Abstract
The increase in carbon dioxide emissions has significantly impacted human society and the global environment. As carbon dioxide is the most abundant and cheap C1 resource, the conversion and utilization of carbon dioxide have received extensive attention from researchers. Among the many carbon dioxide conversion and utilization methods, the reverse water–gas conversion (RWGS) reaction is considered one of the most effective. This review discusses the research progress made in RWGS with various heterogeneous metal catalyst types, covering topics such as catalyst performance, thermodynamic analysis, kinetics and reaction mechanisms, and catalyst design and preparation, and suggests future research on RWGS heterogeneous catalysts.
1. Introduction
With the development of industry and human activities, the concentration of carbon dioxide (CO2) in the global atmosphere is increasing yearly. As the main component of greenhouse gases, the CO2 concentration in the atmosphere continues to rise, causing a series of severe environmental problems such as climate warming, glacier melting, and ocean acidification, seriously threatening the living environment of human beings [1].
At present, fossil fuels are still the main mode for humans to obtain primary energy, and forms of renewable energy, such as solar and wind energy, are still in the development stage and cannot yet fully replace fossil fuels. CO2 is an abundant, inexpensive, non-toxic, non-flammable, and renewable single-carbon structural unit that can be used as a good carbon source.
In recent years, in order to cope with the negative impact of CO2, in addition to developing new energy sources, carbon capture and storage (CCS) and carbon capture and utilization (CCU) technologies have attracted widespread attention due to their high efficiency and easy application in capturing large amounts of CO2 [2]. CCS compresses CO2 from the source into high-density CO2, making it suitable for long-term transportation, storage, and monitoring, but the potential risk of leakage and continuous on-site monitoring are the main disadvantages related to this technology. Meanwhile, CCU uses different strategies to convert carbon dioxide into useful chemical products such as fuels (such as methanol) and materials (polymers) [3,4]. CCU is more useful than CCS because value-added products can be obtained using the captured CO2. In addition, CCU also offers no possibility of carbon dioxide leakage, which makes it promising in terms of sustainability and environmental friendliness [5]. CO2 utilization modes mainly include biological utilization, mineralization utilization, chemical synthesis, etc. In many CO2 utilization technologies, CO2 produces a variety of high−value basic chemicals through catalytic conversion, a process that has been widely studied by researchers. CO2 catalytic conversion technology mainly includes thermal catalysis [6,7,8,9], photocatalysis [10,11], electrocatalysis [12,13], and so on. The research on CO2 catalytic conversion mainly focuses on CO2 catalytic hydrogenation.
CO2 catalytic conversion research focuses on CO2 catalytic hydrogenation, a very green and environmentally friendly method for converting carbon dioxide into high-value substances such as CO, methanol, olefins, and alkanes [14,15]. According to the reaction pathway, the catalytic hydrogenation of CO2 mainly generates CO [16,17], methane [18,19], and hydrocarbons [20,21].
In summary, whether from the perspective of protecting the environment or CO2 utilization, the catalytic hydrogenation of CO2 is a method with broad application prospects to reduce the greenhouse effect and provide energy resources. However, CO2 has a very stable C=O bond and is thermodynamically difficult to activate [22]. Researchers have tried to reduce the activation energy of the CO2 reduction reaction by adding catalysts to achieve the goal of efficient CO2 conversion. Therefore, preparing simple and easily synthesized high-activity catalysts to convert carbon dioxide into high−value−added products is significant for improving the natural environment and addressing the energy demand.
CO2 catalytic hydrogenation to CO is known as the reverse water–gas conversion (RWGS) reaction. The CO produced via this reaction can be used as a syngas, often used to synthesize methanol, higher alcohols, or Fischer–Tropsch fuels. This method is expected to be an effective way to achieve carbon neutrality and cycling and will significantly impact the environment and the future energy mix.
RWGS mainly converts the greenhouse gas CO2 into CO. However, this reaction also produces the byproduct CH4. Therefore, developing an RWGS reaction catalyst with high CO selectivity is necessary to convert CO2 into CO in the RWGS reaction. The RWGS reaction is a typical endothermic reaction. From a thermodynamic point of view, since the RWGS reaction is endothermic, increasing the temperature during the reaction is more conducive to the equilibrium moving in a positive direction in the RWGS reaction. From a kinetic point of view, it has a higher reaction rate at a high temperature. Combined with thermodynamics and kinetics, the RWGS reaction is a process with high energy consumption [23]. At the same time, a high temperature will also lead to catalyst sintering, carbon deposition, and deactivation. Therefore, developing low-temperature, high−activity, and high-selectivity RWGS reaction catalysts has become the focus of current research, and many reviews of this work have been published [1,24,25,26,27,28,29]. Here, we review the research progress made in RWGS with different kinds of heterogeneous metal catalysts, including catalyst design and preparation, catalyst performance, thermodynamic analysis, kinetics and mechanism of reaction, etc., and predict future research on RWGS heterogeneous catalysts.
2. Results and Discussion
2.1. Reaction Mechanism of the Reverse Water–Gas Conversion Reaction
The RWGS reaction has unique advantages as one of the ways to utilize CO2. In order to design a reasonable catalyst, it is important to understand the mechanism of CO formation in the CO2 hydrogenation reaction in detail. The RWGS reaction’s mechanism has always been a topic of debate among researchers, and existing researchers have proposed two RWGS reaction mechanisms, namely a redox mechanism (Figure 1 and Figure 2) and an intermediate species decomposition mechanism (Figure 1 and Figure 3). Which reaction mechanism is followed depends on the type of catalyst or reaction conditions used. The two mechanisms reported in the current literature are still controversial. It is necessary to study the reaction mechanism of various catalyst types for catalyst development.
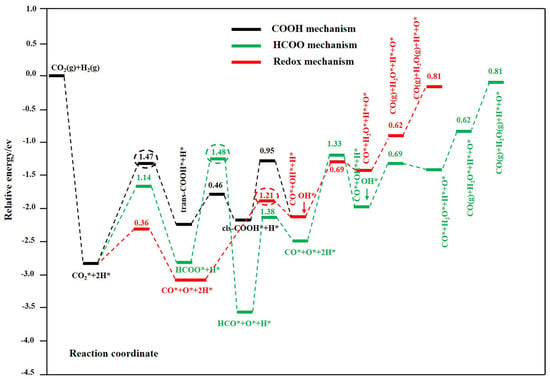
Figure 1.
Potential energy reaction diagram of Cu@Mo2C(001) with preabsorbed OH* species. The associated mechanism via COOH is shown by the black line, which via HCOO is shown by the green line, and the redox mechanism is shown by the red line. The redox mechanism is the most favorable pathway at 300–600 °C. Calculated potential energy profile of the most favorable redox (red line), HCOO (green line), and COOH (black line ) mechanism for the RWGS reaction on the Cu@Mo2C(001) surface. The numbers in the figure are the activation barriers of the elementary steps. The numbers in red, green, and black circles are the activation barriers of the rate–limiting steps in the redox, HCOO, and COOH pathways, respectively. Reprinted with permission from ref [30]. Copyright American Chemical Society 2019.
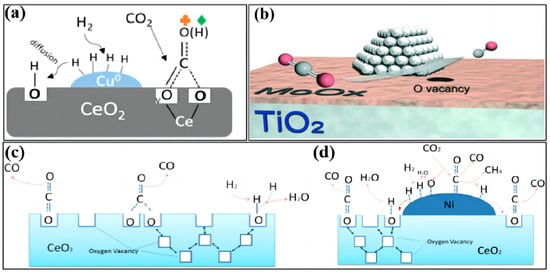
Figure 2.
The redox reaction mechanism of the RWGS reaction over (a) Cu−CeO2 (reprinted with permission from [31]. Copyright American Chemical Society, 2018), (b) Pt/MoOx−TiO2 (reprinted with permission from [32]. Copyright Royal Society of Chemistry, 2021), (c) ceria nanocubes (reprinted with permission from [33]. Copyright Elsevier, 2016), and (d) Ni−CeO2 nanocubes (reprinted with permission from [33]. Copyright Elsevier, 2016).
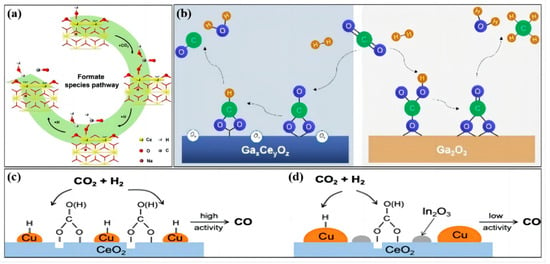
Figure 3.
The associative mechanism of the RWGS reaction over (a) LN−CeO2 (reprinted with permission from [34]. Copyright Elsevier, 2022), (b) Ga2O3−CeO2 (reprinted with permission from [9]. Copyright Wiley, 2022), (c) Cu−CeO2 (reprinted with permission from [35]. Copyright American Chemical Society, 2022), and (d) Cu5In5−CeO2 (reprinted with permission from [35]. Copyright American Chemical Society, 2022).
- (1)
- Redox mechanism
This mechanism mainly refers to the continuous oxidation and reduction of the active species in the catalyst in the CO2 and H2 atmosphere during the RWGS reaction to sustain the catalytic reaction. CO2 is first adsorbed on the catalyst, combining with the active site on the catalyst surface after dissociation to produce CO. The generated active oxygen substances exist on the active site of the catalyst and then continue to reduce the catalyst to generate H2O and release the surface active site to continue participating in the next round of the reaction. H2 is not directly involved in the synthesis of intermediate species. In this process, CO2 dissociation is the rate-limiting step, and the adsorption and desorption steps of CO and H2O can be ignored. The basic reactions of the surface redox mechanism are as follows (X indicates an empty active site with no interaction with the adsorbent):
CO2 + X = CO2·X
CO2·X = CO + O·X
H2 + 2 X = 2 H·X
2 H·X + O·X = H2O + 3 X
Gines and co-workers [36] prepared two catalysts, CuO/ZnO/Al2O3 and CuO/Al2O3, using the co-precipitation method. The mechanism of surface redox was proven via a kinetic experiment based on the RWGS reaction. According to the kinetic experiment results, it was found that the reaction rate of CO2 is controlled by dissociative adsorption. The chromatographic analysis showed that only CO was formed when CO2/N2 was inserted, which indicated that CO2 was dissociated from the active site on the catalyst surface to form CO. In the H2 atmosphere, only H2O was produced, indicating that H2 combines with active oxygen species on the catalyst surface to form H2O. Wang et al. [37] found that the oxygen vacancies that form on the surface of the pre−reduced Au/CeO2 catalyst are oxidized by CO2 to produce CO. This process corresponds to the redox mechanism of RWGS. The author also loaded Au onto different carriers and found through a pulse experiment that the amount of active oxygen on the surface of CeO2 carriers was significantly higher than that on TiO2 carriers. This is because CeO2 has a good oxygen storage and supply capacity and can assist Au atoms to complete the removal and production of surface-reactive oxygen species.
- (2)
- Decomposition mechanism of intermediate species
In the RWGS reaction, the decomposition mechanism of adsorbed intermediate species refers to CO2 and H2 on the catalyst, first being activated to form intermediate species and then decomposed into CO and H2O. In recent years, in most of the literature on RWGS reactions, formates, carbonates, and carbonyl groups were the main intermediate species formed and are considered the key steps for further CO generation.
It has been found in many research reports that there are different intermediates in RWGS reactions with different catalysts, and factors such as the properties of catalysts, the different interactions between metals and carriers, and the differences in reaction conditions lead to the complexity of the decomposition mechanism of intermediate species in RWGS reactions.
Liu et al. [38] conducted a CO2 dissociation experiment to explore the reaction pathway. Combined with the CO2 dissociation experiment results and temperature−programmed surface reaction, CO2 activation can be carried out through relevant intermediate pathways. In order to further explore the active intermediate, in situ diffuse infrared Fourier transform spectroscopy (DRIFTS) is performed. After the injection of CO2 and H2 into the activated 15CuCe catalyst, the formate signal can be detected in addition to the carbonate signal. In the presence of H2, the Cu atom captures the H2 molecule, breaks the H–H bond, and then transfers the H atom to the CO2. Formate structures are formed with the formation of C–H bonds, and these structures are manifested in the intermediates IMA3, IMA3−I, IMA4, and IMA4−I. Based on the above experimental results and density functional theory (DFT) calculation, the authors further concluded that the reaction involved the association mechanism, and the surface formates and carboxylic acid species may be important reaction intermediates. In the reaction process, a large number of surface oxygen vacancies are generated in situ and recycled, forming a synergistic catalytic effect with copper clusters, promoting the activation of CO2 and the formation of active intermediates. The copper clusters and abundant oxygen vacancies in the 15CuCe catalyst undoubtedly create more oxygen−vacancy−active interfaces of metal clusters.
Deng et al. [39] explored the mechanism of a Cu−based slag-based geopolymer microsphere (SGS) catalyst in the RWGS reaction and conducted in situ DRIFTS experiments to observe intermediates generated during the catalytic process. The characteristic bands of bicarbonate and carbonate appeared. The formate band appeared at about 150 °C and then gradually weakened above 200 °C, indicating that formate species formed at low temperatures. The SGS is an alkaline carrier with many surface OH groups. With the increase in temperature, the OH band gradually weakened, indicating that the surface OHs participated in the catalytic process. According to DRIFTS experiment results, we observe that a gas-phase CO band appears above 250 °C, and this band becomes stronger with increasing temperature. However, no formate bands were observed above 250 °C, so it can be inferred that formate species are not intermediates in CO production. Carboxylate bands were found at 1260 cm−1 and 1280 cm−1, so it was determined that the reaction mechanism of a Cu/SGS catalyst in the RWGS reaction is similar to the carboxylate pathway, and *COOH is the intermediate of CO2 to CO. Combined with the results of CO2 TPD, it can be determined that the formation of *COOH comes from the adsorption and activation of CO2 by the abundant OH groups on the SGS carrier, where OHs on the surface of SGS react with CO32− to form HCO3−. H2 is adsorbed on Cu to form H*, and the HCO3− substance reacts with H* on the surface of Cu to form *COOH. In addition, *COOH reacts with H* on the Cu surface to form CO* and OH*, and then CO and H2O dissociate from the Cu surface.
