Abstract
Pectin has recently drawn much attention in biomedical applications due to its distinctive chemical and biological properties. Polymers like pectin with cell-instructive properties are attractive natural biomaterials for tissue repair and regeneration. In addition, bioactive pectin and pectin-based composites exhibit improved characteristics to deliver active molecules. Pectin and pectin-based composites serve as interactive matrices or scaffolds by stimulating cell adhesion and cell proliferation and enhancing tissue remodeling by forming an extracellular matrix in vivo. Several bioactive properties, such as immunoregulatory, antibacterial, anti-inflammatory, anti-tumor, and antioxidant activities, contribute to the pectin’s and pectin-based composite’s enhanced applications in tissue engineering and drug delivery systems. Tissue engineering scaffolds containing pectin and pectin-based conjugates or composites demonstrate essential features such as nontoxicity, tunable mechanical properties, biodegradability, and suitable surface properties. The design and fabrication of pectic composites are versatile for tissue engineering and drug delivery applications. This article reviews the promising characteristics of pectin or pectic polysaccharides and pectin-based composites and highlights their potential biomedical applications, focusing on drug delivery and tissue engineering.
1. Introduction
Recent studies have gained attention on the natural polymer pectin due to its lower price and biological properties that enable it to be used in various pharmacological and biomedical applications [1]. Various developments of simple and more innovative in vitro and in vivo testing to influence immunity, together with manufacturing, purification, and characterization techniques, have immensely contributed to the ongoing research of pectin and pectin-based composites in the food, healthcare, and cosmetic industries for their low toxicity and therapeutic effects. Commercially available pectin satisfies the required specifications and is approved by several Food and Agriculture Organizations for specific applications. Pectin is commercially obtained from the residual part of the plant materials after the extraction of juice (citrus or apple) and sugar (sugar beet). Pectin is an essential part of the cell wall that is needed for the development of plants. Pectin can be efficiently used for drug delivery and tissue engineering through gel beads or microspheres, 3D scaffolds, and membranes.
In all primary cell walls, there exist three significant types of pectic polysaccharides: (i) homogalacturonan, (ii) rhamnogalacturonan-I, and (iii) rhamnogalacturonan-II [2,3,4]—pectin-modifying enzymes and endomembrane system biosynthesis cause the structural complexity and the pectin domains’ heterogeneity. The enzyme pectin methyl esterase can modulate Homogalacturonan. Rhamnogalacturonan-I contains extremely distinct functionally regulated polymers; however, rhamnogalacturonan-II shows a highly stable pectin matrix [2]. Structurally, pectin is classified in a multifunctional family of covalently linked D-galacturonic acid-rich polysaccharides found in terrestrial plants’ primary cell walls [5]. The covalently linked 1-4-alpha-D-galacturonic units are interchangeable with 1-2 attached alpha-L-rhamnopyranosyl remnants that carry saccharide polymers [6]. The galacturonic remnants found in pectin are typically present as salts or methyl esters. The precise chemical structure of pectin is complex to deduce. It depends on the source and conditions they extract in location and other surrounding factors, making their chemical arrangement different. Commercially, pectin is extracted from plant materials such as citrus peel, apple pomace, and sugar beets. The polysaccharide helps to provide intercellular adhesion, rigidity, and mechanical resistance for the cell walls of plants. This support is needed to help plants living in harmful environments related to temperature, pollutants, and other environmental stressors survive. The multifunctional component of pectin has allowed it to provide numerous target sites for chemical modifications [7]. The properties of pectin, such as its nontoxicity, emulsion behavior, diverse chemical composition, biocompatibility, and high stability, enable it to be a commonly used biopolymer. Industrially, pectin is used for various applications such as food manufacturing and biomedical engineering. Biomedical applications of pectin primarily include drug delivery, tissue engineering, and wound healing (Figure 1).
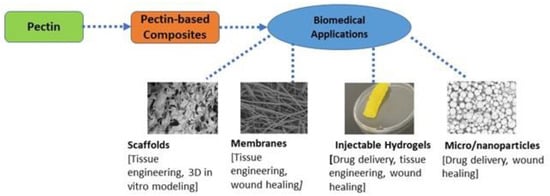
Figure 1.
Several biomedical applications of pectin and pectin-based composites.
The structure of pectin differs and depends on the type of plants and cell types that it develops in. Based on the source that the pectin emerges from, the polymer will vary in size, acetylation type, the degree to which it is esterified, and other variables that are controlled by the differences between the galacturonic acid that lead to the homogalacturonan chain and the side chain type of the rhamnogalacturonan-1 [8]. Rhamnogalacturonan-1 generally forms the branched regions of the pectin polysaccharide, which are the primary carbohydrate chains. Interestingly, Homogalacturonan forms the linear fragment of the polysaccharide, and sometimes, the chain forms the component that rhamnogalacturonan-1 generally makes up (Figure 2). The pectin polysaccharide varies based on the source from which it is groomed and the conditions from which it is extracted [9]. Regardless of the diversity found within the pectin polysaccharide, the structure is classified as canonical. Though pectin was discovered over two hundred years ago, the design of its composition has yet to be ultimately interpreted.
