Influence of Five Drying Methods on Active Compound Contents and Bioactivities of Fresh Flowers from Syringa pubescens Turcz
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Contents of Bioactive Compounds
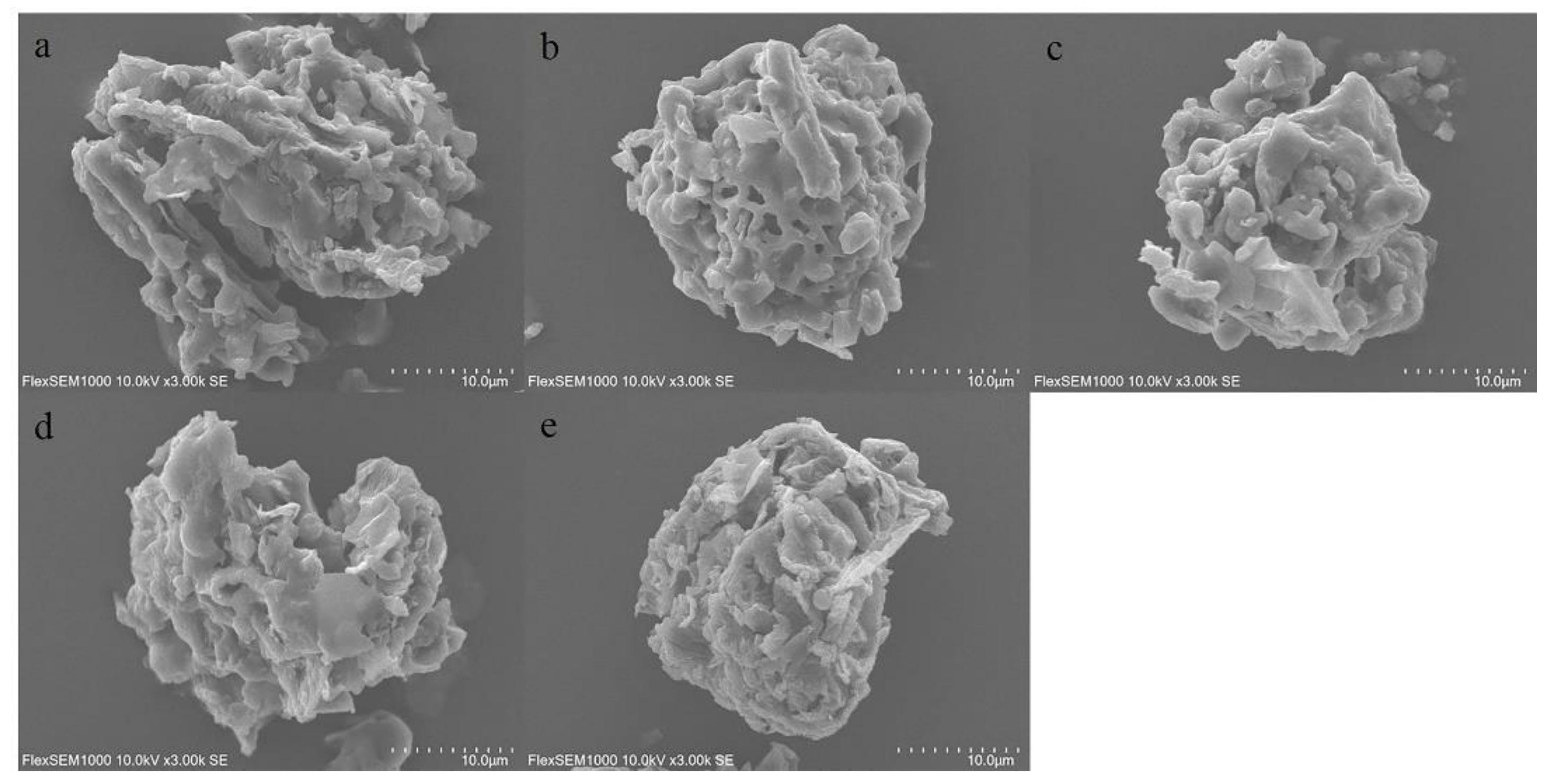
2.2. Scanning Electron Microscopy (SEM) Analysis
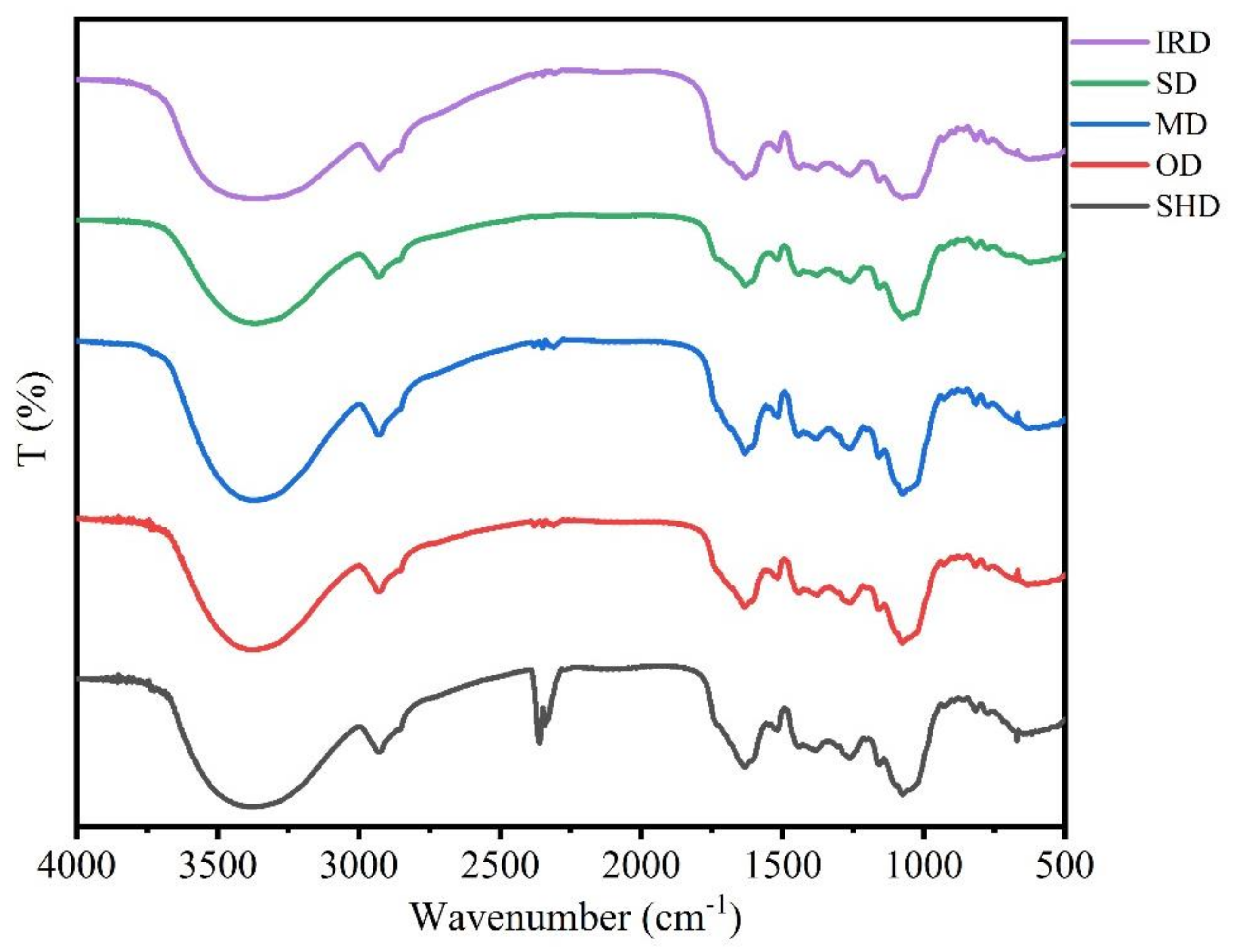
2.3. FT-IR Spectra Analysis
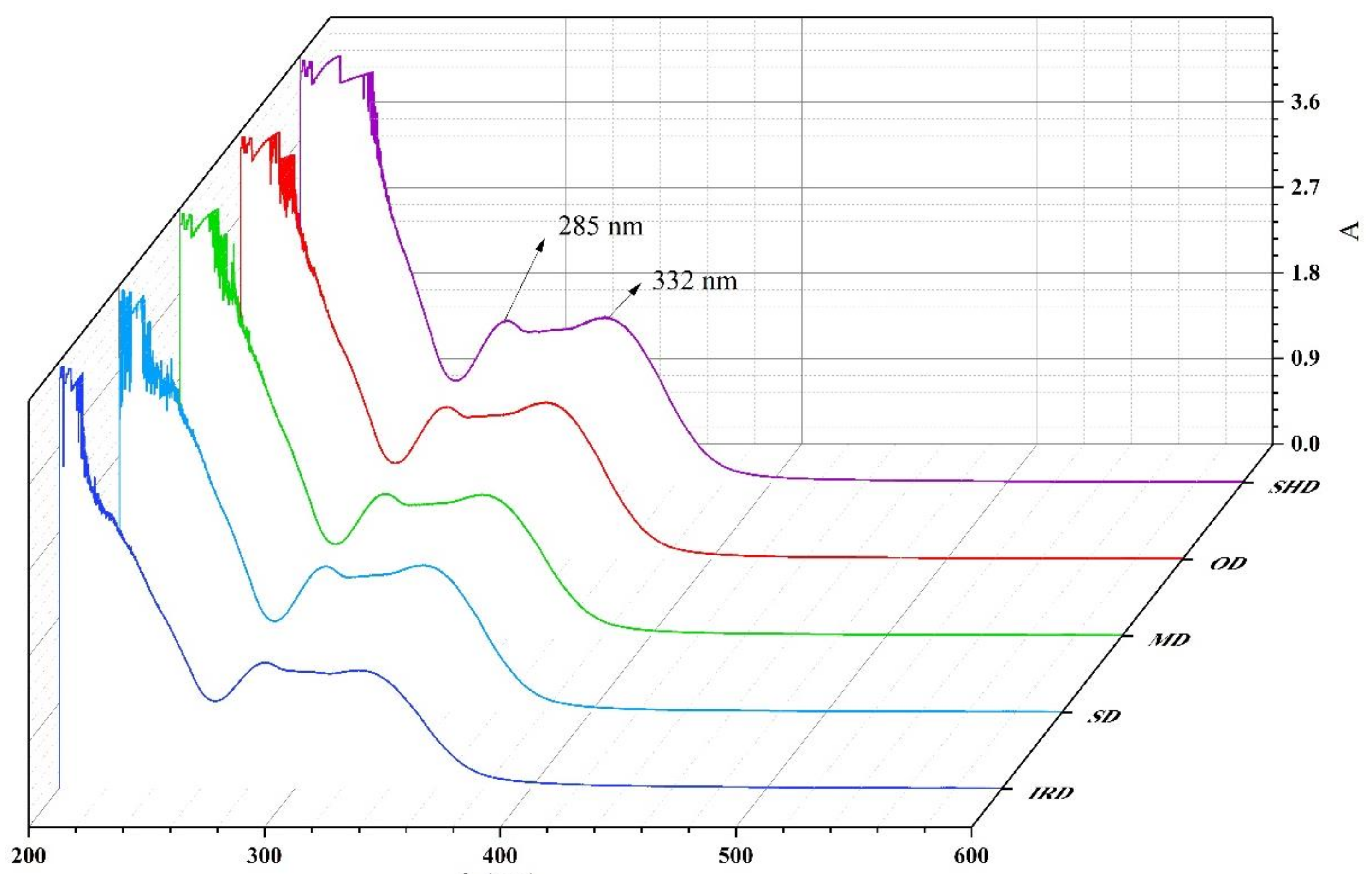
2.4. UV Spectroscopy Analysis
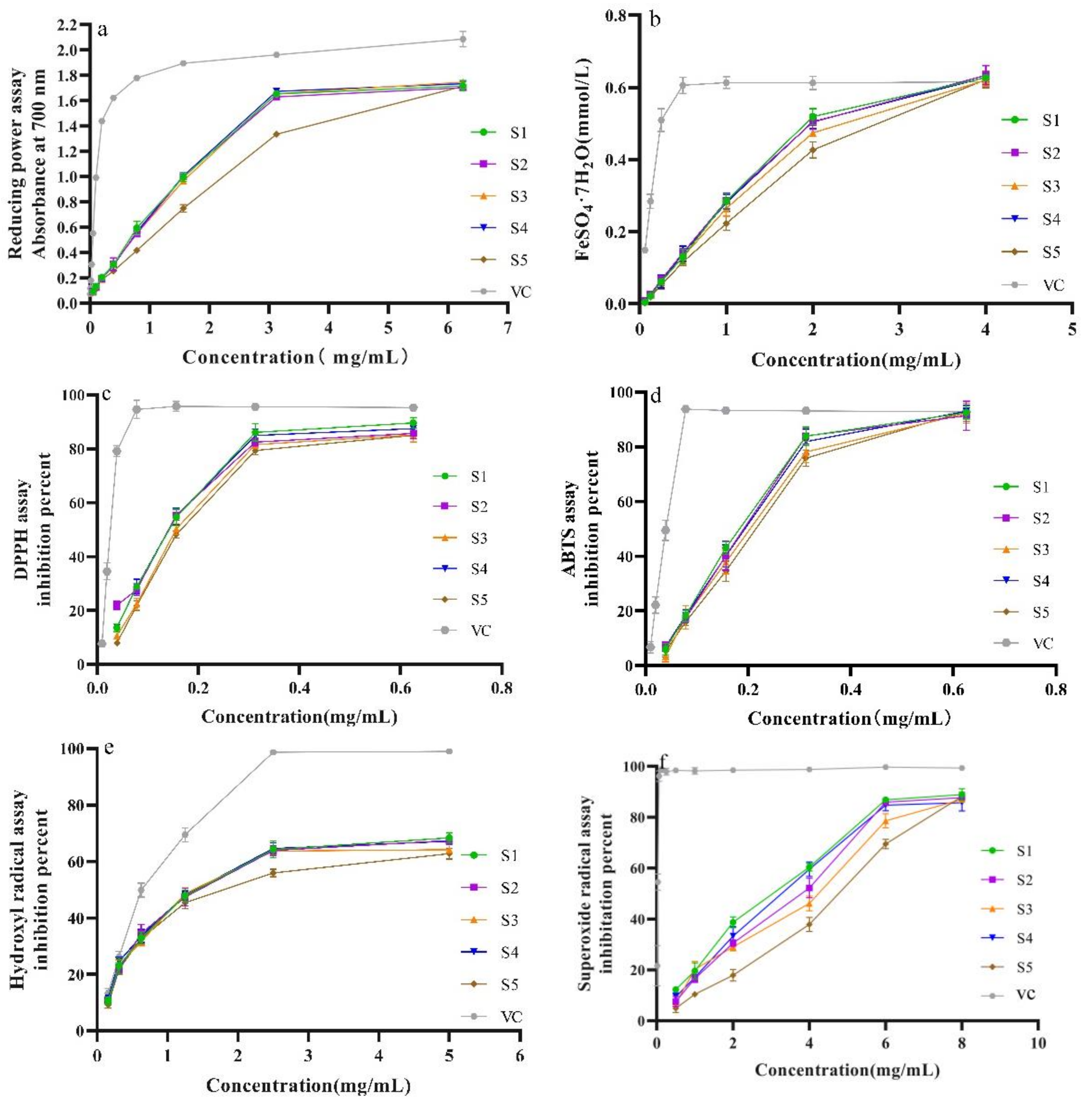
2.5. Antioxidant Activities In Vitro
2.5.1. Influence of Five Drying Methods on Reducing Power
2.5.2. Influence of Different Drying Methods against DPPH, ABTS+ and •OH Scavenging Ability
2.5.3. Influence of Different Drying Methods on Superoxide Radical Scavenging Effect
2.6. Protective Effect of SPF Extract on H2O2-Induced Oxidative Injury in L02 Cells
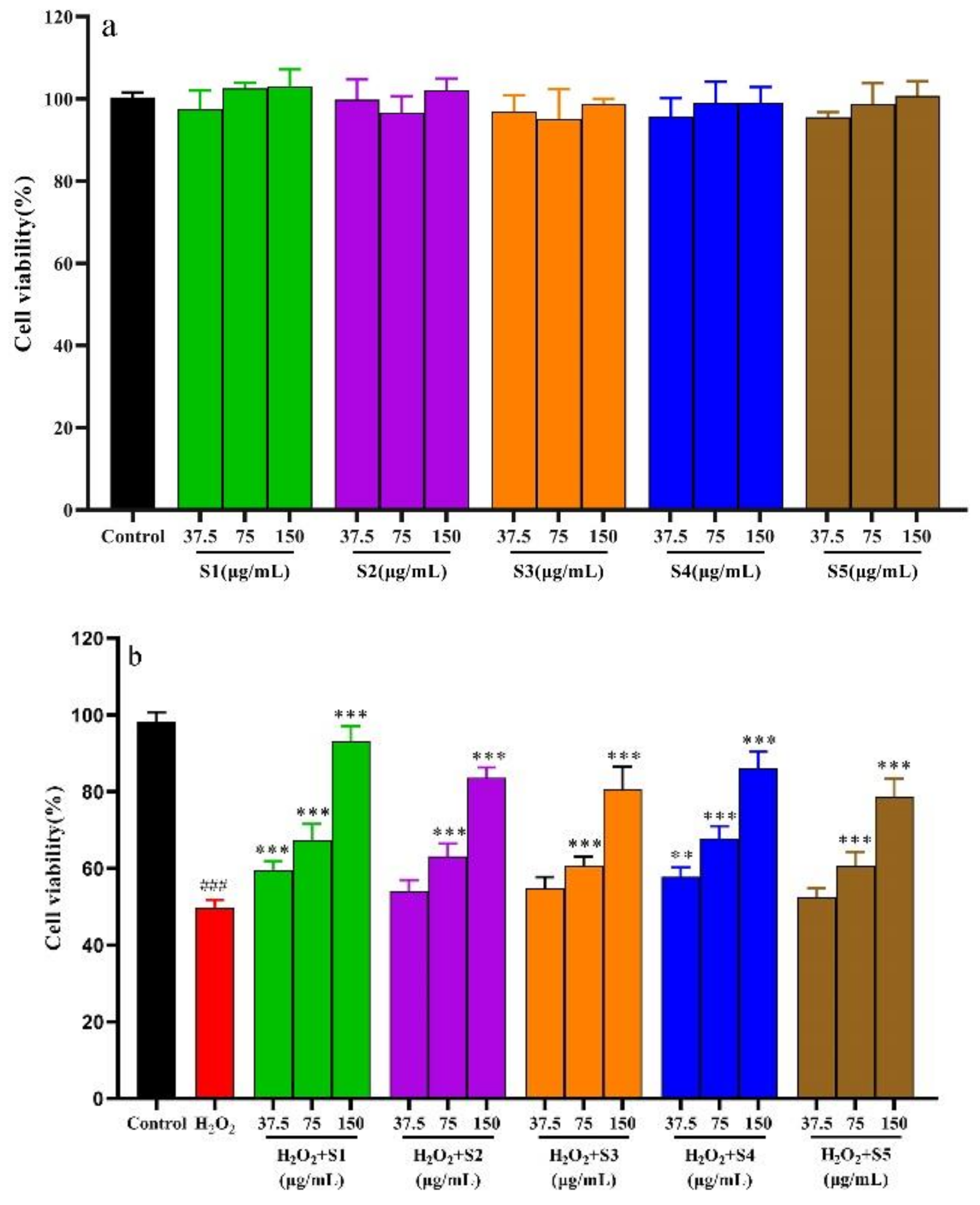
2.6.1. SPF of Cytotoxicity on L02 Cells
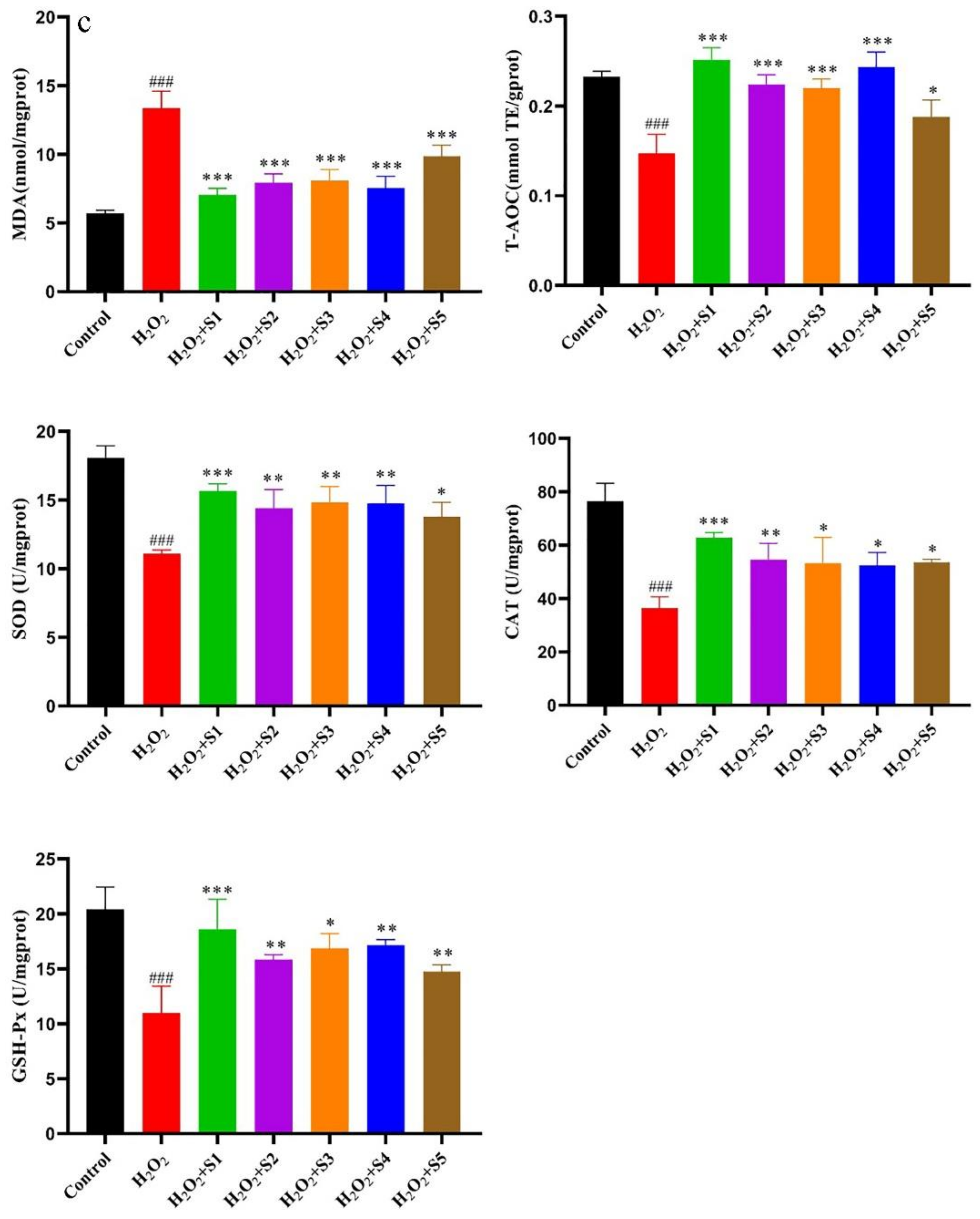
2.6.2. Protective Effect of SPF Extract against Oxidative Damage by H2O2-Induced Cells and Determination of Cells’ Biochemical Indexes
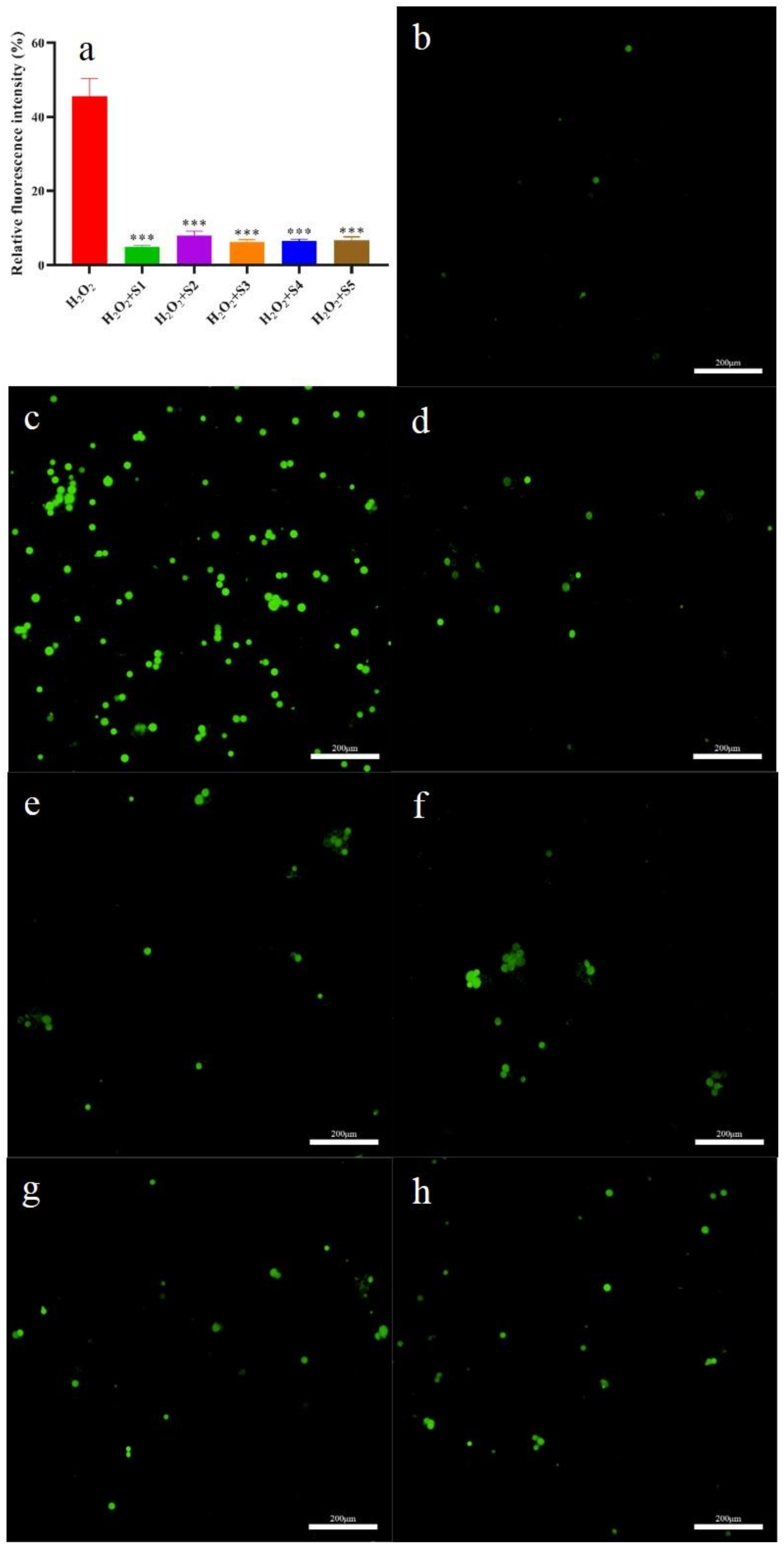
2.6.3. Intracellular ROS Evaluation
2.7. Anti-Inflammatory Activity of SPF
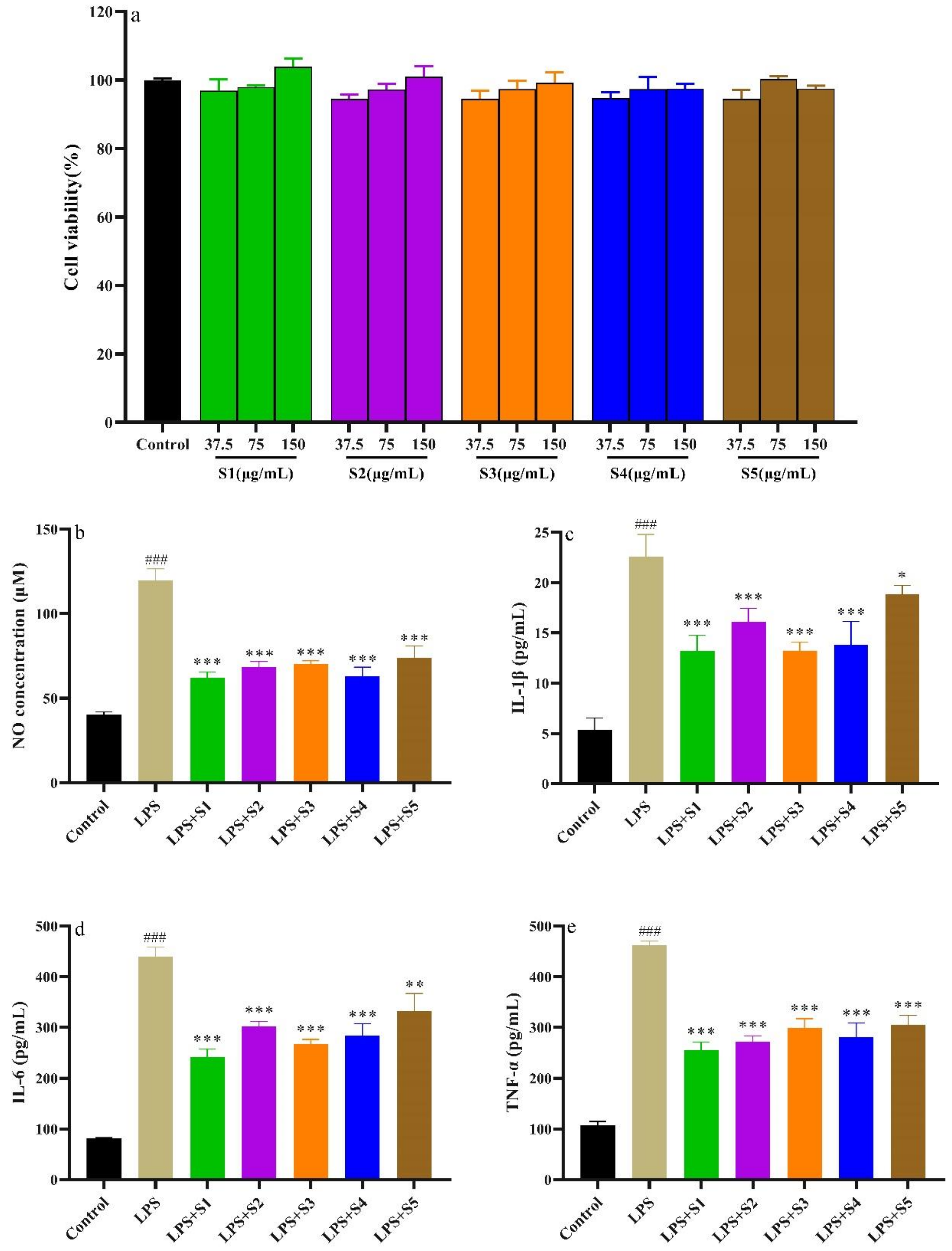
2.7.1. Cytotoxicity of SPF
2.7.2. Determination of NO, IL-6, IL-1β, and TNF-α of RAW.264.7 Cells
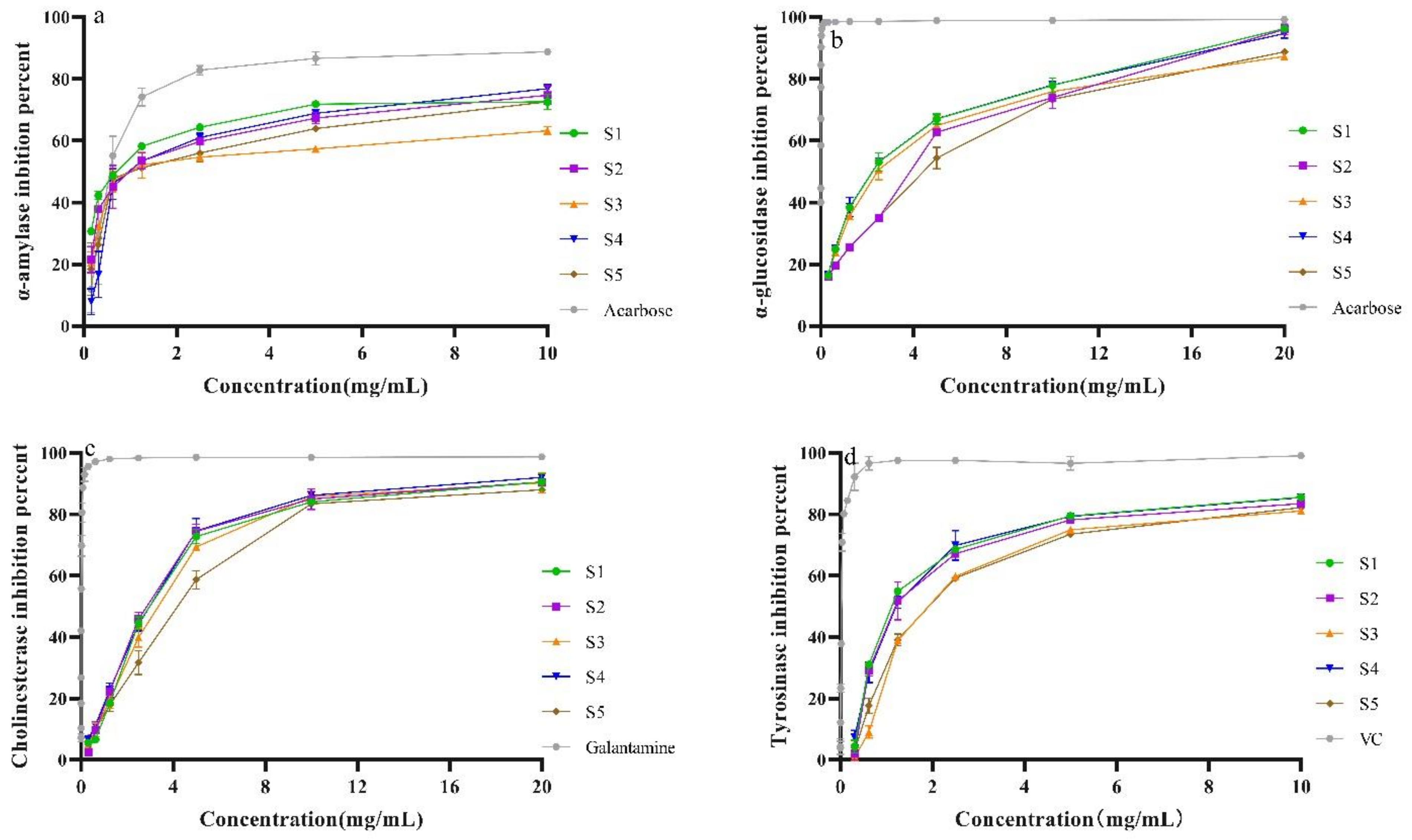
2.8. Enzyme Inhibitory Activity
3. Materials and Methods
3.1. Plant Materials and Reagents
3.2. Drying Methods
3.3. Preparation of the Standard Solution
3.4. Extraction of Bioactive Compounds of the SPF Samples
3.5. Chromatographic Methods
3.6. SEM
3.7. FT-IR Analysis
3.8. UV Spectroscopy
3.9. Assay of Antioxidant Activities
3.9.1. Determination of Reducing Capacity
3.9.2. Assay of the Ferric Reducing Antioxidant Power (FRAP)
3.9.3. DPPH and ABTS Radical Scavenging Assay
3.9.4. Hydroxyl Radical (•OH) Scavenging Assay
3.9.5. Superoxide radical (O2•−) scavenging assay
