Parameter Optimization of Ultrasonic–Microwave Synergistic Extraction of Taxanes from Taxus cuspidata Needles
Abstract
:1. Introduction
2. Results and Discussion
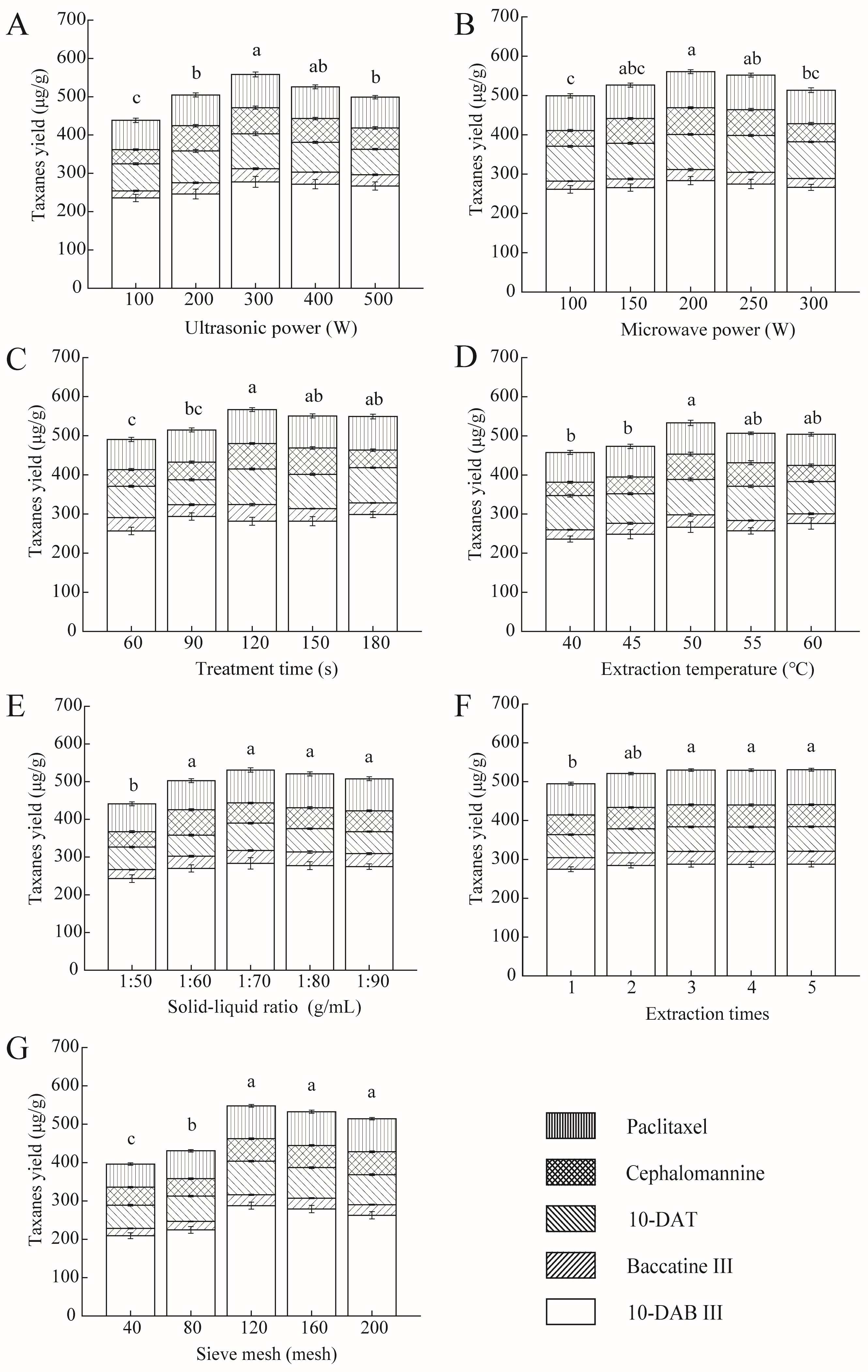
2.1. Single-Factor Experiments
2.1.1. Ultrasonic Power
2.1.2. Microwave Power
2.1.3. Treatment Time
2.1.4. Extraction Temperature
2.1.5. Solid–Liquid Ratio
2.1.6. Number of Extractions
2.1.7. Sieve Mesh Number
2.2. Analysis of PBD Results
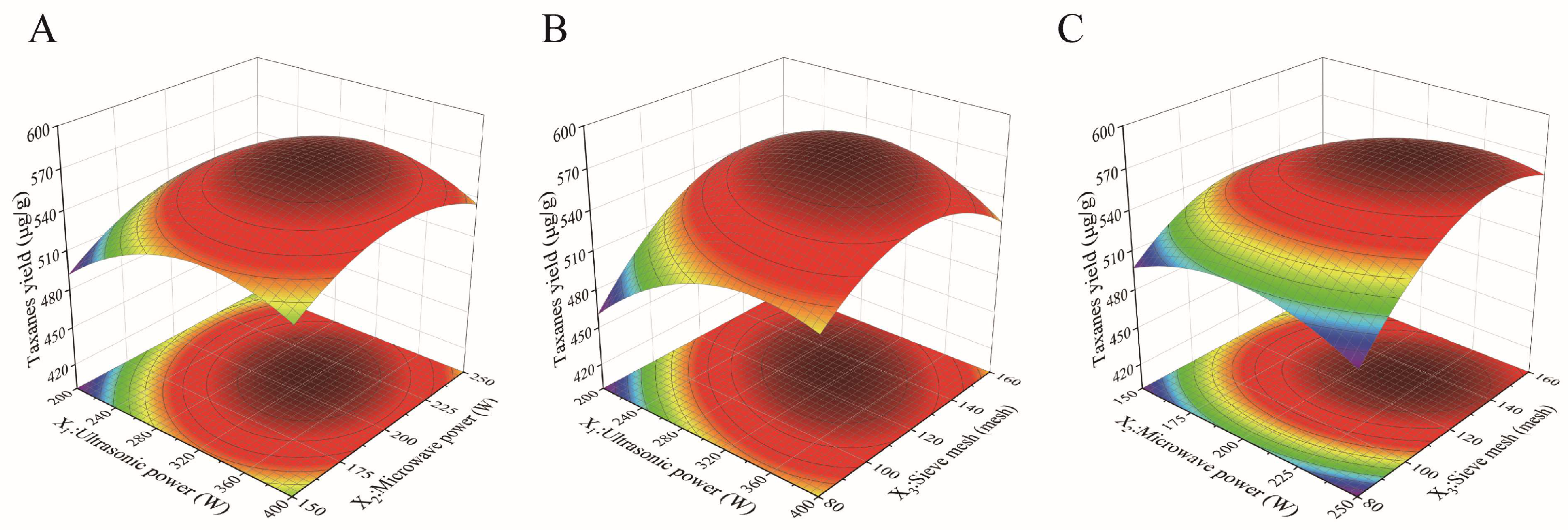
2.3. Analysis of Central Composite Design (CCD) Results
2.4. Comparison of Different Extraction Methods
2.4.1. Taxanes Yield
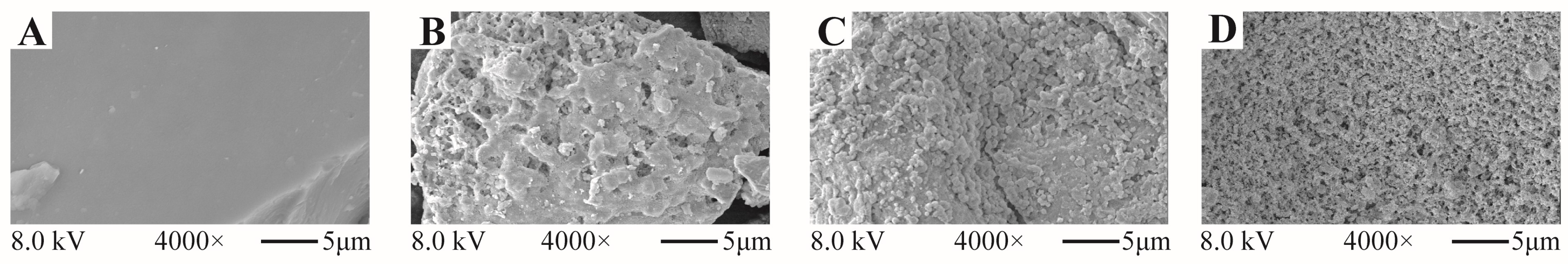
2.4.2. Surface Morphology (SEM)
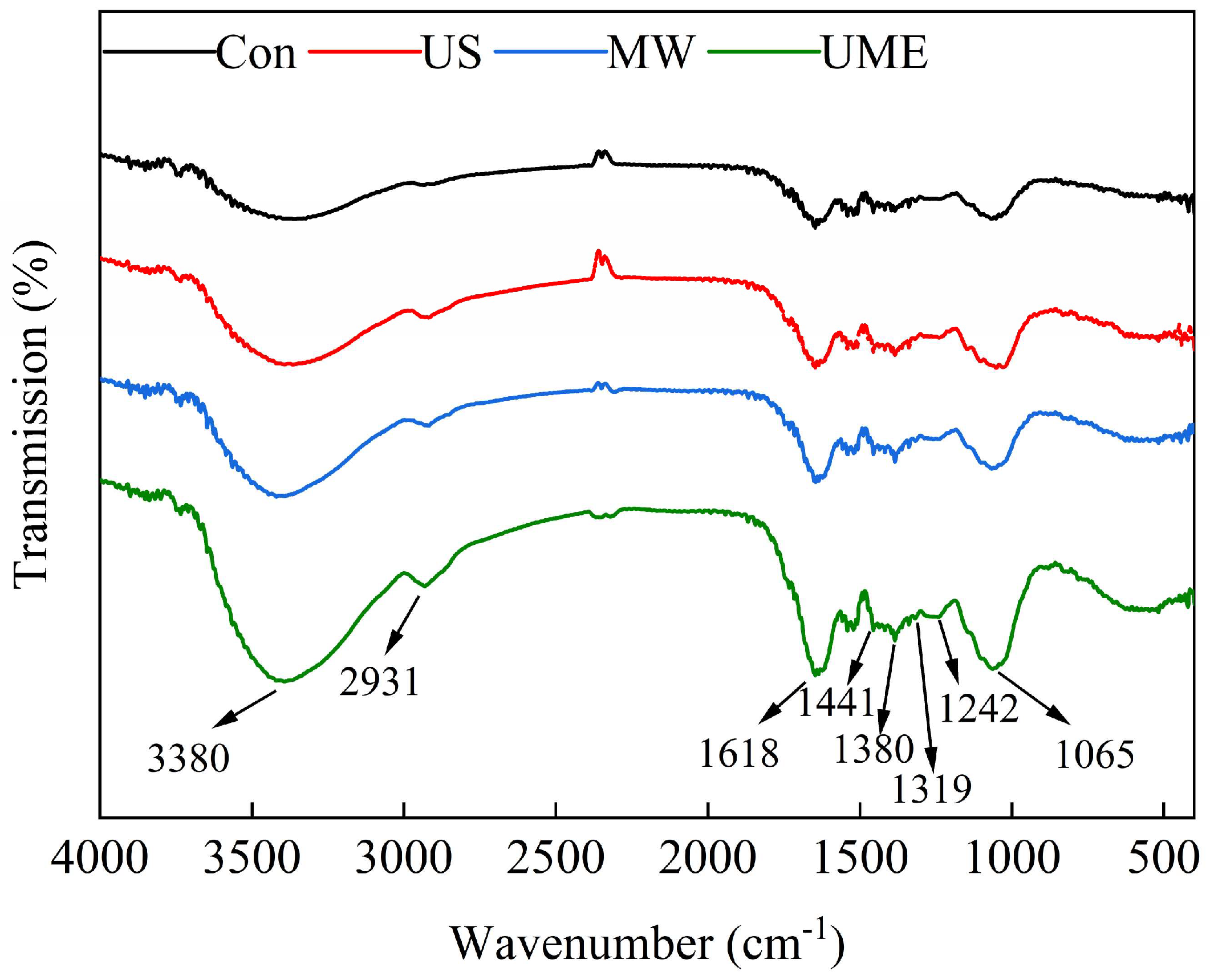
2.4.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4.4. Thermal Properties
3. Materials and Methods
3.1. Materials
3.2. Taxanes Extraction
3.3. Measurement of Taxanes
3.4. Single-Factor Experiments
3.5. PBD
3.6. CCD
3.7. Scanning Electron Microscopy (SEM)
3.8. FTIR of Residues
3.9. Thermal Property Analysis of Residues
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Lu, X.; Zheng, T.; Guo, X.; Chen, Q.; Tang, Z. Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus x media by gas chromatography-mass spectrometry. Open Life Sci. 2021, 16, 287–296. [Google Scholar] [CrossRef]
- Sabzehzari, M.; Zeinali, M.; Naghavi, M.R. Alternative sources and metabolic engineering of Taxol: Advances and future perspectives. Biotechnol. Adv. 2020, 43, 107569. [Google Scholar] [CrossRef]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martinez, R.A.; Canovas-Diaz, M.; de Diego Puente, T. A Compressive Review about Taxol((R)): History and Future Challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Y.; Ni, Z.-Y.; Zhang, M.-L.; Wang, Y.-F.; Shi, Q.-W.; Huo, C.-H.; Sauriol, F.; Kiyota, H.; Gu, Y.-C.; et al. New 6/8/6-Taxanes Isolated from the Heartwood of Taxus cuspidata. Helv. Chim. Acta 2012, 95, 1566–1572. [Google Scholar] [CrossRef]
