Scandium Radioisotopes—Toward New Targets and Imaging Modalities
Abstract
:1. Introduction
2. Scandium Radioisotopes
3. Production of Scandium Radioisotopes
4. Radiochemical Separation
4.1. Separation and Preconcentration of Cyclotron-Produced Scandium
4.2. Separation and Preconcentration of Generator-Produced Scandium
5. Scandium Complexing Ligands
6. Imaging Performance of Scandium-43 and -44
7. Discussion and Further Trends
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dugger, S.A.; Platt, A.; Goldstein, D.B. Drug Development in the Era of Precision Medicine. Nat. Rev. Drug Discov. 2018, 17, 183–196. [Google Scholar] [CrossRef]
- Refardt, J.; Hofland, J.; Kwadwo, A.; Nicolas, G.P.; Rottenburger, C.; Fani, M.; Wild, D.; Christ, E. Theranostics in neuroendocrine tumors: An overview of current approaches and future challenges. Rev. Endocr. Metab. Disord. 2021, 22, 581–594. [Google Scholar] [CrossRef]
- Roesch, F.; Martin, M. Radiometal-theranostics: The first 20 years. J. Radioanal. Nucl. Chem. 2023, 332, 1557–1576. [Google Scholar] [CrossRef]
- Duan, H.; Iagaru, A.; Aparici, C.M. Radiotheranostics—Precision Medicine in Nuclear Medicine and Molecular Imaging. Nanotheranostics 2022, 6, 103–117. [Google Scholar] [CrossRef]
- Mikolajczak, R.; Huclier-Markai, S.; Alliot, C.; Haddad, F.; Szikra, D.; Forgacs, V.; Garnuszek, P. Production of scandium radionuclides for theranostic applications: Towards standardization of quality requirements. EJNMMI Radiopharm. Chem. 2021, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Sitarz, M.; Cussonneau, J.-P.; Matulewicz, T.; Haddad, F. Radionuclide candidates for β+γ coincidence PET: An overview. Appl. Radiat. Isot. 2020, 155, 108898. [Google Scholar] [CrossRef] [PubMed]
- Synowiecki, M.A.; Perk, L.R.; Nijsen, J.F.W. Production of novel diagnostic radionuclides in small medical cyclotrons. EJNMMI Radiopharm. Chem. 2018, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Kilian, K.; Pyrzyńska, K.; Pęgier, M. Comparative Study of Sc(III) Sorption onto Carbon-based Materials. Solvent Extr. Ion Exch. 2017, 35, 450–459. [Google Scholar] [CrossRef]
- Wood, S.A.; Samson, I.M. The aqueous geochemistry of gallium, germanium, indium and scandium. Ore Geol. Rev. 2006, 28, 57–102. [Google Scholar] [CrossRef]
- Feitknecht, W.; Schindler, P. Solubility constants of metal oxides, metal hydroxides and metal hydroxide salts in aqueous solution. Pure Appl. Chem. 1963, 6, 125–206. [Google Scholar] [CrossRef]
- Kondev, F.G.; Wang, M.; Huang, W.J.; Naimi, S.; Audi, G. The NUBASE2020 evaluation of nuclear physics properties. Chin. Phys. C 2021, 45, 030001. [Google Scholar] [CrossRef]
- Szkliniarz, K.; Sitarz, M.; Walczak, R.; Jastrzębski, J.; Bilewicz, A.; Choiński, J.; Jakubowski, A.; Majkowska, A.; Stolarz, A.; Trzcińska, A.; et al. Production of medical Sc radioisotopes with an α particle beam. Appl. Radiat. Isot. 2016, 118, 182–189. [Google Scholar] [CrossRef]
- Minegishi, K.; Nagatsu, K.; Fukada, M.; Suzuki, H.; Ohya, T.; Zhang, M.-R. Production of scandium-43 and -47 from a powdery calcium oxide target via the nat/44Ca(α,x)-channel. Appl. Radiat. Isot. 2016, 116, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Banerjee, D.; Chakraborty, S. Alpha-induced production and robust radiochemical separation of 43Sc as an emerging radiometal for formulation of PET radiopharmaceuticals. Appl. Radiat. Isot. 2023, 199, 110921. [Google Scholar] [CrossRef]