3.9.6. Protective Effect of SPF Extract on H2O2-Induced Oxidative Injury in L02 Cells
SPF of Cytotoxicity on L02
Protective Effect of SPF Extract against Oxidative Damage by H2O2-Induced Cells and Determination of Cells’ Biochemical Indexes
Intracellular ROS Evaluation
3.10. Anti-Inflammatory Property of SPF in LPS-Activated RAW264.7 Cells
3.10.1. Cell Culture and Cytotoxic Effect of SPF
3.10.2. Determination of NO, IL-6, IL-1β and TNF-α of RAW264.7 Cells
3.11. Enzyme Inhibitory Activity
3.11.1. α-Amylase Inhibitory Property Assay
3.11.2. Inhibitory Activity of α-Glucosidase
3.11.3. Inhibitory Activity on Tyrosinase Assay
3.11.4. Cholinesterase Inhibitory Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Zhang, J.; Wang, P.; Zhang, Y.; Yang, Q.; Zhang, Z.; Ma, Z.; Wu, Y.; Ma, J.; Fan, E. Evaluation of alpha-glucosidase inhibition activity and glycosides in the Syringa pubescens Turcz from different geographical origin. Sci. Hortic. 2023, 320, 112198. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Zhang, Y.; Li, M.; Wang, P.; Zhang, J.; Liu, K.; Wu, Y.; Shen, G.; Ma, Z. Simultaneous Determination of Phenylethanoid Glycosides and Antioxidant Activity of Syringa pubescens Turcz. from Different Geographical Origin in China. J. Chromatogr. Sci. 2022, 61, 766–772. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Q.; Wang, D.; Deng, R.; Yu, M.; Wang, X. A comparative study of echinacoside, oleuropein content and antioxidant properties of different solvent extracts from Syringa pubescens Turcz. Pak. J. Pharm. Sci. 2022, 35, 35–40. [Google Scholar] [PubMed]
- Zhang, H. Protective effects of Syringa pubescens extract on liver injury induced by cadmium in mice. Clin. Res. Pract. 2018, 23, 154–156. [Google Scholar]
- He, X.Y.; Shi, J.J.; Gong, R.C.; Miao, T.T. Anti-inflammatory effect of Syringa pubescens Turcz. J. Tonghua Norm. Univ. 2013, 34, 55–57. [Google Scholar]
