Progress of Dispersants for Coal Water Slurry
Abstract
:1. Introduction
2. Classification and Characteristics of CWS Dispersants
| Basis of Classification | Subtype | Example | Characterization | Reference |
|---|---|---|---|---|
| Dissociation properties | Anionic | Lignin-based dispersants, naphthalene-based dispersants | Largest variety, relatively low dispersant price | [41,42] |
| Cationic | Quaternary ammonium salts, heterocycles, and octadecenylamine acetate | Good dispersion | [43] | |
| Amphoteric | Amphoteric polycarboxylic acid dispersant | Better adsorption effect | [44] | |
| Non-ionic | Polyoxyethylene ether and polyoxyethylene/polyoxypropane block polyether surfactants | Easily regulated and controlled, independent of water quality and soluble matter in coal | [45] | |
| Raw material sources | Natural product | Lignin-based dispersants, starch-based dispersants | Low-cost, abundant, and renewable | [36,46] |
| Synthetic | Naphthalene sulfonate-based dispersants, polyolefin-based dispersants | Targeted modification | [34] | |
| Dispersant structure | One-dimensional dispersants | Naphthalene sulfonic acid formaldehyde condensate (NSF), sodium salt of dodecylbenzene sulfonate, and sodium dodecyl sulfate (SDS) | With linear hydrophobic ends | [23,24,25] |
| Two-dimensional dispersants | Sodium polystyrene sulfonate (PSS), polycarboxylic acid-type dispersant | Contains many hydrophilic and hydrophobic groups | [27,28] | |
| Three-dimensional dispersants | Dispersant TAA | Linear and comb structures | [33] |
2.1. Anionic Dispersants
2.1.1. Lignin Dispersants
2.1.2. Humic Acid-Based Dispersants
2.1.3. Naphthalene-Based Dispersants
2.1.4. Polycarboxylic Acid Dispersants
2.2. Cationic Dispersants
2.3. Non-Ionic Dispersants
| Category | Type | Characteristic | Reference |
|---|---|---|---|
| Non-ionic dispersant | Polyoxyethylene ether | Good wettability, permeability, and dispersion | [76] |
| Polyoxyethylene block polyether | High chemical stability | [79] | |
| Polyoxyethylene alkyl alcohol amide | Good stability and hydrolysis resistance | [78] |
3. Mechanism of CWS Dispersants
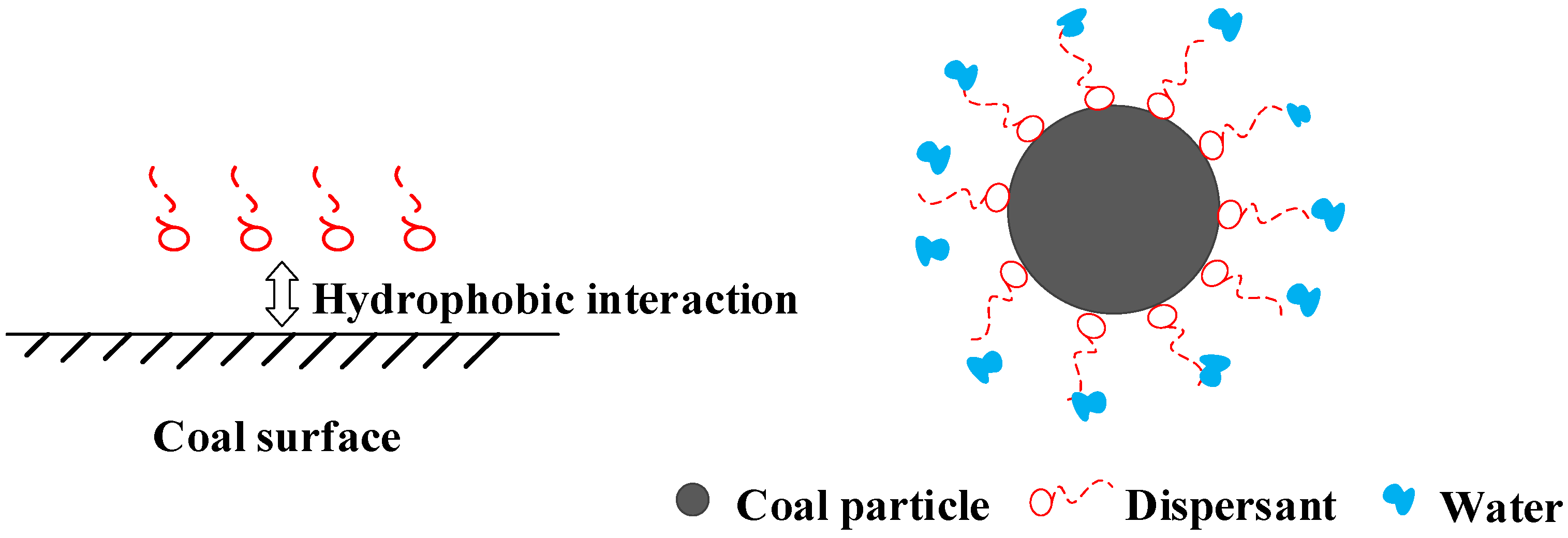
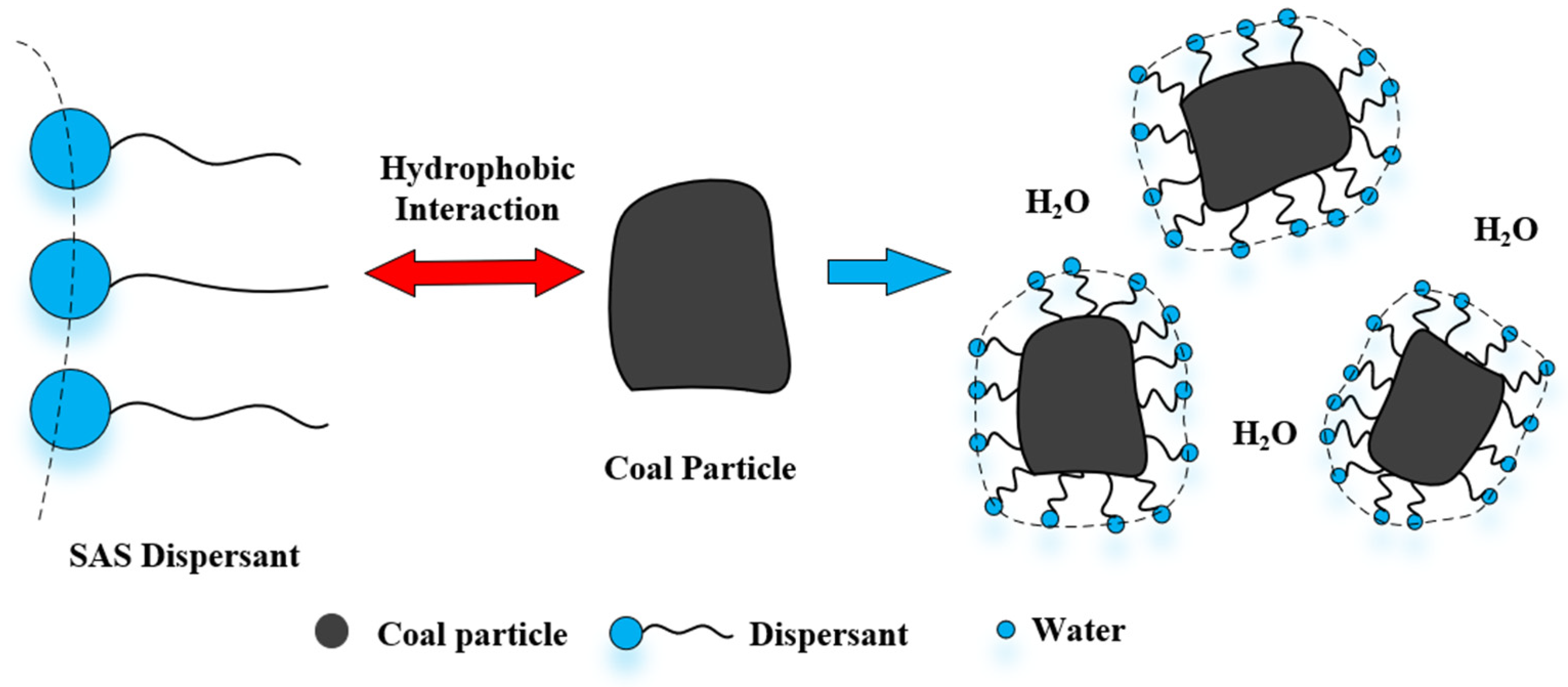
3.1. Wetting and Dispersing
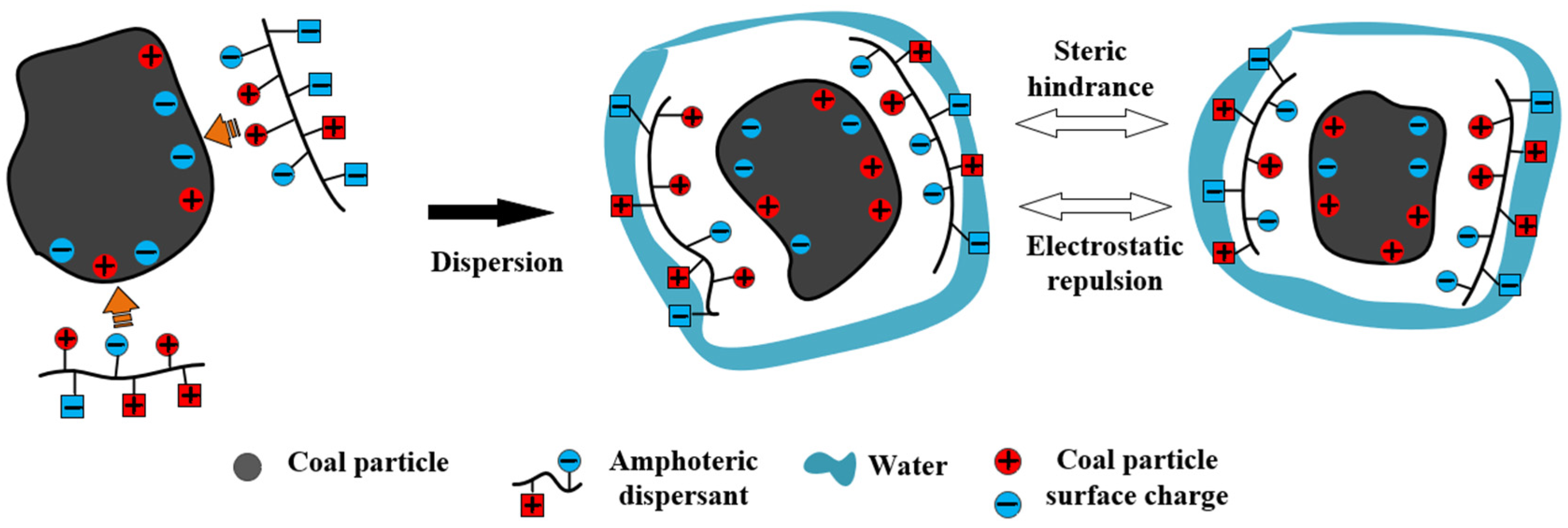
3.2. Electrostatic Repulsion
3.3. Spatial Steric Effects
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pourmahdi, M.; Abdollahi, M.; Nasiri, A. Effect of lignin source and initiation conditions on graft copolymerization of lignin with acrylamide and performance of graft copolymer as additive in water- based drilling fluid. J. Pet. Sci. Eng. 2023, 220, 111253. [Google Scholar] [CrossRef]
- Guo, H.; Yu, Y.; Wang, K.; Yang, Z.; Wang, L.; Xu, C. Kinetic characteristics of desorption and diffusion in raw coal and tectonic coal and their influence on coal and gas outburst. Fuel 2023, 343, 127883. [Google Scholar] [CrossRef]
- Wei, X.; Wang, J.; Ding, Y. Progress and Development Trend of Clean and Efficient Coal Utilization Technology. Bull. Chin. Acad. Sci. 2019, 34, 409–416. [Google Scholar]
