Exploring the Larvicidal and Repellent Potential of Taurus Cedar (Cedrus libani) Tar against the Brown Dog Tick (Rhipicephalus sanguineus sensu lato)
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Extraction of Tar
4.2. Determination of Tar Composition
4.3. Identification and Storing of the Tested Ticks
4.4. Larval Immersion Tests (LIT)
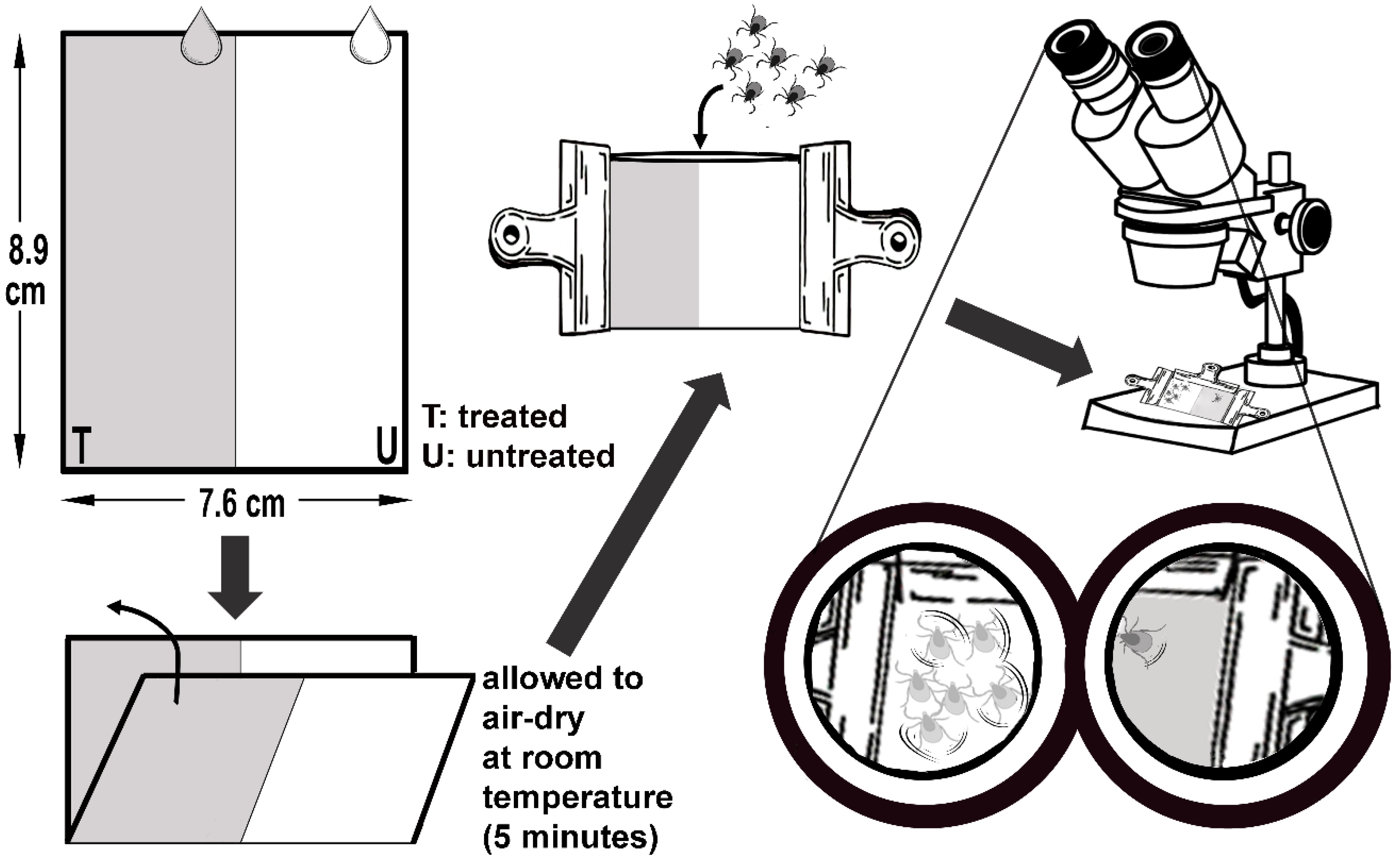
4.5. Larval Repellent Activity Test (LRAT)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hajar, L.; François, L.; Khater, C.; Jomaa, I.; Deque, M.; Cheddadi, R. Cedrus libani (A. Rich.) distribution in Lebanon: Past, present and future. Comptes Rendus Biol. 2010, 333, 622–630. [Google Scholar] [CrossRef]
- Venditti, A.; Maggi, F.; Saab, A.M.; Bramucci, M.; Quassinti, L.; Petrelli, D.; Vitali, L.A.; Lupidi, G.; El Samrani, A.; Borgatti, M.; et al. Antiproliferative, antimicrobial and antioxidant properties of Cedrus libani and Pinus pinea wood oils and Juniperus excelsa berry oil. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2022, 156, 384–395. [Google Scholar] [CrossRef]
- Kargioglu, M.; Cenkci, S.; Serteser, A.; Konuk, M.; Vural, G. Traditional uses of wild plants in the Middle Aegean region of Turkey. Hum. Ecol. 2010, 38, 429–450. [Google Scholar] [CrossRef]
- Kurt, Y.; Işık, K. Comparison of tar produced by traditional and laboratory methods. Stud. Ethno-Med. 2012, 6, 77–83. [Google Scholar] [CrossRef]
- Kurt, Y.; Kaçar, S.M.; Işık, K. Traditional tar production from Cedrus libani a Rich. the Taurus mountains in southern Turkey. Econ. Bot. 2008, 62, 615–620. [Google Scholar] [CrossRef]
- Arı, S.; Kargıoğlu, M.; Temel, M.; Açıkgöz, Ş.; Konuk, M. Afyonkarahisar, Central Western Turkey. J. Ethnobiol. Ethnomed. 2014, 10, 29. [Google Scholar]
- Ninich, O.; Et-Tahir, A.; Kettani, K.; Ghanmi, M.; Aoujdad, J.; El Antry, S.; Ouajdi, M.; Satrani, B. Plant sources, techniques of production and uses of tar: A review. J. Ethnopharmacol. 2022, 285, 114889. [Google Scholar] [CrossRef]
- Gern, L. Die Biologie der Ixodes ricinus Zecke [The biology of the Ixodes ricinus tick]. Ther. Umsch. 2005, 62, 707–712. (In German) [Google Scholar] [CrossRef]