- Wang, X.; Wu, Y.; Li, J.; Wang, A.; Li, G.; Ren, X.; Yin, W. Ultrasound-assisted deep eutectic solvent extraction of echinacoside and oleuropein from Syringa pubescens Turcz. Ind. Crops Prod. 2020, 151, 112442. [Google Scholar] [CrossRef]
- Wu, Y.; Chu, Y.; Yang, Q.; Li, M.; Yu, M.; Deng, R.; Liu, K.; Wang, X.; Fan, E. Response surface methodology optimised solvothermal system enables an efficient extraction of echinacoside and oleuropein from Syringa pubescens Turcz. Phytochem. Anal. 2021, 32, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Ke, Z.; Chai, D.; Miao, Y.; Luo, K.; Li, W. Lycopene, polyphenols and antioxidant activities of three characteristic tomato cultivars subjected to two drying methods. Food Chem. 2021, 338, 128062. [Google Scholar] [CrossRef]
- Ng, Z.X.; Than, M.J.Y.; Yong, P.H. Peperomia pellucida (L.) Kunth herbal tea: Effect of fermentation and drying methods on the consumer acceptance, antioxidant and anti-inflammatory activities. Food Chem. 2021, 344, 128738. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Qin, L.; Shen, J.; He, Z.; Shao, Q.; Lin, D. Drying methods affect bioactive compound contents and antioxidant capacity of Bletilla striata (Thunb.) Reichb.f. flower. Ind. Crops Prod. 2021, 164, 113388. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Stankevičius, M.; Drevinskas, T.; Akuneca, I.; Kaškonas, P.; Bimbiraitė-Survilienė, K.; Maruška, A.; Ragažinskienė, O.; Kornyšova, O.; Briedis, V.; et al. Evaluation of phytochemical composition of fresh and dried raw material of introduced Chamerion angustifolium L. using chromatographic, spectrophotometric and chemometric techniques. Phytochemistry 2015, 115, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Silpa, S.G.; Smitha, G.R.; Ranjitha, K. Drying and packaging methods impact the bacoside profile and microbiological quality of Brahmi herb (Bacopa monnieri L.) during storage. Ind. Crops Prod. 2021, 159, 113064. [Google Scholar] [CrossRef]