- Wan, G.; Yu, J.; Wang, X.; Sun, L. Study on the pyrolysis behavior of coal-water slurry and coal-oil-water slurry. J. Energy Inst. 2022, 100, 10–21. [Google Scholar] [CrossRef]
- Nyashina, G.S.; Kuznetsov, G.V.; Strizhak, P.A. Energy efficiency and environmental aspects of the combustion of coal-water slurries with and without petrochemicals. J. Clean. Prod. 2018, 172, 1730–1738. [Google Scholar] [CrossRef]
- Dmitrienko, M.A.; Strizhak, P.A. Coal-water slurries containing petrochemicals to solve problems of air pollution by coal thermal power stations and boiler plants: An introductory review. Sci. Total Environ. 2018, 613–614, 1117–1129. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, P.; Zhang, W.; Li, J.; Zhang, G. Polycarboxylate adsorption on coal surfaces and its effect on viscosity of coal-water slurries. Powder Technol. 2017, 315, 98–105. [Google Scholar] [CrossRef]
- Tu, Y.; Feng, P.; Ren, Y.; Cao, Z.; Wang, R.; Xu, Z. Adsorption of ammonia nitrogen on lignite and its influence on coal water slurry preparation. Fuel 2019, 238, 34–43. [Google Scholar] [CrossRef]
- Hong, N.; Qiu, X. Structure-Adsorption Behavior-Dispersion Property Relationship of Alkyl Chain Cross-Linked Lignosulfonate with Different Molecular Weights. ACS Omega 2020, 5, 4836–4843. [Google Scholar] [CrossRef]
- Gao, X.R.; Chang, H.H.; Wei, W.L.; Wang, L.Z.; Zhang, Z.L.; Wang, Z.Z. The Influence of the Amount of Dispersants and Slurry Content on the Slurry Ability of Coal Pitch Water Slurry. Energy Sources Part A-Recovery Util. Environ. Eff. 2011, 33, 194–201. [Google Scholar] [CrossRef]
- Huang, J.; Xu, J.; Wang, D.; Li, L.; Guo, X. Effects of Amphiphilic Copolymer Dispersants on Rheology and Stability of Coal Water Slurry. Ind. Eng. Chem. Res. 2013, 52, 8427–8435. [Google Scholar] [CrossRef]
- Li, X.T.; Ma, C.D.; Lyu, J.Q.; He, M.; Wang, J.X.; Wang, Q.B.; Wang, Z.H.; You, X.F.; Li, L. Influence and mechanism of alkali-modified sludge on coal water slurry properties. Environ. Sci. Pollut. Res. 2023, 30, 27372–27381. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, X.L.; Li, G.; Kong, L.X.; Li, H.Z.; Bai, J.; Li, W. Effect of Coal Blending on Ash Fusibility and Slurryability of Xinjiang Low-Rank Coal. Processes 2022, 10, 1693. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Li, N.; Wang, Y.; Li, D.; Cen, K. Effects of Metal Ions in Organic Wastewater on Coal Water Slurry and Dispersant Properties. Energy Fuels 2019, 33, 7110–7117. [Google Scholar] [CrossRef]
- LiZhuang, Z.O.U.; Shuquan, Z.H.U.; Xiaoling, W.; Xiangkun, G.U.O.; Guangwen, C.U.I. Interaction between different CWS dispersants and coal—X Adsorptive characteristics of dispersant on coal surface. J. Fuel Chem. Technol. 2006, 34, 10–14. [Google Scholar]
- Wang, S.; Liu, J.; Wu, H.; Wang, Y.; Li, N. Research status of compound dispersant of coal water slurry. Appl. Chem. Ind. 2017, 46, 1616. [Google Scholar]
- Shen, Y.; Miao, P.; Liu, S.; Gao, J.; Han, X.; Zhao, Y.; Chen, T. Preparation and Application Progress of Imprinted Polymers. Polymers 2023, 15, 2344. [Google Scholar] [CrossRef]
- Konduri, M.K.R.; Fatehi, P. Alteration in interfacial properties and stability of coal water slurry by lignosulfonate. Powder Technol. 2019, 356, 920–929. [Google Scholar] [CrossRef]