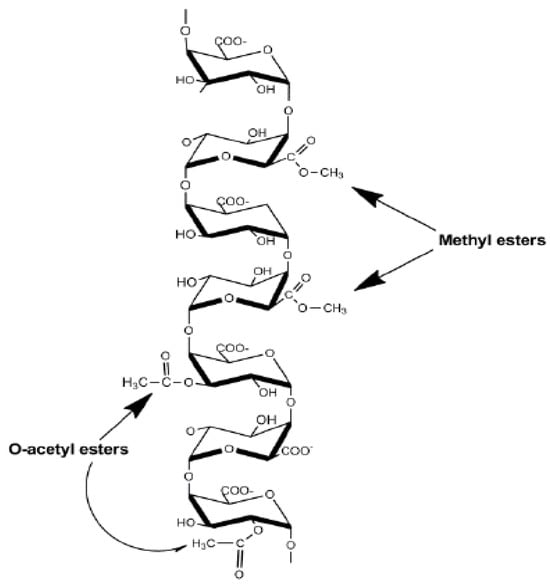
Figure 2.
Homogalacturonan structure of pectin polysaccharides. Homogalacturonan is a linear polymer of α-(1,4)-D galacturonic acid with methyl-esterified at C-6 and acetyl-esterified at positions O-2 and O-3 [10].
The pectin polymer is a core structure alternating alpha-1, 4-linked D-galacturonic acid and alpha-1, 2-L-rhamnose units. The system of pectin regulates the influence that the polysaccharide has on cytokine production; this proves that the elemental characteristics that are found in the polysaccharide are related to its ability to impact cellular environmental conditions. The diversity in the structure of pectin polymers from different plant origins enables it to be used in multiple applications. Pectin extracted from various sources of plants generally has similar structural characteristics, but the structures ultimately differ based on the species and the plant’s physiological stage. With the effect of structural features, the chemical composition of pectin, such as the galacturonic acid proportion, methyl group content, and grade of acetylation, determines the polymer’s function [1].
Immune reactivity is a factor that influences the use of pectin within biomedical and tissue engineering/drug delivery applications [11,12,13]. In applications for pathological conditions, immunomodulators are essential to regulate the body’s distinctive immune response to foreign materials and antigens from foreign or transplanted cells. The purpose of utilizing immunomodulators such as pectin is not to eliminate the immune response but to regulate the reactivity and further the efficiency of the applications that require the modulation of the immune system. Past studies have reported that pectin can weaken inflammatory reactivity by stimulating anti-inflammatory cytokines and decreasing the assembly of proinflammatory cytokines [14].
Due to its structural complexity and diversity, pectin has many applications. Pectin consists of many active functional groups of polysaccharides, enabling them to have much more excellent modification properties than other biopolymers. Pectin is a hydrophilic natural polymer that can absorb or retain much water and exhibit swelling properties. Hydrogels and composite materials can be formed by crosslinking and other techniques, and the matrix structure can be incorporated with various bioactive compounds. Pectin-based smart composites with physical-sensitive (light, temperature, electricity), chemical-sensitive (pH, redox, glucose), and biological-sensitive (enzymes) properties are suitable in the delivery system of bioactive compounds in addition to their suitable biodegradable and biocompatible properties. Due to its broad availability, pectin has become a prominent branch of the research and development of nature-based biomedical and healthcare areas. This article, therefore, aims to review the biological properties of pectin or pectic polysaccharides and summarize their biomedical applications in the emerging fields of drug delivery and tissue engineering, aiming at their expanding usage.
2. Properties of Pectic Polysaccharides
Pectin’s versatile properties allow it to be prospectively used in other applications, like medicine, as a carrier vehicle for drug delivery and a scaffold in tissue engineering or regenerative medicine.
2.1. Immunoregulatory Activity
The structural features of pectin provide a polysaccharide with biological activities such as immunomodulation. Immunomodulation is classified as a group of therapeutic interventions to regulate the immune system. Immunomodulators respond to the immune system by two different mechanisms: immunostimulation and immunosuppression. Immunosuppressive activity occurs on the backbone of pectin polysaccharides [15]. The structural changes in the galacturonic chain of the pectin control the macromolecule’s capacity to reduce immune reactivity [16]. The presence of a high quantity of galacturonic acid residues displays an increased immunosuppression activity. The amount of galacturonic acid residue fragments found on pectin determines the immunomodulatory effect. The injection of a glucan, zymosan, enables the pectin that contains more than 80% of the content of galacturonic acid residues to lower the production of macrophages. The polysaccharides of pectin that have 75% galacturonic acid residues or less do not reduce the gathering of macrophages stimulated by the injection of zymosan. Certain plants that produce pectin contain a significant percentage of galacturonic acid residues, while others do not. Plants with a high quantity of galacturonic acid residues include Potamogeton natans L., pond weeds that produce the pectin called Potamogeton Anand, and Vaccinium oxycoccos L. This cranberry plant produces the pectin called oxycoccusan. Plants that give rise to the pectins with lower than 75% galacturonic acid are those derived from Butomus, derived from Butomaceae, and Lemna, which emerged from Araceae. Table 1 shows pectin’s immunoregulatory activities.

