Versatile Application of TiO2@PDA Modified Filter Paper for Oily Wastewater Treatment
Abstract
:1. Introduction
2. Results
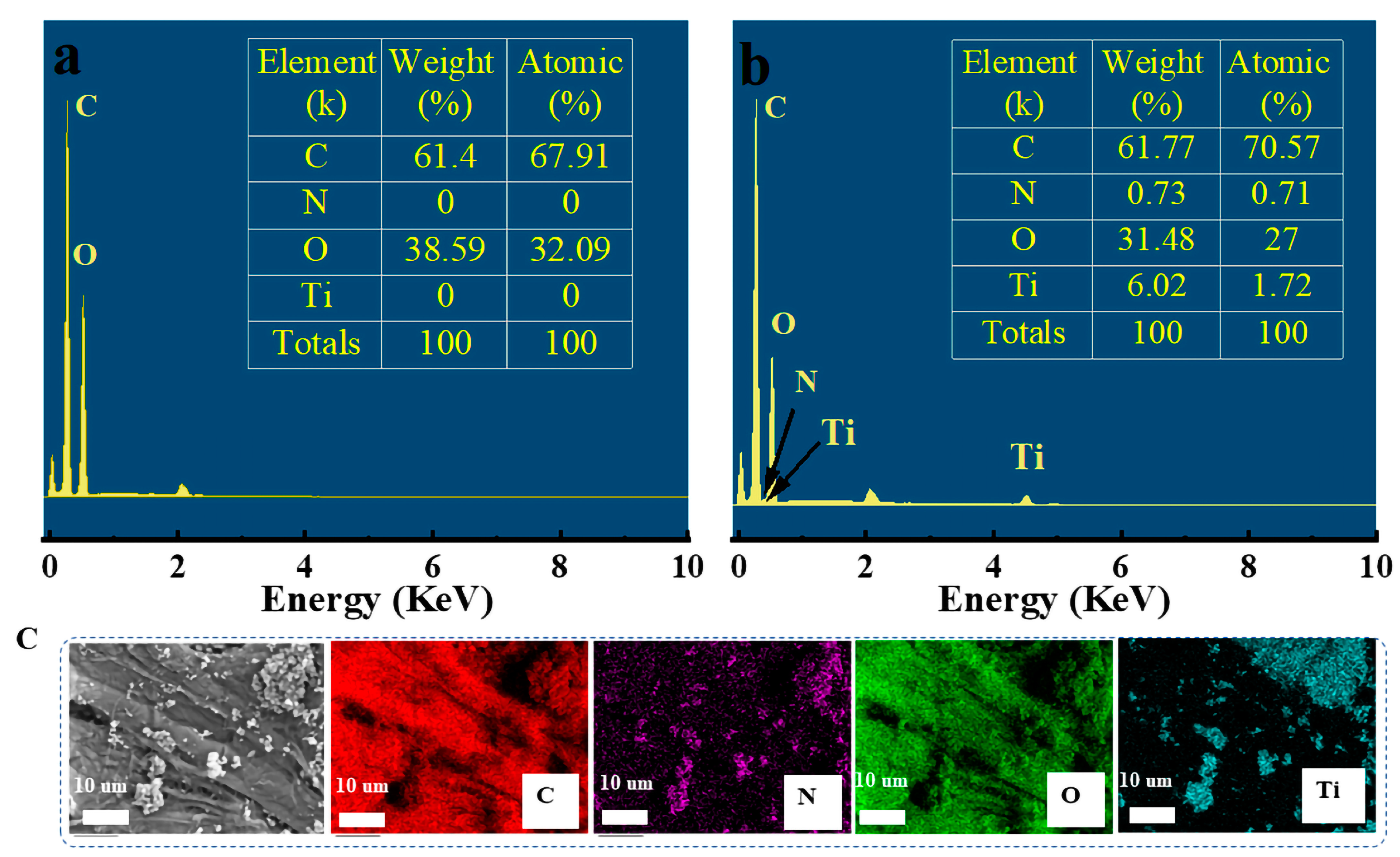
2.1. Morphology Characterization
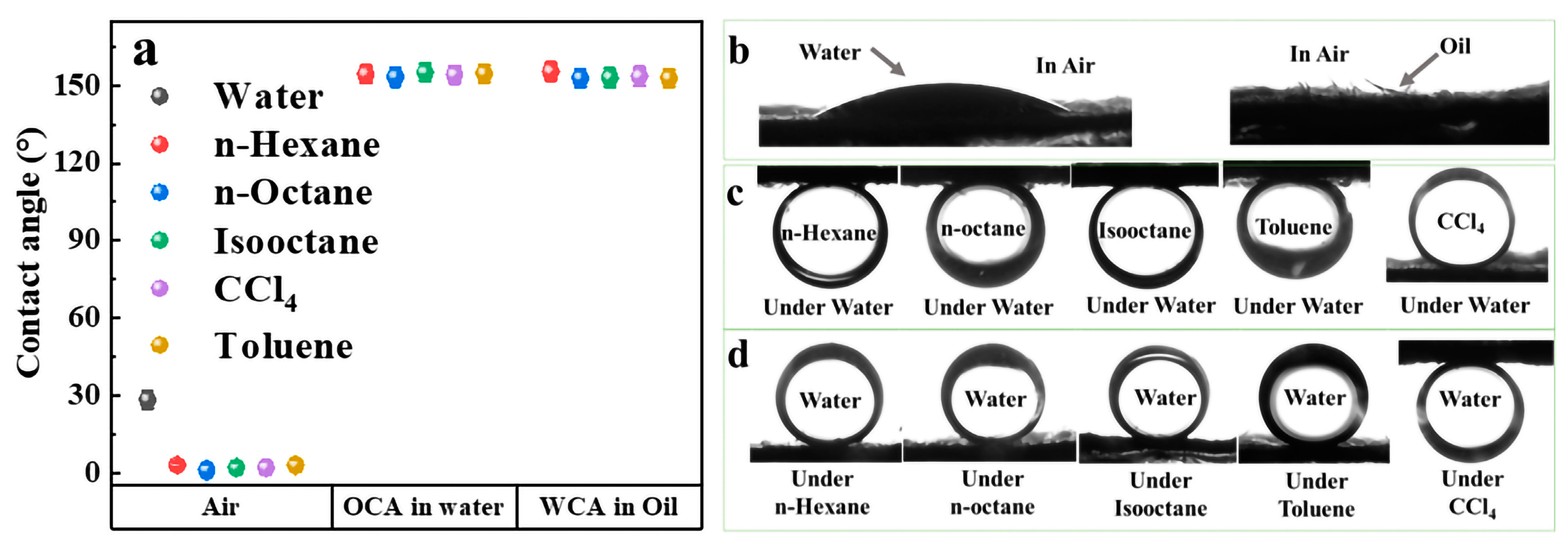
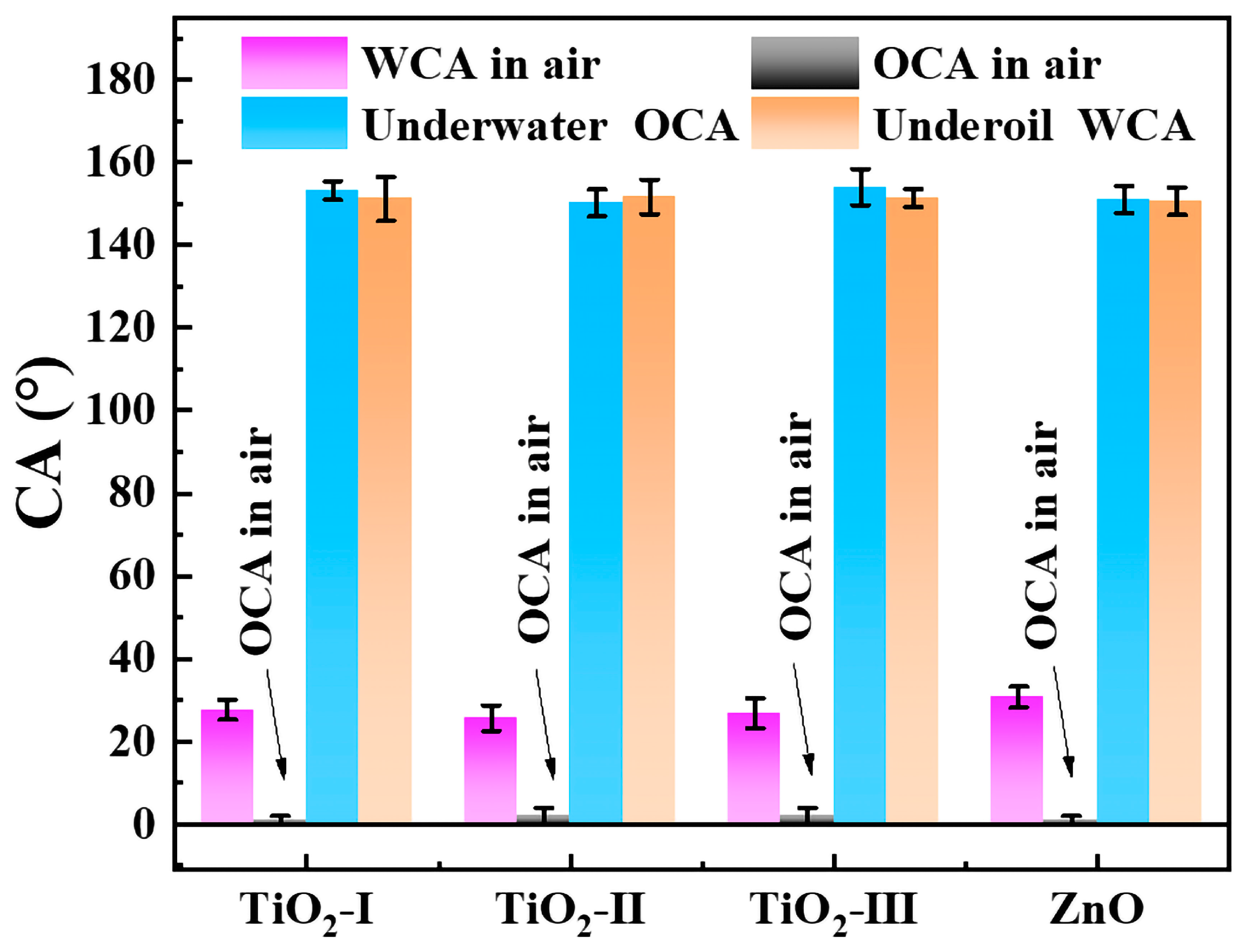
2.2. Wettability
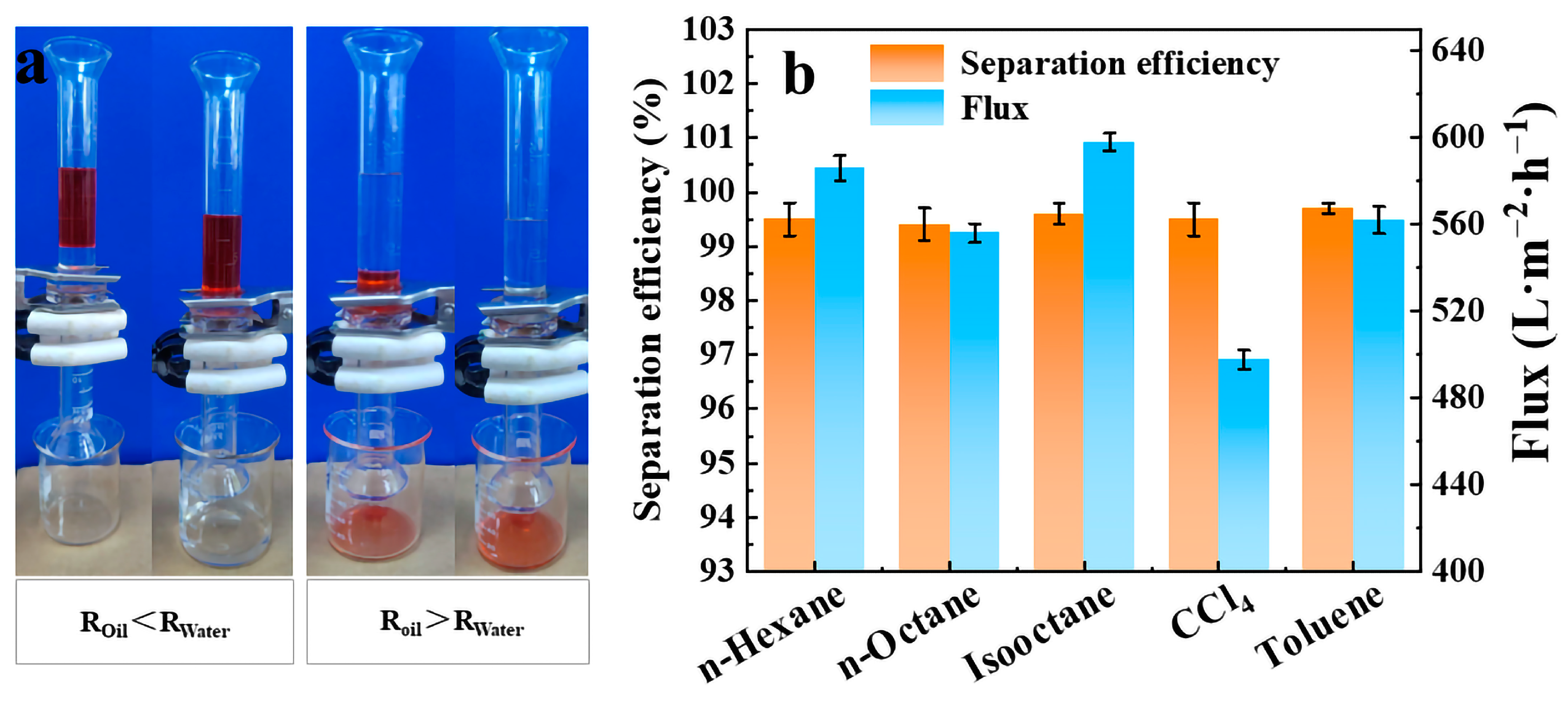
2.3. Oil/Water Separation
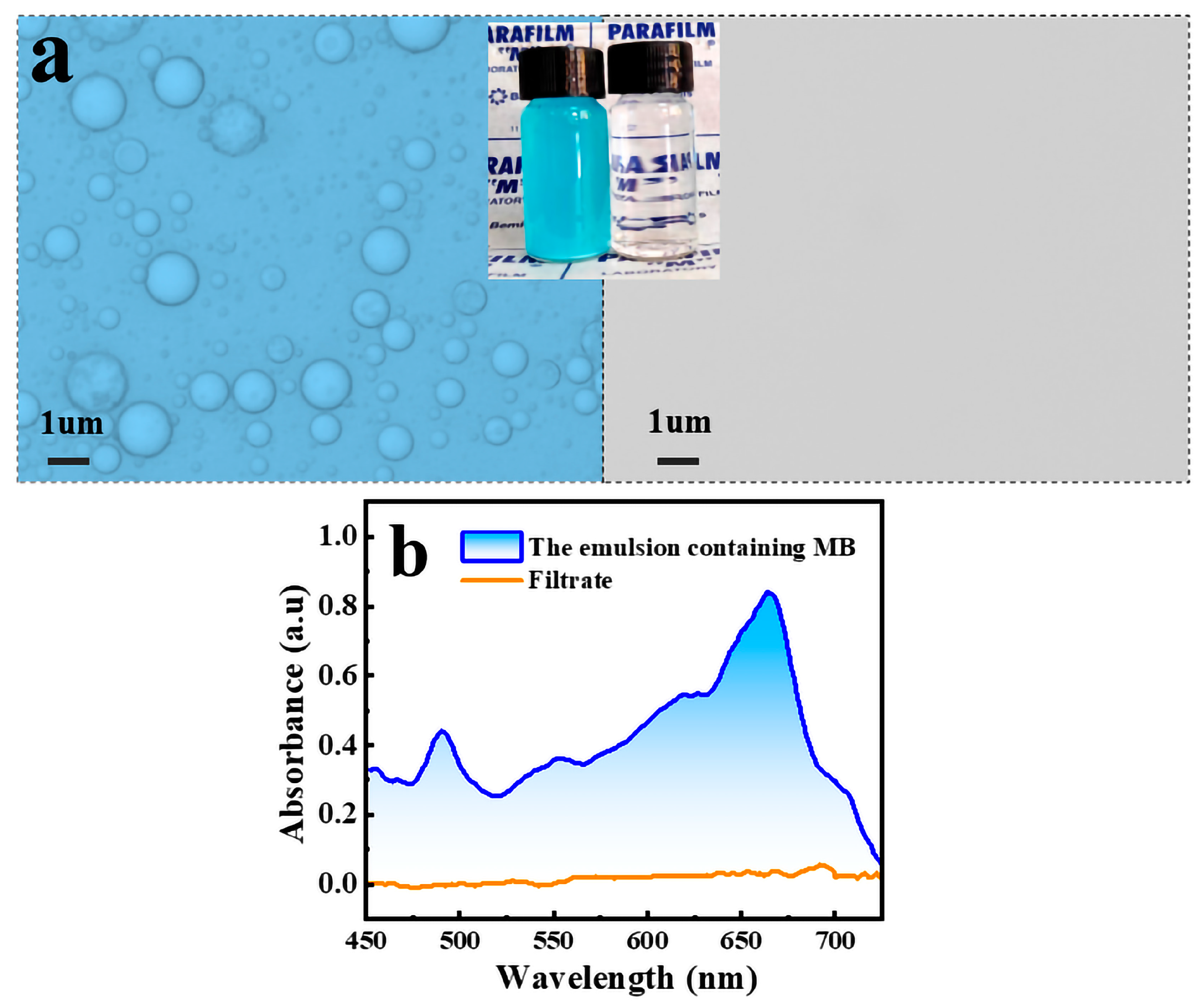
2.4. Emulsion Separation
2.5. In Situ Separation and Purification of Contaminated Emulsion
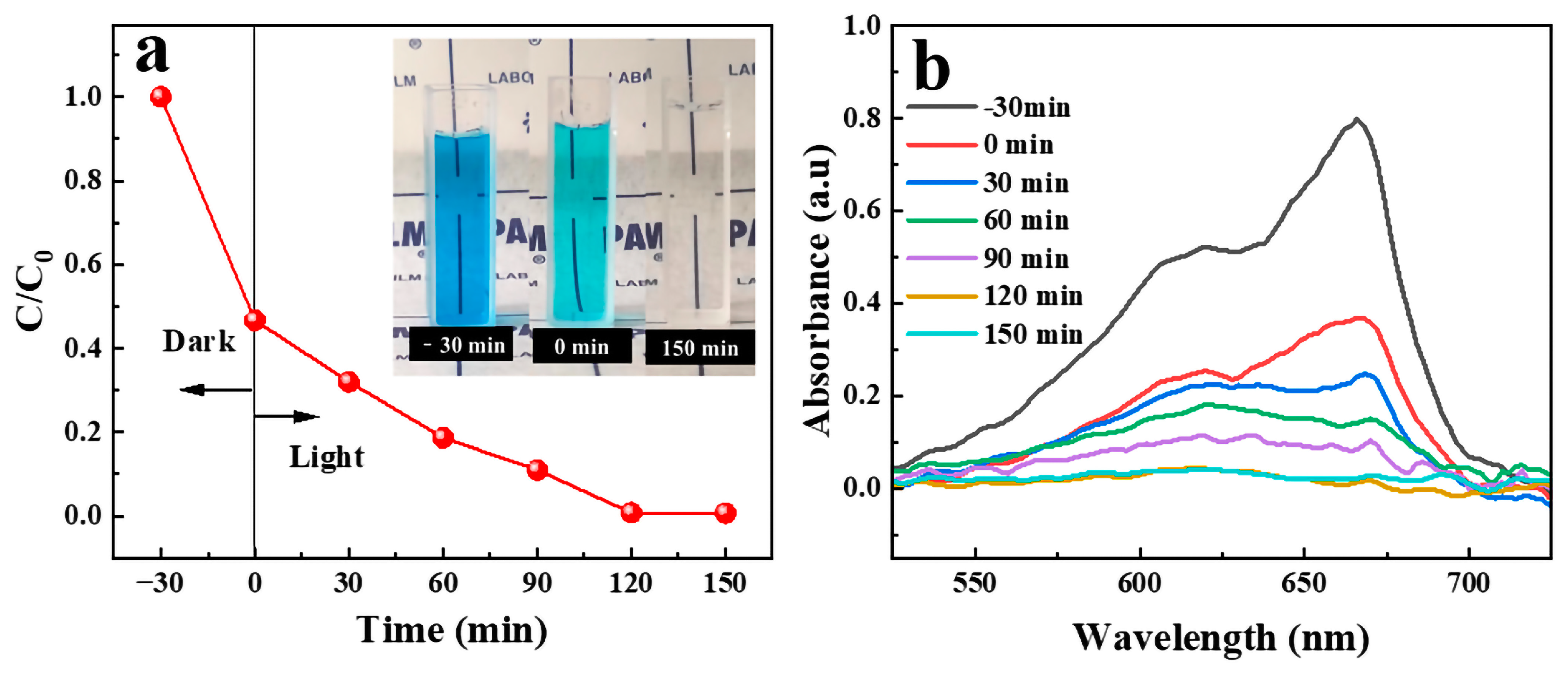
2.6. Degradation of Soluble Contaminant under UV Irradiation
2.7. Generalization of the Developed Method
3. Experimental Section
3.1. Materials
3.2. Preparation of Multifunctional FP
3.3. Oil/Water Separation
3.4. Emulsion Separation
3.5. Water Purification
3.6. Photodegradation
3.7. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, Y.; Wei, P.; Li, F.; Liu, L.; Zhao, X. Liquid-assisted strategy for dual-purpose oil-water separation with super-omniphobic mesh. Chem. Eng. J. 2023, 475, 146094. [Google Scholar] [CrossRef]
- Lincy, V.; Prasannan, A.; Hong, P.-D. Rational design of multifunctional membrane material with underwater superoleophobicity for dye contaminated emulsion separation. J. Membr. Sci. 2021, 639, 119716. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Xing, H.; Fu, Q.; Niu, C.; Lu, L. Advances in Asymmetric Wettable Janus Materials for Oil–Water Separation. Molecules 2022, 27, 7470. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Lou, Y.; Jiang, S.; Fang, G.; Wu, W. Robust paper-based materials for efficient oil–water emulsion separation. Cellulose 2021, 28, 10565–10578. [Google Scholar] [CrossRef]
