Antifungal Constituents of Piper crocatum and Their Activities as Ergosterol Biosynthesis Inhibitors Discovered via In Silico Study Using ADMET and Drug-Likeness Analysis
Abstract
1. Introduction
2. Results
2.1. Structure Elucidation of Compounds 1–3
2.1.1. Structure Determinations of Compound 1
2.1.2. Structure Determination of Compound 2
| No. | 13C NMR | 1H NMR |
|---|---|---|
| δc | δH (Integral, Mult., J = Hz) | |
| 1 | 59.5 | 4.13 (2H, d, 6.5) |
| 2 | 123.1 | 5.39 (1H, t, 7) |
| 3 | 140.4 | - |
| 4 | 39.9 | 1.97 (2H, m) |
| 5 | 32.8 | 1.36 (1H, m) |
| 6 | 37.5 | 1.05 (2H, m) |
| 7 | 25.2 | 1.23 (2H, m) |
| 8 | 37.4 | 1.23 (2H, m) |
| 9 | 36.7 | 1.12 (2H, m) |
| 10 | 29.8 | 1.23 (2H, m) |
| 11 | 37.3 | 1.23 (2H, m) |
| 12 | 24.5 | 1.23 (2H, m) |
| 13 | 24.9 | 1.36 (2H, m) |
| 14 | 39.4 | 1.12 (2H, m) |
| 15 | 28.1 | 1.50 (1H, m) |
| 16 | 22.7 | 0.84 (3H, d, 7) |
| 17 | 22.8 | 0.84 (3H, d, 7) |
| 18 | 16.2 | 1.65 (3H, s) |
| 19 | 32.7 | 1.36 (1H, m) |
| 20 | 19.7 | 0.83 (3H, t, 3) |
| 21 | 19.8 | 0.83 (3H, t, 3) |
2.1.3. Structure Determinations of Compound 3
2.2. Antifungal Assay
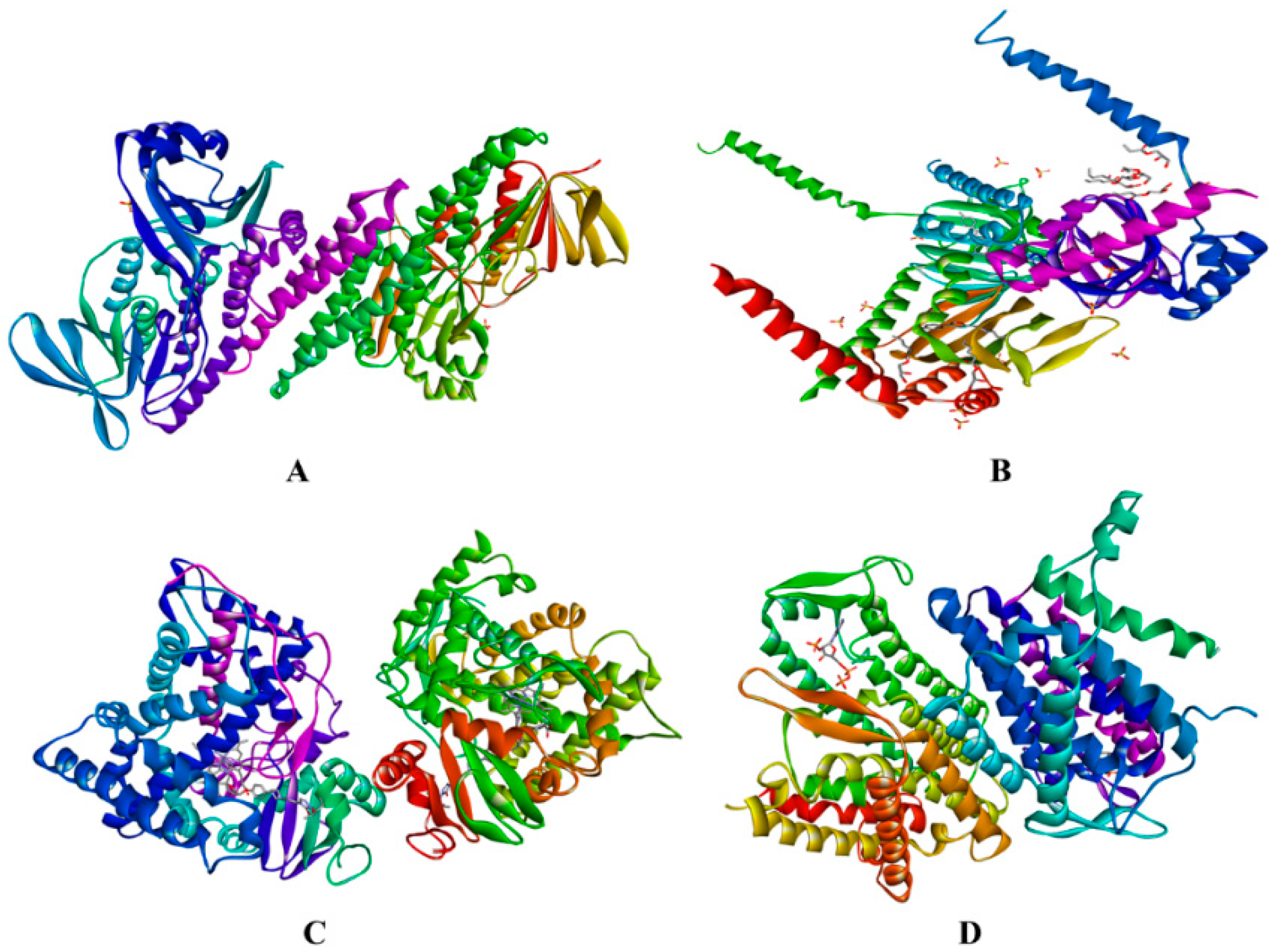
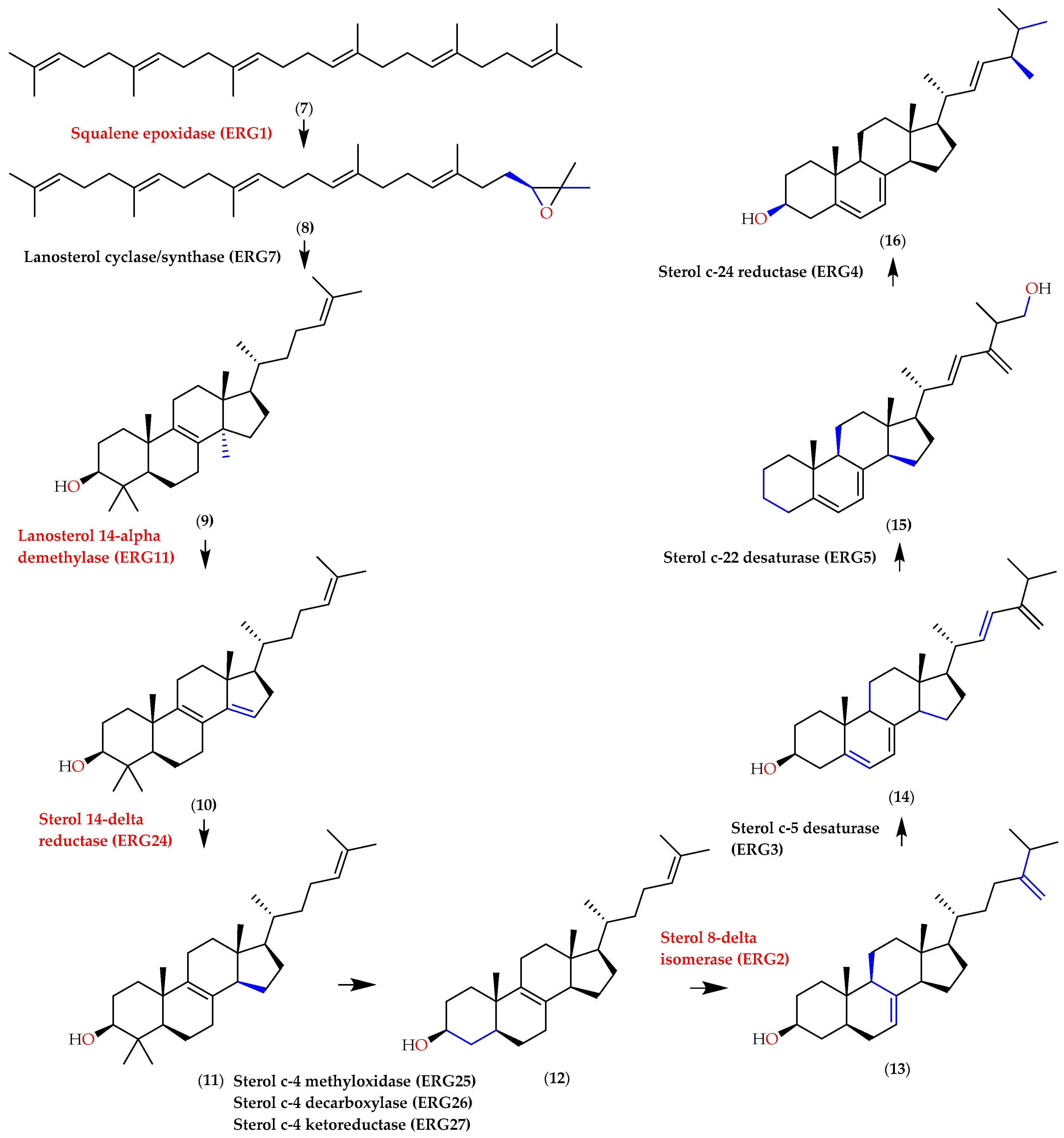
2.3. In Silico Assay
2.4. ADMET and Drug-Likeness Analysis
3. Discussion
4. Materials and Methods
4.1. Material
4.1.1. Plant Material
4.1.2. In Vitro Assay Materials
4.1.3. In Silico Assay Materials
4.1.4. ADMET and Drug-Likeness Analysis
4.1.5. Instruments
4.2. Methods
4.2.1. Preparation of Extracts and Compounds
4.2.2. In Vitro Assay
Structure Determination of Compounds 1–3
Antifungal Assay
4.2.3. In Silico Assay
4.2.4. ADMET and Drug-Likeness Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsai, P.W.; Chen, Y.T.; Hsu, P.C.; Lan, C.Y. Study of Candida albicans and Its Interactions with The Host: A Mini Review. Biomed. J. 2013, 3, 51–64. [Google Scholar] [CrossRef]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida Species: Current Epidemiology, Pathogenicity, Biofilm Formation, Natural Antifungal Products and New Therapeutic Options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Arthington-Skaggs, B.A.; Jradi, H.; Desai, T.; Morrison, C.J. Quantitation of Ergosterol Content: Novel Method for Determination of Fluconazole Susceptibility of Candida albicans. J. Clin. Microbiol. 1999, 37, 3332–3337. [Google Scholar] [CrossRef]
- Flowers, S.A.; Colón, B.; Whaley, S.G.; Schuler, M.A.; David Rogers, P. Contribution of Clinically Derived Mutations in ERG11 to Azole Resistance in Candida albicans. Antimicrob. Agents Chemother. 2015, 59, 450–460. [Google Scholar] [CrossRef] [PubMed]
- French, L.; Horton, J.; Matousek, M. Abnormal Vaginal Discharge: What Does and Does Not Work in Treating Underlying Causes. J. Fam. Pract. 2004, 53, 890–903. [Google Scholar] [PubMed]
- Whaley, S.G.; Rogers, P.D. Azole Resistance in Candida Glabrata. Curr. Infect. Dis. Rep. 2016, 18, 19–21. [Google Scholar] [CrossRef]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-Albicans Candida Species. Front. Microbiol. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Semenyuta, I.V.; Kobzar, O.L.; Hodyna, D.M.; Brovarets, V.S.; Metelytsia, L.O. In Silico Study of 4-Phosphorylated Derivatives of 1,3-Oxazole as Inhibitors of Candida albicans Fructose-1,6-Bisphosphate Aldolase II. Heliyon 2019, 5, e01462. [Google Scholar] [CrossRef] [PubMed]
- Fatmawaty; Anggreni, N.G.M.; Fadhil, N.; Prasasty, V.D. Potential in Vitro and in Vivo Antioxidant Activities from Piper crocatum and Persea Americana Leaf Extracts. Biomed. Pharmacol. J. 2019, 12, 661–667. [Google Scholar] [CrossRef]
- Suri, M.A.; Azizah, Z.; Asra, R. A Review: Traditional Use, Phytochemical And Pharmacological Review Of Red Betel Leaves (Piper crocatum Ruiz & Pav). Asian J. Pharm. Res. Dev. 2021, 9, 159–163. [Google Scholar]
- Rodrigues, R.V.; Lanznaster, D.; Longhi Balbinot, D.T.; Gadotti, V.D.M.; Facundo, V.A.; Santos, A.R.S. Antinociceptive Effect of Crude Extract, Fractions and Three Alkaloids Obtained from Fruits of Piper Tuberculatum. Biol. Pharm. Bull. 2009, 32, 1809–1812. [Google Scholar] [CrossRef]
- Apeh, V.O.; Njoku, O.U.; Nwodo, F.O.C.; Chukwuma, I.F.; Emmanuel, A.A. In Silico Drug-Like Properties Prediction and in Vivo Antifungal Potentials of Citrullus Lanatus Seed Oil against Candida albicans. Arab. J. Chem. 2022, 15, 103578. [Google Scholar] [CrossRef]
- Durant-Archibold, A.A.; Santana, A.I.; Gupta, M.P. Ethnomedical Uses and Pharmacological Activities of Most Prevalent Species of Genus Piper in Panama: A Review. J. Ethnopharmacol. 2018, 217, 63–82. [Google Scholar] [CrossRef] [PubMed]
