Waste-Wood-Isolated Cellulose-Based Activated Carbon Paper Electrodes with Graphene Nanoplatelets for Flexible Supercapacitors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology of ACP−GnP Samples
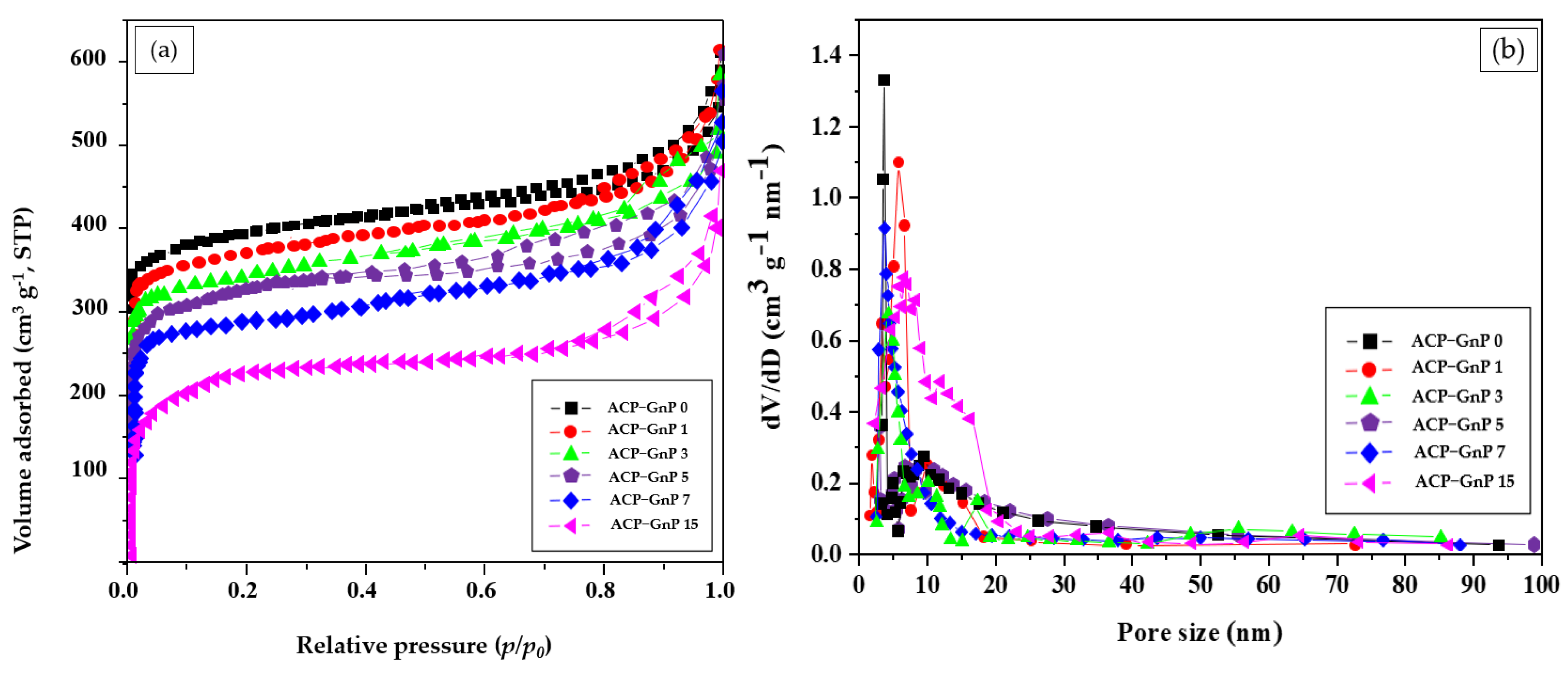
2.2. Textural Properties of ACP−GnP Samples
2.3. Crystallinity of ACP−GnP Samples
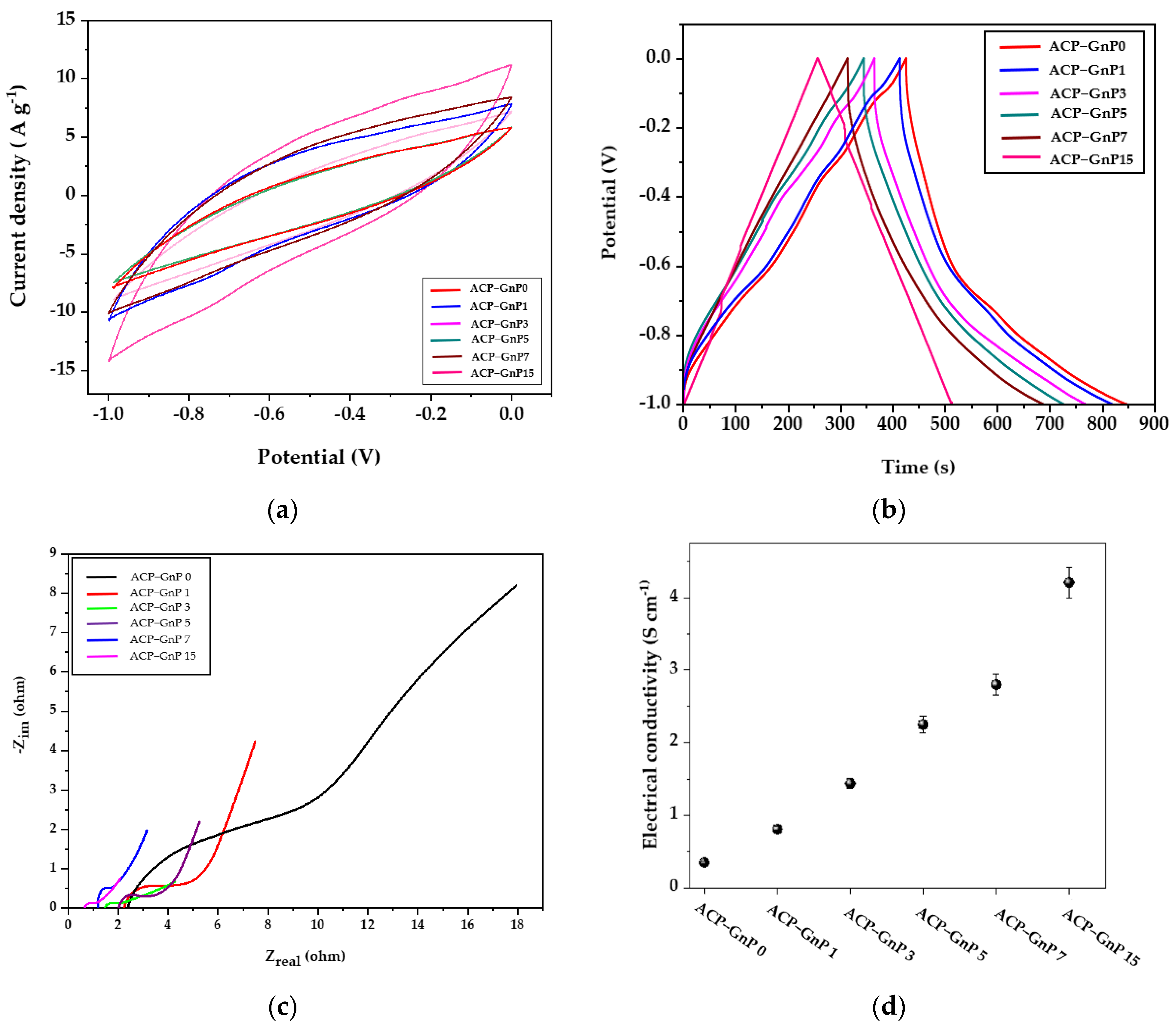
2.4. Electrochemical Performance of ACPs
3. Materials and Methods
3.1. Materials
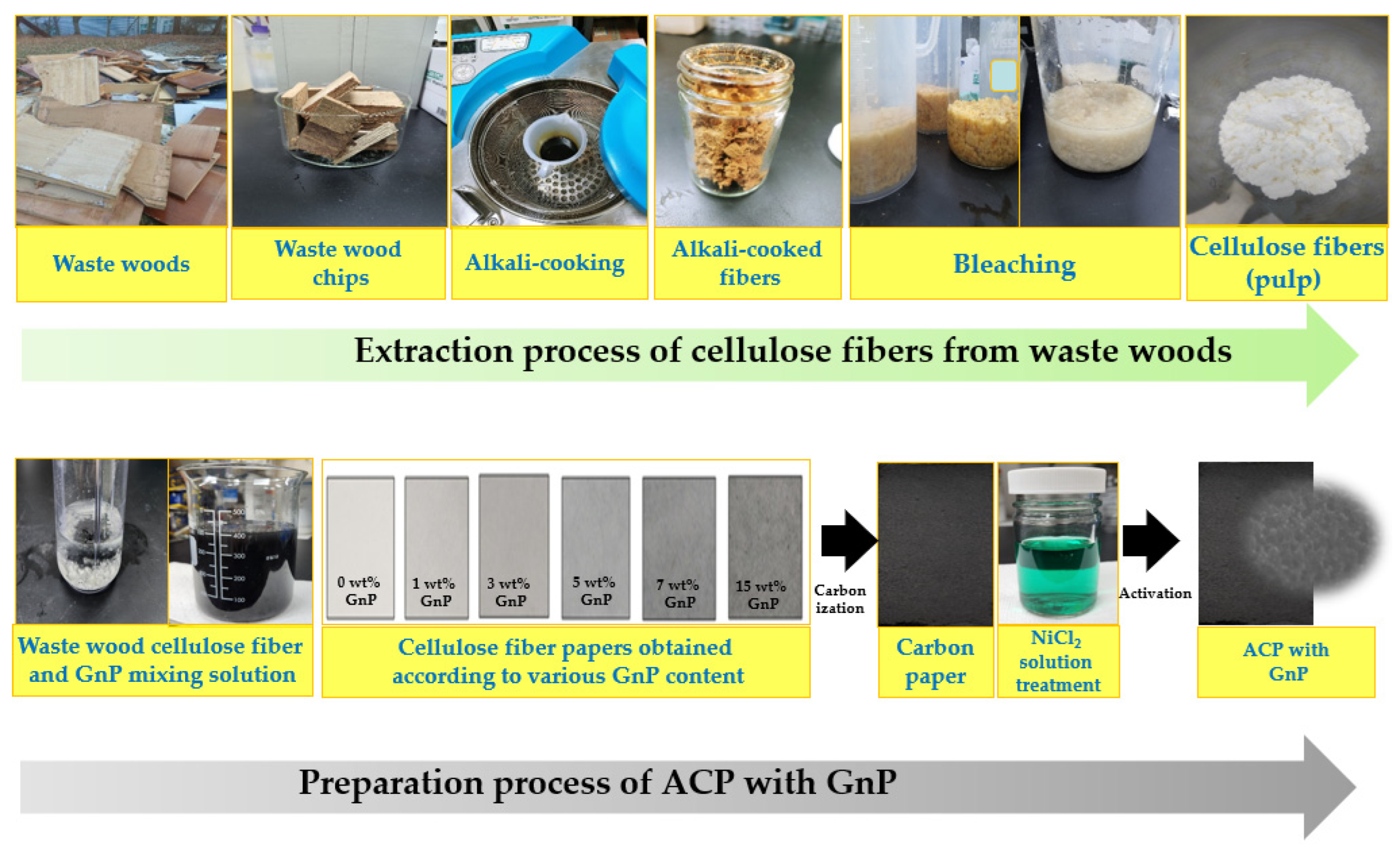
3.2. Preparation of the Activated Carbon Paper Containing Varying GnP Content
3.3. Characterization
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Guo, N.; Li, M.; Sun, X.; Wang, F.; Yang, R. Enzymatic hydrolysis lignin derived hierarchical porous carbon for supercapacitors in ionic liquids with high power and energy densities. Green Chem. 2017, 19, 2595–2602. [Google Scholar] [CrossRef]
- Subramanian, V.; Luo, C.; Stephan, A.M.; Nahm, K.; Thomas, S.; Wei, B. Supercapacitors from activated carbon derived from banana fibers. J. Phys. Chem. C 2007, 111, 7527–7531. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Lee, J.K.Y.; Tian, L.; Srinivasan, M.; Adams, S.; Ramakrishna, S. Electrospun carbon nanofibers and their hybrid composites as advanced materials for energy conversion and storage. Nano Energy 2016, 22, 361–395. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Zheng, P. Solvothermal preparation of micro/nanostructured TiO2 with enhanced lithium storage capability. Mater. Chem. Phys. 2017, 190, 202–208. [Google Scholar] [CrossRef]
- Patsidis, A.C.; Kalaitzidou, K.; Psarras, G.C. Dielectric response, functionality and energy storage in epoxy nanocomposites: Barium titanate vs exfoliated graphite nanoplatelets. Mater. Chem. Phys. 2012, 135, 798–805. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Materials science: Electrochemical capacitors for energy management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef]
- Duy, L.T.; Seo, H. Construction of stretchable supercapacitors using graphene hybrid hydrogels and corrosion-resistant silver nanowire current collectors. Appl. Surf. Sci. 2020, 521, 146–467. [Google Scholar] [CrossRef]
