Thermodynamic and Vibrational Aspects of Peptide Bond Hydrolysis and Their Potential Relationship to the Harmfulness of Infrared Radiation
Abstract
:1. Introduction
2. Theory/Calculations
- (1)
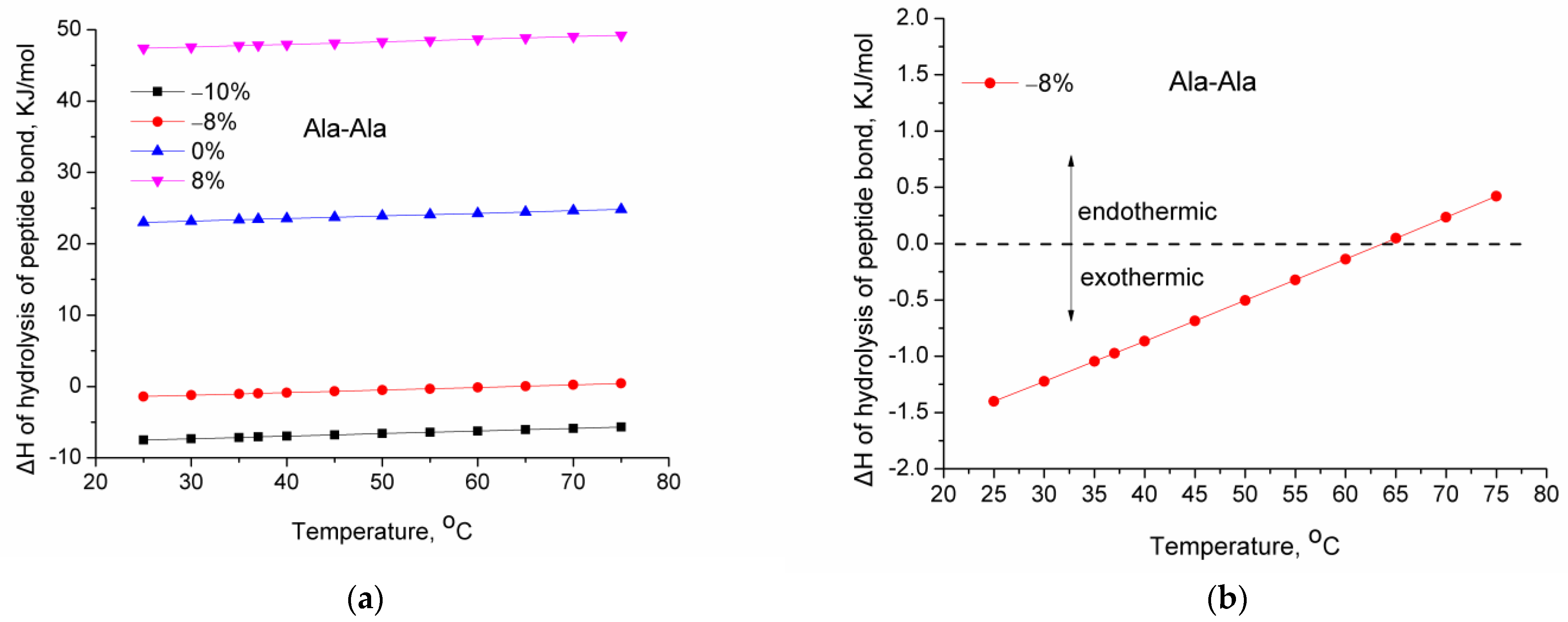
- How does the enthalpy of the hydrolysis reaction change with temperature, and is the hydrolysis reaction favored or not by the increase in temperature?