- De Lourdes Reyes-Escogido, M.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and Pharmacological Aspects of Capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef]
- Rehse, K.; Shahrouri, T. Hydroxylamine Derivatives. Arch. Pharm. Med. Chem. 1998, 365–367. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.; Zhang, H.; Wang, Y.; Nie, S.; Li, C. Quantification of Total Polysaccharides and Triterpenoids in Ganoderma Lucidum and Ganoderma Atrum by Near Infrared Spectroscopy and Chemometrics. Food Chem. 2012, 135, 268–275. [Google Scholar] [CrossRef]
- Tshilanda, D.D.; Onyamboko, D.N.; Babady-Bila, P.; Ngbolua, K.-N.; Tshibangu, D.S.; dia Fita Dibwe, E.; Mpiana, P.T. Anti-Sickling Activity of Ursolic Acid Isolated from the Leaves of Ocimum gratissimum L. (Lamiaceae). Nat. Prod. Bioprospect. 2015, 5, 215–221. [Google Scholar] [CrossRef]
- Erwin; Pusparohmana, W.R.; Safitry, R.D.; Marliana, E.; Usman; Kusuma, I.W. Isolation and Characterization of Stigmasterol and β-Sitosterol from Wood Bark Extract of Baccaurea Macrocarpa Miq. Mull. Arg. Rasayan J. Chem. 2020, 13, 2552–2558. [Google Scholar] [CrossRef]
- Forgo, P.; Kövér, K.E. Gradient Enhanced Selective Experiments in the 1H NMR Chemical Shift Assignment of the Skeleton and Side-Chain Resonances of Stigmasterol, a Phytosterol Derivative. Steroids 2004, 69, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ravipati, A.S.; Reddy, N.; Koyyalamudi, S.R. Biologically Active Compounds from the Genus Uncaria (Rubiaceae), 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 43, ISBN 9780444634306. [Google Scholar]
- Kuete, V. Potential of Cameroonian Plants and Derived Products against Microbial Infections: A Review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef]
- Muslim, S.N.; Hussin, Z.S. Chemical Compounds and Synergistic Antifungal Properties of Thymus Kotschanus Essential Oil Plus Ketoconazole against Candida spp. Gene Rep. 2020, 21, 100916. [Google Scholar] [CrossRef]
- Falcón-Cano, G.; Cabrera-Pérez, M.Á.; Molina, C. ADME Prediction with KNIME: In Silico Aqueous Solubility Consensus Model Based on Supervised Recursive Random Forest Approaches. ADMET DMPK 2020, 8, 251–273. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Nahar, N.; Rashid, R.B.; Chowdhury, A.; Rashid, M.A. Computational Investigations of Physicochemical, Pharmacokinetic, Toxicological Properties and Molecular Docking of Betulinic Acid, a Constituent of Corypha taliera (Roxb.) with Phospholipase A2 (PLA2). BMC Complement. Altern. Med. 2018, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Beaumont, K.; Maurer, T.S.; Di, L. Volume of Distribution in Drug Design. J. Med. Chem. 2015, 58, 5691–5698. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Chen, C.; Yang, J. Predictive Model of Blood-Brain Barrier Penetration of Organic Compounds. Acta Pharmacol. Sin. 2005, 26, 500–512. [Google Scholar] [CrossRef]
- Guttman, Y.; Kerem, Z. Computer-Aided (In Silico) Modeling of Cytochrome P450-Mediated Food–Drug Interactions (FDI). Int. J. Mol. Sci. 2022, 23, 8498. [Google Scholar] [CrossRef]
- El-Shamy, N.T.; Alkaoud, A.M.; Hussein, R.K.; Ibrahim, M.A.; Alhamzani, A.G.; Abou-Krisha, M.M. DFT, ADMET and Molecular Docking Investigations for the Antimcrobial Activity of 6,6’-Diamino-1,1’,3,3’-Tetramethyl-5,5’-(4-Chlorobenzylidene)Bis[Pyrimidine-2,4(1H,3H)-Dione]. Molecules 2022, 27, 620. [Google Scholar] [CrossRef]
- Lister, I.N.E.; Ginting, C.N.; Girsang, E.; Nataya, E.D.; Azizah, A.M.; Widowati, W. Hepatoprotective Properties of Red Betel (Piper crocatum Ruiz and Pav) Leaves Extract Towards H2O2-Induced HepG2 Cells via Anti-Inflammatory, Antinecrotic, Antioxidant Potency. Saudi Pharm. J. 2020, 28, 1182–1189. [Google Scholar] [CrossRef]
- Fadlilah, M. Benefit of Red Betel (Piper crocatum Ruiz & Pav.) As Antibiotics. J. Major. 2015, 4, 71–75. [Google Scholar]
- Safithri, M.; Fahma, F. Potency of Piper crocatum Decoction as an Antihiperglycemia in Rat Strain Sprague Dawley. Hayati J. Biosci. 2008, 15, 45–48. [Google Scholar] [CrossRef][Green Version]
- Suchodolski, J.; Muraszko, J.; Bernat, P.; Krasowska, A. A Crucial Role for Ergosterol in Plasma Membrane Composition, Localisation, and Activity of Cdr1p and H+-ATPase in Candida albicans. Microorganisms 2019, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Dadar, M.; Tiwari, R.; Karthik, K.; Chakraborty, S.; Shahali, Y.; Dhama, K. Candida albicans-Biology, Molecular Characterization, Pathogenicity, and Advances in Diagnosis and Control–An Update. Microb. Pathog. 2018, 117, 128–138. [Google Scholar] [CrossRef]
