Recent Advances in the Reverse Water–Gas Conversion Reaction
Abstract
:1. Introduction
2. Results and Discussion
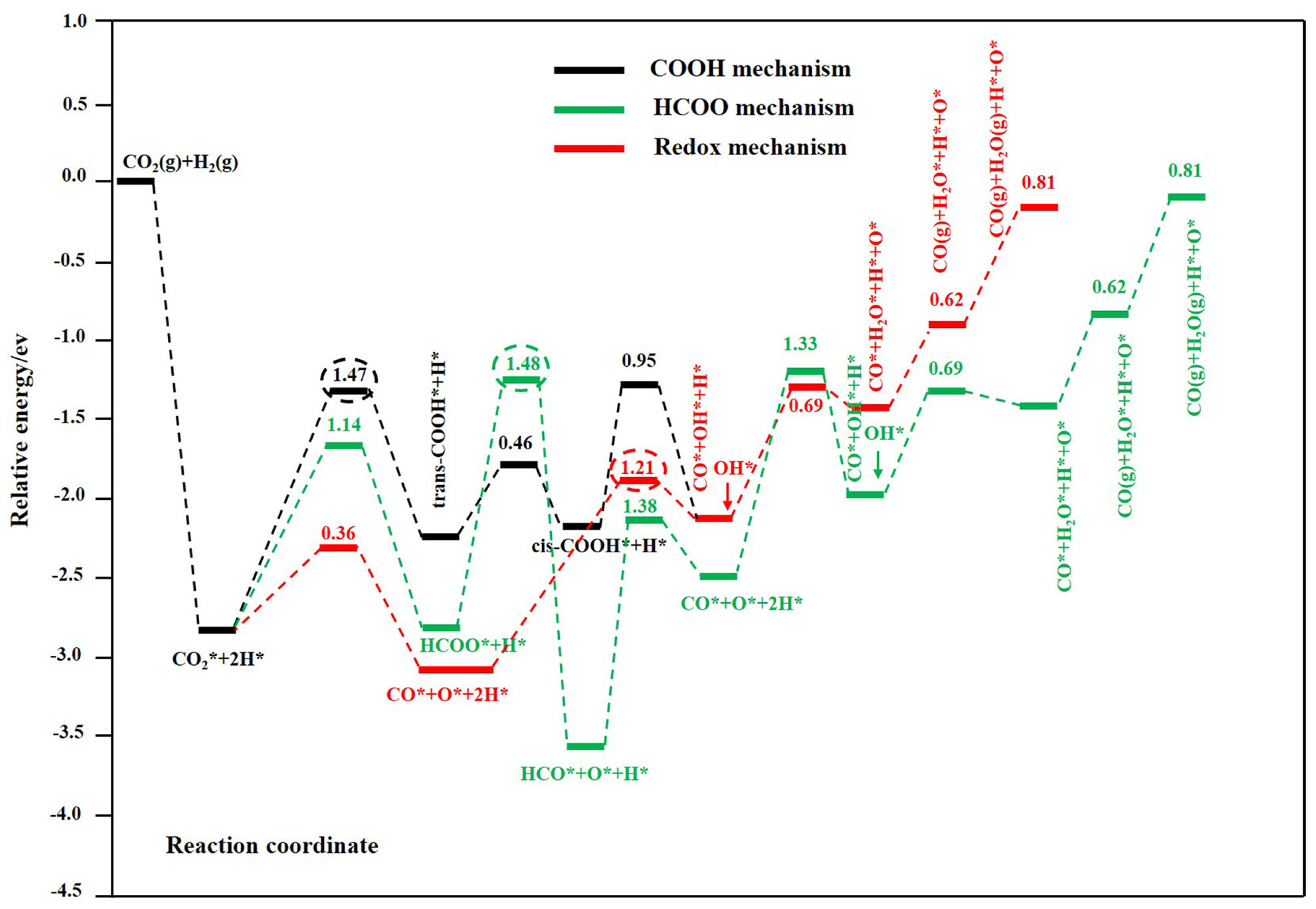
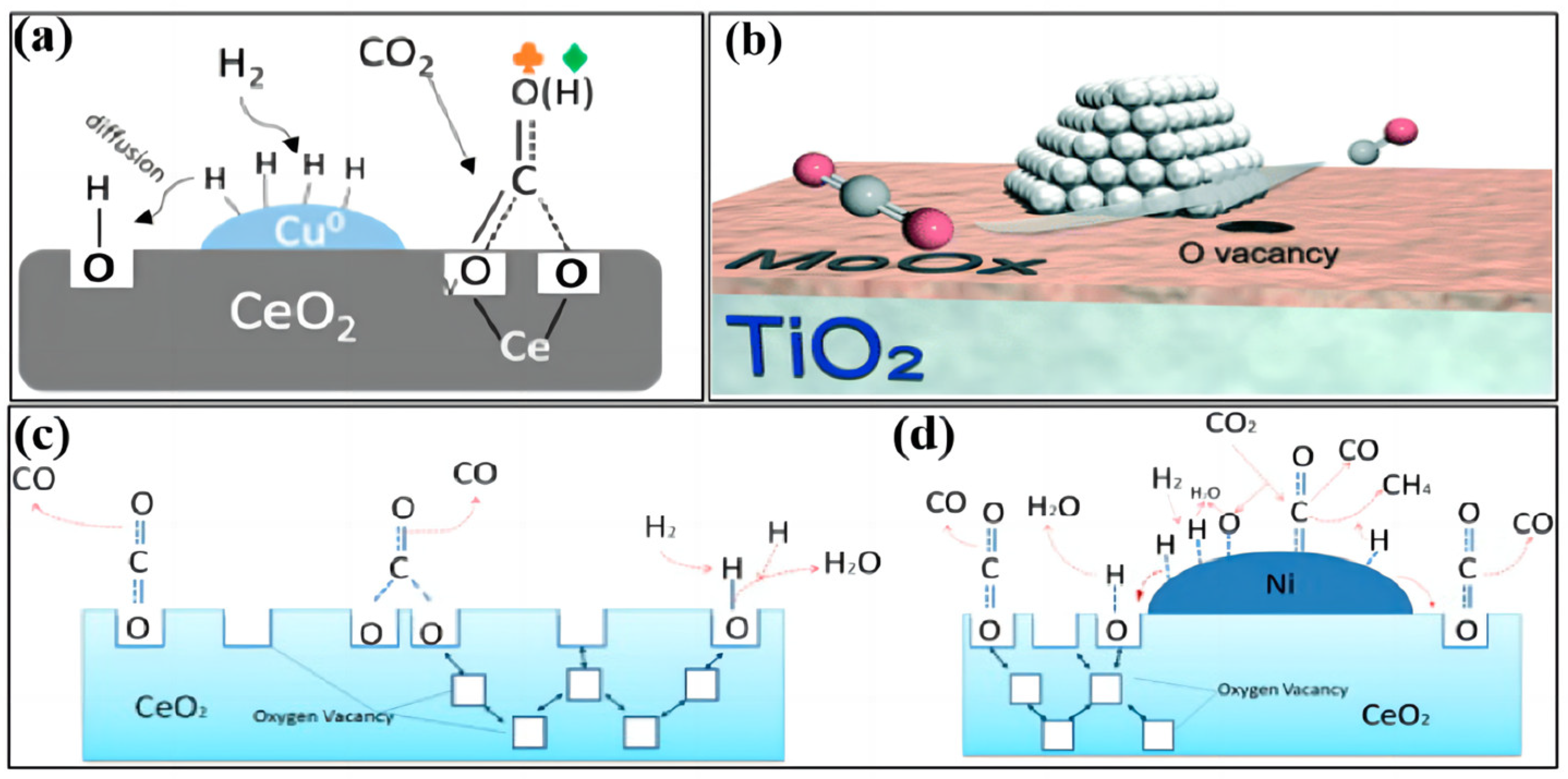
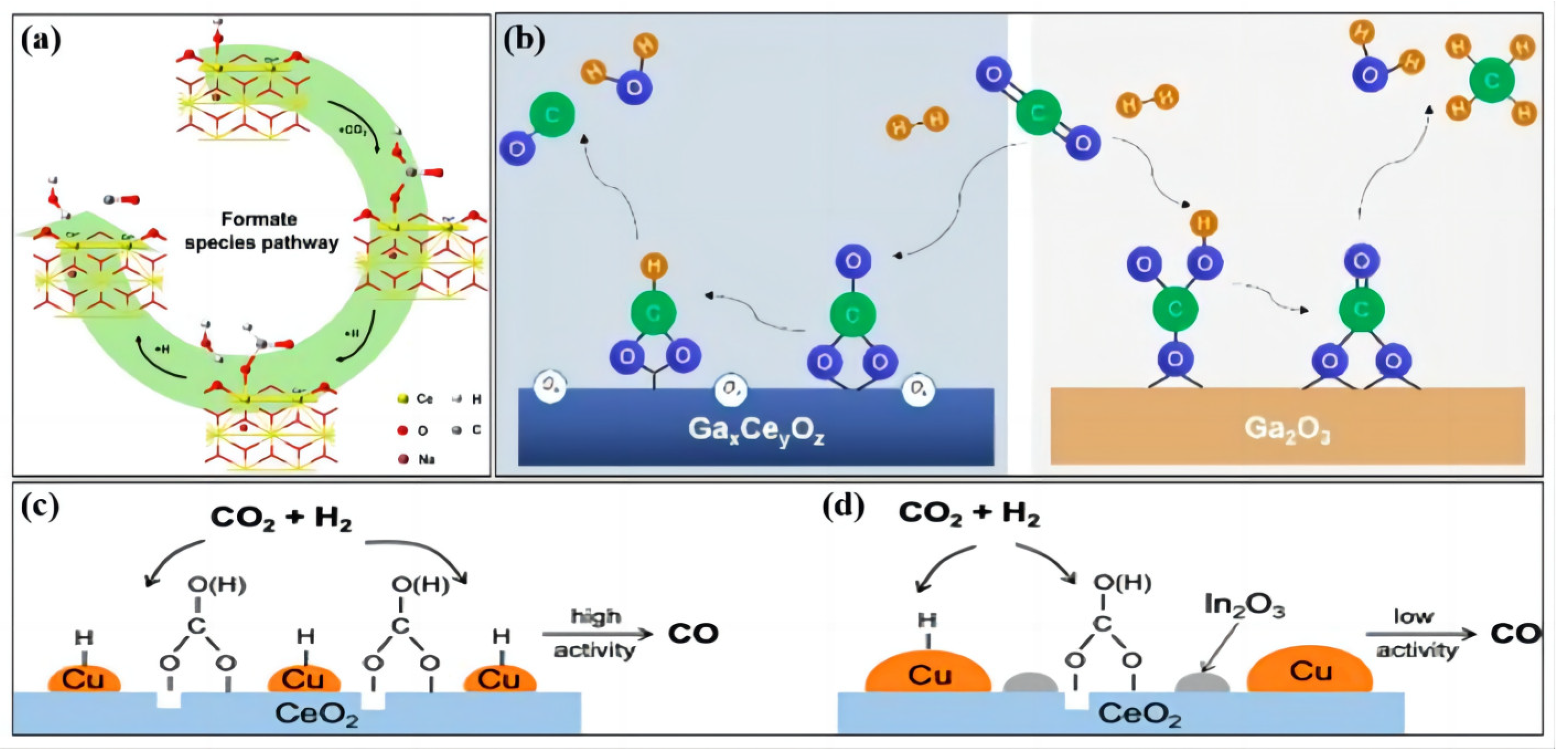
2.1. Reaction Mechanism of the Reverse Water–Gas Conversion Reaction
- (1)
- Redox mechanism
- (2)
- Decomposition mechanism of intermediate species
2.2. Overview of Catalysts of Different Systems in the Reverse Water–Gas Conversion Reaction
2.3. Precious Metal Catalysts
2.3.1. Pt–Based Catalysts
2.3.2. Pd–Based Catalysts
2.3.3. Ru−Based Catalysts
2.3.4. Au−Based Catalysts
2.3.5. Some Other Precious Metal-Based Catalysts
2.4. Non–Precious Metal Catalysts
2.4.1. Ni–Based Catalysts
2.4.2. Fe−Based Catalysts
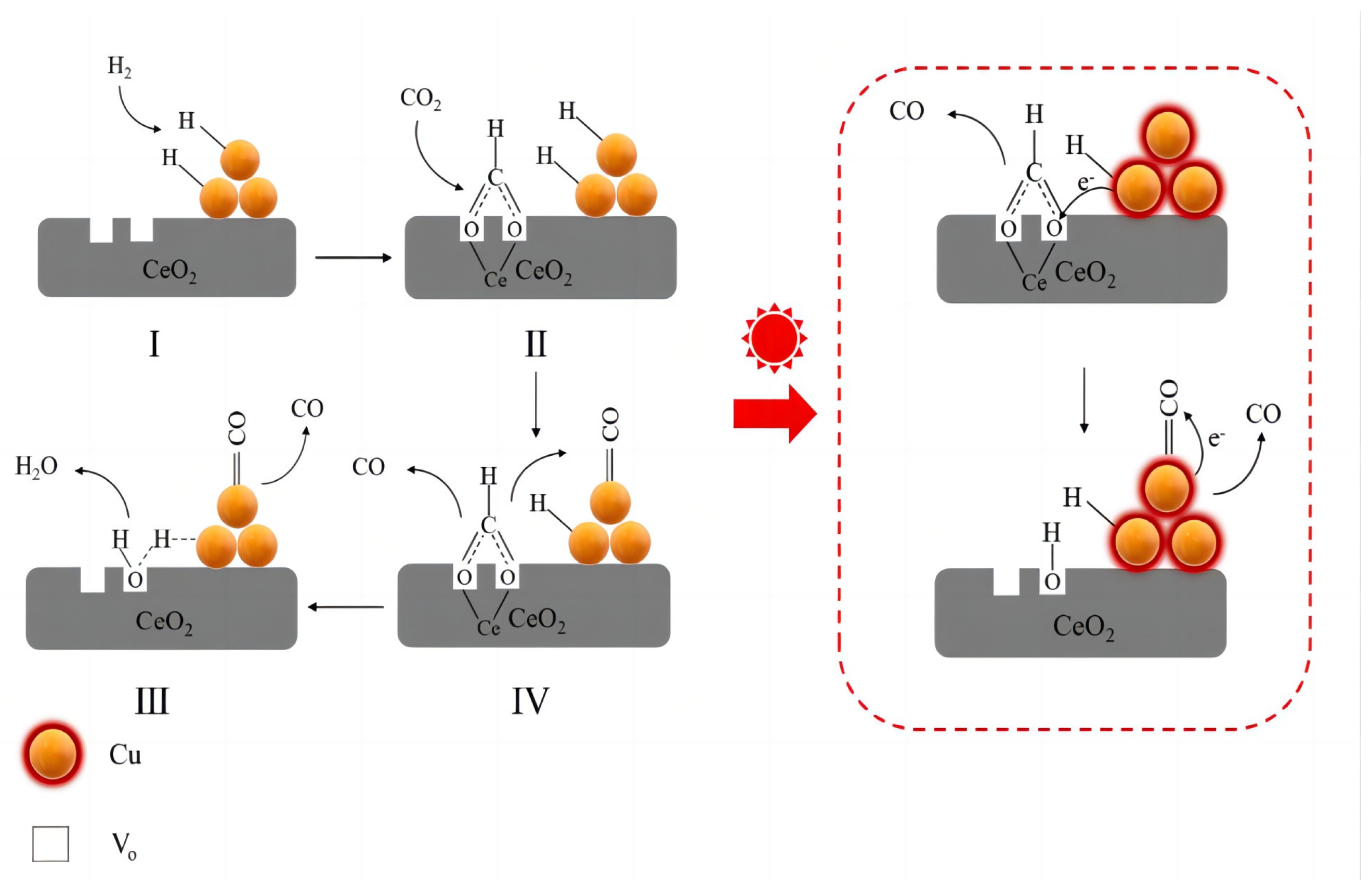
2.4.3. Cu-Based Catalysts
2.4.4. Mo-Based Catalysts
2.5. Other Catalytic Systems
2.5.1. Transition Metal Carbide Catalysts
2.5.2. Perovskite–Type Catalysts
2.6. Catalyst Deactivation in the Reverse Water–Gas Conversion Reaction
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tawalbeh, M.; Javed, R.M.N.; Al-Othman, A.; Almomani, F. The novel contribution of non-noble metal catalysts for intensified carbon dioxide hydrogenation. Energy Convers. Manag. 2023, 279, 116755. [Google Scholar] [CrossRef]
- Yao, X.; Zhong, P.; Zhang, X.; Zhu, L. Business model design for the carbon capture utilization and storage (CCUS) project in China. Energy Policy 2018, 121, 519. [Google Scholar] [CrossRef]
- Tapia, J.F.D.; Lee, J.; Ooi, R.E.H.; Foo, D.C.Y.; Tan, R.R. A review of optimization and decision-making models for the planning of CO2 capture, utilization and storage (CCUS) systems. Sustain. Prod. Consump. 2018, 13, 1. [Google Scholar] [CrossRef]
- Adelung, S.; Dietrich, R.U. Impact of the reverse water gas shift operating conditions on the Power-to-Liquid fuel production cost. Fuel 2022, 317, 123440. [Google Scholar] [CrossRef]
- Atsbha, T.A.; Yoon, T.; Seongho, P.; Lee, C. A review on the catalytic conversion of CO2 using H2 for synthesis of CO, methanol, and hydrocarbons. J. CO2 Util. 2021, 44, 101413. [Google Scholar] [CrossRef]
- Lima, D.D.S.; Dias, Y.R.; Pere z-Lopez, O.W. CO2 methanation over Ni-Al and Co-Al LDH-derived catalysts: The role of basicity. Sustain. Energy Fuels 2020, 4, 5747. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Wiese, K.; Parlinska-Wojtan, M.; Rabeah, J.; Behma, A.; Brückner, J.R. Encapsulation of Ru nanoparticles: Modifying the reactivity toward CO and CO2 methanation on highly active Ru/TiO2 catalysts. Appl. Catal. B-Environ. 2020, 270, 118846. [Google Scholar] [CrossRef]
- Dai, H.; Xiong, S.; Zhu, Y.; Zheng, J.; Huang, L.; Zhou, C.; Deng, J.; Zhang, X. NiCe bimetallic nanoparticles embedded in hexagonal mesoporous silica (HMS) for reverse water gas shift reaction. Chin. Chem. Lett. 2022, 33, 2590. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, A.; Xiong, S.; Xiao, X.; Zhou, C.; Pan, Y. The Catalytic Performance of Ga2O3-CeO2 Composite Oxides over Reverse Water Gas Shift Reaction. ChemCatChem 2022, 14, e202200049. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, R.; Waterhouse, G.I.N.; Zhang, T. Selective photothermal CO2 reduction to CO, CH4, alkanes, alkenes over bimetallic alloy catalysts derived from layered double hydroxide nanosheets. Nano Energy 2022, 102, 107650. [Google Scholar] [CrossRef]
- Xie, B.; Lovell, E.; Tan, T.H.; Jantarang, S.; Yu, M.; Scott, J.; Amal, R. Emerging material engineering strategies for amplifying photothermal heterogeneous CO2 catalysis. J. Energy Chem. 2021, 59, 108. [Google Scholar] [CrossRef]
- Zeng, X.; Tu, Z.; Yuan, L.; Xiao, C.; Wen, Y.; Xiong, K. Two-Dimensional transition metal hexaaminobenzene monolayer single atom catalyst for electrocatalytic Carbon Dioxide Reduction. Nanomaterials 2022, 12, 4005. [Google Scholar] [CrossRef]
- Xue, R.; Ge, P.; Xie, J.; Hu, Z.Y.; Wang, X.K.; Li, P.Q. Controllable CO2 reduction or hydrocarbon oxidation driven by entire solar via silver quantum dots direct photocatalysis. Small 2023, 19, 2207234. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yan, B.H.; Chen, J.G.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Portillo, E.; Gandara, J.; Reina, T.R.; Pastor-Perez, L. Is the RWGS a viable route for CO2 conversion to added value products? A techno-economic study to understand the optimal RWGS conditions. Sci. Total Environ. 2023, 857, 159394. [Google Scholar] [CrossRef]
- Alvarez-Galvan, C.; Lustemberg, P.G.; Oropeza, F.E.; Bashiller-Baeza, B.; Ospina, M.D.; Herranz, M.; Cebollada, J.; Collado, L.; Campos-Martin, J.M.; Pena-O’Shea, V.A.; et al. Highly active and stable Ni/La-doped ceria material for catalytic CO2 reduction by reverse water gas shift reaction. ACS Appl. Mater. Inter. 2022, 14, 50739. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, A.; Irankhah, A.; Aghamiri, S.F. Reverse water gas shift reaction and CO2 mitigation: Nanocrystalline MgO as a support for nickel based catalysts. J. Environ. Chem. Eng. 2018, 6, 4945. [Google Scholar] [CrossRef]
