New Adamantane-Containing Edaravone Conjugates as Potential Neuroprotective Agents for ALS Treatments
Abstract
:1. Introduction
2. Results and Discussion
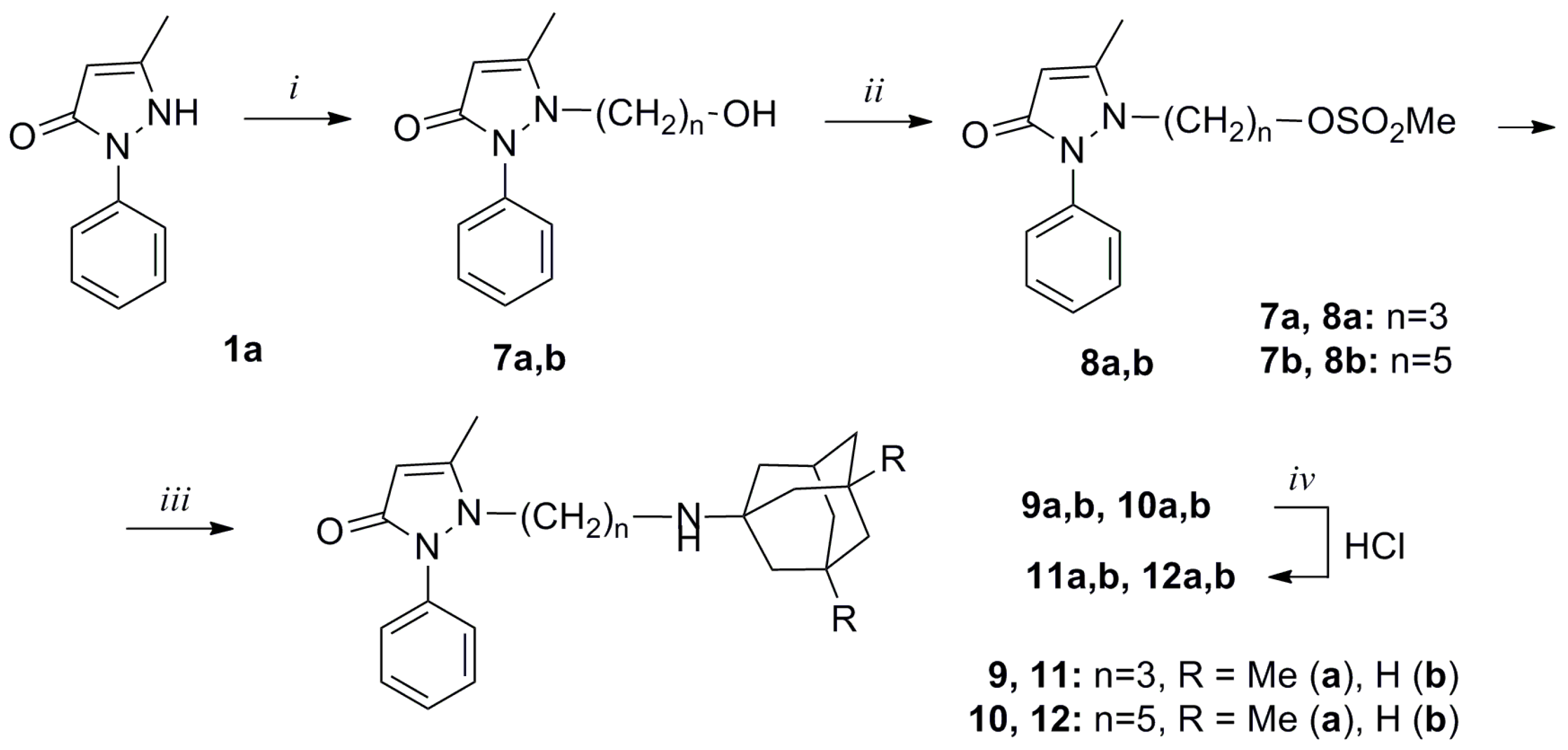
2.1. Synthesis and Structure of New Adamantane-Containing Edaravone Conjugates
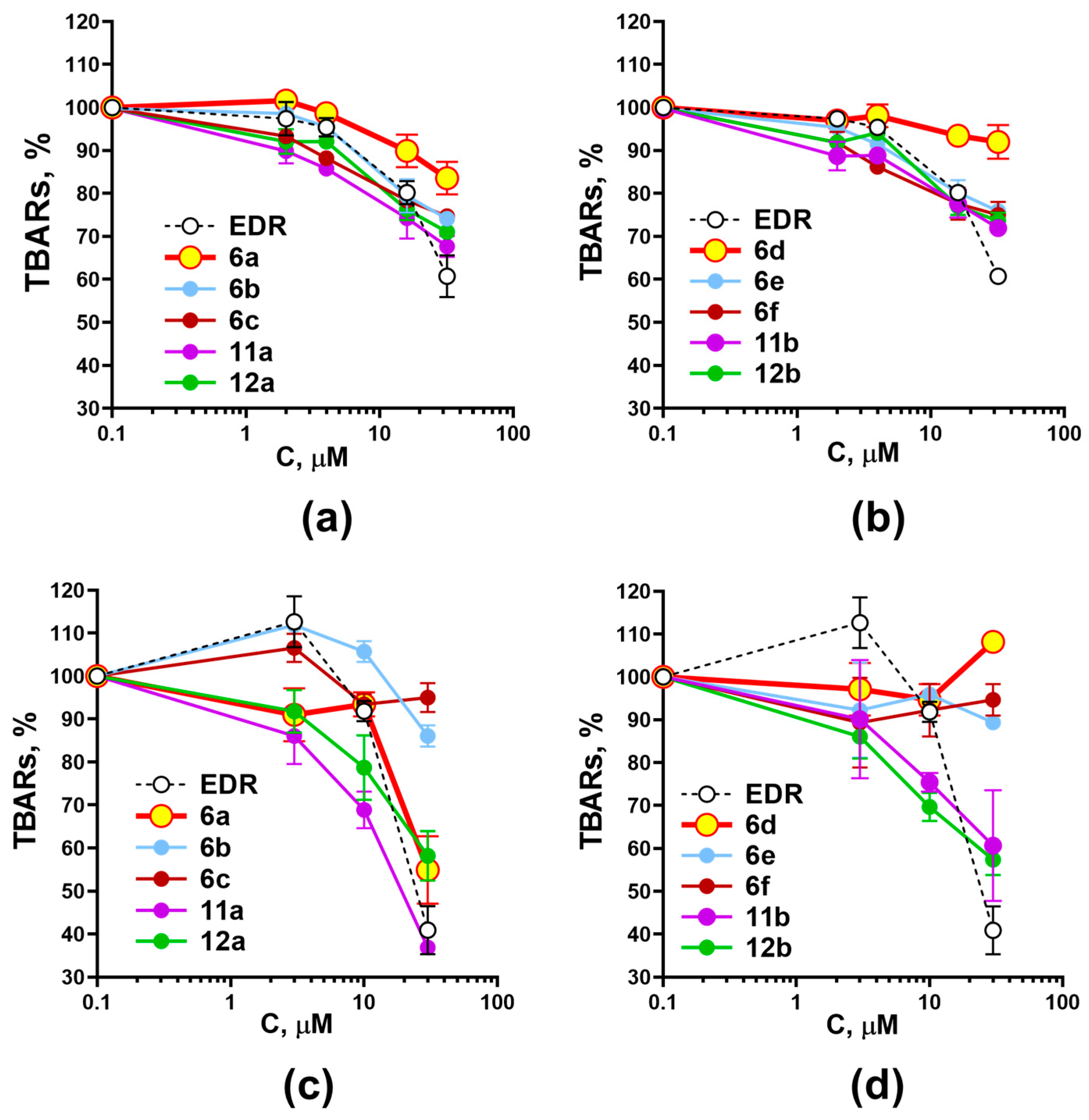
2.2. Investigation of the Effect of Adamantane-Containing Edaravone Conjugates on the LP of Rat-Brain Homogenate
2.3. Investigation of the Effect of Adamantane-Containing Edaravone Conjugates on Fast Sodium Currents
2.4. Anti-Aggregation Properties of New Conjugates of Edaravone and Aminoadamantane Derivatives
3. Materials and Methods
3.1. Investigation of the Effect of Compounds on Lipid Peroxidation in Rat-Brain Homogenate
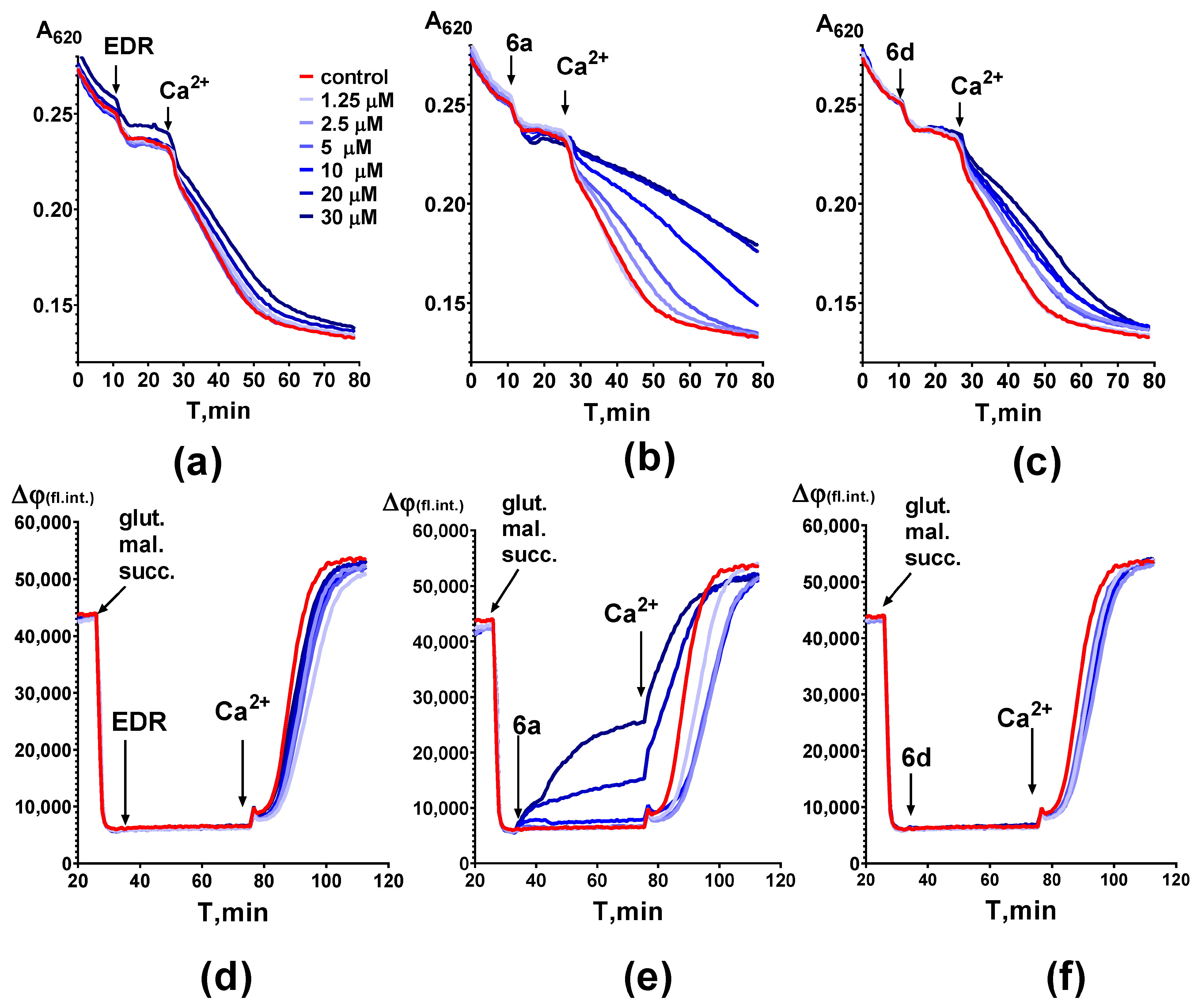
3.2. Investigation of the Effect of the Compounds on Mitochondrial Characteristics
3.3. Evaluation of Fast Sodium Currents
3.4. Anti-Aggregation Properties of Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, R.H.; Al-Chalabi, A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef] [PubMed]
- McAlary, L.; Chew, Y.L.; Lum, J.S.; Geraghty, N.J.; Yerbury, J.J.; Cashman, N.R. Amyotrophic lateral sclerosis: Proteins, proteostasis, prions, and promises. Front. Cell Neurosci. 2020, 14, 581907. [Google Scholar] [CrossRef] [PubMed]
- Blasco, H.; Mavel, S.; Corcia, P.; Gordon, P.H. The glutamate hypothesis in ALS: Pathophysiology and drug development. Curr. Med. Chem. 2014, 21, 3551–3575. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ji, Y.; Wang, W.; Zhang, L.; Chen, Z.; Yu, M.; Sun, H. Amyotrophic lateral sclerosis: Molecular mechanisms, biomarkers, and therapeutic strategies. Antioxidants 2021, 10, 1012. [Google Scholar] [CrossRef]