- Tallei, T.E.; Tumilaar, S.G.; Niode, N.J.; Fatimawali; Kepel, B.J.; Idroes, R.; Effendi, Y.; Sakib, S.A.; Emran, T. Bin Potential of Plant Bioactive Compounds as SARS-CoV-2 Main Protease (Mpro) and Spike (S) Glycoprotein Inhibitors: A Molecular Docking Study. Scientifica 2020, 2020, 6307457. [Google Scholar] [CrossRef]
- Edikresnha, D.; Suciati, T.; Suprijadi; Khairurrijal, K. Freeze-Thawed Hydrogel Loaded by Piper crocatum Extract with In-Vitro Antibacterial and Release Tests. J. Mater. Res. Technol. 2021, 15, 17–36. [Google Scholar] [CrossRef]
- Madhumita, M.; Guha, P.; Nag, A. Bio-Actives of Betel Leaf (Piper betle L.): A Comprehensive Review on Extraction, Isolation, Characterization, and Biological Activity. Phyther. Res. 2020, 34, 2609–2627. [Google Scholar] [CrossRef] [PubMed]
- Lister, N.E.; Viany, R.D.; Nasution, A.N.; Zein, R.; Manjang, Y.; Munaf, E. Antimicrobial Activities of Methanol Extract of Sirih Merah (Piper crocatum L.) Lleaf. J. Chem. Pharm. Res. 2014, 6, 650–654. [Google Scholar]
- Lister, I.N.E.; Ginting, C.N.; Girsang, E.; Armansyah, A.; Marpaung, H.H.; Sinaga, A.P.F.; Handayani, R.A.S.; Rizal, R. Antioxidant Properties of Red Betel (Piper crocatum) Leaf Extract and Its Compounds. J. Nat. Remedies 2019, 19, 198–205. [Google Scholar] [CrossRef]
- Lely, N.; Arifin, H.; Aldi, Y.; Wahyuni, F.S. Anti-Inflammatory Effects of Methanol Extract, Hexane, Ethyl Acetate, and Butanol Fraction of Piper crocatum Ruiz & Pav Leaves on Lipopolysaccharide-Induced RAW 264.7 Cells. Pharmacogn. J. 2021, 13, 1341–1346. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Pessoa, C.; de Moraes, M.O.; de Alencar, N.M.; Mesquita, R.O.; Lima, M.W.; Alves, A.P.N.; Pessoa, O.D.L.; Chaves, J.H.; Silveira, E.R.; et al. In Vivo Growth Inhibition of Sarcoma 180 by Piperlonguminine, an Alkaloid Amide from the Piper Species. J. Appl. Toxicol. 2008, 28, 599–607. [Google Scholar] [CrossRef]
- Lima, C.N.F.; de Lima, L.F.; Correia, D.B.; Machado, S.T.; de Sousa, J.P.; Santos, E.S.; Delmondes, G.; de Menezes, I.R.A.; Felipe, C.F.B.; Coutinho, H.D.M.; et al. Systematic Review: Medicinal Use and Scientific Elucidation of the Piper Genus for the Treatment of Symptoms and Inflammatory Diseases. J. Med. Plants Res. 2020, 14, 62–72. [Google Scholar] [CrossRef]
- Kusuma, S.A.F.; Hendriani, R.; Genta, A. Antimicrobial Spectrum of Red Piper Betel Leaf Extract (Piper crocatum Ruiz & Pav) as Natural Antiseptics Against Airborne Pathogens. J. Pharm. Sci. Res. 2017, 9, 583. [Google Scholar]
- Shin, S.; Pyun, M.S. Anti-Candida Effects of Estragole in Combination with Ketoconazole or Amphotericin B. Phyther. Res. 2004, 18, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmad, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Ocimum Sanctum Essential Oil and Its Active Principles Exert Their Antifungal Activity by Disrupting Ergosterol Biosynthesis and Membrane Integrity. Res. Microbiol. 2010, 161, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Khan, F.G.; Suri, K.A.; Gupta, B.D.; Satti, N.K.; Dutt, P.; Afrin, F.; Qazi, G.N.; Khan, I.A. In Vitro Antifungal Activity of Hydroxychavicol Isolated from Piper betle L. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.O.; De Oliveira Pereira, F.; De Oliveira, W.A.; De Oliveira Lima, E.; Menezes, E.A.; Cunha, F.A.; De Fátima Formiga Melo Diniz, M. Antifungal Activity and Mode of Action of Carvacrol Against Candida albicans Strains. J. Essent. Oil Res. 2013, 25, 138–142. [Google Scholar] [CrossRef]
- Aziz, A.N.; Ibrahim, H.; Rosmy Syamsir, D.; Mohtar, M.; Vejayan, J.; Awang, K. Antimicrobial Compounds from Alpinia Conchigera. J. Ethnopharmacol. 2013, 145, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.C.; Santana, D.B.; Araújo, R.M.; De Paula, J.E.; Do Nascimento, P.C.; Lopes, N.P.; Braz-Filho, R.; Espindola, L.S. Discovery of The Rapanone and Suberonone Mixture as a Motif for Leishmanicidal and Antifungal Applications. Bioorg. Med. Chem. 2014, 22, 135–140. [Google Scholar] [CrossRef]
- De Assis, P.A.; Theodoro, P.N.E.T.; De Paula, J.E.; Araújo, A.J.; Costa-Lotufo, L.V.; Michel, S.; Grougnet, R.; Kritsanida, M.; Espindola, L.S. Antifungal Ether Diglycosides from Matayba guianensis Aublet. Bioorg. Med. Chem. Lett. 2014, 24, 1414–1416. [Google Scholar] [CrossRef]
- Haider, K.I.; Javaid, A. Antifungal Activity and GC-MS Analysis of N-Butanol Extract of Quinoa (Chenopodium quinoa Willd.) Leaves. Bangladesh J. Bot. 2020, 49, 1045–1051. [Google Scholar] [CrossRef]
- Mukungu, N.; Abuga, K.; Mungai, N.; Bosire, K.; Karumi, E. Isolation and Structural Elucidation of Compounds from The Non-Alkaloidal Extract of Nicandra Physaloides and the Antimicrobial Activity of Withanicandrin. East Cent. Afr. J. Pharm. Sci. 2013, 16, 49–53. [Google Scholar]