Table 1.
Pectin’s immunoregulatory activities: source and mechanism of action.
2.2. Anti-Inflammatory Activity
Different degrees of methyl esterification affect the inflammatory properties of pectin. The various degrees of pectin methyl esterification play a role in determining the polysaccharide’s capacity to prevent the functional activity of white blood cells and leukocytes. In observing the influence of methyl esterification on pectin macromolecules, it is essential to analyze the makeup and characteristics of the pectin progenitor’s raw materials and the methods used to isolate the pectin. Table 2 summarizes the anti-inflammatory properties of pectin.

Table 2.
Anti-inflammatory properties of pectin.
2.3. Antibacterial Activity
Biomedical applications of antimicrobial natural systems have gained much attention in recent years. Biodegradable natural products based on pectin, pectin-linoleate, pectin-oleate, and pectin palmitate were reported to inhibit the microbial effect on several bacterial strains, including E. coli and S. aureus. Table 3 shows the reported data on the antibacterial properties of pectin. Table 3 exhibits the antibacterial properties of various pectin.

Table 3.
Antibacterial properties of pectin-based composite materials.
2.4. Anticancer Activity of Pectin and Pectin-Based Composites
Effective cancer treatment, a significant global disease, is highly challenging. Even though there is a substantial advancement in surgery, gene therapy, immunotherapy, chemotherapy, and radiotherapy, the mortality rate due to metastatic cancer is still alarming. Drug resistance of cancer tumor cells and adverse side effects of chemotherapies have been considered the critical drawbacks of cancer treatment. Several in vitro and in vivo studies reported the anti-tumor activity of pectin that showed a decrease in tumor cell adhesion and proliferation and stimulation of cell apoptosis [37]. Table 4 displays the anticancer activity of pectin and other pectin-based composites.

Table 4.
Anticancer activity of pectin and pectin-based composites.
3. Pectin for Drug Delivery Applications
Due to their excellent properties, such as biocompatibility, nontoxicity, flexibility in fabrication, and functionalization, natural polymer pectin hydrogels have gained extensive consideration in drug delivery applications. In the drug delivery system, the degradation rate of the hydrogel carrier is significant when delivering the active substance to the target site [54]. Using pectin within the drug delivery system has broadly been explored because pectin hydrogels can release drugs. Generally, researchers in the drug delivery system want the drug to be safely and efficiently immobilized or covalently attached to a biomaterial vehicle such as pectin [55]. Figure 3 shows the schematic diagram of a drug delivery system using pectin or pectin-based composites. Industrially, when integrated into a drug component, pectin is broadly utilized to treat radioactive isotopes and heavy metal poisoning [56]. Concerning heavy metals, pectin can act as a chelating agent by removing or preventing the interactions of toxic heavy metals within the human body, such as iron, copper, and mercury. When pectin polysaccharides interact with metal ions, esterification, and chelation occur according to the number of non-methyl-esterified galacturonosyl residues. Pectin molecules that are not esterified can form gels when surrounded by bivalent cations.
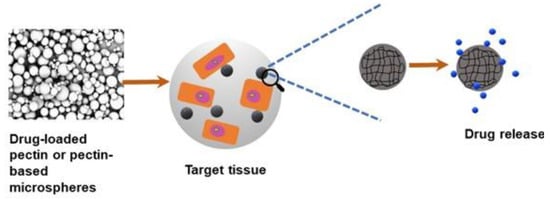
Figure 3.
Schematic diagram of drug delivery approach using pectin or pectin-based microspheres, which release the drug at a controlled rate when injected/implanted to the target tissue in vivo.
For this reason, when ionic crosslinks between galacturonan chains containing six or more adjacent residues increase, the metal binding of pectin molecules expands, and the degree of methyl esterification decreases [57]. The degree of esterification describes the percentage of galacturonic acids that react with methanol and are converted into an ester. There are generally two categories of pectin: high-methoxyl pectin and methoxyl pectin. When calcium surrounds the low-methoxyl pectin, the pectin gains the capacity to form gel because of the ionic crosslinking between the homogalacturonan chains. The egg-box mechanism is classified as one of the few gelation mechanisms that have been discovered [8]. In the egg-box process, six or more contiguous and non-esterified galacturonic residues are contained between each formed homogalacturonan chain within the calcium-crosslinked junction zones [8]. As a result of this interaction, an absorbent polymer network is formed [58]. The degree of methyl esterification of the galacturonosyl residues plays a crucial role in leading the gelation of the pectin polysaccharides and in determining the physical properties of the pectin. The pectin polysaccharides’ ability to form gels is one of the main reasons they are used within developed applications from various areas of the profession, such as physics, chemistry, biochemistry, biotechnology, cryobiology, and medicine [57].