- Guanghua, Z.; Xuedan, Z.H.U.; Pan, X.I.E.; Jingjing, Q.I. Preparation and characterization of new type anion: Coal-water slurry dispersant. Appl. Chem. Ind. 2008, 37, 1267. [Google Scholar]
- Lv, D.M.; Yan, H.; Wu, H.J.; Zheng, Z.Q.; Zhang, X.J.; Li, J.; Gong, S.W. Effect of modification by Gemini cationic surfactant on the properties of slurries prepared with petroleum coke: Experiments and molecular dynamics simulation. Fuel 2022, 317, 123541. [Google Scholar] [CrossRef]
- Li, L.; Zhao, L.Y.; Wang, Y.X.; Wu, J.N.; Meng, G.H.; Liu, Z.Y.; Zhang, J.S.; Hu, B.X.; He, Q.H.; Guo, X.H. Novel Dispersant with a Three-Dimensional Reticulated Structure for a Coal-Water Slurry. Energy Fuels 2018, 32, 8310–8317. [Google Scholar] [CrossRef]
- Zhu, J.F.; Li, J.L.; Liu, R.Q.; Wang, J.Q.; Tang, Y.W.; Zhang, W.B.; Zhang, G.H. Synthesis and evaluation of a multi-block polycarboxylic acid for improving low-rank coal to make the slurry. Colloids Surf. A—Physicochem. Eng. Asp. 2022, 646, 128966. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Z.; Sun, Q.; Liu, K.; Hu, S.; Li, J. Insights into the Dispersion Mechanism of Microfine Coal Particles Modified with Naphthalene Sulfonate Formaldehyde Based on EDLVO Theory. Energy Fuels 2023, 37, 7777–7787. [Google Scholar] [CrossRef]
- Xu, R.; He, Q.; Cai, J.; Pan, Y.; Shen, J.; Hu, B. Effects of chemicals and blending petroleum coke on the properties of low-rank Indonesian coal water mixtures. Fuel Process. Technol. 2008, 89, 249–253. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, T.; Ren, G.; Zhu, Z.; Yang, Y. Research on the Effect of Surfactants on the Biodesulfurization of Coal. Energy Fuels 2017, 31, 8116–8119. [Google Scholar] [CrossRef]
- Yi, F.; Gopan, A.; Axelbaum, R.L. Characterization of coal water slurry prepared for PRB coal. J. Fuel Chem. Technol. 2014, 42, 1167–1171. [Google Scholar] [CrossRef]
- Zhou, M.; Huang, K.; Yang, D.; Qiu, X. Development and evaluation of polycarboxylic acid hyper-dispersant used to prepare high-concentrated coal–water slurry. Powder Technol. 2012, 229, 185–190. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, G.; Liu, G.; Qu, Q.; Li, Y. Investigation on the rheological and stability characteristics of coal–water slurry with long side-chain polycarboxylate dispersant. Fuel Process. Technol. 2014, 118, 187–191. [Google Scholar] [CrossRef]
- Yu, Y.J.; Liu, J.Z.; Hu, Y.X.; Gao, F.Y.; Zhou, J.H.; Cen, K.F. The properties of Chinese typical brown coal water slurries. Energy Sources Part A—Recovery Util. Environ. Eff. 2016, 38, 1176–1182. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Shang, T.; Zhu, J. Synthesis, characterization and application of a dispersant based on rosin for coal-water slurry. Int. J. Min. Sci. Technol. 2014, 24, 695–699. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Oliveira, M.L.S.; Sampaio, C.H.; de Brum, I.A.S.; Hower, J.C. Vanadium and Nickel Speciation in Pulverized Coal and Petroleum Coke Co-combustion. Energy Fuels 2013, 27, 1194–1203. [Google Scholar] [CrossRef]