- Liu, W.C.; Gong, T.; Zhu, P. Advances in exploring alternative Taxol sources. RSC Adv. 2016, 6, 48800–48809. [Google Scholar] [CrossRef]
- Fan, X.-H.; Wang, L.-T.; Chang, Y.-H.; An, J.-Y.; Zhu, Y.-W.; Yang, Q.; Meng, D.; Fu, Y.-j. Application of green and recyclable menthol-based hydrophobic deep eutectic solvents aqueous for the extraction of main taxanes from Taxus chinensis needles. J. Mol. Liq. 2021, 326, 114970. [Google Scholar] [CrossRef]
- Wang, S.J.; Li, C.; Wang, H.J.; Zhong, X.M.; Zhao, J.; Zhou, Y.J. A process optimization study on ultrasonic extraction of paclitaxel from Taxus cuspidata. Prep. Biochem. Biotechnol. 2016, 46, 274–280. [Google Scholar] [CrossRef]
- Luo, H.; Nie, Y.K.; Fu, Y.J.; Zu, Y.G.; Li, S.M.; Liu, W.; Zhang, L.; Luo, M.; Kong, Y.; Li, Z.N. Determination of main taxoids in Taxus species by microwave-assisted extraction combined with LC-MS/MS analysis. J. Sep. Sci. 2009, 32, 192–201. [Google Scholar] [CrossRef]
- Lu, X.; Zheng, Z.; Li, H.; Cao, R.; Zheng, Y.; Yu, H.; Xiao, J.; Miao, S.; Zheng, B. Optimization of ultrasonic-microwave assisted extraction of oligosaccharides from lotus (Nelumbo nucifera Gaertn.) seeds. Ind. Crop. Prod. 2017, 107, 546–557. [Google Scholar] [CrossRef]
- Estrada-Gil, L.; Contreras-Esquivel, J.C.; Flores-Gallegos, C.; Zugasti-Cruz, A.; Govea-Salas, M.; Mata-Gomez, M.A.; Rodriguez-Herrera, R.; Ascacio-Valdes, J.A. Recovery of Bioactive Ellagitannins by Ultrasound/Microwave-Assisted Extraction from Mexican Rambutan Peel (Nephelium lappaceum L.). Molecules 2022, 27, 1592. [Google Scholar] [CrossRef]
- Li, C.; Chen, S.; Sha, J.; Cui, J.; He, J.; Fu, J.; Shen, Y. Extraction and purification of total flavonoids from Eupatorium lindleyanum DC. and evaluation of their antioxidant and enzyme inhibitory activities. Food Sci. Nutr. 2021, 9, 2349–2363. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, C.; Dai, W.; Jing, H.; Du, X.; Peng, M.; Zhang, Y.; Mo, L.; Wang, L.; Chen, X.; et al. Effects of different extraction on the antibacterial and antioxidant activities of phenolic compounds of areca nut (husks and seeds). J. Food Meas. Charact. 