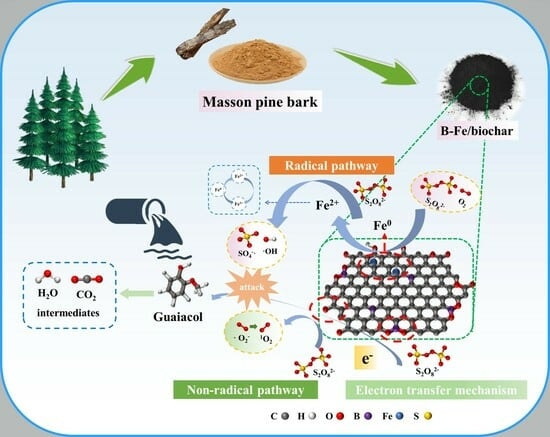
Insights into Enhanced Peroxydisulfate Activation with B and Fe Co-Doped Biochar from Bark for the Rapid Degradation of Guaiacol
Abstract
:1. Introduction
2. Results and Discussion
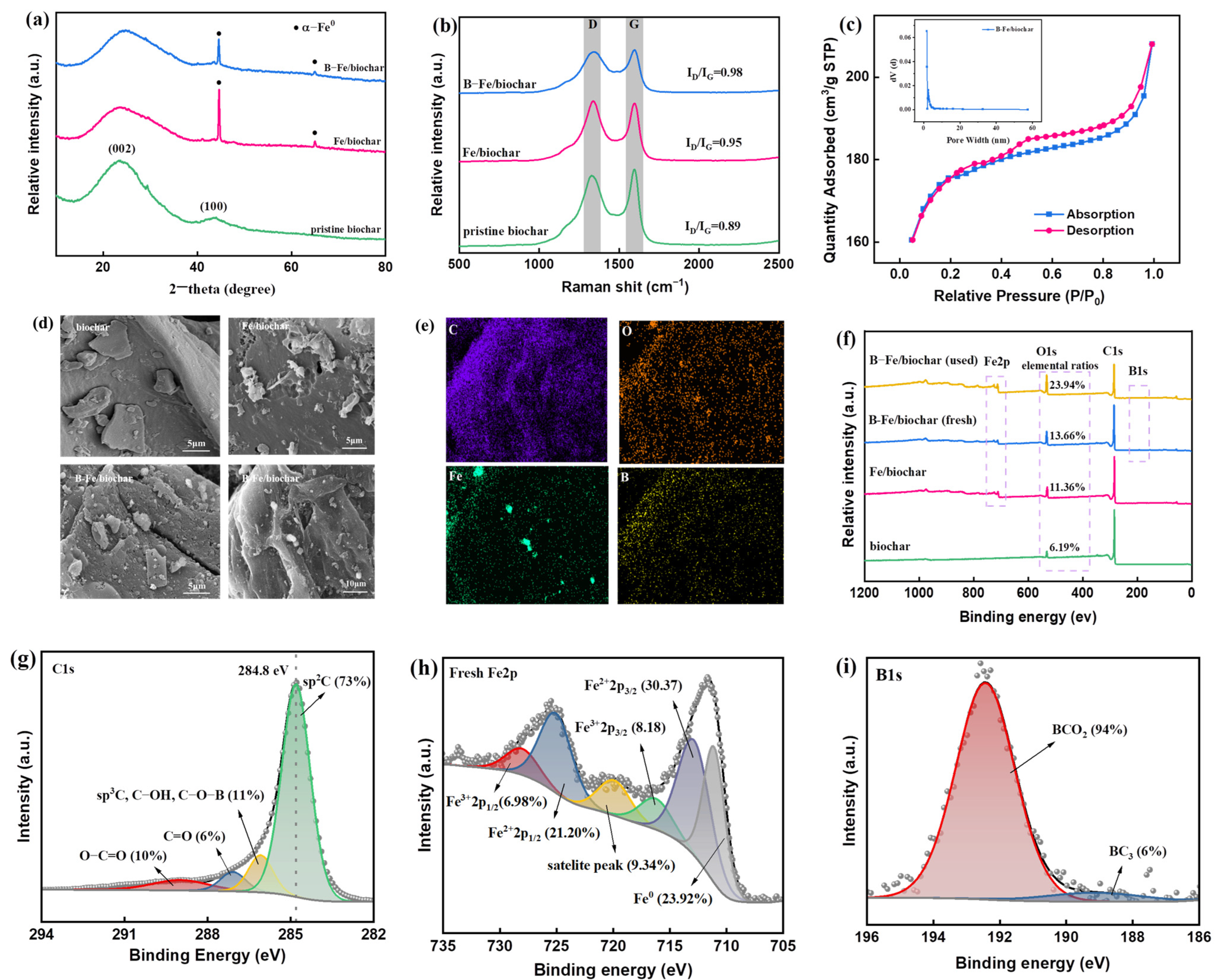
2.1. Characterizations of B-Fe/Biochar
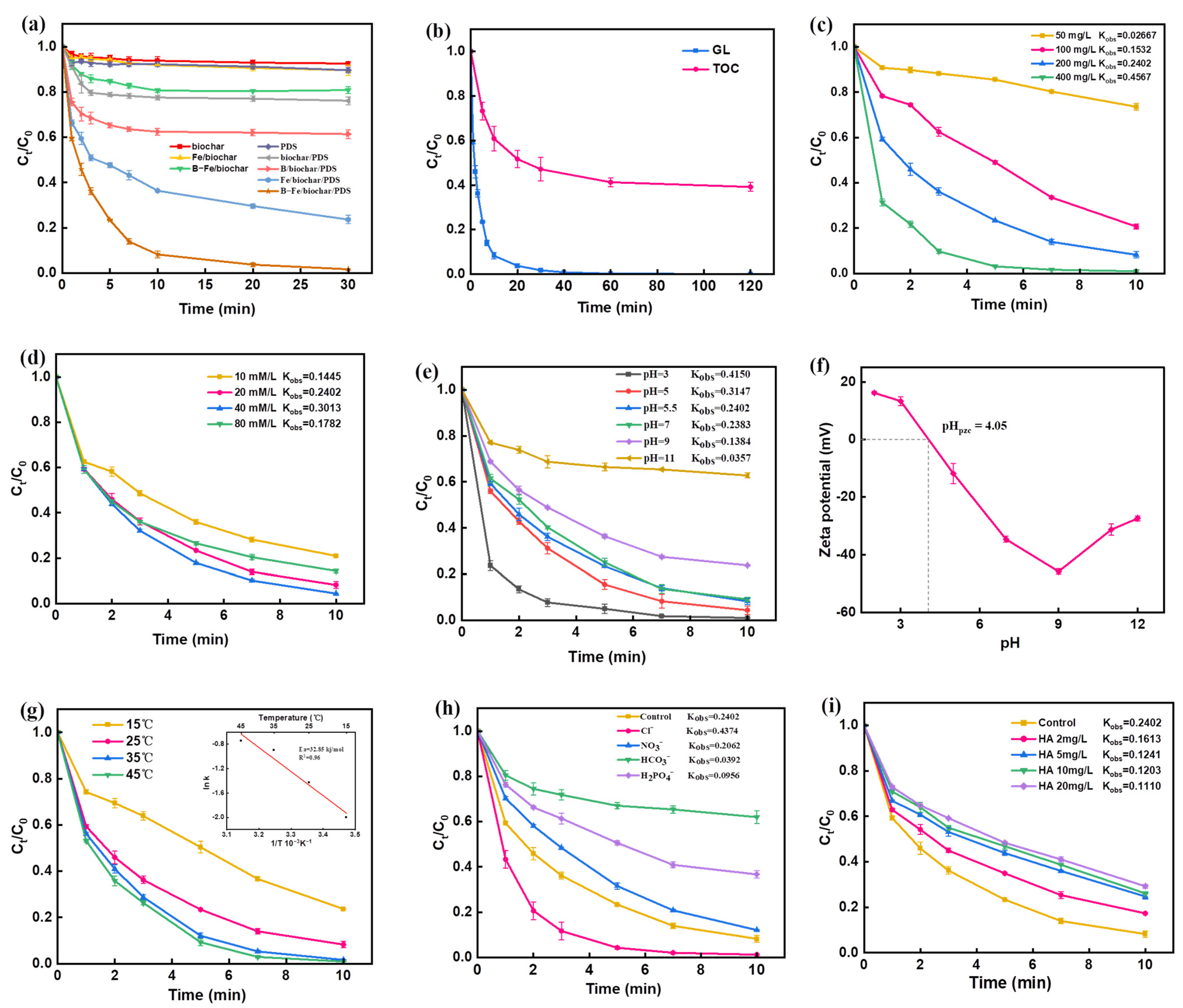
2.2. Catalytic Degradation of GL with B-Fe/Biochar-Activated PDS System
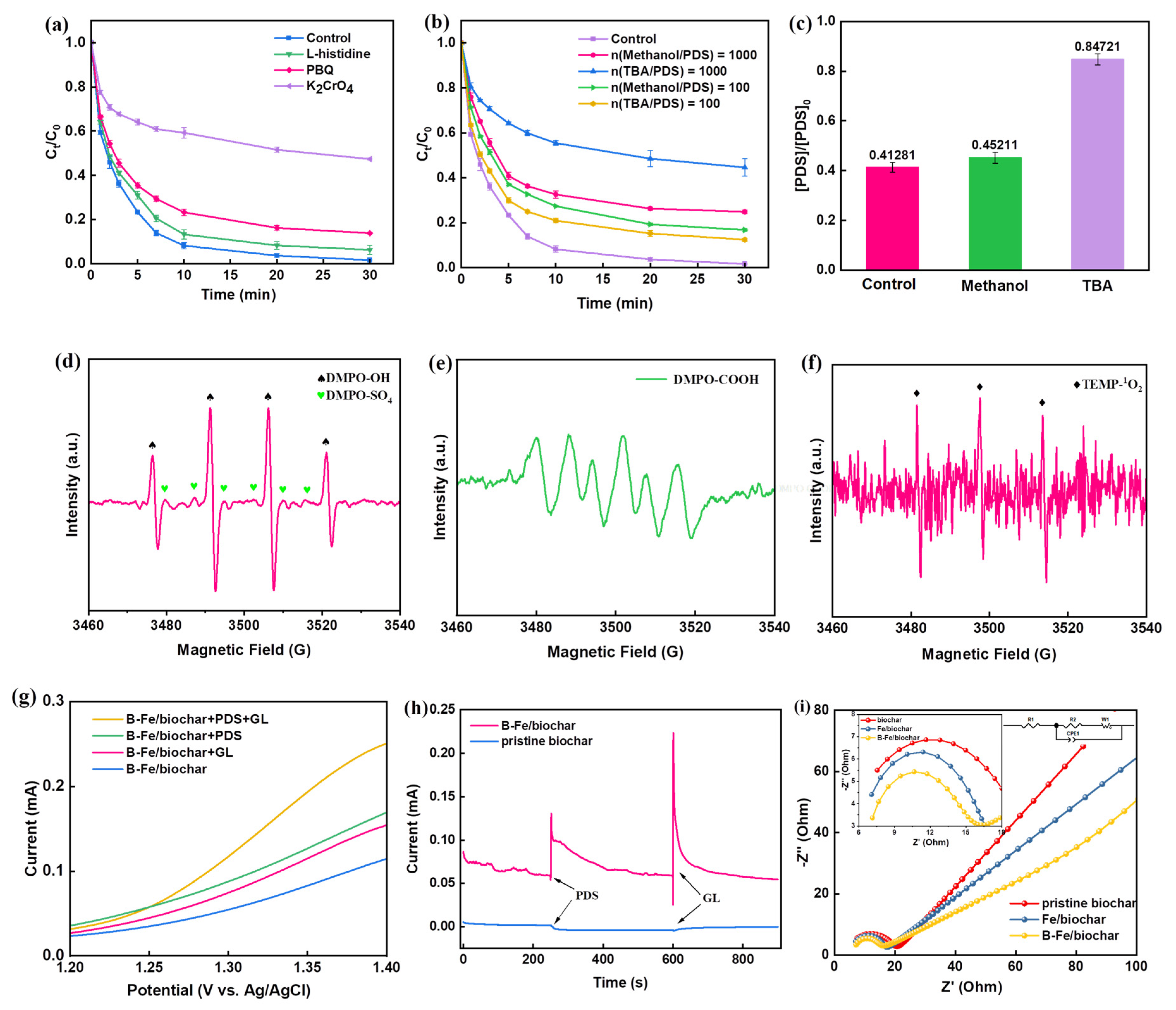
2.3. Activation Mechanism of Persulfate by the B-Fe/Biochar Catalyst
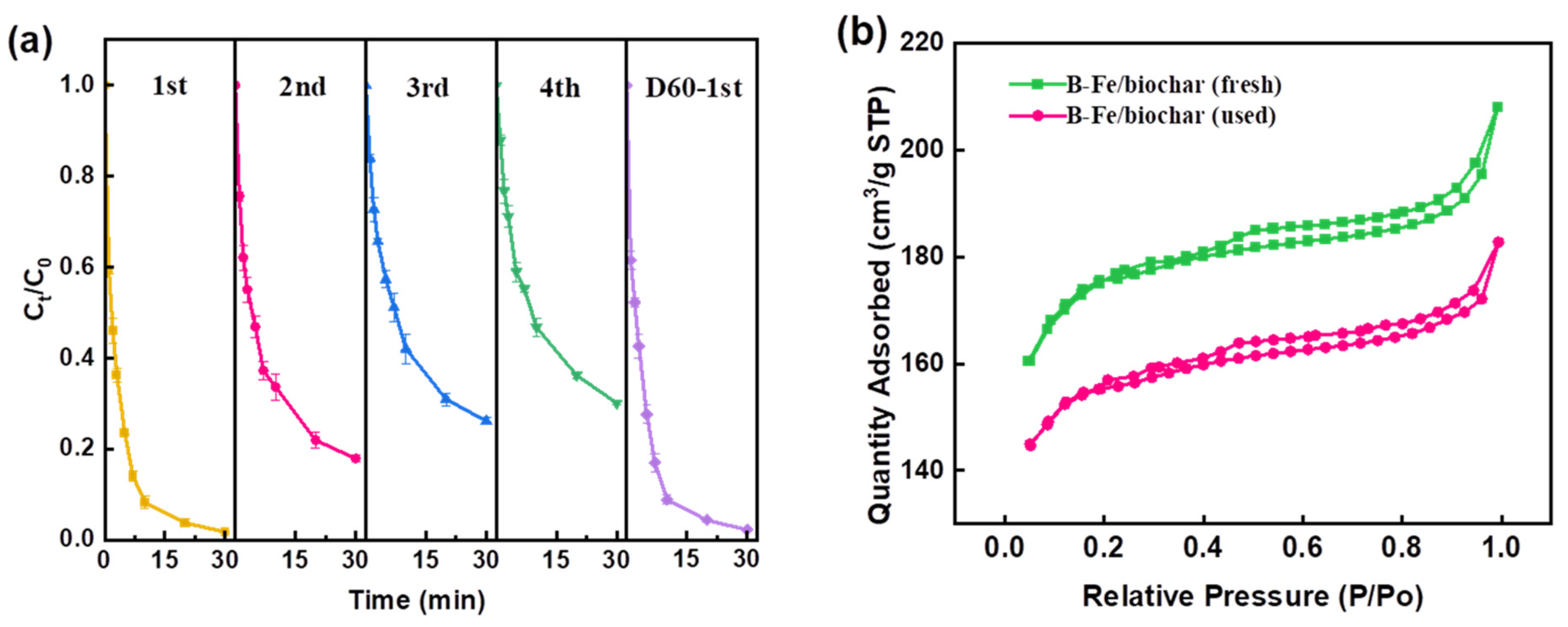
2.4. Stability and Reusability of B-Fe/Biochar Catalyst
3. Material and Methods
3.1. Materials and Chemicals
3.2. Synthesis of Boron and Iron Co-Doped Biochar Catalyst (B-Fe/Biochar)
3.3. Characterization Methods
3.4. Degradation of GL
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vineet, K.; Pradeep, V. A critical review on environmental risk and toxic hazards of refractory pollutants discharged in chlorolign in waste of pulp and paper mills and their remediation approaches for environmental safety. Environ. Res. 2023, 236, 116728. [Google Scholar]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M.N. Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.P.; Liu, Q.J.; Lin, Q.T. The Application Study Progress of Advanced Treatment Technology of Papermaking Wastewater. Trans. China Pulp Pap. 2010, 25, 102–107. [Google Scholar]
- Wang, S.; Li, X.P.; Zhang, A.L.; Jia, C.Y. Study on the Advanced Treatment of Papermaking Effluent by Membrane Separation Technology. Trans. China Pulp Pap. 2013, 28, 15–18. [Google Scholar]
- Xie, L.; Liu, W.J.; Tu, Y. Experimental study on advanced treatment of wastewater from waste paper papermaking by coagulation sedimentation. Chin. J. Environ. Eng. 2010, 4, 2190–2194. [Google Scholar]
- Deng, Y.; Zhao, R.Z. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Choong, Z.; Lin, K.A.; Lisak, G.; Lim, T.; Oh, W. Multi-heteroatom-doped carbocatalyst as peroxymonosulfate and peroxydisulfate activator for water purification: A critical review. J. Hazard. Mater. 2022, 426, 128077. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Bai, X.; Shi, J.; Hu, M.; Chai, J.; Li, K.; Jin, P. Photosensitive Dye as an Ideal Peroxymonosulfate Activator for Efficient Self-Degradation: A Novel Idea of Using Waste to Treat Waste. Molecules 2023, 28, 4237. [Google Scholar] [CrossRef]