- Dang, F.; Zhao, W.; Tong, W.; Yang, P.; Wang, W.; Liu, Y. Extrinsic design of high-performance programmable supercapacitor with large specific areal capacitance. Electrochim. Acta 2023, 463, 142845. [Google Scholar] [CrossRef]
- Li, G.; Ji, Y.; Zuo, D.; Xu, J.; Zhang, H. Carbon electrodes with double conductive networks for high-performance electrical double-layer capacitors. Adv. Compos. Hybrid Mater. 2019, 2, 456–461. [Google Scholar] [CrossRef]
- Kumar, S.; Saeed, G.; Kim, N.H.; Lee, J.H. Fabrication of Co-Ni-Zn ternary Oxide@NiWO4 core-shell nanowire arrays and Fe2O3-CNTs@GF for ultra-high-performance asymmetric supercapacitor. Compos. Part B-Eng. 2019, 176, 107223. [Google Scholar] [CrossRef]
- Huang, P.; Lethien, C.; Pinaudm, S.; Brousse, K.; Laloo, R.; Turq, V.; Respaud, M.; Demortière, A.; Daffos, B.; Taberna, P.L.; et al. On-chip and freestanding elastic carbon films for micro-supercapacitors. Science 2016, 351, 691–695. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Bagal, I.V.; Ryu, S.W.; Kim, D.H. Hybrid material passivation approach to stabilize the silicon nanowires in aqueous electrolyte for high-energy efficient supercapacitor. Chem. Eng. J. 2019, 362, 609–618. [Google Scholar] [CrossRef]
- Ke, Q.Q.; Guan, C.; Zhang, X.; Zheng, M.R.; Zhang, Y.W.; Cai, Y.Q.; Zhang, H.; Wang, J. Surface-charge-mediated formation of H-TiO2@Ni(OH)2 heterostructures for high performance supercapacitors. Adv. Mater. 2017, 29, 1604164. [Google Scholar] [CrossRef]
- Rodríguez-Rego, J.M.; Carrasco-Amador, J.P.; Mendoza-Cerezo, L.; Marcos-Romero, A.C.; Macías-García, A. Guide for the development and evaluation of supercapacitors with the proposal of novel design to improve their performance. J. Energy Storage 2023, 68, 107816. [Google Scholar] [CrossRef]
- Wang, H.; Feng, H.; Li, J. Graphene and graphene-like layered transition metal review. Polym. Eng. Sci. 2022, 62, 269–303. [Google Scholar] [CrossRef]
