Abstract
A rapid synthesis of chiral sulfoxide-functionalized meta-terphenyl derivatives by a 2,5-[C4+C2] ring transformation reaction of pyrylium salts with in situ generated enantiomerically pure α-sulfinylacetaldehydes is described in this paper. This synthetic method demonstrates, for the first time, the use of α-sulfinylacetaldehydes in a reaction sequence initiated by the nucleophilic attack of pyrylium salts by α-sulfinylcarbanions to generate chiral aromatic systems. The method presented shows a broad applicability starting with various methyl sulfoxides and a number of functionalized pyrylium salts, furnishing meta-terphenyls with complex substitution patterns from readily accessible starting compounds.
1. Introduction
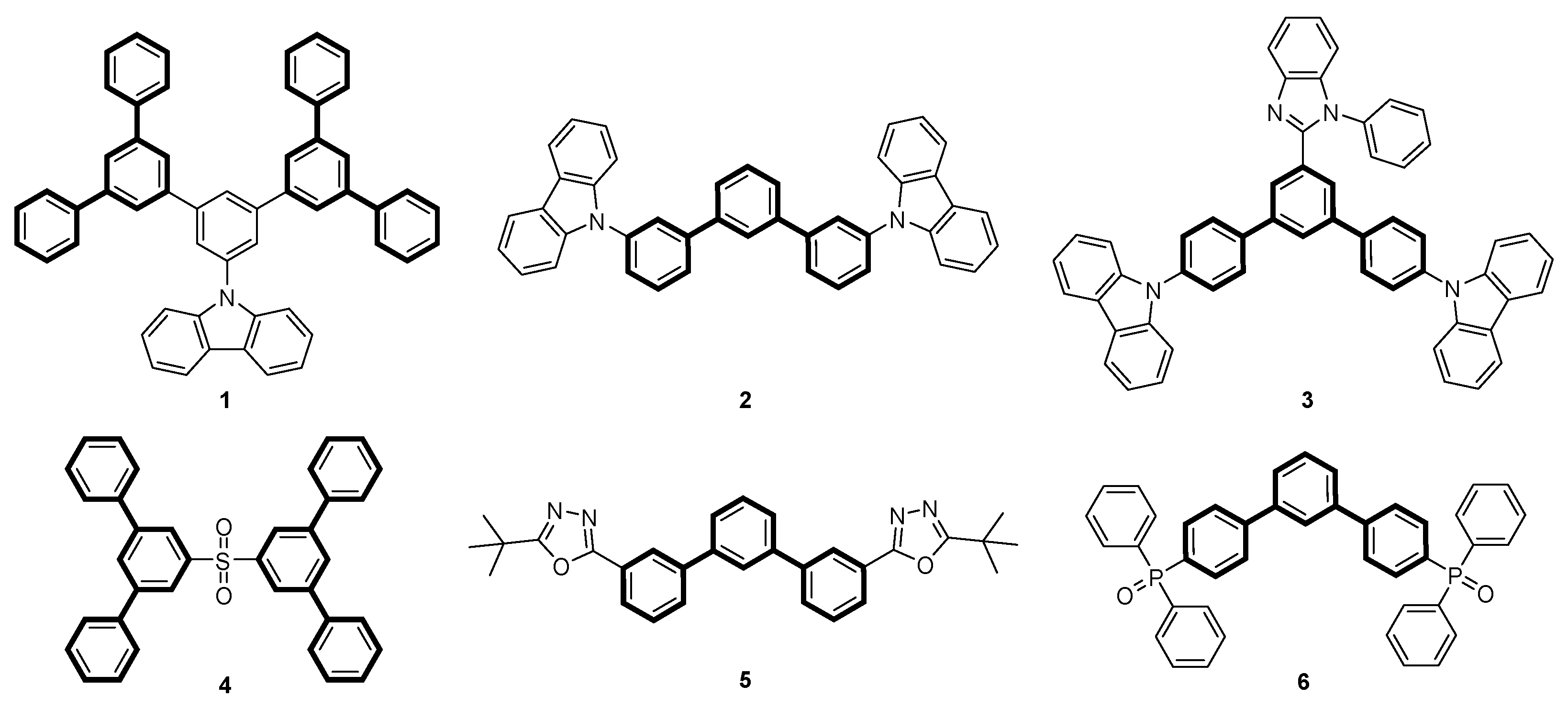
Terphenyls are a class of organic compounds consisting of three interconnected phenyl rings. Depending on the arrangements of the aromatic rings, where substitutions can occur in the ortho-, meta-, or para-positions, different structural isomers and properties can be obtained. The meta-terphenyl skeleton occurs in several natural compounds [1], such as trifucol [2], macranthol [3], and mulberrofuran R [4]. Due to their extensive conjugation, terphenyls exhibit distinct optical [5,6,7,8] and electronic properties [9] that make them valuable for the preparation of various materials, such as organic light-emitting diodes (OLEDs, Figure 1).
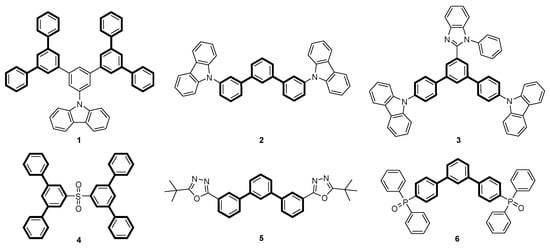
Figure 1.
Examples of host 1–4 [10,11] and electron transport materials 5–6 [12] containing meta-terphenyl units.
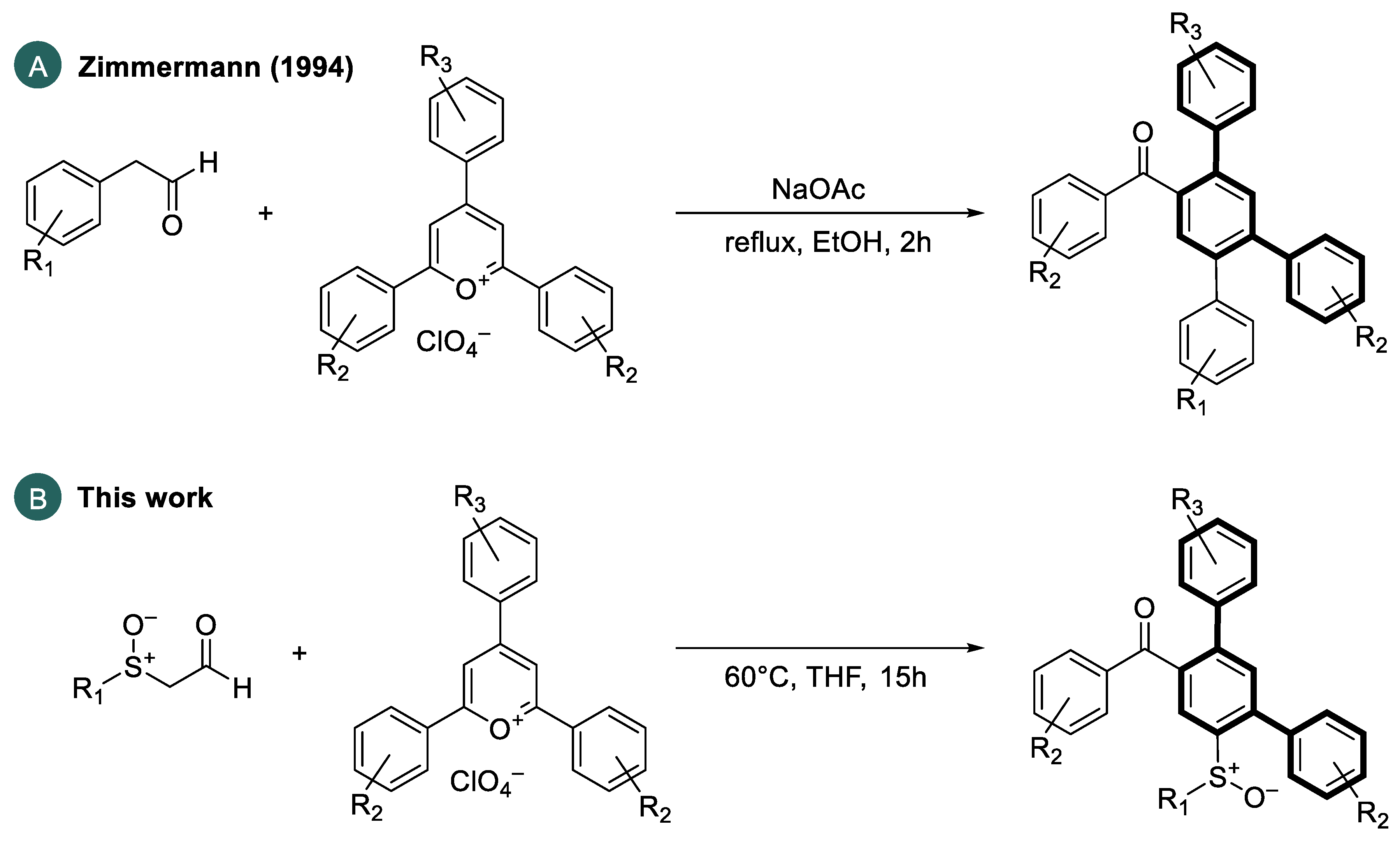
In this case, the meta-terphenyl skeleton is mainly used in host materials. Especially, carbazole-substituted meta-terphenyl derivatives of types 1–3 show promising optoelectronic properties [11,13,14,15]. In addition, Sasabe et al. [10] synthesized a sulfone-bridged meta-terphenyl derivative 4 as a high-performance host material for green and blue OLEDs. In addition, meta-terphenyl derivatives are used in electron transport materials. Examples include m-terphenyloxadiazole 5, synthesized by Wu et al. [5], or m-terphenyldiphenylphosphine oxide 6, prepared by Zhang et al. [12]. An interesting extension of the above-mentioned applications would be the synthesis of chiral, non-racemic polyaromatic systems aimed at the synthesis of materials emitting circularly polarized light (CPL), which is of interest for many optoelectronic applications [16,17,18]. In general, two primary approaches to the preparation of terphenyl compounds can be distinguished: (i) the coupling of dihalobenzene derivatives with aryl metal nucleophiles [19,20,21,22] and (ii) the use of open-chain precursor molecules to form the aromatic rings by concerted or sequential benzannulation reactions [22,23,24]. In 1994, Zimmermann [25] reported a ring transformation reaction of triarylpyrylium salts with aryl acetaldehydes to obtain substituted carbocycles. He converted phenylacetaldehyde or 4-fluorophenylacetaldehyde with functionalized 2,4,6-triarylpyrylium salts in an ethanolic solution in the presence of sodium acetate as a weak base into the corresponding 2,4,5-triarylbenzophenones in high yields (Scheme 1).
