Bench to Bedside Development of [18F]Fluoromethyl-(1,2-2H4)choline ([18F]D4-FCH)
Abstract
:1. Introduction
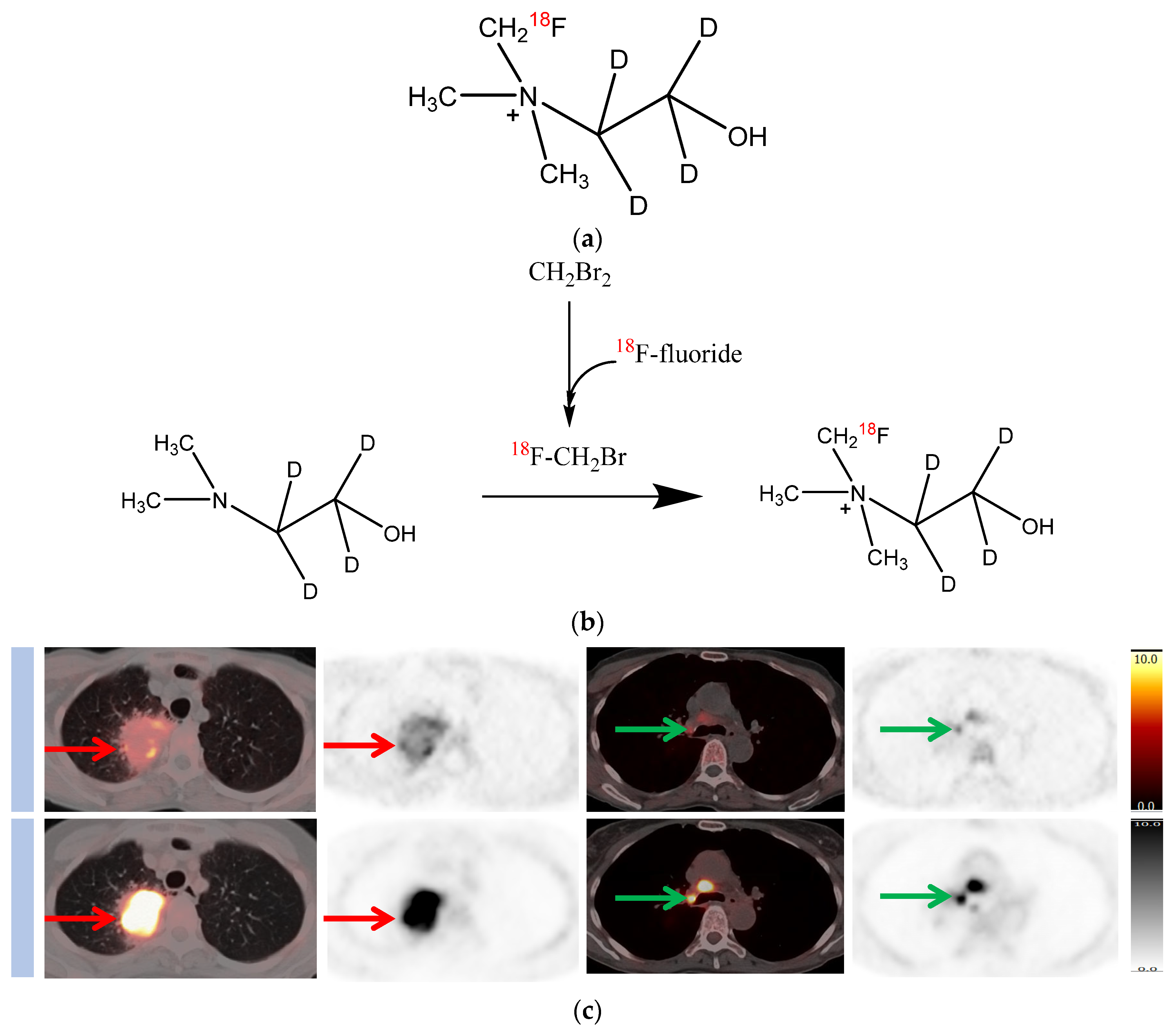
2. Deuterated Choline
3. Synthesis and Stability
4. In Vivo Biodistribution
5. In Vivo Metabolic Stability
6. In Vivo Response Assessment
7. In Vitro and In Vivo Comparison of [11C] and [18F]choline Analogues
8. Healthy Volunteer Biodistribution
9. First In-Patient (Lung Cancer) Evaluation
10. Longitudinal Case Study in a Brain Tumour Patient
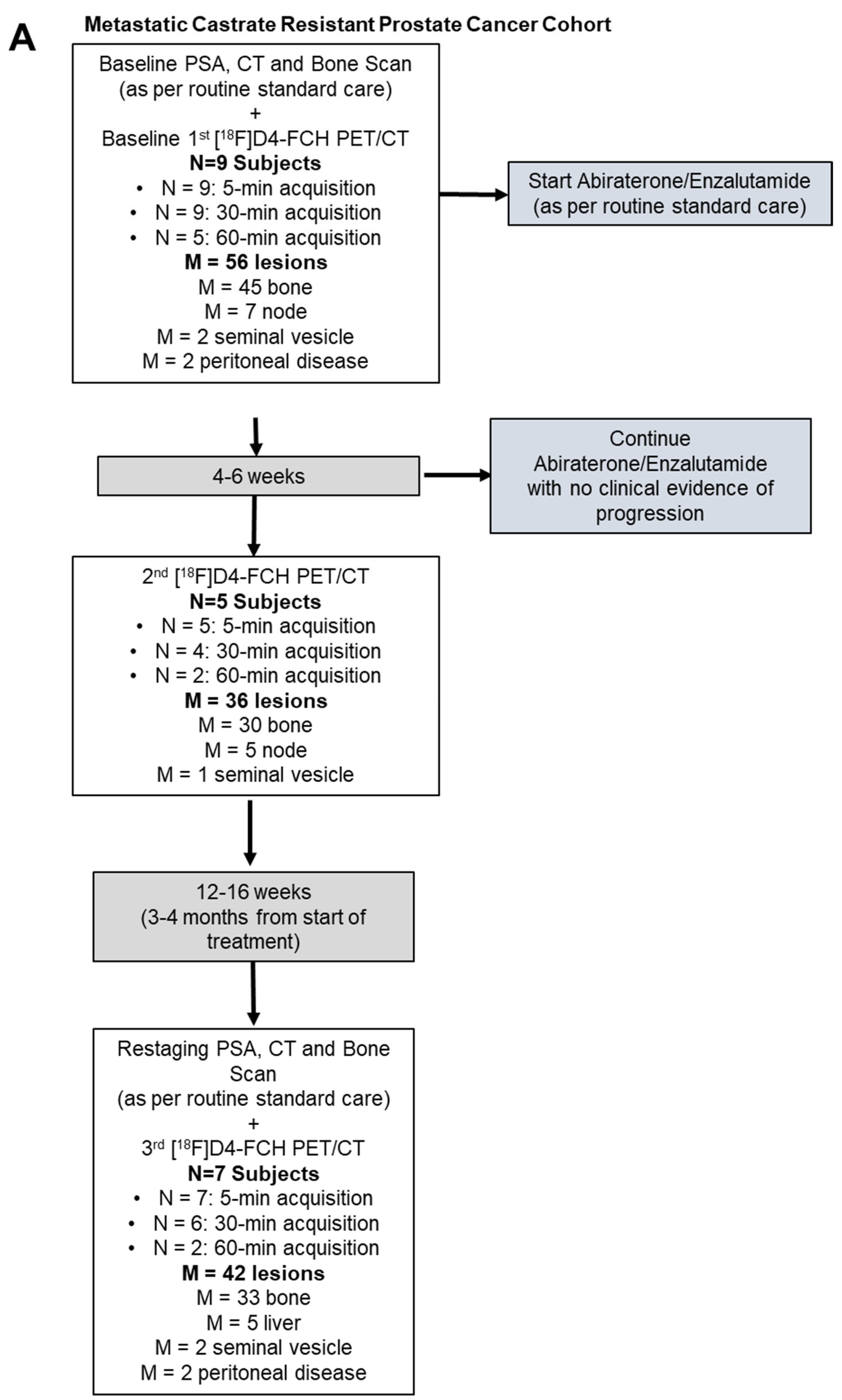
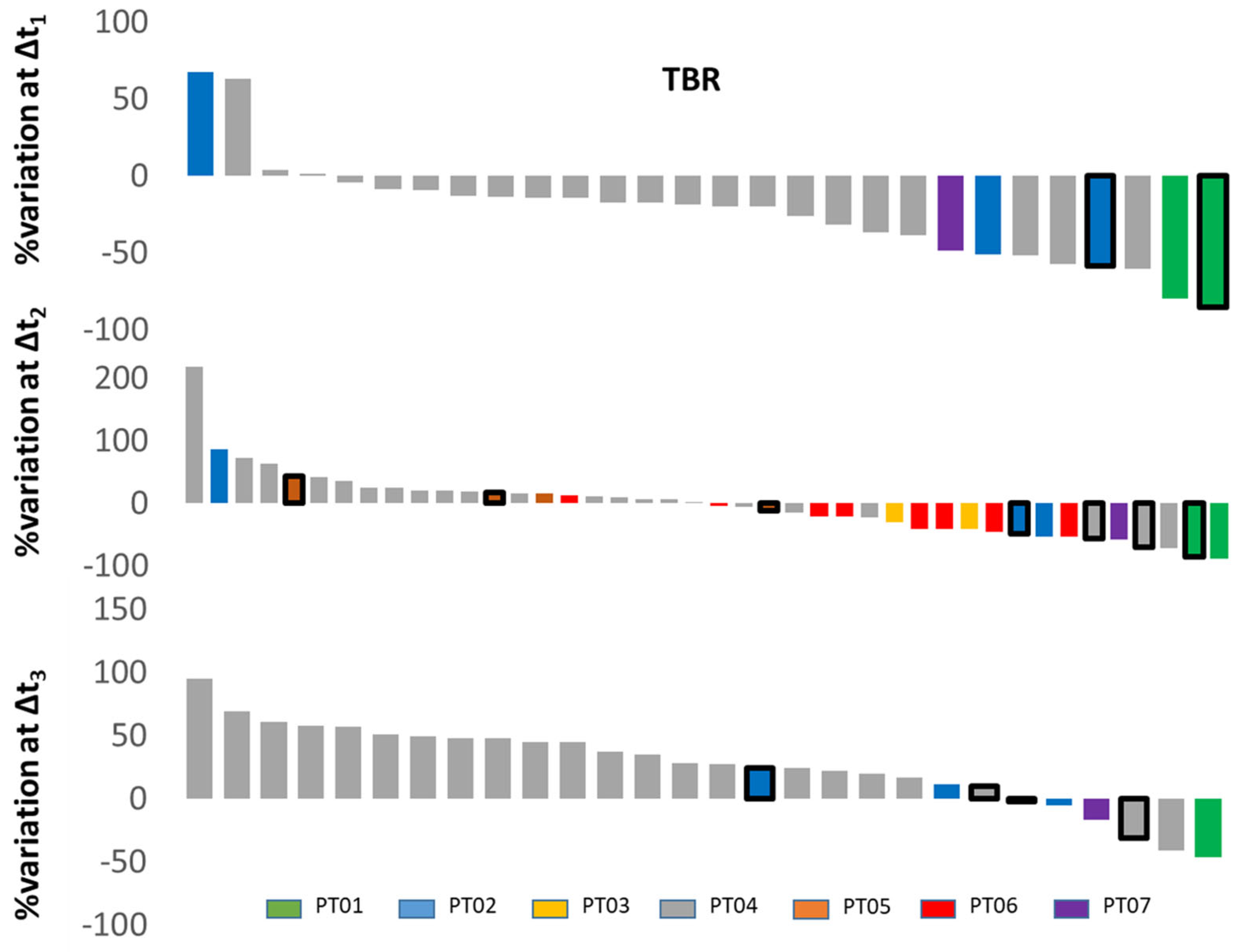
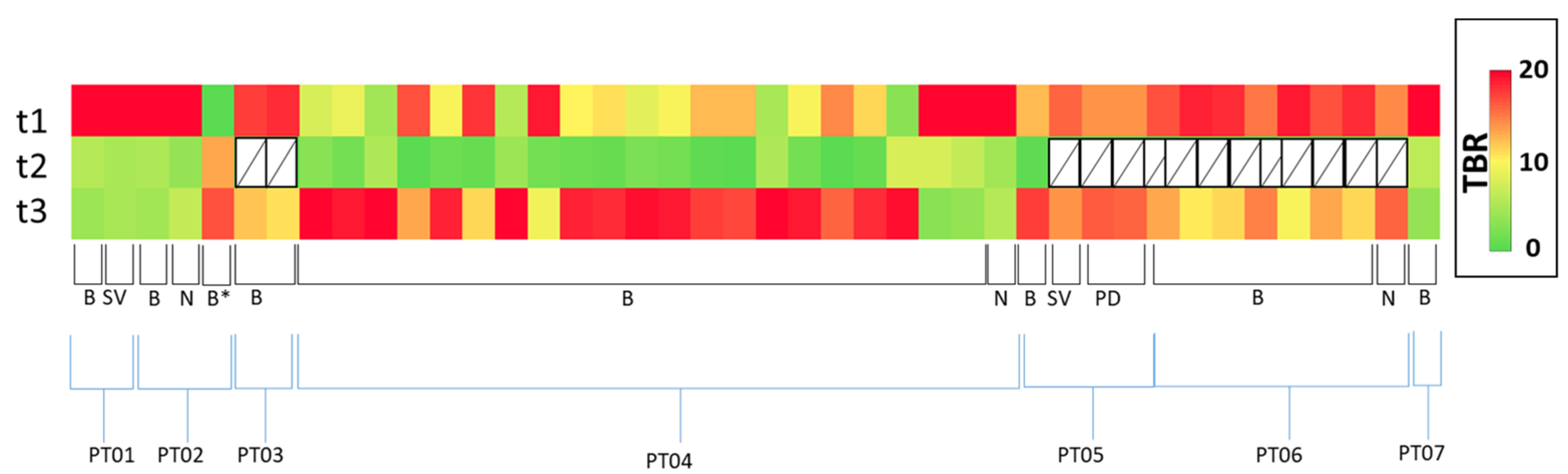
11. Response Evaluation in Metastatic Castrate-Resistant Prostate Cancer (mCRPC): Proof of Concept Study
Patient Characteristics and Optimal Imaging Time for [18F]D4-FCH PET in mCRPC
12. Discussion
13. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeisel, S.H. Dietary choline: Biochemistry, physiology, and pharmacology. Annu. Rev. Nutr. 1981, 1, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Aboagye, E.O.; Bhujwalla, Z.M. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res. 1999, 59, 80–84. [Google Scholar] [PubMed]
- Gibellini, F.; Smith, T.K. The Kennedy pathway--De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 2010, 62, 414–428. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Waki, A.; Obata, A.; Furukawa, T.; Yonekura, Y.; Fujibayashi, Y. Radiolabeled choline as a proliferation marker: Comparison with radiolabeled acetate. Nucl. Med. Biol. 2004, 31, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Ramirez de Molina, A.; Penalva, V.; Lucas, L.; Lacal, J.C. Regulation of choline kinase activity by Ras proteins involves Ral-GDS and PI3K. Oncogene 2002, 21, 937–946. [Google Scholar] [CrossRef]
- Ramirez de Molina, A.; Rodriguez-Gonzalez, A.; Gutierrez, R.; Martinez-Pineiro, L.; Sanchez, J.; Bonilla, F.; Rosell, R.; Lacal, J. Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochem. Biophys. Res. Commun. 2002, 296, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Ramirez de Molina, A.; Banez-Coronel, M.; Gutierrez, R.; Rodriguez-Gonzalez, A.; Olmeda, D.; Megias, D.; Lacal, J.C. Choline kinase activation is a critical requirement for the proliferation of primary human mammary epithelial cells and breast tumor progression. Cancer Res. 2004, 64, 6732–6739. [Google Scholar] [CrossRef]
- Alongi, P.; Laudicella, R.; Lanzafame, H.; Farolfi, A.; Mapelli, P.; Picchio, M.; Burger, I.A.; Iagaru, A.; Minutoli, F.; Evangelista, L. PSMA and Choline PET for the Assessment of Response to Therapy and Survival Outcomes in Prostate Cancer Patients: A Systematic Review from the Literature. Cancers 2022, 14, 1770. [Google Scholar] [CrossRef]
