Amphetamine Derivatives as Potent Central Nervous System Multitarget SERT/NET/H3 Agents: Synthesis and Biological Evaluation
Abstract
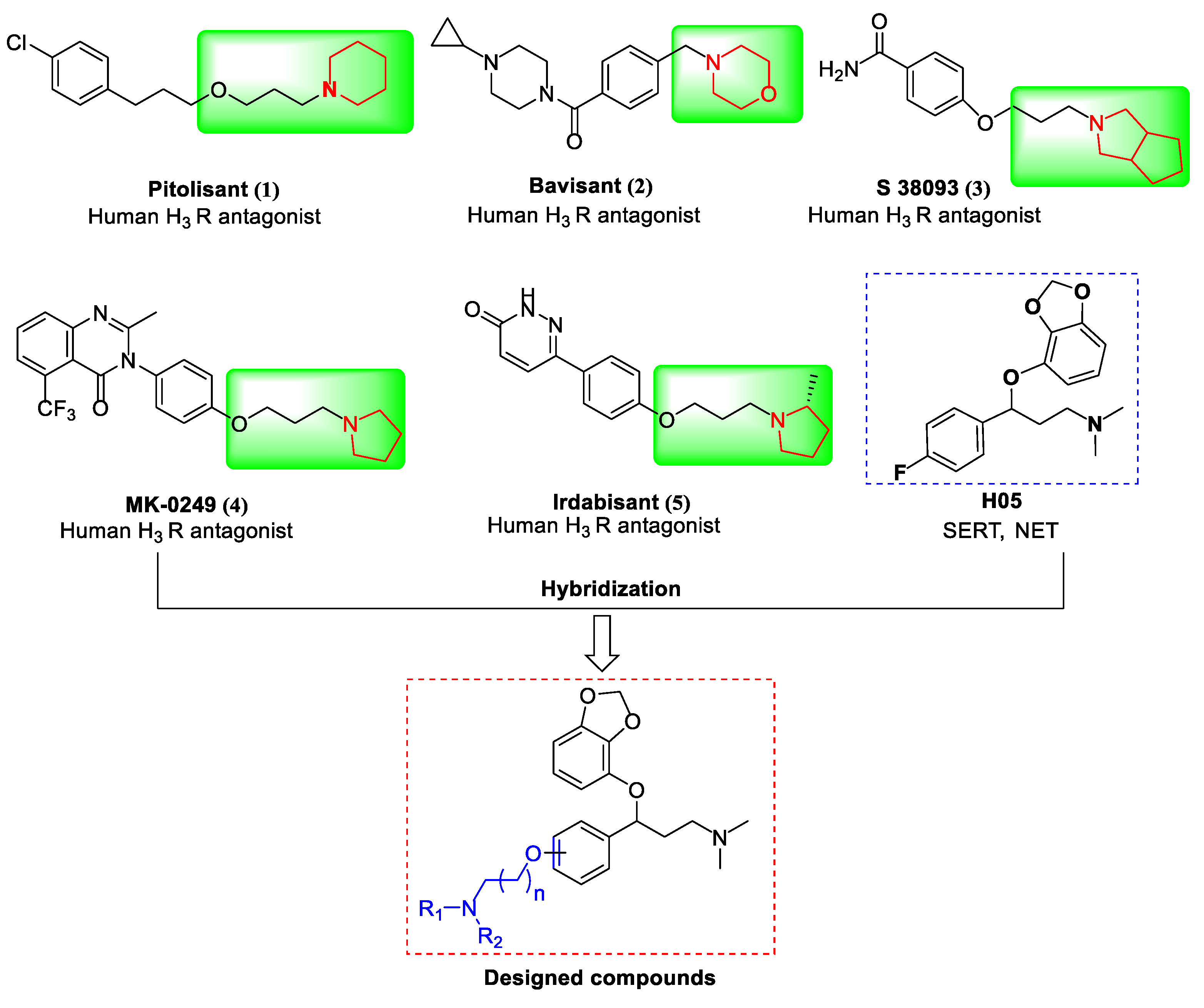
1. Introduction
2. Results and Discussion
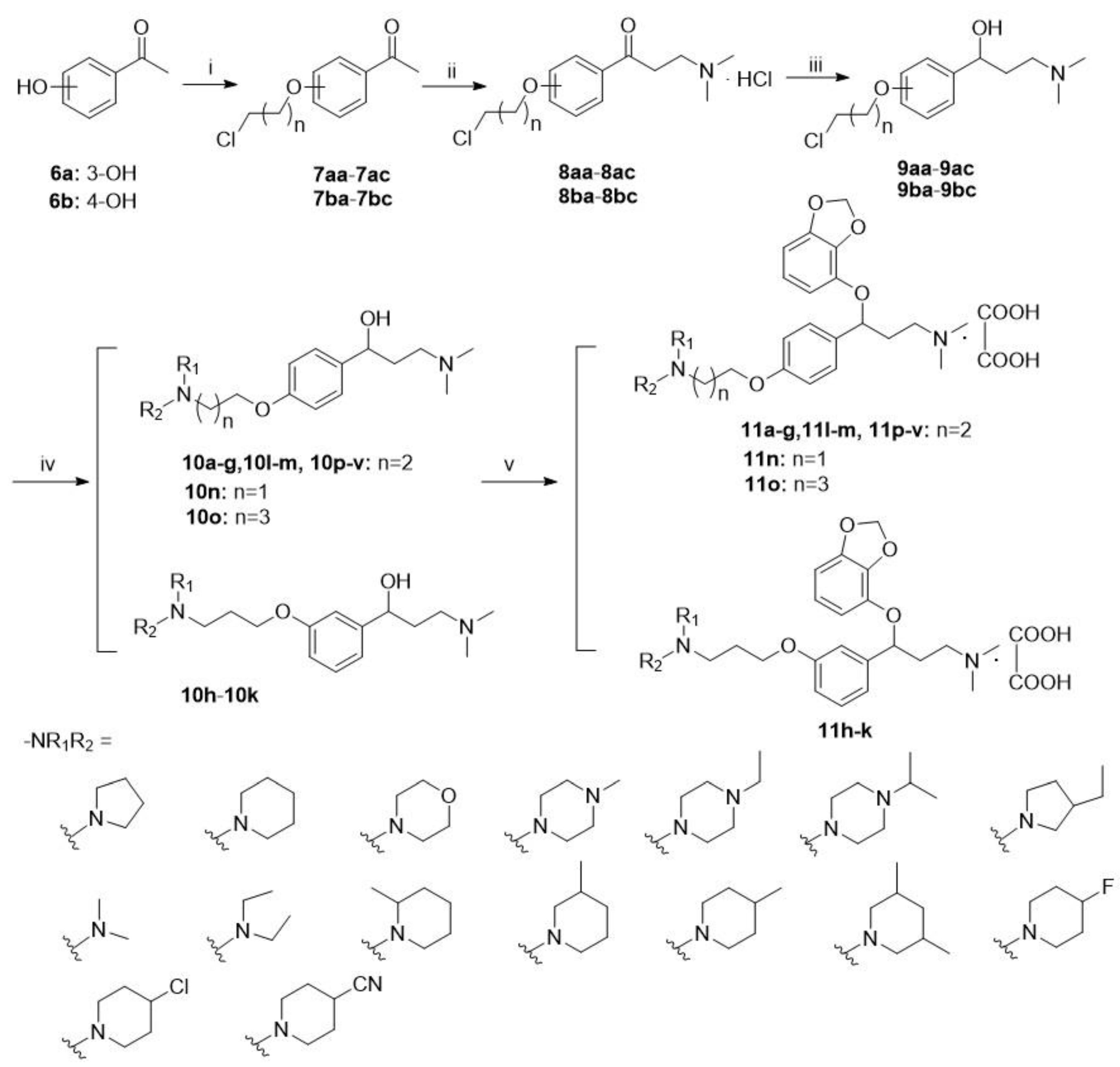
2.1. Chemistry
2.2. In Vitro Study of Target Compounds
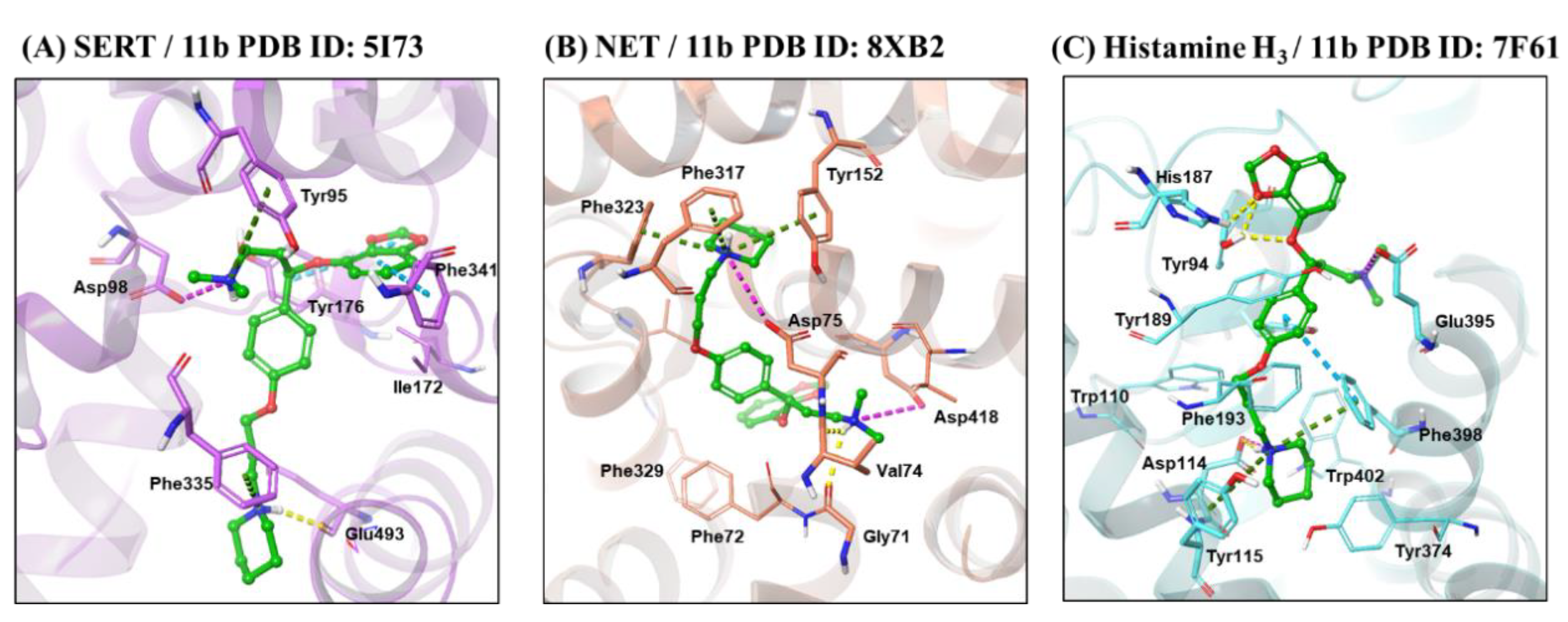
2.3. Molecular Docking
2.4. Intrinsic Activity of Compound 11b
2.5. Acute Toxicity
2.6. In Vivo Behavioral Studies
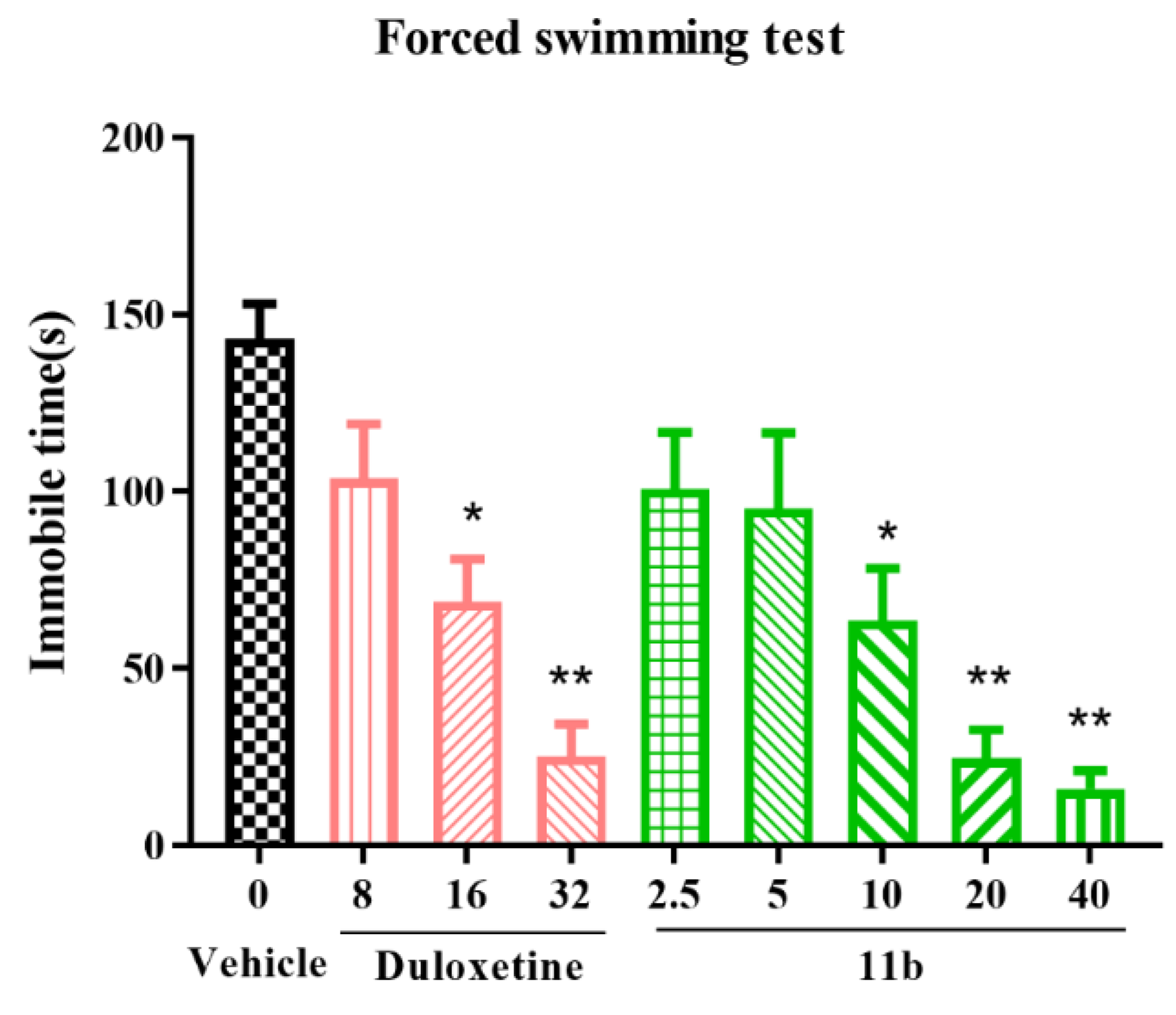
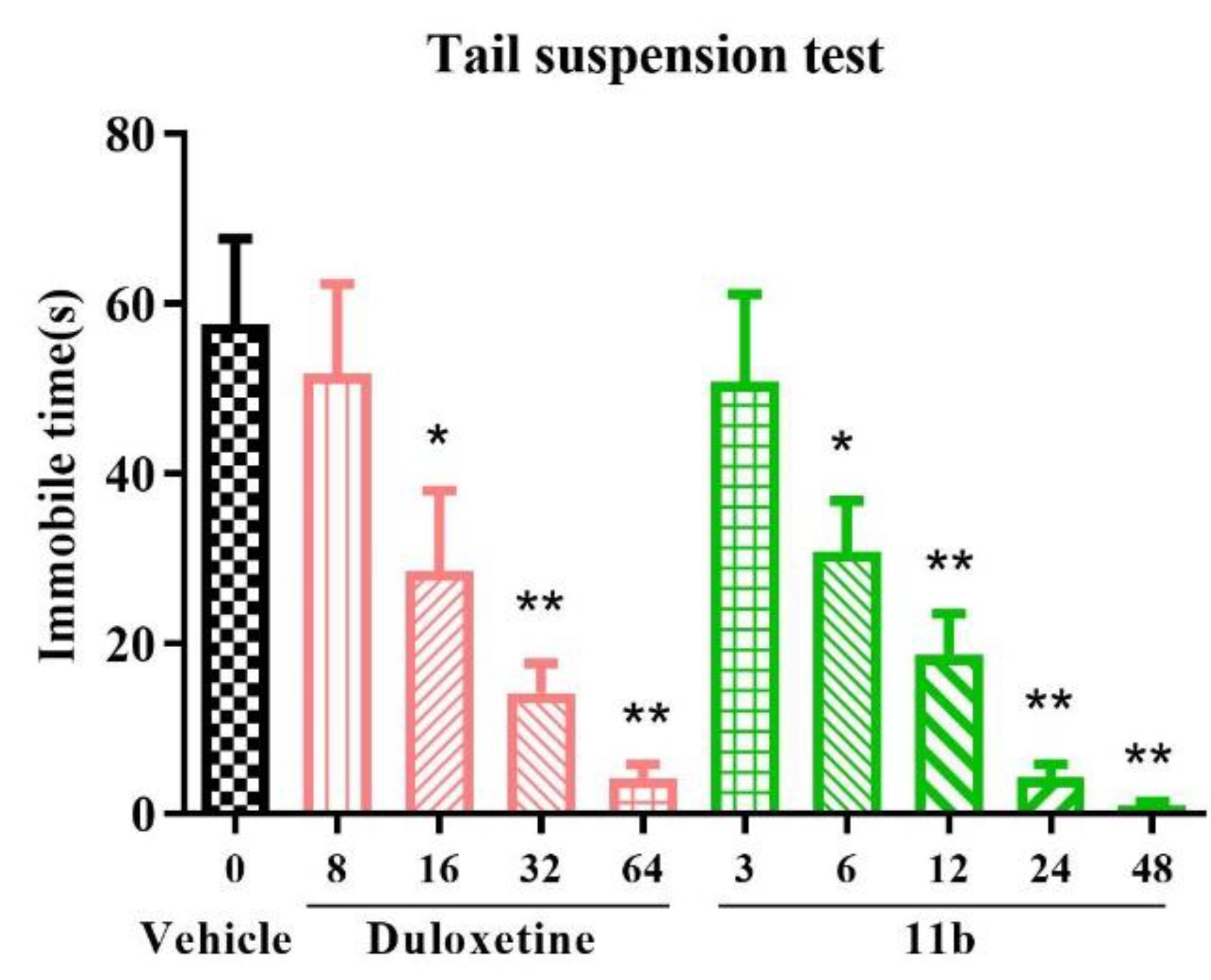
2.6.1. Forced Swimming Test (FST) and Tail Suspension Test (TST) in Mice
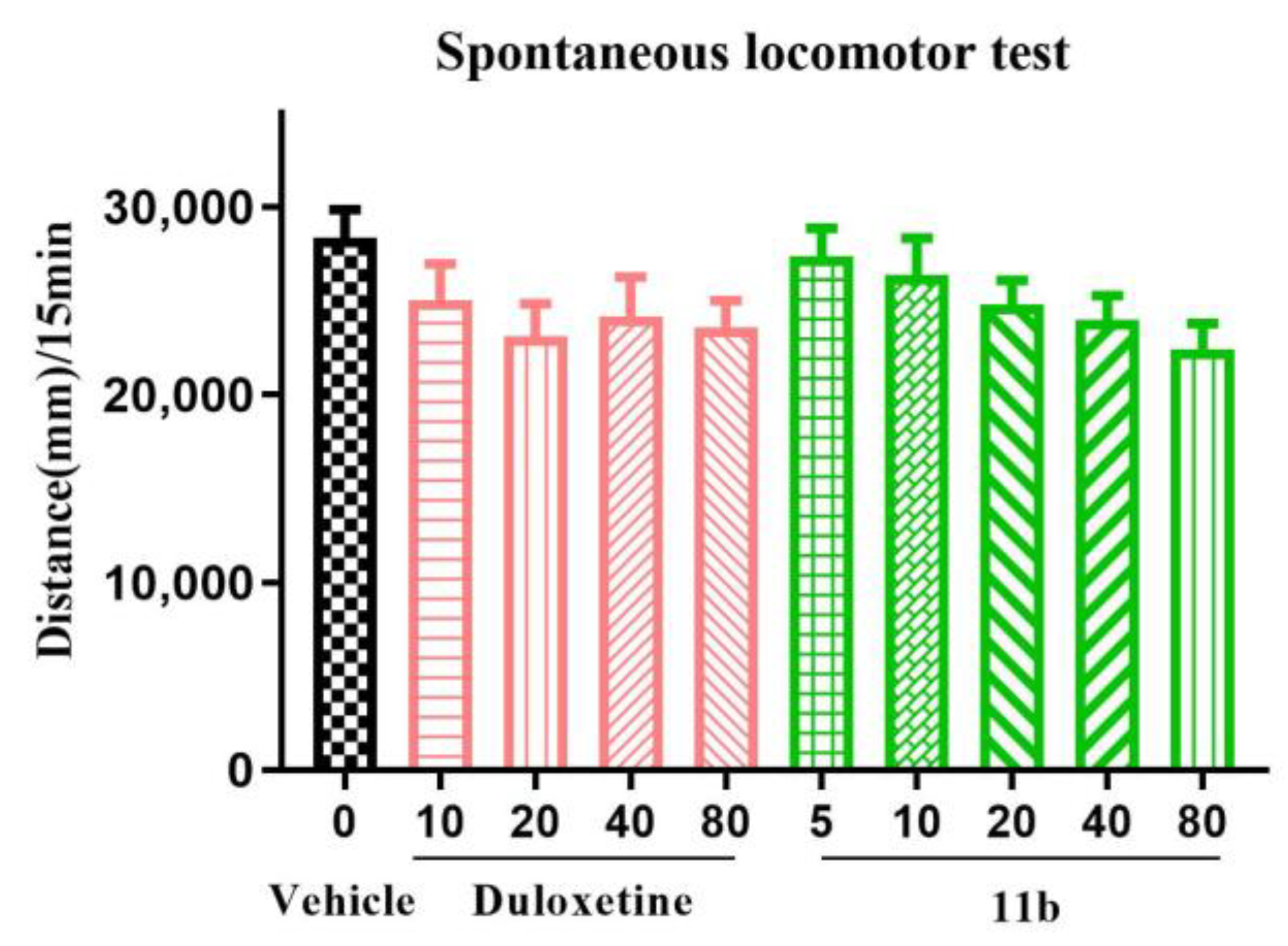
2.6.2. Locomotor Activity Test in Mice
2.7. Selectivity Characterization of Compound 11b
2.8. Pharmacokinetic Study in Rats
3. Materials and Methods
3.1. General Information
3.2. Instrumentation
3.3. Synthesis
3.3.1. General Procedure for the Preparation of Intermediates 7
3.3.2. General Procedure for the Preparation of Intermediates 8
3.3.3. General Procedure for the Preparation of Intermediates 9
3.3.4. General Procedure for the Preparation of Intermediates 10
3.3.5. General Procedure for the Preparation of Target Compound 11
- 3-(benzo[d][1,3]dioxol-4-yloxy)-N,N-dimethyl-3-(4-(3-(pyrrolidin-1-yl)propoxy)phenyl)propan-1-amine oxalate (11a), Pale-white solid; m.p. 138–140 °C; yield 51.2%; 1H NMR (400 MHz, DMSO-d6) δ 7.33 (d, J = 8.2 Hz, 1H), 7.28 (d, J = 8.1 Hz, 1H), 6.92 (d, J = 8.0 Hz, 2H), 6.64 (t, J = 8.1 Hz, 1H), 6.54–6.38 (m, 2H), 6.01–5.89 (m, 2H), 5.45 (dd, J = 8.3, 4.6 Hz, 1H), 4.04 (p, J = 6.9, 6.0 Hz, 2H), 3.41–3.16 (m, 6H), 3.06 (tp, J = 13.3, 7.7, 6.3 Hz, 2H), 2.73 (d, J = 8.9 Hz, 6H), 2.47–2.26 (m, 1H), 2.24–2.02 (m, 3H), 1.99–1.85 (m, 4H). 13C NMR (101 MHz, DMSO-d6) δ 165.24, 158.50, 148.94, 141.69, 136.24, 132.72, 128.07, 122.24, 114.95, 111.75, 103.05, 101.34, 78.21, 65.29, 54.22, 53.42, 51.80, 42.73, 32.71, 25.77, 23.16. HRMS (ESI) m/z: calcd for C25H34N2O4 [M + H]+: 427.2591; found: 427.2592.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-N,N-dimethyl-3-(4-(3-(piperidin-1-yl)propoxy)phenyl)propan-1-amine oxalate (11b), Pale-white solid; m.p. 105–107 °C; yield 43.5%; 1H NMR (400 MHz, DMSO-d6) δ 7.33 (d, J = 8.3 Hz, 2H), 6.91 (d, J = 8.2 Hz, 2H), 6.65 (t, J = 8.1 Hz, 1H), 6.50 (dd, J = 13.3, 8.1 Hz, 2H), 5.97 (d, J = 12.1 Hz, 2H), 5.42 (dd, J = 8.3, 4.6 Hz, 1H), 4.01 (q, J = 7.7, 6.1 Hz, 2H), 3.11 (qt, J = 18.2, 4.6 Hz, 8H), 2.72 (s, 6H), 2.40–2.26 (m, 1H), 2.11 (qd, J = 11.8, 5.7 Hz, 3H), 1.73 (p, J = 5.5 Hz, 4H), 1.61–1.47 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 158.48, 148.93, 141.69, 136.22, 132.72, 128.07, 122.25, 114.94, 111.71, 103.04, 101.34, 78.20, 65.46, 54.19, 53.87, 52.50, 42.71, 32.70, 23.86, 22.99, 21.92. HRMS (ESI) m/z: calcd for: C26H36N2O4 [M + H]+: 441.2748; found:441.2739.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-N,N-dimethyl-3-(4-(3-morpholinopropoxy)phenyl)propan-1-amine oxalate (11c), Pale-white solid; m.p. 169–170 °C; yield 35.4%; 1H NMR (400 MHz, DMSO-d6) δ 7.32 (d, J = 8.4 Hz, 2H), 6.91 (d, J = 8.4 Hz, 2H), 6.65 (t, J = 8.1 Hz, 1H), 6.50 (ddd, J = 13.5, 8.2, 1.0 Hz, 2H), 5.97 (dd, J = 12.1, 1.0 Hz, 2H), 5.42 (dd, J = 8.3, 4.6 Hz, 1H), 3.98 (t, J = 6.2 Hz, 2H), 3.23 (ddt, J = 28.3, 11.8, 5.7 Hz, 2H), 3.12–2.97 (m, 8H), 2.75 (s, 6H), 2.63 (t, J = 7.3 Hz, 2H), 2.34 (d, J = 10.1 Hz, 1H), 2.16 (d, J = 11.9 Hz, 1H), 1.91 (p, J = 6.7 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 158.59, 148.94, 141.67, 136.23, 132.60, 128.07, 122.25, 114.94, 111.73, 103.06, 101.35, 78.18, 65.58, 64.55, 54.21 (d, J = 10.6 Hz), 52.10, 42.64, 32.60, 24.26. HRMS (ESI) m/z: calcd for: C25H34N2O5 [M + H]+: 443.2540; found:443.2545.