- Gao, B.; Li, X.; Ding, K.; Huang, C.; Li, Q.; Chu, P.; Huo, K. Recent progress in nanostructured transition metal nitrides for advanced electrochemical energy storage. J. Mater. Chem. A 2019, 7, 14–37. [Google Scholar] [CrossRef]
- Asghar, A.; Rashid, M.S.; Hussain, S.; Shad, N.A.; Hamza, M.; Chen, Z. Facile hydrothermal synthesis of MoS2 nano-worms-based aggregate as electrode material for high energy density asymmetric supercapacitor. Electrochim. Acta 2023, 465, 143011. [Google Scholar] [CrossRef]
- Arkhipova, E.A.; Ivanov, A.S.; Maslakov, K.I.; Savilov, S.V. Nitrogen-doped mesoporous graphene nanoflakes for high performance ioni;c liquid supercapacitors. Electrochim. Acta 2020, 353, 136463. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Tian, C.; Tan, T.; Xie, Y.; Shi, K.; Li, M.; Fu, H. Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage. RSC Adv. 2012, 2, 4498–4506. [Google Scholar] [CrossRef]
- Arkhipova, E.A.; Novotortsev, R.Y.; Ivanov, A.S.; Maslakov, K.I.; Savilov, S.V. Rice husk-derived activated carbon electrode in redox-active electrolyte—New approach for enhancing supercapacitor performance. J. Energy Storage 2022, 55, 105699. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Zhu, S.; Zhang, H.; Liu, X. Porous carbons derived from desilication treatment and mixed alkali activation of rice husk char for supercapacitors. Energy Sources A 2021, 43, 282–290. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Wu, D.; Bai, X.; Lin, Y.; Wu, T.; Zhang, C.; Chen, D.; Li, H. Improving the supercapcitor performance of activated carbon materials derived from pretreated rice husk. J. Energy Storage 2021, 44, 103432. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, Y.S.; Kwac, L.K.; Shin, H.K. Characterization of activated carbon paper electrodes prepared by rice husk-isolated cellulose fibers for supercapacitor applications. Molecules 2020, 25, 3951. [Google Scholar] [CrossRef] [PubMed]
- Omokafe, S.M.; Adeniyi, A.A.; Igbafen, E.O.; Oke, S.R.; Olubambi, P.A. Fabrication of activated carbon from coconut shells and its electrochemical properties for supercapacitors. Int. J. Electrochem. Sci. 2020, 15, 10854–10865. [Google Scholar] [CrossRef]