- Cutruneo, C.M.N.L.; Oliveira, M.L.S.; Ward, C.R.; Hower, J.C.; de Brum, I.A.S.; Sampaio, C.H.; Kautzmann, R.M.; Taffarel, S.R.; Teixeira, E.C.; Silva, L.F.O. A mineralogical and geochemical study of three Brazilian coal cleaning rejects: Demonstration of electron beam applications. Int. J. Coal Geol. 2014, 130, 33–52. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, X.; Jin, L.E.; Cao, Q.; Li, P. Synthesis and evaluation of a novel dispersant with jellyfish-like 3D structure for preparing coal–water slury. Fuel 2017, 200, 458–466. [Google Scholar] [CrossRef]
- Hu, S.; Li, J.; Liu, K.; Chen, Y. Comparative study on distribution characteristics of anionic dispersants in coal water slurry. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129176. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, G. Synthesis of Benzylated Hydrophobic Modified Starch and Dispersion Property of Coal Water Slurry. Coal Convers. 2020, 43, 81–88. [Google Scholar]
- Zhu, J.; Wang, P.; Li, Y.; Li, J.; Zhang, G. Dispersion performance and mechanism of polycarboxylates bearing side chains of moderate length in coal-water slurries. Fuel 2017, 190, 221–228. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, G.; Li, J.; Zhao, F. Synthesis, adsorption and dispersion of a dispersant based on starch for coal–water slurry. Colloids Surf. A Physicochem. Eng. Asp. 2013, 422, 165–171. [Google Scholar] [CrossRef]
- Shen, J.; Fatehi, P.; Ni, Y.H. Biopolymers for surface engineering of paper-based products. Cellulose 2014, 21, 3145–3160. [Google Scholar] [CrossRef]
- Kontturi, E.; Vuorinen, T. Indirect evidence of supramolecular changes within cellulose microfibrils of chemical pulp fibers upon drying. Cellulose 2009, 16, 65–74. [Google Scholar] [CrossRef]
- Ding, C.; Zhu, X.; Ma, X.; Yang, H. Synthesis and Performance of a Novel Cotton Linter Based Cellulose Derivatives Dispersant for Coal-Water Slurries. Polymers 2022, 14, 1103. [Google Scholar] [CrossRef]
- Chen, X.; Liu, M.; Liu, Y.; Chen, Z. Study on Performance of Masson Pine Alkali Lignin Prepared as a Dispersant of Coal Water Slurry. J. Fujian Norm. Univ. Nat. Sci. Ed. 2013, 29, 63–67. [Google Scholar]
- Zhang, K.; Tian, Y.; Zhang, R.; Shi, G.; Zhou, B.; Zhang, G.; Niu, Y.; Tian, Z.; Gong, J. Interactions of amphiphilic humic acid-based polymers with coal and effect on preparation of coal-water slurry. Powder Technol. 2020, 376, 652–660. [Google Scholar] [CrossRef]
- Ionkin, A.S.; Fish, B.M.; Li, Z.R.; Liang, L.; Lewittes, M.E.; Cheng, L.K.; Westphal, C.; Pepin, J.G.; Gao, F. Quaternary phosphonium salts as cationic selective dispersants in silver conductive pastes for photovoltaic applications. Sol. Energy Mater. Sol. Cells 2014, 124, 39–47. [Google Scholar] [CrossRef]
- Du, L.; Zhang, G.H.; Yang, D.D.; Luo, J.; Liu, Y.W.; Zhang, W.B.; Zhang, C.; Li, J.G.; Zhu, J.F. Synthesis of a novel amphoteric copolymer and its application as a dispersant for coal water slurry preparation. R. Soc. Open Sci. 2021, 8, 201480. [Google Scholar] [CrossRef]
- Yi, S.U.; Shuquan, Z.H.U. Slurryability of single chain alkylphenol polyoxyethylene as coal water slurry dispersant. J. China Coal Soc. 2011, 36, 1396–1400. [Google Scholar]