- Lowis, C.; Ferguson, S.; Paulßen, E.; Hoehr, C. Improved Sc-44 production in a siphon-style liquid target on a medical cyclotron. Appl. Radiat. Isot. 2021, 172, 109675. [Google Scholar] [CrossRef] [PubMed]
- Kurakina, E.S.; Wharton, L.; Hoehr, C.; Orving, C.; Magomedbekov, E.P.; Filosofov, D.; Radchenko, V. Improved separation scheme for 44Sc produced by irradiation of natCa targets with 12.8 MeV protons. Nucl. Med. Biol. 2022, 104–105, 22–27. [Google Scholar] [CrossRef]
- Filosofov, D.V.; Loktionova, N.S.; Rösch, F. A 44Ti/44Sc radionuclide generator for potential application of 44Sc-based PET-radiopharmaceuticals. Radiochim. Acta 2010, 98, 149–156. [Google Scholar] [CrossRef]
- Pupillo, G.; Mou, L.; Boschi, A.; Calzaferri, S.; Canton, L.; Cisternino, S.; De Dominicis, L.; Duatti, A.; Fontana, A.; Haddad, F.; et al. Production of 47Sc with natural vanadium targets: Results of the PASTA project. J. Radioanal. Nucl. Chem. 2019, 322, 1711–1718. [Google Scholar] [CrossRef]
- Snow, M.S.; Foley, A.; Ward, J.L.; Kinlaw, M.T.; Stoner, J.; Carney, K.P. High purity 47Sc production using high-energy photons and natural vanadium targets. Appl. Radiat. Isot. 2021, 178, 109934. [Google Scholar] [CrossRef] [PubMed]
- Domnanich, A.; Eicher, R.; Mueller, C.; Jordi, S.; Yakusheva, V.; Braccini, S.; Behe, M.; Schibli, R.; van der Meulen, N.P. Production and separation of 43Sc for radiopharmaceutical purposes. EJNMMI Radiopharm. Chem. 2017, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.V.; Aluicio-Sarduy, E.; Bradshaw, T.; Hurley, S.A.; Olson, A.P.; Barrett, K.E.; Batterton, J.; Ellison, P.A.; Barnhart, T.E.; Pirasteh, A.; et al. Cyclotron production of 43Sc and 44gSc from enriched 42CaO, 43CaO, and 44CaO targets. Front. Chem. 2023, 11, 1167783. [Google Scholar] [CrossRef]
- Domnanich, K.A.; Müller, C.; Benešová, M.; Dressler, R.; Haller, S.; Köster, U.; Ponsard, B.; Schibli, R.; Türler, A.; van der Meulen, N.P. 47Sc as useful β–-emitter for the radiotheragnostic paradigm: A comparative study of feasible production routes. EJNMMI Radiopharm. Chem. 2017, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.E.; Gajecki, L.; Deri, M.A.; Sanders, V.A. Current State of 44Ti/44Sc Radionuclide Generator Systems and Separation Chemistry. Curr. Radiopharm. 2023, 16, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Pruszyński, M.; Loktionova, N.; Filosofov, D.; Rösch, F. Post-elution processing of 44Ti/44Sc generator-derived 44Sc for clinical application. Appl. Radiat. Isot. 2010, 68, 1636–1641. [Google Scholar] [CrossRef]
- Majkowska-Pilip, A.; Bilewicz, A. Macrocyclic complexes of scandium radionuclides as precursors for diagnostic and therapeutic radiopharmaceuticals. J. Inorg. Biochem. 2011, 105, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Radchenko, V.; Meyer, C.; Engle, J.; Naranjo, C.; Unc, G.; Mastren, T.; Brugh, M.; Birnbaum, E.; John, K.; Nortier, F.; et al. Separation of 44Ti from proton irradiated scandium by using solid-phase extraction chromatography and design of 44Ti/44Sc generator system. J. Chromatogr. A 2016, 1477, 39–46. [Google Scholar] [CrossRef]
- Radchenko, V.; Engle, J.W.; Medvedev, D.G.; Maassen, J.M.; Naranjo, C.M.; Unc, G.A.; Meyer, C.A.; Mastren, T.; Brugh, M.; Mausner, L.; et al. Proton-induced production and radiochemical isolation of 44Ti from scandium metal targets for 44Ti/44Sc generator development. Nucl. Med. Biol. 2017, 50, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Larenkov, A.A.; Makichyan, A.G.; Iatsenko, V.N. Separation of 44Sc from 44Ti in the context of a generator system for radiopharmaceutical purposes with the example of [44Sc]Sc-PSMA-617 and [44Sc]Sc-PSMA-I&T synthesis. Molecules 2021, 26, 6371. [Google Scholar] [CrossRef] [PubMed]