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-N,N-dimethyl-3-(4-(3-(4-methylpiperazin-1-yl)propoxy)phenyl)propan-1-amine oxalate (11d), Pale-white solid; m.p. 155–156 °C; yield 40.8%; 1H NMR (400 MHz, DMSO-d6) δ 7.32 (d, J = 8.2 Hz, 2H), 6.90 (d, J = 8.2 Hz, 2H), 6.65 (t, J = 8.1 Hz, 1H), 6.50 (dd, J = 15.6, 8.1 Hz, 2H), 5.98 (d, J = 12.5 Hz, 2H), 5.42 (dd, J = 8.3, 4.6 Hz, 1H), 3.97 (t, J = 6.2 Hz, 2H), 3.19 (dt, J = 11.8, 6.1 Hz, 2H), 3.11–2.97 (m, 4H), 2.76 (s, 9H), 2.61 (d, J = 10.6 Hz, 6H), 2.33 (d, J = 10.8 Hz, 1H), 2.14 (t, J = 12.1 Hz, 1H), 1.90 (p, J = 6.5 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 158.76, 148.93, 141.67, 136.22, 132.43, 128.06, 122.24, 114.91, 111.72, 103.05, 101.34, 78.17, 65.83, 54.19, 53.84, 52.69, 50.12, 43.09, 42.65, 32.62, 25.95. HRMS (ESI) m/z: calcd for: C26H37N3O4 [M + H]+: 456.2857; found:456.2851.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-3-(4-(3-(4-ethylpiperazin-1-yl)propoxy)phenyl)-N,N-dimethylpropan-1-amine oxalate (11e), Pale-white solid; m.p. 187–189 °C; yield 35.1%; 1H NMR (400 MHz, DMSO-d6) δ 7.32 (d, J = 8.2 Hz, 2H), 6.91 (d, J = 8.2 Hz, 2H), 6.65 (t, J = 8.1 Hz, 1H), 6.50 (dd, J = 14.4, 8.1 Hz, 2H), 5.98 (d, J = 12.5 Hz, 2H), 5.42 (dd, J = 8.3, 4.7 Hz, 1H), 4.05–3.95 (m, 2H), 3.33–3.11 (m, 2H), 3.08 (dt, J = 13.0, 7.4 Hz, 4H), 3.00–2.92 (m, 2H), 2.76 (s, 10H), 2.63 (t, J = 7.4 Hz, 2H), 2.33 (dt, J = 13.9, 7.9 Hz, 1H), 2.21–2.08 (m, 1H), 1.91 (p, J = 6.7 Hz, 2H), 1.16 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 158.74, 148.93, 141.66, 136.23, 132.44, 128.06, 122.25, 114.92, 111.73, 103.06, 101.35, 78.16, 65.78, 54.21, 53.80, 51.00, 50.23, 49.93, 42.65, 32.61, 25.74, 9.77. HRMS (ESI) m/z: calcd for: C27H39N3O4 [M + H]+: 470.3013; found: 470.3017.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-3-(4-(3-(4-isopropylpiperazin-1-yl)propoxy)phenyl)-N,N-dimethylpropan-1-amine oxalate (11f), Pale-white solid; m.p. 160–162 °C; yield 49.3%; 1H NMR (400 MHz, DMSO-d6) δ 7.32 (d, J = 8.4 Hz, 2H), 6.91 (d, J = 8.4 Hz, 2H), 6.65 (t, J = 8.1 Hz, 1H), 6.55–6.50 (m, 1H), 6.50–6.43 (m, 1H), 5.97 (dd, J = 12.1, 1.0 Hz, 2H), 5.42 (dd, J = 8.4, 4.6 Hz, 1H), 3.98 (t, J = 6.2 Hz, 2H), 3.23 (ddt, J = 28.3, 11.8, 5.7 Hz, 2H), 3.12–3.07 (m, 4H), 2.75 (s, 7H), 2.63 (t, J = 7.3 Hz, 2H), 1.91 (p, J = 6.7 Hz, 2H), 1.18 (t, J = 7.2 Hz, 12H). 13C NMR (101 MHz, DMSO-d6) δ 164.66, 158.76, 148.94, 141.68, 132.45, 128.05, 122.24, 114.93, 111.74, 103.06, 101.34, 78.20, 65.85, 56.42, 54.21, 53.86, 50.29, 47.31, 45.88, 42.66, 32.63, 25.93, 17.18, 8.93. HRMS (ESI) m/z: calcd for: C28H41N3O4 [M + H]+: 484.3170; found: 484.3178.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-3-(4-(3-(hexahydrocyclopenta[c]pyrrol-2(1H)-yl)propoxy)phenyl)-N,N-dimethylpropan-1-amine oxalate (11g), Pale-white solid; m.p. 110–112 °C; yield 44.1%; 1H NMR (400 MHz, DMSO-d6) δ 7.33 (d, J = 8.2 Hz, 2H), 6.91 (d, J = 8.2 Hz, 2H), 6.65 (t, J = 8.1 Hz, 1H), 6.50 (dd, J = 13.7, 8.1 Hz, 2H), 5.98 (d, J = 12.4 Hz, 2H), 5.43 (dd, J = 8.4, 4.6 Hz, 1H), 4.06–4.00 (m, 2H), 3.13 (dt, J = 48.0, 6.5 Hz, 4H), 2.74 (s, 10H), 2.33 (d, J = 11.1 Hz, 1H), 2.10 (dt, J = 22.8, 6.0 Hz, 3H), 1.71–1.43 (m, 6H), 1.18 (t, J = 7.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 158.49, 148.93, 141.68, 136.23, 132.68, 128.06, 122.24, 114.93, 111.73, 103.03, 101.35, 78.19, 65.35, 60.23, 58.75, 54.15, 50.94, 42.62, 41.16, 32.61, 31.20, 25.66, 25.03, 21.22, 14.54. HRMS (ESI) m/z: calcd for: C28H38N2O4 [M + H]+: 467.2904; found: 467.2920.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-N,N-dimethyl-3-(3-(3-(pyrrolidin-1-yl)propoxy)phenyl)propan-1-amine oxalate (11h), Pale-white solid; m.p. 104–106 °C; yield 38.9%; 1H NMR (400 MHz, DMSO-d6) δ 7.28 (t, J = 7.9 Hz, 1H), 6.98 (d, J = 7.0 Hz, 2H), 6.86 (d, J = 8.2 Hz, 1H), 6.66 (t, J = 8.1 Hz, 1H), 6.52 (dd, J = 14.6, 8.1 Hz, 2H), 5.99 (d, J = 12.1 Hz, 2H), 5.45 (dd, J = 8.4, 4.1 Hz, 1H), 4.03 (q, J = 7.2 Hz, 2H), 3.32–3.09 (m, 8H), 2.74 (s, 6H), 2.34–2.06 (m, 4H), 1.92 (d, J = 6.5 Hz, 4H). 13C NMR (101 MHz, DMSO-d6) δ 158.86, 148.97, 142.43, 141.76, 136.15, 130.27, 122.32, 118.86, 114.46, 112.58, 111.42, 103.14, 101.43, 78.43, 65.23, 54.14, 53.39, 51.77, 42.69, 32.76, 25.73, 23.17. HRMS (ESI) m/z: calcd for: C25H34N2O4 [M + H]+: 427.2591; found: 427.2586.