2.2. Overview of Catalysts of Different Systems in the Reverse Water–Gas Conversion Reaction
In industrial production, stable catalysts with high activity and selectivity need to be considered at low temperatures. However, designing a catalyst that meets all these criteria simultaneously is challenging. The current catalysts used for RWGS are mainly divided into precious metal catalysts and non-precious metal catalysts. Some representative research results are listed in Table 1.

Table 1.
A brief summary of the RWGS reaction conditions of selected catalysts and their CO2 conversion and CO selectivity.
2.3. Precious Metal Catalysts
Precious metal-supported catalysts are the most common catalysts for RWGS. The main active species are Pt, Pd, Au, Ir, Rh, Ru, etc. The main carriers are SiO2, CeO2, TiO2, and Al2O3, on which precious metals promote the dissociation of H2, while oxide carriers facilitate the breakage of the C=O bond in CO2. The dispersion and chemical state of precious metal nanoparticles are the key factors affecting catalyst performance and significantly impact the adsorption behavior of reactants on the catalyst and the subsequent intermediate species transformation [40].
2.3.1. Pt–Based Catalysts
Supported Pt catalysts [32,41,42,43,60,61,62,63] are widely used in RWGS reactions due to their excellent H2 dissociation and hydrogenation activity.
Chen et al. [42] found that the activation energy of CO production on a Pt/CeO2 catalyst was significantly lower than that on a pure CeO2 catalyst. The calculated TOF values were roughly the same across these Pt/CeO2 catalysts, indicating that the RWGS reaction was insensitive to the size of the anchored Pt particles and the primary crystallinity of the CeO2 carrier. The results of temperature-programmed reduction (TPR) and X-ray photoelectron spectra (XPS) showed that with the addition of Pt, the reducibility of the CeO2 carrier was enhanced, oxygen vacancies were more easily generated, and CO2 activation was accelerated. In addition, using in situ Fourier transform infrared spectroscopy (FT−IR) and temperature-programmed surface reaction-mass spectrometry (TPSR−MS) experiments, we found that redox and dissociation mechanisms co-exist in RWGS reactions on Pt/CeO2 catalysts. CO2 molecules adsorbed at the Ce3+ active site cannot directly generate CO, the same as the previous redox mechanism.
Liu et al. [43] prepared Pt-Re/SiO2 catalysts with different Re contents using co-impregnation and tested the RWGS reaction. The characterization results showed that the oxygenophilic ReOx (0 ≤ x ≤ 3.5) near the Pt particles modified the Pt surface through partial covering and electron interaction, resulting in decreased CO adsorption sites and adsorption strength. At 400 °C, the turnover frequency of the optimal PT−Re/SiO2 catalyst is 3.9 times higher than that of Pt/SiO2, and the apparent activation energy is reduced. The CO selectivity on Pt−Re/SiO2 remains above 96.2% compared to Re/SiO2, which produces large amounts of CH4. The reaction order analysis showed that Pt promoted H2 activation, while oxyphilic ReOx enhanced CO2 adsorption and activation. The peripheral sites of the Pt/ReOx interface have C–O cleavage properties, which can synergistically increase RWGS activity and inhibit the production of CH4.
To improve the potential of plasma effects on H−doped WOy, Ge et al. [62] reported that Mo−doped Pt/WOy (Pt/MoWOy) significantly increases the concentration of dopant (H+) and oxygen vacancies in Pt/HxMoWOy during H2 reduction, promoting the photothermal hydrogenation of CO2 to CO. The developed Pt/HxMoWOy showed excellent catalytic performance (3.1 mmol·h−1·g−1) in the photothermal RWGS reaction at 140 °C, which was superior to the undoped Pt/HxWOy (1.02 mmol·h−1·g−1). The experiment and comprehensive analysis show that the abundant surface-free electrons and oxygen vacancies (VOs) in Pt/HxMoWOy are the reasons for the effective CO2 adsorption and transfer. The characterization of catalysts revealed a reversible redox of Mo and W atoms during RWGS reactions, confirming that oxygen vacancies between Mo and W atoms in Pt/HxMoWOy act as active sites. Pt nanoparticles activate H2 to regenerate oxygen vacancies. In addition, density functional theory calculations show that Mo doping significantly reduces the energy barrier of oxygen vacancy formation in WOy during H2 reduction.
He et al. [63] synthesized a single–atom catalyst, Pt1/SiC, in which the Pt particles are uniformly dispersed on SiC and applied it in the conversion of CO2 via the reverse water–gas shift reaction, exhibiting 100% selectively and 54% CO2 conversion at 900 °C with a H2/CO2 ratio of 1:1. It was found that in the first few hours, the Pt1/SiC catalyst showed excellent stability with negligible decline in activity. However, over time, Pt1/SiC was gradually deactivated. After the reaction lasted 10 h, the CO2 conversion rate remained relatively stable at about 50%. The authors attributed the decrease in catalyst activity to two factors: the poisoning of the Pt1/SiC catalyst caused by CO molecules and the reduction of a small amount of Pt1/SiC.
2.3.2. Pd–Based Catalysts
Many researchers have focused on the design, preparation, and mechanism of Pd catalysts [44,64,65,66,67,68,69,70,71] based on their catalytic performance in the RGWS reaction. Here are some selected examples discussed in detail.
Nelson et al. [64] dispersed Pd on a TiO2 carrier. The results show that in the RWGS reaction, Pd is mainly dispersed on titanium dioxide in the form of isolated atoms. Achieving atomic dispersion requires artificially increasing the absolute surface area of titanium dioxide by an order of magnitude, which can be achieved by physically mixing the catalyst Pd/TiO2 with pure titanium dioxide prior to the RWGS reaction. Kinetic analysis, infrared spectroscopy, X−ray absorption spectroscopy, and scanning electron microscopy showed that the RWGS activity of the Pd/TiO2−0.01 catalyst was very good within 92 h after the in situ dispersion of Pd atoms. The thermodynamic stability of Pd under high-temperature RWGS reaction conditions is related to the Pd–Ti coordination, which is related to the formation of oxygen vacancies and the artificial increase in titanium dioxide’s surface area.
Onodera et al. [65] discovered that, up to 300 °C, Au−doped Pt/CeO2 catalysts, which were created using the electroless plating method, demonstrated comparable CO2 conversion activities to the Pt/CeO2 catalyst. Furthermore, above 250 °C, the CO selectivity of the Au−doped Pt/CeO2 catalysts was significantly higher than that of the Pt/CeO2 catalyst because the addition of the proper amount of Au to the Pt/CeO2 catalyst created a favorable density of state for the RWGS reaction. However, a higher density of state leads to a greater electron donation from the Au-doped Pt/CeO2 catalyst to an antibonding orbital of CO2, which weakens the O−C−O bonds and accelerates the methanation reaction. It is hypothesized that by managing the density of the state close to the Fermi level, the Pt/CeO2 catalyst’s increased CO selectivity could be realized.
RWGS reactivity is enhanced in real reactions by the dual effect of Pd1−FeOx single−atom catalysts (SACs), as Du et al. reported [66]. Unexpectedly, Pd SAs not only offer single active sites but also dynamically carburize the FeOx surface over an extended reaction period. This results in exceptional RWGS activity (42.0 mmol CO·gcat−1·h−1) and CO selectivity (>98%), surpassing the results of other documented Fe–based catalysts. The results of this dynamic carburization show that the atmospheric rWGS reaction produces a highly active Fe5C2 phase with the lowest activation barrier of 27.7 kJ/mol. These results are obtained using cutting–edge characterization techniques. Compared to Pd nanoparticles with a fast FeOx encapsulation that prevents carburization, SACs’ dual effect produces better catalytic performance. The various functions that individual atoms play in actual reactions are revealed by this work, suggesting that SACs have a broader function. During actual reactions, single atoms play a dual role in the formation of a highly active phase of heterogeneous catalysts in addition to serving as independent active sites. It is important for rational designs to take into account both roles.
A series of monodispersed Pd nanoparticles, ranging in size from 2.8 nm to 8.1 nm, were prepared by Yang et al. [67] in a progressive manner. With regard to Pd sizes, the photo−thermal catalytic activity in the RWGS reaction exhibits a volcano−type dependence, with the highest activity occurring at a Pd/TiO2 size of 6.3 nm. The tunable−surface electronic properties, such as the quantum size effect and metal–support interaction, are responsible for the size−modulated activity.
2.3.3. Ru−Based Catalysts
Some researchers have also turned their attention to the research of Ru−based catalysts [45,46,72,73,74,75], which we briefly describe below.
Tang et al. [72] prepared an efficient RWGS catalyst by encapsulating a Ru cluster with a size of 1 nm in a hollow silica shell. The space–confined structure prevents the sintering of Ru clusters, and the permeable silica layer allows the diffusion of gaseous reactants and products. This catalyst with reduced particle sizes not only maintains the excellent activity of Ru in the CO2 hydrogenation reaction but also exhibits close to 100% CO selectivity and excellent stability at 200–500 °C.
Abdel−Mageed et al. [45] investigated the effect of carrier particle size on the performance of highly active Ru/TiO2 catalysts and found that after high−temperature reduction treatment, the selectivity of TiO2 particle size can be controlled from 100% methanation to 100% CO. The comprehensive characterization of the catalysts shows that while the reaction behavior changes, their structure, chemical, and electronic properties also change significantly. The chemical modification of the carrier via oxygen-vacancy formation leads to the electronic modification of the Ru centers around the interface, which in turn affects the reaction behavior of these centers in CO2 reduction reactions, from methanation to RWGS reactions.
Wu et al. [73] reported effective photocatalysis driven by sunlight over Ru clusters supported by MXene that have strong light–absorption capabilities, high sintering resistance, and increased metal loading. Remarkably, MXene-supported Ru clusters demonstrated superior photothermal catalytic performance compared to silica-supported Ru clusters and MXene−supported Ru nanoparticles by 2 and 81 times, respectively. The superior photothermal characteristics of MXene materials and the robust binding between metal clusters and MXene supports are the cornerstones of our approach. Remarkably, the CO production rate of Ru clusters supported by MXene in the batch reactor was 4.0 mol·gRu−1·h−1, one of the highest recorded rates for photothermal RWGS catalysts to date. This approach can, in theory, be expanded to metal catalysts with even greater dispersion to promote the development of effective sunlight–driven single-atom catalysis. Future research will thoroughly examine how light affects catalytic reactions, with the goal of adding more photochemical activation to increase photocatalytic efficiency. Their work shows the great potential of metal cluster catalysis powered only by concentrated solar radiation. Developing catalysts with enhanced reactivity and solar power alone presents excellent potential as a low-carbon chemical technology.
Chen et al. [74] examined the mechanisms of the RWGS reaction on Ru adsorption on a three−fold site of the CeO2(111) surface using first–principles calculations and microkinetic simulations. Microkinetic simulations covering all elementary reaction steps were used to investigate the effects of temperature on the active site and the preferred reaction mechanism. Ru(OH)3−(OH) is the predominant active site at low temperatures, according to the microkinetic simulations. A low barrier to direct CO dissociation is the outcome of this hydroxyl−promoted configuration. RuO3 becomes the predominant active site when the temperature rises due to decreased Ru(OH)3−(OH) abundance. The Mars–van Krevelen mechanism is the one that is favored in such circumstances. Heat makes forming oxygen vacancies easier, which serve as CO2 anchoring sites. When CO2 adsorbs, the oxygen vacancies are restored, increasing the CO2 molecule’s reactivity and facilitating the simultaneous scission of C−O bonds and CO desorption. Ultimately, their research suggests a temperature-dependent RWGS mechanism and emphasizes the impact of surface hydroxyl and oxygen vacancies on RWGS compared to the Ru/CeO2 single−atom catalyst.
2.3.4. Au−Based Catalysts
In addition to the above precious metal catalysts, some Au-based catalysts have also been studied and reported, and the following is a brief introduction.
Abdallah et al. [34] loaded a titanium dioxide and zirconia support with a very low content (<0.1 wt%) of Au, used it for the RWGS reaction, and found that this gold-based catalyst showed high catalytic activity for the RWGS reaction at low temperatures. At 250 °C, the catalytic activity of the Au/TiO2 catalyst is nearly 10 times that of Au/ZrO2. The in situ infrared DRIFT results show that the formate is the main intermediate species on the Au/ZrO2 catalyst, while on the Au/TiO2 catalyst, the reaction is carried out via the formation of hydroxyl carbonyl intermediates. The results of scanning transmission electron microscopy (STEM), STEM−EELS (electron−energy−loss spectrometry), XPS, and EPR in situ indicate that the Au–Ov–Ti3+ interface sites are responsible for the excellent activity of Au/TiO2.
To bridge the knowledge gap regarding the completeness of the plasma–thermal coupling mechanism for Au/γ−Al2O3, Wang et al. [76] studied the photo−thermocoupled catalytic RWGS reactions and their reaction pathway and plasmonic enhancement mechanism. As per the findings, the total reaction is facilitated by both the formate and carboxyl pathways. A low reaction temperature over small Au NPs is proposed to be mediated primarily by the m−formate pathway. Combining resonance and hot-electron energy-transfer mechanisms, spectro–kinetics, and theoretical computation analyses show that the plasmonic energy preferentially moves to HCOO*. As the rate−determining step (RDS) of the entire RWGS reaction, the plasmonic energy facilitates the dehydration of HCOO* to CO.
2.3.5. Some Other Precious Metal-Based Catalysts
In addition to introducing the above precious metal catalysts, here we also introduce these relatively less reported precious metal catalysts, such as Bi, Pt−Re, Re, Rh, Ir, In, etc.
Kang et al. [77] reported a Bi-based photothermal RWGS catalyst. Using a soft template technique, they were able to create Bi single atoms loaded with CeO2 nanosheets that demonstrated exceptional stability and activity for RWGS at 400 °C air corrosion, demonstrating previously unheard-of oxidation resistance. High activity and oxidation resistance for RWGS result from the Bi single atoms’ low energy barrier and their +3 valence state retention during RWGS, as demonstrated via experiments and first−principles calculations. Loading BiOx/CeO2 into a Ti2O3−based photothermal system resulted in a CO generation rate of 31.00 mmol g−1 h−1, four times higher than the state−of−the−art for solar-driven RWGS under 20 sun irradiation units. This was accomplished under three sun units of radiation.