2022, 16, 1502–1515. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, J.; Liu, H.; Brennan, C.S.; Liu, J.; Zou, X. Protective effects of the flavonoid fraction obtained from pomelo fruitlets through ultrasonic-associated microwave extraction against AAPH-induced erythrocyte hemolysis. RSC Adv. 2019, 9, 16007–16017. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Fang, Z. Efficient physical extraction of active constituents from edible fungi and their potential bioactivities: A review. Trends Food Sci. Technol. 2020, 105, 468–482. [Google Scholar] [CrossRef]
- Xu, N.; Sun, Y.H.; Guo, X.L.; Liu, C.; Mao, Q.; Hou, J.M. Optimization of ultrasonic—Microwave synergistic extraction of polysaccharides from Morchella conica. J. Food Process. Pres. 2017, 42, e13423. [Google Scholar] [CrossRef]
- Kwansang, J.; Chen, C.J.; Chaiprateep, E.O. Optimization of water-based ultrasonic-microwave assisted extraction (UMAE) of bioactive compounds from Garcinia mangostana pericarp. J. Complement. Integr. Med. 2022, 19, 219–225. [Google Scholar] [CrossRef]
- Mason, T.J.; Chemat, F.; Vinatoru, M. The Extraction of Natural Products using Ultrasound or Microwaves. Curr. Org. Chem. 2011, 15, 237–247. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, D.; Zhang, R.; Wang, G.; Zhang, Y. Extraction and purification of polyphenols and determination of antioxidant activity. Trans. CSAE 2020, 36, 324–332. [Google Scholar] [CrossRef]
- Banožić, M.; Banjari, I.; Flanjak, I.; Paštar, M.; Vladić, J.; Jokić, S. Optimization of MAE for the Separation of Nicotine and Phenolics from Tobacco Waste by Using the Response Surface Methodology Approach. Molecules 2021, 26, 4363. [Google Scholar] [CrossRef]
- Xu, L.; He, W.; Lu, M.; Yuan, B.; Zeng, M.; Tao, G.; Qin, F.; Chen, J.; Guan, Y.; He, Z. Enzyme-assisted ultrasonic-microwave synergistic extraction and UPLC-QTOF-MS analysis of flavonoids from Chinese water chestnut peels. Ind. Crop. Prod. 2018, 117, 179–186. [Google Scholar] [CrossRef]