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-N,N-dimethyl-3-(3-(3-(piperidin-1-yl)propoxy)phenyl)propan-1-amine oxalate (11i), Pale-white solid; m.p. 81–83 °C; yield 32.6%; 1H NMR(400 MHz, DMSO-d6) δ 7.28 (td, J = 7.8, 1.7 Hz, 1H), 7.01–6.94 (m, 2H), 6.86 (dd, J = 8.1, 2.4 Hz, 1H), 6.66 (t, J = 8.1 Hz, 1H), 6.57–6.47 (m, 2H), 5.99 (dd, J = 11.6, 1.0 Hz, 2H), 5.45 (dd, J = 8.4, 4.3 Hz, 1H), 4.09–3.95 (m, 2H), 3.14–3.09 (m, 4H), 2.73 (d, J = 1.5 Hz, 6H), 2.53–2.49 (m, 4H), 2.30 (dd, J = 19.6, 9.4 Hz, 1H), 2.22 –2.05 (m, 3H), 1.73 (p, J = 5.7 Hz, 4H), 1.60–1.45 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 158.85, 148.96, 142.43, 141.76, 136.14, 130.27, 122.32, 118.85, 114.47, 112.58, 111.42, 103.13, 101.43, 78.44, 65.40, 54.15, 53.83, 52.48, 42.68, 32.76, 23.77, 22.93, 21.90. HRMS (ESI) m/z: calcd for: C26H36N2O4 [M + H]+: 441.2748; found: 441.2749.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-N,N-dimethyl-3-(3-(3-morpholinopropoxy)phenyl)propan-1-amine oxalate (11j), Pale-white solid; m.p. 95–98 °C; yield 43.4%; 1H NMR (400 MHz, DMSO-d6) δ 7.28 (t, J = 8.0 Hz, 1H), 7.01–6.95 (m, 2H), 6.88– 6.82 (m, 1H), 6.67 (t, J = 8.1 Hz, 1H), 6.52 (dd, J = 16.1, 8.1 Hz, 2H), 5.99 (d, J = 11.0 Hz, 2H), 5.45 (dd, J = 8.4, 4.2 Hz, 1H), 4.01 (q, J = 7.3, 6.0 Hz, 2H), 3.77–3.69 (m, 4H), 3.17 (dtd, J = 32.2, 11.9, 4.6 Hz, 2H), 2.95–2.82 (m, 6H), 2.77 (s, 6H), 2.35–2.26 (m, 1H), 2.19 (d, J = 11.3 Hz, 1H), 2.06–1.97 (m, 2H). 13C NMR (101 MHz, Chloroform-d) δ 158.87, 148.88, 142.28, 141.64, 136.07, 130.19, 122.23, 118.71, 114.33, 112.54, 111.34, 103.06, 101.33, 78.29, 65.49, 64.58, 54.05, 52.12, 42.59, 32.61, 24.25. HRMS (ESI) m/z: calcd for: C25H34N2O5 [M + H]+: 443.2540; found: 443.2540.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-3-(3-(3-(4-isopropylpiperazin-1-yl)propoxy)phenyl)-N,N-dimethylpropan-1-amine oxalate (11k), Pale-white solid; m.p. 185–186 °C; yield 42.5%; 1H NMR (400 MHz, DMSO-d6) δ 7.27 (t, J = 8.0 Hz, 1H), 6.97 (dd, J = 7.1, 1.5 Hz, 2H), 6.88–6.81 (m, 1H), 6.71–6.62 (m, 1H), 6.52 (ddd, J = 15.0, 8.2, 1.0 Hz, 2H), 5.99 (dd, J = 10.5, 1.0 Hz, 2H), 5.45 (dd, J = 8.5, 4.4 Hz, 1H), 4.00 (q, J = 6.1 Hz, 2H), 3.33–3.08 (m, 4H), 3.06 (d, J = 5.5 Hz, 3H), 2.76 (s, 9H), 2.65 (t, J = 7.4 Hz, 2H), 2.36–2.28 (m, 1H), 2.19 (d, J = 7.2 Hz, 1H), 1.92 (p, J = 6.7 Hz, 2H), 1.19 (d, J = 6.6 Hz, 7H). 13C NMR (101 MHz, Chloroform-d) δ 159.42, 153.04, 142.88, 134.85, 130.80, 128.29, 127.42, 126.70, 126.43, 126.00, 122.54, 121.07, 118.89, 114.33, 112.98, 107.94, 76.97, 66.10, 57.23, 54.75, 54.15, 50.37, 47.56, 43.10, 33.44, 26.00, 17.34. HRMS (ESI) m/z: calcd for: C28H41N3O4 [M + H]+: 484.3170; found: 484.3159.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-3-(4-(3-(dimethylamino)propoxy)phenyl)-N,N-dimethylpropan-1-amine oxalate (11l), Pale-white solid; m.p. 121–122 °C; yield 35.1%;1H NMR (400 MHz, DMSO-d6) δ 7.33 (d, J = 8.2 Hz, 2H), 6.92 (d, J = 8.2 Hz, 2H), 6.65 (t, J = 8.1 Hz, 1H), 6.50 (dd, J = 12.2, 8.1 Hz, 2H), 5.98 (d, J = 12.0 Hz, 2H), 5.43 (dd, J = 8.3, 4.7 Hz, 1H), 4.01 (t, J = 6.0 Hz, 2H), 3.14 (q, J = 7.7 Hz, 3H), 3.05 (dt, J = 12.1, 6.1 Hz, 1H), 2.73 (d, J = 6.1 Hz, 12H), 2.33 (dt, J = 13.5, 7.4 Hz, 1H), 2.20–2.02 (m, 3H). 13C NMR (101 MHz, DMSO-d6) δ 158.50, 148.93, 141.69, 136.22, 132.72, 128.08, 122.25, 114.94, 111.73, 103.04, 101.35, 78.20, 65.33, 54.46, 54.16, 42.63 (d, J = 6.6 Hz), 32.64, 24.36. HRMS (ESI) m/z: calcd for: C23H32N2O4 [M + H]+: 401.2435; found:401.2529.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-3-(4-(3-(diethylamino)propoxy)phenyl)-N,N-dimethylpropan-1-amine oxalate (11m), Pale-white solid; m.p. 98–100 °C; yield 30.3%; 1H NMR (400 MHz, DMSO-d6) δ 7.33 (d, J = 8.1 Hz, 2H), 6.92 (d, J = 8.0 Hz, 2H), 6.69–6.60 (m, 1H), 6.50 (dd, J = 12.2, 8.1 Hz, 2H), 5.98 (d, J = 12.2 Hz, 2H), 5.43 (dd, J = 8.3, 4.8 Hz, 1H), 4.05–4.00 (m, 2H), 3.16–3.03 (m, 8H), 2.73 (s, 6H), 2.37–2.26 (m, 1H), 2.11 (ddq, J = 22.7, 12.3, 6.4, 5.5 Hz, 3H), 1.18 (dd, J = 5.8, 3.3 Hz, 6H). 13C NMR (101 MHz, DMSO-d6) δ 158.48, 148.93, 141.69, 136.24, 132.72, 128.09, 122.25, 114.95, 111.76, 103.03, 101.36, 78.23, 65.30, 60.25, 54.15, 48.12, 46.45, 45.71, 42.62, 32.60, 23.45, 21.21, 14.53, 8.82 (d, J = 2.8 Hz). HRMS (ESI) m/z: calcd for: C25H36N2O4 [M + H]+: 429.2748; found: 429.2752.