- Liu, B.Z.; Huang, B.R.; Wang, Z.Z.; Tang, L.; Ji, C.H.; Zhao, C. Homogeneous/heterogeneous metal-catalyzed persulfate oxidation technology for organic pollutants elimination: A review. J. Environ. Chem. Eng. 2023, 11, 109586. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Q.; Ji, G.Z.; Lin, A. Degradation of antibiotic pollutants by persulfate activated with various carbon materials. Chem. Eng. J. 2022, 429, 13238. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.X.; Chen, Z.L. Efficient activation of persulfate by metallic sulfide mineral for the efficient removal of pesticides: Performance, radical generation and mechanism. J. Clean. Prod. 2023, 421, 138521. [Google Scholar] [CrossRef]
- Lyu, H.H.; Lim, J.Y.; Zhang, Q.R.; Senadheera, S.S.; Zhang, C.C.; Huang, Q.L.; Ok, Y.S. Conversion of organic solid waste into energy and functional materials using biochar catalyst: Bibliometric analysis, research progress, and directions. Appl. Catal. B Environ. 2024, 340, 123223. [Google Scholar] [CrossRef]
- Zeng, S.Y.; Li, K.Q.; Xu, X.; Zhang, J.Y.; Xue, Y. Efficiently catalytic degradation of tetracycline via persulfate activation with plant-based biochars: Insight into endogenous mineral self-template effect and pyrolysis catalysis. Chemosphere 2023, 337, 139309. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Cheng, S.; Xing, B.; Qu, X.; Shi, C.; Meng, W.; Zhang, C.; Xia, H. Preparation of pyrolysis products by catalytic pyrolysis of poplar: Application of biochar in antibiotic wastewater treatment. Chemosphere 2023, 338, 139519. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Chen, Y.D.; Li, R.X.; Zhang, C.F.; Ge, Y.M.; Cao, G.L.; Ma, M.; Duan, X.G.; Wang, S.B.; Ren, N.Q. N-doped graphitic biochars from C-phycocyanin extracted spirulina residue for catalytic persulfate activation toward nonradical disinfection and organic oxidation. Water Res. 2019, 159, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Renda, S.; Greco, G.; González, B.; Palma, V.; Manyà, J.J. Wheat-Straw-Derived Activated Biochar as a Renewable Support of Ni-CeO2 Catalysts for CO2 Methanation. Sustainability. 2021, 13, 8939. [Google Scholar]
- Dehkhoda, A.M.; Gyenge, E.; Ellis, N. A novel method to tailor the porous structure of koh-activated biochar and its application in capacitive deionization and energy storage. Biomass Bioenergy 2016, 87, 107–121. [Google Scholar] [CrossRef]