- Nava, S.; Guglielmone, A.A.; Mangold, A.J. An overview of systematics and evolution of ticks. Front. Biosci. 2009, 14, 2857–2877. [Google Scholar] [CrossRef]
- Hawman, D.W.; Feldmann, H. Crimean-Congo haemorrhagic fever virus. Nat. Rev. Microbiol. 2023, 21, 463–477. [Google Scholar] [CrossRef]
- Tan, L.P.; Hamdan, R.H.; Hassan, B.N.H.; Reduan, M.F.H.; Okene, I.A.; Loong, S.K.; Khoo, J.J.; Samsuddin, A.S.; Lee, S.H. Rhipicephalus Tick: A Contextual Review for Southeast Asia. Pathogens 2021, 30, 821. [Google Scholar] [CrossRef]
- Dantas-Torres, F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): From taxonomy to control. Vet. Parasitol. 2008, 152, 173–185. [Google Scholar] [CrossRef]
- Aydin, L.; Girisgin, O.; Ozuicli, M.; Girisgin, A.O. Potential risk in public parks: Investigation of the tick species (Acari:Ixodida) in Bursa metropolitan area, Turkey. Ankara Univ. Vet. Fak. Derg. 2020, 67, 393–439. [Google Scholar] [CrossRef]
- Koc, S.; Aydin, L.; Cetin, H. Tick species (Acari: Ixodida) in Antalya city, Turkey: Species diversity and seasonal activity. Parasitol. Res. 2015, 114, 2581–2586. [Google Scholar] [CrossRef]
- Koc, S.; Aydın, L.; Cetin, H. The first study on fipronil, chlorpyrifos-methyl and permethrin resistance in Rhipicephalus sanguineus sensu lato ticks from Turkey. Int. J. Trop. Insect Sci. 2022, 42, 597–602. [Google Scholar] [CrossRef]
- Tucker, N.S.; Kaufman, P.E.; Weeks, E.N. Identification of permethrin and etofenprox cross-tolerance in Rhipicephalus sanguineus sensu lato (Acari: Ixodidae). Pest Manag. Sci. 2019, 75, 2794–2801. [Google Scholar] [CrossRef]
- Becker, S.; Webster, A.; Doyle, R.L.; Martins, J.R.; Reck, J.; Klafke, G.M. Resistance to deltamethrin, fipronil and ivermectin in the brown dog tick, Rhipicephalus sanguineus sensu stricto, Latreille (Acari: Ixodidae). Ticks Tick Borne Dis. 2019, 10, 1046–1050. [Google Scholar] [CrossRef]
- Eiden, A.L.; Kaufman, P.E.; Oi, F.M.; Allan, S.A.; Miller, R.J. Detection of permethrin resistance and fipronil tolerance in Rhipicephalus sanguineus (Acari: Ixodidae) in the United States. J. Med. Entomol. 2015, 52, 429–436. [Google Scholar] [CrossRef]