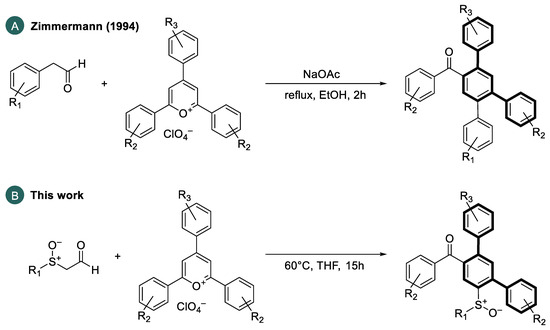
Scheme 1.
(A) Ring transformations of arylacetaldehydes with triarylpyrylium salts by Zimmermann [25]. (B) Use of α-sulfinylacetaldehydes to perform an analogous ring transformation reaction for the preparation of sulfinyl-functionalized meta-terphenyls.
Based on these results, in this paper, we use chiral, enantiomerically pure α-sulfinylacetaldehydes, as nucleophiles to perform an analogous ring transformation with a series of 2,4,6-triarylpyrylium salts to obtain optically active sulfinylated meta-terphenyls.
2. Results and Discussion
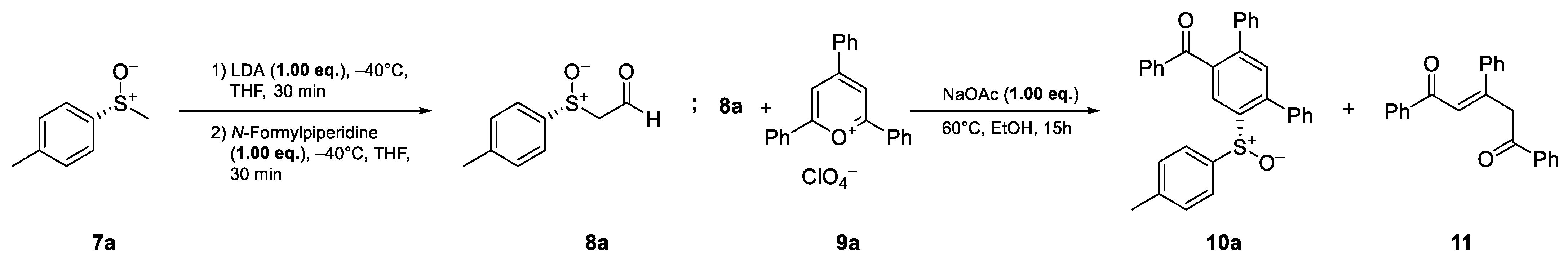
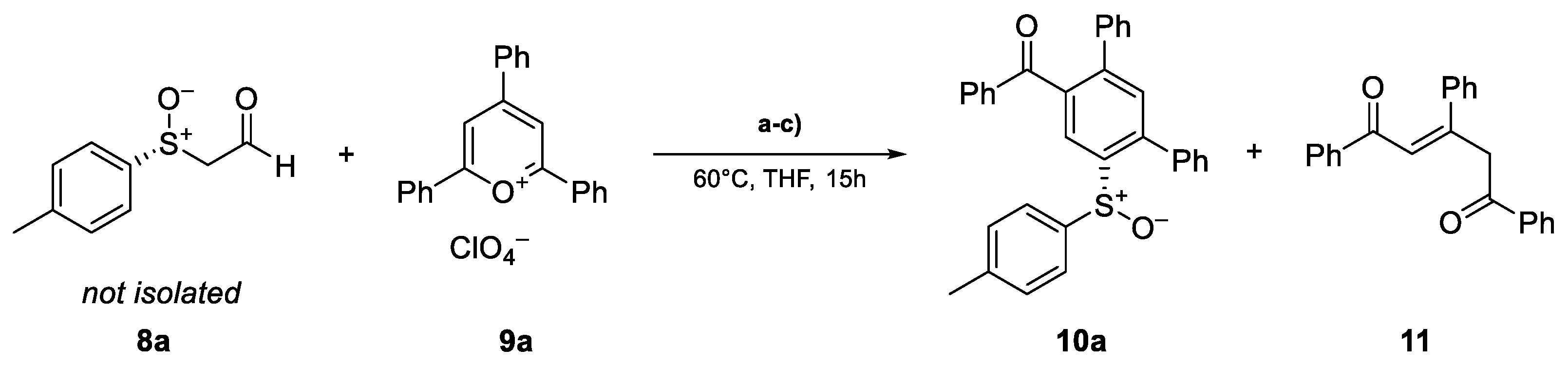
The preparation of enantiomerically pure (R)-p-tolyl methyl sulfoxide ((R)-7a) was conducted using the method developed by Andersen [26] and Solladie [27,28] starting from p-toluene sodium sulfinate. Subsequently, the synthesis of (R)-sulfinylacetaldehyde (8a) was achieved via the deprotonation of (R)-7a with lithiumdiisopropylamide (LDA), followed by a formyl transfer with N-formylpiperidine, according to the method of Pflieger et al. [29]. After the isolation of aldehyde 8a, the ring transformation was performed with 2,4,6-triphenylpyrylium perchlorate (9a) [30] in ethanol in the presence of sodium acetate, analogous to the work of Zimmermann [25]. In this first attempt, the desired cyclization product 10a was obtained at a 20% yield. In addition to the desired product, the open chain 1,4-diketone 11 was isolated at a 28% yield as a side product (Scheme 2, see also Scheme 5).
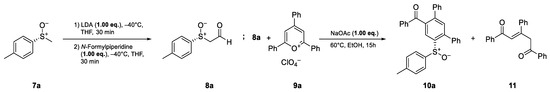
Scheme 2.
Initial experiments employing the isolated sulfinylacetaldehyde 8a.
Due to the instability of 8a [31], we tried to avoid its isolation. After the reaction of lithiated sulfoxide (R)-7a with the formylating reagent, a THF solution at −40 °C was added directly to a room temperature suspension of pyrylium salt 9a in THF and heated to 60 °C overnight. This significantly increased the yield of 10a to 32%. Nevertheless, the by-product 11 was isolated at approximately the same yield as before (Table 1, #1). By adding 4 molecular sieves, its yield was reduced from 26% to 10% (Table 1, #2). Surprisingly, increasing the equivalents of (R)-sulfinylacetaldehyde lowered the yield of the cyclization product dramatically, whereas the yield of the by-product remained unchanged (Table 1, #3). One reason for the low yield seems to be the high concentration of sulfinylacetaldehyde, which is very unstable at elevated temperatures and may undergo self-condensation. By not isolating 8a, it is no longer necessary to use sodium acetate as a base due to the formation of lithiumpiperidide in the course of the reaction. This leads to approximately the same product yield as entry 2, but the yield of the by-product decreases significantly (Table 1, #4). Conducting the reaction with a 1:1.8 excess of the pyrylium salt increased the yield of 10a even further, to 49% (Table 1, #5).

Table 1.
Optimization of reaction conditions a.
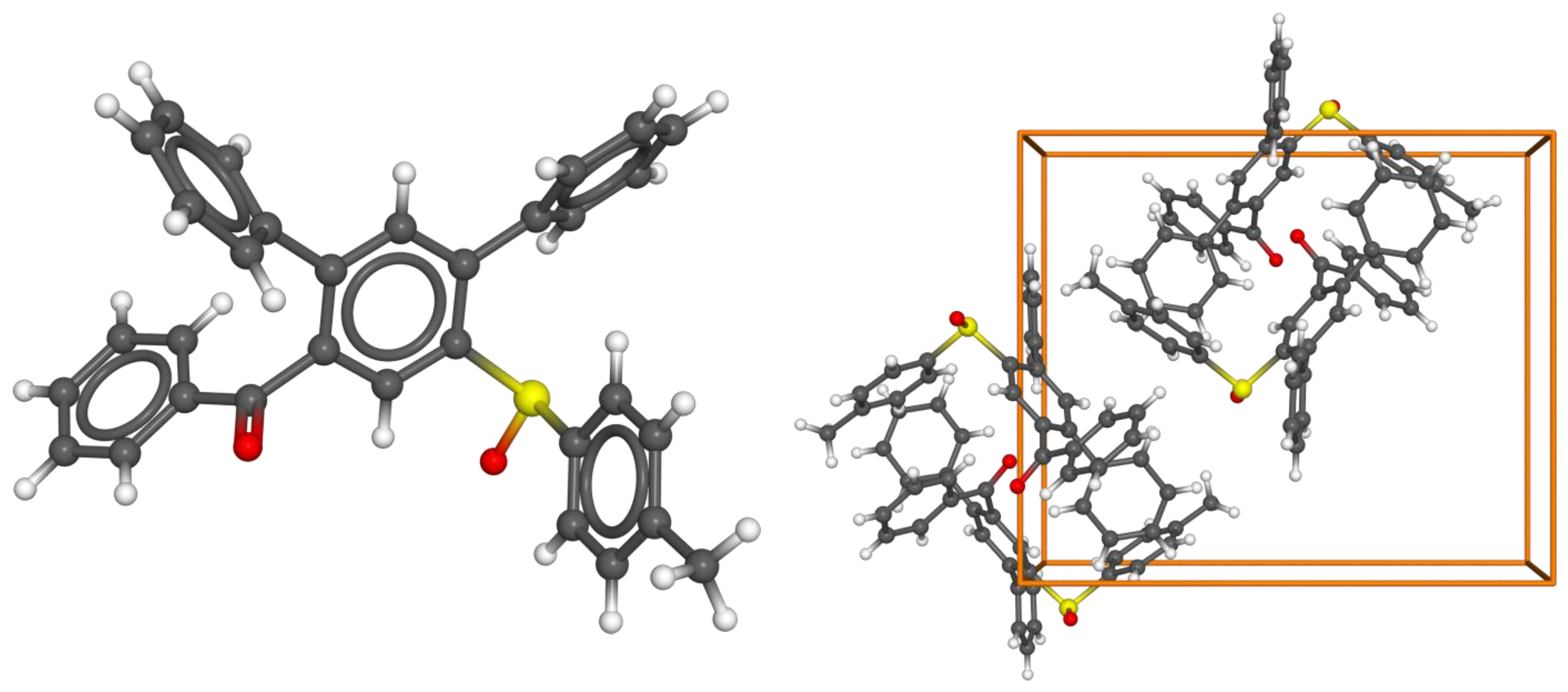
The structure of the standard substrate 10a was confirmed by a single-crystal X-ray analysis (Figure 2, CCDC deposition number 2302385).