- Petersen, E.M.; Rao, R.G.; Vance, B.C.; Tessonnier, J.P. SiO2/SiC supports with tailored thermal conductivity to reveal the effect of surface temperature on Ru-catalyzed CO2 methanation. Appl. Catal. B-Environ. 2021, 286, 119904. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Zhang, S.; An, K.; Ma, Z.; Wang, L.; Liu, Y. Cerium modified Ni-La2O3/ZrO2 for CO2 methanation. J. Energy Chem. 2020, 43, 155. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.Y.; Chen, X.B.; Rui, N.; Betancourt, L.E.; Lin, L.L.; Xu, W.Q.; Sun, C.J.; Abeykoon, A.M.M.; Rodriguez, J.A.; et al. Effects of Zr doping into ceria for the dry reforming of methane over Ni/CeZrO2 catalysts: In situ studies with XRD, XAFS, and AP-XPS. ACS Catal. 2020, 10, 3274. [Google Scholar] [CrossRef]
- Martin, O.; Antonio, J.M.; Cecilia, M.; Sharon, M.; Takuya, F.S.; Roland, H.; Charlotte, D.; Daniel, C.F.; Javier, P.R. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation. Angew. Chem. Int. Ed. 2016, 55, 6261. [Google Scholar] [CrossRef]
- Chen, K.H.; Li, H.R.; He, L.N. Advance and prospective on CO2 activation and transformation strategy. Chin. J. Org. Chem. 2020, 40, 2195. [Google Scholar] [CrossRef]
- Panagiotopoulou, P. Hydrogenation of CO2 over supported noble metal catalysts. Appl. Catal. A-Gen 2017, 542, 63. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kuhn, J.N. CO2 conversion by reverse water gas shift catalysis: Comparison of catalysts, mechanisms and their consequences for CO2 conversion to liquid fuels. RSC Adv. 2016, 6, 49675. [Google Scholar] [CrossRef]
- Su, X.; Yang, X.L.; Zhao, B.; Huang, Y.Q. Designing of highly selective and high-temperature endurable RWGS heterogeneous catalysts: Recent advances and the future directions. J. Energy Chem. 2017, 26, 854. [Google Scholar] [CrossRef]
- Bahmanpour, A.M.; Signorile, M.; Kröcher, O. Recent progress in syngas production via catalytic CO2 hydrogenation reaction. Appl. Catal. B-Environ. 2021, 295, 120319. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Kumar, A.; Khraisheh, M. A Review of CeO2 Supported Catalysts for CO2 Reduction to CO through the Reverse Water Gas Shift Reaction. Catalysts 2022, 12, 1101. [Google Scholar] [CrossRef]
- Pahija, E.; Panaritis, C.; Gusarov, S.; Shadbahr, J.; Bensebaa, F.; Patience, G.; Boffito, D.C. Experimental and Computational Synergistic Design of Cu and Fe Catalysts for the Reverse Water-Gas Shift: A Review. ACS Catal. 2022, 12, 6887. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.L.; Wohlrab, S. Review of CO2 Reduction on Supported Metals (Alloys) and Single-Atom Catalysts (SACs) for the Use of Green Hydrogen in Power-to-Gas Concepts. Catalysts 2022, 12, 16. [Google Scholar] [CrossRef]
- Jing, H.; Li, Q.; Wang, J.; Liu, D.; Wu, K. Theoretical Study of the Reverse Water Gas Shift Reaction on Copper Modified β-Mo2C(001) Surfaces. J. Phys. Chem. C. 2019, 123, 1235. [Google Scholar] [CrossRef]
- Lin, L.; Yao, S.; Liu, Z.; Zhang, F.; Li, N.; Vovchok, D.; Martinez-Arias, A.; Castañeda, R.; Lin, J.; Senanayake, S.D. In situ characterization of Cu/CeO2 nanocatalysts for CO2 hydrogenation: Morphological effects of nanostructured ceria on the catalytic activity. J. Phys. Chem. C. 2018, 122, 12934. [Google Scholar] [CrossRef]
- Mine, S.; Yamaguchi, T.; Ting, K.W.; Maeno, Z.; Siddiki, S.M.A.H.; Oshima, K.; Satokawa, S.; Shimizu, K.; Toyao, T. Reverse water-gas shift reaction over Pt/MoOx/TiO2: Reverse Mars-van Krevelen mechanism via redox of supported MoOx. Catal. Sci. Technol. 2021, 11, 4172. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Xu, H.; Han, Y. Reverse water-gas shift reaction over ceria nanocube synthesized by hydrothermal method. Catal. Commun. 2016, 76, 1. [Google Scholar] [CrossRef]
- Lu, B.; Zhang, T.; Zhang, L.; Xu, Y.; Zhang, Z.; Wu, F.; Li, X.; Luo, C. Promotion effects of oxygen vacancies on activity of Na-doped CeO2 catalysts for reverse water gas shift reaction. Appl. Surf. Sci. 2022, 587, 152881. [Google Scholar] [CrossRef]
- Li, M.; My Pham, T.H.; Ko, Y.; Zhao, K.; Zhong, L.; Luo, W.; Züttel, A. Support-Dependent Cu-In Bimetallic Catalysts for Tailoring the Activity of Reverse Water Gas Shift Reaction. ACS Sustain. Chem. Eng. 2022, 10, 1524. [Google Scholar] [CrossRef]
- Gines, M.J.L.; Marchi, A.J.; Apesteguía, C.R. Kinetic study of the reverse water gas shift reaction over CuO/ZnO/Al2O₃ catalysts. Appl. Catal. A-Gen. 1997, 154, 15. [Google Scholar] [CrossRef]
- Wang, L.C.; Khazaneh, M.T.; Widmann, D.; Behm, R.J. TAP reactor studies of the oxidizing capability of CO2 on a Au/CeO2 catalyst—A first step toward identifying a redox mechanism in the reverse water gas shift reaction. J. Catal. 2013, 302, 20. [Google Scholar] [CrossRef]
- Liu, H.X.; Li, S.Q.; Wang, W.W.; Yu, W.Z.; Zhang, W.J.; Ma, C.; Jia, C.J. Partially sintered copper-ceria as excellent catalyst for the high temperature reverse water gas shift reaction. Nat. Commun. 2022, 13, 867. [Google Scholar] [CrossRef]
- Deng, L.; Su, Q.Q.; Ye, Q.; Wan, H.; He, Y.; Cui, X. Slag-based geopolymer microsphere-supported Cu: A low cost and sustainable catalyst for CO2 hydrogenation. Sustain. Energy Fuels 2022, 6, 1436. [Google Scholar] [CrossRef]
- Matsubu, J.C.; Zhang, S.; Derita, L.; Marinkovic, N.S.; Chen, J.G.; Graham, G.W.; Pan, X.; Christopher, P. Adsorbate mediated strong metal support interactions in oxide supported Rh catalysts. Nat. Chem. 2017, 9, 120. [Google Scholar] [CrossRef]
