Effusanin B Inhibits Lung Cancer by Prompting Apoptosis and Inhibiting Angiogenesis
Abstract
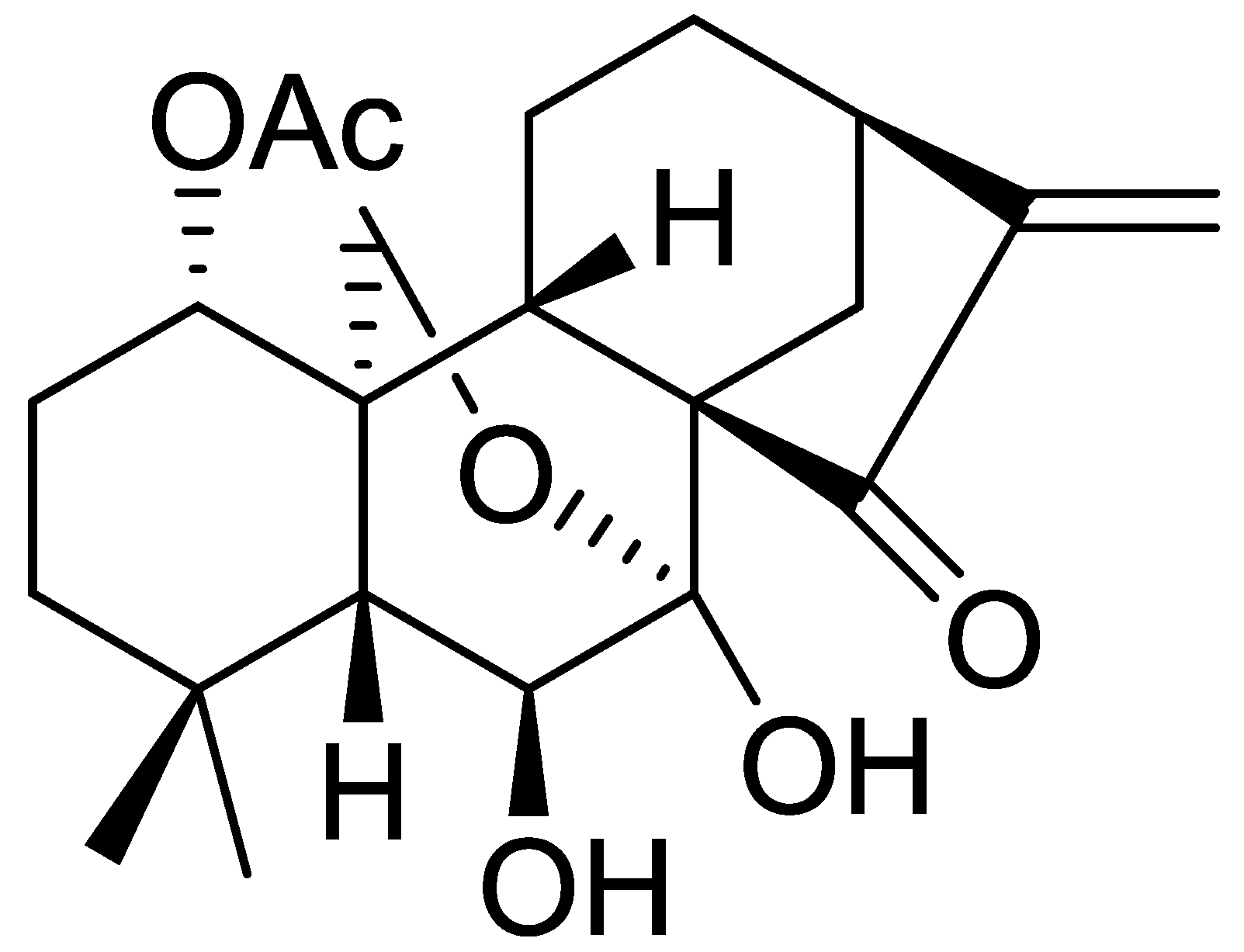
1. Introduction
2. Results
2.1. Effusanin B Inhibited Tumor Cell Proliferation In Vitro
2.2. Apoptotic Effects of A549 Cells Induced by Effusanin B
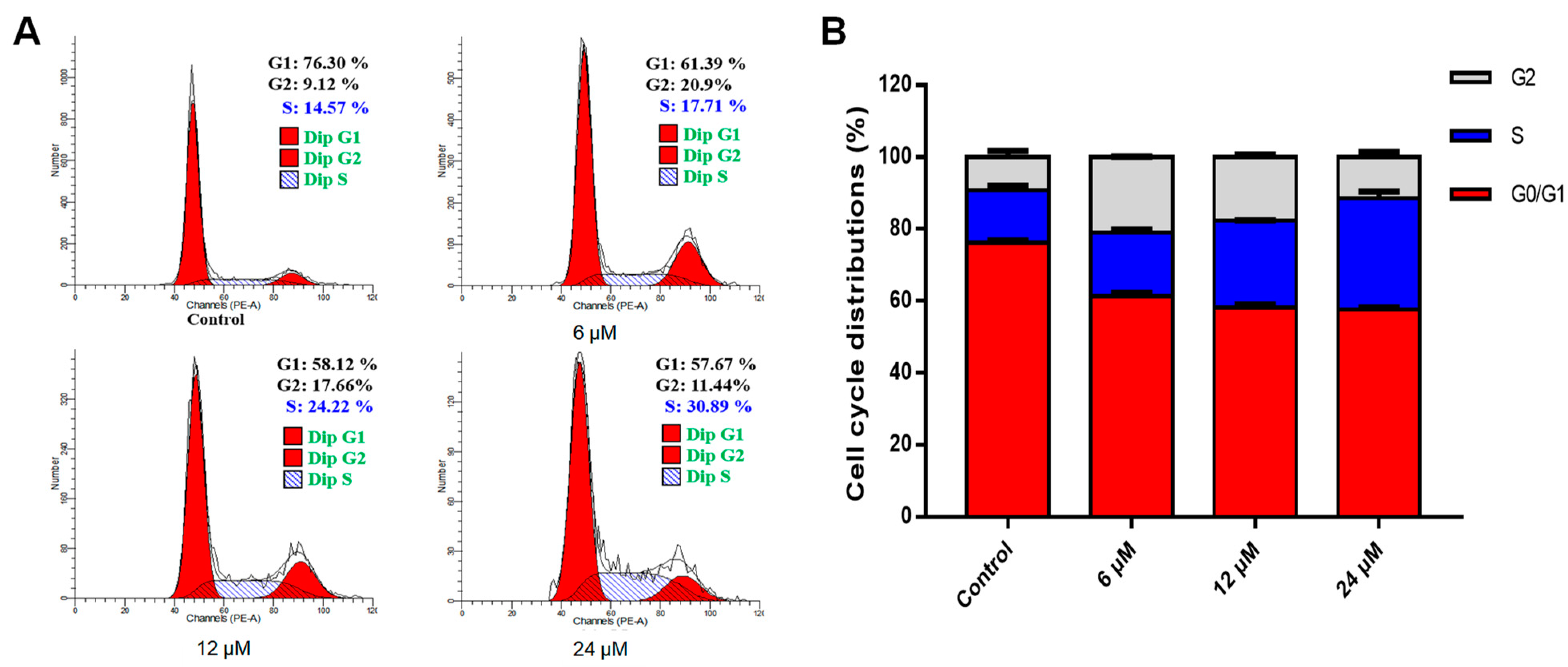
2.3. Effects of Effusanin B on Cell Cycle
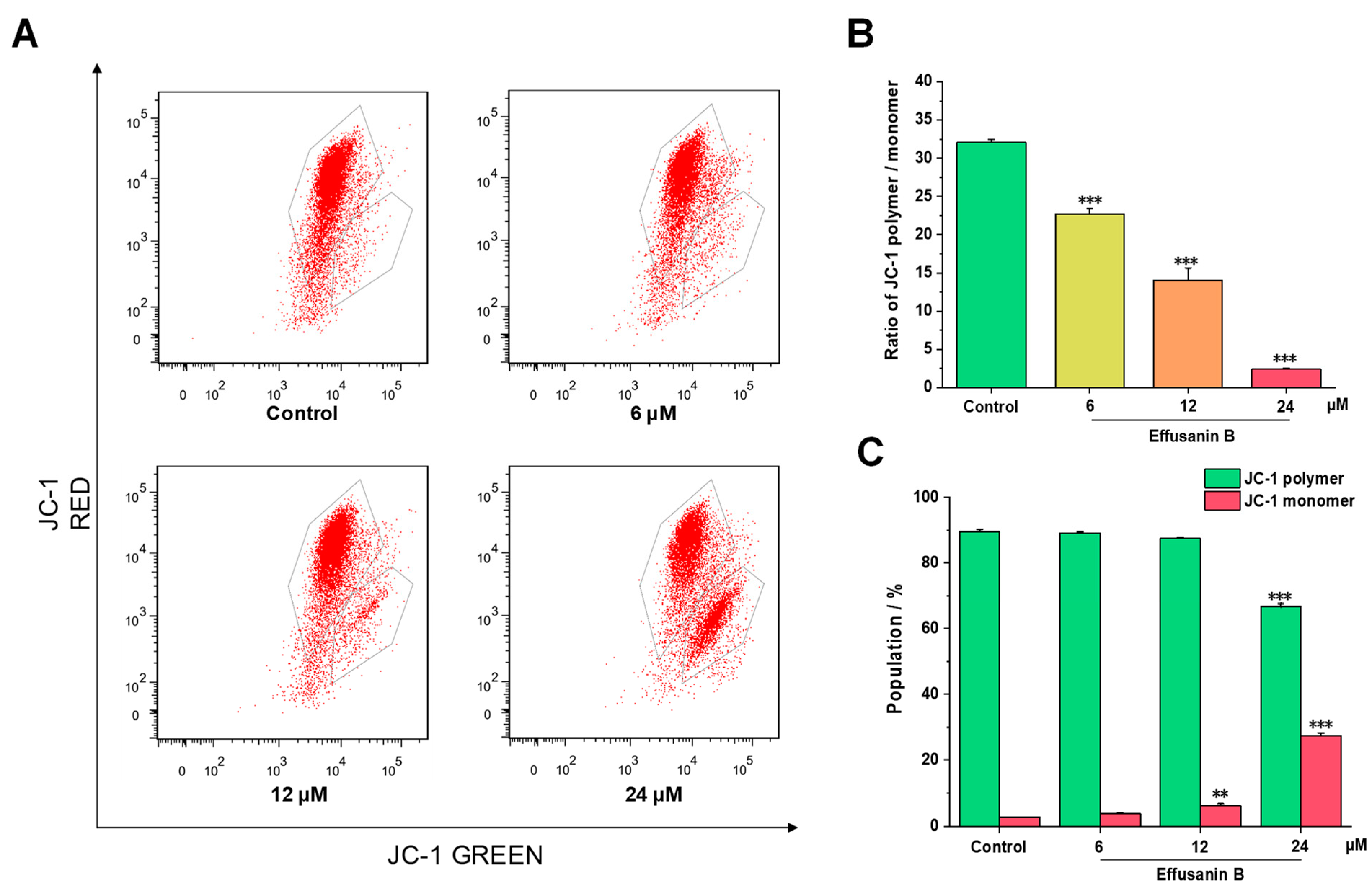
2.4. Effusanin B Induced the MMP
2.5. Effusanin B Induced ROS Generation in A549 Cells
2.6. Effusanin B Affected the Expression of Apoptosis-Related Proteins
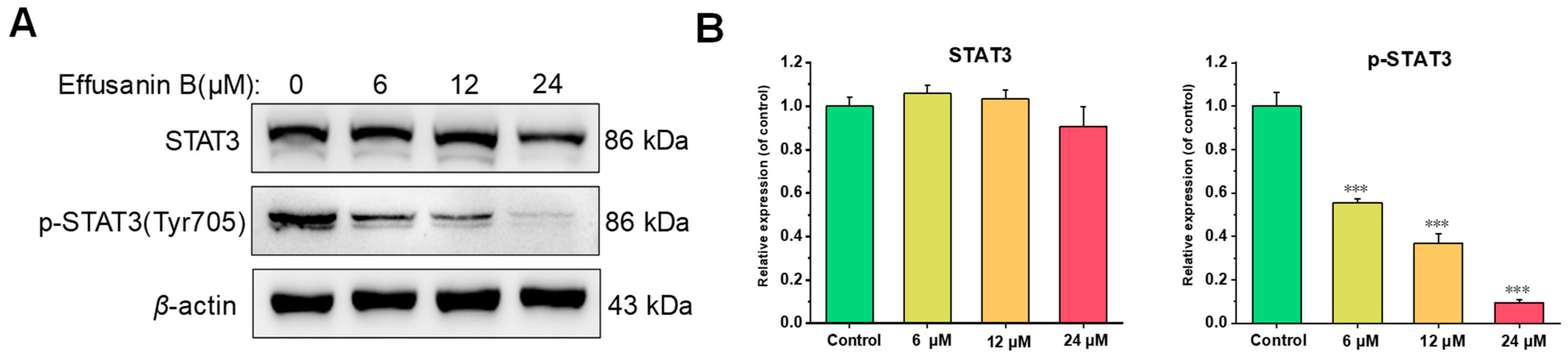
2.7. Effusanin B Regulated STAT3 Signaling Pathway
2.8. Effusanin B Inhibited A549 Cell Metastasis by Regulating the FAK Signaling Pathway
2.9. Effusanin B Possessed In Vivo Antitumor Effects
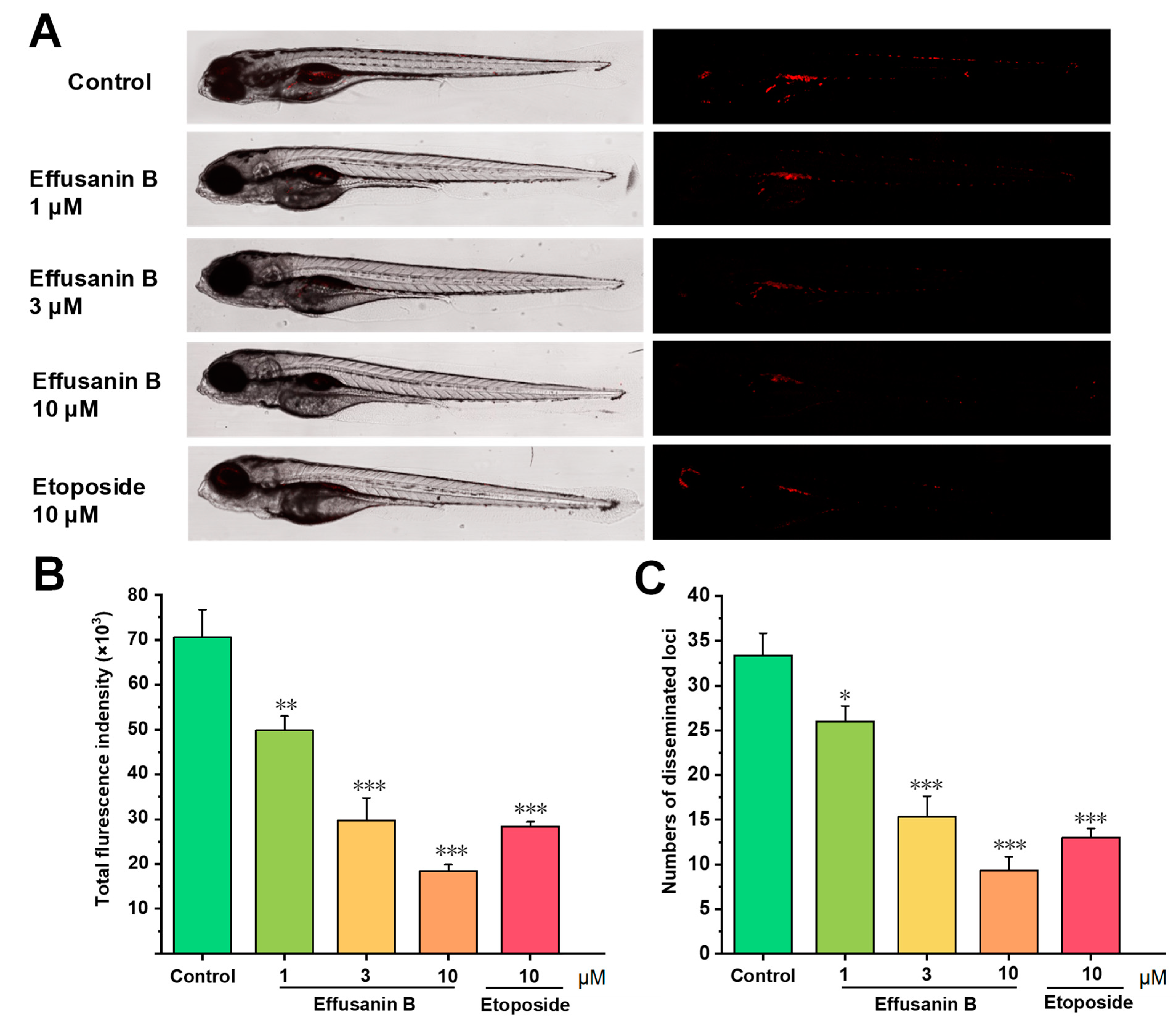
2.10. Effusanin B Blocked Angiogenesis In Vivo
3. Discussion
4. Materials and Methods
4.1. Materials and Cell Culture
4.2. Purification of Effusanin B
4.3. Cytotoxicity Assay
4.4. Apoptosis Analysis
4.5. Cell Cycle Analysis
4.6. Mitochondrial Membrane Potential (MMP) Evaluation
4.7. Measurement of Reactive Oxygen Species (ROS)
4.8. Wound-Scratch Assay
4.9. Western Blotting Analysis
4.10. Zebrafish Husbandry and Maintenance
4.11. In Vivo Antitumor Assay Using Zebrafish Xenografts
4.12. Antiangiogenetic Assay Using a Transgenic Zebrafish Model