- Moshi, M.J.; Joseph, C.C.; Innocent, E.; Nkunya, M.H.H. In Vitro Antibacterial and Antifungal Activities of Extracts and Compounds from Uvaria Scheffleri. Pharm. Biol. 2004, 42, 269–273. [Google Scholar] [CrossRef]
- Alawode, T.T.; Lajide, L.; Olaleye, M.; Owolabi, B. Stigmasterol and β-Sitosterol: Antimicrobial Compounds in the Leaves of Icacina Trichantha Identified by GC–MS. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 10. [Google Scholar] [CrossRef]
- Naqvi, S.F.; Khan, I.H.; Javaid, A. Hexane Soluble Bioactive Components of Chenopodium Murale Stem. J. Weed Sci. Res. 2020, 27, 425–432. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef] [PubMed]
- Ridhay, A.; Noor, A.; Soekamto, N.H.; Harlim, T.; van Altena, I. A Stigmasterol Glycoside from The Root Wood of Melochia Umbellata (Houtt) Stapf Var. Degrabrata K. Indones. J. Chem. 2012, 12, 100–103. [Google Scholar] [CrossRef]
- Seleem, D.; Pardi, V.; Murata, R.M. Review of Flavonoids: A Diverse Group of Natural Compounds with Anti-Candida albicans Activity In Vitro. Arch. Oral Biol. 2017, 76, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Zida, A.; Bamba, S.; Yacouba, A.; Ouedraogo-Traore, R.; Guiguemdé, R.T. Anti-Candida albicans Natural Products, Sources of New Antifungal Drugs: A Review. J. Mycol. Med. 2017, 27, 1–19. [Google Scholar] [CrossRef]
- Siswina, T.; Rustama, M.M.; Sumiarsa, D.; Kurnia, D. Phytochemical Profiling of Piper crocatum and Its Antifungal Activity as Lanosterol 14 Alpha Demethylase CYP51 Inhibitor: A Review. F1000Research 2022, 11, 1115. [Google Scholar] [CrossRef]
- Loeffler, J.; Stevens, D.A. Antifungal Drug Resistance Mechanisms. Clin. Infect. Dis. 2003, 36, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Tanabe, K.; Niimi, M.; Monk, B.C. Candida albicans Drug Resistance–Another Way to Cope with Stress. Microbiology 2007, 153, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Apsari, A.S.; Adiguna, M.S. Resistensi Antijamur Dan Strategi Untuk Mengatasi. Mdvi 2013, 40, 89–95. [Google Scholar]
- Cantón, E.; Espinel-Ingroff, A.; Pemán, J. Trends in Antifungal Susceptibility Testing Using CLSI Reference and Commercial Methods. Expert Rev. Anti. Infect. Ther. 2009, 7, 107–119. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Xia, Y.L.; Ai, S.M.; Liang, J.; Sang, P.; Ji, X.L.; Liu, S.Q. Insights Into Protein–Ligand Interactions: Mechanisms, Models, and Methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- Afriza, D.; Suriyah, W.H.; Ichwan, S.J.A. In Silico Analysis of Molecular Interactions Between The Anti-Apoptotic Protein Survivin and Dentatin, Nordentatin, and Quercetin. J. Phys. Conf. Ser. 2018, 1073, 032001. [Google Scholar] [CrossRef]
- Shawon, J.; Khan, A.M.; Shahriar, I.; Halim, M.A. Improving The Binding Affinity and Interaction of 5-Pentyl-2-Phenoxyphenol Against Mycobacterium Enoyl ACP Reductase by Computational Approach. Inform. Med. Unlocked 2021, 23, 100528. [Google Scholar] [CrossRef]
- Shareef, M.A.; Sirisha, K.; Khan, I.; Sayeed, I.; Jadav, S.S.; Ramu, G.; Kumar, C.G.; Kamal, A.; Babu, B.N. Design, Synthesis, and Antimicrobial Evaluation of 1,4-Dihydroindeno[1,2-c] Pyrazole Tethered Carbohydrazide Hybrids: Exploring Their In Silico ADMET, Ergosterol Inhibition and ROS Inducing Potential. Medchemcomm 2019, 10, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, P.P.; Gupta, V.; Prakash, B. Assessing the Antifungal and Aflatoxin B1 Inhibitory Efficacy of Nanoencapsulated Antifungal Formulation Based on Combination of Ocimum spp. Essential Oils. Int. J. Food Microbiol. 2020, 330, 108766. [Google Scholar] [CrossRef] [PubMed]
- Atkovska, K.; Samsonov, S.A.; Paszkowski-Rogacz, M.; Pisabarro, M.T. Multipose Binding in Molecular Docking. Int. J. Mol. Sci. 2014, 15, 2622–2645. [Google Scholar] [CrossRef]
- Bulusu, G.; Desiraju, G.R. Strong and Weak Hydrogen Bonds in Protein–Ligand Recognition. J. Indian Inst. Sci. 2020, 100, 31–41. [Google Scholar] [CrossRef]
- Morozov, A.V.; Kortemme, T. Potential Functions for Hydrogen Bonds in Protein Structure Prediction and Design. Adv. Protein Chem. 2005, 72, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Ghorai, P.K. Development of Quantum Chemical Method to Calculate Half Maximal Inhibitory Concentration (IC50). Mol. Inform. 2016, 35, 199–206. [Google Scholar] [CrossRef]