- Shi, Q.K.; Deng, S.; Zheng, Y.L.; Du, Y.L.; Li, L.; Yang, S.Z.; Zhang, G.X.; Du, L.; Wang, G.F.; Cheng, M.; et al. The application of transition metal-modified biochar in sulfate radical based advanced oxidation processes. Environ. Res. 2022, 212, 113340. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, L.; Tang, Y. Peroxydisulfate activation using Fe, Co co-doped biochar and synergistic effects on tetracycline degradation. Chem. Eng. J. 2023, 452, 139381. [Google Scholar] [CrossRef]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D. Effect of inorganic, synthetic and naturally occurring chelating agents on Fe(II) mediated advanced oxidation of chlorophenols. Water Res. 2009, 43, 684–694. [Google Scholar] [CrossRef]
- Dong, Z.L.; Zhang, Z.J.; Jiang, Y.; Chu, Y.Q.; Xu, J.Y. Embedding CsPbBr3 perovskite quantum dots into mesoporous TiO2 beads as an S-scheme heterojunction for CO2 photoreduction. Chem. Eng. J. 2022, 433, 133762. [Google Scholar] [CrossRef]
- Fan, X.F.; Yu, Y.L.; Dong, S.J.; Liu, Y.M.; Song, C.W.; Li, Q.B.; Wang, X.F. Heteroatoms-doped biochar derived from deciduous resource as persulfate catalysts for efficient degradation of pheno. J. Water Process Eng. 2022, 481, 102866. [Google Scholar] [CrossRef]
- Dou, J.B.; Cheng, J.; Lu, Z.J.; Tian, Z.Q.; Xu, J.M.; He, Y. Biochar co-doped with nitrogen and boron switching the free radical based peroxydisulfate activation into the electron-transfer dominated nonradical process. Appl. Catal. B Environ. 2022, 301, 120832. [Google Scholar] [CrossRef]
- Ren, W.; Xiong, L.L.; Nie, G.; Zhang, H.; Duan, X.G.; Wang, S.B. Insights into the electrontransfer regime of peroxydisulfate activation on carbon nanotubes: The role of oxygen functional groups. Environ. Sci. Technol. 2020, 54, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jia, Y.; Zhou, M.; Su, X.; Sun, J. High-efficiency degradation of organic pollutants with Fe, N co-doped biochar catalysts via persulfate activation. J. Hazard. Mater. 2020, 397, 122764. [Google Scholar] [CrossRef]
- Jin, Q.Q.; Chen, Z.L.; Chen, Q.; Yan, P.W.; Zhao, S.X.; Shen, J.M.; Li, L.; Guo, F.; Kang, J. Structure activity relationship study of N-doped ligand modified Fe(III)/H2O2 for degrading organic pollutants. J. Hazard. Mater. 2021, 404, 124142. [Google Scholar] [CrossRef]
- Yu, Z.D.; Ma, J.C.; Huang, X.Y.; Lv, Y.C.; Liu, Y.F.; Lin, C.X.; Dou, R.N.; Ye, X.X.; Shi, Y.X.; Liu, M.H. Insights into enhanced peroxydisulfate activation with S doped Fe@C catalyst for the rapid degradation of organic pollutants. J. Colloid Interface Sci. 2022, 610, 24–34. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Ren, N. B-doped graphitic porous biochar with enhanced surface affinity and electron transfer for efficient peroxydisulfate activation. Chem. Eng. J. 2020, 396, 125119. [Google Scholar] [CrossRef]