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, L.; Kwok, H.F. Nanotechnology-based approaches overcome lung cancer drug resistance through diagnosis and treatment. Drug Resist. Updates 2023, 66, 100904. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Shao, S.; Yu, H.; Wang, D.; Li, C.; Wang, S. Rabdosia serra alleviates dextran sulfate sodium salt-induced colitis in mice through anti-inflammation, regulating Th17/Treg balance, maintaining intestinal barrier integrity, and modulating gut microbiota. J. Pharm. Anal. 2022, 12, 824–838. [Google Scholar] [CrossRef] [PubMed]
- Committee for the editing of Chinese Materia Medica, State Administration of Traditional Chinese Medicine. Chinese Materia Medica (VII), 1st ed.; Shanghai Science and Technology Press: Shanghai, China, 1999; pp. 154–156. [Google Scholar]
- Wong, L.L.; Liang, Z.; Chen, H.; Zhao, Z. Rapid differentiation of Xihuangcao from the three Isodon species by UPLC-ESI-QTOF-MS/MS and chemometrics analysis. Chin. Med. 2016, 11, 48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, G.-L.; Xu, W.; Liu, X.-J.; Yan, X.-L.; Chen, J. Two new abietane diterpenoids from the leaves of Rabdosia serra. J. Asian Nat. Prod. Res. 2020, 22, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Q.; Xuan, L.-J. ent-6,7-Secokaurane diterpenoids from Rabdosia serra and their cytotoxic activities. Phytochemistry 2016, 122, 119–125. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, X.; Tian, M.; Ma, Y.; Jin, B.; Gao, W.; Huang, L. Recent progress and new perspectives for diterpenoid biosynthesis in medicinal plants. Med. Res. Rev. 2021, 41, 2971–2997. [Google Scholar] [CrossRef] [PubMed]