RWGS reaction tests were conducted by Liu [43] on Pt−Re/SiO2 catalysts that varied in Re content and were prepared via co–impregnation. The results of the characterization showed that the oxophilic ReOx (0 ≤ x ≤ 3.5) that was close to the Pt particle changed the surface of Pt by partial coverage and electronic interaction, which led to a decrease in the number of sites and a weakening of the strength of CO adsorption. When Pt/Re/SiO2 is optimized (Pt/Re = 1.91), the turnover frequency (2.30 s−1) is 3.9 times higher at 400 °C than when Pt/SiO2 is used under differential conditions, and the apparent activation energy is decreased. Pt–Re/SiO2’s CO selectivity maintained >96.2% under integral conditions, unlike Re/SiO2, which generated a significant amount of CH4. Pt helps to activate H2, while ReOx, an oxophile, helps to activate and adsorb CO2. This was determined using reaction order analysis. The RWGS activity is enhanced while CH4 production is inhibited by the perimeter sites at the Pt/ReOx interface that have balanced hydrogenation and C–O cleavage properties.
Yang et al. [78] clearly demonstrated the close relationship between the size effect, the active site, and the reaction mechanism using a special set of well-defined TiO2-supported Re catalysts and systematic characterizations. Additionally, a size-dependent, wave−like activity of CO2 conversion was found. CO2 hydrogenation is regulated by the RWGS reaction in the size range of a single atom to 1.0 nm. As size increases, the turnover frequency of this reaction falls. Nevertheless, CO2 methanation takes over as the primary reaction and exhibits a size-dependent performance resembling a volcano for clusters larger than 1 point 0 nm. According to the mechanistic analysis, formate pathways in single-atom catalysts allow the perimeter site to control the RWGS reaction. The edge site in the Re cluster, on the other hand, serves as the active site. Here, CO2 undergoes a redox process to reduce CO and then hydrogenates to methane across the edge sites. This finding could contribute to a better mechanistic understanding of structure sensitivity.
In Liu’s work [79], it was successfully constructed to perform the reverse water–gas shift reaction mediated by the diatomic anion Rh2−. At room temperature, the production of a gas−phase H2O molecule and the ion product [Rh2(CO)ads]− were clearly identified, and the desorption of CO from [Rh2(CO)ads]− was the only basic step that needed additional energy to finish the catalysis. Since designing efficient routes to yield H2O from CO2 and H2 is difficult, this experimentally identified Rh2− anion represents the first gas–phase species that can drive the RWGS reaction. Their recently created double-ion trap reactors were used to carry out the reactions, which were then examined using high−precision quantum-chemical calculations, photoelectron spectroscopy, and mass spectrometry. They discovered that the distribution of the final products (D2O and Rh2CO−) and the reactivity were not significantly affected by the order in which the reactants (CO2 or D2) were fed into the reactor. The essential steps to steer the reaction in the direction of the RWGS were revealed with atomic precision.
In Lu’s work [80], a solvent evaporation self-assembly method was used to build a system of Ir species and α−MoC. Because of the synergistic effect of Ir and α–MoC, the catalytic performance of the Ir/MoC catalysts for the RWGS reaction was significantly better than that of pure α−MoC over a wide temperature range (200–500 °C). At 500 °C, 0.1 MPa, and 300,000 mL·g−1·h−1, the ideal 0.5% Ir/MoC catalyst produced a 48.4% CO2 conversion, similar to the equilibrium conversion of 49.9%. Higher than most previously reported values, the CO selectivity and space–time yield of CO over 0.5%Ir/MoC reached 94.0% and 423.1 umol·g−1·s−1, respectively. Furthermore, over a 100 h period, 0.5% Ir/MoC maintained its catalytic properties and showed exceptional stability at elevated temperatures. It was shown via several characterization techniques that the Ir species supported on α−MoC substrates were widely distributed. The stability of the Ir/MoC catalysts was significantly enhanced by the strong metal–support interaction that took place via electron transfer between Ir and α-MoC. Ir single atoms (Ir1) and clusters (Irn) coexisted to form Irn−Ir1−C–Mo synergistic sites between Ir and α−MoC for the Ir/MoC catalysts with Ir loadings > 0.2% (mass fraction). Comparing the 0.5% Ir/MoC catalyst to the other Ir/MoC catalyst, the number of Ir1 species and size of Irn species were higher and smaller, respectively. The exceptional adsorption and activation of CO2 and H2 during the RWGS were facilitated by the optimal electron density conferred upon 0.5% Ir/MoC. The RWGS reaction mechanism was found to occur via a formate pathway in in situ diffuse-reflectance infrared Fourier transform spectroscopy experiments. The creation and breakdown of formate intermediates were noticeably aided, even though the Irn−Ir1−C−Mo synergistic site formation had no effect on the reaction mechanism. The synergistic effect, therefore, effectively increased the catalytic performance of Ir/MoC. This work offers guidelines for creating stable and effective CO2 utilization catalysts.
With In2O3, Mhamane’s work [81] showed a sustainable catalytic CO2 conversion to almost 100% CO selectivity at room pressure. Using less hydrogen in the feed than stoichiometric amounts, it is important to note that high CO yield may be observed at the expense of unwanted methanation; 1:1 and 1:0.67 CO2:H2 ratios show 98–99.6% CO selectivity with 25–38% CO2 conversion between 773 and 873 K. For the reverse water–gas shift reaction, an ideal reactant composition is present when CO2 and H2 conversion occurs under steady-state conditions at 773–873 K, suggesting a 1:1 ratio of adsorbed reactants (with 1:0.67 CO2:H2 feed) on the catalyst surface. In contrast, H2–rich feed compositions indicate the H2–dominated surface. Using near-ambient pressure photoelectron spectroscopy (NAPPES) to investigate surface electronic structure changes under near–operating conditions, the following intriguing results were found: (a) Higher temperatures caused a shift in the valence band to lower binding energy, up to 0.6 eV. (b) Active oxygen vacancy site formation modifies the In2O3 work function and is responsible for the catalyst surface’s observed heterogeneous nature under NAPPES measurement conditions. (c) It is discovered that the aforementioned alterations are reversible upon cessation of the reaction. We found that in the active temperature window of the catalyst supporting the heterogeneous reaction, the vibrational characteristics of the reactant molecules were widened.
Precious metal catalysts (Pt, Pd, Au, Rh, Ru, Ir, etc.) are not easy to deactivate, although they have high catalytic activity and stability. However, due to their high prices and scarce resources, they are limited in large–scale applications in industrialization and are only suitable for laboratory mechanism research. Therefore, efforts are needed to find some alternative catalysts to precious metals.
2.4. Non–Precious Metal Catalysts
Although precious metal catalysts have excellent CO2 reduction performance and stability, their high cost limits their large−scale industrial application. Therefore, the exploration of inorganic non-precious metal catalysts has attracted widespread attention from scientific research staff, and non-precious metal catalysts (Cu, Ni, Fe, Mo, etc.) are often used to replace precious metals because of their low cost and good catalytic activity.
2.4.1. Ni–Based Catalysts
Nickel-based catalysts experience the problems of easy sintering and carbon deposition in the RWGS reaction and have low selectivity for CO. Several examples of design, preparation, and mechanism research on nickel-based catalysts are listed below.
Zhang et al. [48] prepared a sulfur-containing Ni/ZrO2 catalyst to improve CO2 conversion and regulate CO2 hydrogenation selectivity. The effect of carrier size on the catalytic performance of RWGS was investigated. The results show that the 80 nm Ni/ZrO2 sample with a smaller carrier size had higher Ni dispersion and oxygen-vacancy concentration, had more exposed active centers, and displayed improved adsorption and activation ability of CO2.
Gong et al. [82] proved that the interfacial synergism of Ni/Ga2O3 promoted the selective hydrogenation of CO2 to CO (CO selectivity > 95%) in the temperature range of 350–450 °C. Studies show that the synergistic effect of the Ni/Ga2O3 interface significantly affects the adsorption of H2 and CO2, resulting in the formation of different intermediates and products. *HCOO preferentially forms on the Ni surface and is further hydrogenated to CH4 and H2O. In contrast, the Ni/Ga2O3 interface favors the formation of CO with the help of H2. This study provides a highly selective catalyst for the RWGS reaction in the CO2 hydrogenation process and promotes the surface modification of the catalyst to improve the activity and selectivity.
Our group [8] prepared NiCe−HMS and Ni-HMS limited-structure catalysts using the one-pot method and conducted RWGS tests in the 500–750 °C temperature range. Compared with Ni/HMS catalysts prepared via the traditional impregnation method, Ni−HMS and NiCe−HMS showed higher CO selectivity. This is due to the formation of highly dispersed nickel nanoparticles on the surface of the carrier, which inhibits the formation of CH4.
Zhang et al. [83] reported the activity and selectivity of Ni-rich nickel phosphide catalysts for CO2 hydrogenation for the first time. Their findings demonstrate that Ni2P/SiO2 is an extremely effective catalyst for RWGS, yielding more than 80% of CO throughout the entire 350–750 °C temperature range.
Shen et al. [84] discovered that Ni doping (1.0−Ni−CeO) significantly increased the RWGS reaction activity of pure CeO2 at low temperatures. At 300 °C and a WHSV of 60,000 mL·g−1·h−1, the Ni−based mass−specific CO formation rate of 1.0−Ni−CeO2 could reach up to 1542 mmol·gNi−1·h−1. Furthermore, it was established through our experiments and density functional theory calculations that the surface defects serve as the active sites for H2 heterolytic dissociation, which is the RWGS reaction’s rate-determining step. In 1.0−Ni−CeO2, the formed Ce−H species is stable. Furthermore, the superior H2 heterolytic dissociation capacity and high active H− released from the Ce-H species of 1.0−Ni−CeO2 at low temperatures are responsible for its increased catalytic activity and selectivity. Moreover, CO−TPD (temperature−programmed desorption) and CO DRIFTS demonstrated that 1.0−Ni−CeO’s 100% CO selectivity was primarily due to its lower CO affinity. A new reaction mechanism was revealed using systematic in situ DRIFTS analysis. According to this mechanism, CO2 was first adsorbed on the hydroxyl groups of Ce3+−OH rather than on oxygen vacancies, which resulted in the formation of the bicarbonate intermediate (*HCO3). The bicarbonate intermediate was then reduced to formate (*HCOO), and the highly active H− in Ce−H caused the production of CO. This work may open up new avenues for the development of robust, affordable, and efficient catalysts for hydrogenation reactions at low temperatures.
In addition, many other nickel catalysts have been reported [48,49,85,86,87,88,89,90,91,92,93,94,95,96,97,98], including bimetallic [93,94,95,96,97,98] or polymetallic catalysts composed of nickel and other metals.
2.4.2. Fe−Based Catalysts
Fe−based catalysts [50,99,100,101,102,103,104,105], as typical high-temperature catalysts, have also been used in RWGS reaction studies. Here are some selected examples. The carrier−free nano ferric oxide catalyst prepared by Kim et al. has high catalytic activity and stability [50]. Through transmission electron microscope observation, it was found that nano iron oxide did not easily agglomerate during the RWGS reaction. XPS and X–ray diffraction (XRD) show that atomic carbon and oxygen formed due to dissociative chemisorption of CO or CO2 appear to diffuse into many nanoparticles to form Fe oxides and Fe carbides. C and O are produced by the reactants on the surface of the iron oxide, and CO2 and CO are diffused into the body of the iron oxide nanocatalyst. Therefore, during the RWGS reaction, the structure of the catalytically active surface consisting of metal Fe remains unchanged, so the catalytic activity remains stable for a long time. For Fe–based catalysts, it is still a subject of debate as to whether the active phase is metallic iron, iron oxide, or iron carbide.
Bogdan et al. [99] investigated, for the first time, the reduction of CO2 to CO with hydrogen on alumina–supported Co and Fe catalysts under supercritical conditions with CO or CH4 as the target product. The selectivity of the Co/Al2O3 catalyst for methanation is close to 100%, while the Fe/Al2O3 system shows an advantage for hydrogenating CO2 to CO and significantly forms ethane (up to 15%). Compared with the gas–phase reaction, the spatio–temporal yield can be increased by one order of magnitude under supercritical conditions. The difference in the crystal phase characteristics of the iron-containing catalyst leads to the reverse water–gas transfer reaction to produce carbon monoxide, while the reduced iron phase leads to the Fischer–Tropsch reaction to produce a mixture of hydrocarbons. Direct methanation occurs selectively on Co catalysts. Methanol formation was not observed on the studied Fe and Co catalysts.
Zhang et al. [100] found that metallic iron was a better RWGS catalyst than Fe3C. The addition of Cs and Cu can promote the activity of Fe0, hindering the carburization of iron while favoring higher conversion rates and selectivity. When stability tests were performed, the catalyst aged during the RWGS reaction, and a new phase appeared: Fe5C2 (Hagg carbide). For RWGS reactions, Fe5C2 is an excellent catalyst with a higher carbon dioxide conversion rate than samples in which Fe⁰ is the active phase. However, Fe5C2 has a low CO selectivity compared to Fe0-based samples. The results showed that the best activity/selectivity was achieved by fine-tuning the Fe/Fe₅C2 ratio.
Watanabe et al. [101] used H2S as a co–feeding gas to investigate the catalytic activity of Fe/CeO2 on the RWGS reaction and maintained a stable activity for 12 h. In addition, co–feed H2S can be used as a hydrogen source for RWGS.
In Xu’s study [102], the comparison of dynamic and steady−state CO/CO2 hydrogenation performance showed a large amount of FeOx covering the χ−Fe5C2−dominated bulk phase during CO2 hydrogenation. The overall RWGS rate was positively correlated with the surface content of FeOx under differential conditions (CO2 conversion < 10%) and negatively correlated with the surface content of FeOx under integral conditions (CO2 conversion > 10%).
Their findings help us to understand the role of different types and amounts of iron sites on Fe–based catalysts during CO2 hydrogenation. Adjusting the number and properties of different surface sites to achieve optimal intersite synergies may stimulate the development of future multi−site CO2 hydrogenation catalysts.