- Su, X.; Wu, Y.; Li, Y.; Huang, Y.; Liu, Y.; Luo, P.; Zhang, Z. Effect of Different Post-Harvest Processing Methods on the Chemical Constituents of Notopterygium franchetii by an UHPLC-QTOF-MS-MS Metabolomics Approach. Molecules 2019, 24, 3188. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Zhu, X.; Ai, Z.; Ai, Y.; Qiu, F.; Ni, D. Effect of different drying methods on the sensory quality and chemical components of black tea. LWT 2019, 99, 112–118. [Google Scholar] [CrossRef]
- Li, M.; Ai, Z.; Xiao, H.; Mowafy, S.; Pei, Y.; Liu, Y. Improvement of drying efficiency and physicochemical quality of kiwifruit slices using infrared-assisted tilted tray air impingement drying. Dry. Technol. 2023, 41, 1159–1170. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Begum, N.; Xia, P.; Liu, J.; Liang, Z. Effects of Different Processing Methods Based on Different Drying Conditions on the Active Ingredients of Salvia miltiorrhiza Bunge. Molecules 2022, 27, 4860. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, Y.; Huang, J.; Zeng, H.; Zheng, B. Effects of different drying methods on the product quality and volatile compounds of whole shiitake mushrooms. Food Chem. 2016, 197, 714–722. [Google Scholar] [CrossRef]
- Lu, C.-T.; Mei, X.-G. Improvement of phenylethanoid glycosides production by a fungal elicitor in cell suspension culture of Cistanche deserticola. Biotechnol. Lett. 2003, 25, 1437–1439. [Google Scholar] [CrossRef]
- da Silva, A.C.P.; Paiva, J.P.; Diniz, R.R.; dos Anjos, V.M.; Silva, A.B.S.M.; Pinto, A.V.; dos Santos, E.P.; Leitão, A.C.; Cabral, L.M.; Rodrigues, C.R.; et al. Photoprotection assessment of olive (Olea europaea L.) leaves extract standardized to oleuropein: In vitro and in silico approach for improved sunscreens. J. Photochem. Photobiol. B Biol. 2019, 193, 162–171. [Google Scholar] [CrossRef]