- Yu, J.F.; Feng, H.P.; Tang, L.; Pang, Y.; Zeng, G.M.; Lu, Y.; Dong, H.R.; Wang, J.J.; Liu, Y.N.; Feng, C.Y.; et al. Metal-free carbon materials for persulfate-based advanced oxidation process: Microstructure, property and tailoring. Prog. Mater. Sci. 2020, 111, 100654. [Google Scholar] [CrossRef]
- Agnoli, S.; Favaro, M. Doping graphene with boron: A review of synthesis methods, physicochemical characterization, and emerging applications. J. Mater. Chem. A 2016, 4, 5002–5025. [Google Scholar] [CrossRef]
- Wan, Y.T.; Zhang, W.; Yu, Q.; Xiu, G.L. Catalytic degradation of bisphenol A by Fe@B-doped porous carbon. Environ. Pollut. Control. 2021, 43, 1537–1542, 1554. [Google Scholar]
- Pan, G.; Wei, J.; Xu, M.; Li, J.; Wang, L.; Li, Y.; Cui, N.; Li, J.; Wang, Z. Insight into boron-doped biochar as efficient metal-free catalyst for peroxymonosulfate activation: Important role of -O-B-O- moieties. J. Hazard. Mater. 2023, 445, 130479. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.M.; Chen, W.C.; Huang, C.P.; Cheng, J.W.; Dong, C.D. Algae-derived metal-free boron-doped biochar as an efficient bioremediation pretreatment for persistent organic pollutants in marine sediments. J. Clean. Prod. 2022, 336, 130448. [Google Scholar] [CrossRef]
- An, J.; Zhang, G.; Zheng, R.; Wang, P. Removing lignin model pollutants with BiFeO3–g-C3N4 compound as an efficient visible-light-heterogeneous Fenton-like catalyst. J. Environ. Sci. 2016, 48, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Yan, X.F.; Liu, F.; Li, L. Study on Degradation of Guaiacol by Catalytic Ozonation Using AlOOH as Catalyst. Trans. China Pulp Pap. 2017, 32, 17–21. [Google Scholar]
- Xie, Z.C.; Wang, Y.; Wang, P.; Zhang, L. W-Doped TiO2 Preparation and Photocatalytic Degradation of Guaiacol. Appl. Mech. Mater. 2014, 513–517, 33–36. [Google Scholar] [CrossRef]
- Zhu, S.S.; Huang, X.C.; Ma, F.; Wang, L.; Duan, X.G. Catalytic removal of aqueous contaminants on N-doped graphitic biochars: Inherent roles of adsorption and nonradical mechanisms. Environ. Sci. Technol. 2018, 52, 8649–8658. [Google Scholar] [CrossRef]
- Ma, D.M.; Yang, Y.; Liu, B.F.; Xie, G.J.; Chen, C.; Ren, N.Q.; Xing, D.F. Zero-valent iron and biochar composite with high specific surface area via K2FeO4 fabrication enhances sulfadiazine removal by persulfate activation. Chem. Eng. J. 2021, 408, 127992. [Google Scholar] [CrossRef]