- Zhang, K.; Ma, J.; Lyu, B.; Shi, G.; Zhou, B.; Tian, Y. Influence of “tentacle structure” on the properties of jellyfish-like 3D dispersants based on tannic acid for preparing high-concentrated coal–water slurry. Fuel 2020, 274, 117860. [Google Scholar] [CrossRef]
- Li, P.-W.; Yang, D.-J.; Lou, H.-M.; Qiu, X.-Q. Study on the stability of coal water slurry using dispersion-stability analyzer. J. Fuel Chem. Technol. 2008, 36, 524–529. [Google Scholar] [CrossRef]
- Zhang, W.B.; Luo, J.; Huang, Y.; Zhang, C.; Du, L.; Guo, J.; Wu, J.; Zhang, X.; Zhu, J.F.; Zhang, G.H. Synthesis of a novel dispersant with topological structure by using humic acid as raw material and its application in coal water slurry preparation. Fuel 2020, 262, 116576. [Google Scholar] [CrossRef]
- Ra, H.W.; Yoon, S.M.; Mun, T.Y.; Seo, M.W.; Moon, J.H.; Yoon, S.J.; Kim, J.H.; Lee, J.G.; Jung, M.Z.; Lee, J.D. Influence of surfactants and experimental variables on the viscosity characteristics of coal water mixtures. Korean J. Chem. Eng. 2018, 35, 1219–1224. [Google Scholar] [CrossRef]
- Wang, C.; Hou, Y.; Cao, H.; Wang, T.; Zhou, L.; Zhang, K. Preparation and properties of amphoteric polycarboxylate coal tar pitch water slurry dispersant. Appl. Chem. Ind. 2022, 51, 2215. [Google Scholar]
- Yang, D.; Guo, W.; Li, X.; Wang, Y.; Qiu, X. Effects of molecular weight of grafted sulfonated lignin on its dispersion and adsorption properties as a dispersant for coal water slurries. J. Fuel Chem. Technol. 2013, 41, 20–25. [Google Scholar]
- Yang, D.; Li, X.; Li, H.; Jiang, H.; Li, Q. Application of High-Efficiency Bamboo Lignin-Based Dispersant to Coal Phenol-Water Slurry. J. South China Univ. Technology. Nat. Sci. Ed. 2014, 42, 1–7. [Google Scholar]
- Li, P.; Yang, D.; Qiu, X.; Feng, W. Study on Enhancing the Slurry Performance of Coal–Water Slurry Prepared with Low-Rank Coal. J. Dispers. Sci. Technol. 2014, 36, 1247–1256. [Google Scholar] [CrossRef]
- He, W.; Fatehi, P. Preparation of sulfomethylated softwood kraft lignin as a dispersant for cement admixture. RSC Adv. 2015, 5, 47031–47039. [Google Scholar] [CrossRef]
- Wenxin, J.I.; Liqiong, W. Preparation and Study of High Concentration Coal-water-slurry of Ningdong Coal Suited to Texco Gasification Process. J. Henan Norm. Univ. Nat. Sci. 2011, 39, 92–94. [Google Scholar]
- Gong, Z.; Shuai, L. Lignin condensation, an unsolved mystery. Trends Chem. 2023, 5, 163–166. [Google Scholar] [CrossRef]
- Lu, H.Y.; Li, X.F.; Zhang, C.Q.; Li, W.H.; Xu, D.P. beta-Cyclodextrin grafted on alkali lignin as a dispersant for coal water slurry. Energy Sources Part A–Recovery Util. Environ. Eff. 2019, 41, 1716–1724. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, L.; Li, J.; Shang, T.; Xu, H.; Qiang, Y. Synthesis And properties research of sulfonated humic acid grafted copolymer. Coal Convers. 2013, 36, 92–96. [Google Scholar]
- Doskočil, L.; Grasset, L.; Válková, D.; Pekař, M. Hydrogen peroxide oxidation of humic acids and lignite. Fuel 2014, 134, 406–413. [Google Scholar] [CrossRef]