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-N,N-dimethyl-3-(4-(2-(piperidin-1-yl)ethoxy)phenyl)propan-1-amine oxalate (11n), Pale-white solid; m.p. 116–118 °C; yield 33.2%;1H NMR (400 MHz, DMSO-d6) δ 7.35 (d, J = 8.3 Hz, 2H), 6.96 (d, J = 8.5 Hz, 2H), 6.65 (t, J = 8.1 Hz, 1H), 6.51 (dd, J = 11.1, 8.2 Hz, 2H), 5.98 (d, J = 12.3 Hz, 2H), 5.45 (dd, J = 8.3, 4.6 Hz, 1H), 4.32 (t, J = 4.9 Hz, 2H), 4.03 (q, J = 7.1 Hz, 2H), 3.41 (t, J = 4.9 Hz, 2H), 3.22 (dd, J = 11.8, 4.9 Hz, 1H), 3.10 (dt, J = 11.9, 6.1 Hz, 2H), 2.76 (s, 6H), 1.99 (s, 2H), 1.74 (p, J = 5.9 Hz, 5H), 1.18 (t, J = 7.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 164.86, 157.87, 157.77, 148.93, 141.64, 136.20, 133.20, 128.08, 122.26, 115.09, 111.68, 103.06, 101.35, 78.09, 62.68, 60.23, 55.15, 54.14, 52.95, 42.62, 32.63, 22.85, 21.67, 21.22, 14.54. HRMS (ESI) m/z: calcd for: C25H34N2O4 [M + H]+: 427.2591; found: 427.2587.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-N,N-dimethyl-3-(4-(4-(piperidin-1-yl)butoxy)phenyl)propan-1-amine oxalate (11o), Pale-white solid; m.p. 169–171 °C; yield 42.9%; 1H NMR (400 MHz, DMSO-d6) δ 7.31–7.24 (m, 2H), 6.90–6.82 (m, 2H), 6.59 (d, J = 8.0 Hz, 1H), 6.46 (dd, J = 11.3, 7.9 Hz, 2H), 5.93 (d, J = 11.9 Hz, 2H), 5.37 (dd, J = 8.3, 4.8 Hz, 1H), 3.91 (t, J = 5.8 Hz, 2H), 3.13–2.86 (m, 8H), 2.65 (s, 6H), 2.26 (ddt, J = 13.4, 8.9, 5.3 Hz, 1H), 2.07 (ddt, J = 13.3, 10.3, 4.8 Hz, 1H), 1.69 (dp, J = 16.5, 5.1 Hz, 8H), 1.47 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 165.45, 158.79, 149.01, 141.81, 136.29, 132.63, 128.12, 122.31, 115.00, 111.78, 103.08, 101.41, 78.31, 67.31, 56.12, 54.36, 52.46, 42.93, 32.95, 26.49, 23.04, 22.05, 20.79. HRMS (ESI) m/z: calcd for: C27H38N2O4 [M + H]+: 455.2904; found: 455.2906.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-N,N-dimethyl-3-(4-(3-(2-methylpiperidin-1-yl)propoxy)phenyl)propan-1-amine oxalate (11p), Pale-white solid; m.p. 129–131 °C; yield 33.8%; 1H NMR (400 MHz, DMSO-d6) δ 7.34 (d, J = 8.2 Hz, 2H), 6.92 (d, J = 8.2 Hz, 2H), 6.67 (t, J = 8.1 Hz, 1H), 6.52 (dd, J = 12.0, 8.1 Hz, 2H), 5.98 (d, J = 12.2 Hz, 2H), 5.43 (dd, J = 8.3, 4.7 Hz, 1H), 4.07–3.99 (m, 2H), 3.36 (d, J = 11.7 Hz, 2H), 3.21–2.94 (m, 4H), 2.81 (t, J = 12.1 Hz, 2H), 2.69 (s, 6H), 2.31 (d, J = 11.8 Hz, 1H), 2.10 (dt, J = 16.0, 10.4 Hz, 3H), 1.75 (d, J = 13.7 Hz, 2H), 1.60 (d, J = 13.0 Hz, 1H), 1.38 (q, J = 12.4 Hz, 2H), 1.17 (d, J = 6.4 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 158.63, 148.61, 143.77, 136.84, 135.32, 128.78, 125.95, 123.95, 116.18, 114.60, 111.53, 104.55, 101.75, 79.32, 66.55, 56.81, 54.29, 53.76, 50.46, 44.98, 34.61, 33.87, 27.53, 25.78, 23.86, 18.36. HRMS (ESI) m/z: calcd for: C27H38N2O4 [M + H]+: 455.2904; found: 455.2908.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-N,N-dimethyl-3-(4-(3-(3-methylpiperidin-1-yl)propoxy)phenyl)propan-1-amine oxalate (11q), Pale-white solid; m.p. 130–132 °C; yield 37.6%; 1H NMR (400 MHz, DMSO-d6) δ 7.33 (d, J = 8.2 Hz, 2H), 6.91 (d, J = 8.2 Hz, 2H), 6.65 (t, J = 8.1 Hz, 1H), 6.50 (dd, J = 12.0, 8.1 Hz, 2H), 5.98 (d, J = 12.2 Hz, 2H), 5.43 (dd, J = 8.3, 4.7 Hz, 1H), 4.04–4.00 (m, 2H), 3.36 (dd, J = 24.3, 11.6 Hz, 2H), 3.11 (tdd, J = 23.7, 12.0, 7.3 Hz, 4H), 2.73 (s, 8H), 2.46 (d, J = 11.7 Hz, 1H), 2.32 (d, J = 9.8 Hz, 1H), 2.12 (q, J = 7.2, 6.6 Hz, 3H), 1.89 (d, J = 17.1 Hz, 1H), 1.76–1.69 (m, 2H), 1.05 (dd, J = 11.8, 4.7 Hz, 1H), 0.89 (d, J = 6.5 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 158.49, 148.93, 141.69, 136.22, 132.72, 128.07, 122.24, 114.94, 111.71, 103.04, 101.34, 78.18, 65.46, 60.23, 57.97, 54.21, 53.86, 52.01, 42.73 (d, J = 2.2 Hz), 32.73, 30.52, 23.93, 22.69, 21.24, 19.06, 14.56. HRMS (ESI) m/z: calcd for: C27H38N2O4 [M + H]+: 455.2904; found: 455.2903.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-N,N-dimethyl-3-(4-(3-(4-methylpiperidin-1-yl)propoxy)phenyl)propan-1-amine oxalate (11r), Pale-white solid; m.p. 126–128 °C; yield 42.8%; 1H NMR (400 MHz, DMSO-d6) δ 7.32 (d, J = 8.1 Hz, 2H), 6.91 (d, J = 8.1 Hz, 2H), 6.72–6.60 (m, 1H), 6.50 (dd, J = 12.9, 8.1 Hz, 2H), 5.97 (d, J = 12.0 Hz, 2H), 5.42 (t, J = 6.6 Hz, 1H), 4.07–3.99 (m, 2H), 3.36 (d, J = 11.7 Hz, 2H), 3.21–2.94 (m, 4H), 2.81 (t, J = 12.1 Hz, 2H), 2.69 (s, 6H), 2.31 (d, J = 11.8 Hz, 1H), 2.10 (dt, J = 16.0, 10.4 Hz, 3H), 1.75 (d, J = 13.7 Hz, 2H), 1.60 (d, J = 13.0 Hz, 1H), 1.38 (q, J = 12.4 Hz, 2H), 0.92 (d, J = 6.4 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 158.49, 148.93, 141.73, 136.22, 132.78, 128.06, 122.24, 114.93, 111.69, 103.02, 101.33, 78.21, 65.49, 60.23, 54.31, 53.65, 42.92, 32.94, 28.56, 24.18, 21.29 (d, J = 11.2 Hz), 14.56. HRMS (ESI) m/z: calcd for: C27H38N2O4 [M + H]+: 455.2904; found: 455.2912.