- Jain, A.; Tripathi, S.K. Fabrication and characterization of energy storing supercapacitor devices using coconut shell based activated charcoal electrode. Mater. Sci. Eng B 2014, 183, 54–60. [Google Scholar] [CrossRef]
- Deng, M.; Wang, J.; Zhang, Q. Effect of freezing pretreatment on the performance of activated carbon from coconut shell for supercapacitor application. Mater. Lett. 2022, 306, 130934. [Google Scholar] [CrossRef]
- Medagedara, A.D.T.; Waduge, N.M.; Bandara, T.M.W.J.; Wimalasena, I.G.K.J.; Dissanayake, M.; Tennakone, K.; Rajapakse, R.M.G.; Rupasinghe, C.P.; Kumara, G.R.A. Triethylammonium thiocyanate ionic liquid electrolyte-based supercapacitor fabricated using coconut shell-derived electronically conducting activated charcoal electrode material. J. Energy Storage 2022, 55, 105628. [Google Scholar] [CrossRef]
- Chou, T.C.; Huang, C.; Doong, R. Fabrication of hierarchically ordered porous carbons using sugarcane bagasse as the scaffold for supercapacitor applications. Synth. Met. 2014, 194, 29–37. [Google Scholar] [CrossRef]
- Sarkar, S.; Arya, A.; Gaur, U.K.; Gaur, A. Investigations on porous carbon derived from sugarcane bagasse as an electrode material for supercapacitors. Biomass Bioenergy 2020, 142, 105730. [Google Scholar] [CrossRef]
- Wang, X.; Cao, L.; Lewis, R.; Hreid, T.; Zhang, Z.; Wang, H. Biorefining of sugarcane bagasse to fermentable sugars and surface oxygen group-rich hierarchical porous carbon for supercapacitors. Renew. Energy 2020, 162, 2306–2317. [Google Scholar] [CrossRef]
- Du, B.; Chai, L.; Zhu, H.; Cheng, J.; Wang, X.; Chen, X.; Zhou, J.; Sun, R.C. Effective fractionation strategy of sugarcane bagasse lignin to fabricate quality lignin-based carbon nanofibers supercapacitors. Inter. J. Biol. Macromol. 2021, 184, 604–6017. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, C.A.; Menkiti, M.C.; Obiora-Okafo, I.A.; Ezenewa, O.N. Controlled pyrolysis of sugarcane bagasse enhanced mesoporous carbon for improving capacitance of supercapacitor electrode. Biomass Bioenergy 2021, 146, 105996. [Google Scholar] [CrossRef]