- Jovanovic-Malinovska, R.; Kuzmanova, S.; Winkelhausen, E. Application of ultrasound for enhanced extraction of prebiotic oligosaccharides from selected fruits and vegetables. Ultrason. Sonochem. 2015, 22, 446–453. [Google Scholar] [CrossRef]
- Paoli, M.; Bighelli, A.; Castola, V.; Tomi, F.; Casanova, J. Quantification of taxanes in a leaf and twig extract from Taxus baccata L. using 13C-NMR spectroscopy. Magn. Reson. Chem. 2013, 51, 756–761. [Google Scholar] [CrossRef]
- Wang, Y.; Gamage, J.; Zhang, Z. Separation of taxanes from Taxus canadensis using dynamic pressurized liquid extraction. Biotechnol. Bioprocess Eng. 2011, 16, 769–776. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G.; Han, Q.; Yan, X.; Feng, L.; Zhang, R.; Zhang, Y. Extraction of the polyphenols from kiwifruit peel and its effect on melanin in B16 cells. Trans. CSAE 2022, 38, 317–325. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Meng, H.; Li, W.; Wang, S. Ultrasonic Extraction and Separation of Taxanes from Taxus cuspidata Optimized by Response Surface Methodology. Separations 2022, 9, 193. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Li, W.; Tang, Y.; Meng, H.; Wang, S. Improving the extraction yield of taxanes from Taxus cuspidata needles using cold plasma. J. Appl. Res. Med. Aroma. 2023, 34, 100457. [Google Scholar] [CrossRef]
- Wianowska, D.; Hajnos, M.Ł.; Dawidowicz, A.L.; Oniszczuk, A.; Waksmundzka-Hajnos, M.; Głowniak, K. Extraction Methods of 10-Deacetylbaccatin III, Paclitaxel, and Cephalomannine from Taxus baccata L. Twigs: A Comparison. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 589–601. [Google Scholar] [CrossRef]
- Chamutpong, S.; Chen, C.J.; Chaiprateep, E.O. Optimization ultrasonic-microwave-assisted extraction of phenolic compounds from Clinacanthus nutans using response surface methodology. J. Adv. Pharm. Technol. 2021, 12, 190–195. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, X.; Zhang, Q.; Du, X.; Yang, L.; Zu, Y.; Yang, F. Simultaneous synergistic microwave–ultrasonic extraction and hydrolysis for preparation of trans-resveratrol in tree peony seed oil-extracted residues using imidazolium-based ionic liquid. Ind. Crop. Prod. 2016, 94, 266–280. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Meng, H.; Li, W.; Wang, S. Identification and Optimization of a Novel Taxanes Extraction Process from Taxus cuspidata Needles by High-Intensity Pulsed Electric Field. Molecules 2022, 27, 3010. [Google Scholar] [CrossRef]
- Wang, X.; Peng, S.; Peng, M. Extraction of total polyphenols from Eucommia ulmoides oliver leaves assisted by ultrasound-microwave and their antioxidant activity. Chem. Ind. For. Prod. 2018, 38, 85–92. [Google Scholar]
- Zhang, D.; Bao, Y.; Ma, Z.; Zhou, J.; Chen, H.; Lu, Y.; Zhu, L.; Chen, X. Optimization of fermentation medium and conditions for enhancing valinomycin production by Streptomyces sp. ZJUT-IFE-354. Prep. Biochem. Biotechnol. 2022, 53, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, S.; Chen, C.; Lin, J.; Chen, L.; Wang, D.; Hu, J.; Chen, X.; Yang, J.; Li, Y.; et al. Optimization of extraction and bioactivity detection of celery leaf flavonoids using BP neural network combined with genetic algorithm and response. Prep. Biochem. Biotechnol. 2022, 52, 648–656. [Google Scholar] [CrossRef]
- Zhao, G.; Gao, Q.; Hadiatullah, H.; Zhang, J.; Zhang, A.; Yao, Y. Effect of wheat bran steam explosion pretreatment on flavors of nonenzymatic browning products. LWT 2021, 135, 110026. [Google Scholar] [CrossRef]
- Medina, J.D.C.; Woiciechowski, A.; Filho, A.Z.; Nigam, P.S.; Ramos, L.P.; Soccol, C.R. Steam explosion pretreatment of oil palm empty fruit bunches (EFB) using autocatalytic hydrolysis: A biorefinery approach. Bioresour. Technol. 2016, 199, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Wu, M. Extraction and Purification of Paclitaxel and Preparation and Functional Evaluation of Anti-Hepatocellular Carcinoma Targeting Nanoparticles with pH Response. Ph.D. Dissertation, Northeast Forestry University, Harbin, China, 2020. [Google Scholar]