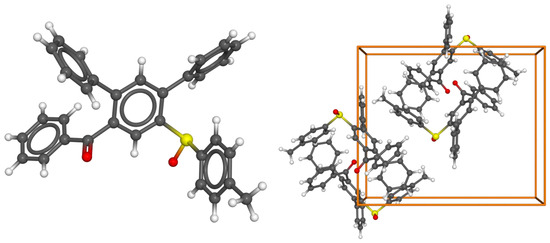
Figure 2.
(left) Molecular structure of 10a. (right) Unit cell (Z = 4, space group P 212121) with anisotropic thermal ellipsoids (50% probability level). The hydrogen atoms were placed geometrically.
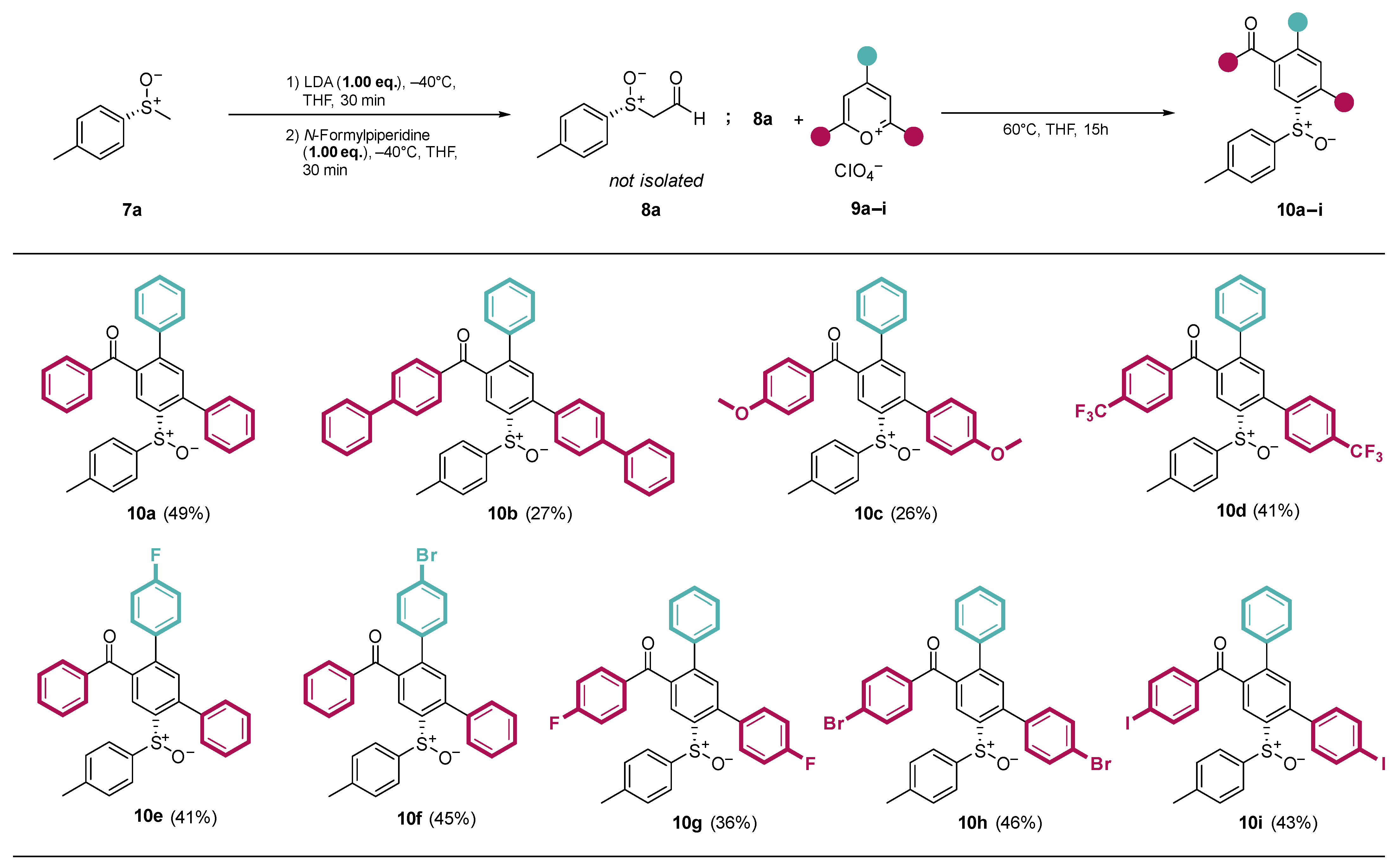
At the optimized reaction conditions, substrate variations were first investigated using a variety of functionalized pyrylium salts 9a–i (Scheme 3). With the exception of 10b and 10c, all products were obtained at yields ranging from 40 to 50%. The pyrylium salts reacting with 10b and 10c with electron-donating substituents (9b-Ph and 9c-OMe) were expected to show a decreased reactivity due to the reduced electrophilicity at the 2-position of the pyrylium salt. Indeed, both donor-substituted products were obtained with reduced yields.
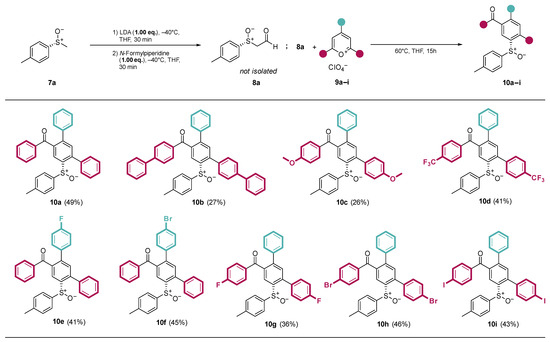
Scheme 3.
Substrate scope and isolated yields of the ring transformation products 10a–i with different pyrylium salts 9a–i.
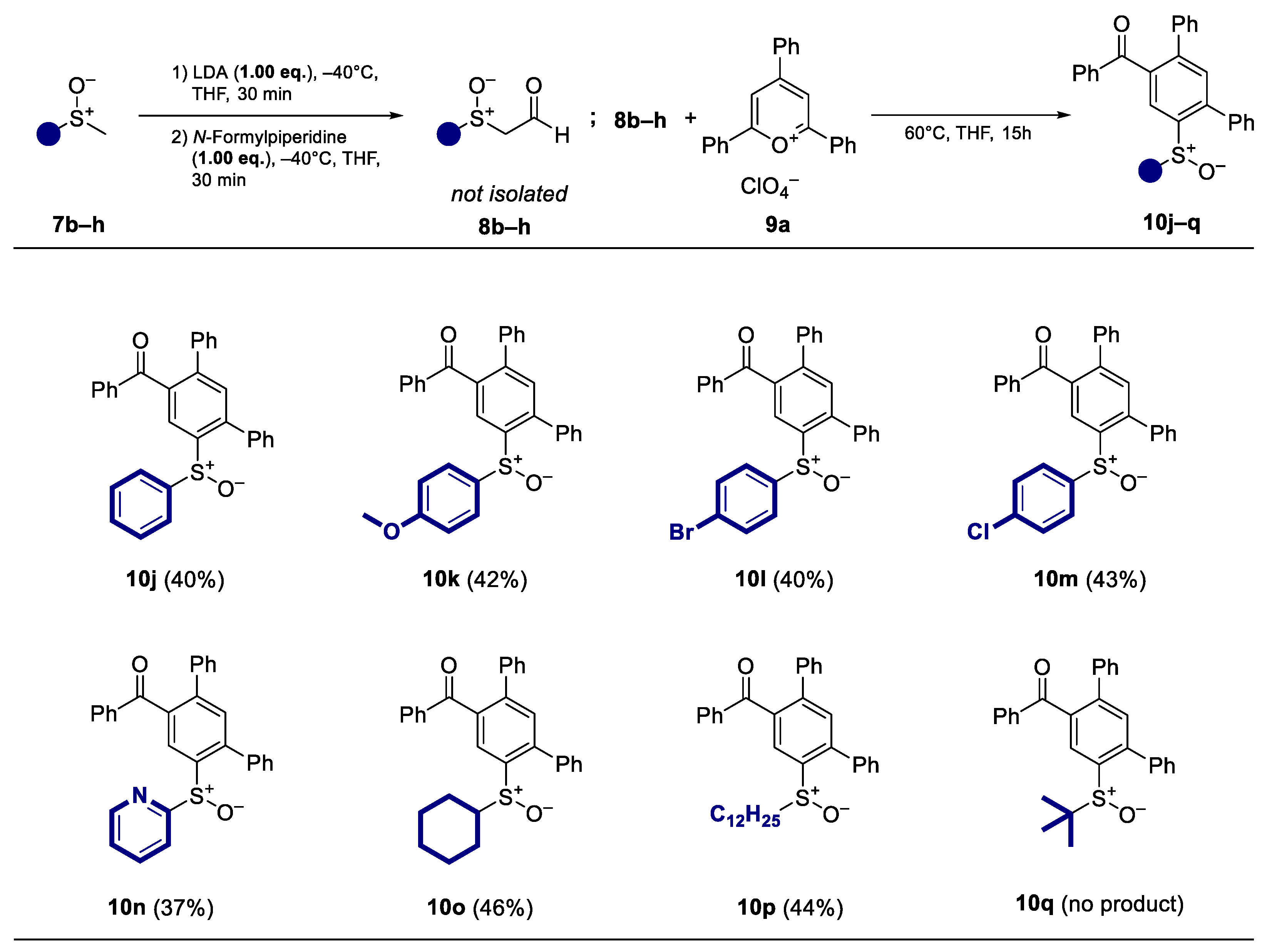
After varying the quantity of pyrylium salts, different racemic methyl sulfoxides 7b–h were tested (Scheme 4). In this case, almost all sulfoxides showed comparable reactivity and the resulting terphenyls were obtained at yields similar to those of the standard substrate (R)-7a. The method is well suited aromatic sulfoxides with electron-donating substituents (10k) as well as with halogens (10l–m). Moreover, heteroaromatic sulfoxides (10n) and aliphatic sulfoxides with additional acidic protons in the α′-position showed comparable reactivity to (R)-7a and were obtained at yields of 40% (10o–p). No product can be isolated with sterically demanding tert-butyl methyl sulfoxide (10q).