Pectin has been used as a nourishing dietary component, and polysaccharides have also been used in drugs to treat diseases that develop from within the digestive system. Unmodified pectin is not digestible [59]. Within the human body’s digestive system, pectin activates the movement and peristalsis of the digestive system. Peristalsis is known as the wave-like movement that occurs for muscle contraction. Pectin can also cleanse the small intestine’s villi, and its gel properties enable it to improve the absorption of food intake and biologically stimulated materials. Pectin has been used as a drug delivery vehicle within the deliveries of colon-specific drugs and hydrogel-based drug delivery systems. Hydrogels generally consist of crosslinked, hydrophilic polymer chains that form three-dimensional networks [60]. In the hydrogel-based drug delivery system, pectin, as a drug delivery vehicle, can release the desired medication at a specific rate and area in the body [6]. In colon-specific delivery, the polymer can prevent specific drugs from traveling into the upper intestines, and instead, the drug is delivered into the colon. The polymer also can control the release of drugs at specific rates that are desired. Pectin’s magnitude of interaction with other diverse biopolymers leads to the production of new composite materials used in applications such as tissue engineering.
4. Pectin for Tissue Engineering Applications
Tissue engineering is the strategy of regenerating damaged tissue using biomaterials (Figure 4). This application aims to use materials such as polymers that can mimic the natural cell formation and aid in the attachment, proliferation, and differentiation of cells [11]. In tissue engineering, damaged tissues are recovered when the body’s cells and the highly porous tissue scaffold are integrated as a template for forming the tissue’s new growth [12]. In tissue engineering, pectin generally acts as a matrix material [13]. Developing synthetic tissue engineering scaffolds that emerge from endogenous or transplanted parent cells gains the capacity to function based on environments that properly integrate signals that restore proper cellular processes.
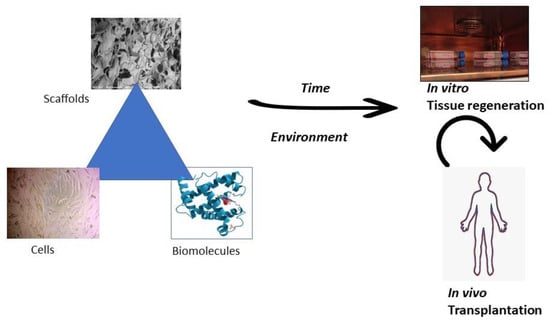
Figure 4.
The fundamental tissue engineering strategy. With appropriate time and environment, the tissue engineering triad consisting of pectin-based scaffolds, autologous cells, and biomolecules contributes to tissue regeneration in vitro and later transplantation in the human body in vivo.
Pectin has several advantages in tissue engineering, such as biodegradability, biocompatibility, low toxicity levels, antibacterial characteristics, and the polymer’s ability to promote controlled drug release. Hundreds of research articles on pectin’s applications in tissue engineering have been documented in the past ten years. Pectin is very attractive in tissue engineering because when it is fabricated into a tissue scaffold, it can control the release of drugs from the scaffold, which accelerates the local regenerative activity [61]. Pectin can also be modified in numerous ways with compounds and other biopolymers. When adjusting the pectin biomaterial for applications such as tissue engineering, it is essential to use biopolymers and compounds that interact well with it and promote the overall efficiency of the composite in combination with it. There are two main ways that interactions can occur between polysaccharides and compounds: (1) repulsion by steric exclusion and (2) attraction between the molecules. When pectin is modified with oligopeptide-arginine-glycine-aspartic, the fabricated composite can improve preosteoblast generation via cell adhesion and differentiation better than pectin alone [52]. Recently, pectin constructed with chitosan has been explored in tissue engineering as a scaffold to regenerate damaged tissue such as bone and skin. Porous pectin-based tissue scaffolds are generally fabricated using standardized techniques such as freeze-drying. Combined, chitosan and pectin create a polyelectrolyte complex that results in a smart scaffold that has improved mechanical resistance, porous microstructures, swelling capacity, stabilized crosslinking, and biocompatibility [62].
Modified pectin has even been explored in regenerating tissue parts such as the ear and nose. A 3D anatomical-shaped scaffold of the ear and nose was developed in a study using the composite of pectin and (3-glycidyloxypropyl) trimethoxysilone (pectin-GpTMS). The pectin–GpTMS greatly benefits as a biomaterial in mimicking different tissues for patient-specific scaffolds [63]. The diverse abilities that pectin has are why it is widely used globally in various biomedical applications. Table 5 demonstrates the application of pectin systems in tissue engineering strategy.

Table 5.
A summary of several pectin systems in a tissue engineering approach.