- Li, J.; Li, Z.; Ma, Q.; Zhou, Y. Enhancement of anthocyanins extraction from haskap by cold plasma pretreatment. Innov. Food Sci. Emerg. Technol. 2023, 84, 103294. [Google Scholar] [CrossRef]
- Kurek, M.A.; Karp, S.; Wyrwisz, J.; Niu, Y. Physicochemical properties of dietary fibers extracted from gluten-free sources: Quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus) and millet (Panicum miliaceum). Food Hydrocoll. 2018, 85, 321–330. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Zhang, Y.; Meng, H.; Li, Z.; Feng, X. Characterization of cellulose from olive pomace extracted by different methods. J. Chin. Inst. Food Sci. Technol. 2022, 22, 306–313. [Google Scholar] [CrossRef]
- Moczkowska, M.; Karp, S.; Niu, Y.; Kurek, M.A. Enzymatic, enzymatic-ultrasonic and alkaline extraction of soluble dietary fibre from flaxseed—A physicochemical approach. Food Hydrocolloid. 2019, 90, 105–112. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, S.; Gao, S.; Guo, W.; Xie, X.; Kawul, G.; Wang, M.; Feng, Y.; Chen, C. Optimization of the composite enzyme extraction of polysaccharide from Erythronium sibiricum bulb and its immunoregulatory activities. Prep. Biochem. Biotechnol. 2022, 52, 681–690. [Google Scholar] [CrossRef]

| A (W) | B (W) | C (s) | D (°C) | E (g/mL) | F | G (Mesh) | Extraction Yields (μg/g) | |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 (400) | −1 (150) | 1 (150) | 1 (55) | −1 (1:50) | 1 (3) | 1 (160) | 510.37 |
| 2 | 1 | −1 | 1 | 1 | 1 (1:70) | −1 (1) | −1 (80) | 453.56 |
| 3 | −1 (200) | 1 (250) | −1 (90) | 1 | 1 | −1 | 1 | 488.16 |
| 4 | 1 | −1 | −1 | −1 (45) | 1 | −1 | 1 | 541.87 |
| 5 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | 473.73 |
| 6 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 470.95 |
| 7 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | 461.16 |
| 8 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 438.77 |
| 9 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | 535.19 |
| 10 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | 570.77 |