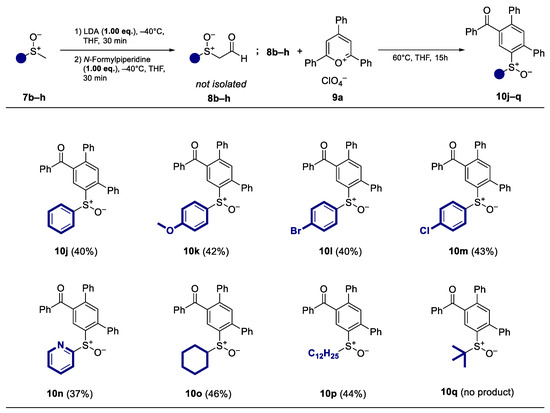
Scheme 4.
Substrate scope and isolated yields of the ring transformation products 10j–q (racemic) with different methyl sulfoxides 7b–h.
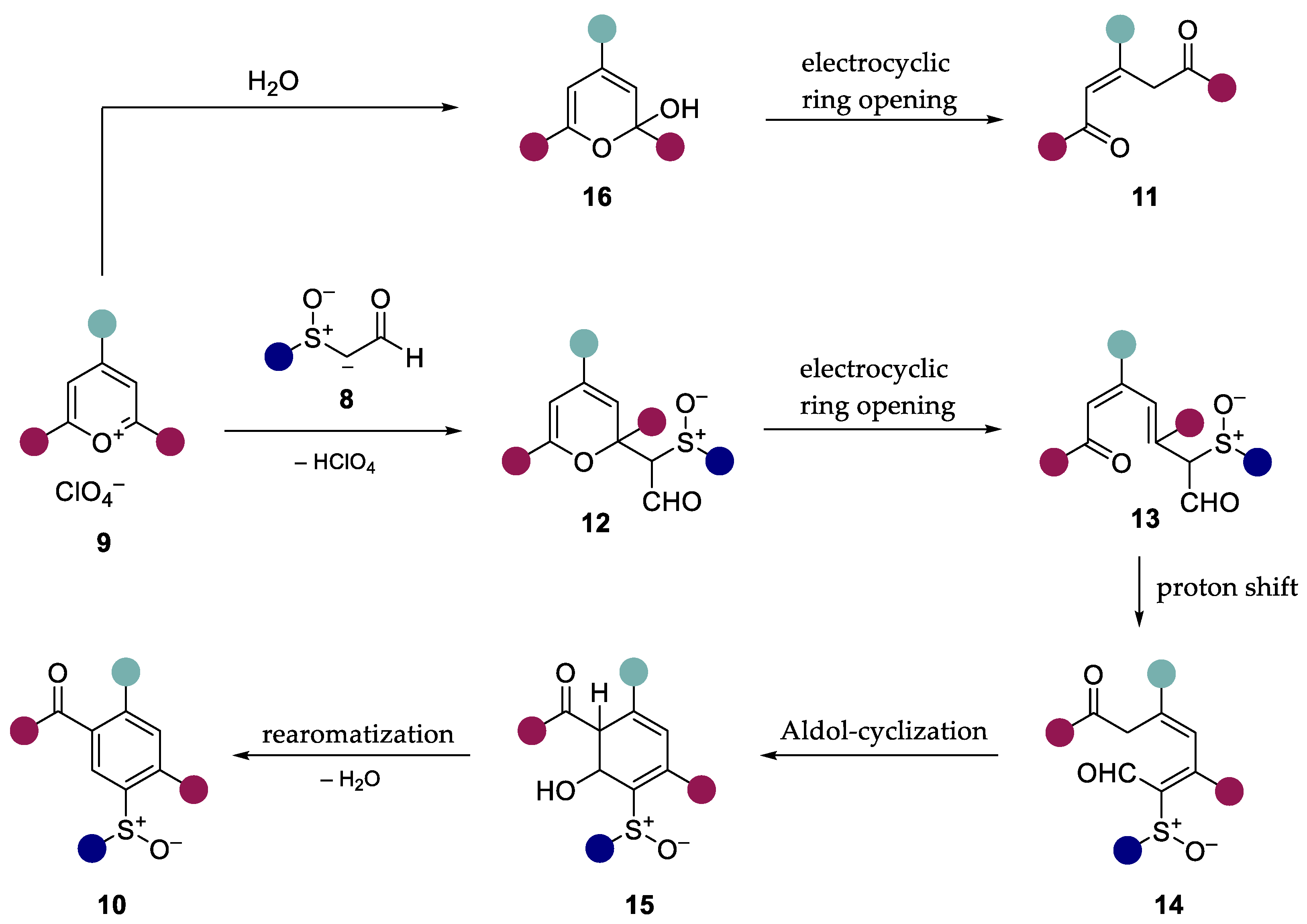
From the results obtained, we assumed that the reaction mechanism described by Zimmermann [25] for phenylacetaldehyde could be applied to our system (Scheme 5). In this case, the carbanion of the α-sulfinylacetaldehydes 8 attacks the preferred 2-position [32] of the pyrylium salt 9. The resulting 2H-pyran 12 then reacts via electrocyclic ring opening to obtain the ketoaldehyde 13 [33]. The intermediate 14 obtained by proton shift reacts with the acidic methylene group in the course of an aldol addition to obtain the intermediate 15. Condensation accompanied by rearomatization yields the ring transformation products 10. In the presence of water, the pyrylium salts hydrolyze to the unstable cyclic hemiacetal 16, which reacts by electrocyclic ring opening to form the open-chain 1,4-diketone 11 [34].
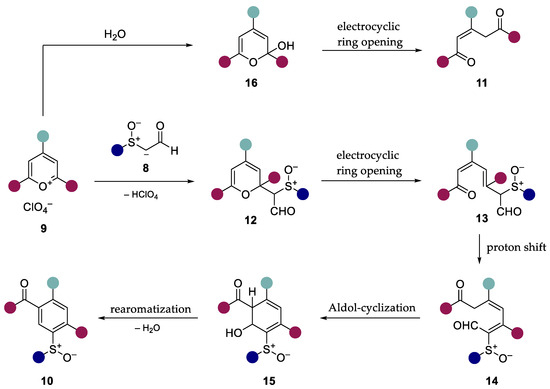
Scheme 5.
Assumed reaction mechanism of the ring transformation reaction of α-sulfinylacet-aldehydes with triarylpyrylium salts based on the work of Zimmermann [25].
3. Materials and Methods
3.1. General Methods
The melting points were determined using a Stuart Smp10 melting point apparatus (Vernon Hills, IL, USA) and were uncorrected. Thin-layer chromatography (TLC) was performed using E. Merck silica gel SilG/UV254 by Macherey Nagel & Co., Düren, Germany (thickness of layer 0.2 mm) and visualized by UV fluorescence quenching. The 1H NMR spectra were recorded on a Bruker DRX 500 spectrometer operating at 500 MHz at 300 K. The 13C NMR spectra were recorded on the same instrument at 125 MHz. The 19F NMR spectra were recorded at 471 MHz. All chemical shifts (δ) are reported in ppm relative to tetramethylsilane (TMS) as the internal standard (δ = 0.00 ppm). The spectra were referenced against the residual solvent signal, as reported in the literature [35]. The fine structure of proton signals was specified as s (singlet), d (doublet), and t (triplet). Quaternary carbons were designated with subscript q. The iR spectra were recorded using a FTIR spectrometer Paragon 1000 (Perkin Elmer LAS GmbH, Rodgau, Germany). The specific optical rotations were determined using a Anton Paar MCP300 Polarimeter in 1 dm cuvettes. ESI-MS measurements were recorded using a Bruker Impact II. Elemental analyses were performed on Vario El from Elementar. The pyrylium salts 9a–i were synthesized using the established methods [30] by the condensation of the corresponding benzaldehydes and acetophenones with phosphorus oxychloride and perchloric acid. The racemic methyl sulfoxides 7b–h were synthesized from the corresponding thiols by methylation followed by oxidation with mCPBA, according to literature procedures [36]. All other reagents were obtained from commercial sources and used without further purification, unless otherwise specified.