- Benabdallah, N.; Zhang, H.; Unnerstall, R.; Fears, A.; Summer, L.; Fassbender, M.; Rodgers, B.E.; Abou, D.; Radchenko, V.; Thorek, D.L.J. Engineering a modular 44Ti/44Sc generator: Eluate evaluation in preclinical models and estimation of human radiation dosimetry. EJNMMI Res. 2023, 13, 6371. [Google Scholar] [CrossRef]
- Gajecki, L.; Marino, C.M.; Cutler, C.S.; Sanders, V.A. Evaluation of hydroxamate-based resins towards a more clinically viable 44Ti/44Sc radionuclide generator. Appl. Radiat. Isot. 2023, 192, 110588. [Google Scholar] [CrossRef] [PubMed]
- Kankanamalage, P.H.; Brossard, T.; Song, J.; Nolen, J.; Rotsch, D.A. Photonuclear production of 47Ca for 47Ca/47Sc generator from natural CaCO3 targets. Appl. Radiat. Isot. 2023, 200, 110943. [Google Scholar] [CrossRef] [PubMed]
- Rane, S.; Harris, J.T.; Starovoitova, V.N. 47Ca production for 47Ca/47Sc generator system using electron linacs. Appl. Radiat. Isot. 2015, 97, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.P.; McAlister, D.R.; Dietz, M.L. Extraction chromatography versus sol vent extraction: How similar are they? Separ. Sci. Technol. 2006, 41, 2163–2182. [Google Scholar] [CrossRef]
- Bertelsen, E.R.; Jackson, J.A.; Shafer, J.C. A survey of extraction chromatographic f-element separations developed by EP Horwitz. Solvent Extr. Ion Exch. 2020, 38, 251–289. [Google Scholar] [CrossRef]
- Pyrzyńska, K.; Kilian, K.; Pęgier, M. Separation and purification of scandium: From industry to medicine. Sep. Purif. Rev. 2019, 48, 65–77. [Google Scholar] [CrossRef]
- Zou, D.; Deng, Y.; Chen, J.; Li, D. A review on solvent extraction of scandium. J. Rare Earths 2022, 40, 1499–1508. [Google Scholar] [CrossRef]
- van der Meulen, N.P.; Hasler, R.; Talip, Z.; Grundler, P.V.; Favaretto, C.; Umbricht, C.A.; Müller, C.; Dellepiane, G.; Carzaniga, T.S.; Braccini, S. Developments toward the Implementation of 44Sc Production at a Medical Cyclotron. Molecules 2020, 25, 4706. [Google Scholar] [CrossRef]
- Alliot, C.; Kerdjoudj, R.; Michel, N.; Haddad, F.; Huclier-Markai, S. Cyclotron production of high purity 44m, 44Sc with deu-terons from 44CaCO3 targets. Nucl. Med. Biol. 2015, 42, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Hoehr, C.; Oehlke, E.; Benard, F.; Lee, C.J.; Hou, X.; Badesso, B.; Ferguson, S.; Miao, Q.; Yang, H.; Buckley, K.; et al. 44gSc production using a water target on a 13MeV cyclotron. Nucl. Med. Biol. 2014, 41, 401–406. [Google Scholar] [CrossRef]
- Kerdjoudj, R.; Priok, M.; Alliot, C.; Kubicek, V.; Havlickova, J.; Roesch, F.; Hermann, P.; Hucler-Markai, S. Scandium(III) com-plexes with a monoposphorous acid analogues: A thermodynamic and radiolabelling study with 44Sc from cyclotron and from 44Ti/44Sc generator. Dalt. Trans. 2016, 45, 1398–1409. [Google Scholar] [CrossRef]
- Walczak, R.; Krajewski, S.; Szkliniarz, K.; Sitarz, M.; Abbas, K.; Choiński, J.; Jakubowski, A.; Jastrzębski, J.; Majkowska, A.; Simonelli, F.; et al. Cyclotron production of 43Sc for PET imaging. EJNMMI Phys. 2015, 2, 33. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.P.; McAlister, D.R.; Bond, A.H.; Barrans, R.E., Jr. Novel extraction of chromatographic resins based on tetraalkyldi-glycolamides: Characterization and potential applications. Solvent Extr. Ion Exch. 2015, 23, 319–344. [Google Scholar] [CrossRef]