| 11 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 470.00 |
| 12 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 435.08 |
| Origin | Square Sum of Dispersion | df | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Model | 19,446.64 | 7 | 2778.09 | 11.36 | 0.0167 |
| A | 3039.35 | 1 | 3039.35 | 12.43 | 0.0243 |
| B | 2351.08 | 1 | 2351.08 | 9.62 | 0.0362 |
| C | 1001.29 | 1 | 1001.29 | 4.10 | 0.1130 |
| D | 1490.55 | 1 | 1490.55 | 6.10 | 0.0690 |
| E | 13.99 | 1 | 13.99 | 0.057 | 0.8227 |
| F | 422.06 | 1 | 422.06 | 1.73 | 0.2592 |
| G | 11,128.32 | 1 | 11,128.32 | 45.52 | 0.0025 |
| Residual | 977.90 | 4 | 244.47 | ||
| Cor total | 20,424.54 | 11 |
| No. | Ultrasonic Power (X1) | Microwave Power (X2) | Sieve Mesh Number (X3) | Extraction Yields (Y) |
|---|---|---|---|---|
| 1 | 1.682 (468) | 0 (200) | 0 (120) | 502.72 |
| 2 | 0 (300) | −1.682 (116) | 0 | 496.97 |
| 3 | 0 | 0 | 0 | 573.99 |
| 4 | 0 | 0 | 0 | 560.99 |
| 5 | 0 | 0 | −1.682 (50) | 440.85 |
| 6 | 1 (400) | 1 (250) | 1 (160) | 518.84 |
| 7 | −1 (200) | −1 (150) | −1 (80) | 427.04 |
| 8 | 0 | 0 | 0 | 559.86 |
| 9 | 0 | 0 | 0 | 574.76 |
| 10 | −1.682 (132) | 0 | 0 | 479.65 |
| 11 | 0 | 0 | 0 | 563.23 |
| 12 | 0 | 0 | 1.682 (190) | 519.32 |
| 13 | 0 | 1.682 (284) | 0 | 520.03 |
| 14 | −1 | −1 | 1 | 474.47 |
| 15 | 0 | 0 | 0 | 585.97 |
| 16 | −1 | 1 | −1 | 442.80 |
| 17 | 1 | −1 | −1 | 504.03 |
| 18 | 1 | −1 | 1 | 473.75 |
| 19 | 1 | 1 | −1 | 491.59 |
| 20 | −1 | 1 | 1 | 531.47 |
| Sources | Sum of Squares | df | Mean Square | F Value | p Values |
|---|---|---|---|---|---|
| Model | 41,781.48 | 9 | 4642.39 | 41.83 | <0.0001 |
| X1 | 1674.67 | 1 | 1674.67 | 15.09 | 0.0030 |
| X2 | 1522.72 | 1 | 1522.72 | 13.72 | 0.0041 |
| X3 | 5143.59 | 1 | 5143.59 | 46.34 | <0.0001 |
| X1X2 | 201.11 | 1 | 201.11 | 1.81 | 0.2080 |
| X1X3 | 2419.82 | 1 | 2419.82 | 21.80 | 0.0009 |
| X2X3 | 1219.46 | 1 | 1219.46 | 10.99 | 0.0078 |
| X12 | 12,101.65 | 1 | 12,101.65 | 109.04 | <0.0001 |
| X22 | 7529.60 | 1 | 7529.60 | 67.84 | <0.0001 |
| X32 | 15,602.74 | 1 | 15,602.74 | 140.58 | <0.0001 |