- Meyer, T. Amyotrophic lateral sclerosis (ALS)—Diagnosis, course of disease and treatment options. Dtsch. Med. Wochenschr. 2021, 146, 1613–1618. [Google Scholar] [CrossRef]
- Jaiswal, M.K. Riluzole and edaravone: A tale of two amyotrophic lateral sclerosis drugs. Med. Res. Rev. 2019, 39, 733–748. [Google Scholar] [CrossRef]
- Shelkovnikova, T.A.; Ustyugov, A.A.; Smirnov, A.P.; Skvortsova, V.I.; Buchman, V.L.; Bachurin, S.O.; Ninkina, N.N. Fus gene mutations associated with familiar forms of amyotrophic lateral sclerosis affect cellular localization and aggregation properties of the encoded protein. Dokl. Biochem. Biophys. 2011, 438, 123–126. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.; Deng, M. New developments and opportunities in drugs being trialed for amyotrophic lateral sclerosis from 2020 to 2022. Front. Pharmacol. 2022, 13, 1054006. [Google Scholar] [CrossRef]
- Bachurin, S.O.; Aksinenko, A.Y.; Makhaeva, G.F.; Shevtsova, E.F. Multipharmacophore strategy in medicinal chemistry for the design of drugs for the treatment of Alzheimer’s and some other neurodegenerative diseases. Russ. Chem. Bull. 2023, 72, 130–147. [Google Scholar] [CrossRef]
- Aksinenko, A.Y.; Goreva, T.V.; Epishina, T.A.; Bachurin, S.O. Synthesis of conjugates of 5-methyl-2-phenylpyrazol-3-ones and 1-aminoadamantanes as potential neuroprotective agents. Russ. Chem. Bull. 2022, 71, 1794–1800. [Google Scholar] [CrossRef]
- Wang, T.; Tomas, D.; Perera, N.D.; Cuic, B.; Luikinga, S.; Viden, A.; Turner, B.J. Ferroptosis mediates selective motor neuron death in amyotrophic lateral sclerosis. Cell Death Differ. 