3.2. General Procedure for the Synthesis of the Ring Transformation Products
The corresponding methyl sulfoxide 7a–h (1.00 eq.) was placed in an oven-dried Schlenk tube under argon and dissolved in dry THF (1.00 mL/mmol). The solution was cooled to −40 °C, and lithium diisopropylamide (1.00 eq.; 2M solution in THF, ethylbenzene, and n-heptane) was added and stirred for 30 min. N-formylpiperidine (1.00 eq.) was then added slowly, and the mixture was stirred for an additional 40 min at −40 °C. In a second Schlenk tube, the 2,4,6-triarylpyrylium salt 9a–i was placed under argon and suspended with dry THF (2.00 mL/mmol). To this, the in situ synthesized α-sulfinylacetaldehyde was quickly added, and the mixture was heated to 60 °C. After 15 h, the mixture was cooled to room temperature, and dichloromethane (10 mL/mmol) was added and transferred to a separatory funnel. The mixture was extracted with water, and the aqueous phase was subsequently re-extracted twice with dichloromethane. The organic phases were combined and dried over magnesium sulfate. After the removal of the solvent, the crude product was purified by column chromatography (for aromatic methyl sulfoxides, 5% ethyl acetate to 20% ethyl acetate in n-pentane; for alkyl methyl sulfoxides, 25% Et2O in n-pentane).
(R)-Phenyl(6′-(p-tolylsulfinyl)-[1,1′:3′,1″-terphenyl]-4′-yl)methanone (10a) prepared from 600 mg (3.89 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield: 49% as an off-white solid. mp 186 °C. Rf = 0.22 (20% ethyl acetate in n-pentane). IR (ATR) (cm−1): 1669, 1041, 697. 1H NMR (500 MHz, Chloroform-d): δ 8.30 (s, 1H), 7.77–7.71 (m, 2H), 7.52–7.40 (m, 4H), 7.39–7.26 (m, 7H), 7.25–7.16 (m, 3H), 7.07–7.02 (m, 2H), 7.00–6.94 (m, 2H), 2.30 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 197.2q, 143.6q, 143.0q, 142.2q, 141.7q, 141.4q, 138.9q (2C), 137.4q, 137.0q, 133.4, 132.4, 130.2, 129.7, 129.6, 129.0, 128.7, 128.7, 128.5, 128.5, 128.1, 125.7, 124.6, 21.5 ppm. MS (ESI) (m/z): 473.16 [M + H]+, 495.14 [M + Na]+, 511.11 [M + K]+, 945.31 [2M + H]+, 967.29 [2M + Na]+, 983.26 [2M + K]+. HRMS (ESI) (m/z): Calcd for C32H24O2S [M + H]+ 473.15698; Found 473.15715. Anal. Calc. for C32H24O2S: C 81.33; H 5.12. Found C 81.32; H 5.34. = +28.27° (c 0.53; acetone).
(R)-[1,1′-biphenyl]-4-yl(4′-(p-tolylsulfinyl)-[1,1′:3′,1″:4″,1″-quaterphenyl]-6′-yl)methanone (10b) prepared from 600 mg (3.89 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield: 27% as an off-white solid. mp 116 °C. Rf = 0.24 (20% ethyl acetate in n-pentane). IR (ATR) (cm−1): 1598, 754, 695. 1H NMR (500 MHz, Chloroform-d): δ 8.34 (s, 1H), 7.85 (d, J = 8.5 Hz, 2H), 7.73–7.64 (m, 4H), 7.63–7.57 (m, 4H), 7.55–7.50 (m, 2H), 7.50–7.44 (m, 3H), 7.42 (d, J = 8.1 Hz, 4H), 7.34 (d, J = 6.6 Hz, 2H), 7.28–7.20 (m, 3H), 7.06 (s, 4H), 2.32 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 196.7q, 146.1q, 143.7q, 143.0q, 141.9q, 141.8q, 141.6q, 141.4q, 140.3q, 139.9q, 139.0q, 138.9q, 136.4q, 135.8q, 132.5, 130.8, 130.1, 129.8, 129.1, 129.0, 129.0, 128.6, 128.4, 128.1, 127.9, 127.4, 127.4, 127.2, 127.2, 125.7, 124.7, 21.5 ppm. MS (ESI) (m/z): 625.21 [M + H]+, 647.20 [M + Na]+, 1249.43 [2M + H]+, 1271.41 [2M + Na]+. HRMS (ESI) (m/z): Calcd for C44H32O2S [M + H]+ 625.21958; Found 625.21987. Anal. Calc. for C44H32O2S: C 84.58; H 5.16. Found C 84.28; H 5.27. = −30.94° (c 0.48; acetone).
(R)-(4-methoxy-6′-(p-tolylsulfinyl)-[1,1′:3′,1″-terphenyl]-4′-yl)(4-methoxyphenyl)methanone (10c) prepared from 600 mg (3.89 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield: 26% as a yellow solid. mp 188 °C. Rf = 0.06 (20% ethyl acetate in n-pentane). IR (ATR) (cm−1): 1594, 1248, 1169, 1026. 1H NMR (500 MHz, Chloroform-d): δ 8.24 (s, 1H), 7.76 (d, J = 9.0 Hz, 2H), 7.37 (s, 1H), 7.32 (m, 2H), 7.30–7.21 (m, 5H), 7.09 (d, J = 8.3 Hz, 2H), 7.05 (d, J = 8.3 Hz, 2H), 6.99 (d, J = 8.8 Hz, 2H), 6.87 (d, J = 9.0 Hz, 2H), 3.92 (s, 3H), 3.87 (s, 3H), 2.33 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 195.8q, 163.8q, 160.0q, 143.4q, 142.8q, 141.7q, 141.6q, 141.5q, 139.1q, 139.0q, 132.6, 132.5, 130.9, 130.1q, 129.9q, 129.7, 128.9, 128.5, 128.0, 125.6, 124.5, 114.1, 113.8, 55.6, 55.5, 21.5 ppm. MS (ESI) (m/z): 533.17 [M + H]+, 555.16 [M + Na]+, 1065.35 [2M + H]+, 1087.33 [2M + Na]+. HRMS (ESI) (m/z): Calcd for C34H28O4S [M + H]+ 533.17811; Found 533.17854. Anal. Calc. for C34H28O4S: C 76.67; H 5.30. Found C 76.33; H 5.12. = +15.19° (c 0.25; acetone).
(R)-(6′-(p-tolylsulfinyl)-4-(trifluoromethyl)-[1,1′:3′,1″-terphenyl]-4′-yl)(4-(trifluoromethyl)phenyl)methanone (10d) prepared from 300 mg (1.95 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield: 41% as an off-white solid. mp 91 °C. Rf = 0.47 (20% ethyl acetate in n-pentane). IR (ATR) (cm−1): 1672, 1322, 1064. 1H NMR (500 MHz, Chloroform-d): δ 8.34 (s, 1H), 7.78 (d, J = 8.1 Hz, 2H), 7.69 (d, J = 8.0 Hz, 2H), 7.58 (d, J = 8.1 Hz, 2H), 7.42 (d, J = 8.0 Hz, 2H), 7.38 (s, 1H), 7.23 (m, 5H), 7.07 (d, J = 8.0 Hz, 2H), 6.99 (d, J = 8.0 Hz, 2H), 2.32 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 196.0q, 143.9q, 143.4q, 142.3q, 141.2q, 140.9q, 140.7q, 139.6q, 138.7q, 138.3q, 134.5q (d, 2JCF = 33 Hz), 132.3, 131.0q (d, 2JCF = 33 Hz), 130.2, 130.0 (2C), 129.0, 128.8, 128.5, 125.9, 125.7 (q, 3JCF = 3.9 Hz), 125.5 (q, 3JCF = 3.9 Hz), 125.3, 124.0q (1JCF = 271 Hz), 123.5q (1JCF = 271 Hz), 21.5 ppm. 19F NMR (471 MHz, Chloroform-d) δ -62.6, -63.2 ppm. MS (ESI) (m/z): 609.13 [M + H]+, 631.11 [M + Na]+, 647.09 [M + K]+, 1217.26 [2M + H]+, 1239.24 [2M + Na]+, 1255.21 [2M + K]+. HRMS (ESI) (m/z): Calcd for C34H22F6O2S [M + H]+ 609.13175; Found 609.13177. Anal. Calc. for C34H22F6O2S: C 67.10; H 3.64. Found C 67.17; H 3.66. = +8.70° (c 0.53; Aceton).