- Flores, R.; Momen, M.A.; Healy, M.R.; Jansone-Popova, S.; Lyon, K.L.; Reinhart, O.; Cheshire, M.C. The coordination chemistry and stoichiometry of extracted diglycolamide complexes of lanthanides in extraction chromatography materials. Solvent Extr. Ion Exch. 2022, 40, 6–27. [Google Scholar] [CrossRef]
- Pourmand, A.; Dauphas, N. Distribution coefficients of 60 elements on TODGA resin: Application to Ca, Lu, Hf, U and Th isotope geochemistry. Talanta 2010, 81, 741–753. [Google Scholar] [CrossRef] [PubMed]
- van der Meulen, N.P.; Bunka, M.; Domnanich, K.A.; Müller, C.; Haller, S.; Vermeulen, C.; Türler, A.; Schibli, R. Cyclotron production of 44Sc: From bench to bedside. Nucl. Med. Biol. 2015, 42, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cheng, C.Y. Separation and purification of scandium by solvent extraction and related technologies: A review. J. Chem. Technol. Biotechnol. 2011, 86, 1237–1246. [Google Scholar] [CrossRef]
- Horwitz, E.P.; Dietz, M.L.; Chiarizia, R.; Diamond, H.; Essling, A.M.; Graczyk, D. Separation and preconcentration of uranium from acidic media by extraction chromatography. Anal. Chim. Acta 1992, 266, 25–37. [Google Scholar] [CrossRef]
- Valdovinos, H.F.; Hernandez, R.; Barnhart, T.E.; Graves, S.; Cai, W.; Nickles, R. Separation of cyclotron-produced 44Sc from a natural calcium target using a dipentyl pentylphosphonate functionalized extraction resin. Appl. Radiat. Isot. 2015, 95, 23–29. [Google Scholar] [CrossRef]
- Misiak, R.; Walczak, R.; Wąs, B.; Bartyzel, M.; Mietelski, J.W.; Bilewicz, A. 47Sc production development by cyclotron irradia-tion of 48Ca. J. Radioanal. Nucl. Chem. 2017, 313, 429–434. [Google Scholar] [CrossRef]
- Krajewski, S.; Cydzik, I.; Abbas, K.; Bulgheromi, F.; Holzwarth, U.; Bilewicz, A. Cyclotron production of 44Sc for clinical application. Radiochim. Acta 2013, 101, 333–338. [Google Scholar] [CrossRef]
- Kilian, K.; Cheda, L.; Sitarz, M.; Szkliniarz, K.; Choiński, J.; Stolarz, A. Separation of 44Sc from natural calcium carbonate targets for synthesis of 44Sc-DOTATATE. Molecules 2018, 23, 1787. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tsujisaka, M.; Zheng, L.; Takano, S.; Sohrin, Y. Application of NOBIAS Chelate-PA 1 resin to the determination of zirconium, niobium, hafnium, and tantalum in seawater. Anal. Sci. 2019, 35, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Hatje, V.; Bruland, K.W.; Flegal, A.R. Determination of rare earth elements after pre-concentration using NOBIAS-chelate PA-1®resin: Method development and application in the San Francisco Bay plume. Mar. Chem. 2014, 160, 34–41. [Google Scholar] [CrossRef]
- Gizawy, M.A.; Aydia, M.I.; Shamsel-Din, H.A.; El-Azony, K.M. Selective separation of no carrier added Sc-47 from reactor irradiated Ca using zirconium vanadate gel for nuclear medical applications. Arab J. Nucl. Sci. Appl. 2022, 55, 1–14. [Google Scholar]
- Wojdowska, W.; Pawlak, D.; Cieszykowska, I.; Żółtowska, M.; Janiak, T.; Barcikowski, T.; Stolarz, A.; Choiński, J.; Parus, J.; Garnuszek, P.; et al. Improved procedures of Sc(OH)(3) precipitation and UTEVA extraction for Sc-44 separation. Nucl. Med. Rev. 2019, 22, 56–59. [Google Scholar]