- Ghidaglia, J.; Golse, N.; Pascale, A.; Sebagh, M.; Besson, F.L. 18F-FDG/18F-Choline Dual-Tracer PET Behavior and Tumor Differentiation in HepatoCellular Carcinoma. A Systematic Review. Front. Med. 2022, 9, 924824. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Kosaka, N.; Kishi, H. PET imaging of prostate cancer using carbon-11-choline. J. Nucl. Med. 1998, 39, 990–995. [Google Scholar] [PubMed]
- Hara, T.; Kosaka, N.; Kishi, H. Development of 18F-fluoroethylcholine for cancer imaging with PET: Synthesis, biochemistry, and prostate cancer imaging. J. Nucl. Med. 2002, 43, 187–199. [Google Scholar]
- DeGrado, T.R.; Coleman, R.E.; Wang, S.; Baldwin, S.W.; Orr, M.D.; Robertson, C.N.; Polascik, T.J.; Price, D.T. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: Initial findings in prostate cancer. Cancer Res. 2001, 61, 110–117. [Google Scholar]
- Bansal, A.; Shuyan, W.; Hara, T.; Harris, R.A.; Degrado, T.R. Biodisposition and metabolism of [18F]fluorocholine in 9L glioma cells and 9L glioma-bearing fisher rats. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Roivainen, A.; Forsback, S.; Gronroos, T.; Lehikoinen, P.; Kahkonen, M.; Sutinen, E.; Minn, H. Blood metabolism of [methyl-11C]choline; implications for in vivo imaging with positron emission tomography. Eur. J. Nucl. Med. 2000, 27, 25–32. [Google Scholar] [CrossRef]
