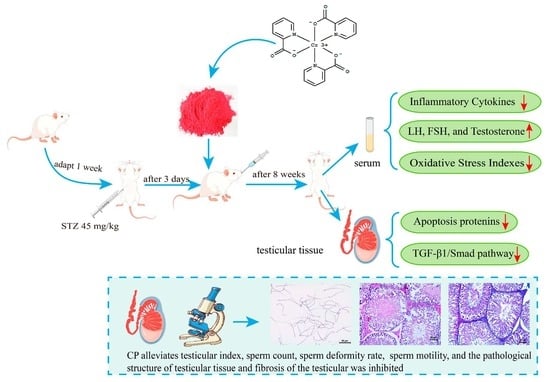
Chromium Picolinate Protects against Testicular Damage in STZ-Induced Diabetic Rats via Anti-Inflammation, Anti-Oxidation, Inhibiting Apoptosis, and Regulating the TGF-β1/Smad Pathway
Abstract
:1. Introduction
2. Results
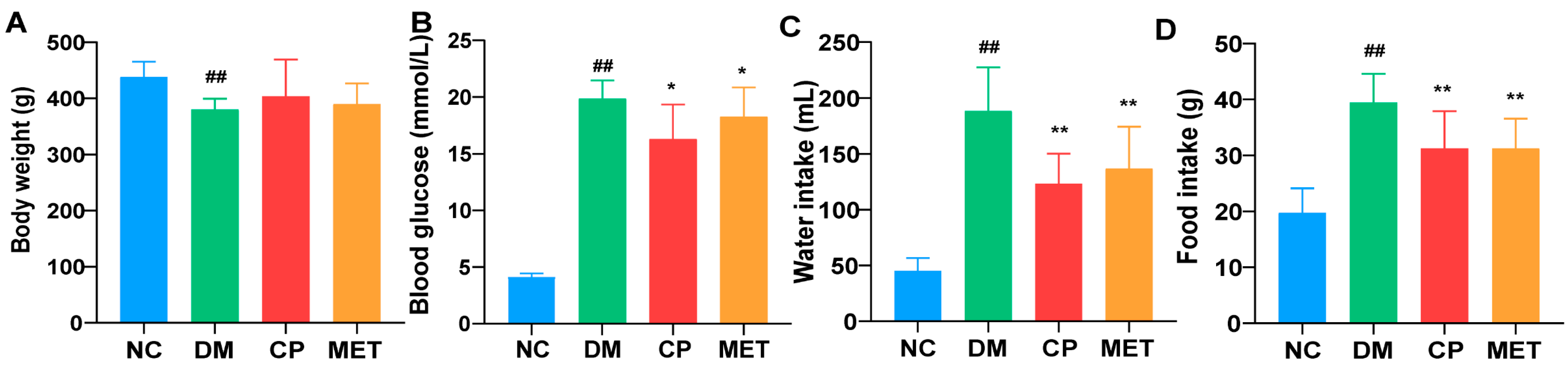
2.1. Effects of Chromium Picolinate on Blood Glucose, Body Weight, Food, and Water Intake of DM Rats
2.2. Effects of Chromium Picolinate on the Testicular Index and Sperm Quality of DM Rats
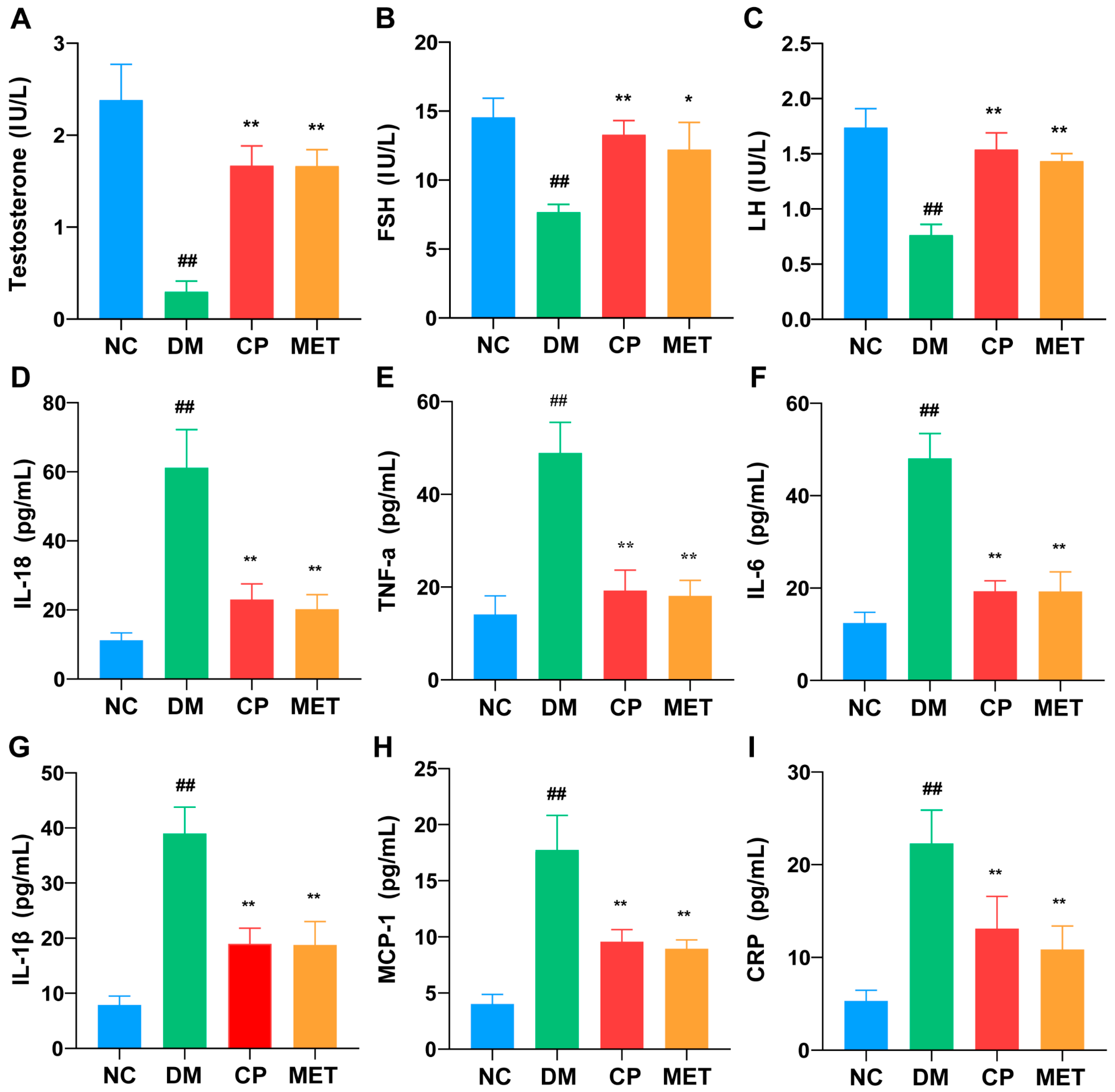
2.3. Effects of Chromium Picolinate on Serum LH, FSH, and Testosterone Levels in DM Rats
2.4. Effects of Chromium Picolinate on Serum Inflammatory Cytokine Levels in DM Rats
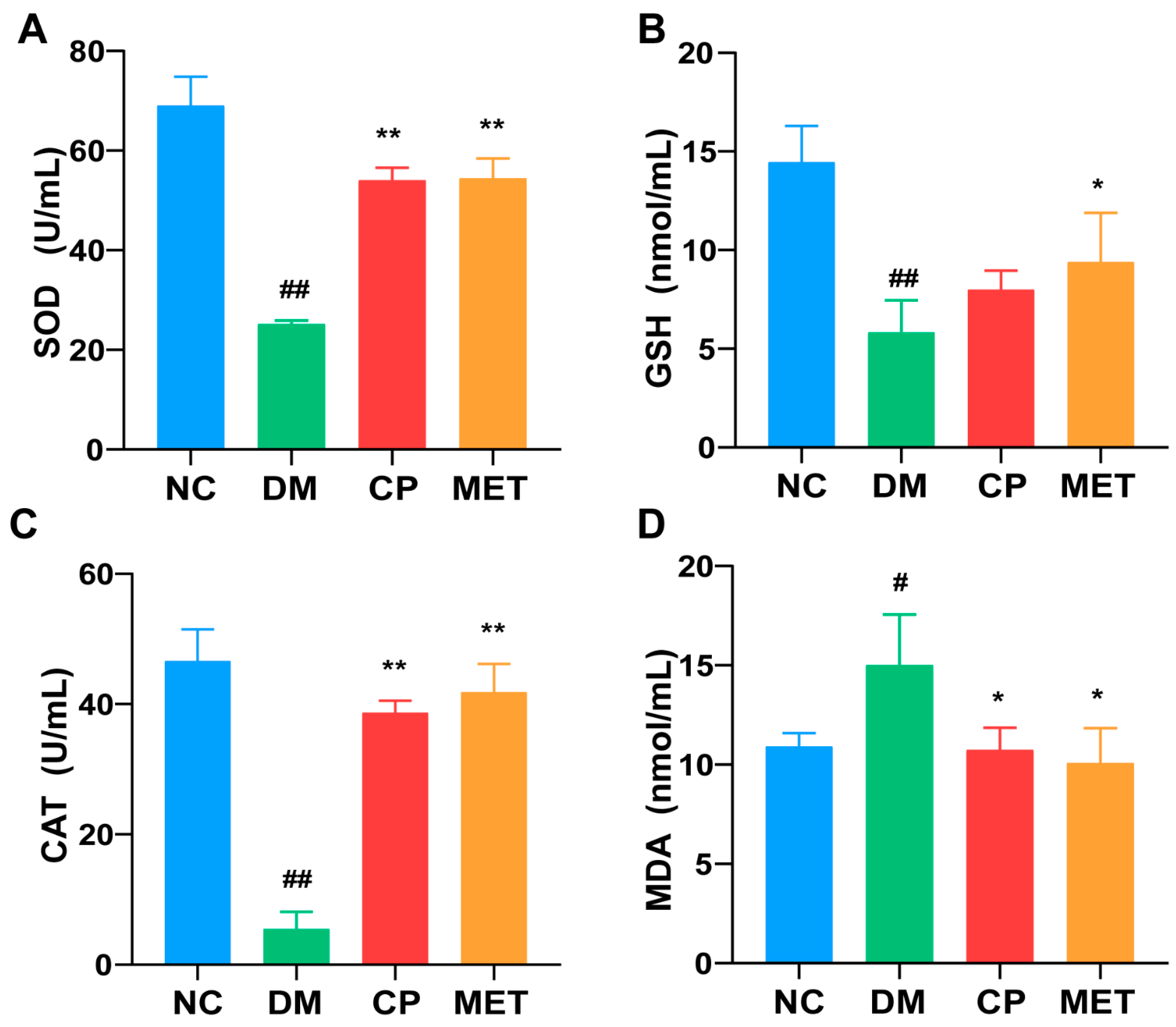
2.5. Effects of Chromium Picolinate on Testicular GSH, SOD, MDA, and CAT Index of DM Rats
2.6. Effect of Chromium Picolinate on the Structure of the Testicular Tissue of Diabetic Rats
2.7. Effect of Chromium Picolinate on the Ultrastructure of the Testis of DM Rats
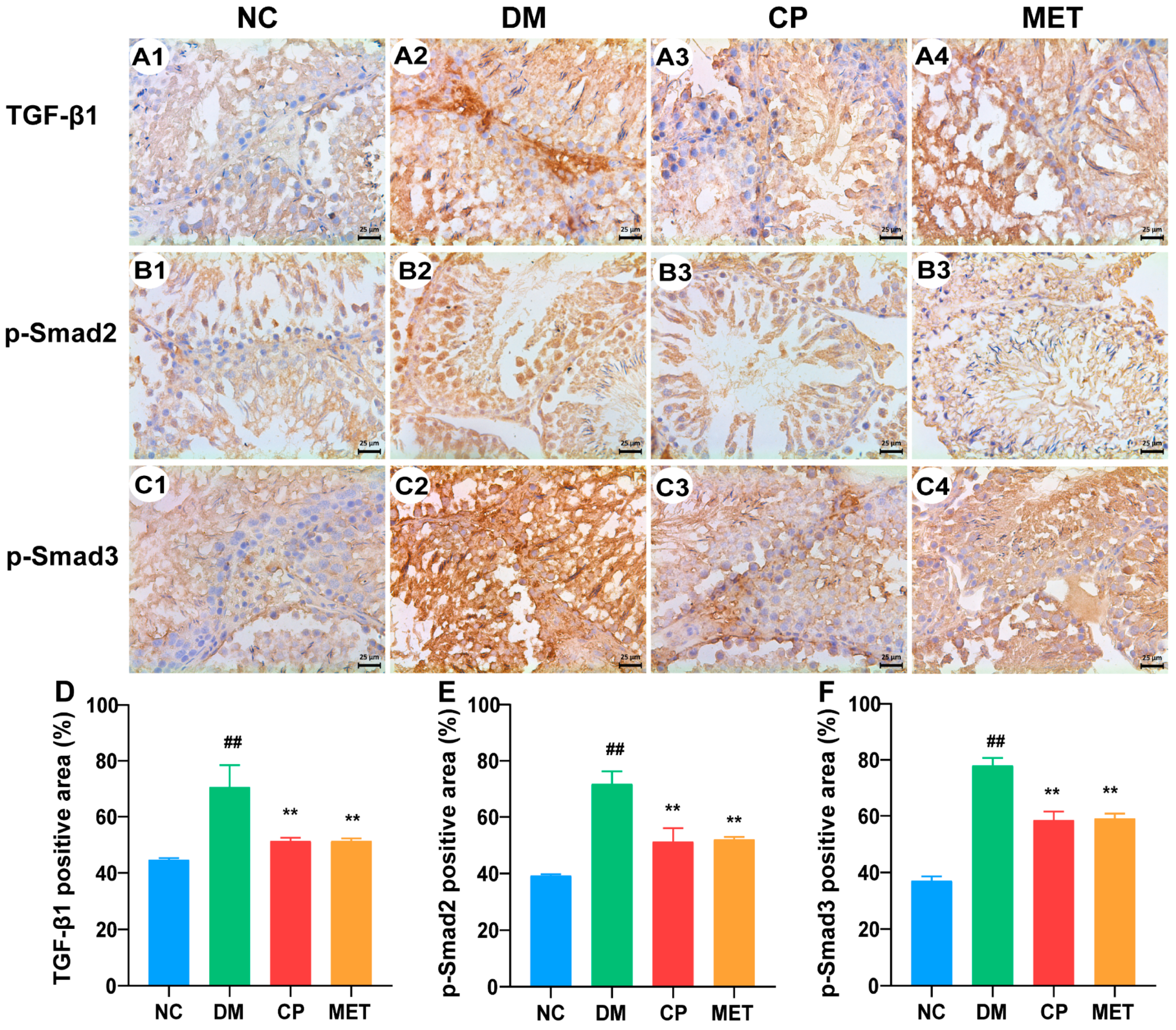
2.8. Effect of Chromium Picolinate on the Testicular Interstitial Fibrosis of Diabetic Rats
2.9. Effect of Chromium Picolinate on Bax, Capase-3, Bcl-2, and NF-κB Protein Expression Levels in the Testis of DM Rats
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Experimental Design
4.3. Sperm Parameters: Sperm Motility Sperm Count and Sperm Deformity Rate Assessment
4.4. Serum LH, FSH, and Testosterone Detection
4.5. Inflammatory Cytokines Detection
4.6. GSH, SOD, MDA, and CAT Index Detection
4.7. Histological Evaluation of the Testes
4.8. Transmission Electron Microscopic Observation of the Testis Ultrastructure
4.9. Testicular Interstitial Fibrosis Evaluation
4.10. Immunohistochemical Analysis of Apoptosis and Fibrosis-Related Proteins in Testis
4.11. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef] [PubMed]