- Yardley, D.A. nab-Paclitaxel mechanisms of action and delivery. J. Control Release 2013, 170, 365–372. [Google Scholar] [CrossRef]
- Wei, J.; Yan, Y.; Chen, X.; Qian, L.; Zeng, S.; Li, Z.; Xu, Z. The Roles of Plant-Derived Triptolide on Non-Small Cell Lung Cancer. Oncol. Res. 2019, 27, 849–858. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, Y.; Liu, F.; Zhao, Y.; Xu, J.; Guo, Y. Trigothysoid N inhibits tumor proliferation migration by targeting mitochondria the STAT3/FAK pathway Arab. J. Chem. 2023, 16, 104930. [Google Scholar]
- Li, Y.; Wang, H.; Liu, W.; Hou, J.; Xu, J.; Guo, Y.; Hu, P. Cratoxylumxanthone C, a natural xanthone, inhibits lung cancer proliferation and metastasis by regulating STAT3 and FAK signal pathways. Front. Pharmacol. 2022, 13, 920422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.; Li, Y.; Li, Y.; Zhang, H.; Song, Z.; Guo, Y. A natural xanthone suppresses lung cancer growth and metastasis by targeting STAT3 and FAK signaling pathways. Phytomedicine 2022, 102, 154118. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, J.; Guo, Y. Chlorahololide D, a Lindenane-Type Sesquiterpenoid Dimer from Chloranthus holostegius Suppressing Breast Cancer Progression. Molecules 2023, 28, 7070. [Google Scholar] [CrossRef] [PubMed]
- Tetsuro, F.; Yoshio, T.; Tetsuro, S.; Akira, U. Structures of effusanins, antibacterial diterpenoids from rabdosia effusa. Chem. Lett. 1980, 9, 1635–1638. [Google Scholar]
- Abbaskhan, A.; Choudhary, M.; Tsuda, Y.; Parvez, M.; Atta ur, R.; Shaheen, F.; Parween, Z.; Zaidi, M.A. A New Diepoxy-ent-kauranoid, Rugosinin, from Isodon rugosus. Planta Med. 2003, 69, 94–96. [Google Scholar] [CrossRef]