- Deng, Y.; Bian, H.; Dai, M.; Liu, X.; Peng, C. Underwater superoleophobic HKUST-1/PDA@SM membrane with excellent stability and anti-fouling performance for oil-in-water emulsion separation. J. Membr. Sci. 2023, 678, 121655. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and superoleophilic PVDF membranes for effective separation of water-in-oil emulsions with high flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, G.; Lang, D.; Qian, Q.; Yang, C.; Wang, J.; Wu, R.; Wang, W. Partially carbonized wastepaper with excellent mechanical strength for oil-water and emulsion separation. J. Taiwan Inst. Chem. Eng. 2023, 145, 104816. [Google Scholar] [CrossRef]
- Xing, W.; Yan, Y.; Wang, C.; Gao, J.; Yu, C.; Yan, Y.; Li, C.; Ma, Z.; Wu, Y. MOFs self-assembled molecularly imprinted membranes with photoinduced regeneration ability for long-lasting selective separation. Chem. Eng. J. 2022, 437, 135128. [Google Scholar] [CrossRef]
- Safarpour, M.; Hosseinpour, S.; Haddad Irani-nezhad, M.; Orooji, Y.; Khataee, A. Fabrication of Ti2SnC-MAX Phase Blended PES Membranes with Improved Hydrophilicity and Antifouling Properties for Oil/Water Separation. Molecules 2022, 27, 8914. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Zhang, D.; Sun, S.; Liu, J.; Li, B.; Shi, Z. The Preparation of Superhydrophobic Polylactic Acid Membrane with Adjustable Pore Size by Freeze Solidification Phase Separation Method for Oil–Water Separation. Molecules 2023, 28, 5590. [Google Scholar] [CrossRef]