- (2)
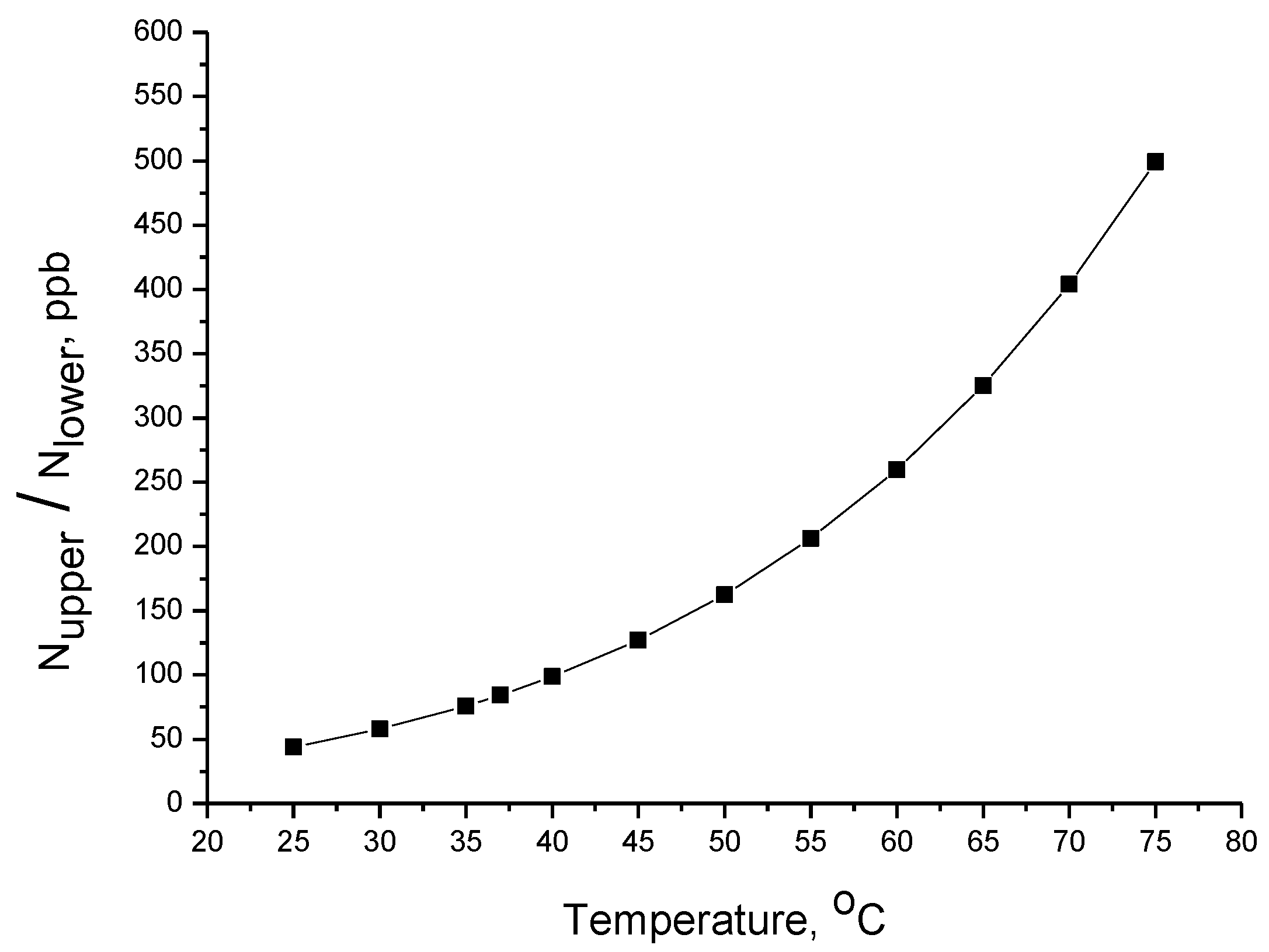
- Can IR radiation provide enough energy to molecules to surpass the activation energy of the hydrolysis reaction?
2.1. Enthalpy of Reaction and Its Variation with Temperature
- : Enthalpy of reaction.
- : Sum of the dissociation energies of the broken bonds.
- : Sum of the dissociation energies of the formed bonds.
- : The stretching frequency in cm−1.
- : The dissociation energy of the bond in Kcal/mol.
- : Constant characteristic of the bond, e.g., 26.7 Kcal/mol for a carbon–nitrogen bond.