- King, G.L. The role of inflammatory cytokines in diabetes and its complications. J. Periodontol. 2008, 79, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Kyathanahalli, C.; Bangalore, S.; Hanumanthappa, K.; Muralidhara. Experimental diabetes-induced testicular damage in prepubertal rats. J. Diabetes 2014, 6, 48–59. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yin, G.; Li, Q.Q.; Zeng, Q.; Duan, J. Diabetes mellitus causes male reproductive dysfunction: A review of the evidence and mechanisms. In Vivo 2021, 35, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- Pavlinkova, G.; Margaryan, H.; Zatecka, E.; Valaskova, E.; Elzeinova, F.; Kubatova, A.; Bohuslavova, R.; Peknicova, J. Transgenerational inheritance of susceptibility to diabetes-induced male subfertility. Sci. Rep. 2017, 7, 4940. [Google Scholar] [CrossRef] [PubMed]
- Apichakan, S.; Supatcharee, A.; Jaturon, B.; Wannisa, S.; Sitthichai, I. Testicular histopathology and phosphorylated protein changes in mice with diabetes induced by multiple-low doses of streptozotocin: An experimental study. Int. J. Reprod. Biomed. 2018, 16, 235–246. [Google Scholar]
- Korejo, N.A.; Wei, Q.W.; Shah, A.H.; Shi, F.X. Effects of concomitant diabetes mellitus and hyperthyroidism on testicular and epididymal histoarchitecture and steroidogenesis in male animals. J. Zhejiang Univ. Sci. B 2016, 17, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Amaral, S.; Oliveira, P.J.; Ramalho-Santos, J. Diabetes and the impairment of reproductive function: Possible role of mitochondria and reactive oxygen species. Curr. Diabetes Rev. 2008, 4, 46–54. [Google Scholar]
- Nna, V.U.; Abu Bakar, A.B.; Ahmad, A.; Eleazu, C.O.; Mohamed, M. Oxidative stress, NF-κB-mediated inflammation and apoptosis in the testes of streptozotocin-induced diabetic rats: Combined protective effects of malaysian propolis and metformin. Antioxidants 2019, 8, 465. [Google Scholar] [CrossRef]
- Qadiri, A.; Mirzaei, B.F.; Hamidian, G.; Zavvari, O.Z.; Ahmadi, M.; Oghbaei, H.; Mehri, K.; Vatankhah, A.M.; Keyhanmanesh, R. Administration of troxerutin improves testicular function and structure in type-1 diabetic adult rats by reduction of apoptosis. Avicenna J. Phytomed. 2019, 9, 374–385. [Google Scholar]