- Gunatilaka, A.A.L.; Kingston, D.G.I. DNA-damaging natural products with potential anticancer activity. Stud. Nat. Prod. Chem. 1997, 20, 457–505. [Google Scholar]
- Osawa, K.; Yasuda, H.; Maruyama, T.; Morita, H.; Takeya, K.; Itokawa, H.; Okuda, K. An investigation of diterpenes from the leaves of Rabdosia trichocarpa and their antibacterial activity against oral microorganisms. Chem. Pharm. Bull. 1994, 42, 922–925. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, Y.; Hao, Z.; Zhang, Y.; Luo, S.; Dang, X.; Sun, R.; Duan, S.; Lv, D.; Jiang, F.; et al. Design, synthesis and antitumor activities of thiazole-containing mitochondrial targeting agents. Bioorg Chem. 2021, 115, 105271. [Google Scholar] [CrossRef]
- De Lima, L.T.F.; de Oliveira Ganzella, F.A.; Cardoso, G.C.; dos Santos Pires, V.; Chequin, A.; Santos, G.L.; de Souza Ramos, E.A. l-carvone decreases breast cancer cells adhesion, migration, and invasion by suppressing FAK activation. Chem. Biol. Interact. 2023, 378, 110480. [Google Scholar]
- Mohammad, N.S.; Nazli, R.; Zafar, H.; Fatima, S. Effects of lipid based Multiple Micronutrients Supplement on the birth outcome of underweight pre-eclamptic women: A randomized clinical trial. Pak. J. Med. Sci. 2022, 38, 219–226. [Google Scholar]
- Dai, X.; Fan, J.; Liu, D.; Li, H.; Shu, L.; Gao, P.; Wang, X. The marine natural product trichobotrysin B inhibits proliferation and promotes apoptosis of human glioma cells via the IL-6-mediated STAT3/JAK signaling pathway. Smart Mater. Struct. 2024, 5, 66–74. [Google Scholar] [CrossRef]