- Mu, Y.; Zhu, K.; Luan, J.; Zhang, S.; Zhang, C.; Na, R.; Yang, Y.; Zhang, X.; Wang, G. Fabrication of hybrid ultrafiltration membranes with improved water separation properties by incorporating environmentally friendly taurine modified hydroxyapatite nanotubes. J. Membr. Sci. 2019, 577, 274–284. [Google Scholar] [CrossRef]
- Zhao, X.; Jia, N.; Cheng, L.; Liu, L.; Gao, C. Dopamine-induced biomimetic mineralization for in situ developing antifouling hybrid membrane. J. Membr. Sci. 2018, 560, 47–57. [Google Scholar] [CrossRef]
- Huang, T.; Cao, S.; Luo, D.; Zhang, N.; Lei, Y.-z.; Wang, Y. Polydopamine-assisted polyethylenimine grafting melamine foam and the application in wastewater purification. Chemosphere 2022, 287, 132054. [Google Scholar] [CrossRef]
- Lin, X.; Chen, Y.; Liu, N.; Cao, Y.; Xu, L.; Zhang, W.; Feng, L. In situ ultrafast separation and purification of oil/water emulsions by superwetting TiO2 nanocluster-based mesh. Nanoscale 2016, 8, 8525–8529. [Google Scholar] [CrossRef]
- Baig, U.; Waheed, A. An efficient and simple strategy for fabricating a polypyrrole decorated ceramic-polymeric porous membrane for purification of a variety of oily wastewater streams. Environ. Res. 2023, 219, 114959. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhan, H.; Li, D.; Tian, H.; Chang, C. Tunicate cellulose nanocrystals modified commercial filter paper for efficient oil/water separation. J. Membr. Sci. 2019, 591, 117362. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, N.; Zhang, Q.; Qu, R.; Liu, Y.; Li, X.; Wei, Y.; Feng, L.; Jiang, L. Thermo-Driven Controllable Emulsion Separation by a Polymer-Decorated Membrane with Switchable Wettability. Angew. Chem. Int. Ed. 2018, 57, 5740–5745. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-F.; Li, P.-P.; Zhang, Y.-P.; Liu, P.-F.; Cui, C.-X.; Wang, J.-C.; Li, X.-J.; Qu, L.-B. Laboratory filter paper from superhydrophobic to quasi-superamphiphobicity: Facile fabrication, simplified patterning and smart application. Cellulose 2019, 26, 3859–3872. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Wang, N.; Chen, D.-L.; Chen, Y.; Chen, M.-J.; Chen, X.-X. Smart Superhydrophobic Filter Paper for Water/Oil Separation and Unidirectional Transportation of Liquid Droplet. Membranes 2022, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Peng, H.; Wu, J.; Wang, Y.; Wang, H.; Liu, Z.; Guo, X. Fabrication of superhydrophobic cellulose/chitosan composite aerogel for oil/water separation. Fibers Polym. 2017, 18, 706–712. [Google Scholar] [CrossRef]
- Xu, X.; Long, Y.; Li, Q.; Li, D.; Mao, D.; Chen, X.; Chen, Y. Modified cellulose membrane with good durability for effective oil-in-water emulsion treatment. J. Clean. Prod. 2019, 211, 1463–1470. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Q. Facile preparation of water-proof paper with tunable surface properties for water/oil separation. Appl. Surf. Sci. 2021, 567, 150738. [Google Scholar] [CrossRef]