- Khosravi, Z.; Sedaghat, R.; Baluchnejadmojarad, T.; Roghani, M. Diosgenin ameliorates testicular damage in streptozotocin-diabetic rats through attenuation of apoptosis, oxidative stress, and inflammation. Int. Immunopharmacol. 2019, 70, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Orman, D.; Vardi, N.; Ates, B.; Taslidere, E.; Elbe, H. Aminoguanidine mitigates apoptosis, testicular seminiferous tubules damage, and oxidative stress in streptozotocin-induced diabetic rats. Tissue Cell 2015, 47, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.C.; Feng, Y.L.; Wang, Y.H.; Kong, L.J.; Zhou, M.S.; Wu, M.M.; Liu, C.Y.; Weng, H.C.; Wang, H.W. Islet transplantation ameliorates diabetes-induced testicular interstitial fibrosis and is associated with inhibition of TGF-β1/Smad2 pathway in a rat model of type 1 diabetes. Mol. Med. Rep. 2021, 23, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Takihara, H.; Naito, K.; Aktuelle, U. Quantitative analysis of testicular interstitial fibrosis after vasectomy in humans. Aktuelle Urol. 2003, 34, 262–264. [Google Scholar]
- Shen, N.; Li, X.; Zhou, T.; Bilal, M.U.; Du, N.; Hu, Y. Shensong yangxin capsule prevents diabetic myocardial fibrosis by inhibiting TGF-β1/smad signaling. J. Ethnopharmacol. 2014, 157, 161–170. [Google Scholar] [CrossRef]
- Li, J.; Kang, S.W.; Kim, J.L.; Sung, H.Y.; Kwun, I.S.; Kang, Y.H. Isoliquiritigenin entails blockade of TGF-β1-smad signaling for retarding high glucose-induced mesangial matrix accumulation. J. Agric. Food Chem. 2010, 58, 3205–3212. [Google Scholar] [CrossRef]
- Gina, J.R.; Nancy, S.W.; Andrea, R.R.; Curtiss, B.C. Chromium as adjunctive treatment for type 2 diabetes. Ann. Pharmacother. 2003, 37, 876–885. [Google Scholar]
- Walter, M. Chromium in human nutrition: A review. J. Nutr. 1993, 123, 622–633. [Google Scholar]
- Amoikon, E.K.; Fernandez, J.M.; Southern, L.L.; Thompson, D.L.; Ward, T.L.; Olcott, B.M. Effect of chromium tripicolinate on growth, glucose tolerance, insulin sensitivity, plasma metabolites, and growth hormone in pigs. J. Anim. Sci. 1995, 73, 1123–1130. [Google Scholar] [CrossRef]