- Traore, M.; Gong, A.; Wang, Y.; Qiu, L.; Bai, Y.; Zhao, W.; Liu, Y.; Chen, Y.; Liu, Y.; Wu, H.; et al. Research progress of rare earth separation methods and technologies. J. Rare Earths 2023, 41, 182–189. [Google Scholar] [CrossRef]
- Hovey, J.L.; Dittrich, T.M.; Allen, M.J. Coordination chemistry of surface-associated ligands for solid-liquid adsorption of ra-re-earth elements. J. Rare Earths 2023, 41, 1–18. [Google Scholar] [CrossRef]
- Loveless, C.S.; Blanco, J.R.; Diehl, G.L.; Elbahrawi, R.T.; Carzaniga, T.S.; Braccini, S.; Lapi, S.E. Cyclotron production and separation of scandium radionuclides from natural titanium metal and titanium dioxide targets. J. Nucl. Med. 2021, 62, 131–136. [Google Scholar] [CrossRef]
- Rotsch, D.A.; Brown, M.A.; Nolen, J.A.; Brossard, T.; Henning, W.F.; Chemerisov, S.D.; Gromov, R.G.; Greene, J. Electron linear accelerator production and purification of scandium-47 from titanium dioxide targets. Appl. Radiat. Isot. 2018, 131, 77–82. [Google Scholar] [CrossRef]
- Deilami-Nezhad, L.; Moghaddam-Banaem, L.; Sadeghi, M.; Asgari, M. Production and purification of Scandium-47: A potential radioisotope for cancer theranostics. Appl. Radiat. Isot. 2010, 118, 124–130. [Google Scholar] [CrossRef]
- Nagy, G.; Szikra, D.; Trencsényi, G.; Fekete, A.; Garai, I.; Giani, A.M.; Negri, R.; Masciocchi, N.; Maiocchi, A.; Uggeri, F.; et al. AAZTA: An Ideal Chelating Agent for the Development of 44Sc PET Imaging Agents. Angew. Chem. Int. Ed. 2017, 56, 2118–2122. [Google Scholar] [CrossRef] [PubMed]
- Sinnes, J.-P.; Nagel, J.; Rösch, F. AAZTA5/AAZTA5-TOC: Synthesis and radiochemical evaluation with 68Ga, 44Sc and 177Lu. EJNMMI Radiopharm. Chem. 2019, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Sinnes, J.-P.; Bauder-Wüst, U.; Schäfer, M.; Moon, E.S.; Kopka, K.; Rösch, F. 68Ga, 44Sc and 177Lu-labeled AAZTA5-PSMA-617: Synthesis, radiolabeling, stability and cell binding compared to DOTA-PSMA-617 analogues. EJNMMI Radiopharm. Chem. 2020, 5, 28. [Google Scholar] [CrossRef]
- Greifenstein, L.; Grus, T.; Nagel, J.; Sinnes, J.P.; Rösch, F. Synthesis and labeling of a squaric acid containing PSMA-inhibitor coupled to AAZTA5 for versatile labeling with 44Sc, 64Cu, 68Ga and 177Lu. Appl. Radiat. Isot. 2020, 156, 108867. [Google Scholar] [CrossRef]
- Ghiani, S.; Hawala, I.; Szikra, D.; Trencsényi, G.; Baranyai, Z.; Nagy, G.; Vágner, A.; Stefania, R.; Pandey, S.; Maiocchi, A. Synthesis, radiolabeling, and pre-clinical evaluation of [44Sc]Sc-AAZTA conjugate PSMA inhibitor, a new tracer for high-efficiency imaging of prostate cancer. EJNMMI 2021, 48, 2351–2362. [Google Scholar] [CrossRef]
- Fersing, C.; Masurier, N.; Rubira, L.; Deshayes, E.; Lisowski, V. AAZTA-Derived Chelators for the Design of Innovative Radiopharmaceuticals with Theranostic Applications. Pharmaceuticals 2022, 15, 234. [Google Scholar] [CrossRef]
- Lepareur, N.A. Cold Kit Labeling: The Future of 68Ga Radiopharmaceuticals? Front. Med. 2022, 9, 812050. [Google Scholar] [CrossRef]
- Satpati, D. Recent Breakthrough in 68Ga-Radiopharmaceuticals Cold Kits for Convenient PET Radiopharmacy. Bioconjugate Chem. 2021, 32, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Baudhuin, H.; Van Bockstal, P.-J.; De Beer, T.; Vaneycken, I.; Bridoux, J.; Raes, G.; Caveliers, V.; Keyaerts, M.; Devoogdt, N.; Lahoutte, T.; et al. Lyophilization of NOTA-sdAbs: First step towards a cold diagnostic kit for 68Ga-labeling. Eur. J. Pharm. Biopharm. 2021, 166, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Dénes, N.; Kis, A.; Szabó, J.P.; Berényi, E.; Garai, I.; Bai, P.; Hajdu, I.; Szikra, D.; Trencsényi, G. Preclinical evaluation of melanocortin-1 receptor (MC1-R) specific 68Ga- and 44Sc-labeled DOTA-NAPamide in melanoma imaging. Eur. J. Pharm. Sci. 2017, 106, 336–344. [Google Scholar] [CrossRef]
