Structural Characteristics and Antioxidant Mechanism of Donkey-Hide Gelatin Peptides by Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Activity of Gelatin Peptides with Different MWs
2.2. Amino Acid Composition of Gelatin Peptides with Different MWs
2.3. Functional Groups and Secondary Structures of Gelatin Peptides with Different MWs
2.4. Sequence Identification and Antioxidant Activity of Synthetic Peptides
2.5. Molecular Dynamics (MD) Simulations and Stability Analysis of PGPAP Binding to Keap1
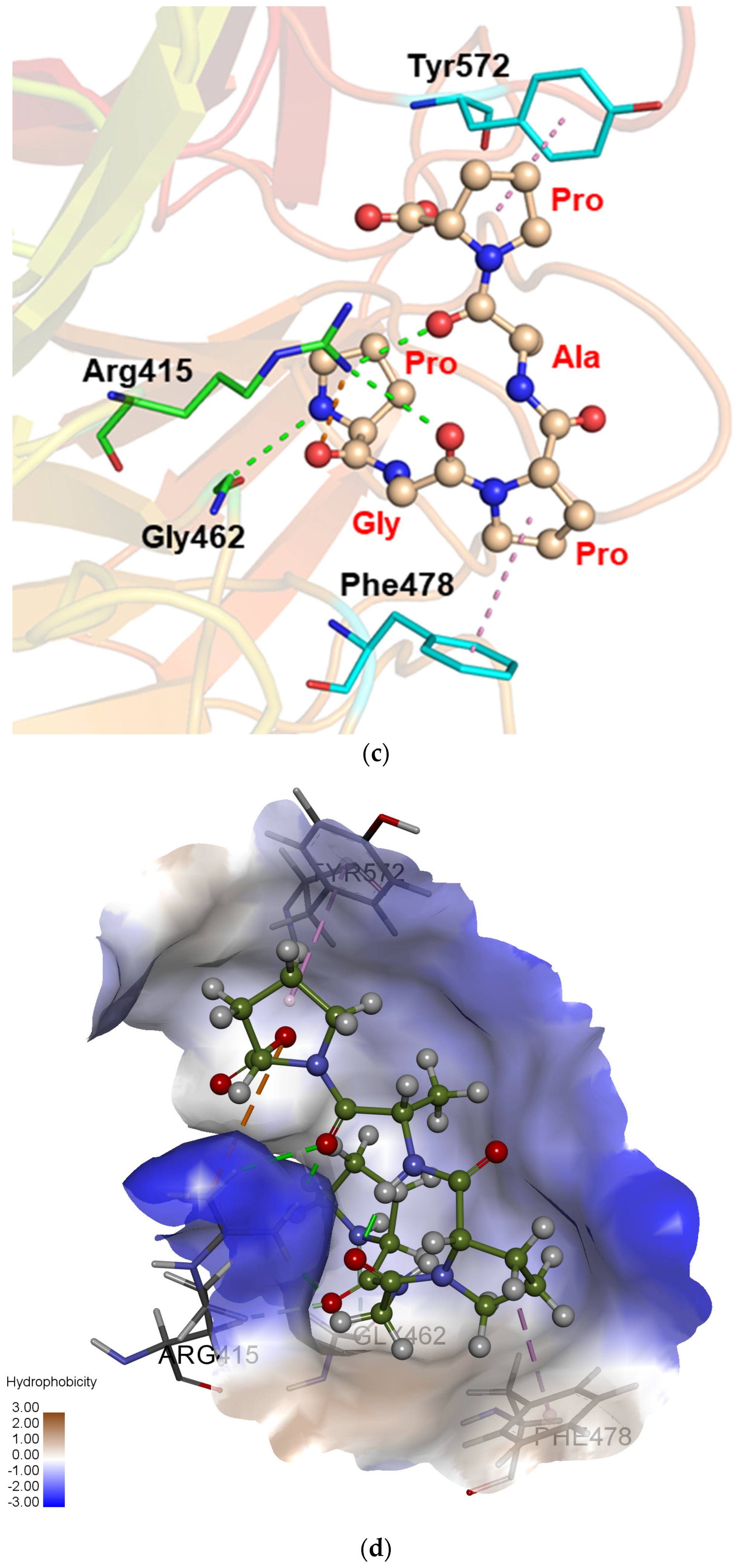
2.6. Molecular Mechanism Analysis of Peptide PGPAP Binding to Keap1
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Gelatin Peptides with Different MWs
3.3. Analysis of the Amino Acid Composition of Gelatin Peptides
3.4. Analysis of the Functional Groups of Gelatin Peptides via FTIR Spectroscopy
3.5. Analysis of the Secondary Structure of Gelatin Peptides via CD
3.6. Determination of the Antioxidant Activity of Gelatin Peptides
3.6.1. DPPH Radical Scavenging Assay of Gelatin Peptides
3.6.2. ABTS Radical Scavenging Assay of Gelatin Peptides
3.7. Peptide Sequence Identification Using Liquid Chromatography (LC)–Tandem Mass Spectrometry (MS/MS)
3.8. Peptide Synthesis
3.9. Antioxidant Activity Tests of Synthetic Peptides
3.10. Molecular Docking of Gelatin Peptides with Keap1
3.11. Molecular Dynamic (MD) Simulations of the PGPAP-Keap1 Complex
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Liang, R.; Cheng, S.; Dong, Y.; Ju, H. Intracellular antioxidant activity and apoptosis inhibition capacity of PEF-treated KDHCH in HepG2 cells. Food Res. Int. 2019, 121, 336–347. [Google Scholar] [CrossRef]
- Xiang, N.; Lyu, Y.; Zhu, X.; Bhunia, A.K.; Narsimhan, G. Effect of physicochemical properties of peptides from soy protein on their antimicrobial activity. Peptides 2017, 94, 10–18. [Google Scholar] [CrossRef]
- Tavakolipour, H. Extraction and evaluation of gelatin from silver carp waste. World J. Fish Mar. Sci. 2011, 3, 10–15. [Google Scholar]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Hu, F.Y.; Wang, Y.M.; Zhang, B.; Deng, S.G.; Wu, C.W. Purification and identification of three novel antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) skin. Food Res. Int. 2015, 73, 124–129. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Bukowski, M.; Mak, P. Identification of antioxidant peptides in enzymatic hydrolysates of carp (Cyprinus carpio) skin gelatin. Molecules 2019, 24, 97. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, Y.; Li, L. Relationship between primary structure or spatial conformation and functional activity of antioxidant peptides from Pinctada fucata. Food Chem. 2018, 264, 108–117. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, L.; Guo, X.; Qi, X.; Qian, H. Influence of the degree of hydrolysis (DH) on antioxidant properties and radical-scavenging activities of peanut peptides prepared from fermented peanut meal. Eur. Food Res. Technol. 2011, 232, 941–950. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Zhao, Z. Effect of whey protein hydrolysates with different molecular weight on fatigue induced by swimming exercise in mice. J. Sci. Food Agric. 2014, 94, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Shen, H.; Luo, Y. Antioxidant activity of hydrolysates and peptide fractions derived from porcine hemoglobin. J. Food Sci. Technol. 2011, 48, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Bougatef, A.; Hajji, M.; Balti, R.; Lassoued, I.; Triki-Ellouz, Y.; Nasri, M. Antioxidant and free radical-scavenging activities of smooth hound (Mustelus mustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chem. 2009, 114, 1198–1205. [Google Scholar] [CrossRef]
- Laakso, S. Inhibition of lipid peroxidation by casein Evidence of molecular encapsulation of 1, 4-pentadiene fatty acids. Biochim. Et Biophys. Acta (BBA)-Lipids Lipid Metab. 1984, 792, 11–15. [Google Scholar] [CrossRef]
- Leung, C.H.; Zhang, J.T.; Yang, G.J.; Liu, H.; Han, Q.B.; Ma, D.L. Emerging screening approaches in the development of Nrf2-Keap1 protein-protein interaction inhibitors. Int. J. Mol. Sci. 2019, 20, 4445. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.; Nie, S.; Ding, L.; Wang, L.; Liu, J.; Liu, W.; Zhang, T. Direct inhibition of Keap1-Nrf2 interaction by egg-derived peptides DKK and DDW revealed by MD and fluorescence polarization. RSC Adv. 2017, 7, 34963–34971. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Purification, identification and characterization of two novel antioxidant peptides from finger millet (Eleusine coracana) protein hydrolysate. Food Res. Int. 2019, 120, 697–707. [Google Scholar] [CrossRef]
- Tonolo, F.; Fiorese, F.; Moretto, L.; Folda, A.; Scalcon, V.; Grinzato, A.; Ferro, S.; Arrigoni, G.; Bindoli, A.; Feller, E.; et al. Identification of new peptides from fermented milk showing antioxidant properties: Mechanism of action. Antioxidants 2020, 9, 117. [Google Scholar] [CrossRef]
- Tonolo, F.; Moretto, L.; Grinzato, A.; Fiorese, F.; Folda, A.; Scalcon, V.; Ferro, S.; Arrigoni, G.; Bellamio, M.; Feller, E.; et al. Fomented soy-derived bioactive peptides selected by a molecular docking approach show antioxidant properties involving the Keap1/Nrf2 pathway. Antioxidants 2020, 9, 1306. [Google Scholar] [CrossRef]