- Qi, S.S.; Zheng, H.X.; Jiang, H.; Yuan, L.P.; Dong, L.C. Protective effects of chromium picolinate against diabetic-induced renal dysfunction and renal fibrosis in streptozotocin-induced diabetic rats. Biomolecules 2020, 10, 398. [Google Scholar] [CrossRef]
- Wojciechowska, J.; Krajewski, W.; Bolanowski, M.; Kręcicki, T.; Zatoński, T. Diabetes and cancer: A review of current knowledge. Exp. Clin. Endocrinol. Diabetes 2016, 124, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, G.O.; Nlekerem, C.M.; Ibukun, E.O.; Kolawole, A.O. Quail (Coturnix japonica) egg yolk bioactive components attenuate streptozotocin-induced testicular damage and oxidative stress in diabetic rats. Eur. J. Nutr. 2018, 57, 2857–2867. [Google Scholar] [CrossRef]
- La-vignera, S.; Condorelli, R.; Vicari, E.; Dagata, R.; Calogero, A.E. Diabetes mellitus and sperm parameters. J. Androl. 2012, 33, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, W.; Wang, J.; Chen, Y.; Huang, W.; Zhu, Y. Taxifolin attenuates diabetic nephropathy in streptozotocin-induced diabetic rats. Am. J. Transl. Res. 2018, 10, 1205–1210. [Google Scholar] [PubMed]
- Liu, Y.; Yang, Z.; Kong, D.; Zhang, Y.; Yu, W.; Zha, W. Metformin ameliorates testicular damage in male mice with streptozotocin-induced type 1 diabetes through the PK2/PKR pathway. Oxid. Med. Cell. Longev. 2019, 2019, 5681701. [Google Scholar] [CrossRef]
- He, W.; Liu, H.; Hu, L.; Wang, Y.; Huang, L.; Liang, A.; Wang, X.; Zhang, Q.; Chen, Y.; Cao, Y.; et al. Icariin improves testicular dysfunction via enhancing proliferation and inhibiting mitochondria-dependent apoptosis pathway in high-fat diet and streptozotocin-induced diabetic rats. Reprod. Biol. Endocrinol. 2021, 19, 168. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, S.; Yan, W.; Ji, L.; Shao, M.; Sun, Z.; He, D.; Zhang, L.; Xia, Z.; Li, X.; et al. Rape bee pollen alleviates renal tissue damage in diabetic rats via anti-inflammation, anti-oxidation, and modulating gut microbiota. eFood 2023, 4, e101. [Google Scholar] [CrossRef]