- Li, W.; Gan, J.; Liu, Y.; Zou, Y.; Zhang, S.; Qu, Y. Platinum and Frustrated Lewis Pairs on Ceria as Dual-Active Sites for Efficient Reverse Water-Gas Shift Reaction at Low Temperatures. Angew. Chem. Int. Ed. 2023, 62, e202305661. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Su, X.; Liang, B.; Yang, X.; Ren, X.; Duan, H.; Huang, Y.; Zhang, T. Identification of relevant active sites and a mechanism study for reverse water gas shift reaction over Pt/CeO2 catalysts. J. Energy Chem. 2016, 25, 1051. [Google Scholar] [CrossRef]
- Liu, Y.X.; Li, L.W.; Zhang, R.Y.; Guo, Y.H.; Wang, H.; Ge, Q.F.; Zhu, X.L. Synergetic enhancement of activity and selectivity for reverse water gas shift reaction on Pt-Re/SiO2 catalysts. J. CO2 Util. 2022, 63, 102128. [Google Scholar] [CrossRef]
- Bhalothia, D.; Yang, S.S.; Yan, C.; Beniwal, A.; Chang, Y.X.; Wu, S.C.; Chen, P.C.; Wang, K.W.; Chen, T.Y. Surface anchored atomic cobalt-oxide species coupled with oxygen vacancies boost the CO-production yield of Pd nanoparticles. Sustain. Energy Fuels 2023, 7, 526. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.; Wiese, K.; Hauble, A.; Bansmann, J.; Rabeah, J.; Parlinska-Wojtan, M.; Bruckner, A.; Behm, R. Steering the selectivity in CO2 reduction on highly active Ru/TiO2 catalysts: Support particle size effects. J. Catal. 2021, 401, 160. [Google Scholar] [CrossRef]
- Vikanova, K.V.; Kustov, A.L.; Makhov, E.A.; Tkachenko, O.P.; Kapustin, G.I.; Kalmykov, K.B.; Mishin, I.V.; Nissenbaum, V.D.; Dunaev, S.F.; Kustov, L.M. Rhenium-contained catalysts based on superacid ZrO2 supports for CO2 utilization. Fuel 2023, 351, 128956. [Google Scholar] [CrossRef]
- Rabee, A.; Zhao, D.; Cisneros, S.; Kreyenschulte, C.R.; Kondratenko, V.; Bartling, S.; Kubis, C.; Kondratenko, E.i.V.; Brückner, A.; Rabeah, J. Role of interfacial oxygen vacancies in low loaded Au-based catalysts for the low temperature reverse water gas shift reaction. Appl. Catal. B-Env. 2023, 321, 122083. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zang, Y.H.; Gao, F.; Qu, J.F.; Gu, J.F.; Lin, X.T. Enhanced catalytic activity of CO2 hydrogenation to CO over sulfur-containing Ni/ZrO2 catalysts: Support size effect. New J. Chem. 2022, 46, 22332. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, K.; Song, Y.; Lee, Y.; Roh, H.; Na, K. Highly CO-selective Ni-MgO-CexZr1-xO2 catalyst for efficient low-temperature reverse water-gas shift reaction. J. Ind. Eng. Chem. 2023, 118, 341. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, S.W.; Yoon, H.S.; Kim, Y.D. Reverse water gas shift reaction catalyzed by Fe nanoparticles with high catalytic activity and stability. J. Ind. Eng. Chem. 2015, 23, 67. [Google Scholar] [CrossRef]
- Zhuang, Y.; Currie, R.; McAuley Kimberley, B.; Simakov, D.S.A. Highly-selective CO2 conversion via reverse water gas shift reaction over the 0.5wt% Ru-promoted Cu/ZnO/Al2O3 catalyst. Appl. Catal. A-Gen. 2019, 575, 74. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Kumar, A.; Khraisheh, M. Combustion synthesis of copper ceria solid solution for CO2 conversion to CO via reverse water gas shift reaction. Int. J. Hydrogen Energy 2022, 47, 41259. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, J.; Sun, X.; Sun, J. Engineering nanointerfaces of Cu-based catalysts for balancing activity and stability of reverse water gas shift reaction. J. CO2 Util. 2023, 71, 102460. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, A.; Zhu, M.; Wu, X.X.; Wang, H.; Zhu, X.L.; Ge, Q.F. Tuning reverse water gas shift and methanation reactions during CO2 reduction on Ni catalysts via surface modification by MoOx. J. CO2 Util. 2021, 52, 101678. [Google Scholar] [CrossRef]
- Okemoto, A.; Harada, M.R.; Ishizaka, T.; Hiyoshi, N.; Soto, K. Catalytic performance of MoO3/FAU zeolite catalysts modified by Cu for reverse water gas shift reaction. Appl. Catal. A Gen. 2020, 592, 117415. [Google Scholar] [CrossRef]
- Ronda-lloret, M.; Yang, L.Q.Q.; Hammerton, M.; Marakatti, V.S.; Tromp, M.; Sofer, Z.; Sepulveda-Escribano, A.; Ramos-Fernandez, E.V.; Delgado, J.J.; Rothenberg, G.; et al. Molybdenum oxide supported on Ti3AlC2 is an active reverse water gas shift catalyst. ACS Sustain. Chem. Eng. 2021, 9, 4957. [Google Scholar]
- Kharaji, A.G.; Shariati, A.; Takassi, M. A novel γ-Alumina supported Fe-Mo bimetallic catalyst for reverse water gas shift reaction. Chin. J. Chem. Eng. 2013, 21, 1007. [Google Scholar] [CrossRef]
- Yang, L.; Pastor-perez, L.; Gu, S.; Sepúlveda-Escribano, A.; Reina, T.R. Highly efficient Ni/CeO2-Al2O₃ catalysts for CO2 upgrading via reverse water gas shift: Effect of selected transition metal promoters. Appl. Catal. B-Environ. 2018, 232, 464. [Google Scholar] [CrossRef]
- Goguet, A.; Meunier, F.; Breen, J.; Burch, R.; Petch, M.; Faurghenciu, A. Study of the origin of the deactivation of a Pt/CeO2 catalyst during reverse water gas shift (RWGS) reaction. J. Catal. 2004, 226, 382. [Google Scholar] [CrossRef]
- Lymperi, A.; Chatzilias, C.; Xydas, F.; Martino, E.; Kyriakou, G.; Katsaounis, A. Electrochemical Promotion of CO2 Hydrogenation Using a Pt/YSZ Fuel Cell Type Reactor. Nanomaterials 2023, 13, 1930. [Google Scholar] [CrossRef]