- Li, X.; Qin, Y.; Jia, Y.; Li, Y.Y.; Zhao, Y.X.; Pan, Y.W.; Sun, J.H. Preparation and application of Fe/biochar (Fe-BC) catalysts in wastewater treatment: A review. Chemosphere 2021, 274, 129766. [Google Scholar] [CrossRef]
- Wang, H.Z.; Guo, W.Q.; Liu, B.H.; Wu, Q.L.; Luo, H.C.; Zhao, Q.; Si, Q.S.; Sseguya, F.; Ren, N.Q. Edge-nitrogenated biochar for efficient peroxydisulfate activation: An electron transfer mechanism. Water Res. 2019, 160, 405–414. [Google Scholar] [CrossRef]
- Wang, S.Y.; Xia, Y.; Tan, L.; Luo, H.P.; Liu, Y.N.; Chen, H.; Jiang, F. Co and N co-doped hierarchical porous carbon as peroxymonosulfate activator for phenol degradation via nonradical pathway mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130121. [Google Scholar] [CrossRef]
- Xu, L.; Fu, B.R.; Sun, Y.; Jin, P.K.; Bai, X.; Jin, X.; Shi, X. Degradation of organic pollutants by Fe/N co-doped biochar via peroxymonosulfate activation: Synthesis, performance, mechanism and its potential for practical application. Chem. Eng. J. 2020, 400, 125870. [Google Scholar] [CrossRef]
- Park, J.H.; Wan, J.J.; Tafti, N.; Delaune, R.D. Removal of Eriochrome Black T by sulfate radical generated from Fe-impregnated biochar/persulfate in Fenton-like reaction. J. Ind. Eng. Chem. 2019, 71, 201–209. [Google Scholar] [CrossRef]
- Montearudo, J.M.; El-taliawy, H.; Duran, A.; Garo, G.; Bester, K. Sono-activated persulfate oxidation of diclofenac: Degradation, kinetics, pathway and contribution of the different radicals involved. J. Hazard. Mater. 2018, 357, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.L.; Zhang, Q.; Zhang, C.; Wang, R.Z.; Deng, R.; Luo, H.; Li, T.; Li, J.; Chen, S.; Liu, C.H. Mn doped magnetic biochar as persulfate activator for the degradation of tetracycline. Chem. Eng. J. 2020, 391, 123532. [Google Scholar] [CrossRef]
- Chen, X.L.; Li, H.; Lai, L.; Zhang, Y.; Chen, Y.; Li, X.; Liu, B.; Wang, H. Peroxymonosulfate activation using MnFe2O4 modified biochar for organic pollutants degradation: Performance and mechanisms. Sep. Purif. Technol. 2023, 308, 122886. [Google Scholar] [CrossRef]
- Hussain, I.; Li, M.; Zhang, Y.; Li, Y.; Huang, S.; Du, X.; Liu, G.; Hayat, W.; Anwar, N. Insights into the mechanism of persulfate activation with nZVI/BC nanocomposite for the degradation of nonylphenol. Chem. Eng. J. 2017, 311, 163–172. [Google Scholar] [CrossRef]