- Umbricht, C.A.; Benešová, M.; Schmid, R.M.; Türler, A.; Schibli, R.; van der Meulen, N.P.; Müller, C. 44Sc-PSMA-617 for radiotheragnostics in tandem with 177Lu-PSMA-617—Preclinical investigations in comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res. 2017, 7, 9. [Google Scholar] [CrossRef]
- Orteca, G.; Sinnes, J.-P.; Rubagotti, S.; Iori, M.; Capponi, P.C.; Piel, M.; Rösch, F.; Ferrari, E.; Asti, M. Gallium-68 and scandium-44 labelled radiotracers based on curcumin structure linked to bifunctional chelators: Synthesis and characterization of potential PET radiotracers. J. Inorg. Biochem. 2020, 204, 110954. [Google Scholar] [CrossRef] [PubMed]
- Siwowska, K.; Guzik, P.; Domnanich, K.A.; Rodríguez, J.M.M.; Bernhardt, P.; Ponsard, B.; Hasler, R.; Borgna, F.; Schibli, R.; Köster, U.; et al. Therapeutic Potential of 47Sc in Comparison to 177Lu and 90Y: Preclinical Investigations. Pharmaceutics 2019, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Bunka, M.; Müller, C.; Vermeulen, C.; Haller, S.; Türler, A.; Schibli, R.; van der Meulen, N.P. Imaging quality of 44Sc in comparison with five other PET radionuclides using Derenzo phantoms and preclinical PET. Appl. Radiat. Isot. 2016, 110, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.V.M.; Gnesin, S.; Strobel, K.; Pérez, M.d.S.; Roos, J.E.; Müller, C.; van der Meulen, N.P. Fifty Shades of Scandium: Comparative Study of PET Capabilities Using Sc-43 and Sc-44 with Respect to Conventional Clinical Radionuclides. Diagnostics 2021, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Rosar, F.; Buchholz, H.-G.; Michels, S.; Hoffmann, M.A.; Piel, M.; Waldmann, C.M.; Rösch, F.; Reuss, S.; Schreckenberger, M. Image quality analysis of 44Sc on two preclinical PET scanners: A comparison to 68Ga. EJNMMI Phys. 2020, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Cussonneau, J.; Abaline, J.; Acounis, S.; Beaupère, N.; Beney, J.; Bert, J.; Bouvier, S.; Briend, P.; Butterworth, J.; Carlier, T.; et al. 3γ medical imaging with a liquid xenon Compton camera and 44Sc radionuclide. Acta Phys. Pol. B 2017, 48, 1661–1667. [Google Scholar] [CrossRef]
- Okumura, Y.; Yoshida, E.; Tashima, H.; Suga, M.; Kawachi, N.; Parodi, K.; Yamaya, T. Sensitivity improvement in 44Sc whole gamma imaging: Simulation study. In Proceedings of the 2018 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Sydney, Australia, 10–17 November 2018; p. 8824536. [Google Scholar] [CrossRef]
- Binder, T.M.; Anagnostatou, V.; Dedes, G.; Kamada, K.; Kang, H.G.; Lovatti, G.; Nitta, M.; Safari, M.; Zoglauer, A.; Parodi, K.; et al. Component characterization and commissioning of a gamma-PET prototype detector system. Front. Phys. 2022, 10, 954204. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, P. Feasibility study of imaging with tissue-scattered triple-γ coincidence events in Compton-PET. J. Instrum. 2022, 17, P05040. [Google Scholar] [CrossRef]