- Ngoh, Y.; Gan, C. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Miguel, M.; Bartolome, B.; López-Fandiño, R. Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis. J. Food Prot. 2004, 67, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, K.; Ukeda, H.; Ochi, H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J. Nutr. Biochem. 2000, 11, 128–131. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Ren, X.J.; Deng, S.G.; Wu, C.W. Purification and characterization of three antioxidant peptides from protein hydrolyzate of croceine croaker (Pseudosciaena crocea) muscle. Food Chem. 2015, 168, 662–667. [Google Scholar] [CrossRef]
- Saito, K.; Jin, D.H.; Ogawa, T.; Muramoto, K.; Hatakeyama, E.; Yasuhara, T.; Nokihara, K. Antioxidative properties of tripeptide libraries prepared by the combinatorial chemistry. J. Agric. Food Chem. 2003, 51, 3668–3674. [Google Scholar] [CrossRef]
- Tamburro, A.M.; Bochicchio, B.; Pepe, A. Dissection of human tropoelastin: Exon-by-exon chemical synthesis and related conformational studies. Biochemistry 2003, 42, 13347–13362. [Google Scholar] [CrossRef]
- Yang, R.; Wang, J.; Lin, S.; Ye, H.; Chen, F. In vitro antioxidant activities of the novel pentapeptides Ser-His-Glu-Cys-Asn and Leu-Pro-Phe-Ala-Met and the relationship between activity and peptide secondary structure. J. Sci. Food Agric. 2017, 97, 1945–1952. [Google Scholar] [CrossRef]
- Liang, R.; Zhang, Z.; Lin, S. Effects of pulsed electric field on intracellular antioxidant activity and antioxidant enzyme regulating capacities of pine nut (Pinus koraiensis) peptide QDHCH in HepG2 cells. Food Chem. 2017, 237, 793–802. [Google Scholar] [CrossRef]
- Liang, R.; Cheng, S.; Lin, S.; Dong, Y.; Ju, H. Validation of Steric Configuration Changes Induced by a Pulsed Electric Field Treatment as the Mechanism for the Antioxidant Activity Enhancement of a Peptide. Food Bioprocess Technol. 2021, 14, 1751–1757. [Google Scholar] [CrossRef]
- Chen, H.M.; Muramoto, K.; Yamauchi, F.; Nokihara, K. Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J. Agric. Food Chem. 1996, 9, 44. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.; Huang, Y.; Wu, X.; Hou, R.; Wang, H.; Wang, N.; Zhang, X. Protection of small molecule corn peptide Leu-Asp-Tyr-Glu from mitochondria against oxidative damage. Chem. J. Chin. Univ. 2004, 25, 1073–1075. Available online: http://www.cjcu.jlu.edu.cn/EN/Y2004/V25/I6/1073 (accessed on 6 June 2023).