- Lee, S.J.; Mulay, P.; Diebolt-Brown, B.; Lackovic, M.J.; Mehler, L.N.; Beckman, J.; Waltz, J.; Prado, J.B.; Mitchell, Y.A.; Higgins, S.A.; et al. Acute illnesses associated with exposure to fipronil-surveillance data from 11 states in the United States, 2001–2007. Clin. Toxicol. 2010, 48, 737–744. [Google Scholar] [CrossRef]
- Cochran, R.C.; Yu, L.; Krieger, R.I.; Ross, J.H. Post application fipronil exposure following use on pets. J. Toxicol. Environ. Health A 2015, 78, 1217–1226. [Google Scholar] [CrossRef]
- Rocha, G.M.; Grisolia, C.K. Why pesticides with mutagenic, carcinogenic and reproductive risks are registered in Brazil. Dev. World Bioeth. 2019, 19, 148–154. [Google Scholar] [CrossRef]
- Deering, K.; Spiegel, E.; Quaisser, C.; Nowak, D.; Rakete, S.; Garí, M.; Bose-O’Reilly, S. Exposure assessment of toxic metals and organochlorine pesticides among employees of a natural history museum. Environ. Res. 2020, 184, 109271. [Google Scholar]
- Nagini, S.; Palrasu, M.; Bishayee, A. Limonoids from neem (Azadirachta indica A. Juss.) are potential anticancer drug candidates. Med. Res. Rev. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Lai, B.; Liu, Y.; Li, C.; Bu, C. Discovery selective acetylcholinesterase inhibitors to control Tetranychus urticae (Acari: Tetranychidae). J. Insect Sci. 2023, 1, 19. [Google Scholar]
- Staub, D.; Debrunner, M.; Amsler, L.; Steffen, R. Effectiveness of a repellent containing DEET and EBAAP for preventing tick bites. Wilderness Environ. Med. 2002, 13, 12–20. [Google Scholar] [CrossRef]
- Rowland, M.; Freeman, T.; Downey, G.; Hadi, A.; Saeed, M. DEET mosquito repellent sold through social marketing provides personal protection against malaria in an area of all-night mosquito biting and partial coverage of insecticide-treated nets: A case-control study of effectiveness. Trop. Med. Int. Health 2004, 9, 343–350. [Google Scholar] [CrossRef]
- Dorman, D.C. Diethyltoluamide (DEET) insect repellent toxicosis. Vet. Clin. N. Am. Small Anim. Pract. 1990, 20, 387–391. [Google Scholar] [CrossRef]
- Robbins, P.J.; Cherniack, M.G. Review of the biodistribution and toxicity of the insect repellent N,N-diethyl-m-toluamide (DEET). J. Toxicol. Environ. Health 1986, 18, 503–525. [Google Scholar] [CrossRef]