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-3-(4-(3-(3,5-dimethylpiperidin-1-yl)propoxy)phenyl)-N,N-dimethylpropan-1-amine oxalate (11s), Pale-white solid; m.p. 140–142 °C; yield 39.7%;1H NMR (400 MHz, DMSO-d6) δ 7.33 (d, J = 7.8 Hz, 2H), 6.91 (d, J = 8.0 Hz, 2H), 6.65 (t, J = 8.0 Hz, 1H), 6.50 (dd, J = 11.9, 8.1 Hz, 2H), 6.00– 5.90 (m, 2H), 5.44 (d, J = 6.8 Hz, 1H), 4.03 (dd, J = 12.6, 6.4 Hz, 3H), 3.36 (t, J = 8.3 Hz, 3H), 3.14 (s, 2H), 2.75 (d, J = 8.8 Hz, 6H), 2.42 (d, J = 12.1 Hz, 2H), 2.15 (s, 2H), 1.92 (s, 1H), 1.73 (d, J = 12.7 Hz, 1H), 1.21 (dt, J = 19.7, 4.7 Hz, 2H), 0.88 (d, J = 6.0 Hz, 7H), 0.78 (q, J = 12.3 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ158.63, 148.60, 143.77, 137.03, 135.31, 127.96, 126.65, 123.95, 115.24, 111.53, 104.30, 101.75, 79.40, 66.45, 60.01, 54.25, 44.86, 39.01, 34.61, 29.12, 27.40, 18.64. HRMS (ESI) m/z: calcd for: C28H40N2O4 [M + H]+: 469.3061; found:469.3064.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-3-(4-(3-(4-fluoropiperidin-1-yl)propoxy)phenyl)-N,N-dimethylpropan-1-amine oxalate (11t), Pale-white solid; m.p. 134–136 °C; yield 50.1%;1H NMR (400 MHz, DMSO-d6) δ 7.31 (t, J = 10.2 Hz, 2H), 6.91 (d, J = 8.4 Hz, 2H), 6.64 (d, J = 8.2 Hz, 1H), 6.50 (dd, J = 13.2, 8.1 Hz, 2H), 5.98 (d, J = 12.1 Hz, 2H), 5.42 (m, 1H), 5.12-4.89 (m,1H),4.02 (m, 2H),3.26–2.96 (m, 8H), 2.74 (s, 6H), 2.46 (d, J = 10.7 Hz, 1H), 2.26 (d, J = 6.7 Hz, 1H), 2.06 (p, J = 5.9 Hz, 2H), 1.73 –1.66 (m, 4H). 13C NMR (101 MHz, DMSO-d6) δ158.60, 148.51, 143.14, 136.07, 134.84, 128.51,126.07, 123.30, 115.74, 113.87, 111.71, 104.21, 100.91, 89.67, 87.88, 79.15, 66.16, 54.34, 50.01, 44.95, 34.71, 30.10, 27.28. HRMS (ESI) m/z: calcd for: C26H35FN2O4 [M + H]+: 459.2654; found: 459.2661.
- 3-(benzo[d][1,3]dioxol-4-yloxy)-3-(4-(3-(4-chloropiperidin-1-yl)propoxy)phenyl)-N,N-dimethylpropan-1-amine oxalate (11u), Pale-white solid; m.p. 145–146 °C; yield 39.4%; 1H NMR (400 MHz, DMSO-d6) δ 7.33 (d, J = 7.4 Hz, 2H), 6.92 (d, J = 7.5 Hz, 2H), 6.65 (t, J = 8.0 Hz, 1H), 6.51 (dd, J = 14.1, 8.1 Hz, 2H), 5.96 (t, J = 14.2 Hz, 2H), 5.46–5.37 (m, 1H), 3.99 (d, J = 2.7 Hz, 2H), 3.26 (ddd, J = 29.4, 14.6, 4.7 Hz, 4H), 3.17–2.97 (m, 6H), 2.76 (s, 6H), 2.35–2.24 (m, 3H), 2.13–2.06 (m, 2H), 2.04–1.96 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 158.62, 148.60, 143.77, 136.91, 135.31, 127.98, 127.70, 123.95, 115.24, 111.72, 104.30, 101.74, 79.32, 66.24, 56.89, 54.47, 54.18, 50.87, 44.98, 34.61, 33.45, 27.28. HRMS (ESI) m/z: calcd for: C26H35ClN2O4 [M + H]+: 475.2358; found:475.2364.
- 1-(3-(4-(1-(benzo[d][1,3]dioxol-4-yloxy)-3-(dimethylamino)propyl)phenoxy)propyl)piperidine-4-carbonitrile oxalate (11v), Pale-white solid; m.p. 148–151 °C; yield 18.3%; 1H NMR (500 MHz, DMSO-d6) δ 7.33 (d, J = 8.2 Hz, 2H), 6.91 (d, J = 8.2 Hz, 2H), 6.65 (t, J = 8.1 Hz, 1H), 6.51 (dd, J = 14.6, 8.1 Hz, 2H), 5.98 (d, J = 12.4 Hz, 2H), 5.43 (t, J = 6.5 Hz, 1H), 3.99 (t, J = 6.1 Hz, 2H), 3.24–3.15 (m, 2H), 3.05 (td, J = 26.1, 23.9, 13.3 Hz, 6H), 2.76 (s, 6H), 2.36–2.29 (m, 1H), 2.20–2.01 (m, 5H), 1.96 (d, J = 11.2 Hz, 2H), 1.27–1.16 (m, 1H). 13C NMR (101 MHz, DMSO-d6) δ158.59, 148.65, 143.73, 137.13, 135.30, 127.71, 123.92, 121.09, 115.23, 111.43, 104.55, 101.65, 79.42, 66.14, 54.39, 52.42, 44.86, 34.61, 27.28, 26.49, 25.91. HRMS (ESI) m/z: calcd for: C27H35N3O4 [M + H]+: 466.2700; found: 466.2739.
3.4. Molecular Docking Study
3.5. Biological Studies
Ethics Statement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marwaha, S.; Palmer, E.; Suppes, T.; Cons, E.; Young, A.H.; Upthegrove, R. Novel and emerging treatments for major depression. Lancet 2023, 401, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.; Li, C.; Duan, S.; Ji, S.; Xu, Y.; Mao, Y.; Wang, H.; Tian, J. Epigenetic Mechanism of 5-HT/NE/DA Triple Reuptake Inhibitor on Adult Depression Susceptibility in Early Stress. Mice. Front. Pharmacol. 2022, 13, 848251. [Google Scholar] [CrossRef] [PubMed]