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D. The Nrf2-Keap1-ARE signaling pathway: The regulation and dual function of Nrf2 in cancer. Antioxid. Redox Signal. 2010, 13, 1623. [Google Scholar] [CrossRef] [PubMed]
- Awuh, J.A.; Haug, M.; Mildenberger, J.; Marstad, A.; Do, C.P.N.; Louet, C.; Stenvik, J.; Steigedal, M.; Damås, J.K.; Halaas, Ø. Keap1 regulates inflammatory signaling in Mycobacterium avium-infected human macrophages. Proc. Natl. Acad. Sci. USA 2015, 112, 4272–4280. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, M.B.Y.; Frušić-Zlotkin, M.; Soroka, Y.; Sasson, S.B.; Bianco-Peled, H.; Bitton, R.; Kohen, R. Nitroxide delivery system for Nrf2 activation and skin protection. Eur. J. Pharm. Biopharm. 2015, 94, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Mcmahon, M.; Chowdhry, S.; Dinkova-Kostova, A.T. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid. Redox Signal. 2010, 13, 1713–1748. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, Y.; Shuian, D.; Liu, J.; Zhao, W. Identification and molecular mechanism of novel immunomodulatory peptides from gelatin hydrolysates: Molecular docking, dynamic simulation, and cell experiments. J. Agric. Food Chem. 2023, 71, 2924–2934. [Google Scholar] [CrossRef]
- Provencher, S.W.; Gloeckner, J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry 1981, 20, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Kozics, K.; Klusová, V.; Srancíková, A.; Mucaji, P.; Slamenová, D.; Hunáková, L.; Kusznierewicz, B.; Horváthová, E. Effects of Salvia officinalis and Thymus vulgaris on oxidant-induced DNA damage and antioxidant status in HepG2 cells. Food Chem. 2013, 141, 2198–2206. [Google Scholar] [CrossRef]