- Tavares, E.M.; Judge, B.S.; Jones, J.S. Bug off! Severe toxicity following inhalational exposure to N, N-diethyl-meta-toluamide (DEET). Am. J. Emerg. Med. 2019, 37, 1395.e3–1395.e4. [Google Scholar] [CrossRef]
- Dorman, D.C.; Buck, W.B.; Trammel, H.L.; Jones, R.D.; Beasley, V.R. Fenvalerate/N,N-diethyl-m-toluamide (Deet) toxicosis in two cats. J. Am. Vet. Med. Assoc. 1990, 196, 100–102. [Google Scholar]
- Mount, M.E.; Moller, G.; Cook, J.; Holstege, D.M.; Richardson, E.R.; Ardans, A. Clinical illness associated with a commercial tick and flea product in dogs and cats. Vet. Hum. Toxicol. 1991, 33, 19–27. [Google Scholar]
- Diaz, J.H. Chemical and Plant-Based Insect Repellents: Efficacy, Safety, and Toxicity. Wilderness Environ. Med. 2016, 27, 153–163. [Google Scholar]
- Wu, W.; Yang, Y.; Feng, Y.; Ren, X.; Li, Y.; Li, W.; Huang, J.; Kong, L.; Chen, X.; Lin, Z.; et al. Study of the repellent activity of 60 essential oils and their main constituents against Aedes albopictus, and nano-formulation development. Insects 2022, 13, 1077. [Google Scholar] [CrossRef]
- Mercimek-Takci, H.A.; Turkmen, F.U.; Sarı, M. Effect of cedar (Cedrus libani A. Rich) tar on bacterial growth. J. Microbiol. Biotech. Food Sci. 2020, 9, 805–808. [Google Scholar] [CrossRef]
- Mercimek-Takci, H.A.; Turkmen, F.U.; Sarı, M. In vitro mutagenic effect of cedar (Cedrus libani A. Rich) tar in the salmonella/microsome assay system. Banat’s J. Biotechnol. 2019, 10, 13–18. [Google Scholar] [CrossRef]
- Koc, S.; Gultekin, Z.N.; Kahraman, S.; Cengiz, A.; Polat, B.; Caliskan, C.; Tufan-Cetin, O.; Cetin, H. Larvicidal and repellent effects of essential oils on the brown dog tick (Rhipicephalus sanguineus sensu lato) with description of new larval repellent activity test method. Exp. Appl. Acarol. 2023. Accepted for publication. [Google Scholar]
- Eller, F.J.; Vander Meer, R.K.; Behle, R.W.; Flor-Weiler, L.B.; Palmquist, D.E. Bioactivity of cedarwood oil and cedrol against arthropod pests. Environ. Entomol. 2014, 43, 762–766. [Google Scholar] [CrossRef]