- Shin, H.K.; Jeun, J.P.; Kim, H.B.; Kang, P.H. Isolation of cellulose fibers from kenaf using electron beam. Radiat. Phys. Chem. 2012, 81, 936–940. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Kim, H.G.; Lee, U.S.; Kwac, L.K.; Lee, S.O.; Kim, Y.S.; Shin, H.K. Electron beam irradiation isolates cellulose nanofiber from Korea “Tall Goldenrod” invasive alien plant. Nanomaterials 2019, 9, 1358. [Google Scholar] [CrossRef] [PubMed]
- Bledzki, A.K.; Gassan, J. Composites reinforced with cellulose based fiber. Prog. Polym. Sci. 1999, 24, 221–274. [Google Scholar] [CrossRef]
- Ng, H.M.; Sin, L.T.; Tee, T.T.; Bee, S.T.; Hue, D.; Low, C.Y.; Rahmat, A.R. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part B 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, C.; Cao, X.; Wang, Q.; Yang, G.; Chen, J. Porous carbon spheres derived from hemicelluloses for supercapacitor application. Inter. J. Mol. Sci. 2022, 23, 7101. [Google Scholar] [CrossRef]
- Schlee, P.; Hosseinaei, O.; Baker, D.; Landmér, A.; Tomani, P.; Mostazo-López, M.J.; Caxorla-Amorós, D.; Herou, S. From waste to wealth: From kraft lignin to free-standing supercapacitors. Carbon 2019, 145, 470–480. [Google Scholar] [CrossRef]
- Hossain, M.U.; Wang, I.; Iris, K.M.; Tsang, D.S.; Poon, C.S. Environmental and technical feasibility study of upcycling wood waste into cement-bonded particle board. Constr. Build. Master. 2018, 173, 474–480. [Google Scholar] [CrossRef]
- Mancini, M.; Rinnan, Å. Near infrared technique as a tool for the rapid assessment of waste wood quality for energy applications. Renew. Energy 2021, 177, 113–123. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Wu, P.-H. Environmental concerns about carcinogenic air toxics produced from waste woods as alternative energy sources. Energy Sources A Recover. Util. Environ. Eff. 2013, 35, 725–732. [Google Scholar] [CrossRef]
- Humar, M.; Jermer, J.; Peek, R. Regulations in the European Union with emphasis on Germany, Sweden and Slovenia. Environ. Impacts Treat. Wood 2006, 6495, 37–57. [Google Scholar] [CrossRef]