(R)-(4″-fluoro-6′-(p-tolylsulfinyl)-[1,1′:3′,1″-terphenyl]-4′-yl)(phenyl)methanone (10e) prepared from 600 mg (3.98 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield: 41% as an off-white solid. mp 164 °C. Rf = 0.20 (20% ethyl acetate in n-pentane). IR (ATR) (cm−1): 1667, 1039. 1H NMR (500 MHz, Chloroform-d): δ 8.29 (s, 1H), 7.77–7.69 (m, 2H), 7.56–7.48 (m, 1H), 7.46–7.41 (m, 3H), 7.40–7.34 (m, 2H), 7.33 (s, 1H), 7.32–7.28 (m, 2H), 7.27–7.22 (m, 2H), 7.04 (d, J = 8.0 Hz, 2H), 7.00–6.94 (m, 2H), 6.94–6.88 (m, 2H), 2.30 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 197.1q, 162.6q (d, 1JCF = 248.0 Hz), 143.1q, 142.5q, 142.3q, 141.8q, 141.3q, 138.8q, 137.3q, 136.9q, 135.0q (d, 4JCF = 3.3 Hz), 133.6, 132.4, 130.7 (3JCF = 8.2 Hz), 130.2, 129.8, 129.6, 128.8, 128.6, 128.5, 126.9, 125.7, 124.6, 115.6 (d, 2JCF = 21.6 Hz), 21.5 ppm. 19F NMR (471 MHz, Chloroform-d) δ -113.8 ppm. MS (ESI) (m/z): 491.15 [M + H]+, 513.13 [M + Na]+, 529.10 [M + K]+, 981.29 [2M + H]+, 1003.28 [2M + Na]+, 1019.24 [2M + K]+. HRMS (ESI) (m/z): Calcd for C32H23FO2S [M + H]+ 491.14756; Found 491.14762. Anal. Calc. for C32H23FO2S: C 78.34; H 4.73. Found C 78.46; H 4.96. = +29.52° (c 0.49; acetone).
(R)-(4″-bromo-6′-(p-tolylsulfinyl)-[1,1′:3′,1″-terphenyl]-4′-yl)(phenyl)methanone (10f) prepared from 600 mg (3.89 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield: 46% as a white solid. mp 167 °C. Rf = 0.21 (20% ethyl acetate in n-pentane). IR (ATR) (cm−1): 1660, 1046. 1H NMR (500 MHz, Chloroform-d): δ 8.29 (s, 1H), 7.77–7.72 (m, 2H), 7.59–7.50 (m, 1H), 7.49–7.34 (m, 7H), 7.32 (s, 1H), 7.31–7.27 (m, 2H), 7.19–7.11 (m, 2H), 7.04 (d, J = 8.0 Hz, 2H), 6.99–6.89 (m, 2H), 2.30 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 196.9q, 143.4q, 142.4q, 142.3q, 141.8q, 141.2q, 138.7q, 137.9q, 137.2q, 136.9q, 133.7, 132.3, 131.7, 130.5, 130.2, 129.8, 129.6, 128.8, 128.8, 128.6, 125.6, 124.6, 122.6q, 21.5 ppm. MS (ESI) (m/z): 551.07 [M + H]+, 573.05 [M + Na]+, 1101.13 [2M + H]+, 1123.11 [2M + Na]+. HRMS (ESI) (m/z): Calcd for C32H23BrO2S [M + H]+ 551.06749; Found 551.06752. Anal. Calc. for C32H23BrO2S: C 69.69; H 4.20. Found C 69.21; H 3.99. = +25.77° (c 0.50; acetone).
(R)-(4-fluoro-6′-(p-tolylsulfinyl)-[1,1′:3′,1″-terphenyl]-4′-yl)(4-fluorophenyl)methanone (10g) prepared from 600 mg (3.89 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield: 36% as an off-white solid. mp 190 °C. Rf = 0.26 (20% ethyl acetate in n-pentane). IR (ATR) (cm−1): 1663, 1594, 1226. 1H NMR (500 MHz, Chloroform-d): δ 8.26 (s, 1H), 7.77–7.64 (m, 2H), 7.29–7.17 (m, 7H), 7.10 (t, J = 8.6 Hz, 2H), 7.06 (d, J = 8.0 Hz, 2H), 6.99 (t, J = 8.6 Hz, 4H), 2.30 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 195.6q, 165.9q (d, 1JCF = 256.1 Hz), 163.12q (d, 1JCF = 248.9 Hz), 143.59q, 143.20q, 142.11q, 141.33q, 141.11q, 138.83q, 138.69q, 133.5q (d, 4JCF = 5.46 Hz), 133.4q (d, 4JCF = 5.95 Hz), 132.7 (d, 3JCF = 9.5 Hz), 132.52, 131.4 (d, 3JCF = 8.1 Hz), 129.92, 128.96, 128.68, 128.33, 125.82, 124.77, 115.8 (d, 2JCF = 22.15 Hz), 115.7 (d, 2JCF = 21.91 Hz), 21.56 ppm. 19F NMR (471 MHz, Chloroform-d) δ -104.2, -112.5 ppm. MS (ESI) (m/z): 509.14 [M + H]+, 531.12 [M + Na]+, 1017.27 [2M + H]+, 1039.25 [2M + Na]+. HRMS (ESI) (m/z): Calcd for C32H22F2O2S [M + H]+ 509.13813; Found 509.13831. Anal. Calc. for C32H22F2O2S: C 75.57; H 4.36. Found C 75.66; H 4.38. = +28.14° (c 0.50; acetone).
(R)-(4-bromo-6′-(p-tolylsulfinyl)-[1,1′:3′,1″-terphenyl]-4′-yl)(4-bromophenyl)methanone (10h) prepared from 600 mg (3.89 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield: 46% as an off-white solid. mp 235 °C. Rf = 0.41 (20% ethyl acetate in n-pentane). IR (ATR) (cm−1): 1667, 1039. 1H NMR (500 MHz, Chloroform-d): δ 8.26 (s, 1H), 7.60–7.51 (m, 4H), 7.47 (d, J = 8.5 Hz, 2H), 7.33 (s, 1H), 7.23 (s, 5H), 7.18 (d, J = 8.5 Hz, 2H), 7.10 (d, J = 8.3 Hz, 2H), 7.03 (d, J = 8.3 Hz, 2H), 2.33 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): 196.1q, 143.7q, 143.1q, 142.2q, 141.2q, 141.0q, 138.7q, 138.5q, 136.2q, 135.7q, 132.3, 131.9, 131.8, 131.5, 131.2, 129.9, 128.9, 128.8q, 128.7, 128.4, 125.7, 124.9, 123.2q, 21.5 ppm. MS (ESI) (m/z): 628.98 [M + H]+, 650.96 [M + Na]+, 666.93 [M + K]+, 1256.95 [2M + H]+, 1278.93 [2M + Na]+. HRMS (ESI) (m/z): Calcd for C32H22Br2O2S [M + H]+ 628.97800; Found 628.97792. Anal. Calc. for C32H22Br2O2S: C 60.97; H 3.52. Found C 60.89; H 3.66. = −19.41° (c 0.50; acetone).
(R)-(4-iodo-6′-(p-tolylsulfinyl)-[1,1′:3′,1″-terphenyl]-4′-yl)(4-iodophenyl)methanone (10i) prepared from 300 mg (1.95 mmol) (R)-p-tolyl methyl sulfoxide ((R)-7a). Yield: 43% as an off-white solid. mp 234 °C. Rf = 0.36 (20% ethyl acetate in n-pentane). IR (ATR) (cm−1): 1659, 1038, 956. 1H NMR (500 MHz, Chloroform-d): δ 8.25 (s, 1H), 7.81–7.75 (m, 2H), 7.73–7.66 (m, 2H), 7.43–7.36 (m, 2H), 7.33 (s, 1H), 7.23 (m, 5H), 7.09 (d, J = 8.0 Hz, 2H), 7.07–7.00 (m, 4H), 2.33 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 196.2q, 143.6q, 142.9q, 142.0q, 141.2q, 140.9q, 138.5q, 138.4q, 137.8, 137.7, 136.7, 136.1, 132.1, 131.2, 131.1, 129.8, 128.8, 128.6, 128.3, 125.6, 124.8, 101.7q, 94.7q, 21.4 ppm. MS (ESI) (m/z): 724.95 [M + H]+, 746.93 [M + Na]+. HRMS (ESI) (m/z): Calcd for C32H22I2O2S [M + H]+ 724.95027; Found 724.95074. Anal. Calc. for C32H22I2O2S: C 53.06; H 3.06. Found C 53.04; H 2.99. = −32.45° (c 0.26; acetone).
Phenyl(6′-(phenylsulfinyl)-[1,1′:3′,1″-terphenyl]-4′-yl)methanone (10j) prepared from 309 mg (2.20 mmol) methylphenylsulfoxide. Yield: 39% as an off-white solid. mp 140 °C. Rf = 0.19 (20% ethyl acetate in n-pentane). IR (ATR) (cm−1): 1663, 1046. 1H NMR (500 MHz, Chloroform-d): δ 8.31 (s, 1H), 7.79–7.72 (m, 2H), 7.56–7.18 (m, 17H), 7.17–7.04 (m, 2H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 197.2q, 144.5q, 143.8q, 142.7q, 142.3q, 139.0q, 138.9q, 137.4q, 137.0q, 133.4, 132.5, 131.2, 130.2, 129.6, 129.1, 129.0, 128.8, 128.8, 128.6, 128.5, 128.1, 125.6, 124.7 ppm. MS (ESI) (m/z): 459.14 [M + H]+. HRMS (ESI) (m/z): Calcd for C31H22O2S [M + H]+ 459.1413; Found 459.1414.
(6′-((4-methoxyphenyl)sulfinyl)-[1,1′:3′,1″-terphenyl]-4′-yl)(phenyl)methanone (10k) prepared from 355 mg (2.09 mmol) 4-methoxyphenylmethylsulfoxide. Yield: 42% as an off-white solid. mp 94 °C. Rf = 0.10 (20% ethyl acetate in n-pentane). IR (ATR) (cm−1): 1664, 1250, 1045, 698. 1H NMR (500 MHz, Chloroform-d): δ 8.35 (s, 1H), 7.77 (d, J = 8.3 Hz, 2H), 7.57–7.48 (m, 1H), 7.45–7.37 (m, 6H), 7.33–7.27 (m, 4H), 7.27–7.16 (m, 3H), 7.02 (d, J = 8.8 Hz, 2H), 6.75 (d, J = 8.9 Hz, 2H), 3.79 (s, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 197.2q, 161.8q, 143.4q, 142.9q, 142.0q, 138.8q, 138.7q, 137.3q, 136.9q, 135.5q, 133.3, 132.3, 130.1, 129.4, 128.9, 128.6, 128.5, 128.4, 128.4, 127.9, 127.7, 124.3, 114.4, 55.4 ppm. MS (ESI) (m/z): 489.15 [M + H]+, 511.13 [M + Na]+, 527.11 [M + K]+, 977.30 [2M + H]+, 999.28 [2M + Na]+, 1015.25 [2M + K]+. HRMS (ESI) (m/z): Calcd for C32H24O3S [M + H]+ 489.15189; Found 489.15195. Anal. Calc. for C32H24O3S: C 78.66; H 4.95. Found C 78.53; H 5.01.