- Wang, C.-F.; Fan, X.; Zhang, F.; Wang, S.-Z.; Zhao, Y.-P.; Zhao, X.-Y.; Zhao, W.; Zhu, T.-G.; Lu, J.-L.; Wei, X.-Y. Characterization of humic acids extracted from a lignite and interpretation for the mass spectra. RSC Adv. 2017, 7, 20677–20684. [Google Scholar] [CrossRef]
- Ge, L.; Li, J. Study on the Influence of Pulping Process on Rheological Properties of Coal Water Slurry. IOP Conf. Ser. Earth Environ. Sci. 2019, 384, 012107. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, Y.; Li, J.; Niu, Y.; Qu, Q. Synthesis and properties of sulfomethlated humic acid dispersant for coal-water-slurry. Mod. Chem. Ind. 2014, 34, 72. [Google Scholar]
- Wu, J.; Zhang, G.; Zhang, W.; Du, L.; Li, J.; Duan, Q.; Liu, J. New humic acid dispersants and their dispersion to coal water slurries. Appl. Chem. Ind. 2018, 47, 2638–2642. [Google Scholar]
- Zhang, K.; Deng, S.; Li, P.; Jin, L.E.; Cao, Q. Synthesis of a novel humic acid-based polycarboxylic dispersant for coal water slurry. Int. J. Green Energy 2016, 14, 205–211. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, G.; Zhu, J. Performance of sulfonated naphthol formaldehyde/naphthalene composites dispersant for coal water slurry. Coal Convers. 2013, 36, 40–43. [Google Scholar]
- Shang, Y.; Wang, X.G.; Lei, X.; Shang, L. Performance of Sodium Ligninsulfonate/naphthalene Composites Dispersant. Coal Convers. 2018, 41, 36. [Google Scholar]
- Qiu, X.Q.; Zhou, M.S.; Yang, D.J.; Lou, H.M.; Ouyang, X.P.; Pang, Y.X. Evaluation of sulphonated acetone-formaldehyde (SAF) used in coal water slurries prepared from different coals. Fuel 2007, 86, 1439–1445. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Li, X.-F.; Zhang, C.-Q.; Chen, J.-Y.; Ma, L.-G.; Li, W.-H.; Xu, D.-P. Experiments and molecular dynamics simulations on the adsorption of naphthalenesulfonic formaldehyde condensates at the coal-water interface. Fuel 2020, 264, 116838. [Google Scholar] [CrossRef]
- Hu, S.; Jiang, F.; Li, J.; Wu, C.; Liu, K.; Chen, Y. Understanding the adsorption behaviors of naphthalene sulfonate formaldehyde in coal water slurry. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127245. [Google Scholar] [CrossRef]
- Zhu, J.F.; Zhang, G.H.; Miao, Z.; Shang, T. Synthesis and performance of a comblike amphoteric polycarboxylate dispersant for coal-water slurry. Colloids Surf. A-Physicochem. Eng. Asp. 2012, 412, 101–107. [Google Scholar] [CrossRef]
- Hong, N.L.; Zhang, S.W.; Yi, C.H.; Qiu, X.Q. Effect of Polycarboxylic Acid Used as High-Performance Dispersant on Low-Rank Coal-Water Slurry. J. Dispers. Sci. Technol. 2016, 37, 415–422. [Google Scholar] [CrossRef]
- Xun, W.; Wu, C.; Li, J.; Yang, C.; Leng, X.; Xin, D.; Li, Y. Effect of Functional Polycarboxylic Acid Superplasticizers on Mechanical and Rheological Properties of Cement Paste and Mortar. Appl. Sci. 2020, 10, 5418. [Google Scholar] [CrossRef]
- Wu, H.; Lv, D.; Nie, Y.; Jiang, T.; Li, J.; Gong, S.; Yan, H. Experimental and computational studies of polycarboxylate dispersant effect on the properties of low-rank coal water slurries. Asia-Pac. J. Chem. Eng. 2022, 17, e2837. [Google Scholar] [CrossRef]
- Acet, N.; Eroglu, D. Electrodeposition of Ni/TiC Nanocomposites in the Presence of a Cationic Dispersant. J. Electrochem. Soc. 2018, 165, D31–D36. [Google Scholar] [CrossRef]