- Gonda, X.; Dome, P.; Neill, J.C.; Tarazi, F.I. Novel antidepressant drugs: Beyond monoamine targets. CNS Spectr. 2023, 28, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Stocking, E.M.; Miller, J.M.; Barbier, A.J.; Wilson, S.J.; Boggs, J.D.; McAllister, H.M.; Wu, J.; Lovenberg, T.W.; Carruthers, N.I.; Wolin, R.L. Synthesis and biological evaluation of diamine-based histamine H3 antagonists with serotonin reuptake inhibitor activity. Bioorg. Med. Chem. Lett. 2007, 17, 3130–3135. [Google Scholar] [CrossRef] [PubMed]
- Bang-Andersen, B.; Ruhland, T.; Jørgensen, M.; Smith, G.; Frederiksen, K.; Jensen, K.G.; Zhong, H.; Nielsen, S.M.; Hogg, S.; Mørk, A.; et al. Discovery of 1-[2-(2,4-dimethylphenylsulfanyl) phenyl] piperazine (Lu AA21004): A novel multimodal compound for the treatment of major depressive disorder. J. Med. Chem. 2011, 54, 3206–3221. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, D.; Kang, S.Y.; Park, W.K.; Kim, H.J.; Jung, M.E.; Son, E.J.; Pae, A.N.; Kim, J.; Lee, J. Arylpiperazine-containing pyrimidine 4-carboxamide derivatives targeting serotonin 5-HT2A, 5-HT2C, and the serotonin transporter as a potential antidepressant. Bioorg. Med. Chem. Lett. 2010, 20, 6439–6442. [Google Scholar] [CrossRef]
- Subbaiah, M.A.M. Triple Reuptake Inhibitors as Potential Therapeutics for Depression and Other Disorders: Design Paradigm and Developmental Challenges. J. Med. Chem. 2018, 61, 2133–2165. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Singh, K.; Bhatia, R.; Kumar, B.; Singh, G.; Monga, V. Design Strategies, Chemistry and Therapeutic Insights of Multi-target Directed Ligands as Antidepressant Agents. Curr. Neuropharmacol. 2022, 20, 1329–1358. [Google Scholar] [CrossRef]
- Ma, H.G.; Huang, B.S.; Zhang, Y. Recent advances in multi-target directed ligands targeting G-protein-coupled receptors. Drug Discov. Today. 2020, 25, 1682–1692. [Google Scholar] [CrossRef]
- Reinhold, J.A.; Mandos, L.A.; Lohoff, F.W.; Rickels, K. Evidence for the use of vilazodone in the treatment of major depressive disorder. Expert. Opin. Pharmacother. 2012, 13, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Katona, C.L.; Katona, C.P. New generation multi-modal antidepressants: Focus on vortioxetine for major depressive disorder. Neuropsychiatr. Dis. Treat. 2014, 10, 349–354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kononoff, V.J.; Nuutinen, S.; Tuominen, M.; Panula, P. Histamine H3 receptor regulates sensorimotor gating and dopaminergic signaling in the striatum. J. Pharmacol. Exp. Ther. 2016, 357, 264–272. [Google Scholar] [CrossRef]
- Łażewska, D.; Kieć-Kononowicz, K. New developments around histamine H3 receptor antagonists/inverse agonists: A patent review (2010–present). Expert. Opin. Ther. Pat. 2014, 24, 89–111. [Google Scholar] [CrossRef]
- Peng, X.; Yang, L.; Liu, Z.; Lou, S.; Mei, S.; Li, M.; Chen, Z.; Zhang, H. Structural basis for recognition of antihistamine drug by human histamine receptor. Nat. Commun. 2022, 13, 6105. [Google Scholar] [CrossRef]
- Qian, H.; Shu, C.; Xiao, L.; Wang, G. Histamine and histamine receptors: Roles in major depressive disorder. Front. Psychiatry 2022, 13, 825591. [Google Scholar] [CrossRef]
- Gao, Z.; Hurst, W.J.; Czechtizky, W.; Hall, D.; Moindrot, N.; Nagorny, R.; Pichat, P.; Stefany, D.; Hendrix, J.A.; George, P.G. Identification and profiling of 3,5-dimethyl-isoxazole-4-carboxylic acid [2-methyl-4-((2S,3’S)-2-methyl-[1,3’]bipyrrolidinyl-1’-yl)phenyl] amide as histamine H3 receptor antagonist for the treatment of depression. Bioorg. Med. Chem. Lett. 2013, 23, 6269–6273. [Google Scholar] [CrossRef] [PubMed]
- Barbier, A.J.; Aluisio, L.; Lord, B.; Qu, Y.; Wilson, S.J.; Boggs, J.D.; Bonaventure, P.; Miller, K.; Fraser, I.; Dvorak, L.; et al. Pharmacological characterization of JNJ-28583867, a histamine H3 receptor antagonist and serotonin reuptake inhibitor. Eur. J. Pharmacol. 2007, 576, 43–54. [Google Scholar] [CrossRef]