2022, 29, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Takayasu, Y.; Nakaki, J.; Kawasaki, T.; Koda, K.; Ago, Y.; Baba, A.; Matsuda, T. Edaravone, a radical scavenger, inhibits mitochondrial permeability transition pore in rat brain. J. Pharmacol. Sci. 2007, 103, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Kiernan, M.C.; Vucic, S. Axonal excitability in amyotrophic lateral sclerosis: Axonal excitability in ALS. Neurother. Rev. Neurother. 2017, 14, 78–90. [Google Scholar] [CrossRef]
- Shibuya, K.; Otani, R.; Suzuki, Y.-i.; Kuwabara, S.; Kiernan, M.C. Neuronal hyperexcitability and free radical toxicity in amyotrophic lateral sclerosis: Established and future targets. Pharmaceuticals 2022, 15, 433. [Google Scholar] [CrossRef]
- Xie, R.; Zheng, D.; Xing, J.; Zhang, X.; Song, Y.; Xie, Y.; Kuang, F.; Dong, H.; You, S.; Xu, H.; et al. Blockade of persistent sodium currents contributes to the riluzole-induced inhibition of spontaneous activity and oscillations in injured DRG neurons. PLoS ONE 2011, 6, e18681. [Google Scholar] [CrossRef] [PubMed]
- Bellingham, M. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: What have we learned in the last decade? CNS Neurosci. Ther. 2011, 17, 4–31. [Google Scholar] [CrossRef]
- Shelkovnikova, T.; Peters, O.; Deykin, A. Fused in sarcoma (FUS) protein lacking nuclear localization signal (NLS) and major RNA binding motifs triggers proteinopathy and severe motor phenotype in transgenic mice. J. Biol. Chem. 2013, 288, 25266–25274. [Google Scholar] [CrossRef] [PubMed]
- Ustyugov, A.; Aksinenko, A.; Steinberg, D.; Lapshina, M.; Nebogatikov, V.; Bachurin, S. Fluorinated γ-carbolines as a promising class of neuroprotective drugs. Rus. Chem. Bull. 2021, 70, 982–986. [Google Scholar] [CrossRef]
- Åkerman, K.; Wikström, M. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976, 68, 191–197. [Google Scholar] [CrossRef]