- Liu, L.; Liu, T.; Tao, W.; Liao, N.; Yan, Q.; Li, L.; Sun, D. Flavonoids from Scutellaria barbata D. Don exert antitumor activity in colorectal cancer through inhibited autophagy and promoted apoptosis via ATF4/sestrin2 pathway. Phytomedicine 2022, 99, 154007. [Google Scholar] [CrossRef]
- Hou, Y.; Pi, C.; Feng, X.; Wang, Y.; Fu, S.; Zhang, X.; Wei, Y. Antitumor Activity In Vivo and Vitro of New Chiral Derivatives of Baicalin and Induced Apoptosis via the PI3K/Akt Signaling Pathway. Mol. Ther. Oncolyt. 2020, 19, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Dadsena, S.; Zollo, C.; García-Sáez, A.J. Mechanisms of mitochondrial cell death. Biochem. Soc. Trans. 2021, 49, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yu, T.; Yang, L.; Liu, T.; Song, Z.; Liu, S.; Tang, C. Polysaccharide from Cordyceps cicadae inhibit mitochondrial apoptosis to ameliorate drug-induced kidney injury via Bax/Bcl-2/Caspase-3 pathway. J. Funct. Foods 2022, 97, 105244. [Google Scholar] [CrossRef]
- Du, H.; He, Y.; Zhu, J.; Zhou, H.; Shao, C.; Yang, J.; Wan, H. Danhong injection alleviates cerebral ischemia-reperfusion injury by inhibiting mitochondria-dependent apoptosis pathway and improving mitochondrial function in hyperlipidemia rats. Biomed. Pharmacother. 2023, 157, 114075. [Google Scholar] [CrossRef]
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef]