- Pazinato, R.; Volpato, A.; Baldissera, M.D.; Santos, R.C.V.; Baretta, D.; Vaucher, R.; Giongo, J.L.; Boligon, A.A.; Stefani, L.M.; DaSilva, A.S. In vitro effect of seven essential oils on the reproduction of the cattle tick Rhipicephalus microplus. J. Adv. Res. 2016, 7, 1029–1034. [Google Scholar] [CrossRef]
- Flor-Weiler, L.B.; Behle, R.W.; Eller, F.J.; Muturi, E.J.; Rooney, A.P. Repellency and toxicity of a CO2-derived cedarwood oil on hard tick species (Ixodidae). Exp. Appl. Acarol. 2022, 86, 299–312. [Google Scholar]
- Luxemburger, C.; Perea, W.A.; Delmas, G.; Pruja, C.; Pecoul, B.; Moren, A. Permethrin-impregnated bed nets for the prevention of malaria in schoolchildren on the Thai-Burmese border. Trans R. Soc. Trop. Med. Hyg. 1994, 88, 155–159. [Google Scholar] [CrossRef]
- Sukumaran, D.; Sharma, A.K.; Wasu, Y.H.; Pandey, P.; Tyagi, V. Knockdown and repellent effect of permethrin-impregnated army uniform cloth against Aedes aegypti after different cycles of washings. Parasitol. Res. 2014, 113, 1739–1747. [Google Scholar] [CrossRef]
- Vang, A.; White, A.V.; Balanay, J.A.G.; Tutor Marcom, R.; Richards, S.L. Evaluation of surface versus total permethrin content in permethrin-treated clothing: Implications for protection against mosquitoes. Pathog. Glob. Health 2022, 116, 365–375. [Google Scholar] [CrossRef]
- Tian, Y.; Taylor, C.E.; Lord, C.C.; Kaufman, P.E. Evidence of permethrin resistance and fipronil tolerance in Rhipicephalus sanguineus s.l. (Acari: Ixodidae) populations from Florida and California. J. Med. Entomol. 2023, 60, 412–416. [Google Scholar] [CrossRef]
- Candelier, K.; Jay-Allemand, C.; Dijoux, R.; Ducruet, R.; Kieny, E.; Aznar, D.; Cayzac, C.; Bidel, L.P.R. Repellent activities against four Ascomycota species and Reticulitermes flavipes of acetonic extractives from sapwood to inner heartwood fractions of Cedrus atlantica. Bois Et Forêts Des. Trop. 2023, 355, 87–98. [Google Scholar] [CrossRef]
- Buneri, I.D.; Yousuf, M.; Attaullah, M.; Afridi, S.; Anjum, S.I.; Rana, H.; Ahmad, N.; Amin, M.; Tahir, M.; Ansari, M.J. A comparative toxic effect of Cedrus deodara oil on larval protein contents and its behavioral effect on larvae of mealworm beetle (Tenebrio molitor) (Coleoptera: Tenebrionidae). Saudi J. Biol. Sci. 2019, 26, 281–285. [Google Scholar] [CrossRef]