- Seuser, G.; Martinelli, M.; Garcia, E.S.; Upton, G.F.; Ayala, M.; Villarreal, J.; Rajabi, Z.; Cronauer, D.C.; Kropf, A.J.; Jacobs, G. Reverse water-gas shift: Na doping of m-ZrO2 supported Pt for selectivity control. Appl. Catal. A-Gen. 2023, 650, 119000. [Google Scholar] [CrossRef]
- Ge, H.; Kuwahara, Y.; Kusu, K.; Kobayashi, H.; Yamashita, H. Enhanced visible-NIR absorption and oxygen vacancy generation of Pt/HxMoWOy by H-spillover to facilitate photothermal catalytic CO2 hydrogenation. J. Mater. Chem. A 2022, 10, 10854. [Google Scholar] [CrossRef]
- He, Y.L.; Huang, D.H. Single-Atom Platinum Catalyst for Efficient CO2 Conversion via Reverse Water Gas Shift Reaction. Molecules 2023, 28, 6630. [Google Scholar] [CrossRef]
- Nelson, N.C.; Chen, L.X.; Meira, D.; Kovarik, L.; Szanyi, J. In situ dispersion of palladium on TiO2 during reverse water gas shift reaction: Formation of atomically dispersed palladium. Angew. Chem. Int. Ed. 2020, 59, 17657. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Miyakea, T.; Sugimasaa, M. Low-temperature RWGS enhancement of Pt1-nAun/CeO2 catalysts and their electronic state. RSC Adv. 2023, 13, 29320. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Qi, R.; Zhang, Y.; Gu, Q.; Xu, X.; Tan, Y.; Liu, X.; Wang, A.; Zhu, B.; Yang, B.; et al. Single-atom-driven dynamic carburization over Pd1-FeOx catalyst boosting CO2 conversion. Chem 2022, 8, 3252. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, T.; Tang, Y.; Jiang, Y.; Kitagawa, H.; Wen, X.; Wang, F. Size-modulated photo-thermal catalytic CO2 hydrogenation performances over Pd nanoparticles. J. Catal. 2023, 424, 22. [Google Scholar] [CrossRef]
- Chen, L.; Allec, S.I.; Nguyen, M.T.; Kovarik, L.; Hoffman, A.S.; Hong, J.; Meira, D.; Shi, H.; Bare, S.R.; Glezakou, V.A.; et al. Dynamic Evolution of Palladium Single Atoms on Anatase Titania Support Determines the Reverse Water-Gas Shift Activity. J. Am. Chem. Soc. 2023, 145, 10847. [Google Scholar] [CrossRef]
- Ni, W.; Zeng, M.; Wang, K.; Lin, Y.; Zhang, Z.; Dai, W.; Fu, X. Photo-thermal catalytic reverse water gas shift reaction over Pd/MaZrOx (M=Sr, SrMn) catalysts driven by Cycle-double sites. J. CO2 Util. 2023, 69, 102413. [Google Scholar] [CrossRef]
- Saravanan, P.K.; Bhalothia, D.; Huang, G.H.; Beniwal, A.; Cheng, M.; Chao, Y.C.; Lin, M.W.; Chen, P.C.; Chen, T.Y. Sub-Millisecond Laser-Irradiation-Mediated Surface Restructure Boosts the CO Production Yield of Cobalt Oxide Supported Pd Nanoparticles. Nanomaterials 2023, 13, 1801. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Wang, K.; Wang, H.; Liu, L.; Wu, X.; Geng, B.; Chu, X.; Song, S.; Zhang, H. Synergism of Ultrasmall Pt Clusters and Basic La2O2CO3 Supports Boosts the Reverse Water Gas Reaction Efficiency. Adv. Energy Mater. 2023, 13, 2203806. [Google Scholar] [CrossRef]
- Tang, R.; Zhu, Z.; Li, C.; Xiao, M.Q.; Wu, Z.Y.; Zhang, D.K.; Zhang, C.C.; Xiao, Y.; Chu, M.Y.; Genest, A.; et al. Ru-catalyzed reverse water gas shift reaction with near unity selectivity and superior stability. ACS Mater. Lett. 2021, 3, 1652. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Shen, J.; Li, C.; Zhang, C.; Feng, K.; Wang, Z.; Wang, X.; Meira, D.M.; Cai, M.; Zhang, D.; et al. Mo2TiC2 MXene-Supported Ru Clusters for Efficient Photothermal Reverse Water-Gas Shift. ACS Nano. 2023, 17, 1550. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Filot, I.A.W.; Hensen, E.J.M. Elucidation of the Reverse Water-Gas Shift Reaction Mechanism over an Isolated Ru Atom on CeO2(111). J. Phys. Chem. C. 2023, 127, 20314. [Google Scholar] [CrossRef]
- Kim, G.; Shin, S.; Choi, Y.; Kim, J.; Kim, G.; Kim, K.; Lee, H. Gas-Permeable Iron-Doped Ceria Shell on Rh Nanoparticles with High Activity and Durability. JACS Au. 2022, 2, 1115. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shao, S.; Liu, Y.; Cao, M.; Yu, J.; Lau, C.H.; Zheng, Y.; Fan, X. DRIFTS-SSITKA-MS investigations on the mechanism of plasmon preferentially enhanced CO2 hydrogenation over Au/γ-Al2O3. Appl. Catal. B-Environ. 2023, 328, 122531. [Google Scholar] [CrossRef]
- Kang, X.; Yuan, D.; Yi, Z.; Yu, C.; Yuan, X.; Liang, B.; Sun, X.; Gao, L.; Wang, S.; Li, Y. Bismuth single atom supported CeO2 nanosheets for oxidation resistant photothermal reverse water gas shift reaction. Catal. Sci. Technol. 2022, 12, 5559. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Y.; Gao, B.; Zhang, L.; Guo, L. Size-Dependent Active Site and Its Catalytic Mechanism for CO2 Hydrogenation Reactivity and Selectivity over Re/TiO2. ACS Catal. 2023, 13, 10364. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Mou, L.; Liu, Q.; Li, Z.; Li, X.; He, S. Reverse water-gas shift reaction catalyzed by diatomic rhodium anions. Phys. Chem. Chem. Phys. 2022, 24, 14616. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, S.; Zhou, H.; Huang, C.; Xia, L.; Liu, X.; Luo, H.; Wang, H. Ir Single Atoms and Clusters Supported on α-MoC as Catalysts for Efficient Hydrogenation of CO2 to CO. Acta Phys-Chim. Sin. 2023, 39, 230202. [Google Scholar]
- Mhamane, N.B.; Chetry, S.; Ranjan, R.; Raja, T.; Gopinath, C.S. Sustainable CO2 Reduction on In2O3 with Exclusive CO Selectivity: Catalysis and In Situ Valence Band Photoelectron Spectral Investigations. ACS Sustain. Chem. Eng. 2022, 10, 3521. [Google Scholar] [CrossRef]