2.4.3. Cu-Based Catalysts
Cu-based catalysts have attracted much attention because of their low price, high activity at relatively low temperatures, and good selectivity to CO. However, since copper is easily sintered or coked, such catalysts are prone to deactivation during the RWGS reaction. In order to overcome these shortcomings, many RGWS reactions catalyzed by Cu–based catalysts have been reported [39,51,52,53,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131], and we will describe some representative examples in detail.
Ai et al. [106] discovered that the grinding process could enhance the amount and distribution of Cu0 species on the surface and that the Cu content could control the amount and distribution of Cu0 species’ crystallinity. Moreover, the low crystallinity of Cu0 species could encourage the exposure of CO2–hydrogenation active sites.
Zhuang et al. [51] added Ru to the Cu/ZnO/Al2O3 catalyst for the RWGS reaction. The CO2 conversion rate was more than two times higher than that of the Cu/ZnO/Al2O3 catalyst. At the same time, the stability of the catalyst was significantly improved. Through XRD, scanning electron microscope–energy−dispersive spectroscopy (SEM–EDS), scanning transmission electron microscope–energy–dispersive spectroscopy (STEM–EDS), and TPR characterization, it was found that this may be due to the formation of Ru–Cu core–shell nanoparticles. In addition, the interaction between Ru and the support is also an important factor affecting catalyst selectivity.
Liu et al. [38] constructed stable copper clusters in a Cu/CeO2 catalyst with a high copper loading capacity of 15 wt%. Under very harsh reaction conditions, CeO2 nanorods were partially sintered, forming 2D and 3D copper clusters on their surfaces. This partially sintered catalyst exhibits unparalleled activity and excellent durability at high temperatures. The interaction between copper and CeO2 ensures that the copper clusters are stably anchored to the CeO2 surface. The large number of in situ surface-oxygen vacancies causes a synergistic effect with adjacent copper clusters, which promotes the reaction.
Deng et al. [39] prepared catalysts with different copper loads using the simple impregnation method via NaOH−activated slag-polymer microspheres (SGSs) as the carriers. The results show that the 10% Cu/SGS catalyst has better CO2 conversion performance. At 550 °C, the CO2 conversion and CO selectivity of the 10% Cu/SGS catalyst reached 48% and 96%, respectively. The results show that the high performance of the catalyst is mainly due to the interaction between copper and the SGS carriers and the adsorption and activation of CO2 by the alkaline center on the SGS carriers.
Liu et al. [107] loaded Cu onto titanium dioxide nanotubes (TiNT) and titanium dioxide nanoparticles (TiNP), and the RWGS activities of the two catalysts were different. The activity of the nanoparticle carrier is very low, and the content of Cu hardly changes the activity, but the activity of the nanotube carrier is very high and has three different behaviors as the surface density of copper increases. At a low surface density, an active Cu−O−Ti site with low apparent activation energy is formed. At a higher surface density, copper nanoparticles are formed on the surface of TiNT, and the reaction barrier is reduced. At moderate surface densities, the metal copper clusters are engulfed by a TiOx overlay formed during hydrogen treatment, similar to the overlay formed by classical strong metal carrier interactions (SMSI). These reducing layers are significantly more active against RWGS than the initial TiNT surface but have similar activation barriers, higher than those on exposed copper and TiNP surfaces. SMSI is an important concept in heterogeneous catalysis, but it will inevitably sacrifice catalytic activity due to over-coverage.
González–Arias et al. [108] prepared a series of Cu−MnOx catalysts and found that the improvement in catalyst performance was due to the addition of Mn to enhance the dispersion of Cu and increase the surface alkali concentration. Under standard RWGS conditions, the highest reaction rate of the catalyst is related to the improvement of Cu dispersion and the composition of the highly active Cu−MnOx domains. It is worth noting that the change in the optimal copper–manganese ratio is a function of the RWGS reaction conditions.
Ebrahimi et al. [52] prepared a Cu/CeO2 solid solution using the combustion synthesis method. The produced catalyst exhibited high activity, stability, and 100% CO selectivity in the RWGS reaction, and these catalytic activities were attributed to the excess oxygen vacancy on the CeO2 surface, which could promote CO2 conversion. The high activity of this catalyst is due to the synergistic effect between the active Cu0 and Ce3+−oxygen vacancy.
Yang et al. [109] reported the reduction reaction of carbon dioxide catalyzed by Cu−CeO2 under visible light and speculated regarding a reaction mechanism induced by the localized surface plasmon resonance effect with the assistance of visible light. The specific reaction process is divided into four steps [Figure 4]: (I) hot electrons and hot holes are produced via the plasmon effect of Cu when visible light is present; (II) hydrogen dissociation and spillover are accelerated via the hot holes’ reaction with hydrogen; (III) CO is produced via the hot electrons attacking and transferring to the intermediate species; and (IV) vibrant light exposure causes the oxygen vacancies to regenerate.
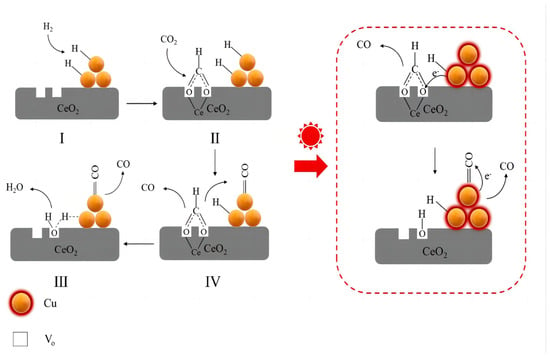
Figure 4.
The proposed mechanism of the RWGS reaction under visible−light irradiation.
Zhang et al. [53] studied a variety of strategies to effectively regulate nanointerfaces from different aspects, such as carrier composition, Cu preparation parameters, and pretreatment and reaction conditions, in order to balance the activity and stability of Cu/TiO2 catalysts in RWGS reactions. The results show that due to the reducibility of TiO2 and the limited electron transfer from TiO2 to Cu, only doping Zn in the TiO2 carrier can inhibit the formation of nanointerfaces during the reduction treatment. Cu coverage on Zn−modified TiO2 is significantly decreased, but the catalytic activity is increased by 44%, and the stability is unchanged.
In addition to the specific examples mentioned above, many other researchers have reported interesting works [39,53,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131] on copper catalysts, mainly focusing on catalyst design and mechanism studies.
2.4.4. Mo-Based Catalysts
Molybdenum is more abundant and cheaper than precious metals, increasing the potential for large−scale industrial applications of molybdenum−based catalysts. Molybdenum−containing catalysts are widely used, including Ni−Mo catalysts [132], molybdenum sulfide catalysts [133], MoOx catalysts [54], supported Mo-based catalysts [55,134], new Mo2C and Mo2N catalysts [135], etc. A few representative examples are described below.
Xin et al. [136] found that during the CO2 hydrogenation reaction, Ru−MoO3 formed a strong metal−support interaction (SMSI) state between the metal and the carrier at 250 °C, which was conducive to CO2 selective hydrogenation to form CO. During the reaction, Ru nanoparticles promote the reduction of MoO3 to form an active MoO3−x coating with oxygen vacancies, which migrates to the surface of Ru nanoparticles to form a coating structure (Ru@MoO3−x). The resulting SMSI state changes the catalytic performance of the catalyst from 100% methanation at the Ru site on the exposed surface to 99% CO formation via the quasi-MvK (Mars–van Krevelen) mechanism in the MoO3−x layer. Selective regulation is achieved through different SMSI states in the CO2 hydrogenation reaction.
Ronda [56] et al. took advantage of the high thermal stability and excellent electrical conductivity of the MAX phase to prepare a MoO3/Ti3AlC2 catalyst to increase its intrinsic RWGS activity. When molybdenum oxide is loaded on the MAX phase of the Ti3AlC2, the low surface area of the MAX phase leads to the formation of large MoO3 rods with blocky characteristics. The presence of electron-rich Ti3AlC2 enhanced the redox characteristics of MoO3 in the RWGS reaction, resulting in a high degree of surface reduction and the formation of many oxygen vacancies. Therefore, the catalyst showed the highest activity in catalytic experiments. When the MAX phase acts as a carrier, the electron-rich Mo site is an ideal activation site for CO2 via electron transfer to the CO2 antibond orbital, an interaction that weakens the C−O bond and favors reduction to CO. The catalyst is selective to CO, thus inhibiting the formation of methane and coke.
Zhang et al. [54] found that the surface modification of Ni by MoOx can regulate RWGS and methanation reactions. The addition of MoOx improves the dispersion of Ni through strong interaction, and the partially reduced MoOx modifies the surface of Ni particles using covering and electron modification, which enhances the desorption of CO on the surface. The addition of a large amount of Mo (Mo/Ni ratio of 1) causes the reaction to shift to RWGS and the CO selectivity to be greater than 94%. Kharaji et al. [57] incorporated Mo into the Fe/Al2O3 catalyst and found that Mo greatly improved the RWGS catalytic activity and CO selectivity due to the Fe−O−Mo structure formed in the Fe2(MoO4)3 phase of the Fe−Mo/Al2O3 catalyst. The presence of the Fe−O−Mo structure causes Fe2(MoO4)3 to have a lower reducibility, which effectively inhibits the reduction in Fe oxides. On the other hand, the existence of the Fe–O–Mo structure promotes the flow of electrons from Fe to Mo, resulting in the Fe species being in an electron–deficient state and forming a positive surface charge, which is detrimental to the adsorption of CO and effectively inhibiting the further hydrogenation of CO. As an additive and active component, Mo shows good CO selectivity, but its CO2 conversion needs to be improved.
Zhang et al. [137] reported the preparation of Cu−Cs−MO2C using Cu as an accelerator and Cs as a dopant to improve the conversion and selectivity of the catalyst, respectively. It was found that the increase in CO2 conversion was due to the addition of copper to provide more active sites for the catalyst, such as Cu+ and Cu0. Cs was due to its significant positive electric properties that create electron perturbations on the catalyst surface, thus enhancing catalytic performance. Its addition can improve CO selectivity, especially at low temperatures. In addition, the carbon–doped catalyst appears to be activated in situ due to the recarburizing phenomenon, which results in the catalyst being fairly stable in continuous operation.
Zhang et al. [138] used silica as the carrier to prepare a Mo−P−SiO2 catalyst and found that the catalyst was completely oxidized through characterization. It was found that the high selectivity of carbon monoxide was due to the MoP phase generated on the surface of the silica carrier, which was more conducive to the direct conversion of carbon dioxide into carbon monoxide through MoP(0001). Based on the potential energy−surface profile, methane generation on the MoP(0001) surface requires higher energy than carbon monoxide. In other words, the MoP(0001) surface is more selective for producing CO than CH4.
Yuan et al. [139] reported the application of Ni−doped MoS2 in CO2 hydrogenation into methanol and prepared a series of MoS2/NiX catalysts. Through DFT calculations, they found that MoS2/Ni–catalyzed CO2 hydrogenation follows the oxidation–reduction pathway, and the optimal adsorption structures and barrier energies of different intermediates were shown in the article. CO2 and H2 are directly dissociated from CO*, O*, and 2H* by an energy barrier of 0.63 eV. The large adsorption energy indicates that CO* has a strong interaction with MoS2 (Eads = −1.27 eV) and is more likely to react further on the carrier without releasing CO.
2.5. Other Catalytic Systems
2.5.1. Transition Metal Carbide Catalysts
Transition metal carbide catalysts (TMCs) are also promising and attractive catalysts; after the introduction of carbon, the electronic properties of these catalysts change, meaning that their activity becomes similar to that of precious metal catalysts, with a strong H2 dissociation and CO bond-breaking ability, and showing high activity in the CO2 hydrogenation to CO reaction. Therefore, it has been reported that catalysts such as vanadium carbide [140], tungsten carbide [141,142], cobalt carbide [143], and molybdenum carbide [126,144,145,146,147,148,149] have superior catalytic activity and CO selectivity when applied to RWGS reactions.
Juneau et al. reported K-promoted tungsten carbide with porous γ−Al2O3 as the carrier for RWGS in 2019 [141]. Later, they [142] found that micellar-based silica coating technology could prepare a 10 nm tungsten carbide nanoparticle catalyst. The high activity and CO selectivity of nano-WXC carburizing observed at 1000 °C may be due to the fact that the coated silica maintains a particle size of 10 nm during carburizing, thus promoting the formation of surface carbides. Compared with the catalyst prepared via the impregnation method, the synthesis method affects the formation degree of W × C and the reactivity of RWGS. In Zhang’s [143] paper, they described a mechanism for controlling the formation and quantity of carboxylate species on hollow cubic Co3O4 (without Mn), thereby modifying the surface energy and crystal growth rate. At 270 °C, where CO2 conversion was almost at its thermodynamic limits at a space velocity of 60,000 mL gcat–1 h–1, Co2C nanoprisms demonstrated excellent activity in RWGS. Reaction mechanisms and kinetics studies were used to correlate the catalytic performance of Co2C nanoprisms with highly active surfaces (020) and (101), as well as double reaction pathways (redox and formate routes). This work shows great potential for bridging RWGS and sequential cascade reactions, and it offers a way to design and modulate the morphologies of transition metal carbides. Reddy [144] synthesized Mo2C using the direct carbonization of Mo precursors. Under 723 K and 1 atmospheric pressure with a molar ratio of CO2/H2 = 1:3, the conversion of CO2 catalyzed by Mo2C can reach 57%, and the CO selectivity can reach 62%. Marquart et al. [145] prepared four catalysts using three different synthesis processes with good catalytic activity. The results show that CO2 forms adsorbed oxygen surface species by dissociating on the carbide surface. Above 850 K, the carbides oxidize into the oxide phase MoO2 or MoO3. The different catalysts all exhibit CO2 conversion rates higher than 30%, and the CO selectivity is 99% even at high H2/CO2 ratios. Although the new molybdenum carbide catalyst has excellent activity in the RWGS reaction, its preparation method is difficult compared with other catalysts.