- Chaudhary, A.; Sharma, P.; Nadda, G.; Tewary, D.K.; Singh, B. Chemical composition and larvicidal activities of the Himalayan cedar, Cedrus deodara essential oil and its fractions against the diamondback moth, Plutella xylostella. J. Insect Sci. 2011, 11, 157. [Google Scholar] [CrossRef]
- Reddy, S.G.; Kirti Dolma, S.; Koundal, R.; Singh, B. Chemical composition and insecticidal activities of essential oils against diamondback moth, Plutella xylostella (L.) (Lepidoptera: Yponomeutidae). Nat. Prod. Res. 2016, 30, 1834–1838. [Google Scholar] [CrossRef]
- Cetin, H.; Kurt, Y.; Isik, K.; Yanikoglu, A. Larvicidal effect of Cedrus libani seed oils on mosquito Culex pipiens. Pharm. Biol. 2009, 47, 665–668. [Google Scholar] [CrossRef][Green Version]
- Kala, S.; Sogan, N.; Naik, S.N.; Agarwal, A.; Kumar, J. Impregnation of pectin-cedarwood essential oil nanocapsules onto mini cotton bag improves larvicidal performances. Sci. Rep. 2020, 24, 14107. [Google Scholar] [CrossRef]
- Egenberg, I.M.; Holtekjølen, A.K.; Lundanes, E. Characterisation of naturally and artificially weathered pine tar coatings by visual assessment and gas chromatography-mass spectrometry. J. Cult. Herit. 2003, 4, 221–241. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Saab, A.; Tundis, R.; Statti, G.A.; Lampronti, I.; Menichini, F.; Gambari, R.; Cinatl, J.; Doerr, H.W. Phytochemical analysis and in vitro evaluation of the biological activity against herpes simplex virus type 1 (HSV-1) of Cedrus libani A. Rich. Phytomedicine 2008, 15, 79–83. [Google Scholar] [CrossRef]
- Paoli, M.; Nam, A.M.; Castola, V.; Casanova, J.; Bighelli, A. Chemical variability of the wood essential oil of Cedrus atlantica Manetti from Corsica. Chem. Biodivers. 2011, 8, 344–351. [Google Scholar] [CrossRef]
- Singh, D.; Agarwal, S.K. Himachalol and β-himachalene: Insecticidal principles of himalayan cedarwood oil. J. Chem. Ecol. 1988, 14, 1145–1151. [Google Scholar] [CrossRef]
- Aydın, L. Güney Marmara Bölgesi Ruminantlarında Görülen Kene Türleri ve Yayılışları. Ph.D. Thesis, University of Uludag, Bursa, Turkey, 1994. [Google Scholar]