- Koroglu Aydın, P.; Karabulut-Bulan, O.; Bugan, I.; Turkyilmaz, I.B.; Altun, S.; Yanardag, R. The protective effect of metformin against testicular damage in diabetes and prostate cancer model. Cell Biochem. Funct. 2022, 40, 60–70. [Google Scholar] [CrossRef]
- Maresch, C.C.; Stute, D.C.; Alves, M.G.; Oliveira, P.F.; Kretser, D.M.; Linn, T. Diabetes-induced hyperglycemia impairs male reproductive function: A systematic review. Hum. Reprod. Update 2018, 24, 86–105. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Chen, C.; Wang, J.; Xie, W.; Wang, M.; Li, X.; Zhang, X. Purple potato (Solanum tuberosum L.) anthocyanins attenuate alcohol-induced hepatic injury by enhancing antioxidant defense. J. Nat. Med. 2016, 70, 45–53. [Google Scholar] [CrossRef]
- Qi, S.; He, J.; Dong, L.; Yuan, L.P.; Wu, J.; Zu, Y.X.; Zheng, H. Cyanidin-3-glucoside from black rice prevents renal dysfunction 503 and renal fibrosis in streptozotocin-diabetic rats. J. Funct. Foods 2020, 72, 104062. [Google Scholar] [CrossRef]
- Shi, G.J.; Zheng, J.; Han, X.X.; Jiang, Y.P.; Li, Z.M.; Wu, J.; Chang, Q.; Niu, Y.; Sun, T.; Li, Y.X.; et al. Lycium barbarum polysaccharide attenuates diabetic testicular dysfunction via inhibition of the PI3K/Akt pathway-mediated abnormal autophagy in male mice. Cell Tissue Res. 2018, 374, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lin, S.; Lv, Q.; Yang, Q.; Wu, G.; Hu, J.; Yang, J. Taurine recovers testicular steroidogenesis and spermatogenesis in streptozotocin-induced diabetic rats. Adv. Exp. Med. Biol. 2017, 975, 801–811. [Google Scholar] [PubMed]
- Schoeller, E.L.; Schon, S.; Moley, K.H. The effects of type 1 diabetes on the hypothalamic, pituitary and testes axis. Cell Tissue Res. 2012, 349, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.F.; Murray, F.T.; Drylie, D.D. Interstitial compartment pathology and spermatogenic disruption in testes from impotent diabetic men. Anat. Rec. 1985, 213, 53–62. [Google Scholar] [CrossRef]
- Al-Megrin, W.A.; El-Khadragy, M.F.; Hussein, M.H.; Mahgoub, S.; Abdel-Mohsen, D.M.; Taha, H.; Bakkar, A.A.A.; Abdel-Moneim, A.E.; Amin, H.K. Green Coffea arabica extract ameliorates testicular injury in high-fat diet/streptozotocin-induced diabetes in rats. J. Diabetes Res. 2020, 2020, 6762709. [Google Scholar] [CrossRef]