- Ly, K.S.; Letavic, M.A.; Keith, J.M.; Miller, J.M.; Stocking, E.M.; Barbier, A.J.; Bonaventure, P.; Lord, B.; Jiang, X.; Boggs, J.D.; et al. Synthesis and biological activity of piperazine and diazepane amides that are histamine H3 antagonists and serotonin reuptake inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 39–43. [Google Scholar] [CrossRef]
- Letavic, M.A.; Keith, J.M.; Jablonowski, J.A.; Stocking, E.M.; Gomez, L.A.; Ly, K.S.; Miller, J.M.; Barbier, A.J.; Bonaventure, P.; Boggs, J.D.; et al. Novel tetrahydroisoquinolines are histamine H3 antagonists and serotonin reuptake inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 1047–1051. [Google Scholar] [CrossRef]
- Xu, X.; Wei, Y.; Guo, Q.; Zhao, S.; Liu, Z.; Xiao, T.; Liu, Y.; Qiu, Y.; Hou, Y.; Zhang, G.; et al. Pharmacological Characterization of H05, a Novel Serotonin and Noradrenaline Reuptake Inhibitor with Moderate 5-HT2A Antagonist Activity for the Treatment of Depression. J. Pharmacol. Exp. Ther. 2018, 365, 624–635. [Google Scholar] [CrossRef] [PubMed]
- de Biase, S.; Pellitteri, G.; Gigli, G.L.; Valente, M. Evaluating pitolisant as a narcolepsy treatment option. Expert. Opin. Pharmacother. 2021, 22, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Weisler, R.H.; Pandina, G.J.; Daly, E.J.; Cooper, K.; Gassmann-Mayer, C. Randomized clinical study of a histamine H3 receptor antagonist for the treatment of adults with attention-deficit hyperactivity disorder. CNS Drugs. 2012, 26, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Panayi, F.; Sors, A.; Bert, L.; Martin, B.; Rollin-Jego, G.; Billiras, R.; Carrié, I.; Albinet, K.; Danober, L.; Rogez, N.; et al. In vivo pharmacological profile of S 38093, a novel histamine H3 receptor inverse agonist. Eur. J. Pharmacol. 2017, 803, 1–10. [Google Scholar] [CrossRef]
- Herring, W.J.; Wilens, T.E.; Adler, L.A.; Baranak, C.; Liu, K.; Snavely, D.B.; Lines, C.R.; Michelson, D. Randomized controlled study of the histamine H3 inverse agonist MK-0249 in adult attention-deficit/hyperactivity disorder. J. Clin. Psychiatry 2012, 73, 891–898. [Google Scholar] [CrossRef]
- Raddatz, R.; Hudkins, R.L.; Mathiasen, J.R.; Gruner, J.A.; Flood, D.G.; Aimone, L.D.; Le, S.; Schaffhauser, H.; Duzic, E.; Gasior, M.; et al. CEP-26401 (irdabisant), a potent and selective histamine H₃ receptor antagonist/inverse agonist with cognition-enhancing and wake-promoting activities. J. Pharmacol. Exp. Ther. 2012, 340, 124–133. [Google Scholar] [CrossRef]
- Gill, H.; Gill, B.; El-Halabi, S.; Chen-Li, D.; Lipsitz, O.; Rosenblat, J.D.; Van Rheenen, T.E.; Rodrigues, N.B.; Mansur, R.B.; Majeed, A.; et al. Antidepressant Medications and Weight Change: A Narrative Review. Obesity 2020, 28, 2064–2072. [Google Scholar] [CrossRef]
- Calvi, A.; Fischetti, I.; Verzicco, I.; Belvidere Murri, M.; Zanetidou, S.; Volpi, R.; Coghi, P.; Tedeschi, S.; Amore, M.; Cabassi, A. Antidepressant Drugs Effects on Blood Pressure. Front. Cardiovasc. Med. 2021, 8, 704281. [Google Scholar] [CrossRef]
- Stahl, S.M. Selective Histamine H1 Antagonism: Novel Hypnotic and Pharmacologic Actions Challenge Classical Notions of Antihistamines. CNS Spectr. 2008, 13, 1027–1038. [Google Scholar] [CrossRef]
- Aronow, W.S.; Shamliyan, T.A. Effects of antidepressants on QT interval in people with mental disorders. Arch. Med. Sci. 2020, 16, 727–741. [Google Scholar] [CrossRef]
- Lewis, N.C.; Ainslie, P.N.; Atkinson, G.; Jones, H.; Grant, E.J.; Lucas, S.J. Initial orthostatic hypotension and cerebral blood flow regulation: Effect of α1-adrenoreceptor activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R147–R154. [Google Scholar] [CrossRef]
- Moorthy, N.S.N.; Ramos, M.J.; Fernandes, P.A. Human ether-a-go-go-related gene channel blockers and its structural analysis for drug design. Curr. Drug Targets 2013, 14, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research Approval Package for: Application Number 21-427. Pharmacology Review(s) #3. pp. 29–45. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/021427_s000_Cymbalta_Pharmr_P2.pdf (accessed on 3 November 2024).