- Smith, G.; Zhao, Y.; Leyton, J.; Shan, B.; Nguyen, Q.D.; Perumal, M.; Turton, D.; Arstad, E.; Luthra, S.K.; Robins, E.G.; et al. Radiosynthesis and pre-clinical evaluation of [18F]fluoro-[1,2-(2)H(4)]choline. Nucl. Med. Biol. 2011, 38, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Gadda, G. pH and deuterium kinetic isotope effects studies on the oxidation of choline to betaine-aldehyde catalyzed by choline oxidase. Biochim. Biophys. Acta 2003, 1650, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Leyton, J.; Smith, G.; Zhao, Y.; Perumal, M.; Nguyen, Q.D.; Robins, E.; Arstad, E.; Aboagye, E.O. [18F]fluoromethyl-[1,2-2H4]-choline: A novel radiotracer for imaging choline metabolism in tumors by positron emission tomography. Cancer Res. 2009, 69, 7721–7728. [Google Scholar] [CrossRef] [PubMed]
- Witney, T.H.; Alam, I.S.; Turton, D.R.; Smith, G.; Carroll, L.; Brickute, D.; Twyman, F.J.; Nguyen, Q.D.; Tomasi, G.; Awais, R.O.; et al. Evaluation of deuterated 18F- and 11C-labeled choline analogs for cancer detection by positron emission tomography. Clin. Cancer Res. 2012, 18, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Challapalli, A.; Sharma, R.; Hallett, W.A.; Kozlowski, K.; Carroll, L.; Brickute, D.; Twyman, F.; Al-Nahhas, A.; Aboagye, E.O. Biodistribution and radiation dosimetry of deuterium-substituted 18F-fluoromethyl-[1,2-2H4]choline in healthy volunteers. J. Nucl. Med. 2014, 55, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Dubash, S.; Inglese, M.; Mauri, F.; Kozlowski, K.; Trivedi, P.; Arshad, M.; Challapalli, A.; Barwick, T.; Al-Nahhas, A.; Stanbridge, R.; et