- Gong, J.; Chu, M.Y.; Guan, W.H.; Liu, Y.; Zhong, Q.X.; Cao, M.H.; Xu, Y. Regulating the interfacial synergy of Ni/Ga2O₃ for CO2 hydrogenation toward the reverse water gas shift reaction. Ind. Eng. Chem. Res. 2021, 60, 9448. [Google Scholar] [CrossRef]
- Zhang, Q.; Pastor-P´erez, L.; Villora-Pico, J.J.; Joyce, M.; Sepúlveda-Escribano, A.; Duyar, M.S.; Reina, T.R. Ni-Phosphide catalysts as versatile systems for gas-phase CO2 conversion: Impact of the support and evidences of structure-sensitivity. Fuel 2022, 323, 124301. [Google Scholar] [CrossRef]
- Shen, H.; Dong, Y.; Yang, S.; He, Y.; Wang, Q.; Cao, Y.; Wang, W.; Wang, T.; Zhang, Q.; Zhang, H. Identifying the roles of Ce3+-OH and Ce-H in the reverse water-gas shift reaction over highly active Ni-doped CeO2 catalyst. Nano Res. 2022, 15, 5831. [Google Scholar] [CrossRef]
- Ranjbar, A.; Aghamiri, S.F.; Irankhah, A. Effect of MgO/Al2O3 ratio in the support of mesoporous Ni/MgO-Al2O3 catalysts for CO2 utilization via reverse water gas shift reaction. Int. J. Hydrogen Energy 2023, 48, 19115. [Google Scholar] [CrossRef]
- Zagoraios, D.; Kokkinou, N.; Kyriakou, G.; Katsaounis, A. Electrochemical control of the RWGS reaction over Ni nanoparticles deposited on yttria stabilized zirconia. Catal. Sci. Technol. 2022, 12, 1869. [Google Scholar] [CrossRef]
- Lozano-Reis, P.; Prats, H.; Sayós, R.; Illas, F. Limitations of free energy diagrams to predict the catalytic activity: The reverse water gas shift reaction catalyzed by Ni/TiC. J. Catal. 2023, 425, 203. [Google Scholar] [CrossRef]
- Rutherford, B.; Panaritis, C.; Pahija, E.; Couillard, M.; Patarachao, B.; Shadbahr, J.; Bensebaa, F.; Patience, G.S.; Boffito, D.C. Ni nanoparticles on Co3O4 catalyze the reverse water-gas shift with 95% CO selectivity at 300 °C. Fuel 2023, 348, 128235. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, Z.; Wu, X.; Xiong, W.; Ding, J.; Zhuang, Z.; Huang, W. Ni Single Atoms Confined in Nitrogen-Doped Carbon Nanotubes for Active and Selective Hydrogenation of CO2 to CO. ACS Catal. 2023, 13, 7132. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Huang, C.; Chen, C.; Choojun, K.; Sooknoi, T.; Tian, H.; Lin, Y. Reversal of methanation-oriented to RWGS-oriented Ni/SiO2 catalysts by the exsolution of Ni2+ confined in silicalite-1. Green Chem. 2023, 25, 7582. [Google Scholar] [CrossRef]
- Wei, A.; Zhang, R.; Qin, Y.; Wang, H.; Zhu, X.; Ge, Q. Theoretical Insight into Tuning CO2 Methanation and Reverse Water Gas Shift Reactions on MoOx-Modified Ni Catalysts. J. Phys. Chem. C. 2022, 126, 18078. [Google Scholar] [CrossRef]
- Arpini, B.H.; Braga, A.H.; Borges, L.R.; Vidinha, P.; Gonçalves, R.V.; Szanyi, J.; Rossi, L.M. Tuning CO2 Hydrogenation Selectivity by N-Doped Carbon Coating over Nickel Nanoparticles Supported on SiO2. ACS Sustain. Chem. Eng. 2022, 10, 2331. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Chen, L.; Li, Y. Breaking the Linear Scaling Relationship of the Reverse Water-Gas-Shift Reaction via Construction of Dual-Atom Pt-Ni Pairs. ACS Catal. 2023, 13, 3735. [Google Scholar] [CrossRef]
- Błaszczak, P.; Zając, M.; Ducka, A.; Matlak, K.; Wolanin, B.; Wang, S.; Mandziak, A.; Bochentyn, B.; Jasiński, P. High-temperature Co-electrolysis of CO2/H2O and direct methanation over Co-impregnated SOEC. Bimetallic synergy between Co and Ni. Int. J. Hydrogen Energy 2022, 47, 35017. [Google Scholar] [CrossRef]
- Merkouri, L.; Saché, E.; Pastor-Pérez, L.; Duyar, M.S.; Reina, T.R. Versatile Ni-Ru catalysts for gas phase CO2 conversion: Bringing closer dry reforming, reverse water gas shift and methanation to enable end-products flexibility. Fuel 2022, 315, 123097. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, R.; Liu, Y.; Wu, X.; Wang, H.; Ge, Q.; Zhu, X. Blocking Methanation during Reverse Water Gas Shift Reaction on Ni/SiO2 Catalysts by Surface Ag. ChemCatChem 2022, 15, e202201284. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Li, J.; Wang, Z. In-Ni Intermetallic Compounds Derived from Layered Double Hydroxides as Efficient Catalysts toward the Reverse Water Gas Shift Reaction. ACS Catal. 2022, 12, 4026. [Google Scholar] [CrossRef]
- Cai, W.; Yin, J.; Hu, C.; Han, H.; Ma, J.; Cao, Y.; Zhao, Y. Fe-Co-Ni Trimetallic Catalysts with MOFs as Precursor for CO2 Hydrogenation to C2-C4 Hydrocarbons: Insight Into the Influence of Ni. Catal Lett. 2023, 153, 2718. [Google Scholar] [CrossRef]
- Bogdan, V.I.; Koklin, A.E.; Kustov, A.L.; Pokusaeva, Y.A.; Bogdan, T.V.; Kustov, L.M. Carbon Dioxide Reduction with Hydrogen on Fe, Co Supported Alumina and Carbon Catalysts under Supercritical Conditions. Molecules 2021, 26, 2883. [Google Scholar] [CrossRef]
- Zhang, Q.; Pastor-perez, L.; Wang, Q.; Reina, T.R. Conversion of CO2 to added value products via rwgs using Fe-promoted catalysts: Carbide, metallic Fe or a mixture. Energy Chem. 2022, 66, 635. [Google Scholar] [CrossRef]
- Watanabe, R.; Karasawa, F.; Yokoyama, C.; Oshima, K.; Kishida, M.; Hori, M.; Ono, Y.; Satokawa, S.; Verma, P.; Fukuhara, C. Highly stable Fe/CeO2 catalyst for the reverse water gas shift reaction in the presence of H2S. RSC Adv. 2023, 13, 11525. [Google Scholar] [CrossRef]