- Ren, J.; Tao, F.; Liu, L.; Wang, X.; Cui, Y. A novel TiO2@stearic acid/chitosan coating with reversible wettability for controllable oil/water and emulsions separation. Carbohydr. Polym. 2020, 232, 115807. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, Y.; Yu, F.; Zhang, H. In-situ construction of MOFs-based superhydrophobic/superoleophilic coating on filter paper with self-cleaning and antibacterial activity for efficient oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126976. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhang, L.; Zhang, M.; He, G.; Sun, Z. Facile fabrication of a low adhesion, stable and superhydrophobic filter paper modified with ZnO microclusters. Appl. Surf. Sci. 2019, 496, 143743. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, Z.; Wang, J.; Wang, P.; Li, X.; Long, R.; Wang, Q. Chemically stable NH2-MIL-125(Ti)/Sep/PDA composite membranes with high-efficiency for oil/water emulsions separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 646, 128899. [Google Scholar] [CrossRef]
- Chen, G.; Chen, S.; Zhang, X.; Yang, F.; Fu, J. Twofold bioinspiration of TiO2-PDA hybrid fabrics with desirable robustness and remarkable polar/nonpolar liquid separation performance. Front. Mater. Sci. 2021, 15, 124–137. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Pan, G.; Li, B. A dynamic biointerface controls mussel adhesion. Science 2023, 382, 763–764. [Google Scholar] [CrossRef]
- Du, J.; Zhou, C.; Yang, Z.; Cheng, J.; Shen, Y.; Zeng, X.; Tan, L.; Dong, L. Conversion of solid Cu2(OH)2CO3 into HKUST-1 metal-organic frameworks: Toward an under-liquid superamphiphobic surface. Surf. Coat. Technol. 2019, 363, 282–290. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Liu, S.; Zhang, Y.; Du, Z.; Qu, L. Bio-inspired fabrication of fire-retarding, magnetic-responsive, superhydrophobic sponges for oil and organics collection. Appl. Clay Sci. 2019, 172, 19–27. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Dual-channel separation system based on platanus fruit-like Ni@Ni(OH) hierarchical architecture for fast, efficient and continuous light/heavy oil–water separation. J. Ind. Eng. Chem. 2019, 74, 208–215. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Liu, S.; Zhang, Y.; Qu, L. Fabrication of superhydrophobic marigold shape LDH films on stainless steel meshes via in-situ growth for enhanced anti-corrosion and high efficiency oil-water separation. Appl. Clay Sci. 2019, 182, 105292. [Google Scholar] [CrossRef]
- Xiong, L.; Yang, Y.; Mai, J.; Sun, W.; Zhang, C.; Wei, D.; Chen, Q.; Ni, J. Adsorption behavior of methylene blue onto titanate nanotubes. Chem. Eng. J. 2010, 156, 313–320. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Dai, L.; Si, C.-L. Mussel-Inspired Cellulose-Based Nanocomposite Fibers for Adsorption and Photocatalytic Degradation. ACS Sustain. Chem. Eng. 2018, 6, 15756–15763. [Google Scholar]
- Baosheng, R.; Rui, W.; Jinzhi, R.; Jianquan, L.; Qifeng, C. Preparation and photocatalytic properties of nano TiO2/carbonized plant fiber composites. Acta Mater. Compos. Sin. 2020, 37, 1138–1147. [Google Scholar]
- Serpone, N.; Emeline, A.V.; Kuznetsov, V.N.; Ryabchuk, V.K. Second Generation Visible-Light-Active Photocatalysts: Preparation, Optical Properties, and Consequences of Dopants on the Band Gap Energy of TiO2. In Environmentally Benign Photocatalysts; Springer: New York, NY, USA, 2010; pp. 35–111. [Google Scholar]