- Ince, C.; Tayançlı, S. Recycling waste wood in cement mortars towards the regeneration of sustainable environment. Constr. Build. Mater. 2021, 299, 123891. [Google Scholar] [CrossRef]
- Zolin, L.; Nair, J.R.; Beneventi, D.; Bella, F.; Destro, M.; Jagdale, P.; Cannavaro, I.; Tagliaferro, A.; Haussy, D.; Geobaldo, F.; et al. A simple route toward next- gen green energy storage concept by nanofibres-based self-supporting electrodes and a solid polymeric design. Carbon 2016, 107, 811–822. [Google Scholar] [CrossRef]
- Reina, M.; Scalia, A.; Auxilia, G.; Fontana, M.; Bella, F.; Ferrero, S.; Lamberti, A. Boosting electric double layer capacitance in laser-induced graphene-based supercapacitors. Adv. Sustain. Syst. 2022, 6, 100228. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, L.; Du, T.; Ren, B.; Xu, Y.; Wang, S.; Miao, J.; Liu, Z. A review of carbon materials for supercapacitors. Mater. Des. 2022, 221, 111017. [Google Scholar] [CrossRef]
- Ahmed, J.; Bher, A.; Auras, R.; Al-Zuwayed, S.A.; Joseph, A.; Mulla, M.F.; Alazemi, A. Morphological, thermo-mechanical, and barrier properties of coextruded multilayer polylactide composite films reinforced with graphene nanoplatelets and encapsulated thyme essential oil. Food Packag. Shelf Life 2023, 40, 101179. [Google Scholar] [CrossRef]
- Cheng, S.; Guo, X.; Tan, P.; Lin, M.; Cai, J.; Zhou, Y.; Zhao, D.; Cai, W.; Zhang, Y.; Zhang, X. Aligning graphene nanoplates coplanar in polyvinyl alcohol by using a rotating magnetic field to fabricate thermal interface materials with high through-plane thermal conductivity. Compos. Part B 2023, 264, 110916. [Google Scholar] [CrossRef]
- Sesuk, T.; Tammawat, P.; Jivaganont, P.; Somton, K.; Limthongkul, P.; Kobsiriphat, W. Activated carbon derived from coconut coir pitch as high performance supercapacitor electrode material. J. Energy Storage 2019, 25, 100910. [Google Scholar] [CrossRef]
- Mo, R.J.; Zhao, Y.; Zhao, M.M.; Wu, M.; Wang, C.; Li, J.P.; Kuga, S.; Huang, Y. Graphene-like porous carbon from sheet cellulose as electrodes for supercapacitors. Chem. Eng. J. 2018, 346, 104–112. [Google Scholar] [CrossRef]