5. Conclusions and Future Prospects
This review summarizes the important biological properties and recent development of pectin and pectin-based composites in biomedical applications. Pectin and pectin-based composites are attractive for biomedical applications due to their nontoxicity, biocompatibility, biodegradability, anti-tumor, antibacterial, and anticancer characteristics. These materials also have excellent chemical reactivity and emulsification properties, which make them widely used and an advanced candidate for drug delivery and tissue engineering applications. Developing functionalized scaffolds for bone tissue, skin, biological valves, and injectable scaffolds is being explored, promising natural macromolecular materials for medical applications. However, further investigations are needed to explore insights into the roles of bioactivity of pectin in vivo. A lack of available research on the mechanisms of pectin’s anticancer protection mechanisms, as well as clinical trials, has restricted pectin’s application in medicine and drug development. A combination of in vitro and in vivo degradation kinetics, information on digested products, and the mechanisms of actions could further illuminate how pectin can be further technologically explored to expand its applications in the clinical setting of the biomedical and tissue engineering field.
Funding
There are no funding sources associated with this manuscript.
Data Availability Statement
No new data were created.
Acknowledgments
The author acknowledges the support from the Texas Undergraduate Medical Academy, Prairie View A&M University, USA.
Conflicts of Interest
The author declares no conflict of interest.
References
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant. Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, L.; Li, Y.; Chen, Q.; Wang, L.; Farag, M.A.; Liu, L.; Zhan, S.; Wu, Z.; Liu, L. Soy protein isolate-citrus pectin composite hydrogels induced by TGase and ultrasonic treatment: Potential targeted delivery system for probiotics. Food Hydrocoll. 2023, 143, 108901. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, P.; Liu, Y.; Wu, Z.; Wang, L.; Liu, L. Citrus pectin research advances: Derived as a biomaterial in the construction and applications of micro/nano-delivery systems. Food Hydrocoll. 2022, 133, 107910. [Google Scholar] [CrossRef]
- Coimbra, P.; Ferreira, P.; de Sousa, H.C.; Batista, P.; Rodrigues, M.A.; Correia, I.J.; Gil, M.H. Preparation and chemical and biological characterization of a pectin/chitosan polyelectrolyte complex scaffold for possible bone tissue engineering applications. Int. J. Biol. Macromol. 2011, 48, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, L.; Bianco-Peled, H. Pectin-chitosan physical hydrogels as potential drug delivery vehicles. Int. J. Biol. Macromol. 2017, 101, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomater. 2018, 66, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, Y.; Khozhaenko, E.; Kovalev, V.; Khotimchenko, M. Cerium binding activity of pectins isolated from the seagrasses Zostera marina and Phyllospadix iwatensis. Mar. Drugs 2012, 10, 834–848. [Google Scholar] [CrossRef]
- Pérez, S.; Mazeau, K.; Hervé du Penhoat, C. The three-dimensional structures of the pectic polysaccharides. Plant Physiol. Biochem. 2000, 38, 37–55. [Google Scholar] [CrossRef]
- Marisol, O.-V.; Emmanuel, A.-H.; Irasema, V.-A.; Miguel ÁngelMarisol, M.-T. Plant Cell Wall Polymers: Function, Structure and Biological Activity of Their Derivatives. In Polymerization; Ailton De Souza, G., Ed.; IntechOpen: Rijeka, Croatia, 2012; p. 4. [Google Scholar]
- Cuijpers, V.M.; Walboomers, X.F.; Jansen, J.A. Scanning electron microscopy stereoimaging for three-dimensional visualization and analysis of cells in tissue-engineered constructs: Technical note. Tissue Eng. Part. C Methods 2011, 17, 663–668. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Kumar, P.T.; Ramya, C.; Jayakumar, R.; Nair, S.K.; Lakshmanan, V.K. Drug delivery and tissue engineering applications of biocompatible pectin–chitin/nano CaCO3 composite scaffolds. Colloids Surf. B Biointerfaces 2013, 106, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Salman, H.; Bergman, M.; Djaldetti, M.; Orlin, J.; Bessler, H. Citrus pectin affects cytokine production by human peripheral blood mononuclear cells. Biomed. Pharmacother. 2008, 62, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.V.; Markov, P.A.; Popova, G.Y.; Nikitina, I.R.; Efimova, L.; Ovodov, Y.S. Anti-inflammatory activity of low and high methoxylated citrus pectins. Biomed. Prev. Nutr. 2013, 3, 59–63. [Google Scholar] [CrossRef]