- Aydın, L. Distribution and species of ticks on ruminants in the southern Traditional Tar Production from the Anatolian Black Pine [Pinus nigra Arn. subsp. pallasiana (Lamb.) Holmboe var. pallasiana] and its usages in Marmara region. Acta Parasitol. Turc. 2000, 24, 194–200. [Google Scholar]
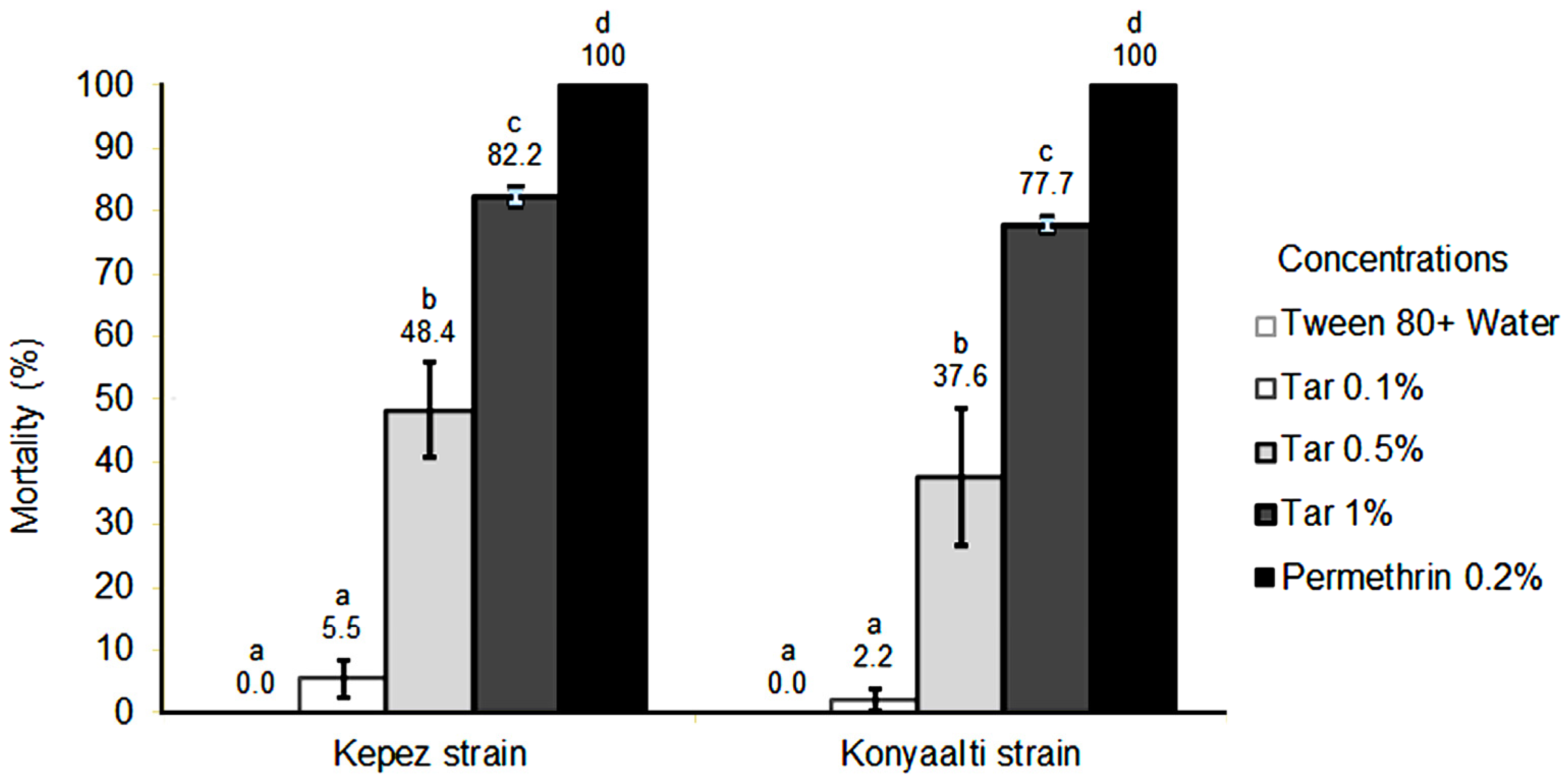
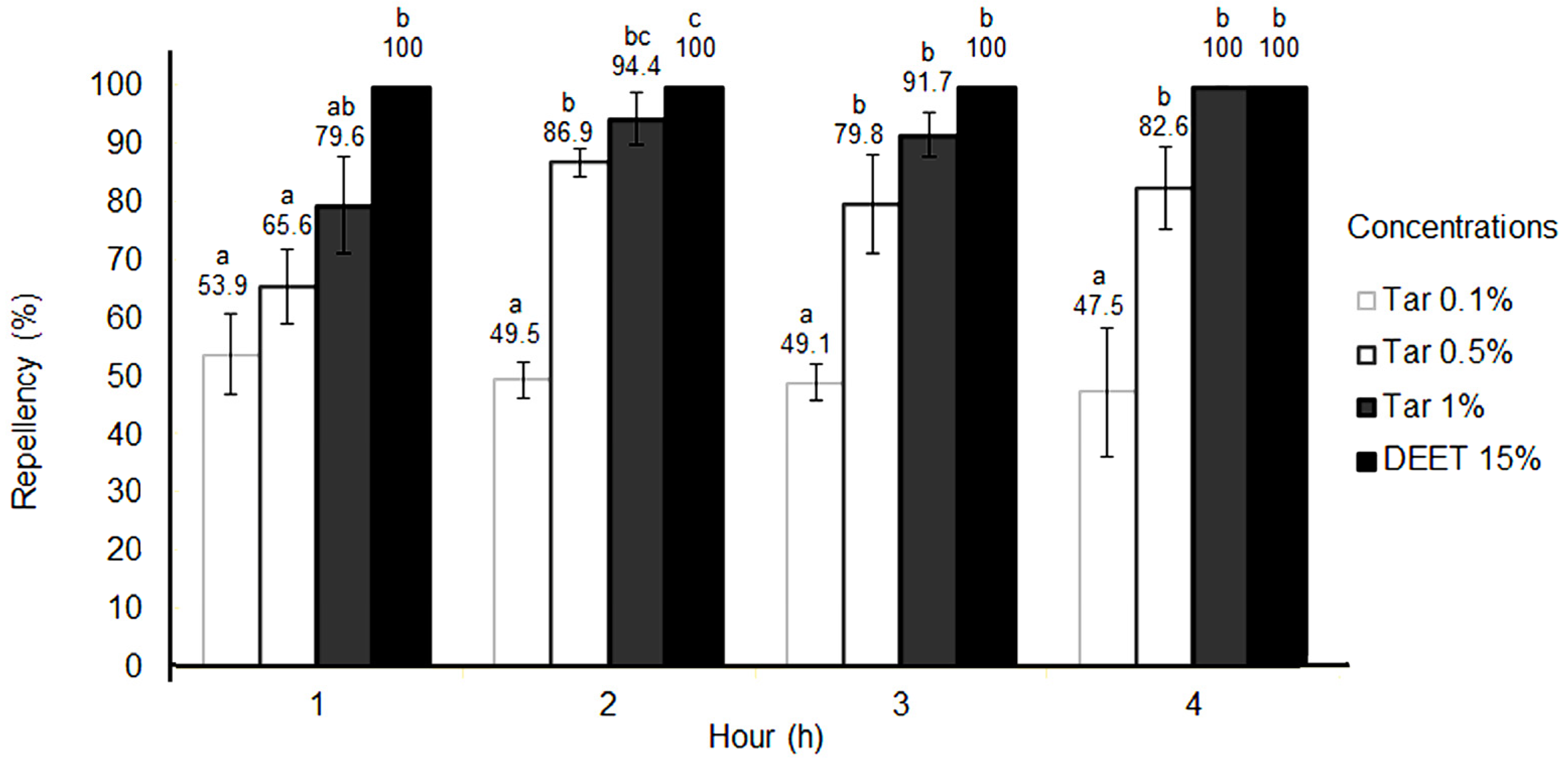
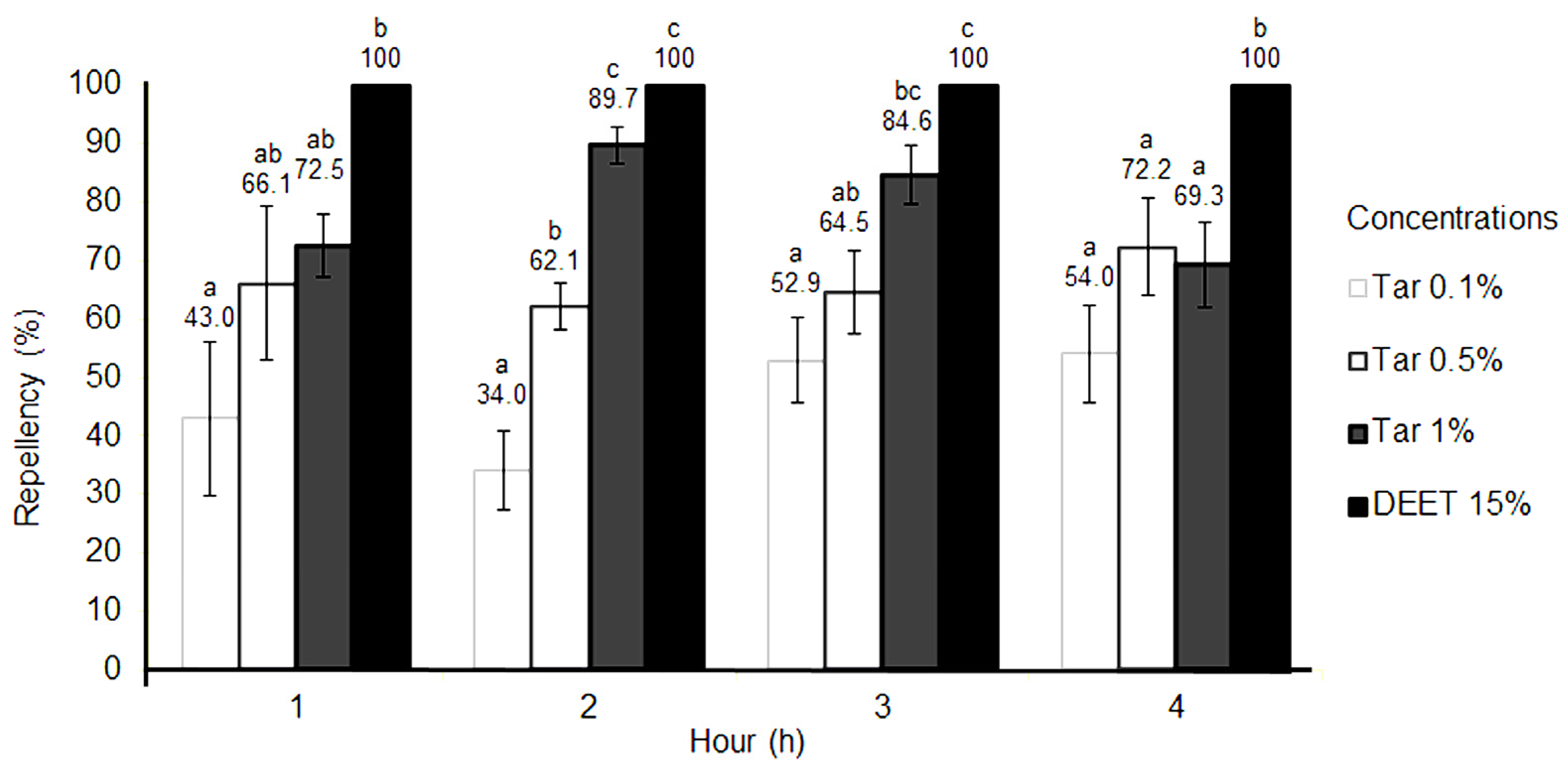
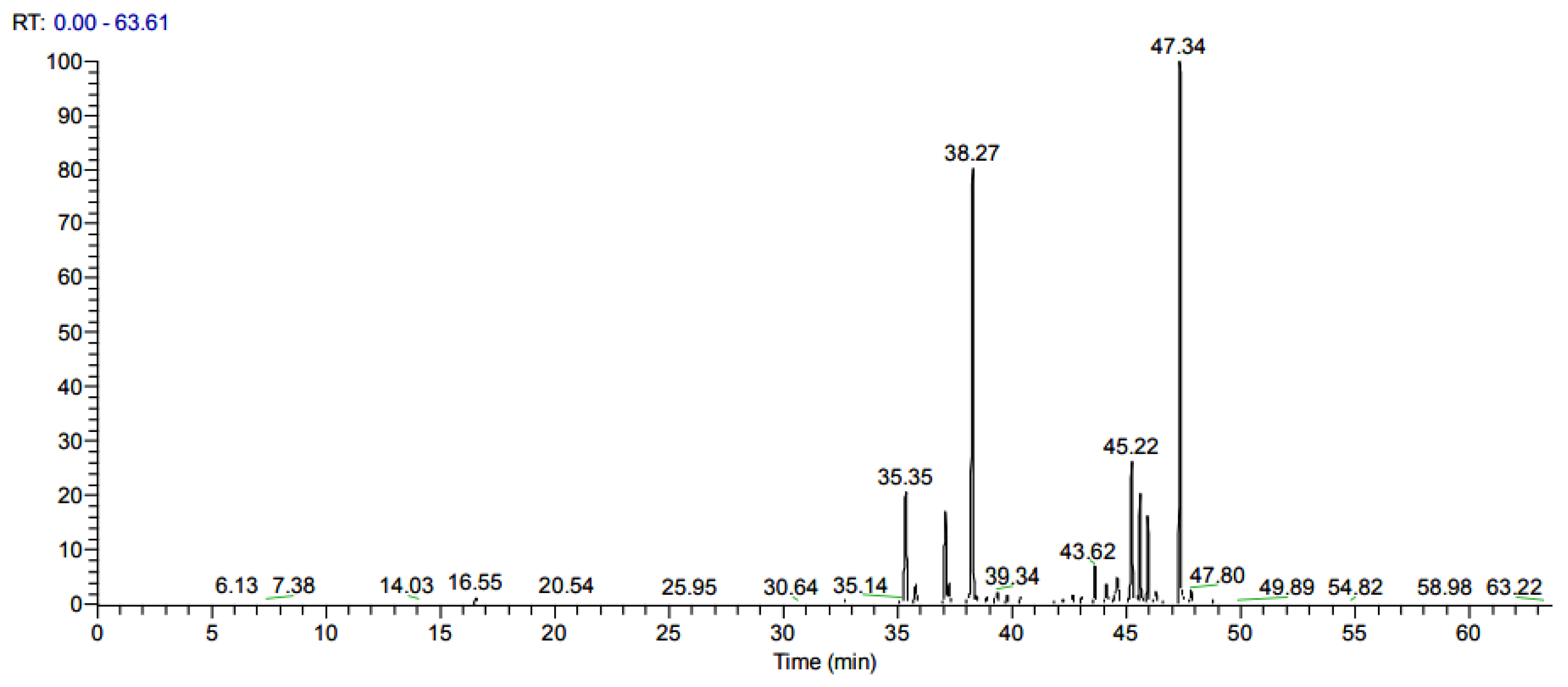
| Strain | LC50 (%) | Confidence Limits (%) | LC90 (%) | Confidence Limits (%) | Chi-Square | p-Value |
|---|---|---|---|---|---|---|
| Kepez | 0.47 | 0.40–0.55 | 1.52 | 1.20–2.12 | 1.38 | 0.239 |
| Konyaalti | 0.58 | 0.50–0.67 | 1.63 | 1.30–2.30 | 1.82 | 0.177 |
| No | NIST Library Similarity (%) | Retention Time (min) | Substances | Composition Rate (%) |
|---|---|---|---|---|
| 1 | 90.9 | 6.13 | α-Pinene | 0.09 |
| 2 | 70.7 | 7.66 | m-Ethyl-toluene | 0.09 |
| 3 | 81.2 | 10.47 | 1,2,3-Trimethylbenzene | 0.06 |
| 4 | 72.3 | 12.31 | m-Methylstyrene | 0.06 |
| 5 | 75.9 | 12.88 | p-Cymene | 0.06 |
| 6 | 85.3 | 13.29 | dI-Limonene | 0.07 |
| 7 | 90.3 | 14.03 | 1-Methyl-4-isopropenylbenzene | 0.21 |
| 8 | 90.5 | 15.46 | Guaiacol | 0.16 |
| 9 | 85.7 | 16.07 | (2-Methyl-1-butenyl)-benzene | 0.02 |
| 10 | 89.4 | 16.55 | 4-Acetyl-1-methylcyclohexene | 0.53 |
| 11 | 84.7 | 18.84 | 3-Methylindene | 0.03 |
| 12 | 81.3 | 19.79 | p-Methylacetophenone | 0.02 |
| 13 | 72.8 | 20.16 | p-Menth-1-en-8-ol | 0.03 |
| 14 | 81.6 | 20.54 | Creosol | 0.27 |
| 15 | 81.2 | 20.93 | α,α,α-Trimethylstyrene | 0.01 |
| 16 | 79.9 | 22.68 | 2-Methyl-1,2-dihydronaphthalene | 0.02 |
| 17 | 91.4 | 25.95 | p-Ethylguaiacol | 0.10 |
| 18 | 81.9 | 26.92 | α-Longipinene | 0.04 |
| 19 | 74.8 | 28.31 | 2-Methoxy-4-propyl-phenol | 0.03 |
| 20 | 91.1 | 30.64 | Junipene | 0.29 |
| 21 | 87.1 | 32.16 | ç-Cadinene | 0.16 |
| 22 | 92.6 | 32,82 | Glcycl-L-proline | 1.47 |
| 23 | 84.3 | 33.48 | 6-Methyl-2-p-tolyl-heptane | 0.13 |
| 24 | 78.0 | 35.14 | Exo-8-(2-Propeny)-endo-8-methyl-3-oxabicyclo[4.2.0]oct-5-ene | 0.11 |
| 25 | 94.5 | 35.35 | α-Himachalene | 5.28 |
| 26 | 96.8 | 37.08 | Longifolene-(V4) | 6.66 |
| 27 | 74.2 | 37.24 | 1,2,3,4,4a,7-Hexahydro-1,6-dimethyl-4-(1-methylethyl)-naphthalene | 1.19 |
| 28 | 87.6 | 37.61 | α-Curcumene | 0.12 |