- Xu, M.; Cao, C.; Xu, J. Understanding kinetically interplaying reverse water-gas shift and Fischer-Tropsch synthesis during CO2 hydrogenation over Fe-based catalysts. Appl. Catal. A-Gen. 2022, 641, 118682. [Google Scholar] [CrossRef]
- Malhi, H.S.; Sun, C.; Zhang, Z.; Liu, Y.; Liu, W.; Ren, P.; Tu, W.; Han, Y. Catalytic consequences of the decoration of sodium and zinc atoms during CO2 hydrogenation to olefins over iron-based catalyst. Catal. Today 2022, 387, 28. [Google Scholar] [CrossRef]
- Fedorov, A.; Lund, H.; Kondratenko, V.A.; Kondratenko, E.V.; Linke, D. Elucidating reaction pathways occurring in CO2 hydrogenation over Fe-based catalysts. Appl. Catal. B-Environ. 2023, 328, 122505. [Google Scholar] [CrossRef]
- Panek, B.; Kierzkowska-Pawlak, H.; Uznański, P.; Nagy, S.; Nagy-Trembošová, V.; Tyczkowski, J. The Role of Carbon Nanotube Deposit in Catalytic Activity of FeOX-Based PECVD Thin Films Tested in RWGS Reaction. Catalysts 2023, 13, 1302. [Google Scholar] [CrossRef]
- Ai, X.; Xie, H.; Chen, S.; Zhang, G.; Xu, B.; Zhou, G. Highly dispersed mesoporous Cu/γ-Al2O3 catalyst for RWGS reaction. Int. J. Hydrogen Energy 2022, 47, 14884. [Google Scholar] [CrossRef]
- Liu, C.; Nauert, S.L.; Alsina, M.A.; Wang, D.; Grant, A.; He, K.; Weitz, E.; Nolan, M.; Gray, K.A.; Notestein, J.M. Role of surface reconstruction on Cu/TiO2 nanotubes for CO2 conversion. Appl. Catal. B Env. 2019, 255, 117754. [Google Scholar] [CrossRef]
- Gonzalez-arias, J.; Gonzalez-Castano, M.; Sanchez, M.E.; Cara-Jimenez, J.; Arellano-Garcia, H. Valorization of biomass-derived CO2 residues with Cu-MnOx catalysts for RWGS reaction. Renew. Energy 2022, 182, 443. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, M.; Wang, K.; Yue, X.; Chen, X.; Dai, W.; Fu, X. Visible light-assisted thermal catalytic reverse water gas reaction over Cu-CeO2: The synergistic of hot electrons and oxygen vacancies induced by LSPR effect. Fuel 2022, 315, 123186. [Google Scholar] [CrossRef]
- Dehghanpoor, S.; Sedaghat, M.H.; Bakhtyari, A.; Makarem, M.A.; Rahimpour, M.R. A Feasibility Study of the Conversion of Petrochemical Off-Gas Streams to Methanol Over CuO/ZnO/Al2O3 Catalyst. Top. Catal. 2022. [Google Scholar] [CrossRef]
- Xiong, W.; Wu, Z.; Chen, X.; Ding, J.; Ye, A.; Zhang, W.; Huang, W. Active copper structures in ZnO-Cu interfacial catalysis: CO2 hydrogenation to methanol and reverse water-gas shift reactions. Sci. China Chem. 2023. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Kumar, A.; Khraisheh, M. Combustion synthesis of lanthanum oxide supported Cu, Ni, and CuNi nanoparticles for CO2 conversion reaction. Int. J. Hydrogen Energy 2023, 48, 24580. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, L.; Pan, W.B.; Shen, Y.Y.; Li, H. Composition effect in CuZr nanoparticles for CO2 conversion to CH3OH. Mater. Chem. Phys. 2022, 283, 125994. [Google Scholar] [CrossRef]
- Hu, R.; Wang, T.; Li, H.; Zhu, Y.; Wang, Y.; Wen, F.; Xing, E.; Wu, Y.; Da, Z. Cu and Cu-Fe Bi-Metal Nanoparticles Encapsulated in Hollow S-1 Zeolite for Reverse Water Gas Shift Reaction. Catalysts 2023, 13, 1037. [Google Scholar] [CrossRef]
- Gao, Y.; Xiong, K.; Zhu, B. Design of Cu/MoOx for CO2 Reduction via Reverse Water Gas Shift Reaction. Catalysts 2023, 13, 684. [Google Scholar] [CrossRef]
- Tarifa, P.; González-Castaño, M.; Cazaña, F.; Monzón, A.; Arellano-García, H. Development of one-pot Cu/cellulose derived carbon catalysts for RWGS reaction. Fuel 2022, 319, 123707. [Google Scholar] [CrossRef]
- Zuo, J.; Na, W.; Zhang, P.; Yang, X.; Wen, J.; Zheng, M.; Wang, H. Enhanced activity of CexZr1-xO2 solid solutions supported Cu-based catalysts for hydrogenation of CO2 to methanol. Mol. Catal. 2022, 526, 112357. [Google Scholar] [CrossRef]
- Hu, M.; Hu, H.; Tang, S.; Pan, Z. Enhanced CuAl2O4 Catalytic Activity via Alkalinization Treatment toward High CO2 Conversion during Reverse Water Gas Shift Reaction. Catalysts 2022, 12, 1511. [Google Scholar] [CrossRef]
- Cui, X.; Liu, Y.; Yan, W.; Xue, Y.; Mei, Y.; Li, J.; Gao, X.; Zhang, H.; Zhu, S.; Niu, Y.; et al. Enhancing methanol selectivity of commercial Cu/ZnO/Al2O3 catalyst in CO2 hydrogenation by surface silylation. Appl. Catal. B-Environ. 2023, 339, 123099. [Google Scholar] [CrossRef]
- Querido, A.R.; Goncalves, L.P.L.; Kolen’ko, Y.V.; Pereira, M.F.R.; Soares, O.S.G.P. Enhancing the performance of Cu catalysts for the reverse water-gas shift reaction using N-doped CNT-ZnO composite as support. Catal. Sci. Technol. 2023, 13, 3606. [Google Scholar] [CrossRef]
- Pahija, E.; Panaritis, C.; Rutherford, B.; Couillard, M.; Patarachao, B.; Shadbahr, J.; Bensebaa, F.; Patience, G.S.; Boffito, D.C. FeOx nanoparticle doping on Cu/Al2O3 catalysts for the reverse water gas shift. J. CO2 Util. 2022, 64, 102155. [Google Scholar] [CrossRef]
- Wang, J.; Couillard, M.; Baranova, E.A. Insight into Electrochemical Promotion of Cu/Co3O4 Catalysts for the Reverse Water Gas Shift Reaction. ChemCatChem 2023, 15, e202201514. [Google Scholar] [CrossRef]