- Alcaraz, L.; Fernández, A.L.; Garcíai-Díaz, I.; López, F.A. Preparation and characterization of activated carbons from winemaking wastes and their adsorption of methylene blue. Adsorpt. Sci. Technol. 2018, 36, 1331–1351. [Google Scholar] [CrossRef]
- Darabut, A.M.; Lobko, Y.; Yakovlev, Y.; Rodríguez, M.G.; Veltruská, K.; Šmíd, B.; Kúš, P.; Nováková, J.; Dopita, M.; Vorokhta, M.; et al. Influence of thermal treatment on the structure and electrical conductivity of thermally expanded graphite. Adv. Powder Technol. 2022, 33, 103884. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Sadiq, N.M.; Abdullah, R.M.; Tahir, D.A.; Jameel, D.A.; Hamad, S.M.; Abdullah, O.G. Design of biodegradable polymer blend electrolytes with decoupled ion motion for EDLC device application: Electrical and electrochemical properties. Results Phys. 2023, 51, 106692. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.J.; Chae, S.-H.; Lee, J.J.; Lee, M.S.; Yoon, W.; Kwac, L.K.; Kim, H.G.; Shin, H.K. Waste-Wood-Isolated Cellulose-Based Activated Carbon Paper Electrodes with Graphene Nanoplatelets for Flexible Supercapacitors. Molecules 2023, 28, 7822. https://doi.org/10.3390/molecules28237822
Lee JJ, Chae S-H, Lee JJ, Lee MS, Yoon W, Kwac LK, Kim HG, Shin HK. Waste-Wood-Isolated Cellulose-Based Activated Carbon Paper Electrodes with Graphene Nanoplatelets for Flexible Supercapacitors. Molecules. 2023; 28(23):7822. https://doi.org/10.3390/molecules28237822
Chicago/Turabian StyleLee, Jung Jae, Su-Hyeong Chae, Jae Jun Lee, Min Sang Lee, Wonhyung Yoon, Lee Ku Kwac, Hong Gun Kim, and Hye Kyoung Shin. 2023. "Waste-Wood-Isolated Cellulose-Based Activated Carbon Paper Electrodes with Graphene Nanoplatelets for Flexible Supercapacitors" Molecules 28, no. 23: 7822. https://doi.org/10.3390/molecules28237822
APA StyleLee, J. J., Chae, S.-H., Lee, J. J., Lee, M. S., Yoon, W., Kwac, L. K., Kim, H. G., & Shin, H. K. (2023). Waste-Wood-Isolated Cellulose-Based Activated Carbon Paper Electrodes with Graphene Nanoplatelets for Flexible Supercapacitors. Molecules, 28(23), 7822. https://doi.org/10.3390/molecules28237822