2.5.2. Perovskite–Type Catalysts
Mixed metal oxides with ABO3-type perovskite structures acting as carriers of rare earth metals and transition metals are considered to be very promising catalytic ceramic materials [150]. The catalytic properties of perovskites can be modulated by changing the properties of A and B ions or inserting dopants into the structure. Perovskite oxides have attracted the attention of researchers due to their high mobility of oxygen and ability to stabilize unusual cationic oxidation states, as well as their thermal stability at high temperatures [151]. Alkali metals, alkaline earth metals, and rare earth metals are commonly used as accelerators, which can change the acid–base properties of the catalyst, improve the dispersion of the active species, and increase the strength of the interaction between the active species and the carrier. Kim et al. [152] added Y, Zn, and Ce to BaZrO3 and discussed the influence of dopants on the catalytic activity. At 600 °C for 5 h, all catalysts showed stable catalytic properties. Among them, the BaZr0.8Y0.16Zn0.04O3 (BZYZ) catalyst showed excellent activity with a CO2 conversion rate of 37.5% and CO selectivity of 97%. Ce insertion improved the ionic conductivity of BZYZ, but additional Ce did not have any positive effect on the catalytic activity of the RWGS reaction. Jo et al. [153] examined the effects of controlled template removal and nanocasting on La0.8Sr0.2FeO3 (LSF). The nano−cast LSF has a mesoporous structure due to using the SBA−15 template, and further etching enhances its textural characteristics. Characterization verified the high dispersion of metal–active sites on nano–cast LSF. RWGS operating at 600 °C used LSF as oxygen carriers with varying silica contents. It was discovered that the CO yield rose as the silica residue in LSF decreased; however, the catalyst’s mesoporous structure would be destroyed by excessive etching, removing too much silica (<5 wt%). An LSF with a 10 wt% silicon content outperforms RWGS. The surface−oxygen−to−lattice−oxygen atomic ratio could be a crucial factor in determining the reaction’s reactivity. The photothermal catalyst Ni/LaInO3 designed by Yu et al. [154] can convert CO2 into nearly uniform CO in a H2−rich environment with a high generation rate, which promotes the adsorption and activation of CO2. Due to the photoelectric effect, the RWGS reaction time can be significantly reduced. This study provides a new idea for designing efficient photothermal catalysts to maximize the use of solar energy in the future and lays the foundations for regulating product selectivity during catalytic CO2 hydrogenation.
In heterogeneous catalysis, the catalytic performance of bifunctional catalysts is often better than that of single-component catalysts. The shape, size, and distribution of catalytic particles also have a significant influence on the activity of the catalyst. Kopac et al. [155] theoretically studied the effects of bifunctional groups on RWGS reactions in detail. Density functional theory was used to calculate the energy diagram of RWGS on three surfaces (copper (111), SrTiO3 surface, and Cu/SrTiO3 interface between two solids). These three surfaces were combined using mesoscale dynamics Monte Carlo simulations to study the turnover and yield of CO production as a function of particle size. The results show that the reaction speed at the interface is faster. However, in addition to the interface, the binding of copper and carrier sites further accelerates the RWGS reaction, suggesting that the catalyst’s bifunction is manifested in more complex interactions between phases rather than just interface effects, such as hydrogen spillover. The authors found three different effects, namely an electronic effect, a synergistic effect, and a geometric effect, and a smaller Cu on the carrier SrTiO3 showed a higher CO formation rate.
2.6. Catalyst Deactivation in the Reverse Water–Gas Conversion Reaction
Under high-temperature conditions, the catalyst used for RWGS reaction has poor thermal stability or weak interactions between the active component and the carrier, which leads to sintering and agglomeration of the catalyst at high temperatures and decreases the activity of the catalyst [58]. In addition, carbon deposition is also an important cause of catalyst deactivation. Carbon deposition, as the name suggests, means that the surface of the catalyst is covered by a certain form of carbon, and carbon is mainly formed via carbon-containing substances in the raw material breaking bonds on the surface of the catalyst or first coking and then dehydrogenation. These carbon species cover the active site, thus reducing catalyst activity. Therefore, the stability of the catalyst at high temperatures is extremely important for the RWGS reaction.
Yang et al. [58] mixed Fe into Ni/CeO2–Al2O3, and the NiFe/CeO2–Al2O3 catalyst showed an excellent CO2 conversion rate in the stability test at 800,000 mL·g1·h−1 and 700 °C. FeOx greatly enhances Ni dispersion on the surface, which helps to provide higher activity in the reaction. In addition, the FeOx–Ni interaction leads to electron enrichment of Ni surface atoms, and higher electron density is conducive to CO2 adsorption. The 0.3CuMgAl–LDH–400 catalyst prepared using the hydrothermal method by Chen et al. [156] showed high stability during the RWGS reaction, and the activity remained unchanged for more than 30 h, which was due to the fact that the use of LDH as a carrier improved the dispersion of Cu and the presence of more alkaline sites. On the contrary, the catalytic activity of 0.3CuMgAl–IMP–400 prepared using impregnation decreased slightly after 20 h of the reaction. The results show that the catalyst prepared with LDH as the support has better stability.
Goguet et al. [59,157] studied the deactivation process of Pt/CeO2 catalysts. The stability test results show that the initial CO2 conversion rate is stable at 13.7%, and the CO selectivity is greater than 99%. As the reaction progresses, its catalytic activity begins to decrease gradually, possibly due to the catalyst’s carbon deposition or the metal’s sintering. In order to clarify the deactivation principle, the TPO cycle test was performed on the Pt/CeO2 catalyst before and after the reaction. The results show a linear relationship between the degree of deactivation of the Pt/CeO2 catalyst and the amount of carbon deposition. The place where carbon deposition occurs is the active part of the reaction. With the increase in time, the active part is gradually covered by carbon deposition, which leads to catalyst deactivation.
3. Conclusions
Converting carbon dioxide into high-value-added chemicals and fuels is a potential path to a carbon-neutral future. The reverse water–gas conversion reaction converts carbon dioxide into carbon monoxide and produces syngas, followed by methanation and the preparation of alcohols using Fischer–Tropsch synthesis, which is a promising method for carbon dioxide utilization. However, due to the endothermic reaction at low temperatures, the strong exothermic methanation reaction is competitive with the reverse water–gas change reaction. Although a high temperature is conducive to the forward reaction, the energy consumption of the reaction is too high. Therefore, developing catalysts capable of high conversion and selectivity at lower temperatures in the future will be a research focus for scientists. Transition metals ought to be the main subject of future research on the elemental makeup of active RWGS catalysts. Future resource shortages are inevitable, but they can be minimized by avoiding reliance on noble and rare elements. Targeting less expensive components will also lower overall expenses. Common elements like Cu, Fe, and Mo are important for further RWGS research because they can significantly increase catalytic activity, particularly when combined with metal oxides as a support. Future theoretical and computational investigations, in addition to experimental catalytic testing (i.e., DFT and general kinetic models), are required to facilitate the preparation of catalysts and to investigate the energetics of intermediates at various points within the catalytic structure through mechanism studies. Throughout the catalytic process, the catalyst’s deactivation and stability will be taken into account. Finally, for the development of catalysts with high CO2 conversion rates and high CO selectivity, we can proceed based on the following aspects: (1) improving the preparation method for catalysts to improve the dispersion of active components and (2) modifying the carrier to increase the dispersion of metal species and adjust the interaction between the active component and the carrier. In addition, we may be able to promote the development of low-carbon industrial catalysis using light-driven chemical reactions and develop and design suitable catalysts to gradually transition RWGS reactions from thermal reactions to photocatalytic processes.
Author Contributions
Writing—original draft, C.Z., J.Z. and Y.F. Writing—review and editing, H.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by support from the 2023 Jiangsu Degree and Postgraduate Education Teaching Reform project (JGKT23_C085), the 2023 Jiangsu Graduate Research and Practice Innovation plan (KYCX23_3469), the Funding for School-level Research projects of Yancheng Institute of Technology (No. xjr2019007 and No. xjr2023031), the 2023 Excellent Graduation Project (Thesis) Cultivation project of Yancheng Institute of Technology, and the Natural Science Foundation of Sichuan (No. 2023NSFSC1103).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tawalbeh, M.; Javed, R.M.N.; Al-Othman, A.; Almomani, F. The novel contribution of non-noble metal catalysts for intensified carbon dioxide hydrogenation. Energy Convers. Manag. 2023, 279, 116755. [Google Scholar] [CrossRef]
- Yao, X.; Zhong, P.; Zhang, X.; Zhu, L. Business model design for the carbon capture utilization and storage (CCUS) project in China. Energy Policy 2018, 121, 519. [Google Scholar] [CrossRef]
- Tapia, J.F.D.; Lee, J.; Ooi, R.E.H.; Foo, D.C.Y.; Tan, R.R. A review of optimization and decision-making models for the planning of CO2 capture, utilization and storage (CCUS) systems. Sustain. Prod. Consump. 2018, 13, 1. [Google Scholar] [CrossRef]
- Adelung, S.; Dietrich, R.U. Impact of the reverse water gas shift operating conditions on the Power-to-Liquid fuel production cost. Fuel 2022, 317, 123440. [Google Scholar] [CrossRef]
- Atsbha, T.A.; Yoon, T.; Seongho, P.; Lee, C. A review on the catalytic conversion of CO2 using H2 for synthesis of CO, methanol, and hydrocarbons. J. CO2 Util. 2021, 44, 101413. [Google Scholar] [CrossRef]
- Lima, D.D.S.; Dias, Y.R.; Pere z-Lopez, O.W. CO2 methanation over Ni-Al and Co-Al LDH-derived catalysts: The role of basicity. Sustain. Energy Fuels 2020, 4, 5747. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Wiese, K.; Parlinska-Wojtan, M.; Rabeah, J.; Behma, A.; Brückner, J.R. Encapsulation of Ru nanoparticles: Modifying the reactivity toward CO and CO2 methanation on highly active Ru/TiO2 catalysts. Appl. Catal. B-Environ. 2020, 270, 118846. [Google Scholar] [CrossRef]
- Dai, H.; Xiong, S.; Zhu, Y.; Zheng, J.; Huang, L.; Zhou, C.; Deng, J.; Zhang, X. NiCe bimetallic nanoparticles embedded in hexagonal mesoporous silica (HMS) for reverse water gas shift reaction. Chin. Chem. Lett. 2022, 33, 2590. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, A.; Xiong, S.; Xiao, X.; Zhou, C.; Pan, Y. The Catalytic Performance of Ga2O3-CeO2 Composite Oxides over Reverse Water Gas Shift Reaction. ChemCatChem 2022, 14, e202200049. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, R.; Waterhouse, G.I.N.; Zhang, T. Selective photothermal CO2 reduction to CO, CH4, alkanes, alkenes over bimetallic alloy catalysts derived from layered double hydroxide nanosheets. Nano Energy 2022, 102, 107650. [Google Scholar] [CrossRef]