- Boehler, R.M.; Graham, J.G.; Shea, L.D. Tissue engineering tools for modulation of the immune response. Biotechniques 2011, 51, 239–240, 242, 244. [Google Scholar] [CrossRef] [PubMed]
- Daguet, D.; Pinheiro, I.; Verhelst, A.; Possemiers, S.; Marzorati, M. Arabinogalactan and fructooligosaccharides improve the gut barrier function in distinct areas of the colon in the Simulator of the Human Intestinal Microbial Ecosystem. J. Funct. Foods 2016, 20, 369–379. [Google Scholar] [CrossRef]
- Vogt, L.M.; Sahasrabudhe, N.M.; Ramasamy, U.; Meyer, D.; Pullens, G.; Faas, M.M.; Venema, K.; Schols, H.A.; De Vos, P. The impact of lemon pectin characteristics on TLR activation and T84 intestinal epithelial cell barrier function. J. Funct. Foods 2016, 22, 398–407. [Google Scholar] [CrossRef]
- Ho, G.T.; Zou, Y.F.; Aslaksen, T.H.; Wangensteen, H.; Barsett, H. Structural characterization of bioactive pectic polysaccharides from elderflowers (Sambuci flos). Carbohydr. Polym. 2016, 135, 128–137. [Google Scholar] [CrossRef]
- Kapoor, S.; Dharmesh, S.M. Pectic Oligosaccharide from tomato exhibiting anticancer potential on a gastric cancer cell line: Structure-function relationship. Carbohydr. Polym. 2017, 160, 52–61. [Google Scholar] [CrossRef]
- Liu, Z.; Dang, J.; Wang, Q.; Yu, M.; Jiang, L.; Mei, L.; Shao, Y.; Tao, Y. Optimization of polysaccharides from Lycium ruthenicum fruit using RSM and its antioxidant activity. Int. J. Biol. Macromol. 2013, 61, 127–134. [Google Scholar] [CrossRef]
- Peng, Q.; Xu, Q.; Yin, H.; Huang, L.; Du, Y. Characterization of an immunologically active pectin from the fruits of Lycium ruthenicum. Int. J. Biol. Macromol. 2014, 64, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Leivas, C.L.; Nascimento, L.F.; Barros, W.M.; Santos, A.R.; Iacomini, M.; Cordeiro, L.M. Substituted galacturonan from starfruit: Chemical structure and antinociceptive and anti-inflammatory effects. Int. J. Biol. Macromol. 2016, 84, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Peng, L.; Lei, Z.; Jia, X.; Zou, J.; Yang, Y.; He, X.; Zeng, N. Traditional Uses, Phytochemical Constituents and Pharmacological Properties of Averrhoa carambola L.: A Review. Front. Pharmacol. 2021, 12, 699899. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, S.; Ksouri, R.; Falleh, H.; Pichette, A.; Abdelly, C.; Legault, J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem. 2012, 132, 943–947. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Abdelhamid, A.; Rihouey, C.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Optimized extraction of pectin-like polysaccharide from Suaeda fruticosa leaves: Characterization, antioxidant, anti-inflammatory and analgesic activities. Carbohydr. Polym. 2018, 185, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Sherry, C.L.; Kim, S.S.; Dilger, R.N.; Bauer, L.L.; Moon, M.L.; Tapping, R.I.; Fahey, G.C., Jr.; Tappenden, K.A.; Freund, G.G. Sickness behavior induced by endotoxin can be mitigated by the dietary soluble fiber, pectin, through up-regulation of IL-4 and Th2 polarization. Brain Behav. Immun. 2010, 24, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, G.E.; Winnischofer, S.M.B.; Ramirez, M.I.; Iacomini, M.; Cordeiro, L.M.C. The influence of sweet pepper pectin structural characteristics on cytokine secretion by THP-1 macrophages. Food Res. Int. 2017, 102, 588–594. [Google Scholar] [CrossRef]
- Pedrosa, L.F.; Raz, A.; Fabi, J.P. The Complex Biological Effects of Pectin: Galectin-3 Targeting as Potential Human Health Improvement? Biomolecules 2022, 12, 289. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, X.J.; Jiang, Y.; Zhou, Z. Citrus pectin derived silver nanoparticles and their antibacterial activity. Inorg. Nano-Met. Chem. 2017, 47, 15–20. [Google Scholar] [CrossRef]
- Gupta, V.K.; Pathania, D.; Asif, M.; Sharma, G. Liquid phase synthesis of pectin–cadmium sulfide nanocomposite and its photocatalytic and antibacterial activity. J. Mol. Liq. 2014, 196, 107–112. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, G.; Thakur, R. Pectin @ zirconium (IV) silicophosphate nanocomposite ion exchanger: Photo catalysis, heavy metal separation and antibacterial activity. Chem. Eng. J. 2015, 267, 235–244. [Google Scholar] [CrossRef]