al. Spatial heterogeneity of radiolabeled choline positron emission tomography in tumors of patients with non-small cell lung cancer: First-in-patient evaluation of [18F]fluoromethyl-(1,2-(2)H(4))-choline. Theranostics 2020, 10, 8677–8690. [Google Scholar] [CrossRef]
- Li, Y.; Inglese, M.; Dubash, S.; Barnes, C.; Brickute, D.; Braga, M.C.; Wang, N.; Beckley, A.; Heinzmann, K.; Allott, L.; et al. Consideration of Metabolite Efflux in Radiolabelled Choline Kinetics. Pharmaceutics 2021, 13, 1246. [Google Scholar] [CrossRef] [PubMed]
- Trousil, S.; Kaliszczak, M.; Schug, Z.; Nguyen, Q.D.; Tomasi, G.; Favicchio, R.; Brickute, D.; Fortt, R.; Twyman, F.J.; Carroll, L.; et al. The novel choline kinase inhibitor ICL-CCIC-0019 reprograms cellular metabolism and inhibits cancer cell growth. Oncotarget 2016, 7, 37103–37120. [Google Scholar] [CrossRef] [PubMed]
- Mazarico, J.M.; Sanchez-Arevalo Lobo, V.J.; Favicchio, R.; Greenhalf, W.; Costello, E.; Carrillo-de Santa Pau, E.; Marques, M.; Lacal, J.C.; Aboagye, E.; Real, F.X. Choline Kinase Alpha (CHKalpha) as a Therapeutic Target in Pancreatic Ductal Adenocarcinoma: Expression, Predictive Value, and Sensitivity to Inhibitors. Mol. Cancer Ther. 2016, 15, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Radiation dose to patients from radiopharmaceuticals (addendum 2 to ICRP publication 53). Ann. ICRP 1998, 28, 1–126.