- Yu, Z.; Kan, R.; Ji, H.; Wu, S.; Zhao, W.; Shuian, D.; Liu, J.; Li, J. Identification of tuna protein-derived peptides as potent SARSCoV-2 inhibitors via molecular docking and molecular dynamic simulation. Food Chem. 2021, 342, 128366. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Lill, M.A.; Danielson, M.L. Computer-aided drug design platform using PyMOL. J. Comput.-Aided Mol. Des. 2011, 25, 13–19. [Google Scholar] [CrossRef]
- Spoel, D.V.D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
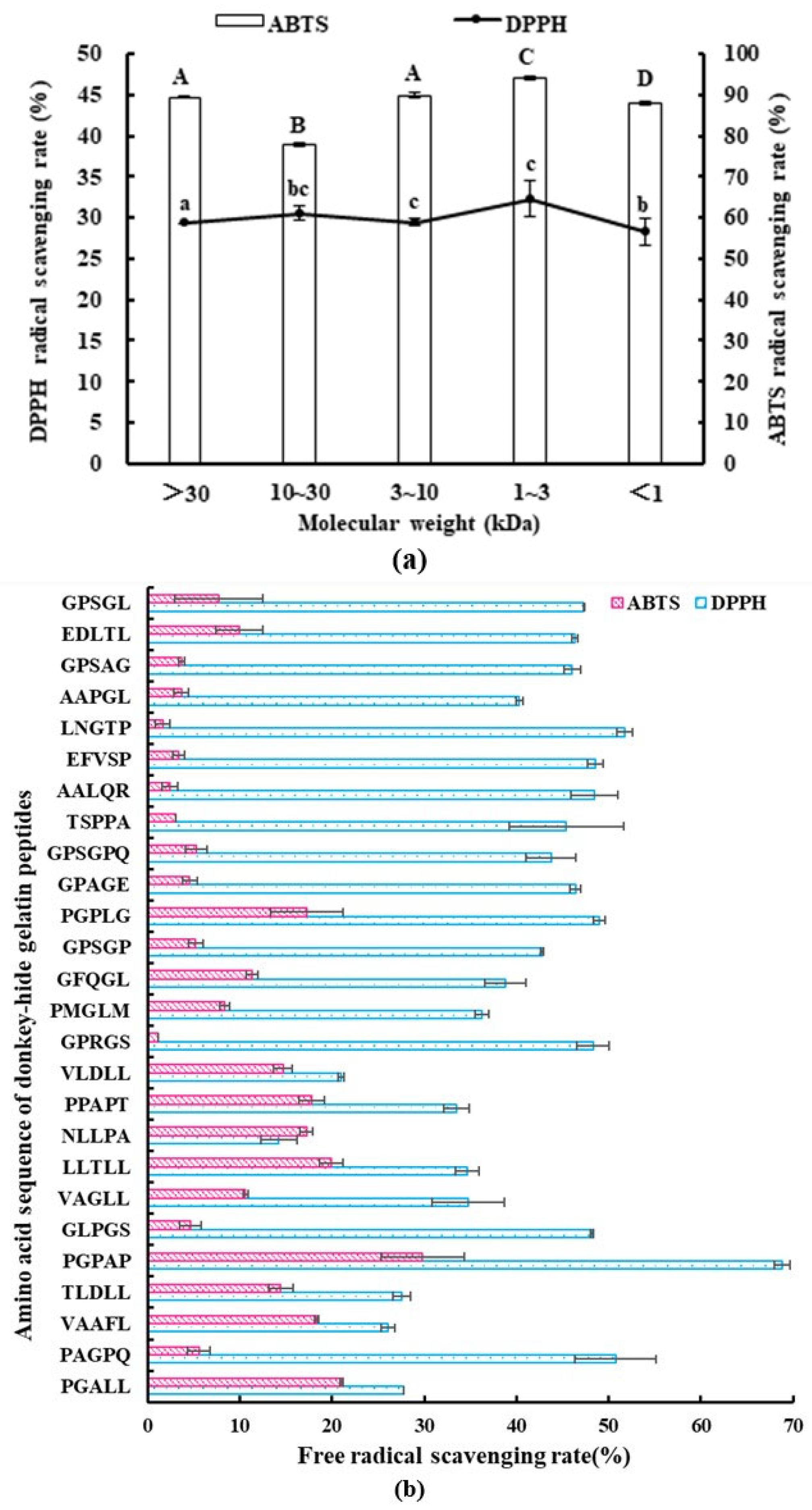





| (a) | ||||||
| No. | Amino Acid Type | Amino Acid Content of Donkey-Hide Gelatin Peptides (mg/g) | ||||
| >30 kDa | 10–30 kDa | 3–10 kDa | 1–3 kDa | <1 kDa | ||
| 1 | Asn (N) | 73.189 | 63.137 | 61.07 | 60.278 | 50.186 |
| 2 | Thr (T) | 29.513 | 25.766 | 27.857 | 26.285 | 23.272 |
| 3 | Ser (S) | 39.896 | 34.747 | 34.871 | 35.864 | 33.833 |
| 4 | Glu (E) | 107.354 | 90.651 | 86.697 | 88.686 | 78.885 |
| 5 | Gly (G) | 128.919 | 118.312 | 124.697 | 129.384 | 115.45 |
| 6 | Ala (A) | 63.607 | 60.372 | 56.137 | 59.293 | 59.261 |
| 7 | Cys (C) | 11.416 | 11.579 | 15.318 | 14.41 | 9.948 |
| 8 | Val (V) | 25.303 | 23.089 | 21.879 | 22.12 | 21.671 |
| 9 | Met (M) | 23.675 | 22.583 | 20.419 | 21.044 | 20.839 |
| 10 | Ile (I) | 32.001 | 31.48 | 33.731 | 35.121 | 29.606 |
| 11 | Leu (L) | 36.751 | 33.759 | 32.873 | 35.278 | 32.613 |
| 12 | Tyr (Y) | 16.381 | 13.858 | 14.759 | 15.526 | 12.439 |
| 13 | Phe (F) | 28.169 | 26.427 | 26.334 | 25.897 | 24.547 |
| 14 | His (H) | 10.438 | 9.922 | 9.249 | 9.64 | 8.856 |
| 15 | Lys (K) | 43.891 | 41.852 | 39.377 | 39.551 | 39.326 |
| 16 | Arg (R) | 60.535 | 55.137 | 44.898 | 51.678 | 50.074 |
| 17 | Pro (P) | 113.935 | 96.871 | 92.763 | 95.652 | 96.794 |
| (b) | ||||||
| No. | Amino Acid Sequence | Leading Razor Protein | Protein Names | Gene Names | Score | |
| 1 | GPSGL | A0A8C4MG85 | Obscurin, cytoskeletal calmodulin and titin-interacting RhoGEF | OBSCN | 61.28 | |
| 2 | EDLTL | 57.71 | ||||
| 3 | GPSAG | 52.86 | ||||
| 4 | AAPGL | 25.12 | ||||
| 5 | LNGTP | 25.08 | ||||
| 6 | EFVSP | 24.47 | ||||