- and : Two frequencies of stretching vibration of the same bond in cm−1 corresponding to bonds of different energy and .
- : The difference of the two frequencies () in cm−1.
- : The difference in the bond energy () in Kcal/mol.
- : The difference in the bond energy () in KJ/mol.
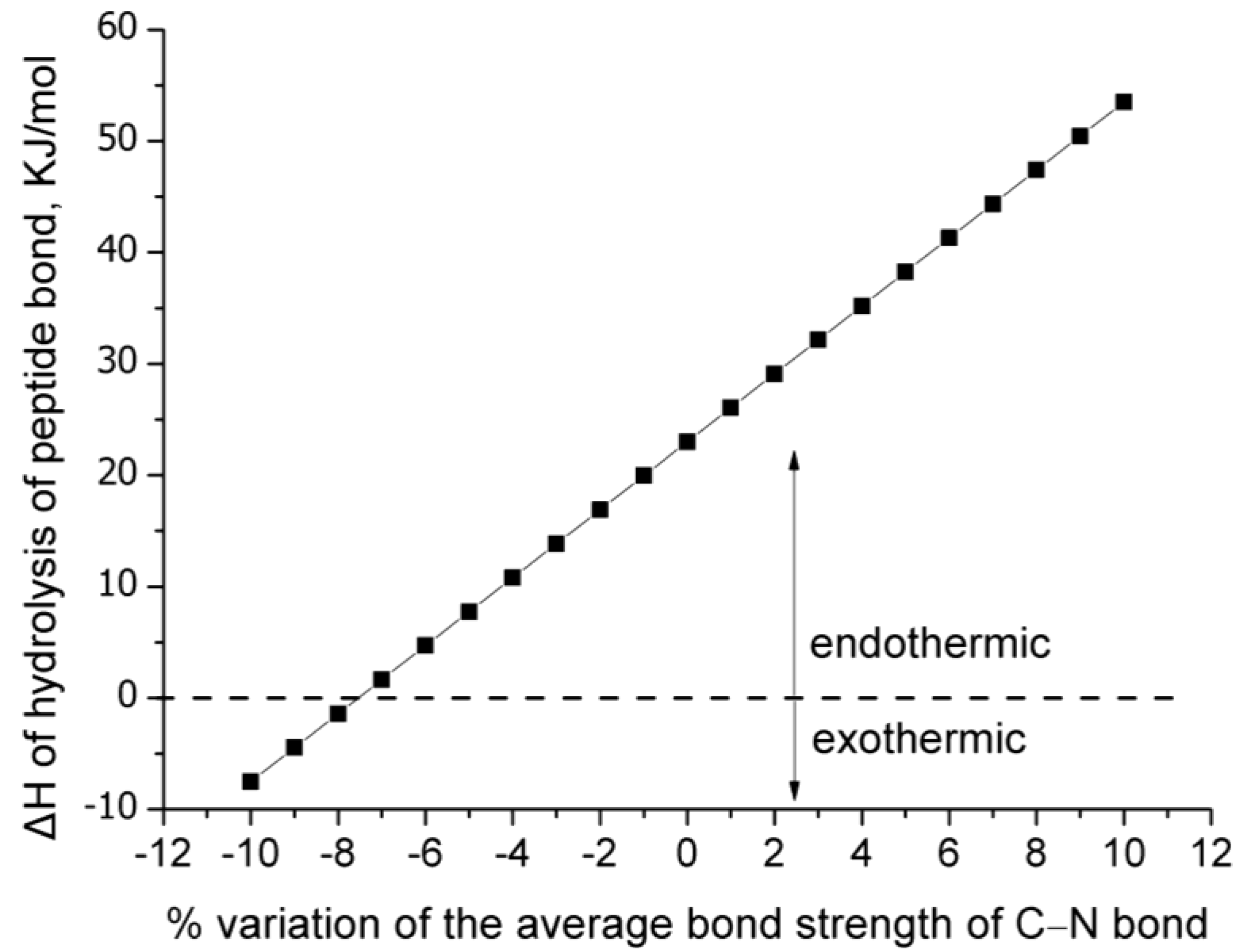
- : The standard enthalpy of reaction at 298 K (25 °C). This value was calculated multiple times from Equation (1) using the average bond strength literature values and by varying by ±10% the average bond strength of the C–N bond.