- Putri, S.A.; Nur Shadrina, A.A.; Julaeha, E.; Kurnia, D. Potential Nevadensin from Ocimum Basilicum as Antibacterial Agent against Streptococcus Mutans: In Vitro and In Silico Studies. Comb. Chem. High Throughput Screen. 2022, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pagadala, N.S.; Syed, K.; Tuszynski, J. Software for Molecular Docking: A Review. Biophys. Rev. 2017, 9, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Tevyashova, A.N.; Olsufyeva, E.N.; Solovieva, S.E.; Printsevskaya, S.S.; Reznikova, M.I.; Trenin, A.S.; Galatenko, O.A.; Treshalin, I.D.; Pereverzeva, E.R.; Mirchink, E.P.; et al. Structure-Antifungal Activity Relationships of Polyene Antibiotics of the Amphotericin B Group. Antimicrob. Agents Chemother. 2013, 57, 3815–3822. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.Y.; Zhu, B.W.; Wang, X.D.; Qin, L.; Li, D.M.; Miao, L.; Murata, Y. Stability of Polyhydroxylated 1,4-Naphthoquinone Pigment Recovered from Spines of Sea Urchin Strongylocentrotus Nudus. Int. J. Food Sci. Technol. 2012, 47, 1479–1486. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The Antibacterial Properties of Phenolic Isomers, Carvacrol and Thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial Activity of Carvacrol Related to Its Chemical Structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Calvo, J.M.; Barbero, G.R.; Guerrero-Vásquez, G.; Durán, A.G.; Macías, M.; Rodríguez-Iglesias, M.A.; Molinillo, J.M.G.; Macías, F.A. Synthesis, Antibacterial and Antifungal Activities of Naphthoquinone Derivatives: A Structure–Activity Relationship Study. Med. Chem. Res. 2016, 25, 1274–1285. [Google Scholar] [CrossRef]
- Moebius, F.F.; Bermoser, K.; Reiter, R.J.; Hanner, M.; Glossmann, H. Yeast Sterol C8-C7 Isomerase: Identification and Characterization of a High-Affinity Binding Site for Enzyme Inhibitors. Biochemistry 1996, 35, 16871–16878. [Google Scholar] [CrossRef]
- Odds, F.C.; Brown, A.J.P.; Gow, N.A.R. Antifungal Agents: Mechanisms of Action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Tavangar, P.; Keighobadi, M. An Overview of Azoles Targeting Sterol 14α-Demethylase for Antileishmanial Therapy. Eur. J. Med. Chem. 2017, 135, 241–259. [Google Scholar] [CrossRef]
- Adams, C.P.; Brantner, V.V. Spending on New Drug Development. Health Econ. 2010, 19, 130–141. [Google Scholar] [CrossRef] [PubMed]
- DiMasi, J.A.; Hansen, R.W.; Grabowski, H.G. The Price of Innovation: New Estimates of Drug Development Costs. J. Health Econ. 2003, 22, 151–185. [Google Scholar] [CrossRef]
- Sagar Patil, P. International Journal of Advanced Research in Biological Sciences Drug Discovery and ADMET Process: A Review. Int. J. Adv. Res. Biol. Sci 2016, 3, 181–192. [Google Scholar]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. AdmetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. AdmetSAR 2.0: Web-Service for Prediction and Optimization of Chemical ADMET Properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. PkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A.; Omran, G.; Abd-Alhaseeb, M.; Houssen, M. The Anti-Tumor Effect of Stigmasterol on Sorafenib Treated Human Breast Cancer Cell Lines. Res. Sq. 2021, 1–14. [Google Scholar]
- Kangsamaksin, T.; Chaithongyot, S.; Wootthichairangsan, C.; Hanchaina, R.; Tangshewinsirikul, C.; Svasti, J. Lupeol and Stigmasterol Suppress Tumor Angiogenesis and Inhibit Cholangiocarcinoma Growth in Mice via Downregulation of Tumor Necrosis Factor-A. PLoS ONE 2017, 12, e0189628. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.M.; Muratov, E.; Fourches, D.; Strickland, J.; Kleinstreuer, N.; Andrade, C.H.; Tropsha, A. Predicting Chemically-Induced Skin Reactions. Part I: QSAR Models of Skin Sensitization and Their Application to Identify Potentially Hazardous Compounds. Toxicol. Appl. Pharmacol. 2015, 284, 262–272. [Google Scholar] [CrossRef]
- Hosoya, K.I.; Ohtsuki, S.; Terasaki, T. Recent Advances in the Brain-to-Blood Efflux Transport Across the Blood-Brain Barrier. Int. J. Pharm. 2002, 248, 15–29. [Google Scholar] [CrossRef]
- Jaworska, J.; Harol, A.; Kern, P.S.; Gerberick, G.F. Integrating Non-Animal Test Information into an Adaptive Testing Strategy-Skin Sensitization Proof of Concept Case. Altern. Anim. Exp. 2011, 28, 211–225. [Google Scholar]
- Johansen, J.D.; Frosch, P.J.; Lepoittevin, J.P. Contact Dermatitis: Fifth Edition. Contact Dermat. 2011, 1–1262. [Google Scholar] [CrossRef]
- MacKay, C.; Davies, M.; Summerfield, V.; Maxwell, G. From Pathways to People_ Applying the Adverse Outcome Pathway (AOP) for Skin Sensitization to Risk Assessment. Altern. Anim. Exp. 2013, 30, 473–486. [Google Scholar]
- Aeby, P.; Ashikaga, T.; Bessou-Touya, S.; Schepky, A.; Gerberick, F.; Kern, P.; Marrec-Fairley, M.; Maxwell, G.; Ovigne, J.M.; Sakaguchi, H.; et al. Identifying and Characterizing Chemical Skin Sensitizers without Animal Testing: Colipa’s Research and Method Development Program. Toxicol. Vitr. 2010, 24, 1465–1473. [Google Scholar] [CrossRef]