- Liang, X.; Zhao, Y.; Guo, N.; Yang, Q. Heterogeneous activation of peroxymonosulfate by Co3O4 loaded biochar for efficient degradation of 2,4-dichlorophenoxyacetic acid. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127152. [Google Scholar] [CrossRef]
- Song, H.; Li, Q.; Ye, Y.; Pan, F.; Zhang, D.; Xia, D. Degradation of cephalexin by persulfate activated with magnetic loofah biochar: Performance and mechanism. Sep. Purif. Technol. 2021, 272, 118971. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, W.; Wang, X.; Tong, W.; Li, P.; Zhou, H.; Wang, Y.; Zhang, Y. Molten salt induced nitrogen-doped biochar nanosheets as highly efficient peroxymonosulfate catalyst for organic pollutant degradation. Environ. Pollut. 2020, 260, 114053. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Jiang, S.M. One-step synthesis of biochar supported nZVI composites for highly efficient activating persulfate to oxidatively degrade atrazine. Chem. Eng. J. 2021, 420, 129868. [Google Scholar] [CrossRef]
- Lutze, H.V.; Kerlin, N.; Schmidt, T.C. Sulfate radical-based water treatment in presence of chloride: Formation of chlorate, inter-conversion of sulfate radicals into hydroxyl radicals and influence of bicarbonate. Water Res. 2015, 72, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.L.; Liu, Z.; Pan, S.Y.; Zhao, C.; Wang, Z.W.; Gao, B.Y.; Li, Q. Activation of peroxydisulfate via Fe@sulfur-doped carbon-supported nanocomposite for degradation of norfloxacin: Efficiency and mechanism. Chem. Eng. J. 2023, 460, 141729. [Google Scholar] [CrossRef]
- Wang, J.; Liao, Z.W.; Ifthikar JShi, L.; Chen, Z.Q.; Chen, Z.L. One-step preparation and application of magnetic sludge-derived biochar on acid orange 7 removal via both adsorption and persulfate based oxidation. RSC Adv. 2017, 7, 18696–18706. [Google Scholar] [CrossRef]
- Ghauch, A.; Tuqan, A.M. Oxidation of bisoprolol in heated persulfate/H2O systems: Kinetics and products. Chem. Eng. J. 2012, 183, 162–171. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, C.S.; Tu, Y.J.; Huang, Y.H.; Zhang, H. Heterogeneous degradation of organic pollutants by persulfate activated by CuO-Fe3O4: Mechanism, stability, and effects of pH and bicarbonate ions. Environ. Sci. Technol. 2015, 49, 6838–6845. [Google Scholar] [CrossRef]
- Weng, C.H.; Tao, H. Highly efficient persulfate oxidation process activated with Fe0 aggregate for decolorization of reactive azo dye Remazol Golden Yellow. Arab. J. Chem. 2018, 11, 1292–1300. [Google Scholar] [CrossRef]