| 7 | AALQR | 24.30 | ||||
| 8 | TSPPA | 20.54 | ||||
| 9 | GPSGPQ | B9VR89 | Collagen alpha-2 type I chain | COL1A2 | 162.47 | |
| 10 | GPAGE | 63.06 | ||||
| 11 | PGPLG | 55.36 | ||||
| 12 | GPSGP | 50.40 | ||||
| 13 | GFQGL | 32.63 | ||||
| 14 | PMGLM | 26.57 | ||||
| 15 | GPRGS | 22.12 | ||||
| 16 | VLDLL | A0A8C4MF50 | Ubiquitin protein ligase E3 component n-recognin 4 | UBR4 | 43.99 | |
| 17 | PPAPT | 38.19 | ||||
| 18 | NLLPA | 33.54 | ||||
| 19 | LLTLL | 23.49 | ||||
| 20 | VAGLL | 22.99 | ||||
| 21 | GLPGS | A0A8C4L3F1 | SZT2 subunit of KICSTOR complex | SZT2 | 56.75 | |
| 22 | PGPAP | 61.37 | ||||
| 23 | TLDLL | A0A8C4LPB4 | Neurobeachin like 2 | NBEAL2 | 45.11 | |
| 24 | VAAFL | 28.74 | ||||
| 25 | PAGPQ | A0A8C4KVK0 | Dynein heavy chain domain 1 | DNHD1 | 81.36 | |
| 26 | PGALL | A0A8C4MZE5 | Zinc finger homeobox 4 | ZFHX4 | 25.12 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, R.; Xu, L.; Fan, C.; Cao, L.; Guo, X. Structural Characteristics and Antioxidant Mechanism of Donkey-Hide Gelatin Peptides by Molecular Dynamics Simulation. Molecules 2023, 28, 7975. https://doi.org/10.3390/molecules28247975
Liang R, Xu L, Fan C, Cao L, Guo X. Structural Characteristics and Antioxidant Mechanism of Donkey-Hide Gelatin Peptides by Molecular Dynamics Simulation. Molecules. 2023; 28(24):7975. https://doi.org/10.3390/molecules28247975
Chicago/Turabian StyleLiang, Rong, Le Xu, Chen Fan, Lele Cao, and Xingfeng Guo. 2023. "Structural Characteristics and Antioxidant Mechanism of Donkey-Hide Gelatin Peptides by Molecular Dynamics Simulation" Molecules 28, no. 24: 7975. https://doi.org/10.3390/molecules28247975
APA StyleLiang, R., Xu, L., Fan, C., Cao, L., & Guo, X. (2023). Structural Characteristics and Antioxidant Mechanism of Donkey-Hide Gelatin Peptides by Molecular Dynamics Simulation. Molecules, 28(24), 7975. https://doi.org/10.3390/molecules28247975