- Hassan, E.A.; Abou Elseoud, W.S.; Abo-Elfadl, M.T.; Hassan, M.L. New pectin derivatives with antimicrobial and emulsification properties via complexation with metal-terpyridines. Carbohydr. Polym. 2021, 268, 118230. [Google Scholar] [CrossRef] [PubMed]
- Supreetha, R.; Bindya, S.; Deepika, P.; Vinusha, H.; Hema, B. Characterization and biological activities of synthesized citrus pectin-MgO nanocomposite. Results Chem. 2021, 3, 100156. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, P.; Zhan, Y.; Shi, X.; Lin, J.; Du, Y.; Li, X.; Deng, H. Pectin/lysozyme bilayers layer-by-layer deposited cellulose nanofibrous mats for antibacterial application. Carbohydr. Polym. 2015, 117, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Rosas, M.I.; Morales-Castro, J.; Cubero-Márquez, M.; Salvia-Trujillo, L.; Martín-Belloso, O. Antimicrobial activity of nanoemulsions containing essential oils and high methoxyl pectin during long-term storage. Food Control 2017, 77, 131–138. [Google Scholar] [CrossRef]
- Nisar, T.; Yang, X.; Alim, A.; Iqbal, M.; Wang, Z.; Guo, Y. Physicochemical responses and microbiological changes of bream (Megalobrama ambycephala) to pectin-based coatings enriched with clove essential oil during refrigeration. Int. J. Biol. Macromol. 2019, 124, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, Z.; Leng, J.; Liu, D.; Hao, M.; Gao, X.; Tai, G.; Zhou, Y. The inhibitory effects and mechanisms of rhamnogalacturonan I pectin from potato on HT-29 colon cancer cell proliferation and cell cycle progression. Int. J. Food Sci. Nutr. 2013, 64, 36–43. [Google Scholar] [CrossRef]
- Donadio, J.L.S.; d Prado, S.B.R.; Rogero, M.M.; Fabi, J.P. Effects of pectins on colorectal cancer: Targeting hallmarks as a support for future clinical trials. Food Funct. 2022, 13, 11438–11454. [Google Scholar] [CrossRef]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Modified sugar beet pectin induces apoptosis of colon cancer cells via an interaction with the neutral sugar side-chains. Carbohydr. Polym. 2016, 136, 923–929. [Google Scholar] [CrossRef]
- Ogutu, F.O.; Mu, T.-H.; Sun, H.; Zhang, M. Ultrasonic Modified Sweet Potato Pectin Induces Apoptosis like Cell Death in Colon Cancer (HT-29) Cell Line. Nutr. Cancer 2018, 70, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Delphi, L.; Sepehri, H. Apple pectin: A natural source for cancer suppression in 4T1 breast cancer cells in vitro and express p53 in mouse bearing 4T1 cancer tumors, in vivo. Biomed. Pharmacother. 2016, 84, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Leclere, L.; Fransolet, M.; Cote, F.; Cambier, P.; Arnould, T.; Cutsem, P.V.; Michiels, C. Heat-Modified Citrus Pectin Induces Apoptosis-Like Cell Death and Autophagy in HepG2 and A549 Cancer Cells. PLoS ONE 2015, 10, e0115831. [Google Scholar] [CrossRef] [PubMed]
- Prado, S.B.; Ferreira, G.F.; Harazono, Y.; Shiga, T.M.; Raz, A.; Carpita, N.C.; Fabi, J.P. Ripening-induced chemical modifications of papaya pectin inhibit cancer cell proliferation. Sci. Rep. 2017, 7, 16564. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Diao, J.; Wang, Y.; Sun, S.; Zhang, H.; Liu, Y.; Wang, Y.; Cao, J. A New Water-Soluble Nanomicelle Formed through Self-Assembly of Pectin–Curcumin Conjugates: Preparation, Characterization, and Anticancer Activity Evaluation. J. Agric. Food Chem. 2017, 65, 6840–6847. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mei, M.; Xu, Y.; Shi, S.; Wang, S.; Wang, H. Versatile functionalization of pectic conjugate: From design to biomedical applications. Carbohydr. Polym. 2023, 306, 120605. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gou, Y.; Li, W.; Zhang, P.; Chen, J.; Wu, H.; Hu, F.; Cheng, W. Activation of Intrinsic Apoptotic Signaling Pathway in A549 Cell by a Pectin Polysaccharide Isolated from Codonopsis pilosula and Its Selenized Derivative. J. Carbohydr. Chem. 2015, 34, 475–489. [Google Scholar] [CrossRef]
- Gaikwad, D.; Shewale, R.; Patil, V.; Mali, D.; Gaikwad, U.; Jadhav, N. Enhancement in in vitro anti-angiogenesis activity and cytotoxicity in lung cancer cell by pectin-PVP based curcumin particulates. Int. J. Biol. Macromol. 2017, 104, 656–664. [Google Scholar] [CrossRef]
- Ogbonna, C.; Kavaz, D. Development of novel silver-apple pectin nanocomposite beads for antioxidant, antimicrobial and anticancer studies. Biologia 2022, 77, 879–891. [Google Scholar] [CrossRef]