- Álvarez-Hernández, D.; Marín-Sánchez, M.; Lobo-Andrades, L.; Azancot, L.; Bobadilla, L.F.; Ivanova, S.; Centeno, M.A. Low-temperature reverse water gas-shift reaction over highly efficient Cu-hydrotalcites: Mechanistic insights on the role of malachite phase. Catal. Today. 2023, 422, 114235. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Lu, S.; Li, J.; Zhang, H.; Su, X.; Xue, F.; Cao, B.; Fang, T. Mechanism of CO2 hydrogenation over a Zr1-Cu single-atom catalyst. New J. Chem. 2022, 11, 5043–5051. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, X.; Ke, Q. Mechanism of CO2 hydrogenation to methanol on the W-doped Rh(111) surface unveiled by first-principles calculation. Colloids Surf. A 2022, 638, 128332. [Google Scholar] [CrossRef]
- Jurado, A.; Morales-García, A.; Viñes, F.; Illas, F. Molecular Mechanism and Microkinetic Analysis of the Reverse Water Gas Shift Reaction Heterogeneously Catalyzed by the Mo2C MXene. ACS Catal. 2022, 12, 15658. [Google Scholar] [CrossRef]
- Ortner, N.; Zhao, D.; Mena, H.; Weiß, J.; Lund, H.; Bartling, S.; Wohlrab, S.; Armbruster, U.; Kondratenko, E.V. Revealing Origins of Methanol Selectivity Loss in CO2 Hydrogenation over CuZn-Containing Catalysts. ACS Catal. 2023, 13, 60. [Google Scholar] [CrossRef]
- Phey, M.L.P.; Abdullah, T.A.T.; Ali, U.F.M.; Mohamud, M.Y.; Ikram, M.; Nabgan, W. Reverse water gas shift reaction over a Cu/ZnO catalyst supported on regenerated spent bleaching earth (RSBE) in a slurry reactor: The effect of the Cu/Zn ratio on the catalytic activity. RSC Adv. 2023, 5, 3039. [Google Scholar] [CrossRef]
- Zhang, W.; Vidal-López, A.; Comas-Vives, A. Theoretical study of the catalytic performance of Fe and Cu single-atom catalysts supported on Mo2C toward the reverse water-gas shift reaction. Front. Chem. 2023, 11, 1144189. [Google Scholar] [CrossRef] [PubMed]
- Zafar, F.; Zhao, R.; Ali, M.; Park, Y.M.; Roh, H.; Gao, X.; Tian, J.; Bae, J.W. Unprecedented contributions of In2O3 promoter on ordered mesoporous Cu/Al2O3 for CO2 hydrogenation to oxygenates. Chem. Eng. J. 2022, 439, 135649. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; Fang, Z.; Zhou, H.; Zhu, J.; Li, Y.; Huang, S.; Lin, W.; Zhang, Y. Unveiling the role of adsorbed hydrogen in tuning the catalytic activity of CO2 conversion to methanol at Cu/TiC surfaces. J. CO2 Util. 2023, 72, 102515. [Google Scholar] [CrossRef]
- Chen, W.; Maugé, F.; Van Gestel, J.; Nie, H.; Li, D.; Long, X. Effect of modification of the alumina acidity on the properties of supported Mo and CoMo sulfide catalysts. J. Cat. 2013, 304, 47. [Google Scholar] [CrossRef]
- Sameer, S.; Singh, G.; Gahtori, J.; Goyal, R.; Ghosh, I.K.; Barrabes, N.; Bordoloi, A. Molybdenum sulfide embedded mesoporous N-Doped carbon as a noble metal-free highly selective catalyst for conversion of CO2 to CO. J. Environ. Chem. Eng. 2022, 10, 108988. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, K.; Li, Z.; Yang, B.; Yan, B.; Luo, K.H. Defect-Driven Efficient Selective CO2 Hydrogenation with Mo-Based Clusters. JACS Au. 2023, 3, 2736. [Google Scholar] [CrossRef] [PubMed]
- Dasireddy, V.D.B.C.; Vengust, D.; Likozar, B.; Kovač, J.; Mrzel, A. Production of syngas by CO2 reduction through reverse water gas shift (RWGS) over catalytically active molybdenum based carbide, nitride and composite nanowires. Renew. Energy 2021, 176, 251. [Google Scholar] [CrossRef]
- Xin, H.; Lin, L.; Li, R.; Li, D.; Song, T.Y.; Mu, R.T.; Fu, Q.; Bao, X.H. Overturning CO2 hydrogenation selectivity with high activity via reaction induced strong metal support Interactions. J. Am. Chem. Soc. 2022, 144, 4874. [Google Scholar] [CrossRef]
- Zhang, Q.; Pastor-Pérez, L.; Jin, W.; Gu, S.; Reina, T.R. Understanding the promoter effect of Cu and Cs over highly effective β-Mo2C catalysts for the reverse water-gas shift reaction. Appl. Catal. B-Env. 2019, 244, 889. [Google Scholar] [CrossRef]
- Zhang, Q.; Bown, M.; Pastor-Pérez, L.; Duyar, M.S.; Reina, T.R. CO2 Conversion via Reverse Water Gas Shift Reaction Using Fully Selective Mo-P Multicomponent Catalysts. Ind. Eng. Chem. Res. 2022, 61, 12857. [Google Scholar] [CrossRef]
- Yuan, Y.; Qi, L.; Gao, Z.; Guo, T.; Zhai, D.; He, Y.; Ma, J.; Guo, Q. Performance Exploration of Ni-Doped MoS2 in CO2 Hydrogenation to Methanol. Molecules 2023, 28, 5796. [Google Scholar] [CrossRef]
- Pajares, A.; Prats, H.; Romero, A.; Viñes, F.; Piscina, P.R.; Sayós, R.; Homs, N.; Illas, F. Critical effect of carbon vacancies on the reverse water gas shift reaction over vanadium carbide catalysts. Appl. Catal. B-Environ. 2020, 267, 118719. [Google Scholar] [CrossRef]
- Morse, J.R.; Juneau, M.; Baldwin, J.W.; Porosoff, M.D.; Willauer, H.D. Alkali promoted tungsten carbide as a selective catalyst for the reverse water gas shift reaction. J. CO2 Util. 2020, 35, 38. [Google Scholar] [CrossRef]
- Juneau, M.; Yaffe, D.; Liu, R.; Agwara, J.N.; Porosoff, M.D. Establishing tungsten carbides as active catalysts for CO2 Hydrogenation. Nanoscale 2022, 14, 16458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Luo, H.; Wu, Z.; Wei, B.; Shao, Z.; Huang, C.; Hua, K.; Xia, L.; Li, J.; et al. Morphological Modulation of Co2C by Surface-Adsorbed Species for Highly Effective Low-Temperature CO2 Reduction. ACS Catal. 2022, 12, 8544–8557. [Google Scholar] [CrossRef]
- Reddy, K.P.; Dama, S.; Mhamane, N.B.; Ghosalya, M.K.; Raja, T.; Satyanarayana, C.V.; Gopinath, C.S. Molybdenum carbide catalyst for the reduction of CO2 to CO: Surface science aspects by nappes and catalysis studies. Dalton Trans. 2019, 48, 12199. [Google Scholar] [CrossRef]
