | |
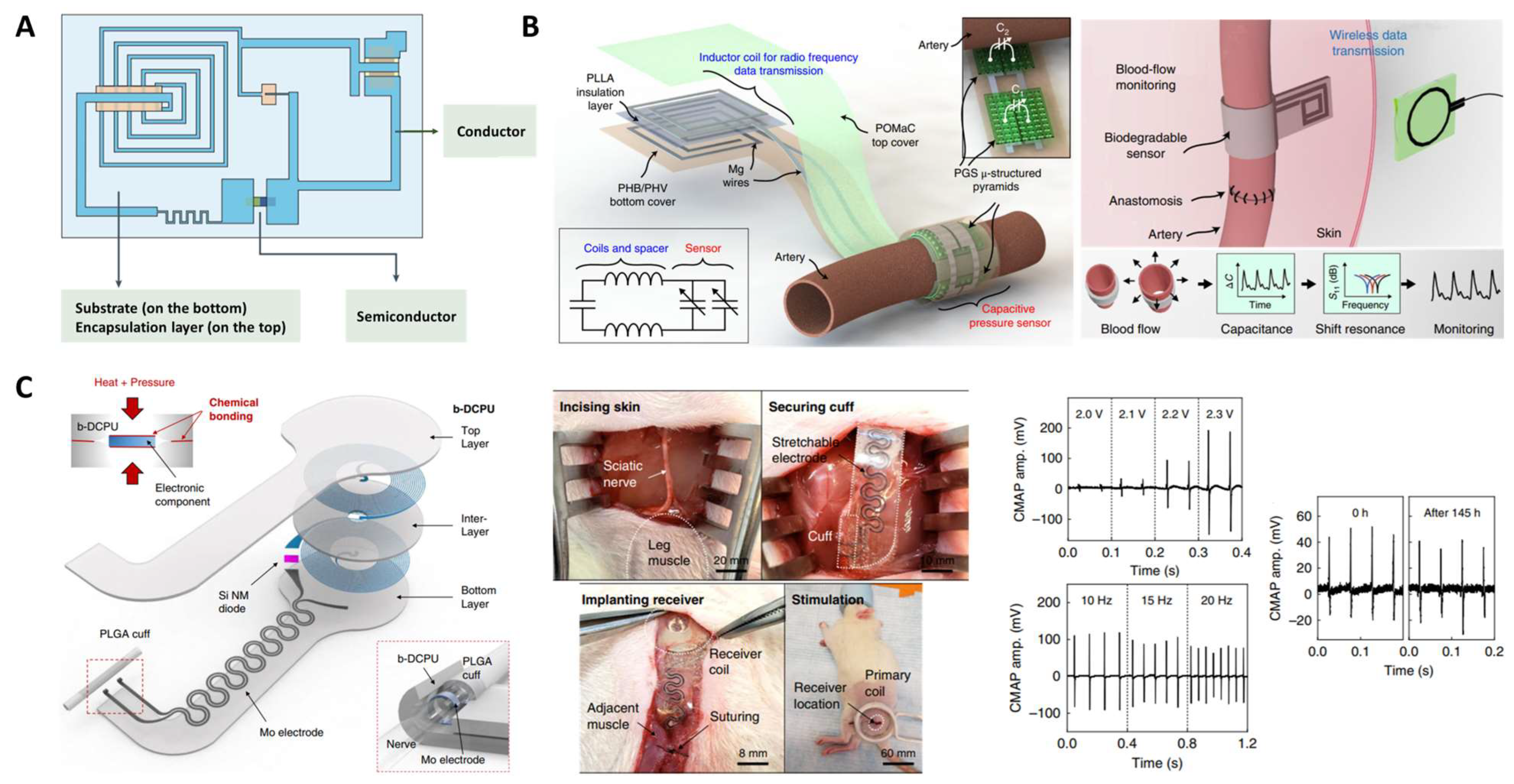
| Bioelectronic | Semiconductor device | PCL | elastic matrix | ||
| Pressure sensor | PGS POMaC, PHB/PHV PLLA | dielectric layer packaging layer spacer | [153] | ||
| Stimulation device | PCL | substrate and encapsulant | [52] | ||
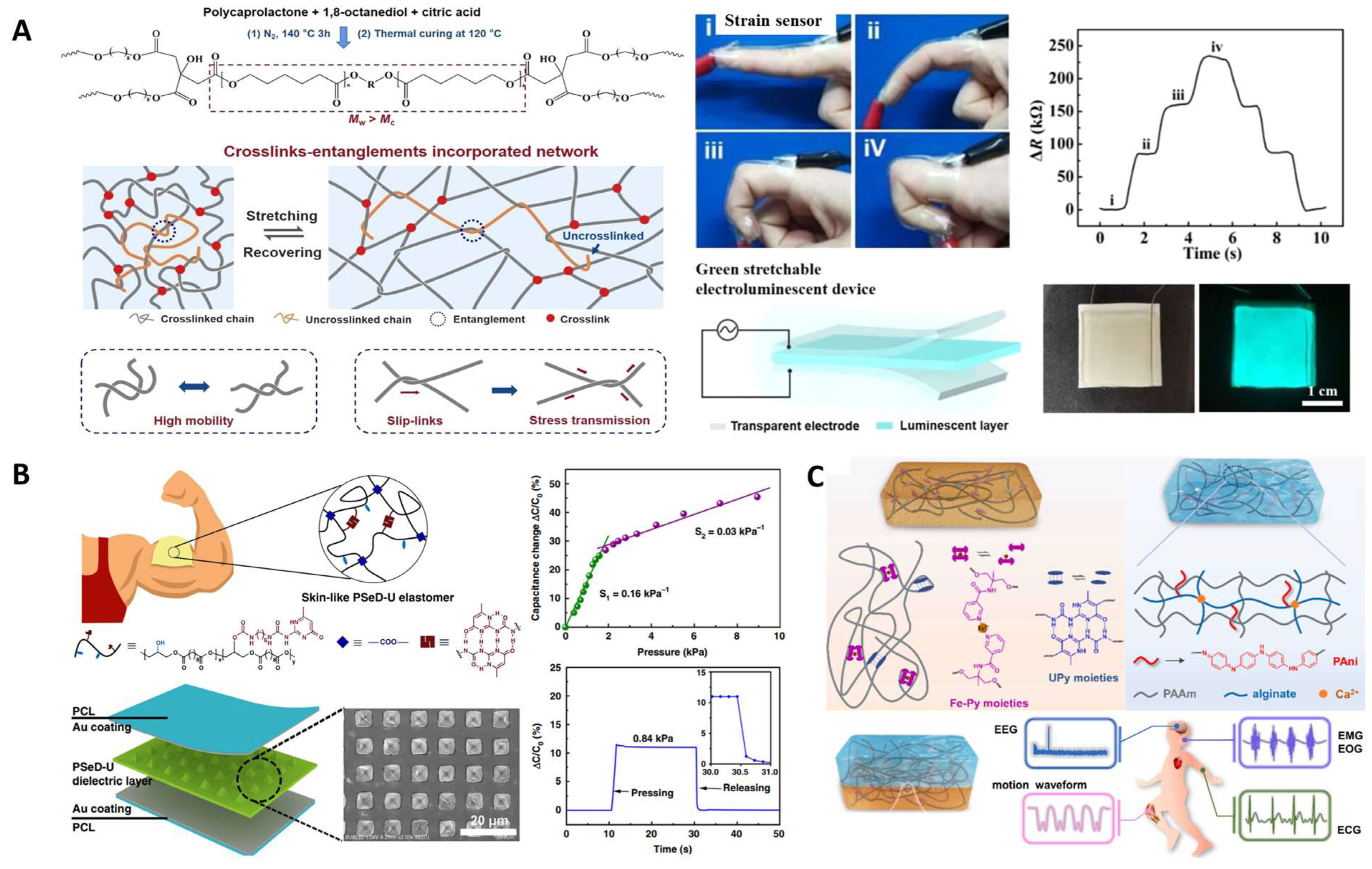
| Piezoresistive sensor | PGS | piezoresistive layer | CNTs | [159] | |
| PSeD-U | piezoresistive layer | Au | [53] | ||
| Strain sensor | PGS-urethane | sensor layer | PEDOT: PSS-functionalized CNTs | [130] | |
| POCL | elastic matrix | [EMI]+[TFSI]− | [157] | ||
| PSeHCD | elastic matrix | PEDOT: PSS | [156] | ||
| Electronic device | G-PLCL | elastic layer with high modulus and stretchability | conductive hydrogel | [161] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhong, W. Recent Progress in Advanced Polyester Elastomers for Tissue Engineering and Bioelectronics. Molecules 2023, 28, 8025. https://doi.org/10.3390/molecules28248025
Zhao Y, Zhong W. Recent Progress in Advanced Polyester Elastomers for Tissue Engineering and Bioelectronics. Molecules. 2023; 28(24):8025. https://doi.org/10.3390/molecules28248025
Chicago/Turabian StyleZhao, Yawei, and Wen Zhong. 2023. "Recent Progress in Advanced Polyester Elastomers for Tissue Engineering and Bioelectronics" Molecules 28, no. 24: 8025. https://doi.org/10.3390/molecules28248025
APA StyleZhao, Y., & Zhong, W. (2023). Recent Progress in Advanced Polyester Elastomers for Tissue Engineering and Bioelectronics. Molecules, 28(24), 8025. https://doi.org/10.3390/molecules28248025