- Xie, B.; Lovell, E.; Tan, T.H.; Jantarang, S.; Yu, M.; Scott, J.; Amal, R. Emerging material engineering strategies for amplifying photothermal heterogeneous CO2 catalysis. J. Energy Chem. 2021, 59, 108. [Google Scholar] [CrossRef]
- Zeng, X.; Tu, Z.; Yuan, L.; Xiao, C.; Wen, Y.; Xiong, K. Two-Dimensional transition metal hexaaminobenzene monolayer single atom catalyst for electrocatalytic Carbon Dioxide Reduction. Nanomaterials 2022, 12, 4005. [Google Scholar] [CrossRef]
- Xue, R.; Ge, P.; Xie, J.; Hu, Z.Y.; Wang, X.K.; Li, P.Q. Controllable CO2 reduction or hydrocarbon oxidation driven by entire solar via silver quantum dots direct photocatalysis. Small 2023, 19, 2207234. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yan, B.H.; Chen, J.G.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Portillo, E.; Gandara, J.; Reina, T.R.; Pastor-Perez, L. Is the RWGS a viable route for CO2 conversion to added value products? A techno-economic study to understand the optimal RWGS conditions. Sci. Total Environ. 2023, 857, 159394. [Google Scholar] [CrossRef]
- Alvarez-Galvan, C.; Lustemberg, P.G.; Oropeza, F.E.; Bashiller-Baeza, B.; Ospina, M.D.; Herranz, M.; Cebollada, J.; Collado, L.; Campos-Martin, J.M.; Pena-O’Shea, V.A.; et al. Highly active and stable Ni/La-doped ceria material for catalytic CO2 reduction by reverse water gas shift reaction. ACS Appl. Mater. Inter. 2022, 14, 50739. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, A.; Irankhah, A.; Aghamiri, S.F. Reverse water gas shift reaction and CO2 mitigation: Nanocrystalline MgO as a support for nickel based catalysts. J. Environ. Chem. Eng. 2018, 6, 4945. [Google Scholar] [CrossRef]
- Petersen, E.M.; Rao, R.G.; Vance, B.C.; Tessonnier, J.P. SiO2/SiC supports with tailored thermal conductivity to reveal the effect of surface temperature on Ru-catalyzed CO2 methanation. Appl. Catal. B-Environ. 2021, 286, 119904. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Zhang, S.; An, K.; Ma, Z.; Wang, L.; Liu, Y. Cerium modified Ni-La2O3/ZrO2 for CO2 methanation. J. Energy Chem. 2020, 43, 155. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.Y.; Chen, X.B.; Rui, N.; Betancourt, L.E.; Lin, L.L.; Xu, W.Q.; Sun, C.J.; Abeykoon, A.M.M.; Rodriguez, J.A.; et al. Effects of Zr doping into ceria for the dry reforming of methane over Ni/CeZrO2 catalysts: In situ studies with XRD, XAFS, and AP-XPS. ACS Catal. 2020, 10, 3274. [Google Scholar] [CrossRef]
- Martin, O.; Antonio, J.M.; Cecilia, M.; Sharon, M.; Takuya, F.S.; Roland, H.; Charlotte, D.; Daniel, C.F.; Javier, P.R. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation. Angew. Chem. Int. Ed. 2016, 55, 6261. [Google Scholar] [CrossRef]
- Chen, K.H.; Li, H.R.; He, L.N. Advance and prospective on CO2 activation and transformation strategy. Chin. J. Org. Chem. 2020, 40, 2195. [Google Scholar] [CrossRef]
- Panagiotopoulou, P. Hydrogenation of CO2 over supported noble metal catalysts. Appl. Catal. A-Gen 2017, 542, 63. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kuhn, J.N. CO2 conversion by reverse water gas shift catalysis: Comparison of catalysts, mechanisms and their consequences for CO2 conversion to liquid fuels. RSC Adv. 2016, 6, 49675. [Google Scholar] [CrossRef]
- Su, X.; Yang, X.L.; Zhao, B.; Huang, Y.Q. Designing of highly selective and high-temperature endurable RWGS heterogeneous catalysts: Recent advances and the future directions. J. Energy Chem. 2017, 26, 854. [Google Scholar] [CrossRef]
- Bahmanpour, A.M.; Signorile, M.; Kröcher, O. Recent progress in syngas production via catalytic CO2 hydrogenation reaction. Appl. Catal. B-Environ. 2021, 295, 120319. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Kumar, A.; Khraisheh, M. A Review of CeO2 Supported Catalysts for CO2 Reduction to CO through the Reverse Water Gas Shift Reaction. Catalysts 2022, 12, 1101. [Google Scholar] [CrossRef]
- Pahija, E.; Panaritis, C.; Gusarov, S.; Shadbahr, J.; Bensebaa, F.; Patience, G.; Boffito, D.C. Experimental and Computational Synergistic Design of Cu and Fe Catalysts for the Reverse Water-Gas Shift: A Review. ACS Catal. 2022, 12, 6887. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.L.; Wohlrab, S. Review of CO2 Reduction on Supported Metals (Alloys) and Single-Atom Catalysts (SACs) for the Use of Green Hydrogen in Power-to-Gas Concepts. Catalysts 2022, 12, 16. [Google Scholar] [CrossRef]
- Jing, H.; Li, Q.; Wang, J.; Liu, D.; Wu, K. Theoretical Study of the Reverse Water Gas Shift Reaction on Copper Modified β-Mo2C(001) Surfaces. J. Phys. Chem. C. 2019, 123, 1235. [Google Scholar] [CrossRef]
- Lin, L.; Yao, S.; Liu, Z.; Zhang, F.; Li, N.; Vovchok, D.; Martinez-Arias, A.; Castañeda, R.; Lin, J.; Senanayake, S.D. In situ characterization of Cu/CeO2 nanocatalysts for CO2 hydrogenation: Morphological effects of nanostructured ceria on the catalytic activity. J. Phys. Chem. C. 2018, 122, 12934. [Google Scholar] [CrossRef]
- Mine, S.; Yamaguchi, T.; Ting, K.W.; Maeno, Z.; Siddiki, S.M.A.H.; Oshima, K.; Satokawa, S.; Shimizu, K.; Toyao, T. Reverse water-gas shift reaction over Pt/MoOx/TiO2: Reverse Mars-van Krevelen mechanism via redox of supported MoOx. Catal. Sci. Technol. 2021, 11, 4172. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Xu, H.; Han, Y. Reverse water-gas shift reaction over ceria nanocube synthesized by hydrothermal method. Catal. Commun. 2016, 76, 1. [Google Scholar] [CrossRef]
- Lu, B.; Zhang, T.; Zhang, L.; Xu, Y.; Zhang, Z.; Wu, F.; Li, X.; Luo, C. Promotion effects of oxygen vacancies on activity of Na-doped CeO2 catalysts for reverse water gas shift reaction. Appl. Surf. Sci. 2022, 587, 152881. [Google Scholar] [CrossRef]
- Li, M.; My Pham, T.H.; Ko, Y.; Zhao, K.; Zhong, L.; Luo, W.; Züttel, A. Support-Dependent Cu-In Bimetallic Catalysts for Tailoring the Activity of Reverse Water Gas Shift Reaction. ACS Sustain. Chem. Eng. 2022, 10, 1524. [Google Scholar] [CrossRef]
- Gines, M.J.L.; Marchi, A.J.; Apesteguía, C.R. Kinetic study of the reverse water gas shift reaction over CuO/ZnO/Al2O₃ catalysts. Appl. Catal. A-Gen. 1997, 154, 15. [Google Scholar] [CrossRef]
- Wang, L.C.; Khazaneh, M.T.; Widmann, D.; Behm, R.J. TAP reactor studies of the oxidizing capability of CO2 on a Au/CeO2 catalyst—A first step toward identifying a redox mechanism in the reverse water gas shift reaction. J. Catal. 2013, 302, 20. [Google Scholar] [CrossRef]
- Liu, H.X.; Li, S.Q.; Wang, W.W.; Yu, W.Z.; Zhang, W.J.; Ma, C.; Jia, C.J. Partially sintered copper-ceria as excellent catalyst for the high temperature reverse water gas shift reaction. Nat. Commun. 2022, 13, 867. [Google Scholar] [CrossRef]
- Deng, L.; Su, Q.Q.; Ye, Q.; Wan, H.; He, Y.; Cui, X. Slag-based geopolymer microsphere-supported Cu: A low cost and sustainable catalyst for CO2 hydrogenation. Sustain. Energy Fuels 2022, 6, 1436. [Google Scholar] [CrossRef]
- Matsubu, J.C.; Zhang, S.; Derita, L.; Marinkovic, N.S.; Chen, J.G.; Graham, G.W.; Pan, X.; Christopher, P. Adsorbate mediated strong metal support interactions in oxide supported Rh catalysts. Nat. Chem. 2017, 9, 120. [Google Scholar] [CrossRef]
- Li, W.; Gan, J.; Liu, Y.; Zou, Y.; Zhang, S.; Qu, Y. Platinum and Frustrated Lewis Pairs on Ceria as Dual-Active Sites for Efficient Reverse Water-Gas Shift Reaction at Low Temperatures. Angew. Chem. Int. Ed. 2023, 62, e202305661. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Su, X.; Liang, B.; Yang, X.; Ren, X.; Duan, H.; Huang, Y.; Zhang, T. Identification of relevant active sites and a mechanism study for reverse water gas shift reaction over Pt/CeO2 catalysts. J. Energy Chem. 2016, 25, 1051. [Google Scholar] [CrossRef]
- Liu, Y.X.; Li, L.W.; Zhang, R.Y.; Guo, Y.H.; Wang, H.; Ge, Q.F.; Zhu, X.L. Synergetic enhancement of activity and selectivity for reverse water gas shift reaction on Pt-Re/SiO2 catalysts. J. CO2 Util. 2022, 63, 102128. [Google Scholar] [CrossRef]
- Bhalothia, D.; Yang, S.S.; Yan, C.; Beniwal, A.; Chang, Y.X.; Wu, S.C.; Chen, P.C.; Wang, K.W.; Chen, T.Y. Surface anchored atomic cobalt-oxide species coupled with oxygen vacancies boost the CO-production yield of Pd nanoparticles. Sustain. Energy Fuels 2023, 7, 526. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.; Wiese, K.; Hauble, A.; Bansmann, J.; Rabeah, J.; Parlinska-Wojtan, M.; Bruckner, A.; Behm, R. Steering the selectivity in CO2 reduction on highly active Ru/TiO2 catalysts: Support particle size effects. J. Catal. 2021, 401, 160. [Google Scholar] [CrossRef]
- Vikanova, K.V.; Kustov, A.L.; Makhov, E.A.; Tkachenko, O.P.; Kapustin, G.I.; Kalmykov, K.B.; Mishin, I.V.; Nissenbaum, V.D.; Dunaev, S.F.; Kustov, L.M. Rhenium-contained catalysts based on superacid ZrO2 supports for CO2 utilization. Fuel 2023, 351, 128956. [Google Scholar] [CrossRef]
- Rabee, A.; Zhao, D.; Cisneros, S.; Kreyenschulte, C.R.; Kondratenko, V.; Bartling, S.; Kubis, C.; Kondratenko, E.i.V.; Brückner, A.; Rabeah, J. Role of interfacial oxygen vacancies in low loaded Au-based catalysts for the low temperature reverse water gas shift reaction. Appl. Catal. B-Env. 2023, 321, 122083. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zang, Y.H.; Gao, F.; Qu, J.F.; Gu, J.F.; Lin, X.T. Enhanced catalytic activity of CO2 hydrogenation to CO over sulfur-containing Ni/ZrO2 catalysts: Support size effect. New J. Chem. 2022, 46, 22332. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, K.; Song, Y.; Lee, Y.; Roh, H.; Na, K. Highly CO-selective Ni-MgO-CexZr1-xO2 catalyst for efficient low-temperature reverse water-gas shift reaction. J. Ind. Eng. Chem. 2023, 118, 341. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, S.W.; Yoon, H.S.; Kim, Y.D. Reverse water gas shift reaction catalyzed by Fe nanoparticles with high catalytic activity and stability. J. Ind. Eng. Chem. 2015, 23, 67. [Google Scholar] [CrossRef]
- Zhuang, Y.; Currie, R.; McAuley Kimberley, B.; Simakov, D.S.A. Highly-selective CO2 conversion via reverse water gas shift reaction over the 0.5wt% Ru-promoted Cu/ZnO/Al2O3 catalyst. Appl. Catal. A-Gen. 2019, 575, 74. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Kumar, A.; Khraisheh, M. Combustion synthesis of copper ceria solid solution for CO2 conversion to CO via reverse water gas shift reaction. Int. J. Hydrogen Energy 2022, 47, 41259. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, J.; Sun, X.; Sun, J. Engineering nanointerfaces of Cu-based catalysts for balancing activity and stability of reverse water gas shift reaction. J. CO2 Util. 2023, 71, 102460. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, A.; Zhu, M.; Wu, X.X.; Wang, H.; Zhu, X.L.; Ge, Q.F. Tuning reverse water gas shift and methanation reactions during CO2 reduction on Ni catalysts via surface modification by MoOx. J. CO2 Util. 2021, 52, 101678. [Google Scholar] [CrossRef]
- Okemoto, A.; Harada, M.R.; Ishizaka, T.; Hiyoshi, N.; Soto, K. Catalytic performance of MoO3/FAU zeolite catalysts modified by Cu for reverse water gas shift reaction. Appl. Catal. A Gen. 2020, 592, 117415. [Google Scholar] [CrossRef]
- Ronda-lloret, M.; Yang, L.Q.Q.; Hammerton, M.; Marakatti, V.S.; Tromp, M.; Sofer, Z.; Sepulveda-Escribano, A.; Ramos-Fernandez, E.V.; Delgado, J.J.; Rothenberg, G.; et al. Molybdenum oxide supported on Ti3AlC2 is an active reverse water gas shift catalyst. ACS Sustain. Chem. Eng. 2021, 9, 4957. [Google Scholar]
- Kharaji, A.G.; Shariati, A.; Takassi, M. A novel γ-Alumina supported Fe-Mo bimetallic catalyst for reverse water gas shift reaction. Chin. J. Chem. Eng. 2013, 21, 1007. [Google Scholar] [CrossRef]
- Yang, L.; Pastor-perez, L.; Gu, S.; Sepúlveda-Escribano, A.; Reina, T.R. Highly efficient Ni/CeO2-Al2O₃ catalysts for CO2 upgrading via reverse water gas shift: Effect of selected transition metal promoters. Appl. Catal. B-Environ. 2018, 232, 464. [Google Scholar] [CrossRef]
- Goguet, A.; Meunier, F.; Breen, J.; Burch, R.; Petch, M.; Faurghenciu, A. Study of the origin of the deactivation of a Pt/CeO2 catalyst during reverse water gas shift (RWGS) reaction. J. Catal. 2004, 226, 382. [Google Scholar] [CrossRef]