- Lv, W.; Booz, G.W.; Wang, Y.; Fan, F.; Roman, R.J. Inflammation and renal fibrosis: Recent developments on key signaling molecules as potential therapeutic targets. Eur. J. Pharmacol. 2018, 820, 65–76. [Google Scholar] [CrossRef]
- Tang, S.-J.; Shao, C.-X.; Yang, Y.; Ren, R.; Jin, L.; Hu, D.; Xu, J. The antitumor effect of mycelia extract of the medicinal macrofungus Inonotus hispidus on HeLa cells via the mitochondrial-mediated pathway. J. Ethnopharmacol. 2023, 311, 116407. [Google Scholar] [CrossRef]
- Tait, S.W.G.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef]
- Morris, J.L.; Gillet, G.; Prudent, J.; Popgeorgiev, N. Bcl-2 Family of Proteins in the Control of Mitochondrial Calcium Signalling: An Old Chap with New Roles. Int. J. Mol. Sci. 2021, 22, 3730. [Google Scholar] [CrossRef]
- Amin, T.; Sharma, R.P.; Mir, K.B.; Slathia, N.; Chhabra, S.; Tsering, D.; Goswami, A. Quinoxalinone substituted pyrrolizine (4h)-induced dual inhibition of AKT and ERK instigates apoptosis in breast and colorectal cancer by modulating mitochondrial membrane potential. Eur. J. Pharmacol. 2023, 957, 175945. [Google Scholar] [CrossRef]
- Chen, X.J.; Liu, Z.B.; Li, X.; Pu, X.M.; Mei, M.J.; Pu, X.Y.; Wang, X.G.; Hao, J.J.; Zhang, F.; Qiu, B.; et al. 3-Hydroxymorindone from Knoxia roxburghii (Spreng.) M. A. Rau induces ROS-mediated mitochondrial dysfunction cervical cancer cells apoptosis via inhibition of PI3K/AKT/NF-κB signaling pathway. J. Funct. Foods 2023, 103, 105498. [Google Scholar] [CrossRef]