- Wu, L.B.; Lin, Q.T.; Fu, H.Y.; Luo, H.Y.; Zhong, Q.F.; Li, J.Q.; Chen, Y.J. Role of sulfide-modified nanoscale zero-valent iron on carbon nanotubes in nonradical activation of peroxydisulfate. J. Hazard. Mater. 2022, 422, 126949. [Google Scholar] [CrossRef]
- Pang, K.F.; Sun, W.; Ye, F.; Yang, L.H.; Pu, M.J.; Yang, C.; Zhang, Q.C.; Niu, J.F. Sulfur-modified chitosan derived N, S-co-doped carbon as a bifunctional material for adsorption and catalytic degradation sulfamethoxazole by persulfate. J. Hazard. Mater. 2022, 424, 127270. [Google Scholar] [CrossRef]
- Xu, K.H.; Lin, Q.T.; Fan, X.D.; Zheng, J.L.; Liu, Y.X.; Ma, Y.J.; He, J. Enhanced degradation of sulfamethoxazole by activation of peroxodisulfate with red mud modified biochar: Synergistic effect between adsorption and nonradical activation. Chem. Eng. J. 2023, 460, 141578. [Google Scholar] [CrossRef]
- Guan, C.; Jiang, J.; Pang, S.; Luo, C.; Ma, J.; Zhou, Y.; Yang, Y. Oxidation kinetics of bromophenols by nonradical activation of peroxydisulfate in the presence of carbon nanotube and formation of brominated polymeric products. Environ. Sci. Technol. 2017, 51, 10718–10728. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, S.X.; Yu, M.W.; Xu, H.; Lv, J.G.; Yang, S.S.; Zhu, X.T.; Li, L. One-pot pyrolysis method for synthesis of Fe/N co-doped biochar as an effective peroxymonosulfate activator for RhB degradation. J. Taiwan Inst. Chem. Eng. 2021, 128, 209–219. [Google Scholar] [CrossRef]
- Yu, J.F.; Tang, L.; Pang, Y.; Zeng, G.M.; Wang, J.J.; Deng, Y.C.; Liu, Y.N.; Feng, H.P.; Chen, S.; Ren, X.Y. Magnetic nitrogen-doped sludge-derived biochar catalysts for persulfate activation: Internal electron transfer mechanism. Chem. Eng. J. 2019, 364, 146–159. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Alnashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, H.J.; Jeong, J.; Lee, J.; Park, N.; Lee, C. Activation of persulfates by carbon nanotubes: Oxidation of organic compounds by nonradical mechanism. Chem. Eng. J. 2015, 266, 28–33. [Google Scholar] [CrossRef]
- Tang, L.; Liu, Y.I.; Wang, J.J. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism. Appl. Catal. B-Environ. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, L.L.; Huang, Y.N.; Wang, W.K.; Xu, J. Boron regulates catalytic sites of biochar to enhance the formation of surface-confined complex for improved peroxydisulfate activation. Chemosphere 2022, 301, 134690. [Google Scholar] [CrossRef]