- : The enthalpy of reaction at temperature T.
- : The difference in the molar heat capacities of the products and the reactants (by considering the stoichiometry of the reaction).
2.2. Aspects Related to Activation Energy
- : Number of molecules in the upper energy state.
- : Number of molecules in the lower energy state.
- : The difference in energy of the two states ().
- : Absolute temperature.
- : Boltzmann’s constant.
3. Results and Discussion
4. Further Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holzer, A.M.; Athar, M.; Elmets, C.A. The Other End of the Rainbow: Infrared and Skin. J. Investig. Dermatol. 2010, 130, 1496–1499. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Krutmann, J. Photoprotection of human skin beyond ultraviolet radiation. Photodermatol. Photoimmunol. Photomed. 2014, 30, 167–174. [Google Scholar] [CrossRef]
- Schroeder, P.; Haendeler, J.; Krutmann, J. The role of near infrared radiation in photoaging of the skin. Exp. Gerontol. 2008, 43, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, J.; Sliney, D. Physical and Biological Hazards of the Workplace; Stave, G., Wald, P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Cho, S.; Shin, M.H.; Kim, Y.K.; Seo, J.-E.; Lee, Y.M.; Park, C.-H.; Chung, J.H. Effects of Infrared Radiation and Heat on Human Skin Aging in vivo. J. Investig. Dermatol. Symp. Proc. 2009, 14, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Schieke, S.M.; Schroeder, P.; Krutmann, J. Cutaneous effects of infrared radiation: From clinical observations to molecular response mechanisms. Photodermatol. Photoimmunol. Photomed. 2003, 19, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Chung, J.H. Thermal aging: A new concept of skin aging. J. Dermatol. Sci. Suppl. 2006, 2, S13–S22. [Google Scholar] [CrossRef]
- White, R.H. Hydrolytic stability of biomolecules at high temperatures and its implication for life at 250 °C. Nature 1984, 310, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, P.; Lademann, J.; Darvin, M.E.; Stege, H.; Marks, C.; Bruhnke, S.; Krutmann, J. Infrared Radiation-Induced Matrix Metalloproteinase in Human Skin: Implications for Protection. J. Investig. Dermatol. 2008, 128, 2491–2497. [Google Scholar] [CrossRef]
- Schieke, S.M.; Stege, H.; Kürten, V.; Grether-Beck, S.; Sies, H.; Krutmann, J. Infrared-A Radiation-Induced Matrix Metalloproteinase 1 Expression is Mediated Through Extracellular Signal-regulated Kinase 1/2 Activation in Human Dermal Fibroblasts. J. Investig. Dermatol. 2002, 119, 1323–1329. [Google Scholar] [CrossRef]
- Zumdahl, S.S.; Zumdahl, S.A.; DeCoste, D.J. Chemistry; Cengage Learning: Boston, MA, USA, 2018. [Google Scholar]
- Cremer, D.; Wu, A.; Larsson, A.; Kraka, E. Some Thoughts about Bond Energies, Bond Lengths, and Force Constants. Mol. Model. Annu. 2000, 6, 396–412. [Google Scholar] [CrossRef]
- Zavitsas, A.A. Quantitative relationship between bond dissociation energies, infrared stretching frequencies, and force constants in polyatomic molecules. J. Phys. Chem. 1987, 91, 5573–5577. [Google Scholar] [CrossRef]
- Atkins, P.W.; De Paula, J.; Keeler, J. Atkins’ Physical Chemistry, 11th ed.; Oxford University Press: New York, NY, USA, 2018. [Google Scholar]
- Available online: https://webbook.nist.gov/ (accessed on 20 August 2023).