(6′-((4-bromophenyl)sulfinyl)-[1,1′:3′,1″-terphenyl]-4′-yl)(phenyl)methanone (10l) prepared from 438 mg (2.00 mmol) 4-bromophenylmethylsulfoxid. Yield: 43% as a white solid. mp 92 °C. Rf = 0.34 (20% ethyl acetate in n-pentane). IR (ATR) (cm−1): 1664, 1048, 697. 1H NMR (500 MHz, Chloroform-d): δ 8.27 (s, 1H), 7.76–7.68 (m, 2H), 7.53–7.43 (m, 4H), 7.39 (s, 1H), 7.38–7.26 (m, 6H), 7.25–7.18 (m, 5H), 7.03–6.95 (m, 2H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 197.1q, 144.0q, 143.0q, 142.4q, 142.1q, 139.1q, 138.7q, 137.5q, 137.2q, 136.9q, 133.5, 132.6, 130.1, 129.6, 129.3, 129.0, 128.9, 128.6, 128.5, 128.2, 126.9, 124.5 ppm. MS (ESI) (m/z): 537.05 [M + H]+, 559.03 [M + Na]+, 575.00 [M + K]+, 1073.09 [2M + H]+, 1095.07 [2M + Na]+. HRMS (ESI) (m/z): Calcd for C31H21BrO2S [M + H]+ 537.05184; Found 537.05130. Anal. Calc. for C31H21BrO2S: C 75.52; H 4.29. Found C 75.48; H 4.32.
(6′-((4-chlorophenyl)sulfinyl)-[1,1′:3′,1″-terphenyl]-4′-yl)(phenyl)methanone (10m) prepared from 350 mg (2.00 mmol) 4-chlorophenylmethylsulfoxid. Yield: 40 % as a white solid. mp 122 °C. Rf = 0.34 (20% ethyl acetate in n-pentane). IR (ATR) (cm−1): 1664, 1049, 697. 1H NMR (500 MHz, Chloroform-d): δ 8.26 (s, 1H), 7.72 (d, J = 8.0 Hz, 2H), 7.53–7.43 (m, 4H), 7.41–7.30 (m, 7H), 7.28 (m, 2H), 7.25–7.19 (m, 3H), 6.92 (d, J = 8.0 Hz, 2H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 197.1q, 144.0q, 143.7q, 142.3q, 142.1q, 139.1q, 138.7q, 137.2q, 136.9q, 133.5, 132.6, 132.3, 130.1, 129.6, 129.0, 128.9, 128.6, 128.5, 128.2, 127.0, 125.8q, 124.5 ppm. MS (ESI) (m/z): 493.10 [M + H]+, 515.08 [M + Na]+, 531.05 [M + K]+, 985.19 [2M + H]+, 1007.17 [2M + Na]+. HRMS (ESI) (m/z): Calcd for C31H21ClO2S [M + H]+ 493.10236; Found 493.10232. Anal. Calc. for C31H21ClO2S: C 69.28; H 3.94. Found C 69.42; H 3.80.
Phenyl(6′-(pyridin-2-ylsulfinyl)-[1,1′:3′,1″-terphenyl]-4′-yl)methanone (10n) prepared from 300 mg (2.12 mmol) 2-pyridylmethylsulfoxid. Yield: 36% as a white solid. mp 110 °C. Rf = 0.04 (20% ethyl acetate in n-pentane). IR (ATR) (cm−1): 1659, 1048, 689. 1H NMR (500 MHz, Chloroform-d): δ 8.44 (m, 1H), 7.80 (s, 1H), 7.78–7.69 (m, 2H), 7.65–7.58 (m, 2H), 7.56–7.52 (m, 2H), 7.42 (s, 1H), 7.41–7.32 (m, 4H), 7.24–7.14 (m, 5H), 7.14–7.06 (m, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 196.9q, 165.5q, 150.0, 144.4q, 144.2q, 142.1q, 138.9q (2C), 138.1, 137.6q, 136.9q, 133.3, 132.5, 130.5, 130.1, 129.0, 128.7, 128.5, 128.4, 128.4, 128.2, 127.3, 124.7, 120.2 ppm. MS (ESI) (m/z): 460.13 [M + H]+. HRMS (ESI) (m/z): Calcd for C30H21NO2S [M + H]+ 460.1366; Found 460.1368. Anal. Calc. for C30H21NO2S: C 78.41; H 4.61; N 3.05. Found C 78.35; H 4.67; N 3.07.
(6′-(cyclohexylsulfinyl)-[1,1′:3′,1″-terphenyl]-4′-yl)(phenyl)methanone (10o) prepared from 304 mg (2.08 mmol) cyclohexylmethylsulfoxid. Yield: 46% as a white solid. mp 160 °C. Rf = 0.22 (20% ethyl acetate in n-pentane). IR (ATR) (cm−1): 2926, 1664, 1046, 697. 1H NMR (500 MHz, Chloroform-d): δ 8.11 (s, 1H), 7.77–7.69 (m, 2H), 7.52–7.42 (m, 7H), 7.38–7.32 (m, 4H), 7.28–7.20 (m, 3H), 2.27 (m, 1H), 1.84–1.73 (m, 1H), 1.70–1.61 (m, 1H), 1.60–1.50 (m, 2H), 1.48–1.16 (m, 3H), 1.16–0.99 (m, 3H), 0.92–0.81 (m, 1H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 197.3q, 143.6q, 142.1q, 139.3q, 138.9q, 138.6q, 137.6q, 137.0q, 133.4, 132.3, 130.1, 129.3, 129.0, 128.9, 128.7, 128.6, 128.4, 128.1, 126.0, 60.7, 27.3, 25.8, 25.3, 25.3, 22.8 ppm. MS (ESI) (m/z): 465.18 [M + H]+, 487.17 [M + Na]+, 503.14 [M + K]+, 951.35 [2M + Na]+. HRMS (ESI) (m/z): Calcd for C31H28O2S 465.18828; Found 465.18810. Anal. Calc. for C31H28O2S: C 80.14; H 6.07. Found C 80.22; H 6.02.
(6′-(dodecylsulfinyl)-[1,1′:3′,1″-terphenyl]-4′-yl)(phenyl)methanone (10p) prepared from 465 mg (2.00 mmol) 1-dodecylmethylsulfoxid. Yield: 44% as a yellow oil. Rf = 0.40 (20% ethyl acetate in n-pentane). IR (ATR) (cm−1): 2922, 1666, 1047, 697. 1H NMR (500 MHz, Chloroform-d): δ 8.23 (s, 1H), 7.78 (d, J = 8.5 Hz, 2H), 7.58–7.45 (m, 6H), 7.45–7.33 (m, 4H), 7.28 (dd, J = 14.7, 7.7 Hz, 3H), 2.59 (dd, J = 14.0, 8.0 Hz, 1H), 2.56–2.39 (m, 1H), 1.72–1.58 (m, 1H), 1.54–1.41 (m, 1H), 1.39–1.07 (m, 18H), 0.93 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (126 MHz, Chloroform-d): δ 197.3q, 143.6q, 141.3q, 141.3q, 139.0q, 138.9q, 137.3q, 137.0q, 133.4, 132.3, 130.1, 129.1, 129.1, 129.0, 128.9, 128.6, 128.5, 128.4, 128.1, 125.0, 54.9, 32.0, 29.7 (2C), 29.6, 29.4, 29.4, 29.0, 28.3, 22.8, 22.2, 14.2 ppm. MS (ESI) (m/z): 551.30 [M + H]+, 573.28 [M + Na]+, 1001.59 [2M + H]+, 1123.57 [2M + Na]+. HRMS (ESI) (m/z): Calcd for C37H43O2S [M + H]+ 551.29783; Found 551.29773. Anal. Calc. for C37H43O2S: C 80.68; H 7.69. Found C 80.38; H 7.75.
4. Conclusions
In summary, we developed an efficient synthesis for the preparation of highly substituted meta-terphenyls bearing both a sulfoxide moiety and an acyl group at the central ring starting from readily available methyl sulfoxides 7a–h and triarylpyrylium perchlorates 9a–i. Starting from chiral, enantiomerically pure (R)-p-tolyl methyl sulfoxide ((R)-7a), the optically active derivatives 10a–i were prepared. The method is broadly applicable to a variety of aromatic and alkyl methyl sulfoxides and differently functionalized pyrylium salts. All new compounds were fully characterized by spectroscopic methods. The crystal structural analysis of 10a completed the structural evidence (see Supplementary Materials).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28227590/s1. NMR spectra and crystallographic data.