| 29 | 84.4 | 38.06 | ç-Muurolene | 0.11 |
| 30 | 97.2 | 38.27 | β-Himachalene | 29.16 |
| 31 | 96.3 | 38.62 | Cuparene | 0.28 |
| 32 | 81.6 | 38.83 | 8,9-Dehydro-neoisolongifolene | 0.46 |
| 33 | 86.4 | 39.34 | 9.10-Dehydro-cycloisolongifolene | 0.54 |
| 34 | 88.0 | 39.78 | α-Calacorene | 0.10 |
| 35 | 82.6 | 42.71 | cis-α-Bisabolene | 0.38 |
| 36 | 85.6 | 41.93 | Longiborneol | 0.26 |
| 37 | 89.2 | 43.03 | Dodecylbenzene | 0.28 |
| 38 | 90.1 | 43.62 | α-Bisabolol | 1.94 |
| 39 | 80.9 | 44.10 | Veridiflorol | 1.38 |
| 40 | 82.8 | 44.49 | α-Turmerone | 0.57 |
| 41 | 81.4 | 45.22 | ar-Turmerone | 8.82 |
| 42 | 82.3 | 45.59 | β-Turmerone | 5.12 |
| 43 | 97.4 | 47.37 | α-Atlantone | 28.70 |
| 44 | Others | 4.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koc, S.; Gultekin, Z.N.; Kahraman, S.; Cengiz, A.; Polat, B.; Caliskan, C.; Yildirim, T.; Tufan-Cetin, O.; Cetin, H. Exploring the Larvicidal and Repellent Potential of Taurus Cedar (Cedrus libani) Tar against the Brown Dog Tick (Rhipicephalus sanguineus sensu lato). Molecules 2023, 28, 7689. https://doi.org/10.3390/molecules28237689
Koc S, Gultekin ZN, Kahraman S, Cengiz A, Polat B, Caliskan C, Yildirim T, Tufan-Cetin O, Cetin H. Exploring the Larvicidal and Repellent Potential of Taurus Cedar (Cedrus libani) Tar against the Brown Dog Tick (Rhipicephalus sanguineus sensu lato). Molecules. 2023; 28(23):7689. https://doi.org/10.3390/molecules28237689
Chicago/Turabian StyleKoc, Samed, Zeynep Nur Gultekin, Sevval Kahraman, Aysegul Cengiz, Burak Polat, Cansu Caliskan, Tolga Yildirim, Ozge Tufan-Cetin, and Huseyin Cetin. 2023. "Exploring the Larvicidal and Repellent Potential of Taurus Cedar (Cedrus libani) Tar against the Brown Dog Tick (Rhipicephalus sanguineus sensu lato)" Molecules 28, no. 23: 7689. https://doi.org/10.3390/molecules28237689
APA StyleKoc, S., Gultekin, Z. N., Kahraman, S., Cengiz, A., Polat, B., Caliskan, C., Yildirim, T., Tufan-Cetin, O., & Cetin, H. (2023). Exploring the Larvicidal and Repellent Potential of Taurus Cedar (Cedrus libani) Tar against the Brown Dog Tick (Rhipicephalus sanguineus sensu lato). Molecules, 28(23), 7689. https://doi.org/10.3390/molecules28237689