- Lymperi, A.; Chatzilias, C.; Xydas, F.; Martino, E.; Kyriakou, G.; Katsaounis, A. Electrochemical Promotion of CO2 Hydrogenation Using a Pt/YSZ Fuel Cell Type Reactor. Nanomaterials 2023, 13, 1930. [Google Scholar] [CrossRef]
- Seuser, G.; Martinelli, M.; Garcia, E.S.; Upton, G.F.; Ayala, M.; Villarreal, J.; Rajabi, Z.; Cronauer, D.C.; Kropf, A.J.; Jacobs, G. Reverse water-gas shift: Na doping of m-ZrO2 supported Pt for selectivity control. Appl. Catal. A-Gen. 2023, 650, 119000. [Google Scholar] [CrossRef]
- Ge, H.; Kuwahara, Y.; Kusu, K.; Kobayashi, H.; Yamashita, H. Enhanced visible-NIR absorption and oxygen vacancy generation of Pt/HxMoWOy by H-spillover to facilitate photothermal catalytic CO2 hydrogenation. J. Mater. Chem. A 2022, 10, 10854. [Google Scholar] [CrossRef]
- He, Y.L.; Huang, D.H. Single-Atom Platinum Catalyst for Efficient CO2 Conversion via Reverse Water Gas Shift Reaction. Molecules 2023, 28, 6630. [Google Scholar] [CrossRef]
- Nelson, N.C.; Chen, L.X.; Meira, D.; Kovarik, L.; Szanyi, J. In situ dispersion of palladium on TiO2 during reverse water gas shift reaction: Formation of atomically dispersed palladium. Angew. Chem. Int. Ed. 2020, 59, 17657. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Miyakea, T.; Sugimasaa, M. Low-temperature RWGS enhancement of Pt1-nAun/CeO2 catalysts and their electronic state. RSC Adv. 2023, 13, 29320. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Qi, R.; Zhang, Y.; Gu, Q.; Xu, X.; Tan, Y.; Liu, X.; Wang, A.; Zhu, B.; Yang, B.; et al. Single-atom-driven dynamic carburization over Pd1-FeOx catalyst boosting CO2 conversion. Chem 2022, 8, 3252. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, T.; Tang, Y.; Jiang, Y.; Kitagawa, H.; Wen, X.; Wang, F. Size-modulated photo-thermal catalytic CO2 hydrogenation performances over Pd nanoparticles. J. Catal. 2023, 424, 22. [Google Scholar] [CrossRef]
- Chen, L.; Allec, S.I.; Nguyen, M.T.; Kovarik, L.; Hoffman, A.S.; Hong, J.; Meira, D.; Shi, H.; Bare, S.R.; Glezakou, V.A.; et al. Dynamic Evolution of Palladium Single Atoms on Anatase Titania Support Determines the Reverse Water-Gas Shift Activity. J. Am. Chem. Soc. 2023, 145, 10847. [Google Scholar] [CrossRef]
- Ni, W.; Zeng, M.; Wang, K.; Lin, Y.; Zhang, Z.; Dai, W.; Fu, X. Photo-thermal catalytic reverse water gas shift reaction over Pd/MaZrOx (M=Sr, SrMn) catalysts driven by Cycle-double sites. J. CO2 Util. 2023, 69, 102413. [Google Scholar] [CrossRef]
- Saravanan, P.K.; Bhalothia, D.; Huang, G.H.; Beniwal, A.; Cheng, M.; Chao, Y.C.; Lin, M.W.; Chen, P.C.; Chen, T.Y. Sub-Millisecond Laser-Irradiation-Mediated Surface Restructure Boosts the CO Production Yield of Cobalt Oxide Supported Pd Nanoparticles. Nanomaterials 2023, 13, 1801. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Wang, K.; Wang, H.; Liu, L.; Wu, X.; Geng, B.; Chu, X.; Song, S.; Zhang, H. Synergism of Ultrasmall Pt Clusters and Basic La2O2CO3 Supports Boosts the Reverse Water Gas Reaction Efficiency. Adv. Energy Mater. 2023, 13, 2203806. [Google Scholar] [CrossRef]
- Tang, R.; Zhu, Z.; Li, C.; Xiao, M.Q.; Wu, Z.Y.; Zhang, D.K.; Zhang, C.C.; Xiao, Y.; Chu, M.Y.; Genest, A.; et al. Ru-catalyzed reverse water gas shift reaction with near unity selectivity and superior stability. ACS Mater. Lett. 2021, 3, 1652. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Shen, J.; Li, C.; Zhang, C.; Feng, K.; Wang, Z.; Wang, X.; Meira, D.M.; Cai, M.; Zhang, D.; et al. Mo2TiC2 MXene-Supported Ru Clusters for Efficient Photothermal Reverse Water-Gas Shift. ACS Nano. 2023, 17, 1550. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Filot, I.A.W.; Hensen, E.J.M. Elucidation of the Reverse Water-Gas Shift Reaction Mechanism over an Isolated Ru Atom on CeO2(111). J. Phys. Chem. C. 2023, 127, 20314. [Google Scholar] [CrossRef]
- Kim, G.; Shin, S.; Choi, Y.; Kim, J.; Kim, G.; Kim, K.; Lee, H. Gas-Permeable Iron-Doped Ceria Shell on Rh Nanoparticles with High Activity and Durability. JACS Au. 2022, 2, 1115. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shao, S.; Liu, Y.; Cao, M.; Yu, J.; Lau, C.H.; Zheng, Y.; Fan, X. DRIFTS-SSITKA-MS investigations on the mechanism of plasmon preferentially enhanced CO2 hydrogenation over Au/γ-Al2O3. Appl. Catal. B-Environ. 2023, 328, 122531. [Google Scholar] [CrossRef]
- Kang, X.; Yuan, D.; Yi, Z.; Yu, C.; Yuan, X.; Liang, B.; Sun, X.; Gao, L.; Wang, S.; Li, Y. Bismuth single atom supported CeO2 nanosheets for oxidation resistant photothermal reverse water gas shift reaction. Catal. Sci. Technol. 2022, 12, 5559. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Y.; Gao, B.; Zhang, L.; Guo, L. Size-Dependent Active Site and Its Catalytic Mechanism for CO2 Hydrogenation Reactivity and Selectivity over Re/TiO2. ACS Catal. 2023, 13, 10364. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Mou, L.; Liu, Q.; Li, Z.; Li, X.; He, S. Reverse water-gas shift reaction catalyzed by diatomic rhodium anions. Phys. Chem. Chem. Phys. 2022, 24, 14616. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, S.; Zhou, H.; Huang, C.; Xia, L.; Liu, X.; Luo, H.; Wang, H. Ir Single Atoms and Clusters Supported on α-MoC as Catalysts for Efficient Hydrogenation of CO2 to CO. Acta Phys-Chim. Sin. 2023, 39, 230202. [Google Scholar]
- Mhamane, N.B.; Chetry, S.; Ranjan, R.; Raja, T.; Gopinath, C.S. Sustainable CO2 Reduction on In2O3 with Exclusive CO Selectivity: Catalysis and In Situ Valence Band Photoelectron Spectral Investigations. ACS Sustain. Chem. Eng. 2022, 10, 3521. [Google Scholar] [CrossRef]
- Gong, J.; Chu, M.Y.; Guan, W.H.; Liu, Y.; Zhong, Q.X.; Cao, M.H.; Xu, Y. Regulating the interfacial synergy of Ni/Ga2O₃ for CO2 hydrogenation toward the reverse water gas shift reaction. Ind. Eng. Chem. Res. 2021, 60, 9448. [Google Scholar] [CrossRef]
- Zhang, Q.; Pastor-P´erez, L.; Villora-Pico, J.J.; Joyce, M.; Sepúlveda-Escribano, A.; Duyar, M.S.; Reina, T.R. Ni-Phosphide catalysts as versatile systems for gas-phase CO2 conversion: Impact of the support and evidences of structure-sensitivity. Fuel 2022, 323, 124301. [Google Scholar] [CrossRef]
- Shen, H.; Dong, Y.; Yang, S.; He, Y.; Wang, Q.; Cao, Y.; Wang, W.; Wang, T.; Zhang, Q.; Zhang, H. Identifying the roles of Ce3+-OH and Ce-H in the reverse water-gas shift reaction over highly active Ni-doped CeO2 catalyst. Nano Res. 2022, 15, 5831. [Google Scholar] [CrossRef]
- Ranjbar, A.; Aghamiri, S.F.; Irankhah, A. Effect of MgO/Al2O3 ratio in the support of mesoporous Ni/MgO-Al2O3 catalysts for CO2 utilization via reverse water gas shift reaction. Int. J. Hydrogen Energy 2023, 48, 19115. [Google Scholar] [CrossRef]
- Zagoraios, D.; Kokkinou, N.; Kyriakou, G.; Katsaounis, A. Electrochemical control of the RWGS reaction over Ni nanoparticles deposited on yttria stabilized zirconia. Catal. Sci. Technol. 2022, 12, 1869. [Google Scholar] [CrossRef]
- Lozano-Reis, P.; Prats, H.; Sayós, R.; Illas, F. Limitations of free energy diagrams to predict the catalytic activity: The reverse water gas shift reaction catalyzed by Ni/TiC. J. Catal. 2023, 425, 203. [Google Scholar] [CrossRef]
- Rutherford, B.; Panaritis, C.; Pahija, E.; Couillard, M.; Patarachao, B.; Shadbahr, J.; Bensebaa, F.; Patience, G.S.; Boffito, D.C. Ni nanoparticles on Co3O4 catalyze the reverse water-gas shift with 95% CO selectivity at 300 °C. Fuel 2023, 348, 128235. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, Z.; Wu, X.; Xiong, W.; Ding, J.; Zhuang, Z.; Huang, W. Ni Single Atoms Confined in Nitrogen-Doped Carbon Nanotubes for Active and Selective Hydrogenation of CO2 to CO. ACS Catal. 2023, 13, 7132. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Huang, C.; Chen, C.; Choojun, K.; Sooknoi, T.; Tian, H.; Lin, Y. Reversal of methanation-oriented to RWGS-oriented Ni/SiO2 catalysts by the exsolution of Ni2+ confined in silicalite-1. Green Chem. 2023, 25, 7582. [Google Scholar] [CrossRef]
- Wei, A.; Zhang, R.; Qin, Y.; Wang, H.; Zhu, X.; Ge, Q. Theoretical Insight into Tuning CO2 Methanation and Reverse Water Gas Shift Reactions on MoOx-Modified Ni Catalysts. J. Phys. Chem. C. 2022, 126, 18078. [Google Scholar] [CrossRef]
- Arpini, B.H.; Braga, A.H.; Borges, L.R.; Vidinha, P.; Gonçalves, R.V.; Szanyi, J.; Rossi, L.M. Tuning CO2 Hydrogenation Selectivity by N-Doped Carbon Coating over Nickel Nanoparticles Supported on SiO2. ACS Sustain. Chem. Eng. 2022, 10, 2331. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Chen, L.; Li, Y. Breaking the Linear Scaling Relationship of the Reverse Water-Gas-Shift Reaction via Construction of Dual-Atom Pt-Ni Pairs. ACS Catal. 2023, 13, 3735. [Google Scholar] [CrossRef]
- Błaszczak, P.; Zając, M.; Ducka, A.; Matlak, K.; Wolanin, B.; Wang, S.; Mandziak, A.; Bochentyn, B.; Jasiński, P. High-temperature Co-electrolysis of CO2/H2O and direct methanation over Co-impregnated SOEC. Bimetallic synergy between Co and Ni. Int. J. Hydrogen Energy 2022, 47, 35017. [Google Scholar] [CrossRef]
- Merkouri, L.; Saché, E.; Pastor-Pérez, L.; Duyar, M.S.; Reina, T.R. Versatile Ni-Ru catalysts for gas phase CO2 conversion: Bringing closer dry reforming, reverse water gas shift and methanation to enable end-products flexibility. Fuel 2022, 315, 123097. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, R.; Liu, Y.; Wu, X.; Wang, H.; Ge, Q.; Zhu, X. Blocking Methanation during Reverse Water Gas Shift Reaction on Ni/SiO2 Catalysts by Surface Ag. ChemCatChem 2022, 15, e202201284. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Li, J.; Wang, Z. In-Ni Intermetallic Compounds Derived from Layered Double Hydroxides as Efficient Catalysts toward the Reverse Water Gas Shift Reaction. ACS Catal. 2022, 12, 4026. [Google Scholar] [CrossRef]
- Cai, W.; Yin, J.; Hu, C.; Han, H.; Ma, J.; Cao, Y.; Zhao, Y. Fe-Co-Ni Trimetallic Catalysts with MOFs as Precursor for CO2 Hydrogenation to C2-C4 Hydrocarbons: Insight Into the Influence of Ni. Catal Lett. 2023, 153, 2718. [Google Scholar] [CrossRef]
- Bogdan, V.I.; Koklin, A.E.; Kustov, A.L.; Pokusaeva, Y.A.; Bogdan, T.V.; Kustov, L.M. Carbon Dioxide Reduction with Hydrogen on Fe, Co Supported Alumina and Carbon Catalysts under Supercritical Conditions. Molecules 2021, 26, 2883. [Google Scholar] [CrossRef]
- Zhang, Q.; Pastor-perez, L.; Wang, Q.; Reina, T.R. Conversion of CO2 to added value products via rwgs using Fe-promoted catalysts: Carbide, metallic Fe or a mixture. Energy Chem. 2022, 66, 635. [Google Scholar] [CrossRef]
- Watanabe, R.; Karasawa, F.; Yokoyama, C.; Oshima, K.; Kishida, M.; Hori, M.; Ono, Y.; Satokawa, S.; Verma, P.; Fukuhara, C. Highly stable Fe/CeO2 catalyst for the reverse water gas shift reaction in the presence of H2S. RSC Adv. 2023, 13, 11525. [Google Scholar] [CrossRef]
- Xu, M.; Cao, C.; Xu, J. Understanding kinetically interplaying reverse water-gas shift and Fischer-Tropsch synthesis during CO2 hydrogenation over Fe-based catalysts. Appl. Catal. A-Gen. 2022, 641, 118682. [Google Scholar] [CrossRef]
- Malhi, H.S.; Sun, C.; Zhang, Z.; Liu, Y.; Liu, W.; Ren, P.; Tu, W.; Han, Y. Catalytic consequences of the decoration of sodium and zinc atoms during CO2 hydrogenation to olefins over iron-based catalyst. Catal. Today 2022, 387, 28. [Google Scholar] [CrossRef]
- Fedorov, A.; Lund, H.; Kondratenko, V.A.; Kondratenko, E.V.; Linke, D. Elucidating reaction pathways occurring in CO2 hydrogenation over Fe-based catalysts. Appl. Catal. B-Environ. 2023, 328, 122505. [Google Scholar] [CrossRef]
- Panek, B.; Kierzkowska-Pawlak, H.; Uznański, P.; Nagy, S.; Nagy-Trembošová, V.; Tyczkowski, J. The Role of Carbon Nanotube Deposit in Catalytic Activity of FeOX-Based PECVD Thin Films Tested in RWGS Reaction. Catalysts 2023, 13, 1302. [Google Scholar] [CrossRef]
- Ai, X.; Xie, H.; Chen, S.; Zhang, G.; Xu, B.; Zhou, G. Highly dispersed mesoporous Cu/γ-Al2O3 catalyst for RWGS reaction. Int. J. Hydrogen Energy 2022, 47, 14884. [Google Scholar] [CrossRef]
- Liu, C.; Nauert, S.L.; Alsina, M.A.; Wang, D.; Grant, A.; He, K.; Weitz, E.; Nolan, M.; Gray, K.A.; Notestein, J.M. Role of surface reconstruction on Cu/TiO2 nanotubes for CO2 conversion. Appl. Catal. B Env. 2019, 255, 117754. [Google Scholar] [CrossRef]