- Luque, P.A.; Garrafa-Gálvez, H.E.; Nava, O.; Olivas, A.; Martínez-Rosas, M.E.; Vilchis-Nestor, A.R.; Villegas-Fuentes, A.; Chinchillas-Chinchillas, M.J. Efficient sunlight and UV photocatalytic degradation of Methyl Orange, Methylene Blue and Rhodamine B, using Citrus×paradisi synthesized SnO2 semiconductor nanoparticles. Ceram. Int. 2021, 47, 23861–23874. [Google Scholar] [CrossRef]
- Choudhary, K.; Saini, R.; Purohit, L.P. Controllable synthesis of Ce-doped ZnO: TiO2 nanospheres for photocatalytic degradation of MB dye and levofloxacin under sunlight light irradiation. Opt. Mater. 2023, 143, 114167. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Mersal, G.A.M.; Zuo, J.; Li, J.; Wang, Q.; Feng, Y.; Liu, J.; Liu, Z.; Wang, B.; et al. “Several birds with one stone” strategy of pH/thermoresponsive flame-retardant/photothermal bactericidal oil-absorbing material for recovering complex spilled oil. J. Mater. Sci. Technol. 2022, 128, 82–97. [Google Scholar] [CrossRef]
- Chen, X.-X.; Zhang, Y.-P.; Du, H.-L.; Wan, L.; Zuo, C.-G.; Qu, L.-B. “Two birds with one stone” strategy for oil/water separation based on Ni foams assembled using metal–organic frameworks. New J. Chem. 2023, 47, 14289–14296. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Yang, J.-H.; Li, L.-L.; Cui, C.-X.; Li, Y.; Liu, S.-Q.; Zhou, X.-M.; Qu, L.