- Wang, W.; Li, C.; Chen, Z.; Zhang, J.; Ma, L.; Tian, Y.; Yu, J. Novel diosgenin–amino acid–benzoic acid mustard trihybrids exert antitumor effects via cell cycle arrest and apoptosis. J. Steroid Biochem. Mol. Biol. 2022, 216, 106038. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Z.; Li, J.; Zhang, H.; Jia, Y.; Liu, J. DP from Euphorbia fischeriana S. mediated apoptosis in leukemia cells via the PI3k/Akt signaling pathways. J. Ethnopharmacol. 2021, 279, 113889. [Google Scholar] [CrossRef]
- Thangam, R.; Senthilkumar, D.; Suresh, V.; Sathuvan, M.; Sivasubramanian, S.; Pazhanichamy, K.; Sivaraman, J. Induction of ROS-Dependent Mitochondria-Mediated Intrinsic Apoptosis in MDA-MB-231 Cells by Glycoprotein from Codium decorticatum. J. Agric. Food Chem. 2014, 62, 3410–3421. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, A.J.; Ye, S.-K. Highlighted STAT3 as a potential drug target for cancer therapy. BMB Rep. 2019, 52, 415–423. [Google Scholar] [CrossRef]
- Xu, Y.H.; Lu, S. A meta-analysis of STAT3 and phospho-STAT3 expression and survival of patients with non-small-cell lung cancer. Eur. J. Surg. Oncol. 2014, 40, 311–317. [Google Scholar] [CrossRef]
- Shen, Y.; Cai, H.; Ma, S.; Zhu, W.; Zhao, H.; Li, J.; Xiao, Z. Telocinobufagin Has Antitumor Effects in Non-Small-Cell Lung Cancer by Inhibiting STAT3 Signaling. J. Nat. Prod. 2022, 85, 765–775. [Google Scholar] [CrossRef]
- Banerjee, K.; Resat, H. Constitutive activation of STAT3 in breast cancer cells: A review. Int. J. Cancer 2016, 138, 2570–2578. [Google Scholar] [CrossRef]