- Badelin, V.G.; Kulikov, O.V.; Vatagin, V.S.; Udzig, E.; Zielenkiewicz, A.; Zielenkiewicz, W.; Krestov, G.A. Physico-chemical properties of peptides and their solutions. Thermochim. Acta 1990, 169, 81–93. [Google Scholar] [CrossRef]
- Colthup, N.; Daly, L.; Wiberley, S. Introduction to Infrared and Raman Spectroscopy, 3rd ed.; Academic Press Inc.: San Diego, CA, USA, 1990. [Google Scholar]
- Banwell, C. Fundamentals of Molecular Spectroscopy; McGraw-Hill Book Company Europe: Berkshire, UK, 1983. [Google Scholar]
- Beć, K.B.; Huck, C.W. Breakthrough Potential in Near-Infrared Spectroscopy: Spectra Simulation. A Review of Recent Developments. Front. Chem. 2019, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Choppin, G.R.; Violante, M.R. Near-Infrared Studies of the Structure of Water. III. Mixed Solvent Systems. J. Chem. Phys. 1972, 56, 5890–5898. [Google Scholar] [CrossRef]
- Rawitscher, M.; Wadsö, I.; Sturtevant, J.M. Heats of Hydrolysis of Peptide Bonds. J. Am. Chem. Soc. 1961, 83, 3180–3184. [Google Scholar] [CrossRef]
- Dobry, A.; Sturtevant, J.M. Heats of Hydrolysis of Amide and Peptide Bonds. J. Biol. Chem. 1952, 195, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Rekharsky, M.V.; Nemykina, E.V.; Erokhin, A.S. Thermochemistry of N-C bonds hydrolysis in amides, peptides, N-acetyl amino acids and high-energy N-C bonds hydrolysis in N-acetyl imidazole and urea. Int. J. Biochem. 1992, 24, 861–867. [Google Scholar] [CrossRef]
- Radzicka, A.; Wolfenden, R. Rates of Uncatalyzed Peptide Bond Hydrolysis in Neutral Solution and the Transition State Affinities of Proteases. J. Am. Chem. Soc. 1996, 118, 6105–6109. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Jiao, Y.; Cao, F.; Liu, H. Radiation-induced Cell Death and Its Mechanisms. Health Phys. 2022, 123, 376–386. [Google Scholar] [CrossRef]
- Temenoff, J.; Mikos, A. Biomaterials: The Intersection of Biology and Materials Science; Pearson Education Inc.: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Cherstvy, A.G.; Kornyshev, A.A. DNA Melting in Aggregates: Impeded or Facilitated? J. Phys. Chem. B 2005, 109, 13024–13029. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, F.; Pietrini, A.; Longo, M.; Comez, L.; Petrillo, C.; Sacchetti, F.; Paciaroni, A. Melting of DNA Nonoriented Fibers: A Wide-Angle X-ray Diffraction Study. J. Phys. Chem. B 2014, 118, 3785–3792. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, N. DNA melting in the presence of molecular crowders. PCCP 2017, 19, 19452–19460. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsioptsias, C. Thermodynamic and Vibrational Aspects of Peptide Bond Hydrolysis and Their Potential Relationship to the Harmfulness of Infrared Radiation. Molecules 2023, 28, 7902. https://doi.org/10.3390/molecules28237902
Tsioptsias C. Thermodynamic and Vibrational Aspects of Peptide Bond Hydrolysis and Their Potential Relationship to the Harmfulness of Infrared Radiation. Molecules. 2023; 28(23):7902. https://doi.org/10.3390/molecules28237902
Chicago/Turabian StyleTsioptsias, Costas. 2023. "Thermodynamic and Vibrational Aspects of Peptide Bond Hydrolysis and Their Potential Relationship to the Harmfulness of Infrared Radiation" Molecules 28, no. 23: 7902. https://doi.org/10.3390/molecules28237902
APA StyleTsioptsias, C. (2023). Thermodynamic and Vibrational Aspects of Peptide Bond Hydrolysis and Their Potential Relationship to the Harmfulness of Infrared Radiation. Molecules, 28(23), 7902. https://doi.org/10.3390/molecules28237902