- Grech-Sollars, M.; Ordidge, K.L.; Vaqas, B.; Davies, C.; Vaja, V.; Honeyfield, L.; Camp, S.; Towey, D.; Mayers, H.; Peterson, D.; et al. Imaging and Tissue Biomarkers of Choline Metabolism in Diffuse Adult Glioma: 18F-Fluoromethylcholine PET/CT, Magnetic Resonance Spectroscopy, and Choline Kinase alpha. Cancers 2019, 11, 1969. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Bubendorf, L.; Schopfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef]
- Challapalli, A.; Barwick, T.; Tomasi, G.; Doherty, M.O.; Contractor, K.; Stewart, S.; Al-Nahhas, A.; Behan, K.; Coombes, C.; Aboagye, E.O.; et al. Exploring the potential of [11C]choline-PET/CT as a novel imaging biomarker for predicting early treatment response in prostate cancer. Nucl. Med. Commun. 2014, 35, 20–29. [Google Scholar] [CrossRef]
- Inazu, M. Choline transporter-like proteins CTLs/SLC44 family as a novel molecular target for cancer therapy. Biopharm. Drug Dispos. 2014, 35, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef]
- Glunde, K.; Bhujwalla, Z.M. Metabolic tumor imaging using magnetic resonance spectroscopy. Semin. Oncol. 2011, 38, 26–41. [Google Scholar] [CrossRef]
- Treglia, G.; Giovannini, E.; Di Franco, D.; Calcagni, M.L.; Rufini, V.; Picchio, M.; Giordano, A. The role of positron emission tomography using carbon-11 and fluorine-18 choline in tumors other than prostate cancer: A systematic review. Ann. Nucl. Med. 2012, 26, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Umbehr, M.H.; Muntener, M.; Hany, T.; Sulser, T.; Bachmann, L.M. The Role of 11C-Choline and 18F-Fluorocholine Positron Emission Tomography (PET) and PET/CT in Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2013, 64, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Cucchetti, A.; Piscaglia, F.; Caturelli, E.; Benvegnu, L.; Vivarelli, M.; Ercolani, G.; Cescon, M.; Ravaioli, M.; Grazi, G.L.; Bolondi, L.; et al. Comparison of recurrence of hepatocellular carcinoma after resection in patients with cirrhosis to its occurrence in a surveilled cirrhotic population. Ann. Surg. Oncol. 2009, 16, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Lencioni, R. mRECIST for HCC: Performance and novel refinements. J. Hepatol. 2020, 72, 288–306. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, J.H.; Kim, S.K.; Kang, K.W.; Park, K.W.; Choi, J.I.; Lee, W.J.; Kim, C.M.; Nam, B.H. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J. Nucl. Med. 2008, 49, 1912–1921. [Google Scholar] [CrossRef]
- Zukotynski, K.A.; Emmenegger, U.; Hotte, S.; Kapoor, A.; Fu, W.; Blackford, A.L.; Valliant, J.; Benard, F.; Kim, C.K.; Markowski, M.C.; et al. Prospective, Single-Arm Trial Evaluating Changes in Uptake Patterns on Prostate-Specific Membrane Antigen-Targeted 18F-DCFPyL PET/CT in Patients with Castration-Resistant Prostate Cancer Starting Abiraterone or Enzalutamide. J. Nucl. Med. 2021, 62, 1430–1437. [Google Scholar] [CrossRef]