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef] [PubMed]
- Petit-Demouliere, B.; Chenu, F.; Bourin, M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology 2005, 177, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.A.; Green, E.M.; Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 2016, 532, 334–339. [Google Scholar] [CrossRef]
- Ji, W.; Miao, A.; Liang, K.; Liu, J.; Qi, Y.; Zhou, Y.; Duan, X.; Sun, J.; Lai, L.; Wu, J.X. Substrate binding and inhibition mechanism of norepinephrine transporter. Nature 2024, 633, 473–479. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, K.; Dou, F.; Hao, C.; Zhang, Y.; Cao, X.; Gao, L.; Xiong, J.; Liu, X.; Liu, B.F.; et al. Isoquinolinone derivatives as potent CNS multi-receptor D2/5-HT1A/5-HT2A/5-HT6/5-HT7 agents: Synthesis and pharmacological evaluation. Eur. J. Med. Chem. 2020, 207, 112709. [Google Scholar] [CrossRef]
- Marlon, C.; Gin, H.; Lawrence, A.B.; Zhan, C.C.; Erica, J.G.; Madhavi, P.; Marina, S.; Arlene, M.; Tracy, C.; Jill, W.; et al. Pharmacological characterization of A-960656, a histamine H3 receptor antagonist with efficacy in animal models of osteoarthritis and neuropathic pain. Eur. J. Pharm. 2012, 684, 87–94. [Google Scholar]
- Fabrizio, M.; Paolo, C.; Roberto, A.; Roberto, B.; Barbara, B.; Michela, B.; Letizia, B.; Giorgio, B.; Simone, B.; Anna, C.; et al. 1-(Aryl)-6-[alkoxyalkyl]-3-azabicyclo[3.1.0]hexanes and 6-(Aryl)-6-[alkoxyalkyl]-3-azabicyclo[3.1.0] hexanes: A New Series of Potent and Selective Triple Reuptake Inhibitors. J. Med. Chem. 2010, 53, 2534–2551. [Google Scholar]
- Frecentese, F.; Fiorino, F.; Perissutti, E.; Severino, B.; Magli, E.; Esposito, A.; De Angelis, F.; Massarelli, P.; Nencini, C.; Viti, B.; et al. Efficient microwave combinatorial synthesis of novel indolic arylpiperazine derivatives as serotoninergic ligands. Eur. J. Med. Chem. 2010, 45, 752–759. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Y.; Chen, Y.; Oiu, Y.; Yu, M.; Xu, X.; Liu, X.; Liu, B.F.; Zhang, G. Synthesis and biological evaluation of fused tricyclic heterocycle piperazine (piperidine) derivatives as potential Multireceptor atypical Antipsychotics. J. Med. Chem. 2018, 61, 10017–10039. [Google Scholar] [CrossRef]
- Kagermeier, N.; Werner, K.; Keller, M.; Baumeister, P.; Bernhardt, G.; Seifert, R.; Buschauer, A. Dimeric carbamoylguanidine-type histamine H2 receptor ligands: A new class of potent and selective agonists. Bioorg Med. Chem. 2015, 23, 3957–3969. [Google Scholar] [CrossRef]
- Tayebatim, S.K.; Codini, M.; Gallai, V.; Mannino, F.; Parnetti, L.; Ricci, A.; Sarchielli, P.; Amenta, F. Radioligand binding assay of M1-M5 muscarinic cholinergic receptor subtypes in human peripheral blood lymphocytes. J. Neuroimmunol. 1999, 99, 224–229. [Google Scholar] [CrossRef]
- Cao, X.; Chen, Y.; Zhang, Y.; Qiu, Y.; Yu, M.; Xu, X.; Liu, X.; Liu, B.F.; Zhang, G. Synthesis and biological evaluation of new 6-hydroxypyridazinone benzisoxazoles: Potential multi-receptor-targeting atypical antipsychotics. Eur. J. Med. Chem. 2016, 124, 713–728. [Google Scholar] [CrossRef]
- Buchstaller, H.P.; Siebert, C.D.; Steinmetz, R.; Frank, I.; Berger, M.L.; Gottschlich, R.; Leibrock, J.; Krug, M.; Steinhilber, D.; Noe, C.R. Synthesis of Thieno[2,3-b]Pyridinones Acting as Cytoprotectants and as Inhibitors of [3H]Glycine Binding to the N-Methyl-D-aspartate (NMDA) Receptor. J. Med. Chem. 2006, 49, 864–871. [Google Scholar] [CrossRef]
- Bruce, R.D. An up-and-down procedure for acute toxicity testing. Fundam. Appl. Toxicol. 1985, 5, 151–157. [Google Scholar] [CrossRef]
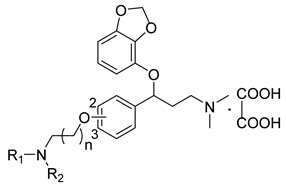 | ||||
| Compound | Structure | Receptor Affinity Ki ± SEM (nM) a | ||
| SERT | NET | H3 | ||
| 11a |  | 10.3 ± 0.9 | 19.2 ±2.3 | 4.2 ± 0.3 |
| 11b |  | 7.8 ± 0.9 | 10.5 ± 1.2 | 5.5 ± 0.4 |
| 11c | 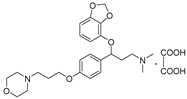 | 43.2 ± 3.8 | 32.6 ± 2.7 | 26.2 ± 2.1 |
| 11d | 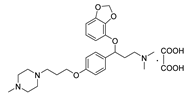 | 52.3 ± 2.8 | 86.8 ± 9.1 | 278.2 ± 30.1 |
| 11e |  | 39.6 ± 3.3 | 66.9 ± 5.1 | 351.5 ± 32.3 |
| 11f | 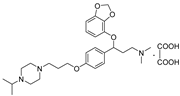 | 35.7 ± 3.1 | 57.9 ± 6.4 | 233.9 ± 21.8 |
| 11g | 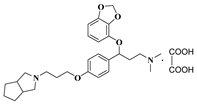 | 31.3 ± 4.0 | 48.2 ± 4.2 | 10.6 ± 1.7 |
| 11h |  | 191.6 ± 18.8 | 144.2 ± 13.5 | 202.4 ± 23.6 |
| 11i |  | 236.4 ± 26.1 | 105.4 ± 9.8 | 118.6 ± 10.5 |
| 11j |  | 465.4 ± 39.8 | 152.9 ± 12.7 | 170.3 ± 15.9 |
| 11k |  | 163.2 ± 17.6 | 111 ± 10.3 | 421.5 ± 39.4 |
| 11l | 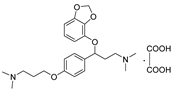 | 17.1 ± 1.6 | 114.9 ± 12.3 | 958.1 ± 87.6 |
| 11m |  | 28.4 ± 11.3 | 85.2 ± 9.1 | 744.6 ± 68.3 |
| 11n | 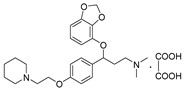 | 26.1 ± 2.3 | 37.3 ± 4.2 | 1021.5 ± 98.3 |
| 11o | 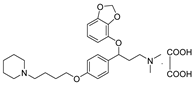 | 49.2 ± 4.8 | 64.6 ± 5.5 | 835.3 ± 79.1 |
| 11p | 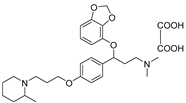 | 20.6 ± 1.9 | 35.4 ± 3.2 | 21.8 ± 2.3 |
| 11q | 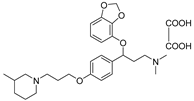 | 12.1 ± 1.6 | 20.7 ± 2.1 | 3.2 ± 0.2 |
| 11r | 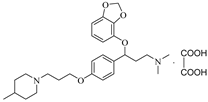 | 27.1 ± 1.6 | 38.3 ± 3.5 | 11.5 ± 1.0 |
| 11s | 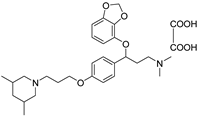 | 30.3 ± 2.6 | 25.1 ± 2.8 | 18.2 ± 1.9 |
| 11t | 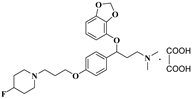 | 34.2 ± 2.8 | 42.5 ± 4.6 | 88.6 ± 9.1 |
| 11u | 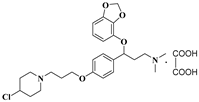 | 39.5 ± 4.0 | 50.2 ± 4.9 | 133.7 ± 3.0 |
| 11v | 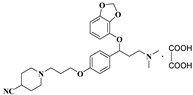 | 60.5 ± 5.7 | 48.6 ± 6.4 | 115.4 ± 12.8 |
| Duloxetine | 9.2 ± 1.1 | 31.5 ± 3.4 | >1000 | |
| Pitolisant | >1000 | >1000 | 0.58 ± 0.03 | |
| Compound | Receptor Affinity Ki ± SEM (nM) a | (IC50, nM) | |
|---|---|---|---|
| α1 | H1 | hERG | |
| 11a | 428.4 ± 35.3 | 241.8 ± 30.5 | 819.1 ± 90.4 |
| 11b | 1165.2 ± 125.8 | 896.9 ± 96.3 | 1000.8 ± 112.1 |
| 11q | 306.7 ± 28.1 | 624.6 ± 57.7 | 779.6 ± 68.5 |
| Duloxetine | 1008.3 ± 97.8 | 1122.6 ± 121.5 | 1407.7 ± 153.8 |
| Receptor | Compd | Activation (10 μM, %) (n = 3) | EC50 (nM) | Inhibition (10 μM, %) (n = 3) | IC50 (nM) |
|---|---|---|---|---|---|
| SERT | Citalopram | 101.2 ± 1.8 | 8.9 | ||
| 11b | 4.4 ± 0.3 | 98.5 ± 3.0 | 20.1 | ||
| NET | Nisoxetine | 99.8 ± 1.9 | 37.4 | ||
| 11b | 2.7 ± 0.2 | 100.1 ± 1.2 | 49.6 | ||
| H3 | Histamine | 101.3 ± 2.7 | 14.1 | ||
| Pitolisant | 99.3 ± 2.5 | 2.45 | |||
| 11b | 5.3 ± 0.6 | 101.5 ± 2.0 | 0.72 |
| Dose (mg/kg) | Cmax(ng/mL) | Tmax(h) | T1/2(h) | AUC(0–t)(ng·h/mL) | AUC(0–∞) (ng·h/mL) | MRTlast (h) | F (%) |
|---|---|---|---|---|---|---|---|
| 2 (iv) | 224.4 | 0.08 | 1.3 | 261.4 | 265.1 | 1.2 | - |
| 20 (po) | 133.9 | 2.0 | 2.6 | 941.8 | 944.1 | 5.4 | 35.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Ren, L.; Wang, D.; Luo, J.; Xu, C.; Feng, J.; Qiu, Y.; Xu, X.; Chen, G. Amphetamine Derivatives as Potent Central Nervous System Multitarget SERT/NET/H3 Agents: Synthesis and Biological Evaluation. Molecules 2024, 29, 5240. https://doi.org/10.3390/molecules29225240
Li Q, Ren L, Wang D, Luo J, Xu C, Feng J, Qiu Y, Xu X, Chen G. Amphetamine Derivatives as Potent Central Nervous System Multitarget SERT/NET/H3 Agents: Synthesis and Biological Evaluation. Molecules. 2024; 29(22):5240. https://doi.org/10.3390/molecules29225240
Chicago/Turabian StyleLi, Quxiang, Lili Ren, Dongli Wang, Junyong Luo, Changda Xu, Jian Feng, Yufan Qiu, Xiangqing Xu, and Guoguang Chen. 2024. "Amphetamine Derivatives as Potent Central Nervous System Multitarget SERT/NET/H3 Agents: Synthesis and Biological Evaluation" Molecules 29, no. 22: 5240. https://doi.org/10.3390/molecules29225240
APA StyleLi, Q., Ren, L., Wang, D., Luo, J., Xu, C., Feng, J., Qiu, Y., Xu, X., & Chen, G. (2024). Amphetamine Derivatives as Potent Central Nervous System Multitarget SERT/NET/H3 Agents: Synthesis and Biological Evaluation. Molecules, 29(22), 5240. https://doi.org/10.3390/molecules29225240