- Wang, C.; Gao, X.; Santhanam, R.K.; Chen, Z.; Chen, Y.; Xu, L.; Wang, C.; Ferri, N.; Chen, H. Effects of polysaccharides from Inonotus obliquus and its chromium (III) complex on advanced glycation end-products formation, α-amylase, α-glucosidase activity and H2O2-induced oxidative damage in hepatic L02 cells. Food Chem. Toxicol. 2018, 116, 335–345. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Kang, M.-C.; Lee, H.H.L.; Cho, C.H.; Choi, I.; Park, Y.; Lee, S.-H. Antioxidant Effects of Turmeric Leaf Extract against Hydrogen Peroxide-Induced Oxidative Stress In Vitro in Vero Cells and In Vivo in Zebrafish. Antioxidants 2021, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Li, X.; Hu, W.; Zhang, L.; Xu, X.; Wu, H.; Ji, L. Hepatoprotective effect of phenylethanoid glycosides from Incarvillea compacta against CCl4-induced cytotoxicity in HepG2 cells. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 617–625. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Pavathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Carlson, N.G.; Wieggel, W.A.; Chen, J.; Bacchi, A.; Rogers, S.W.; Gahring, L.C. Inflammatory Cytokines IL-1α, IL-1β, IL-6, and TNF-α Impart Neuroprotection to an Excitotoxin Through Distinct Pathways1. J. Immunol. 1999, 163, 3963–3968. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, M.R.M.; Passos, F.R.S.; Monteiro, B.S.; Gandhi, S.R.; Heimfarth, L.; Lima, B.S.; Nascimento, Y.M.; Duarte, M.C.; Araujo, A.A.S.; Menezes, I.R.A.; et al. HPLC-DAD-UV analysis, anti-inflammatory and anti-neuropathic effects of methanolic extract of Sideritis bilgeriana (lamiaceae) by NF-κB, TNF-α, IL-1β and IL-6 involvement. J. Ethnopharmacol. 2021, 265, 113338. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, J.; Yang, W.; Chen, S.; Liu, D.; Fang, H.; Zhang, H.; Ye, X. Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef]
- Altun, M.L.; Yılmaz, B.S.; Orhan, I.E.; Citoglu, G.S. Assessment of cholinesterase and tyrosinase inhibitory and antioxidant effects of Hypericum perforatum L. (St. John’s wort). Ind. Crops Prod. 2013, 43, 87–92. [Google Scholar] [CrossRef]