- Ahmed, H.H.; Abd, M.D.; Abdel, A.E.; Hadeer, A.A. Pre-clinical study for the antidiabetic potential of selenium nanoparticles. Biol. Trace Elem. Res. 2017, 177, 267–280. [Google Scholar] [CrossRef]
- Roy, S.; Metya, S.K.; Rahaman, N.; Sannigrahi, S.; Ahmed, F. Ferulic acid in the treatment of post-diabetes testicular damage: Relevance to the down regulation of apoptosis correlates with antioxidant status via modulation of TGF-β1, IL-1β and Akt signalling. Cell Biochem. Funct. 2014, 32, 115–124. [Google Scholar] [CrossRef]
- Christian, H.; Alena, R.; Elizabeth, W.; Chen, J.; Schneuder, A.; Long, S.A.; Wei, S.; Rawlings, R.; Kinsman, M.; Evanko, S.P.; et al. Enhanced T cell responses to IL-6 in type 1 diabetes are associated with early clinical disease and increased IL-6 receptor expression. Sci. Transl. Med. 2016, 8, 356ra119. [Google Scholar]
- Maresch, C.C.; Stute, D.C.; Ludlow, H.; Hammers, H.P.; Kretser, D.M.; Hedher, M.P.; Linn, T. Hyperglycemia is associated with reduced testicular function and activin dysregulation in the Ins2 Akita+/− mouse model of type 1 diabetes. Mol. Cell. Endocrinol. 2017, 446, 91–101. [Google Scholar] [CrossRef]
- Nasiri, K.; Akbari, A.; Nimrouzi, M.; Ruyvaran, M.; Mohamadian, A. Safflower seed oil improves steroidogenesis and spermatogenesis in rats with type II diabetes mellitus by modulating the genes expression involved in steroidogenesis, inflammation and oxidative stress. J. Ethnopharmacol. 2021, 275, 114–139. [Google Scholar] [CrossRef] [PubMed]
- Atta, M.S.; Almadaly, E.A.; El-Far, A.H. Thymoquinone defeats diabetes-induced testicular damage in rats targeting antioxidant, inflammatory and aromatase expression. Int. J. Mol. Sci. 2017, 18, 919. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhan, M.; Li, J.; Zhang, W.; Shang, X. Lycopene alleviates lipopolysaccharide-induced testicular injury in rats by activating the PPAR signaling pathway to integrate lipid metabolism and the inflammatory response. Transl. Androl. Urol. 2023, 12, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.; Aktas, C.; Erboga, M. Protective effects of quercetin against apoptosis and oxidative stress in streptozotocin-induced diabetic rat testis. Food Chem. Toxicol. 