- Nie, C.Y.; Dai, Z.H.; Liu, W.J.; Duan, X.G.; Wang, C.Y.; Lai, B.; Ao, Z.M.; Wang, S.B.; An, T.C. Criteria of active sites in nonradical persulfate activation process from integrated experimental and theoretical investigations: Boron-nitrogen-co-doped nanocarbon-mediated peroxydisulfate activation as an example. Environ. Sci. Nano 2020, 7, 1899–1911. [Google Scholar] [CrossRef]
- Ahn, Y.Y.; Bae, H.; Kim, H.I.; Kim, S.H.; Kim, J.H.; Lee, S.G.; Lee, J. Surface-loaded metal nanoparticles for peroxymonosulfate activation: Efficiency and mechanism reconnaissance. Appl. Catal. B-Environ. 2019, 241, 561–569. [Google Scholar] [CrossRef]
- Ren, X.Y.; Wang, J.J.; Yu, J.F.; Song, B.; Feng, H.P.; Shen, M.C.; Zhang, H.; Zou, J.J.; Zeng, G.M.; Tang, L.; et al. Waste valorization: Transforming the fishbone biowaste into biochar as an efficient persulfate catalyst for degradation of organic pollutant. J. Clean. Prod. 2021, 291, 125225. [Google Scholar] [CrossRef]
- Chen, C.; Ma, T.F.; Shang, Y.N.; Gao, B.Y.; Jin, B.; Dan, H.B.; Li, Q.; Yue, Q.Y.; Li, Y.W.; Wang, Y.; et al. In-situ pyrolysis of Enteromorpha as carbocatalyst for catalytic removal of organic contaminants: Considering the intrinsic N/Fe in Enteromorpha and non-radical reaction. Appl. Catal. B-Environ. 2019, 250, 382–395. [Google Scholar] [CrossRef]
- Liang, C.J.; Huang, C.F.; Mohanty, N.; Kurakalva, R.M. A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 2008, 73, 1540–1543. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhu, Y.; Bian, H.; Song, L.; Liu, Y.; Lv, Y.; Ye, X.; Lin, C.; Li, X. Insights into Enhanced Peroxydisulfate Activation with B and Fe Co-Doped Biochar from Bark for the Rapid Degradation of Guaiacol. Molecules 2023, 28, 7591. https://doi.org/10.3390/molecules28227591
Huang J, Zhu Y, Bian H, Song L, Liu Y, Lv Y, Ye X, Lin C, Li X. Insights into Enhanced Peroxydisulfate Activation with B and Fe Co-Doped Biochar from Bark for the Rapid Degradation of Guaiacol. Molecules. 2023; 28(22):7591. https://doi.org/10.3390/molecules28227591
Chicago/Turabian StyleHuang, Jian, Yu Zhu, Huiyang Bian, Liang Song, Yifan Liu, Yuancai Lv, Xiaoxia Ye, Chunxiang Lin, and Xiaojuan Li. 2023. "Insights into Enhanced Peroxydisulfate Activation with B and Fe Co-Doped Biochar from Bark for the Rapid Degradation of Guaiacol" Molecules 28, no. 22: 7591. https://doi.org/10.3390/molecules28227591
APA StyleHuang, J., Zhu, Y., Bian, H., Song, L., Liu, Y., Lv, Y., Ye, X., Lin, C., & Li, X. (2023). Insights into Enhanced Peroxydisulfate Activation with B and Fe Co-Doped Biochar from Bark for the Rapid Degradation of Guaiacol. Molecules, 28(22), 7591. https://doi.org/10.3390/molecules28227591