- Jia, X.; Yang, Y.; Wang, Q.; Tian, Y.; Hong, Y.; Tian, M.; Tang, D. Phytochemical composition, antioxidant, anti-tyrosinase, anti-cholinesterase, and anti-inflammatory activities of Rhynchanthus beesianus rhizome extracts. Arab. J. Chem. 2023, 16, 104952. [Google Scholar] [CrossRef]
- Saeedi, M.; Khezri, K.; Seyed Zakaryaei, A.; Mohammadamini, H. A comprehensive review of the therapeutic potential of α-arbutin. Phytother. Res. 2021, 35, 4136–4154. [Google Scholar] [CrossRef]
- Khattabi, L.; Boudiar, T.; Bouhenna, M.M.; Chettoum, A.; Chebrouk, F.; Chader, H.; Lozano-Sánchez, J.; Segura-Carretero, A.; Nieto, G.; Akkal, S. RP-HPLC-ESI-QTOF-MS Qualitative Profiling, Antioxidant, Anti-Enzymatic, Anti-Inflammatory, and Non-Cytotoxic Properties of Ephedra alata Monjauzeana. Foods 2022, 11, 145. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, V.; Anand, P.; Kumar, V.; Ranjan Dwivedi, A.; Kumar, V. Advancements in the development of multi-target directed ligands for the treatment of Alzheimer’s disease. Biorg. Med. Chem. 2022, 61, 116742. [Google Scholar] [CrossRef]
- Li, Q.; Mo, J.; Xiong, B.; Liao, Q.; Chen, Y.; Wang, Y.; Xing, S.; He, S.; Lyu, W.; Zhang, N.; et al. Discovery of Resorcinol-Based Polycyclic Structures as Tyrosinase Inhibitors for Treatment of Parkinson’s Disease. ACS Chem. Neurosci. 2022, 13, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Q.; Wu, Y.; Chen, G.; Yue, W.; Liang, Q. Response Surface Optimized Ultrasonic-Assisted Extraction of Flavonoids from Sparganii Rhizoma and Evaluation of Their in Vitro Antioxidant Activities. Molecules 2012, 17, 6769–6783. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Xue, J.; Fan, E. Plant Phenolics Extraction from Flos Chrysanthemi: Response Surface Methodology Based Optimization and the Correlation Between Extracts and Free Radical Scavenging Activity. J. Food Sci. 2017, 82, 2726–2733. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Z.; Wang, F.; Liu, L.; Wei, Y.; Li, J.; Zhang, L.; Zheng, K.; Wu, L.; Men, X.; et al. Extraction, characterization, and biological activities of exopolysaccharides from plant root soil fungus Fusarium merismoides A6. Braz. J. Microbiol. 2023, 54, 199–211. [Google Scholar] [CrossRef]