2012, 50, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Koh, P.O. Streptozotocin-induced diabetes increases the interaction of Bad/Bcl-XL and decreases the binding of pBad/14-3-3 in rat testis. Life Sci. 2008, 81, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.G.; Tan, Y.; Dai, J.Y.; Li, B.; Guo, L.P.; Cui, J.W.; Wang, G.J.; Shi, X.; Zhang, X.; Mellen, N.; et al. Exacerbation of diabetes-induced testicular apoptosis by zinc deficiency is most likely associated with oxidative stress, p38 MAPK activation, and p53 activation in mice. Toxicol. Lett. 2010, 200, 100–106. [Google Scholar] [CrossRef] [PubMed]
- AlAmri, O.D.; Albeltagy, R.S.; Akabawy, M.A.; Mahgoub, S.; Abdel-Mohsen, D.M.; Abdel-Mohsen, A.E.; Amin, H.K. Investigation of antioxidant and anti-inflammatory activities as well as the renal protective potential of green coffee extract in high fat-diet/streptozotocin-induced diabetes in male albino rats. J. Funct. Foods 2020, 71, 103996. [Google Scholar] [CrossRef]
- Cayan, S.; Dusmez, D.; Bozlu, M.; Gorur, S.; Akbay, E. Human testicular mast cells and fibrosis in infertile men. Fertil. Steril. 2001, 76, S258. [Google Scholar] [CrossRef]
- Voelker, J.; Berg, P.H.; Sheetz, M.; Duffin, K.; Shen, T.; Moser, B.; Greene, T.; Blumenthal, S.S.; Rychlik, I.; Yagil, Y.; et al. Anti-TGF-β1 antibody therapy in patients with diabetic nephropathy. J. Am. Soc. Nephrol. 2017, 28, 953–962. [Google Scholar] [CrossRef]
- Salama, N.; Tsuji, M.; Tamura, M.; Kagawa, S. Transforming growth factor (beta1) in testes of aged and diabetic rats: Correlation with testicular function. Arch. Androl. 2001, 47, 217–226. [Google Scholar] [CrossRef]
- Kabel, A.M. Zinc/alogliptin combination attenuates testicular toxicity induced by doxorubicin in rats: Role of oxidative stress, apoptosis and TGF-β1/NF-κB signaling. Biomed. Pharmacother. 2018, 97, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Marion, L.; Petra, K. Integration of the TGF-β pathway into the cellular signalling network. Cell. Signal. 2002, 14, 977–988. [Google Scholar]