- Sharma, S.; Baran, J.; Chug, N.; Curceanu, C.; Czerwiński, E.; Dadgar, M.; Dulski, K.; Eliyan, K.; Gajos, A.; Gupta-Sharma, N.; et al. Efficiency determination of J-PET: First plastic scintillators-based PET scanner. EJNMMI Phys. 2023, 10, 28. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Production of Emerging Radionuclides towards Theranostic Applications: Copper-61, Scandium-43 and -44, and Yttrium-86; IAEA-TECDOC-1955; International Atomic Energy Agency: Vienna, Austria, 2021. [Google Scholar]
- Jalilian, A.R.; Gizawy, M.A.; Alliot, C.; Takacs, S.; Chakarborty, S.; Rovais, M.R.A.; Pupillo, G.; Nagatsu, K.; Park, J.H.; Khandaker, M.U.; et al. IAEA Activities on 67Cu, 186Re, 47Sc Theranostic Radionuclides and Radiopharmaceuticals. Curr. Radiopharm. 2021, 14, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.S.; Bailey, E.; Kumar, V.; Schwarz, S.W.; Bom, H.H.-S.; Hatazawa, J.; Paez, D.; Orellana, P.; Louw, L.; Mut, F.; et al. Global Issues of Radiopharmaceutical Access and Availability: A Nuclear Medicine Global Initiative Project. J. Nucl. Med. 2021, 62, 422–430. [Google Scholar] [CrossRef] [PubMed]

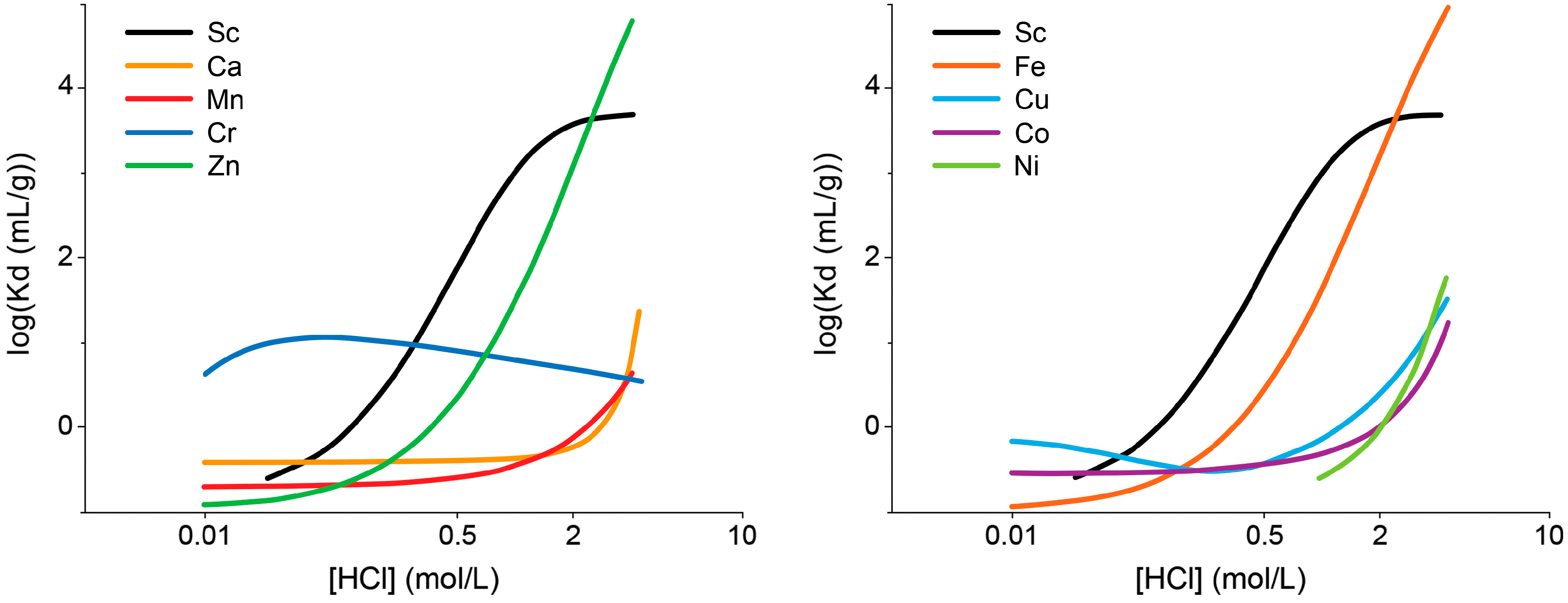
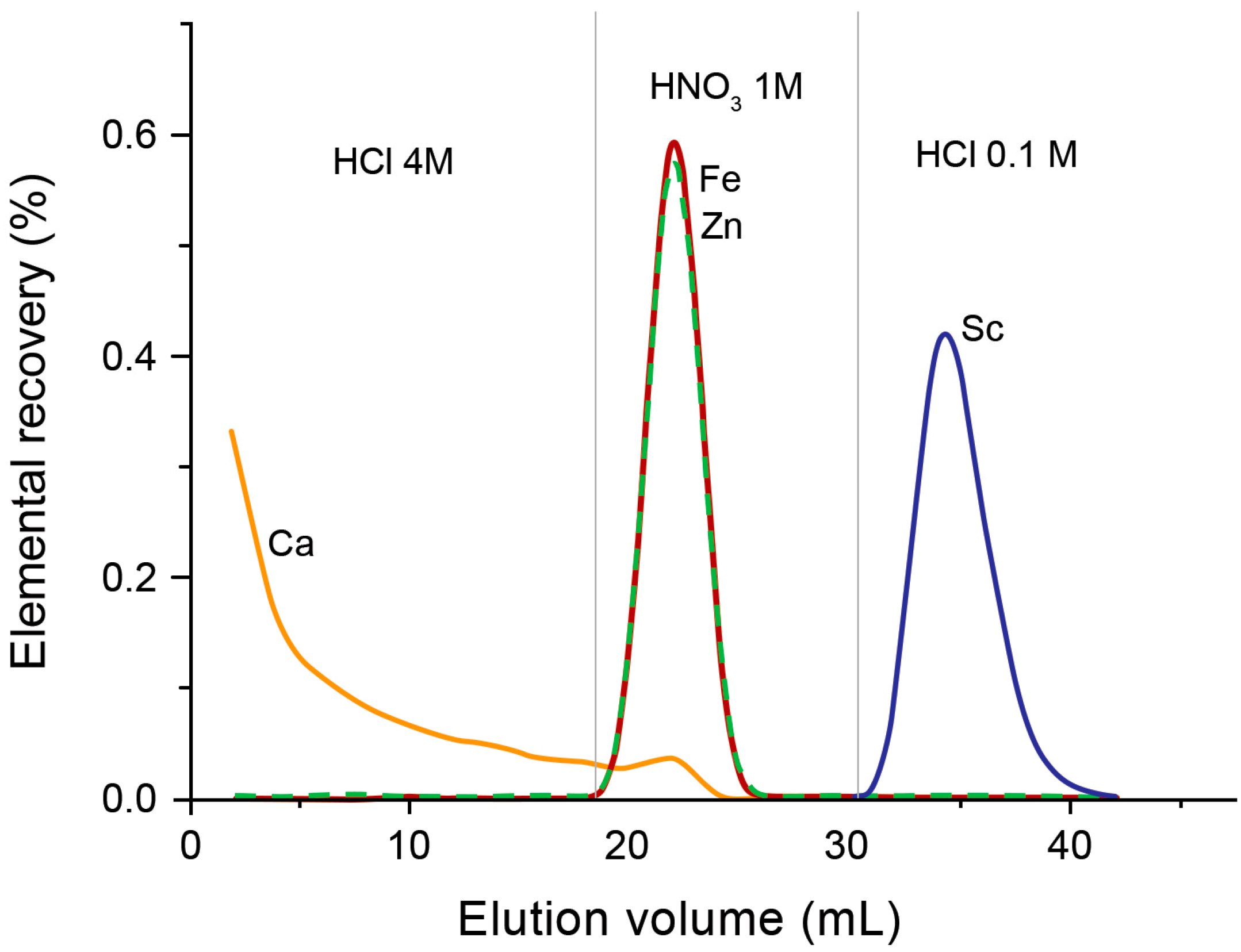
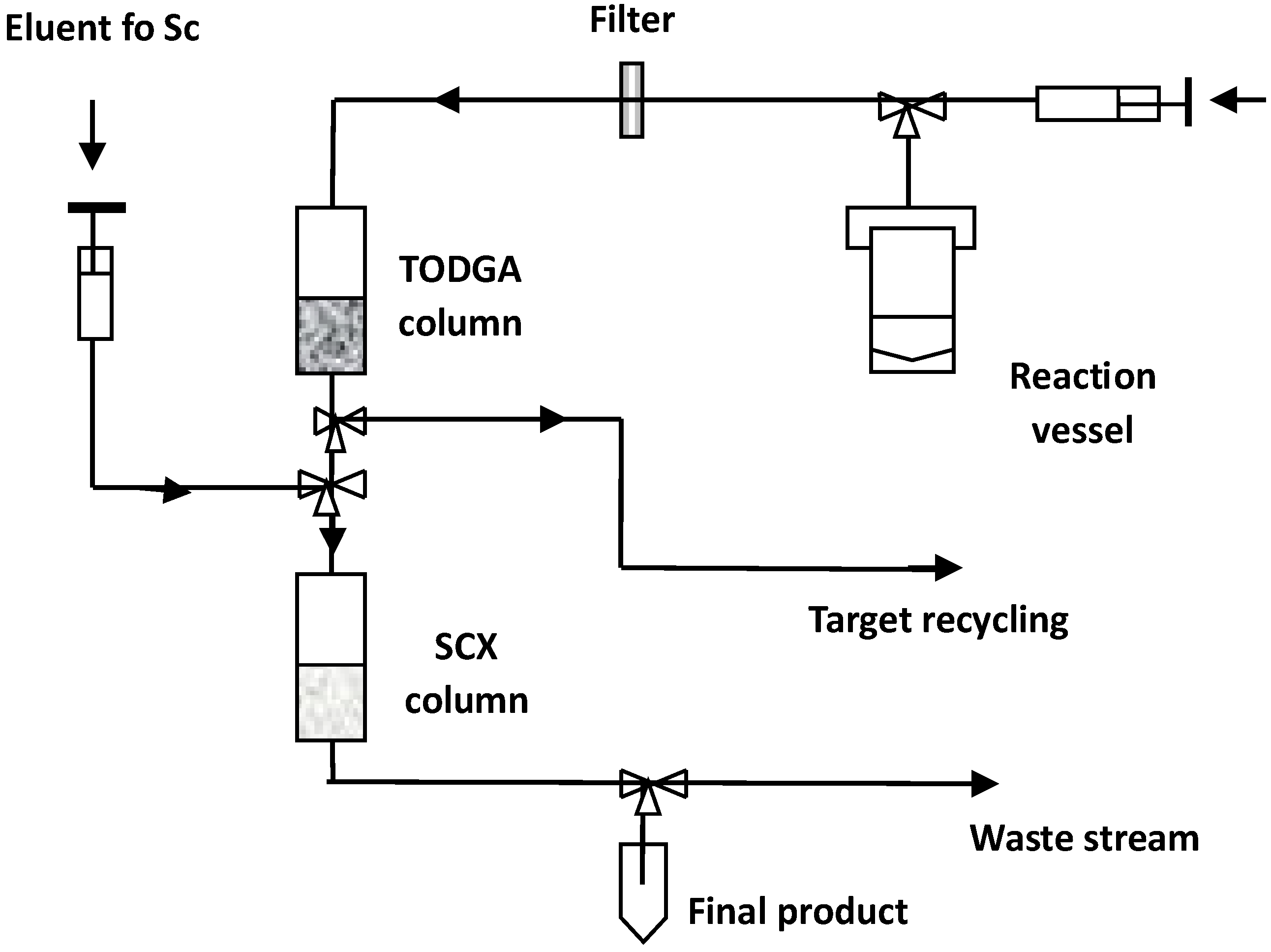
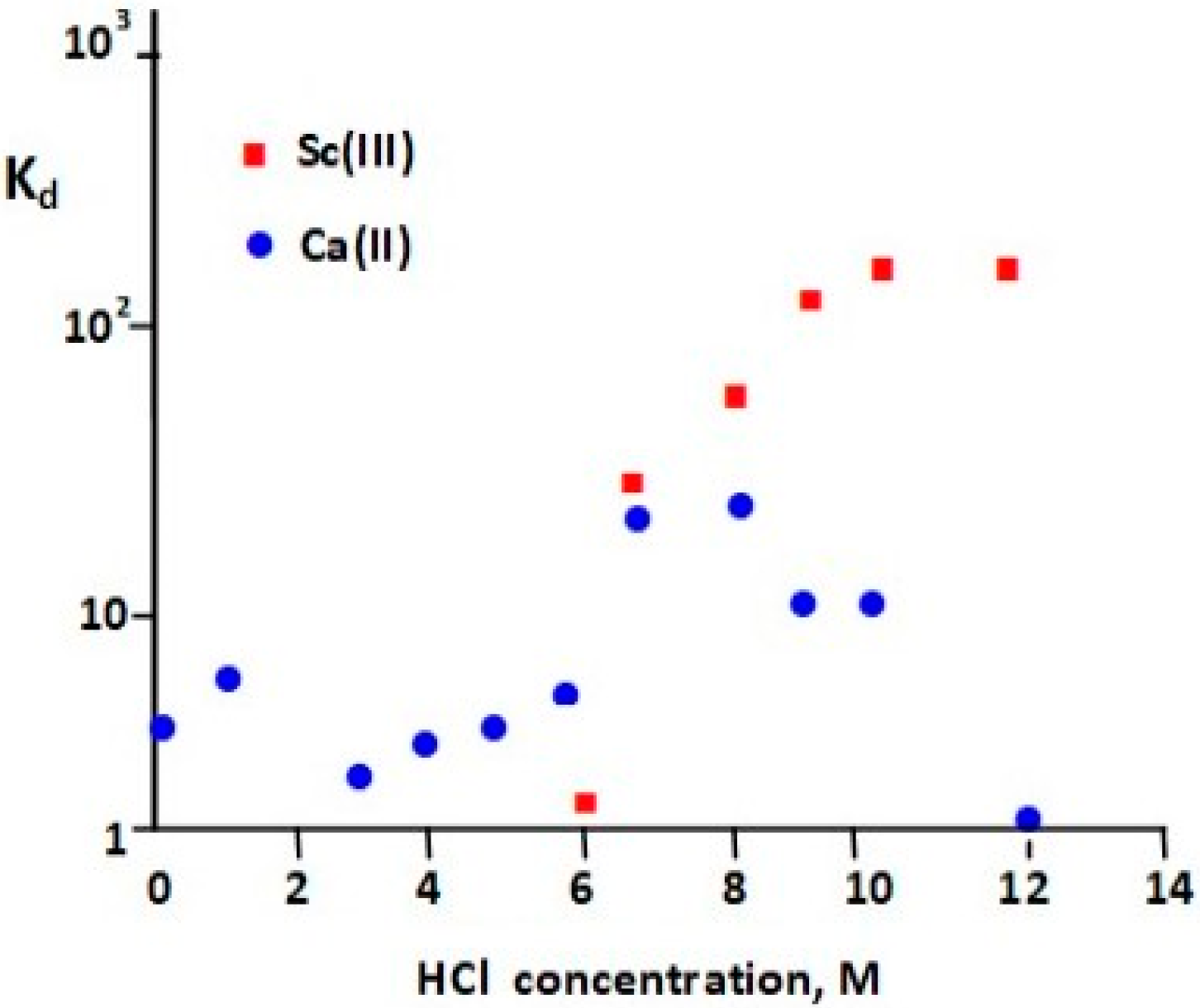
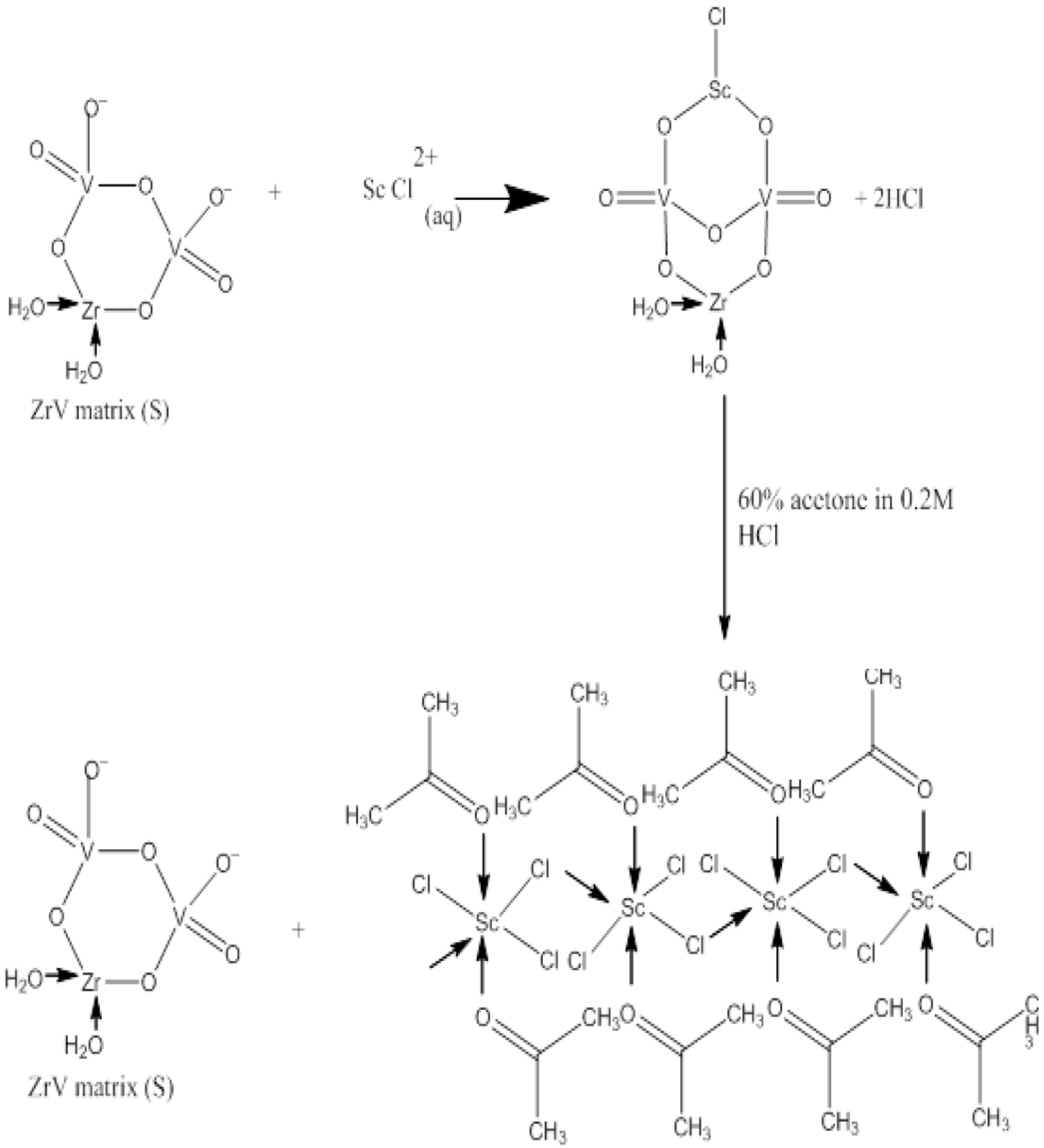

| Isotope | Irradiation Data | Activity | Radionuclidic Purity (%) | Ref. | ||
|---|---|---|---|---|---|---|
| Reaction | Abundance (%) | Beam Energy (MeV) | ||||
| Target materials with natural isotopic abundance | ||||||
| Scandium-43 | 40Ca(α,n)43Ti- > 43Sc | 96.9 | 12.5–17.5 | 240 MBq/µAh | >98.9 | [12] |
| 40Ca(α,n)43Ti- > 43Sc | 96.9 | 34 | 54.8 MBq/µAh | 99.7 | [13] | |
| 96.9 | 28 | 102.7 MBq/µAh | 99.9 | [14] | ||
| Scandium-44 | 44Ca(p,n)44Sc | 2.1 | 12 | 6.2 MBq/µAh | N/a | [15] |
| 44Ca(p,n)44Sc | 2.1 | 12.8 | 10.0 MBq/µAh | N/a | [16] | |
| 45Sc(p,2n)Ti44- > 44Sc | 100 | Generator | 185 MBq/elution | N/a | [17] | |
| Scandium-47 | 51V(p,x)47Sc | 99.75 | 20–30 | 1.7 MBq/µAh | [18] | |
| 51V(γ,p)47Sc | 99.75 | 20 | N/a | >99.99 | [19] | |
| 51V(γ,p)47Sc | 99.75 | 38 | 3.7 GBq | 98.2 | [19] | |
| Isotopically enriched target materials | ||||||
| Scandium-43 | 46Ti(p,α)43Sc | 97 | 15.1 | 225 MBq | >98.2 | [20] |
| 42Ca(d,n)43Sc | 93.58 | 5.8 | 30.4 MBq/µAh | 99.4 | [21] | |
| 43Ca(p,n)43Sc | 83.9 | 13.6 | 229.0 MBq/µAh | 87.8 | [21] | |
| Scandium-44 | 44Ca(p,n)44gSc | 98.9 | 13.6 | 433.3 MBq/µAh | 99.7 | [21] |
| 43Ca(d,n)44gSc | 83.9 | 5.8 | 34.4 MBq/µAh | 98.1 | [21] | |
| Scandium-47 | 46Ca(n, γ)47Ca- > 47Sc | 5.0 | 2.14 GBq | 99.99 | [22] | |
| 47Ti(n,p)47Sc | 95.7 | 4.9 MBq | 88–99 | [22] | ||
| Target Dissolution | Separation from the Target | Sc Preconcentration | Recovery of Sc (%) | Impurities (mg/L) | Ref. | ||
|---|---|---|---|---|---|---|---|
| Sorbent | Cleaning | Sorbent | Eluent for Sc | ||||
| 3 M HCl | TODGA (70 mg) | 4 mL of 0.1 M HCl |
|
(pH 4.5–5.0)
(pH 0–0.5) | 98 | Ca ~4.5, Pb < 0.7, Al, Zn < 1, Cu < 0.02 (comparable for both resins) | [45] |
| 1 M HNO3 | TODGA (87 mg) | 4 mL of 0.1 M HCl | DGA (43 mg) | 700 µL of 0.05 M HCl | Not reported | Traces of 88Y | [37] |
| 4 M HCl | TODGA (300 mg) | 20 mL of 4 M HCl then 12 mL 1 M HNO3 | ― | 10 mL of 0.1 M HCl | 88 ± 3 | Al + Fe = 1.14 | [38] |
| 6 M HCl | TEHDGA (300 mg) | 10 mL of 0.1 M HCl | Dowex 50Wx8 (NH4+ form, 140 mg) | 300 µL of 0.1 M NH4-α-HIB | 95 ± 3 | Al 0.06, Fe, Ni 0.03, Zn 0.007, Ca, Ni, Mn < LOD | [16] |
| 3 M HCl | TODGA (70 mg) | 4 mL of 0.1 M HCl | Bond Elut SCX (100 mg) | 700 µL of 5 M NaCl/ 0.13 M HCl (pH 0–0.5) | 90.4 ± 5.5 | 88Y 0.19% | [20] |