- Huang, B.; Ban, X.; He, J.; Zeng, H.; Zhang, P.; Wang, Y. Hepatoprotective and antioxidant effects of the methanolic extract from Halenia elliptica. J. Ethnopharmacol. 2010, 131, 276–281. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.; Zhuang, S.; Wen, Y.; Cheng, W.; Zeng, Z.; Jiang, T.; Tang, C. Effect of Anoectochilus roxburghii flavonoids extract on H2O2—Induced oxidative stress in LO2 cells and D-gal induced aging mice model. J. Ethnopharmacol. 2020, 254, 112670. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Xu, L.; Jia, Y.; Xue, Z.; Zhang, M.; Phisalaphong, M.; Chen, H. Ultrasound-assisted modified pectin from unripe fruit pomace of raspberry (Rubus chingii Hu): Structural characterization and antioxidant activities. LWT 2020, 134, 110007. [Google Scholar] [CrossRef]
- Zhen, D.; Xuan, T.-Q.; Hu, B.; Bai, X.; Fu, D.-N.; Wang, Y.; Wu, Y.; Yang, J.; Ma, Q. Pteryxin attenuates LPS-induced inflammatory responses and inhibits NLRP3 inflammasome activation in RAW264.7 cells. J. Ethnopharmacol. 2022, 284, 114753. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S. A comparative study of three drying methods on the phenolic profile and biological activities of Salvia absconditiflora. J. Food Meas. Charact. 2019, 13, 162–168. [Google Scholar] [CrossRef]
- Moonrungsee, N.; Shimamura, T.; Kashiwagi, T.; Jakmunee, J.; Higuchi, K.; Ukeda, H. Sequential injection spectrophotometric system for evaluation of mushroom tyrosinase-inhibitory activity. Talanta 2012, 101, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G. A study on in vitro enzyme inhibitory properties of Asphodeline anatolica: New sources of natural inhibitors for public health problems. Ind. Crops Prod. 2016, 83, 39–43. [Google Scholar] [CrossRef]