- Gonzalez, C.; Matzkin, M.; Frungieri, M.; Terradas, C.; Ponzio, R.; Puigdomenech, E.; Levalle, O.; Calandra, R.S.; Gonzalez-Calvar, S.I. Expression of the TGF-beta1 system in human testicular pathologies. Reprod. Biol. Endocrinol. 2010, 8, 148. [Google Scholar] [CrossRef] [PubMed]



| Score | Description |
|---|---|
| 1 | No cells |
| 2 | Sertoli cells without germ cells |
| 3 | Only spermatogonia |
| 4 | Only a few spermatocytes |
| 5 | Many spermatocytes |
| 6 | Only a few early spermatids |
| 7 | Many early spermatids without differentiation |
| 8 | Few late spermatids |
| 9 | Many late spermatids |
| 10 | Full spermatogenesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Hu, Y.; Shao, M.; Chen, S.; Qi, S. Chromium Picolinate Protects against Testicular Damage in STZ-Induced Diabetic Rats via Anti-Inflammation, Anti-Oxidation, Inhibiting Apoptosis, and Regulating the TGF-β1/Smad Pathway. Molecules 2023, 28, 7669. https://doi.org/10.3390/molecules28227669
Zheng H, Hu Y, Shao M, Chen S, Qi S. Chromium Picolinate Protects against Testicular Damage in STZ-Induced Diabetic Rats via Anti-Inflammation, Anti-Oxidation, Inhibiting Apoptosis, and Regulating the TGF-β1/Smad Pathway. Molecules. 2023; 28(22):7669. https://doi.org/10.3390/molecules28227669
Chicago/Turabian StyleZheng, Hongxing, Yingjun Hu, Mengli Shao, Simin Chen, and Shanshan Qi. 2023. "Chromium Picolinate Protects against Testicular Damage in STZ-Induced Diabetic Rats via Anti-Inflammation, Anti-Oxidation, Inhibiting Apoptosis, and Regulating the TGF-β1/Smad Pathway" Molecules 28, no. 22: 7669. https://doi.org/10.3390/molecules28227669
APA StyleZheng, H., Hu, Y., Shao, M., Chen, S., & Qi, S. (2023). Chromium Picolinate Protects against Testicular Damage in STZ-Induced Diabetic Rats via Anti-Inflammation, Anti-Oxidation, Inhibiting Apoptosis, and Regulating the TGF-β1/Smad Pathway. Molecules, 28(22), 7669. https://doi.org/10.3390/molecules28227669