- Perlovich, G.L.; Proshin, A.N.; Volkova, T.V.; Kurkov, S.V.; Grigoriev, V.V.; Petrova, L.N.; Bachurin, S.O. Novel isothiourea derivatives as potent neuroprotectors and cognition enhancers: Synthesis, biological and physicochemical properties. J. Med. Chem. 2009, 9, 1845–1852. [Google Scholar] [CrossRef]
- Kwiatkowski, T.; Bosco, D.; Leclerc, L.; Tamrazian, E.; Vanderburg, C.; Russ, C.; Gilchrist, J.; Kasarskis, E.; Munsat, T.; Valdmanis, P.; et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 2009, 323, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Renton, A.; Majounie, E.; Waite, A.; Simón-sánchez, J.; Rollinson, S.; Gibbs, J.; Schymick, J.; Laaksovirta, H.; Van Swieten, J.; Kaganovich, A.; et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011, 72, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.P.; Brown, R.H., Jr.; Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhai, H.; Bigio, E. FUS-immunoreactive inclusions are a common feature in sporadic and non-SOD1 familial amyotrophic lateral sclerosis. Ann. Neurol. 2010, 67, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.; Rüb, U.; Orantes, M.; Braak, H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef] [PubMed]




| Substances | Effect on Fast Na-Currents IC50, μM | Substances | Effect on Fast Na-Currents IC50, μM |
|---|---|---|---|
6a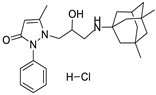 | 7.8 ± 1.1 | 6d | 6.9 ± 1.5 |
6b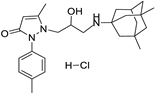 | 7.1 ± 1.1 | 6e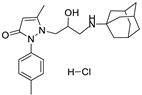 | 10.9 ± 1.3 |
6c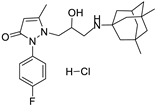 | 17.2 ± 1.6 | 6f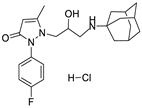 | 9.5 ± 1.0 |
11a | 11.8 ± 1.2 | 11b | 16.2 ± 1.3 |
12a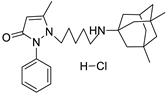 | 5.9 ± 0.8 | 12b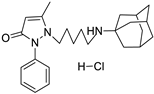 | 9.9 ± 1.1 |
| Reference agents | |||