| Residual | 1109.85 | 10 | 110.99 | ||
| Lack of fit | 586.63 | 5 | 117.33 | 1.12 | 0.4516 |
| pure error | 523.22 | 5 | 104.64 | ||
| Cor total | 42,891.33 | 19 |
| Methods | Yield (μg/g) | |||||
|---|---|---|---|---|---|---|
| 10-DAB III | Baccatine III | 10-DAT | Cephalomannine | Paclitaxel | Taxanes | |
| US | 271.38 ± 9.37 b | 31.69 ± 1.75 a | 78.45 ± 6.58 b | 44.10 ± 1.96 b | 77.25 ± 6.34 a | 502.87 ± 26.00 b |
| MW | 230.31 ± 7.28 c | 25.54 ± 1.83 b | 52.05 ± 2.09 c | 37.35 ± 2.21 c | 57.43 ± 5.35 b | 402.68 ± 18.76 c |
| UME | 289.78 ± 9.55 a | 31.70 ± 1.79 a | 98.60 ± 2.11 a | 59.45 ± 2.46 a | 90.79 ± 13.62 a | 570.32 ± 29.53 a |
| Treatment | Ton (°C) | Tend (°C) | Tpeak (°C) | ∆H (J/g) |
|---|---|---|---|---|
| Con | 298.40 ± 0.13 | 326.12 ± 0.42 | 314.54 ± 0.35 | −7.92 ± 0.67 |
| US | 299.35 ± 0.21 | 323.13 ± 0.14 | 310.65 ± 0.17 | −5.86 ± 0.13 |
| MW | 300.45 ± 0.15 | 324.12 ± 0.16 | 310.01 ± 0.16 | −5.69 ± 0.13 |
| UME | 298.40 ± 0.22 | 319.49 ± 0.23 | 307.40 ± 0.46 | −5.19 ± 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Zhang, Y.; Li, W.; Tang, Y.; Wang, S. Parameter Optimization of Ultrasonic–Microwave Synergistic Extraction of Taxanes from Taxus cuspidata Needles. Molecules 2023, 28, 7746. https://doi.org/10.3390/molecules28237746
Zhao Z, Zhang Y, Li W, Tang Y, Wang S. Parameter Optimization of Ultrasonic–Microwave Synergistic Extraction of Taxanes from Taxus cuspidata Needles. Molecules. 2023; 28(23):7746. https://doi.org/10.3390/molecules28237746
Chicago/Turabian StyleZhao, Zirui, Yajing Zhang, Wenlong Li, Yuanhu Tang, and Shujie Wang. 2023. "Parameter Optimization of Ultrasonic–Microwave Synergistic Extraction of Taxanes from Taxus cuspidata Needles" Molecules 28, no. 23: 7746. https://doi.org/10.3390/molecules28237746
APA StyleZhao, Z., Zhang, Y., Li, W., Tang, Y., & Wang, S. (2023). Parameter Optimization of Ultrasonic–Microwave Synergistic Extraction of Taxanes from Taxus cuspidata Needles. Molecules, 28(23), 7746. https://doi.org/10.3390/molecules28237746