- Karlberg, A.T.; Bergström, M.A.; Börje, A.; Luthman, K.; Nilsson, J.L.G. Allergic Contact Dermatitis-Formation, Structural Requirements, and Reactivity of Skin Sensitizers. Chem. Res. Toxicol. 2008, 21, 53–69. [Google Scholar] [CrossRef]
- Saint-Mezzard, P.; Rosieres, A.; Krasteva, M.; Berard, F.; Dubois, B.; Kaiserlian, D.; Nicolas, J.-F. Allergic Contact Dermatitis. Eur. J. Dermatol. 2004, 14, 284–295. [Google Scholar] [CrossRef]
- Hennino, A.; Saint-Mezard, P.; Nicolas, J.F.; Vocanson, M.; Dubois, B.; Chavagnac, C.; Kaiserlian, D. Update on the Pathophysiology with Special Emphasis on CD8 Effector T Cells and CD4 Regulatory T Cells. An. Bras. Dermatol. 2005, 80, 335–350. [Google Scholar] [CrossRef]
- El-Din, H.M.A.; Loutfy, S.A.; Fathy, N.; Elberry, M.H.; Mayla, A.M.; Kassem, S.; Naqvi, A. Molecular Docking Based Screening of Compounds against VP40 from Ebola Virus. Bioinformation 2016, 12, 192–196. [Google Scholar] [CrossRef]
- Drwal, M.N.; Banerjee, P.; Dunkel, M.; Wettig, M.R.; Preissner, R. ProTox: A Web Server for the In Silico Prediction of Rodent Oral Toxicity. Nucleic Acids Res. 2014, 42, 53–58. [Google Scholar] [CrossRef]
- Kurnia, D.; Putri, S.A.; Tumilaar, S.G.; Zainuddin, A.; Dharsono, H.D.A.; Amin, M.F. In Silico Study of Antiviral Activity of Polyphenol Compounds from Ocimum Basilicum by Molecular Docking, ADMET, and Drug-Likeness Analysis. Adv. Appl. Bioinforma. Chem. 2023, 16, 37–47. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lomrado, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 3, 3–25. [Google Scholar] [CrossRef]
- Prasanth, D.S.N.B.K.; Murahari, M.; Chandramohan, V.; Panda, S.P.; Atmakuri, L.R.; Guntupalli, C. In Silico Identification of Potential Inhibitors from Cinnamon against Main Protease and Spike Glycoprotein of SARS-CoV-2. J. Biomol. Struct. Dyn. 2021, 39, 4618–4632. [Google Scholar] [CrossRef]
- Bennet, D.; Kim, S. A Transdermal Delivery System to Enhance Quercetin Nanoparticle Permeability. J. Biomater. Sci. Polym. Ed. 2013, 24, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Bank, P.D. 5V5Z Lanosterol 14 Alpha Demethylase in Candida albicans. Available online: https://www.rcsb.org/structure/5v5z (accessed on 13 November 2023).
- Vlainić, J.; Jović, O.; Kosalec, I.; Vugrek, O.; Čož-Rakovac, R.; Šmuc, T. In Vitro Confirmation of Siramesine as a Novel Antifungal Agent with in Silico Lead Proposals of Structurally Related Antifungals. Molecules 2021, 26, 3504. [Google Scholar] [CrossRef]
- Knowledgebase, U. UniProtKB C7SEV3. Available online: https://www.uniprot.org/uniprotkb (accessed on 13 November 2023).
- National Center for Biotechnology Information PubChem Compound. Available online: http://www.ncbi.nlm.nih.gov/pccompound (accessed on 13 November 2023).
- Abutaha, N.; Almutairi, B.O. Exploring The Therapeutic Potential of GC–MS Separated Compounds from Dracaena Cinnabari against Dengue Virus and Aedes Aegypti Using In Silico Tools. J. King Saud Univ. Sci. 2023, 35, 102478. [Google Scholar] [CrossRef]
- Cockerill, F.R.; Wikler, M.A.; Alder, J.; Dudley, M.N.; Elliopoulos, G.M.; Ferraro, M.J.; Hardy, D.J.; Hecht, D.W. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 9th ed.; Clinical and Laboratory Institute: Malvern, PA, USA, 2012; Volume 32, ISBN 1562387839. [Google Scholar]
- Kurnia, D.; Hutabarat, G.S.; Windaryanti, D.; Herlina, T.; Herdiyati, Y.; Satari, M.H. Potential Allylpyrocatechol Derivatives as Antibacterial Agent against Oral Pathogen of S. Sanguinis ATCC 10,556 and as Inhibitor of MurA Enzymes: In Vitro and in Silico Study. Drug Des. Devel. Ther. 2020, 14, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.A.; Da Sousa, J.P.; De Luna Freire Pessôa, H.; De Freitas, A.F.R.; Coutinho, H.D.M.; Alves, L.B.N.; Lima, E.O. Ocimum Basilicum: Antibacterial Activity and Association Study with Antibiotics against Bacteria of Clinical Importance. Pharm. Biol. 2016, 54, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Dallakyan, S.; Olson, A. Small-Molecule Library Screening by Docking with PyRx. In Chemical Biology: Methods and Protocols, Methods in Molecular Biology; Humana Press: New York, NY, USA, 2015; Volume 1263, pp. 243–250. ISBN 978-1-4939-2268-0. [Google Scholar]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
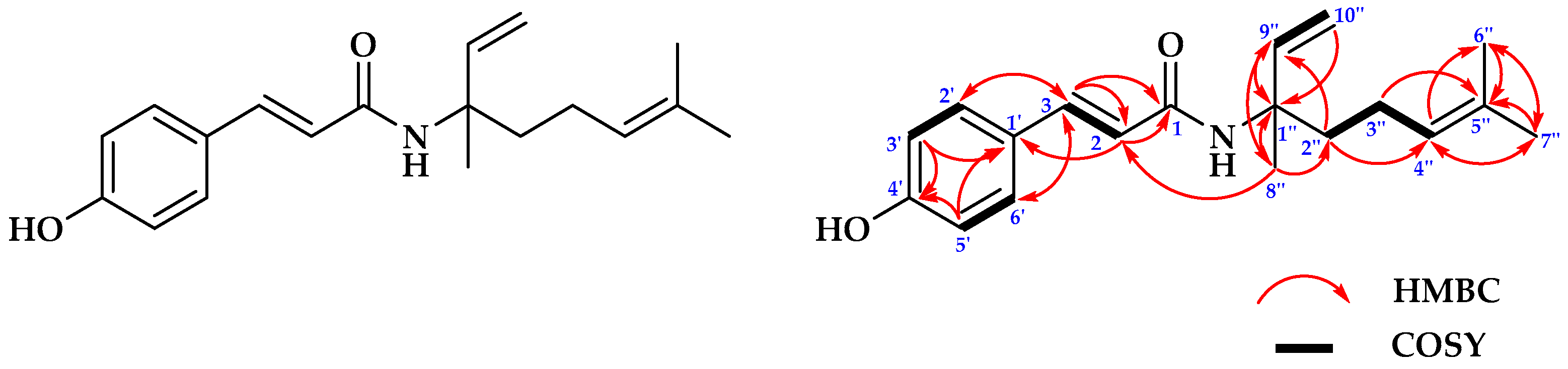
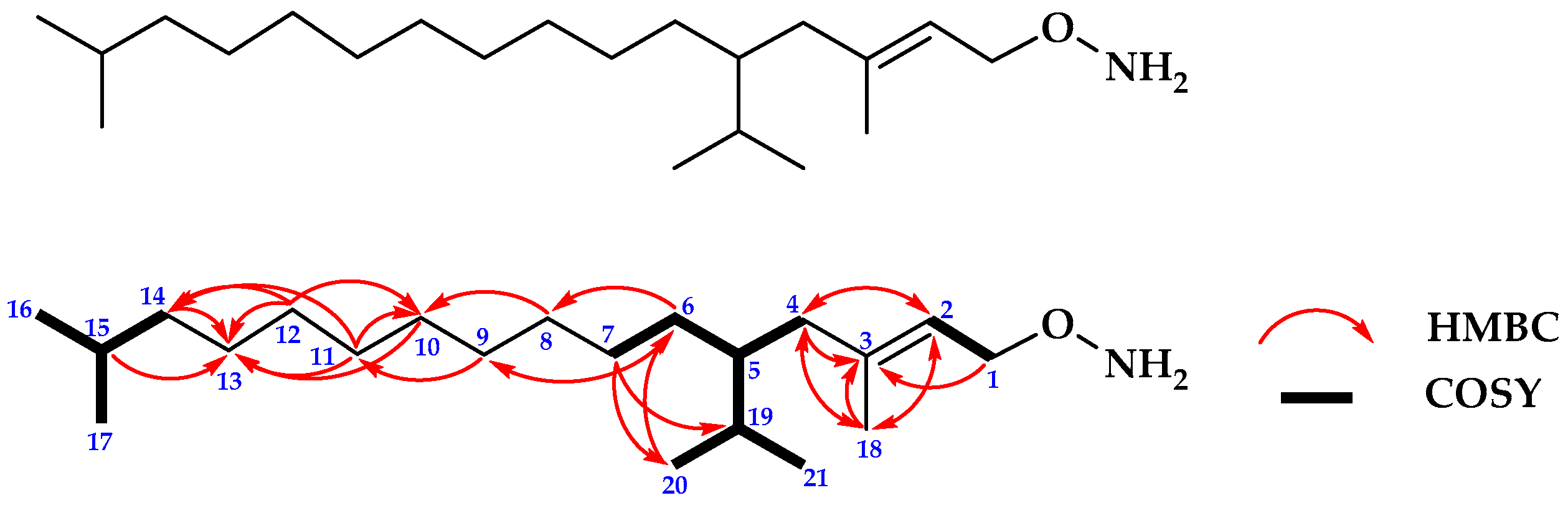
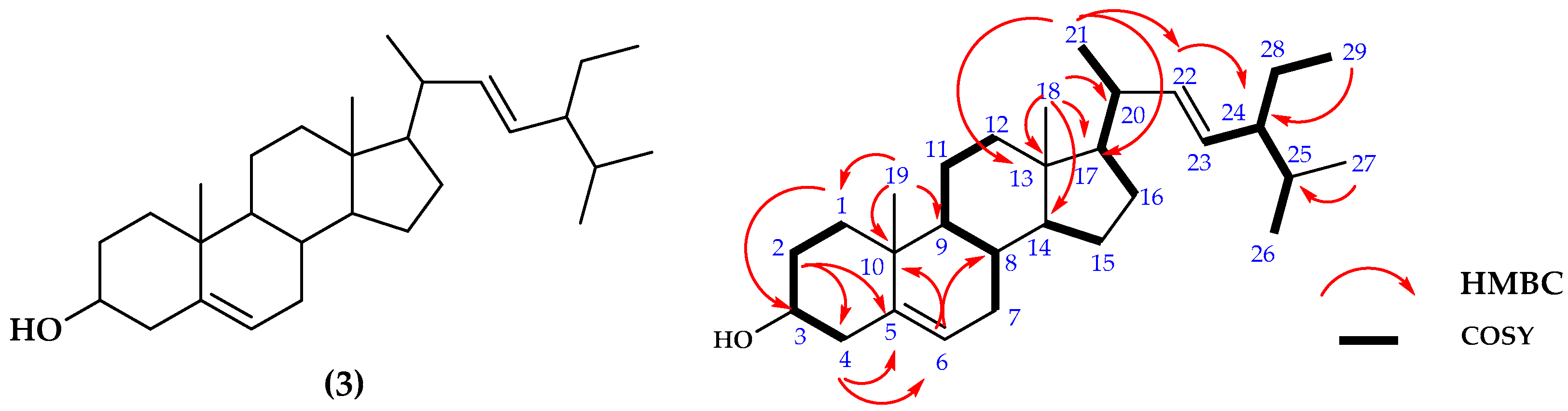

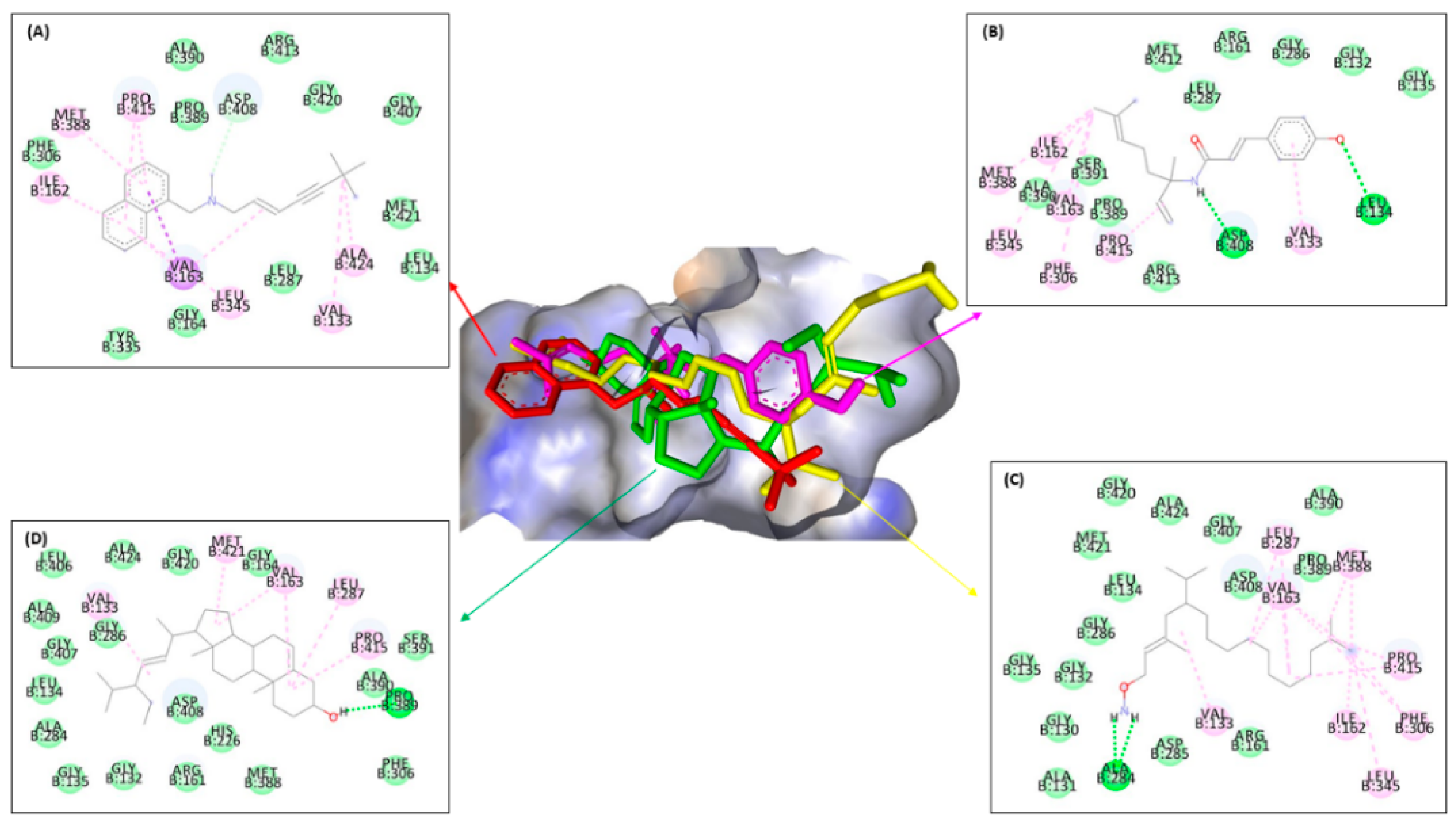


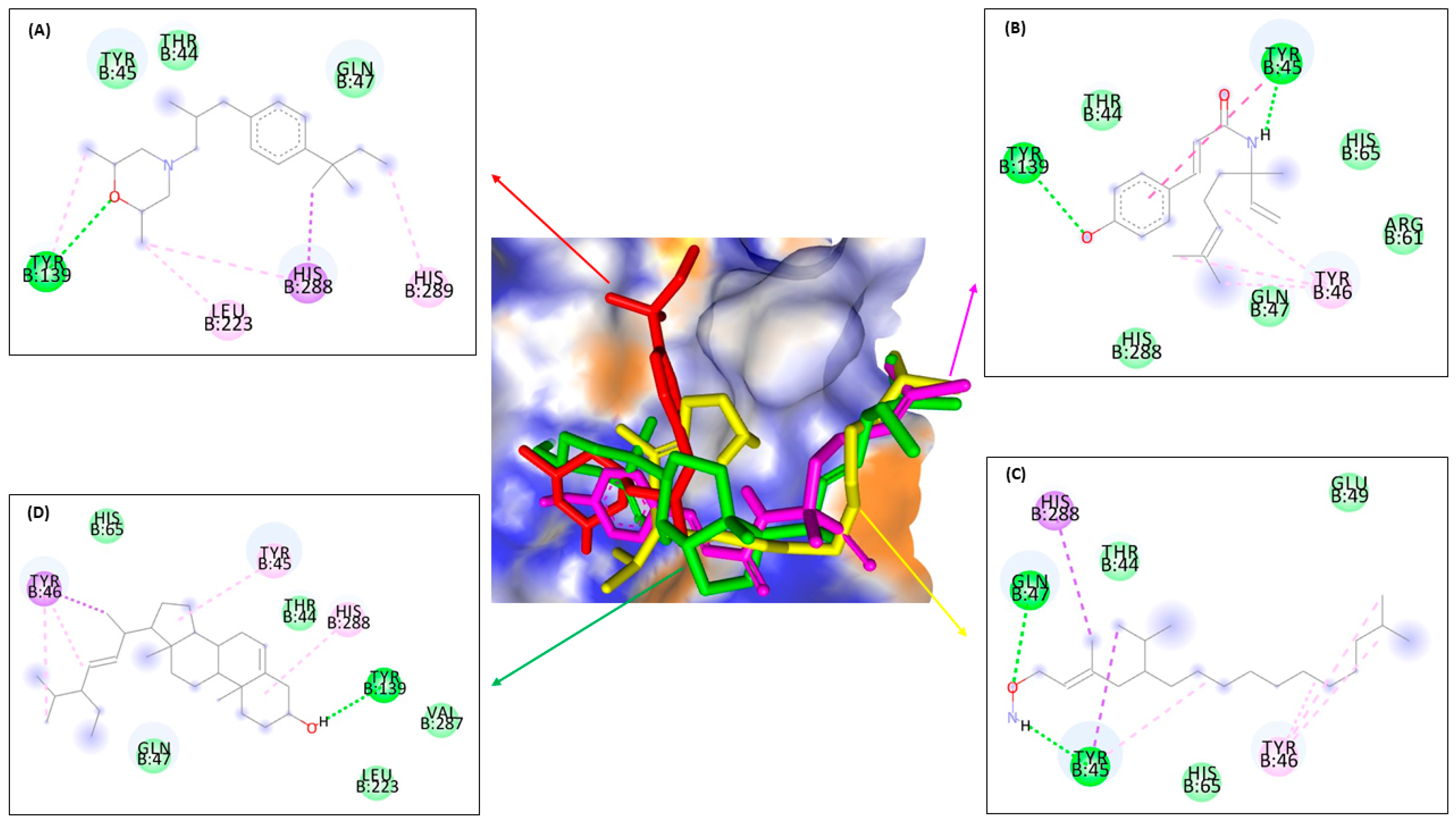

| No. | 13C NMR | 1H NMR |
|---|---|---|
| δc | δH (Integral, Mult., J = Hz) | |
| 1 | 166.5 | - |
| 2 | 117.0 | 6.25 (1H, d, 16) |
| 3 | 143.9 | 7.54 (1H, d, 16) |