- Gonzalez-arias, J.; Gonzalez-Castano, M.; Sanchez, M.E.; Cara-Jimenez, J.; Arellano-Garcia, H. Valorization of biomass-derived CO2 residues with Cu-MnOx catalysts for RWGS reaction. Renew. Energy 2022, 182, 443. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, M.; Wang, K.; Yue, X.; Chen, X.; Dai, W.; Fu, X. Visible light-assisted thermal catalytic reverse water gas reaction over Cu-CeO2: The synergistic of hot electrons and oxygen vacancies induced by LSPR effect. Fuel 2022, 315, 123186. [Google Scholar] [CrossRef]
- Dehghanpoor, S.; Sedaghat, M.H.; Bakhtyari, A.; Makarem, M.A.; Rahimpour, M.R. A Feasibility Study of the Conversion of Petrochemical Off-Gas Streams to Methanol Over CuO/ZnO/Al2O3 Catalyst. Top. Catal. 2022. [Google Scholar] [CrossRef]
- Xiong, W.; Wu, Z.; Chen, X.; Ding, J.; Ye, A.; Zhang, W.; Huang, W. Active copper structures in ZnO-Cu interfacial catalysis: CO2 hydrogenation to methanol and reverse water-gas shift reactions. Sci. China Chem. 2023. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Kumar, A.; Khraisheh, M. Combustion synthesis of lanthanum oxide supported Cu, Ni, and CuNi nanoparticles for CO2 conversion reaction. Int. J. Hydrogen Energy 2023, 48, 24580. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, L.; Pan, W.B.; Shen, Y.Y.; Li, H. Composition effect in CuZr nanoparticles for CO2 conversion to CH3OH. Mater. Chem. Phys. 2022, 283, 125994. [Google Scholar] [CrossRef]
- Hu, R.; Wang, T.; Li, H.; Zhu, Y.; Wang, Y.; Wen, F.; Xing, E.; Wu, Y.; Da, Z. Cu and Cu-Fe Bi-Metal Nanoparticles Encapsulated in Hollow S-1 Zeolite for Reverse Water Gas Shift Reaction. Catalysts 2023, 13, 1037. [Google Scholar] [CrossRef]
- Gao, Y.; Xiong, K.; Zhu, B. Design of Cu/MoOx for CO2 Reduction via Reverse Water Gas Shift Reaction. Catalysts 2023, 13, 684. [Google Scholar] [CrossRef]
- Tarifa, P.; González-Castaño, M.; Cazaña, F.; Monzón, A.; Arellano-García, H. Development of one-pot Cu/cellulose derived carbon catalysts for RWGS reaction. Fuel 2022, 319, 123707. [Google Scholar] [CrossRef]
- Zuo, J.; Na, W.; Zhang, P.; Yang, X.; Wen, J.; Zheng, M.; Wang, H. Enhanced activity of CexZr1-xO2 solid solutions supported Cu-based catalysts for hydrogenation of CO2 to methanol. Mol. Catal. 2022, 526, 112357. [Google Scholar] [CrossRef]
- Hu, M.; Hu, H.; Tang, S.; Pan, Z. Enhanced CuAl2O4 Catalytic Activity via Alkalinization Treatment toward High CO2 Conversion during Reverse Water Gas Shift Reaction. Catalysts 2022, 12, 1511. [Google Scholar] [CrossRef]
- Cui, X.; Liu, Y.; Yan, W.; Xue, Y.; Mei, Y.; Li, J.; Gao, X.; Zhang, H.; Zhu, S.; Niu, Y.; et al. Enhancing methanol selectivity of commercial Cu/ZnO/Al2O3 catalyst in CO2 hydrogenation by surface silylation. Appl. Catal. B-Environ. 2023, 339, 123099. [Google Scholar] [CrossRef]
- Querido, A.R.; Goncalves, L.P.L.; Kolen’ko, Y.V.; Pereira, M.F.R.; Soares, O.S.G.P. Enhancing the performance of Cu catalysts for the reverse water-gas shift reaction using N-doped CNT-ZnO composite as support. Catal. Sci. Technol. 2023, 13, 3606. [Google Scholar] [CrossRef]
- Pahija, E.; Panaritis, C.; Rutherford, B.; Couillard, M.; Patarachao, B.; Shadbahr, J.; Bensebaa, F.; Patience, G.S.; Boffito, D.C. FeOx nanoparticle doping on Cu/Al2O3 catalysts for the reverse water gas shift. J. CO2 Util. 2022, 64, 102155. [Google Scholar] [CrossRef]
- Wang, J.; Couillard, M.; Baranova, E.A. Insight into Electrochemical Promotion of Cu/Co3O4 Catalysts for the Reverse Water Gas Shift Reaction. ChemCatChem 2023, 15, e202201514. [Google Scholar] [CrossRef]
- Álvarez-Hernández, D.; Marín-Sánchez, M.; Lobo-Andrades, L.; Azancot, L.; Bobadilla, L.F.; Ivanova, S.; Centeno, M.A. Low-temperature reverse water gas-shift reaction over highly efficient Cu-hydrotalcites: Mechanistic insights on the role of malachite phase. Catal. Today. 2023, 422, 114235. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Lu, S.; Li, J.; Zhang, H.; Su, X.; Xue, F.; Cao, B.; Fang, T. Mechanism of CO2 hydrogenation over a Zr1-Cu single-atom catalyst. New J. Chem. 2022, 11, 5043–5051. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, X.; Ke, Q. Mechanism of CO2 hydrogenation to methanol on the W-doped Rh(111) surface unveiled by first-principles calculation. Colloids Surf. A 2022, 638, 128332. [Google Scholar] [CrossRef]
- Jurado, A.; Morales-García, A.; Viñes, F.; Illas, F. Molecular Mechanism and Microkinetic Analysis of the Reverse Water Gas Shift Reaction Heterogeneously Catalyzed by the Mo2C MXene. ACS Catal. 2022, 12, 15658. [Google Scholar] [CrossRef]
- Ortner, N.; Zhao, D.; Mena, H.; Weiß, J.; Lund, H.; Bartling, S.; Wohlrab, S.; Armbruster, U.; Kondratenko, E.V. Revealing Origins of Methanol Selectivity Loss in CO2 Hydrogenation over CuZn-Containing Catalysts. ACS Catal. 2023, 13, 60. [Google Scholar] [CrossRef]
- Phey, M.L.P.; Abdullah, T.A.T.; Ali, U.F.M.; Mohamud, M.Y.; Ikram, M.; Nabgan, W. Reverse water gas shift reaction over a Cu/ZnO catalyst supported on regenerated spent bleaching earth (RSBE) in a slurry reactor: The effect of the Cu/Zn ratio on the catalytic activity. RSC Adv. 2023, 5, 3039. [Google Scholar] [CrossRef]
- Zhang, W.; Vidal-López, A.; Comas-Vives, A. Theoretical study of the catalytic performance of Fe and Cu single-atom catalysts supported on Mo2C toward the reverse water-gas shift reaction. Front. Chem. 2023, 11, 1144189. [Google Scholar] [CrossRef] [PubMed]
- Zafar, F.; Zhao, R.; Ali, M.; Park, Y.M.; Roh, H.; Gao, X.; Tian, J.; Bae, J.W. Unprecedented contributions of In2O3 promoter on ordered mesoporous Cu/Al2O3 for CO2 hydrogenation to oxygenates. Chem. Eng. J. 2022, 439, 135649. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; Fang, Z.; Zhou, H.; Zhu, J.; Li, Y.; Huang, S.; Lin, W.; Zhang, Y. Unveiling the role of adsorbed hydrogen in tuning the catalytic activity of CO2 conversion to methanol at Cu/TiC surfaces. J. CO2 Util. 2023, 72, 102515. [Google Scholar] [CrossRef]
- Chen, W.; Maugé, F.; Van Gestel, J.; Nie, H.; Li, D.; Long, X. Effect of modification of the alumina acidity on the properties of supported Mo and CoMo sulfide catalysts. J. Cat. 2013, 304, 47. [Google Scholar] [CrossRef]
- Sameer, S.; Singh, G.; Gahtori, J.; Goyal, R.; Ghosh, I.K.; Barrabes, N.; Bordoloi, A. Molybdenum sulfide embedded mesoporous N-Doped carbon as a noble metal-free highly selective catalyst for conversion of CO2 to CO. J. Environ. Chem. Eng. 2022, 10, 108988. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, K.; Li, Z.; Yang, B.; Yan, B.; Luo, K.H. Defect-Driven Efficient Selective CO2 Hydrogenation with Mo-Based Clusters. JACS Au. 2023, 3, 2736. [Google Scholar] [CrossRef] [PubMed]
- Dasireddy, V.D.B.C.; Vengust, D.; Likozar, B.; Kovač, J.; Mrzel, A. Production of syngas by CO2 reduction through reverse water gas shift (RWGS) over catalytically active molybdenum based carbide, nitride and composite nanowires. Renew. Energy 2021, 176, 251. [Google Scholar] [CrossRef]
- Xin, H.; Lin, L.; Li, R.; Li, D.; Song, T.Y.; Mu, R.T.; Fu, Q.; Bao, X.H. Overturning CO2 hydrogenation selectivity with high activity via reaction induced strong metal support Interactions. J. Am. Chem. Soc. 2022, 144, 4874. [Google Scholar] [CrossRef]
- Zhang, Q.; Pastor-Pérez, L.; Jin, W.; Gu, S.; Reina, T.R. Understanding the promoter effect of Cu and Cs over highly effective β-Mo2C catalysts for the reverse water-gas shift reaction. Appl. Catal. B-Env. 2019, 244, 889. [Google Scholar] [CrossRef]
- Zhang, Q.; Bown, M.; Pastor-Pérez, L.; Duyar, M.S.; Reina, T.R. CO2 Conversion via Reverse Water Gas Shift Reaction Using Fully Selective Mo-P Multicomponent Catalysts. Ind. Eng. Chem. Res. 2022, 61, 12857. [Google Scholar] [CrossRef]
- Yuan, Y.; Qi, L.; Gao, Z.; Guo, T.; Zhai, D.; He, Y.; Ma, J.; Guo, Q. Performance Exploration of Ni-Doped MoS2 in CO2 Hydrogenation to Methanol. Molecules 2023, 28, 5796. [Google Scholar] [CrossRef]
- Pajares, A.; Prats, H.; Romero, A.; Viñes, F.; Piscina, P.R.; Sayós, R.; Homs, N.; Illas, F. Critical effect of carbon vacancies on the reverse water gas shift reaction over vanadium carbide catalysts. Appl. Catal. B-Environ. 2020, 267, 118719. [Google Scholar] [CrossRef]
- Morse, J.R.; Juneau, M.; Baldwin, J.W.; Porosoff, M.D.; Willauer, H.D. Alkali promoted tungsten carbide as a selective catalyst for the reverse water gas shift reaction. J. CO2 Util. 2020, 35, 38. [Google Scholar] [CrossRef]
- Juneau, M.; Yaffe, D.; Liu, R.; Agwara, J.N.; Porosoff, M.D. Establishing tungsten carbides as active catalysts for CO2 Hydrogenation. Nanoscale 2022, 14, 16458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Luo, H.; Wu, Z.; Wei, B.; Shao, Z.; Huang, C.; Hua, K.; Xia, L.; Li, J.; et al. Morphological Modulation of Co2C by Surface-Adsorbed Species for Highly Effective Low-Temperature CO2 Reduction. ACS Catal. 2022, 12, 8544–8557. [Google Scholar] [CrossRef]
- Reddy, K.P.; Dama, S.; Mhamane, N.B.; Ghosalya, M.K.; Raja, T.; Satyanarayana, C.V.; Gopinath, C.S. Molybdenum carbide catalyst for the reduction of CO2 to CO: Surface science aspects by nappes and catalysis studies. Dalton Trans. 2019, 48, 12199. [Google Scholar] [CrossRef]
- Marquart, W.; Raseale, S.; Prieto, G.; Zimina, A.; Fisher, N. CO2 reduction over Mo2C-based catalysts. ACS Catal. 2021, 11, 1624. [Google Scholar] [CrossRef]
- Holder, C.F.; Morse, J.R.; Barboun, P.M.; Shabaev, A.R.; Baldwin, J.W.; Willauer, H.D. Evaluating metal oxide support effects on the RWGS activity of Mo2C catalysts. Catal. Sci. Technol. 2023, 13, 2685. [Google Scholar] [CrossRef]
- Pajares, A.; Liu, X.; Busacker, J.R.; Piscina, P.R.D.L.; Homs, N. Supported Nanostructured MoxC Materials for the Catalytic Reduction of CO2 through the Reverse Water Gas Shift Reaction. Nanomaterials 2022, 12, 3165. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Trigueros, A.; Caccia, M.; Caccia, J. Tuning Activity and Selectivity in Catalyzed Reactions of Environmental and Industrial Importance with a High-Surface Area, Mesoporous Mo2C Catalyst. Chem. Mater. 2022, 34, 6232. [Google Scholar] [CrossRef]
- Liu, R.; Chen, C.; Chu, W.; Sun, W. Unveiling the Origin of Alkali Metal (Na, K, Rb, and Cs) Promotion in CO2 Dissociation over Mo2C Catalysts. Materials 2022, 15, 3775. [Google Scholar] [CrossRef]
- Bhavani, A.G.; Kim, W.Y.; Lee, J.S. Barium substituted lanthanum manganite perovskite for CO2 reforming of methane. ACS Catal 2013, 3, 1537. [Google Scholar] [CrossRef]
- Royer, S.; Alamdari, H.; Duprez, D.; Kaliaguine, S. Oxygen storage capacity of La1-xA′BO3 perovskites (with A’=Sr, Ce; B=Co, Mn) relation with catalytic activity in the CH4 oxidation reaction. Appl. Catal. B Environ. 2005, 58, 273. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, J.L.; Park, E.J.; Kim, Y.D.; Uhm, S. Dopant effect of barium zirconate-based perovskite-type catalysts for the intermediate temperature reverse water gas shift reaction. ACS Catal. 2014, 4, 3117. [Google Scholar] [CrossRef]
- Jo, A.; Kim, Y.; Lim, H.S.; Lee, M.; Kang, D.; Lee, J.W. Controlled template removal from nanocast La0.8Sr0.2FeO3 for enhanced CO2 conversion by reverse water gas shift chemical looping. J. CO2 Util. 2022, 56, 101845. [Google Scholar] [CrossRef]
- Yu, J.; Muhetaer, A.; Gao, X.; Zhang, Z.; Yang, Y.; Li, Q.; Chen, L.; Liu, H.; Xu, D. Highly Active Hydrogen-rich Photothermal Reverse Water Gas Shift Reaction on Ni/LaInO3 Perovskite Catalysts with Near-unity Selectivity. Angew. Chem. Int. Ed. 2023, 62, e202303135. [Google Scholar] [CrossRef] [PubMed]
- Kopac, D.; Likozar, B.; Hus, M. How size matters: Electronic, cooperative, and geometric effect in perovskite supported copper catalysts for CO2 reduction. ACS Catal. 2020, 10, 4102. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, H.F.; Cai, J.Y.; Li, Z. Highly Efficient CO2 to CO transformation over Cu-based catalyst derived from a CuMgAl-Layered Double Hydroxide (LDH). ChemCatChem 2021, 13, 656. [Google Scholar] [CrossRef]
- Goguet, A.; Meunier, F.C.; Tibiletti, D.; Breen, J.P.; Burch, R. Spectrokinetic investigation of reverse water gas shift reaction Intermediates over a Pt/CeO2 Catalyst. J. Phys. Chem. B 2004, 108, 20240. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).