-B. Facile Fabrication of Superhydrophobic Copper- Foam and Electrospinning Polystyrene Fiber for Combinational Oil–Water Separation. Polymers 2019, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhao, X.; Jia, N.; Cheng, L.; Liu, L.; Gao, C. Superwetting Oil/Water Separation Membrane Constructed from In Situ Assembled Metal–Phenolic Networks and Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2020, 12, 10000–10008. [Google Scholar] [CrossRef]
- Sun, X.; Yan, L.; Xu, R.; Xu, M.; Zhu, Y. Surface modification of TiO2 with polydopamine and its effect on photocatalytic degradation mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 199–209. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.-H.; Zhang, Y.-P.; Wan, L.; Chen, X.-X.; Yuan, P.; Qu, L.-B. Versatile Application of TiO2@PDA Modified Filter Paper for Oily Wastewater Treatment. Molecules 2023, 28, 7903. https://doi.org/10.3390/molecules28237903
Zhao C-H, Zhang Y-P, Wan L, Chen X-X, Yuan P, Qu L-B. Versatile Application of TiO2@PDA Modified Filter Paper for Oily Wastewater Treatment. Molecules. 2023; 28(23):7903. https://doi.org/10.3390/molecules28237903
Chicago/Turabian StyleZhao, Chang-Hua, Yu-Ping Zhang, Li Wan, Xin-Xin Chen, Pei Yuan, and Ling-Bo Qu. 2023. "Versatile Application of TiO2@PDA Modified Filter Paper for Oily Wastewater Treatment" Molecules 28, no. 23: 7903. https://doi.org/10.3390/molecules28237903
APA StyleZhao, C.-H., Zhang, Y.-P., Wan, L., Chen, X.-X., Yuan, P., & Qu, L.-B. (2023). Versatile Application of TiO2@PDA Modified Filter Paper for Oily Wastewater Treatment. Molecules, 28(23), 7903. https://doi.org/10.3390/molecules28237903