- Marquart, W.; Raseale, S.; Prieto, G.; Zimina, A.; Fisher, N. CO2 reduction over Mo2C-based catalysts. ACS Catal. 2021, 11, 1624. [Google Scholar] [CrossRef]
- Holder, C.F.; Morse, J.R.; Barboun, P.M.; Shabaev, A.R.; Baldwin, J.W.; Willauer, H.D. Evaluating metal oxide support effects on the RWGS activity of Mo2C catalysts. Catal. Sci. Technol. 2023, 13, 2685. [Google Scholar] [CrossRef]
- Pajares, A.; Liu, X.; Busacker, J.R.; Piscina, P.R.D.L.; Homs, N. Supported Nanostructured MoxC Materials for the Catalytic Reduction of CO2 through the Reverse Water Gas Shift Reaction. Nanomaterials 2022, 12, 3165. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Trigueros, A.; Caccia, M.; Caccia, J. Tuning Activity and Selectivity in Catalyzed Reactions of Environmental and Industrial Importance with a High-Surface Area, Mesoporous Mo2C Catalyst. Chem. Mater. 2022, 34, 6232. [Google Scholar] [CrossRef]
- Liu, R.; Chen, C.; Chu, W.; Sun, W. Unveiling the Origin of Alkali Metal (Na, K, Rb, and Cs) Promotion in CO2 Dissociation over Mo2C Catalysts. Materials 2022, 15, 3775. [Google Scholar] [CrossRef]
- Bhavani, A.G.; Kim, W.Y.; Lee, J.S. Barium substituted lanthanum manganite perovskite for CO2 reforming of methane. ACS Catal 2013, 3, 1537. [Google Scholar] [CrossRef]
- Royer, S.; Alamdari, H.; Duprez, D.; Kaliaguine, S. Oxygen storage capacity of La1-xA′BO3 perovskites (with A’=Sr, Ce; B=Co, Mn) relation with catalytic activity in the CH4 oxidation reaction. Appl. Catal. B Environ. 2005, 58, 273. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, J.L.; Park, E.J.; Kim, Y.D.; Uhm, S. Dopant effect of barium zirconate-based perovskite-type catalysts for the intermediate temperature reverse water gas shift reaction. ACS Catal. 2014, 4, 3117. [Google Scholar] [CrossRef]
- Jo, A.; Kim, Y.; Lim, H.S.; Lee, M.; Kang, D.; Lee, J.W. Controlled template removal from nanocast La0.8Sr0.2FeO3 for enhanced CO2 conversion by reverse water gas shift chemical looping. J. CO2 Util. 2022, 56, 101845. [Google Scholar] [CrossRef]
- Yu, J.; Muhetaer, A.; Gao, X.; Zhang, Z.; Yang, Y.; Li, Q.; Chen, L.; Liu, H.; Xu, D. Highly Active Hydrogen-rich Photothermal Reverse Water Gas Shift Reaction on Ni/LaInO3 Perovskite Catalysts with Near-unity Selectivity. Angew. Chem. Int. Ed. 2023, 62, e202303135. [Google Scholar] [CrossRef] [PubMed]
- Kopac, D.; Likozar, B.; Hus, M. How size matters: Electronic, cooperative, and geometric effect in perovskite supported copper catalysts for CO2 reduction. ACS Catal. 2020, 10, 4102. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, H.F.; Cai, J.Y.; Li, Z. Highly Efficient CO2 to CO transformation over Cu-based catalyst derived from a CuMgAl-Layered Double Hydroxide (LDH). ChemCatChem 2021, 13, 656. [Google Scholar] [CrossRef]
- Goguet, A.; Meunier, F.C.; Tibiletti, D.; Breen, J.P.; Burch, R. Spectrokinetic investigation of reverse water gas shift reaction Intermediates over a Pt/CeO2 Catalyst. J. Phys. Chem. B 2004, 108, 20240. [Google Scholar] [CrossRef]
| Catalyst | Reaction Gas Ratio, H2/CO2/Inert Gas | Temperature °C | Reaction Airspeed mL·gcat−1mL·h−1 | CO2 Conversion Rate % | CO Selectivity % |
|---|---|---|---|---|---|
| Cu/CeO2 [37] | 3:1:0 | 600 °C | 400,000 | 50 | 100 |
| Cu/SGS [38] | 4:1:0 | 550 °C | 3600 | 48 | 96 |
| Pt/CeO2 [40] | 9:9:2 | 500 °C | 30,000 | 30 | - |
| Ptcluster/PN−CeO2 [41] | 3:1:0 | 300 °C | 12,000 | 17.5 | 99.9 |
| Pt1/SiC [32] | 1:1:0 | 900 °C | - | 54 | 100 |
| Pt−Re/SiO2 [42] | 4:1:5 | 400 °C | 60,000 | 24.3 | 96.2 |
| Pt/SiO2 [43] | 1:4:5 | 400 °C | 60,000 | 12.1 | 100 |
| CoPd-CoOOV [44] | 3:1:0 | 300 °C | - | - | 94 |
| Ni/ZrO2 [45] | 4:1:4 | 500 °C | 13,500 | 27.6 | 100 |
| Re2O7/SZ [46] | 4:1:0 | 400 °C | - | 18 | 95 |
| Ni/Ga2O2 [47] | 4:1:5 | 450 °C | 60,000 | 40 | 95 |
| Fe−oxide [48] | 1:1:0 | 600 °C | 24,000 | 38 | >85 |
| Ni−MgO−Ce0.8Zr0.2O2 [49] | 63:21:16 | 250 °C | 50,000 | 4.5 | 90.5 |
| Cs/Fe,O [50] | 4:1:0 | 450 °C | 12,000 | 58 | 75 |
| Cu/MnOx [51] | 12:3:5 | 550 °C | 60,000 | 55.5 | 100 |
| 1 wt.%Cu−CeO2 [52] | 4:1:0 | 600 °C | - | 70 | 100 |
| MoO3/FAU [53] | 12.5:12.5:10 | 500 °C | 7500 | 15 | 100 |
| Ru@MoO3-x [54] | 9:3:88 | 250 °C | 100,000 | 45 | >99 |
| MoO3/TiAlC2 [55] | 4:1:0 | 550 °C | 15,000 | 30 | - |
| Cu−Cs−Mo2C [56] | 4:1:0 | 600 °C | 12,000 | - | 100 |
| Mo−P−Si2O [57] | 4:1:0 | 550 °C | 12,000 | 18 | 100 |
| Ni/CeO2−Al2O3 [58] | 4:1:0 | 750 °C | 30,000 | 59 | 94 |
| 2%Pt−CeO2 [59] | 4:1:0 | 290 °C | - | 27.1 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Zhang, J.; Fu, Y.; Dai, H. Recent Advances in the Reverse Water–Gas Conversion Reaction. Molecules 2023, 28, 7657. https://doi.org/10.3390/molecules28227657
Zhou C, Zhang J, Fu Y, Dai H. Recent Advances in the Reverse Water–Gas Conversion Reaction. Molecules. 2023; 28(22):7657. https://doi.org/10.3390/molecules28227657
Chicago/Turabian StyleZhou, Changjian, Jiahao Zhang, Yuqing Fu, and Hui Dai. 2023. "Recent Advances in the Reverse Water–Gas Conversion Reaction" Molecules 28, no. 22: 7657. https://doi.org/10.3390/molecules28227657
APA StyleZhou, C., Zhang, J., Fu, Y., & Dai, H. (2023). Recent Advances in the Reverse Water–Gas Conversion Reaction. Molecules, 28(22), 7657. https://doi.org/10.3390/molecules28227657