| H2O | TODGA (50 mg) | 3 times 5 mL of 6 M HCl | ― | 2.5 mL of 0.05 M HCl | 88 ± 6 | Ca 6.0, Zn 5.4, Fe 1.1, Pb 0.38, Al 0.18 | [39] |
| 4 M HCl | TODGA | 10 mL of 1 M HNO3 | ― | 0.40 mL of 0.1 M HCl | Not reported | Al + Fe (1.14 ± 0.66) | [40] |
| 3 M HCl | TODGA (70 mg) | 2–3 mL of 0.1 M HCl | Dowex 50 (100 mg, H+ form) | 1 M CH3COONH4, pH 4 | 87 | Fe 0.56, Ca < 1 | [41] |
| 9 M HCl | UTEVA (50 mg) | 5 mL of 9 M HCl | ― | 0.4 mL of H2O | 79 | Fe < 0.001, Ca < 1 | |
| 1 M HCl | Chelex 100 (Na+ form) | 30 mL of 0.01 M HCl | ― | 0.4 mL of 1 M HCl | 85 | Fe 10.5, Ca < 1 | |
| 9 M HCl | UTEVA (50 mg) | 5 mL of 9 M HCl | ― | 0.40 mL of H2O | >80 | Fe < 0.001 | [42] |
| 12 M HCl | UTEVA (50 mg) | 5 mL of 9 M HCl | ― | 0.40 mL of 1 M HCl | >80 | Ca 82.2, Fe 5.2, Zn 4.7, Al 0.17, Ni 1.7, Mn 0.11 | [48] |
| 10 M HCl | UTEVA (100 mg) | 5 mL of 10 M HCl | ― | 0.3 mL of H2O | >80 | Ca, Fe, Zn, Ni, Al, Mn < 0.005 | [12] |
| 1 M HCl | Chelex 100 | 30 mL of 0.01 M HCl | ― | 2 mL of 1 M HCl | 85 | Fe 0.99, Ca < 1 | [49] |
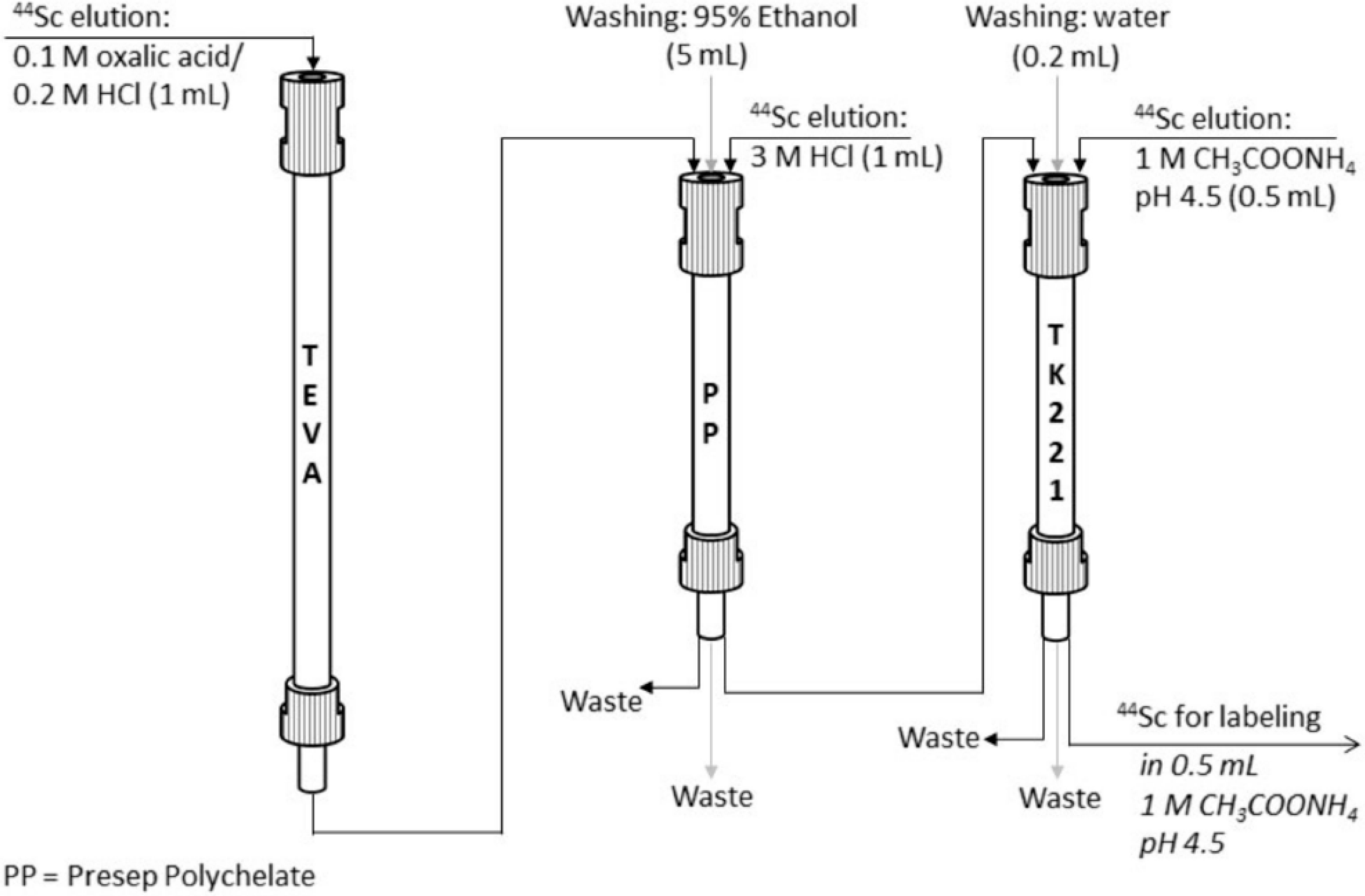
| UTEVA (70 mg) | 2 mL of 11 M HCl | AG50Wx4 (H+ form) | 1 M CH3COONH4, pH 4.0 | 93 | Not reported | [55] | |
| 0.1 M HCl | Chelex 100 (Na+ form) | 30 mL of 0.01 M HCl | ― | 1 M HCl | >70 | Fe 0.99, Ca < 1 | [50] |
| 2 M HCL | Nobias Chelate PA1 (10 mg) | Formic buffer pH 3.0 | ― | 0.1 mL of 2 M HCl | 94.9 ± 2.8 | Al 0.09, Ca 1.34, Cu 0.02, Fe 0.005, Mn 0.004, Ni 0.013, Pb 1.03, Zn 0.13 | [51] |
| 0.01 M HCl | Zirconium vanadate gel (600 mg) | 0.001 M HNO3 | ― | 0.2 M HCl with 60% acetone | 88 ± 2.2 | Ca ≤ 0.05 | [54] |
| Target Dissolution | Separation from the Target | Recovery of Sc (%) | Impurities | Ref. | ||
|---|---|---|---|---|---|---|
| Sorbent | Cleaning | Eluent | ||||
| 6 M HCl | TEHDGA (5 mL) | 4M HCl | 0.1 M HCl | Not reported | Not reported | [26] |
| Zr resin, hydroxamate groups (1 mL) | ― | 6 M HCl/0.65 M H2O2 | >94 | Not reported | ||
| Concentrated H2SO4 | TODGA | 6 M HNO3 then 6 M HCl | 0.1 M HCl or HNO3 | >90 | As 0.72, Zn 5.3, Fe 3.5, Ti 9.1, Al 0.27, Ca 4.5 (µg) | [59] |
| NH4HF2 (200 mg) in 3 mL of 12 M HCl | TEHDGA (110 mg) | 7 M HCl, 7 M HNO3 | 10 mL of 0.1 M HCl | 94 | Fe 1.0, Cu 0.8, Zn 1.3, V 0.5, Al 0.8 (mg/L) | [58] |
| 6 M HCl | AG1-X8 (Cl− form) | 1 M HCl | 20 mL of 0.005 M H2C2O4/0.07 M HCl | 97 | Not reported | [17] |
| 6 M HCl | AG50Wx8 (53 mg, H+ form) | 20 mL of 0.1 M H2C2O4/0.2 M HCl | 2–3 mL of 0.25 M CH3COONH4 (pH 4) | ~90 | Not reported | [24] |
| 1 mL of hot H2SO4 concentrated + 50 mg (NH4)2SO4 + 0.1 mL of H2O2 | Dowex 50WX8, (3 g, H+ form) | 2 M HNO3 | 100 mL of 4 M HCl + 0.1 M HF | >90 | Ti 0.05 mg/L | [60] |
| TEVA (150 mg) | 0.1 M H2C2O4/ 0.2 M HCl | 95 | [28] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilian, K.; Pyrzyńska, K. Scandium Radioisotopes—Toward New Targets and Imaging Modalities. Molecules 2023, 28, 7668. https://doi.org/10.3390/molecules28227668
Kilian K, Pyrzyńska K. Scandium Radioisotopes—Toward New Targets and Imaging Modalities. Molecules. 2023; 28(22):7668. https://doi.org/10.3390/molecules28227668
Chicago/Turabian StyleKilian, Krzysztof, and Krystyna Pyrzyńska. 2023. "Scandium Radioisotopes—Toward New Targets and Imaging Modalities" Molecules 28, no. 22: 7668. https://doi.org/10.3390/molecules28227668
APA StyleKilian, K., & Pyrzyńska, K. (2023). Scandium Radioisotopes—Toward New Targets and Imaging Modalities. Molecules, 28(22), 7668. https://doi.org/10.3390/molecules28227668