| 1′ | 127.4 | - |
| 2′ | 129.9 | 7.39 (1H, d, 8.5) |
| 3′ | 115.9 | 6.83 (1H, d, 8.5) |
| 4′ | 157.7 | - |
| 5′ | 115.9 | 6.83 (1H, d, 8.5) |
| 6′ | 129.9 | 7.39 (1H, d, 8.5) |
| 1″ | 83.1 | - |
| 2″ | 40.1 | 1.83 & 1.91 (1H, m) |
| 3″ | 22.5 | 2.02 (2H, dd, 7.5; 16.25) |
| 4″ | 123.9 | 5.10 (1H, t, 2) |
| 5″ | 131.9 | - |
| 6″ | 25.8 | 1.66 (3H, s) |
| 7″ | 17.7 | 1.60 (3H, s) |
| 8″ | 23.8 | 1.60 (3H, s) |
| 9″ | 141.9 | 6.02 (1H, dd, 11; 17.5) |
| 10″ | 113.2 | 5.16 (2H, dd, 16.5; 11) |
| - | - | 1.71 |
| - | - | 5.83 |
| No. | 13C NMR | 1H NMR |
|---|---|---|
| δc | δH (Integral, Mult., J = Hz) | |
| 1 | 37.3 | 1.82 (2H, m) |
| 2 | 31.9 | 1.5 9(2H, m) |
| 3 | 71.9 | 3.51 (1H, m) |
| 4 | 42.4 | 2.26 (2H, dd, 2:2) |
| 5 | 140.8 | - |
| 6 | 121.8 | 5.33 (1H, t, 2) |
| 7 | 31.7 | 1.98 (-) |
| 8 | 31.9 | 1.59 (-) |
| 9 | 50.2 | 0.99 (-) |
| 10 | 36.2 | - |
| 11 | 21.3 | 1.44 (-) |
| 12 | 39.7 | 1.28 (-) |
| 13 | 42.3 | - |
| 14 | 56.9 | 1.03 (-) |
| 15 | 24.4 | 1.50 (-) |
| 16 | 29.0 | 1.23 (-) |
| 17 | 56.0 | 1.14 (-) |
| 18 | 12.0 | 0.65 (-) |
| 19 | 19.1 | 0.99 (-) |
| 20 | 40.62 | 1.23 (-) |
| 21 | 23.1 | 1.05 (3H, d, 7) |
| 22 | 138.4 | 5.13 (1H, dd, 8.5; 15) |
| 23 | 129.3 | 5.00 (1H, dd, 8.5; 15.4) |
| 24 | 51.3 | 1.50 (-) |
| 25 | 31.9 | 1.49 (-) |
| 26 | 21.2 | 0.78 (3H, d, 7.5) |
| 27 | 19.5 | 0.83 (3H, d, 1) |
| 28 | 25.5 | 1.15 (-) |
| 29 | 12.1 | 0.83 (-) |
| Compounds | Inhibition Zone (mm) at Concentrations (% w/v) | Concentrations (% w/v) | |||
|---|---|---|---|---|---|
| 2.5 | 5 | 10 | MIC | MFC | |
| Compound 1 | 8.9 | 10.0 | 11.9 | 0.46 | 1.8 |
| Compound 2 | 9.4 | 12.4 | 13.0 | 0.62 | 2.5 |
| Compound 3 | 9.7 | 12.8 | 14.5 | 0.31 | 1.2 |
| Ketoconazole (pc) [5,22] | 30.0 | 31.3 | 32.2 | 0.00005 | 0.0001 |
| Methanol (nc) | 0 | 0 | 0 | nm | nm |
| Sterile water (nc) | 0 | 0 | 0 | nm | nm |
| Ligands | Binding Affinity/ΔG (Kcal/mol) | Inhibition Constant/Ki (μM) | ||||||
|---|---|---|---|---|---|---|---|---|
| ERG1 | ERG2 | ERG11 | ERG24 | ERG1 | ERG2 | ERG11 | ERG24 | |
| Positive Control | −7.36 | −10.99 | −10.09 | −5.25 | 4.06 | 8.7 × 10−3 | 4 × 10−2 | 140.73 |
| Ligand 1 | −7.86 | −9.18 | −8.38 | −5.33 | 1.75 | 1.8 × 10−1 | 7.2 × 10−1 | 122.90 |
| Ligand 2 | −6.77 | −8.97 | −7.68 | −4.06 | 10.94 | 2.6 × 10−1 | 2.37 | 1060 |
| Ligand 3 | −11.14 | −12.78 | −12.00 | −6.89 | 6.8 × 10−3 | 4 × 10−4 | 1.6 × 10-3 | 8.88 |
| Type of Interaction | Residues | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERG1 | ERG2 | ERG11 | ERG24 | |||||||||||||
| Compounds | Compounds | Compounds | Compounds | |||||||||||||
| Terbina- Fine | 1 | 2 | 3 | Amorol- Fine | 1 | 2 | 3 | Keto- Conazole | 1 | 2 | 3 | Amorol- Fine | 1 | 2 | 3 | |
| Conventional | - | Asp B:408, Leu B:134 | Ala B:284 | Pro B:389 | - | Glu B:172, Thr B:181 | Asp B:126, Val B:152, Ile B:124 | - | Tyr B:64 | His B:377, Pro B:375, Met B:508 | His B:377, Met B:508, Pro B:375 | Gly B:307 | Tyr B:139 | Tyr B:139, Tyr B:45 | Gln B:47, Tyr B:45 | - |
| Carbon | Asp B:408 | - | - | - | Asp B:126, Tyr B:120 | - | - | - | - | Leu B:376 | Ser B:507, Leu B:376 | - | - | - | - | - |
| π–donor hydrogen bond | Tyr B:103 | Tyr B:103 | - | - | Ser B:378 | - | - | - | - | - | - | - | ||||
| π-sigma | Val B:163 | - | - | - | Tyr B:103 | His B:135, Phe B:107 | His B:154, Tyr B:206 | Tyr B:103, Phe B:107 | Leu B:87 | Phe B:380 | - | - | His B:288 | - | His B:288, Tyr B:45 | Tyr B:46 |
| π–π stacked | - | - | - | - | Ala B:98, Leu B:95, Tyr B:206, Ile B:178 | Trp B:89, Trp B:164, Val B:162 | - | - | - | His B:377 | - | - | - | - | - | - |
| π–π T-shaped | - | - | - | - | - | - | - | - | - | - | - | - | - | Tyr B:45 | - | - |
| Alkyl | Ala B:424, Val B:133 | Ile B:162, Met B:388, Leu B:345, Phe B:306, Val B:163, Pro B:415 | Leu B:287, Val B:163, Met B:388, Pro B:415, Ile B:162, Phe B:306, Leu B:345 | Val B:133, Met B:421, Val B:163, Leu B:287, Pro B:145 | Trp B:164, His B:154, Trp B:89, Ile B:124, Val B:162 | Val B:162, Phe B:133 | Ile B:178, Tyr B:120, Ala B:185, Ile B:124 | Trp B:164, Trp B:89, Phe B:107 | Met B:508, Val B:509, Thr B:311 | Tyr B:401, Lys B:90 | Phe B:228, Tyr B:118, Phe B:380, Phe B:233, Met B: 508, Leu B:376 | His B:377 | Tyr B:139, Leu B:223, His B:289 | - | - | - |
| π-alkyl | Ile B:162, Met B:388, Pro B:415 | Val B:133 | Val B:133 | - | Ala B:185 | Ala B:185 | Ile B:178, Leu B:105, Leu B:182, Tyr B:103, Thr B:181, Tyr B:120 | Leu B:182, Tyr B:206, Leu B:95, Ala B:98, Tyr B:103 | Leu B:376 | Phe B:233, Val B:234, Leu B:88 | - | Leu B:376 | His B:288 | Tyr B:46 | Tyr B;45, Tyr B:46 | Tyr B:46, Tyr B:45, His B:288 |