- Hofman, M.S.; Hicks, R.J.; Maurer, T.; Eiber, M. Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls. Radiographics 2018, 38, 200–217. [Google Scholar] [CrossRef]
- Contractor, K.; Challapalli, A.; Barwick, T.; Winkler, M.; Hellawell, G.; Hazell, S.; Tomasi, G.; Al-Nahhas, A.; Mapelli, P.; Kenny, L.M.; et al. Use of [11C]choline PET-CT as a noninvasive method for detecting pelvic lymph node status from prostate cancer and relationship with choline kinase expression. Clin. Cancer Res. 2011, 17, 7673–7683. [Google Scholar] [CrossRef]
- Treglia, G.; Pereira Mestre, R.; Ferrari, M.; Bosetti, D.G.; Pascale, M.; Oikonomou, E.; De Dosso, S.; Jermini, F.; Prior, J.O.; Roggero, E.; et al. Radiolabelled choline versus PSMA PET/CT in prostate cancer restaging: A meta-analysis. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 127–139. [Google Scholar]
- Meller, B.; Bremmer, F.; Sahlmann, C.O.; Hijazi, S.; Bouter, C.; Trojan, L.; Meller, J.; Thelen, P. Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI Res. 2015, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Oruc, Z.; Guzel, Y.; Ebinc, S.; Komek, H.; Kucukoner, M.; Kaplan, M.A.; Oruc, I.; Urakci, Z.; Isikdogan, A. Efficacy of 68Ga-PSMA PET/CT-derived whole-body volumetric parameters in predicting response to second-generation androgen receptor axis-targeted therapy, and the prognosis in metastatic hormone-refractory prostate cancer patients. Nucl. Med. Commun. 2021, 42, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Svec, P.; Novy, Z.; Kucka, J.; Petrik, M.; Sedlacek, O.; Kuchar, M.; Liskova, B.; Medvedikova, M.; Kolouchova, K.; Groborz, O.; et al. Iodinated Choline Transport-Targeted Tracers. J. Med. Chem. 2020, 63, 15960–15978. [Google Scholar] [CrossRef]
- Quak, E.; Cavarec, M.; Ciappuccini, R.; Girault, G.; Rouzier, R.; Lequesne, J. Detection, resection and cure: A systematic review and meta-analysis of 18F-choline PET in primary hyperparathyroidism. Q. J. Nucl. Med. Mol. Imaging 2023, 67, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Reizine, E.; Chalaye, J.; Mule, S.; Regnault, H.; Perrin, C.; Calderaro, J.; Laurent, A.; Amaddeo, G.; Kobeiter, H.; Tacher, V.; et al. Utility of Early Posttreatment PET/CT Evaluation Using FDG or 18F-FCH to Predict Response to 90Y Radioembolization in Patients with Hepatocellular Carcinoma. AJR Am. J. Roentgenol. 2022, 218, 359–369. [Google Scholar] [CrossRef]
- Filippi, L.; Bagni, O.; Notarianni, E.; Saltarelli, A.; Ambrogi, C.; Schillaci, O. PET/CT with 18F-choline or 18F-FDG in Hepatocellular Carcinoma Submitted to 90Y-TARE: A Real-World Study. Biomedicines 2022, 10, 2996. [Google Scholar] [CrossRef]
- Lacal, J.C.; Zimmerman, T.; Campos, J.M. Choline Kinase: An Unexpected Journey for a Precision Medicine Strategy in Human Diseases. Pharmaceutics 2021, 13, 788. [Google Scholar] [CrossRef] [PubMed]
- Study of Intravenous TCD-717 in Patients with Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT01215864 (accessed on 10 April 2023).