- Routray, A.; Senapati, P.K.; Padhy, M.; Das, D.; Mohapatra, R.K. Effect of mixture of a non-ionic and a cationic surfactant for preparation of stabilized high concentration coal water slurry. Int. J. Coal Prep. Util. 2022, 42, 925–940. [Google Scholar] [CrossRef]
- Slaczka, A.; Piszczynski, Z. Effect of selected additives on the stability and rheology of Coal-Water Slurry Fuels (CWSF). Gospod. Surowcami Miner. -Miner. Resour. Manag. 2008, 24, 363–373. [Google Scholar]
- Li, L.; Ma, C.; Hu, S.; He, M.; Yu, H.; Wang, Q.; Cao, X.; You, X. Effect of the benzene ring of the dispersant on the rheological characteristics of coal-water slurry: Experiments and theoretical calculations. Int. J. Min. Sci. Technol. 2021, 31, 515–521. [Google Scholar] [CrossRef]
- Zhao, L.A.; Wang, T.; Guo, K. Study on Rheological Parameters and Hydraulic Gradient of High Concentration Coal Water Slurry. Coal Convers. 2020, 43, 88–96. [Google Scholar]
- Rao, H.; Li, W.; Zhao, F.; Song, Y.; Liu, H.; Zhu, L.; Chen, H. Electrodeposition of High-Quality Ni/SiC Composite Coatings by Using Binary Non-Ionic Surfactants. Molecules 2023, 28, 3344. [Google Scholar] [CrossRef]
- Zhou, C.; Cao, Q.; Chang, H. Influence of dispersant structure on the performance of coal—Water slurry. China Surfactant Deterg. Cosmet. 2015, 45, 404–408. [Google Scholar]
- Miao, Z.; Li, T.; Meng, X.; Chu, R.; Zhang, Y.; Wu, G. Effect of Gemini dispersing additive on performances of coal water slurry prepared from low-rank coal. J. China Univ. Min. Technol. 2013, 42, 1054–1059. [Google Scholar]
- Zhu, J.; Wang, P.; Zhang, G.; Li, J. Synthesis andproperties of comb-like polycarboxylates AMPS/MAA/MAAMPEG. J. Funct. Mater. 2017, 48, 5205–5210. [Google Scholar]
- Guo, J.; Zhang, G.; Zhang, W.; Zhu, J.; Wu, J.; Du, L. Effect on surface hydrophobic modification and slurribility of lignite coal by alkyl ketone dimer. Chem. Ind. Eng. Prog. 2019, 38, 4705–4711. [Google Scholar]
- Zhang, G.; Yang, D.; Zhang, W.; Du, L.; Ren, J.; Ni, M.; Liu, J.; Luo, J. Preparation and properties of polyamide double comb type amphoteric coal water slurry dispersant. Appl. Chem. Ind. 2021, 50, 2088–2094. [Google Scholar]
- Qin, Y.; Yang, D.; Guo, W.; Qiu, X. Investigation of grafted sulfonated alkali lignin polymer as dispersant in coal–water slurry. J. Ind. Eng. Chem. 2015, 27, 192–200. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, S.; Cui, L.; Xu, E.; Chen, H.; Meng, X.; Wu, G. Eco-friendly utilization of microplastics for preparing coal water slurry: Rheological behavior and dispersion mechanism. J. Clean. Prod. 2022, 330, 129881. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.; Pisupati, S.V.; Li, D.; Wang, Z.; Cheng, J. Dispersion mechanism of coal water slurry prepared by mixing various high-concentration organic waste liquids. Fuel 2021, 287, 119340. [Google Scholar] [CrossRef]
- Li, Y.; Xia, W.; Wen, B.; Xie, G. Filtration and dewatering of the mixture of quartz and kaolinite in different proportions. J. Colloid Interface Sci. 2019, 555, 731–739. [Google Scholar] [CrossRef]