| Drying Method | Bioactive Compounds | ||||||
|---|---|---|---|---|---|---|---|
| Salidroside | Ayringin | Echinacoisde | Forsythoside B | Verbascoside | Isoacteoside | Oleuropein | |
| SHD | 2.471 ± 0.049 a | 0.149 ± 0.015 b | 11.734 ± 0.360 b | 11.351 ± 0.218 b | 4.486 ± 0.097 b | 0.077 ± 0.001 a | 21.490 ± 0.242 c |
| OD | 2.478 ± 0.072 a | 0.161 ± 0.005 b | 9.555 ± 0.056 d | 11.983 ± 0.040 a | 5.073 ± 0.022 a | 0.079 ± 0.001 a | 21.401 ± 0.117 c |
| MD | 2.646 ± 0.172 a | 0.028 ± 0.004 c | 10.422 ± 0.138 c | 9.256 ± 0.137 c | 2.817 ± 0.009 d | 0.052 ± 0.007 b | 31.735 ± 0.310 a |
| SD | 1.903 ± 0.062 c | 0.317 ± 0.040 a | 13.824 ± 0.121 a | 9.472 ± 0.170 c | 2.924 ± 0.030 c | 0.073 ± 0.003 a | 25.968 ± 0.261 b |
| IRD | 2.212 ± 0.070 b | 0.018 ± 0.007 c | 4.737 ± 0.189 e | 5.891 ± 0.058 d | 2.041 ± 0.016 e | 0.050 ± 0.017 b | 14.674 ± 0.038 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Zhang, J.; Wu, Y.; Zhang, Z.; Wang, X.; Ma, J. Influence of Five Drying Methods on Active Compound Contents and Bioactivities of Fresh Flowers from Syringa pubescens Turcz. Molecules 2023, 28, 7803. https://doi.org/10.3390/molecules28237803
Xu W, Zhang J, Wu Y, Zhang Z, Wang X, Ma J. Influence of Five Drying Methods on Active Compound Contents and Bioactivities of Fresh Flowers from Syringa pubescens Turcz. Molecules. 2023; 28(23):7803. https://doi.org/10.3390/molecules28237803
Chicago/Turabian StyleXu, Weidong, Jiameng Zhang, Yanfang Wu, Zichen Zhang, Xinsheng Wang, and Junying Ma. 2023. "Influence of Five Drying Methods on Active Compound Contents and Bioactivities of Fresh Flowers from Syringa pubescens Turcz" Molecules 28, no. 23: 7803. https://doi.org/10.3390/molecules28237803
APA StyleXu, W., Zhang, J., Wu, Y., Zhang, Z., Wang, X., & Ma, J. (2023). Influence of Five Drying Methods on Active Compound Contents and Bioactivities of Fresh Flowers from Syringa pubescens Turcz. Molecules, 28(23), 7803. https://doi.org/10.3390/molecules28237803