Author Contributions
Conceptualization, D.B. and M.R.; synthetic work, D.B.; X-ray crystallography, K.H.; writing—original draft preparation, review, and editing, D.B. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, J.-K. Natural Terphenyls: Developments since 1877. Chem. Rev. 2006, 106, 2209–2223. [Google Scholar] [CrossRef] [PubMed]
- Glombitza, K.-W.; Rauwald, H.-W.; Eckhardt, G. Fucole, polyhydroxyoligophenyle aus Fucus vesiculosus. Phytochemistry 1975, 14, 1403–1405. [Google Scholar] [CrossRef]
- Kouno, I.; Hashimoto, A.; Kawano, N.; Yang, C.-S. New Sesqui-neolignan from the Pericarps of Illicium macranthum. Chem. Pharm. Bull. 1989, 37, 1291–1292. [Google Scholar] [CrossRef][Green Version]
- Taro, N.; Hideki, K.; Kazuhiro, T.; Toshio, F. Structure of Mulberrofuran R, a Novel 2-Arylbenzofuran Derivative from the Cultivated Mulberry Tree (Morus lhou Koidz.). Heterocycles 1987, 26, 759. [Google Scholar] [CrossRef]
- Wu, C.-A.; Chou, H.-H.; Shih, C.-H.; Wu, F.-I.; Cheng, C.-H.; Huang, H.-L.; Chao, T.-C.; Tseng, M.-R. Synthesis and physical properties of meta-terphenyloxadiazole derivatives and their application as electron transporting materials for blue phosphorescent and fluorescent devices. J. Mater. Chem. 2012, 22, 17792–17799. [Google Scholar] [CrossRef]
- Kamath, L.; Manjunatha, K.B.; Shettigar, S.; Umesh, G.; Narayana, B.; Samshuddin, S.; Sarojini, B.K. Investigation of third-order nonlinear and optical power limiting properties of terphenyl derivatives. Opt. Laser Technol. 2014, 56, 425–429. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, B.; Gao, Y.; Chen, H.; Dong, B.; Xu, Y.; Li, J.; Wang, H.; Li, W. Triplet collection for highly efficient single-emitting-layer pure fluorescent WOLED based thermally activated delayed fluorescent host of acridine/sulfone derivative. Opt. Mater. 2020, 110, 110510. [Google Scholar] [CrossRef]
- Lee, C.W.; Lee, J.Y. A hole transport material with ortho- linked terphenyl core structure for high power efficiency in blue phosphorescent organic light-emitting diodes. Org. Electron. 2014, 15, 399–404. [Google Scholar] [CrossRef]
- Liao, H.-R.; Lin, Y.-J.; Chou, Y.-M.; Luo, F.-T.; Wang, B.-C. Theoretical study of optical and electronic properties of p-terphenyls containing cyano substituents as promising light-emitting materials. J. Lumin. 2008, 128, 1373–1378. [Google Scholar] [CrossRef]
- Sasabe, H.; Seino, Y.; Kimura, M.; Kido, J. A m-Terphenyl-Modifed Sulfone Derivative as a Host Material for High-Efficiency Blue and Green Phosphorescent OLEDs. Chem. Mater. 2012, 24, 1404–1406. [Google Scholar] [CrossRef]
- Su, S.-J.; Cai, C.; Kido, J. RGB Phosphorescent Organic Light-Emitting Diodes by Using Host Materials with Heterocyclic Cores: Effect of Nitrogen Atom Orientations. Chem. Mater. 2011, 23, 274–284. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, B.; Tan, J.; Mu, G.; Yi, W.; Lv, X.; Zhuang, S.; Liu, W.; Wang, L. Optimized electron-transport material based on m-terphenyl-diphenylphosphine oxide with the harmonious compatibility of high ET and electron mobility for highly efficient OLEDs. J. Mater. Chem. C 2017, 5, 8516–8526. [Google Scholar] [CrossRef]
- Jiang, W.; Duan, L.; Qiao, J.; Zhang, D.; Dong, G.; Wang, L.; Qiu, Y. Novel star-shaped host materials for highly efficient solution-processed phosphorescent organic light-emitting diodes. J. Mater. Chem. 2010, 20, 6131–6137. [Google Scholar] [CrossRef]
- Sasabe, H.; Pu, Y.-J.; Nakayama, K.-I.; Kido, J. m-Terphenyl-modified carbazole host material for highly efficient blue and green PHOLEDS. Chem. Commun. 2009, 6655–6657. [Google Scholar] [CrossRef]
- Gong, S.; Zhao, Y.; Yang, C.; Zhong, C.; Qin, J.; Ma, D. Tuning the Photophysical Properties and Energy Levels by Linking Spacer and Topology between the Benzimidazole and Carbazole Units: Bipolar Host for Highly Efficient Phosphorescent OLEDs. J. Phys. Chem. C 2010, 114, 5193–5198. [Google Scholar] [CrossRef]
- Han, J.; Guo, S.; Lu, H.; Liu, S.; Zhao, Q.; Huang, W. Recent Progress on Circularly Polarized Luminescent Materials for Organic Optoelectronic Devices. Adv. Opt. Mater. 2018, 6, 1800538. [Google Scholar] [CrossRef]
- Takaishi, K.; Yamamoto, T.; Hinoide, S.; Ema, T. Helical Oligonaphthodioxepins Showing Intense Circularly Polarized Luminescence (CPL) in Solution and in the Solid State. Chem. Eur. J. 2017, 23, 9249–9252. [Google Scholar] [CrossRef]
- Rickhaus, M.; Bannwart, L.M.; Neuburger, M.; Gsellinger, H.; Zimmermann, K.; Häussinger, D.; Mayor, M. Inducing Axial Chirality in a “Geländer” Oligomer by Length Mismatch of the Oligomer Strands. Angew. Chem. Int. Ed. 2014, 53, 14587–14591. [Google Scholar] [CrossRef]
- Al-Zoubi, R.M.; Al-Jammal, W.K.; El-Khateeb, M.Y.; McDonald, R. Synthesis of Diiodinated Biphenyls and Iodinated meta-Terphenyls by Regioselective Suzuki–Miyaura Cross-Coupling Reactions of 5-Substituted 1,2,3-Triiodobenzenes. Eur. J. Org. Chem. 2015, 2015, 3374–3384. [Google Scholar] [CrossRef]
- Antelo Miguez, J.M.; Adrio, L.A.; Sousa-Pedrares, A.; Vila, J.M.; Hii, K.K. A Practical and General Synthesis of Unsymmetrical Terphenyls. J. Org. Chem. 2007, 72, 7771–7774. [Google Scholar] [CrossRef]
- Camacho, D.H.; Salo, E.V.; Guan, Z. Synthesis and Structure of m-Terphenyl-Based Cyclophanes with Nitrogen Intra-annular Functional Groups. Org. Lett. 2004, 6, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Adrio, L.A.; Míguez, J.M.A.; Hii, K.K. Synthesis of Terphenyls. Org. Prep. Proced. Int. 2009, 41, 331–358. [Google Scholar] [CrossRef]
- Poudel, T.N.; Tamargo, R.J.I.; Cai, H.; Lee, Y.R. Recent Progress in Transition-Metal-Free, Base-Mediated Benzannulation Reactions for the Synthesis of a Diverse Range of Aromatic and Heteroaromatic Compounds. Asian J. Org. Chem. 2018, 7, 985–1005. [Google Scholar] [CrossRef]
- Nagahata, S.; Takei, S.; Ueno, S. One-Pot Synthesis of Multiarylated Benzophenones via [3+2+1] Benzannulation of Ketones, Alkynes, and α,β-Unsaturated Carbonyls. J. Org. Chem. 2022, 87, 10377–10384. [Google Scholar] [CrossRef]
- Zimmermann, T. Ringtransformationen heterocyclischer Verbindungen. VII. 2,4,5-Triaryl-benzophenone aus 2,4,6-Triaryl-pyryliumsalzen und Arylacetaldehyden: Erste Pyryliumringtransformationen mit Aldehyden als Kohlenstoffnucleophile. J. Prakt. Chem./Chem.-Ztg. 1994, 336, 303–306. [Google Scholar] [CrossRef]
- Andersen, K.K. Synthesis of (+)-ethyl p-tolyl sulfoxide from (−)-menthyl (−)-p-toluenrsulfinate. Tetrahedron Lett. 1962, 3, 93–95. [Google Scholar] [CrossRef]
- Solladié, G. Asymmetric synthesis using nucleophilic reagents containing a chiral sulfoxide group. Synthesis 1981, 185–196. [Google Scholar] [CrossRef]
- Solladié, G.; Hutt, J.; Girardin, A. Improved Preparation of Optically Active Methyl p-Tolyl Sulfoxide. Synthesis 1987, 173. [Google Scholar] [CrossRef]
- Pflieger, P.; Mioskowski, C.; Salaun, J.P.; Weissbart, D.; Durst, F. Synthesis of optically active α-sulfinylacetaldehyde. Tetrahedron Lett. 1988, 29, 6775–6778. [Google Scholar] [CrossRef]
- Vidal, M.; Rezende, M.C.; Pastene, C.; Aliaga, C.; Domínguez, M. Solvatochromism of conjugated 4-N,N-dimethylaminophenyl-pyridinium donor–acceptor pairs. New J. Chem. 2018, 42, 4223–4231. [Google Scholar] [CrossRef]
- Banfi, L.; Colombo, L.; Gennari, C.; Annunziata, R.; Cozzi, F. Stereospecific synthesis of chiral.alpha.-sulfinylhydrazones. Synthesis 1982, 829–831. [Google Scholar] [CrossRef]
- Balaban, A.T.; Nenitzescu, C.D. 699 Reaction of pyrylium salts with alkali cyanides. J. Chem. Soc. 1961, 3566–3572. [Google Scholar] [CrossRef]
- Kuthan, J. Pyrans, Thiopyrans, and Selenopyrans. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press: Cambridge, MA, USA, 1983; Volume 34, pp. 145–303. [Google Scholar]
- Williams, A. Hydrolysis of pyrylium salts. Kinetic evidence for hemiacetal intermediates. J. Am. Chem. Soc. 1971, 93, 2733–2737. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Shimada, R.; Ogiwara, Y. Indium-Catalyzed Deoxygenation of Sulfoxides with Hydrosilanes. Asian J. Org. Chem. 2021, 10, 845–850. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).