| Setting | Findings/Comments |
|---|---|
| In vitro stability [15], 2011 |
|
| In vivo biodistribution [15], 2011 |
|
| In vivo response assessment [17], 2009 | At day 10 after drug treatment, compared with the pretreatment group, the following observations were seen:
|
| In vitro and in vivo comparison [18], 2012 |
|
| Human biodistribution [19], 2014 |
|
| First in-patient evaluation (lung cancer) [20], 2020 |
|
| Impact of hypoxia on D4-FCH kinetics [21], 2021 |
|
| Prostate cancer response assessment (Current Study) |
|
| Pt No. | Age (year) | Metastatic Site (Number) | Drug Used | Baseline Parameters | 3-Month Parameters | Other Clinical Parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSA (ng/mL) | TBR | PSA (ng/mL) | TBR | PCWG3 (Subsequent Treatment) | Alive/Dead | PFS (m) | OS (m) | ||||||||||
| 5 min p.i. | 30 min p.i. | 5 min p.i. | 30 min p.i. | ||||||||||||||
| Sum | Sumw | Sum | Sumw | Sum | Sumw | Sum | Sumw | ||||||||||
| 1 | 75 | left SV, right iliac bone. | Enzalutamide | 95.25 | 28.45 | 14.31 | 38.83 | 19.42 | 1.08 | 3.80 | 1.98 | 4.89 | 1.34 | SD (Docetaxel) | Alive | 34.63 | 48.43 |
| 2 | 73 | node (1), T8 bone. | Enzalutamide | 10.54 | 19.80 | 13.98 | 17.48 | 10.81 | 2.22 | 11.37 | 5.55 | 8.76 | 1.18 | PR ∏ (Docetaxel) | Dead | 11.93 | 29.17 |
| 3 | 58 | bone (sacrum and scapula) | Abiraterone | 9.67 | 13.42 | 7.87 | 13.56 | 7.73 | 3.02 | 6.17 | 3.74 | 8.91 | 1.73 | SD (Docetaxel) | Alive | 47.73 | 53.80 |
| 4 | 63 | multiple bone mets (19), nodes (3) | Abiraterone | 33.44 | 170.51 | 8.65 | 191.21 | 9.52 | 14.5 | 181.36 | 9.60 | 211.00 | 0.81 | SD (Carboplatin/Etoposide) | Dead | 9.13 | 29.53 |
| 5 | 80 | left SV, peritoneal lesions (2), C3 bone (1) | Abiraterone | 198 | 27.91 | 7.75 | 23.91 | 6.45 | 63.67 | 30.91 | 8.29 | 27.90 | 1.06 | SD (Cabazitaxel) | Dead | 14.47 | 38.67 |
| 6 | 74 | bone mets (7), node (1) | Enzalutamide | 228.82 | 18.51 | 3.34 | 21.25 | 3.26 | 92.95 | 14.88 | 2.33 | 14.88 | 2.17 | SD (Docetaxel) | Dead | 12.13 | 28.10 |
| 7 | 84 | right iliac bone (1). | Abiraterone | 27.19 | 2.62 | 2.62 | 4.10 | 4.10 | 8.17 | 1.38 | 1.38 | 1.69 | 1.69 | SD (‡ Nil) | Dead | 25.60 | 25.80 |
| 8 | 74 | nodes (2) | Enzalutamide | 22.54 | 7.70 | 3.50 | 6.62 | 3.50 | 14.90 | N/A | N/A | N/A | N/A | SD (Enzalutamide) | Alive | 36.00 | 36.00 |
| 9 | 69 | bone (2) | Enzalutamide | 94.47 | 97.32 | 8.56 | 98.73 | 8.77 | 32.78 | N/A | N/A | N/A | N/A | N/A († Treatment break) | Alive | 34.00 | 36.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Challapalli, A.; Barwick, T.D.; Dubash, S.R.; Inglese, M.; Grech-Sollars, M.; Kozlowski, K.; Tam, H.; Patel, N.H.; Winkler, M.; Flohr, P.; et al. Bench to Bedside Development of [18F]Fluoromethyl-(1,2-2H4)choline ([18F]D4-FCH). Molecules 2023, 28, 8018. https://doi.org/10.3390/molecules28248018
Challapalli A, Barwick TD, Dubash SR, Inglese M, Grech-Sollars M, Kozlowski K, Tam H, Patel NH, Winkler M, Flohr P, et al. Bench to Bedside Development of [18F]Fluoromethyl-(1,2-2H4)choline ([18F]D4-FCH). Molecules. 2023; 28(24):8018. https://doi.org/10.3390/molecules28248018
Chicago/Turabian StyleChallapalli, Amarnath, Tara D. Barwick, Suraiya R. Dubash, Marianna Inglese, Matthew Grech-Sollars, Kasia Kozlowski, Henry Tam, Neva H. Patel, Mathias Winkler, Penny Flohr, and et al. 2023. "Bench to Bedside Development of [18F]Fluoromethyl-(1,2-2H4)choline ([18F]D4-FCH)" Molecules 28, no. 24: 8018. https://doi.org/10.3390/molecules28248018
APA StyleChallapalli, A., Barwick, T. D., Dubash, S. R., Inglese, M., Grech-Sollars, M., Kozlowski, K., Tam, H., Patel, N. H., Winkler, M., Flohr, P., Saleem, A., Bahl, A., Falconer, A., De Bono, J. S., Aboagye, E. O., & Mangar, S. (2023). Bench to Bedside Development of [18F]Fluoromethyl-(1,2-2H4)choline ([18F]D4-FCH). Molecules, 28(24), 8018. https://doi.org/10.3390/molecules28248018