- Hu, P.; Liang, L. The role of hydrophobic interaction in the heterocoagulation between coal and quartz particles. Miner. Eng. 2020, 154, 106421. [Google Scholar] [CrossRef]
- Song, H.; Cao, Z.; Xie, W.; Cheng, F.; Gasem, K.A.M.; Fan, M. Improvement of dispersion stability of filler based on fly ash by adding sodium hexametaphosphate in gas-sealing coating. J. Clean. Prod. 2019, 235, 259–271. [Google Scholar] [CrossRef]
- Huang, X.-T.; Xiao, W.; Zhao, H.-B.; Cao, P.; Hu, Q.-X.; Qin, W.-Q.; Zhang, Y.-S.; Qiu, G.-Z.; Wang, J. Hydrophobic flocculation flotation of rutile fines in presence of styryl phosphonic acid. Trans. Nonferrous Met. Soc. China 2018, 28, 1424–1432. [Google Scholar] [CrossRef]
- Yoon, R.-H.; Flinn, D.H.; Rabinovich, Y.I. Hydrophobic Interactions between Dissimilar Surfaces. J. Colloid Interface Sci. 1997, 185, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Z.; Tu, Y.; Wang, J.; Li, J. Study on properties of coal-sludge-slurry prepared by sludge from coal chemical industry. Powder Technol. 2020, 366, 552–559. [Google Scholar] [CrossRef]
- Hu, S.X.; Chen, Y.M.; Wu, C.N.; Li, J.G.; Liu, K. The performance and dispersing mechanism of anionic dispersants in slurries prepared by upgraded coal. Colloids Surf. A-Physicochem. Eng. Asp. 2020, 606, 125450. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, D.; Zhou, M. Effect of sodium tripolyphosphate on cws dispersants. Coal Convers. 2011, 34, 29–34. [Google Scholar]
- Shuai, W.; Wang, S.; Sun, T.; Yin, H.; Zu, Y.; Yao, G.; Li, Z.; Qi, Z.; Zhong, M. Improving the steric hindrance effect of linear sulfonated acetone-formaldehyde dispersant and its performance in coal-water slurry. RSC Adv. 2022, 12, 35508–35516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qiu, X.; Wang, W. Development of coal water slurry additives. Mod. Chem. Ind. 2004, 24, 16–19. [Google Scholar]
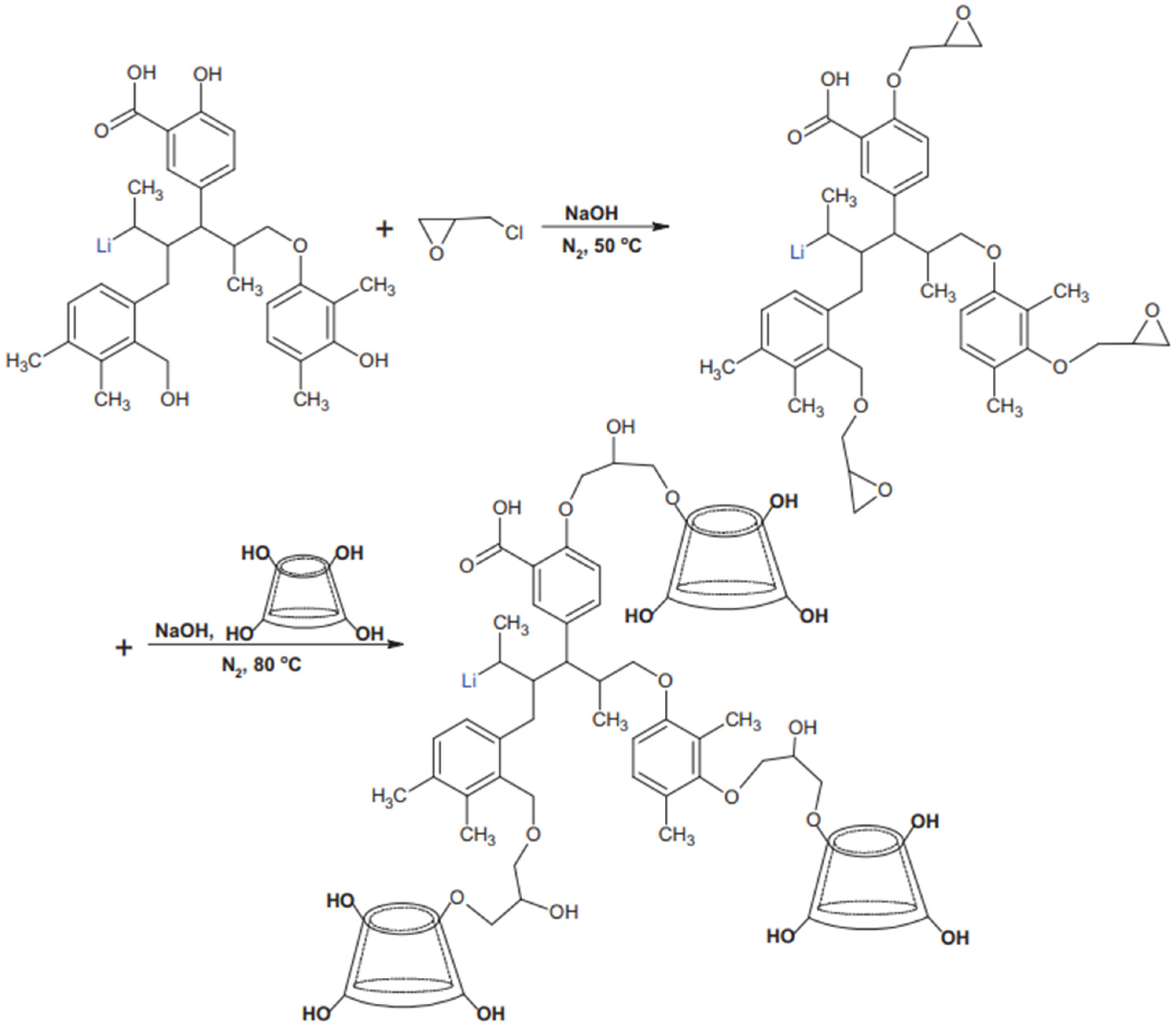
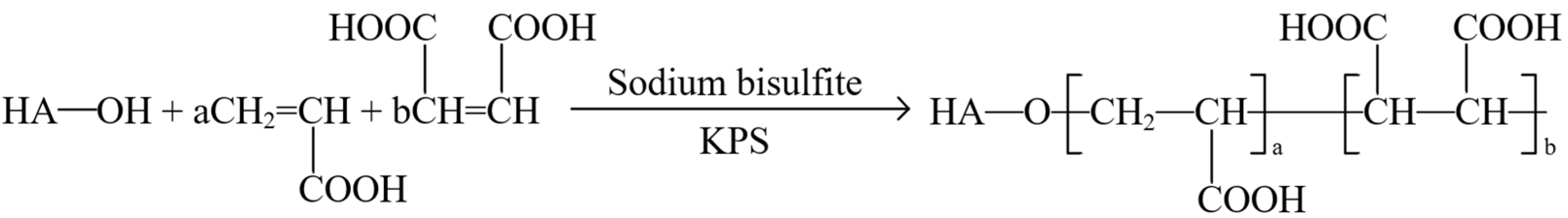


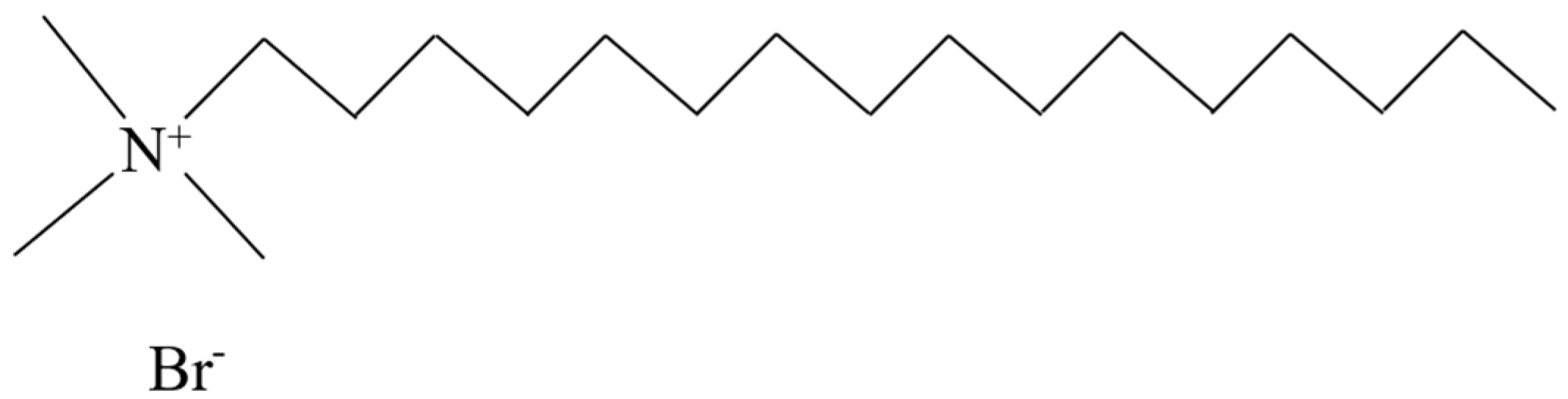
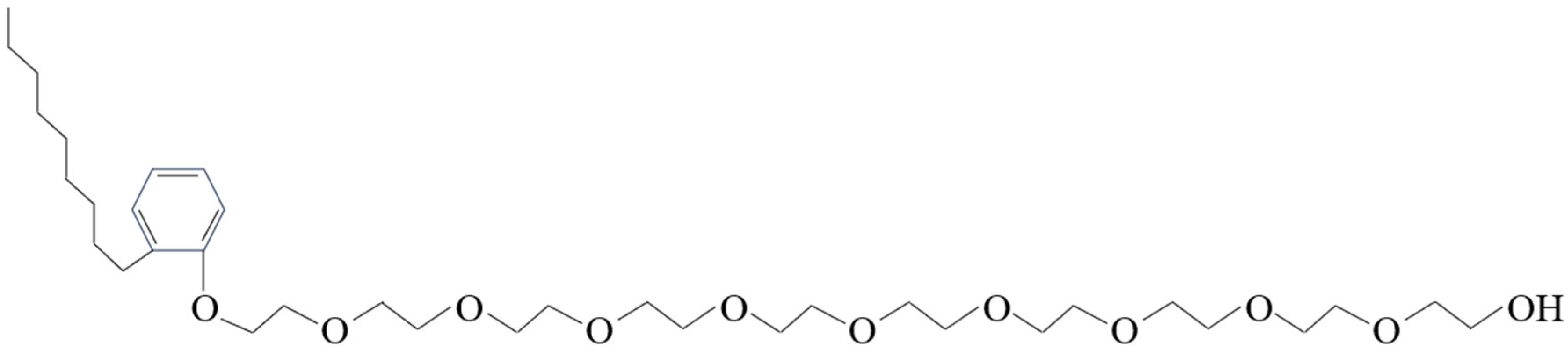

| Category | Type | Characteristic | Reference |
|---|---|---|---|
| Anionic dispersant | Lignin dispersant | Low cost and good stability | [47] |
| Humic acid-based dispersants | Good viscosity reduction effect and poor stability | [42,48] | |
| Naphthalene-based dispersants | Good liquidity and poor stability | [47,49] | |
| Polycarboxylic acid dispersants | Good performance, low pollution | [27,50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, S.; Liu, N.; Wei, B.; An, T. Progress of Dispersants for Coal Water Slurry. Molecules 2023, 28, 7683. https://doi.org/10.3390/molecules28237683
Liu X, Wang S, Liu N, Wei B, An T. Progress of Dispersants for Coal Water Slurry. Molecules. 2023; 28(23):7683. https://doi.org/10.3390/molecules28237683
Chicago/Turabian StyleLiu, Xiaotian, Shan Wang, Ning Liu, Bo Wei, and Tian An. 2023. "Progress of Dispersants for Coal Water Slurry" Molecules 28, no. 23: 7683. https://doi.org/10.3390/molecules28237683
APA StyleLiu, X., Wang, S., Liu, N., Wei, B., & An, T. (2023). Progress of Dispersants for Coal Water Slurry. Molecules, 28(23), 7683. https://doi.org/10.3390/molecules28237683