Memantine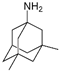 | 12.8 ± 1.7 | Amantadine | >100 |
Edaravone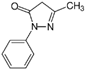 | >100 | Riluzole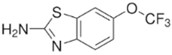 | 15.3 ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapshina, M.A.; Shevtsova, E.F.; Grigoriev, V.V.; Aksinenko, A.Y.; Ustyugov, A.A.; Steinberg, D.A.; Maleev, G.V.; Dubrovskaya, E.S.; Goreva, T.V.; Epishina, T.A.; et al. New Adamantane-Containing Edaravone Conjugates as Potential Neuroprotective Agents for ALS Treatments. Molecules 2023, 28, 7567. https://doi.org/10.3390/molecules28227567
Lapshina MA, Shevtsova EF, Grigoriev VV, Aksinenko AY, Ustyugov AA, Steinberg DA, Maleev GV, Dubrovskaya ES, Goreva TV, Epishina TA, et al. New Adamantane-Containing Edaravone Conjugates as Potential Neuroprotective Agents for ALS Treatments. Molecules. 2023; 28(22):7567. https://doi.org/10.3390/molecules28227567
Chicago/Turabian StyleLapshina, Maria A., Elena F. Shevtsova, Vladimir V. Grigoriev, Aleksey Yu. Aksinenko, Aleksey A. Ustyugov, Daniil A. Steinberg, Grigoriy V. Maleev, Elena S. Dubrovskaya, Tatiana V. Goreva, Tatiana A. Epishina, and et al. 2023. "New Adamantane-Containing Edaravone Conjugates as Potential Neuroprotective Agents for ALS Treatments" Molecules 28, no. 22: 7567. https://doi.org/10.3390/molecules28227567
APA StyleLapshina, M. A., Shevtsova, E. F., Grigoriev, V. V., Aksinenko, A. Y., Ustyugov, A. A., Steinberg, D. A., Maleev, G. V., Dubrovskaya, E. S., Goreva, T. V., Epishina, T. A., Zamoyski, V. L., Makhaeva, G. F., Fisenko, V. P., Veselov, I. M., Vinogradova, D. V., & Bachurin, S. O. (2023). New Adamantane-Containing Edaravone Conjugates as Potential Neuroprotective Agents for ALS Treatments. Molecules, 28(22), 7567. https://doi.org/10.3390/molecules28227567