- Fan, Z.; Xu, Q.; Wang, C.; Lin, X.; Zhang, Q.; Wu, N. A tropomyosin-like Meretrix meretrix Linnaeus polypeptide inhibits the proliferation and metastasis of glioma cells via microtubule polymerization and FAK/Akt/MMPs signaling. Int. J. Biol. Macromol. 2020, 145, 154–164. [Google Scholar] [CrossRef]
- Zou, L.; Liu, X.; Li, J.; Li, W.; Zhang, L.; Li, J.; Zhang, J. Tetramethylpyrazine Enhances the Antitumor Effect of Paclitaxel by Inhibiting Angiogenesis and Inducing Apoptosis. Front. Pharmacol. 2019, 10, 707. [Google Scholar] [CrossRef]
- Olejarz, W.; Kubiak-Tomaszewska, G.; Chrzanowska, A.; Lorenc, T. Exosomes in Angiogenesis and Anti-angiogenic Therapy in Cancers. Int. J. Mol. Sci. 2020, 21, 5840. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Li, Y.; Xing, H.; Cao, R.; Jin, X.; Xu, J.; Guo, Y. Effusanin B Inhibits Lung Cancer by Prompting Apoptosis and Inhibiting Angiogenesis. Molecules 2023, 28, 7682. https://doi.org/10.3390/molecules28237682
Hou J, Li Y, Xing H, Cao R, Jin X, Xu J, Guo Y. Effusanin B Inhibits Lung Cancer by Prompting Apoptosis and Inhibiting Angiogenesis. Molecules. 2023; 28(23):7682. https://doi.org/10.3390/molecules28237682
Chicago/Turabian StyleHou, Jiantong, Ying Li, Honghong Xing, Ruyu Cao, Xiaomeng Jin, Jing Xu, and Yuanqiang Guo. 2023. "Effusanin B Inhibits Lung Cancer by Prompting Apoptosis and Inhibiting Angiogenesis" Molecules 28, no. 23: 7682. https://doi.org/10.3390/molecules28237682
APA StyleHou, J., Li, Y., Xing, H., Cao, R., Jin, X., Xu, J., & Guo, Y. (2023). Effusanin B Inhibits Lung Cancer by Prompting Apoptosis and Inhibiting Angiogenesis. Molecules, 28(23), 7682. https://doi.org/10.3390/molecules28237682