- Suganya, K.S.U.; Govindaraju, K.; Kumar, V.G.; Karthick, V.; Parthasarathy, K. Pectin mediated gold nanoparticles induce apoptosis in mammary adenocarcinoma cell lines. Int. J. Biol. Macromol. 2016, 93, 1030–1040. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Mosalam, F.M.; Ghorab, M.; Hanora, A.; Elbarbary, A.M. Antimicrobial, antioxidant and anticancer activities of zinc nanoparticles prepared by natural polysaccharides and gamma radiation. Int. J. Biol. Macromol. 2018, 107, 2298–2311. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, M.; Dziadek, K.; Salagierski, S.; Drozdowska, M.; Serafim, A.; Stancu, I.; Szatkowski, P.; Kopec, A.; Rajzer, I.; Douglas, T.E.; et al. Newly crosslinked chitosan- and chitosan-pectin-based hydrogels with high antioxidant and potential anticancer activity. Carbohydr. Polym. 2022, 290, 119486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, L.; Liu, L.; Wu, Z.; Pan, D.; Liu, L. Recent Advances of Stimuli-Responsive Polysaccharide Hydrogels in Delivery Systems: A Review. J. Agric. Food Chem. 2022, 70, 6300–6316. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Yang, Y.; Zhou, Z.; Bo, Y.; Wang, Y.; He, Y.; Wang, D.; Qin, J. Pectin-based injectable and biodegradable self-healing hydrogels for enhanced synergistic anticancer therapy. Acta Biomater. 2021, 131, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Munarin, F.; Guerreiro, S.G.; Grellier, M.A.; Tanzi, M.C.; Barbosa, M.A.; Petrini, P.; Granja, P.L. Pectin-based injectable biomaterials for bone tissue engineering. Biomacromolecules 2011, 12, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, O.; Khudyakov, A.; Sergushkina, M.; Solomina, O.; Polezhaeva, T. Pectins as a universal medicine. Fitoterapia 2020, 146, 104676. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.A.; Ishii, T.; Albersheim, P.; Darvill, A.G. Rhamnogalacturonan II: Structure and function of a borate crosslinked cell wall pectic polysaccharide. Annu. Rev. Plant Biol. 2004, 55, 109–139. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Liang, L.; Fan, X.; Yu, Z.; Hotchkiss, A.T.; Wilk, B.J.; Eliaz, I. The role of modified citrus pectin as an effective chelator of lead in children hospitalized with toxic lead levels. Altern. Ther. Health Med. 2008, 14, 34–38. [Google Scholar]
- Eswaramma, S.; Reddy, N.S.; Rao, K.K. Phosphate crosslinked pectin based dual responsive hydrogel networks and nanocomposites: Development, swelling dynamics, and drug release characteristics. Int. J. Biol. Macromol. 2017, 103, 1162–1172. [Google Scholar] [CrossRef]
- Rambhia, K.J.; Ma, P.X. Controlled drug release for tissue engineering. J. Control. Release 2015, 219, 119–128. [Google Scholar] [CrossRef]
- Bombaldi de Souza, F.C.; Bombaldi de Souza, R.F.; Drouin, B.; Mantovani, D.; Moraes, Â.M. Comparative study on complexes formed by chitosan and different polyanions: Potential of chitosan-pectin biomaterials as scaffolds in tissue engineering. Int. J. Biol. Macromol. 2019, 132, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Lapomarda, A.A.; De Acutis, A.; Chiesa, I.; Fortunato, G.M.; Montemurro, F.; De Maria, C.; Belmonte, M.M.; Gottardi, R.; Vozzi, G. Pectin-GPTMS-Based Biomaterial: Towards a Sustainable Bioprinting of 3D scaffolds for Tissue Engineering Application. Biomacromolecules 2020, 21, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Mubarok, W.; Elvitigala, K.C.M.L.; Kotani, T.; Sakai, S. Visible light photocrosslinking of sugar beet pectin for 3D bioprinting applications. Carbohydr. Polym. 2023, 316, 121026. [Google Scholar] [CrossRef] [PubMed]
- Akshata, C.R.; Harichandran, G.; Murugan, E. Effect of pectin on the crystallization of strontium substituted HA for bone reconstruction application. Colloids Surf. B Biointerfaces 2023, 226, 113312. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Chávez, M.L.; Claudio-Rizo, J.A.; Caldera-Villalobos, M.; Cabrera-Munguía, D.A.; Becerra-Rodríguez, J.J.; Rodríguez-Fuentes, N. Novel bioactive collagen-polyurethane-pectin scaffolds for potential application in bone regenerative medicine. Appl. Surf. Sci. Adv. 2022, 11, 100317. [Google Scholar] [CrossRef]
- Hu, Z.; Cheng, J.; Xu, S.; Cheng, X.; Zhao, J.; Kenny Low, Z.W.; Chee, P.L.; Lu, Z.; Zheng, L.; Kai, D. PVA/pectin composite hydrogels inducing osteogenesis for bone regeneration. Mater. Today Bio 2022, 16, 100431. [Google Scholar] [CrossRef] [PubMed]
- Suliman, S.; Mieszkowska, A.; Folkert, J.; Rana, N.; Mohamed-Ahmed, S.; Fuoco, T.; Finne-Wistrand, A.; Dirscherl, K.; Jørgensen, B.; Mustafa, K.; et al. Immune-instructive copolymer scaffolds using plant-derived nanoparticles to promote bone regeneration. Inflamm. Regen. 2022, 42, 12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).