| Van der Waals | Phe B:306, Ala B:390, Pro B:389, Arg B:413, Gly B:420, Gly B:407, Met B:421, | Ala B:390, Ser B:391, Pro B:389, Arg B:413, Met B:412, Leu B:287, Arg B:161, Gly B:286, | Gly B:135, Gly B:420, Met B:421, Leu B:134, Gly B:286, Gly B:132, Gly B:130, | Leu B:406, Ala B:409, Gly B:407, Gly B:286, Leu B:134, Ala B:284, Gly B:135, | Glu B:172, Thr B:181, Leu B:182, Leu B:105, Ser B:117 | Thr B:160, Gln B:135, Val B:152, Ser B:117, Asp B:126, Val B:84, Tyr B:120, | Phe B:133, Val B:162, Ser B:117, Trp B:164, Phe B:107, Trp B:89, Leu B:95, | Ser B:117, Tyr B:120, Phe B:184, Val B:84, Ala B:185, Thr B:181, Leu B:105, | Gln B:66, Gly B:65, Pro B:230, His B:377, Ser B:378, Phe B:228, His B:310, | Met B:92, Leu B:87, Tyr B:64, Ser B:507, Val B:509, Ser B:378, Pro B:230, | Tyr B:64, Ser B:378, Pro B:230, Phe B:126, Gly B:307, Thr B:122, Leu B:121, | Phe B:380, Pro B:230, Ser B:507, Ser B:378, Pro B:375, Val B:509, His B:310, | Tyr B:45, Thr B:44, Gln B:47 | Thr B:44, His B:65, Arg B:61, Gln B:47, His B:288 | Thr B:44, Glu B:49, His B:65 | His B:65, Thr B:44, Val B:287, Leu B:223, Gln B:47 |
| Leu B:134, Leu B:287, Gly B:164, Tyr B:335 | Gly B:132, Gly B:135 | Ala B:131, Asp B:285, Arg B:161, Ala B:390, Pro B:389, Asp B:408, Gly B:407, Ala B:424 | Gly B:132, Arg B:161, Asp B:408, His B:226, Met B:388, Phe B:306, Ala B:390, Ser B:391, Gly B:164, Gly B:420, Ala B:424 | Leu B:182, Ile B:178, Leu B:105, Ile B:124 | Phe B:184, Glu B:172, Val B:153, Ser B:125 | Met B:93, Ile B:178, Ala B:98, Thr B:181, Glu B:172, Asp B:126 | Phe B:126, Gly B:307, Thr B:311, Leu B:121, Leu B:88, Phe B:380, Phe B:233, Lys B:90, Ser B:507, Tyr B:505, Ser B:506 | Lys B:90, Ala B:117 | Val B:509, Val B:510 | Thr B:311, Gly B:308, Phe B:228, Leu B:121, Met B:508, Phe B:233, Gly B:65, Tyr B:64, Tyr B:505, Ser B:506, Leu B:87 | ||||||
| Pharmacokinetic Properties | Parameters | Ligands | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Absorption | Water solubility | −4.674 log mol/L | −7.66 log mol/L | −6.673 log mol/L |
| Intestinal absorption | 89.583% absorbed | 90.281% absorbed | 96.151% absorbed | |
| Skin permeability | −2.7 log Kp | −2.792 log Kp | −2.781 log Kp | |
| Distribution | Volume distribution (VDss) | 0.366 log L/kg | 0.11 log L/kg | 0.18 log L/kg |
| BBB permeability | −0.062 log BB | −0.414 log BB | 0.799 log BB | |
| CNS permeability | −2.12 log PS | −1.36 log PS | −1.737 log PS | |
| Metabolism | Inhibitor of: | Yes | Yes | No |
| CYP1A2 | ||||
| CYP2C19 | Yes | No | No | |
| CYP2C9 | Yes | No | No | |
| CYP2D6 | No | No | No | |
| CYP3A4 | No | No | No | |
| Excretion | Total Clearance | 0.418 log mL/min/kg | 1.861 log mL/min/kg | 0.618 log mL/min/kg |
| Acute oral toxicity | Lethal dose 50% | 2.331 mol/kg | 1.654 mol/kg | 2.375 mol/Kg |
| Skin sensitization | No | Yes | No | |
| Parameters | Ligands | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Molecular mass (<500 Daltons) | 299.41 | 325.57 | 412.69 |
| Hydrogen bond donors/HBD (<5) | 2 | 1 | 1 |
| Hydrogen bond acceptors/HBA (<10) | 3 | 2 | 1 |
| LogP (<5) | 4.6 | 7.35 | 7.8 |
| Molecular refractivity (40–130) | 93.52 | 106.38 | 132.76 |
| Rotatable bonds | 8 | 15 | 5 |
| Topological polar surface area | 49.33 | 35.25 | 20.23 |
| Violation | 0 | 1 | 2 |
| Drug-likeness | Yes | Yes | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siswina, T.; Rustama, M.M.; Sumiarsa, D.; Apriyanti, E.; Dohi, H.; Kurnia, D. Antifungal Constituents of Piper crocatum and Their Activities as Ergosterol Biosynthesis Inhibitors Discovered via In Silico Study Using ADMET and Drug-Likeness Analysis. Molecules 2023, 28, 7705. https://doi.org/10.3390/molecules28237705
Siswina T, Rustama MM, Sumiarsa D, Apriyanti E, Dohi H, Kurnia D. Antifungal Constituents of Piper crocatum and Their Activities as Ergosterol Biosynthesis Inhibitors Discovered via In Silico Study Using ADMET and Drug-Likeness Analysis. Molecules. 2023; 28(23):7705. https://doi.org/10.3390/molecules28237705
Chicago/Turabian StyleSiswina, Tessa, Mia Miranti Rustama, Dadan Sumiarsa, Eti Apriyanti, Hirofumi Dohi, and Dikdik Kurnia. 2023. "Antifungal Constituents of Piper crocatum and Their Activities as Ergosterol Biosynthesis Inhibitors Discovered via In Silico Study Using ADMET and Drug-Likeness Analysis" Molecules 28, no. 23: 7705. https://doi.org/10.3390/molecules28237705
APA StyleSiswina, T., Rustama, M. M., Sumiarsa, D., Apriyanti, E., Dohi, H., & Kurnia, D. (2023). Antifungal Constituents of Piper crocatum and Their Activities as Ergosterol Biosynthesis Inhibitors Discovered via In Silico Study Using ADMET and Drug-Likeness Analysis. Molecules, 28(23), 7705. https://doi.org/10.3390/molecules28237705









